

Arguing For and Against Genetic Engineering
- Share on Facebook
- Share on Pinterest
- Share on LinkedIn
- Share on WhatsApp
- Share via Email
Table of Contents
Harvard philosopher Michael Sandel recently spoke at Stanford on the subject of his new book, The Case against Perfection: Ethics in the Age of Genetic Engineering. He focused on the “ethical problems of using biomedical technologies to determine and choose from the genetic material of human embryos,” an issue that has inspired much debate.
Having followed Sandel’s writings on genetic enhancement for several years, I think that this issue deserves special thought. For many years, the specter of human genetic engineering has haunted conservatives and liberals alike. Generally, their main criticisms run thus:
First, genetic engineering limits children’s autonomy to shape their own destinies. Writer Dinesh D’Souza articulates this position in a 2001 National Review Online article: “If parents are able to remake a child’s genetic makeup, they are in a sense writing the genetic instructions that shape his entire life. If my parents give me blue eyes instead of brown eyes, if they make me tall instead of medium height, if they choose a passive over an aggressive personality, their choices will have a direct, lifelong effect on me.” In other words, genetic enhancement is immoral because it artificially molds people’s lives, often pointing their destinies in directions that they themselves would not freely choose. Therefore, it represents a fundamental violation of their rights as human beings.
Second, some fear that genetic engineering will lead to eugenics. In a 2006 column, writer Charles Colson laments: “British medical researchers recently announced plans to use cutting-edge science to eliminate a condition my family is familiar with: autism. Actually, they are not ‘curing’ autism or even making life better for autistic people. Their plan is to eliminate autism by eliminating autistic people. There is no in utero test for autism as there is for Down syndrome…[Prenatal] testing, combined with abortion-on-demand, has made people with Down syndrome an endangered population…This utilitarian view of life inevitably leads us exactly where the Nazis were creating a master race. Can’t we see it?” The logic behind this argument is that human genetic enhancement perpetuates discrimination against the disabled and the “genetically unfit,” and that this sort of discrimination is similar to the sort that inspired the eugenics of the Third Reich.
A third argument is that genetic engineering will lead to vast social inequalities. This idea is expressed in the 1997 cult film Gattaca, which portrays a society where the rich enjoy genetic enhancements—perfect eyesight, improved height, higher intelligence—that the poor cannot afford. Therefore, the main character Vincent, a man from a poor background who aspires to be an astronaut, finds it difficult to achieve his goal because he is short-sighted and has a “weak heart.” This discrepancy is exacerbated by the fact that his brother, who is genetically-engineered, enjoys perfect health and is better able to achieve his dreams. To many, Gattaca is a dystopia where vast gaps between the haves and have-nots will become intolerable, due to the existence of not just material, but also genetic inequalities.
The critics are right that a world with genetic engineering will contain inequalities. On the other hand, it is arguable that a world without genetic engineering, like this one, is even more unequal. In Gattaca, a genetically “fit” majority of people can aspire to be astronauts, but an unfortunate “unfit” minority cannot. In the real world, the situation is the other way round: the majority of people don’t have the genes to become astronauts, and only a small minority with perfect eyesight and perfect physical fitness—the Neil Armstrong types—would qualify.
The only difference is that in the real world, we try to be polite about the unpleasant realities of life by insisting that the Average Joe has, at least theoretically, a Rocky-esque chance of becoming an astronaut. In that sense, our covert discrimination is much more polite than the overt discrimination of the Gattaca variety. But it seems that our world, where genetic privilege exists naturally among a tiny minority, could conceivably be less equal (and less socially mobile) than a world with genetic engineering, where genetic enhancements would be potentially available to the majority of people, giving them a chance to create better futures for themselves. Supporters of human genetic engineering thus ask the fair question: Are natural genetic inequalities, doled out randomly and sometimes unfairly by nature, more just than engineered ones, which might be earned through good old fashioned American values like hard work, determination, and effort?
“But,” the critics ask, “wouldn’t genetic engineering lead us to eugenics?” The pro-genetic engineering crowd thinks not. They suggest that genetic engineering, if done on a purely decentralized basis by free individuals and couples, will not involve any form of coercion. Unlike the Nazi eugenics program of the 1930s, which involved the forced, widespread killing of “unfit” peoples and disabled babies, the de facto effect of genetic engineering is to cure disabilities, not kill the disabled. This is a key moral difference. As pointed out by biologist Robert Sinsheimer, genetic engineering would “permit in principle the conversion of all the ‘unfit’ to the highest genetic level.” Too often, women choose to abort babies because pre-natal testing shows that they have Down syndrome or some other ailment. If anything, genetic engineering should be welcomed by pro-life groups because by converting otherwise-disabled babies into normal, healthy ones, it would reduce the number of abortions.
In addition, the world of Gattaca, for all its faults, features a world that, far from being defined along Hitler-esque racial lines, has in fact transcended racism. Being blond-haired and blue-eyed loses its racially elitist undertones because such traits are easily available on the genetic supermarket. Hair color, skin color, and eye color become a subjective matter of choice, no more significant than the color of one’s clothes. If anything, genetic engineering will probably encourage, not discourage, racial harmony and diversity.
It is true that genetic engineering may limit children’s autonomy to shape their own destinies. But it is equally true that all people’s destinies are already limited by their natural genetic makeup, a makeup that they are born with and cannot change. A short person, for example, would be unlikely to join the basketball team because his height makes it difficult for him to compete with his tall peers. An ugly person would be unable to achieve her dream of becoming a famous actress because the lead roles are reserved for the beautiful. A myopic kid who wears glasses will find it difficult to become a pilot. A student with an IQ of 75 will be unlikely to get into Harvard however hard he tries. In some way or another, our destinies are limited by the genes we are born with.
In this sense, it is arguable that genetic engineering might help to level the playing field. Genetic engineering could give people greater innate capacity to fulfill their dreams and pursue their own happiness. Rather than allow peoples’ choices to be limited by their genetic makeup, why not give each person the capability of becoming whatever he or she wants to, and let his or her eventual success be determined by effort, willpower, and perseverance? America has long represented the idea that people can shape their own destinies. To paraphrase Dr. King, why not have a society where people are judged not by the genes they inherit, but by the content of their character?
Looking at both sides, the genetic engineering controversy does raise questions that should be answered, not shouted down. Like all major scientific advances, it probably has some negative effects, and steps must be taken to ameliorate these outcomes. For example, measures should also be taken to ensure that genetic engineering’s benefits are, at least to some extent, available to the poor. As ethicists Maxwell Mehlman and Jeffrey Botkin suggest in their book Access to the Genome: The Challenge to Equality, the rich could be taxed on genetic enhancements, and the revenue from these taxes could be used to help pay for the genetic enhancement of the poor. To some extent, this will help to ameliorate the unequal effects of genetic engineering, allowing its benefits to be more equitably distributed. In addition, caution must be taken in other areas, such as ensuring that the sanctity of human life is respected at all times. In this respect, pro-life groups like Focus on the Family can take a leading role in ensuring that scientific advances do not come at the expense of moral ethics.
At the same time, we should not allow our fear of change to prevent our society from exploring this promising new field of science, one that promises so many medical and social benefits. A strategy that defines itself against the core idea of scientific progress cannot succeed. Instead of attempting to bury our heads in the sand, we should seek to harness genetic engineering for its positive benefits, even as we take careful steps to ameliorate its potential downsides.
Stanford's Censorship: A Culture of Censorship
Stanford’s censorship: the end of the stanford internet observatory, stanford, what did you expect.
The rampage in Main Quad and the president’s office was as predictable as it is contemptible. By allowing disorder to fester, the University has earned its humiliation.
Stanford's Rigged Housing “Accommodations”
We use cookies to enhance our website for you. Proceed if you agree to this policy or learn more about it.
- Essay Database >
- Essays Samples >
- Essay Types >
- Argumentative Essay Example
Genetic Engineering Argumentative Essays Samples For Students
11 samples of this type
Do you feel the need to check out some previously written Argumentative Essays on Genetic Engineering before you begin writing an own piece? In this free directory of Genetic Engineering Argumentative Essay examples, you are given a thrilling opportunity to examine meaningful topics, content structuring techniques, text flow, formatting styles, and other academically acclaimed writing practices. Adopting them while composing your own Genetic Engineering Argumentative Essay will definitely allow you to finalize the piece faster.
Presenting superb samples isn't the only way our free essays service can aid students in their writing efforts – our authors can also compose from scratch a fully customized Argumentative Essay on Genetic Engineering that would make a solid basis for your own academic work.
Free Argumentative Essay About Genetically Modified Foods
The definition of genetically modified food (gmf), example of argumentative essay on gmos are a health risk to american consumers, the pros of genetically modified foods argumentative essays example.
Introduction
Don't waste your time searching for a sample.
Get your argumentative essay done by professional writers!
Just from $10/page
The Need To Regulate GM Foods Argumentative Essay Sample
Genetically modified food good or bad argumentative essay examples, genetically modified food: good or bad, free genetically modified plants argumentative essay sample, perfect model argumentative essay on “should scientists be allowed to conduct and publish research on engineering virulent flu, example of argumentative essay on genetically modified plants, genetic engineering should be supported argumentative essay examples, critique: optimized facility design for biotech facilities argumentative essay examples, argumentative essay on genetically modified foods.
Password recovery email has been sent to [email protected]
Use your new password to log in
You are not register!
By clicking Register, you agree to our Terms of Service and that you have read our Privacy Policy .
Now you can download documents directly to your device!
Check your email! An email with your password has already been sent to you! Now you can download documents directly to your device.
or Use the QR code to Save this Paper to Your Phone
The sample is NOT original!
Short on a deadline?
Don't waste time. Get help with 11% off using code - GETWOWED
No, thanks! I'm fine with missing my deadline
- Search Menu
- Sign in through your institution
- Volume 12, Issue 1, 2024 (In Progress)
- Volume 11, Issue 1, 2023
- Advance articles
- Editor's Choice
- Virtual Issues
- Clinical Briefs
- ISEMPH Prizes
- Why Publish
- Author Guidelines
- Submission Site
- Open Access
- Calls for Papers
- About Evolution, Medicine, and Public Health
- About the International Society for Evolution, Medicine and Public Health
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- For Reviewers
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, human enhancement, genetic engineering, conclusions.
- < Previous
Human enhancement: Genetic engineering and evolution
- Article contents
- Figures & tables
- Supplementary Data
Mara Almeida, Rui Diogo, Human enhancement: Genetic engineering and evolution, Evolution, Medicine, and Public Health , Volume 2019, Issue 1, 2019, Pages 183–189, https://doi.org/10.1093/emph/eoz026
- Permissions Icon Permissions
Genetic engineering opens new possibilities for biomedical enhancement requiring ethical, societal and practical considerations to evaluate its implications for human biology, human evolution and our natural environment. In this Commentary, we consider human enhancement, and in particular, we explore genetic enhancement in an evolutionary context. In summarizing key open questions, we highlight the importance of acknowledging multiple effects (pleiotropy) and complex epigenetic interactions among genotype, phenotype and ecology, and the need to consider the unit of impact not only to the human body but also to human populations and their natural environment (systems biology). We also propose that a practicable distinction between ‘therapy’ and ‘enhancement’ may need to be drawn and effectively implemented in future regulations. Overall, we suggest that it is essential for ethical, philosophical and policy discussions on human enhancement to consider the empirical evidence provided by evolutionary biology, developmental biology and other disciplines.
Lay Summary: This Commentary explores genetic enhancement in an evolutionary context. We highlight the multiple effects associated with germline heritable genetic intervention, the need to consider the unit of impact to human populations and their natural environment, and propose that a practicable distinction between ‘therapy’ and ‘enhancement’ is needed.
There are countless examples where technology has contributed to ameliorate the lives of people by improving their inherent or acquired capabilities. For example, over time, there have been biomedical interventions attempting to restore functions that are deficient, such as vision, hearing or mobility. If we consider human vision, substantial advances started from the time spectacles were developed (possibly in the 13th century), continuing in the last few years, with researchers implanting artificial retinas to give blind patients partial sight [ 1–3 ]. Recently, scientists have also successfully linked the brain of a paralysed man to a computer chip, which helped restore partial movement of limbs previously non-responsive [ 4 , 5 ]. In addition, synthetic blood substitutes have been created, which could be used in human patients in the future [ 6–8 ].
The progress being made by technology in a restorative and therapeutic context could in theory be applied in other contexts to treat non-pathological conditions. Many of the technologies and pharmaceutical products developed in a medical context to treat patients are already being used by humans to ‘enhance’ some aspect of their bodies, for example drugs to boost brain power, nutritional supplements, brain stimulating technologies to control mood or growth hormones for children of short stature. Assistive technology for disabled people, reproductive medicine and pharmacology, beside their therapeutic and restorative use, have a greater potential for human ‘enhancement’ than currently thought. There are also dual outcomes as some therapies can have effects that amount to an enhancement as for example, the artificial legs used by the South African sprinter Oscar Pistorius providing him with a competitive advantage.
This commentary will provide general ethical considerations on human enhancement, and within the several forms of so-called human biomedical enhancement, it will focus on genetic engineering, particularly on germline (heritable) genetic interventions and on the insights evolutionary biology can provide in rationalizing its likely impact. These insights are a subject often limited in discussions on genetic engineering and human enhancement in general, and its links to ethical, philosophical and policy discussions, in particular [ 9 ]. The rapid advances in genetic technology make this debate very topical. Moreover, genes are thought to play a very substantial role in biological evolution and development of the human species, thus making this a topic requiring due consideration. With this commentary, we explore how concepts based in evolutionary biology could contribute to better assess the implications of human germline modifications, assuming they were widely employed. We conclude our brief analysis by summarizing key issues requiring resolution and potential approaches to progress them. Overall, the aim is to contribute to the debate on human genetic enhancement by looking not only at the future, as it is so often done, but also at our evolutionary past.
The noun ‘enhancement’ comes from the verb ‘enhance’, meaning ‘to increase or improve’. The verb enhance can be traced back to the vulgar Latin inaltiare and late Latin inaltare (‘raise, exalt’), from ‘ altare ’ (‘make high’) and altus (‘high’), literally ‘grown tall’. For centuries human enhancement has populated our imagination outlined by stories ranging from the myths of supernormal strengths and eternal life to the superpowers illustrated by the 20th century comic books superheroes. The desire of overcoming normal human capacities and the transformation to an almost ‘perfect’ form has been part of the history of civilization, extending from arts and religion to philosophy. The goal of improving the human condition and health has always been a driver for innovation and biomedical developments.
In the broadest sense, the process of human enhancement can be considered as an improvement of the ‘limitations’ of a ‘natural version’ of the human species with respect to a specific reference in time, and to different environments, which can vary depending on factors such as, for example, climate change. The limitations of the human condition can be physical and/or mental/cognitive (e.g. vision, strength or memory). This poses relevant questions of what a real or perceived human limitation is in the environment and times in which we are living and how it can be shifted over time considering social norms and cultural values of modern societies. Besides, the impact that overcoming these limitations will have on us humans, and the environment, should also be considered. For example, if we boost the immune system of specific people, this may contribute to the development/evolution of more resistant viruses and bacteria or/and lead to new viruses and bacteria to emerge. In environmental terms, enhancing the longevity of humans could contribute to a massive increase in global population, creating additional pressures on ecosystems already under human pressure.
Two decades ago, the practices of human enhancement have been described as ‘biomedical interventions that are used to improve human form or functioning beyond what is necessary to restore or sustain health’ [ 10 ]. The range of these practices has now increased with technological development, and they are ‘any kind of genetic, biomedical, or pharmaceutical intervention aimed at improving human dispositions, capacities, or well-being, even if there is no pathology to be treated’ [ 11 ]. Practices of human enhancement could be visualized as upgrading a ‘system’, where interventions take place for a better performance of the original system. This is far from being a hypothetical situation. The rapid progress within the fields of nanotechnology, biotechnology, information technology and cognitive science has brought back discussions about the evolutionary trajectory of the human species by the promise of new applications which could provide abilities beyond current ones [ 12 , 13 ]. If such a possibility was consciously embraced and actively pursued, technology could be expected to have a revolutionary interference with human life, not just helping humans in achieving general health and capabilities commensurate with our current ones but helping to overcome human limitations far beyond of what is currently possible for human beings. The emergence of new technologies has provided a broader range of potential human interventions and the possibility of transitioning from external changes to our bodies (e.g. external prosthesis) to internal ones, especially when considering genetic manipulation, whose changes can be permanent and transmissible.
The advocates of a far-reaching human enhancement have been referred to as ‘transhumanists’. In their vision, so far, humans have largely worked to control and shape their exterior environments (niche construction) but with new technologies (e.g. biotechnology, information technology and nanotechnology) they will soon be able to control and fundamentally change their own bodies. Supporters of these technologies agree with the possibility of a more radical interference in human life by using technology to overcome human limitations [ 14–16 ], that could allow us to live longer, healthier and even happier lives [ 17 ]. On the other side, and against this position, are the so-called ‘bioconservatives’, arguing for the conservation and protection of some kind of ‘human essence’, with the argument that it exists something intrinsically valuable in human life that should be preserved [ 18 , 19 ].
There is an ongoing debate between transhumanists [ 20–22 ] and bioconservatives [ 18 , 19 , 23 ] on the ethical issues regarding the use of technologies in humans. The focus of this commentary is not centred on this debate, particularly because the discussion of these extreme, divergent positions is already very prominent in the public debate. In fact, it is interesting to notice that the ‘moderate’ discourses around this topic are much less known. In a more moderate view, perhaps one of the crucial questions to consider, independently of the moral views on human enhancement, is whether human enhancement (especially if considering germline heritable genetic interventions) is a necessary development, and represents an appropriate use of time, funding and resources compared to other pressing societal issues. It is crucial to build space for these more moderate, and perhaps less polarized voices, allowing the consideration of other positions and visions beyond those being more strongly projected so far.
Ethical and societal discussions on what constitutes human enhancement will be fundamental to support the development of policy frameworks and regulations on new technological developments. When considering the ethical implications of human enhancement that technology will be available to offer now and in the future, it could be useful to group the different kinds of human enhancements in the phenotypic and genetic categories: (i) strictly phenotypic intervention (e.g. ranging from infrared vision spectacles to exoskeletons and bionic limbs); (ii) somatic, non-heritable genetic intervention (e.g. editing of muscle cells for stronger muscles) and (iii) germline, heritable genetic intervention (e.g. editing of the C–C chemokine receptor type 5 (CCR5) gene in the Chinese baby twins, discussed later on). These categories of enhancement raise different considerations and concerns and currently present different levels of acceptance by our society. The degree of ethical, societal and environmental impacts is likely to be more limited for phenotypic interventions (i) but higher for genetic interventions (ii and iii), especially for the ones which are transmissible to future generations (iii).
The rapid advances in technology seen in the last decades, have raised the possibility of ‘radical enhancement’, defined by Nicholas Agar, ‘as the improvement of human attributes and abilities to levels that greatly exceed what is currently possible for human beings’ [ 24 ]. Genetic engineering offers the possibility of such an enhancement by providing humans a profound control over their own biology. Among other technologies, genetic engineering comprises genome editing (also called gene editing), a group of technologies with the ability to directly modify an organism’s DNA through a targeted intervention in the genome (e.g. insertion, deletion or replacement of specific genetic material) [ 25 ]. Genome editing is considered to achieve much greater precision than pre-existing forms of genetic engineering. It has been argued to be a revolutionary tool due to its efficiency, reducing cost and time. This technology is considered to have many applications for human health, in both preventing and tackling disease. Much of the ethical debate associated with this technology concerns the possible application of genome editing in the human germline, i.e. the genome that can be transmitted to following generations, be it from gametes, a fertilized egg or from first embryo divisions [ 26–28 ]. There has been concern as well as enthusiasm on the potential of the technology to modify human germline genome to provide us with traits considered positive or useful (e.g. muscle strength, memory and intelligence) in the current and future environments.
Genetic engineering: therapy or enhancement and predictability of outcomes
To explore some of the possible implications of heritable interventions we will take as an example the editing (more specifically ‘deletion’ using CRISPR genome editing technology) of several base pairs of the CCR5 gene. Such intervention was practised in 2018 in two non-identical twin girls born in China. Loss of function mutations of the CCR5 had been previously shown to provide resistance to HIV. Therefore, the gene deletion would be expected to protect the twin baby girls from risk of transmission of HIV which could have occurred from their father (HIV-positive). However, the father had the infection kept under control and the titre of HIV virus was undetectable, which means that risk of transmission of HIV infection to the babies was negligible [ 29 ].
From an ethical ground, based on current acceptable practices, this case has been widely criticized by the scientific community beside being considered by many a case of human enhancement intervention rather than therapy [ 29 , 30 ]. One of the questions this example helps illustrate is that the ethical boundary between a therapy that ‘corrects’ a disorder by restoring performance to a ‘normal’ scope, and an intervention that ‘enhances’ human ability outside the accepted ‘normal’ scope, is not always easy to draw. For the sake of argument, it could be assumed that therapy involves attempts to restore a certain condition of health, normality or sanity of the ‘natural’ condition of a specific individual. If we take this approach, the question is how health, normality and sanity, as well as natural per se, are defined, as the meaning of these concepts shift over time to accommodate social norms and cultural values of modern societies. It could be said that the difficulty of developing a conceptual distinction between therapy and enhancement has always been present. However, the potential significance of such distinction is only now, with the acceleration and impact of technological developments, becoming more evident.
Beyond ethical questions, a major problem of this intervention is that we do not (yet?) know exactly the totality of the effects that the artificial mutation of the CCR5 may have, at both the genetic and phenotypic levels. This is because we now know that, contrary to the idea of ‘one gene-one trait’ accepted some decades ago, a gene—or its absence—can affect numerous traits, many of them being apparently unrelated (a phenomenon also known as pleiotropy). That is, due to constrained developmental interactions, mechanisms and genetic networks, a change in a single gene can result in a cascade of multiple effects [ 31 ]. In the case of CCR5, we currently know that the mutation offers protection against HIV infection, and also seems to increase the risk of severe or fatal reactions to some infectious diseases, such as the influenza virus [ 32 ]. It has also been observed that among people with multiple sclerosis, the ones with CCR5 mutation are twice as likely to die early than are people without the mutation [ 33 ]. Some studies have also shown that defective CCR5 can have a positive effect in cognition to enhance learning and memory in mice [ 34 ]. However, it’s not clear if this effect would be translated into humans. The example serves to illustrate that, even if human enhancement with gene editing methods was considered ethically sound, assessing the totality of its implications on solid grounds may be difficult to achieve.
Genetic engineering and human evolution: large-scale impacts
Beyond providing the opportunity of enhancing human capabilities in specific individuals, intervening in the germline is likely to have an impact on the evolutionary processes of the human species raising questions on the scale and type of impacts. In fact, the use of large-scale genetic engineering might exponentially increase the force of ‘niche construction’ in human evolution, and therefore raise ethical and practical questions never faced by our species before. It has been argued that natural selection is a mechanism of lesser importance in the case of current human evolution, as compared to other organisms, because of advances in medicine and healthcare [ 35 ]. According to such a view, among many others advances, natural selection has been conditioned by our ‘niche-construction’ ability to improve healthcare and access to clean water and food, thus changing the landscape of pressures that humans have been facing for survival. An underlying assumption or position of the current debate is that, within our human species, the force of natural selection became minimized and that we are somehow at the ‘end-point’ of our evolution [ 36 ]. If this premise holds true, one could argue that evolution is no longer a force in human history and hence that any human enhancement would not be substituting itself to human evolution as a key driver for future changes.
However, it is useful to remember that, as defined by Darwin in his book ‘On the Origin of the Species’, natural selection is a process in which organisms that happen to be ‘better’ adapted to a certain environment tend to have higher survival and/or reproductive rates than other organisms [ 37 ]. When comparing human evolution to human genetic enhancement, an acceptable position could be to consider ethically sound those interventions that could be replicated naturally by evolution, as in the case of the CCR5 gene. Even if this approach was taken, however, it is important to bear in mind that human evolution acts on human traits sometimes increasing and sometimes decreasing our biological fitness, in a constant evolutionary trade-off and in a contingent and/or neutral—in the sense of not ‘progressive’—process. In other worlds, differently from genetic human enhancement, natural selection does not ‘ aim ’ at improving human traits [ 38 ]. Human evolution and the so-called genetic human enhancement would seem therefore to involve different underlying processes, raising several questions regarding the implications and risks of the latter.
But using genetic engineering to treat humans has been proposed far beyond the therapeutic case or to introduce genetic modifications known to already occur in nature. In particular, when looking into the views expressed on the balance between human evolution and genetic engineering, some argue that it may be appropriate to use genetic interventions to go beyond what natural selection has contributed to our species when it comes to eradicate vulnerabilities [ 17 ]. Furthermore, when considering the environmental, ecological and social issues of contemporary times, some suggest that genetic technologies could be crucial tools to contribute to human survival and well-being [ 20–22 ]. The possible need to ‘engineer’ human traits to ensure our survival could include the ability to allow our species to adapt rapidly to the rate of environmental change caused by human activity, for which Darwinian evolution may be too slow [ 39 ]. Or, for instance, to support long-distance space travel by engineering resistance to radiation and osteoporosis, along with other conditions which would be highly advantageous in space [ 40 ].
