🚨🚨 Rapid Response Legal Fund: Your gift matched $1-for-$1 Donate
🚨🚨 Rapid Response Legal Fund: Your gift matched $1-for-$1
our Stories
October 21, 2021

The Clean Water Case of the Century

The nation's highest court sided with clean water advocates in a decades-long legal dispute involving a wastewater treatment plant, its pollution discharges, and a partially dead coral reef in Hawaiʻi.
“This decision is a huge victory for clean water,” said David Henkin , the Earthjustice attorney who argued the case before the U.S. Supreme Court.
Our Clients Hawaiʻi Wildlife Fund, Sierra Club-Maui Group, Surfrider Foundation, West Maui Preservation Association Read the Supreme Court Decision
In 2020, the U.S. Supreme Court issued its decision solidifying the Clean Water Act’s place as one of the nation’s most effective environmental laws.
The following year, in the first application of the Supreme Court's test, the Hawai‘i district court reaffirmed protections for the nation's waters.
What started as a local water pollution case could have had disastrous repercussions for clean water across the United States.
What did the U.S. Supreme Court decide?
The U.S. Supreme Court's decision leaves in place vital protections for the nation’s oceans, rivers, and lakes.
The court found that point source discharges to navigable waters through groundwater are regulated under the Clean Water Act. In its decision on County of Maui v. Hawai ʻ i Wildlife Fund , the court held that the Clean Water Act “require[s] a permit if the addition of the pollutants through groundwater is the functional equivalent of a direct discharge from the point source into navigable waters.”
In other words, the Clean Water Act prohibits unpermitted discharge of pollution “into navigable waters, or when the discharge reaches the same result through roughly similar means.”
In doing so, the Court rejected the Trump administration’s polluter-friendly position in the clearest of terms: “We do not see how Congress could have intended to create such a large and obvious loophole in one of the key regulatory innovations of the Clean Water Act.”
The opinion was written by Justice Breyer with a vote of 6-3; with Chief Justice Roberts joining the opinion, along with Ginsburg, Sotomayor, Kagan, and Kavanaugh. (Learn about what happens next with this case, following the Supreme Court's decision.)
Abigail Dillen, President of Earthjustice, explains what happened and what the ruling means:
It’s stunning to think how close we came to a world where industries could just point their pipes straight down into groundwater to dispense of their pollution indirectly into clean water without repercussion. — Abbie Dillen (@AbbieDillen) April 23, 2020
What happened during oral arguments at the U.S. Supreme Court?
Earthjustice attorney David Henkin presented oral arguments in November, before the nine Justices of the U.S. Supreme Court in County of Maui v. Hawaiʻi Wildlife Fund
The Justices posed tough questions to both sides. (Read the transcript.) A summary of the hearing:
- The County’s interpretation of the Clean Water Act is that a “point source” (such as a pipe) must be the thing that delivers pollution for it to be regulated. That, once in groundwater or not straight from the “point source,” the Clean Water Act does not regulate that discharge.
- A few Justices feared the County’s position would create a roadmap for polluters to evade regulation. Justice Breyer asked: What if we end the pipe five feet from the ocean?
- Justice Kagan doubled down, saying nobody would get a permit if they could cut the pipe a few feet short.
- But the Justices also asked Earthjustice attorney Henkin: What should be a definition for a limit to what is regulated? Would this interpretation mean homeowners' septic tanks that leach through groundwater to a river need to get permits under the Clean Water Act (or face stiff fines).
- Henkin explained that pollution that is “traceable” and a “proximate cause” would be regulated and that for three decades, U.S. EPA had gone with that interpretation without millions of homeowners on the hook for pollution from their septic tanks. ( More on the back and forth over the Justice’s questions on limits. )
- The County also deflected responsibility back to the states, saying state groundwater permitting, grant programs, etc., are sufficient to regulate that flows from a point source (such as Maui County’s wastewater wells) through groundwater and to a protected body of water.
- Justice Sotomayor interjected that that’s a problem because it presumes the state will regulate that pollution. Justice Kagan also stated that this case isn’t about relying on state backstops.
- The attorney for the U.S. Government made an analogy about spiking a punch with whiskey . Henkin deftly turned it back around.
The attorney for the U.S. Government made an analogy about spiking a punch with whiskey. Henkin deftly turned it back around. @Earthjustice ’s Sam Sankar explains here. pic.twitter.com/XZrQIjAQM3 — Earthjustice (@Earthjustice) November 6, 2019
- Justices generally seemed to reject the County's extreme position that only pollution direct from a point source is regulated, so pollution sprayed through air or that travels over ground would also be free of Clean Water Act regs. But they seemed unsure how far to go.
- At the end, Justice Sotomayor brought it home asking: What current regulations exist that stop the county from polluting the ocean? It’s definitely happening, and Maui County says the Clean Water Act shouldn't stop them — so are they just going to get away with it? What is being done to stop this?
. @Earthjustice attorney David Henkin gives his final thoughts on defending America's clean water in front of the Supreme Court, likely the biggest day of his career. #CleanWaterActIntact pic.twitter.com/b6k9I8nflc — Earthjustice (@Earthjustice) November 6, 2019
What’s County of Maui v. Hawai‘i Wildlife Fund about?
At its most basic level, this case was about whether a wastewater treatment facility in Maui is violating the Clean Water Act by polluting the ocean indirectly through groundwater.
Since the 1980s, Maui’s Lahaina wastewater treatment facility has been discharging millions of gallons daily of treated sewage into groundwater that reaches the waters off Kahekili Beach, a favorite local snorkeling spot. Depending on local geological conditions, groundwater, which is any water that exists beneath the land’s surface , can flow into major waterways like rivers, streams, and, in the Maui case, the ocean.
In 2012, after years of complaints from the community and unsuccessful negotiations with county officials over the destruction the pollution has caused to the reef and marine life, Earthjustice sued Maui County on behalf of four Maui community groups — Hawaiʻi Wildlife Fund , Sierra Club-Maui Group , Surfrider Foundation , and West Maui Preservation Association .
What is the legal history of this case, before it reached the Supreme Court?
Prior to the U.S. Supreme Court's Apr. 23 decision, two courts ruled in favor of Earthjustice and its clients. In 2016, the U.S. Environmental Protection Agency also agreed with the courts that Maui County was acting illegally.
The county doesn’t dispute that its wastewater pollution reaches the ocean.
Instead, it argued that the discharge of pollution from the facility’s wells does not require Clean Water Act permits because the pollutants do not flow directly into the Pacific Ocean, but indirectly through groundwater. Both the district court and the Ninth Circuit appeals court rejected the county’s claims.
“At bottom, this case is about preventing the county from doing indirectly that which it cannot do directly,” the Ninth Circuit ruled in 2018.
The District of Hawaiʻi court added in its 2014 ruling on the same issue that: “[Maui County’s claim] would, of course, make a mockery of [the Clean Water Act’s regulatory scheme] if [the] authority to control pollution was limited to the bed of the navigable stream itself. The tributaries which join to form the river could then be used as open sewers as far as federal regulation was concerned. No less can be said for groundwater flowing directly into the ocean.”
But Maui County wasn’t giving up. In Feb. 2019, it successfully petitioned the United States Supreme Court to hear the case, an act which now endangers clean water protections writ large.
On Sept. 20, 2019, the Maui County Council voted to settle County of Maui v. Hawaiʻi Wildlife Fund , a decision intended to avoid a standoff at the U.S. Supreme Court that could jeopardize clean water across the United States. But the County of Maui had to officially submit the paperwork to settle the case.
Why does this case matter beyond Maui?
If the Supreme Court had sided with Maui County and overturned the Ninth Circuit’s ruling , it would have allowed industry to freely pollute U.S. waters as long as the pollution isn’t directly discharged into a water source.
Over the past four decades, the U.S. EPA and states across the country have used their Clean Water Act authority to prevent a variety of industries — including wastewater treatment facilities, chemical plants, concentrated animal feeding operations, mines, and oil and gas waste-treatment facilities — from contaminating the nation’s waters via groundwater.
Industry groups are closely watching this case, and the list of groups that have filed amicus briefs to the county’s claims is a who’s who of polluters.
A Supreme Court decision reversing the Ninth Circuit’s ruling would have blown a hole in the Clean Water Act. It would have essentially allowed groundwater to “launder” pollution, allowing polluters to evade responsibility even if their waste contaminates clean water. This was the perverse logic underlying Maui County’s claim that it doesn’t need a permit as long as its pollution runs through the groundwater before reaching the ocean.
Earthjustice attorney David Henkin finds this contention “absurd.”
“According to Maui County, a polluter can avoid the law by taking a pipeline that discharges waste directly into the ocean and cutting it ten feet short of the shoreline,” Henkin said.
Instead of discharging waste directly into the ocean, the polluter is discharging waste onto the beach that then makes its way into the ocean.
“At the end of the day, the water is still polluted,” says Henkin. “And, under the county’s twisted logic, the polluter would get off scot-free.”
Who is on the county’s side?
The list of groups that support Maui County’s efforts to gut the Clean Water Act include Kinder Morgan , Energy Transfer Partners (the company behind the Dakota Access Pipeline ), the U.S. Chamber of Commerce, American Fuel & Petrochemical Manufacturers, National Mining Association, and industrial agricultural business organizations.
The U.S. EPA under the Trump administration has also done an about-face to side with these industries. In April, the agency reversed four decades of agency guidance that the Clean Water Act does regulate discharges of pollution that reach our nation’s waters through groundwater.
4,600 miles due east of Maui, gasoline is flowing into Browns Creek, South Carolina, via contaminated soil and groundwater. Kinder Morgan says that isn’t the corporation’s problem. Justice for the community could hinge on the outcome of this Supreme Court case. Read the story of the small town of Belton, Anderson County.
Who is on the side of clean water?
Eleven different groups that include former U.S. EPA administrators and officials from multiple administrations, 13 states, two counties facing similar pollution, a Native American tribe, craft brewers , law professors, aquatic scientists and scientific societies, and clean water advocates filed briefs in support of Earthjustice and its Maui community clients.
“As the amicus briefs vividly illustrate, this case pits those who are committed to the protection of life-giving, clean water against the Trump administration and polluting industries that want free rein to use groundwater as a sewer to dump their waste and toxic discharges into our nation’s lakes, rivers, and oceans,” Earthjustice attorney David Henkin says.
What happened after the Supreme Court decision?
The case went back to the Ninth Circuit, which then sent it back to the district court.
The next step in the case was for the lower court to decide whether Maui’s discharges meet the new test established by the Supreme Court: whether the sewage plant discharges to the groundwater, through which the sewage migrates inevitably and inexorably to the ocean a quarter mile away, are the functional of direct discharges to the ocean.
On Oct. 20, 2021, the Hawai‘i district court did just that — reaffirming protections for the nation's waters in the first application of the Supreme Court's Maui test.
The court denied the County of Maui’s request to reconsider the court’s Jul. 26, 2021, decision that the county must get a Clean Water Act permit for injection wells at the Lahaina Wastewater Reclamation Facility in West Maui.
“As the first court to apply the Supreme Court’s test, the court sent a strong message of hope to communities seeking to protect their oceans, rivers, and lakes from polluters like Maui County that are fouling those life-giving waters by using groundwater as a sewer,” said Earthjustice attorney David Henkin.
Why does this case matter to me?
Maui County’s argument was not only absurd, it was extremely dangerous. If the Supreme Court had ruled in the county’s favor, it would have jeopardized clean water across the country.
If you care about clean water, then you should care about this case.
At Earthjustice, we’re a nonprofit in the business of building a better future for our planet.
Which is why your support is so crucial.
We stand alongside hundreds of public-interest clients at the frontlines of the fight for a better today and tomorrow. Case by case, our lawyers face off against deep-pocketed interests — and we win.
Our lawyers measure success in clean air, clean water, and safeguards for communities across the country.
Every one of our clients gets top-tier legal representation, free of charge. We can’t keep fighting for our planet without your help . Whether you give $5 or $500, this will be the best investment you make today.
Established in 1988, Earthjustice's Mid-Pacific regional office in Honolulu focuses on environmental and community health issues, including ensuring water is a public trust and achieving a cleaner energy future.
Mid-Pacific Office
Established in 1988, Earthjustice's Mid-Pacific Office, located in Honolulu, Hawaiʻi, works on a broad range of environmental and community health issues, including to ensure water is a public trust and to achieve a cleaner energy future.
The legal case: Lahaina Injection Well
“Take a deep breath — but not too deep if you live near a coal ash dumpsite.”
The Stories to read on Air and Water
- Trump Prioritizes Allegiance over Qualifications with EPA Pick
- Trump Is More Prepared This Time. We Are Too.
- Take Action Urge the Environmental Protection Agency to authorize this crucial clean air rule today
What you need to know this week
- What I Wish I’d Known Before Getting a Tattoo
- Project 2025 Means More Environmental Injustice. We’ll Fight Back.
- Project 2025 Means Undoing Climate Solutions. We’ll Fight Back.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 22 September 2023
US drinking water quality: exposure risk profiles for seven legacy and emerging contaminants
- Ronnie Levin 1 ,
- Cristina M. Villanueva 2 , 3 , 4 , 5 ,
- Daniel Beene 6 , 7 ,
- Angie L. Cradock 1 ,
- Carolina Donat-Vargas 2 , 3 , 4 ,
- Johnnye Lewis 6 ,
- Irene Martinez-Morata 8 ,
- Darya Minovi 9 ,
- Anne E. Nigra 8 ,
- Erik D. Olson 10 ,
- Laurel A. Schaider 11 ,
- Mary H. Ward 12 &
- Nicole C. Deziel 13
Journal of Exposure Science & Environmental Epidemiology volume 34 , pages 3–22 ( 2024 ) Cite this article
21k Accesses
20 Citations
163 Altmetric
Metrics details
Advances in drinking water infrastructure and treatment throughout the 20 th and early 21 st century dramatically improved water reliability and quality in the United States (US) and other parts of the world. However, numerous chemical contaminants from a range of anthropogenic and natural sources continue to pose chronic health concerns, even in countries with established drinking water regulations, such as the US.
Objective/Methods
In this review, we summarize exposure risk profiles and health effects for seven legacy and emerging drinking water contaminants or contaminant groups: arsenic, disinfection by-products, fracking-related substances, lead, nitrate, per- and polyfluorinated alkyl substances (PFAS) and uranium. We begin with an overview of US public water systems, and US and global drinking water regulation. We end with a summary of cross-cutting challenges that burden US drinking water systems: aging and deteriorated water infrastructure, vulnerabilities for children in school and childcare facilities, climate change, disparities in access to safe and reliable drinking water, uneven enforcement of drinking water standards, inadequate health assessments, large numbers of chemicals within a class, a preponderance of small water systems, and issues facing US Indigenous communities.
Research and data on US drinking water contamination show that exposure profiles, health risks, and water quality reliability issues vary widely across populations, geographically and by contaminant. Factors include water source, local and regional features, aging water infrastructure, industrial or commercial activities, and social determinants. Understanding the risk profiles of different drinking water contaminants is necessary for anticipating local and general problems, ascertaining the state of drinking water resources, and developing mitigation strategies.
Impact statement
Drinking water contamination is widespread, even in the US. Exposure risk profiles vary by contaminant. Understanding the risk profiles of different drinking water contaminants is necessary for anticipating local and general public health problems, ascertaining the state of drinking water resources, and developing mitigation strategies.
Similar content being viewed by others
Cross-national challenges and strategies for PFAS regulatory compliance in water infrastructure

A national survey of lead and other metal(loids) in residential drinking water in the United States
Perspectives from the Society for Pediatric Research: contaminants of water and children’s health: Can we do better?
Introduction.
In 2010, the United Nations General Assembly explicitly recognized the human right to affordable and safe drinking water. The 20th and early 21st centuries saw major advances in the provision of reliable water to the developed and developing world. Achieving universal access to basic drinking water remains a critical global health goal. As access to drinking water becomes more widespread, concerns about chronic health issues from chemical contamination of drinking water provided through modern water systems become more paramount. Understanding the risk profiles of different drinking chemical contaminants is a necessary basis for assessing the state of drinking water resources.
In this narrative review, we begin with an overview of the configuration and governance of US public drinking water systems. For comparison, we briefly summarize the World Health Organization’s (WHO’s) approach to drinking water guidelines. To illustrate the variety of risks that may occur, we describe the exposure risk profiles of seven commonly-occurring chemical contaminants each affecting millions of Americans: arsenic, disinfection by-products, fracking-related substances, lead, nitrate, PFAS and uranium. We selected these contaminants to represent legacy (i.e., well-known contaminants that are generally regulated) and emerging chemicals (i.e., contaminants more recently identified as environmental health threats and often lacking regulations), threats to ground and surface water, issues of local and regional contamination, natural and anthropogenic sources, organic and inorganics, and those related treatment and distribution features or social determinants (that is, the nonmedical factors that influence health outcomes such as income, race or ethnicity, housing and employment and the wider set of forces and systems shaping the conditions of daily life). We conclude with a discussion of cross-cutting issues and challenges: aging and deteriorating water infrastructure; problems related to children, schools, and childcare settings; climate change; disparities in access to clean, reliable, safe drinking water; inconsistent regulatory enforcement; inadequate or outdated health assessments; preponderance of small systems; large numbers of substances within chemical groups; uneven enforcement of US drinking water standards; and disenfranchisement of Indigenous communities.
