- Skip to content
- Skip to navigation
- [email protected]
- +44(0) 161 906 7500


Cutting-edge clinical research for adults and children
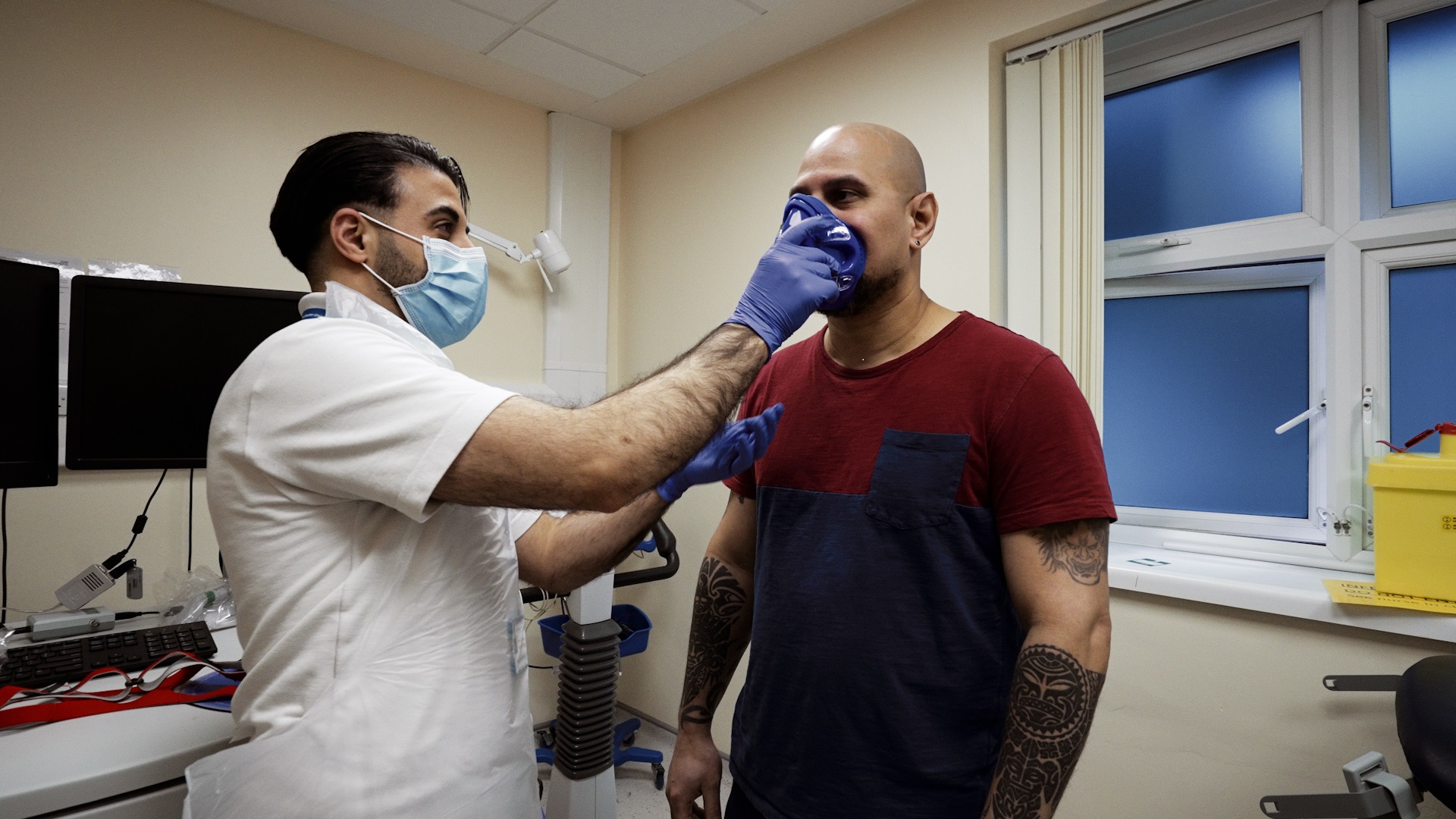
World-class clinical research facilities
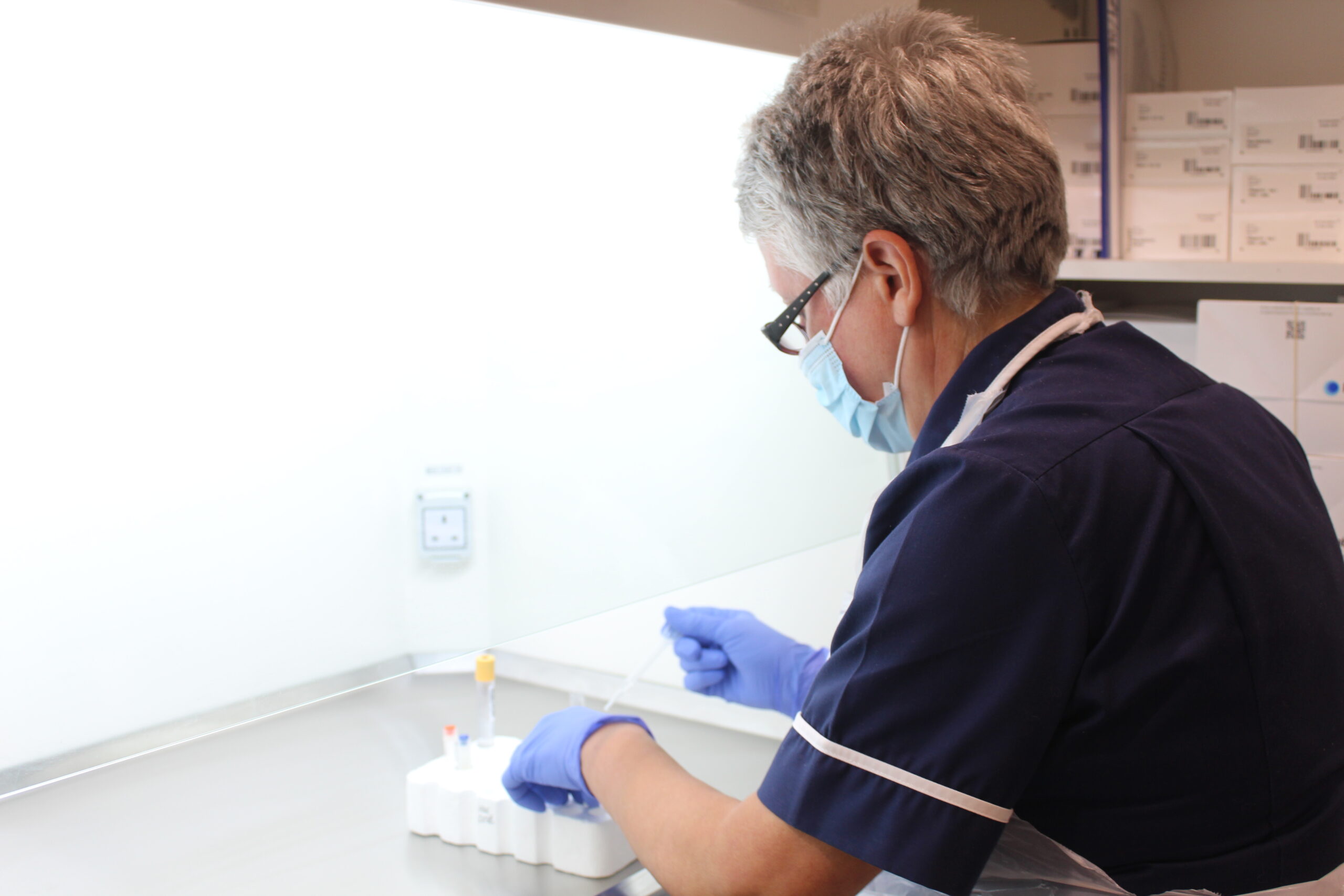
Involving the people of Greater Manchester in clinical research
About manchester crf.
“A world-class integrated Clinical Research Facility that enhances translation of scientific advances through experimental medicine research, and provides opportunities for people of all ages and backgrounds across Greater Manchester to take part in research”
Our research, our impact
Transforming the lives of people with cystic fibrosis: CF modulator therapies, from trial to patient.
ESCAPE: study of COVID-19 seroprevalence