When considering the ethical and societal merits of these propositions, it is useful to consider how proto-forms of enhancement has been approached by past human societies. In particular, it can be argued that humans have already employed—as part of our domestication/‘selective breeding’ of other animals—techniques of indirect manipulation of genomes on a relatively large scale over many millennia, albeit not on humans. The large-scale selective breeding of plants and animals over prehistoric and historic periods could be claimed to have already shaped some of our natural environment. Selective breeding has been used to obtain specific characteristics considered useful at a given time in plants and animals. Therefore, their evolutionary processes have been altered with the aim to produce lineages with advantageous traits, which contributed to the evolution of different domesticated species. However, differently from genetic engineering, domestication possesses inherent limitations in its ability to produce major transformations in the created lineages, in contrast with the many open possibilities provided by genetic engineering.
When considering the impact of genetic engineering on human evolution, one of questions to be considered concerns the effects, if any, that genetic technology could have on the genetic pool of the human population and any implication on its resilience to unforeseen circumstances. This underlines a relevant question associated with the difference between ‘health’ and biological fitness. For example, a certain group of animals can be more ‘healthy’—as domesticated dogs—but be less biologically ‘fit’ according to Darwin’s definition. Specifically, if such group of animals are less genetically diverse than their ancestors, they could be less ‘adaptable’ to environmental changes. Assuming that, the human germline modification is undertaken at a global scale, this could be expected to have an effect, on the distribution of genetically heritable traits on the human population over time. Considering that gene and trait distributions have been changing under the processes of evolution for billions of years, the impact on evolution will need to be assessed by analysing which genetic alterations have been eventually associated with specific changes within the recent evolutionary history of humans. On this front, a key study has analysed the implications of genetic engineering on the evolutionary biology of human populations, including the possibility of reducing human genetic diversity, for instance creating a ‘biological monoculture’ [ 41 ]. The study argued that genetic engineering will have an insignificant impact on human diversity, while it would likely safeguard the capacity of human populations to deal with disease and new environmental challenges and therefore, ensure the health and longevity of our species [ 41 ]. If the findings of this study were considered consistent with other knowledge and encompassing, the impact of human genetic enhancements on the human genetic pool and associated impacts could be considered secondary aspects. However, data available from studies on domestication strongly suggests that domestication of both animals and plans might lead to not only decreased genetic diversity per se, but even affect patterns of variation in gene expression throughout the genome and generally decreased gene expression diversity across species [ 42–44 ]. Given that, according to recent studies within the field of biological anthropology recent human evolution has been in fact a process of ‘self-domestication’ [ 45 ], one could argue that studies on domestication could contribute to understanding the impacts of genetic engineering.
Beyond such considerations, it is useful to reflect on the fact that human genetic enhancement could occur on different geographical scales, regardless of the specific environment and geological periods in which humans are living and much more rapidly than in the case of evolution, in which changes are very slow. If this was to occur routinely and on a large scale, the implications of the resulting radical and abrupt changes may be difficult to predict and its impacts difficult to manage. This is currently highlighted by results of epigenetics studies, and also of the microbiome and of the effects of pollutants in the environment and their cumulative effect on the development of human and non-human organisms alike. Increasingly new evidence indicates a greater interdependence between humans and their environments (including other microorganisms), indicating that modifying the environment can have direct and unpredictable consequences on humans as well. This highlight the need of a ‘systems level’ approach. An approach in which the ‘bounded body’ of the individual human as a basic unit of biological or social action would need to be questioned in favour of a more encompassing and holistic unit. In fact, within biology, there is a new field, Systems Biology, which stresses the need to understand the role that pleiotropy, and thus networks at multiple levels—e.g. genetic, cellular, among individuals and among different taxa—play within biological systems and their evolution [ 46 ]. Currently, much still needs to be understood about gene function, its role in human biological systems and the interaction between genes and external factors such as environment, diet and so on. In the future if we do choose to genetically enhance human traits to levels unlikely to be achieved by human evolution, it would be crucial to consider if and how our understanding of human evolution enable us to better understand the implications of genetic interventions.
New forms of human enhancement are increasingly coming to play due to technological development. If phenotypic and somatic interventions for human enhancement pose already significant ethical and societal challenges, germline heritable genetic intervention, require much broader and complex considerations at the level of the individual, society and human species as a whole. Germline interventions associated with modern technologies are capable of much more rapid, large-scale impacts and seem capable of radically altering the balance of humans with the environment. We know now that beside the role genes play on biological evolution and development, genetic interventions can induce multiple effects (pleiotropy) and complex epigenetics interactions among genotype, phenotype and ecology of a certain environment. As a result of the rapidity and scale with which such impact could be realized, it is essential for ethical and societal debates, as well as underlying scientific studies, to consider the unit of impact not only to the human body but also to human populations and their natural environment (systems biology). An important practicable distinction between ‘therapy’ and ‘enhancement’ may need to be drawn and effectively implemented in future regulations, although a distinct line between the two may be difficult to draw.
In the future if we do choose to genetically enhance human traits to levels unlikely to be achieved by human evolution, it would be crucial to consider if and how our understanding of humans and other organisms, including domesticated ones, enable us to better understand the implications of genetic interventions. In particular, effective regulation of genetic engineering may need to be based on a deep knowledge of the exact links between phenotype and genotype, as well the interaction of the human species with the environment and vice versa .
For a broader and consistent debate, it will be essential for technological, philosophical, ethical and policy discussions on human enhancement to consider the empirical evidence provided by evolutionary biology, developmental biology and other disciplines.
This work was supported by Fundação para a Ciência e a Tecnologia (FCT) of Portugal [CFCUL/FIL/00678/2019 to M.A.].
Conflict of interest : None declared.
Pham P , Roux S , Matonti F et al. Post-implantation impedance spectroscopy of subretinal micro-electrode arrays, OCT imaging and numerical simulation: towards a more precise neuroprosthesis monitoring tool . J Neural Eng 2013 ; 10 : 046002 .
Google Scholar
Maghami MH , Sodagar AM , Lashay A et al. Visual prostheses: the enabling technology to give sight to the blind . J Ophthal Vis Res 2014 ; 9 : 494 – 505 .
Weitz AC , Nanduri D , Behrend MR et al. Improving the spatial resolution of epiretinal implants by increasing stimulus pulse duration . Sci Transl Med 2015 ; 7 : 318ra203.
Bouton CE , Shaikhouni A , Annetta NV et al. Restoring cortical control of functional movement in a human with quadriplegia . Nature 2016 ; 533 : 247 – 50 .
Geddes L. First paralysed person to be ‘reanimated’ offers neuroscience insights. Technique moves man’s arm by decoding his thoughts and electrically stimulating his own muscles . Nat News 2016 ; 533 .
Squires JE. Artificial blood . Science 2002 ; 295 : 1002 – 5 .
Lowe KC. Blood substitutes: from chemistry to clinic . J Mater Chem 2006 ; 16 : 4189 – 96 .
Moradi S , Jahanian-Najafabadi A , Roudkenar MH. Artificial blood substitutes: first steps on the long route to clinical utility . Clin Med Insights Blood Disord 2016 ; 9 : 33 – 41 .
Powell R , Kahane G , Savulescu J. Evolution, genetic engineering, and human enhancement . Philos Technol 2012 ; 25 : 439 – 58 .
Parens E (ed.). Enhancing Human Traits: Ethical and Social Implications . Washington, DC : Georgetown University Press , 1998 .
Google Preview
Giubilini A , Sanyal S. Challenging human enhancement. In: Clarke S , Savulescu J , Coady T et al. (eds). The Ethics of Human Enhancement: Understanding the Debate . Oxford : Oxford University Press , 2016 .
Elliott C. Better Than Well: American Medicine Meets the American Dream . New York, NY : WWW Norton & Company, Inc ., 2003 .
Kramer P. Listening to Prozac . London : Fourth Estate , 1994 .
Moravec H. Mind Children: The Future of Robot and Human Intelligence . Cambridge : Harvard University Press , 1990 .
Bostrom N. Human genetic enhancements: a transhumanist perspective . J Value Inq 2003 ; 37 : 493 – 506 .
Kurzweil R. The Singularity is Near: When Humans Transcend Biology . New York, NY : Viking , 2005 .
Harris J. Enhancing Evolution: The Ethical Case for Making Better People . Princeton, NJ : Princeton University Press , 2010 .
Fukuyama F. Our Posthuman Future: Consequences of the Biotechnology Revolution . New York, NY : Picador , 2002 .
Sandel M. The Case Against Perfection: Ethics in the Age of Genetic Engineering . Cambridge : The Belknap Press of Harvard University Press , 2007 .
Savulescu J , Persson I. The perils of cognitive enhancement and the urgent imperative to enhance the moral character of humanity . J Appl Philos 2008 ; 25 : 162 – 77 .
Buchanan A. Beyond Humanity . Oxford : Oxford University Press , 2011 .
Persson I , Savulescu J. Moral enhancement, freedom, and the god machine . Monist 2012 ; 95 : 399 – 421 .
Leon K. Ageless bodies, happy souls: biotechnology and the pursuit of perfection . New Atlantis 2003 ; 1 : 9 – 28 .
Agar N. Humanity’s End: Why We Should Reject Radical Enhancement . Cambridge : MIT Press , 2010 .
Gaj T , Gersbach CA , Barbas CF III ,. ZFN, TALEN, and CRISPR/Cas based methods for genome engineering . Trends Biotechnol 2013 ; 3 : 397 – 405 .
Baltimore D , Berg P , Botchan M et al. Biotechnology. A prudent path forward for genomic engineering and germline gene modification . Science 2015 ; 348 : 36 – 8 .
Otieno MO. CRISPR/Cas9 human genome editing: challenges, ethical concerns and implications . J Clin Res Bioeth 2015 ; 6 : 253 .
Ishii T. Germline genome-editing research and its socio-ethical implications . Trends Mol Med 2015 ; 21 : 473 – 81 .
Bionews.org.uk. First Genome-edited Babies: A Very Different Perception of Ethics , 2018 . https://www.bionews.org.uk/page_140060 (27 August 2019, date last accessed).
Cyranoski D. CRISPR-baby scientist fails to satisfy his critics . Nat News 2018 ; 564 : 13 – 4 .
Galis F , Metz JA. Evolutionary novelties: the making and breaking of pleiotropic constraints . Integr Comp Biol 2007 ; 47 : 409 – 19 .
Falcon A , Cuevas MT , Rodriguez-Frandsen A et al. CCR5 deficiency predisposes to fatal outcome in influenza virus infection . J Gen Virol 2015 ; 96 : 2074 – 8 .
Gade-Andavolu R , Comings DE , MacMurray J et al. Association of CCR5 Δ32 deletion with early death in multiple sclerosis . Genet Med 2004 ; 6 : 126 – 31 .
Zhou M , Greenhill S , Huang S et al. CCR5 is a suppressor for cortical plasticity and hippocampal learning and memory . eLife 2016 ; 5 : e20985 .
Tibayrenc M , Ayala FJ (eds). On Human Nature: Biology, Psychology, Ethics, Politics, and Religion . London : Academic Press , 2017 .
Baldi P. The Shattered Self: The End of Natural Evolution . Cambridge : MIT Press , 2001 .
Darwin C. On the Origin of Species by Means of Natural Selection, or, the Preservation of Favoured Races in the Struggle for Life . London : J. Murray , 1859 .
Gould SJ. The Structure of Evolutionary Theory . Belknap, NY : Harvard University Press , 2002 .
Rees M. Our Final Century: Will the Humans Race Survive the Twenty-first Century? Eastbourne : Gardners Books , 2003 .
Nuffield Council on Bioethics. Genome Editing: An Ethical Review . London : Nuffield Council on Bioethics , 2016 .
Powell R. The evolutionary biological implications of human genetic engineering . J Med Philos 2012 ; 37 : 204 – 26 .
Liu W , Chen L , Zhang S et al. Decrease of gene expression diversity during domestication of animals and plants . BMC Evol Biol 2019 ; 19 : 1 – 11 .
Fages A , Hanghøj K , Khan N et al. Tracking five millennia of horse management with extensive ancient genome time series . Cell 2019 ; 177 : 1419 – 35 .
Zhang J , Wang X , Yao J et al. Effect of domestication on the genetic diversity and structure of Saccharina japonica populations in China . Sci Rep 2017 ; 7 : 42158 .
Theofanopoulou C , Gastaldon S , O’Rourke T et al. Self-domestication in Homo sapiens : insights from comparative genomics . PLoS One 2018 ; 13 : e0196700 .
Capra F , Luisi PL. The Systems View of Life . Cambridge : Cambridge University Press , 2014
- genetic engineering
| Month: | Total Views: |
|---|---|
| September 2019 | 51 |
| October 2019 | 571 |
| November 2019 | 512 |
| December 2019 | 474 |
| January 2020 | 492 |
| February 2020 | 712 |
| March 2020 | 680 |
| April 2020 | 615 |
| May 2020 | 609 |
| June 2020 | 599 |
| July 2020 | 605 |
| August 2020 | 632 |
| September 2020 | 842 |
| October 2020 | 1,322 |
| November 2020 | 1,583 |
| December 2020 | 1,688 |
| January 2021 | 1,536 |
| February 2021 | 2,019 |
| March 2021 | 3,077 |
| April 2021 | 2,893 |
| May 2021 | 2,223 |
| June 2021 | 1,434 |
| July 2021 | 906 |
| August 2021 | 911 |
| September 2021 | 1,393 |
| October 2021 | 2,158 |
| November 2021 | 2,377 |
| December 2021 | 1,736 |
| January 2022 | 1,315 |
| February 2022 | 1,783 |
| March 2022 | 2,522 |
| April 2022 | 1,946 |
| May 2022 | 1,552 |
| June 2022 | 789 |
| July 2022 | 675 |
| August 2022 | 486 |
| September 2022 | 919 |
| October 2022 | 1,462 |
| November 2022 | 1,427 |
| December 2022 | 1,216 |
| January 2023 | 1,372 |
| February 2023 | 1,740 |
| March 2023 | 2,320 |
| April 2023 | 2,195 |
| May 2023 | 1,646 |
| June 2023 | 1,066 |
| July 2023 | 940 |
| August 2023 | 1,070 |
| September 2023 | 1,283 |
| October 2023 | 1,953 |
| November 2023 | 1,886 |
| December 2023 | 1,684 |
| January 2024 | 1,871 |
| February 2024 | 2,312 |
| March 2024 | 2,729 |
| April 2024 | 2,752 |
| May 2024 | 2,034 |
| June 2024 | 1,117 |
| July 2024 | 854 |
| August 2024 | 1,048 |
| September 2024 | 741 |
Email alerts
Citing articles via, affiliations.
- Online ISSN 2050-6201
- Copyright © 2024 International Society for Evolution, Medicine, and Public Health
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Home — Essay Samples — Science — Technology & Engineering — Genetic Engineering
Essays on Genetic Engineering
What makes a good genetic engineering essay topic.
When it comes to writing a captivating genetic engineering essay, the topic you choose is paramount. It not only grabs the reader's attention but also allows for effective exploration of the subject matter. So, how can you brainstorm and select a standout essay topic? Here are some recommendations:
- Brainstorm: Kickstart your ideas by brainstorming topics related to genetic engineering. Consider the latest advancements, ethical concerns, controversial issues, or potential future applications. Jot down any ideas that come to mind.
- Research: Once you have a list of potential topics, conduct thorough research to gather relevant information and understand different perspectives. This will help you evaluate the feasibility and depth of each topic.
- Consider Interest: Choose a topic that genuinely piques your interest. Writing about something you are passionate about will make the entire process more enjoyable and motivate you to delve deeper into the subject matter.
- Relevance: Ensure that the chosen topic is relevant to genetic engineering. It should align with the scope of the subject and allow you to explore various aspects related to it.
- Uniqueness: Strive for a unique and imaginative topic that stands out from the ordinary. Steer clear of generic subjects and instead focus on specific areas or emerging trends within genetic engineering.
- Controversy: Controversial topics often generate more interest and discussion. Consider exploring ethical dilemmas, potential risks, or societal impacts of genetic engineering to add a thought-provoking element to your essay.
- Depth and Scope: Assess the depth and scope of each topic. Make sure it provides enough material for a comprehensive essay without being too broad or too narrow.
- Audience Appeal: Keep your target audience in mind. Choose a topic that would captivate readers, whether they are experts in the field or individuals with limited knowledge about genetic engineering.
- Originality: Strive for originality in your topic selection. Look for unique angles, lesser-known areas, or innovative applications of genetic engineering that can make your essay stand out.
- Personal Connection: If possible, choose a topic that connects with your personal experiences or future aspirations. This will enhance your engagement and make your essay more meaningful.
Igniting Thought: The Finest Genetic Engineering Essay Topics
Below are some of the most captivating genetic engineering essay topics to consider:
- Genetic Engineering and the Future of Human Evolution
- The Ethical Dilemmas of Designer Babies
- Genetic Engineering in Agriculture: Balancing Benefits and Concerns
- CRISPR-Cas9: Unleashing Revolutionary Potential in Genetic Engineering
- The Potential of Genetic Engineering in Cancer Treatment
- Genetic Engineering's Role in Creating Sustainable Food Sources
- Genetic Engineering and Animal Welfare: Navigating Ethical Considerations
- Genetic Engineering and its Impact on Biodiversity
- The Social and Economic Implications of Genetic Engineering
- Genetic Engineering's Influence on Human Longevity
- Enhancing Athletic Performance: The Power of Genetic Engineering
- Genetic Engineering Techniques for Disease Prevention and Treatment
- Genetic Engineering's Role in Environmental Conservation
- Genetic Engineering and the Preservation of Endangered Species
- The Psychological and Societal Effects of Genetic Engineering
- The Pros and Cons of Genetic Engineering for Non-Medical Purposes
- Exploring the Potential Risks and Benefits of Genetic Engineering in Space Exploration
- Genetic Engineering and the Creation of Biofuels
- The Morality of Genetic Engineering: Insights from Religious and Philosophical Perspectives
- Genetic Engineering's Role in Combating Climate Change
Thought-Provoking Genetic Engineering Essay Questions
Consider these stimulating questions for your genetic engineering essay:
- How does genetic engineering impact the concept of natural selection?
- What are the potential consequences of genetic engineering on human genetic diversity?
- Is it ethically justifiable to use genetic engineering for cosmetic purposes?
- How does genetic engineering contribute to the development of personalized medicine?
- What are the social implications of genetically modifying animals for human consumption?
- How does the use of genetic engineering in agriculture affect food security?
- Should genetic engineering be used to resurrect extinct species?
- What are the potential risks and benefits of genetically modifying viruses for medical purposes?
- How does genetic engineering influence the balance between individual rights and societal well-being?
- Can genetic engineering be the solution to eradicating genetic diseases?
Provocative Genetic Engineering Essay Prompts
Here are some imaginative and engaging prompts for your genetic engineering essay:
- Imagine a world where genetic engineering has eliminated all hereditary diseases. Discuss the potential benefits and drawbacks of such a scenario.
- You have been granted the ability to genetically engineer one aspect of yourself. What would you choose and why?
- Write a fictional story set in a future where genetic engineering is widespread and explore the consequences it has on society.
- Reflect on the ethical considerations of genetically modifying animals for entertainment purposes, such as creating glow-in-the-dark pets.
- Create a persuasive argument for or against the use of genetic engineering in enhancing human intelligence.
Answering Your Genetic Engineering Essay Queries
Q: Can I write about the history of genetic engineering?
A: Absolutely! Exploring the historical context of genetic engineering can provide valuable insights and set the foundation for your essay.
Q: How can I make my genetic engineering essay engaging for readers with limited scientific knowledge?
A: Simplify complex concepts and terminologies, provide relevant examples, and use relatable analogies to help readers grasp the information more easily.
Q: Can I express my personal opinion in a genetic engineering essay?
A: Yes, expressing your personal opinion is encouraged as long as you support it with logical reasoning and evidence from reputable sources.
Q: Are there any potential risks associated with genetic engineering that I should discuss in my essay?
A: Yes, incorporating a discussion on the potential risks and ethical concerns surrounding genetic engineering is essential to provide a balanced perspective.
Q: Can I include interviews or case studies in my genetic engineering essay?
A: Absolutely! Interviews or case studies can add depth and real-life examples to support your arguments and make your essay more compelling.
Remember, when writing your genetic engineering essay, let your creativity shine through while maintaining a formal and engaging tone.
The 4 Forces of Evolution: a Comprehensive Analysis
The ethics of genetic engineering, made-to-order essay as fast as you need it.
Each essay is customized to cater to your unique preferences
+ experts online
Genetic Engineering
Ethical issues of genetic engineering, the dangers of genetic engineering to humanity, the use and ethics of genetic engineering, let us write you an essay from scratch.
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
The Potential and Consequences of Genetic Engineering
The issue of the use of genetic modification of humans, reasons why genetic engineering should be banned, genetic engineering: an overview of the dna/rna and the crispr/cas9 technology, get a personalized essay in under 3 hours.
Expert-written essays crafted with your exact needs in mind
Review of Human Germline Engineering
Positional cloning of genetic disorders, engineering american society: the lesson of eugenics, bioethical issues related to genetic engineering, cloning and ethical controversies related to it, genetic editing as a possibility of same-sex parents to have children, adhering to natural processes retains the integrity of a natural human race , genetically modified organisms: soybeans, gene silencing to produce milk with reduced blg proteins, the role of crispr-cas9 gene drive in mosquitoes, the life of gregor mendel and his contributions to science, eugenics, its history and modern development, morphological operation hsv color space tree detetction, cytogenetics: analysis of comparative genomic hybridization and its implications, genetically engineered eucalyptus tree and crispr, review of the process of dna extraction, review of the features of the process of cloning, heterologous gene expression as an approach for fungal secondary metabolite discovery, review of the genetic algorithm searches, genetic engineering: clustered regularly interspaced short palindromic repeats.
Genetic engineering (also called genetic modification) is a process that uses laboratory-based technologies to alter the DNA makeup of an organism.
Genetic engineering as the direct manipulation of DNA by humans outside breeding and mutations has only existed since the 1970s. In 1972, Paul Berg created the first recombinant DNA molecules by combining DNA from the monkey virus SV40 with that of the lambda virus. The first field trials of genetically engineered plants occurred in France and the US in 1986, tobacco plants were engineered to be resistant to herbicides.
It is a set of technologies used to change the genetic makeup of cells, including the transfer of genes within and across species boundaries to produce improved or novel organisms. New DNA is obtained by either isolating and copying the genetic material of interest using recombinant DNA methods or by artificially synthesising the DNA. Used in research and industry, genetic engineering has been applied to the production of cancer therapies, brewing yeasts, genetically modified plants and livestock, and more.
Relevant topics
- Engineering
- Mathematics in Everyday Life
- Space Exploration
- Time Travel
- Stephen Hawking
- Charles Darwin
- Natural Selection
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
132 Genetic Engineering Essay Topic Ideas & Examples
Welcome to our list of genetic engineering essay topics! Here, you will find everything from trending research titles to the most interesting genetic engineering topics for presentation. Get inspired with our writing ideas and bonus samples!
🔝 Top 10 Genetic Engineering Topics for 2024
🏆 best genetic engineering topic ideas & essay examples, ⭐ good genetic engineering research topics, 👍 simple & easy genetic engineering essay topics, ❓ genetic engineering discussion questions, 🔎 genetic engineering research topics, ✅ genetic engineering project ideas.
- Ethical Issues of Synthetic Biology
- CRISPR-Cas9 and Its Applications
- Progress and Challenges in Gene Therapy
- Applications of Gene Editing in Animals
- The Process of Genetic Engineering in Plants
- Genetic Engineering for Human Enhancement
- Genetic Engineering for Improving Crop Yield
- Regulatory Issues of Genetic Editing of Embryos
- Gene Silencing in Humans through RNA Interference
- Gene Drive Technology for Controlling Invasive Species
- Changing the world: Genetic Engineering Effects Genes used in genetic engineering have a high impact on health and disease, therefore the inclusion of the genetic process alters the genes that influence human behavior and traits.
- The Ethical Issues of Genetic Engineering Many people have questioned the health risks that arise from genetically modified crops, thus it is the politicians who have to ensure that the interests of the people are met and their safety is assured. […]
- Is Genetically Engineered Food the Solution to the World’s Hunger Problems? However, the acceptance of GMO’s as the solution to the world’s food problem is not unanimously and there is still a multitude of opposition and suspicion of their use.
- Human Genetic Engineering: Key Principles and Issues There are many options for the development of events in the field of genetic engineering, and not all of them have been studied. To conclude, human genetic engineering is one of the major medical breakthroughs, […]
- Mitochondrial Diseases Treatment Through Genetic Engineering Any disorders and abnormalities in the development of mitochondrial genetic information can lead to the dysfunction of these organelles, which in turn affects the efficiency of intracellular ATP production during the process of cellular respiration.
- Genetic Engineering: Is It Ethical to Manipulate Life? In the case of more complex operations, genetic engineering can edit existing genes to turn on or off the synthesis of a particular protein in the organism from which the gene was taken.
- Biotechnology and Genetic Engineering Apart from that, there are some experiments that cannot be ethically justified, at least in my opinion, for example, the cloning of human being or the attempts to find the gene for genius.