US public drinking water systems
In the US, there are about 150,000 public water systems (PWSs), i.e., those that serve at least 15 service connections or an average of at least 25 people for at least 60 days a year (Table 1 ). They may be owned publicly, by a governmental or semi-governmental entity, or privately. A community water system (CWS) serves the same population over the course of the year, while a noncommunity system, such as a restaurant or campground, serves different populations. CWSs account for one-third of PWSs (~49,600 of 150,000) but serve about 320 million Americans, approximately 95% of the US population.
CWSs are not evenly distributed across service-size populations (Table 1 ). Most CWSs (91%) are small-medium, serving under 10,000 people each, but together only serve 16% of the US population (52 million people), while the largest 9% of CWSs provide water to 83% of the US population (267 million people) [ 1 ]. Water source is not evenly divided between surface and groundwater systems; while 77% of CWSs are supplied by groundwater, they only serve 28% of the population (Table 2 ). In addition to the 320 million Americans served by PWSs for at least some of their water, >43 million people (~15% of the US population) rely on domestic (private) wells for residential drinking water [ 2 ]. This review focuses primarily on CWSs, including small and very small systems, but the large number of non-community systems and private wells poses additional and unique challenges. An added complexity with regard to exposure and health assessments is that many people are served by multiple water systems at home, work, school, and other locations; consequently, the total population served by PWSs in the US exceeds the estimated population.
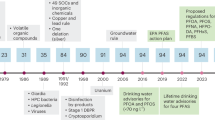
Under the US Safe Drinking Water Act (SDWA) [ 3 ], the US Environmental Protection Agency (EPA) sets legal limits for contaminants in public drinking water known as the Maximum Contaminant Level (MCL). For each regulated contaminant, EPA sets a health-based Maximum Contaminant Level Goal (MCLG), the level at which an adult can regularly consume drinking water over a lifetime with an adequate margin of safety. The MCL reflects the level closest to the MCLG that CWSs can feasibly achieve using the best available technology; the 1996 Amendments to the SDWA allow EPA to use cost-benefit analysis to set an MCL that is less stringent than is feasible. If EPA decides setting a numerical MCL for a contaminant is infeasible, EPA can issue a Treatment Technique instead. EPA has promulgated standards for about 100 contaminants in drinking water (Table 3 ) [ 4 ]. This is a small fraction of the approximately 700 identified disinfection by-products [ 5 ], 1200 chemicals reportedly used or produced by fracking [ 6 ], 14,700 PFAS [ 7 ], and other chemicals in commercial use. While not all of these compounds are likely to be present in drinking water, this suggests that EPA’s current regulatory structure may be missing many chemical contaminants of concern. EPA uses the Unregulated Contaminant Monitoring Program to collect occurrence data for up to 30 contaminants suspected to be present in drinking water, but that do not have health-based standards. Every five years, EPA reviews the contaminant list to determine if any should be considered for regulation.
Global drinking water standards
The WHO does not recommend a uniform international enforceable standard for drinking water. Instead, WHO advocates a local risk-benefit approach (qualitative or quantitative) for establishing national standards and regulations according to local needs and resources [ 8 ]. WHO therefore issues guidance for developing national and regional drinking water standards, recommends periodic review of these standards, and suggests that updates can be made readily. While WHO has issued guidelines for numerous drinking water contaminants, none are legal or enforceable standards, as WHO is not a regulatory body. However, many countries rely on WHO guidelines as the basis for their drinking water standards [ 8 ]. Countries and territories that specify their own parameter values for drinking-water quality do so in a variety of formats: regulations, standards, specifications, laws, decrees, requirements, and norms. With very few exceptions, the majority set regulatory values equal to or more stringent than the WHO Guideline [ 9 , 10 ].
Contaminant profiles
Sources and health effects.
Inorganic arsenic is a known human carcinogen causally associated with cancers of the skin, bladder, and lungs, and epidemiologic evidence supports a potential association with cancers of the kidney, breast, pancreas, and liver [ 11 , 12 ]. Chronic exposure is also associated with respiratory disease, cardiovascular disease, adverse birth outcomes, metabolic disorders and diabetes, impaired immunological functioning, kidney disease, and adverse neurocognitive outcomes [ 13 , 14 ]. Risk of some outcomes including cancer likely persist for chronic exposure to water arsenic at concentrations at or below EPA’s MCL of 10 µg/L [ 15 , 16 ].
Exposure risk profile
The significant spatial variability in water arsenic concentrations across the US reflects variability in geologic, biogeochemical, hydrologic, and climatic conditions that influence geogenic arsenic prevalence in bedrock and solubility, (e.g., arid oxidizing environments in the Southwestern US, and humid reducing environments and alkaline pH in the Northeast) [ 17 ]. Arsenic is detectable in over 50% of CWSs contributing to the EPA’s Six Year Review database (>36,000 CWSs) [ 18 ]. Approximately 2.6% of CWSs report arsenic concentrations exceeding the MCL (10 µg/L). An estimated 85% of PWSs rely on groundwater sources. Predicted arsenic levels in domestic wells are strongly associated with CWS concentrations using the same groundwater resources [ 19 ]. Hardrock mining processes and mine waste also contribute to surface and groundwater arsenic in the Southwest and Great Plains regions of the US, especially near Indigenous communities [ 20 ].
Significant sociodemographic and regional inequalities in CWS arsenic exposures have been identified across the US. In 2009–2011, mean arsenic concentrations were 1.70 µg/L nationwide and were highest in: systems serving communities categorized as Semi-Urban, Hispanic (3.40 µg/L); communities in the Southwestern US (3.18 µg/L); communities with less than 500 residents; communities reliant on groundwater sources; and incarcerated populations in the Southwest (2006–2011, mean 6.41 µg/L) [ 18 ]. At the county level, a higher proportion of Indigenous or Hispanic/Latino residents was associated with higher CWS arsenic concentrations, after adjustment for socioeconomic vulnerability [ 21 ]. A California study similarly found that higher proportions of Latino residents were associated with significantly higher CWS arsenic concentrations [ 22 ]. Similarly, a higher proportion of non-Hispanic Black residents was also associated with higher CWS arsenic concentrations in the Southwestern US, while concentrations were inversely associated with higher proportions of non-Hispanic White residents both nationwide and regionally. Higher county-level high school diploma attainment was associated with lower CWS arsenic concentrations; this association was modified by region, likely reflecting regional/local differences in socioeconomic context and drinking water infrastructure [ 23 ].
US regulatory context
Evidence from EPA violation records, routine compliance monitoring records, and urinary biomarkers of water arsenic exposure indicates that public water arsenic exposure declined significantly following the reduction of EPA’s MCL from 50 to 10 µg/L (effective 2006), consistent with the WHO guideline of 10 µg/L [ 18 , 24 , 25 , 26 ]. The largest exposure reductions occurred for Mexican-American residents (36% reduction, compared to 17% overall) and for CWSs with the highest baseline arsenic concentrations, including those serving New England (mean 37% reduction) and Alaska and Hawaii (mean 24% reduction).
EPA’s MCLG of 0 µg/L for arsenic reflects that there is no safe level of exposure to carcinogens, and mounting epidemiologic evidence supports that the current MCL is inadequate to protect human health. Denmark and the states of New Jersey and New Hampshire set more health protective MCLs of 5 µg/L, and water utilities in the Netherlands voluntarily adopted 1 µg/L. In setting the 5 µg/L MCL, the New Jersey Department of Environmental Protection (NJDEP) cited the National Academy of Sciences' 2001 report that a water arsenic concentration of 0.003 µg/L is estimated to result in a one-in-one-million excess lifetime risk of lung and bladder cancer [ 27 ]. NJDEP considered available testing and treatment technologies but not cost-benefit analysis [ 22 ]. Significant uncertainties and a lack of overall scientific consensus remain regarding the risk assessment for inorganic arsenic. The current EPA Integrated Risk Information System (IRIS) cancer slope factor for arsenic (1.5 per mg/kg bodyweight-day) relates to skin cancer only, while the 2010 proposed slope factor (25.7 per mg/kg bodyweight-day) corresponds to combined lung and bladder cancers and reflects increased susceptibility for women; synergistic effects for tobacco smoking are not considered in either [ 28 ].
Unique and shared challenges
Climate change poses significant challenges to reducing water arsenic concentrations, especially in the Southwest where water arsenic levels are already high. Wildfires and extended drought conditions caused by climate change are likely to concentrate arsenic and other inorganic contaminants as water levels decrease in groundwater [ 19 , 29 ]. Additional epidemiologic studies of drinking water arsenic at low- to moderate levels relevant for US populations, especially investigating cancer and cardiovascular disease, in diverse US populations would further inform risk assessment efforts.
Disinfection by-products
Disinfection of drinking water is necessary to prevent waterborne infections. However, disinfectants are highly reactive and interact with organic matter, bromide, nitrogenous compounds and other precursors to form unintended disinfection by-products (DBPs) [ 30 , 31 ]. DBP concentrations can vary substantially based on source water, disinfectant and treatment practice. Chlorine is a cost-effective disinfectant widely used worldwide, and trihalomethanes (THMs) followed by haloacetic acids (HAAs) are the most prevalent chlorination by-products. THMs comprise chloroform, dibromochloromethane, bromodichloromethane, and bromoform [ 32 ]. Chloroform is reported to be the dominant THM (up to 90%) in many areas worldwide, while brominated THMs are the more abundant species elsewhere, such as in Middle East countries, associated with high concentrations of bromide ions in raw water [ 33 ]. Local bromide discharges from industrial sources, including coal-fired power plants, oil and gas extraction activities, and textile mills, will impact DBP risks [ 34 ].
Alternative disinfectants may decrease THM formation but may encourage formation of other DBPs, such as chlorate and chlorite (from chlorine dioxide), nitrogenous DBPs (chloramines), bromate (ozone), iodinated and brominated aromatic DBP (chlorine dioxide). Chloramines are widely used in the US as an alternative to chlorine to reduce THM formation. Some of these DBPs show higher toxicity at lower concentrations than THMs and HAAs [ 33 ]. Among the ~700 identified DBPs, only 4 THMs, 5 HAAs, bromate, chlorate, and chlorite are currently regulated in the US and/or the European Union (EU) (Table 3 ) [ 35 , 36 ].
Long-term exposure to THMs, as a marker of DBP exposure, has been consistently associated with bladder cancer risk in epidemiological studies [ 37 ]. DBP exposure has also been linked to pregnancy and reproductive outcomes, but evidence is mixed [ 38 , 39 ]. The WHO International Agency for Research on Cancer has classified chloroform and bromodichloromethane as possible human carcinogens [ 40 ]. Iodinated DBPs are more toxic in mammalian cells than their chlorinated and brominated analogues [ 41 ]. Iodinated DBPs may be among the most genotoxic and cytotoxic DBPs, with iodoacetic acids potentially the most genotoxic of all DBPs [ 42 ]. Endocrine disruption and adverse reproductive and developmental impacts are also seen [ 43 , 44 , 45 ]. Some nitrogenous DBPs, including haloacetonitriles, haloacetamides, and halonitromethanes are more toxic and carcinogenic than THMs and HAA [ 43 , 46 ]. In particular, the toxicity of haloacetamides is estimated to be 142 times higher than HAAs [ 47 ].
DBP formation generally is higher in surface than groundwater systems due to higher levels of natural organic matter. The composition of organic matter and other water constituents in raw water affects which DBPs are formed. In particular, hydrophobic organic matter (e.g., high molecular weight organic materials) has higher potential to form THMs, nitrogenous DBPs and aromatic DBPs than hydrophilic organic matter. Occurrence of bromide and iodide ions promotes the formation of brominated and iodinated DBPs, respectively, while ammonia in water favors nitrogenous DBP formation [ 46 ].
Seasonal fluctuations (involving variations of surface water quality and water temperature) influence DBP formation. DBPs tend to show higher concentrations in summer than winter and certain DBP groups exhibit distinct seasonal patterns. Hydrophobic natural organic matter is positively correlated with air temperature whereas the hydrophilic natural organic matter shows a reverse trend [ 48 ]. Temperature increases the reaction rate between organic matter and chlorine, increasing THM formation. HAA formation, however, increases with temperature up to 20 °C then decreases. High temperatures also increase THM volatilization. Likewise, high pH enhances THM formation as well as the hydrolysis of other DBPs into THMs and HAAs, while low pH increases HAA formation [ 33 , 49 , 50 ].
THM and HAA formation increases with disinfectant dose and residence time in the distribution system, so distal parts of the distribution system generally have higher DBP levels. DBP exceedances occur more often in small systems, which have fewer resources for treatment to reduce DBP levels, such as filtering organic matter prior to disinfection [ 51 ]. DBP formation in the distribution system may be higher with polyethylene pipes [ 52 ] and with increasing diameter and pipe age [ 53 ].
Tap water is also used for showering, bathing, dishwashing and cooking. While exposure to non-volatile DBPs occurs predominantly through ingestion, exposure to volatile and skin-permeable DBPs (e.g., THMs) also occurs through dermal absorption and inhalation [ 32 , 54 , 55 ]. Exposure to THMs in swimming pools has been evaluated (measuring THMs in blood, exhaled air and urine), but the relative importance of different exposure routes remains inconclusive [ 56 ].
The pervasive presence of DBPs in drinking water poses significant concerns for human health. Even regulated DBPs lack comprehensive toxicological evidence, and while data indicate that some unregulated DBPs may pose greater risks that those currently regulated, the majority of emerging DBPs remain poorly understood. Furthermore, the potential interactions among the 700 identified DBPs in drinking water, have not been adequately examined individually or in mixtures. Synergistic effects of climate change (e.g., increasing temperature, more frequent and severe flooding and droughts), acidification of soil and surface water, land use change and other anthropogenic pressures all impact water quality and consequently, water treatment and DBP formation [ 57 ]. Precipitation and temperature are the main climate factors affecting water quality. The increase in atmospheric temperature and warming of surface waters is linked to eutrophication and increased microbial activity and dissolved organic carbon. Drought has been shown to significantly impact water chemistry, including higher levels of hydrophilic organic matter [ 58 ]. Ultimately, all these factors impact the concentration and composition of organic matter and consequently, DBP formation [ 57 ]. Aging and outdated water infrastructure and the large number of small systems are also challenges.

Fracking-related substances (Unconventional oil and gas development)
Unconventional oil and gas development (UOGD), commonly called “fracking”, is a method for extracting oil and natural gas from deep, low permeable geologic formations, requiring more intense stimulation compared to more accessible, conventional reservoirs [ 59 ]. In the US, there are approximately 150,000 active UOG wells, and more than 9 million people rely on drinking-water sources located within 1.6 km (1 mile) of a UOG well [ 59 ]. Water contamination from UOGD remains a major community concern [ 60 ].
Fracturing fluids and wastewater used or produced by UOGD may contain toxic, radioactive, endocrine-disrupting, and/or carcinogenic chemicals [ 6 , 61 ]. Potential water contamination events include surface spills of fracturing fluids or wastewater at the well site, release of improperly treated wastewater, and leaks in well infrastructure [ 62 ]. An estimated 1–4% of UOG wells have reported spills [ 63 , 64 ] and, based on Pennsylvania data, approximately 20% have a non-administrative violation [ 65 ]. These are uncommon events, and multiple groundwater monitoring studies conducted in regions with UOGD have not found evidence of widespread contamination [ 66 , 67 , 68 , 69 ]. However, specific instances of groundwater and surface water impairments have been identified [ 70 , 71 , 72 ].
Numerous epidemiologic studies have observed an increased risk of adverse health effects including adverse perinatal outcomes, childhood cancer incidence, hospitalizations, asthma exacerbations, mental health issues, and mortality in the elderly in relation to proximity to UOGD sites [ 59 ]. The extent to which these associations may be related to water is unclear, because most studies have used aggregate proximity-based metrics to assign exposures, which are not specific to any hazard [ 59 ]. A few epidemiologic studies have focused specifically on the drinking water exposure pathway. Two studies of pediatric health outcomes applied a novel water-pathway specific metric that restricts the analysis to UOGD wells that are hydrologically connected to the watershed of a residence [ 66 , 73 ]. This exposure metric is most relevant for groundwater. Another study found that proximity of community drinking water sources to UOGD wells was associated with greater likelihood of a variety of adverse birth outcomes [ 74 , 75 ]. More research is needed to understand whether the increased health risks observed in populations living in the vicinity of UOGD are attributable to drinking-water exposures, other hazards, or a combination of factors.
