Covidence website will be inaccessible as we upgrading our platform on Monday 23rd August at 10am AEST, / 2am CEST/1am BST (Sunday, 15th August 8pm EDT/5pm PDT)

How to write a search strategy for your systematic review
Home | Blog | How To | How to write a search strategy for your systematic review
Practical tips to write a search strategy for your systematic review
With a great review question and a clear set of eligibility criteria already mapped out, it’s now time to plan the search strategy. The medical literature is vast. Your team plans a thorough and methodical search, but you also know that resources and interest in the project are finite. At this stage it might feel like you have a mountain to climb.
The bottom line? You will have to sift through some irrelevant search results to find the studies that you need for your review. Capturing a proportion of irrelevant records in your search is necessary to ensure that it identifies as many relevant records as possible. This is the trade-off of precision versus sensitivity and, because systematic reviews aim to be as comprehensive as possible, it is best to favour sensitivity – more is more.
By now, the size of this task might be sounding alarm bells. The good news is that a range of techniques and web-based tools can help to make searching more efficient and save you time. We’ll look at some of them as we walk through the four main steps of searching for studies:
- Decide where to search
- Write and refine the search
- Run and record the search
- Manage the search results
Searching is a specialist discipline and the information given here is not intended to replace the advice of a skilled professional. Before we look at each of the steps in turn, the most important systematic reviewer pro-tip for searching is:
Pro Tip – Talk to your librarian and do it early!
1. decide where to search .
It’s important to come up with a comprehensive list of sources to search so that you don’t miss anything potentially relevant. In clinical medicine, your first stop will likely be the databases MEDLINE , Embase , and CENTRAL . Depending on the subject of the review, it might also be appropriate to run the search in databases that cover specific geographical regions or specialist areas, such as traditional Chinese medicine.
In addition to these databases, you’ll also search for grey literature (essentially, research that was not published in journals). That’s because your search of bibliographic databases will not find relevant information if it is part of, for example:
- a trials register
- a study that is ongoing
- a thesis or dissertation
- a conference abstract.
Over-reliance on published data introduces bias in favour of positive results. Studies with positive results are more likely to be submitted to journals, published in journals, and therefore indexed in databases. This is publication bias and systematic reviews seek to minimise its effects by searching for grey literature.
2. Write and refine the search
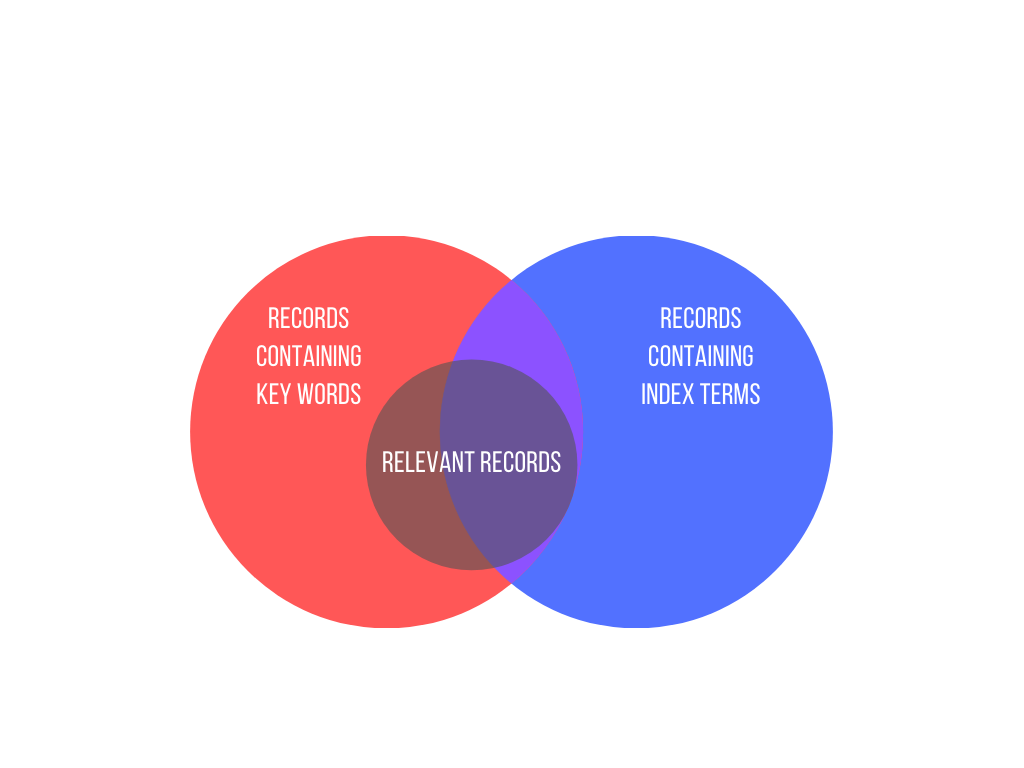
Search terms are derived from key concepts in the review question and from the inclusion and exclusion criteria that are specified in the protocol or research plan.
Keywords will be searched for in the title or abstract of the records in the database. They are often truncated (for example, a search for therap* to find therapy, therapies, therapist). They might also use wildcards to allow for spelling variants and plurals (for example, wom#n to find woman and women). The symbols used to perform truncation and wildcard searches vary by database.
Index terms
Using index terms such as MeSH and Emtree in a search can improve its performance. Indexers with subject area expertise work through databases and tag each record with subject terms from a prespecified controlled vocabulary.
This indexing can save review teams a lot of time that would otherwise be spent sifting through irrelevant records. Using index terms in your search, for example, can help you find the records that are actually about the topic of interest (tagged with the index term) but ignore those that contain only a brief mention of it (not tagged with the index term).
Indexers assign terms based on a careful read of each study, rather than whether or not the study contains certain words. So the index terms enable the retrieval of relevant records that cannot be captured by a simple search for the keyword or phrase.
Use a combination
Relying solely on index terms is not advisable. Doing so could miss a relevant record that for some reason (indexer’s judgment, time lag between a record being listed in a database and being indexed) has not been tagged with an index term that would enable you to retrieve it. Good search strategies include both index terms and keywords.
Let’s see how this works in a real review! Figure 2 shows the search strategy for the review ‘Wheat flour fortification with iron and other micronutrients for reducing anaemia and improving iron status in populations’. This strategy combines index terms and keywords using the Boolean operators AND, OR, and NOT. OR is used first to reach as many records as possible before AND and NOT are used to narrow them down.
- Lines 1 and 2: contain MeSH terms (denoted by the initial capitals and the slash at the end).
- Line 3: contains truncated keywords (‘tw’ in this context is an instruction to search the title and abstract fields of the record).
- Line 4: combines the three previous lines using Boolean OR to broaden the search.
- Line 11: combines previous lines using Boolean AND to narrow the search.
- Lines 12 and 13: further narrow the search using Boolean NOT to exclude records of studies with no human subjects.

Writing a search strategy is an iterative process. A good plan is to try out a new strategy and check that it has picked up the key studies that you would expect it to find based on your existing knowledge of the topic area. If it hasn’t, you can explore the reasons for this, revise the strategy, check it for errors, and try it again!
3. Run and record the search
Because of the different ways that individual databases are structured and indexed, a separate search strategy is needed for each database. This adds complexity to the search process, and it is important to keep a careful record of each search strategy as you run it. Search strategies can often be saved in the databases themselves, but it is a good idea to keep an offline copy as a back-up; Covidence allows you to store your search strategies online in your review settings.
The reporting of the search will be included in the methods section of your review and should follow the PRISMA guidelines. You can download a flow diagram from PRISMA’s website to help you log the number of records retrieved from the search and the subsequent decisions about the inclusion or exclusion of studies. The PRISMA-S extension provides guidance on reporting literature searches.
It is very important that search strategies are reproduced in their entirety (preferably using copy and paste to avoid typos) as part of the published review so that they can be studied and replicated by other researchers. Search strategies are often made available as an appendix because they are long and might otherwise interrupt the flow of the text in the methods section.
4. Manage the search results
Once the search is done and you have recorded the process in enough detail to write up a thorough description in the methods section, you will move on to screening the results. This is an exciting stage in any review because it’s the first glimpse of what the search strategies have found. A large volume of results may be daunting but your search is very likely to have captured some irrelevant studies because of its high sensitivity, as we have already seen. Fortunately, it will be possible to exclude many of these irrelevant studies at the screening stage on the basis of the title and abstract alone 😅.
Search results from multiple databases can be collated in a single spreadsheet for screening. To benefit from process efficiencies, time-saving and easy collaboration with your team, you can import search results into a specialist tool such as Covidence. A key benefit of Covidence is that you can track decisions made about the inclusion or exclusion of studies in a simple workflow and resolve conflicting decisions quickly and transparently. Covidence currently supports three formats for file imports of search results:
- EndNote XML
- PubMed text format
- RIS text format
If you’d like to try this feature of Covidence but don’t have any data yet, you can download some ready-made sample data .
And you’re done!
There is a lot to think about when planning a search strategy. With practice, expert help, and the right tools your team can complete the search process with confidence.
This blog post is part of the Covidence series on how to write a systematic review.
Sign up for a free trial of Covidence today!
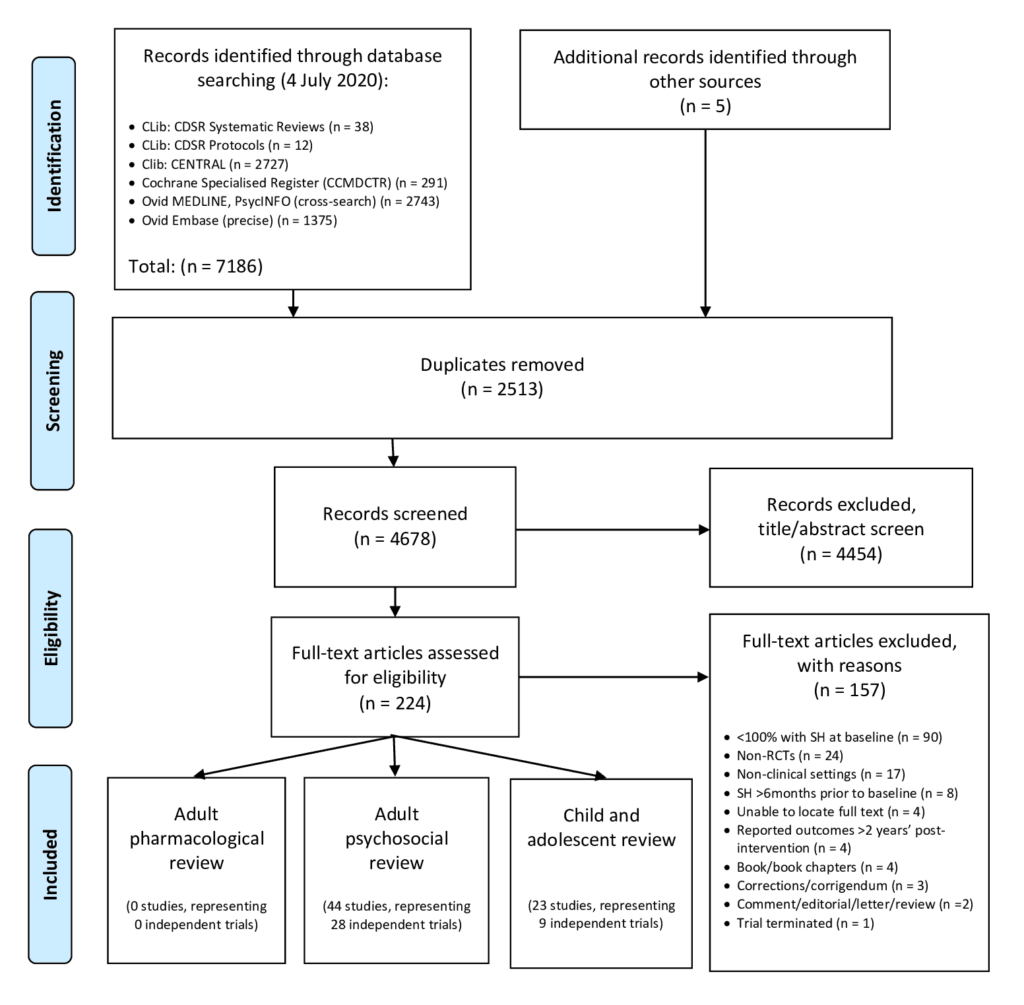
[1] Witt KG, Hetrick SE, Rajaram G, Hazell P, Taylor Salisbury TL, Townsend E, Hawton K. Pharmacological interventions for self‐harm in adults . Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No.: CD013669. DOI: 10.1002/14651858.CD013669.pub2. Accessed 02 February 2021

Laura Mellor. Portsmouth, UK
Perhaps you'd also like....

Top 5 Tips for High-Quality Systematic Review Data Extraction
Data extraction can be a complex step in the systematic review process. Here are 5 top tips from our experts to help prepare and achieve high quality data extraction.

How to get through study quality assessment Systematic Review
Find out 5 tops tips to conducting quality assessment and why it’s an important step in the systematic review process.

How to extract study data for your systematic review
Learn the basic process and some tips to build data extraction forms for your systematic review with Covidence.
Better systematic review management
Head office, working for an institution or organisation.
Find out why over 350 of the world’s leading institutions are seeing a surge in publications since using Covidence!
Request a consultation with one of our team members and start empowering your researchers:
By using our site you consent to our use of cookies to measure and improve our site’s performance. Please see our Privacy Policy for more information.
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Systematic Reviews
Constructing a search strategy and searching for evidence.
Aromataris, Edoardo PhD; Riitano, Dagmara BHSC, BA
Edoardo Aromataris is the director of synthesis science at the Joanna Briggs Institute in the School of Translational Health Science, University of Adelaide, South Australia, where Dagmara Riitano is a research officer. Contact author: Edoardo Aromataris, [email protected] . The authors have disclosed no potential conflicts of interest, financial or otherwise.
The Joanna Briggs Institute aims to inform health care decision making globally through the use of research evidence. It has developed innovative methods for appraising and synthesizing evidence; facilitating the transfer of evidence to health systems, health care professionals, and consumers; and creating tools to evaluate the impact of research on outcomes. For more on the institute's approach to weighing the evidence for practice, go to http://joannabriggs.org/jbi-approach.html .
Overview
This article is the third in a new series on the systematic review from the Joanna Briggs Institute, an international collaborative supporting evidence-based practice in nursing, medicine, and allied health fields. The purpose of the series is to show nurses how to conduct a systematic review—one step at a time. This article details the major considerations surrounding search strategies and presents an example of a search using the PubMed platform (pubmed.gov).
The third article in a series from the Joanna Briggs Institute details how to develop a comprehensive search strategy for a systematic review.
The systematic literature review, widely regarded as the gold standard for determining evidence-based practice, is increasingly used to guide policy decisions and the direction of future research. The findings of systematic reviews have greater validity than those of other types of reviews because the systematic methods used seek to minimize bias and increase rigor in identifying and synthesizing the best available evidence on a particular question. It's therefore important that when you search for evidence, you attempt to find all eligible studies and consider them for inclusion in your review. 1
One rule of thumb we use when beginning a search for evidence to support a systematic review: if you don't find the evidence, it can't be reviewed! Unfortunately, there is no prescriptive approach to conducting a comprehensive search. But searching is an art that can be cultivated and practiced. It involves several standard processes, such as developing search strings, searching across bibliographic citation databases that index health care research, looking for “gray,” or unpublished, literature, and hand searching.
GETTING STARTED
Developing a search strategy is an iterative process—that is, it involves continual assessment and refinement. As keywords or key terms are used in a search, their usefulness will be determined by the search results. Consequently, searching for evidence is sometimes considered more of an art than a science. It's therefore unlikely that two people, whether they are clinicians or librarians, will develop an identical search strategy or yield identical results from a search on the same review question.
The time required to conduct a search for a systematic review will also vary. It's dependent on the review question, the breadth of the evidence base, and the scope of the proposed search as stated in the review protocol. Narrow searches will often be adequate when investigating a topic requiring a few specific keywords, such as when you're searching only for randomized controlled trials (RCTs) conducted in a single population with a rare disorder. A narrow search will be less resource intensive than a search conducted when the review question is broader or the search relies on general keywords (such as education , prevention , or experience ). And while it may seem important conceptually to use a general keyword (such as safety in a search for articles on medical errors, for example), in practice it will add few relevant studies beyond those identified using more specific terms (such as error or harm ).