- Genetic Engineering in the Movie “Gattaca” by Niccol This would not be right at all since a person should be responsible for their own life and not have it dictated to them as a result of a societal construct created on the basis […]
- Religious vs Scientific Views on Genetic Engineering With the need to increase the global economy, the field of agriculture is one among the many that have been used to improve the commercial production to take care of the global needs for food […]
- Genetic Engineering Using a Pglo Plasmid The objective of this experiment is to understand the process and importance of the genetic transformation of bacteria in real time with the aid of extrachromosomal DNA, alternatively referred to as plasmids.
- Managing Diabetes Through Genetic Engineering Genetic engineering refers to the alteration of genetic make-up of an organism through the use of techniques to introduce a new DNA or eliminate a given hereditable material. What is the role of genetic engineering […]
- The Role of Plant Genetic Engineering in Global Security Although it can be conveniently stated that the adequacy, abundance and reliability of the global food supply has a major role to play in the enhancement of human life, in the long run, they influence […]
- Significance of Human Genetic Engineering The gene alteration strategy enables replacing the specific unwanted genes with the new ones, which are more resistant and freer of the particular ailment, hence an essential assurance of a healthy generation in the future.
- Is the World Ready for Genetic Engineering? The process of manipulating genes has brought scientists to important discoveries, among which is the technology of the production of new kinds of crops and plants with selected characteristics. The problem of the advantages and […]
- Genome: Bioethics and Genetic Engineering Additionally, towards the end of the documentary, the narrator and some of the interviewed individuals explain the problem of anonymity that is also related to genetic manipulations.
- Is Genetic Engineering an Environmentally Sound Way to Increase Food Production? According to Thomas & Earl and Barry, genetic engineering is environmentally unsound method of increasing food production because it threatens the indigenous species.
- Gattaca: Ethical Issues of Genetic Engineering Although the world he lives in has determined that the only measure of a man is his genetic profile, Vincent discovers another element of man that science and society have forgotten.
- A Major Milestone in the Field of Science and Technology: Should Genetic Engineering Be Allowed? The most controversial and complicated aspect of this expertise is Human Genetic Engineering- whereby the genotype of a fetus can be altered to produce desired results.
- Genetic Engineering Is Ethically Unacceptable However, the current application of genetic engineering is in the field of medicine particularly to treat various genetic conditions. However, this method of treatment has various consequences to the individual and the society in general.
- Designer Genes: Different Types and Use of Genetic Engineering McKibben speaks of Somatic Gene Therapy as it is used to modify the gene and cell structure of human beings so that the cells are able to produce certain chemicals that would help the body […]
- A Technique for Controlling Plant Characteristics: Genetic Engineering in the Agriculture A cautious investigation of genetic engineering is required to make sure it is safe for humans and the environment. The benefit credited to genetic manipulation is influenced through the utilization of herbicide-tolerant and pest-safe traits.
- The Dangers of Genetic Engineering and the Issue of Human Genes’ Modification In this case, the ethics of human cloning and human genes’ alteration are at the center of the most heated debates. The first reason to oppose the idea of manipulation of human genes lies in […]
- Genetically Engineered Food Against World Hunger I support the production of GMFs in large quality; I hold the opinion that they can offer a lasting solution to food problems facing the world.
- Genetic Engineering in Food: Development and Risks Genetic engineering refers to the manipulation of the gene composition of organisms, to come up with organisms, which have different characteristics from the organic ones.
- Genetic Engineering in the Workplace The main purpose of the paper is to evaluate and critically discuss the ethical concerns regarding the implementation of genetic testing in the workplace and to provide potential resolutions to the dilemmas.
- Designer Babies Creation in Genetic Engineering The creation of designer babies is an outcome of advancements in technology hence the debate should be on the extent to which technology can be applied in changing the way human beings live and the […]
- Genetic Engineering and Eugenics Comparison The main idea in genetic engineering is to manipulate the genetic make-up of human beings in order to shackle their inferior traits. The concept of socially independent reproduction is replicated in both eugenics and genetic […]
- The Film “Gattaca” and Genetic Engineering In the film, it is convincing that in the near future, science and technology at the back of genetic engineering shall be developed up to the level which makes the film a reality.
- Future of Genetic Engineering and the Concept of “Franken-Foods” This is not limited to cows alone but extends to pigs, sheep, and poultry, the justification for the development of genetically modified food is based on the need to feed an ever growing population which […]
- Ecological Effects of the Release of Genetically Engineered Organisms Beneficial soil organisms such as earthworms, mites, nematodes, woodlice among others are some of the soil living organisms that are adversely affected by introduction of genetically engineered organisms in the ecosystem since they introduce toxins […]
- Benefits of Genetic Engineering as a Huge Part of People’s Lives Genetic Engineering is said to question whether man has the right to manipulate the course and laws of nature and thus is in constant collision with religion and the beliefs held by it regarding life.
- Perfect Society: The Effects of Human Genetic Engineering
- Genetic Engineering and Forensic Criminal Investigations
- Biotechnology Assignment and Genetic Engineering
- Genetic Engineering and Genetically Modified Organisms
- Bio-Ethics and the Controversy of Genetic Engineering
- Health and Environmental Risks of Genetic Engineering in Food
- Genetic Engineering and the Risks of Enforcing Changes on Organisms
- Genetic Engineering and How It Affects Globel Warming
- Cloning and Genetic Engineering in the Food Animal Industry
- Genetic Engineering and Its Impact on Society
- Embryonic Research, Genetic Engineering, & Cloning
- Genetic Engineering: Associated Risks and Possibilities
- Issues Concerning Genetic Engineering in Food Production
- Genetic Engineering, DNA Fingerprinting, Gene Therapy
- Cloning: The Benefits and Dangers of Genetic Engineering
- Genetic Engineering, History, and Future: Altering the Face of Science
- Islamic and Catholic Views on Genetic Engineering
- Gene Therapy and Genetic Engineering: Should It Be Approved in the US
- Exploring the Real Benefits of Genetic Engineering in the Modern World
- Genetic Engineering and Food Security: A Welfare Economics Perspective
- Identify the Potential Impact of Genetic Engineering on the Future Course of Human Immunodeficiency Virus
- Genetic Engineering and DNA Technology in Agricultural Productivity
- Human Genetic Engineering: Designing the Future
- Genetic Engineering and the Politics Behind It
- The Potential and Consequences of Genetic Engineering
- Genetic Engineering and Its Effect on Human Health
- The Moral and Ethical Controversies, Benefits, and Future of Genetic Engineering
- Gene Therapy and Genetic Engineering for Curing Disorders
- Genetic Engineering and the Human Genome Project
- Ethical Standards for Genetic Engineering
- Genetic Engineering and Cryonic Freezing: A Modern Frankenstein
- The Perfect Child: Genetic Engineering
- Genetic Engineering and Its Effects on Future Generations
- Agricultural Genetic Engineering: Genetically Modified Foods
- Genetic Engineering: The Manipulation or Alteration of the Genetic Structure of a Single Cell or Organism
- Analysing Genetic Engineering Regarding Plato Philosophy
- The Dangers and Benefits of Human Cloning and Genetic Engineering
- Genetic Engineering: Arguments of Both Proponents and Opponents and a Mediated Solution
- Genetic and How Genetic Engineering Is Diffusing Individualism
- Finding Genetic Harmony With Genetic Engineering
- What Is Genetic Engineering?
- Do You Think Genetically Modified Food Could Harm the Ecosystems of the Areas in Which They Grow?
- How Agricultural Research Systems Shape a Technological Regime That Develops Genetic Engineering?
- Can Genetic Engineering for the Poor Pay Off?
- How Does Genetic Engineering Affect Agriculture?
- Do You Think It’s Essential to Modify Genes to Create New Medicines?
- How Can Genetic Engineering Stop Human Suffering?
- Can Genetic Engineering Cure HIV/AIDS in Humans?
- How Has Genetic Engineering Revolutionized Science and the World?
- Do You Think Genetic Engineering Is Playing God and That We Should Leave Life as It Was Created?
- What Are Some Advantages and Disadvantages of Genetic Engineering?
- How Will Genetic Engineering Affect the Human Race?
- When Does Genetic Engineering Go Bad?
- What Are the Benefits of Human Genetic Engineering?
- Does Genetic Engineering Affect the Entire World?
- How Does the Christian Faith Contend With Genetic Engineering?
- What Are the Ethical and Social Implications of Genetic Engineering?
- How Will Genetic Engineering Impact Our Lives?
- Why Should Genetic Engineering Be Extended?
- Will Genetic Engineering Permanently Change Our Society?
- What Are People Worried About Who Oppose Genetic Engineering?
- Do You Worry About Eating GM (Genetically Modified) Food?
- What Do You Think of the Idea of Genetically Engineering New Bodily Organs to Replace Yours When You Are Old?
- Should Genetic Engineering Go Ahead to Eliminate Human Flaws, Such as Violence, Jealousy, Hate, Etc?
- Does the Government Have the Right to Limit How Far We Modify Ourselves?
- Why Is Genetic Food Not Well Accepted?
- What Is the Best in the Genetic Modification of Plants, Plant Cell, or Chloroplasts and Why?
- How Do You Feel About Human Gene Editing?
- Does Climate Change Make the Genetic Engineering of Crops Inevitable?
- What Do You Think About Plant Genetic Modification?
- Gene Drives and Pest Control
- The Benefits of Genetically Modified Organisms
- Challenges of Gene Editing for Rare Genetic Diseases
- The Use of Genetic Engineering to Treat Human Diseases
- Ethical Considerations and Possibilities of Designer Babies
- How Genetic Engineering Can Help Restore Ecosystems
- Basic Techniques and Tools for Gene Manipulation
- Latest Advancements in Genetic Engineering and Genome Editing
- Will Engineering Resilient Organisms Help Mitigate Climate Change?
- Creation of Renewable Resources through Genetic Engineering
- Genetic Engineering Approach to Drought and Pest Resistance
- Genetic Engineering Use in DNA Analysis and Identification
- Synthetic Microorganisms and Biofactories for Sustainable Bioproduction
- Stem Cells’ Potential for Regenerative Medicine
- The Role of Genetic Modification in Vaccine Development
- Can Genetic Engineering Help Eradicate Invasive Species Responsibly?
- Genetic Engineering for Enhancing the Body’s Defense Mechanisms
- Advancements in Transplantation Medicine and Creating Bioengineered Organs
- Genetic Editing of Microbes for Environmental Cleanup
- Is It Possible to Develop Living Detection Systems?
- Infertility Essay Topics
- Bioethics Titles
- Genetics Research Ideas
- Epigenetics Essay Titles
- Morality Research Ideas
- Stem Cell Essay Titles
- Biochemistry Research Topics
- Evolution Topics
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, February 26). 132 Genetic Engineering Essay Topic Ideas & Examples. https://ivypanda.com/essays/topic/genetic-engineering-essay-topics/
"132 Genetic Engineering Essay Topic Ideas & Examples." IvyPanda , 26 Feb. 2024, ivypanda.com/essays/topic/genetic-engineering-essay-topics/.
IvyPanda . (2024) '132 Genetic Engineering Essay Topic Ideas & Examples'. 26 February.
IvyPanda . 2024. "132 Genetic Engineering Essay Topic Ideas & Examples." February 26, 2024. https://ivypanda.com/essays/topic/genetic-engineering-essay-topics/.
1. IvyPanda . "132 Genetic Engineering Essay Topic Ideas & Examples." February 26, 2024. https://ivypanda.com/essays/topic/genetic-engineering-essay-topics/.
Bibliography
IvyPanda . "132 Genetic Engineering Essay Topic Ideas & Examples." February 26, 2024. https://ivypanda.com/essays/topic/genetic-engineering-essay-topics/.
IvyPanda uses cookies and similar technologies to enhance your experience, enabling functionalities such as:
- Basic site functions
- Ensuring secure, safe transactions
- Secure account login
- Remembering account, browser, and regional preferences
- Remembering privacy and security settings
- Analyzing site traffic and usage
- Personalized search, content, and recommendations
- Displaying relevant, targeted ads on and off IvyPanda
Please refer to IvyPanda's Cookies Policy and Privacy Policy for detailed information.
Certain technologies we use are essential for critical functions such as security and site integrity, account authentication, security and privacy preferences, internal site usage and maintenance data, and ensuring the site operates correctly for browsing and transactions.
Cookies and similar technologies are used to enhance your experience by:
- Remembering general and regional preferences
- Personalizing content, search, recommendations, and offers
Some functions, such as personalized recommendations, account preferences, or localization, may not work correctly without these technologies. For more details, please refer to IvyPanda's Cookies Policy .
To enable personalized advertising (such as interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. These partners may have their own information collected about you. Turning off the personalized advertising setting won't stop you from seeing IvyPanda ads, but it may make the ads you see less relevant or more repetitive.
Personalized advertising may be considered a "sale" or "sharing" of the information under California and other state privacy laws, and you may have the right to opt out. Turning off personalized advertising allows you to exercise your right to opt out. Learn more in IvyPanda's Cookies Policy and Privacy Policy .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Gen Fam Med
- v.21(3); 2020 May
Ethical considerations of gene editing and genetic selection
Jodie rothschild.
1 Rothschild Biomedical Communications, Seattle WA, USA
For thousands of years, humans have felt the need to understand the world around them—and ultimately manipulate it to best serve their needs. There are always ethical questions to address, especially when the manipulation involves the human genome. There is currently an urgent need to actively pursue those conversations as commercial gene sequencing and editing technologies have become more accessible and affordable. This paper explores the ethical considerations of gene editing (specifically germline) and genetic selection—including the hurdles researchers will face in trying to develop new technologies into viable therapeutic options.
1. BACKGROUND
1.1. gene editing.
Artificial manipulation of genes is a relatively new science, and a number of watershed moments have provided the foundation for the current state of genetic engineering. Researchers first discovered that nonspecific alterations to Drosophila DNA could be introduced using radiation 1 and chemicals 2 in 1927 and 1947, respectively. Greater understanding of the structure of the DNA molecule (such as the work of Watson, Crick, and Franklin, leading to the discovery of DNA’s double‐helix structure 3 ) and the cellular processes that govern its transcription, translation, replication, and repair (such as the function of ligases 4 and restriction enzymes 5 ) led to the first splicing experiments 6 and, ultimately, the first recombinant DNA 7 in the early 1970s. DNA recombination techniques were used extensively in the budding yeast Saccharomyces cerevisiae 8 , 9 beginning in the early 1980s, allowing researchers to study functional eukaryotic genomics. And in a significant advancement, the development of polymerase chain reaction (PCR) allowed scientists to amplify DNA, producing millions of copies from a single strand. 10
Around the same time, a number of laboratories created the first transgenic mice, 11 and about five years later, the first knockout mice were created. 12 Targeted gene editing was further advanced by the discovery that engineered endonucleases could create site‐specific double‐stranded breaks (DSBs), which in turn induce homologous recombination (HR), 13 , 15 the most common type of homology‐directed repair (HDR). When the Human Genome Project was declared complete in 2003, 15 it became possible to identify (and thus, theoretically, target) any human gene of interest.
The three main techniques for gene editing involve molecules that recognize and bind to specific DNA sequences; researchers can use custom molecules to affect genetic and epigenetic changes on essentially any gene. For example, these molecules can be combined with endonucleases, creating DSBs which can be repaired using either nonhomologous end joining (NHEJ), which often results in small random indel mutations, or HDR, which, when donor DNA with homology to either side of the cleavage site is present, can be used to create new or “repaired” versions of a target gene. The site‐specific DNA recognition molecule can also be combined with an effector molecule to up‐ or downregulate gene expression.
1.1.1. ZFPs/ZFNs
In the late 1970s and early 1980s, there was a large focus on understanding transcription factor IIIA (TFIIIA), the first eukaryotic transcription factor to be described. In 1983, researchers determined that zinc is required for TFIIIA function, 16 and in 1985 came the discovery that the zinc‐binding portions of the proteins are actually repeating motifs, independently folded to create finger‐like domains that grip the DNA. 17 This class of proteins is now referred to as zinc finger proteins (ZFPs), and several similar proteins have been discovered in the proteomes of a number of different organisms. Because each zinc finger recognizes three base pairs, 18 , 19 , 20 a peptide can be created to recognize a target gene by joining the appropriate zinc fingers in a linear fashion.
A 1994 paper describes a ZFP that was engineered to recognize and suppress an oncogene, as well as a ZFP that acted (in a different cell system) as a promoter of another gene by recognizing its activation domain. 21 The same paper suggests that ZFPs can be bound to effector proteins as a means of controlling gene expression.
Building on this idea, researchers fused a ZFP to the nonspecific cleavage domain of the Fok1 restriction enzyme. 22 The resulting heterodimer, known as a zinc finger nuclease (ZFN), can recognize a specific DNA sequence and produce a targeted DSB. As previously mentioned, these DSB can either be repaired via NHEJ, resulting in small indels, or HDR, which can be harnessed to insert an alternate or repaired gene. Fok1 must dimerize, so ZFNs must be created in pairs (one targeting the 3’ strand and the other targeting the 5’ strand) which improves target specificity—though efficiency remains relatively low (G‐rich sequences are especially difficult to target).
Ex vivo and in vivo delivery of ZFNs is relatively easy given their small size and the small size of the ZFN cassettes (which allows for the use of a variety of vectors). However, while ZFNs were certainly novel at the time they were developed, they are incredibly difficult and expensive to engineer, making them less practical in general than newer technologies.
1.1.2. TALEs/TALENs
In 2009, two different laboratories described a newly identified DNA‐binding motif: the transcription activator‐like effector (TALE), a protein secreted by the plant pathogen Xanthomonas . 23 , 24 Each TALE includes a DNA‐binding region composed of tandem repeats with repeat‐variable diresidues (RVDs) at positions 12 and 13; each RVD recognizes an individual nucleotide.
Like ZFPs, synthetic TALEs can be designed to affect gene regulation, 25 combined with effector proteins, or fused to endonucleases 26 , 27 , 28 to create TALE nucleases (TALENs); as with ZFNs, because Fok1 is the endonuclease used, TALENs must be created in pairs.
TALE nucleases are much larger than ZFNs, and so can be more difficult to deliver efficiently (especially in vivo). However, for myriad reasons (including the nature of their relative interactions with the DNA and the fact that each RVD recognizes a single base), TALE‐based chimeras (especially TALENs) can be built with higher specificity and greater targeting capacity than ZFP‐based chimeras. In addition, TALENs can be produced significantly more cheaply, easily, and with greater efficiency than ZFNs.
1.1.3. CRISPR‐Cas
In 1987, a laboratory in Osaka accidentally discovered an unusual palindromic repeat sequence in the E. coli genome they were studying, unique in that it was regularly interspaced. 29 These DNA motifs were further identified in various bacterial genomes by multiple laboratories over the next 20 years; their function, however, was still unknown. By 2005, three groups had independently determined that the spacer sequences were actually derived from phage DNA, 30 , 31 , 32 and the possibility of the genes playing a role in bacterial immunity was first suggested. 30 , 33 By this time, the scientific community referred to this unusual array as clustered regularly interspaced short palindromic repeats, or CRISPR. Meanwhile, researchers in the Netherlands had identified several other genes located near the CRISPR locus that appeared to be functionally associated with the CRISPR genes; 34 these would turn out to be the CRISPR associated proteins (Cas) that make up an integral part of the CRISPR‐Cas system.
In 2007, the CRISPR‐Cas system was identified as being a prokaryotic defense against pathogens. 35 As a part of a self‐/non–self‐determination mechanism of adaptive immunity, prokaryotes integrate a segment (generally 32‐38 base pairs) of phage DNA into their own genome, creating the spacers in the CRISPR arrays. After the CRISPR genes are transcribed, endoribonucleases cleave the resulting CRISPR RNA (pre‐crRNA), resulting in shorter RNA units composed of a single spacer sequence and the palindromic repeat (crRNA); depending on the organism, a trans‐activating crRNA (tracrRNA) may also be transcribed. The RNA forms a ribonucleoprotein (RNP) complex with the associated Cas proteins; any phage DNA containing the spacer sequence will be identified by the guiding RNA and cleaved by the endonuclease function of the Cas protein(s). The protospacer is the homologous sequence in the invading DNA, and is followed by a short protospacer adjacent motif (PAM); because the PAM is not incorporated in the CRISPR array, the CRISPR‐Cas complex is able to recognize the foreign DNA as non‐self (and thus will not cleave the prokaryotic cell's own DNA). 36
In 2012, Jennifer Doudna, Emmanuelle Charpentier, and others on their team engineered a synthetic chimera of the tracrRNA and crRNA (now known as single guide RNA, or sgRNA), which was able to direct Cas9 to create a targeted, site‐specific double‐stranded break. 37 By 2013, investigators had established that the CRISPR‐Cas9 was an effective, facile, and multiplexable method of editing the human genome. 38 , 39 , 40 , 41
Newer CRISPR‐based editing methods do not reply on unpredictable NHEJ or donor DNA. For example, endonuclease‐deficient Cas proteins can be fused to base‐editing enzymes; 42 first described in 2016, researchers have recently reported a high‐fidelity base editor with no off‐target mutations (OTMs). 43 Epigenetic techniques are also being explored using CRISPR‐Cas technology, 44 , 45 including linking endonuclease‐deficient Cas proteins to effector molecules. And prime editing addresses genetic disorders caused by multibase variances (such as sickle‐cell and Tay‐Sachs); in this case, the impaired Cas9 is fused to an engineered reverse transcriptase. 46
ZFNs and TALENs do maintain some advantages: CRISPR requires a PAM sequence, and sgRNA spacer sequences are usually only about 20 base pairs, meaning an inherently reduced targeting capacity (though researchers have recently begun exploring the effects of increased sgRNA length on cleavage efficiency and target specificity 47 ). CRISPR vectors are also necessary larger, making delivery more difficult. Overall, however, CRISPR is generally the preferred method of genetic and epigenetic manipulation, especially as improvements are made to the technology. CRISPR’s main advantage over its predecessors lies in the fact that rather than a complex protein as the DNA recognition molecule, the CRISPR system relies on a guide RNA. CRISPR kits are thus significantly cheaper, easier, and more efficiently produced than either ZFNs or TALENs.
1.2. Gene selection
Genetic selection happens in nature—natural selection is the mechanism that drives Darwinian evolution. Humans have also been practicing artificial selection for thousands of years, selecting for phenotypic traits when breeding plants and animals. New technologies have been developed over the last 53 years that allow selection of an embryo based on various criteria such as sex, ploidy, and polymorphisms.
1.2.1. Preimplantation genetic testing
Preimplantation genetic testing (PGT) encompasses various techniques used to screen embryos prior to transfer. Originally all referred to as preimplantation genetic diagnosis (PGD), there are actually three types of PGT: aneuploidy detection, now called PGT‐A; monogenic disorder detection, now called PGT‐M; and structural rearrangement detection, now called PGT‐SR.
Preimplantation genetic testing was ideated eleven years before the birth of the first in vitro fertilization (IVF) baby in 1978. Rabbit blastocysts were stained and observed using a fluorescence microscope; screening for sex chromatin allowed for the identification of the female embryos. 48 Because of the mutagenic potential of the preparation, the embryos were not implanted; a year later, cells from the trophoblasts of rabbit blastocysts were stained and sorted for sex, and the biopsied embryos transferred and allowed to grow to full term (at which point sex was confirmed). 49
Researchers then began to explore various methods of extracting a single embryonic cell for PGT: a blastomere biopsy (BB) removed during cleavage stage, 50 trophectoderm biopsy (TB), 51 and polar body biopsy. 52 Meanwhile, polymerase chain reaction (PCR) was developed in 1985 and quickly recognized as a potential tool for PGT when it was used to amplify the portion of the β‐globin locus that includes the Dde I site (absence of which is diagnostic for sickle‐cell anemia). 53 The blastomere biopsy technique and PCR were brought together in 1990 when two human pregnancies were established using sex selected embryos to eliminate the risk of inheriting recessive x‐linked conditions. 54
Fluorescence in situ hybridization (FISH) was the first cytogenetic technique to be used for PGT. Fluorochrome‐labeled site‐specific probes were hybridized to sample DNA, revealing aneuploidy and translocations; in 1993, two laboratories used FISH to identify X‐chromosomes, Y‐chromosomes, and aneuploidy. 55 , 56 However, the technique was limited by the number of chromosomes that could be assessed and by its inability to detect monogenic disorders.
Researchers then turned to comparative genomic hybridization (CGH) in 1999. 57 , 58 CGH can be thought of as competitive FISH: Sample and reference DNA are each labeled with a different color fluorophore, denatured, and allowed to hybridize to a metaphase spread. The DNA is then microscopically analyzed for differences in fluorescence intensity, indicating copy‐number variation (CNV).
While it was a vast improvement over its predecessor, CGH was time‐consuming (requiring embryos to be freeze‐thawed), labor‐intensive, and limited in its sensitivity. The next generation of CGH technology, array CGH (aCGH), addressed these limitations. 59 Like traditional CGH, aCGH allows for 24‐chromosome analysis; however, rather than human observation, fluorescence intensity evaluation is performed by a computer, locus by locus, with high specificity and resolution.