In the US, UOGD often occurs in rural areas where homes are served by private (domestic) drinking water wells, which are not subject to federal regulations and monitoring [ 76 ], or by small water systems. Therefore, available data are quite limited, and the data that are available tend to focus on the few chemicals that are regulated, which are only a tiny subset of the approximately 1200 chemicals reportedly used or produced by UOGD.
Although chemical disclosure policies have improved, researchers still lack a complete and consistent inventory of chemicals used in UOGD [ 77 ]. Furthermore, contaminants also include transformation products and naturally occurring chemicals mobilized during the development process [ 78 ]. Some researchers have mined existing voluntary reporting databases and created their own datasets [ 79 ].
Proximity to UOGD wells, often emphasized for sampling and in risk estimation, may be an inadequate surrogate for predicting exposure given geologic heterogeneity and topographical variations producing the complex flow paths and stochastic nature of contamination events [ 66 ]. Applying physically based hydrological models demonstrates that unlike the typical circular buffers used in exposure and epidemiologic studies, the groundwater capture zones exhibit more of a surfboard shape [ 80 ]; better modeling the capture zone could improve identification of homes more vulnerable to contamination and inform sampling locations. In addition, application of machine learning techniques to available monitoring data can help identify hotspots [ 81 , 82 ].
Finally, information on the locations and descriptions of where violations and spills occur is not available in real time and therefore timely sampling in response to a potential contamination event is not feasible. In addition, violations data are not available in a consistent format across states, posing another challenge to multi-state research. Further, chemicals disperse at varying rates (particularly in groundwater), so a single collected sample may be unlikely to coincide with release of a plume of multiple contaminants, potentially necessitating repeated measures.
Lead is a widely used element with thousands of applications. So closely associated with the conveyance of water, the very word ‘plumbing’ derives from its Latin name, plumbum. Lead is also highly toxic and associated with adverse health endpoints across virtually all body systems, including nervous, cardiovascular, renal, immunological, hematological and reproductive/developmental systems in men and women, in adults and children [ 83 , 84 , 85 ]. Lead has been classified as a probable human carcinogen by EPA since 1988 based on rodent toxicology data [ 83 ].
Lead is usually a corrosion by-product in drinking water related to water’s natural corrosivity and lead’s extensive use in plumbing components such as pipes, solder, brass and bronze [ 86 ], faucets [ 87 , 88 ], galvanized steel pipe coatings [ 89 , 90 ], valves and meters. Lead has been progressively restricted from plumbing use in the US during the past few decades, but its durability means that an estimated 9.2 million lead pipes installed in the late 1800’s to early 1900’s remain servicing US homes [ 91 , 92 ].And until banned in 1986 [ 93 ], lead solder joining copper pipes was practically ubiquitous in the US.
Studies evaluating multiple lead exposure sources from within the house found that, when present, lead pipes contribute the most lead to drinking water [ 94 ]. Lead pipes carrying water from the water main to the residence (lead service lines) and lead pipes inside homes were often installed in cities that expanded greatly during the Industrial Revolution [ 95 ] therefore home age and the history of the urban area are factors to consider. However, lead pipes continued to be installed in the US until banned in 1986, including locales such as Chicago that required lead service line installation until then. Beginning in the 1930s, copper pipes replaced lead pipes as the most common residential piping material; lead solder was used to join them. The combination of copper and lead produces galvanic corrosion that is associated with high lead leaching potential and elevated water lead levels (WLLs).
All water is corrosive, but the degree of corrosivity varies. Principal factors include pH, alkalinity and hardness of the water [ 96 ]. Seasonality is evident in WLLs [ 97 , 98 ]. The warmer the temperature the greater the potential for lead leaching; consumption of drinking water also increases in the summer.
EPA issued the Lead and Copper Rule (LCR) in 1991. Lead contamination of tap water relates mostly to water corrosivity and corrosion within the lead service line and residence; and water lead levels vary due to stagnation time, temperature, extent of lead plumbing components, and other factors. Citing some of these factors, EPA decided not to set an MCL for lead. EPA’s Lead and Copper Rule (LCR), instead, established a Treatment Technique requiring systems to control the corrosiveness of their water. If more than 10% of the tap water samples exceed the Action Level (AL) of 0.015 mg/L, water systems must take additional steps. The AL was based upon feasibility; it is not enforceable and exceeding it is not a violation of the SDWA. In January 2021, EPA revised the LCR [ 99 ] and almost immediately agreed to review it to address shortcomings [ 100 ]. The MCLG is zero based upon both lead’s carcinogenicity and that no safe level of exposure has been determined.
The SDWA only regulates PWSs. Homes with private wells, which are not covered under the SDWA, are less likely to have lead pipes than older urban areas. On the other hand, they may be more vulnerable to use of leaded solder and are less likely to use a corrosion inhibitor even with very acidic water; they may also have lead in their water pumps [ 101 ]. One study found that WLLs from private wells may be higher than those in adjacent public water systems [ 102 ].
Numerous studies show the clinical significance of exposures to even low WLLs across a range of effects. WLLs are associated with cognitive performance in children [ 103 ], renal function in dialysis patients [ 104 ], adverse birth outcomes [ 105 ], iron deficiency in patients with End Stage Kidney Disease [ 106 ], and the likelihood of juvenile delinquency [ 107 ]. Water-lead outbreaks are attested to raise general population BLLs [ 108 , 109 ] and BLLs are also associated with WLLs in non-crisis circumstances [ 110 ]. Violations of the LCR are common. The Government Accountability Office (GAO) reported that at least 10% of the water systems subject to the LCR had at least one open violation of the rule [ 111 ]. This is likely an underestimate as GAO has repeatedly found that EPA’s enforcement data are incomplete, especially related to compliance with the LCR [ 111 , 112 , 113 ]. This is consistent with EPA’s finding that only 8% of LCR treatment technique violations reported to states are passed on to EPA. Similarly, a 2016 report found that over 5300 CWSs serving an estimated 18 million Americans violated the LCR that year [ 114 ]. A later study found that 186 million Americans receive water from CWSs with detectable lead contamination [ 115 ]. A familiar pattern is evident in violations of the LCR in the US: minoritized and low-income communities bear an increased risk of receiving poorer quality drinking water and of having lead pipes [ 116 , 117 , 118 ].
Climate change effects include the acidification of the natural world, reducing the pH in air, water and soil. Lower pH is associated with increased lead mobility and bioavailability [ 119 , 120 , 121 ]. Hence, climate change and global warming may increase water lead levels both through thermal and biochemical mechanisms as well as increasing lead’s mobility and bioavailability. Global warming will likely increase water consumption, also.
Disparities exist in lead exposure, enforcement issues and problems with school drinking water. US lead exposures from drinking water appear to be widely underestimated related to systematic poor monitoring, reporting and enforcement [ 122 ]. Less data are available on lead contamination of drinking water outside the US, but there is evidence of underestimation of lead contamination in EU drinking water, also [ 123 ]. The WHO provisional guideline value is 10 μg/l, set in 2011; sampling protocols differ between the US and WHO [ 124 ]. Until the remaining 9.2 million lead pipes are replaced and effective corrosion control is widely adopted, drinking water will remain a significant lead exposure source.
Nitrate in drinking water
Nitrate levels in water resources have increased worldwide from applications of inorganic fertilizer and animal manure in agricultural areas [ 125 ]. Contamination sources also include septic systems that do not effectively remove nitrogen and discharges from wastewater treatment plants [ 126 ], as well as atmospheric deposition of nitrogen oxides and fertilizer use on lawns, golf courses, and parks.
Private wells typically have higher nitrate concentrations than CWSs due to their shallower depth. High nitrate concentrations (near/exceeding the MCL) are most common in shallow (<100 feet) private wells located in agricultural areas because of nearby nitrogen sources (fertilizer use, animal operations, septic systems) [ 127 ]. Treatment of private wells is the responsibility of the property owner, leading to racial/ethnic, rural/urban, and socioeconomic disparities in access to safe drinking water. Some states provide resources, such as subsidized water test kits, to private well users. However, state-level regulation of private wells varies dramatically, and private well users are often unaware of the resources available to them [ 128 ].
A 2019 study [ 129 ] evaluating nitrate exposures in US CWSs estimated that about 5.6 million people were exposed to water with ≥5 mg nitrate-nitrogen (NO 3 -N)/L (more than half the MCL of 10 mg NO 3 -N/L) between 2010 and 2014. Hispanic/Latino residents were more likely to be served by CWSs with elevated nitrate levels. Disproportionately high exposure to nitrate-contaminated water among Hispanic/Latino communities has also been identified in the Yakima Valley of Washington State and San Joaquin Valley of California, among other areas [ 130 ]. Additional research has documented cases of poor water quality, including high nitrate concentrations, in drinking water used by migrant worker communities, Alaska Native villages and other Tribal lands, and in colonias along the US-Mexico border [ 130 ]. Limited measurement data characterizing residents using private wells presents challenges but recent advances in exposure modeling have proved useful for identifying exposure disparities [ 131 , 132 , 133 ].
US regulatory context and health concerns
The US recommended standard for nitrate in drinking water was originally set in 1962 by the US Public Health Service as 10 mg NO 3 -N/L, based on infant methemoglobinemia. EPA’s subsequent MCL only considered this outcome and was based on a no-adverse-effect concentration for drinking water used to prepare formula for infants <6 months of age with no margin of safety; other health effects were not considered [ 134 , 135 ]. The MCL has not been revised since it was promulgated in 1975 [ 135 ]. The literature investigating the health effects of nitrate exposure has expanded greatly since the MCL was set [ 136 ]. Nitrate ingested from drinking water may increase the risk of birth defects and some cancers because nitrate is a precursor in the formation of N-nitroso compounds (NOC), many of which are teratogens and carcinogens. NOC are formed in the body (a process called endogenous nitrosation) when nitrate is consumed in the absence of antioxidants that inhibit their formation [ 137 ]. Among epidemiologic studies with individual-level data, seven studies (in Australia, Canada, California, Texas, and Denmark) found increased central nervous system (CNS) malformations in children whose mothers consumed drinking water with high nitrate concentrations during pregnancy. In six studies, the increase in CNS malformations occurred at levels below the MCL [ 136 , 138 , 139 , 140 ]. Studies of spontaneous abortion, fetal growth, and birth weight have been more limited and had mixed results [ 136 , 139 , 140 ].
In 2006, the International Agency for Research on Cancer (IARC) concluded that when ingested under conditions that result in endogenous nitrosation, nitrate and nitrite are probably carcinogenic to humans [ 137 ]. Since the IARC review, there have been more than 20 studies of incident cancer mostly in the US and Europe. Colorectal cancer is the most well-studied, with four of five studies finding increased risks [ 136 , 141 ]. Studies of other incident cancers were fewer; however, positive associations at levels below the MCL were observed for cancers of the bladder [ 136 , 142 ], kidney [ 136 ], childhood and adolescent/young adult brain [ 143 , 144 , 145 ], ovary and thyroid [ 136 ].
Unlike nitrate in drinking water, nitrate naturally present in food (mostly in fruits and vegetables) is consumed together with antioxidants, vitamins and polyphenols that inhibit endogenous nitrosation [ 146 ]. In controlled longitudinal feeding studies, high nitrate intake through consumption of high-nitrate vegetables such as beets and dietary supplements has been shown to reduce hypertension and may play a role in the protective effect of vegetables on cardiovascular disease risk [ 147 , 148 ]. However, hypertension and nitrate ingestion through drinking water sources has not been studied. Clinical and subclinical hypothyroidism have been linked to higher nitrate intake from drinking water in several studies [ 136 ] and deserve further study.
Additional studies of cancers, thyroid disease, and birth outcomes/defects that have shown the most consistent associations with drinking water nitrate are needed to further elucidate risks below the MCL. Evaluating subgroups with higher endogenous nitrosation will improve inference. Methods for quantifying the nitrate-reducing bacteria in the oral microbiome and characterizing genetic variation in N-nitroso compound metabolism hold promise for identifying these high-risk groups in epidemiologic studies.
Over the past several decades, nitrate levels in many ground and surface waters increased despite efforts to reduce nitrogen inputs [ 127 ]. Nitrate concentrations are expected to increase in aquifers used for drinking water as the contamination in shallow groundwater moves to deeper aquifers [ 127 ]. Disparities in exposure, an outdated health assessment, and large numbers of contaminated small systems and private wells are among the shared challenges related to nitrate. Efforts to understand the disproportionate impacts of nitrate exposure will help inform future policy and regulations to limit sources of nitrate contamination, decrease exposure, and alleviate public health harms.
PFAS (per- and polyfluoroalkyl substances)
Per- and polyfluoroalkyl substances (PFAS) are a major class of contaminants of concern in drinking water across the US and globally. PFAS as a class are generally considered “emerging” although some individual chemicals have state-level MCLs or have been phased out of commercial production may be classified as “legacy” pollutants. PFAS exposures are widespread; according to the National Health and Nutrition Examination Survey (NHANES), over 98% of the US population has detectable levels of PFAS in their blood [ 149 ]. In areas with contaminated water supplies, drinking water is a major contributor to PFAS exposure, although exposures can also come from diet, consumer products, and building materials. Dubbed “forever chemicals” because of their extreme persistence in the environment, PFAS also raise concerns due to far-reaching toxicity and bioaccumulation potential, with certain long-chain PFAS having human half-lives in blood on the order of years [ 150 ]. Exposures to some PFAS have been associated with adverse health outcomes across many major systems in the body, including immunotoxicity, dyslipidemia, changes in thyroid hormone levels, decreased birth weight, and testicular and kidney cancer [ 151 , 152 , 153 ]. Much of the available toxicological and epidemiological evidence is based on a relatively small number of long-chain PFAS (often those considered “legacy” PFAS), especially perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), which have been phased out of production in the US and Europe, but reportedly are still being produced internationally and imported into the US in consumer goods [ 154 ]. However, a growing body of evidence raises concerns about the toxicity of “emerging” alternative PFAS being used as replacements [ 155 , 156 ].
The highest levels of PFAS in drinking water have been found close to industrial facilities where PFAS are manufactured or processed and sites with discharges of aqueous film forming foam (AFFF) at military bases, major airports, and other fire training areas [ 157 ]. PFAS are widely used in consumer items, such as stain-resistant carpets and upholstery, food packaging, apparel, and cosmetics. An increasing number of PFAS contamination sites have been linked to waste disposal, including land-applied biosolids, effluent from wastewater treatment plants and septic systems, and landfill leachate [ 158 ].
PFAS have increasingly been detected in PWSs as analytical sensitivity has improved and testing has become more widespread. In the third round of EPA’s Unregulated Contaminant Monitoring Rule (UCMR3) in 2013–2015, which included six (mainly legacy) PFAS compounds, only 4% of PWSs reported detections above minimum reporting limits (MRLs) [ 157 , 159 ]. However, this testing greatly underestimated the extent of PFAS in PWSs as the MRLs were relatively high (10–90 ng/L) and most smaller PWSs (≤10,000 customers) were not included. A more recent analysis estimated that 18–80 million Americans are served by PWSs delivering ≥10 ng/L of PFOS and PFOA and that the water of 200 million Americans has concentrations of PFOS and PFOA ≥ 1 ng/L [ 160 , 161 ]. In 2023, EPA estimated that from 70 to 94 million people in the US are exposed to six PFAS of concern in their drinking water at elevated levels [ 162 ]. Private wells also can be vulnerable to PFAS contamination, even in areas without industrial activity [ 126 ] but little testing has been conducted on private wells [ 163 ]. PFAS contamination of drinking water supplies is emerging as an environmental justice concern. A recent analysis of monitoring data from 7873 CWSs in 18 US states found that detection of several PFAS is positively associated with the number of PFAS sources and proportions of people of color (Hispanic/Latino, non-Hispanic Black) who are served by these water systems. There are also disparities in the extent of PFAS testing; a smaller proportion of Tribal PWSs were included in the UCMR 3 testing compared to non-Tribal PWSs, and this difference will likely persist in the UCMR 5 testing currently underway [ 164 ].
Understanding the characteristics of PWSs most likely to have PFAS can help prioritize PFAS testing in areas where drinking water is most vulnerable. In the UCMR 3 testing, detection frequencies were twice as high among PWSs that relied on groundwater sources compared to surface water, although this testing found that short-chain PFAS were more frequently found in surface water systems [ 157 ]. These patterns vary in different regions of the world; nationwide testing of Swedish drinking water found that detection frequencies in surface water systems were twice as high as for groundwater systems [ 165 ]. In a 2022 study of groundwater in the eastern US, PFAS were detected in 60% of public supply wells and 20% of private wells, and PFAS detections were correlated with nearby urban land use, tritium (a marker of recent recharge), volatile organic compounds, and pharmaceuticals [ 166 ].