When beginning the search for evidence, you should conduct a few small searches as a test of various search terms and combinations of terms. An ideal search strategy is both sensitive and specific: a sensitive search will recall relevant studies, while a specific search will exclude irrelevant studies. A search that is overly sensitive may capture all the necessary studies but may require a labor-intensive vetting of unnecessary studies at the stage of study selection. A search that is overly specific will yield fewer results but is always subject to the risk that important studies may have been omitted.
Finding help. Given the complexity of the many indexing languages and rules governing the various databases, we recommend that early in the process you make use of an experienced research librarian who can examine your search strategy and help you choose citation databases relevant to your review question. If you can't easily access the services of a research librarian, there are many online tutorials that can help. A Google search—for example, “How do I search using PubMed?”—will reveal sites containing helpful hints and training developed by the U.S. National Library of Medicine (NLM) and librarians from across the globe.
DEVELOPING THE SEARCH STRATEGY
A review protocol with a clearly defined review question and inclusion criteria will provide the foundation for your search strategy. Before embarking on the search, you will need to understand the review question and what information you'll need to address it. For example, it's important to consider the type of data being sought (quantitative, qualitative, economic), the types of studies that report the data (RCTs, cohort studies, ethnographic studies), and the limits or restrictions you'll apply (publication date or language). This will shorten the time required to search and help to ensure that the information retrieved is both relevant and valid.
Once you've determined the review question, you'll need to identify the key terms articulated in the question and the protocol and create a logic grid or concept map. In a logic grid for a review on the effectiveness of an intervention, for example, each column represents a discrete concept that is generally aligned with each element of the PICO mnemonic— P opulation, I ntervention, C omparison intervention, and O utcome measures.

Consider an example using the following review question: “Is animal-assisted therapy more effective than music therapy in managing aggressive behavior in elderly people with dementia?” Within this question are the four PICO concepts: elderly patients with dementia (population), animal-assisted therapy (intervention), music therapy (comparison intervention), and aggressive behavior (outcome measures) (see Table 1 for an example of a logic grid).

Keywords or free-text words. The first formal step in all searches is to determine any alternative terms or synonyms for the identified concepts in the logic grid. Normally, you'll identify these terms—often referred to as keywords or free-text words—within the literature itself. Perhaps you'll start with a simple search using the terms dementia and animal-assisted therapy or music therapy and aggressive behavior . By looking at the titles and abstracts of the retrieved articles, you can find key terms used in the literature, as well as key concepts that are important to your question. For instance, is the term animal-assisted therapy used synonymously with the term pet therapy ? Furthermore, retrieving and reading a few relevant studies of any design—such as an experimental study or a traditional literature review on the topic—will further aid in identifying any commonly used terms.
When developing your search strategy, note that most search platforms (such as Ovid or EBSCOhost) used to access databases (such as MEDLINE) search for the exact terms entered in the database, including any misspellings. This means that to conduct a comprehensive search, you should enter as many relevant key terms as possible. Important articles may be overlooked if all relevant synonyms for a concept aren't included, as some authors may refer to the same concept using a different term (such as heart attack instead of myocardial infarction ). Such differences notwithstanding, you may find that including a relevant but broad term may retrieve many irrelevant studies.
Expanding on the logic grid shown in Table 1 , Table 2 now contains the keywords chosen from scanning the titles and abstracts of retrieved articles in your initial search. Column one contains terms relating to dementia , the defining feature of the population of interest; columns two and three contain terms relating to animal-assisted therapy and music therapy , the intervention and comparator of interest; and column four contains terms relating to aggressive behavior , the outcome of interest. Placing the terms into a logic grid illustrates how the related concepts or synonyms will combine to construct the final search string.
Index terms or subject headings. Comprehensive search strategies should consist of both keywords or free-text words and index terms, which are used by some major bibliographic databases to describe the content of each published article using a “controlled vocabulary”—that is, a list of standard terms that categorize articles based on their content (such terms will vary from database to database). For example, PubMed uses medical subject heading (MeSH) terms, the controlled vocabulary of MEDLINE. 2 MeSH terms are categorized within 16 main “trees” (such as anatomy, organisms, diseases, drugs, and chemicals), each of which branches from the broadest to the most specific terms.
To determine whether index terms exist for the concepts you've identified in your review question, you can search for each term in the MeSH database (selected from the drop-down list on the PubMed home page). For example, by entering dementia , PubMed will identify relevant MeSH terms that include Dementia and Alzheimer Disease . By selecting Dementia , you'll see the term's tree, including the subcategories listed below it, such as Lewy Body Disease .
As was the case when identifying key terms to use in the search strategy, it is also recommended that an initial, simple search using a few key concepts ( dementia AND animal-assisted therapy or dementia AND music therapy AND aggressive behavior ) be performed in PubMed to identify index terms. The aim is to retrieve a few relevant articles to see how they were indexed using the controlled vocabulary. Once the results are displayed, you can scroll through the citations and click on the title of any eligible article to view its details. From here, follow the link to the article's MeSH terms and examine which ones were used to describe the article's content. Repeat this process with a number of different articles to determine whether similar indexing terms have been used.

The terms in the logic grid can now be updated with the MeSH terms you have chosen from those listed with each retrieved article (see Table 3 ). The [mh] that appears next to these terms in the grid is the search-field descriptor that stands for “MeSH headings.” It's worth noting that “Entry Terms” under each search term's MeSH listing (if one is available) can also be examined for suggestions of alternative terms that can be searched in titles and abstracts.
Because new articles in PubMed are not indexed immediately, and because indexing is a manual, subjective process susceptible to human variation, it's important to also search for the key terms in the titles and abstracts of articles—in other words, for free-text or keywords—to capture any articles that could be missed by using index terms (such as MeSH headings) alone. For example, if we did not search for free-text words and did not include the index term Bonding, Human Pet (a MeSH term), we might miss an important article that wasn't indexed under the MeSH term Animal-Assisted Therapy .

By adding the search-field descriptor [tiab] (meaning “title/abstract”) to a search term, you can direct PubMed to search the title and abstract field code for these terms. A number of other search-field descriptors can be used as well, such as [au] for “author” and [pt] for “publication type.” 2 Using a search-field descriptor such as [tw] (“text word”) is often preferred over [tiab] for systematic reviews because the former searches in the title and abstract of articles as well as across a greater number of fields and will return a greater number of results for the same search query. Shortcuts or “wildcard” characters can also be used to account for different terminology or spelling. For example, PubMed allows truncation searching, in which an asterisk can substitute for any word's beginning or ending (for instance, a search for therap* will retrieve articles with the words therapy and therapeutic ). Search-field descriptors and wildcard characters should be applied to any newly identified keywords and index terms in the logic grid (see Table 4 ).
Once all search terms, including both free-text words and indexing terms, have been collected and finalized, a second search can then be undertaken across all selected citation databases. Initially, the key terms and synonyms within each column in the logic grid are combined using “OR.” (Most databases use some form of Boolean logic—search terms connected by the Boolean operators “OR” and “AND,” among others.) This will direct the database to find articles containing any of the search terms within the indicated fields. To do this in PubMed, select the “Advanced” search box and clear the search history. Copy and paste the first set of terms into PubMed and run the search.
For example, an initial search for articles related to different types of dementia might look like this:
“Dementia [tw] OR Alzheimer [tw] OR Huntington* [tw] OR Kluver [tw] OR Lewy [tw] OR Dementia [mh] OR Alzheimer disease [mh]"
This search could yield more than 100,000 citations. Following this, clear the search box and repeat the process with search terms from the second column in Table 4 . It is easier to search each column of the logic grid individually—particularly if each column contains an extensive list of search terms—rather than combining all the search sets in one go. Furthermore, by running each search successively you can determine if a component of the search string is producing many irrelevant results and easily adjust the search strategy. In our example, if you add the term aggress* [tw] to capture aggressive and aggression in the title or abstract, you will get an overwhelming number of irrelevant results because these terms are also used to describe the spread of certain cancers.
Once you complete the searches aligned to each concept, click on the “Advanced” option again. This allows for display of the “search history” and for a ready combination of the individual searches using the Boolean operators “AND” and “OR.” Using this method, parentheses are automatically placed around each set of terms to maintain the logical structure of the search. For example, the search for articles on animal-assisted therapy versus music therapy to treat aggression in patients with dementia might look like this:
“(Dementia [tw] OR Alzheimer [tw] OR Huntington* [tw] OR Kluver [tw] OR Lewy [tw] OR Dementia [mh] OR Alzheimer disease [mh]) AND (Animal assisted therapy [tw] OR Animal assisted activit* [tiab] OR Animal assisted intervention* [tiab] OR Animal therapy [tw] OR Pet therapy [tw] OR Dog therapy [tw] OR Dog assisted therapy [tw] OR Canine assisted therapy [tw] OR Aquarium [tiab] OR Animal Assisted Therapy [mh] OR Pets [mh] OR Dogs [mh] OR Cats [mh] OR Birds [mh] OR Bonding, Human-Pet [mh] OR Animals, Domestic [mh]) OR (Music* [tw] OR Music therapy [tw] OR Singing [tw] OR Sing [tw] OR Auditory stimulat* [tw] OR Music [mh] OR Music Therapy [mh] OR Acoustic Stimulation [mh] OR Singing [mh]) AND (Aggression [tw] OR Neuropsychiatric [tiab] OR Apathy inventory [tiab] OR Cornell scale [tiab] OR Cohen Mansfield [tiab] OR BEHAVE-AD [tiab] OR CERAD-BRSD [tiab] OR Behavior* [tiab] OR Behaviour* [tiab] OR Aggression [mh] OR Personality inventory [mh] OR Psychomotor agitation [mh])"
Once the final search has been conducted, you can further refine search results by publication date, study groups, language, or any other limits appropriate to the review topic by selecting the relevant filter (left-hand side of the screen in PubMed) from the range available. PubMed also provides predefined search filters that restrict search results to specific clinical study categories or subject matters (such as clinical queries). You will have determined the date range for the search at the protocol development stage. Given that your aim is to summarize the evidence surrounding a particular question, you should justify any limits to the publication date of included studies in the background section of the protocol. The chosen time frame will vary depending on the review question. For example, reviewers may impose a start date for a search that coincides with the introduction of a new intervention and the advent of the preceding clinical research on it.
The structure of the search strategy will remain the same regardless of the search platform used to search a database. But since most major databases use a unique controlled vocabulary to index their articles, the indexing terms will need to be adapted to each database; in most cases the key terms remain the same across different databases. These differences in indexing terms are the main reason it is not recommended to search bibliographic citation databases for a systematic review using a federated search engine or platform—that is, one that searches multiple databases and sources at once.
You should also be aware that the platforms used to search citation databases often use different wildcard characters or commands. For this reason, beginning searchers should use the online tutorials and help pages of the various platforms and databases. For example, while Ovid's search platform can also be used to search the MEDLINE database, the terms used for truncation searching are quite different: an asterisk (*) is used for unlimited truncation within PubMed and a dollar symbol ($) in Ovid. Moreover, in Ovid the question mark (?) wildcard can be used within or at the end of a word to substitute for one character or no characters ( behavio?r will retrieve articles with the words behaviour and behavior ); the number sign (#) wildcard can substitute for a single character ( wom#n will retrieve articles with both woman and women ). The use of wildcards for substitution of characters is not supported in PubMed.
Because searching is an iterative process, you won't want to predetermine when it will end. Consequently, it is important to look at the results of the search continually as you develop the search strategy to determine whether the results are relevant. One way to do this is to check if already identified relevant articles are being captured by the search. If not, the search strategy will need to be modified accordingly.
Once the search is complete, the results can be exported to bibliographic management software such as EndNote or Reference Manager. These tools are useful for organizing the search results, removing duplicate citations, and selecting studies (the next step of the systematic review process, to be discussed in the next article in this series).
WHERE TO SEARCH?
Developing the search strategy and search filters for use within each database is an important and time-consuming part of the search process, often more so than the search itself! Another important consideration is where to search. A search for a systematic review should be comprehensive and attempt to identify all of the available evidence. This can be an enormous undertaking.
Generally, a systematic review to inform health care practice and policy should search the major medical databases including MEDLINE from the NLM in North America and searchable through PubMed, and Embase, a product of Elsevier that indexes many European biomedical journals; the controlled vocabulary for Embase is searchable through Emtree, which also contains all MeSH terms ( www.elsevier.com/online-tools/embase/emtree ). Nurses undertaking systematic reviews will find that much literature relevant to nursing practice is also available in the Cumulative Index to Nursing and Allied Health Literature (CINAHL) database by EBSCO. Beyond these, there are many others: Web of Science, PsycINFO, Scopus, JSTOR, Academic Search Premier, Academic Onefile, the Cochrane Nursing Care Field trials register, and the list goes on.
You should establish which databases index articles relevant to the topic at hand. Some databases have a specific topic focus, such as PsycINFO, which should be searched for a question related to mental health. The JBI Database of Systematic Reviews and Implementation Reports is, as the name suggests, a repository for systematic reviews and would be unnecessary for most review searches (systematic reviews rarely include other systematic reviews among their inclusion criteria). Similarly, a quick Google search (“What information is in… ?”) to establish the content and coverage of other databases is worthwhile and will help in identifying unnecessary overlap in the search strategy.
Hand searching. You may also wish to consider more traditional means of locating evidence. Screening the reference lists of studies already selected for inclusion in the review is often a valuable means of identifying other pertinent studies. Similarly, hand searching specific journals is often used by systematic review authors to locate studies. Journals selected for hand searching should be identified as relevant from database or preliminary searching; the likelihood is that these journals may contain relevant studies. Because hand searching can be an onerous task, it's recommended that no more than two or three relevant journals should be hand searched for a review.
Finding experts is another method of locating evidence. While contacting authors to clarify details of studies and to request data are relatively common pursuits for the systematic reviewer during the appraisal and extraction processes, doing so to identify relevant studies can also be useful. Such experts can often provide papers that even a comprehensive search may have failed to identify.
SHADES OF GRAY
Systematic reviews that purport to have conducted a comprehensive search should have made some attempt to search for gray literature. The International Conference on Grey Literature in Luxembourg defined it in 1997 (and expanded on it in 2004) as literature “produced at all levels of government, academic, business and industry in electronic and print formats not controlled by commercial publishing.” 3 However, this definition is often broadened to include any study or paper that has not been formally published or peer reviewed. Gray literature often appears in the form of government or institution reports and newsletters and even in blogs, conference proceedings, census reports, or nonindependent research papers. As a result, these reports or manuscripts are often not as widely available and are generally more difficult to locate.
Nonetheless, the inclusion of gray literature in systematic reviews has emerged as an important adjunct to commercially published research, as it often reflects a source of timely or alternative information that can help to minimize publication bias and provide a more accurate and thorough account of the evidence. 4, 5
There are three common ways to search for gray literature. The first involves searching or browsing the Web sites of organizations relevant to the review question (such as the World Health Organization or the National Institute for Health and Care Excellence). The second involves searching databases that collate and index gray literature. Although gray literature is rarely indexed, two commonly used sources are OpenGrey ( www.opengrey.eu ), an open access database to gray literature from Europe, and the Grey Literature Report ( www.greylit.org ), a bimonthly report from the New York Academy of Medicine. Reviewers will find that such databases do not have an extensive or advanced search capability, and therefore searching them is often limited to the use of a few critical keywords. Furthermore, they lack indexing or subject headings; without this feature a search can be quite time consuming. The third approach is to use online search engines. Search engines such as Google do not use a controlled vocabulary and so performing a simple search of a few select keywords is best. Such sites will yield a large number of results. To make results more manageable, you can try limiting the search to terms that appear on the title page of an article only 6 or by using keywords that limit the results to specific documents (such as guidelines). Searches can also be limited by language or sources (for example, adding site:gov to a Google search will limit results to government Web sites). An example of a tool that can also help is the federated search engine MedNar ( http://mednar.com/mednar ) that searches across a range of government and organizational sites, as well as commercial databases.