A number of other cytogenetic techniques for comprehensive chromosome screening (CCS) have since been developed: digital PCR (or dPCR, wherein a sample‐containing PCR solution is separated into tens of thousands of droplets and the reaction occurs separately in each partition), which can detect CNV, aneuploidy, mutations, and rare sequences; quantitative real‐time PCR (qPCR), in which a preamplification step prior to real‐time PCR allows for rapid detection of aneuploidy in all 24 chromosomes; single nucleotide polymorphism (SNP) array (which involves hybridizing fluorescent nucleotide probes to sample DNA and comparing the resulting fluorescence to a bioinformatic reference), which can detect imbalanced translocation, aneuploidy, and monogenic (and some multifactorial) disease; and next‐generation sequencing (NGS), the high‐throughput, massively parallel DNA sequencing technologies that allow for significantly quicker and cheaper sequencing than the Sanger method and make it possible to screen for everything from SNPs to aneuploidy.
Researchers and IVF laboratories use different combinations of FISH and/or the various CCS techniques.
1.2.2. Other prenatal testing
Often, IVF is not feasible, necessitating postimplantation prenatal testing (when indicated by family history and other risk factors). Amniocentesis, chorionic villus sampling (CVS), and percutaneous umbilical cord sampling (PUBS) were initially paired with karyotyping, which can detect sex, aneuploidy, and some types of structural chromosomal disorders. Karyotyping was superseded by chromosomal microarray techniques (aCGH and SNP array) and, more recently, low‐pass genome sequencing, as these technologies allow detection of CNVs as well as aneuploidy. 60
Amniocentesis is a procedure in which an ultrasound‐guided needle is inserted transabdominally in order to aspirate amniotic fluid. Applications of amniocentesis extend beyond genetic testing, such as assessment of fetal lung maturity, detection of Rh incompatibility, and decompression of polyhydramnios (accumulation of amniotic fluids).
Prior to 15 weeks’ gestation, the prenatal testing method of choice is CVS, a technique that involves analysis of samples taken from placental tissue. The CVS procedure is ultrasound‐guided and can be performed either transabdominally or transcervically (associated with higher miscarriage rates). CVS carries the risks of miscarriage, amniotic fluid leakage, and limb reduction defects and is limited by the possibility of placental mosaicism and maternal cell contamination.
Percutaneous umbilical cord sampling is a rarely used procedure, performed between 24 and 32 weeks’ gestation, in which fetal blood from the umbilical cord is obtained. Because of the high potential for complications, PUBS is generally reserved for cases in which the pregnancy is deemed high‐risk for genetic disorders and other testing methods (amniocentesis, CVS, and ultrasound) are unable to provide the needed information or have been inconclusive. PUBS is also used to provide more information about fetal health (such as blood gas levels and infection).
In 1997, the presence of cell‐free fetal DNA (cffDNA) in maternal blood was established using PCR amplification with Y‐chromosome probes. 61 This led to the development of noninvasive prenatal testing (NIPT) of cffDNA. NIPT has been shown to be an accurate and sensitive technique for the detection of some aneuploidies (such as trisomy 21 62 ), less so for others. 63 Because cffDNA comes from the placenta, placental mosaicism can result in inaccurate results. Further, NIPT detects all cell‐free DNA in the mother's blood, including her own; maternal mosaicism or malignancies can also contribute to inaccuracies. As such, NIPT is considered a screening test, rather than a diagnostic test.
2. ETHICS OF GENE EDITING
On November 25, 2018, news broke that Jiankui He of Southern University of Science and Technology in Shenzhen, China had registered a clinical trial in which he planned to implant human embryos which had been modified using CRISPR‐Cas9. 64 Within days, the world learned that not only had edited embryos been implanted, two baby girls, Lulu and Nana, had already been born. 65
He used CRISPR‐Cas9 to create a nonspecific sequence alteration in the CCR5 gene. CCR5 is a seven‐transmembrane–spanning G protein–coupled CC chemokine (β chemokine) receptor. When expressed on the surface of a human T cell, CCR5 is the main coreceptor (along with CD4) for the human immunodeficiency virus (HIV). A naturally occurring 32–base pair deletion (with heterozygote allele frequencies of about 10% in people with European origin), known as CCR5∆32 , has been shown to disable the protein; 66 heterozygosity of the CCR5∆32 allele has been shown to slow disease progression, while homozygosity significantly increases disease resistance. He's goal was to knock out CCR5 , with the desired outcome of creating HIV‐resistant babies (it should be noted that HIV infection in CCR5∆32 +/+ individuals has been increasingly reported, associated with X4‐trophic HIV strains—that is, strains that rely exclusively on coreceptor CXCR4 for endocytosis, rather than CCR5 67 ).
He presented the details of his investigation 68 at the Second International Summit on Human Genome Editing, being held “to discuss scientific, medical, ethical, and governance issues associated with recent advances in human gene‐editing research.” 69 While his manuscript describing the trial was not accepted by any publications, excerpts are available to the public, and various media outlets (and some experts) have been able to view the paper and supplementary data in their entirety. Enough is now known about He's work that it can serve as the basis of a conversation about the ethics surrounding germline gene editing. There are a number of issues—those inherent in the technologies themselves, as well as scientific hurdles that need to be overcome—before initiating clinical trials, to ensure that they are carried out as ethically as possible.
2.1. Not all sequence variations are created equally
CCR5∆32 has been researched extensively, but is one of only a few CCR5 variants studied. In his abstract, He claims that his team has reproduced this natural variant, but this is not the case: Two embryos were implanted, one of which (Nana) had frameshift mutations on both alleles (a 1–base pair insertion and a 4–base pair deletion, respectively) and the other of which (Lulu) showed a 15–base pair deletion on only one allele. Frameshift mutations have a high probability of disrupting protein structure (and thus function). The 15–base pair deletion, however, will result in five missing amino acids when the protein is translated, and its effect on the protein's function is unknown. He's team could have frozen the embryos, duplicated the sequence alterations in other cell lines, and tested whether or not the genetic changes actually conferred disease resistance, before actually implanting the embryos, but it does not appear that they made an effort to fully understand the actual effect of the alterations they had made. 64 With all of the risks associated with the CRISPR editing process, embryos should not be implanted if the scientists are unsure of the effects.
2.2. Mosaicism
A CRISPR‐Cas vector is inserted into a zygote soon after fertilization. If the CRISPR‐induced mutagenesis only occurred during the single‐cell stage, each successive round of cleavage would yield genetically identical cells. However, while the half‐life of the Cas proteins themselves may not be long, the vectors will remain and continue to be transcribed for days. During this time, the embryo will continue to divide, eventually forming a blastocyst of a few hundred cells. Uneven distribution of the plasmid and the RNPs means that there is a significant potential for mosaicism.
In He's laboratory, three to five cells were removed from each blastocyst, and their genomes sequenced. If Lulu's embryo were made up of identical cells (with one wild type allele and one with a 15–base pair deletion) as He had reported, the Sanger chromatogram should have shown two sets of peaks, approximately the same height. However, it appears likely that there were actually three different combinations of alleles: two normal copies, one normal copy and one with a 15–base pair deletion, and one normal copy and an unknown large insertion. Similarly, while Nana's embryo should have shown two alterations, the Sanger chromatogram revealed three. 70
The suspicion of mosaicism is borne out when sequencing of samples from the cord blood, umbilical cords, and placentas are reviewed. Just as with the embryo sequencing, rampant mosaicism is evident. It is reasonable to assume that the girls’ bodies are mosaic as well, but for an unknown reason, He's team did not test any cells from the girls themselves. 70 There is therefore the possibility that not all of the cells in Nana's body will have modifications to both CCR5 alleles, meaning it is possible that Nana is not actually resistant to HIV.
Mosaicism can have myriad effects: Even a few mutated cells in an organ can cause disease, a single cell can develop into a tumor, and any allelic variation in germ cells will be inherited by the following generation. There is no way to sequence a cell's genome without destroying the cell itself; as such, it is currently impossible to rule out mosaicism in a blastocyst.
2.3. Off‐target effects
As efficient as CRISPR is, there is a high probability of OTMs. He's team reported that in addition to the CCR5 gene edits, there was only one OTM, a 1–base pair insertion in a noncoding region of Chromosome 1 in Lulu's genome. This was based on their relatively limited sequencing, however; as noted above, mosaicism cannot be ruled out. (It should also be noted that there were flaws in the sequencing itself, so there may be other alterations that were missed in the screening, on top of the mosaicism. 70 )
2.4. Other consequences of target gene modification
When undertaking to knock out a gene in an embryo, it is vital to understand all of the functions of that gene.
CCR5 is a chemokine receptor that mediates leukocyte chemotaxis, and thus helps mount immune response. It is therefore unsurprising that homozygosity for the CCR5∆32 variant has been shown to be significantly correlated with more symptomatic infection and higher mortality rates in patients with West Nile virus, 71 influenza A, 71 , 72 and tick‐borne encephalitis. 73 It has also been shown to be associated with upregulation of certain CC chemokine ligands, and in turn associated with progressive reduction in survival time for patients with multiple sclerosis (MS). 74 Is it ethical to create a sequence variation that confers resistance to one illness, while increasing the likelihood of succumbing to another?
Public health conversations will need to change as well. It is possible, for example, that some with a CCR5 edit will engage in riskier sexual practices, or that some with a PCSK9 edit (which is associated with decreased levels of low‐density lipoprotein cholesterol, or LDL, in the blood 75 , 76 ) will be less likely to make behavioral changes such as increased exercise and diet modification.
2.5. Which genes/diseases to target?
Many who have viewed He's work have questioned why he chose to focus on CCR5 and HIV resistance. HIV prevalence in China is relatively low, 77 and current treatments can keep viral loads at almost undetectable levels. He stated that his research could help tamp down the HIV/AIDS epidemic; the most hard‐hit areas (such as Africa), however, would likely not gain much benefit from gene‐editing technologies.
Per a December 2018 poll, 78 Americans draw the line at so‐called enhancement, but favor the use of genetic engineering to address disease and disability. Which diseases and disabilities to target, however, is still an open discussion. Some questions that may help inform that decision: Should there be a focus on infectious disease resistance? Only fatal conditions? Will we decide that there is a need to quantify the degree of suffering? If an effective treatment already exists, should we still seek prevention through genetic modification? Is childhood versus adulthood onset of illness an important factor? Not all sequence variants are guaranteed to cause disease (eg, BRCA genes); should they be considered? What about orphan diseases? And should certain types of disabilities be prioritized over others?
2.6. OTHER ISSUES
2.6.1. clinical research ethics.
The history of research using human subjects has been blemished by unethical treatment of the subjects themselves. From Imperial Japan to the Tuskegee Institute, examples of atrocities committed in the name of medical science can be found across the world. As a result, a number of guidelines have been developed to facilitate ethical research going forward. 79 Nazi Germany engaged in abominable human experimentation during World War II; in 1947, the Nuremberg Military Tribunal (during which Nazi physicians and administrators were tried for war crimes and crimes against humanity) resulted in the Nuremberg Code, a statement aimed at preventing such abuses in the future. Stemming from a reaction to the same offenses, the World Medical Association produced Ethical Principles for Medical Research Involving Human Subjects —known as the Declaration of Helsinki—in 1964 (it has been modified a few times since), which in 1982 was adapted by the Council for International Organizations of Medical Sciences into a manual, Proposed International Ethical Guidelines for Biomedical Research Involving Human Subjects , as a guideline for World Health Organization (WHO) member countries. (Individual countries have created their own guidelines, as well. In the United States, for example, the National Research Act of 1974 established the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research—often referred to as the Belmont commission—which issued the 1979 Ethical Principles and Guidelines for the Protection of Human Subjects of Research , now known as the Belmont Report. The National Research Act of 1974 also established a set of regulations regarding human subject research; by 1991, after various updates and additions, the government decided that the regulations should become a “Common Rule” covering all federally connected research, codified in Title 45, Part 46 of the Code of Federal Regulations.)
This article will not delve into all of He's ethical missteps as regards his research (such as the fact that he registered the trial with the Chinese Clinical Trial Registry in November 2018, after the twins had already been born). However, there are a few key issues, directly addressed by at least one of these major reports, which can be considered in terms of guiding future ethics discussions regarding gene‐editing clinical trials.
First and foremost, clinical trial participants should be informed of all of the associated risks and benefits. There is significant ambiguity as to the informed consent process in He's study. First, the experiment was misleadingly couched as an HIV vaccine trial. 65 , 80 It is also unclear how much the parents in He's study understood about risks such as mosaicism or increased susceptibility to other infections. The investigator is responsible for keeping participants informed throughout the study; it does not appear that in this case they were informed of the mosaicism present in both embryos (in his presentation at the human genome editing summit, He said only that the parents had been informed about one OTM 68 and does not even mention the mosaicism in his manuscript 70 ). It is likely that were the parents fully informed, they would have decided not to implant one or both embryos. This raises another question: Should parents be the ones to make such a decision, or is that the responsibility of the researchers and doctors?
The selection of the study participants is an issue, as well: The first inclusion criterion is that the participants must be a married couple wherein the husband is HIV‐positive and the wife is HIV‐negative. 81 Father‐to‐child transmission of HIV is rare (especially when the father is on antiretroviral therapy and the mother is on preexposure prophylaxis), but it is possible; for this reason, many couples opt for sperm washing (to separate the sperm from the virus), followed by either in vitro fertilization (IVF) or intrauterine implantation (IUI). While there is no explicit law against HIV‐positive parents accessing these procedures, it is unlikely that it would be approved by hospitals’ ethics committees. 82 Chinese couples often travel to other countries (such as Thailand) for the procedure, but it can cost hundreds of thousands of yuan. 83 The couples selected for He's study may have seen participation as their only chance to have children—indeed, He described the father as having “lost hope for life.” 68 This makes them particularly vulnerable to exploitation. (This may also have informed their decision to implant both embryos rather than just the one that had alterations to both alleles.)
Study participants should be able to voluntarily withdraw from the research at any point; He's informed consent form stipulates that were the couple to withdraw from the study (at any point between the implantation of the embryo in the first IVF cycle and 28 days postbirth), they would be responsible for reimbursing the laboratory for all project costs (and that if reimbursement was not received within 10 calendar days of withdrawal, a substantial fine—more than the average annual income of a Chinese citizen—would be imposed). 80 This is sufficiently cost prohibitive as to prevent a subject from withdrawing from the study.
It is unclear whether He's research actually underwent an ethics review process: In his manuscript, He claims that the Medical Ethics Committee of the Shenzhen Harmonicare Women's and Children's Hospital approved the study in March 2017, but only elaborates by stating that his team was “… told that the committee held a comprehensive discussion of risks and benefits… During the study, the director of the ethics committee was constantly updated about the state of the clinical trial.” 84 The hospital has since denied that the study was reviewed at all, and claims that the signatures on the approval were forged. 85 What is clear is that a number of regulations were violated or circumvented, including the guidelines for embryo research which allow an edited embryo to be cultured for no more than 14 days and prohibit its implantation, 86 as well as the aforementioned limitations on assisted reproductive services for HIV‐positive parents. 82 It is likely that He switched blood samples and kept many of the IVF technicians and obstetricians in the dark as to the nature of the study to get around these issues. 87
Finally, it is reasonable to consider whether He was qualified to be the investigator on such a trial: He had published one paper about CRISPR (in 2010, before human gene editing was an application of the technology), his background was in physics (he crossed over into biophysics), and he had no medical training. This is especially concerning as biohackers have made available both the equipment and the basic blueprints for home CRISPR editing (see the Other Perspectives section)—including advice on how to obtain human embryos and eventually implant them. 88
2.6.2. Socioeconomic disparities
Multiple polls have shown that the majority of people around the world are opposed to the use of genetic engineering of embryos for enhancement, such as athletic ability and intelligence, or for altering physical characteristics, such as eye color and height. 89 It is easy to conceive of the risk of a new age of eugenics.
But even the application of genetic modification to address medical needs holds the potential for establishing inequality. The technology will remain incredibly expensive for some time, prohibitively so for most people. CCR5 edits lie in an ill‐defined area between medical need and enhancement; an unfair health advantage will be established if such modifications are only accessible to the wealthy. Other kinds of edits may mean the difference between life and death; should potentially life‐saving therapies only be available to those with financial means? Put another way, should those individuals on one side of the growing socioeconomic gap be the only ones protected from the suffering that comes with illnesses such as Alzheimer's disease, Huntington disease, or cystic fibrosis?
2.6.3. Possible stigma
Especially while the concept is still novel, it is difficult to predict how society will feel about gene‐edited babies. Will Nana and Lulu face any sort of backlash? Conversely, if and when gene editing becomes commonplace, will there be a stigma associated with not having been edited in some way, such as still being susceptible to various infectious diseases? Might children like Lulu be less accepted for not carrying a desired modification? He wanted to spare HIV‐infected individuals’ children the stigma and discrimination their parents endured; 90 it is possible that having edited genes has replaced one potential stigma with another.
2.6.4. Insurance
Because gene editing will be a tool to cure and prevent illness, insurance coverage will be an important part of the conversation. First, will insurance cover the editing itself? If so, will germline versus somatic cell editing be an important distinction? Will coverage be based on the targeted illness or disability (and expected associated costs)? And who will decide which edits are considered medically necessary and which are considered elective?
Once babies born from edited embryos are born, more questions arise. Will those whose genes have not been edited to prevent certain illnesses be considered to have preexisting conditions? Will they be expected to pay more for coverage? On the other side of the coin, will those who have had their genes edited (especially when the technology is first rolled out) pay more because of possible off‐target risks or potential negative consequences of editing (eg, the increased susceptibility to influenza associated with CCR5 editing)?
2.6.5. Other perspectives
A full discussion of ethics requires a balanced presentation of various points of view.
There are those who object to continued research into gene editing, especially in zygotes, for myriad reasons. For example, some feel that gene editing is “playing God” and that it is not man's role to make changes to the basic building blocks of humanity; others are concerned about the potential that the technology, once perfected, could be co‐opted to produce designer babies; there is the consideration that opening a market for human eggs for research could lead to exploitation of disadvantaged women; and still others have concerns similar to those who are opposed to embryonic stem cell research—such as the conviction that embryos should not be created for the purpose of research, or that un‐implanted embryos (which they consider potential life) should not be destroyed.
There are also those who believe that not only should research continue, but that even nascent technology such as gene editing should be accessible to the public. 91 Known as biohackers, these scientists and activists laud the efforts like Jiankui He's. 88 Educational and laboratory materials are currently available to essentially anyone. It is even possible to purchase CRISPR kits, 92 and while a new California law requires that such kits are labeled “not for self‐administration” 93 there are currently no laws prohibiting people from doing just that—in fact, the owner of one company was investigated by the California Medical Board for unlicensed practice of medicine after injecting himself with CRISPR, but the investigation was dropped after four months with “no further action… anticipated.” 94
3. GLOBAL DISCUSSIONS ON GERMLINE EDITING
While it is impossible to mandate that all countries follow the same set of guidelines, it is possible to establish guiding principles for the risk‐benefit analyses and ethical discussions each country will undertake in developing their own regulatory framework. Because science moves faster than regulation, the scientific community as a whole can also use these principles to help guide ethically charged research decisions where no regulations yet exist. To that end, various groups have been meeting all over the world to try to come to a consensus on how to proceed with germline editing research and the potential clinical applications thereof.

3.1. Before He’s announcement
In 2015, the International Bioethics Committee (IBC), part of the United Nations Educational, Scientific, and Cultural Organization (UNESCO), released the Report of the IBC on Updating its Reflection on the Human Genome and Human Rights. 95 The report considers other technologies as well, but “recommends a moratorium on genome editing of the human germline.”
Also in 2015, investigators in China announced that they had successfully used CRISPR to edit a nonviable human embryo. 96 This inspired the first International Summit on Human Gene Editing, held December 2015 in Washington, D.C. Hosted by the US National Academy of Sciences, US National Academy of Medicine, the Royal Society of the UK, and the Chinese Academy of Sciences, the summit brought together more than 3500 stakeholders (500 in person and 3000 online) from around the world to discuss human gene editing. At the end of the summit, the organizing committee released a statement advising ongoing global engagement and discussion, and outlined their conclusions regarding gene editing: 97 “(i)ntensive basic and preclinical research is clearly needed and should proceed, subject to appropriate legal and ethical rules and oversight…”; “(m)any promising and valuable clinical applications of gene editing are directed at altering genetic sequences only in somatic cells… [and] they can be… evaluated within existing and evolving regulatory frameworks for gene therapy…”; and “(g)ene editing might also be used, in principle, to make genetic alterations in gametes or embryos…” The statement goes on to address the ethical, legal, and scientific questions surrounding germline editing that have yet to be answered, and warns:
It would be irresponsible to proceed with any clinical use of germline editing unless and until (a) the relevant safety and efficacy issues have been resolved, based on appropriate understanding and balancing of risks, potential benefits, and alternatives, and (b) there is broad societal consensus about the appropriateness of the proposed application. Moreover, any clinical use should proceed only under appropriate regulatory oversight. At present, these criteria have not been met for any proposed clinical use: the safety issues have not yet been adequately explored; the cases of most compelling benefit are limited; and many nations have legislative or regulatory bans on germline modification. However, as scientific knowledge advances and societal views evolve, the clinical use of germline editing should be revisited on a regular basis.
While this statement in no way gives a green light for trials such as He's, it also does not call for an outright moratorium. In March 2017, another Chinese team published the results of the first use of CRISPR in viable human embryos. 98 Less than two years later, He's work was revealed to the world.
3.2. After He’s announcement
The article about He's trial was published the day before the second International Summit on Human Gene Editing. As they had at the first summit, organizers released a concluding statement on the last day. Surprisingly, not only does the statement again fall short of calling for a moratorium on clinical use of gene editing, the language is even softer than that of the first summit statement:
The variability of effects produced by genetic changes makes it difficult to conduct a thorough evaluation of benefits and risks. Nevertheless, germline genome editing could become acceptable in the future if these risks are addressed and if a number of additional criteria are met. These criteria include strict independent oversight, a compelling medical need, an absence of reasonable alternatives, a plan for long‐term follow‐up, and attention to societal effects. Even so, public acceptability will likely vary among jurisdictions, leading to differing policy responses. The organizing committee concludes that the scientific understanding and technical requirements for clinical practice remain too uncertain and the risks too great to permit clinical trials of germline editing at this time. Progress over the last three years and the discussions at the current summit, however, suggest that it is time to define a rigorous, responsible translational pathway toward such trials.
In December 2018, seeing the need for a more substantial framework of regulatory guidance, the WHO established the Advisory Committee on Developing Global Standards for Governance and Oversight of Human Genome Editing, “a global, multi‐disciplinary expert panel to examine the scientific, ethical, social and legal challenges associated with human genome editing… tasked to advise and make recommendations on appropriate institutional, national, regional and global governance mechanisms for human genome editing.” 99 They have established the Human Genome Editing Registry to collect information on human clinical trials involving genome editing, and the WHO has supported the advisory committee's interim recommendation that “it would be irresponsible at this time for anyone to proceed with clinical applications of human germline genome editing.” 100
In 2019, the US National Academies of Medicine and Science, together with the Royal Society, convened the International Commission on the Clinical Use of Human Germline Genome Editing. The goal of commission is: 101
… with the participation of science and medical academies around the world, to develop a framework for scientists, clinicians, and regulatory authorities to consider when assessing potential clinical applications of human germline genome editing. The framework will identify a number of scientific, medical, and ethical requirements that should be considered, and could inform the development of a potential pathway from research to clinical use—if society concludes that heritable human genome editing applications are acceptable.
The commission's final report is scheduled to be released in the spring of 2020.
As the science progresses, there are clearly significant conversations yet to be had.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Rothschild J. Ethical considerations of gene editing and genetic selection . J Gen Fam Med . 2020; 21 :37–47. 10.1002/jgf2.321 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 20 April 2022
Beyond safety: mapping the ethical debate on heritable genome editing interventions
- Mara Almeida ORCID: orcid.org/0000-0002-0435-6296 1 &
- Robert Ranisch ORCID: orcid.org/0000-0002-1676-1694 2 , 3
Humanities and Social Sciences Communications volume 9 , Article number: 139 ( 2022 ) Cite this article
23k Accesses
16 Citations
9 Altmetric
Metrics details
- Medical humanities
- Science, technology and society
Genetic engineering has provided humans the ability to transform organisms by direct manipulation of genomes within a broad range of applications including agriculture (e.g., GM crops), and the pharmaceutical industry (e.g., insulin production). Developments within the last 10 years have produced new tools for genome editing (e.g., CRISPR/Cas9) that can achieve much greater precision than previous forms of genetic engineering. Moreover, these tools could offer the potential for interventions on humans and for both clinical and non-clinical purposes, resulting in a broad scope of applicability. However, their promising abilities and potential uses (including their applicability in humans for either somatic or heritable genome editing interventions) greatly increase their potential societal impacts and, as such, have brought an urgency to ethical and regulatory discussions about the application of such technology in our society. In this article, we explore different arguments (pragmatic, sociopolitical and categorical) that have been made in support of or in opposition to the new technologies of genome editing and their impact on the debate of the permissibility or otherwise of human heritable genome editing interventions in the future. For this purpose, reference is made to discussions on genetic engineering that have taken place in the field of bioethics since the 1980s. Our analysis shows that the dominance of categorical arguments has been reversed in favour of pragmatic arguments such as safety concerns. However, when it comes to involving the public in ethical discourse, we consider it crucial widening the debate beyond such pragmatic considerations. In this article, we explore some of the key categorical as well sociopolitical considerations raised by the potential uses of heritable genome editing interventions, as these considerations underline many of the societal concerns and values crucial for public engagement. We also highlight how pragmatic considerations, despite their increasing importance in the work of recent authoritative sources, are unlikely to be the result of progress on outstanding categorical issues, but rather reflect the limited progress on these aspects and/or pressures in regulating the use of the technology.
