An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Antioxidants (Basel)
- PMC10669910


Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications
Muhammad nouman shaukat.
1 Department of Agriculture, Food and Environment, University of Catania, Via S. Sofia 100, 95123 Catania, Italy; [email protected]
Akmal Nazir
2 Department of Food Science, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain P.O. Box 15551, United Arab Emirates; [email protected]
Biagio Fallico
Associated data.
Not applicable.
Ginger is an herbaceous and flowering plant renowned for its rhizome, which is widely employed as both a spice and an herb. Since ancient times, ginger has been consumed in folk medicine and traditional cuisines for its favorable health effects. Different in vitro and in vivo studies have disclosed the advantageous physiological aspects of ginger, primarily due to its antioxidant, anti-inflammatory, antimicrobial, and anti-carcinogenic properties. These health-promoting features are linked to the variety of bioactive compounds that are present in ginger. Following the advancement in consumer awareness and the industrial demand for organic antioxidants and functional ingredients, the application of ginger and its derivatives has been broadly investigated in a wide range of food products. The prominent features transmitted by ginger into different food areas are antioxidant and nutraceutical values (bakery); flavor, acceptability, and techno-functional characteristics (dairy); hedonic and antimicrobial properties (beverages); oxidative stability, tenderization, and sensorial attributes (meat); and shelf life and sensorial properties (film, coating, and packaging). This review is focused on providing a comprehensive overview of the tendencies in the application of ginger and its derivatives in the food industry and concurrently briefly discusses the beneficial aspects and processing of ginger.
1. Introduction
Ginger ( Zingiber officinale Roscoe) plant, producing an irregular bumpy rhizome, is extensively cultivated in the southeast region of Asia. Accurate information regarding ginger plant’s origin is not available due to a very long cultivation history in this region. In ancient Greek history (40–90 AD), the Greeks were familiar to the dietary and culinary uses of ginger. In the 13th century, during his visit to China and Sumatra, Marco Polo became acquainted with ginger and imported it to Europe. In the same era, the Arabs transported the ginger from India to East Africa, while the Portuguese spread it to West Africa [ 1 ].
It is an important dietary ingredient in daily South Asian cuisine and traditional Chinese medicine. In the entire history, ginger rhizome, commonly known simply as ginger, is not merely utilized as a spice or herb to impart various food attributes like flavor, aroma, food preservation, and nutritional reform, but it has also been administered for the mitigation of various ailments such as asthma, flu, indigestion, and gastrointestinal discomfort [ 2 ].
Tropical and subtropical climates are favorable for ginger cultivation because it cannot combat very low temperatures. When ginger is utilized as a vegetable or for the production of ginger preserve, pickles, candies, and beverages, the recommended harvesting time is about 4–5 months after plantation, whereas when its intended employment is dried ginger and for the manufacturing of bleached and dehydrated ginger or functional products like ginger oil and oleoresin, the recommended harvesting period is around 8–10 months [ 3 ]. The height of a ginger plant is usually around 2 feet with yellow bloomed flowers characterized by a pungent smell. The underground stem or rhizome of ginger, attributed with a hot and strong spicy aroma flavor, used for medicinal and culinary purposes, is also one of the most abundantly used spices belonging to the Zingiberaceae family [ 3 , 4 ]. The ginger plant has a very close relevancy to other herbal plants like cardamom ( Elettaria cardamomum ) and turmeric ( Curcuma longa ) as they are members of the same family ( Zingiberaceae ). Ginger comprises carbohydrates (60–70%), water (9–12%), proteins (9%), lipids (3–6%), ash (8%), crude fiber (3–8%), and essential oil (2–3%). Apart from these macronutrients, ginger also contains various bioactive compounds and some mineral elements like sodium, potassium, calcium, magnesium, and phosphorus [ 5 ]. There is also a possibility of synthesizing new compounds when there is any alteration in the ginger’s growth cycles, for example, during the storage of ginger. The time period, humidity level, and temperature are also the influencing factors of the normal composition of ginger [ 6 ]. The presence of ketones in the chemical composition bestows the unique spicy aroma to these plants [ 7 ].
Ginger has been the subject of extensive research in the past owing to its possible health advantages. Several authors have reviewed the available literature and discussed the bioactive compounds present in ginger, the techniques used for their extraction, health promoting features, the encapsulation of ginger bioactives, and some specific or brief food applications of ginger and its derivatives [ 6 , 8 , 9 , 10 , 11 , 12 , 13 ]. However, there is a lack of reviews regarding the application of ginger to different food categories. It is also imperatively important to mention that the primary objective of incorporating ginger and its derivatives into a food product is to improve the nutraceutical value and shelf life of the relevant product behind which the lead character, directly or indirectly, belongs to the antioxidant compounds and their antioxidant activities. Therefore, the present paper is designed to furnish a comprehensive review of ginger application in different food sectors, while also briefly discussing the bioactive components, the effect of processing and extraction methods, and potential health benefits.
2. Bioactive Compounds in Ginger
The nutritional and nutraceutical benefits of ginger are closely associated with the presence of various bioactive compounds. These bioactive compounds, reported in different studies, are classified into three classes: gingerols, volatile oils, and diarylheptanoids [ 14 ]. The chemical components of ginger include volatiles (geraniol, borneol, terpineol, zingiberenol, cineole, limonene, camphene, curcumene, zingiberol, geranyl acetate, linalool, α-Farnesene, α-sesquiphellandrene, α-zingiberene, β-bisabolene, β-elemene, and β-phellandrene) and non-volatiles (gingerols, shogaols, zingerone, and paradols). These non-volatile phytochemicals, having pungent odors and flavors, are the characteristic bioactive constituents of ginger. The distinctive aroma flavor of ginger is the resultant blend of these three non-volatile active compounds (gingerol, shogaol, and zingerone), which constitute one to three percent of fresh ginger by weight. There are two common components, vanillyl (4-hydroxy-3-methoxphenyl) and the ketone functional group, that are found in every pungent compound of ginger [ 7 , 15 ]. The chemical structures of the major bioactive compounds that are present in ginger extract and essential oil are presented in Figure 1 .

Chemical structures of major bioactive compounds present in ginger.
Gingerol is a pungent oil, yellow in color, that is also found in crystalline solid form with a low melting point. Gingerols are classified into 4-, 6-, 8-, 10-, and 12-gingerol based on the length of the unbranched alkaline chain. 6-Gingerol is the most abundantly available type of gingerol, while considerable amounts of 8-gingerol and 10-gingerol are also found in fresh ginger [ 16 , 17 ]. 6-Gingerol is a moderately pungent and vital active compound of ginger with anti-inflammatory, anti-bacterial, and anti-carcinogenic properties. 6-Gingerol is reported to have different health-beneficial properties like anti-carcinogenic, antioxidant, anti-inflammatory, cardiotonic and hypotensive, anti-emetic, anti-pyretic, anti-rheumatic, anti-ulcer, anti-prostaglandin, and many other properties. 8-Gingerol is the most pungent and one of the most active phytochemicals in ginger. It has been cited with many health benefits by various researchers [ 18 , 19 ].
Just like the 6-gingerol in gingerols, 6-shogaol is the most amply available and most effective type of shogaol in ginger. 6-Shogaol is also described with many pharmacological and health characteristics like anti-inflammatory, anti-ulcer, anti-prostaglandin, anti-carcinogenic, anti-invasive, and many other properties. 6-Shogaol has a very powerful expectorant or anti-tissue effect, which may assist in reducing blood pressure. Some studies also disclosed that 6-shogaol, the dehydration product of 6-giongerol, is probably more functionally active than its precursor [ 20 , 21 , 22 ]. Zingerone, also known as vanillyl acetone, is a bioactive compound of ginger that is available in crystalline solid form. Its chemical makeup is very similar to other flavor compounds like eugenol and vanillin [ 23 ].
The bioactive composition of ginger essential oil, mainly comprising the volatile oils of ginger, is different than ginger extract. The functional compounds of ginger essential oil are classified into two main groups: monoterpenes and sesquiterpene hydrocarbons. The chemical composition of ginger essential oil is also affected by the nature (fresh or dried) and source of ginger rhizome [ 5 ]. The most abundantly available bioactive compounds of ginger essential oil are α -curcumene, α -zingiberene, geranial, β-sesquiphellandrene, β-bisabolene, and neral. Ginger essential oil has also been characterized by a diversified extent of bioactivity and health-promoting properties [ 24 ].
3. Effect of Processing and Extraction Process on Ginger Bioactive Compounds
Due to its high moisture (ranging from 85–95%), fresh ginger rhizome is susceptible to spoilage and decay. During storage, the time and temperature have remarkable impacts on the bioactivity, phytochemical profile, and overall quality of ginger rhizome. To avoid postharvest losses and to maintain the quality of fresh food commodities, different processing and preservation techniques such as drying, salting, canning, freezing, vacuum packaging, etc., are practiced in the food industry [ 25 ]. Among these processing technologies, different drying methods with a lot of variations and advancements have been employed for ginger processing. Apart from a longer shelf life, dried ginger is also superior to fresh ginger because of its better compatibility and yield for the extraction of bioactive compounds. The advanced and latest drying technologies including freeze-drying and infrared heating are advantageous over conventional drying methods but confined due to some limitations (such as the processing time, higher energy demand, and expensive equipment). A supreme quality product could also be obtained by optimizing the processing parameters, like the temperature and time, under controlled climate conditions [ 26 ].
The selection of an appropriate processing technique along with optimum processing parameters is essential to obtain the desired end product. The processing conditions could lead towards changes in the phytochemical content, bioactive profile, and overall makeup of ginger ( Figure 2 ) [ 27 ]. For instance, during drying, more pungent compounds, i.e., shogaols, are produced during the dehydration process. Zingerone, a mild spicy and pungent component with a sweet aroma, is derived during the cooking of ginger. Gingerols are destabilized during the thermal or drying process, and also during storage, which results in a mutation at the C-5 of gingerol through the removal of the OH group. The removal of this OH group results in the production of a double bond between the carbon positions of C-4 and C-5. Therefore, shogaols can be produced from the dehydration of heat-unstable β-hydroxyl ketone and gingerdiones to form an α, β-unsaturated ketone [ 28 ].

Effect of processing on ginger bioactives and its constituents [ 6 ].
Gingerols are sensitive to some processing and storage conditions like thermal processing, acidic environments, air, light, and prolonged storage. When gingerols undergo these conditions, there is a possibility of the transformation of gingerols into shogaols [ 29 ]. Studies also revealed that during the dehydration process of ginger, the quantity of 6-gingerol decreased with the increase in 6-shogaol [ 30 ]. Moreover, the microbial metabolism of shogaol generated the β-ketone hydroxyl deoxygenated group of products named paradols. During the heating of fresh ginger, zingerone is formed from the gingerols through a retro aldol reaction [ 23 ].
The chemical composition and yield of ginger extract and ginger essential oil are also influenced by the extraction method. Advanced and green extraction techniques are preferred over classical extraction techniques [ 11 ]. The extraction techniques with low temperature requirements such as supercritical fluid extractions, ultrasound-assisted extractions, and enzyme-assisted extractions are usually employed to avoid the degradation or conversion of heat-sensitive bioactive compounds. The extracts acquired from these extraction processes are relatively rich in gingerols, antioxidants, and total phenols. Meanwhile, microwave-assisted extraction, pressurized liquid extraction, and acidic solvent extraction are useful to obtain 6-shogaol-rich extracts due to the possible transformation of 6-gingerol to 6-shogaol under stress conditions. Microwave-assisted and ultrasound-assisted extraction render an economical edge of being time-efficient, while supercritical fluid extraction is acknowledged for the safety and quality of the product. [ 9 , 10 ]. If a comparison is drawn on the basis of the phenolic profile, conventional solvent extraction using water and ethanol as solvents resulted in total phenolic contents of 104.7 ± 4.5 mg GAE/100 g and 650.44 ± 27.32 mg GAE/100 g of dry weight, respectively [ 31 , 32 ], while the extract of freeze-dried ginger powder produced through sonication came up with a total phenolic content of 931.94 ± 0.02 mg GAE/100 g of dry weight [ 27 ].
Furthermore, a synergistic effect with a superior quality product could also be achieved through the consolidation of these extraction techniques, and this is the most emerging approach in the field of extraction to maximize the extraction efficiency through the synchronization of two or more advanced extraction technologies [ 33 ]. For instance, the efficacy of using different natural deep eutectic solvents (NADESs) as green extraction solvents has also been evaluated in combination with ultrasound-assisted extraction. This consolidation has emerged successfully with favorable results as the total phenolic content of the extract obtained from this venture was 2010 ± 26 mg GAE/100 g of dried ginger powder, which is remarkably higher than the previously described extraction techniques [ 34 ].
4. Beneficial Aspects of Bioactive Compounds
As mentioned above, the functional and bioactive compounds present in ginger are associated with multiple health benefits and advantageous characteristics. The exploitation and characterization of the versatile biological and functional competencies of ginger are also crucial for further research. These multifaceted bioactivities of ginger bioactives and their modes of action are discussed here in this section.
4.1. Antioxidant Activity
Any disturbance or adjustment with the cell’s antioxidant defense system can lead to the excessive production of Reactive Oxygen Species (ROS), and eventually, to an increase in oxidative stress. For the recovery of normal levels of free radicals and oxidative stress, various natural antioxidants have been analyzed for the neutralization of free radicals and encounter oxidative stress for the prevention of different health disorders [ 35 ]. The maintenance of a normal ROS level in a living organism is very important for normal functioning, which can be achieved through the provision of hydrogen atoms or electrons to free radicals to hinder the ROS production and convert them into antioxidant lipid complexes or thermally stable products [ 36 ].
In the past two decades, the exploration of natural plant resources has become very attractive and beneficial, most probably due to the presence of bioactives, less toxicity, and the lower production costs [ 37 ]. Ginger bioactive compounds have significant antioxidant potential to restore or maintain normal oxidative stress levels. Ginger leaves were reported to have more antioxidative potential than ginger rhizome and ginger flowers [ 32 , 38 ]. The higher in vitro antioxidant activity (88.93 ± 0.03% DPPH; 88.23 ± 0.98% ABTS) was noted in 6-gingerol. The daily oral administration of 6-gingerol (50–75 mg/Kg body weight) to mice for three weeks enhanced their blood glucose levels, reduced their oxidative stress, and also improved their insulin signaling. A higher antioxidant activity of shogaols was recorded as 89.01 ± 0.6% and 90.2 ± 0.11% for ABTS and DPPH, respectively [ 39 , 40 ]. The highest antioxidant potential of 6-shogaol compared to other phenols of ginger is due to the existence of a special functional group (α, β-unsaturated carbonyl), which is efficiently involved in the regulation of glutathione. Glutathione is the most amply available type of thiol in animal cells, and it is engaged in the cell detoxification mechanism against detrimental substances and intracellular redox management [ 41 ].
4.2. Anti-Inflammatory Activity
Inflammation is a phase in the natural healing process of the body to combat intruders for the prevention of infection or injury. The basic aim of inflammation is to abrogate cellular damage, withdraw and absorb necrotic cells and tissues, and regain the stability and conformity of the intracellular environment [ 42 ]. When cells go through severe inflammation, a large number of mononuclear immune cells are penetrated, which stimulates the production of inflammatory interleukins, cytokines, and tumor necrosis factors including inflammation-promoting cytokines, such as tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), which are very significant for the inflammation process. A recent study focused on the anti-inflammatory characteristics of ginger mentioned that ginger consumption could substantially lower the TNF-α serum levels but has a minor impact on IL-6 [ 43 ].
It was also observed that the anti-neuroinflammatory capacity of gingerols enhanced with the increase in the alkyl chain length as 10-gingerol’s anti-neuroinflammatory activity was higher than the other active compounds of ginger [ 44 ]. Furthermore, different in vivo studies have also supported these claims through the practical implication of ginger bioactives. Nanoparticles derived from ginger that were orally administered to mice, having acute and chronic colitis, at the dosage rate of 0.3mg/mice on a daily basis for one week efficiently halted intestinal inflammation by decreasing the pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6 and increasing the levels of anti-inflammatory cytokines like IL-10 (Interleukin-10) and IL-22 (Interleukin-22). In mice suffering with dextran sulfate sodium-induced colitis, when 6-shogaol-equipped nanoparticles (equivalent to 15 mg/kg of 6-shogaol) were orally administered on a daily basis for a week, they not only diminished the colitis symptoms but also boosted the healing process [ 45 , 46 ].
4.3. Antimicrobial Activity
Currently, chemical and synthetic antimicrobials are being replaced with natural and organic antimicrobials derived from plants, animals, and microorganisms, both in health management and in food preservation. These biologically derived antimicrobials from plant sources are preferred due to their multi-oriental management, multi-targeted approach, drug resistance, and lower toxicity [ 47 ]. Ginger and its derivatives have been appraised for their tremendous anti-bacterial, anti-viral, and anti-fungal activities [ 48 ]. Microorganisms tend to make biofilm as their strong defense against antimicrobials. A study carried out by Chakotiya et al. [ 49 ] reported that ginger has shown an inhibitory action against Psedomonas aeruginosa (a multi-drug-resistant strain) by disturbing the membrane stability and preventing biofilm formation.
Ginger essential oil possesses excellent anti-bacterial activity against food-borne pathogenic bacteria such as Escherichia coli and Staphylococcus aureus . A study reported the bactericidal mechanism of ginger essential oil through the demolishment of the cell membrane activity and through intervening with the energy metabolism because of some macromolecular elements like proteins and nucleic acids [ 50 ]. Another study expressed that fresh ginger oil had a moderate antimicrobial effect on Candida , Aspergillus niger , and P. aeruginosa and a mild effect against Saccharomyces cerevisiae , and no inhibition was detected against Bacillus subtilis , Trichoderma spp., and Pencillium spp. Ginger oil obtained from dried ginger was observed to have higher anti-bacterial activity against P. aeruginosa [ 51 ]. There is a close correlation between anti-bacterial and antioxidant properties as the blocking of antioxidant activity could result in the detention of anti-bacterial activity. Processing and storage conditions could also be made changes with functional attributes of ginger resulting in difference in antioxidant and anti-bacterial activities [ 52 ].
4.4. Anti-Carcinogenic Potential
Cancer is one of the major causes of death in the world, and in 2020, approximately 19 million cancer cases were reported, out of which about 10 million cases worldwide were fatal [ 53 ]. In recent years, ginger has been extensively investigated for its anti-carcinogenic activities against different types of cancer, like cervical, breast, colorectal, and prostate cancers [ 54 , 55 ]. Chronic inflammation plays a key function in carcinogenesis as it has a close connection with all crucial stages of cancer formation [ 56 ]. Ginger and its active compounds have been identified with anti-carcinogenic properties against all stages of cancer development including cancer initiation, promotion, progression, and drug resistance. An in vitro study disclosed that a fraction loaded with polyphenols derived from dried ginger powder restrained the progression of gastric adenocarcinoma cells and colorectal cancer cells [ 57 ].
In vivo and in vitro studies were carried out to evaluate the action mechanism and cytotoxic effects of ginger against prostate cancer [ 58 ]. It was reported that 6-gingerol, 6-shogaol, 10-gingerol, and 10-shogaol showed considerable anti-proliferative effects on prostate cancer cells in humans through the deregulation of the protein expression of MRP1 (multi-drug resistance-associated protein 1) and GSTπ (Glutathione-S-transferase). The proliferation of PC-3 prostate cancer cells was synergistically inhibited through the bifold consolidation of ginger bioactives like 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol [ 59 ]. 6-Gingerol stimulated the increase in ROS formation in human gastric adenocarcinoma (AGS) cells, which promote apoptosis and reduce the mitochondrial membrane potential [ 60 ]. The in vitro application of 6-shogaol (10–20 μM) to PC12 cells for 24 h improvised the phase II antioxidant compounds at the pheochromocytoma cell line in mice, which effectively inhibited the proliferation of oxidative stress in PC12 cells. On the contrary, 6-gingerol failed to protect the PC12 cells from the oxidative stress, possibly due to the absence of an α, β-unsaturated ketone, which is an indicator of the α, β-unsaturated ketone unit’s importance in cytoprotection [ 61 ].
4.5. Neuroprotective Activity
There is a possibility of an onset of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, and old people are at an especially high risk. Many recent studies have expressed that ginger possesses anti-neuroinflammatory capacity and has a significant role in memory function, which might be useful in the management and inhibition of neurodegenerative diseases [ 62 , 63 ]. It was also disclosed that 10-gingerol from fresh ginger exhibits powerful anti-neuroinflammatory activity, proven through a lipopolysaccharide-activated BV2 microglia culture model. 10-Gingerol restricted the articulation of pro-inflammatory genes by inhibiting NF-κB activation, which ultimately led to a decrease in the levels of IL-6, IL-1β, NO, and TNF-α. [ 44 ]. The phytochemicals derived from ginger such as gingerols, shogaols, and others, at a concentration of 20 μM, have been reported with strong neuroprotection capacities, especially against Alzheimer’s disease. Ginger could lower the Aβ-species and cerebral plaques, inhibit the cerebral inflammation, amend the tau protein, and restrain Aβ-induced apoptosis through different pathways [ 64 ].