In the absence of enforceable federal drinking water standards, a regulatory patchwork emerged as some individual states adopted their own regulations. In 2016, EPA lowered its non-enforceable health advisories for PFOA and PFOS from 400 ng/L and 200 ng/L, respectively, to 70 ng/L for the two compounds individually or combined. Between 2016 and 2022, 18 US states adopted health advisories or enforceable standards at levels lower than 70 ng/L for PFOA and PFOS and/or for other PFAS compounds, mainly in the range of 10–20 ng/L for several PFAS compounds, individually or combined [ 167 ]. In 2022, EPA issued drastically stricter interim health advisories of 0.004 ng/L for PFOA and 0.02 ng/L for PFOS, noting “the levels at which negative health outcomes could occur are much lower than previously understood,” and finalized health advisories for two other PFAS [ 168 ]. In March 2023, EPA issued long-awaited draft MCLs of 4 ng/L for both PFOA and PFOS (individually), reflecting the “lowest feasible quantitation level.” [ 162 ] At the same time, EPA also issued a third MCL for four additional PFAS (PFHxS, PFNA, PFBS, and GenX chemicals) using a Hazard Index value of 1. The Hazard Index approach is routinely applied in risk assessment settings, but this represents the first proposed use for setting drinking water MCLs and represents a step towards more of a class-based approach by moving beyond a one-at-a-time approach to limiting PFAS in drinking water.
PFAS pose numerous challenges for drinking water providers and regulators. With an estimated 14,700 compounds classified as PFAS [ 7 ], the full extent of PFAS in water is likely underestimated by current analytical methods, which typically target only 20-30 compounds. The fifth cycle of UCMR (UCMR 5) will include 29 PFAS, including a range of both legacy and newer alternative PFAS [ 169 ]. Methods to estimate total PFAS (e.g., extractable organofluorine) or that target certain precursor compounds (e.g., total oxidizable precursor assay) are not widely applied to drinking water. Although thousands of water systems have discovered PFAS contamination, the full extent is unknown as testing has been inconsistent [ 160 ].
The cost of PFAS monitoring and treatment itself places substantial financial burdens on PWSs, especially those serving small and low-income communities. For example, the Hyannis Water System in Barnstable, MA, which serves 14,000 customers and includes environmental justice neighborhoods, has spent over $20 million to install granular activated carbon (GAC) treatment on 11 groundwater wells and will incur annual operating costs of $800,000 [ 170 ]. The cost for some large water systems could exceed $1 billion over time [ 171 ]. The most common PFAS treatment methods used by PWSs (GAC, ion exchange, reverse osmosis) are non-destructive, creating substantial quantities of contaminated filter media and wastes. High-temperature incineration under carefully-controlled monitoring and conditions has been reported in limited studies to largely break down PFAS and regenerate GAC filter media for reuse [ 172 ]. However, additional in-depth study is needed to confirm the efficacy of incineration/regeneration at fully destroying PFAS in spent GAC media under a variety of conditions, and use of such incineration has raised concerns about the sustainability of long-distance transport of spent GAC and air emissions from incineration potentially exacerbating exposures in environmental justice communities. New destruction methods such as super critical water oxidation are currently not commercially available, but in the future may provide a path forward to treat contaminated media and waste [ 173 ].
Many scientists, regulators, and advocates support class-based approaches to restricting PFAS [ 174 ]. Others, including industry representatives, have argued that applying a class-based approach to PFAS in drinking water is complicated by a lack of toxicity data for many PFAS and the different potencies of individual PFAS compounds. Shared challenges also include the lack of health assessments and occurrence data for the enormous class of PFAS that continues to grow literally daily. Finally, addressing PFAS contamination will require a concerted effort to limit PFAS manufacturing to avoid new sources of PFAS and new regulatory approaches that assess individual and combinations of PFAS, enhanced and more widespread testing to understand the true scope of water contamination, and development of new remediation methods to degrade PFAS without creating new exposure risks.
Uranium (U) occurs naturally in the earth’s crust, with water contamination resulting from geochemical processes. Exposure to uranium in the US population is widespread; 74% of NHANES participants from 2001 to 2010 had detectable concentrations of U in their urine. Urine is the preferred biomarker to assess chronic exposure in populations with constant exposures, as previous studies have identified a good correlation between urine U and environmental U in water, air and food [ 175 , 176 , 177 , 178 ]. Drinking water remains the main route of U exposure in the US [ 179 ].
In the human body, uranium is rapidly distributed and accumulates in the bone and the kidneys, which are the main target organs that have been used in determining chemical toxicity for water standards [ 180 ]. Alpha radiation from uranium decay is classified as carcinogenic, and increasing epidemiological evidence shows that exposure to uranium in its metallic form is associated with chronic kidney disease, as well as neurologic, reproductive and cardiovascular toxicity [ 181 , 182 , 183 ]. While most epidemiologic studies have been conducted in occupational populations with high levels of exposure [ 184 , 185 ], recent work on community level exposures in Indigenous communities exposed to uranium mine waste has identified more sensitive endpoints including cardiovascular disease and immune dysfunction [ 186 , 187 ], and is supported by laboratory and animal uranium exposure model studies [ 188 , 189 ]. Prior development of drinking water standards incorporated the long-held assumption that ingested uranium is very poorly absorbed from the gut. However, more recent studies suggest the higher resulting exposure of regulatory immune cells lining the gut may still result in central dysregulation of the immune system [ 190 ]. Thus, additional epidemiological studies are needed to better understand and characterize the adverse health effects of uranium drinking water exposure at the moderate and low exposure levels common in the general population, as well as to identify vulnerable subpopulations.
Uranium mobilization is influenced by the redox environment and increases in oxic groundwaters and in the presence of carbonate complexes, which can lead to the persistence of uranium in drinking water even after treatment to remove chemical contaminants [ 191 , 192 ]. U is consistently found co-occurring with other metals in groundwater, mostly arsenic and selenium [ 193 , 194 , 195 , 196 ]. Anthropogenic activities, including mining of uranium ore, producing phosphate fertilizers, and military operations, can lead to increased uranium contamination in drinking water [ 177 , 181 , 197 ]. Depleted uranium (DU), a radioactive byproduct obtained from uranium enrichment and primarily derived from human activities, is one of the contributors to increased U exposure through drinking contaminated water and inhalation of DU aerosols. DU is used for the production of military and hospital equipment and has a half-life of millions of years [ 177 ]. Exposure to DU has been associated with renal, neurological and adverse developmental effects in previous observational studies [ 198 , 199 ]. As a result, U concentrations in water supplies and air are highest in regions with natural geogenic uranium presence and redox conditions that facilitate its release, as well as intense mining, industrial, and military activities.
U is widely detected in private domestic wells and CWSs across the US. According to data from the US National Water Information System (NWIS), 50% of domestic wells in the US have detectable concentrations of U, with ~4% of wells exceeding the EPA MCL of 30 µg/L [ 200 ]. Uranium is also detected in 63.1% of regulated CWSs, with ~2% of CWSs exceeding the MCL [ 193 ]. Nationwide, CWSs reliant on groundwater have higher mean U concentrations (4.67 µg/L), compared to those reliant on surface water (1.79 µg/L) [ 193 ]. Of the 161,000 abandoned hard rock mines in the Western US states, U was the second highest prevalence of primary ore mined, creating the expectation of regionally higher concentrations of uranium in those regions, regardless of surface or groundwater sources, resulting from both anthropogenic contamination and natural mineralization [ 20 ].
Several sociodemographic and geographical inequalities in U concentrations at the CWS level have been documented. Nationwide, the mean concentration of uranium in 2000–2011 was 4.4 µg/L [ 193 ]. Higher mean concentrations of uranium were detected in CWSs serving populations less than 500 (5.04 µg/L), those serving communities characterized as “Semi-Urban Hispanic” (10.04 µg/L), and CWSs serving communities in the Central Midwest (8.04 µg/L) and Southwest (9.13 µg/L) regions [ 193 ]. At the county level, a recent nationwide study identified that higher proportions of residents who self-identify as Hispanic/Latino, American Indian and Alaskan Native were associated with higher uranium in CWSs, after adjustment for income and education [ 201 ]. Previous studies in the Navajo Nation have documented higher concentrations of uranium and other toxic metals in drinking water sources compared to the rest of the US [ 194 , 195 , 196 ]. At least 12% of unregulated water sources in the Navajo Nation have uranium levels above the MCL of 30 µg/L [ 202 ]. Consistent with the exposure data, epidemiological studies have identified 2-3-fold higher urinary levels of uranium among Navajo people compared to the general population levels documented in NHANES [ 203 ], and urinary uranium concentration among pregnant women living on Navajo Nation in the Southwest are 2.67 to 2.80 times higher compared to the general US population [ 156 ]. Exposures related to private wells are a key challenge.
Cross-cutting exposure issues
The array of exposure risk profiles for these seven different but widespread contaminants reveals a constellation of common elements, listed in Table 4 and discussed below.
Aging, deteriorating water infrastructure
Much US water infrastructure was first installed in the late Victorian period when the influx of workers to cities demanded an enormous housing boom. It is now over 100 years old, and some is closer to 150 years old. Even much of the infrastructure installed later into the 20 th century is now past its design life. The American Society of Civil Engineers gave America’s drinking water infrastructure a “C-” grade, highlighting a water main break every two minutes and an estimated 6 billion gallons of treated water lost each day [ 204 ]. Pipe breaks and leaks also reduce water pressure potentially causing back-siphoning of bacteria and other contaminants into the system [ 205 ]. Unlined cast iron especially can be plagued by biofilms that can harbor pathogens if not carefully maintained [ 205 , 206 ]. Additionally, EPA recently estimated that there are 9.2 million lead service lines nationally [ 92 ]. The lack of funding and prioritization for replacing these lead pipes has resulted in a slow pace of replacement posing public health risks particularly to vulnerable populations such as inner-city children [ 115 , 207 , 208 ].
Most US drinking water treatment plants provide conventional treatment including coagulation, sedimentation, sand filtration, and chlorination. EPA’s most recent Community Water System Survey found that less than 10% of drinking water treatment plants use modern technologies such as ion exchange, granular activated carbon (GAC), ozone, UV disinfection, or membranes [ 209 , 210 ]. Half of the groundwater-supplied water treatment facilities provide no treatment other than disinfection [ 209 , 210 ].
Deteriorated and outdated infrastructure can present health risks because it can introduce contaminants into the water (e.g., with lead service lines or DBPs), the impaired integrity of distribution system pipes can allow for contamination and recontamination. Outdated or poorly maintained treatment also may be inadequate to meet the challenges of contaminated source waters and may introduce contaminants into finished waters. This article documents that millions of US residents consume drinking water containing chemical contaminants that often may pose significant health risks ranging from cancer to neurological disease and other sequelae. In addition, while we have not examined microbiological risks from drinking water in detail, the US Centers for Disease Control and Prevention (CDC) and others have estimated that 4-32 million cases of gastrointestinal illness each year are waterborne [ 211 , 212 , 213 ], associated with emergency department visits, hospitalizations, and deaths, and incurring billions of dollars in direct healthcare costs; the precise contribution of drinking water to these illnesses is sometimes difficult to confirm [ 214 ]. Aging infrastructure is a major risk for microbial contamination due to broken and leaking pipes, poor water pressure, uncontrolled biofilms, etc.
Fixing these challenges will be expensive. EPA’s most recent assessment, published in 2023, estimated that $625 billion is needed to maintain and improve the nation’s drinking water infrastructure over the next 20 years [ 92 ]. This may be a substantial underestimate; the American Water Works Association estimated that repairing, updating and replacing crumbling drinking water infrastructure will cost at least $1 trillion over the next 25 years [ 215 ].
As Table 1 shows, 91% of CWSs serve under 10,000 people. Special challenges arise for small and rural water systems, and particularly those serving disadvantaged communities, as they have limited resources and often lack the technically-trained staff and the economies of scale to address system problems. The condition of water infrastructure relates largely to social determinants of health.
Children, schools and childcare settings
Children, particularly those who are very young, are especially vulnerable to many contaminants commonly found in drinking water such as lead, arsenic, and nitrates [ 216 ]. Contamination can occur due to source water contamination, water delivery infrastructure or plumbing components containing lead, and/or inadequate water treatment, testing, and remediation practices. Some exposures can have severe and long-lasting health consequences [ 216 ]. Nationally, 1.5–2% of households are likely to have elevated concentrations of metal(loids), including lead, arsenic, and copper, in their drinking water [ 217 ].
In 2019, more than half of US children aged 5 or under (59%) not enrolled in kindergarten were in a non-parental care arrangement, where they are likely to consume water or food prepared on-site. Most of these children were cared for in a day-care, preschool, or similar facility (62%) or received care in a private home (20%) [ 218 ]. Additionally, nearly 50 million students attend school in the US, of which, in 2021, the majority (49.5 million) were in public school systems [ 219 ]. Schools that have their own water systems are regulated under Federal laws [ 36 ]. A GAO report found that 18% of reported exceedances of the Action Level occurred in schools and day care centers with their own water supplies; GAO considers this an underestimate [ 111 ].
However, most schools (89%) obtain their drinking water from a CWS [ 220 ]. Oversight and testing for contaminants in drinking water in educational settings like schools or childcare settings have historically been left to the states [ 218 , 221 ]. Lead concentrations are strongly related to how long the water has stagnated, so schools and childcare facilities -- where the water can sit in pipes for 12 or more hours overnight and longer on weekends, holidays, and vacations – can present high potential exposure risks. Data collected in schools in US states and the District of Columbia have shown detectable levels of lead in school drinking water, including several exceedances of the Action Level of 15 µg/l [ 222 , 223 , 224 ]. Testing in 4005 childcare facilities in North Carolina found at least 1 tap water source exceeded 1 µg/l and 10 µg/l at 56% and 12% of facilities, respectively [ 225 ]. Reliance on well water may also be a risk factor for elevated lead concentrations in some educational facilities [ 225 ].
Several national efforts are underway to address lead in drinking water in educational settings. In 2016, as part of the Water Infrastructure Improvements for the Nation (WIIN) Act, a program was authorized to make funding available to states, territories, and Tribes to assist local agencies in voluntary testing for lead contamination in drinking water at schools and childcare facilities. This program was expanded via the Bipartisan Infrastructure Law in 2021, to make funding available for installation of filters or other remediation actions in response to testing. In addition, EPA’s Office of Ground Water and Drinking Water has developed resources to assist state programs and individual schools and childcare facilities in their efforts to reduce lead in drinking water. As of January 2023, the EPA reports testing in more than 12,500 educational facilities serving more than 3.5 million persons, enabling needed remediation to ensure lead-safe drinking water in educational settings [ 226 ].
As part of EPA’s recent Lead and Copper Rule Revisions (LCRR), over a 5-year period, CWSs must conduct sampling at 20% of elementary schools and 20% of childcare facilities per year and conduct sampling at secondary schools on request for 1 testing cycle [ 99 ]. For elementary schools, CWSs must test 5 outlets per school; CWSs must only test 2 outlets at childcare centers. Any follow up testing after the first test after 5 years is only upon request from the school. There is no mandatory notification of results to the parents, teachers, or children, and what actions, if any, the school system or CWS will undertake is unclear.
Climate change
Climate change is likely to increase the occurrence of intense droughts, water scarcity, severe fires, rising sea levels, flooding, melting polar ice, and catastrophic storms [ 227 ]. These will have direct and indirect effects on the provision and quality of drinking water. Droughts will increase demand and reduce supply for water. Wildfires require water for control and can also pollute water sources. Higher temperatures will make higher water efficiency increasingly important to public water systems [ 228 ]. Rising sea levels increase intrusion of salt water into coastal aquifers and can contaminate near-coastal surface and ground water supplies. Chemical pollutants and pesticides that became airborne and deposited in glaciers, including banned persistent organic pollutants such as polychlorinated biphenyls; melting glaciers are now discharging the chemicals back into the surroundings and water bodies [ 229 ]. Global warming increases water temperatures and thus water lead levels, at the same time that higher temperatures will increase water consumption [ 121 ]. The acidification (lowered pH) anticipated for all environmental media will increase lead mobility and bioavailability.