Other sources of gray literature can be found in numerous guides developed to assist researchers. For example, the Canadian Agency for Drugs and Technologies in Health's Grey Matters provides an extensive list of gray literature sources that can be searched. 7 Developed with the systematic reviewer in mind, the tool kit provides a checklist that aids users in documenting the search process and in ensuring it has been conducted in a standardized way.
REPORTING THE SEARCH STRATEGY
The final consideration is reporting the details of the search strategy, including the filters (such as language, date limits) and databases and other sources used. A hallmark of a systematic review is its reproducibility; another researcher should be able to review the same question and arrive at similar conclusions. Without a transparent reporting of the search strategy—one that allows readers to assess the quality of the search and its sources, and in turn, make a judgment on the likely credibility of the review 8, 9 —this would not be possible.
Most journals that publish systematic reviews now espouse the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses; online at www.prisma-statement.org ), which dictate that the full search strategy for at least one major database should be reported in an appendix and published along with the review. 10 Online repositories of systematic reviews, such as the JBI Database of Systematic Reviews and Implementation Reports and the Cochrane Database of Systematic Reviews , allow for publication of all the search filters and strategies across the databases and sites used. A systematic reviewer will appreciate that reporting only the search filters used is inadequate. The methods section of a review should list all of the bibliographic citation databases searched, ideally with the platform used to search them, as well as the dates they were searched and any limits used. The results of the search should be adequately reported, as well; this is often quite simple to convey in a flow diagram, which is also detailed in the PRISMA guidelines. 10
Once the search is complete and the results from each source have been exported, the next step, study selection, can begin. This is where titles, abstracts, and sometimes the full text of studies found are screened against the inclusion and exclusion criteria. This step of the process will be the focus of the next article in this series.
evidence; gray literature; literature search; review question; systematic review
- + Favorites
- View in Gallery
Readers Of this Article Also Read
The systematic review: an overview, evidence-based practice: step by step: igniting a spirit of inquiry, evidence-based practice: step by step: the seven steps of evidence-based..., evidence-based practice, step by step: asking the clinical question: a key step ..., developing the review question and inclusion criteria.

Systematic Review
- Library Help
- What is a Systematic Review (SR)?
- Steps of a Systematic Review
- Framing a Research Question
Developing a Search Strategy
- Searching the Literature
- Managing the Process
- Meta-analysis
- Publishing your Systematic Review
Workshop materials
- PICO Worksheet
- Search Strategy Example
- Search Strategy Presentation Slides
- Search strings for demo
Find step-by-step instructions on how to develop a search strategy on p. 44
Errors in search strategies
Salvador-Oliván, J. A., Marco-Cuenca, G., & Arquero-Avilés, R. (2019). Errors in search strategies used in systematic reviews and their effects on information retrieval . Journal of the Medical Library Association : JMLA , 107 (2), 210–221. https://doi.org/10.5195/jmla.2019.567 .
- Search Term Harvesting
- Text Mining Tools
- Search Filters / Hedges
- Documenting
- Blogs & Discussion Lists
|
Image: |
Begin brainstorming search terms by using the following techniques: supplied by the principal investigator or found through preliminary searches. from . and . Use database tools (e.g. , index, subject headings) to find controlled vocabulary terms; to locate word variants or synonyms, tools to find Medical Subject Headings ( terms or "implicit" keywords. to generate a few options for your initial research topic and narrow it down to a specific population, geographical location, disease, etc. You may explore similar tools, or to identify additional search terms.Look for relevant and/or frequently occurring terms. List all terms in an Excel . Example: Learn how to . Test out the new feature: . Try as an alternative to conventional "advanced search." Instead of entering Boolean strings into one-dimensional search boxes, queries are formulated by manipulating objects on a two-dimensional canvas. This eliminates syntax errors, makes the query semantics more transparent, and offers new ways to collaborate, share, and optimize search strategies and best practices. |
Translating search strategies across databases
- ChatGPT Ask ChatGPT using this prompt, "Covert this search into terms appropriate for the [name] database." Further reading: Wang, S., Scells, H., Koopman, B., & Zuccon, G. (2023). Can ChatGPT write a good boolean query for systematic review literature search?. arXiv preprint arXiv:2302.03495.
- LitSonar Use the Help section for further guidance on how to use this tool (https://litsonar.com/help). Capable of searching eight different databases simultaneously
- Polyglot Use the Polyglot tool to translate search strings from PubMed across multiple databases. Access the tool's tutorial for more information (https://sr-accelerator.com/#/help/polyglot).
- MEDLINE Transpose Use this tool to translate your MEDLINE (PubMed) search to MEDLINE (Ovid) format or vice versa.
- Database Syntax Guide Guide to translating syntax for multiple databases. From Cochrane.
_____________________________________________________________
Take control of your search and turn off Pubmed's Automatic Term Mapping (ATM) ! It will not include all variant terminology and automatically explodes MeSH terms. Not using ATM allows for clearer documentation of the search method.
For more information on Automatic Term Mapping, watch the video below .
Further readings
- Burns, C. S., Nix, T., Shapiro, R. M., & Huber, J. T. (2021). MEDLINE search retrieval issues: A longitudinal query analysis of five vendor platforms. PLoS ONE , 16 (5), e0234221. https://doi.org/10.1371/journal.pone.0234221
|
Image by , Assistant Professor, SCEM, Mangaluru | These tools can help you with building your search strategy. |
- PubMed Pub ReMiner - Text mining for PubMed to look at commonalities between MeSH terms and keywords
- Go PubMed - Text mining tool for PubMed or MeSH terms. This article explains the features of this text mining tool.
- PubVenn - This tool enables you to explore PubMed using venn diagrams. Also, try Search Workbench .
- Yale MeSH Analyzer - Watch this tutorial (7 min.). This tool allows users to enter up to 20 PubMed ID numbers, which it uses to aggregate the metadata from the associated articles into a spreadsheet. For systematic reviews, it is useful in search strategy development to quickly aggregate the Medical Subject Heading (MeSH) terms associated with relevant articles. While it only works for PubMed, it can be useful for developing searches in medical-adjacent fields, such as psychology, nutrition, and animal health.
- NCBI MeSH on Demand identifies MeSH® terms in your submitted text (abstract or manuscript). MeSH on Demand also lists PubMed similar articles relevant to your submitted text.
- HelioBLAST - This tool finds text records that are similar to the submitted query. Your query is searched against the citations (abstract and titles) in Medline/PubMed and the top matching articles are returned in the results.
- Coremine - It is ideal for those seeking an overview of a complex subject while allowing the possibility to "drill down" to specific details. Instructions
- Carrot2 - This tool can automatically organize search results into topics. It can query PubMed and allows boolean searching.
- SWIFT-Review - Desktop text mining tool specific to systematic reviews. To obtain your free license for SWIFT Review, simply browse to the Sciome Software web page to login and/or create your SWIFT-Review account.
- Voyant - General text mining (this is the download). For the web version go to http://voyant-tools.org
- TerMine - General text mining
- JSTOR Text Analyzer - Recommends journal articles in JSTOR relevant to text.
- CREBP-SRA Word Frequency Analyser (WFA) - This tool helps determine which words you should use to construct and refine a search strategy
- Medline Ranker requires a set of known relevant records with PubMed identifiers and a test set of records (e.g. search results from a highly sensitive search). Medline Ranker sorts the records in the test set and presents those that were most similar to the relevant records first. Medline Ranker also provides a list of discriminating terms which discriminate relevant records from non-relevant records.
_________________________________________________________________________
For more information on text mining tools - review and comparison, read the following article:
Paynter, R., Bañez, L. L., Berliner, E., Erinoff, E., Lege-Matsuura, J., Potter, S., & Uhl, S. (2016). EPC methods: an exploration of the use of text-mining software in systematic reviews .
|
Image modified from , image #24 | Search hedges are vetted strategies created by expert searchers
If you edit a filter, note this in the manuscript. Example: “We used a prognosis filter based on that developed by Smith (2015).” |
You might limit to a particular publication type in Pubmed. See a full list of Pubmed publication types .
- Cochrane Handbook Part 2, Section 6.4.11 provides search filters to limit to randomized controlled trials in Medline/PubMed, Medline/Ovid, and Embase
- McMaster - Filters by the Hedges team
- PubMed Systematic Review Filter Search Strategy
- Search Filters from Univ. of Texas School of Public Health
Hedges by Topic (in alphabetical order )
- Prady, S. L., Uphoff, E. P., Power, M., & Golder, S. (2018). Development and validation of a search filter to identify equity-focused studies: Reducing the number needed to screen. BMC Medical Research Methodology, 18 (1), 106. https://doi.org/10.1186/s12874-018-0567-x
- Health Risk Assessment by Vicky Tessier at the INSPQ
- Effectiveness of Interventions
- van der Mierden, S., Hooijmans, C. R., Tillema, A. H., Rehn, S., Bleich, A., & Leenaars, C. H. (2022). Laboratory animals search filter for different literature databases: PubMed, Embase, Web of Science and PsycINFO. Laboratory animals , 56 (3), 279–286. https://doi.org/10.1177/00236772211045485
|
Image by | To find any nesting errors, use and check the option. Check your search strategy for any errors using the checklist below. |
- Updated Press Checklist (2015) See page 41-42
- IOM Standards for Systematic Reviews
- PRESS Checklist

McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement . Journal of Clinical Epidemiology, 75 , 40-46.
|
Image by | Keep track of all your search terms and search strategies that you have used for each database. You will need this information as supplemental material to accompany your manuscript. about how to present your search strategy: vs vs
|
Systematic Literature Review Worksheet
Use the Database Search Log to record your search terms, search strategy and databases searched.
Guidance on Reporting Systematic Reviews
Cochrane strongly encourages that review authors include a study flow diagram as recommended by the PRISMA statement.
- PRISMA Flow Diagram
- PRISMA Flow Diagram Generator
- PRISMA Checklist
Other checklists include:
- ARRIVE and DSPC for animal studies
- MOOSE - meta-analysis of observational studies in epidemiology
- STARLITE - general health policy and clinical practice
- TIDier-PHP - population health and policy interventions
Examples of documented search methodologies:
- Full search strategies for all database searches provided in the Appendices:
Bath, P. & Krishnan, K. (2014). Interventions for deliberately altering blood pressure in acute stroke . Cochrane Database of Systematic Reviews, 10.
- A summary of sources searched and keywords used in the Sources section:
McIntyre, S, Taitz, D, Keogh, J, Goldsmith, S, Badawi, N & Blair, E. (2013). A systematic review of risk factors for cerebral palsy in children born at term in developed countries . Developmental Medicine & Child Neurology, 55( 6), 499-508.
|
Image by | Learn from other experienced searchers and get professional advice from the library community. |
- ACRL Systematic Reviews & Related Methods Interest Group [email protected]
- Cindy Schmidt's Blog: PubMed Search Strategies This blog has been created to share PubMed search strategies. Search strategies posted here are not perfect. They are posted in the hope that others will benefit from the work already put into their creation and/or will offer suggestions for improvements.
- MedTerm Search Assist from the University of Pittsburgh By librarians for librarians - A database to share biomedical terminology and strategies for comprehensive searches.
- MLA expert searching discussion list [email protected] - This discussion list often discusses subject strategies and sometimes search filters.
- PRESS Forum This closed wiki-based forum is a place for librarians to request reviews of systematic review search strategies, and to review the searches of others.
- << Previous: Framing a Research Question
- Next: Searching the Literature >>
- Last Updated: Aug 26, 2024 12:37 PM
- URL: https://lib.guides.umd.edu/SR
Search Strategies for [Systematic] Literature Reviews
- First Online: 11 August 2022
Cite this chapter

- Rob Dekkers 4 ,
- Lindsey Carey 5 &
- Peter Langhorne 6
2422 Accesses
4 Citations
After setting review questions as discussed in the previous chapter, the search for relevant publications is the next step of a literature review.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
JEL is the abbreviation of the ‘Journal of Economics Literature’, published by the American Economic Association, which launched this coding system.
Actually, Schlosser et al. ( 2006 , p. 571 ff.) call it ‘traditional pearl growing.’ The term ‘classical’ pearl growing has been adopted to ensure consistency throughout the book.
The wording ‘topical bibliography’ by Schlosser et al. ( 2006 , p. 574) has been replaced with ‘topical survey’ in order to connect better to the terminology in this book.
Webster and Watson ( 2002 , p. xvi) call it forward searching and backward searching rather than snowballing. See Table 5.3 for the nomenclature used in the book for search strategies.