Similar content being viewed by others
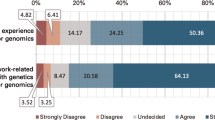
Genetics experience impacts attitudes towards germline gene editing: a survey of over 1500 members of the public

Between desire and fear: a qualitative interview study exploring the perspectives of carriers of a genetic condition on human genome editing
The interplay of ethics and genetic technologies in balancing the social valuation of the human genome in unesco declarations, introduction.
The ability to alter a sequence of genetic material was initially developed in microorganisms during the 1970s and 1980s (for an overview: Walters et al., 2021 ). Since then, technological advances have allowed researchers to alter DNA in different organisms by introducing a new gene or by modifying the sequence of bases in the genome. The manipulation of the genome of living organisms (typically plants) continues a course that science embraced more than 40 years ago, and may ultimately allow, if not deliberately curtailed by societal decisions, the possibility of manipulating and controlling genetic material of other living species, including humans.
Genetic engineering can be used in a diverse range of contexts, including research (e.g., to build model organisms), pharmacology (e.g., for insulin production) and agriculture (e.g., to improve crop resistance to environmental pressures such as diseases, or to increase yield). Beyond these applications, modern genetic engineering techniques such as genome editing technologies have the potential to be an innovative tool in clinical interventions but also outside the clinical realm. In the clinical context, genome editing techniques are expected to help in both disease prevention and in treatment (Porteus, 2019 ; Zhang, 2019 ). Nevertheless, genome editing technology raises several questions, including the implications of its use for human germline cells or embryos, since the technology’s use could facilitate heritable genome editing interventions (Lea and Niakan, 2019 ). This possible use has fuelled a heated debate and fierce opposition, as illustrated by the moratoriums proposed by researchers and international institutions on the use of the technology (Lander et al., 2019 ; Baltimore et al., 2015 ; Lanphier et al., 2015 ). Heritable human germline modifications are currently prohibited under various legislations (Baylis et al., 2020 ; Ledford, 2015 ; Isasi et al., 2016 ; König, 2017 ) and surveys show public concerns about such applications, especially without clear medical justification (e.g., Gaskell et al., 2017 ; Jedwab et al., 2020 ; Scheufele et al., 2017 ; Blendon et al., 2016 ).
To analyse some implications of allowing heritable genome editing interventions in humans, it is relevant to explore underlying values and associated ethical considerations. Building on previous work by other authors (e.g., Coller, 2019 ; de Wert et al., 2018 ; van Dijke et al., 2018 ; Mulvihill et al., 2017 ; Ishii, 2015 ), this article aims to provide context to the debates taking place and critically analyse some of the major pragmatic, categorical and sociopolitical considerations raised to date in relation to human heritable genome editing. Specifically, we explore some key categorical and sociopolitical considerations to underline some of the possible barriers to societal acceptance, key outstanding questions requiring consideration, and possible implications at the individual and collective level. In doing so, we hope to highlight the predominance of pragmatic arguments in the scientific debate regarding the permissible use of heritable genome editing interventions compared to categorical arguments relevant to broader societal debate.
Human genome editing: a brief history of CRISPR/Cas9
Human genome editing is an all-encompassing term for technologies that are aimed at making specific changes to the human genome. In humans, these technologies can be used in embryos or germline cells as well as somatic cells (Box 1 ). Concerning human embryos or germline cells, the intervention could introduce heritable changes to the human genome (Lea and Niakan, 2019 ; Vassena et al., 2016 ; Wolf et al., 2019 ). In contrast, an intervention in somatic cells is not intended to result in changes to the genome of subsequent generations. It is worth noting that intergenerational effects occur only when the modified cells are used to establish a pregnancy which is carried to term. Thus, a distinction has been made between germline genome editing (GGE), which may only affect in vitro embryos in research activity, and heritable genome editing (HGE), which is used in reproductive medicine (e.g., Baylis et al., 2020 ). HGE could be used to prevent the transmission of serious genetic disease; however, other applications could be imagined, e.g., creating genetic resistance or even augmenting human functions.
In the last decade, prominent technical advances in genome engineering methods have taken place, including the zinc-finger nucleases (ZFNs) and TAL effector nucleases (TALENs), making human genome modification a tangible possibility (Gaj et al., 2013 ; Li et al., 2020 ; Gupta and Musunuru, 2014 ). In 2012, a study showed that the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), combined with an enzyme called Cas9, could be used as a genome‐editing tool in human cell culture (Jinek et al., 2012 ). In 2013, the use of CRISPR/Cas9 in mammalian cells was described, demonstrating the application of this tool in the genome of living human cells (Cong et al., 2013 ). In 2014, CRISPR/Cas9 germline modifications were first used in non-human primates, resulting in the birth of gene-edited cynomolgus monkeys (Niu et al., 2014 ). This was followed in 2015 by the first-ever public reported case of genome modification in non-viable human embryos (tripronuclear zygotes) (Liang et al., 2015 ). This study has caused broad concerns in the scientific community (Bosley et al., 2015 ) with leading journals rejecting publication for ethical reasons. Five years after these initial experiments were conducted, more than 10 papers have been published reporting the use of genome editing tools on human preimplantation embryos (for an overview: Niemiec and Howard, 2020 ).
Compared to counterpart genome technologies (e.g., ZFNs and TALENs), CRISPR/Cas9 is considered by many a revolutionary tool due to its efficiency and reduced cost. More specifically, CRISPR/Cas9 seems to provide the possibility of a more targeted and effective intervention in the genome involving the insertion, deletion, or replacement of genetic material (Dance, 2015 ). The potential applicability of CRISPR/Cas9 technique is considered immense, since it can be used on all type of organisms, from bacteria to plants, non-human cells, and human cells (Barrangou and Horvath, 2017 ; Hsu et al., 2014 ; Doudna and Charpentier, 2014 ; Zhang, 2019 ).
Box 1 Difference associated with germline cells and somatic cells.
For the purposes of the analysis presented in this article, one of the main differences is the heritability of genes associated with either type of cell. Germline cells include spermatozoa, oocytes, and their progenitors (e.g., embryonic cells in early development), which can give rise to a new baby carrying a genetic heritage coming from the parents. Thus, germline are those cells in an organism which are involved in the transfer of genetic information from one generation to the next. Somatic cells, conversely, constitute many of the tissues that form the body of living organisms, and do not pass on genetic traits to their progeny.
Germline interventions: the international debate
As a reaction to the 2015 study with CRISPR/Cas9, several commentaries by scientists were published regarding the future use of the technology (e.g., Bosley et al., 2015 ; Lanphier et al., 2015 ; Baltimore et al., 2015 ). Many of them focused on germline applications, due to the possibility of permanent, heritable changes to the human genome and its implications for both individuals and future generations. These commentaries included position statements calling for great caution in the use of genome editing techniques for heritable interventions in humans and suggested a voluntary moratorium on clinical germline applications of CRISPR/Cas9, at least until a broad societal understanding and consensus on their use could be reached (Brokowski, 2018 ; Baltimore et al., 2015 ; Lander, 2015 ). Such calls for a temporary ban were often seen as reminiscent of the “Asilomar ban” on recombinant DNA technology in the mid-1970s (Guttinger, 2017 ). Other commentaries asked for research to be discouraged or halted all together (Lanphier et al., 2015 ). More firmly, the United States (US) National Institutes of Health (NIH) released a statement indicating that the NIH would not fund research using genome editing technologies on human embryos (Collins, 2015 ).
In December 2015, the first International Summit on Human Gene Editing took place, hosted by the US National Academy of Sciences, the US National Academy of Medicine, the UK Royal Society, and the Chinese Academy of Sciences (NASEM). The organizing committee issued a statement about appropriate uses of the technology that included the following: “It would be irresponsible to proceed with any clinical use of germline editing unless and until (i) the relevant safety and efficacy issues have been resolved, based on appropriate understanding and balancing of risks, potential benefits, and alternatives, and (ii) there is broad societal consensus about the appropriateness of the proposed application” (NASEM, 2015 ).
Following this meeting, initiatives from different national bodies were organized to promote debate on the ethical issues raised by the new genome editing technologies and to work towards a common framework governing the development and permissibility of their use in humans. This included an ethical review published in 2016 by the Nuffield Council on Bioethics, addressing conceptual and descriptive questions concerning genome editing, and considering key ethical questions arising from the use of the technology in both human health and other contexts (Nuffield Council on Bioethics, 2016 ). In 2017, a committee on human genome editing set up by the US National Academy of Sciences (NAS) and the National Academy of Medicine (NAM) carried out a so-called consensus study “Human Genome Editing: Science, Ethics, and Governance” (NASEM, 2017 ). This study put forward a series of recommendations on policies and procedures to govern human applications of genome editing. Specifically, the study concluded that HGE could be justified under specific conditions: “In some situations, heritable genome editing would provide the only or the most acceptable option for parents who desire to have genetically related children while minimizing the risk of serious disease or disability in a prospective child” (NASEM, 2017 ). The report stimulated much public debate and was met with support and opposition since it was seen as moving forward on the permissibility of germline editing in the clinical context (Ranisch and Ehni, 2020 ; Hyun and Osborn, 2017 ).
Following the report in 2016, the Nuffield Council on Bioethics published a second report in 2018. Similar to the NASEM 2017 report, this report emphasizes the value of procreative freedom and stresses that in some cases HGE might be the only option for couples to conceive genetically related, healthy offspring. In this document, the Nuffield Council on Bioethics maintains that there are no categorical reasons to prohibit HGE. However, it highlights three kinds of interests that should be recognized when discussing prospective HGE. They are related to individuals directly affected by HGE (parents or children), other parts of society, and future generations of humanity. In this context, two ethical principles are highlighted as important to guide future evaluations of the HGE use in specific interventions: “(...) to influence the characteristics of future generations could be ethically acceptable, provided if, and only if, two principles are satisfied: first, that such interventions are intended to secure, and are consistent with, the welfare of a person who may be born as a consequence, and second, that any such interventions would uphold principles of social justice and solidarity (…)” (Nuffield Council on Bioethics, 2018 ). This report was met with criticism for (implicitly) advocating genetic heritable interventions might be acceptable even beyond the boundaries of therapeutic uses. This is particularly controversial and goes well beyond the position previously reached by the NASEM report (which limited permissible uses of genome editing at preventing the transmission of genetic variants associated to diseases) (Drabiak, 2020 ). On the other hand, others have welcomed the report and, within it, the identification of explicit guiding ethical principles helpful in moving forward the debate on HGE (Gyngell et al., 2019 ).
As a follow-up to the 2015 conference, a second International Summit on Human Gene Editing was scheduled for November 2018 in Hong Kong (National Academies of Sciences, Engineering, and Medicine, 2019 ). The event, convened by the Hong Kong Academy of Sciences, the UK Royal Society, the US National Academy of Sciences and the US National Academy of Medicine, was supposed to focus on the prospects of HGE. Just before the Summit began, news broke that He Jiankui, a Chinese researcher and invited speaker at the Summit, created the world’s first genetically edited babies resulting from the use of CRISPR/Cas9 in embryos (Regalado, 2018 ; Lovell-Badge, 2019 ). Although an independent investigation of the case is still pending, his experiments have now been reviewed in detail by some scholars (e.g., Greely, 2019 , 2021 ; Kirksey, 2020 ; Davies, 2020 ; Musunuru, 2019 ). These experiments were globally criticized, since they did not follow suitable safety procedures or ethical guidelines (Wang and Yang, 2019 ; Lovell-Badge, 2019 ; Krimsky, 2019 ), nor considered the recommendations previously put forward by international reports (NASEM, 2017 ; Nuffield Council on Bioethics, 2018 ) and legal frameworks (Araki and Ishii, 2014 ; Isasi et al., 2016 ). Different reactions were triggered, including another call by scientists for a global moratorium on clinical human genome editing, to allow time for international discussions to take place on its appropriate uses (Lander et al., 2019 ) or an outright ban on the technology (Botkin, 2019 ). There were also calls for a measured analysis of the possible clinical applications of human genome editing, without the imposition of a moratorium (Daley et al., 2019 ; Dzau et al., 2018 ).
Most countries currently have legal frameworks to ban or severely restrict the use of heritable genome editing technologies (Araki and Ishii, 2014 ; Isasi et al., 2016 ; Baylis et al., 2020 ). However, since He’s experiment, the possibility that researchers might still attempt (with some likelihood of success) to use the technology in human embryos, became a growing concern, particularly since some scientists have already announced their interest in further clinical experiments (Cyranoski, 2019 ). For many, He’s experiments highlighted the ongoing risks associated with the use of modern genome editing technology without proper safety protocols and regulatory frameworks at an international level (Ranisch et al., 2020 ). This has triggered the need to develop clear and strict regulations to be implemented if these tools are to be used in the future. This incident also led to the formation of several working groups, including the establishment of an international commission on the Clinical Use of Human Germline Genome Editing set up by the US National Academy of Medicine, the US National Academy of Sciences, and the UK’s Royal Society. In 2020, the commission published a comprehensive report on HGE, proposing a translational pathway from research to clinical use (National Academy of Medicine, National Academy of Sciences, and the Royal Society, 2020 ). Likewise, a global expert Advisory Committee was established by the World Health Organization (WHO) with the goal of developing recommendations on governance mechanisms for human genome editing. Although the committee insisted in an interim recommendation that “it would be irresponsible at this time for anyone to proceed with clinical applications of human germline genome editing” (WHO, 2019 ), it did not express fundamental concerns on the possibility that some forms of HGE will one day become a reality. In 2021, the WHO’s Advisory Committee issued some publications, including a “Framework for governance” report and a “Recommendations” report (WHO, 2021 ). Building on a set of procedural and substantive values and principles, the “Framework for Governance” report discusses a variety of tools and institutions necessary for developing appropriate national, transnational, and international governance and oversight mechanisms for HGE. Specifically, the report considers the full spectrum of possible applications of human genome editing (including epigenetic editing and human enhancement) and addresses specific challenges associated with current, possible and speculative scenarios. These range from somatic gene therapy for the prevention of serious hereditary diseases to potentially more controversial applications reminiscent of the He Jiankui case (e.g., the use of HGE in reproductive medicine outside regulatory controls and oversight mechanisms). Additionally, the “Recommendations” report proposes among other things whistleblowing mechanisms to report illegal or unethical research. It also highlights the need for a global human genome editing registry, that should also cover basic and preclinical research on different applications of genetic manipulation, including HGE. The report also emphasises the need of making possible benefits of human genome editing widely accessible.
The idea of a human genome editing registry has also been supported by the European Group on Ethics in Science and New Technologies (EGE), an advisory board to the President of the European Commission. After an initial statement on genome editing published in 2016, still calling for a moratorium on editing of human embryos (EGE, 2016 ), the EGE published a comprehensive Opinion in 2021 (EGE, 2021 ). Although the focus of this report is on the moral issues surrounding genome editing in animals and plants, HGE is also discussed. Similar to the WHO Advisory Committee, the EGE recommends for HGE not to be introduced prematurely into clinical application and that measures should be taken to prevent HGE’s use for human enhancement.
Overall, when reviewing reports and initiatives produced since 2015, common themes and trajectories can be identified. A key development is the observation that the acceptance of the fundamental permissibility of such interventions appears to be increasing. This constitutes an important change from previous positions, reflecting the fact that human germline interventions have long been considered a ‘red line’ or at least viewed with deep scepticism (Ranisch and Ehni, 2020 ). In particular, while there is agreement that it would be premature to bring HGE into a clinical context, key concerns expressed by authoritative international bodies and committees are now associated with acceptable uses of the technology, rather than its use per se. Consideration is now being given to the conditions and objectives under which germline interventions could be permissible, instead of addressing the fundamental question of whether HGE may be performed at all. The question of permissibility is often linked to the stage of technological development. These developments are remarkable, since the key ethical aspects of genome editing are now frequently confined to questions of safety or cost–benefit ratios, rather than categorical considerations.
Another common issue can also be found in recent reports: the question of involving society in the debate. There is consensus on the fact that the legitimacy and governance of HGE should not be left solely to scientists and other experts but should involve society more broadly. Since germline interventions could profoundly change the human condition, the need for a broad and inclusive public debate is frequently emphasized (Iltis et al., 2021 ; Scheufele et al., 2021 ). The most striking expression of the need for public engagement and a “broad societal consensus” can be found in the final statement by the 2015 International Summit on Human Gene Editing organizing committee, as previously quoted (NASEM, 2015 ). Furthermore, the EGE and others also stresses the need for an inclusive societal debate before HGE can be considered permissible.
The pleas for public engagement are, however, not free of tension. For example, the NASEM’s 2017 report was criticised for supporting HGE bypassing the commitment for the broad societal consensus (Baylis, 2017 ). Regarding HGE, some argue that only a “small but vocal group of scientists and bioethicists now endorse moving forward” (Andorno et al., 2020 ). Serious efforts to engage the public on the permissibility and uses of HGE have yet to be made. This issue not only lacks elaboration on approaches to how successful public participation can occur, but also how stop short of presenting views on how to translate the public’s views into ethical considerations and policy (Baylis, 2019 ).
Potential uses of heritable genome editing technology
HGE is expected to allow a range of critical interventions: (i) preventing the transmission of genetic variants associated with severe genetic conditions (mostly single gene disorders); (ii) reducing the risk of common diseases (mostly polygenic diseases), with the promise of improving human health; and (iii) enhancing human capabilities far beyond what is currently possible for human beings, thereby overcoming human limitations. The identification of different classes of potential interventions has shifted the debate to the applications considered morally permissible beyond the acceptable use of HGE (Dzau et al., 2018 ). Specifically, there are differences in the limits of applicability suggested by some of the key cornerstone publications discussed above. For example, the NASEM ( 2017 ) report suggests limiting the use of HGE to the transmission of genetic variants linked to severe conditions, although in a very regulated context. In a very similar way, the 2020 report from the International Commission on the Clinical Use of Human Germline Genome Editing suggests that the initial clinical use of HGE should be limited to the prevention of serious monogenic diseases. By contrast, the 2018 Nuffield Council on Bioethics Report does not seem to limit the uses of genome editing to specific applications, though suggests that applications should be aligned with fundamental guiding ethical principles and need to have followed public debate (Savulescu et al., 2015 ). The same report also discusses far-reaching and speculative uses of HGE that might achieve “other outcomes of positive value” (Nuffield Council on Bioethics, 2018 ). Some of these more speculative scenarios include “built-in genetic resistance or immunity to endemic disease”; “tolerance for adverse environmental conditions” and “supersenses or superabilities” (Nuffield Council on Bioethics, 2018 , p. 47).
There have been different views on the value of HGE technology. Some consider that HGE should be permissible in the context of therapeutic applications, since it can provide the opportunity to treat and cure diseases (Gyngell et al., 2017 ). For example, intervention in severe genetic disorders is considered as therapeutic and hence morally permissible, or even obligatory. Others consider HGE to be more like a public health measure, which could be used to reduce the prevalence of a disease (Schaefer, 2020 ). However, others maintain that reproductive uses of HGE are not therapeutic because there is no individual in a current state of disease which needs to be treated, rather a prospective individual to be born with a specific set of negative prospective traits (Rulli, 2019 ).
Below, HGE is discussed in the context of reproductive uses and conditions of clinical advantage over existent reproductive technologies. The HGE applications are explored regarding their potential for modifying one or more disease-related genes relevant to the clinical context. Other uses associated with enhancement of physical and mental characteristics, which are considered non-clinical (although the distinction is sometimes blurred), are also discussed.
Single gene disorders
An obvious application of HGE interventions is to prevent the inheritance of genetic variants known to be associated with a serious disease or condition. Its potential use for this purpose could be typically envisaged through assisted reproduction, i.e., as a process to provide reproductive options to couples or individuals at risk of transmitting genetic conditions to their offspring. Critics of this approach often argue that other assisted reproductive technologies (ARTs) and preimplantation screening technologies e.g., preimplantation genetic diagnosis (PGD), not involving the introduction of genetic modifications to germline cells, are already available for preventing the transmission of severe genetic conditions (Lander, 2015 ; Lanphier et al., 2015 ). These existent technologies aim to support prospective parents in conceiving genetically related children without the condition that affect them. In particular, PGD involves the creation of several embryos by in vitro fertilization (IVF) treatment that will be tested for genetic anomalies before being transferred to the uterine cavity (Sermon et al., 2004 ). In Europe, there is a range in the regulation of the PGD technology with most countries having restrictions of some sorts (Soini, 2007 ). The eligibility criteria for the use of PGD also vary across countries, depending on the range of heritable genetic diseases for which it can be used (Bayefsky, 2016 ).
When considering its effectiveness, PGD presents specific limitations, which include the rare cases in which either both prospective parents are homozygous carriers of a recessive genetic disease, or one of the parents is homozygous for a dominant genetic disease (Ranisch, 2020 ). In these cases, all embryos produced by the prospective parents will be affected by the genetic defect, and therefore it will not be possible to select an unaffected embryo after PGD. Currently, beyond adoption of course, the options available for these prospective parents include the use of a third-party egg or sperm donors.
Overall, given the rarity of cases in which it is not applicable, PGD is thought to provide a reliable option to most prospective parents for preventing severe genetic diseases to be transmitted to their offspring, except in very specific cases. HGE interventions have been suggested to be an alternative method to avoid single gene disorders in the rare cases in which selection techniques such as PGD cannot be used (Ranisch, 2020 ). It has also been proposed to use tools such as CRISPR/Cas9 to edit morphologically suitable but genetically affected embryos, and thus increase the number of embryos available for transfer (de Wert et al., 2018 ; Steffann et al., 2018 ). Moreover, HGE interventions are considered by some as a suitable alternative to PGD, even when the use of PGD could be possible. One argument in this respect is that, although not leading to the manifestation of the disease, the selected embryos can still be carriers of it. In this respect, differently from PGD, HGE interventions can be used to eliminate unwanted, potential future consequences of genetic diseases (i.e., by eliminating the critical mutation carried out in the selected embryo), with the advantage of reducing the risks of further propagation of the disease in subsequent future generations (Gyngell et al., 2017 ).
Overall, HGE interventions are thought to offer a benefit over PGD in some situations by providing a broader range of possible interventions, as well as by providing a larger number of suitable embryos. The latter effect is usually important in the cases where unaffected embryos are small in number, making PGD ineffective (Steffann et al., 2018 ). Whether these cases provide a reasonable ground to justify research and development on the clinical use of HGE remain potentially contentious. Some authors have suggested that the number of cases in which PGD cannot be effectively used to prevent transmission of genetic disorders is so marginal that clinical application of HGE could hardly be justified (Mertes and Pennings, 2015 ). Particularly when analyzing economic considerations (i.e., the allocation of already scarce resources towards clinical research involving expensive techniques with limited applicability) and additional risks associated with direct interventions. In either case of HGE being used as an alternative or a complementary tool to PGD, PGD will most likely still be used to identify those embryos that would manifest the disease and would hence require subsequent HGE.
The PGD technique, however, is not itself free of criticism and possible moral advantages of HGE over PGD have also been explored (Hammerstein et al., 2019 ; Ranisch, 2020 ). PGD remains ethically controversial since, identifying an unaffected embryo from the remaining embryos (which will not be used and ultimately discarded) amounts to the selection of ‘healthy’ embryos rather than ‘curing’ embryos affected by the genetic conditions. On the other hand, given a safe and effective application of the technology, the use of HGE is considered by many morally permissible to prevent the transmission of genetic variants known to be associated with serious illness or disability (de Miguel Beriain, 2020 ). One question that remains is whether HGE and PGD have a differing or equal moral permissibility or, at least, comparable. On issues including human dignity and autonomy, it was argued that HGE and PGD interventions can be considered as equally morally acceptable (Hammerstein et al., 2019 ). This equal moral status was, however, only valid if HGE is used under the conditions of existent gene variants in the human gene pool and to promote the child health’s best interest in the context of severe genetic diseases (Hammerstein et al., 2019 ). Because of selection and ‘therapy’, moral assessments resulted in HGE interventions being considered to some extent preferable to PGD, once safety is carefully assessed (Gyngell et al., 2017 ; Cavaliere, 2018 ). Specifically, PGD’s aim is selective and not ‘therapeutic’, which could be said to contradict the aims of traditional medicine (MacKellar and Bechtel, 2014 ). In contrast to PGD’s selectivity, HGE interventions are seen as ‘pre-emptively therapeutic’, and therefore closer to therapy than PGD (Cavaliere, 2018 ). However, it is also argued that HGE does not have curative aims, and thus it is not a therapeutic application, as there is no patient involved in the procedure to be cured (Rulli, 2019 ). On balance, there appears to be no consensus on which of the approaches, HGE and PGD, is morally a better strategy to prevent the transmission of single gene disorders, with a vast amount of literature expressing diverse positions when considering different scenarios (Delaney, 2011 ; Gyngell et al., 2017 ; Cavaliere, 2018 ; Ranisch, 2020 ; Rehmann-Sutter, 2018 ; Sparrow, 2021 ).