4.6. Cardiovascular Protection
Worldwide, the dominant cause of premature deaths (17.9 million people per year) is cardiovascular disease [ 65 ]. Various studies revealed that ginger can efficiently provide protection against cardiovascular diseases by reducing the levels of blood pressure and blood lipids. The level of high-density lipoprotein cholesterol (HDL-C), also known as good cholesterol due to its defensive stature against cardiovascular diseases, increased in the serum, while a decrease in body weight was observed in mice on a high-fat diet supplemented with 0.5% of lyophilized ginger extract for a period of about two weeks. Ginger extract also facilitated high-density lipoprotein (HDL) production through enhancing the levels of lecithin–cholesterol acyltransferase RNA and apoliprotein A-1 in the liver. Another study also supported the role of ginger extract in the reduction in low-density lipoprotein (LDL) and total cholesterol, and the integrated daily oral application of aqueous ginger extract (250 mg/kg of feed) for about a month and aerobic exercise increased the HDL levels in high-fat-diet-fed rats [ 66 , 67 ].
4.7. Anti-Obese Activity
Obesity or overweight is a great concern throughout the world, and it emerges from the disturbance in the energy metabolism. Basically, there are two types of adipose (fat) tissues classified on the basis of their arrangements and activities. The core function of white adipose tissues is to serve as energy depositories, while brown adipose tissues are responsible for metabolism improvement and energy expenditure. The transformation of white adipose tissues to brown adipose tissues is a novel approach to tackle obesity. 6-Gingerol could stimulate the cell browning via an AMPK-dependent approach in 3T3-L1 adipocytes [ 68 , 69 ]. Gingerenone A was discovered as a better performer than gingerol and shogaol with efficient inhibition activity against adipogenesis and fat accumulation in 3T3-L1 preadipocyte cells. In this study, the oral administration of Gingerenone A (50 mg/kg of bodyweight) on a daily basis for 15 weeks also influenced the fatty acid metabolism through the in vivo activation of AMPK, debilitating diet-induced obesity [ 70 ].
4.8. Anti-Diabetic Effect
Diabetes mellitus is a chronic metabolic disease characterized by the insufficiency of insulin and/or insulin resistance, resulting in an uncontrolled increase in the blood glucose level. Persistent hyperglycemia could promote protein glycation and the generation of advanced glycation end products (AGEs). An in vitro study revealed that 6-gingerol and 6-shogaol inhibited the proliferation of diabetic complexities and confined the methylglycoxal (MGO), the precursor of AGEs, to hinder AGE production [ 71 ]. The oral supplementation of 6-gingerol (200 mg/kg of corn oil) on a day-to-day basis for 28 days to type 2 diabetic mice stimulated glycogen synthase 1 and improved the GLUT4 (glucose transporter type 4) cell membrane presentation, which raised the glycogen storage levels in the skeletal muscles [ 72 ].
5. Food Applications
Currently, there is a growing trend towards healthier and functional food products as consumers are more conscious about healthy lifestyles and daily intakes. Due to prospering recognition and interest in functional food products, the scope for the exploration, characterization, and potential utilization of functional food ingredients and bioactive compounds has also magnified. Ginger, being a valuable functional ingredient and marvelous source of bioactives, has also been integrated into a variety of food products. Here, the food applications of ginger in its different forms (powder, juice, extract, and essential oil) will be discussed to provide a comprehensive and broader prospective of its employment. The impact of ginger incorporation on the sensorial attributes of various food products is also discussed below ( Figure 3 ).
5.1. Bakery Products
A variety of studies have been carried out to use ginger as a potential food additive in bakery products ( Table 1 ). For instance, the supplementation of ginger powder at a rate of 2–4% was reported, which can promise feasible textural, rheological, and antioxidant properties along with a better mineral content in bread [ 73 ]. Balestra et al. [ 74 ] formulated a functional bread using ginger with acceptable physicochemical and sensorial characteristics. They found that up to 3% of ginger powder can be incorporated into the bread without compromising the rheological properties of the dough and bread. Another study also came up with similar findings over the functional, physicochemical, and sensory properties of bread fortified with ginger powder but with a different optimized level of ginger powder (1%) than the previous study [ 75 ]. Moreover, Almasodi [ 76 ] studied the addition of ginger powder in bread as well as in rusk. They concluded that the incorporation of up to 3% and 6% of ginger powder resulted in satisfactory hedonic and textural properties for bread and rusk, respectively. In another study, garlic and ginger powders at different concentrations were also incorporated in wheat bread. Besides functionality improvement, it also increased the shelf life of the bread from 5 days to 7 days at an ambient temperature [ 77 ]. Ginger and turmeric have inhibitory effects on the growth of Rhizopus stolonifer on bread, but ginger may impart unfavorable flavor characteristics to the bread [ 78 ].
The aforementioned studies mainly dealt with the addition of ginger to produce functional bread. However, in some parts of the world, distinct baking products exist wherein ginger is used as a dominant flavoring ingredient. For instance, gingerbread is a traditional bakery product in the Czech Republic, particularly in the town of Pardubice, where ginger is the prevalent flavor. It is produced in a variety of shapes, types, and textures ranging from a crispy biscuit to a soft cake [ 79 , 80 ]. Hence, ginger has been practiced as a flavor enhancer, solely or in a combination, for a long time, but it is largely dependent on the nature of the food product, the form of the ginger to be incorporated, their mutual compatibility, and the sensorial preferences of the consumers.
Lee et al. [ 81 ] proposed that functional cookies can be prepared with a 4% incorporation of ginger powder, as this concentration was found to have overall acceptability and a very bland effect on the quality characteristics. Kaushal et al. [ 82 ] also investigated the functional and sensorial properties of cookies with the addition of ginger powder. The functional and organoleptic properties of cookies supplemented with 12% ginger powder were very favorable compared to the controls. In another investigation, Yang et al. [ 83 ] also evaluated the effect of ginger substitution on the acrylamide content in dough and biscuit properties. Ground ginger addition at the rate of 1% was reported with favorable structural and color characteristics and acceptable sensorial characteristics with a 6.2% decrease in the acrylamide content. The substitution of ginger at higher rates decreased the acrylamide content, but the hedonic properties were not feasible. In principle, gingerols reduced the acrylamide content through inhibition where the phenol hydroxyl group of gingerol played a vital role. Kumari and Gupta [ 84 ] also utilized ginger powder and some other functional plant ingredients in the production of value-added cookies. Acceptable organoleptic characteristics were found with 2.5% of ginger powder.
The functional, nutritional, antioxidant, and sensorial properties of millet flour and ginger powder-formulated cookies were investigated by Marak et al. [ 85 ]. This study reported that ginger flour incorporation of up to 10% was found satisfactory for the textural and sensorial properties of cookies, and it also efficiently improved the functional and nutritional values of cookies with a potential lead towards shelf life extension. When cumin and ginger were utilized for the preparation of cookies, a minimal difference was observed in the dough development as compared to the control [ 86 ]. Both potential ingredients were integrated in concentrations of up to 5% in soft wheat cookies, but there was not any substantial effect on the end product. Keeping in view the acceptability and feasibility indicators, these concentrations were also recommended for commercial scale production. The antimicrobial effect of ginger was also evaluated through the addition of ginger powder into biscuits. Aerobic bacteria were decreased proportionally by increasing the amount of ginger powder in biscuits. Functional biscuits with significant activity against bacteria and mold could be produced with around a 2.5% addition of ginger powder without any compromise on the quality characteristics [ 87 ]. Adebayo-Oyetoro et al. [ 88 ] also prepared ginger spiced cookies with around a 3% addition of ginger powder.
Some important studies on the application of ginger and its derivatives to bakery products.
| Derivative | Product | Positive Effect | Reference |
|---|---|---|---|
| Ginger Powder | Wheat Bread | ↑ Antioxidant Activity; Minerals; Total Phenolics; Textural Properties; Shelf Life; Sensorial Characteristics; ↓ Microbial Growth | [ , ] |
| Cookies | ↑ Antioxidants; Phenolic Contents; Shelf Life; ↓ Acrylamide Content; Microbial Growth; ≈ Sensorial Characteristics | [ , , , ] | |
| Cake | ↑ Antioxidant Activity; Total Phenolics, Shelf Life; Sensorial Properties; ↓ Bacterial and Fungal growth | [ ] | |
| Ginger Juice | Wheat Flour Muffin | ↑ Total Phenolics; Antioxidants; Sensorial Characteristics; ≈ Textural Attributes | [ ] |
| Ginger Extract | Baked Bars | ↑ Antioxidants; Phenolic Contents; Antioxidant Retention during Storage; Flavor and Color; ≈ Overall Acceptability | [ ] |
↑ = Increased/Improved, ↓ = Lower/Decreased, ≈ = Negligible/Unchanged.
To a large extent, Almasodi [ 89 ] successfully incorporated ginger powder in butter cake and biscuits at the rates of 5% and 10%, respectively. Storage studies revealed that, apart from providing a nutritional boost, ginger powder also supplemented bakery products with natural antimicrobial compounds. In another recipe, the water content was substituted with ginger juice at three different concentrations from 10% to 30% to analyze the quality attributes of muffins [ 90 ]. Significant differences were observed in the color, texture, and taste with the increasing amount of ginger juice. Ginger juice at 10% of the water used in the recipe was recommended as the optimum amount for muffin production. The conventional and supercritical extracts of ginger were also analyzed through supplementation in baked bars at 3% and 0.3% concentrations, respectively [ 91 ]. The supercritical extract performed better than the conventional extract to improve and maintain the phenolic contents and antioxidant activities in storage duration. A sensorial evaluation also declared the supercritical ginger extract to be better than the control and conventional extract.
As per the foregoing studies, ginger powder is the most widely employed form of ginger in bakery products, followed by ginger juice. The pertinent amount of ginger powder and processing conditions are the key factors in the acquirement of balanced functional bakery items without any alteration in the desirable characteristics of the product. So, bakery products with admirable nutraceutical values could also be produced through the precise supplementation of ginger.
5.2. Dairy Products
There is also a considerable potential for innovation and improvement in the dairy sector, where ginger, an active ingredient, has also contributed ( Table 2 ). The supplementation of ginger powder (1%) has been displayed as a bovine yogurt product with an enhanced nutritional value, better quality characteristics, and ease in the production process [ 92 ]. Ginger powder is also formulated with dromedary milk to prepare and characterize a functional yogurt product. The resultant product showed very similar results to the previous study (bovine milk yogurt) with the recommendation of 1% ginger powder as an optimum supplementation extent. The water holding capacity, viability of the starter culture, and rate of pH reduction was increased, while the fermentation time and flow behavior was decreased, leading towards an acceptable yogurt from dromedary milk [ 93 ]. Ethiopian cottage cheese, ‘Ayib’, was also treated with ginger and garlic powders to analyze their impacts on the chemical arrangement and sensorial characteristics of the product [ 94 ]. Ayib treated with garlic powder was described with better overall acceptability than ginger powder, and further investigations were suggested to explore the biochemical and microbial properties of both powders.
A novel approach was also attempted to evaluate the effects of ginger, cinnamon, and carob powders on spray-dried milk powders and their agglomerates [ 95 ]. Milk powder with added ginger powder relatively performed better in terms of solubility, hygroscopicity, flowability, and cohesiveness. The incorporation of these powders significantly reduced the solubility time of milk powders, while the water activities and moisture levels of the resultant products were also within adequate extents, feasible for the safe storage of these milk powders. In another investigation, an Indigenous sub-continental sweet dairy product, Peda, was also prepared, analyzed, and finally optimized (2%) utilizing ginger powder [ 96 ]. The inclusion of ginger powder into Peda not only increased the total solid, total sugar, and ash contents, but also improved its shelf life by reducing the total plate count.
Apart from ginger powder, the effect of ginger juice on the physicochemical and hedonic attributes of yogurt was also investigated at different concentrations. An inverse relationship was observed among the amount of ginger juice, the viability of the starter culture, and the hardness of the product. Based on the finding of this study and the sensorial characteristics of the product, the ginger juice addition at the rate of 4% ( v / v ) was regarded as the suitable concentration in yogurt production [ 97 ], while Allam et al. [ 98 ] reported that ginger juice (2%) incorporation into fermented milk products reduced the coagulation time and accelerated the syneresis process.
Jadhav et al. [ 99 ] formulated a functional milkshake product via the inclusion of ginger juice at four different concentrations from 2.5% to 10% ( v / v ). The increase in the ginger juice concentration was inversely proportional to the fat, protein, and total solid contents of the product. Considering the sensorial properties and other attributes, a 2.5% ginger juice addition in the milkshake was regarded as an optimum value. Gaur et al. [ 100 ] also successfully developed an herbal milk product by optimizing the concentrations of herbal ingredients such as ginger, turmeric, and tulsi (holy basil).
Several studies were also designed for the potential exploitation of ginger into an herbal ice cream product. Herbal ice cream could be manufactured employing 4% ginger juice ( w / w ) with a better hedonic response than all other treatments including the control. Ginger juice improved the product’s resistance against melting but lowered the fat and total solid contents in the herbal ice cream [ 101 ]. Similar findings were also reported in another investigation [ 102 ]. The incorporation of ginger juice has a pertinent effect on the thermal properties but no substantial influence on the freezing characteristics of ice cream [ 103 ]. Mule et al. [ 104 ] efficiently employed ginger juice, aloe vera juice, and L. acidophilus -based probiotic culture to produce probiotic ice cream at the optimized levels of 3%, 6%, and 7% ( w / w ), respectively.
The fortification of ginger extract into fermented dairy products is another approach to bestow the favorable characteristics of ginger to the products. Ginger extract, at an 8% concentration of the low-fat yogurt formulation, not only boosted the phenolic contents and antioxidant activities, but also played a vital role in the retention of these properties [ 105 ]. The addition of ginger extract also lowers the syneresis process in the yogurt. During the storage period, there was not any remarkable effect on the acidity, but the viscosity of the product was decreased with the incorporation of ginger extract.
In two other investigations, herbal yogurt was developed using ginger extract and evaluated for its antioxidant capacity and sensorial properties. Ginger was regarded as a better source of antioxidant properties than beet root, while the herbal yogurt containing ginger extract (2%) and goat milk was characterized with the highest antioxidant activity than other milk sources (buffalo and cow). A sensorial analysis revealed that herbal yogurt augmented with ginger extract (1%) was superior in color and appearance, while garlic extract (0.2%) was responsible for a better taste and flavor [ 106 , 107 ].
Ginger extract in combination with gum arabic has also been exploited in the manufacturing of a yogurt-based traditional Iranian drink, Doogh [ 108 ]. The synbiotic dairy product with maximal overall acceptability and advanced probiotic status was developed with a blend of ginger and gum arabic at the concentrations of 0.25% and 0.5%, respectively. In another attempt to produce synbiotic labneh through the efficient utilization of ginger and probiotics, an effective inhibitory effect on some food-borne pathogens was detected along with an enhanced phenolic profile and superior overall acceptability of the end product [ 109 ].
The ethical, economical, and supply chain aspects of the calf rennet compelled dairy experts to explore other potential sources of milk coagulation. Ginger and its derivatives were also analyzed as potential coagulants in cheese processing. Ginger crude extract emerged as an auspicious coagulant in soft un-ripened cheese manufactured from camel milk [ 110 ]. Ginger extract did not perform as good as commercial camel chymosin but rendered a crucial task in the value addition of the product. Further studies were also proposed to explore the coagulation capability of pure coagulation enzymes from ginger. Peshawari cheese, a traditional cheese in the Khyber Pakhtunkhwa province of Pakistan, was prepared utilizing ginger protease compared to calf rennet [ 111 ]. In a comparison between ginger protease and calf rennet, there was not any pertinent difference observed in terms of the physicochemical and microbiological properties of the Peshawari cheese. In fact, ginger protease accelerated the proteolysis and improved the organoleptic characteristics of the cheese.
Ginger extract has been productively employed in soft cheese manufacturing as a functional ingredient. Ginger extract addition promoted the quality attributes of soft cheese by reducing the pH, oxidative rancidity, and microbial load while increasing the proteolysis, cohesiveness, and smoothness of the product. Ginger extract-fortified cheese also earned higher scores for texture, flavor, and overall acceptability than the control. Ginger extract (4%) coupled with the pickling of the soft cheese was reported with superior quality characteristics [ 112 ].
Herbal ice cream with health-promoting attributes and a reduced sugar content could also be prepared by formulating ginger extract and xylitol [ 113 ]. The physical characteristics of the product were not altered significantly by incorporating herbal ingredients, but the antioxidant activity and phenolic contents were improved, and an alluring appearance was imparted to the final product. Another study was also conducted to characterize the volatile compounds of mature ginger rhizome and its potential utilization to develop a functional ice cream product [ 114 ]. The maturity of ginger rhizome exhibited a direct and inverse relationship with zingiberene and ar-curcumin, respectively. A value-added functional ice cream with enviable sensorial characteristics and innovative ginger flavoring could be produced by incorporating the gingerols from freeze-dried ginger extract.
In another study, different forms of ginger such as ginger juice, paste, candy, and powder were formulated into ice cream to analyze its physicochemical and hedonic attributes. The addition of ginger juice and paste decreased the fat, total solid, and overrun contents, while the incorporation of ginger candy and powder increased the fat, total solid, and overrun contents, but the phenolic contents and antioxidant capacity were increased in all inclusions. The product’s resistance to melting improved in all preparations of the ginger, and the optimized incorporation level of all ginger forms were 4% paste, 6% juice, 10% candy, and 1% powder [ 115 ].
A novel approach of utilizing ginger essential oil in yogurt preparation was also successfully applied to evaluate the quality characteristics of the final product. Ginger essential oil enrichment (1000 ppm) lowered the syneresis and pH, while it enhanced the shelf life of the product and improved the overall acceptance of the product [ 116 ]. Efforts were also made for the efficient employment of essential oils from ginger, thyme, and clove in soft cheese formulation [ 117 ]. The inhibitory effects of these essential oils were reported against different food-borne pathogens, and the sensorial attributes were also improved with the addition of these essential oils.
Some important investigations on the incorporation of ginger and its derivatives in dairy products.
| Derivative | Product | Positive Effect | Reference |
|---|---|---|---|
| Ginger Powder | Yogurt | ↑ Antioxidant Activity; Phenolic Contents; Total Solids; Viscosity; Textural Properties, Storage Stability; ↓ Syneresis; pH; Time; Energy; ≈ Sensorial Characteristics | [ , ] |
| PEDA | ↑ Functionality; Shelf Life; ↓ Microbial Growth; Cost | [ ] | |
| Ginger Juice | Yogurt | ↑ Total Phenolic Contents; Antioxidant Total Solids; Viscosity; ↓ Coagulation Time; pH | [ , ] |
| Ice Cream | ↑ Antioxidant Activity; Phenolic Content; Sensorial Characteristics; Melting Resistance | [ , , ] | |
| Ginger Extract | Yogurt | ↑ Antioxidants; Phenolic Contents; Sensorial Properties; ↓ Syneresis; pH | [ , ] |
| Labneh | ↑ Antimicrobial Activity; Antioxidants; Phenolic Contents; Sensorial Characteristics; ↓ Microbial Growth; pH | [ ] | |
| Cheese | ↑ Antioxidant Activity; Total Phenols; Oxidative Stability; Storage Stability; Sensorial Characteristics; Coagulation ↓ Microbial Growth | [ , ] | |
| Ice Cream | ↑ Antioxidants; Total Phenolic Contents; Sensorial Properties; ↓ Microbial Count; ≈ Viscosity; Overrun | [ , ] | |
| Ginger Protease | Cheese | ↑ Proteolysis; Functionality; Coagulation; Sensorial Properties | [ ] |
| Ginger Essential Oil | Soft Cheese | ↑ Antioxidants; Phenolic Contents; Sensorial Characteristics; ↓ Microbial Growth | [ ] |
In conclusion, ginger’s diversified applications in dairy products have demonstrated its vast potential as a functional ingredient. Among the different forms of ginger, ginger extract came up as an extensively employed form, followed by ginger juice, powder, and essential oil. Ginger extract exhibited its aptitude for improving the overall techno-functional characteristics of dairy products and offering persuasive coagulation properties for cheese manufacturing. While the use of all forms of ginger proved to be effective in elevating the functionality and efficacy of dairy products, at the same time, some quality and hedonic characteristics were also compromised, but the incorporation of ginger extract was identified with higher functional and physicochemical improvements and minimal quality defects.
5.3. Beverages
Like other food sectors, the incorporation of functional food ingredients including ginger has also been investigated in beverages ( Table 3 ). In the manufacturing of a ready-to-drink ginger–cocoa beverage, ginger powder and cocoa were optimized at an 8% concentration with low-fat cocoa powder, respectively, as higher concentrations of ginger and fat interfere with two decisive quality factors, sedimentation and viscosity [ 118 ]. A successful attempt was also made to develop a ready-to-drink and ready-to-reconstitute ginger-based beverage with superior functionality and a 6-month shelf life at an ambient temperature [ 119 ].
Ginger extract in combination with lemon juice rendered an excellent job as a natural preservative in a carrot–mandarin RTS drink. During storage, a few modifications were observed in the physicochemical properties, but the product characteristics remained consistent to a large extent [ 120 ]. A ginger and Indian gooseberry-based herbal beverage was also prepared and investigated by replacing sugar with aspartame, an artificial sweetener [ 121 ]. Liu et al. [ 122 ] recommended atmospheric pressure sterilization for a ginger-flavored beverage due to its feasibility and capability to retain the important volatile compounds.
Fresh ginger extract was adequately exploited in the production of a ginger-based functional carbonated beverage. The functionality and sensorial characteristics of the drink were improved with the addition of ginger rhizome extract, but the shelf life was largely associated with the carbonation level of the product [ 123 ]. In another study, supercritical extraction was mentioned as a superior extraction approach among conventional and ultrasonic extraction techniques, while mint was detected with marginally better phenolic and antioxidant profiles than ginger. A ginger–mint drink of high nutraceutical value was prepared at 0.4% concentrations of each extract with favorable physicochemical and hedonic characteristics [ 124 ].