Strategic partnerships: AvroBio

Addressing health inequalities through Manchester CRF infrastructure
Be part of research.

Be Part of Research
Research changes lives but it can only happen thanks to people who volunteer for studies. Whether you are living with a condition or simply want to make a difference you can help.
Manchester CRF is dedicated to providing opportunities for people of all ages and backgrounds to take part in, and helps shape and design, clinical research.

MAC Clinical Research is a full-service, global, pharmaceutical contract research organization (CRO), with science at the heart of everything we do. Read More…
- MAC Leadership
- Our History
- Working Together
- Corporate Social Responsibility
The Home of Clinical Trials
Effective solutions to successfully deliver your clinical trials.


Clinical Research Services
- Project Management
- Clinical Operations
- Clinical Trial Feasibility
- Pharmacovigilance
- Regulatory Affairs
- Vendor Management
- Digital Solutions
- Medical Monitoring
Site & Patient Services
- Participant Recruitment
- Participant Retention
- Global Investigator Sites
- Sleep Laboratory
- Laboratory Services
- Early Phase Unit
- Psychedelic Testing Suites
- Dedicated Research Sites
- Memory Assessment Research Clinics
Pharmaceutical Services
- Nationwide Logistical Coverage

Methods & Technology
MAC conduct clinical trials for most of the worlds leading pharma and biotechs. Read More…
Scientific Solutions
We offer an array of scientific solutions to support any clinical trial. Read More…
- Human Models of Disease
- Clinical Assessments and Procedures
- Sleep Assessments
- Psychedelics
- Training Services
- Quality Assurance
- Adaptive Trials
- Remote Clinical Trial Support

Strategic Consulting
MAC’s flexible strategic consulting can support your project at any stage. Read More…

Therapeutic Expertise
- Therapeutic Areas
- Case Studies
- Timeline Recovery

- Meetings & Conferences
- Resource Library
Make a Difference
- Participate In A Clinical Trial

MHRA Accredited Phase 1 Unit
Browse menu.
The MAC Clinical Research Early Phase Unit (EPU) is an MHRA accredited Phase 1 centre located within and supported by the Manchester University Foundation Trust. Our EPU conducts First in Human (FIH) studies in both patients and healthy volunteers, working across a whole range of therapeutic areas.
Our experience and expertise in participant recruitment for studies conducted at our EPU showcases our ability to conduct large, early-phase studies in specific patient populations, where in-patient stays and safety monitoring are required.
Safety is our watchword, and our spacious unit was built with this in mind. We are situated in a building dedicated to the advancement of medical science, equipped with a state-of-the-art Mortara Cardiac Telemetry system, and can send 24-hour data online we also provide prompt review of 24-hour Holter monitoring, with direct access to a cardiology team if there are any anomalies.

Our staff are our strength, and our EPU is approved by the Faculty of Pharmaceutical Medicine (FPM) as a training centre for physicians who are studying to become accredited FIH Investigators. We have 7 on-site physicians Manchester, as well as senior nurses with extensive experience in clinical research. All of our medical and study staff are supported by a team of Clinical Trial Assistants (CTAs), most of whom have or are pursuing Masters’ or Doctorate degrees or equivalent training.
Clinical research continues to become more scientifically complex and more tightly controlled. Many studies involve “umbrella” protocols, which endeavour to combine FIH with First-in-Patient (FIP) studies. Our extensive experience with this type of study, as well as with operationalising adaptive designs, enables our EPU to manage studies involving high-level scientific techniques, such as EEG, surgical insertion of temperature probes, and pharmacodynamic (PD) parameters. We don’t have to divide patients into cohorts (unless specified in the protocol) and offer clients/Sponsors total flexibility with the ability to run the unit with just 1 patient in-house, if necessary.
MAC Early Phase Unit Overview:
- Thirty-two beds for high-intensity safety monitoring arranged in four bays of eight beds
- 4 Individual rooms in one area – flexible use as screening, consulting or overnight rooms
- 2 x 3 bedded rooms (can house patient and carer)
- Patient lounge, eatery and kitchen
- Nurse alarms at each bed
- Nurses station giving a full 360 degree view of bays
- 8 channel 5 and 10 lead Mortara Telemetry system wireless and centrally monitored. Automated 12-lead ECG capture on studies and transmit information to central over-reader
- MAC is the only private company which has 24/7 emergency support from hospital resuscitation team, all physicians ALS trained, all clinical staff ILS trained
- Dedicated laboratory area, alarmed temperature monitoring throughout building with dial-out in case of temperature issues

Privacy Overview