Polygenetic conditions
HGE is also argued to have the potential to be used in other disorders which have a polygenic disposition and operate in combination with environmental influences (Gyngell et al., 2017 , 2019 ). Many common diseases, which result from the involvement of several genes and environmental factors, fall into this category. Examples of common diseases of this type includes diabetes, coronary artery disease and different types of cancers, for which many of the genes involved were identified by studies of genome wide association (e.g., Wheeler and Barroso, 2011 ; Peden and Farral, 2011 ). These diseases affect the lives of millions of people globally, severely impacting health and often leading to death. Furthermore, these diseases have a considerable burden on national health systems. Currently, many of these diseases are controlled through pharmaceutical products, although making healthier life choices about diet and exercise can also contribute to preventing and managing some of them. Despite the interest, the use of PGD in polygenic conditions would hardly be feasible, due to the number of embryos needed to select the preferred genotype and available polygenic predictors (Karavani et al., 2019 ; Shulman and Bostrom, 2014 ).
In theory, HGE could be a potentially useful tool to target different genes and decrease the susceptibility to multifactorial conditions in current and future generations. The application of HGE to polygenic conditions is often argued by noting that the range of applicability of the technique (well beyond single gene disorders) would justify and outweigh the cost needed to develop it. However, to do so, a more profound knowledge of genetic interactions, of the role of genes and environmental factors in diverse processes would be needed to be able to modify such interconnected systems with limited risk to the individual (Lander, 2015 ). Besides, it is now understood that, depending on the genetic background, individuals will have different risks of developing polygenetic diseases (risk-associated variants), but hardly any certainty of it. In other words, although at the population level there would most likely be an incidence of the disease, it is not possible to be certain of the manifestation of the disease in any specific individual. As a result, the benefits of targeting a group of genes associated to a disease in a specific individual would have to be assessed in respect to the probability of incidence of the disease. The risk-benefit ratio for HGE is considerably increased for polygenic conditions compared to monogenic disorders. Additionally, the risks of adverse effects, e.g., off-target effects, increases with the number of genes targeted for editing. The latter effects make the potential benefits of HGE in polygenic diseases more uncertain than in single gene disorders.
Genetic enhancement
A widespread concern regarding the use of HGE is that such interventions could be used not only to prevent serious diseases, but also to enhance desirable genetic traits. Currently, our knowledge on how to genetically translate information into specific phenotypes is very limited and some argue that it might never be technically feasible to achieve comprehensive genetic enhancements using current gene editing technologies (Janssens, 2016 ; Ranisch, 2021 ). Similar to many diseases, in which different genetic and other factors are involved, many of the desirable traits to be targeted by any enhancement will most likely be the result of a combination of several different genes influenced by environment and context. Moreover, the implications for future generations of widespread genetic interventions in the human population and its potential impact on our evolutionary path are difficult to assess (Almeida and Diogo, 2019 ). Nevertheless, others argue that genetic enhancement through HGE could be possible in the near future (de Araujo, 2017 ).
There has been much discussion regarding the meaning of the terms and the conceptual or normative difference between ‘therapy’ and ‘enhancement’ (for an early discussion: Juengst, 1997 ; Parens, 1998 ). There are mainly three different meanings of ‘enhancement’ used in the literature. First, ‘enhancement’ is sometimes used to refer to measures that go beyond therapy or prevention of diseases, i.e., that transcend goals of medicine. Second, ‘enhancement’ is used to refer to measures that equip a human with traits or capacities that they typically do not possess. In both cases, the term points to equally controversial and contrasting concepts: on the one hand, those of ‘health’, ‘disease’ or ‘therapy’, and on the other, those of ‘normality’ or ‘naturalness’. Third, ‘enhancement’ is sometimes also used as an umbrella-term describing all measures that have a positive effect on a person’s well-being. According to this definition, the cure, or prevention of a disease is then also not opposed to an enhancement. Here again, this use refers to the controversial concept of ‘well-being’ or a ‘good life’.
It is beyond the scope of this article to provide a detailed review of the complex debate about enhancement (for an overview: Juengst and Moseley, 2019 ). However, three important remarks can be made: first, although drawing a clear line between ‘enhancement’ and ‘therapy’ (or ‘normality’, etc.) will always be controversial, some cases can be clearly seen as human enhancement. This could include modifications to augment human cognition, like having a greater memory, or increasing muscle mass to increase strength, which are not considered essential for human health (de Araujo, 2017 ).
Second, it is far from clear whether a plausible account of human enhancement would, in fact, be an objectivist account. While authors suggest that there is some objectivity regarding the conditions that constitute a serious disease (Habermas, 2003 ), the same might not be true for what constitutes an improvement of human functioning. It may rather turn out that an enhancement for some might be seen as a dis-enhancement for others. Furthermore, the use of the HGE for enhancement purposes can be considered at both an individual and a collective level (Gyngell and Douglas, 2015 ; Almeida and Diogo, 2019 ), with a range of ethical and biological implications. If HGE is to be used for human enhancement, this use will be in constant dependence on what we perceive as ‘normal’ functioning or as ‘health’. Therefore, factors such as cultural and societal norms will have an impact on where such boundaries are drawn (Almeida and Diogo, 2019 ).
Third, it should be noted that from an ethical perspective the conceptual question of what enhancement is, and what distinguishes it from therapy, is less important than whether this distinction is ethically significant in the first place. In this context, it was pointed out that liberal positions in bioethics often doubt that the distinction between therapy and enhancement could play a meaningful role in determining the limits of HGE (Agar, 1998 ). The consideration of genetic intervention for improving or adding traits considered positive by individuals have raised extreme positions. Some welcome the possibility to ameliorate the human condition, whilst others consider it an alarming attempt to erase aspects of our common human ‘nature’. More specifically, some authors consider HGE a positive step towards allowing humans the opportunity to obtain beneficial traits that otherwise would not be achievable through human reproduction, thus providing a more radical interference in human life to overcome human limitations (de Araujo, 2017 ; Sorgner, 2018 ). The advocates of this position are referred to as ‘bioliberals’ or ‘transhumanists’ (Ranisch and Sorgner, 2014 ), and its opponents are referred to as ‘bioconservatives’ (Fukuyama, 2002 ; Leon, 2003 ; Sandel, 2007 ). Transhumanism supports the possibility of humans taking control of their biology and interfering in their evolution with the use of technology. Bioconservatism defends the preservation and protection of ‘human essence’ and expresses strong concerns about the impact of advanced technologies on the human condition (Ranisch and Sorgner, 2014 ).
For the general public, HGE used in a clinical context seems to be less contentious compared when used as a possible human enhancement tool. Specifically, some surveys indicate that the general-public typically exhibits a reduced support for the use of genome editing interventions for enhancement purposes compared to therapeutic purposes (Gaskell et al., 2017 ; Scheufele et al., 2017 ). In contrast, many technologies and pharmaceutical products developed in the medical context to treat patients are already being used by individuals to ‘enhance’ some aspect of their bodies. Some examples include drugs to boost brain power, nutritional supplements, and brain-stimulating technologies to control mood, even though their efficiency and safety is not clear. This could suggest that views on enhancement may vary depending on the context and on what is perceived as an enhancement by individuals. It may be informative to carry out detailed population studies to explore whether real ethical boundaries and concerns exist, or whether these are purely the result of the way information is processed and perceived.
Heritable genome editing: Mapping the ethical debate
Even though genome editing methods have only been developed in the last decade, the normative implication of interventions into the human germline have been discussed since the second half of the 20th century (Walters et al., 2021 ). Some even argue that, virtually, all the ethical issues raised by genetic engineering were already being debated at that time (Paul, 2005 ). This includes questions about the distinction between somatic and germline interventions, as well as between therapy and enhancement (e.g., Anderson, 1985 ). Nevertheless, as it has been widely noted, it is difficult to draw clear lines between these two categories (e.g., McGee, 2020 ; Juengst, 1997 ), and alternative frameworks have been proposed, particularly in the context of HGE (Cwik, 2020 ). Other questions include the normative status of human nature (e.g., Ramsey, 1970 ), the impossibility of consent from future generations (e.g., Lappe, 1991 ), possible slippery slopes towards eugenics (e.g., Howard and Rifkin, 1977 ), or implications for justice and equality (e.g., Resnik, 1994 ).
When discussing the ethics of HGE, roughly three types of considerations can be distinguished: (i) pragmatic, (ii) sociopolitical, and iii) categorical (Richter and Bacchetta, 1998 ; cf. Carter, 2002 ). Pragmatic considerations focus on medical or technological aspects of HGE, such as the safety or efficacy of interventions, risk–benefit ratio, possible alternatives or the feasibility of responsible translational research. Such considerations largely depend on the state of science and are thus always provisional. For example, if high-risk technologies one day evolve into safe and reliable technologies, some former pragmatic considerations may become obsolete. Sociopolitical aspects, on the other hand, are concerned with the possible societal impact of technologies, e.g., how they can promote or reduce inequalities, support or undermine power asymmetries, strengthen, or threaten democracy. Similar to pragmatic considerations, sociopolitical reasons depend on specific contexts and empirical factors. However, these are in a certain sense ‘outside’ the technology—even though technologies and social realities often have a symbiotic relationship. While sociopolitical considerations can generate strong reasons against (or in favour of) implementing certain technologies, most often these concerns could be mitigated by policies or good governance. Categorical considerations are different and more akin to deontic reasons. They emphasise categorical barriers to conduct certain deeds. It could be argued, for instance, that the integrity of the human genome or the impossibility to obtain consent from future generation simply rule out certain options to modify human nature. Such categorical considerations may persist despite technological advances or changing sociopolitical conditions.
Comparing the bioethical literature on genetic engineering from the last century with the ongoing discussions shows a remarkable shift in the ethical deliberation. In the past, scholars from the field of medical ethics, as well as policy reports, used to focus on possible categorical boundaries for germline interventions and on possible sociopolitical consequences of such scenarios. For instance, the influential 1982 report “Splicing Life” from the US President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioural Research prominently discussed concerns about ‘playing God’ against the prospects of genetically engineering human beings, as well as possible adverse consequences of such interventions. Although this study addresses potential harms, pragmatic arguments played only a minor role, possibly due to the technical limitations at the time.
With the upcoming availability of effective genome editing techniques, the focus on the moral perspective seems to have been reversed. Increasingly, the analysis of the permissibility of germline interventions is confined to questions of safety and efficacy. This is demonstrated by the 2020 consensus study report produced by an international commission convened by the US National Academy of Medicine, the US National Academy of Sciences, and the UK’s Royal Society, which aimed at defining a translational pathway for HGE. Although the report recognizes that HGE interventions does not only raise pragmatic questions, ethical aspects were not explicitly addressed (National Academy of Medicine, National Academy of Sciences, and the Royal Society, 2020 ).
Similarly, in 2019, a report on germline interventions published by the German Ethics Council (an advisory body to the German government and parliament) emphasizes that the “previous categorical rejection of germline interventions” could not be maintained (Deutscher Ethikrat, 2019 , p. 5). The German Ethics Council continues to address ethical values and societal consequences of HGE. However, technical progress and the development of CRISPR/Cas9 tools seem to have changed the moral compass in the discussion about germline interventions.
For a comprehensive analysis of HGE to focus primarily on pragmatic arguments such as safety or efficacy would be inadequate. In recent years, developments in the field of genome editing have occurred at an incredibly fast pace. At the same time, there are still many uncertainties about the efficacy of the various gene editing methods and unexpected effects in embryo editing persist (Ledford, 2015 ). Social and political implication also remain largely unknown. To date, it has been virtually impossible to estimate how deliberate interventions into the human germline could shape future societies and to conduct a complete analysis of the safety aspects of germline interventions.
Moreover, as the EGE notes, we should be cautious not to limit the complex process of ethical decision-making to pragmatic aspects such as safety. The “‘safe enough’ narrative purports that it is enough for a given level of safety to be reached in order for a technology to be rolled out unhindered, and limits reflections on ethics and governance to considerations about safety” (EGE, 2021 , p. 20). Consequently, the EGE has highlighted the need to engage with value-laden concepts such as ‘humanness’, ‘naturalness’ or ‘human diversity’ when determining the conditions under which HGE could be justified. Even if a technology has a high level of safety, its application may still contradict ethical values or lead to undesirable societal consequences. Efficacy does not guarantee compatibility with well-established ethical values or cultural norms.
While concepts such as ‘safety’ or ‘risk’ are often defined in scientific terms, this does not take away the decision of what is ethically desirable given the technical possibilities. As Hurlbut and colleagues put it in the context of genome editing: “Limiting early deliberation to narrowly technical constructions of risk permits science to define the harms and benefits of interest, leaving little opportunity for publics to deliberate on which imaginations need widening, and which patterns of winning and losing must be brought into view” (Hurlbut et al., 2015 ). Therefore, if public engagement is to be taken seriously, cultural norms and values of those affected by technologies must also be considered (Klingler et al., 2022 ). This, however, means broadening the narrow focus on pragmatic reasons and allowing categorical as well as sociopolitical concerns in the discourse. Given the current attention on pragmatic reasons in current debates on HGE, it is therefore beneficial to revisit the categorical and sociopolitical concerns that remain unresolved. The following sections provide an overview of relevant considerations that can arise in the context of HGE and that underline many of the societal concerns and values crucial for public engagement.
Human genome ‘integrity’
Heritability seems to be one of the foremost considerations regarding germline genome editing, as it raises relevant questions on a ‘natural’ human genome and its role in ‘human nature’ (Bayertz, 2003 ). This follows an ongoing philosophical debate on ‘human nature’, at least as defined by the human genome. This has ensued a long debate on the value of the human genome and normative implications associated with its modification (e.g., Habermas, 2003 ). Although a comprehensive discussion of these topics goes beyond the scope of this paper, the human genome is viewed by many as playing an important role in defining ‘human nature’ and providing a basis for the unity of the human species (for discussion: Primc, 2019 ). Considering the implications for the individual and the collective, some affirm the right of all humans to inherit an unmodified human genome. For some authors, germline modification is considered unethical, e.g., a “line that should not be crossed” (Collins, 2015 ) or a “crime against humanity” (Annas et al., 2002 ).
The Universal Declaration on the Human Genome and Human Rights (UDHGHR) states that “the human genome underlies the fundamental unity of all members of the human family, as well as the recognition of their inherent dignity and diversity. In a symbolic sense, it is the heritage of humanity” (Article 1, UNESCO, 1997 ). The human genome is viewed as our uniquely human collective ‘heritage’ that needs to be preserved and protected. Critics of heritable genetic interventions argue that germline manipulation would disrupt this natural heritage and therefore would threaten human rights and human equality (Annas, 2005 ). Heritable human genome editing creates changes that can be heritable to future generations. For many, this can represent a threat to the unity and identity of the human species, as these modifications could have an impact on the human’s gene pool. Any alterations would then affect the evolutionary trajectory of the human species and, thus, its unity and identity.
However, the view of the human genome as a common heritage is confronted with observations of the intrinsic dynamism of the genome (Scally, 2016 ). Preservation of the human genome, at least in its current form, would imply that the genome is static. However, the human genome is dynamic and, at least in specific periods of environmental pressure, must have naturally undergone change, as illustrated by human evolution (Fu and Akey, 2013 ). The genome of any individual includes mutations that have occurred naturally. Most of them seem to be neither beneficial nor detrimental to the ability of an individual to live or to his/her health. Others can be detrimental and limiting to their wellbeing. It has been shown that, on average, each human genome has 60 new mutations compared to their parents (Conrad et al., 2011 ). At the human population level, a human genome can have in average 4.1–5 million variants compared to the ‘reference’ genome (Li and Sadler, 1991 ; Genomes Project C, 2015 ). The reference genome itself is thus a statistical entity, representing the statistic distribution of the probability of different gene variants in the whole genome. Human genomic variation is at the basis of the differences in the various physical traits present in humans (e.g., eye colour, height, etc.), as well as specific genetic diseases. Thus, the human population is comprised of genomes with a pattern of variants and not of ‘one’ human genome that needs to be preserved (Venter et al. 2001 ). The human genome has naturally been undergoing changes throughout human history. An essentialist view of nature seems to be the basis for calling for the preservation of genome integrity. However, in many ways, this view is intrinsically challenged by the interpretation portrayed by evolutionary biology of our genetic history already more than a century ago. Nevertheless, despite the dynamic state of the human genome, this in itself cannot justify the possibility of modifying the human genome. It is also worth considering that the integrity of the human genome could also be perceived in a ‘symbolic’ rather than biological literal meaning. Such an interpretation would not require a literally static genome over time, but instead suggest a boundary between ‘naturally’ occurring variation and ‘artificially’ induced change. This is rather a version of the ‘natural’/unnatural argument, rather than an argument for a literally unchanged genetic sequence.
The modification of the human genome raises complex questions about the characterization of the human species genome and if there should be limits on interfering with it. The options to modify the human genome could range from modifying only the genes that are part of the human gene pool (e.g., those genes involved in severe genetic diseases such as Huntington’s disease) to adding new variants to the human genome. Regarding variants which are part of the common range of variation found in the human population (although it is not possible to know all the existent variations), the question becomes whether HGE could also be used in any of them (e.g., even the ones providing some form of enhancement) or only in disease-associated variants and thus be restricted to the prevention of severe genetic diseases. In both cases, the integrity of the human genome is expected to be maintained with no disruption to human lineage. However, it could be argued that this type of modification is defending a somewhat conservative human nature argument, since it is considering that a particular genetic make-up is ‘safe’ or would not involve any relevant trade-offs. In contrast, a different conclusion could be drawn on the integrity of the human genome when introducing genotypical and phenotypical traits that do not lie within the common range of variation found in the population (Cwik, 2020 ). In all cases, since the implications of the technology are intergenerational and consequently, it will be important to carry out an assessment of the risks that we, as a species, are willing to take when dealing with disease and promoting health. For this, we will need to explore societal views, values and cultural norms associated with the human genome, as well as possibly existing perceptions of technology tampering with ‘nature’. To support such an assessment, it would be useful to draw on a firm concept of human nature and the values it implies, beyond what is implied by genetic aspects.
Human dignity
In several of the legally binding and non-binding documents addressing human rights in the biomedical field, human dignity is one of the key values emphasized. There are concerns that heritable genome interventions might conflict with the value of human dignity (Calo, 2012 ; Melillo, 2017 ). The concerns are considered in the context of preserving the human genome (Nordberg et al. 2020 ). More specifically, the recommendation on Genetic Engineering by the Council of Europe (1982) states that “ the rights to life and to human dignity protected by Articles 2 and 3 of the European Convention on Human Rights imply the right to inherit a genetic pattern which has not been artificially changed” (Assembly, 1982 ). This is supported by the Oviedo Convention on Human Rights and Biomedicine (1997), where Article 13 prohibits any genetic intervention with the aim of introducing a modification in the genome of any descendants. The Convention is the only international legally binding instrument that covers human germline modifications among the countries which have ratified it (Council of Europe, 1997 ). However, there have been some authors disputing the continued ban proposed by the Oviedo Convention (Nordberg et al. 2020 ). Such authors have focused on the improvements of safety and efficacy of the technology in contrast to authors focusing on its value for human dignity (Baylis and Ikemoto, 2017 ; Sykora and Caplan, 2017 ). The latter authors seem to highlight the concept of human dignity to challenge heritable interventions to the human genome.
But a question in debate has been to demonstrate how ‘human dignity’, described in such norms, relates to heritable genome interventions. The concept of the human genome as common genetic heritage, distinguishing humans from other species seems one of the main principles implied by such norms. In this view, the human genome determines who belongs to the human species and who does not, and thus confers an individual the dignity of being a human by association. This creates an inherent and strong link between the concept of human genome and the concept of human dignity and its associated legal rights (Annas, 2005 ). It could be argued that a genetic modification to an individual may make it difficult for him/her to be recognized as a human being and therefore, preservation of the human genome being important for human dignity to be maintained. This simple approach, or at least interpretation, however, ignores the fact that the human genome is not a fixed or immutable entity, as exemplified by human evolution (as discussed in the previous section). As a result, the view that HGE interventions are inherently inadmissible based on the need to preserve human dignity is contested (Beriain, 2018 ; Raposo, 2019 ). More broadly, the idea that biological traits are the basis for equality and dignity, supporting the need for the human genome to be preserved, is often challenged (Fenton, 2008 ).
It is argued that to fully assess the impact of the HGE interventions on human dignity, it will be necessary to have a better understanding of the concept of human dignity in the first place (Häyry, 2003 ; Cutas, 2005 ). For some, however, human dignity is a value that underlies questions of equality and justice. Thus, the dignity-based arguments could uncover relevant questions in the discussion of ethical implications on modifying the human genome (Segers and Mertes, 2020 ). In the Nuffield Council on Bioethics Report (2018) principles of social justice and solidarity, as well as welfare, are used to guide the debate on managing HGE interventions. Similarly, the concept of human dignity could, therefore, provide the platform upon which consideration of specific values could be discussed, broaden the debate on HGE to values shared by society.
Right of the child: informed consent
In many modern societies, every individual, including children, have the rights to autonomy and self-determination. Therefore, each person is entitled to decide for themselves in decisions relating to their body. These rights are important for protecting the physical integrity of a person. When assessing the implication of allowing individuals to take (informed) decisions relative to the use of heritable genetic interventions on someone else’s body, it is useful to reflect on the maturity of existing medical practices and, more broadly, on the additional complexities associated with the heritability of any such intervention.
In modern health-care systems, informed consent provides the opportunity for an individual to exercise autonomy and make an informed decision about a medical procedure, based on their understanding of the benefits and risks of such procedure. Informed consent is thus a fundamental principle in medical (research) ethics when dealing with human subjects (Beauchamp and Childress, 2019 ).
Heritable genome interventions present an ethical constraint on the impossibility of future generations of providing consent to an intervention on their genome (Smolenski, 2015 ). In other words, future generations cannot be involved in a decision which could limit their autonomy, since medical or health-related decisions affecting them are placed on the present generation (and, in the case of a child to be born, more specifically, on his/her parents). However, many other actions taken by parents of young children also intentionally influence the lives of those children and have been doing so for millennia (Ranisch, 2017 ). Although these actions may not involve altering their genes, many of such actions can have a long-lasting impact on a child’s life (e.g., education and diet). However, it could be argued that they do not have the irreversible effect that HGE will have in the child and future generations. In cases where parents act to expand the life choices of their children by eliminating disease (e.g., severe genetic diseases), this would normally be thought to outweigh any possible restriction on autonomy. In these cases, if assuming HGE benefits will outweigh risks regarding safety and efficacy, the use of HGE could be expected to contribute to the autonomy of the child, as him/her would be able in the future to have a better life, not constrained by the limitations of the disease. As a result, even if it is accepted that these technologies may in one way reduce the autonomy of future generations, some believe that this will often be outweighed by other effects increasing autonomy (Gyngell et al., 2017 ). In other words, it is reasonable to suppose that, when taken by parents based on good information and understanding of risks and impacts, the limitation in the autonomy of unborn children associated with heritable genetic interventions would be compensated by the beneficial effects of increasing their autonomy when born (Gyngell et al., 2017 ).
It has often been emphasized that possible genetic interventions must not curtail the future possibilities of offspring to live their lives according to their own idea of a good life. This view originated in the liberal tradition and is associated with the “right to an open future”, defended by Joel Feinberg ( 1992 ). That is an anticipatory autonomy right that parents can violate, even though the offspring could exercise it only in the future. Feinberg has discussed the right to an open future in the context of religious education. However, various authors have applied this argument to the question of permissible and desirable genetic interventions (Buchanan et al., 2000 ; Glover, 2006 ; Agar, 1998 ). Accordingly, germline modifications or selection would have to allow the offspring to have a self-determined choice of life plans. It would therefore be necessary to provide offspring with genetic endowments that represent the so-called all-purpose goods. These goods are “useful and valuable in carrying out nearly any plan of life or set of aims that humans typically have” (Buchanan et al., 2000 , p. 167). While this claim is certainly appealing, in reality it will be difficult to identify phenotypes that will only broaden and do not narrow the spectrum of life plans. Take, for example, body size: a physique favourable for a basketball player would at the same time be less favourable in successfully riding horses as a professional jockey and vice versa. Increasing some opportunities often means reducing other ones.
The arguments of informed consent and open future need to be explored outside the realm of severe genetic diseases by considering other scenarios (including scenarios of genetic enhancement). Hereby, the effects of the interventions on the autonomy of future generations can be assessed more comprehensively. As for enhancement, decisions outside the realm of health can be more controversial, as the traits that parents see fit to generate enhancement may inadvertently condition a child’s choices in the future in an undesirable way.
If HGE is to be used, questions on how the consent and information should be provided to parents to fully equip them to decide in the best interests of the child will need to be assessed (Evitt et al., 2015 ). This is evident if considering the informed consent used in the study conducted by He Jiankui. One of the many criticisms of the study was the inadequacy of the informed consent process provided to the parents, which did not meet regulatory or ethical standards (Krimsky, 2019 ; Kirksey, 2020 ). This raises questions on how best to achieve ethical and regulatory compliance regarding informed consent in applications of HGE (Jonlin, 2020 ).