Ginger is also a vital ingredient in Zobo drink, a traditional and indigenous Nigerian drink prepared from Hibiscus sabdariffa flowers [ 125 ]. The fortification of Zobo drink with ginger and garlic extracts substantially reduced the microbial load. The degradation of sensorial features with storage was observed in the control samples, while the samples that incorporated ginger and garlic not only had improved sensorial attributes but also maintained these characteristics during storage [ 126 ].
Ginger has also been formulated in various functional beverages in the form of blends with different spices, herbs, fruits, and other food ingredients. A soybean, ginger, and mango-based functional drink was developed to effectively exploit the nutritional and functional values of these ingredients, which were consequently attributed with feasible sensorial properties and overall acceptability [ 127 ]. An optimum formulation ( v / v ) was furnished for a functional beverage utilizing ginger extract (15.1%), tamarind extract (9.9%), turmeric extract (5.0%), sugar solution (40%), and water (30%).
Ginger extract, Kencur extract, and their combination blend were incorporated into an instant temulawak beverage, an indigenous Indonesian drink, to evaluate their impacts on the physicochemical and sensorial attributes of the final product. The inclusion of ginger extract in a concentration of up to 15% enhanced the flavor, aroma, and overall acceptance of the Temulawak drink [ 128 ]. Another study associated ginger extract with an improvement in the sensorial properties, but there was no significant advancement in the antioxidant activity with the ginger addition to a cocoa, ginger, and hibiscus flower-based beverage product [ 129 ]. A ginger-based beverage with hyperthermic activity was also found to be efficient against peripheral skin extremities in women experiencing mild cold sensitivity [ 130 ].
The integration of two different food ingredients could ameliorate the defective quality attributes of the intended food product. The fusion of a better coloring feature from tea and a favorable flavoring attribute from ginger resulted in a superior acceptable beverage product [ 131 ]. During an antioxidant evaluation in tea-ginger extract, the powder concentration was reported as the most influential process parameter than the time and temperature [ 132 ]. The supercritical ginger extract (0.3%) was preferred over the conventional ginger extract in iced ginger tea and functional coffee substitute production due to its exceptional performance, safety, purity, and overall acceptability [ 133 , 134 ]. Ginger extract (10%) was formulated and optimized with non-decaffeinated Robusta coffee (40%) with an improved functional value and without any adverse effects on the sensorial and quality characteristics [ 135 ].
A few important studies on the addition of ginger and its derivatives to beverage products.
| Derivative | Product | Positive Effect | Reference |
|---|---|---|---|
| Ginger Powder | Cocoa Beverage | ↑ Total Phenolic Contents; Antioxidant Activity; Viscosity | [ ] |
| Ginger Extract | Carrot and Kinnow RTS Drink | ↑ Antioxidant Activity; Preservation; ↓ Microbial Growth; ≈ Sensorial Properties | [ ] |
| Zobo Drink | ↑ Functionality; Shelf Life; Sensorial Properties; ↓ Microbial Count | [ ] | |
| Black Tea | ↑ Antioxidant Activity; Phenolic Contents; Sensorial Properties | [ ] | |
| Ginger Iced Tea | ↑ Nutraceutical Compounds; Shelf Life; Organoleptic Attributes | [ ] | |
| Robusta Coffee | ↑ Antioxidant Activity; Phenolic Contents; ≈ Sensorial Properties | [ ] |
The above-mentioned research findings have provided insight into the exploitation of ginger in beverage products. Even in beverages, the employment of ginger extract seems to dominate over the rest of the ginger derivatives due to its better assimilation and conducive characteristics. The incorporation of ginger into different drinks not only augmented but also maintained the shelf life, nutraceutical value, and antimicrobial activity.
5.4. Meat and Fish Products
Several investigations and experiments were also conducted to figure out the effects of ginger and its derivatives in various forms on meat and fish products ( Table 4 ). Rabbit meat burger patties were fortified with ginger powder at two different concentrations (1% and 2%) and significantly improvised the fatty acid profile by accelerating the polyunsaturated fatty acids [ 136 ]. Ginger powder also increased the antioxidant capacity and reduced the lipid peroxidation, subsequently catering a meat product with a better shelf life and enriched nutritional status. Mancini et al. [ 137 ] also designed an experiment to enhance the quality of pork burgers through ginger powder incorporation. The physiochemical attributes, antioxidant capacity, fatty acid profile, microbial growth, and lipid oxidation of the ginger powder-enriched pork burger were evaluated, and the results were in agreement with the previous study. In another investigation, ginger was designated as a potential alternative of artificial antioxidants in pasteurized canned meat products [ 138 ]. Ginger and papain powders were also competently exploited as natural tenderizing agents in buffalo calf meat [ 139 ].
A comparative analysis was conducted to assess the effects of three different spices as potential organic preservatives in raw chicken meat products. Clove powder (0.2%), even in a very small quantity, compared to ginger (3%) and garlic (2%) pastes performed very well to improve the oxidative stability, physicochemical properties, and antimicrobial activities in raw chicken meat with refrigerated storage [ 140 ]. Ginger and pineapple juices had also been examined as promising marinating agents in raw and cooked goat meat [ 141 ]. Ginger juice not only provided oxidative stability but also developed the hedonic features by according a bright red color and strong marinating odor and reducing the gamey flavor of Saanen goat meat. In another investigation, papain juice was described as a better tenderizing agent than ginger and pineapple juices as it induced a mild disruption in the muscles of goose meat [ 142 ]. Fermented ginger paste was characterized along 25 metabolites with substantial antimicrobial activity and its application on chicken meat efficiently enhanced the shelf life through a significant reduction in the microbial load [ 143 ]. Similar findings were also reported by Abdel-Naeem and Mohamed [ 144 ] regarding the application of ginger and papain extracts in camel meat patties. Sukada et al. [ 145 ] stipulated that pineapple extract had superior hedonic characteristics than papaya leaf and ginger extracts when practiced on Bali beef through the immersion method.
It was revealed that the competence level of ginger was as good as sodium ascorbate to combat oxidative rancidity, while the textural and sensorial properties of meat products with ginger were better than synthetic antioxidants [ 146 ]. Ginger extract also performed efficiently in the case of smoked buffalo meat as the microbial, physicochemical, and sensorial attributes did not exhibit any remarkable changes during the storage period. Ginger extract not only regulated and maintained the microbial count within certain limits but also enhanced the functionality of the cooked meat products.
Smoked mackerel fish was treated with ginger extract to evaluate its influence on the shelf life and hedonic characteristics [ 147 ]. Ginger extract (5%) significantly improved the stability and functional value of the smoked fish through its antimicrobial and antioxidative features. The sensorial quality of fish with the incorporation of ginger extract was also better and more persistent than other treatments even during storage. Mattje et al. [ 148 ] disclosed that the antioxidant activity of supercritical ginger extract is greater than ginger essential oil, which, consequently, was detected in the treatment of tilapia fish burgers with ginger. Supercritical ginger extract also enhanced the shelf life of fish burgers from 6 to 8 days, but it disturbed the overall perception due its unfavorable spicy flavor.
A variety of synthetic tenderizers and proteolytic enzymes had been engaged in the tenderization of meat. Currently, natural tenderizers are in demand due to certain constraints of synthetic tenderizers such as safety issues, unpleasant tastes, and over-tenderization. Ginger extract (5%) was recommended as a potential tenderizer for Yak meat as it enhanced the cooking yield, tenderness, and lipid oxidative stability during storage [ 149 , 150 ].
Researchers had also adopted a distinct approach to improve the meat quality through feeding ginger, as a potential dietary supplement, to the animals. Ginger and its derivatives in various forms were highly effective in inducing favorable characteristics such as lowering the cholesterol and improving the egg functionality and carcass quality in poultry animals [ 151 , 152 ]. The dietary augmentation of rabbits was also carried out through ginger powder supplementation. This augmentation improved the resistance to lipid oxidation without causing any intervention to the productive functions of the rabbits [ 153 ]. The administration of red ginger as an herbal–biotic feed ingredient (1.5%) competently boosted the productive activity and carcass quality in broilers [ 154 ]. Ginger extract loaded with gingerol was effectively employed to derive its health-promoting features for the prosperity of heat-stressed broilers [ 155 ]. Healthy and good-quality meat could be produced through catering ginger and rosemary essential oils to New Zealand white (NZW) rabbits. The provision of both essential oils (0.5%) promoted the feed exploitation, growth efficiency, and meat quality in growing rabbits [ 156 ]. Meanwhile, essential oils from different spices and herbs including ginger were exploited for their potential applications as natural preservatives to improve the oxidative stability in meat and meat products [ 157 ].
Some important studies on the addition of ginger and its derivatives to meat and fish products.
| Derivative | Product | Positive Effect | Reference |
|---|---|---|---|
| Ginger Powder | Rabbit Burgers | ↑ Antioxidant Activity; Fatty Acid Profile; Shelf Life; ↓ Lipid Peroxidation | [ ] |
| Pork Burger | ↑ Antioxidant Activity; PUFA; Oxidative Stability; Sensorial Characteristics; ↓ Microbial Load | [ ] | |
| Shredded Ginger | Canned Pork | ↑ Antioxidant Activity; Tenderness; Organoleptic Properties; ↓ Microbial Load | [ ] |
| Ginger Paste | Chicken Meat | ↑ Antioxidants; Antimicrobial Activity; Oxidative Stability ↓ Microbial Load | [ , ] |
| Ginger Juice | Goat Meat | ↑ Antioxidant Activity; Tenderness; Oxidative Resistance; Storage Stability; Overall Acceptability; ↓ Gamey Odor | [ ] |
| Beef | ↑ Antioxidant Activity; Tenderness; Sensorial Properties; ↓ Cooking Loss | [ ] | |
| Ginger Extract | Camel Meat Patties | ↑ Antioxidants; Lipid Stability; Collagen Solubility; Sensorial Properties | [ ] |
| Smoked Buffalo Meat | ↑ Antioxidant Activity; Tenderness; Sensorial Characteristics; ↓ Cooking Loss; Microbial Load | [ ] | |
| Smoked Fish | ↑ Antioxidants; Shelf Life; Organoleptic Attributes; ↓ Mold Count; Oxidative Rancidity | [ ] | |
| Tilapia Fish Burger | ↑ Antioxidant Activity; Shelf Life; ↓ Lipid Oxidation | [ ] | |
| Yak Meat | ↑ Antioxidant Activity; Tenderness; Oxidative Resistance; Cooking Yield; ↓ Microbial Load | [ ] | |
| Gingerol Rich Extract | Chicken Feed | ↑ Antioxidant Activity; Bioactive Compounds; Meat Quality | [ ] |
↑ = Increased/Improved, ↓ = Lower/Decreased
As per the aforementioned studies, ginger has an extensive application potential in the meat industry, embellishing meat products with an array of favorable characteristics. The incorporation of ginger into meat products competently ameliorated the nutritional value, oxidative stability, lipid profile, and antimicrobial activity of the meat products. The ability of ginger to provide elegant antioxidant and tenderization activities without compromising the sensorial and textural attributes of meat products has also provided another priority lead over artificial antioxidants and tenderizing agents. The inclusion of ginger in the diets of animals as a dietary supplement has also emerged as a novel and efficient approach, which remarkably improves the fatty acid profile and carcass quality of the animals.

Sensory evaluation of different food products with incorporated ginger. ( A ) Pork burgers with added ginger powder [ 137 ]. ( B ) Comparison of ginger-based beverage with common coffee types. 1—traditional coffee; 2—ginger-based beverage; 3—instant coffee; 4—espresso coffee; 5—American/filter coffee [ 134 ]. ( C ) Different formulations of ginger-supplemented cookies. Ginger powder with concentrations of 0, 2, 4, 6, 8, 10, 12, and 14% for T1 to T8, respectively [ 82 ]. ( D ) Cottage cheese supplemented with natural ginger extract at a concentration of 1% to 10% of the total volume of the cottage cheese [ 158 ].
5.5. Film, Coating, and Packaging
Ginger and its bioactive compounds have also been examined for their potential applications as edible coatings, films, or active ingredients in packaging ( Table 5 ). The use of glycerol as a green solvent was also practiced to extract the bioactives from ginger powder [ 159 ]. The resultant glycerol ginger extract (GGE) was incorporated in a chitosan-based edible coating for walnut kernels. This coating formulation was effective against the lipid oxidation and fungal spoilage of walnuts under storage conditions. Another study disclosed that the combination of ginger and garlic is a more efficient preservative approach than other famous preservatives such as BHT and Nisin [ 160 ]. The application of ginger–garlic extract in the vacuum packaging of Herring fish fillets increases its stability against oxidation and microbial spoilage and also imparts favorable sensorial attributes to the product.
Whey protein isolate (WPI) coating on Kashar cheese was characterized with water and fat barrier attributes, which were significantly developed with the addition of 1.5% ( v / v ) ginger essential oil, regarded as whey protein isolate ginger (WPIG) coating [ 161 ]. WPIG coating was also found to be more competent in the shelf life extension of Kashar cheese due to the anti-bacterial activity of ginger essential oil against E. coli and S. aureus , while Tsironi et al. [ 162 ] also expressed that WPI films enriched with 1% ginger essential oil efficiently prevented the microbial spoilage of minced lamb meat with acceptable sensorial properties until 8 days of refrigerated storage.
Sodium alginate/agar (SA) coating enhanced the shelf life and maintained the quality attributes of beef; moreover, these characteristics were substantially improved with the incorporation of ginger essential oil (SAG) into edible coating [ 163 ]. Compared to the control, these coating formulations, SA and SAG, extended the shelf life of beef at a chilling temperature by 3 to 6 days and 9 days, respectively, through their counter actions against lipid oxidation and microbial decay. Ginger essential oil- fortified (6%) sodium caseinate-based edible coating fabricated through nanoemulsion efficiently improved the shelf life of raw chicken meat [ 164 ]. This edible coating significantly enhanced the microbial resistance and embellished the sensorial characteristics of the chicken meat, but no remarkable increment in antioxidant activity was observed. Researchers also developed a peculiar coating formula based on apple peel extract and zein loaded with ginger essential oil to prolong the shelf life of raw chicken meat [ 165 ].
Fish sarcoplasmic protein and chitosan composite films enriched with ginger essential oil (up to 3%) were analyzed for their physicochemical and antioxidant features [ 166 ]. Ginger essential oil (GEO) nanoemulsion furnished the composite films with better elongation at break, antioxidant, and water barrier properties, while on the other hand, GEO was also liable to accord the inferior thermal and mechanical properties. In another investigation, a blend containing equal concentrations of ginger and cinnamon essential oils was incorporated into chitosan films for its potential utilization in pork packaging. These composite films were reported with better thickness and opacity, restricted the lipid oxidation and microbial spoilage, and enhanced the antioxidant activity in pork slices stored at a refrigeration temperature [ 167 ]. The two different studies also evaluated the effects of ginger and cinnamon essential oil (EO) supplementation on soy protein isolate (SPI) and sodium caseinate-based edible films [ 168 , 169 ]. The water vapor permeability was almost unchanged with both EOs, while the optical features were significantly affected by the cinnamon essential oil. In SPI films, cinnamon EO was more resistant and stretchable than ginger EO, while both EOs were incompetent to cater any improvements in the lipid oxidation stability in sodium caseinate film.
Zhang et al. [ 170 ] also formulated a Tilapia fish skin gelatin, ginger EO, and ZnO nanoparticle-based microemulsion nanofilm for the shelf life extension of fresh meat where ginger EO was responsible for better mechanical characteristics and improved functionality status with elevated antioxidant and antimicrobial properties. Researchers have also struggled to employ ginger and its bioactive compounds in active packaging to prolong the shelf life of food products. Chitosan-based bio-nanocomposite enriched with ginger EO (1%) was effectively exploited to extend the shelf life of poultry without any compromise on the sensorial properties [ 171 ].
It is evident from the foregoing literature that ginger oleoresins have promising applications in edible films, coatings, and the packaging sector, where ginger essential oil has better and extensive utilizations than ginger extract. The consolidation of ginger significantly improved the oxidative stabilities, antimicrobial activities, and sensorial properties of the intended food products. Ginger also performed better than the synthetic preservatives, improved the shelf life, and retained the quality attributes of the products.
Some important studies on the application of ginger and its derivatives to films, coatings, and packaging.
| Derivative | Product | Positive Effects | Reference |
|---|---|---|---|
| Ginger Extract | Chitosan-Based Edible Coating (Walnut) | ↑ Antioxidant Activity; Shelf Life; Oxidative Stability; ↓ Fungal Spoilage | [ ] |
| Garlic Extract cum Vacuum Packaging (Fish Fillet) | ↑ Antioxidant Activity; Shelf Life; Oxidative Stability; Sensorial Properties; ↓ Microbial Spoilage | [ ] | |
| Ginger Essential Oil | Whey Protein Isolate Coating (Cheese) | ↑ Anti-bacterial Activity; Shelf Life; Water and Fat Barrier Properties | [ ] |
| Edible Coating (Chicken Meat) | ↑ Shelf Life; Sensorial Characteristics ↓ Microbial Growth; ≈ Antioxidant Activity | [ ] | |
| Fish Protein and Chitosan Composite Films | ↑ Antioxidant Activity; Shelf Life; Water Barrier Properties | [ ] | |
| Cinnamon EO Chitosan Films (Pork) | ↑ Antioxidant Activity; Shelf Life; Film Thickness; ↓ Microbial Spoilage; Lipid Oxidation | [ ] |
6. Conclusions and Future Prospectus
Ginger, with strong antioxidant, antimicrobial, and other functional activities, has a great potential for employment in the food industry. In this review, many studies have been presented regarding the acquirement of the bioactive compounds of ginger in different forms such as powder, extract, juice, paste, and essential oil. The application of ginger and its derivatives in a variety of food products has been extensively investigated and reported with auspicious results. Among all of the derivatives of ginger, ginger extract was found to be a more practicable, efficient, and versatile form of ginger for food applications. Ginger essential oil is another efficient variant of ginger, but its food applications are limited because of its strong aroma and immiscibility with certain food products.
These studies have also revealed another positive aspect of ginger, which is that it could be employed in a wide range of food products, covering almost all leading sectors of the food industry. The incorporation of ginger, in its various forms, thoroughly improved the functionality and bioactivity of the intended food products, but the physicochemical and sensory properties of these products were also compromised in a legitimate number of studies.
Further studies mainly focused on the efficacy, optimization, and assimilation of ginger bioactives into food products without compromising the physicochemical characteristics and sensory profiles of the products must be carried out. The probable synergistic effect of ginger bioactives with other compounds and toxicity studies of different compounds in their purest forms should also be investigated to ensure the safe use of these functional compounds.
Funding Statement
This research was funded by the University of Catania, Linea 2-Project, ‘RiDARE’.
Author Contributions
Conceptualization, M.N.S. and B.F.; writing—original draft preparation, M.N.S.; writing—review and editing, M.N.S., A.N. and B.F.; visualization, A.N.; supervision, B.F.; funding acquisition, B.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
REVIEW article
A critical review of ginger’s ( zingiber officinale ) antioxidant, anti-inflammatory, and immunomodulatory activities.

- 1 Nutrition Science Department, Faculty of Medicine, Universitas Diponegoro, Semarang, Indonesia
- 2 Center of Nutrition Research (CENURE), Universitas Diponegoro, Semarang, Indonesia
- 3 Food Quality and Design, Wageningen University & Research, Wageningen, Netherlands
Ginger ( Zingiber officinale ) is a rhizome that has been used as a healthy herbal plant for years. Ginger’s chemical components are recognized to provide beneficial health effects, namely as antioxidants and anti-inflammatory agents with the potential to operate as immunomodulators. This literature review covers numerous publications concerning ginger’s immunomodulatory potential, associated with antioxidant and anti-inflammatory effects in modifying the body’s immune system. Pathophysiology of oxidative stress and inflammation were introduced before diving deep down into the herbal plants as an immunomodulator. Ginger’s antioxidant and anti-inflammatory properties are provided by gingerol, shogaols, paradol, and zingerone. Ginger’s antioxidant mechanism is linked to Nrf2 signaling pathway activation. Its anti-inflammatory mechanism is linked to Akt inhibition and NF-KB activation, triggering the release of anti-inflammatory cytokines while reducing proinflammatory cytokines. Ginger consumption as food and drink was also explored. Overall, ginger and its active components have been shown to have strong antioxidant properties and the potential to reduce inflammation. Challenges and future prospects of ginger are also elaborated for future development. Future collaborations between researchers from various fields, including chemists, biologists, clinicians, pharmacists, and the food industry, are required further to investigate the effect of ginger on human immunity. Collaboration between researchers and industry can help accelerate the advancement of ginger applications.
1 Introduction
Ginger ( Zingiber officinale ), belonging to the Zingiberaceae family, has been widely used as a spice in various foods and beverages worldwide. In Southeast Asia, ginger has long been used as a traditional medicine to treat digestive problems, sore throats, coughs, fevers, and so on ( 1 ). The biological activity of ginger comes from the content of volatile and non-volatile compounds. The volatile components in ginger are essential oils with a distinctive aroma, which include sesquiterpenes and monoterpenoids. In contrast, the non-volatile components give ginger a pungent, spicy taste, including gingerol, shogaol, zingerone, and paradol. In fresh ginger, the main component is gingerol, which will then be converted to shogaol, zingerone, and paradol in ginger-based products ( 2 ).