Aguillo IF (2012) Is Google Scholar useful for bibliometrics? A webometric analysis. Scientometrics 91(2):343–351. https://doi.org/10.1007/s11192-011-0582-8
Bardia A, Wahner-Roedler DL, Erwin PL, Sood A (2006) Search strategies for retrieving complementary and alternative medicine clinical trials in oncology. Integr Cancer Ther 5(3):202–205. https://doi.org/10.1177/1534735406292146
Bates MJ (1989) The design of browsing and berrypicking techniques for the online search interface. Online Rev 13(5):407–424
Google Scholar
Bates MJ (2007) What is browsing—really? A model drawing from behavioural science research. Inform Res 20(4). http://informationr.net/ir/12-4/paper330.html
Benzies KM, Premji S, Hayden KA, Serrett K (2006) State-of-the-evidence reviews: advantages and challenges of including grey literature. Worldviews Evid Based Nurs 3(2):55–61. https://doi.org/10.1111/j.1741-6787.2006.00051.x
Bernardo M, Simon A, Tarí JJ, Molina-Azorín JF (2015) Benefits of management systems integration: a literature review. J Clean Prod 94:260–267. https://doi.org/10.1016/j.jclepro.2015.01.075
Beynon R, Leeflang MM, McDonald S, Eisinga A, Mitchell RL, Whiting P, Glanville JM (2013) Search strategies to identify diagnostic accuracy studies in MEDLINE and EMBASE. Cochrane Database Syst Rev (9). https://doi.org/10.1002/14651858.MR000022.pub3
Bolton JE (1971) Small firms—report of the committee of inquiry on small firms (4811). London
Boluyt N, Tjosvold L, Lefebvre C, Klassen TP, Offringa M (2008) Usefulness of systematic review search strategies in finding child health systematic reviews in MEDLINE. Arch Pediatr Adolesc Med 162(2):111–116. https://doi.org/10.1001/archpediatrics.2007.40
Booth A, Noyes J, Flemming K, Gerhardus A, Wahlster P, van der Wilt GJ, Rehfuess E (2018) Structured methodology review identified seven (RETREAT) criteria for selecting qualitative evidence synthesis approaches. J Clinic Epidemiol 99:41–52. https://doi.org/10.1016/j.jclinepi.2018.03.003
Chesbrough H (2012) Open innovation: where we’ve been and where we’re going. Res Technol Manag 55(4):20–27. https://doi.org/10.5437/08956308X5504085
Chesbrough HW (2003) Open innovation: the new imperative for creating and profiting from technology. Harvard Business School Press, Boston
Conn VS, Valentine JC, Cooper HM, Rantz MJ (2003) Grey literature in meta-analyses. Nurs Res 52(4):256–261
de la Torre Díez I, Cosgaya HM, Garcia-Zapirain B, López-Coronado M (2016) Big data in health: a literature review from the year 2005. J Med Syst 40(9):209. https://doi.org/10.1007/s10916-016-0565-7
Dekkers R, Hicks C (2019) How many cases do you need for studies into operations management? Guidance based on saturation. In: Paper presented at the 26th EurOMA conference, Helsinki
Dekkers R, Koukou MI, Mitchell S, Sinclair S (2019) Engaging with open innovation: a Scottish perspective on its opportunities, challenges and risks. J Innov Econ Manag 28(1):193–226. https://doi.org/10.3917/jie.028.0187
Dieste O, Grimán A, Juristo N (2009) Developing search strategies for detecting relevant experiments. Empir Softw Eng 14(5):513–539. https://doi.org/10.1007/s10664-008-9091-7
Eady AM, Wilczynski NL, Haynes RB (2008) PsycINFO search strategies identified methodologically sound therapy studies and review articles for use by clinicians and researchers. J Clin Epidemiol 61(1):34–40. https://doi.org/10.1016/j.jclinepi.2006.09.016
Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, Antes G (1997) Language bias in randomised controlled trials published in English and German. The Lancet 350(9074):326–329. https://doi.org/10.1016/S0140-6736(97)02419-7
Eisenhardt KM (1989) Agency theory: an assessment and review. Acad Manag Rev 14(1):57–74. https://doi.org/10.5465/AMR.1989.4279003
Finfgeld-Connett D, Johnson ED (2013) Literature search strategies for conducting knowledge-building and theory-generating qualitative systematic reviews. J Adv Nurs 69(1):194–204. https://doi.org/10.1111/j.1365-2648.2012.06037.x
Frederick JT, Steinman LE, Prohaska T, Satariano WA, Bruce M, Bryant L, Snowden M (2007) Community-based treatment of late life depression: an expert panel-informed literature review. Am J Prev Med 33(3):222–249. https://doi.org/10.1016/j.amepre.2007.04.035
Glanville J, Kaunelis D, Mensinkai S (2009) How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. Int J Technol Assess Health Care 25(4):522–529. https://doi.org/10.1017/S0266462309990523
Godin K, Stapleton J, Kirkpatrick SI, Hanning RM, Leatherdale ST (2015) Applying systematic review search methods to the grey literature: a case study examining guidelines for school-based breakfast programs in Canada. Syst Rev 4(1):138. https://doi.org/10.1186/s13643-015-0125-0
Green BN, Johnson CD, Adams A (2006) Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med 5(3):101–117. https://doi.org/10.1016/S0899-3467(07)60142-6
Greenhalgh T, Peacock R (2005) Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ 331(7524):1064–1065. https://doi.org/10.1136/bmj.38636.593461.68
Grégoire G, Derderian F, le Lorier J (1995) Selecting the language of the publications included in a meta-analysis: is there a Tower of Babel bias? J Clin Epidemiol 48(1):159–163
Gross T, Taylor AG, Joudrey DN (2015) Still a lot to lose: the role of controlled vocabulary in keyword searching. Catalog Classific Q 53(1):1–39. https://doi.org/10.1080/01639374.2014.917447
Grosso G, Godos J, Galvano F, Giovannucci EL (2017) Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr 37(1):131–156. https://doi.org/10.1146/annurev-nutr-071816-064941
Gusenbauer M, Haddaway NR (2020) Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res Synthesis Methods 11(2):181–217. https://doi.org/10.1002/jrsm.1378
Haddaway NR, Bayliss HR (2015) Shades of grey: two forms of grey literature important for reviews in conservation. Biol Cons 191:827–829. https://doi.org/10.1016/j.biocon.2015.08.018
Haddaway NR, Collins AM, Coughlin D, Kirk S (2015) The role of Google Scholar in evidence reviews and its applicability to grey literature searching. PLoS One 10(9):e0138237. https://doi.org/10.1371/journal.pone.0138237
Harari MB, Parola HR, Hartwell CJ, Riegelman A (2020) Literature searches in systematic reviews and meta-analyses: a review, evaluation, and recommendations. J Vocat Behav 118:103377. https://doi.org/10.1016/j.jvb.2020.103377
Harzing A-WK, van der Wal R (2008) Google Scholar as a new source for citation analysis. Ethics Sci Environ Politics 8(1):61–73. https://doi.org/10.3354/esep00076
Hausner E, Guddat C, Hermanns T, Lampert U, Waffenschmidt S (2016) Prospective comparison of search strategies for systematic reviews: an objective approach yielded higher sensitivity than a conceptual one. J Clin Epidemiol 77:118–124. https://doi.org/10.1016/j.jclinepi.2016.05.002
Hausner E, Waffenschmidt S, Kaiser T, Simon M (2012) Routine development of objectively derived search strategies. Syst Rev 1(1):19. https://doi.org/10.1186/2046-4053-1-19
Havill NL, Leeman J, Shaw-Kokot J, Knafl K, Crandell J, Sandelowski M (2014) Managing large-volume literature searches in research synthesis studies. Nurs Outlook 62(2):112–118. https://doi.org/10.1016/j.outlook.2013.11.002
Hildebrand AM, Iansavichus AV, Haynes RB, Wilczynski NL, Mehta RL, Parikh CR, Garg AX (2014) High-performance information search filters for acute kidney injury content in PubMed, Ovid Medline and Embase. Nephrol Dial Transplant 29(4):823–832. https://doi.org/10.1093/ndt/gft531
Hinckeldeyn J, Dekkers R, Kreutzfeldt J (2015) Productivity of product design and engineering processes—unexplored territory for production management techniques? Int J Oper Prod Manag 35(4):458–486. https://doi.org/10.1108/IJOPM-03-2013-0101
Hopewell S, Clarke M, Lefebvre C, Scherer R (2007) Handsearching versus electronic searching to identify reports of randomized trials. Cochrane Database Syst Rev (2):MR000001. https://doi.org/10.1002/14651858.mr000001.pub2
Isckia T, Lescop D (2011) Une analyse critique des fondements de l’innovation ouverte. Rev Fr Gest 210(1):87–98
Jackson JL, Kuriyama A (2019) How often do systematic reviews exclude articles not published in English? J Gen Intern Med 34(8):1388–1389. https://doi.org/10.1007/s11606-019-04976-x
Jennex ME (2015) Literature reviews and the review process: an editor-in-chief’s perspective. Commun Assoc Inf Syst 36:139–146. https://doi.org/10.17705/1CAIS.03608
Jensen MC, Meckling WH (1976) Theory of the firm: managerial behavior, agency costs and ownership structure. J Financ Econ 3(4):305–360. https://doi.org/10.1016/0304-405X(76)90026-X
Jüni P, Holenstein F, Sterne J, Bartlett C, Egger M (2002) Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol 31(1):115–123. https://doi.org/10.1093/ije/31.1.115
Kennedy MM (2007) Defining a literature. Educ Res 36(3):139. https://doi.org/10.3102/0013189x07299197
Koffel JB (2015) Use of recommended search strategies in systematic reviews and the impact of librarian involvement: a cross-sectional survey of recent authors. PLoS One 10(5):e0125931. https://doi.org/10.1371/journal.pone.0125931
Koffel JB, Rethlefsen ML (2016) Reproducibility of search strategies is poor in systematic reviews published in high-impact pediatrics, cardiology and surgery journals: a cross-sectional study. PLoS One 11(9):e0163309. https://doi.org/10.1371/journal.pone.0163309
Lawal AK, Rotter T, Kinsman L, Sari N, Harrison L, Jeffery C, Flynn R (2014) Lean management in health care: definition, concepts, methodology and effects reported (systematic review protocol). Syst Rev 3(1):103. https://doi.org/10.1186/2046-4053-3-103
Levay P, Ainsworth N, Kettle R, Morgan A (2016) Identifying evidence for public health guidance: a comparison of citation searching with Web of Science and Google Scholar. Res Synthesis Methods 7(1):34–45. https://doi.org/10.1002/jrsm.1158
Li L, Smith HE, Atun R, Tudor Car L (2019) Search strategies to identify observational studies in MEDLINE and Embase. Cochrane Database Syst Rev (3). https://doi.org/10.1002/14651858.MR000041.pub2
Linton JD, Thongpapanl NT (2004) Ranking the technology innovation management journals. J Prod Innov Manag 21(2):123–139. https://doi.org/10.1111/j.0737-6782.2004.00062.x
Lokker C, McKibbon KA, Wilczynski NL, Haynes RB, Ciliska D, Dobbins M, Straus SE (2010) Finding knowledge translation articles in CINAHL. Studies Health Technol Inform 160(2):1179–1183
Lu Z (2011) PubMed and beyond: a survey of web tools for searching biomedical literature. Database. https://doi.org/10.1093/database/baq036
MacSuga-Gage AS, Simonsen B (2015) Examining the effects of teacher—directed opportunities to respond on student outcomes: a systematic review of the literature. Educ Treat Child 38(2):211–239. https://doi.org/10.1353/etc.2015.0009
Mahood Q, Van Eerd D, Irvin E (2014) Searching for grey literature for systematic reviews: challenges and benefits. Res Synthesis Methods 5(3):221–234. https://doi.org/10.1002/jrsm.1106
Marangunić N, Granić A (2015) Technology acceptance model: a literature review from 1986 to 2013. Univ Access Inf Soc 14(1):81–95. https://doi.org/10.1007/s10209-014-0348-1
Mc Elhinney H, Taylor B, Sinclair M, Holman MR (2016) Sensitivity and specificity of electronic databases: the example of searching for evidence on child protection issues related to pregnant women. Evid Based Midwifery 14(1):29–34
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C (2016) PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 75:40–46. https://doi.org/10.1016/j.jclinepi.2016.01.021
McManus RJ, Wilson S, Delaney BC, Fitzmaurice DA, Hyde CJ, Tobias S, Hobbs FDR (1998) Review of the usefulness of contacting other experts when conducting a literature search for systematic reviews. Br Med J 317(7172):1562–1563 https://doi.org/10.1136/bmj.317.7172.1562
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi (2014) PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res 14(1):579. https://doi.org/10.1186/s12913-014-0579-0
Mitnick BM (1973) Fiduciary rationality and public policy: the theory of agency and some consequences. In: Paper presented at the annual meeting of the American political science association, New Orleans, LA
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, Rabb D (2012) The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care 28(2):138–144. https://doi.org/10.1017/S0266462312000086
Neuhaus C, Daniel HD (2008) Data sources for performing citation analysis: an overview. J Document 64(2):193–210. https://doi.org/10.1108/00220410810858010
O’Mara-Eves A, Thomas J, McNaught J, Miwa M, Ananiadou S (2015) Using text mining for study identification in systematic reviews: a systematic review of current approaches. Syst Rev 4(1):5. https://doi.org/10.1186/2046-4053-4-5
Ogilvie D, Foster CE, Rothnie H, Cavill N, Hamilton V, Fitzsimons CF, Mutrie N (2007) Interventions to promote walking: systematic review. BMJ 334(7605):1204. https://doi.org/10.1136/bmj.39198.722720.BE
Onetti A (2019) Turning open innovation into practice: trends in European corporates. J Bus Strateg 42(1):51–58. https://doi.org/10.1108/JBS-07-2019-0138
Papaioannou D, Sutton A, Carroll C, Booth A, Wong R (2010) Literature searching for social science systematic reviews: consideration of a range of search techniques. Health Info Libr J 27(2):114–122. https://doi.org/10.1111/j.1471-1842.2009.00863.x
Pappas C, Williams I (2011) Grey literature: its emerging importance. J Hosp Librariansh 11(3):228–234. https://doi.org/10.1080/15323269.2011.587100
Pearson M, Moxham T, Ashton K (2011) Effectiveness of search strategies for qualitative research about barriers and facilitators of program delivery. Eval Health Prof 34(3):297–308. https://doi.org/10.1177/0163278710388029
Piggott-McKellar AE, McNamara KE, Nunn PD, Watson JEM (2019) What are the barriers to successful community-based climate change adaptation? A review of grey literature. Local Environ 24(4):374–390. https://doi.org/10.1080/13549839.2019.1580688
Piller F, West J (2014) Firms, users, and innovations: an interactive model of coupled innovation. In: Chesbrough HW, Vanhaverbeke W, West J (eds) New frontiers in open innovation. Oxford University Press, Oxford, pp 29–49
Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J (2017) Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ 359:j5024. https://doi.org/10.1136/bmj.j5024
Priem RL, Butler JE (2001) Is the resource-based “view” a useful perspective for strategic management research? Acad Manag Rev 26(1):22–40. https://doi.org/10.5465/amr.2001.4011928
Relevo R (2012) Effective search strategies for systematic reviews of medical tests. J Gener Internal Med 27(1):S28–S32. https://doi.org/10.1007/s11606-011-1873-8
Rethlefsen ML, Farrell AM, Osterhaus Trzasko LC, Brigham TJ (2015) Librarian co-authors correlated with higher quality reported search strategies in general internal medicine systematic reviews. J Clin Epidemiol 68(6):617–626. https://doi.org/10.1016/j.jclinepi.2014.11.025
Rewhorn S (2018) Writing your successful literature review. J Geogr High Educ 42(1):143–147. https://doi.org/10.1080/03098265.2017.1337732
Rietjens JA, Bramer WM, Geijteman EC, van der Heide A, Oldenmenger WH (2019) Development and validation of search filters to find articles on palliative care in bibliographic databases. Palliat Med 33(4):470–474. https://doi.org/10.1177/0269216318824275
Rogers M, Bethel A, Abbott R (2018) Locating qualitative studies in dementia on MEDLINE, EMBASE, CINAHL, and PsycINFO: a comparison of search strategies. Res Synthesis Methods 9(4):579–586. https://doi.org/10.1002/jrsm.1280
Rosenstock TS, Lamanna C, Chesterman S, Bell P, Arslan A, Richards M, Zhou W (2016) The scientific basis of climate-smart agriculture: a systematic review protocol. CGIAR, Copenhagen
Ross SA (1973) The economic theory of agency: the principal’s problem. Am Econ Rev 63(2):134–139
Rosumeck S, Wagner M, Wallraf S, Euler U (2020) A validation study revealed differences in design and performance of search filters for qualitative research in PsycINFO and CINAHL. J Clin Epidemiol 128:101–108. https://doi.org/10.1016/j.jclinepi.2020.09.031
Rowley J, Slack F (2004) Conducting a literature review. Manag Res News 27(6):31–39. https://doi.org/10.1108/01409170410784185
Rudestam K, Newton R (1992) Surviving your dissertation: a comprehensive guide to content and process. Sage, London
Salgado EG, Dekkers R (2018) Lean product development: nothing new under the sun? Int J Manag Rev 20(4):903–933. https://doi.org/10.1111/ijmr.12169
Savoie I, Helmer D, Green CJ, Kazanjian A (2003) BEYOND MEDLINE: reducing bias through extended systematic review search. Int J Technol Assess Health Care 19(1):168–178. https://doi.org/10.1017/S0266462303000163
Schlosser RW, Wendt O, Bhavnani S et al (2006) Use of information-seeking strategies for developing systematic reviews and engaging in evidence-based practice: the application of traditional and comprehensive Pearl growing. A review. Int J Language Commun Disorders 41(5):567–582. https://doi.org/10.1080/13682820600742190
Schryen G (2015) Writing qualitative IS literature reviews—guidelines for synthesis, interpretation, and guidance of research. Commun Assoc Inf Syst 34:286–325. https://doi.org/10.17705/1CAIS.03712
Shishank S, Dekkers R (2013) Outsourcing: decision-making methods and criteria during design and engineering. Product Plan Control Manage Oper 24(4–5):318–336. https://doi.org/10.1080/09537287.2011.648544
Silagy CA, Middleton P, Hopewell S (2002) Publishing protocols of systematic reviews comparing what was done to what was planned. JAMA 287(21):2831–2834. https://doi.org/10.1001/jama.287.21.2831
Soldani J, Tamburri DA, Van Den Heuvel W-J (2018) The pains and gains of microservices: a systematic grey literature review. J Syst Softw 146:215–232. https://doi.org/10.1016/j.jss.2018.09.082
Stevinson C, Lawlor DA (2004) Searching multiple databases for systematic reviews: added value or diminishing returns? Complement Ther Med 12(4):228–232. https://doi.org/10.1016/j.ctim.2004.09.003
Swift JK, Wampold BE (2018) Inclusion and exclusion strategies for conducting meta-analyses. Psychother Res 28(3):356–366. https://doi.org/10.1080/10503307.2017.1405169
Swift JK, Callahan JL, Cooper M, Parkin SR (2018) The impact of accommodating client preference in psychotherapy: a meta-analysis. J Clin Psychol 74(11):1924–1937. https://doi.org/10.1002/jclp.22680
Tanon AA, Champagne F, Contandriopoulos A-P, Pomey M-P, Vadeboncoeur A, Nguyen H (2010) Patient safety and systematic reviews: finding papers indexed in MEDLINE, EMBASE and CINAHL. Qual Saf Health Care 19(5):452–461. https://doi.org/10.1136/qshc.2008.031401
Tillett S, Newbold E (2006) Grey literature at the British library: revealing a hidden resource. Interlend Document Supply 34(2):70–73. https://doi.org/10.1108/02641610610669769
Trott P, Hartmann D (2009) Why ‘open innovation’ is old wine in new bottles. Int J Innov Manag 13(4):715–736. https://doi.org/10.1142/S1363919609002509
vom Brocke J, Simons A, Riemer K, Niehaves B, Plattfaut R, Cleven A (2015) Standing on the shoulders of giants: challenges and recommendations of literature search in information systems research. Commun Assoc Inf Syst 37:205–224. https://doi.org/10.17705/1CAIS.03709
Webster J, Watson RT (2002) Analyzing the past to prepare for the future: writing a literature review. MIS Q 26(2):xiii–xxiii
Wellington JJ, Bathmaker A, Hunt C, McCulloch G, Sikes P (2005) Succeeding with your doctorate. Sage, Thousand Oaks
Wilczynski NL, Haynes RB (2007) EMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviews. J Clin Epidemiol 60(1):29–33. https://doi.org/10.1016/j.jclinepi.2006.04.001
Wilczynski NL, Marks S, Haynes RB (2007) Search strategies for identifying qualitative studies in CINAHL. Qual Health Res 17(5):705–710. https://doi.org/10.1177/1049732306294515
Wohlin C, Prikladnicki R (2013) Systematic literature reviews in software engineering. Inf Softw Technol 55(6):919–920. https://doi.org/10.1016/j.infsof.2013.02.002
Wong SS-L, Wilczynski NL, Haynes RB (2006) Optimal CINAHL search strategies for identifying therapy studies and review articles. J Nurs Scholarsh 38(2):194–199. https://doi.org/10.1111/j.1547-5069.2006.00100.x
Zhang L, Ajiferuke I, Sampson M (2006) Optimizing search strategies to identify randomized controlled trials in MEDLINE. BMC Med Res Methodol 6(1):23. https://doi.org/10.1186/1471-2288-6-23
Download references
Author information
Authors and affiliations.