Disparities in access to clean, reliable, safe drinking water
Racial and ethnic disparities in some drinking water contaminant exposures, such as arsenic, uranium, lead and nitrate, are widely documented at the national, regional, or local levels in the US. These disparities mimic the inequities evident in housing, education, employment, earnings, health care, criminal justice, and environmental burdens and are likely underlined by structural racism [ 230 , 231 ]. These inequities impact many health outcomes through sustained exposures to toxic environmental assaults, including drinking water contaminants, socioeconomic factors, and psychosocial stressors [ 232 , 233 ]. The term ‘structural racism’ refers to the “totality of ways in which societies foster racial discrimination, through mutually reinforcing inequitable systems (in housing, education, employment, earnings, benefits, credit, media, health care, criminal justice, and so on).” [ 231 , 234 ] Structural racism operates through a complex, multilevel, interactive mechanism driven by factors across the natural, built and sociopolitical environment [ 235 , 236 ]. While common patterns are evident for several particular drinking water contaminants and for overall drinking water system violation rates, the specific mechanisms producing these disparities vary across geographic regions and drinking water contaminants, rural versus urban environments, as well as other aspects of communities’ sociodemographic make-up [ 237 , 238 ].
Major drivers of disparities in drinking water contaminants include selective enforcement of drinking water regulations, the exclusion of minorities in unincorporated areas from municipal boundaries and regulated water services (‘underbounding’ of communities of color), the direct withholding of resources and infrastructure investments, and the linguistic isolation of communities [ 116 , 130 , 235 ]. General childhood lead exposure, to which drinking water is now a major contributor, demonstrates the effect of structural racism in creating and reinforcing health disparities [ 18 , 239 ]. The well-documented and publicized events in Flint, Michigan, resulted from intentional changes in water treatment and water supply sources, selective infrastructure investments and inappropriate tap sampling for compliance monitoring [ 15 , 102 ].
Nationwide studies have also identified county-level racial and ethnic composition as a proxy for higher concentrations of other toxic chemicals in public drinking water [ 201 , 240 ]. After considering socioeconomic, educational, and social vulnerability factors, communities with a higher proportion of non-Hispanic Black, Hispanic/Latino, Indigenous, and other minoritized racial and ethnic population groups, are exposed to higher levels of arsenic and uranium in their drinking water, two toxic metals with no known safe level of exposure [ 193 , 201 ]. Similarly, nitrate concentrations are highest in CWSs serving communities with higher proportions of Hispanic/Latino residents [ 129 ], and PFAS were more frequently detected in CWSs serving higher proportions of Hispanic/Latinx and non-Hispanic Black residents [ 163 ]. Mounting evidence supports that current US public drinking water infrastructure, management, and regulatory action does not adequately protect communities of color from elevated contaminant exposures across a wide range of contaminants [ 116 , 193 , 201 , 241 ].
Inadequate health assessments
The US government uses risk assessments to determine priorities among competing needs, including health and environmental requirements. Outdated or inadequate health assessments bias governmental decisions and generate spurious results causing poor choices in determining priorities. The inadequacy of the assessments of drinking water contaminants discussed in this article include both outdated health data and the huge number of substances that have no health assessments at all. For widespread drinking water contaminants such as nitrate, lead and DBPs, the outdated health evaluations reflected in the drinking water standards mean that millions of Americans may have unsafe exposures, including in CWSs complying with current EPA standards. For classes of drinking water contaminants such as most DBPs, fracking-related substances and PFAS, identification and characterization of all of the chemical constituents has still not even occurred.
Large number of substances
DBPs, fracking-related substances and PFAS constitute categories of separate chemicals predicted to number in excess of 15,000. Neither the full identity nor characterization of these substances are currently known, so health data are largely unknown also. However, in each category, sufficient toxicity and epidemiological data are available to suggest enormous potential health risks for the millions of exposed Americans. In addition, many of these substances are difficult and/or expensive to monitor for raising the added specter of financial burden. Of course, these will bear disproportionate burdens on low-income and disadvantaged communities.
Preponderance of small water systems
Small water systems are generally defined as those serving 10,000 or fewer customers [ 242 ]. More than 90% of US CWSs are small. (Table 1 ) Many small systems face financial and operational challenges in providing drinking water that meets EPA standards [ 242 ]. Particular risks or complications for small systems include geographic dispersion and long distances, limited operational and technical resources, inadequate treatment capacity, affordability constraints), strained management demands, and communication needs [ 243 ]. The SDWA authorizes the potential use of Small System Variances to address small system challenges; these may, of course, constitute local exposure risks. Specifically, in 2002, Congress required EPA to re-evaluate EPA’s Small System Variances policy due to the high cost of arsenic treatment in small communities; in response, EPA may alter its affordability guidelines [ 243 ]. In addition, small systems may be at increased vulnerability to a variety of attacks, including contamination with deadly agents; physical attacks, such as the release of toxic gaseous chemicals; and cyberattacks [ 244 ]. Small systems face increased risks related to nitrate, DBPs, fracking and arsenic contamination. Furthermore, climate change will impose a larger burden on small and financially constrained water systems related to economies of scale and constrained resources.
Uneven enforcement of US drinking water standards
EPA has established national primary drinking water standards for about 100 contaminants [ 36 ]; many were set under specific Congressional mandates [ 36 , 245 ]. After EPA establishes an MCL, states can obtain “Primacy” to implement and enforce them, upon approval by EPA [ 245 ]. Each state and recognized Tribal government may apply to EPA for Primacy, formally known as Primary Enforcement Authority, by showing that it has adopted drinking water standards as stringent as the EPA standards and has the authority and capacity to implement and enforce those standards. Once a Primacy agency has received EPA approval, it has the primary responsibility for administrating and enforcing regulations. EPA retains oversight authority to ensure that Primacy agencies are complying with federal rules, and EPA also can file an enforcement action if a Primacy agency has failed to do so. Forty-nine states have Primacy for drinking water, although EPA continues to have Primacy in Wyoming, the District of Columbia, and in Indian country with the exception of the Navajo Nation (the only Tribe with Primacy) [ 246 ].
Enforcement of federal drinking water standards is inconsistent across the US [ 116 , 117 ]. and violations of the EPA drinking water standards are frequent. EPA documents over 40,000 violations annually for US drinking water systems, of which about 2600 PWSs faced formal enforcement actions (such as an administrative order) in 2022 for current and past violations. More than 27,000 violators reportedly received “informal” enforcement such as a reminder or warning letter [ 247 ]. These likely underestimate actual violations. An EPA audit published in 2008, for example, showed that only 8% of treatment technique violations of the LCR contained in state files were reported to the EPA [ 248 ]. A 2011 Government Accountability Office review confirmed widespread under-reporting of drinking water violations, with only 16–72% of violations reported [ 113 ]. The Natural Resources Defense Council (NRDC) reviewed EPA-reported violations and determined that in 2015 alone, there were more than 80,000 reported violations of the SDWA by >18,000 CWSs serving nearly 77 million people [ 117 ]. Another NRDC survey found that that in 2018, 2019 and 2020, 28 million people were served by 7595 CWSs with 12,892 lead violations [ 115 ].
Drinking water violations and inconsistent government response and resolution of violations disproportionately affect low-income communities and communities of color [ 116 , 241 ]. A 2017 study found that in communities with higher populations of black and Hispanic individuals, drinking water health violations are more common, and that in the poorest of communities race and ethnicity matter most in determining drinking water quality [ 116 ]. A 2018 national study similarly found that areas with greater populations of low socio-economic status, minority populations, and uninsured populations were more likely to have initial and repeat drinking water violations [ 249 ]. Race, ethnicity and spoken language of the population have been identified as the strongest factors for drinking water violations and slow resolution of these violations [ 116 ].
Water access and safety for US Indigenous communities
Indigenous communities in the US face significant challenges in accessing and ensuring the quality of their drinking water. Despite their status as sovereign nations under both the SDWA and the Clean Water Act (CWA), many Tribes continue to struggle with establishing water quality standards under the CWA to protect surface waters on Tribal lands from upstream or local water pollution discharges. This has implications for the health of Tribal populations, and absent Tribal adoption of CWA water quality standards for their surface waters, many Tribal governments lack authority to enforce those standards to protect the source waters used by water systems serving their populations.
In addition, primary authority to oversee drinking water systems can be secured by Tribes through the same process by which EPA approves Primacy to States. The resource and monetary challenges associated with obtaining Primacy on Tribal lands are compounded by aging infrastructure and the significant distances over which clean water needs to be delivered to serve remote Tribal populations. Currently, approximately 80 Tribes have been delegated authority to establish their own CWA water quality standards to protect their surface waters [ 250 ]. However, full Primacy under the SDWA has been granted to only one Tribe to date: the Navajo Nation has been granted Primacy to regulate the operation of 170 PWSs on their lands (a mix of Tribal and privately-owned systems).
More than 1000 water systems serve over 1 million people in “Indian Country”, underscoring the lack of access to regulated water supplies for more than 50% of the Indigenous population. The estimated Indigenous population of the US, based on the 2020 Census, is 4.4 million of American Indian or Alaska Native lineage alone and 7 million including those with mixed race (of the former, 2.7 million live within Tribal reservations) (Fig. 1 ). Access to regulated drinking water in homes varies significantly across Tribes. For the Navajo Nation, the largest Indigenous population living on reservation (~170,000), up to 30% of reservation households lacked access to regulated water within their homes in 2022 [ 251 ].
Tribal population within Tribal reservation boundaries in the US by census tract.
Compliance and access with regard to Tribal water systems has improved from 2009 to 2021, but when comparing Tribal systems to the US as a whole, the magnitude of disparities has been virtually unchanged. In 2009, PWSs serving US Tribal populations had nearly double the percentage of reported significant health-based violations as did those in the US as a whole. Of those reported significant health-based violations in 2009 (~14% of Tribal systems vs. ~5% of all US systems), 40% of Tribal violations were chemical contaminants, compared to 20% of all US system violations [ 252 ]. In 2021, the overall rates of significant health-based violations have decreased for both groups, although Tribal systems continue to have approximately twice the percentages of systems with significant health-based violations (6% Tribal vs 3% all US, 2021) [ 253 ]. Furthermore, as discussed above, there is often substantial under-reporting of violations to EPA’s database.
The biggest difference relates to infrastructure disparities faced by Tribes. In 2021, for instance, many of the health-based violations in Tribal systems involved infrastructure and resource disparities, such as water sources in proximity to failing septic systems with pathogen contamination risk and other sanitary issues constituting violations of EPA’s Ground Water Rule; in the rest of the US, chemical contamination is more common. As Tribes assume increasing regulatory authority, access to clean sources and adequate water for Tribal systems remains challenging. As of January 2023, $580 million in the US has been authorized for 15 Tribes to settle water rights issues, and for pumping and water distribution [ 253 ]. Water access is challenged by the long distances to reach homes and both mineralization and the legacy of anthropogenic contamination by industries such as mining. In the Western US, home to more than 50% of the Indigenous population, we estimated that 600,000 Indigenous people live within 10 km of at least one of the 161,000 documented abandoned hard rock mines [ 20 , 254 ]. EPA estimates these sources of mixtures of heavy metals have contaminated more than 40% of the surface waters in the Western US. Without access to regulated PWS or CWS water, the reliance on unregulated sources for drinking water traditionally has been on surface sources, private, or livestock wells. These unregulated sources also evidence impacts from mineralization and abandoned mines, creating clusters of sources exceeding MCLs for multiple heavy metals including arsenic and uranium, as well as barium, cesium, and other metals [ 202 ].
Conclusions
While the US and many other countries have greatly reduced acute risks from drinking water microbial contamination, chemical contamination of drinking water is associated with a wide range of chronic adverse health impacts including cancer and developmental, neurological and reproductive effects. A full understanding of pollutant-specific exposures and risks is hindered by limited availability of data on the occurrence, concentrations, and toxicity of these diverse legacy and emerging chemical contaminants. Further, there are many complex factors that influence the exposure and risk profiles of chemical contaminants in drinking water, including well depth, hydrogeological factors, types of distribution systems and disinfection treatments, and socioeconomic and demographic characteristics of impacted populations. The seven contaminants and contaminant groups presented here represent a tiny fraction of the thousands of regulated and unregulated chemical agents present in drinking water. This review illustrates the complexities of the array of chemical hazards in our drinking water and highlights the need for a concerted effort to invest in upgrading our drinking water infrastructure, strengthen drinking water standards, develop and implement enhanced water treatment, collect and disseminate monitoring data, and require more stringent chemical safety testing to support the welfare of all US residents. Furthermore, our analysis underscores that a focus on source water protection would be more effective than the post-hoc treatment that is now necessary to protect public health.
EPA (Environmental Protection Agency). Factoids: drinking water and ground water statistics for 2007. Vol. EPA 816-K-07-004. Washington DC: Environmental Protection Agency; 2007.
USGS (US Geological Survey). Domestic (private) supply wells. Reston, VA: US Geological Survey; 2019.
US Congress. Title XIV of the public health service act: safety of public water systems (safe drinking water act). Vol. P.L. 116–92, Washington DC: US Congress; 1994.
EPA (US Environmental Protection Agency). National primary drinking water regulations. Available at https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations Accessed Feb 20 2023.
Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res Rev Mutat Res. 2007;636:178–242.
Article CAS Google Scholar
Elliott EG, Ettinger AS, Leaderer BP, Bracken MB, Deziel NC. A systematic evaluation of chemicals in hydraulic-fracturing fluids and wastewater for reproductive and developmental toxicity. J Exp Sci Environ Epidemiol. 2017;27:90–99.
EPA (US Environmental Protection Agency). PFAS master list of PFAS substances. Washington DC: US Environmental Protection Agency; 2021.
WHO (World Health Organization). Guidelines for drinking ‑ water quality. 4th ed. incorporating the first and second addenda. Geneva, Switzerland: WHO; 2022.
Villanueva CM, Evlampidou I, Ibrahim F, Donat-Vargas C, Valentin A, Tugulea A-M, et al. Global assessment of chemical quality of drinking water: the case of trihalomethanes. Water Res. 2023;230:119568.
Article CAS PubMed Google Scholar
WHO. A global overview of national regulations and standards for drinking-water quality. Geneva, Switzerland: WHO; 2021.
IARC (International Agency for Research on Cancer). Some drinking-water disinfectants and contaminants, including arsenic. Vol. 84, Lyon, France: International Agency for Research on Cancer Working Group; 2004.
Ramasamy SLJ. Chapter 4: Arsenic risk assessment. In: Handbook of arsenic toxicology. Washington DC: Academic Press; 2014.
National Research Council. Critical aspects of EPA’s IRIS assessment of inorganic arsenic: interim report. Washington DC: National Academies Press; 2013.
Quansah R, Armah F, Essumang D, Luginaah I, Clarke E, Marfoh K, et al. Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environ Health Perspect. 2015;123:412–21.
Article PubMed PubMed Central Google Scholar
Nigra A, Moon K, Jones M, Sanchez T, Navas-Acien A. Urinary arsenic and heart disease mortality in NHANES 2003-2014. Environ Res. 2021;200:111387.
Article CAS PubMed PubMed Central Google Scholar
García-Esquinas E, Pollán M, Umans J, Francesconi KA, Goessler W, Guallar E, et al. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study. Cancer Epidemiol Prev Biomark. 2013;22:1944–53.
Article Google Scholar
Ayotte J, Medalie L, Qi S, Backer L, Nolan B. Estimating the high-arsenic domestic-well population in the conterminous United States. Environ Sci Technol. 2017;51:12443–54.
Nigra A, Chen Q, Chillrud S, Wang L, Harvey D, Mailloux B, et al. Inequalities in public water arsenic concentrations in counties and community water systems across the United States, 2006-2011. Environ Health Perspect. 2020;128:127001.
Spaur M, Lombard M, Ayotte J, Harvey DE, Bostick BC, Chillrud SN, et al. Associations between private well water and community water supply arsenic concentrations in the conterminous United States. Sci Total Environ. 2021;787:147555.
Lewis J, Hoover J, MacKenzie D. Mining and environmental health disparities in Native American communities. Curr Environ Health Rep. 2017;4:130–41.
Martinez-Morata I, Bostick B, Conroy-Ben O, Duncan DT, Jones MR, Spaur M, et al. Nationwide geospatial analysis of county-level racial/ethnic composition and public drinking water arsenic and uranium. Nat Commun. 2022;13:7461.
Pace C, Balazs C, Bangia K, Depsky N, Renteria A, Morello-Frosch R, et al. Inequities in drinking water quality among domestic well communities and community water systems, California, 2011‒2019. Am J Public Health. 2022;112:88–97.
Nigra A, Cazacu-De Luca A, Navas-Acien A. Socioeconomic vulnerability and public water arsenic concentrations across the US. Environ Pollut. 2022;313:120113.
Nigra A, Sanchez T, Nachman K, Harvey D, Chillrud SN, Graziano JH, et al. The effect of the Environmental Protection Agency maximum contaminant level on arsenic exposure in the USA from 2003 to 2014: an analysis of the National Health and Nutrition Examination Survey (NHANES). Lancet Public Health. 2017;2:e513–e521.
Welch B, Smit E, Cardenas A, Hystad P, Kile M. Trends in urinary arsenic among the U.S. population by drinking water source: Results from the National Health and Nutritional Examinations Survey 2003-2014. Environ Res. 2018;162:8–17.