The immune system is critical to the body’s physiological and immunological function. Therefore, any immune system disturbance can result in various diseases ( 3 , 4 ). The immune system is a complex framework made up primarily of leukocytes and various immune components such as antibodies, proteins, and cytokines that serve as the first line of defense against a wide range of potentially harmful substances in the environment. The interaction of these different immune components makes it easier to establish an optimal immune response ( 5 ). In order to prevent various disorders, the immune system identifies and eliminates pathogens while inducing inflammation, cell or tissue damage, cell death, and wound healing. Immune-mediated diseases, metabolic disorders, and inflammatory diseases can occur if homeostasis is not maintained, as well as susceptibility to infectious diseases such as COVID-19 ( 6 , 7 ).
Degenerative diseases, such as diabetes mellitus and hypertension, cause high mortality rates. Degenerative diseases also increase the severity of infectious diseases. Oxidative stress due to free radicals from the environment is still a threat during a pandemic, so it can disrupt the immune system. Therefore, the easiest and most immediate way to prevent or reduce the transmission of infectious diseases is through clean and healthy living behaviors, social restrictions, and increasing body immunity ( 8 ).
The body’s immune function is essential for prevention, disease recovery, and reducing the risk of infectious and chronic disease. Increasing the body’s immunity can be done by eating balanced nutritious foods supplemented by vitamins, minerals, and bioactive compounds. Herbal plants contain bioactive compounds that can boost the immune system. In addition, herbal plants can also provide therapeutic benefits and have the potential to have antioxidant activity that can suppress free radicals and prevent oxidative stress and inflammation in the body ( 8 ).
Immunomodulators are substances that help to regulate the immune system ( 9 ). Immunomodulators are available in both endogenous and exogenous forms, with the mechanism often based on antioxidant properties. Some natural plant antioxidants have been shown to have immunomodulatory properties including alkaloids, flavonoids, flavones, flavonols, flavanols, isoflavones, quinones, terpenoid ( 10 ). Immunomodulators derived from antioxidants help maintain health by regulating the oxidant-antioxidant balance and immune response. Antioxidants work both directly and indirectly by eliminating reactive oxygen-nitrogen species and increasing the body’s antioxidant balance ( 11 ). Many plants’ bioactive compounds have been investigated for their potential benefits as immunomodulators ( 9 ).
Various studies have explored ginger’s biological activity, the active components that contribute, and their mechanism of action ( 12 ). In vitro and in vivo research demonstrate that ginger crude extract has biological activities such as antioxidant, anti-inflammatory, antibacterial, antiviral, and anticancer properties ( 1 ). Some recent papers showed the different aspects of ginger. The therapeutic role of ginger has been studied by Kausar et al., including antioxidant effect, anti-nausea effect, anti-inflammatory effect, anti-cancer effect, cardiovascular effect, and anti-diabetic effect ( 13 ).
The recent review by Mao ( 1 ), discussed the bioactive compound and bioactivities of ginger, including antioxidant activity, anti-inflammatory activity, antimicrobial activity, neuroprotection, anti-obesity activity, anti-diabetic activity, protective effect against respiratory disorders, also the other bioactivities of ginger (hepatoprotective and antiallergic effects) ( 1 ). Jalali ( 14 ) in a systematic review and meta-analysis, describe ginger supplementation’s effect on inflammatory and oxidative stress markers. A recent study by Ghafoor ( 15 ) shows the effect of drying methods on the total phenolic and antioxidant activity. Morvaridzadeh ( 16 ) in a systematic review and meta-analysis, studied the effect of ginger supplementation on oxidative stress parameters.
Based on this existing background information, this review will consider the antioxidant and anti-inflammatory role of ginger in the immunomodulatory system since it is a relevant but underexposed factor.
Scientific information on Ginger’s ( Zingiber officinale ) Antioxidant, Anti-Inflammatory and Immunomodulatory Activities and their bioactive compounds were gathered. Keywords used including Zingiber officinale antioxidant, Zingiber officinale anti-inflamatory, Zingiber officinale Immunomodulatory. A literature search was conducted using Google Scholar. Published scientific papers from respected publisher such as ACS, Frontiers, ScienceDirect, Springer, Emerald, and others was used.
3 Discussion
3.1 ginger ( zingiber officinale ).
Ginger ( Zingiber officinale ) has been widely used as a spice in various foods and beverages around the world. In Southeast Asia in particular, ginger has also long been used as a traditional medicine to treat digestive problems, sore throats, coughs, fevers, and so on ( 1 ). While in China, it is known as an herbal plant that is widely used in traditional medicine ( 17 ). In Indonesia, ginger has long been known and used as a spice, medicinal plant, as well as flavor and aroma in various foods and beverages. Ginger is a rhizome plant that has been developed in the medicinal industry as perfume and traditional herbal medicine. Young ginger is eaten as fresh vegetables, processed into pickles, and used as an ingredient in beverages ( 18 ).
Ginger is a seasonal herbaceous plant, pseudo-trunked and erect with a height of between 30 cm to 1 m, leaf length of 15–23 mm, leaf width of 8–15 mm, and 2–4 mm long hairy petioles. Morphologically, the ginger plant consists of roots, rhizomes, stems, leaves, and flowers. Ginger has rhizome roots that can last a long time in the soil and can produce new shoots to replace dead leaves and stems. Ginger rhizome branched irregularly, coarsely fibrous, spreading flat and pale-yellow inside. Roots grow from the bottom of the rhizome while shoots will grow from the top of the rhizome ( 18 ). In fact, ginger has different shapes and flavors based on its type ( Table 1 ).
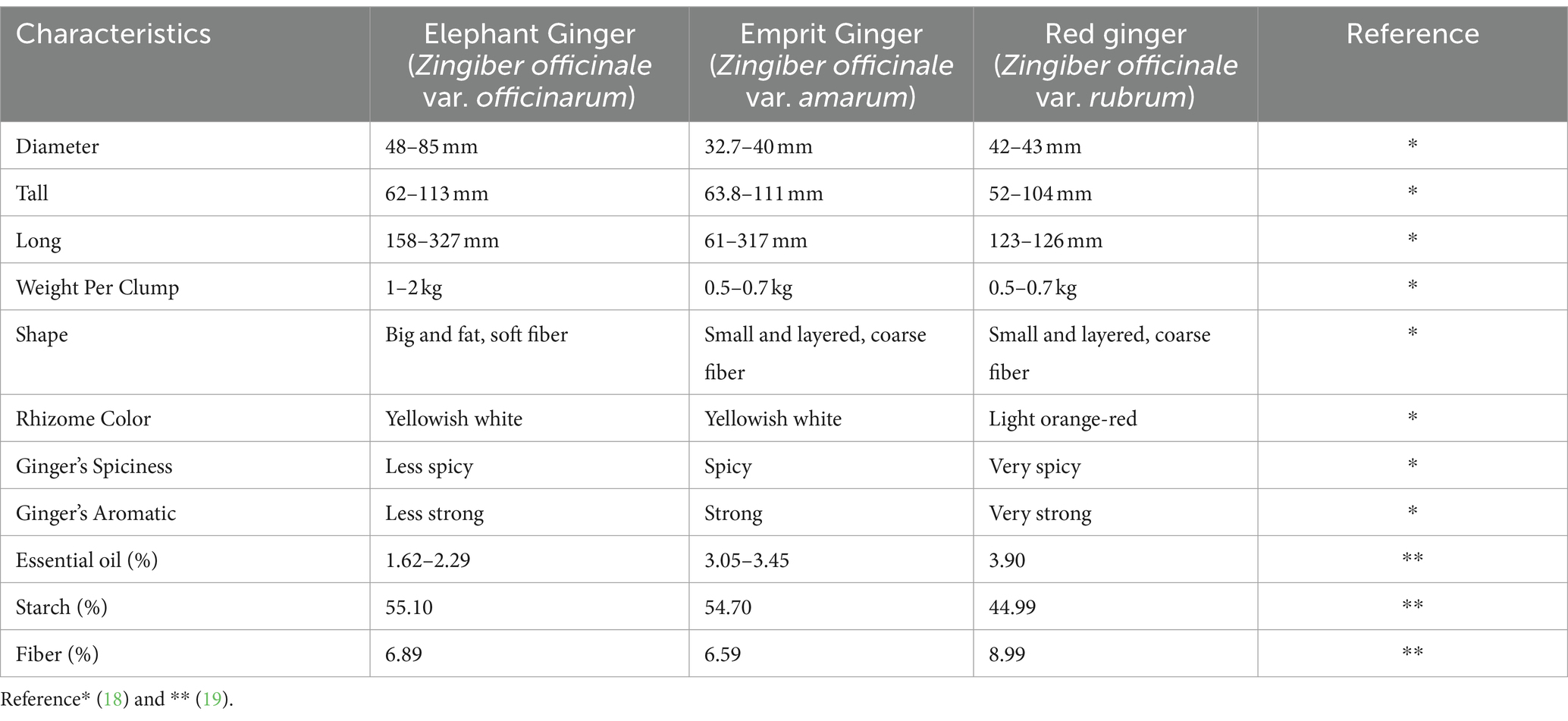
Table 1 . Comparison of the morphology and content between different ginger’s variety.
Ginger has a lot of active compounds such as phenolics and terpenoids. Ginger contains phenolic elements such as gingerol, shogaol, zingerone, and paradol. The major polyphenols in fresh ginger are gingerols. Ginger also contains a variety of phenolic chemicals and terpenoid components, which are regarded to be the major ingredients of ginger essential oil. Ginger also contains carbohydrates, lipids, organic acids, fiber, and other nutrients ( Table 2 ) ( 1 ).
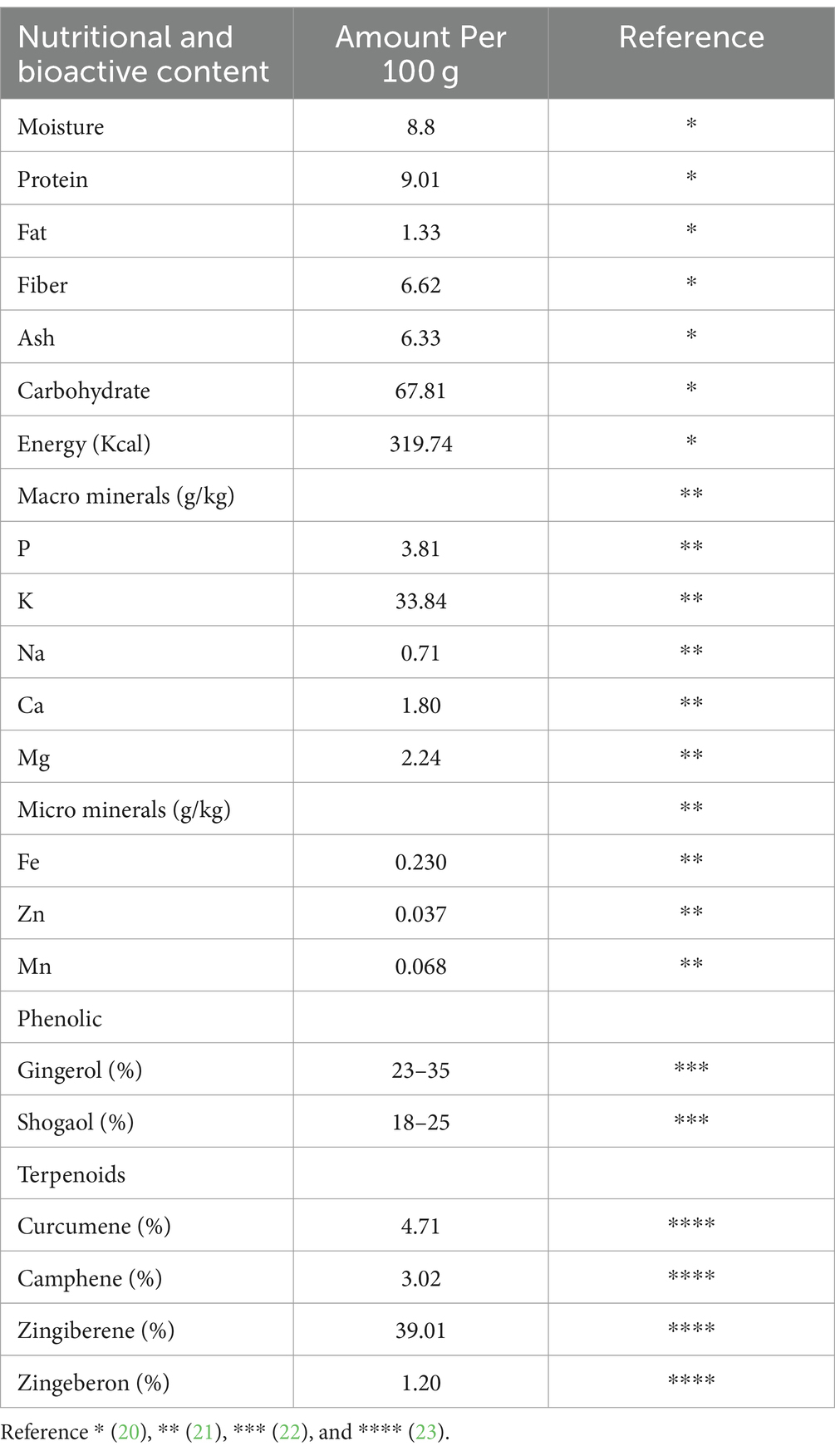
Table 2 . Nutrition content of ginger.
3.1.1 Elephant ginger ( Zingiber officinale var. officinarum )
Elephant ginger is commonly utilized in culinary applications and herbal beverages due to its large rhizome, a milder odor and a relatively lower fiber content and concentration of essential oil compared to other varieties of ginger. Its essential oil comprises 45 components, including 1,8-cineole (6.4%), ar-curcumene (2.75%), camphene (6.48%), citral (16.19%), fernesene (3.8%), linalool (2.57%), methylheptenone (2.33%), sabinene (6.19%), z-citral (neral) (11.84%), α-cedrene (4.72%), α-pinene (3.22%), β-bisabolene (2.28%), β-myrcene (2.48%), and β-sesquiphellandrene (3.35%). Chemical structure of each components can be observed in Figure 1 . A previous study revealed that elephant ginger infusion extraction yielded the lowest phenolic content (7.6400 ± 0.21 mgGAE/g) and exhibited the least antioxidant activity (61.7000 ± 1.51) based on the DPPH method when compared with two other ginger variations ( 24 , 25 ).
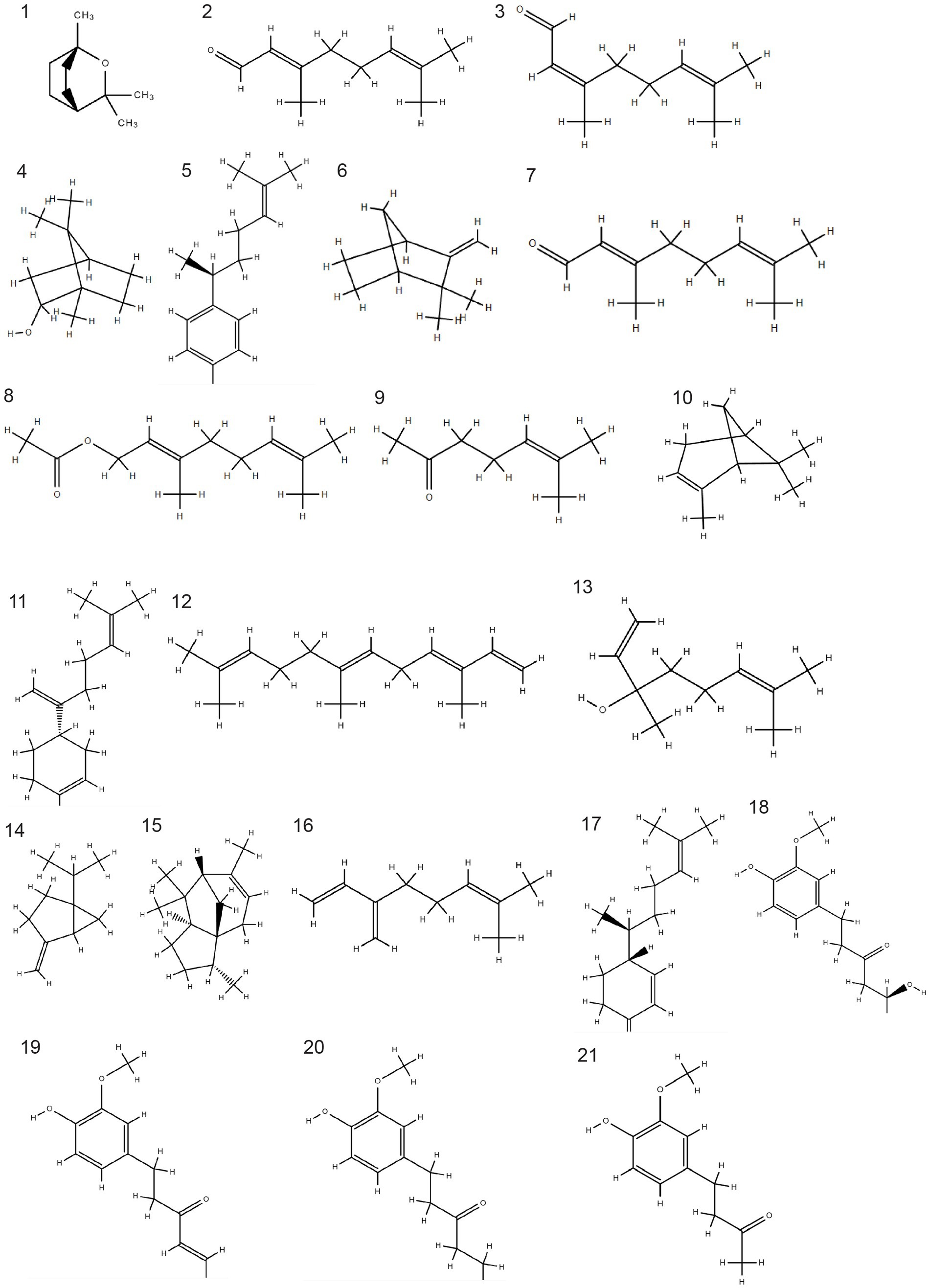
Chemical structure of bioactive components in three varieties of ginger. 1. 1,8-cineole, 2. geranial, 3. neral, 4. borneol, 5 ar-curcumene, 6. camphene, 7. citral, 8. geranyl acetate, 9. methyl heptanone, 10. α-pinene, 11. Β-bisabolene, 12. fernesene, 13. linalool, 14. sabinene, 15. α-cedrene, 16. β-myrcene, 17. β-sesquiphellandrene, 18. gingerol, 19. shogaol, 20. paradol, 21. zingerone. The SDF coordinate generated by PubChem and the chemical structure was build using Chemspider .
3.1.2 Emprit ginger ( Zingiber officinale var. amarum )
Emprit ginger is also utilized for culinary and herbal applications albeit with distinct characteristics compared to elephant ginger. Unlike elephant ginger, emprit ginger features a smaller rhizome, beige skin color, sharper odor, and higher fiber content. Notably, emprit ginger boasts a greater concentration of essential oil than elephant ginger. Its essential oil profile comprises 38 chemical constituents, including notable components such as 1,8-cineole (7.95%), 1,8-p-methandiene (2.74%), ar-curcumene (6.86%), Bornyl acetate (3.21%), camphene (10.14%), citral (16.19%), geranyl acetate (2.24%), methylheptenone (2.44%), z-citral (12.23%), α-pinene (2.5%), and β-bisabolene (2.64%). Chemical structure of each components can be observed in Figure 1 . A preceding study observed that emprit ginger infusion extraction exhibited higher phenolic content (9.5033 ± 0.35 mgGAE/g) and displayed greater antioxidant activity (70.4333 ± 0.25) based on the DPPH method compared to elephant ginger ( 24 , 25 ).
3.1.3 Red ginger ( Zingiber officinale var. rubrum )
Red ginger is generally utilized for dietary supplementation and traditional remedies, characterized by its relatively smaller rhizome size, red skin color, sharp aroma, and higher fiber content, as well as boasting the highest concentration of essential oil among ginger varieties. The essential oil derived from red ginger notably comprises a significant percentage of monoterpenoids (60.6%), primarily including 1.8-cineole (15.1%), geranial (12%), neral (7.1%), and borneol (6.8%). Sesquiterpene constituents are present in lower proportions compared to monoterpenes, with ar-curcumene (16.9%), β-sesquiphellandrene (6.8%), and β-bisabolene (6.4%) being the dominant components. Chemical structure of each components can be observed in Figure 1 .
Previous research has indicated that red ginger exhibits the highest phenolic content in ginger infusion extraction methods (12.2533 ± 0.13 mgGAE/g) ( 24 ). Similarly, according to the DPPH method, red ginger demonstrates the highest antioxidant activity in ginger infusion extraction methods (70.4333 ± 0.25%) among the three ginger variations studied ( 24 , 25 ). Additionally, Table 3 provides a detailed comparison of the chemical constituents present in the three ginger varieties.