University of Glasgow, Glasgow, UK
Rob Dekkers
Glasgow Caledonian University, Glasgow, UK
Lindsey Carey
Prof. Peter Langhorne
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Dekkers, R., Carey, L., Langhorne, P. (2022). Search Strategies for [Systematic] Literature Reviews. In: Making Literature Reviews Work: A Multidisciplinary Guide to Systematic Approaches. Springer, Cham. https://doi.org/10.1007/978-3-030-90025-0_5
Download citation
DOI : https://doi.org/10.1007/978-3-030-90025-0_5
Published : 11 August 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-90024-3
Online ISBN : 978-3-030-90025-0
eBook Packages : Education Education (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Open access
- Published: 14 August 2018
Defining the process to literature searching in systematic reviews: a literature review of guidance and supporting studies
- Chris Cooper ORCID: orcid.org/0000-0003-0864-5607 1 ,
- Andrew Booth 2 ,
- Jo Varley-Campbell 1 ,
- Nicky Britten 3 &
- Ruth Garside 4
BMC Medical Research Methodology volume 18 , Article number: 85 ( 2018 ) Cite this article
209k Accesses
227 Citations
117 Altmetric
Metrics details
Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving readers clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence.
Information specialists and review teams appear to work from a shared and tacit model of the literature search process. How this tacit model has developed and evolved is unclear, and it has not been explicitly examined before.
The purpose of this review is to determine if a shared model of the literature searching process can be detected across systematic review guidance documents and, if so, how this process is reported in the guidance and supported by published studies.
A literature review.
Two types of literature were reviewed: guidance and published studies. Nine guidance documents were identified, including: The Cochrane and Campbell Handbooks. Published studies were identified through ‘pearl growing’, citation chasing, a search of PubMed using the systematic review methods filter, and the authors’ topic knowledge.
The relevant sections within each guidance document were then read and re-read, with the aim of determining key methodological stages. Methodological stages were identified and defined. This data was reviewed to identify agreements and areas of unique guidance between guidance documents. Consensus across multiple guidance documents was used to inform selection of ‘key stages’ in the process of literature searching.
Eight key stages were determined relating specifically to literature searching in systematic reviews. They were: who should literature search, aims and purpose of literature searching, preparation, the search strategy, searching databases, supplementary searching, managing references and reporting the search process.
Conclusions
Eight key stages to the process of literature searching in systematic reviews were identified. These key stages are consistently reported in the nine guidance documents, suggesting consensus on the key stages of literature searching, and therefore the process of literature searching as a whole, in systematic reviews. Further research to determine the suitability of using the same process of literature searching for all types of systematic review is indicated.
Peer Review reports
Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving review stakeholders clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence.
Information specialists and review teams appear to work from a shared and tacit model of the literature search process. How this tacit model has developed and evolved is unclear, and it has not been explicitly examined before. This is in contrast to the information science literature, which has developed information processing models as an explicit basis for dialogue and empirical testing. Without an explicit model, research in the process of systematic literature searching will remain immature and potentially uneven, and the development of shared information models will be assumed but never articulated.
One way of developing such a conceptual model is by formally examining the implicit “programme theory” as embodied in key methodological texts. The aim of this review is therefore to determine if a shared model of the literature searching process in systematic reviews can be detected across guidance documents and, if so, how this process is reported and supported.
Identifying guidance
Key texts (henceforth referred to as “guidance”) were identified based upon their accessibility to, and prominence within, United Kingdom systematic reviewing practice. The United Kingdom occupies a prominent position in the science of health information retrieval, as quantified by such objective measures as the authorship of papers, the number of Cochrane groups based in the UK, membership and leadership of groups such as the Cochrane Information Retrieval Methods Group, the HTA-I Information Specialists’ Group and historic association with such centres as the UK Cochrane Centre, the NHS Centre for Reviews and Dissemination, the Centre for Evidence Based Medicine and the National Institute for Clinical Excellence (NICE). Coupled with the linguistic dominance of English within medical and health science and the science of systematic reviews more generally, this offers a justification for a purposive sample that favours UK, European and Australian guidance documents.
Nine guidance documents were identified. These documents provide guidance for different types of reviews, namely: reviews of interventions, reviews of health technologies, reviews of qualitative research studies, reviews of social science topics, and reviews to inform guidance.
Whilst these guidance documents occasionally offer additional guidance on other types of systematic reviews, we have focused on the core and stated aims of these documents as they relate to literature searching. Table 1 sets out: the guidance document, the version audited, their core stated focus, and a bibliographical pointer to the main guidance relating to literature searching.
Once a list of key guidance documents was determined, it was checked by six senior information professionals based in the UK for relevance to current literature searching in systematic reviews.
Identifying supporting studies
In addition to identifying guidance, the authors sought to populate an evidence base of supporting studies (henceforth referred to as “studies”) that contribute to existing search practice. Studies were first identified by the authors from their knowledge on this topic area and, subsequently, through systematic citation chasing key studies (‘pearls’ [ 1 ]) located within each key stage of the search process. These studies are identified in Additional file 1 : Appendix Table 1. Citation chasing was conducted by analysing the bibliography of references for each study (backwards citation chasing) and through Google Scholar (forward citation chasing). A search of PubMed using the systematic review methods filter was undertaken in August 2017 (see Additional file 1 ). The search terms used were: (literature search*[Title/Abstract]) AND sysrev_methods[sb] and 586 results were returned. These results were sifted for relevance to the key stages in Fig. 1 by CC.
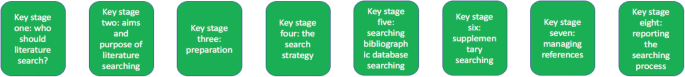
The key stages of literature search guidance as identified from nine key texts
Extracting the data
To reveal the implicit process of literature searching within each guidance document, the relevant sections (chapters) on literature searching were read and re-read, with the aim of determining key methodological stages. We defined a key methodological stage as a distinct step in the overall process for which specific guidance is reported, and action is taken, that collectively would result in a completed literature search.
The chapter or section sub-heading for each methodological stage was extracted into a table using the exact language as reported in each guidance document. The lead author (CC) then read and re-read these data, and the paragraphs of the document to which the headings referred, summarising section details. This table was then reviewed, using comparison and contrast to identify agreements and areas of unique guidance. Consensus across multiple guidelines was used to inform selection of ‘key stages’ in the process of literature searching.
Having determined the key stages to literature searching, we then read and re-read the sections relating to literature searching again, extracting specific detail relating to the methodological process of literature searching within each key stage. Again, the guidance was then read and re-read, first on a document-by-document-basis and, secondly, across all the documents above, to identify both commonalities and areas of unique guidance.
Results and discussion
Our findings.
We were able to identify consensus across the guidance on literature searching for systematic reviews suggesting a shared implicit model within the information retrieval community. Whilst the structure of the guidance varies between documents, the same key stages are reported, even where the core focus of each document is different. We were able to identify specific areas of unique guidance, where a document reported guidance not summarised in other documents, together with areas of consensus across guidance.
Unique guidance
Only one document provided guidance on the topic of when to stop searching [ 2 ]. This guidance from 2005 anticipates a topic of increasing importance with the current interest in time-limited (i.e. “rapid”) reviews. Quality assurance (or peer review) of literature searches was only covered in two guidance documents [ 3 , 4 ]. This topic has emerged as increasingly important as indicated by the development of the PRESS instrument [ 5 ]. Text mining was discussed in four guidance documents [ 4 , 6 , 7 , 8 ] where the automation of some manual review work may offer efficiencies in literature searching [ 8 ].
Agreement between guidance: Defining the key stages of literature searching
Where there was agreement on the process, we determined that this constituted a key stage in the process of literature searching to inform systematic reviews.
From the guidance, we determined eight key stages that relate specifically to literature searching in systematic reviews. These are summarised at Fig. 1 . The data extraction table to inform Fig. 1 is reported in Table 2 . Table 2 reports the areas of common agreement and it demonstrates that the language used to describe key stages and processes varies significantly between guidance documents.
For each key stage, we set out the specific guidance, followed by discussion on how this guidance is situated within the wider literature.
Key stage one: Deciding who should undertake the literature search
The guidance.
Eight documents provided guidance on who should undertake literature searching in systematic reviews [ 2 , 4 , 6 , 7 , 8 , 9 , 10 , 11 ]. The guidance affirms that people with relevant expertise of literature searching should ‘ideally’ be included within the review team [ 6 ]. Information specialists (or information scientists), librarians or trial search co-ordinators (TSCs) are indicated as appropriate researchers in six guidance documents [ 2 , 7 , 8 , 9 , 10 , 11 ].
How the guidance corresponds to the published studies
The guidance is consistent with studies that call for the involvement of information specialists and librarians in systematic reviews [ 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 ] and which demonstrate how their training as ‘expert searchers’ and ‘analysers and organisers of data’ can be put to good use [ 13 ] in a variety of roles [ 12 , 16 , 20 , 21 , 24 , 25 , 26 ]. These arguments make sense in the context of the aims and purposes of literature searching in systematic reviews, explored below. The need for ‘thorough’ and ‘replicable’ literature searches was fundamental to the guidance and recurs in key stage two. Studies have found poor reporting, and a lack of replicable literature searches, to be a weakness in systematic reviews [ 17 , 18 , 27 , 28 ] and they argue that involvement of information specialists/ librarians would be associated with better reporting and better quality literature searching. Indeed, Meert et al. [ 29 ] demonstrated that involving a librarian as a co-author to a systematic review correlated with a higher score in the literature searching component of a systematic review [ 29 ]. As ‘new styles’ of rapid and scoping reviews emerge, where decisions on how to search are more iterative and creative, a clear role is made here too [ 30 ].
Knowing where to search for studies was noted as important in the guidance, with no agreement as to the appropriate number of databases to be searched [ 2 , 6 ]. Database (and resource selection more broadly) is acknowledged as a relevant key skill of information specialists and librarians [ 12 , 15 , 16 , 31 ].
Whilst arguments for including information specialists and librarians in the process of systematic review might be considered self-evident, Koffel and Rethlefsen [ 31 ] have questioned if the necessary involvement is actually happening [ 31 ].
Key stage two: Determining the aim and purpose of a literature search
The aim: Five of the nine guidance documents use adjectives such as ‘thorough’, ‘comprehensive’, ‘transparent’ and ‘reproducible’ to define the aim of literature searching [ 6 , 7 , 8 , 9 , 10 ]. Analogous phrases were present in a further three guidance documents, namely: ‘to identify the best available evidence’ [ 4 ] or ‘the aim of the literature search is not to retrieve everything. It is to retrieve everything of relevance’ [ 2 ] or ‘A systematic literature search aims to identify all publications relevant to the particular research question’ [ 3 ]. The Joanna Briggs Institute reviewers’ manual was the only guidance document where a clear statement on the aim of literature searching could not be identified. The purpose of literature searching was defined in three guidance documents, namely to minimise bias in the resultant review [ 6 , 8 , 10 ]. Accordingly, eight of nine documents clearly asserted that thorough and comprehensive literature searches are required as a potential mechanism for minimising bias.
The need for thorough and comprehensive literature searches appears as uniform within the eight guidance documents that describe approaches to literature searching in systematic reviews of effectiveness. Reviews of effectiveness (of intervention or cost), accuracy and prognosis, require thorough and comprehensive literature searches to transparently produce a reliable estimate of intervention effect. The belief that all relevant studies have been ‘comprehensively’ identified, and that this process has been ‘transparently’ reported, increases confidence in the estimate of effect and the conclusions that can be drawn [ 32 ]. The supporting literature exploring the need for comprehensive literature searches focuses almost exclusively on reviews of intervention effectiveness and meta-analysis. Different ‘styles’ of review may have different standards however; the alternative, offered by purposive sampling, has been suggested in the specific context of qualitative evidence syntheses [ 33 ].
What is a comprehensive literature search?
Whilst the guidance calls for thorough and comprehensive literature searches, it lacks clarity on what constitutes a thorough and comprehensive literature search, beyond the implication that all of the literature search methods in Table 2 should be used to identify studies. Egger et al. [ 34 ], in an empirical study evaluating the importance of comprehensive literature searches for trials in systematic reviews, defined a comprehensive search for trials as:
a search not restricted to English language;
where Cochrane CENTRAL or at least two other electronic databases had been searched (such as MEDLINE or EMBASE); and
at least one of the following search methods has been used to identify unpublished trials: searches for (I) conference abstracts, (ii) theses, (iii) trials registers; and (iv) contacts with experts in the field [ 34 ].
Tricco et al. (2008) used a similar threshold of bibliographic database searching AND a supplementary search method in a review when examining the risk of bias in systematic reviews. Their criteria were: one database (limited using the Cochrane Highly Sensitive Search Strategy (HSSS)) and handsearching [ 35 ].
Together with the guidance, this would suggest that comprehensive literature searching requires the use of BOTH bibliographic database searching AND supplementary search methods.
Comprehensiveness in literature searching, in the sense of how much searching should be undertaken, remains unclear. Egger et al. recommend that ‘investigators should consider the type of literature search and degree of comprehension that is appropriate for the review in question, taking into account budget and time constraints’ [ 34 ]. This view tallies with the Cochrane Handbook, which stipulates clearly, that study identification should be undertaken ‘within resource limits’ [ 9 ]. This would suggest that the limitations to comprehension are recognised but it raises questions on how this is decided and reported [ 36 ].
What is the point of comprehensive literature searching?
The purpose of thorough and comprehensive literature searches is to avoid missing key studies and to minimize bias [ 6 , 8 , 10 , 34 , 37 , 38 , 39 ] since a systematic review based only on published (or easily accessible) studies may have an exaggerated effect size [ 35 ]. Felson (1992) sets out potential biases that could affect the estimate of effect in a meta-analysis [ 40 ] and Tricco et al. summarize the evidence concerning bias and confounding in systematic reviews [ 35 ]. Egger et al. point to non-publication of studies, publication bias, language bias and MEDLINE bias, as key biases [ 34 , 35 , 40 , 41 , 42 , 43 , 44 , 45 , 46 ]. Comprehensive searches are not the sole factor to mitigate these biases but their contribution is thought to be significant [ 2 , 32 , 34 ]. Fehrmann (2011) suggests that ‘the search process being described in detail’ and that, where standard comprehensive search techniques have been applied, increases confidence in the search results [ 32 ].