Foster S, Pennino M, Compton J, Leibowitz S, Kile M. Arsenic drinking water violations decreased across the United States following revision of the maximum contaminant level. Environ Sci Technol. 2019;53:11478–85.
National Research Council Subcommittee to Update the Arsenic in Drinking Water R. Arsenic in drinking water: 2001 update. Washington (DC): National Academies Press (US); 2001.
EPA (US Environmental Protection Agency). Integrated Risk Information System. Arsenic, inorganic (CASRN 7440-38-2). https://iris.epa.gov/ChemicalLanding/&substance_nmbr=278 .
Lombard M, Daniel J, Jeddy Z, Hay L, Ayotte J. Assessing the impact of drought on arsenic exposure from private domestic wells in the conterminous United States. Environ Sci Technol. 2021;55:1822–31.
Wagner ED, Plewa MJ. CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: an updated review. J Environ Sci. 2017;58:64–76.
Jeong CH, Wagner ED, Siebert VR, Anduri S, Richardson SD, Daiber EJ, et al. Occurrence and toxicity of disinfection byproducts in European drinking waters in relation with the HIWATE epidemiology study. Environ Sci Technol. 2012;46:12120–8.
Villanueva CM, Cordier S, Font-Ribera L, Salas LA, Levallois P. Overview of disinfection by-products and associated health effects. Curr Environ Health Rep. 2015;2:107–15.
Kumari M, Gupta S. Occurrence and exposure to trihalomethanes in drinking water: a systematic review and meta-analysis. Exposure Health. 2022;14:915–939.
Good KD, VanBriesen JM. Power plant bromide discharges and downstream drinking water systems in Pennsylvania. Environ Sci Technol. 2017;51:11829–38.
European Union. Directive 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Off J Eur Union. 2020;435:1–62.
EPA (US Environmental Protection Agency). National primary drinking water regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations . Accessed Feb 12 2023.
Costet N, Villanueva C, Jaakkola J, Kogevinas M, Cantor K, King W, et al. Water disinfection by-products and bladder cancer: is there a European specificity? A pooled and meta-analysis of European case–control studies. Occup Environ Med. 2011;68:379–85.
Säve-Söderbergh M, Toljander J, Donat-Vargas C, Berglund M, Åkesson A. Exposure to drinking water chlorination by-products and fetal growth and prematurity: a nationwide register-based prospective study. Environ Health Perspect. 2020;128:57006.
Article PubMed Google Scholar
Patelarou E, Kargaki S, Stephanou EG, Nieuwenhuijsen M, Sourtzi P, Gracia E, et al. Exposure to brominated trihalomethanes in drinking water and reproductive outcomes. Occup Environ Med. 2011;68:438–45.
IARC (International Agency for Research on Cancer). Trichloroethylene and other chlorinated agents. IARC Monographs on the evaluation of carcinogenic risks to humans. Vol. 106. Lyon, France: International Agency for Research on Cancer; 2012.
Zhang Z, Zhu Q, Huang C, Yang M, Li J, Chen Y, et al. Comparative cytotoxicity of halogenated aromatic DBPs and implications of the corresponding developed QSAR model to toxicity mechanisms of those DBPs: Binding interactions between aromatic DBPs and catalase play an important role. Water Res. 2020;170:115283.
Richardson SD, Fasano F, Ellington JJ, Crumley FG, Buettner KM, Evans JJ, et al. Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water. Environ Sci Technol. 2008;42:8330–8.
Allen JM, Plewa MJ, Wagner ED, Wei X, Bokenkamp K, Hur K, et al. Drivers of disinfection byproduct cytotoxicity in U.S. drinking water: should other DBPs be considered for regulation? Environ Sci Technol. 2022;56:392–402.
Save-Soderbergh M, Toljander J, Donat-Vargas C, Akesson A. Drinking water disinfection by-products and congenital malformations: a nationwide register-based prospective study. Environ Health Perspect. 2021;129:97012.
Grellier J, Bennett J, Patelarou E, Smith R, Toledano M, Rushton L, et al. Exposure to disinfection by-products and adverse birth outcomes related to fetal growth and prematurity–a systematic review and meta-analysis. Epidemiology. 2009;20:S67.
Kali S, Khan M, Ghaffar MS, Rasheed S, Waseem A, Iqbal MM, et al. Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: a comprehensive review. Environ Pollut. 2021;281:116950.
Ding S, Chu W, Krasner SW, Yu Y, Fang C, Xu B, et al. The stability of chlorinated, brominated, and iodinated haloacetamides in drinking water. Water Res. 2018;142:490–500.
Zhao R, Reckhow DA, Becker WC, Schindler S. Seasonal variation of disinfection byproduct precursors in a large water supply. J Am Water Works Assoc. 2018;110:15–32.
Kumari M, Gupta S. Cumulative human health risk analysis of trihalomethanes exposure in drinking water systems. J Environ Manag. 2022;321:115949.
Souaya EM, Abdullah AM, Mossad M. Seasonal variation of trihalomethanes levels in greater cairo drinking water. Modern Chem Appl. 2015;3:2.
EPA (US Environmental Protection Agency). Stage 2 disinfectants and disinfection byproducts rule (DBPR) and consecutive system in-depth analysis. Washington DC: US Environmental Protection Agency; 2019.
Hao T, Miao M, Cheng X, Dou Y, Zhang M, Li Y. The effects of polypropylene microplastics on the DBP formation under the chlorination and chloramination processes. Chemosphere. 2022;303:135102.
Mompremier R, Fuentes Mariles ÓA, Becerril Bravo JE, Ghebremichael K. Study of the variation of haloacetic acids in a simulated water distribution network. Water Supply. 2019;19:88–96.
Villanueva CM, Font-Ribera L. Health impact of disinfection by-products in swimming pools. Ann dell’Istituto Super di sanita. 2012;48:387–96.
Weaver WA, Li J, Wen Y, Johnston J, Blatchley MR, Blatchley ER III. Volatile disinfection by-product analysis from chlorinated indoor swimming pools. Water Res. 2009;43:3308–18.
Erdinger L, Kühn KP, Kirsch F, Feldhues R, Fröbel T, Nohynek B, et al. Pathways of trihalomethane uptake in swimming pools. Int J Hyg Environ Health. 2004;207:571–5.
Anderson LE, DeMont I, Dunnington DD, Bjorndahl P, Redden DJ, Brophy MJ, et al. A review of long-term change in surface water natural organic matter concentration in the northern hemisphere and the implications for drinking water treatment. Sci Total Environ. 2022;858:159699.
Strock KE, Saros JE, Nelson SJ, Birkel SD, Kahl JS, McDowell WH. Extreme weather years drive episodic changes in lake chemistry: implications for recovery from sulfate deposition and long-term trends in dissolved organic carbon. Biogeochemistry. 2016;127:353–65.
Deziel N, Clark CJ, Casey JA, et al. Assessing exposure to unconventional oil and gas development: strengths, challenges, and implications for epidemiologic research. Curr Environ Health Rep. 2022;9:436–50.
Clark CJ, Warren JL, Kadan-Lottick N, Ma X, Bell ML, Saiers JE, et al. Community concern and government response: Identifying socio-economic and demographic predictors of oil and gas complaints and drinking water impairments in Pennsylvania. Energy Res Soc Sci. 2021;76:102070.
Elliott EG, Trinh P, Ma X, Leaderer BP, Ward MH, Deziel NC. Unconventional oil and gas development and risk of childhood leukemia: assessing the evidence. Sci Total Environ. 2017;576:138–47.
Vidic RD, Brantley SL, Vandenbossche JM, Yoxtheimer D, Abad JD. Impact of shale gas development on regional water quality. Science. 2013;340:1235009.
Clancy SA, Worrall F, Davies RJ, Gluyas JG. The potential for spills and leaks of contaminated liquids from shale gas developments. Sci Total Environ. 2018;626:1463–73.
Maloney KO, Baruch-Mordo S, Patterson LA, Nicot JP, Entrekin SA, Fargione JE, et al. Unconventional oil and gas spills: Materials, volumes, and risks to surface waters in four states of the U.S. Sci Total Environ. 2017;581-582:369–77.
Brantley SL, Yoxtheimer D, Arjmand S, Grieve P, Vidic R, Pollak J, et al. Water resource impacts during unconventional shale gas development: the Pennsylvania experience. Int J Coal Geol. 2014;126:140–56.
Clark CJ, Johnson NP, Soriano M Jr, Warren JL, Sorrentino KM, Kadan-Lottick NS, et al. Unconventional oil and gas development exposure and risk of childhood acute lymphoblastic leukemia: a case–control study in Pennsylvania, 2009–2017. Environ Health Perspect. 2022;130:087001.
Wen T, Niu X, Gonzales M, Zheng G, Li Z, Brantley SL. Big groundwater data sets reveal possible rare contamination amid otherwise improved water quality for some analytes in a region of marcellus shale development. Environ Sci Technol. 2018;52:7149–59.
McMahon PB, Lindsey BD, Conlon MD, Hunt AG, Belitz K, Jurgens BC, et al. Hydrocarbons in Upland Groundwater, Marcellus Shale Region, Northeastern Pennsylvania and Southern New York, U.S.A. Environ Sci Technol. 2019;53:8027–35.
Xiong B, Soriano MA, Gutchess KM, Hoffman N, Clark CJ, Siegel HG, et al. Groundwaters in Northeastern Pennsylvania near intense hydraulic fracturing activities exhibit few organic chemical impacts. Environ Sci: Process Impacts. 2022;24:252–64.
CAS PubMed Google Scholar
Akob DM, Mumford AC, Orem W, Engle MA, Klinges JG, Kent DB, et al. Wastewater disposal from unconventional oil and gas development degrades stream quality at a West Virginia Injection Facility. Environ Sci Technol. 2016;50:5517–25.
DiGiulio DC, Jackson RB. Impact to underground sources of drinking water and domestic wells from production well stimulation and completion practices in the Pavillion, Wyoming, Field. Environ Sci Technol. 2016;50:4524–36.
Bonetti P, Leuz C, Michelon G. Large-sample evidence on the impact of unconventional oil and gas development on surface waters. Science. 2021;373:896–902.
Gaughan C, Sorrentino KM, Liew Z, Johnson NP, Clark CJ, Soriano M Jr, et al. Residential proximity to unconventional oil and gas development and birth defects in Ohio. Environ Res. 2023;229:115937.
Hill EL. Shale gas development and infant health: evidence from Pennsylvania. J Health Econ. 2018;61:134–50.
Hill E, Ma L. Shale gas development and drinking water quality. Am Econ Rev. 2017;107:522–5.
Lee D, Murphy HM. Private wells and rural health: groundwater contaminants of emerging concern. Curr Environ Health Rep. 2020;7:129–39.
Elsner M, Hoelzer K. Quantitative survey and structural classification of hydraulic fracturing chemicals reported in unconventional gas production. Environ Sci Technol. 2016;50:3290–314.
Santos I, Hildenbrand ZL, Schug KA. A review of analytical methods for characterizing the potential environmental impacts of unconventional oil and gas development. Anal Chem. 2019;91:689–703.
Sumner AJ, Plata DL. A geospatially resolved database of hydraulic fracturing wells for chemical transformation assessment. Environ Sci Process Impacts. 2020;22:945–55.
Soriano MA Jr, Siegel HG, Gutchess KM, Clark CJ, Li Y, Xiong B, et al. Evaluating domestic well vulnerability to contamination from unconventional oil and gas development sites. Water Resour Res. 2020;56:e2020WR028005.
Shaheen SW, Wen T, Herman A, Brantley SL. Geochemical evidence of potential groundwater contamination with human health risks where hydraulic fracturing overlaps with extensive legacy hydrocarbon extraction. Environ Sci Technol. 2022;56:10010–9.
Wen T, Liu M, Woda J, Zheng G, Brantley SL. Detecting anomalous methane in groundwater within hydrocarbon production areas across the United States. Water Res. 2021;200:117236.
EPA (US Environmental Protection Agency). Integrated Science Assessment (ISA) for Lead. Washington DC: Environmental Protection Agency; 2013.
National Toxicology Program (US HHS). NTP Monograph: Health Effects of Low Level Lead. In: Rooney AA, Boyles AL, Taylor K, editors. US Department of Health and Human Services. 2012. https://ntp.niehs.nih.gov/sites/default/files/ntp/ohat/lead/final/monographhealtheffectslowlevellead_newissn_508.pdf
Lanphear BP, Lowry JA, Ahdoot S, Baum CR, Bernstein AS, Bole A, et al. Prevention of childhood lead toxicity. Pediatrics. 2016;138:e20161493.
Elfland C, Scardina P, Edwards M. Lead‐contaminated water from brass plumbing devices in new buildings. J Am Water Works Assoc. 2010;102:66–76.
Cartier C, Nour S, Richer B, Deshommes E, Prévost M. Impact of water treatment on the contribution of faucets to dissolved and particulate lead release at the tap. Water Res. 2012;46:5205–16.
Deshommes E, Trueman B, Douglas I, Huggins D, Laroche L, Swertfeger J, et al. Lead levels at the tap and consumer exposure from legacy and recent lead service line replacements in six utilities. Environ Sci Technol. 2018;52:9451–9.
Clark B, Masters S, Edwards M. Lead release to drinking water from galvanized steel pipe coatings. Environ Eng Sci. 2015;32:713–21.
Ng DQ, Chen CY, Lin YP. A new scenario of lead contamination in potable water distribution systems: Galvanic corrosion between lead and stainless steel. Sci Total Environ. 2018;637-638:1423–31.
EPA (US Environmental Protection Agency). Lead service line replacement. Washington DC: US Environmental Protection Agency; 2022.
EPA (US Environmental Protection Agency). Drinking water infrastructure needs survey and assessment sixth report to congress, 2018. Washington DC: US Environmental Protection Agency; 2018.
EPA (US Environmental Protection Agency). Use of lead free pipes, fittings, fixtures, solder, and flux for drinking water. Washington DC: US Environmental Protection Agency; 2022.
Jarvis P, Quy K, Macadam J, Edwards M, Smith M. Intake of lead (Pb) from tap water of homes with leaded and low lead plumbing systems. Sci Total Environ. 2018;644:1346–56.
Troesken W. The great lead water pipe disaster. Cambridge MA: MIT Press; 2006.
Levin R. Reducing lead in drinking water: a benefit analysis. Vol. EPA 230-0986-019. US Environmental Protection Agency; 1986.
Ngueta G, Prévost M, Deshommes E, Abdous B, Gauvin D, Levallois P. Exposure of young children to household water lead in the Montreal area (Canada): the potential influence of winter-to-summer changes in water lead levels on children’s blood lead concentration. Environ Int. 2014;73:57–65.
Masters S, Welter G, Edwards M. Seasonal variations in lead release to potable water. Environ Sci Technol. 2016;50:5269–77.
EPA (US Environmental Protection Agency): Lead and Copper Rule Revision. Washington DC: US Environmental Protection Agency; 2021.
EPA (Environmental Protection Agency). Review of the national primary drinking water regulation: lead and copper rule revisions (LCRR). Washington DC: Environmental Protection Agency; 2021.
EPA (Environmental Protection Agency). Update on lead leaching from submersible water well pumps and private drinking water systems. Washington DC: Office of Water; 1995.
Gibson J, Fisher M, Clonch A, MacDonald J, Cook P. Children drinking private well water have higher blood lead than those with city water. Proc Natl Acad Sci. 2020;117:16898–907.
Lu W, Levin R, Schwartz J. Lead contamination of public drinking water and academic achievements among children in Massachusetts: a panel study. BMC Public Health. 2022;22:107.
Danziger J, Mukamal KJ, Weinhandl E. Associations of community water lead concentrations with hemoglobin concentrations and erythropoietin-stimulating agent use among patients with advanced CKD. J Am Soc Nephrol. 2021;32:2425–34.
Dave DM, Yang M. Lead in drinking water and birth outcomes: a tale of two water treatment plants. J Health Econ. 2022;84:102644.
Danziger J, Mukamal KJ. Levels of lead in residential drinking water and iron deficiency among patients with end stage kidney disease. Kidney360. 2022;3:1210.
Gibson J, MacDonald J, Fisher M, Chen X, Pawlick A, Cook P. Early life lead exposure from private well water increases juvenile delinquency risk among US teens. Proc Natl Acad Sci. 2022;119:e2110694119.
Edwards M, Triantafyllidou S, Best D. Elevated blood lead in young children due to lead-contaminated drinking water: Washington, DC, 2001−2004. Environ Sci Technol. 2009;43:1618–23.
Hanna-Attisha M, LaChance J, Sadler RC, Champney, Schnepp A. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106:283–90.