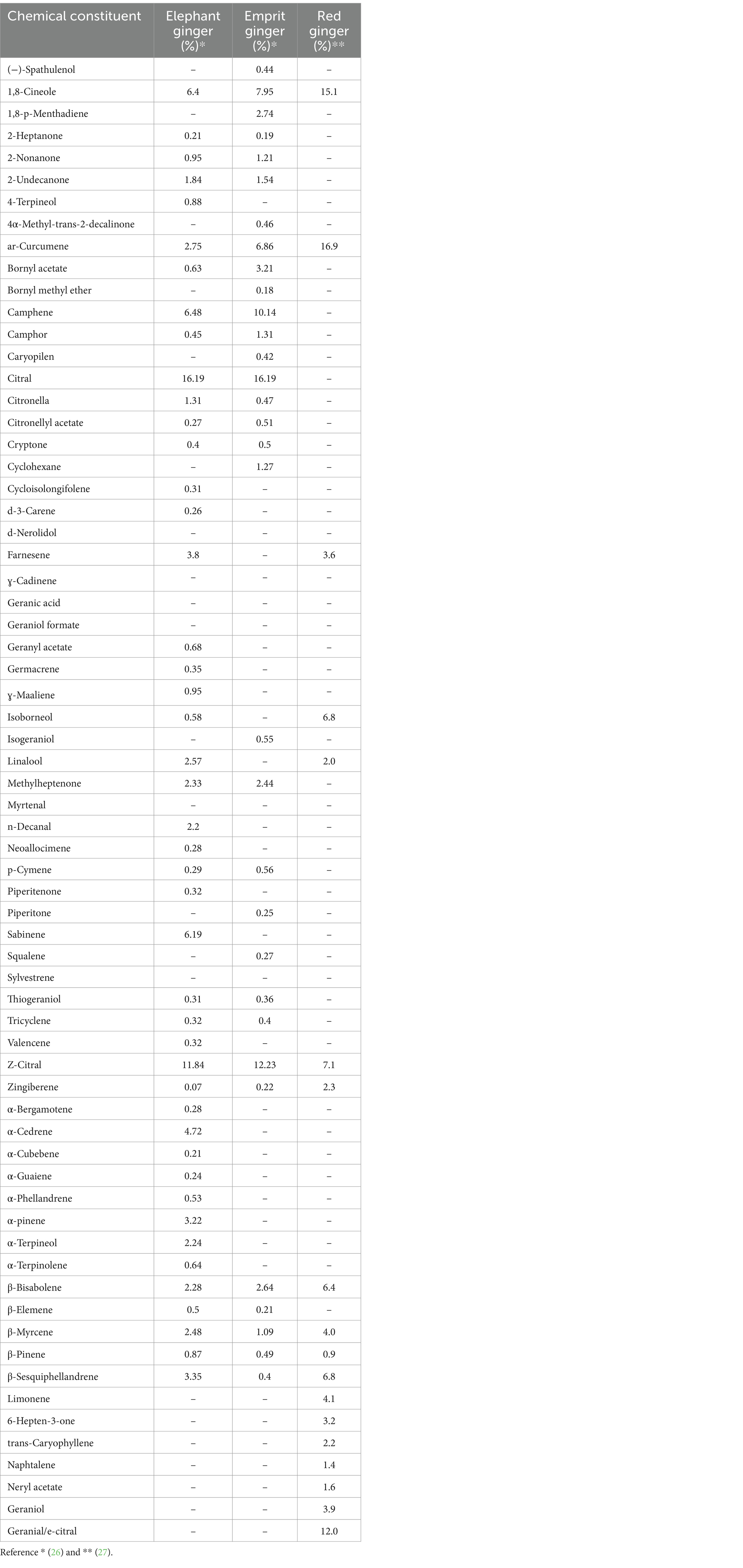
Table 3 . Bioactive constituents in three ginger varieties.
3.2 Immunomodulators
The immune system works as the body’s defense against exposure to foreign substances by recognizing and killing these foreign substances. The immune system is divided into innate and adaptive immune systems, which determine the ability to fight off harmful agents. Innate immunity, which includes epithelial protection, antimicrobial proteins, peptides, humoral and cellular components is recognized as the first line of defense against pathogens. Leukocytes produced ROS to destroy the engulfed pathogen ( 28 ). The innate immune system activates the adaptive immune system, which aids in foreign antigen recognition ( 29 ). The adaptive immune responses consist of B lymphocytes with antigens, T lymphocytes, and regulatory T cells (T-reg) ( 30 ).
The most important cytokines in the body are T-lymphocytes. These cells possess antigen receptors on their cell surfaces that allow them to recognize invading pathogens. T cells are divided into CD4 and CD8. Helper T cells are T lymphocytes that express CD4. This subgroup is further separated into T helper 1 (Th1) and T helper 2 (Th2) cells, referred to as Th1-type cytokines and Th2-type cytokines. Th1-type cytokines are responsible for destroying intracellular parasites by triggering a pro-inflammatory response. Interferon (IFN-γ), tumor necrosis factor (TNF-α), interleukin-1 (IL-1), and IL-18 are examples of Th1 cytokines ( 31 ). Th2-type cytokines, on the other hand, have a more complicated mode of action. Th2 cytokines include IL-4, IL-5, IL-9, and IL-13, all of which are linked to the immunoglobulin response; IL-6, which is pro-inflammatory; and IL-10, which is anti-inflammatory. As a result, a balance of Th1 and Th2 responses is required to fight pathogens effectively while preventing uncontrolled inflammation ( 32 ). Activation of the Nuclear Factor Kappa B (NF-κB) and COX 2 signaling pathways can also increase the concentration of pro-inflammatory cytokines and the activator cascade to activate other cytokines that induce inflammation ( 33 ).
Immunity can be maintained and enhanced by consuming vitamins, minerals, and active compounds from nature that are effective immunomodulators. Antioxidant and anti-inflammatory properties may contribute and play a role as an immunomodulator. Antioxidants neutralize free radicals and prevent oxidative stress, ultimately maintaining the immune system. Anti-inflammatory is also linked with antioxidants to regulate free radical production as well as regulate cytokine pro-inflammation to prevent inflammation. The pathophysiology of oxidative stress and inflammation is essential in understanding the role of antioxidants and anti-inflammatories as immunomodulators.
3.2.1 Pathophysiology of oxidative stress
Oxidative stress can be induced by free radical production. Free radicals are compounds that contain one or more unpaired electrons that are highly reactive and able to oxidize lipids, proteins, carbohydrates, and DNA ( 34 ). Free radicals can be reactive oxygen or nitrogen species (ROS/RNS), referred to as Reactive Oxygen-Nitrogen Species (RONS) ( 35 ). All aerobic cells can generate RONS as a by-product of oxidation and cell metabolic processes during respiration, cell reproduction, maximum physical activity, inflammation (inflammation), and exposure to external contaminants. Superoxide radicals (O 2 −), hydroxyl radicals (OH−), peroxyl radicals (ROO−), hydrogen peroxide radicals (H 2 O 2 ), singlet oxygen radicals (½O 2 ), nitric oxide radicals (NO−), peroxynitrite radicals (ONOO−), hypochlorous acid radicals (HOCl), and fat oxidation products are all reaction product of RONS ( 35 , 36 ).
The major contributor of superoxide radicals (O 2 −) is NADPH oxidase, which is synthesized during cellular respiration by reducing one-electron oxygen molecules with electrons delivered by NADPH. Most O 2 radicals were transformed into hydrogen peroxide (H 2 O 2 ) radicals by Superoxide Dismutase (SOD). Because it lacks unpaired electrons, H 2 O 2 radicals are not free radicals but can become hydroxyl ions (OH − ), which via the Fenton or Haber-Weiss reaction, are highly reactive radicals. The hydroxyl radical (OH−) is very reactive, particularly with phospholipids in cell membranes and proteins. In neutrophils, H 2 O 2 may combine with chloride and MPO to form hypochlorous acid radicals, the primary ROS that damage cellular proteins. L-arginine can stimulate three major isoforms of NO synthase: epithelial NO synthase, which is involved in vasodilation and vascular regulation; neuron NOS, which is involved in intracellular signaling; and receptor NOS, which is stimulated in response to various endotoxin or cytokine signal activations. Finally, O 2 radicals can interact with NO radicals to synthesize reactive substances such as peroxynitrite radicals (ONOO−) ( 35 , 36 ).
Exogenous RONS contributors include air and water pollution, tobacco, alcohol, heavy or transition metals, drugs (e.g., cyclosporine, tacrolimus, gentamicin, and bleomycin), industrial solvents, unhealthy food (e.g., smoked meats, used oils, and fats), radiation, and bacterial, fungal, and viral infections ( 35 , 36 ).

3.2.2 Pathophysiology of inflammation
Inflammation plays a vital role in the body’s response to a disturbance or infection. The inflammatory response is regulated by mediators released by cells around the site of infection and the circulatory system (immune cells). Mediators, including cytokines, proteins, and inflammatory enzymes, can indicate inflammation-related diseases. The various cytokines have their respective sources and roles in inflammation ( Table 4 ).

Table 4 . Cytokines’ various, sources, and their roles in inflammation.
Severe infectious disease can trigger uncontrolled cytokine production, called a cytokine storm. The excessive number of immune cells at the site of infection causes cell and tissue damage, organ failure, and even death ( 38 ). In addition, oxidative stress is also closely related to inflammation and is bidirectional. The formation of RONS triggers inflammation, but on the other hand, inflammation also triggers the formation of RONS, which ends in oxidative stress, as described in Figure 2 ( 33 ). Therefore, antioxidants play a crucial role in preventing inflammation and preventing or suppressing the onset of oxidative stress and cell tissue damage. Antioxidants can limit the production of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor (TNF-α) ( 36 ). In addition, antioxidants increase the effectiveness of white blood cells maintaining the immune system and increasing the body’s resistance to diseases.
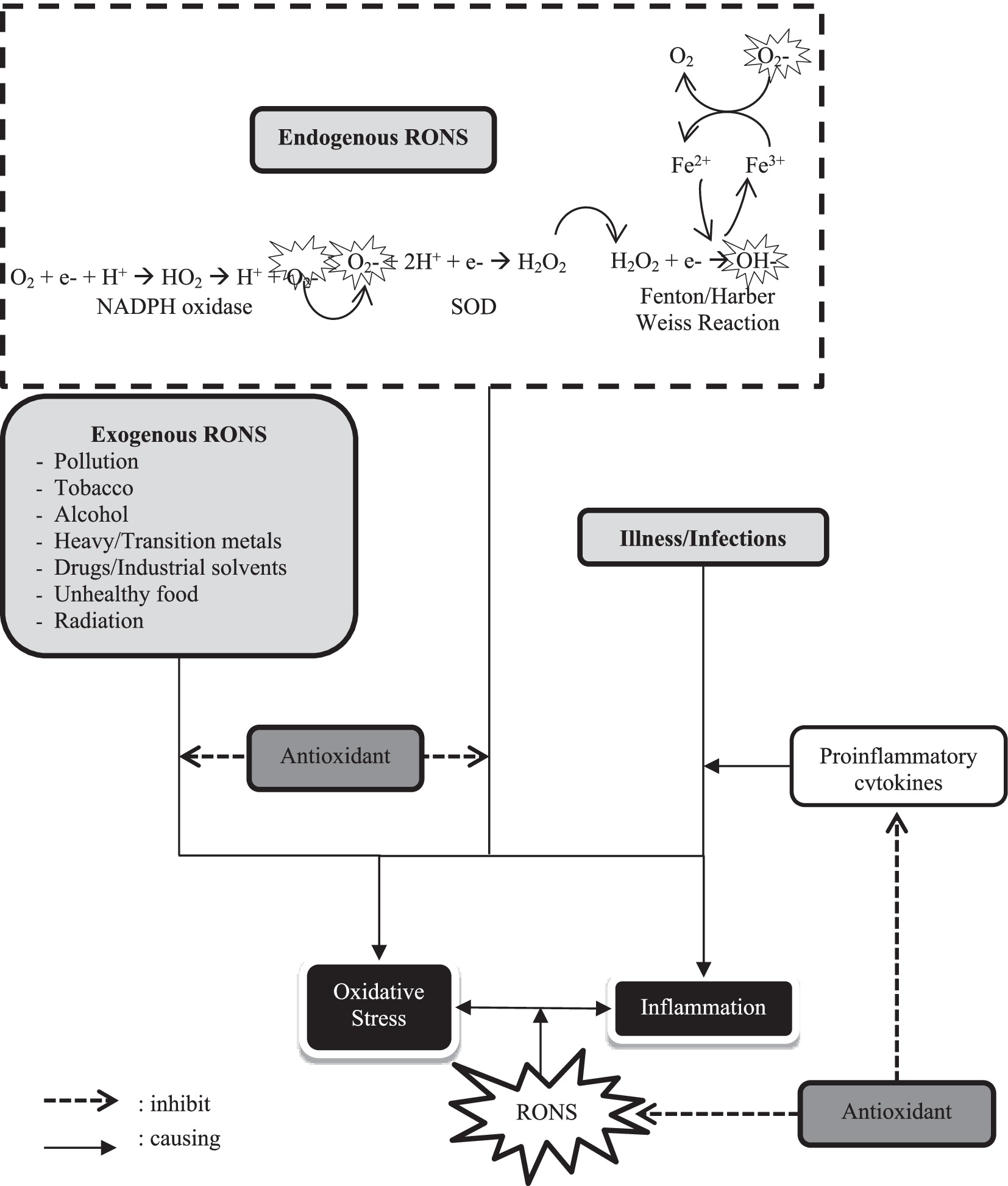
Figure 2 . Oxidative-inflammatory stress relationship and the role of antioxidants. (O 2 −, superoxide radical; SOD, superoxide dismutase; H 2 O 2 , hydrogen peroxide; OH−, hydroxyl radical; RONS, reactive oxygen-nitrogen species).
Respiration and metabolism produce O 2 − which is neutralized by SOD into H 2 O 2 . It can become OH− through Fenton/Harber Weiss Reaction. All those mechanisms belong to endogenous RONS production. Exogenous RONS, including pollution, tobacco, metals, drugs, etc., with endogenous RONS might be causing oxidative stress. Illness/infection can trigger inflammation by proinflammatory cytokines increasing. RONS play a role in bidirectional causes both stress oxidative and inflammatory. Antioxidants can inhibit exogenous and endogenous RONS as well as regulate proinflammatory cytokines, so it has potential as an immunomodulator.
3.3 Herbal plants as immunomodulator
Several endogenous and exogenous factors contribute to immunosuppression and immunostimulation during the homeostatic state, influencing innate and adaptive immune responses ( 10 ). Immune response modulation regulates immune response in therapeutic and preventive efforts against causative agents ( 39 , 40 ). immunomodulators use is currently one of the most pressing issues in the treatment of various diseases.
The use of plant-derived bioactive compounds has become one of the most active areas of research for natural immunomodulators ( 41 ). Natural immunomodulators are becoming popular due to their broad application in disease prevention and treatment via changes in immune response or oxidant-antioxidant status ( 42 ).
Antioxidants function as a defense in protecting the body’s biological systems from the toxicity of free radicals. Antioxidants have two different mechanisms: endogenous and exogenous, as described in Figure 3 .
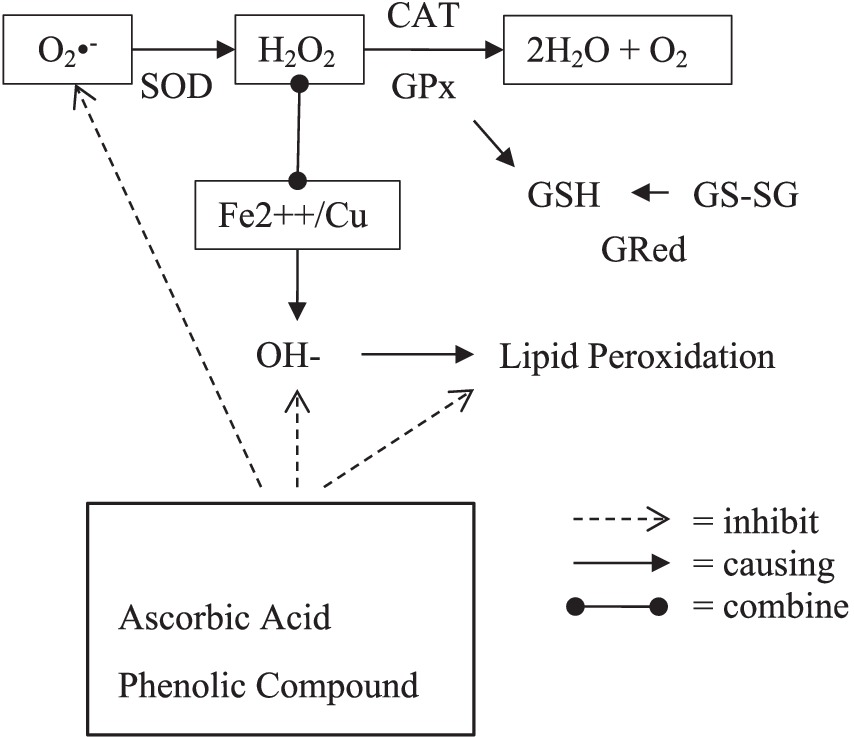
Figure 3 . Mechanism of endogenous and exogenous antioxidant. (O 2 • − , superoxide radical ion; H 2 O 2 , hydrogen peroxide; OH−, hydroxyl radical; SOD, superoxide dismutase; GPx, glutathione peroxidase; GSH, glutathione; GS-SG, glutathione disulfide; GRed, glutathione reductase).
The body may synthesize its antioxidants (endogenous antioxidants) in biological systems, both enzymatic and non-enzymatic routes. Superoxide Dismutase (SOD), Catalase (CAT), and Glutathione Peroxidase (GPx) are forms of enzymatic antioxidants ( 35 , 36 ).
SOD enzymes in the cytosol and mitochondria convert superoxide radicals (O 2 −) into hydrogen peroxide (H 2 O 2 ) catalyzed by the enzymes Catalase (CAT) and Glutathione Peroxidase (GPx). The CAT enzyme uses one H 2 O 2 molecule as an electron donor substrate and one H 2 O 2 molecule as an electron acceptor substrate so that 2H 2 O 2 molecules are neutralized into 2H 2 O and O 2, thereby preventing the production of hydroxyl radicals. In erythrocytes and other tissues, the GPx enzyme catalyzes the destruction of H 2 O 2 and lipid hydroperoxides using reduced glutathione (GSH), protecting membrane lipids and hemoglobin from H 2 O 2 oxidation, and preventing hemolysis caused by hydrogen peroxide attack. GSH will be oxidized to Glutathione Disulfide (GS-SG). In order for GSH to continue to be available to help the GPx enzyme work, this GS-SG must be reduced again to GSH. This function is played by the enzyme Glutathione Reductase (GRed). H 2 O 2, which is not converted to H 2 O, can form reactive hydroxyl radicals (OH) when reacted with transition metal ions (Fe 2++ /Cu). These hydroxyl radicals are more reactive and dangerous because they can cause cell damage through the peroxidation of lipids, proteins, and DNA.
On the other hand, the body does not have enzymes to convert hydroxyl radicals into safe molecules for cells. These hydroxyl radicals are more reactive and dangerous because they can cause cell damage through the peroxidation of lipids, proteins, and DNA. Glutathione-S-transferase and glucose-6-phosphate dehydrogenase are two different other antioxidant enzymes. Non-enzymatic antioxidants interact with RONS and stop the chain reactions of free radicals in the blood, such as tocopherols and carotenoids ( 35 ).
Increased pollution causes excessive ROS production, so endogenous antioxidants are not enough to neutralize free radicals. Therefore, the body needs antioxidants from daily food and drinks ( 36 ). Exogenous antioxidants are antioxidants that come from outside the body and are ingested, such as ascorbic acid (vitamin C), which scavenges hydroxyl and superoxide radical anions; tocopherols (vitamin E), which fight cell membrane lipid peroxidation; and phenolic antioxidants, which include stilbene derivatives (resveratrol, phenolic acids, and flavonoids), oil lecithin, selenium, and zinc ( 35 ).
During exogenous antioxidant intake deficiency, the body has the potential to experience an imbalance between the number of free radicals and the amount of antioxidants called oxidative stress. In the long term, this condition can cause cell damage that can lead to various diseases such as cancer, heart disease, cataracts, premature aging, and other degenerative diseases ( 36 ).
The antioxidant defense system consists of free radical reduction, antioxidant enzyme activation and maintenance, enhancement of other antioxidant mechanisms, and specific immunomodulatory effects ( 42 ). Plants that act as natural immunomodulators can boost immunity by activating innate immune responses such as activating immune cellular components, changing cytokine profiles, and reducing infection and inflammation. This plant’s immunomodulatory properties are linked to its bioactive compounds ( 10 ).
Herbal plants are plants or medicinal plants that can be used for traditional treatment of diseases. The use of herbal plants has been empirically proven and used to maintain a healthy body. In addition, herbal plants are also used by the community in cooking, such as the use of spices. Herbal plants are known to contain many secondary metabolites such as flavonoids, alkaloids, tannins, and triterpenoids ( 17 ). Flavonoids, for example, are phenolic chemical derivatives that can bind RONS, restrict the operation of enzymes that make RONS, and form chelates with metals that stimulate the synthesis of RONS, preventing RONS interactions with normal cells such as lipid peroxidation and DNA damage. Herbal plants are found in the form of roots, rhizomes, tubers, bark, stems, leaves, flowers, fruits, and seeds, and are frequently utilized by the community, such as curcuma, cinnamon, bay leaf, etc. Ginger rhizome is a form of rhizome that has been trusted and utilized for years. Its effectiveness is said to be as a carminative (trigger flatulence) to relieve cold symptoms, and improve appetite, and has herbal benefits to cure coughs, heartburn, dizziness, nausea, and influenza.