Does comprehensive literature searching work?
Egger et al., and other study authors, have demonstrated a change in the estimate of intervention effectiveness where relevant studies were excluded from meta-analysis [ 34 , 47 ]. This would suggest that missing studies in literature searching alters the reliability of effectiveness estimates. This is an argument for comprehensive literature searching. Conversely, Egger et al. found that ‘comprehensive’ searches still missed studies and that comprehensive searches could, in fact, introduce bias into a review rather than preventing it, through the identification of low quality studies then being included in the meta-analysis [ 34 ]. Studies query if identifying and including low quality or grey literature studies changes the estimate of effect [ 43 , 48 ] and question if time is better invested updating systematic reviews rather than searching for unpublished studies [ 49 ], or mapping studies for review as opposed to aiming for high sensitivity in literature searching [ 50 ].
Aim and purpose beyond reviews of effectiveness
The need for comprehensive literature searches is less certain in reviews of qualitative studies, and for reviews where a comprehensive identification of studies is difficult to achieve (for example, in Public health) [ 33 , 51 , 52 , 53 , 54 , 55 ]. Literature searching for qualitative studies, and in public health topics, typically generates a greater number of studies to sift than in reviews of effectiveness [ 39 ] and demonstrating the ‘value’ of studies identified or missed is harder [ 56 ], since the study data do not typically support meta-analysis. Nussbaumer-Streit et al. (2016) have registered a review protocol to assess whether abbreviated literature searches (as opposed to comprehensive literature searches) has an impact on conclusions across multiple bodies of evidence, not only on effect estimates [ 57 ] which may develop this understanding. It may be that decision makers and users of systematic reviews are willing to trade the certainty from a comprehensive literature search and systematic review in exchange for different approaches to evidence synthesis [ 58 ], and that comprehensive literature searches are not necessarily a marker of literature search quality, as previously thought [ 36 ]. Different approaches to literature searching [ 37 , 38 , 59 , 60 , 61 , 62 ] and developing the concept of when to stop searching are important areas for further study [ 36 , 59 ].
The study by Nussbaumer-Streit et al. has been published since the submission of this literature review [ 63 ]. Nussbaumer-Streit et al. (2018) conclude that abbreviated literature searches are viable options for rapid evidence syntheses, if decision-makers are willing to trade the certainty from a comprehensive literature search and systematic review, but that decision-making which demands detailed scrutiny should still be based on comprehensive literature searches [ 63 ].
Key stage three: Preparing for the literature search
Six documents provided guidance on preparing for a literature search [ 2 , 3 , 6 , 7 , 9 , 10 ]. The Cochrane Handbook clearly stated that Cochrane authors (i.e. researchers) should seek advice from a trial search co-ordinator (i.e. a person with specific skills in literature searching) ‘before’ starting a literature search [ 9 ].
Two key tasks were perceptible in preparing for a literature searching [ 2 , 6 , 7 , 10 , 11 ]. First, to determine if there are any existing or on-going reviews, or if a new review is justified [ 6 , 11 ]; and, secondly, to develop an initial literature search strategy to estimate the volume of relevant literature (and quality of a small sample of relevant studies [ 10 ]) and indicate the resources required for literature searching and the review of the studies that follows [ 7 , 10 ].
Three documents summarised guidance on where to search to determine if a new review was justified [ 2 , 6 , 11 ]. These focused on searching databases of systematic reviews (The Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE)), institutional registries (including PROSPERO), and MEDLINE [ 6 , 11 ]. It is worth noting, however, that as of 2015, DARE (and NHS EEDs) are no longer being updated and so the relevance of this (these) resource(s) will diminish over-time [ 64 ]. One guidance document, ‘Systematic reviews in the Social Sciences’, noted, however, that databases are not the only source of information and unpublished reports, conference proceeding and grey literature may also be required, depending on the nature of the review question [ 2 ].
Two documents reported clearly that this preparation (or ‘scoping’) exercise should be undertaken before the actual search strategy is developed [ 7 , 10 ]).
The guidance offers the best available source on preparing the literature search with the published studies not typically reporting how their scoping informed the development of their search strategies nor how their search approaches were developed. Text mining has been proposed as a technique to develop search strategies in the scoping stages of a review although this work is still exploratory [ 65 ]. ‘Clustering documents’ and word frequency analysis have also been tested to identify search terms and studies for review [ 66 , 67 ]. Preparing for literature searches and scoping constitutes an area for future research.
Key stage four: Designing the search strategy
The Population, Intervention, Comparator, Outcome (PICO) structure was the commonly reported structure promoted to design a literature search strategy. Five documents suggested that the eligibility criteria or review question will determine which concepts of PICO will be populated to develop the search strategy [ 1 , 4 , 7 , 8 , 9 ]. The NICE handbook promoted multiple structures, namely PICO, SPICE (Setting, Perspective, Intervention, Comparison, Evaluation) and multi-stranded approaches [ 4 ].
With the exclusion of The Joanna Briggs Institute reviewers’ manual, the guidance offered detail on selecting key search terms, synonyms, Boolean language, selecting database indexing terms and combining search terms. The CEE handbook suggested that ‘search terms may be compiled with the help of the commissioning organisation and stakeholders’ [ 10 ].
The use of limits, such as language or date limits, were discussed in all documents [ 2 , 3 , 4 , 6 , 7 , 8 , 9 , 10 , 11 ].
Search strategy structure
The guidance typically relates to reviews of intervention effectiveness so PICO – with its focus on intervention and comparator - is the dominant model used to structure literature search strategies [ 68 ]. PICOs – where the S denotes study design - is also commonly used in effectiveness reviews [ 6 , 68 ]. As the NICE handbook notes, alternative models to structure literature search strategies have been developed and tested. Booth provides an overview on formulating questions for evidence based practice [ 69 ] and has developed a number of alternatives to the PICO structure, namely: BeHEMoTh (Behaviour of interest; Health context; Exclusions; Models or Theories) for use when systematically identifying theory [ 55 ]; SPICE (Setting, Perspective, Intervention, Comparison, Evaluation) for identification of social science and evaluation studies [ 69 ] and, working with Cooke and colleagues, SPIDER (Sample, Phenomenon of Interest, Design, Evaluation, Research type) [ 70 ]. SPIDER has been compared to PICO and PICOs in a study by Methley et al. [ 68 ].
The NICE handbook also suggests the use of multi-stranded approaches to developing literature search strategies [ 4 ]. Glanville developed this idea in a study by Whitting et al. [ 71 ] and a worked example of this approach is included in the development of a search filter by Cooper et al. [ 72 ].
Writing search strategies: Conceptual and objective approaches
Hausner et al. [ 73 ] provide guidance on writing literature search strategies, delineating between conceptually and objectively derived approaches. The conceptual approach, advocated by and explained in the guidance documents, relies on the expertise of the literature searcher to identify key search terms and then develop key terms to include synonyms and controlled syntax. Hausner and colleagues set out the objective approach [ 73 ] and describe what may be done to validate it [ 74 ].
The use of limits
The guidance documents offer direction on the use of limits within a literature search. Limits can be used to focus literature searching to specific study designs or by other markers (such as by date) which limits the number of studies returned by a literature search. The use of limits should be described and the implications explored [ 34 ] since limiting literature searching can introduce bias (explored above). Craven et al. have suggested the use of a supporting narrative to explain decisions made in the process of developing literature searches and this advice would usefully capture decisions on the use of search limits [ 75 ].
Key stage five: Determining the process of literature searching and deciding where to search (bibliographic database searching)
Table 2 summarises the process of literature searching as reported in each guidance document. Searching bibliographic databases was consistently reported as the ‘first step’ to literature searching in all nine guidance documents.
Three documents reported specific guidance on where to search, in each case specific to the type of review their guidance informed, and as a minimum requirement [ 4 , 9 , 11 ]. Seven of the key guidance documents suggest that the selection of bibliographic databases depends on the topic of review [ 2 , 3 , 4 , 6 , 7 , 8 , 10 ], with two documents noting the absence of an agreed standard on what constitutes an acceptable number of databases searched [ 2 , 6 ].
The guidance documents summarise ‘how to’ search bibliographic databases in detail and this guidance is further contextualised above in terms of developing the search strategy. The documents provide guidance of selecting bibliographic databases, in some cases stating acceptable minima (i.e. The Cochrane Handbook states Cochrane CENTRAL, MEDLINE and EMBASE), and in other cases simply listing bibliographic database available to search. Studies have explored the value in searching specific bibliographic databases, with Wright et al. (2015) noting the contribution of CINAHL in identifying qualitative studies [ 76 ], Beckles et al. (2013) questioning the contribution of CINAHL to identifying clinical studies for guideline development [ 77 ], and Cooper et al. (2015) exploring the role of UK-focused bibliographic databases to identify UK-relevant studies [ 78 ]. The host of the database (e.g. OVID or ProQuest) has been shown to alter the search returns offered. Younger and Boddy [ 79 ] report differing search returns from the same database (AMED) but where the ‘host’ was different [ 79 ].
The average number of bibliographic database searched in systematic reviews has risen in the period 1994–2014 (from 1 to 4) [ 80 ] but there remains (as attested to by the guidance) no consensus on what constitutes an acceptable number of databases searched [ 48 ]. This is perhaps because thinking about the number of databases searched is the wrong question, researchers should be focused on which databases were searched and why, and which databases were not searched and why. The discussion should re-orientate to the differential value of sources but researchers need to think about how to report this in studies to allow findings to be generalised. Bethel (2017) has proposed ‘search summaries’, completed by the literature searcher, to record where included studies were identified, whether from database (and which databases specifically) or supplementary search methods [ 81 ]. Search summaries document both yield and accuracy of searches, which could prospectively inform resource use and decisions to search or not to search specific databases in topic areas. The prospective use of such data presupposes, however, that past searches are a potential predictor of future search performance (i.e. that each topic is to be considered representative and not unique). In offering a body of practice, this data would be of greater practicable use than current studies which are considered as little more than individual case studies [ 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 ].
When to database search is another question posed in the literature. Beyer et al. [ 91 ] report that databases can be prioritised for literature searching which, whilst not addressing the question of which databases to search, may at least bring clarity as to which databases to search first [ 91 ]. Paradoxically, this links to studies that suggest PubMed should be searched in addition to MEDLINE (OVID interface) since this improves the currency of systematic reviews [ 92 , 93 ]. Cooper et al. (2017) have tested the idea of database searching not as a primary search method (as suggested in the guidance) but as a supplementary search method in order to manage the volume of studies identified for an environmental effectiveness systematic review. Their case study compared the effectiveness of database searching versus a protocol using supplementary search methods and found that the latter identified more relevant studies for review than searching bibliographic databases [ 94 ].
Key stage six: Determining the process of literature searching and deciding where to search (supplementary search methods)
Table 2 also summaries the process of literature searching which follows bibliographic database searching. As Table 2 sets out, guidance that supplementary literature search methods should be used in systematic reviews recurs across documents, but the order in which these methods are used, and the extent to which they are used, varies. We noted inconsistency in the labelling of supplementary search methods between guidance documents.
Rather than focus on the guidance on how to use the methods (which has been summarised in a recent review [ 95 ]), we focus on the aim or purpose of supplementary search methods.
The Cochrane Handbook reported that ‘efforts’ to identify unpublished studies should be made [ 9 ]. Four guidance documents [ 2 , 3 , 6 , 9 ] acknowledged that searching beyond bibliographic databases was necessary since ‘databases are not the only source of literature’ [ 2 ]. Only one document reported any guidance on determining when to use supplementary methods. The IQWiG handbook reported that the use of handsearching (in their example) could be determined on a ‘case-by-case basis’ which implies that the use of these methods is optional rather than mandatory. This is in contrast to the guidance (above) on bibliographic database searching.
The issue for supplementary search methods is similar in many ways to the issue of searching bibliographic databases: demonstrating value. The purpose and contribution of supplementary search methods in systematic reviews is increasingly acknowledged [ 37 , 61 , 62 , 96 , 97 , 98 , 99 , 100 , 101 ] but understanding the value of the search methods to identify studies and data is unclear. In a recently published review, Cooper et al. (2017) reviewed the literature on supplementary search methods looking to determine the advantages, disadvantages and resource implications of using supplementary search methods [ 95 ]. This review also summarises the key guidance and empirical studies and seeks to address the question on when to use these search methods and when not to [ 95 ]. The guidance is limited in this regard and, as Table 2 demonstrates, offers conflicting advice on the order of searching, and the extent to which these search methods should be used in systematic reviews.
Key stage seven: Managing the references
Five of the documents provided guidance on managing references, for example downloading, de-duplicating and managing the output of literature searches [ 2 , 4 , 6 , 8 , 10 ]. This guidance typically itemised available bibliographic management tools rather than offering guidance on how to use them specifically [ 2 , 4 , 6 , 8 ]. The CEE handbook provided guidance on importing data where no direct export option is available (e.g. web-searching) [ 10 ].
The literature on using bibliographic management tools is not large relative to the number of ‘how to’ videos on platforms such as YouTube (see for example [ 102 ]). These YouTube videos confirm the overall lack of ‘how to’ guidance identified in this study and offer useful instruction on managing references. Bramer et al. set out methods for de-duplicating data and reviewing references in Endnote [ 103 , 104 ] and Gall tests the direct search function within Endnote to access databases such as PubMed, finding a number of limitations [ 105 ]. Coar et al. and Ahmed et al. consider the role of the free-source tool, Zotero [ 106 , 107 ]. Managing references is a key administrative function in the process of review particularly for documenting searches in PRISMA guidance.
Key stage eight: Documenting the search
The Cochrane Handbook was the only guidance document to recommend a specific reporting guideline: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 9 ]. Six documents provided guidance on reporting the process of literature searching with specific criteria to report [ 3 , 4 , 6 , 8 , 9 , 10 ]. There was consensus on reporting: the databases searched (and the host searched by), the search strategies used, and any use of limits (e.g. date, language, search filters (The CRD handbook called for these limits to be justified [ 6 ])). Three guidance documents reported that the number of studies identified should be recorded [ 3 , 6 , 10 ]. The number of duplicates identified [ 10 ], the screening decisions [ 3 ], a comprehensive list of grey literature sources searched (and full detail for other supplementary search methods) [ 8 ], and an annotation of search terms tested but not used [ 4 ] were identified as unique items in four documents.
The Cochrane Handbook was the only guidance document to note that the full search strategies for each database should be included in the Additional file 1 of the review [ 9 ].
All guidance documents should ultimately deliver completed systematic reviews that fulfil the requirements of the PRISMA reporting guidelines [ 108 ]. The guidance broadly requires the reporting of data that corresponds with the requirements of the PRISMA statement although documents typically ask for diverse and additional items [ 108 ]. In 2008, Sampson et al. observed a lack of consensus on reporting search methods in systematic reviews [ 109 ] and this remains the case as of 2017, as evidenced in the guidance documents, and in spite of the publication of the PRISMA guidelines in 2009 [ 110 ]. It is unclear why the collective guidance does not more explicitly endorse adherence to the PRISMA guidance.
Reporting of literature searching is a key area in systematic reviews since it sets out clearly what was done and how the conclusions of the review can be believed [ 52 , 109 ]. Despite strong endorsement in the guidance documents, specifically supported in PRISMA guidance, and other related reporting standards too (such as ENTREQ for qualitative evidence synthesis, STROBE for reviews of observational studies), authors still highlight the prevalence of poor standards of literature search reporting [ 31 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 ]. To explore issues experienced by authors in reporting literature searches, and look at uptake of PRISMA, Radar et al. [ 120 ] surveyed over 260 review authors to determine common problems and their work summaries the practical aspects of reporting literature searching [ 120 ]. Atkinson et al. [ 121 ] have also analysed reporting standards for literature searching, summarising recommendations and gaps for reporting search strategies [ 121 ].
One area that is less well covered by the guidance, but nevertheless appears in this literature, is the quality appraisal or peer review of literature search strategies. The PRESS checklist is the most prominent and it aims to develop evidence-based guidelines to peer review of electronic search strategies [ 5 , 122 , 123 ]. A corresponding guideline for documentation of supplementary search methods does not yet exist although this idea is currently being explored.