Mulhern R, Roostaei J, Schwetschenau S, Pruthi T, Campbell C, Gibson JM. A new approach to a legacy concern: evaluating machine-learned Bayesian networks to predict childhood lead exposure risk from community water systems. Environ Res. 2022;204:112146.
GAO (General Accounting Office). Drinking water: Additional data and statistical analysis may enhance EPA’s oversight of the lead and copper rule. GAO-17-424. 2017. https://www.gao.gov/products/gao-17-424 .
GAO (General Accounting Office). Drinking water: EPA should strengthen ongoing efforts to ensure that consumers are protected from lead contamination. GAO-06-148. 2006. https://www.gao.gov/products/gao-06-148 .
GAO (Government Accountability Office). Drinking water: unreliable state data limit EPA’s ability to target enforcement priorities and communicate water systems’ performance. Vol. GAO-11-381. Washington, DC: Government Accountability Office; 2011.
Ganim S. 5,300 U.S. water systems are in violation of lead rules. CNN; Atlanta, GA. June 28, 2016. https://www.cnn.com/2016/06/28/us/epa-lead-in-u-s-watersystems/index.html .
Pullen-Fedinick K. Millions served by water systems detecting lead. Washington DC: Natural Resources Defense Council; 2021.
Fedinick K, Taylor S, Roberts M, Moore R, Olson E. Watered down justice. Washington DC: Natural Resource Defense Council Report; 2019.
Fedinick K, Wu M, Panditharatne M, Olson E. Threats on tap: widespread violations highlight need for investment in water infrastructure. Washington DC: Natural Resources Defense Council; 2017.
EDF (Environmental Defense Fund), American University School of Public Affairs: lead pipes and environmental justice. Washington, DC: Environmental Defense Fund; 2020. https://www.edf.org/sites/default/files/u4296/LeadPipe_EnvironJustice_AU%20and%20EDF%20Report.pdf .
Neina D. The role of soil pH in plant nutrition and soil remediation. Appl Environ soil Sci. 2019;2019:1–9.
Sokolova IM, Lannig G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: implications of global climate change. Clim Res. 2008;37:181–201.
Levin R, Zilli Vieira CL, Mordarski DC, Rosenbaum MH. Lead seasonality in humans, animals, and the natural environment. Environ Res. 2020;180:108797.
Levin R, Brown MJ, Kashtock ME, Jacobs DE, Whelan EA, Rodman J, et al. Lead exposures in US children, 2008: implications for prevention. Environ Health Perspect. 2008;116:1285–93.
Hayes C, Skubala N. Is there still a problem with lead in drinking water in the European Union? J Water Health. 2009;7:569–80.
WHO. Water Sanitation and Health: Lead. https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/chemical-hazards-in-drinking-water/lead . Accessed 6 May 2023.
Fields S. Global nitrogen: cycling out of control. Environ Health Perspect. 2004: A556-A563. https://doi.org/10.1289/ehp.112-a556 .
Schaider LA, Ackerman JM, Rudel RA. Septic systems as sources of organic wastewater compounds in domestic drinking water wells in a shallow sand and gravel aquifer. Sci Total Environ. 2016;547:470–81.
Dubrovsky NM, Hamilton PA. The quality of our nation’s water: nutrients in the nation’s streams and groundwater; national findings and implications. https://pubs.usgs.gov/fs/2010/3078/pdf/fs20103078.pdf : US Geological Survey, National Water-Quality Assessment Program; 2010.
Minovi DSK. Tainted Tap: nitrate pollution, factory farms, and drinking water in Maryland and beyond. Washington DC: Center for Progressive Reform; 2020. https://cpr-assets.s3.amazonaws.com/documents/Tainted-Tap-FINAL-102120.pdf .
Schaider LA, Swetschinski L, Campbell C, Rudel RA. Environmental justice and drinking water quality: are there socioeconomic disparities in nitrate levels in U.S. drinking water? Environ Health. 2019;18:3.
VanDerslice J. Drinking water infrastructure and environmental disparities: evidence and methodological considerations. Am J Public Health. 2011;101:S109–114.
Ransom K, Nolan B, Stackelberg P, Belitz K, Fram M. Machine learning predictions of nitrate in groundwater used for drinking supply in the conterminous United States. Sci Total Environ. 2022;807:151065.
Wheeler DC, Nolan BT, Flory AR, DellaValle CT, Ward MH. Modeling groundwater nitrate concentrations in private wells in Iowa. Sci Total Environ. 2015;536:481–8.
Messier KP, Wheeler DC, Flory AR, Jones RR, Patel D, Nolan BT, et al. Modeling groundwater nitrate exposure in private wells of North Carolina for the Agricultural Health Study. Sci Total Environ. 2019;655:512–9.
ATSDR (Agency for Toxic Substance and Disease Registry). Toxicological profile for nitrate and nitrite. Atlanta, GA: US Department of Health; 2021. https://www.atsdr.cdc.gov/toxprofiles/tp204.pdf .
EPA (US Environmental Protection Agency), National Interim Primary Drinking Water Regulations, 40 Fed. Reg. 59566, 59570 (Dec. 24, 1975)(adopting 40 C.F.R. 141.11(b)), Washington, DC, USA.
Ward MH, Jones RR, Brender JD, de Kok TM, Weyer PJ, Nolan BT, et al. Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health. 2018;15:1557.
IARC (International Agency for Research on Cancer). Ingested nitrates and nitrites, and some cyanobacterial peptide toxins. Vol 94. Lyon: IARC; 2010.
Stayner LT, Jensen AS, Schullehner J, Coffman VR, Trabjerg BB, Olsen J, et al. Nitrate in drinking water and risk of birth defects: Findings from a cohort study of over one million births in Denmark. Lancet Reg Health Eur. 2022;14:100286.
Coffman VR, Sondergaard Jensen A, Trabjerg BB, Pedersen CB, Hansen B, Sigsgaard T, et al. Prenatal exposure to nitrate from drinking water and the risk of preterm birth: a Danish nationwide cohort study. Environ Epidemiol. 2022;6:e223.
Coffman VR, Jensen AS, Trabjerg BB, Pedersen CB, Hansen B, Sigsgaard T, et al. Prenatal exposure to nitrate from drinking water and markers of fetal growth restriction: a population-based study of nearly one million Danish-Born children. Environ Health Perspect. 2021;129:27002.
Jones RR, DellaValle CT, Weyer PJ, Robien K, Cantor KP, Krasner S, et al. Ingested nitrate, disinfection by-products, and risk of colon and rectal cancers in the Iowa Women’s Health Study cohort. Environ Int. 2019;126:242–51.
Barry KH, Jones RR, Cantor KP, Beane Freeman LE, Wheeler DC, Baris D, et al. Ingested nitrate and nitrite and bladder cancer in Northern New England. Epidemiology. 2020;31:136–44.
Zumel-Marne A, Castano-Vinyals G, Kundi M, Alguacil J, Cardis E. Environmental factors and the risk of brain tumours in young people: a systematic review. Neuroepidemiology. 2019;53:121–41.
Stayner LT, Schullehner J, Semark BD, Jensen AS, Trabjerg BB, Pedersen M, et al. Exposure to nitrate from drinking water and the risk of childhood cancer in Denmark. Environ Int. 2021;155:106613.
Mueller BA, Nielsen SS, Preston-Martin S, Holly EA, Cordier S, Filippini G, et al. Household water source and the risk of childhood brain tumours: results of the SEARCH International Brain Tumor Study. IntJ Epidemiol. 2004;33:1209–16.
Mirvish SS. Inhibition by vitamins C and E of in vivo nitrosation and vitamin C occurrence in the stomach. Eur J Cancer Prev. 1996;5:131–6.
Rajendra A, Bondonno NP, Rainey-Smith SR, Gardener SL, Hodgson JM, Bondonno CP. Potential role of dietary nitrate in relation to cardiovascular and cerebrovascular health, cognition, cognitive decline and dementia: a review. Food Funct. 2022;13:12572–89.
Ahluwalia A, Gladwin M, Coleman GD, Hord N, Howard G, Kim-Shapiro DB, et al. Dietary nitrate and the epidemiology of cardiovascular disease: report from a National Heart, Lung, and Blood Institute Workshop. J Am Heart Assoc. 2016;5:e003402.
Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong LY. Legacy and alternative per- and polyfluoroalkyl substances in the U.S. general population: paired serum-urine data from the 2013-2014 National Health and Nutrition Examination Survey. Environ Int. 2019;131:105048.
Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75:46–51.
ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for perfluoroalkyls. Prevention USDoHaHSCfDCa. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2021.
Crawford L, Halperin SA, Dzierlenga MW, Skidmore B, Linakis MW, Nakagawa S, et al. Systematic review and meta-analysis of epidemiologic data on vaccine response in relation to exposure to five principal perfluoroalkyl substances. Environ Int. 2023;172:107734.
Costello E, Rock S, Stratakis N, Eckel SP, Walker DI, Valvi D, et al. Exposure to per-and polyfluoroalkyl substances and markers of liver injury: a systematic review and meta-analysis. Environ Health Perspect. 2022;130:046001.
Air Force Civil Engineer Center. About PFOS/PFOA. Air Force Civil Engineer Center Lackland, TX. https://www.afcec.af.mil/WhatWeDo/Environment/Perfluorinated-Compounds/
Rice PA, Aungst J, Cooper J, Bandele O, Kabadi SV. Comparative analysis of the toxicological databases for 6:2 fluorotelomer alcohol (6:2 FTOH) and perfluorohexanoic acid (PFHxA). Food Chem Toxicol. 2020;138:111210.
Pelch KE, Reade A, Kwiatkowski CF, Merced-Nieves FM, Cavalier H, Schultz K, et al. The PFAS-Tox database: a systematic evidence map of health studies on 29 per- and polyfluoroalkyl substances. Environ Int. 2022;167:107408.
Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. Detection of poly-and perfluoroalkyl substances (PFASs) in US drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett. 2016;3:344–50.
Guelfo JL, Marlow T, Klein DM, Savitz DA, Frickel S, Crimi M, et al. Evaluation and management strategies for per-and polyfluoroalkyl substances (PFASs) in drinking water aquifers: perspectives from impacted US Northeast communities. Environ Health Perspect. 2018;126:065001.
EPA (US Environmental Protection Agency). Unregulated contaminant monitoring rule (UCMR) 3 data summary. Washington, D.C.: US Environmental Protection Agency: 2017.
Andrews DQ, Naidenko OV. Population-wide exposure to per-and polyfluoroalkyl substances from drinking water in the United States. Environ Sci Technol Lett. 2020;7:931–6.
Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Exposure Sci Environ Epidemiol. 2019;29:131–47.
EPA (US Environmental Protection Agency). Per- and Polyfluoroalkyl Substances National Primary Drinking Water Regulation. 2023. https://www.regulations.gov/document/EPA-HQ-OW-2022-0114-0027 .
Liddie JM, Schaider LA, Sunderland EM. Sociodemographic factors are associated with the abundance of PFAS sources and detection in US Community Water Systems. Environ Sci Technol. 2023;57:7902–12.
Mok K, Salvatore D, Powers M, Brown P, Poehlein M, Conroy-Ben O, et al. Federal PFAS testing and tribal public water systems. Environ Health Perspect. 2022;130:127701.
Banzhaf S, Filipovic M, Lewis J, Sparrenbom CJ, Barthel R. A review of contamination of surface-, ground-, and drinking water in Sweden by perfluoroalkyl and polyfluoroalkyl substances (PFASs). Ambio. 2017;46:335–46.
McMahon PB, Tokranov AK, Bexfield LM, Lindsey BD, Johnson TD, Lombard MA, et al. Perfluoroalkyl and polyfluoroalkyl substances in groundwater used as a source of drinking water in the eastern United States. Environ Sci Technol. 2022;56:2279–88.
Interstate Technology Regulatory Council. PFAS standards, PFAS water and soils values table. Washington DC: Interstate Technology Regulatory Council. https://pfas-1.itrcweb.org/ .
EPA (US Environmental Protection Agency). Technical fact sheet: drinking water health advisories for four PFAS (PFOA, PFOS, GenX chemicals, and PFBS). EPA 822-F-22-02. Office of Water. Washington DC: 2022. https://www.epa.gov/system/files/documents/2022-06/technical-factsheet-four-PFAS.pdf .
EPA (US Environmental Protection Agency). Fifth unregulated contaminant monitoring rule. 2023. https://www.epa.gov/dwucmr/fifth-unregulated-contaminant-monitoring-rule . In. 2023.
PFAS Interagency Task Force. PFAS in the commonwealth of Massachusetts. Final report of the PFAS Interagency Task Force. Boston, MA: PFAS Interagency Task Force; 2022.
Cordner A, Goldenman G, Birnbaum LS, Brown P, Miller MF, Mueller R, et al. The true cost of PFAS and the benefits of acting now. Environ Sci Technol. 2021;55:9630–3.
DiStefano R, Feliciano T, Mimna RA, Redding AM, Matthis J. Thermal destruction of PFAS during full-scale reactivation of PFAS-laden granular activated carbon. Remediation J. 2022;32:231–8.
EPA (US Environmental Protection Agency). Potential PFAS destruction technology: supercritical water oxidation. Washington, D.C.: US Environmental Protection Agency; 2021.
Cousins I, Goldenman G, Herzke D, Lohmann R, Miller M, Ng CA, et al. The concept of essential use for determining when uses of PFASs can be phased out. Environ Sci Proc Imp. 2019;21:1803–15.
CAS Google Scholar
Karpas Z, Paz-Tal O, Lorber A, Salonen L, Komulainen H, Auvinen A, et al. Urine, hair, and nails as indicators for ingestion of uranium in drinking water. Health Phys. 2005;88:229–42.
Muikku M, Puhakainen M, Heikkinen T, Ilus T. The mean concentration of uranium in drinking water, urine, and hair of the occupationally unexposed Finnish working population. Health Phys. 2009;96:646–54.
Agency for Toxic Substances and Disease Registry (ATSDR) Keith S, Faroon O, Roney N, Scinicariello F, Wilbur S, Ingerman L, et al. Toxicological profile for uranium. Atlanta (GA): Registry Agency for Toxic Substances and Disease Registry; 2013.
Okaneku J, Vearrier D, Mckeever R, Lasala G, Greenberg MI. Urine uranium concentrations and renal function in residents of the United States—2001 to 2010. Clin Toxicol. 2015;53:931–4.
Bjørklund G, Semenova Y, Pivina L, Dadar M, Rahman MM, Aaseth J, et al. Uranium in drinking water: a public health threat. Arch Toxicol. 2020;94:1551–60.
Vicente-Vicente L, Quiros Y, Pérez-Barriocanal F, López-Novoa JM, López-Hernández FJ, Morales AI. Nephrotoxicity of uranium: pathophysiological, diagnostic and therapeutic perspectives. Toxicol Sci. 2010;118:324–47.
Ma M, Wang R, Xu L, Xu M, Liu S. Emerging health risks and underlying toxicological mechanisms of uranium contamination: Lessons from the past two decades. Environ Int. 2020;145:106107.
Corlin L, Rock T, Cordova J, Woodin M, Durant JL, Gute DM, et al. Health Effects and Environmental Justice Concerns of Exposure to Uranium in Drinking Water. Curr Environ Health Rep. 2016;3:434–42.
Dinocourt C, Legrand M, Dublineau I, Lestaevel P. The neurotoxicology of uranium. Toxicology. 2015;337:58–71.
Zablotska L, Fenske N, Schnelzer M, Zhivin S, Laurier D, Kreuzer M. Analysis of mortality in a pooled cohort of Canadian and German uranium processing workers with no mining experience. Int Arch Occup Environ Health. 2018;91:91–103.
Kelly-Reif K, Sandler DP, Shore D, Schubauer, Berigan MK, Troester MA, et al. Radon and cancer mortality among underground uranium miners in the Příbram region of the Czech Republic. Am J Ind Med. 2020;63:859–67.
Hund L, Bedrick EJ, Miller C, Huerta G, Nez T, Ramone S, et al. A Bayesian framework for estimating disease risk due to exposure to uranium mine and mill waste on the Navajo Nation. J R Stat Soc Ser A Stat Soc. 2015;178:1069–91.
Erdei E, Shuey C, Pacheco B, Cajero M, Lewis J, Rubin RL. Elevated autoimmunity in residents living near abandoned uranium mine sites on the Navajo Nation. J Autoimmun. 2019;99:15–23.
Harmon ME, Lewis J, Miller C, Hoover J, Ali A-MS, Shuey C, et al. Residential proximity to abandoned uranium mines and serum inflammatory potential in chronically exposed Navajo communities. J Exposure Sci Environ Epidemiol. 2017;27:365–71.
Zychowski KE, Kodali V, Harmon M, Tyler CR, Sanchez B, Ordonez Suarez Y, et al. Respirable uranyl-vanadate-containing particulate matter derived from a legacy uranium mine site exhibits potentiated cardiopulmonary toxicity. Toxicological Sci. 2018;164:101–14.