3.4 Ginger as immunomodulators
Overproduction of free radicals (RONS) plays an important role in the development of various diseases and triggers inflammation. Several studies have found that ginger has been shown to have potential as a natural immunomodulator through both antioxidant and anti-inflammation properties, or by their modulation to other immune components ( Table 5 ), so that it can prevent oxidative stress and inflammation ( 1 ). It is known that ginger contains antioxidants with an IC50 value of 4.25 g/mL. This means that ginger has a good antioxidant effect (IC50 < 5 mg/mL). Therefore, this ginger rhizome is one of the herbal plants that is widely used by the community ( 17 ).
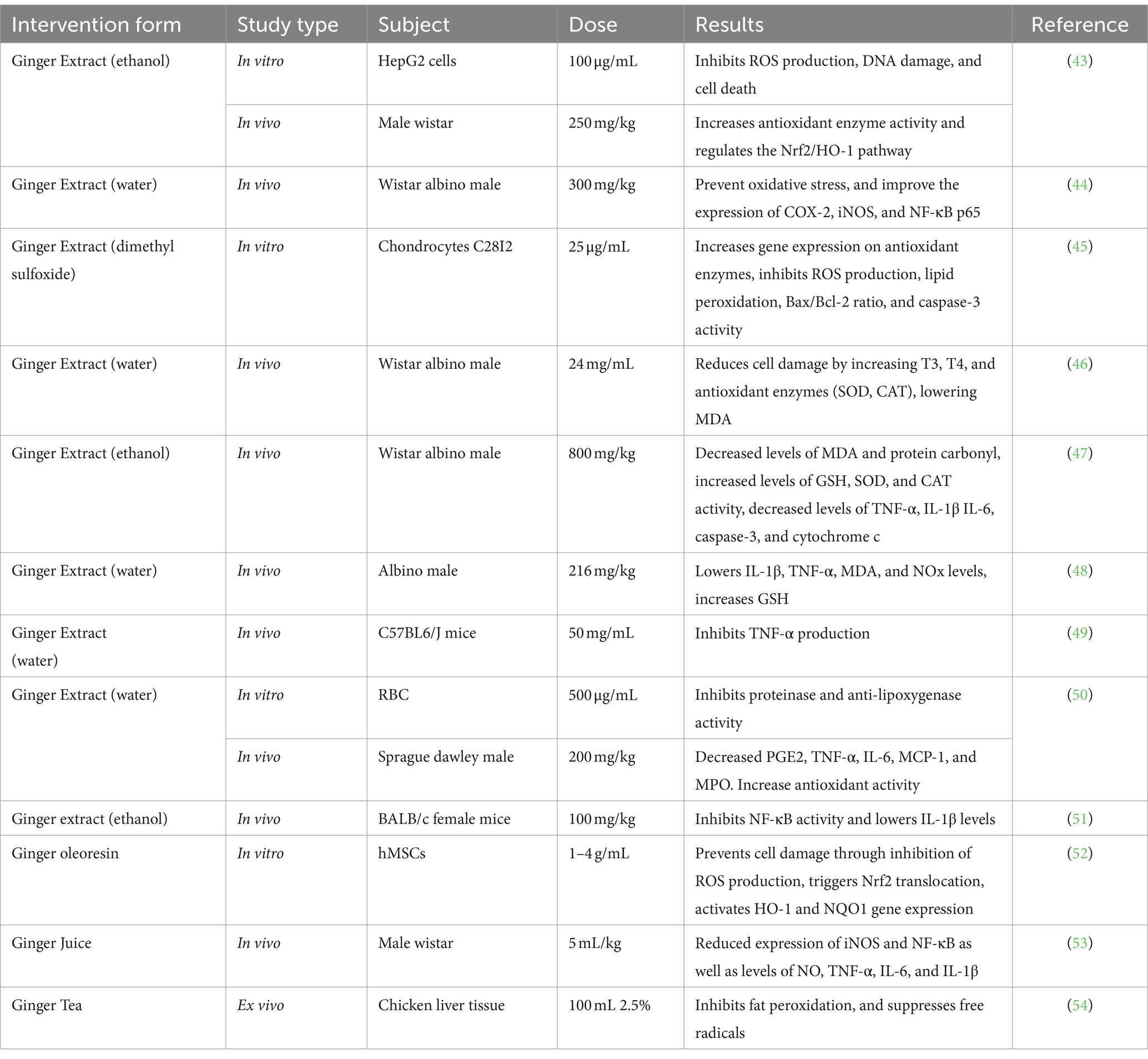
Table 5 . Ginger’s antioxidant and anti-inflammatory immunomodulators.
Ginger’s antioxidant potential mechanism ( Figure 4 ), related to bioactive components such as gingerol, shogaol, zingerone, and paradol. Inflammation is a defensive reaction of the body that arises after microbial invasion, exposure to antigens, or cellular and tissue injury. It entails intricate interactions among different cell types, mediators, receptors, and signaling pathways. Inflammation and oxidative stress can reciprocally influence each other, operating through the anti-inflammatory and antioxidant pathways. Immune cells, particularly T cells, govern the anti-inflammatory pathway. Th1 cell differentiation occurs subsequent to antigen recognition in the presence of IFNγ and IL-12. Th1 cells instigate cellular immunity, crucial for combatting intracellular pathogens. However, uncontrolled Th1 cell-related responses may provoke immunopathological reactions. In times of stress, the body naturally increases the number of Th1 cells. Elevated Th1 cell counts are observed in various diseases, such as multiple sclerosis. IL-12 also significantly contributes to Th1 cell differentiation. A robust correlation exists between IFNγ levels and enhanced activation of T lymphocytes, particularly CD4+ and CD8+ cells. IFNγ also promotes macrophage differentiation and regulates inducible nitric oxide synthase (iNOS) expression in dendritic cells and macrophages. Ginger modulates Th1 regulation by inhibiting IL-12 synthesis by macrophages, thereby affecting antigen presentation, T cell activation, and IFNγ and IL-12 secretion by T cells. Conversely, T lymphocytes transform into Th2 cells following antigen recognition in the presence of IL-4. Th2-specific transcription factors, like GATA 3 binding protein, stimulate Th2-related cytokine production (e.g., IL-4, IL-5, and IL-13). Th2 cells also induce B cells to generate immunoglobulins, fostering humoral immunity. Increased Th2 cell presence can curtail inflammation ( 55 , 56 ).
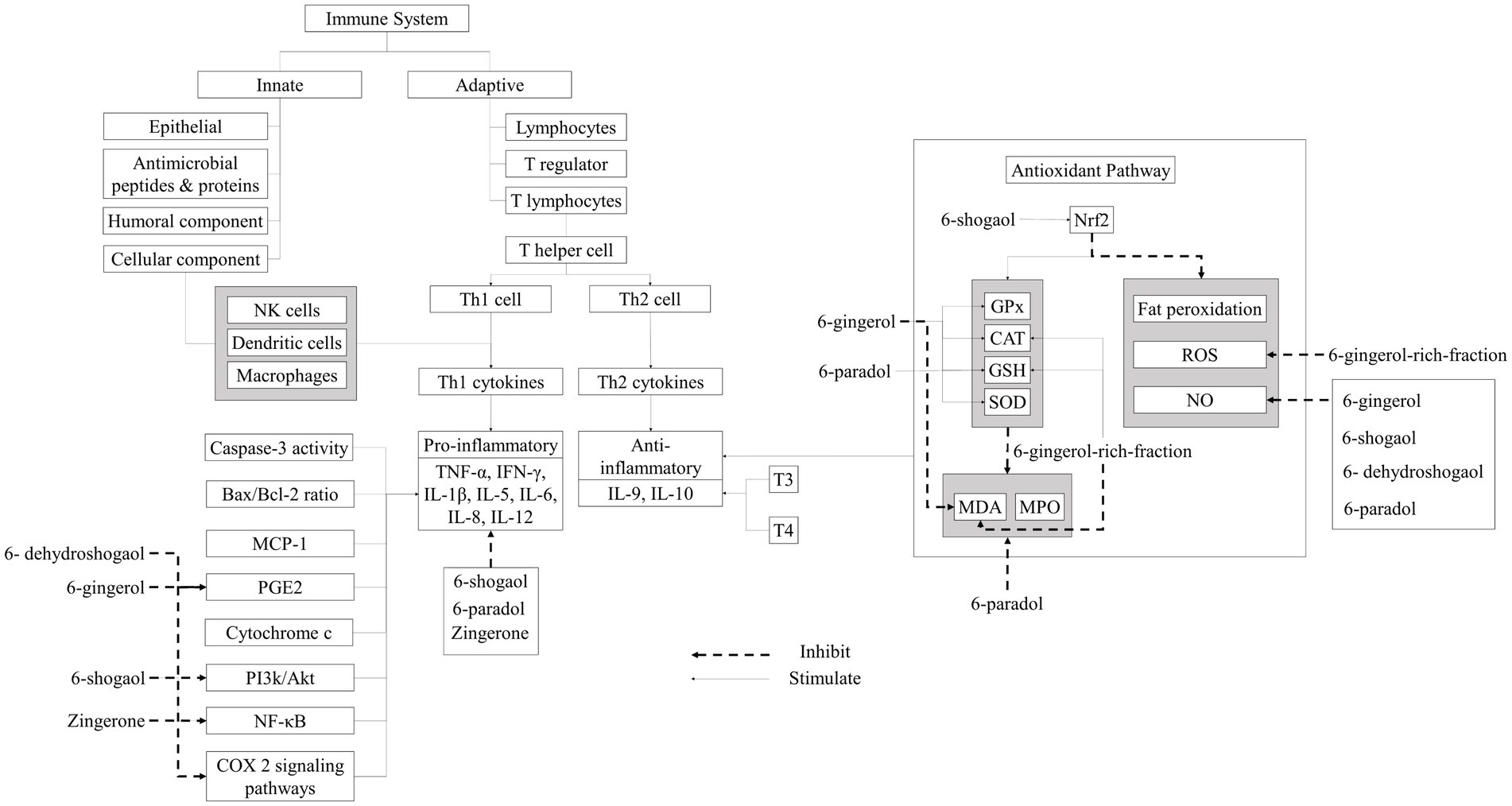
Figure 4 . The potential of ginger as an immunomodulator.
Elevated levels of proinflammatory cytokines (e.g., IL-1β, IL-6, IL-23, IL-33, TNFα, and IL-17) and reduced levels of anti-inflammatory cytokines (such as IL-10, IL-27, and TGFβ) are commonly termed cytokine dysregulation. This imbalance is often observed in individuals facing stress conditions induced by free radicals or pathogenic infections. Dysregulation in cytokine production may contribute to the development of diseases associated with oxidative stress. Ginger is recognized for its anti-inflammatory properties, inhibiting cytokine formation linked with Th1 and Th2 cells and suppressing IgE production. Ginger modulates Th1-type cytokines (including IL-12, IFNγ, and TNF-α) and Th2-type cytokines (like IL-4 and IL-13), thereby reducing T lymphocyte activation and expansion. It also diminishes IFNγ production by T lymphocytes, suppresses IL-2 secretion by stimulated T lymphocytes, and interferes with IL-2 receptor signaling. Studies have shown ginger’s capacity to lower levels of IL-1, IL-6, and TNFα, as well as inflammatory markers like C-reactive protein. Ginger also inhibits NF-кB activation, decreases TNFα, IL-6, and IL-1β expression, and mitigates ROS production. Additionally, ginger suppresses the expression of iNOS, IL-6, and IL-8 through NF-κB inhibition ( 55 , 56 ).
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are potent instigators of oxidative stress, provoking inflammatory responses. These radicals inflict damage upon essential biological molecules like lipids, proteins, DNA, and polysaccharides through peroxidation and nitrosation processes. This disturbance impairs normal cellular function and disrupts neuronal homeostatic equilibrium, ultimately culminating in apoptosis. Oxidative stress also impairs mitochondrial function, impeding adenosine triphosphate transport along the axon, potentially leading to neurodegeneration. Additionally, oxidative stress contributes to the escalation of inflammatory reactions via Nuclear Factor Light Chain-Enhancer (NF-κB) activation. Nitric oxide (NO) induces COX-2 expression, thereby elevating prostaglandin E2 synthesis. Cellular defenses against oxidative stress predominantly rely on the nuclear factor erythroid 2-related factor 2 (Nrf2), which orchestrates the expression of various antioxidant proteins and detoxification enzymes, including those involved in glutathione (GSH) synthesis, crucial for maintaining cellular homeostasis ( 55 , 56 ).
Ginger displays antioxidant properties attributed to compounds like shogaol, gingerol, zingerone, and paradol, thereby diminishing ROS production. Ginger initiates the replenishment of antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), elevates glutathione levels, prevents lipid peroxidation, inhibits NO production, and neutralizes hydroxyl radicals. Furthermore, ginger notably suppresses iNOS expression, reduces the occurrence of caspase-3 positive cells, and diminishes TNF-α expression, thereby inhibiting ROS production and MAPK-related signaling. Additionally, it enhances the expression of specific antioxidant elements such as glutathione, heme oxygenase-1, and quinone-1 through Nrf2 activation. Moreover, ginger inhibits Bax apoptosis protein expression and prevents the expression of H202, malondialdehyde (MDA), and myeloperoxidase (MPO), also activated B cells’ phosphatidylinositol-3-kinase (PI3K) and protein kinase B (Akt), thus exerting a protective effect against cell damage induced by oxidative stress and inflammation ( 55 , 56 ).
According to several studies on ginger antioxidants and anti-inflammatory ( Table 4 ), ginger extract modulates the expression of Nrf2 target genes such as heme oxygenase-1 (HO-1), metallothionein 1 (MT1), aldo-keto reductase 1B10 (AKR1B10), ferritin light chain (FTL), and glutamyltransferase-like activity 4 (GGTLA4). This enhances the production of enzymatic antioxidants the same as SOD, CAT, and GPx, as well as the production of GSH. Increased antioxidant enzymes can reduce RONS synthesis as well as malondialdehyde (MDA) and myeloperoxide levels (MPO). Finally, ginger extract has been shown to reduce oxidative stress, lipid peroxidation, DNA damage, and cell death ( 43 – 51 , 57 ).
Ginger tea is also known to suppress free radicals and inhibit fat peroxidation ( 54 ). Ginger oleoresin is also known to prevent cell damage by inhibiting ROS production, triggering Nrf2 translocation, and activating HO-1 and NQO1 gene expression ( 52 ). Studies related to the anti-inflammatory ability of ginger have shown that ginger extract can inhibit NF-ΚB and Akt thereby improving the expression of Cyclooxygenase-2 (COX-2) and iNOS in macrophage cells. Cyclooxygenase-2 (COX-2) is an enzyme that plays a role in the production of prostaglandin E2 (PGE2). Ginger extract can also reduce proinflammatory cytochrome IL-1β IL-6, TNF-α, cytochrome c, PGE2, and MCP-1. Ginger extract can inhibit the Bax/Bcl-2 ratio and caspase-3 activity and reduce cell damage by increasing T3 and T4. Ginger juice is known to inhibit NF-ΚB, thereby improving iNOS expression. Proinflammatory cytochromes such as TNF-, IL-6, and IL-1β are also inhibited to significantly reduce NO levels ( 53 ). NO works as a vasodilator that widens blood vessels so that the movement of immune cells to the site of infection becomes easier.
The body is constantly in an equilibrium state between free radical formation and elimination. Natural defense system against Catalase, glutathione reductase, SOD antioxidant enzymes, glutathione, urate, coenzyme Q, or exogenous stimuli such as bioactive substances like β-carotene, are all parts of the body’s natural defensive system against free radicals. Because of the neutrophil-produced free radicals, the immune system’s defense against infections serves as one of the sources of ROS generation. Micronutrient deficiency was known to cause a deterioration in immune function, both innate and adaptive, thereby rendering the body susceptible to pathogen attack as a consequence of immune imbalance. Thus, it was critical to consume bioactive compounds that serve as antioxidants in order to maintain a balanced immune response ( 58 ).
Ginger contains bioactive compounds, such as gingerol, shogaol, paradol, and zingerone. Those bioactive compounds were shown to have immunomodulatory properties due to their antioxidant, anti-inflammatory, and regulatory properties of innate and adaptive immune response components. These modulatory activities include inflammatory cytokines suppression and stimulation, antioxidative activity through ROS scavenging activity and enhancement of endogenous antioxidant defenses, and modulation of pro-inflammatory and anti-inflammatory signaling pathways. The potent bioactive components of ginger have been studied and shown that have antioxidant and anti-inflammatory activities as can be observed in Table 6 . Some important components will be described in the next sub-sections.
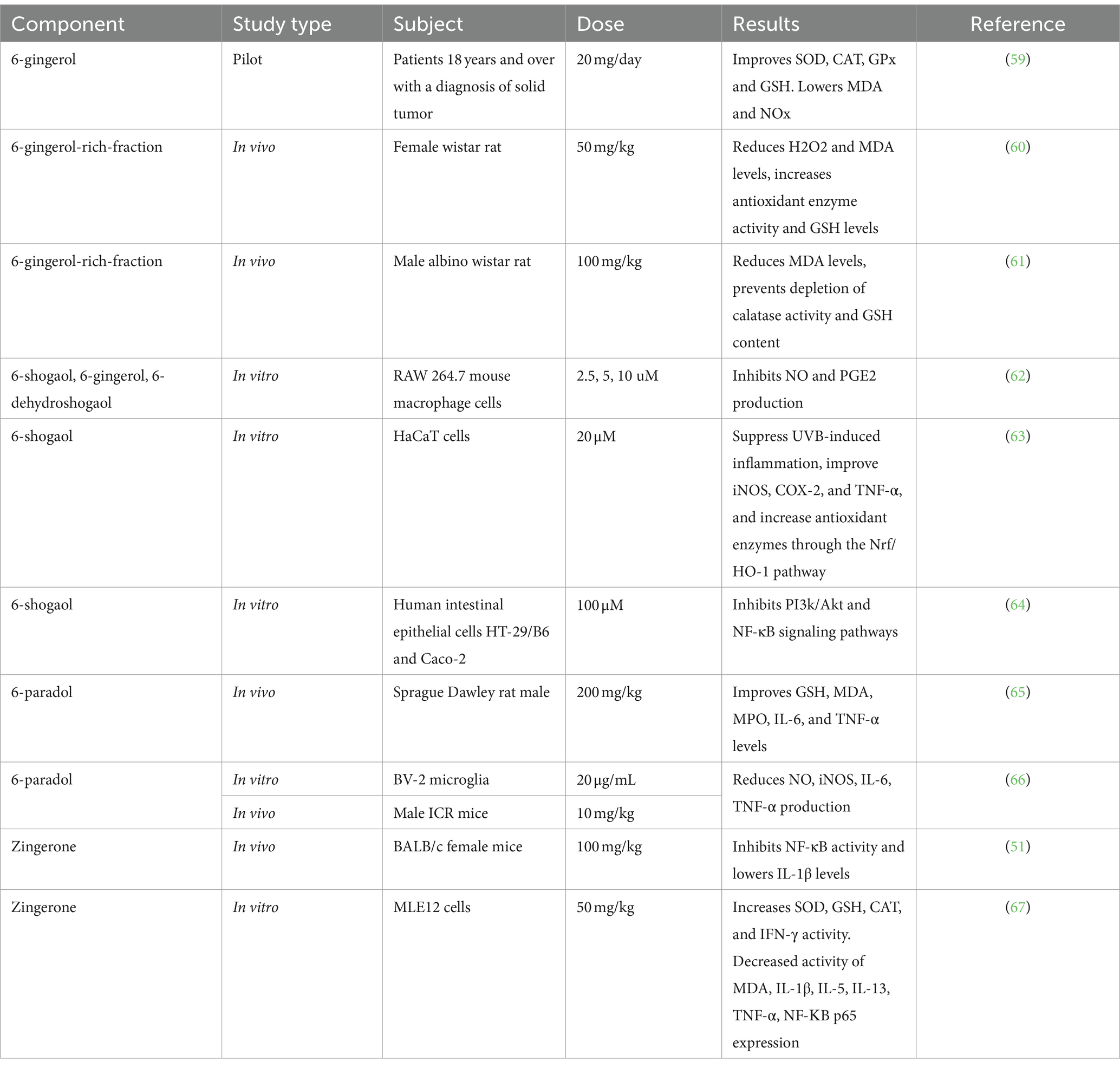
Table 6 . Ginger’s potential antioxidant and anti-inflammatory component.
3.4.1 Gingerol
Gingerol (23–35%) is a compound contained in ginger oleoresin which has a pale yellow color and is unstable ( 68 ). This gingerol gives a spicy taste to fresh ginger which is a phenolic compound ( 69 ). Gingerol is very susceptible to thermal decomposition ( 70 ). The compound 6-gingerol is reported to have antioxidant and anti-inflammatory properties ( 60 , 71 ). The ability of gingerols, especially 6-gingerol, has been shown to increase SOD, CAT, GPx, and GSH levels, as well as reduce MDA and NOx in patients diagnosed with solid tumors aged 18 years and over. Ginger extract (20 mg/day) as a daily supplement for patients receiving chemotherapy can increase antioxidants compared to the placebo group. Ginger supplementation can increase antioxidant activity and reduce oxidative levels in patients ( 59 ). The 6-gingerol-rich fraction (50 mg/kg) can also reduce H 2 O 2 and MDA levels, as well as increase antioxidant enzyme activity and rat GSH levels in in vivo studies ( 60 ). The ability of the 6-gingerol-rich fraction has also been known to reduce MDA levels and prevent depletion of catalase activity and GSH content in mice in 100 mg/kg dose ( 61 ). In vitro studies on mouse macrophages also demonstrated the ability of 6-gingerol to inhibit NO and PGE2 production ( 62 ).