How the reporting of the literature searching process corresponds to critical appraisal tools is an area for further research. In the survey undertaken by Radar et al. (2014), 86% of survey respondents (153/178) identified a need for further guidance on what aspects of the literature search process to report [ 120 ]. The PRISMA statement offers a brief summary of what to report but little practical guidance on how to report it [ 108 ]. Critical appraisal tools for systematic reviews, such as AMSTAR 2 (Shea et al. [ 124 ]) and ROBIS (Whiting et al. [ 125 ]), can usefully be read alongside PRISMA guidance, since they offer greater detail on how the reporting of the literature search will be appraised and, therefore, they offer a proxy on what to report [ 124 , 125 ]. Further research in the form of a study which undertakes a comparison between PRISMA and quality appraisal checklists for systematic reviews would seem to begin addressing the call, identified by Radar et al., for further guidance on what to report [ 120 ].
Limitations
Other handbooks exist.
A potential limitation of this literature review is the focus on guidance produced in Europe (the UK specifically) and Australia. We justify the decision for our selection of the nine guidance documents reviewed in this literature review in section “ Identifying guidance ”. In brief, these nine guidance documents were selected as the most relevant health care guidance that inform UK systematic reviewing practice, given that the UK occupies a prominent position in the science of health information retrieval. We acknowledge the existence of other guidance documents, such as those from North America (e.g. the Agency for Healthcare Research and Quality (AHRQ) [ 126 ], The Institute of Medicine [ 127 ] and the guidance and resources produced by the Canadian Agency for Drugs and Technologies in Health (CADTH) [ 128 ]). We comment further on this directly below.
The handbooks are potentially linked to one another
What is not clear is the extent to which the guidance documents inter-relate or provide guidance uniquely. The Cochrane Handbook, first published in 1994, is notably a key source of reference in guidance and systematic reviews beyond Cochrane reviews. It is not clear to what extent broadening the sample of guidance handbooks to include North American handbooks, and guidance handbooks from other relevant countries too, would alter the findings of this literature review or develop further support for the process model. Since we cannot be clear, we raise this as a potential limitation of this literature review. On our initial review of a sample of North American, and other, guidance documents (before selecting the guidance documents considered in this review), however, we do not consider that the inclusion of these further handbooks would alter significantly the findings of this literature review.
This is a literature review
A further limitation of this review was that the review of published studies is not a systematic review of the evidence for each key stage. It is possible that other relevant studies could help contribute to the exploration and development of the key stages identified in this review.
This literature review would appear to demonstrate the existence of a shared model of the literature searching process in systematic reviews. We call this model ‘the conventional approach’, since it appears to be common convention in nine different guidance documents.
The findings reported above reveal eight key stages in the process of literature searching for systematic reviews. These key stages are consistently reported in the nine guidance documents which suggests consensus on the key stages of literature searching, and therefore the process of literature searching as a whole, in systematic reviews.
In Table 2 , we demonstrate consensus regarding the application of literature search methods. All guidance documents distinguish between primary and supplementary search methods. Bibliographic database searching is consistently the first method of literature searching referenced in each guidance document. Whilst the guidance uniformly supports the use of supplementary search methods, there is little evidence for a consistent process with diverse guidance across documents. This may reflect differences in the core focus across each document, linked to differences in identifying effectiveness studies or qualitative studies, for instance.
Eight of the nine guidance documents reported on the aims of literature searching. The shared understanding was that literature searching should be thorough and comprehensive in its aim and that this process should be reported transparently so that that it could be reproduced. Whilst only three documents explicitly link this understanding to minimising bias, it is clear that comprehensive literature searching is implicitly linked to ‘not missing relevant studies’ which is approximately the same point.
Defining the key stages in this review helps categorise the scholarship available, and it prioritises areas for development or further study. The supporting studies on preparing for literature searching (key stage three, ‘preparation’) were, for example, comparatively few, and yet this key stage represents a decisive moment in literature searching for systematic reviews. It is where search strategy structure is determined, search terms are chosen or discarded, and the resources to be searched are selected. Information specialists, librarians and researchers, are well placed to develop these and other areas within the key stages we identify.
This review calls for further research to determine the suitability of using the conventional approach. The publication dates of the guidance documents which underpin the conventional approach may raise questions as to whether the process which they each report remains valid for current systematic literature searching. In addition, it may be useful to test whether it is desirable to use the same process model of literature searching for qualitative evidence synthesis as that for reviews of intervention effectiveness, which this literature review demonstrates is presently recommended best practice.
Abbreviations
Behaviour of interest; Health context; Exclusions; Models or Theories
Cochrane Database of Systematic Reviews
The Cochrane Central Register of Controlled Trials
Database of Abstracts of Reviews of Effects
Enhancing transparency in reporting the synthesis of qualitative research
Institute for Quality and Efficiency in Healthcare
National Institute for Clinical Excellence
Population, Intervention, Comparator, Outcome
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Setting, Perspective, Intervention, Comparison, Evaluation
Sample, Phenomenon of Interest, Design, Evaluation, Research type
STrengthening the Reporting of OBservational studies in Epidemiology
Trial Search Co-ordinators
Booth A. Unpacking your literature search toolbox: on search styles and tactics. Health Information & Libraries Journal. 2008;25(4):313–7.
Article Google Scholar
Petticrew M, Roberts H. Systematic reviews in the social sciences: a practical guide. Oxford: Blackwell Publishing Ltd; 2006.
Book Google Scholar
Institute for Quality and Efficiency in Health Care (IQWiG). IQWiG Methods Resources. 7 Information retrieval 2014 [Available from: https://www.ncbi.nlm.nih.gov/books/NBK385787/ .
NICE: National Institute for Health and Care Excellence. Developing NICE guidelines: the manual 2014. Available from: https://www.nice.org.uk/media/default/about/what-we-do/our-programmes/developing-nice-guidelines-the-manual.pdf .
Sampson M. MJ, Lefebvre C, Moher D, Grimshaw J. Peer Review of Electronic Search Strategies: PRESS; 2008.
Google Scholar
Centre for Reviews & Dissemination. Systematic reviews – CRD’s guidance for undertaking reviews in healthcare. York: Centre for Reviews and Dissemination, University of York; 2009.
eunetha: European Network for Health Technology Assesment Process of information retrieval for systematic reviews and health technology assessments on clinical effectiveness 2016. Available from: http://www.eunethta.eu/sites/default/files/Guideline_Information_Retrieval_V1-1.pdf .
Kugley SWA, Thomas J, Mahood Q, Jørgensen AMK, Hammerstrøm K, Sathe N. Searching for studies: a guide to information retrieval for Campbell systematic reviews. Oslo: Campbell Collaboration. 2017; Available from: https://www.campbellcollaboration.org/library/searching-for-studies-information-retrieval-guide-campbell-reviews.html
Lefebvre C, Manheimer E, Glanville J. Chapter 6: searching for studies. In: JPT H, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions; 2011.
Collaboration for Environmental Evidence. Guidelines for Systematic Review and Evidence Synthesis in Environmental Management.: Environmental Evidence:; 2013. Available from: http://www.environmentalevidence.org/wp-content/uploads/2017/01/Review-guidelines-version-4.2-final-update.pdf .
The Joanna Briggs Institute. Joanna Briggs institute reviewers’ manual. 2014th ed: the Joanna Briggs institute; 2014. Available from: https://joannabriggs.org/assets/docs/sumari/ReviewersManual-2014.pdf
Beverley CA, Booth A, Bath PA. The role of the information specialist in the systematic review process: a health information case study. Health Inf Libr J. 2003;20(2):65–74.
Article CAS Google Scholar
Harris MR. The librarian's roles in the systematic review process: a case study. Journal of the Medical Library Association. 2005;93(1):81–7.
PubMed PubMed Central Google Scholar
Egger JB. Use of recommended search strategies in systematic reviews and the impact of librarian involvement: a cross-sectional survey of recent authors. PLoS One. 2015;10(5):e0125931.
Li L, Tian J, Tian H, Moher D, Liang F, Jiang T, et al. Network meta-analyses could be improved by searching more sources and by involving a librarian. J Clin Epidemiol. 2014;67(9):1001–7.
Article PubMed Google Scholar
McGowan J, Sampson M. Systematic reviews need systematic searchers. J Med Libr Assoc. 2005;93(1):74–80.
Rethlefsen ML, Farrell AM, Osterhaus Trzasko LC, Brigham TJ. Librarian co-authors correlated with higher quality reported search strategies in general internal medicine systematic reviews. J Clin Epidemiol. 2015;68(6):617–26.
Weller AC. Mounting evidence that librarians are essential for comprehensive literature searches for meta-analyses and Cochrane reports. J Med Libr Assoc. 2004;92(2):163–4.
Swinkels A, Briddon J, Hall J. Two physiotherapists, one librarian and a systematic literature review: collaboration in action. Health Info Libr J. 2006;23(4):248–56.
Foster M. An overview of the role of librarians in systematic reviews: from expert search to project manager. EAHIL. 2015;11(3):3–7.
Lawson L. OPERATING OUTSIDE LIBRARY WALLS 2004.
Vassar M, Yerokhin V, Sinnett PM, Weiher M, Muckelrath H, Carr B, et al. Database selection in systematic reviews: an insight through clinical neurology. Health Inf Libr J. 2017;34(2):156–64.
Townsend WA, Anderson PF, Ginier EC, MacEachern MP, Saylor KM, Shipman BL, et al. A competency framework for librarians involved in systematic reviews. Journal of the Medical Library Association : JMLA. 2017;105(3):268–75.
Cooper ID, Crum JA. New activities and changing roles of health sciences librarians: a systematic review, 1990-2012. Journal of the Medical Library Association : JMLA. 2013;101(4):268–77.
Crum JA, Cooper ID. Emerging roles for biomedical librarians: a survey of current practice, challenges, and changes. Journal of the Medical Library Association : JMLA. 2013;101(4):278–86.
Dudden RF, Protzko SL. The systematic review team: contributions of the health sciences librarian. Med Ref Serv Q. 2011;30(3):301–15.
Golder S, Loke Y, McIntosh HM. Poor reporting and inadequate searches were apparent in systematic reviews of adverse effects. J Clin Epidemiol. 2008;61(5):440–8.
Maggio LA, Tannery NH, Kanter SL. Reproducibility of literature search reporting in medical education reviews. Academic medicine : journal of the Association of American Medical Colleges. 2011;86(8):1049–54.
Meert D, Torabi N, Costella J. Impact of librarians on reporting of the literature searching component of pediatric systematic reviews. Journal of the Medical Library Association : JMLA. 2016;104(4):267–77.
Morris M, Boruff JT, Gore GC. Scoping reviews: establishing the role of the librarian. Journal of the Medical Library Association : JMLA. 2016;104(4):346–54.
Koffel JB, Rethlefsen ML. Reproducibility of search strategies is poor in systematic reviews published in high-impact pediatrics, cardiology and surgery journals: a cross-sectional study. PLoS One. 2016;11(9):e0163309.
Article PubMed PubMed Central CAS Google Scholar
Fehrmann P, Thomas J. Comprehensive computer searches and reporting in systematic reviews. Research Synthesis Methods. 2011;2(1):15–32.
Booth A. Searching for qualitative research for inclusion in systematic reviews: a structured methodological review. Systematic Reviews. 2016;5(1):74.
Article PubMed PubMed Central Google Scholar
Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health technology assessment (Winchester, England). 2003;7(1):1–76.
Tricco AC, Tetzlaff J, Sampson M, Fergusson D, Cogo E, Horsley T, et al. Few systematic reviews exist documenting the extent of bias: a systematic review. J Clin Epidemiol. 2008;61(5):422–34.
Booth A. How much searching is enough? Comprehensive versus optimal retrieval for technology assessments. Int J Technol Assess Health Care. 2010;26(4):431–5.
Papaioannou D, Sutton A, Carroll C, Booth A, Wong R. Literature searching for social science systematic reviews: consideration of a range of search techniques. Health Inf Libr J. 2010;27(2):114–22.
Petticrew M. Time to rethink the systematic review catechism? Moving from ‘what works’ to ‘what happens’. Systematic Reviews. 2015;4(1):36.
Betrán AP, Say L, Gülmezoglu AM, Allen T, Hampson L. Effectiveness of different databases in identifying studies for systematic reviews: experience from the WHO systematic review of maternal morbidity and mortality. BMC Med Res Methodol. 2005;5
Felson DT. Bias in meta-analytic research. J Clin Epidemiol. 1992;45(8):885–92.
Article PubMed CAS Google Scholar
Franco A, Malhotra N, Simonovits G. Publication bias in the social sciences: unlocking the file drawer. Science. 2014;345(6203):1502–5.
Hartling L, Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. Grey literature in systematic reviews: a cross-sectional study of the contribution of non-English reports, unpublished studies and dissertations to the results of meta-analyses in child-relevant reviews. BMC Med Res Methodol. 2017;17(1):64.
Schmucker CM, Blümle A, Schell LK, Schwarzer G, Oeller P, Cabrera L, et al. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS One. 2017;12(4):e0176210.
Egger M, Zellweger-Zahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet (London, England). 1997;350(9074):326–9.
Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health technology assessment (Winchester, England). 2003;7(41):1–90.
Pham B, Klassen TP, Lawson ML, Moher D. Language of publication restrictions in systematic reviews gave different results depending on whether the intervention was conventional or complementary. J Clin Epidemiol. 2005;58(8):769–76.
Mills EJ, Kanters S, Thorlund K, Chaimani A, Veroniki A-A, Ioannidis JPA. The effects of excluding treatments from network meta-analyses: survey. BMJ : British Medical Journal. 2013;347
Hartling L, Featherstone R, Nuspl M, Shave K, Dryden DM, Vandermeer B. The contribution of databases to the results of systematic reviews: a cross-sectional study. BMC Med Res Methodol. 2016;16(1):127.
van Driel ML, De Sutter A, De Maeseneer J, Christiaens T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. J Clin Epidemiol. 2009;62(8):838–44.e3.
Buchberger B, Krabbe L, Lux B, Mattivi JT. Evidence mapping for decision making: feasibility versus accuracy - when to abandon high sensitivity in electronic searches. German medical science : GMS e-journal. 2016;14:Doc09.
Lorenc T, Pearson M, Jamal F, Cooper C, Garside R. The role of systematic reviews of qualitative evidence in evaluating interventions: a case study. Research Synthesis Methods. 2012;3(1):1–10.
Gough D. Weight of evidence: a framework for the appraisal of the quality and relevance of evidence. Res Pap Educ. 2007;22(2):213–28.
Barroso J, Gollop CJ, Sandelowski M, Meynell J, Pearce PF, Collins LJ. The challenges of searching for and retrieving qualitative studies. West J Nurs Res. 2003;25(2):153–78.
Britten N, Garside R, Pope C, Frost J, Cooper C. Asking more of qualitative synthesis: a response to Sally Thorne. Qual Health Res. 2017;27(9):1370–6.
Booth A, Carroll C. Systematic searching for theory to inform systematic reviews: is it feasible? Is it desirable? Health Info Libr J. 2015;32(3):220–35.
Kwon Y, Powelson SE, Wong H, Ghali WA, Conly JM. An assessment of the efficacy of searching in biomedical databases beyond MEDLINE in identifying studies for a systematic review on ward closures as an infection control intervention to control outbreaks. Syst Rev. 2014;3:135.
Nussbaumer-Streit B, Klerings I, Wagner G, Titscher V, Gartlehner G. Assessing the validity of abbreviated literature searches for rapid reviews: protocol of a non-inferiority and meta-epidemiologic study. Systematic Reviews. 2016;5:197.
Wagner G, Nussbaumer-Streit B, Greimel J, Ciapponi A, Gartlehner G. Trading certainty for speed - how much uncertainty are decisionmakers and guideline developers willing to accept when using rapid reviews: an international survey. BMC Med Res Methodol. 2017;17(1):121.
Ogilvie D, Hamilton V, Egan M, Petticrew M. Systematic reviews of health effects of social interventions: 1. Finding the evidence: how far should you go? J Epidemiol Community Health. 2005;59(9):804–8.
Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. Int J Technol Assess Health Care. 2003;19(4):591–603.