Medina S, Lauer FT, Castillo EF, Bolt AM, Ali A-MS, Liu KJ, et al. Exposures to uranium and arsenic alter intraepithelial and innate immune cells in the small intestine of male and female mice. Toxicol Appl Pharmacol. 2020;403:115155.
Stewart B, Mayes MA, Fendorf S. Impact of uranyl-calcium-carbonato complexes on uranium(VI) adsorption to synthetic and natural sediments. Environ Sci Technol. 2010;44:928–34.
Stanley D, Wilkin RT. Solution equilibria of uranyl minerals: Role of the common groundwater ions calcium and carbonate. J Hazard Matter. 2019;377:315–20.
Ravalli F, Yu Y, Bostick BC, Chillrud SN, Schilling K, Basu A, et al. Sociodemographic inequalities in uranium and other metals in community water systems across the USA, 2006–11: a cross-sectional study. Lancet Planet Health. 2022;6:e320–e330.
Jones L, Credo J, Parnell R, Ingram JC. Dissolved Uranium And Arsenic In Unregulated Groundwater Sources – Western Navajo Nation. J Contemp Water Res Educ. 2020;169:27–43.
Ingram J, Jones L, Credo J, Rock T. Uranium and arsenic unregulated water issues on Navajo lands. J Vac Sci Technol. 2020;38:031003.
Credo J, Torkelson J, Rock T, Ingram JC. Quantification of elemental contaminants in unregulated water across Western Navajo Nation. Int J Environ Res Public Health. 2019;16:2727.
WoldeGabriel G, Boukhalfa H, Ware SD, Cheshire M, Reimus P, Heikoop J, et al. Characterization of cores from an in-situ recovery mined uranium deposit in Wyoming: Implications for post-mining restoration. Chem Geol. 2014;390:32–45.
Hindin R, Brugge D, Panikkar B. Teratogenicity of depleted uranium aerosols: a review from an epidemiological perspective. Environ Health. 2005;4:1–19.
Arzuaga X, Rieth SH, Bathija A, Cooper GS. Renal effects of exposure to natural and depleted uranium: a review of the epidemiologic and experimental data. J Toxicol Environ Health Part B. 2010;13:527–45.
Focazio M, Tipton D, Dunkle S, Shapiro S, Geiger LH. The chemical quality of self-supplied domestic well water in the United States. Groundw Monit Remediat. 2006;26:92–104.
Martinez-Morata I, Bostick BC, Conroy-Ben O, Duncan DT, Jones MR, Spaur M, et al. Nationwide geospatial analysis of county racial and ethnic composition and public drinking water arsenic and uranium. Nat Commun. 2022;13:7461.
Hoover J, Gonzales M, Shuey C, Barney Y, Lewis J. Elevated arsenic and uranium concentrations in unregulated water sources on the Navajo Nation, USA. Expo Health. 2017;9:113–24.
Scammell M, Sennett C, Laws RL, Rubin RL, Brooks DR, Amador JJ, et al. Urinary metals concentrations and biomarkers of autoimmunity among Navajo and Nicaraguan Men. Int J Environ Res Public Health. 2020;17:5263.
ASCE (American Society of Civil Engineers). Report card for America’s infrastructure: drinking water. Reston, VA: American Society of Civil Engineers; 2021.
Speight V. Water-distribution systems: the next frontier, in technologies for clean water. In: The bridge, linking engineering and society. Washington, DC: National Academy of Engineering of the National Academies; 2008.
Wingender J, Flemming H-C. Biofilms in drinking water and their role as reservoirs for pathogens. Int J Hyg Environ health. 2011;214.6:417–23.
Olson E, Stubblefield A. Lead pipes are widespread and used in every state. Washington, DC: Natural Resources Defense Council; 2021.
Baehler KJ, McGraw M, Aquino MJ, Heslin R, McCormick L, Neltner T. Full lead service line replacement: a case study of equity in environmental remediation. Sustainability. 2022;14:352.
EPA (US Environmental Protection Agency). Community water system survey, Volume I: Overview, 2006, 15–17. Washington, DC: USEPA; 2009.
EPA (US Environmental Protection Agency). 2006 Community water system survey, Volume II: detailed tables and survey methodology, 2009, Table 43. Washington, DC: US Environmental Protection Agency; 2009.
Colford JJ, Roy S, Beach MJ, Hightower A, Shaw SE, Wade TJ. A review of household drinking water intervention trials and an approach to the estimation of endemic waterborne gastroenteritis in the United States. J Water Health. 2006;4:71–88.
Messner M, Shaw S, Regli S, Rotert K, Blank V, Soller J. An approach for developing a national estimate of waterborne disease due to drinking water and a national estimate model application. J Water Health. 2006;4:201–40.
Reynolds K, Mena KD, Gerba CP. Risk of waterborne illness via drinking water in the United States. Rev Environ Contam Toxicol. 2008;102:117–58.
Collier SA, Deng L, Adam EA, Benedict KM, Beshearse EM, Blackstock AJ, et al. Estimate of burden and direct healthcare cost of infectious waterborne disease in the United States. Emerg Infect Dis. 2021;27:140.
AWWA (American Water Works Association). Buried no longer: confronting America’s water infrastructure challenge. Washington, DC: American Water Works Association; 2012.
Bantol K, Brumberg HL, Shah SI, Javier JR. Perspectives from the Society for Pediatric Research: contaminants of water and children’s health: Can we do better? Pediatr Res. 2020;88:535–43.
Bradham KD, Nelson CM, Sowers TD, Lytle DA, Tully J, Schock MR, et al. A national survey of lead and other metal (loids) in residential drinking water in the United States. J Exp Sci Environ Epidemiol. 2023;33:160–167.
DOE (Department of Education). NCES Early Childhood Program Participation: 2019 (NCES 2020-075REV), Table 1. 2019. https://nces.ed.gov/fastfacts/display.asp?id=4 , Accessed 20 January 2023.
U.S. Department of Education, National Center for Education Statistics, Common Core of Data (CCD), State Nonfiscal Survey of Public Elementary/Secondary Education, 2021-22 Preliminary; and Department of Defense Education Activity (DoDEA) Data Center, Enrollment Data, 2021, retrieved July 7, 2022, from https://www.dodea.edu/datacenter/enrollment.cfm .
Jones SE, Fisher CJ, Greene BZ, Hertz MF, Pritzl J. Healthy and safe school environment, part I: results from the School Health Policies and Programs Study 2006. J Sch Health. 2007;77:522–43.
U.S. Government Accountability Office. Childcare facilities: Federal agencies need to enhance monitoring and collaboration to help assure drinking water is safe from lead. Vol. GAO-20- 597, Washington, D.C.: U.S. Government Accountability Office; 2020. Available at: https://www.gao.gov/assets/gao-20-597.pdf . Accessed September 8 2023.
Lambrinidou Y, Triantafyllidou S, Edwards M. Failing our children: Lead in u.s. school drinking water. N Solut. 2010;20:25–47.
Young A, Nichols M. Beyond Flint: Excessive lead levels found in almost 2,000 water systems across all 50 states. USA Today; March 11, 2016. Available at https://www.usatoday.com/story/news/2016/03/11/nearly-2000-water-systems-fail-lead-tests/81220466/ . Accessed September 8 2023.
Cradock A, Barrett JL, Poole MK, Flax CN, Vollmer L, Hecht C. Lead concentrations in US school drinking water: testing programs, prevalence, and policy opportunities, 2016‒2018. Am J Public Health. 2022;112:S679–S689.
Redmon JH, Kondash A, Norman E, Johnson J, Levine K, McWilliams A, et al. Lead levels in tap water at licensed North Carolina Child Care Facilities, 20202021. Am J Public Health. 2022;112:S695–S705.
EPA (US Environmental Protection Agency). School and child care lead testing and reduction grant program summary data. US Environmental Protection Agency; Available at https://www.epa.gov/ground-water-and-drinkingwater/school-and-child-care-lead-testing-and-reduction-grant-program . Accessed September 8 2023.
United Nations, Climate Action. What is Climate CHange? https://www.un.org/en/climatechange/what-is-climate-change . Accessed June 12 2023.
EPA (US Environmental Protection Agency). Water efficiency and conservation resources for small drinking water systems. https://www.epa.gov/dwcapacity/water-efficiency-and-conservation-resources-small-drinking-water-systems . 2023. Accessed May 12 2023.
Sharma BM, Nizzetto L, Bharat GK, Tayal S, Melymuk L, Sáňka O, et al. Melting Himalayan glaciers contaminated by legacy atmospheric depositions are important sources of PCBs and high-molecular-weight PAHs for the Ganges floodplain during dry periods. Environ Pollut. 2015;206:588–96.
Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–63.
Bailey ZD, Feldman JM, Bassett MT. How structural racism works - racist policies as a root cause of U.S. Racial Health Inequities. N Engl J Med. 2021;384:768–73.
Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10:e0138511.
Cushing L, Morello-Frosch R, Wander M, Pastor M. The haves, the have-nots, and the health of everyone: the relationship between social inequality and environmental quality. Annu Rev Public Health. 2015;36:193–209.
Adkins-Jackson PB, Chantarat T, Bailey ZD, Ponce NA. Measuring structural racism: a guide for epidemiologists and other health researchers. Am J Epidemiol. 2022;191:539–47.
Balazs CL, Ray I. The drinking water disparities framework: on the origins and persistence of inequities in exposure. Am J Public Health. 2014;104:603–11.
Marsh B, Parnell A, Joyner A. Institutionalization of racial inequality in local political geographies. Urban Geogr. 2010;31:691–709.
Doyle JT, Kindness L, Realbird J, Eggers MJ, Camper AK. Challenges and opportunities for tribal waters: addressing disparities in safe public drinking water on the crow reservation in Montana, USA. Int J Environ Res Public Health. 2018;15:567.
Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114:1150–3.
Gostin LO. Lead in the water: a tale of social and environmental injustice. JAMA. 2016;315:2053–4.
Switzer D, Teodoro MP. Class, race, ethnicity, and justice in safe drinking water compliance. Soc Sci Q. 2018;99:524–35.
Switzer D, Teodoro MP. The color of drinking water. J Am Water Works Assoc. 2017;109:40–45.
EPA (US Environmental Protection Agency): Small Drinking Water System Variances. https://www.epa.gov/sdwa/small-drinking-water-system-variances . 2023.
EPA (US Environmental Protection Agency). Water system owner roles and responsibilities: a best practices guide. EPA 816-F-06-036. 2006. https://www.epa.gov/sites/default/files/2015-04/documents/epa816f06036.pdf .
US Cybersecurity & Infrastructure Security Agency. Water and wastewater systems. https://www.cisa.gov/topics/critical-infrastructure-security-and-resilience/criticalinfrastructure-sectors/water-and-wastewater-sector . Accessed July 2023.
US Congress: Safe Drinking Water Act §1452(h), 42 U.S.C. §300j-12(h). Washington DC: 2018.
EPA (US Environmental Protection Agency). Enforcement and compliance history online, safe drinking water act (SDWA) resources and FAQs. Accessed May 12 2023.
EPA (US Environmental Protection Agency). Analyze trends: EPA/state drinking water dashboard. https://echo.epa.gov/trends/comparative-maps-dashboards drinking-water-dashboard?yearview=CY&view=activity&criteria=basic&state=National . 2023. Accessed July 2023.
EPA (US Environmental Protection Agency). 2006 drinking water data quality analysis and action plan for state reported public water system data in the EPA Safe Drinking Water Information System/Federal Version (SDWIS/FED). Vol. EPA 816-R-07-010; Washington, DC: EPA; 2008.
McDonald YJ, Jones NE. Drinking water violations and environmental justice in the United States, 2011-2015. Am J Public Health. 2018;108:1401–7.
EPA (US Environmental Protection Agency). Tribes Approved for Treatment as a State (TAS). US Environmental Protection Agency; 2023. https://www.epa.gov/tribal/tribes-approved-treatment-state-tas .
Neset K, Ritter T, Hanson R, Hargrove R. Effective partnering increases access to water on the Navajo Nation. Mil Eng. 2022;114:62–65.
Google Scholar
EPA (US Environmental Protection Agency). Safe Drinking Water Information System (SDWIS) Federal Reporting Services. https://www.epa.gov/ground-water-and-drinking-water/safe-drinking-water-information-system-sdwis-federal-reporting . 2022.
Naishadham S. 15 Native American tribes to receive $580 million in federal money for water rights settlement. Washington, DC,: PBS NewsHour; 2023. https://www.watereducation.org/aquafornia-news/15-native-american-tribes-receive-580-million-federal-money-water-rights-settlement .
GAO (Government Accountability Office). Hardrock mining: information on abandoned mines and value and coverage of financial assurances on BLM land. Wasginton, DC: United States Government Accountabiltiy Office; 2008.
Download references
Acknowledgements
The authors appreciate the substantive and editorial suggestions of 2 anonymous reviewers.
ALC is supported in part by the Centers for Disease Control and Prevention (U48DP006376). NCD is supported in part by the Yale University Superfund Research Program, which is supported by a grant from the National Institute of Environmental Health Sciences, National Institutes of Health under award number P42ES033815. MHW is supported by the Intramural Research Program of the National Cancer Institute (Project Z01 CP01012522), National Institutes of Health. Irene Martinez-Morata is supported by a fellowship from La Caixa Foundation (ID100010434), fellowship code LCF/BQ/AA20/11820032. AEN is supported by NIH OD and NIDCR grant DP5OD031849, NICHD grant P2CHD058486, and NIEHS grants P30ES009089 and P42ES033719. JL and DB are currently supported by the following National Institutes of Health Awards: National Institute of Environmental Health Sciences (NIEHS) P42ES025589; the Office of the Director UH3 OD023344; and NIEHS and the National Institute on Minority Health and Health Disparities P50 MD015706. LS is supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (Award Numbers P42ES027706, R01ES028311). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and affiliations.
Harvard TH Chan School of Public Health, Boston, MA, USA
Ronnie Levin & Angie L. Cradock
ISGlobal, Barcelona, Spain
Cristina M. Villanueva & Carolina Donat-Vargas
CIBER epidemiología y salud pública (CIBERESP), Madrid, Spain
Universitat Pompeu Fabra (UPF), Barcelona, Spain
IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain
- Cristina M. Villanueva
Community Environmental Health Program, College of Pharmacy, University of New Mexico Health Sciences Center, Albuquerque, NM, USA
Daniel Beene & Johnnye Lewis
University of New Mexico Department of Geography & Environmental Studies, Albuquerque, NM, USA
Daniel Beene
Department of Environmental Health Sciences, Columbia University Mailman School of Public Health, New York, NY, USA
Irene Martinez-Morata & Anne E. Nigra
Center for Science and Democracy, Union of Concerned Scientists, Washington, DC, USA
Darya Minovi
Natural Resources Defense Council, Washington, DC, USA
Erik D. Olson
Silent Spring Institute, Newton, MA, USA
Laurel A. Schaider
Occupational and Environmental Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD, USA
- Mary H. Ward
Yale School of Public Health, New Haven, CT, USA
- Nicole C. Deziel
You can also search for this author in PubMed Google Scholar
Contributions
RL: article concept, writing, editing; CMV: article writing, editing; DRB: writing; ALC: writing; CDV: writing; JL: writing; IMM: writing; DM: writing; AEN: writing; EDO: writing, editing; LAS: writing, editing; MHW: writing; NCD: article writing, concept development, editing.
Corresponding author
Correspondence to Ronnie Levin .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Reportingsummary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Levin, R., Villanueva, C.M., Beene, D. et al. US drinking water quality: exposure risk profiles for seven legacy and emerging contaminants. J Expo Sci Environ Epidemiol 34 , 3–22 (2024). https://doi.org/10.1038/s41370-023-00597-z
Download citation
Received : 27 February 2023
Revised : 16 August 2023
Accepted : 17 August 2023
Published : 22 September 2023
Issue Date : January 2024
DOI : https://doi.org/10.1038/s41370-023-00597-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Drinking water
- Exposure risk profiles
- Social determinants
- Water infrastructure
- Water contaminants
- Environmental disparities
This article is cited by
Public drinking water contaminant estimates for birth cohorts in the environmental influences on child health outcomes (echo) cohort.
- Tessa R. Bloomquist
- Anne E. Nigra
Journal of Exposure Science & Environmental Epidemiology (2024)
Assessing exposure and health consequences of chemicals in drinking water in the 21st Century
Associations of per- and polyfluoroalkyl substances with uterine leiomyomata incidence and growth: a prospective ultrasound study.
- Lauren A. Wise
- Chad M. Coleman
- Amelia K. Wesselink
Drinking water source and exposure to regulated water contaminants in the California Teachers Study cohort
- Danielle N. Medgyesi
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