3.4.2 Shogaol
Shogaol (18–25%) is a constituent present in ginger, especially when ginger undergoes a drying or cooking process. The constituent shogaol may be made by the dehydrating reaction of gingerol, this causes the ginger to lose its pungency when cooked. Based on in vitro studies, 6-shogaol with 20 uM doses can suppress UVB-induced inflammation, improve iNOS, COX-2, and TNF-α, and increase antioxidant enzymes through the Nrf2 pathway in HaCaT cell culture, also can be a healing agent in cells undergoing oxidation so that it can prevent aging caused by free radicals ( 63 ). In human intestinal epithelial cells, 6-shogaol (100 uM) can also inhibit PI3k/Akt and NF-κB signaling pathways ( 64 ). 6-dehydroshogaol in 2.5 μM, 5 μM, and 10 μM doses the dehydrogenated form of shogaol is also known to have the ability to inhibit the production of NO and PGE2 in mouse macrophage cells ( 62 ).
3.4.3 Paradol
Paradol is a derivative compound from gingerol and shogaol in ginger which has been known to have antioxidant and anti-inflammatory activity with no specific amount. 6-paradol can improve the levels of GSH, MDA, MPO, IL-6, and TNF-α by 200 mg/kg doses in mice ( 65 ). 6-paradol could reduce the production of NO, IL-6, and TNF-α in cultured BV-2 microglia cells by 20 ug/mL doses and prevent cell damage in mice by doses 10 mg/kg doses. Its mechanism is related to the inhibition of cyclooxygenase-2 enzyme production which modulates T cell immune response, including TNF-α as Th1 cytokines and IL-6 as Th2 cytokines. Paradol also known that have inhibition activity of iNOS upregulation which reduces NO, MDA, MPO production and increases GSH, so it can be prevent any cell damage ( 66 ).
3.4.4 Zingerone
Zingerone is the primary flavor component of ginger which gives ginger its sweet taste when cooked. Fresh ginger does not contain zingerone, but is produced by cooking or drying ginger, which causes an inverse aldol reaction to gingerols with no specific amount ( 72 ). Zingerone proved to be a highly efficient free radical scavenger by inhibiting enzymes involved in the formation of RONS. In vivo studies show that zingerone can inhibit NF-ΚB activity and decrease IL-1β levels by 100 mg/kg doses in mice ( 51 ). In addition, it is also known that zingerone can increase the activity of SOD, GSH, CAT, and IFN-γ, and decrease the level of MDA, IL-1β, IL-4, IL-5, IL-13, TNF-, NF-ΚB p65 expression and p-IκB based on in vitro studies on MLE12 cell cultures by 50 mg/kg doses. Zingerone also support asthma therapy by decreasing NF-ΚB and activating the Nrf2/HO-1 signaling pathway through AMPK ( 67 ).
3.5 Ginger consumption as food and drink
Ginger’s potential as an antioxidant and anti-inflammatory will be effective only when consumed in adequate amounts. Ademosun, Omoba ( 73 ) mentioned that ginger was commonly consumed in Nigeria as a vegetable, spices, condiments, and drink. A commercial ginger drink containing 25 mg ginger root and other ingredients containing 6.89 mg GAE/L of total phenol and total flavonoid of 1.06 QE/L; on the other hand lab scale ginger drink containing ginger diluted with 200 mL water containing more than twice the total phenol, and more than five times the total flavonoids. Those bioactive compounds were responsible for generating a DPPH radical scavenging ability of about 30% from commercial drinks, and about 50% for 100% ginger drinks. Meanwhile, Naqvi, Thomson ( 74 ) used ginger powder to inject sous vide beef steak to 116% raw weight at 2 g/L ginger concentration to increase flavor. On the other hand Zagórska, Czernicka-Boś ( 75 ) review that ginger in home cooking has diverse final bioactivity depending on the cooking method used. Stewed ginger had TEAC (Trolox equivalent antioxidant capacity) of 19.3 μmol/g. meanwhile fried ginger without oil in a nonstick pan for 15 min producing ginger with IC 50 of 950 μg/mL. The lack of agreement on the unit used to measure ginger antioxidant properties or integrated research that includes multiple ginger processing methods makes the interested parties have difficulties estimating the real benefit of consuming ginger with a certain processing method.
3.6 Challenges and future prospects of ginger
There is always a potential for ginger to be commercialized as an herbal product ( Figure 5 ). Nowadays, many people are selling herbal products in online shops, and it is increasing rapidly. Additionally, the herbal market is going to be high if the selection of products follows the trends, flows properly, and sells in the right time. The entrepreneurial spirit/thinking should go beyond anything other than the ability to mix or produce products. In Indonesia, herbal products based on ginger have been sold not only as traditional medicines or herbal drinks but also as processed food like candy and traditional cake. There should be consideration in having a proper and intensive education for developing ginger’s product in terms of production method, packaging, the stability of the ginger’s component, and shelf life of the herbal product. So, the products could be consumed, and the benefit of ginger is still contained in herbal product-processed food ( 76 ).
Figure 5 . Challenges of ginger’s commercialization as herbal products.
This review demonstrates that ginger contains various bioactive compounds that can be used as nutritional/therapeutic agents to modulate the immune system, inflammatory processes, antioxidant or redox balance, or immune components in general. To obtain a specific benefit from ginger, specific phytochemical compounds should be extracted at a particular condition to obtain an optimal yield ( 77 ).
The immune system’s ability to prevent diseases is strongly influenced by the host’s nutritional status. The discussion in this review suggested that ginger can be used as a potent immunomodulator and, when ingested, could enhance both cell-mediated and humoral immunity of the host, either through their antioxidant or anti-inflammation properties. Ginger has the capacity to enhance the pharmaceutical, food, medical, and agricultural industries. Ginger is becoming increasingly popular due to increased public health awareness due to its high nutritional content and health advantages.
Ginger-based nutraceuticals can be used as a supplement to help manage disease and maintain a healthy lifestyle. Other ginger-based products are also encouraged to be developed to create more diverse functional foods that can reach a broader consumer. Furthermore, ginger-based supplements can be developed as single compounds or phytocomplexes in the pharmaceutical industry. Ginger-based supplements or nutraceuticals can be used as human interventions as a complementary treatment in immune-related diseases after pre-clinical and clinical research.
Therefore, future collaborations between researchers from various fields, including chemists, biologists, clinicians, pharmacists, and the food industry, are required further to investigate the effect of ginger on human immunity. Collaboration between researchers and industry can help accelerate the advancement of ginger applications.
4 Conclusion
In conclusion, ginger has various bioactive components, such as gingerol, shogaol, zingerone, and paradol, known to have antioxidant and anti-inflammatory activities. Ginger extract, ginger juice, ginger tea, and ginger oleoresin have also been shown to have potential as immunomodulators through antioxidant and anti-inflammatory pathways. Bioactive compounds in ginger inhibit pro-inflammatory responses, increase levels of anti-inflammatory cytokines, and promote signaling pathways related to inflammation prevention on the anti-inflammatory pathway. Bioactive compounds in ginger are able to improve oxidative stress tolerance by eliminating ROS and lowering oxidative stress parameters, increasing antioxidant enzymes, and increasing antioxidant capacity.
Ginger’s ability to prevent oxidative stress and other diseases related to inflammation gives ginger the potential to be developed into various functional food products with education for developing ginger’s product. Furthermore, further research is needed regarding the toxicity of ginger and the daily consumption recommendation for humans.
Author contributions
FA: Writing – review & editing, Writing – original draft, Project administration, Methodology, Funding acquisition, Conceptualization. GA: Writing – review & editing, Writing – original draft, Project administration, Funding acquisition, Conceptualization. AA: Writing – review & editing, Writing – original draft, Conceptualization. VF: Writing – review & editing, Writing – original draft, Supervision.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded from the “Matching Fund” Funding Assistance Program Year 2021, based on the Cooperation Agreement between the Directorate General of Higher Education Institutions Ministry of Education, Culture, Research, and Technology of Indonesia’s Ministry of Education, Culture, Research, and Technology with Universitas Diponegoro Number 3077/E3/PKS.08/KL/2021. This work also funded by Staff Exchange/Research Fellowship Through World Class University Program by Universitas Diponegoro Program Year 2023, contract Number 174/UN7.A/HK/IV/2023.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mao, Q-Q, Xu, X-Y, Cao, S-Y, Gan, R-Y, Corke, H, Beta, T, et al. Bioactive compounds and bioactivities of ginger ( Zingiber officinale roscoe). Food Secur . (2019) 8:185. doi: 10.3390/foods8060185
PubMed Abstract | Crossref Full Text | Google Scholar
2. Wohlmuth, H, Leach, DN, Smith, MK, and Myers, SP. Gingerol content of diploid and tetraploid clones of ginger ( Zingiber officinale roscoe). J Agric Food Chem . (2005) 53:5772–8. doi: 10.1021/jf050435b
3. Agita, A, and Alsagaff, MT. Inflammation, immunity, and hypertension. Acta Med Indones . (2017) 49:158.
Google Scholar
4. Falkowski, L, Buddenkotte, J, and Datsi, A. Epigenetics in T-cell driven inflammation and cancer. Semin Cell Dev Biol . (2024) 154:250–60. doi: 10.1016/j.semcdb.2023.01.008
5. Strzelec, M, Detka, J, Mieszczak, P, Sobocińska, MK, and Majka, M. Immunomodulation—a general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Front Immunol . (2023) 14:10.3389/fimmu.2023.1127704. doi: 10.3389/fimmu.2023.1127704
Crossref Full Text | Google Scholar
6. Paragh, G, Seres, I, Harangi, M, and Fülöp, P. Dynamic interplay between metabolic syndrome and immunity. J Camps, (Ed.). Oxidative stress and inflammation in non-communicable diseases—molecular mechanisms and perspectives in therapeutics . Cham: Springer International Publishing; (2014). 171–190
7. Li, Q, Wang, Y, Sun, Q, Knopf, J, Herrmann, M, Lin, L, et al. Immune response in COVID-19: what is next? Cell Death Differ . (2022) 29:1107–22. doi: 10.1038/s41418-022-01015-x
8. Thirumdas, R, Kothakota, A, Pandiselvam, R, Bahrami, A, and Barba, FJ. Role of food nutrients and supplementation in fighting against viral infections and boosting immunity: a review. Trends Food Sci Technol . (2021) 110:66–77. doi: 10.1016/j.tifs.2021.01.069
9. Venkatalakshmi, P, Vadivel, V, and Brindha, P. Role of phytochemicals as immunomodulatory agents: a review. Int J Green Pharmacy . (2016) 10:600. doi: 10.22377/ijgp.v10i1.600
10. Jantan, I, Ahmad, W, and Bukhari, SNA. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front Plant Sci . (2015) 6:e655. doi: 10.3389/fpls.2015.00655
11. Ortuño-Sahagún, D, Zänker, K, Rawat, AKS, Kaveri, SV, and Hegde, P. Natural Immunomodulators. J Immunol Res . (2017) 2017:7529408. doi: 10.1155/2017/7529408
12. Balai Besar Penelitian dan Pengembangan Pascapanen Pertanian . Bahan Pangan Potensial untuk Anti Virus dan Imun Booster Balai Besar Penelitian dan Pengembangan Pascapanen Pertanian . Bogor (2020).
13. Kausar, T, Anwar, S, Hanan, E, Yaseen, M, Aboelnaga, SM, and Azad, Z. Therapeutic role of ginger ( Zingiber officinale )-a review. J Pharmaceut Res Int . (2021) 33:9–16. doi: 10.9734/jpri/2021/v33i29B31584
14. Jalali, M, Mahmoodi, M, Moosavian, SP, Jalali, R, Ferns, G, Mosallanezhad, A, et al. The effects of ginger supplementation on markers of inflammatory and oxidative stress: a systematic review and meta-analysis of clinical trials. Phytother Res . (2020) 34:1723–33. doi: 10.1002/ptr.6638
15. Ghafoor, K, Al Juhaimi, F, Özcan, MM, Uslu, N, Babiker, EE, and Mohamed Ahmed, IA. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger ( Zingiber officinale ) rhizome as affected by drying methods. LWT Food Sci Technol . (2020) 126:109354. doi: 10.1016/j.lwt.2020.109354
16. Morvaridzadeh, M, Sadeghi, E, Agah, S, Fazelian, S, Rahimlou, M, Kern, FG, et al. Effect of ginger ( Zingiber officinale ) supplementation on oxidative stress parameters: a systematic review and meta-analysis. J Food Biochem . (2021) 45:e13612. doi: 10.1111/jfbc.13612
17. Artini, KS, and Veranita, W. Tamanam herbal untuk meningkatkan sistem imun tubuh: literature review. Jurnal Farmasetis . (2021) 10:15–20. doi: 10.32583/farmasetis.v10i1.1383
18. Setyawan, B. Peluang Usaha Budi Daya Jahe . Yogyakarta: Pustaka Baru Press (2014).
19. Setyaningrum, HD, and Saparinto, C. JAHE . Jakarta: Penebar Swadaya Grup (2015).
20. Kefale, B, Delele, MA, Fanta, SW, and Mekonnen, AS. Nutritional, physicochemical, functional, and textural properties of red pepper ( Capsicum annuum L.), red onion ( Allium cepa ), ginger ( Zingiber officinale ), and garlic ( Allium sativum ): Main ingredients for the preparation of spicy foods in Ethiopia. J Food Qual . (2023) 2023:1–13. doi: 10.1155/2023/3916692
21. Alam, MA, Saleh, M, Mohsin, GM, Nadirah, TA, Aslani, F, Rahman, MM, et al. Evaluation of phenolics, capsaicinoids, antioxidant properties, and major macro-micro minerals of some hot and sweet peppers and ginger land-races of Malaysia. J Food Process Preserv . (2020) 44:e14483. doi: 10.1111/jfpp.14483
22. Bischoff-Kont, I, and Fürst, R. Benefits of ginger and its constituent 6-Shogaol in inhibiting inflammatory processes. Pharmaceuticals . (2021) 14:571. doi: 10.3390/ph14060571
23. Yang, Z-N, Yang, W, Peng, Q, He, Q, Feng, Y, Luo, S, et al. Volatile phytochemical composition of rhizome of ginger after extraction by headspace solid-phase microextraction, petrol ether extraction and steam distillation extraction. Bangladesh J Pharmacol . (2009) 4:136–43. doi: 10.3329/bjp.v4i2.3232
24. Mahmudati, N, Wahyono, P, and Djunaedi, D. Antioxidant activity and total phenolic content of three varieties of ginger ( Zingiber officinale ) in decoction and infusion extraction method. J Phys Conf Ser . (2020) 1567:22028. doi: 10.1088/1742-6596/1567/2/022028
25. Pujiati, SA, and Prafitasari, NF. The quality test of fermented ginger drink (ginger ale) produced from various types of Indonesian Ginger. In: Proceedings of the 2nd International Conference on Education and Technology (ICETECH 2021) . Madiun: Atlantis Press. (2022).