Discrimination of people with disabilities
For many years, there has been an effort to develop selective reproduction technologies to prevent genetic diseases or conditions leading to severe disabilities. These forms of reproductive genetic disease prevention are based on effectively filtering and eradicating embryos or foetuses affected by genetic diseases. There are divergent views regarding the use of these technologies. For example, the disability rights movement argues that the use of technologies such as prenatal testing (PNT) and PGD discriminates against people living with a disability (Scully, 2008 ; Asch and Barlevy, 2012 ). The key arguments presented supporting this view are: (i) the limited value of a genetic trait in respect to the life of an embryo (Parens and Asch, 2000 ) and (ii) the ‘expressivist’ argument (Buchanan, 1996 ; Shakespeare, 2006 ). The first argument is based on the critique that a disabling trait is viewed as being more significant than the life of an embryo/foetus. This argument was initially used in the context of prenatal testing and selective termination, and has also been applied in the context of new technologies like PGD (Parens and Asch, 2000 ). The second, the ‘expressivist’ argument, argues that the use of these technologies expresses negative or discriminatory views on the disabling conditions they are targeting and subsequently on the people living with these conditions (Asch and Wasserman, 2015 ). The expressivist argument, however, has been challenged by stressing the importance of differentiating between the disability itself and the people living with disability (Savulescu, 2001 ). The technology’s use is aimed at reducing the incidence of disability, and it does not have a position of value on the people that have a specific condition.
When applying the same arguments to the use of HGE in comparison with other forms of preventing heritable genetic diseases, some important considerations can be made. Regarding the first argument, in contrast to selective reproduction technologies, HGE may allow the removal of the disabled trait with the aim of ensuring survival of the affected embryo. However, most likely, PGD would be used before and after the editing of the embryos to help the identification of the ones requiring intervention and verifying the efficiency of the genetic intervention (de Miguel Beriain, 2018 ; Ranisch, 2020 ). Similarly, the expressivist argument continues to be challenged if the application of human HGE is envisaged in the context of severe genetic diseases (e.g., Tay-Sachs and Huntington’s disease). It has been argued that the choice to live without a specific genotype neither implies discriminating people living with a respective condition nor considering the life of people living with the disease not worth living or less valuable (Savulescu, 2001 ). In other words, the expressivist argument is not a valid or a sufficiently strong ethical argument for prospective parents not to have the option to have a future child without a genetic disease.
It is worth noting that the debate on the use of reproduction technologies for the prevention of genetic diseases is not at all new, and that modern HGE techniques only serve to highlight ethical concerns that have been expressed for a long time. In the case of preventing genetic diseases, the application of both arguments to HGE intervention could be considered not to provide sufficiently strong ethical arguments to limit the use of the technology in the future. However, it is worth exploring whether scientific innovations like HGE are either ameliorating or reinvigorating ethical concerns expressed so far, for example in creating a future that respects or devalues disability as a part of the human condition. Perhaps even more importantly, given their potential spectrum of possible intervention and efficacy, it is important to reflect on whether the broad use of HGE could have an impact on concepts of disability and ‘normality’ as a whole distorting an already unclear ethical line between clinical and non-clinical interventions. Moreover, research work exploring the relationship between disability and identity indicated that personhood with disability can be an important component to people’s identity and interaction with the world. In the case of heritable human genome editing, it is not yet known how this technology will impact the notions of identity and personhood in people who had their germline genome modified (Boardman and Hale, 2018 ). For further progress on these issues public engagement might be important to gather different views and perceptions on the issue.
Justice and equality
Beside the limits of applicability, another common ethical concern associated with the use of genome editing technologies, as with many new technologies, is the question of accessibility (Baumann, 2016 ). Due to the large investments that will need to be made for continuing development of the technology, there is a (perceived) risk of it becoming an expensive technology that only a few wealthy individuals in any population (and/or only citizens in comparatively rich countries) can access. In addition, there is concern that patenting of genome editing technologies will delay widespread access or lead to unequal distribution of corresponding benefits (Feeney et al., 2018 ). This may, consequently, contribute to further increases in existing disparities, since individuals or countries with the means of accessing better health treatments may have economic advantages (Bosley et al., 2015 ). This could enhance inequality at different levels, depending on the limits of applicability of the technology. Taken to its extreme, the use of the technology could allow germline editing to create and distinguish classes of individuals that could be defined by the quality of their manipulated genome.
The concern that the possibility of germline interventions in humans could entrench or even increase inequalities has accompanied the discussion about ethics of genetic interventions from the very beginning until today (e.g. Resnik, 1994 ). In ‘Remaking Eden’ Lee Silver envisioned a divided future society, consisting of a genetically enhanced class, the “genRich”, and a genetic underclass, the “naturals” (Silver, 1997 ). Françoise Baylis recently echoed such concerns regarding future HGE interventions, namely that “unequal access to genome-editing technologies will both accentuate the vagaries of the natural lottery and introduce an unjust genetic divide that mirrors the current unjust economic and social divide between rich and poor individuals” (Baylis, 2019 , p. 67). At the same time, the possibility to genetically intervene in the ‘natural lottery’ has also been associated with the hope of countering natural inequalities and increase equality of opportunities. Robert Sinsheimer may be among the first to envision such a ‘new’ individualistic type of ‘eugenics’ that “would permit in principle the conversion of all of the unfit to the highest genetic level” (Sinsheimer, 1969 , p. 13). More recently, in the book ‘From chance to choice: Genetics and justice’ (2000) it is argued that “equality of opportunity will sometimes require genetic interventions and that the required interventions may not always be limited to the cure or prevention of disease” (Buchanan et al., 2000 , p. 102). When discussing issues related to justice and equality, it will be important to involve a broad spectrum of stakeholders to better evaluate the economic effects of the commercialization of the technology.
Conclusions
With ongoing technological developments and progress with guiding and regulating its acceptable use, the possibility of HGE interventions in the human genome is closer than ever to becoming a reality. The range of HGE applicability can go from preventing the transmission of genetic variants associated with severe genetic conditions (mostly single gene disorders but also, to a lesser extent, polygenic diseases) to genetic enhancements. The permissibility of HGE has often been considered on the basis of possible uses, with therapeutic uses generally considered more acceptable than non-therapeutic ones (including human enhancement). When compared with other technologies with similar therapeutic uses (e.g., PGD) already in use, HGE presents similarities and differences. However, from an ethical acceptability perspective, there is currently no consensus on whether HGE is more or less acceptable than PGD.
An important conclusion of this study is that, along with the technological development of genome germline editing techniques, a shift in the focus of analyses on its applicability has been observed. More specifically, the emphasis on pragmatic considerations seems to have increased substantially compared with the previous emphasis on categorical and sociopolitical arguments. Many of the most recent publications from authoritative advisory committees and institutions discuss the permissibility of HGE interventions primarily on the basis of pragmatic arguments, in which safety and efficacy are the main focus. Since germline interventions could profoundly change the human condition, the need for a broad and inclusive public debate on this topic has also been frequently emphasized. However, limited consideration has been given to approaches to carry out such action effectively, and on how to consider their outcomes in relevant policies and regulations.
It is currently not entirely clear whether: (i) the pragmatic position championed by such authoritative sources builds on the premise that the ethical debate has reached sufficient maturity to allow a turning point; (ii) the lack of progress has somewhat hampered further consideration of issues still considered controversial; (iii) regulatory pressure is somewhat de facto pushing forward the introduction of such technologies despite critical, unresolved ethical issues. Based on the analysis presented in this paper, a combination of the latter factors (ii and iii) seems more likely. In engaging the public in societal debates on the acceptability of such technologies, unresolved questions are likely to re-emerge. Specifically, it is possible that categorical and sociopolitical considerations will gain renewed focus during public engagement. In other words, when involving the public in discussions on HGE, it is possible that cultural values and norms, not only questions of safety and efficacy, will re-emerge as crucial to the acceptance of the technology (What is meant by natural? What is understood by humanity? etc.).
HGE interventions put into question specific biological and moral views of individuals, including views on the value of the human genome, on human dignity, on informed consent, on disability and on societal equality and justice. The range of ethical issues affected by the introduction of such technology, often still characterised by non-convergent, and at times conflicting, positions, illustrate the importance of further consideration of these issues in future studies and public engagement activities. As a result, society’s moral uncertainties will need to be assessed further to support the regulation of HGE technologies and form a well-informed and holistic view on how they can serve society’s common goals and values.
Data availability
This statement is not applicable.
Agar N (1998) Liberal eugenics. Public Aff Q 12(2):137–155
PubMed Google Scholar
Almeida M, Diogo R (2019) Human enhancement: genetic engineering and evolution. Evol Med Public Health 1:183–189. https://doi.org/10.1093/emph/eoz026
Article Google Scholar
Anderson WF (1985) Human Gene Therapy: scientific and ethical considerations. J Med Philos 10(3):275–291. https://doi.org/10.1093/jmp/10.3.275
Article CAS PubMed Google Scholar
Andorno R, Baylis F, Darnovsky M, Dickenson D, Haker H, Hasson K et al. (2020) Geneva statement on heritable human genome editing: the need for course correction. Trends Biotechnol 38(4):351–354
Annas GJ (2005) Bioethics: crossing human rights and health law boundaries. Oxford University Press, New York, NY
Google Scholar
Annas GJ, Andrews LB, Isasi RM (2002) Protecting the endangered human: toward an international treaty prohibiting cloning and inheritable alterations. Am J Law Med 28(2–3):151–178
Article PubMed Google Scholar
Araki M, Ishii T (2014) International regulatory landscape and integration of corrective genome editing into in vitro fertilization. Reprod Biol Endocrinol 12(1):108–120
Article CAS PubMed PubMed Central Google Scholar
Asch A, Barlevy D (2012) Disability and genetics: a disability critique of pre-natal testing and pre-implantation genetic diagnosis. eLS. Wiley, Chichester
Asch A, Wasserman D (2015) Reproductive testing for disability. In: Arras JD, Fenton E, Kukla R (eds.) Routledge companion to bioethics. Routledge, London
Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G et al. (2015) A prudent path forward for genomic engineering and germline gene modification. Science 348(6230):36. https://doi.org/10.1126/science.aab1028
Article ADS CAS PubMed PubMed Central Google Scholar
Baltimore D, Baylis F, Berg P et al. (2015) On human gene editing: international summit statement. News release, December 3, International summit on human gene editing. http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=12032015a
Barrangou R, Horvath P (2017) A decade of discovery: CRISPR functions and applications. Nat Microbiol 2(17092):1–9. https://doi.org/10.1038/nmicrobiol.2017.92
Article CAS Google Scholar
Baumann M (2016) CRISPR/Cas9 genome editing: new and old ethical issues arising from a revolutionary technology. Nanoethics 10:139–59
Bayefsky MJ (2016) Comparative preimplantation genetic diagnosis policy in Europe and the USA and its implications for reproductive tourism. Reprod Biomed Soc Online 3:41–47
Bayertz K (2003) Human nature: how normative might it be? J Med Philos 28(2):131–150. https://doi.org/10.1076/jmep.28.2.131.14210
Baylis F (2017) Human germline genome editing and broad societal consensus. Nat Hum Behav 1:0103
Baylis F (2019) Human genome editing: our future belongs to all of us. Issues Sci Technol 35:42–44
Baylis F, Ikemoto L (2017) The Council of Europe and the prohibition on human germline genome editing. EMBO Rep 8(12):2084–2085. https://doi.org/10.15252/embr.201745343
Baylis F, Darnovsky M, Hasson K, Krahn TM (2020) Human Germ Line and Heritable Genome Editing: the global policy landscape. CRISPR J 3(5):365–377. https://doi.org/10.1089/crispr.2020.0082 . PMID: 33095042
Beauchamp TL, Childress JF (2019) Principles of biomedical ethics. Oxford University Press, USA
Blendon RJ, Gorski MT, Benson JM (2016) The public and the gene-editing revolution. New Engl J Med 374(15):1406–1411. https://doi.org/10.1056/NEJMp1602010
Boardman FK, Hale R (2018) How do genetically disabled adults view selective reproduction? Impairment, identity, and genetic screening. Mol Genet Genom Med 6(6):941–956
Bosley KS, Botchan M, Bredenoord AL, Carroll D, Charo RA, Charpentier E et al. (2015) CRISPR germline engineering: the community speaks. Nat Biotechnol 33(5):478–486. https://doi.org/10.1038/nbt.3227
Botkin JR (2019) The case for banning heritable genome editing. Genet Med 22:487–489
Brokowski C (2018) Do CRISPR germline ethics statements cut it? CRISPR J 1(2):115–125. https://doi.org/10.1089/crispr.2017.0024
Article PubMed PubMed Central Google Scholar
Buchanan A (1996) Choosing who will be disabled: genetic intervention and the morality of inclusion. Soc Philos Policy 13:18–46
Buchanan A, Brock DW, Daniels N, Wikler D (2000) From chance to choice: genetics and justice. Cambridge University Press
Calo Z (2012) Human dignity and health law: personhood in recent bioethical debates. Notre Dame J Law Ethics Public Policy 26:473–499
Carter L (2002) The ethics of germ line gene manipulation—a five dimensional debate. Monash Bioeth Rev 21(4):S66–S81. https://doi.org/10.1007/BF03351288
Cavaliere G (2018) Genome editing and assisted reproduction: curing embryos, society or prospective parents? Med Health Care Phil 21(2):215–25
Coller BS (2019) Ethics of human genome editing. Annu Rev Med 27(70):289–305. https://doi.org/10.1146/annurev-med-112717-094629
Council of Europe (1997) Convention for the protection of human rights and dignity of the human being with regard to the application of biology and medicine: convention on human rights and biomedicine. COE, Oviedo
Collins, F. (2015) Director, National Institutes of Health, https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-funding-research-using-gene-editing-technologieshuman-embryos
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N et al. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Conrad DF, Keebler JE, DePristo MA, Lindsay SJ, Zhang Y, Casals F et al. (2011) Variation in genome-wide mutation rates within and between human families. Nat Genet 43(7):712–4. https://doi.org/10.1038/ng.862
Cutas DE (2005) Looking for the meaning of dignity in the bioethics convention and the cloning protocol. Health Care Anal 13(4):303–313
Cwik B (2020) Revising, correcting, and transferring genes. Am J Bioeth 20(8):7–18
Cyranoski D (2019) Russian biologist plans more CRISPR-edited babies. Nature 570(7760):145–147
Article ADS CAS PubMed Google Scholar
Daley GQ, Lovell-Badge R, Steffann J (2019) After the storm—a responsible path for genome editing. N Engl J Med 380:897–899
de Araujo M (2017) Editing the genome of human beings: CRISPR-Cas9 and the ethics of genetic enhancement. J Evol Technol 27(1):24–42. http://jetpress.org/v27.1/araujo.pdf
Dance A (2015) Core concept: CRISPR gene editing. Proc Natl Acad Sci USA 112:6245–6246
Davies K (2020) Editing humanity: the CRISPR revolution and the new era of genome editing. Pegasus Books, New York, NY
Delaney JJ (2011) Possible people, complaints, and the distinction between genetic planning and genetic engineering. J Med Ethics 37(7):410–414
Deutscher Ethikrat, (2019) Intervening in the Human Germline. https://www.ethikrat.org/en/publications/publication-details/?tx_wwt3shop_detail%5Bproduct%5D=119&tx_wwt3shop_detail%5Baction%5D=index&tx_wwt3shop_detail%5Bcontroller%5D=Products&cHash=25e88ad52f8b75d311510a9bf7a8dc86
Doudna JA, Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346(6213):1258096. https://doi.org/10.1126/science.1258096
Drabiak K (2020) The Nuffield Council’s green light for genome editing human embryos defies fundamental human rights law. Bioethics 34:223–227
Dzau VJ, McNutt M, Bai C (2018) Wake-up call from Hong Kong. Science 362(6420):1215. https://doi.org/10.1126/science.aaw3127
de Miguel Beriain I (2020) Gene editing and disabled people: a response to Felicity Boardman. J Community Genet 11(3):241–243
de Miguel Beriain I (2018) Human dignity and gene editing. EMBO Rep 19:e46789
de Wert G, Heindryckx B, Pennings G, Clarke A, Eichenlaub-Ritter U, van El CG (2018) Responsible innovation in human germline gene editing: background document to the recommendations of ESHG and ESHRE. Eur J Hum Genet 26(4):450–470. https://doi.org/10.1038/s41431-017-0077-z
EGE (2016) Statement on gene editing. https://ec.europa.eu/info/sites/default/files/research_and_innovation/ege/gene_editing_ege_statement.pdf
EGE (2021) Ethics of genome editing. https://ec.europa.eu/info/sites/default/files/research_and_innovation/ege/ege_ethics_of_genome_editing-opinion_publication.pdf
Evitt NH, Mascharak S, Altman RB (2015) Human germline crispr-cas modification: toward a regulatory framework. Am J Bioeth 15(12):25–29
Feeney O, Cockbain J, Morrison M, Diependaele L, Van Assche K, Sterckx S (2018) Patenting foundational technologies: lessons from CRISPR and other core biotechnologies. Am J Bioeth 18(12):36–48
Fenton E (2008) Genetic enhancement – a threat to human rights? Bioethics 22(1):1–7 https://doi.org/10.1111/j.1467-8519.2007.00564.x
Feinberg J (1992) The child’s right to an open future. In: Feinberg J (ed.) Freedom and fulfillment: philosophical essays. Princeton University Press, Princeton, pp. 6–97
Fu W, Akey JM (2013) Selection and adaptation in the human genome. Annu Rev Genom Hum Genet 14:467–89
Fukuyama F (2002) Our posthuman future: consequences of the biotechnology revolution. Picador, New York, NY
Gaj T, Gersbach CA, Barbas III CF (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31(7):397–405
Gaskell G, Bard I, Allansdottir A, da Cunha RV, Eduard P, Hampel J et al. (2017) Public views on gene editing and its uses. Nat Biotechnol 35(11):1021–1023. https://doi.org/10.1038/nbt.3958
Genomes Project C et al. (2015) A global reference for human genetic variation. Nature 526(7571):68–74
Article ADS CAS Google Scholar
Greely HT (2019) Human germline genome editing: an assessment. CRISPR J 2(5):253–265. https://doi.org/10.1089/crispr.2019.0038
Glover J (2006) Choosing children: genes, disability, and design. Oxford University Press, Oxford
Book Google Scholar
Greely HT (2021) CRISPR people: the science and ethics of editing humans. MIT Press, Cambridge, MA; London, UK
Gupta RM, Musunuru K (2014) Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin Investig 124(10):4154–4161. https://doi.org/10.1172/JCI72992
Gyngell C, Douglas T, Savulescu J (2017) The ethics of germline gene editing. J Appl Philosy 34(4):498–513
Gyngell C, Bowman-Smart H, Savulescu J (2019) Moral reasons to edit the human genome: picking up from the Nuffield report. J Med Ethics 0:1–10. https://doi.org/10.1136/medethics-2018-105084
Gyngell C, Douglas T (2015) Stocking the genetic supermarket: reproductive genetic technologies and collective action problems. Bioethics 29(4):241–250
Guttinger S (2017) Trust in science: CRISPR-Cas9 and the ban on human germline editing. Sci Eng Eth 1–20. https://doi.org/10.1007/s11948-017-9931-1
Habermas J (2003) The future of human nature. Polity Press, Cambridge
Hammerstein AL, Eggel M, Biller-Andorno N (2019) Is selecting better than modifying? An investigation of arguments against germline gene editing as compared to preimplantation genetic diagnosis. BMC Med Eth 83:20
Häyry M (2003) Philosophical arguments for and against human reproductive cloning. Bioethics 17(5–6):447–460
Howard T, Rifkin J (1977) Who should play God? The artificial creation of life and what it means for the future of the human race. Dell Publ. Co
Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157(6):1262–1278. https://doi.org/10.1016/j.cell.2014.05.010
Hurlbut JB, Saha K, Jasanoff S (2015) CRISPR democracy: gene editing and the need for inclusive deliberation. Issues Sci Technol 32(1):25–32
Hyun I, Osborn C (2017) Query the merits of embryo editing for reproductive research now. Nat Biotechnol 35(11):1023–1025. https://doi.org/10.1038/nbt.4000 . PMID: 29121025
Iltis AS, Hoover S, Matthews KRW (2021) Public and stakeholder engagement in developing human heritable genome editing policies: what does it mean and what should it mean? Front Political Sci 3. https://www.frontiersin.org/article/10.3389/fpos.2021.730869
Isasi R, Kleiderman E, Knoppers BM (2016) Genetic technology regulation: editing policy to fit the genome? Science 351(6271):337–339. https://doi.org/10.1126/science.aad6778
Ishii T (2015) Germline genome-editing research and its socio-ethical implications. Trends Mol Med 21(8):473–481. https://doi.org/10.1016/j.molmed.2015.05.006
Janssens AC (2016) Designing babies through gene editing: science or science fiction? Genet Med 18(12):1186–1187. https://doi.org/10.1038/gim.2016.28
Jedwab A, Vears DF, Tse C, Gyngell C (2020) Genetics experience impacts attitudes towards germline gene editing: a survey of over 1500 members of the public. J Hum Genetics65(12):1055–1065
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) Programmable dual‐RNA‐guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Jonlin EC (2020) Informed consent for human embryo genome editing. Stem Cell Rep 14(4):530–537. https://doi.org/10.1016/j.stemcr.2020.03.010
Juengst ET (1997) Can enhancement be distinguished from prevention in genetic medicine? J Med Philos 22(2):125–142
Juengst, ET, Moseley D (2019) Human enhancement. In: Zalta EN (ed.) The Stanford encyclopedia of philosophy (Summer 2019 Edition), Metaphysics Research Lab, Philosophy Department, Stanford University, Stanford
Karavani E, Zuk O, Zeevi D, Barzilai N, Stefanis NC, Hatzimanolis A et al. (2019) Screening human embryos for polygenic traits has limited utility. Cell 179(6):1424–1435
Kirksey E (2020) The Mutant Project: inside the global race to genetically modify humans. St. Martin’s Press
Klingler C, Wiese L, Arnason G, Ranisch R (2022) Public engagement with brain organoid research and application: lessons from genome editing. Am J Bioeth Neurosci 13(2):98–100. https://doi.org/10.1080/21507740.2022.2048733
König H (2017) The illusion of control in germline-engineering policy. Nat Biotechnol 35(6):502–506. https://doi.org/10.1038/nbt.3884
Krimsky S (2019) Ten ways in which He Jiankui violated ethics. Nat Biotechnol 37(1):19–20. https://doi.org/10.1038/nbt.4337
Lander ES (2015) Brave new genome. N Engl J Med 373(1):5–8
Lander ES, Baylis F, Zhang F, Charpentier E, Berg P, Bourgain C et al. (2019) Adopt a moratorium on heritable genome editing. Nature 567(7747):165–168. https://doi.org/10.1038/d41586-019-00726-5
Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J (2015) Don’t edit the human germ line. Nature 519(7544):410–1. https://doi.org/10.1038/519410a
Lappe M (1991) Ethical issues in manipulating the human germ line. J Med Philos 16(6):621–639. https://doi.org/10.1093/jmp/16.6.621
Lea RA, Niakan KK (2019) Human germline genome editing. Nat Cell Biol 21(12):1479–1489. https://doi.org/10.1038/s41556-019-0424-0
Ledford H (2015) The landscape for human genome editing. Nature 526(7573):310–311
Leon K (2003) Ageless bodies, happy souls: biotechnology and the pursuit of perfection. New Atlantis 1:9–28
Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X (2020) Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther 5(1):1–23
Li WH, Sadler LA (1991) Low nucleotide diversity in man. Genetics 129(2):513–23
Liang P, Xu Y, Zhang X et al. (2015) CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 6(5):363–372
Lovell-Badge R (2019) CRISPR babies: a view from the centre of the storm. Development 146(3):dev175778
MacKellar C, Bechtel C eds. (2014) The ethics of the new eugenics. Berghahn Books, New York, Oxford
McGee A (2020) Using the therapy and enhancement distinction in law and policy. Bioethics 34(1):70–80
Melillo TR (2017) Gene editing and the rise of designer babies. Vand J Trans Law 50:757–790
Mertes H, Pennings G (2015) Modification of the embryo’s genome: more useful in research than in the clinic. Am J Bioeth 15(12):52–53. https://doi.org/10.1080/15265161.2015.1103813
Mulvihill JJ, Capps B, Joly Y, Lysaght T, Zwart HAE, Chadwick R, International Human Genome Organisation Committee of Ethics Law and Society (2017) Ethical issues of CRISPR technology and gene editing through the lens of solidarity. Br Med Bull 122(1):17–29. https://doi.org/10.1093/bmb/ldx002
Musunuru K (2019) The CRISPR generation: the story of the world’s first gene-edited babies. BookBaby.