26. Diki Prayugo, W, Ria, M, Siti Uswatun, H, and Diah, LA. Chemical constituents, antibacterial activity and mode of action of elephant ginger ( Zingiber officinale var. officinale) and Emprit ginger rhizome ( Zingiber officinale var. amarum) essential oils. Pharm J . (2020) 12:404–9. doi: 10.5530/pj.2020.12.62
27. Rinanda, T, Isnanda, RP, and Zulfitri,. Chemical analysis of red ginger ( Zingiber officinale roscoe var rubrum) essential oil and its anti-biofilm activity against Candida albicans . Nat Prod Commun . (2018) 13:1934578X1801301. doi: 10.1177/1934578X1801301206
28. Paiva, CN, and Bozza, MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal . (2014) 20:1000–37. doi: 10.1089/ars.2013.5447
29. Rajeev, KT. Introductory chapter: immunity and immunomodulation. KT Rajeev and SB Prakash, (Eds.), Immune Response Activation and Immunomodulation . Rijeka: IntechOpen; (2019), p. Ch. 1
30. Sharpe, M, and Mount, N. Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech . (2015) 8:337–50. doi: 10.1242/dmm.018036
31. Bartold, PM, and Van Dyke, TE. Host modulation: controlling the inflammation to control the infection. Periodontol . (2017) 75:317–29. doi: 10.1111/prd.12169
32. Shang, Q, Yu, X, Sun, Q, Li, H, Sun, C, and Liu, L. Polysaccharides regulate Th1/Th2 balance: a new strategy for tumor immunotherapy. Biomed Pharmacother . (2024) 170:115976. doi: 10.1016/j.biopha.2023.115976
33. Miles, EA, and Calder, PC. Effects of Citrus fruit juices and their bioactive components on inflammation and immunity: a narrative review. Front Immunol . (2021) 12:12. doi: 10.3389/fimmu.2021.712608
34. Asri, W. Peran Antioksidan Bagi Kesehatan. Ind J Biotechnol Med . (2014) 3:59–68. doi: 10.22435/jbmi.v3i2.1659
35. Liguori, I, Russo, G, Curcio, F, Bulli, G, Aran, L, Della-Morte, D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging . (2018) 13:757–72. doi: 10.2147/cia.S158513
36. Pisoschi, AM, and Pop, A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem . (2015) 97:55–74. doi: 10.1016/j.ejmech.2015.04.040
37. Chen, L, Deng, H, Cui, H, Fang, J, Zuo, Z, Deng, J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget . (2018) 9:7204–18. doi: 10.18632/oncotarget.23208
38. Ragab, D, Salah Eldin, H, Taeimah, M, Khattab, R, and Salem, R. The COVID-19 cytokine storm; what we know so far. Front Immunol . (2020) 11:11. doi: 10.3389/fimmu.2020.01446
39. Rasheed, HMF, Rasheed, F, Qureshi, AW, and Jabeen, Q. Immunostimulant activities of the aqueous methanolic extract of Leptadenia pyrotechnica, a plant from Cholistan desert. J Ethnopharmacol . (2016) 186:244–50. doi: 10.1016/j.jep.2016.03.039
40. Razali, FN, Sinniah, SK, Hussin, H, Zainal Abidin, N, and Shuib, AS. Tumor suppression effect of Solanum nigrum polysaccharide fraction on breast cancer via immunomodulation. Int J Biol Macromol . (2016) 92:185–93. doi: 10.1016/j.ijbiomac.2016.06.079
41. Shukla, S, Bajpai, VK, and Kim, M. Plants as potential sources of natural immunomodulators. Rev Environ Sci Biotechnol . (2014) 13:17–33. doi: 10.1007/s11157-012-9303-x
42. Ajith, Y, Dimri, U, Dixit, SK, Singh, SK, Gopalakrishnan, A, Madhesh, E, et al. Immunomodulatory basis of antioxidant therapy and its future prospects: an appraisal. Inflammopharmacology . (2017) 25:487–98. doi: 10.1007/s10787-017-0393-5
43. Vipin, AV, Raksha Rao, K, Kurrey, NK, Anu-Appaiah, KA, and Venkateswaran, G. Protective effects of phenolics rich extract of ginger against aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed Pharmacother . (2017) 91:415–24. doi: 10.1016/j.biopha.2017.04.107
44. Hamza, AA, Heeba, GH, Hamza, S, Abdalla, A, and Amin, A. Standardized extract of ginger ameliorates liver cancer by reducing proliferation and inducing apoptosis through inhibition oxidative stress/inflammation pathway. Biomed Pharmacother . (2021) 134:111102. doi: 10.1016/j.biopha.2020.111102
45. Hosseinzadeh, A, Bahrampour Juybari, K, Fatemi, MJ, Kamarul, T, Bagheri, A, Tekiyehmaroof, N, et al. Protective effect of ginger ( Zingiber officinale roscoe) extract against oxidative stress and mitochondrial apoptosis induced by interleukin-1β in cultured chondrocytes. Cells Tissues Organs . (2017) 204:241–50. doi: 10.1159/000479789
46. Al-Amoudi, WM. Toxic effects of lambda-cyhalothrin, on the rat thyroid: involvement of oxidative stress and ameliorative effect of ginger extract. Toxicol Rep . (2018) 5:728–36. doi: 10.1016/j.toxrep.2018.06.005
47. Al Hroob, AM, Abukhalil, MH, Alghonmeen, RD, and Mahmoud, AM. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed Pharmacother . (2018) 106:381–9. doi: 10.1016/j.biopha.2018.06.148
48. Mansour, DF, Abdallah, HMI, Ibrahim, BMM, Hegazy, RR, Esmail, RSE, and Abdel-Salam, LO. The carcinogenic agent Diethylnitrosamine induces early oxidative stress, inflammation and proliferation in rat liver, stomach and Colon: protective effect of ginger extract. Asian Pac J Cancer Prev . (2019) 20:2551–61. doi: 10.31557/apjcp.2019.20.8.2551
49. Ueno, N, Hasebe, T, Kaneko, A, Yamamoto, M, Fujiya, M, Kohgo, Y, et al. TU-100 (Daikenchuto) and ginger ameliorate anti-CD3 antibody induced T cell-mediated murine enteritis: microbe-independent effects involving Akt and NF-κB suppression. PLoS One . (2014) 9:e97456. doi: 10.1371/journal.pone.0097456
50. Ezzat, SM, Ezzat, MI, Okba, MM, Menze, ET, and Abdel-Naim, AB. The hidden mechanism beyond ginger ( Zingiber officinale Rosc.) potent in vivo and in vitro anti-inflammatory activity. J Ethnopharmacol . (2018) 214:113. doi: 10.1016/j.jep.2017.12.019
51. Hsiang, C-Y, Lo, H-Y, Huang, H-C, Li, C-C, Wu, S-L, and Ho, T-Y. Ginger extract and zingerone ameliorated trinitrobenzene sulphonic acid-induced colitis in mice via modulation of nuclear factor-κB activity and interleukin-1β signalling pathway. Food Chem . (2013) 136:170–7. doi: 10.1016/j.foodchem.2012.07.124
52. Ji, K, Fang, L, Zhao, H, Li, Q, Shi, Y, Xu, C, et al. Ginger oleoresin alleviated γ-ray irradiation-induced reactive oxygen species via the Nrf2 protective response in human mesenchymal stem cells. Oxidative Med Cell Longev . (2017) 2017:1480294. doi: 10.1155/2017/1480294
53. Famurewa, AC, Ekeleme-Egedigwe, CA, Onwe, CS, Egedigwe, UO, Okoro, CO, Egedigwe, UJ, et al. Ginger juice prevents cisplatin-induced oxidative stress, endocrine imbalance and NO/iNOS/NF-κB signalling via modulating testicular redox-inflammatory mechanism in rats. Andrologia . (2020) 52:e13786. doi: 10.1111/and.13786
54. Singh, KG, Sonia, S, and Konsoor, N. In-vitro and ex-vivo studies on the antioxidant, anti-inflammatory and antiarthritic properties of Camellia sinensis , Hibiscus rosa sinensis, Matricaria chamomilla , Rosa SP., Zingiber officinale tea extracts. Int J Pharm Sci Res . (2018) 9:3543. doi: 10.13040/IJPSR.0975-8232.9(8).3543-51
55. Jafarzadeha, A, and Nemati, M. Therapeutic potentials of ginger for treatment of multiple sclerosis: a review with emphasis on its immunomodulatory, anti-inflammatory and antioxidative properties. J Neuroimmunol . (2018) 324:54–75. doi: 10.1016/j.jneuroim.2018.09.003
56. Ozkur, M, Benlier, N, Takan, I, Vasileiou, C, Georgakilas, AG, Pavlopoulou, A, et al. Ginger for healthy aging: a systematic review on current evidence of its antioxidant, anti-inflammatory, and anticancer properties. Oxidative Med Cell Longev . (2022) 2022:1–16. doi: 10.1155/2022/4748447
57. Mohammed, ET, Hashem, KS, Ahmed, AE, Aly, MT, Aleya, L, and Abdel-Daim, MM. Ginger extract ameliorates bisphenol a (BPA)-induced disruption in thyroid hormones synthesis and metabolism: involvement of Nrf-2/HO-1 pathway. Sci Total Environ . (2020) 703:134664. doi: 10.1016/j.scitotenv.2019.134664
58. Brambilla, D, Mancuso, C, Scuderi, MR, Bosco, P, Cantarella, G, Lempereur, L, et al. The role of antioxidant supplement in immune system, neoplastic, and neurodegenerative disorders: a point of view for an assessment of the risk/benefit profile. Nutr J . (2008) 7:29. doi: 10.1186/1475-2891-7-29
59. Danwilai, K, Konmun, J, Sripanidkulchai, B, and Subongkot, S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: a pilot study. Cancer Manag Res . (2017) 9:11–8. doi: 10.2147/cmar.S124016
60. Abolaji, AO, Ojo, M, Afolabi, TT, Arowoogun, MD, Nwawolor, D, and Farombi, EO. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem Biol Interact . (2017) 270:15–23. doi: 10.1016/j.cbi.2017.03.017
61. Saiah, W, Halzoune, H, Djaziri, R, Tabani, K, Koceir, EA, and Omari, N. Antioxidant and gastroprotective actions of butanol fraction of Zingiber officinale against diclofenac sodium-induced gastric damage in rats. J Food Biochem . (2018) 42:e12456. doi: 10.1111/jfbc.12456
62. Zhang, G, Nitteranon, V, Chan, LY, and Parkin, KL. Glutathione conjugation attenuates biological activities of 6-dehydroshogaol from ginger. Food Chem . (2013) 140:1–8. doi: 10.1016/j.foodchem.2013.02.073
63. Chen, F, Tang, Y, Sun, Y, Veeraraghavan, VP, Mohan, SK, and Cui, C. 6-shogaol, a active constiuents of ginger prevents UVB radiation mediated inflammation and oxidative stress through modulating NrF2 signaling in human epidermal keratinocytes (HaCaT cells). J Photochem Photobiol B Biol . (2019) 197:111518. doi: 10.1016/j.jphotobiol.2019.111518
64. Luettig, J, Rosenthal, R, Lee, I-FM, Krug, SM, and Schulzke, JD. The ginger component 6-shogaol prevents TNF-α-induced barrier loss via inhibition of PI3K/Akt and NF-κB signaling. Mol Nutr Food Res . (2016) 60:2576–86. doi: 10.1002/mnfr.201600274
65. Rafeeq, M, Murad, HAS, Abdallah, HM, and El-Halawany, AM. Protective effect of 6-paradol in acetic acid-induced ulcerative colitis in rats. BMC Complement Med Therapies . (2021) 21:28. doi: 10.1186/s12906-021-03203-7
66. Gaire, BP, Kwon, OW, Park, SH, Chun, K-H, Kim, SY, Shin, DY, et al. Neuroprotective effect of 6-Paradol in focal cerebral ischemia involves the attenuation of Neuroinflammatory responses in activated microglia. PLoS One . (2015) 10:e0120203. doi: 10.1371/journal.pone.0120203
67. Zhu, Y, Wang, C, Luo, J, Hua, S, Li, D, Peng, L, et al. The protective role of Zingerone in a murine asthma model via activation of the AMPK/Nrf2/HO-1 pathway. Food Funct . (2021) 12:3120–31. doi: 10.1039/D0FO01583K
68. Choi, YY, Kim, MH, Hong, J, Kim, S-H, and Yang, WM. Dried ginger (Zingiber officinalis) inhibits inflammation in a lipopolysaccharide-induced mouse model. Evid Based Complement Alternat Med . (2013) 2013:914563. doi: 10.1155/2013/914563
69. Chen, Z, Zhang, L, Lv, Y, Qu, S, Liu, W, Wang, K, et al. A genome assembly of ginger ( Zingiber officinale roscoe) provides insights into genome evolution and 6-gingerol biosynthesis. Plant J . (2024) 118:682–95. doi: 10.1111/tpj.16625
70. Arablou, T, Aryaeian, N, Valizadeh, M, Sharifi, F, Hosseini, A, and Djalali, M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr . (2014) 65:515–20. doi: 10.3109/09637486.2014.880671
71. Tzeng, T-F, Liou, S-S, Chang, CJ, and Liu, I-M. 6-Gingerol protects against nutritional steatohepatitis by regulating key genes related to inflammation and lipid metabolism. Nutrients . (2015) 7:999–1020. doi: 10.3390/nu7020999
72. Rajan, I, Narayanan, N, Rabindran, R, Jayasree, PR, and Manish Kumar, PR. Zingerone protects against stannous chloride-induced and hydrogen peroxide-induced oxidative DNA damage in vitro. Biol Trace Elem Res . (2013) 155:455–9. doi: 10.1007/s12011-013-9801-x
73. Ademosun, MT, Omoba, OS, and Olagunju, AI. Antioxidant properties, glycemic indices, and carbohydrate hydrolyzing enzymes activities of formulated ginger-based fruit drinks. J Food Biochem . (2021) 45:e13324. doi: 10.1111/jfbc.13324
74. Naqvi, ZB, Thomson, PC, Campbell, MA, Latif, S, Legako, JF, McGill, DM, et al. Sensory and physical characteristics of M. Biceps femoris from older cows using ginger powder (Zingibain) and sous vide cooking. Food Secur . (2021) 10:81936. doi: 10.3390/foods10081936
75. Zagórska, J, Czernicka-Boś, L, Kukula-Koch, W, Iłowiecka, K, and Koch, W. Impact of thermal processing on the selected biological activities of ginger rhizome-a review. Molecules . (2023) 28:412. doi: 10.3390/molecules28010412
76. HFM, Pranoto, MDPTG, Puteri, and Ringoringo, VS, (Eds.), ABG point of view in lemongrass and ginger potency for commercialization as herbal with anti-diabetic claim in Indonesia. The 6th International Conference of Food, Agriculture, and Natural Resource (IC-FANRES 2021); (2022), Jakarta, Indonesia: Atlantis Press
77. Hargono, H, Pradhita, F, and Aulia, MP. Pemisahan Gingerol Dari Rimpang Jahe Segar Melalui Proses Ekstraksi Secara Batch. Majalah Ilmiah Momentum . (2013) 9:16–21. doi: 10.36499/jim.v9i2.920
Keywords: ginger, immunomodulators, immune system, antioxidant, anti-inflammatory
Citation: Ayustaningwarno F, Anjani G, Ayu AM and Fogliano V (2024) A critical review of Ginger’s ( Zingiber officinale ) antioxidant, anti-inflammatory, and immunomodulatory activities. Front. Nutr . 11:1364836. doi: 10.3389/fnut.2024.1364836
Received: 03 January 2024; Accepted: 24 May 2024; Published: 06 June 2024.
Reviewed by:
Copyright © 2024 Ayustaningwarno, Anjani, Ayu and Fogliano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fitriyono Ayustaningwarno, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Search Menu
Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Article Collection Archive
- Author Guidelines
- Submission Site
- Open Access
- Call for Papers
- Why Publish?
- About Nutrition Reviews
- About International Life Sciences Institute
- Editorial Board
- Early Career Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

- < Previous
Therapeutic health effects of ginger ( Zingiber officinale ): updated narrative review exploring the mechanisms of action
- Article contents
- Figures & tables
- Supplementary Data
Megan Crichton, Skye Marshall, Wolfgang Marx, Elizabeth Isenring, Anna Lohning, Therapeutic health effects of ginger ( Zingiber officinale ): updated narrative review exploring the mechanisms of action, Nutrition Reviews , Volume 81, Issue 9, September 2023, Pages 1213–1224, https://doi.org/10.1093/nutrit/nuac115
- Permissions Icon Permissions
Ginger ( Zingiber officinale ) has been investigated for its potentially therapeutic effect on a range of chronic conditions and symptoms in humans. However, a simplified and easily understandable examination of the mechanisms behind these effects is lacking and, in turn, hinders interpretation and translation to practice, and contributes to overall clinical heterogeneity confounding the results. Therefore, drawing on data from nonhuman trials, the objective for this narrative review was to comprehensively describe the current knowledge on the proposed mechanisms of action of ginger on conferring therapeutic health effects in humans. Mechanistic studies support the findings from human clinical trials that ginger may assist in improving symptoms and biomarkers of pain, metabolic chronic disease, and gastrointestinal conditions. Bioactive ginger compounds reduce inflammation, which contributes to pain; promote vasodilation, which lowers blood pressure; obstruct cholesterol production, which regulates blood lipid profile; translocate glucose transporter type 4 molecules to plasma membranes to assist in glycemic control; stimulate fatty acid breakdown to aid weight management; and inhibit serotonin, muscarinic, and histaminergic receptor activation to reduce nausea and vomiting. Additional human trials are required to confirm the antimicrobial, neuroprotective, antineoplastic, and liver- and kidney-protecting effects of ginger. Interpretation of the mechanisms of action will help clinicians and researchers better understand how and for whom ginger may render therapeutic effects and highlight priority areas for future research.
- gastrointestinal diseases
- chronic disease
- pharmacokinetics
- nausea and vomiting
- narrative review
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Short-term Access
To purchase short-term access, please sign in to your personal account above.
Don't already have a personal account? Register
| Month: | Total Views: |
|---|---|
| January 2023 | 46 |
| February 2023 | 63 |
| March 2023 | 63 |
| April 2023 | 18 |
| May 2023 | 25 |
| June 2023 | 24 |
| July 2023 | 7 |
| August 2023 | 53 |
| September 2023 | 79 |
| October 2023 | 59 |
| November 2023 | 49 |
| December 2023 | 55 |
| January 2024 | 43 |
| February 2024 | 49 |
| March 2024 | 37 |
| April 2024 | 56 |
| May 2024 | 25 |
| June 2024 | 20 |
| July 2024 | 25 |
| August 2024 | 21 |
| September 2024 | 386 |
| October 2024 | 17 |
Email alerts
Citing articles via.
- X (formerly Twitter)
- Recommend to your Library
Affiliations
- Online ISSN 1753-4887
- Print ISSN 0029-6643
- Copyright © 2024 International Life Sciences Institute
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A critical review of Ginger's ( Zingiber officinale ) antioxidant, anti-inflammatory, and immunomodulatory activities
Affiliations.
- 1 Nutrition Science Department, Faculty of Medicine, Universitas Diponegoro, Semarang, Indonesia.
- 2 Center of Nutrition Research (CENURE), Universitas Diponegoro, Semarang, Indonesia.
- 3 Food Quality and Design, Wageningen University & Research, Wageningen, Netherlands.
- PMID: 38903613
- PMCID: PMC11187345
- DOI: 10.3389/fnut.2024.1364836
Ginger ( Zingiber officinale ) is a rhizome that has been used as a healthy herbal plant for years. Ginger's chemical components are recognized to provide beneficial health effects, namely as antioxidants and anti-inflammatory agents with the potential to operate as immunomodulators. This literature review covers numerous publications concerning ginger's immunomodulatory potential, associated with antioxidant and anti-inflammatory effects in modifying the body's immune system. Pathophysiology of oxidative stress and inflammation were introduced before diving deep down into the herbal plants as an immunomodulator. Ginger's antioxidant and anti-inflammatory properties are provided by gingerol, shogaols, paradol, and zingerone. Ginger's antioxidant mechanism is linked to Nrf2 signaling pathway activation. Its anti-inflammatory mechanism is linked to Akt inhibition and NF-KB activation, triggering the release of anti-inflammatory cytokines while reducing proinflammatory cytokines. Ginger consumption as food and drink was also explored. Overall, ginger and its active components have been shown to have strong antioxidant properties and the potential to reduce inflammation. Challenges and future prospects of ginger are also elaborated for future development. Future collaborations between researchers from various fields, including chemists, biologists, clinicians, pharmacists, and the food industry, are required further to investigate the effect of ginger on human immunity. Collaboration between researchers and industry can help accelerate the advancement of ginger applications.
Keywords: anti-inflammatory; antioxidant; ginger; immune system; immunomodulators.
Copyright © 2024 Ayustaningwarno, Anjani, Ayu and Fogliano.
PubMed Disclaimer
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Chemical structure of bioactive components…
Chemical structure of bioactive components in three varieties of ginger. 1. 1,8-cineole, 2.…
Oxidative-inflammatory stress relationship and the…
Oxidative-inflammatory stress relationship and the role of antioxidants. (O 2 −, superoxide radical;…
Mechanism of endogenous and exogenous…
Mechanism of endogenous and exogenous antioxidant. (O 2 • − , superoxide radical…
The potential of ginger as…
The potential of ginger as an immunomodulator.
Challenges of ginger’s commercialization as…
Challenges of ginger’s commercialization as herbal products.
Similar articles
- Zingiber Officinale Roscoe: The Antiarthritic Potential of a Popular Spice-Preclinical and Clinical Evidence. Szymczak J, Grygiel-Górniak B, Cielecka-Piontek J. Szymczak J, et al. Nutrients. 2024 Mar 5;16(5):741. doi: 10.3390/nu16050741. Nutrients. 2024. PMID: 38474869 Free PMC article. Review.
- Zingiber officinale var. rubrum : Red Ginger's Medicinal Uses. Zhang S, Kou X, Zhao H, Mak KK, Balijepalli MK, Pichika MR. Zhang S, et al. Molecules. 2022 Jan 25;27(3):775. doi: 10.3390/molecules27030775. Molecules. 2022. PMID: 35164040 Free PMC article. Review.
- Heat treatments of ginger root modify but not diminish its antioxidant activity as measured with multiple free radical scavenging (MULTIS) method. Sueishi Y, Masamoto H, Kotake Y. Sueishi Y, et al. J Clin Biochem Nutr. 2019 Mar;64(2):143-147. doi: 10.3164/jcbn.18-41. Epub 2018 Dec 5. J Clin Biochem Nutr. 2019. PMID: 30936626 Free PMC article.
- A subcritical water extract of soil grown Zingiber officinale Roscoe: Comparative analysis of antioxidant and anti-inflammatory effects and evaluation of bioactive metabolites. Razak AM, Zakaria SNA, Abdul Sani NF, Ab Rani N, Hakimi NH, Mohd Said M, Tan JK, Gan HK, Mad Nordin MF, Makpol S. Razak AM, et al. Front Pharmacol. 2023 Feb 8;14:1006265. doi: 10.3389/fphar.2023.1006265. eCollection 2023. Front Pharmacol. 2023. PMID: 36843947 Free PMC article.
- Ginger and its constituents in asthma: a mini-review. Ahmed Abdelmawgood I, Sayed AM, Mohamed OA, Ali Ramadan S, Waleed Farg J, Saad W, Sayed Hamdy R, Sharaf B, Ashry H, Kotb MA. Ahmed Abdelmawgood I, et al. J Asthma. 2024 Jun 8:1-10. doi: 10.1080/02770903.2024.2361779. Online ahead of print. J Asthma. 2024. PMID: 38805387 Review.
- 10-Gingerol Increases Antioxidant Enzymes and Attenuates Lipopolysaccharide-Induced Inflammation by Modulating Adipokines in 3T3-L1 Adipocytes. Preciado-Ortiz ME, Martínez-López E, Pedraza-Chaverri J, Medina-Campos ON, Rodríguez-Echevarría R, Reyes-Pérez SD, Rivera-Valdés JJ. Preciado-Ortiz ME, et al. Antioxidants (Basel). 2024 Sep 7;13(9):1093. doi: 10.3390/antiox13091093. Antioxidants (Basel). 2024. PMID: 39334752 Free PMC article.
- Mao Q-Q, Xu X-Y, Cao S-Y, Gan R-Y, Corke H, Beta T, et al. . Bioactive compounds and bioactivities of ginger (Zingiber officinale roscoe). Food Secur. (2019) 8:185. doi: 10.3390/foods8060185, PMID: - DOI - PMC - PubMed
- Wohlmuth H, Leach DN, Smith MK, Myers SP. Gingerol content of diploid and tetraploid clones of ginger (Zingiber officinale roscoe). J Agric Food Chem. (2005) 53:5772–8. doi: 10.1021/jf050435b, PMID: - DOI - PubMed
- Agita A, Alsagaff MT. Inflammation, immunity, and hypertension. Acta Med Indones. (2017) 49:158. - PubMed
- Falkowski L, Buddenkotte J, Datsi A. Epigenetics in T-cell driven inflammation and cancer. Semin Cell Dev Biol. (2024) 154:250–60. doi: 10.1016/j.semcdb.2023.01.008, PMID: - DOI - PubMed
- Strzelec M, Detka J, Mieszczak P, Sobocińska MK, Majka M. Immunomodulation—a general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Front Immunol. (2023) 14:10.3389/fimmu.2023.1127704. doi: 10.3389/fimmu.2023.1127704 - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding, linkout - more resources, full text sources.
- Europe PubMed Central
- Frontiers Media SA
- PubMed Central

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

IMAGES
VIDEO
COMMENTS
This study focused on randomized clinical trials investigating the efficacy of ginger to improve human health as well as to support human disease. For that reason, any paper that reported the effectiveness of ginger in clinical aspects was included in this study.
This literature review covers numerous publications concerning ginger’s immunomodulatory potential, associated with antioxidant and anti-inflammatory effects in modifying the body’s immune system.
This review is focused on providing a comprehensive overview of the tendencies in the application of ginger and its derivatives in the food industry and concurrently briefly discusses the beneficial aspects and processing of ginger.
Clinical applications of ginger with an expectation of clinical benefits are receiving significant attention. This systematic review aims to provide a comprehensive discussion in terms of the clinical effects of ginger in all reported areas.
This literature review covers numerous publications concerning ginger’s immunomodulatory potential, associated with antioxidant and anti-inflammatory effects in modifying the body’s immune system. Pathophysiology of oxidative stress and inflammation were introduced before diving deep down into the herbal plants as an immunomodulator.
A recent umbrella review of the therapeutic health effects of ginger acknowledged high interest in this topic, identifying 24 systematic reviews published from 2015 to 2021. 5 However, a simplified and easily understandable discussion of the mechanisms behind these effects is lacking.
Ginger (Zingiber officinale) is a rhizome that has been used as a healthy herbal plant for years. Ginger's chemical components are recognized to provide beneficial health effects, namely as antioxidants and anti-inflammatory agents with the potential to operate as immunomodulators.
In this umbrella review of systematic reviews we aimed to determine the therapeutic effects and safety of any type of ginger from the Zingiber family administered in oral form compared with any comparator or baseline measures on any health and well-being outcome in humans.
Authors: Rossi Indiarto. Universitas Padjadjaran. Edy Subroto. Universitas Padjadjaran. Angeline. Selly. Citations (10) References (76) Abstract. Ginger is a spice type used by rhizome. Ginger...
Our study found ginger is a promising herbal medicine for health care, which is beneficial for nausea and vomiting, metabolic syndrome and pain. Abstract. Objectives.