National Academies of Sciences, Engineering, and Medicine (2015) International summit on human gene editing: a global discussion. The National Academies Press, Washington
National Academy of Sciences, National Academy of Medicine (2017) Human genome editing: science, ethics and governance. The National Academies Press, Washington, DC
National Academy of Medicine, National Academy of Sciences, and the Royal Society (2020) Heritable human genome editing. The National Academies Press, Washington, DC
Niemiec E, Howard HC (2020) Ethical issues related to research on genome editing in human embryos. Comput Struct Biotechnol J 18:887–896
National Academies of Sciences, Engineering, and Medicine (2019) Second International Summit on human genome editing: continuing the global discussion: proceedings of a workshop in brief. The National Academies Press, Washington
Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L et al. (2014) Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156(4):836–843
Nordberg A, Minssen T, Feeney O, de Miguel Beriain I, Galvagni L, Wartiovaara K (2020) Regulating germline editing in assisted reproductive technology: an EU cross‐disciplinary perspective. Bioethics 34(1):16–32
Nuffield Council on Bioethics (2016) Genome Editing: an ethical review. https://www.nuffieldbioethics.org/publications/genome-editing-an-ethical-review
Nuffield Council on Bioethics (2018) Genome editing and human reproduction: social and ethical issues. http://nuffieldbioethics.org/project/genome-editing-human-reproduction
Parens E, Asch A (2000) The disability rights critique of prenatal testing: reflections and recommendations. In: Parens E, Asch A (eds.) Prenatal testing and disability rights. Georgetown University Press, Washington
Parliamentary Assembly (1982) Recommendation on genetic engineering. In Recommendation 934. Council of Europe
Paul D (2005) Genetic engineering and eugenics: the uses of history. In: Baillie HW, Casey TK (eds.) Is human nature obsolete? Genetics, bioengineering, and the future of the human condition. MIT Press, Cambridge Mass
Peden JF, Farrall M (2011) Thirty-five common variants for coronary artery disease: the fruits of much collaborative labour. Hum Mol Genet 20:R198–205
Porteus MH (2019) A new class of medicines through DNA editing. New Engl J Med 380(10):947–959. https://doi.org/10.1056/NEJMra1800729
Parens ET (Ed.) (1998) Enhancing human traits: ethical and social implications. Georgetown University Press, Washington, DC
Primc N (2020) Do we have a right to an unmanipulated genome? The human genome as the common heritage of mankind Bioethics 34(1):41–48
Article MathSciNet PubMed Google Scholar
Ramsey P (1970) Fabricated man: the ethics of genetic control. Yale University Press, New Haven
Ranisch R (2017) Germline genome editing and the functions of consent. Am J Bioeth 17(12):27–29
Ranisch R (2020) Germline genome editing versus preimplantation genetic diagnosis: Is there a case in favour of germline interventions? Bioethics 34:60–69. https://doi.org/10.1111/bioe.12635
Ranisch R, Ehni HJ (2020) Fading red lines? Bioethics of germline genome editing. Bioethics 34(1):3–6. https://doi.org/10.1111/bioe.12709
Ranisch R, Rudolph T, Cremer HJ, Knoepffler N (2020) Ordo-responsibility for germline gene editing. CRISPR J 3(1):37–43
Ranisch R (2021) When CRISPR meets fantasy: transhumanism and the military in the age of gene editing. In: Transhumanism: the proper guide to a posthuman condition or a dangerous idea?. Springer, Cham, pp. 111–120
Ranisch R, Sorgner SL (2014) Introducing post-and transhumanism. In: Ranisch & Sorgner (eds.) Post-and transhumanism: an introduction. pp. 7–27. Peter Lang Group AG, Switzerland
Raposo VL (2019) Gene editing, the mystic threat to human dignity. Bioeth Inq 16:249–257. https://doi.org/10.1007/s11673-019-09906-4
Regalado A (2018) EXCLUSIVE: Chinese scientists are creating CRISPR babies. MIT Technol Rev. https://www.technologyreview.com/s/612458/exclusive-chinese-scientists-are-creating-crispr-babies/ . Accessed 4 Aug 2021
Rehmann-Sutter C (2018) Why human germline editing is more problematic than selecting between embryos: ethically considering intergenerational relationships. New Bioeth 24(1):9–25. https://doi.org/10.1080/20502877.2018.1441669
Resnik D (1994) Debunking the slippery slope argument against human germ-line gene therapy. J Med Philos 19(1):23–40. https://doi.org/10.1093/jmp/19.1.23
Richter G, Bacchetta MD (1998) Interventions in the human genome: some moral and ethical considerations. J Med Philos 23(3):303–317. https://doi.org/10.1076/jmep.23.3.303.2581
Rulli T (2019) Reproductive CRISPR does not cure disease. Bioethics 33:1072–1082
Sandel M (2007) The case against perfection: ethics in the age of genetic engineering. The Belknap Press of Harvard University Press, Cambridge
Savulescu J (2001) Procreative beneficence: why we should select the best children. Bioethics 15(5–6):413–26
Savulescu J, Pugh J, Douglas T, Gyngell C (2015) The moral imperative to continue gene editing research on human embryos. Protein Cell 6:476–479
Scally A (2016) The mutation rate in human evolution and demographic inference. Curr Opin Genet Dev 41:36–43
Schaefer GO (2020) Can reproductive genetic manipulation save lives? Med Health Care Philos 23(3):381–386
Scheufele DA, Xenos MA, Howell EL, Rose KM, Brossard D, Hardy BW (2017) U.S. attitudes on human genome editing. Science 357(6351):553–554. https://doi.org/10.1126/science.aan3708
Scheufele DA, Krause NM, Freiling I, Brossard D (2021) What we know about effective public engagement on CRISPR and beyond. Proc Natl Acad Sci USA 118(22). https://doi.org/10.1073/pnas.2004835117
Scully JL (2008) Disability and genetics in the era of genomic medicine. Nat Rev Genet 9(10):797–802
Segers S, Mertes H (2020) Does human genome editing reinforce or violate human dignity? Bioethics 34(1):33–40
Sermon K, Van Steirteghem A, Liebaers I (2004) Preimplantation genetic diagnosis. Lancet 363(9421):1633–1641. https://doi.org/10.1016/S0140-6736(04)16209-0
Shakespeare T (2006) Disability rights and wrongs. Routledge, London
Shulman C, Bostrom N (2014) Embryo selection for cognitive enhancement: curiosity or game-changer? Glob Policy 5(1):85–92. https://doi.org/10.1111/1758-5899.12123
Silver LM (1997) Remaking Eden: cloning and beyond in a brave new world. William Morrow, New York, NY
Sinsheimer RL (1969). The prospect for designed genetic change. Am Sci, 57(1):134–142. http://www.jstor.org/stable/27828443
Smolenski J (2015) Crispr/cas9 and germline modification: new difficulties in obtaining informed consent. Am J Bioeth 15(12):35–37
Soini S (2007) Preimplantation genetic diagnosis (PGD) in Europe: diversity of legislation a challenge to the community and its citizens. Med Law 26(2):309–323
ADS CAS PubMed Google Scholar
Sorgner SL (2018) Genes, CRISPR/Cas 9, and posthumans. In: Sinaci M and Sorgner SL (eds.) Ethics of emerging biotechnologies, pp. 5–17. Trivent Publishing
Sparrow R (2021) Human germline genome editing: on the nature of our reasons to genome edit Am J Bioeth 19:1–12
Steffann J, Jouannet P, Bonnefont JP, Chneiweiss H, Frydman N (2018) Could failure in preimplantation genetic diagnosis justify editing the human embryo genome? Cell Stem Cell 22(4):481–482. https://doi.org/10.1016/j.stem.2018.01.004
Sykora P, Caplan A (2017) The Council of Europe should not reaffirm the ban on germline genome editing in humans. EMBO Rep 18:1871–1872. https://doi.org/10.15252/embr.201745246
UNESCO (1997) Universal declaration on the human genome and human rights. UNESCO, Paris
van Dijke I, Bosch L, Bredenoord AL, Cornel M, Repping S, Hendriks S (2018) The ethics of clinical applications of germline genome modification: a systematic review of reasons Hum Reprod 33(9):1777–1796
Vassena R, Heindryckx B, Peco R, Pennings G, Raya A, Sermon K, Veiga A (2016) Genome engineering through CRISPR/Cas9 technology in the human germline and pluripotent stem cells. Hum Reprod Update 22(4):411–419. https://doi.org/10.1093/humupd/dmw005
Venter JC et al. (2001) The sequence of the human genome. Science 291(5507):1304–51
Walters L, Cook-Deegan RM, Adashi EY (2021) Governing heritable human genome editing: a textual history and a proposal for the future. CRISPR J 4(4):469–476
Wang H, Yang H (2019) Gene-edited babies: what went wrong and what could go wrong. PLoS Biol 17(4). https://doi.org/10.1371/journal.pbio.3000224
Wheeler E, Barroso I (2011) Genome-wide association studies and type 2 diabetes. Brief Funct Genom 10:52–60
WHO (2019) Statement on governance and oversight of human genome editing. https://www.who.int/news/item/26-07-2019-statement-on-governance-and-oversight-of-human-genome-editing
WHO (2021) Human genome editing: position paper. https://www.who.int/publications/i/item/9789240030404
Wolf DP, Mitalipov PA, Mitalipov SM (2019) Principles of and strategies for germline gene therapy. Nat Med 25(6):890–897
Zhang F (2019) Development of CRISPR-Cas systems for genome editing and beyond. Q Rev Biophys 52. https://doi.org/10.1017/s0033583519000052
Download references
Acknowledgements
This work was supported by Fundação para Ciência e a Tecnologia (FCT) of Portugal [UIDP/00678/2020 to M.A]. We thank Dr. Michael Morrison for his comments and Dr. Gustav Preller for his proofreading of this manuscript.
Author information
Authors and affiliations.
Centro de Filosofia das Ciências da Universidade de Lisboa, Campo Grande, 1749-016, Lisbon, Portugal
Mara Almeida
Faculty of Health Sciences Brandenburg, University of Potsdam, Karl-Liebknecht-Str. 24-25, House 16, 14476, Potsdam, Golm, Germany
- Robert Ranisch
Research Unit “Ethics of Genome Editing”, Institute for Ethics and History of Medicine, University of Tübingen, Gartenstr. 47, 72074, Tübingen, Germany
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Mara Almeida .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical approval
An ethical approval is not applicable.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Almeida, M., Ranisch, R. Beyond safety: mapping the ethical debate on heritable genome editing interventions. Humanit Soc Sci Commun 9 , 139 (2022). https://doi.org/10.1057/s41599-022-01147-y
Download citation
Received : 06 August 2021
Accepted : 28 March 2022
Published : 20 April 2022
DOI : https://doi.org/10.1057/s41599-022-01147-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Clinical translation of wireless soft robotic medical devices.
- Tianlu Wang
- Metin Sitti
Nature Reviews Bioengineering (2024)
An analysis of different concepts of “identity” in the heritable genome editing debate
- Ying-Qi Liaw
Medicine, Health Care and Philosophy (2024)
Modular Ontologies for Genetically Modified People and their Bioethical Implications
- Robert Sladek
NanoEthics (2024)
The need to set explicit goals for human germline gene editing public dialogues
- Wendy P. Geuverink
- Diewertje Houtman
- Sam R. Riedijk
Journal of Community Genetics (2024)
Initial heritable genome editing: mapping a responsible pathway from basic research to the clinic
- Katharina Trettenbach
- Gardar Arnason
Medicine, Health Care and Philosophy (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

Genetic Engineering Essay & Paragraph

An Introductory Essay on Genetic Engineering for Students
By: Haque ; Words: 450; For class 9-12
Introduction: Genetic Engineering is a recent development of science. It is the artificial manipulation, modification, and recombination of DNA or other nucleic acid molecules in order to modify an organism or population of organisms.
Scope of Genetic Engineering: The term genetic engineering initially meant any of a wide range of techniques for the modification or manipulation of organisms through the processes of heredity reproduction. As such, the term included both artificial selection and all the interventions of biomedical techniques. Among them are artificial insemination, in vitro fertilization (e.g., “test-tube” babies), sperm banks, cloning, and gene manipulation. But the term now denotes the narrower field of recombinant DNA technology, or gene cloning, in which DNA molecules from two or more sources are combined either within cells or in vitro and are then inserted into host organisms in which they are able to multiply. Gene cloning is used to produce new genetic combinations that are of value to science, medicine, agriculture, or industry.
Development of Genetic Engineering: A big step forward in the development of genetic engineering was the discovery of restriction enzymes in 1968 by the Swiss microbiologist Werner Arber. In 1969, American molecular Biologist Hamilton O. Smith invented type II restriction enzymes. These enzymes are essential to genetic engineering for their ability to cleave a specific site within the DNA. This invention took the research of genetic engineering a long way. Based on Smith’s work, the American molecular biologist Daniel Nathans helped advance the technique of DNA recombination in 1970 -71 and demonstrated that type II enzymes could be useful in genetic studies. American biochemists Stanley N. Cohen and Herbert W. Boyer pioneered genetic engineering in 1973. They were among the first to cut DNA into fragments, rejoin different fragments, and insert the new genes into E. coli bacteria, which then reproduced.
Uses and Prospect of Genetic Engineering: Genetic engineering has advanced our understanding regarding many theoretical and practical aspects of gene function and organization. Using recombinant DNA techniques, bacteria have been created that are capable of synthesizing human insulin, human growth hormone, alpha interferon, a hepatitis B vaccine, and other medically useful substances. Plants may be genetically adjusted to enable them to fix nitrogen, to have resistance power against a hostile environment and certain diseases. Moreover, genetic diseases in living things can possibly be corrected by replacing “bad” genes with “normal” ones.
Conclusion: In spite of the endless prospect of genetic engineering, special concern has been focused on its achievements for fear that genetic engineering might result in the introduction of new unfavorable and possibly dangerous traits into micro-organisms. Such new traits may include resistance to antibiotics, production of toxins, or a tendency to cause disease. So, it is necessary to be cautious in the use of genetic engineering.
An Argumentative Essay on Pros & Cons of Genetic Engineering
Words: 1000 | For College Students | 10-06-’22
Genetic Engineering: Prospects and Hazards
Genetic engineering is when the genetic makeup of an organism is altered by inserting, deleting, or changing specific pieces of DNA. When conducting genetic engineering, the organisms that have their genetic makeup altered are referred to as genetically modified organisms or GMOs for short. During the process of genetic engineering, a piece or several pieces of DNA are altered to change a characteristic of the organism. If DNA is inserted, it can come from another individual with the desired characteristic, it can come from a different species or it could be artificially produced.
It is exhilarating to think of the transplantation of genes. The possibilities are endless. In the field of biotechnology, genetic engineering paved the way for xeno-transplantation, or the process of transplanting living tissues or organs from animals to humans or vice versa. The research revealed the possibility of using pig organs as replacements for human hearts and kidneys, considering that they have similar physiology and size. It also led to tissue engineering which is now considered an alternative to the replacement of cartilage, cerebrospinal shunts, heart valves, and other organs. Suffice to say that plenty of things can be achieved with genome editing.
Plants lead the list of items or species that are genetically modified. Companies that want to create a sweeter tomato, bigger cherries, and herbicide-resistant crops can do so through GM. There may be health and safety concerns attached to genetically engineered food and crops, but proponents as- sure that the breeding process is only an extension of the natural way. After all, the tissues used for the cell culture still come from a living organism. Because it is now possible to produce food and crops that are bigger and grow faster, resistant to disease, can thrive in different environments, or can be customized based on the soil composition and availability of water in a location, world hunger could be minimized if not completely eliminated. But there is still a question of what genetically modified crops can do to human bodies, their effects, and their long-term impact.
Gene mutation is a revolting concept. Imagine what the world would be like if diseases are taken out of the equation. If bad genes inherited from parents are detected and then removed, the next generation will be healthier and would live longer. Any genetic mutation caused by environmental mutagens may also be corrected through genetic engineering. When a human body is made less susceptible to infections, mutations can be kept under control, and diseases, such as cystic fibrosis and Alzheimer’s, would be no more. Genetic engineering enables researchers to isolate the exact gene that is causing diseases and illnesses, giving them insights into the cause and possible cures that can be initiated.
With the possibility of making the disease a thing of the past, everyone has higher chances of living a longer, fuller, healthier life. In fact, studies in genetic engineering showed that it has the ability to increase the life span of human beings anywhere between 100 and150 years, and this only involves slowing down the aging process by changing a healthy individual’s genome. Combined with other engineering processes, this can be more than possible. These include treating infectious diseases by implanting genes with antigen and antiviral proteins. Genetic engineering also allows desirable traits of certain organisms to be pinpointed and then integrated into others’ DNA. It also acts as an aid for genetics, enabling pharmaceutical companies to produce highly graded products that can help fight illnesses.
Another benefit of genetic engineering is realized in the production of valuable proteins. Recombinant DNA made possible the use of bacteria to produce proteins of medical importance. One such example is that of genetically engineered human insulin which is of great importance and is now marketed throughout the world.
Even scientists themselves believe that genetic engineering can have irreversible side effects, especially with hereditarily modified genes. After all, the process at the present uses viral factors to carry functional genes to the human body. Viral genes as they are, they are likely to leave certain side effects. Also, where the functional genes are placed in the genome is not exactly known. In the event that they replace other important genes instead of the mutated ones, other forms of diseases or health conditions are likely to develop. Is the world equipped to battle new illnesses that may turn out to be deadlier than ever?
Genetically modified wild rice is added with better carotene, which is needed by the human body to make vitamin A. This provides a perfect solution for vitamin A deficiency. Unfortunately, there are worries that GM organisms might actually be harmful to people. The added beta carotene levels aren’t high enough to even make a difference as well. By engineering plants to be resistant to pesticides and herbicides, we necessarily affect the web of life. Herbicide-resistant crops, on the other hand, may reduce the quantity of herbicide requirements, but it can lead to the growth of weeds that are resistant to herbicide and the loss of weed species that are essential to animal food and shelter. Suffice to say that modifying genes can have uncertain effects on humans and the environment.
In the case of transgenic biotechnology, blending animal and human DNA can have uncertain effects, including the creation of entities that possess degrees of intelligence or sentience atypical in non-human animals. Many also believe that there are health risks associated with genetically modified foods as well as in the experimental use of animals, long-term environmental impact, increased suffering of transgenic organisms, and the possible creation of new diseases.
Genetic engineering is likely to put biodiversity in danger because engineering specific traits into select species threaten the planet’s biodiversity by upsetting the natural balance. It will also adversely affect agriculture. Also, the promise to overcome worldwide hunger with the help of genetic engineering is not credible. There will be tough problems witnessed in patenting genetically modified items. A middle path is required to be put in place to accept genetic engineering for the betterment of the world.
Genetic Engineering Paragraph, 100 Words
By: Haque ; For class 9-10/SSC; 11-03-’22
Genetic engineering is one of the recent wonders of modern science. It is the manipulation, modification, and recombination of DNA or other nucleic acid molecules in an organism. Genetic engineering has opened the door to the mysterious world of genes. Using recombinant DNA techniques, bacteria have been created that are capable of synthesizing human insulin, human growth hormone, alpha interferon, a hepatitis B vaccine, and other medically useful substances. Plants can be genetically adapted so that they can fix nitrogen, withstand adverse environments and certain diseases. Even genetic diseases of humans, plants, or other organisms can be cured by replacing their “bad” genes with “normal” genes. In short, genetic engineering is a field of endless possibilities for us.
About the Author
A teacher, writer and blogger, started allparagraph noting students search online for paragraphs on various topics, short and simple essays , edifying stories and other materials of study . In composing these lessons we have tried to use as simple language as possible, keeping young students in mind. If you find any text inappropriate, please let us know so we can make it more useful through necessary corrections and modifications. Thank you!
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Adblock Detected!
Please help us run the website by disabling your ad blocker..

COMMENTS
A third argument is that genetic engineering will lead to vast social inequalities. This idea is expressed in the 1997 cult film Gattaca, which portrays a society where the rich enjoy genetic enhancements—perfect eyesight, improved height, higher intelligence—that the poor cannot afford. Therefore, the main character Vincent, a man from a ...
Example Of Argumentative Essay On Genetically Modified Plants. Genetically engineered organisms especially genetically modified plants and products derived from them have received debate of acceptance or rejection all over the world. The overall reaction is miscellaneous everywhere for the research, growth, production and use of GMOs.
Techné 9:2 Winter 2005 Chapman, Genetic Engineering: the unnatural argument / 84 'encoded' by the organism's DNA sequence: being able to change that DNA is the key to having control over what a living organism is. The rationale behind genetic engineering is that nature should be altered to suit
Considering the facts concerning genetic engineering, the benefits which can be achieved far outweigh the potential risks with regard to both the reduction in human suffering and the likely increase in lifespan. Get Help With Your Essay. If you need assistance with writing your essay, our professional essay writing service is here to help!
Genetic engineering opens new possibilities for biomedical enhancement requiring ethical, societal and practical considerations to evaluate its implications for human biology, human evolution and our natural environment. In this Commentary, we consider human enhancement, and in particular, we explore genetic enhancement in an evolutionary context.
Genetic Engineering. 2 pages / 835 words. Genetic engineering, also known as genetic modification, is the direct manipulation of DNA to alter an organism's characteristics (phenotype) in a particular way. It is a set of technologies used to change the genetic makeup of cells to produce improved or novel organisms.
Genetic Engineering Argumentative Essay. 938 Words 4 Pages. The turn of the century has ushered in many great inventions that have changed humanities lives for the better. Technologies such as virtual reality, smart phones and driverless cars. One of these up and coming technologies is genetic engineering. Genetic engineering, even though ...
2019. Playing with genes: The good, the bad and the uglyGenetic technologies1—the ability to manipulate and trans-form the properties of cells, seeds, microbes, insects, plants, animals and even ...
Argumentative Essay On Genetic Engineering. Decent Essays. 844 Words. 4 Pages. Open Document. Genetic Engineering. Genetic engineering has become increasingly normalized in today's society, and people are exposed to this technology now more than ever before. Most people are aware that food companies practice genetic engineering on their ...
Genetic Engineering and Eugenics Comparison. The main idea in genetic engineering is to manipulate the genetic make-up of human beings in order to shackle their inferior traits. The concept of socially independent reproduction is replicated in both eugenics and genetic […] The Film "Gattaca" and Genetic Engineering.
Genetic Engineering Argumentative Essay. Improved Essays. 713 Words; 3 Pages; Open Document. Essay Sample Check Writing Quality. Show More. Though there are many arguments on both sides as to whether or not genetic engineering is a beneficial or detrimental concept for society, I believe that overall, it is a noticeably important idea that ...
774 Words. 4 Pages. Open Document. Genetic engineering is "the ability to change human nature" (Fukuyama 671), to please parents. Genetic engineering is a way to change genes of children who have a disease or whose parents want them to have a certain appearance. When a person is expecting a child or wanting to have a child, what if they ...
Persuasive Essay On Human Genetic Engineering. 1069 Words | 5 Pages. Kevin Almazan Mrs. Hubbard Senior English March 12, 2018 Human Genetic Engineering Human Genetic Engineering is a great idea for scientists because they are able to give lots of impact on people's lives and the human race.
This paper explores the ethical considerations of gene editing (specifically germline) and genetic selection—including the hurdles researchers will face in trying to develop new technologies into viable therapeutic options. Keywords: ethical, ethics, gene editing, genetic selection. Go to: 1. BACKGROUND.
Persuasive Essay On Genetic Engineering Genetic engineering is currently a growing field in which people are obsessing over. This is new and upcoming technology that combines genetic and Nanotechnological enhancements, which completes the direct manipulation of DNA to alter an organism's characteristics in a particular way.
Genetic engineering has provided humans the ability to transform organisms by direct manipulation of genomes within a broad range of applications including agriculture (e.g., GM crops), and the ...
It has opened the door to the mysterious world of genes. Here is an introductory essay on genetic engineering for students in 450 words, is an argumentative essay on pros and cons of genetic engineering entitled 'Genetic Engineering: Prospects and Hazards', and finally is a genetic engineering paragraph in 100 words.
Genetic Engineering. Genetic engineering is when organisms are genetically modified through altering the base codes of DNA. This can be done through taking out the specific DNA strand that wants or needs to be changed and replacing that with another alike. But there are some complications to it like how do they know that the DNA they have ...
Genetic Engineering Persuasive Essay. In this rapidly changing world, scientists are creating new ways to improve the human race. They have turned to genetic engineering to accomplish the job. Genetic engineering is adding new DNA to an organism manually with the purpose of trying to produce traits that are not originally in the organism, and ...
Genetic Engineering Argumentative Essay. Superior Essays. 1573 Words; 7 Pages; Open Document. Essay Sample Check Writing Quality. Show More. Genetic engineering is the farthest humanity seems to have come in playing God. By purposely interfering with the code of life, the human species began an arduous journey in the hopes of enhancing human ...
Genetic Engineering Argumentative Essay. Decent Essays. 559 Words; 3 Pages; Open Document. The concern of agriculture and food production has been a concern even before the climate changes. The security of our agriculture production sustaining is worrisome. As a result of our concerns, genetic engineering provides a possible solution to improve ...
January 11th, 2017. Genetic Engineering Essay. Genetic engineering is a powerful and dangerous technology. Sometimes called genetic modification, genetic engineering is the process of altering the DNA in an organism's genome. Editing the sequence of nucleotides can sometimes lead to extreme harmful effects on the human race, while on the ...
Genetic engineering is the science of manipulating genes in some way. There are many different opinions on this subject. Genetic engineering could improve diseases or create a " designer child". On the other hand, it is not natural ,could have unintended consequences, and is sometimes not viewed as moral.