Pearson M, Moxham T, Ashton K. Effectiveness of search strategies for qualitative research about barriers and facilitators of program delivery. Eval Health Prof. 2011;34(3):297–308.
Levay P, Raynor M, Tuvey D. The Contributions of MEDLINE, Other Bibliographic Databases and Various Search Techniques to NICE Public Health Guidance. 2015. 2015;10(1):19.
Nussbaumer-Streit B, Klerings I, Wagner G, Heise TL, Dobrescu AI, Armijo-Olivo S, et al. Abbreviated literature searches were viable alternatives to comprehensive searches: a meta-epidemiological study. J Clin Epidemiol. 2018;102:1–11.
Briscoe S, Cooper C, Glanville J, Lefebvre C. The loss of the NHS EED and DARE databases and the effect on evidence synthesis and evaluation. Res Synth Methods. 2017;8(3):256–7.
Stansfield C, O'Mara-Eves A, Thomas J. Text mining for search term development in systematic reviewing: A discussion of some methods and challenges. Research Synthesis Methods.n/a-n/a.
Petrova M, Sutcliffe P, Fulford KW, Dale J. Search terms and a validated brief search filter to retrieve publications on health-related values in Medline: a word frequency analysis study. Journal of the American Medical Informatics Association : JAMIA. 2012;19(3):479–88.
Stansfield C, Thomas J, Kavanagh J. 'Clustering' documents automatically to support scoping reviews of research: a case study. Res Synth Methods. 2013;4(3):230–41.
PubMed Google Scholar
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579.
Andrew B. Clear and present questions: formulating questions for evidence based practice. Library Hi Tech. 2006;24(3):355–68.
Cooke A, Smith D, Booth A. Beyond PICO: the SPIDER tool for qualitative evidence synthesis. Qual Health Res. 2012;22(10):1435–43.
Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al. Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model. Health technology assessment (Winchester, England). 2006;10(36):iii-iv, xi-xiii, 1–154.
Cooper C, Levay P, Lorenc T, Craig GM. A population search filter for hard-to-reach populations increased search efficiency for a systematic review. J Clin Epidemiol. 2014;67(5):554–9.
Hausner E, Waffenschmidt S, Kaiser T, Simon M. Routine development of objectively derived search strategies. Systematic Reviews. 2012;1(1):19.
Hausner E, Guddat C, Hermanns T, Lampert U, Waffenschmidt S. Prospective comparison of search strategies for systematic reviews: an objective approach yielded higher sensitivity than a conceptual one. J Clin Epidemiol. 2016;77:118–24.
Craven J, Levay P. Recording database searches for systematic reviews - what is the value of adding a narrative to peer-review checklists? A case study of nice interventional procedures guidance. Evid Based Libr Inf Pract. 2011;6(4):72–87.
Wright K, Golder S, Lewis-Light K. What value is the CINAHL database when searching for systematic reviews of qualitative studies? Syst Rev. 2015;4:104.
Beckles Z, Glover S, Ashe J, Stockton S, Boynton J, Lai R, et al. Searching CINAHL did not add value to clinical questions posed in NICE guidelines. J Clin Epidemiol. 2013;66(9):1051–7.
Cooper C, Rogers M, Bethel A, Briscoe S, Lowe J. A mapping review of the literature on UK-focused health and social care databases. Health Inf Libr J. 2015;32(1):5–22.
Younger P, Boddy K. When is a search not a search? A comparison of searching the AMED complementary health database via EBSCOhost, OVID and DIALOG. Health Inf Libr J. 2009;26(2):126–35.
Lam MT, McDiarmid M. Increasing number of databases searched in systematic reviews and meta-analyses between 1994 and 2014. Journal of the Medical Library Association : JMLA. 2016;104(4):284–9.
Bethel A, editor Search summary tables for systematic reviews: results and findings. HLC Conference 2017a.
Aagaard T, Lund H, Juhl C. Optimizing literature search in systematic reviews - are MEDLINE, EMBASE and CENTRAL enough for identifying effect studies within the area of musculoskeletal disorders? BMC Med Res Methodol. 2016;16(1):161.
Adams CE, Frederick K. An investigation of the adequacy of MEDLINE searches for randomized controlled trials (RCTs) of the effects of mental health care. Psychol Med. 1994;24(3):741–8.
Kelly L, St Pierre-Hansen N. So many databases, such little clarity: searching the literature for the topic aboriginal. Canadian family physician Medecin de famille canadien. 2008;54(11):1572–3.
Lawrence DW. What is lost when searching only one literature database for articles relevant to injury prevention and safety promotion? Injury Prevention. 2008;14(6):401–4.
Lemeshow AR, Blum RE, Berlin JA, Stoto MA, Colditz GA. Searching one or two databases was insufficient for meta-analysis of observational studies. J Clin Epidemiol. 2005;58(9):867–73.
Sampson M, Barrowman NJ, Moher D, Klassen TP, Pham B, Platt R, et al. Should meta-analysts search Embase in addition to Medline? J Clin Epidemiol. 2003;56(10):943–55.
Stevinson C, Lawlor DA. Searching multiple databases for systematic reviews: added value or diminishing returns? Complementary Therapies in Medicine. 2004;12(4):228–32.
Suarez-Almazor ME, Belseck E, Homik J, Dorgan M, Ramos-Remus C. Identifying clinical trials in the medical literature with electronic databases: MEDLINE alone is not enough. Control Clin Trials. 2000;21(5):476–87.
Taylor B, Wylie E, Dempster M, Donnelly M. Systematically retrieving research: a case study evaluating seven databases. Res Soc Work Pract. 2007;17(6):697–706.
Beyer FR, Wright K. Can we prioritise which databases to search? A case study using a systematic review of frozen shoulder management. Health Info Libr J. 2013;30(1):49–58.
Duffy S, de Kock S, Misso K, Noake C, Ross J, Stirk L. Supplementary searches of PubMed to improve currency of MEDLINE and MEDLINE in-process searches via Ovid. Journal of the Medical Library Association : JMLA. 2016;104(4):309–12.
Katchamart W, Faulkner A, Feldman B, Tomlinson G, Bombardier C. PubMed had a higher sensitivity than Ovid-MEDLINE in the search for systematic reviews. J Clin Epidemiol. 2011;64(7):805–7.
Cooper C, Lovell R, Husk K, Booth A, Garside R. Supplementary search methods were more effective and offered better value than bibliographic database searching: a case study from public health and environmental enhancement (in Press). Research Synthesis Methods. 2017;
Cooper C, Booth, A., Britten, N., Garside, R. A comparison of results of empirical studies of supplementary search techniques and recommendations in review methodology handbooks: A methodological review. (In Press). BMC Systematic Reviews. 2017.
Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ (Clinical research ed). 2005;331(7524):1064–5.
Article PubMed Central Google Scholar
Hinde S, Spackman E. Bidirectional citation searching to completion: an exploration of literature searching methods. PharmacoEconomics. 2015;33(1):5–11.
Levay P, Ainsworth N, Kettle R, Morgan A. Identifying evidence for public health guidance: a comparison of citation searching with web of science and Google scholar. Res Synth Methods. 2016;7(1):34–45.
McManus RJ, Wilson S, Delaney BC, Fitzmaurice DA, Hyde CJ, Tobias RS, et al. Review of the usefulness of contacting other experts when conducting a literature search for systematic reviews. BMJ (Clinical research ed). 1998;317(7172):1562–3.
Westphal A, Kriston L, Holzel LP, Harter M, von Wolff A. Efficiency and contribution of strategies for finding randomized controlled trials: a case study from a systematic review on therapeutic interventions of chronic depression. Journal of public health research. 2014;3(2):177.
Matthews EJ, Edwards AG, Barker J, Bloor M, Covey J, Hood K, et al. Efficient literature searching in diffuse topics: lessons from a systematic review of research on communicating risk to patients in primary care. Health Libr Rev. 1999;16(2):112–20.
Bethel A. Endnote Training (YouTube Videos) 2017b [Available from: http://medicine.exeter.ac.uk/esmi/workstreams/informationscience/is_resources,_guidance_&_advice/ .
Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. Journal of the Medical Library Association : JMLA. 2016;104(3):240–3.
Bramer WM, Milic J, Mast F. Reviewing retrieved references for inclusion in systematic reviews using EndNote. Journal of the Medical Library Association : JMLA. 2017;105(1):84–7.
Gall C, Brahmi FA. Retrieval comparison of EndNote to search MEDLINE (Ovid and PubMed) versus searching them directly. Medical reference services quarterly. 2004;23(3):25–32.
Ahmed KK, Al Dhubaib BE. Zotero: a bibliographic assistant to researcher. J Pharmacol Pharmacother. 2011;2(4):303–5.
Coar JT, Sewell JP. Zotero: harnessing the power of a personal bibliographic manager. Nurse Educ. 2010;35(5):205–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Sampson M, McGowan J, Tetzlaff J, Cogo E, Moher D. No consensus exists on search reporting methods for systematic reviews. J Clin Epidemiol. 2008;61(8):748–54.
Toews LC. Compliance of systematic reviews in veterinary journals with preferred reporting items for systematic reviews and meta-analysis (PRISMA) literature search reporting guidelines. Journal of the Medical Library Association : JMLA. 2017;105(3):233–9.
Booth A. "brimful of STARLITE": toward standards for reporting literature searches. Journal of the Medical Library Association : JMLA. 2006;94(4):421–9. e205
Faggion CM Jr, Wu YC, Tu YK, Wasiak J. Quality of search strategies reported in systematic reviews published in stereotactic radiosurgery. Br J Radiol. 2016;89(1062):20150878.
Mullins MM, DeLuca JB, Crepaz N, Lyles CM. Reporting quality of search methods in systematic reviews of HIV behavioral interventions (2000–2010): are the searches clearly explained, systematic and reproducible? Research Synthesis Methods. 2014;5(2):116–30.
Yoshii A, Plaut DA, McGraw KA, Anderson MJ, Wellik KE. Analysis of the reporting of search strategies in Cochrane systematic reviews. Journal of the Medical Library Association : JMLA. 2009;97(1):21–9.
Bigna JJ, Um LN, Nansseu JR. A comparison of quality of abstracts of systematic reviews including meta-analysis of randomized controlled trials in high-impact general medicine journals before and after the publication of PRISMA extension for abstracts: a systematic review and meta-analysis. Syst Rev. 2016;5(1):174.
Akhigbe T, Zolnourian A, Bulters D. Compliance of systematic reviews articles in brain arteriovenous malformation with PRISMA statement guidelines: review of literature. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2017;39:45–8.
Tao KM, Li XQ, Zhou QH, Moher D, Ling CQ, Yu WF. From QUOROM to PRISMA: a survey of high-impact medical journals' instructions to authors and a review of systematic reviews in anesthesia literature. PLoS One. 2011;6(11):e27611.
Wasiak J, Tyack Z, Ware R. Goodwin N. Jr. Poor methodological quality and reporting standards of systematic reviews in burn care management. International wound journal: Faggion CM; 2016.
Tam WW, Lo KK, Khalechelvam P. Endorsement of PRISMA statement and quality of systematic reviews and meta-analyses published in nursing journals: a cross-sectional study. BMJ Open. 2017;7(2):e013905.
Rader T, Mann M, Stansfield C, Cooper C, Sampson M. Methods for documenting systematic review searches: a discussion of common issues. Res Synth Methods. 2014;5(2):98–115.
Atkinson KM, Koenka AC, Sanchez CE, Moshontz H, Cooper H. Reporting standards for literature searches and report inclusion criteria: making research syntheses more transparent and easy to replicate. Res Synth Methods. 2015;6(1):87–95.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6.
Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62(9):944–52.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed). 2017;358.
Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34.
Relevo R, Balshem H. Finding evidence for comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. 2011;64(11):1168–77.
Medicine Io. Standards for Systematic Reviews 2011 [Available from: http://www.nationalacademies.org/hmd/Reports/2011/Finding-What-Works-in-Health-Care-Standards-for-Systematic-Reviews/Standards.aspx .
CADTH: Resources 2018.
Download references
Acknowledgements
CC acknowledges the supervision offered by Professor Chris Hyde.
This publication forms a part of CC’s PhD. CC’s PhD was funded through the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (Project Number 16/54/11). The open access fee for this publication was paid for by Exeter Medical School.
RG and NB were partially supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula.
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and affiliations.
Institute of Health Research, University of Exeter Medical School, Exeter, UK
Chris Cooper & Jo Varley-Campbell
HEDS, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK
Andrew Booth
Nicky Britten
European Centre for Environment and Human Health, University of Exeter Medical School, Truro, UK
Ruth Garside
You can also search for this author in PubMed Google Scholar
Contributions
CC conceived the idea for this study and wrote the first draft of the manuscript. CC discussed this publication in PhD supervision with AB and separately with JVC. CC revised the publication with input and comments from AB, JVC, RG and NB. All authors revised the manuscript prior to submission. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Chris Cooper .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:.
Appendix tables and PubMed search strategy. Key studies used for pearl growing per key stage, working data extraction tables and the PubMed search strategy. (DOCX 30 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Cooper, C., Booth, A., Varley-Campbell, J. et al. Defining the process to literature searching in systematic reviews: a literature review of guidance and supporting studies. BMC Med Res Methodol 18 , 85 (2018). https://doi.org/10.1186/s12874-018-0545-3
Download citation
Received : 20 September 2017
Accepted : 06 August 2018
Published : 14 August 2018
DOI : https://doi.org/10.1186/s12874-018-0545-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Literature Search Process
- Citation Chasing
- Tacit Models
- Unique Guidance
- Information Specialists
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
University of Tasmania, Australia
Systematic reviews for health: building search strategies.
- Handbooks / Guidelines for Systematic Reviews
- Standards for Reporting
- Registering a Protocol
- Tools for Systematic Review
- Online Tutorials & Courses
- Books and Articles about Systematic Reviews
- Finding Systematic Reviews
- Critical Appraisal
- Library Help
- Bibliographic Databases
- Grey Literature
- Handsearching
- Citation Searching
- 1. Formulate the Research Question
- 2. Identify the Key Concepts
- 3. Develop Search Terms - Free-Text
- 4. Develop Search Terms - Controlled Vocabulary
- 5. Search Fields
- 6. Phrase Searching, Wildcards and Proximity Operators
- 7. Boolean Operators
- 8. Search Limits
- 9. Pilot Search Strategy & Monitor Its Development
- 10. Final Search Strategy
- 11. Adapt Search Syntax
- Documenting Search Strategies
- Handling Results & Storing Papers
Building Search Strategies
Building search strategies is part of the search for studies step..
This tab offers a step-by-step guide to developing a search strategy for a systematic review. The theoretical explanation for each of the steps (left column) is supplemented with an example (right column).
It is recommended to work through the steps sequentially, starting with Step 1 .

Steps of Building Search Strategies
These are the steps required when developing a comprehensive search strategy for a systematic review:
1. Formulate the research question
2. Identify the key concepts
3. Develop search terms - free-text terms
4. Develop search terms - controlled vocabulary terms
5. Search fields
6. Phrase searching, wildcards and proximity operators
7. Boolean operators
8. Search limits
9. Pilot search strategy and monitor its development
10. Final search strategy
11. Adapt search syntax for different databases
Templates / Helpsheets
- Template for Systematic Review search
To illustrate each step for developing a search strategy, the example used in the following 11 steps is (with a slight adaptation):
Aromataris, E & Riitano, D 2014, 'Systematic reviews: Constructing a search strategy and searching for evidence', AJN The American Journal of Nursing , vol. 114, no. 5, pp. 49-56.
Additional Resources
A subsection of the Evidence-Based Information Special Interest Group (EBI-SIG) with the European Association of Health Information and Libraries (EAHIL) are working on a project to create living open access Library of Search Strategy Resources :
- Library of Search Strategy Resources (LSSR)
SuRe Info is a web resource that provides research-based information relating to information retrieval aspects of producing systematic reviews. The resource is kept up-to-date by an international team of experienced information specialists.
- SuRe Info - Summarised Research in Information Retrieval for HTA
Other Australian universities have developed extensive guides on building search strategies:
- Plan your search strategy (University of Adelaide)
- Systematic reviews: Search strategy (University of Newcastle)
These videos from the Medical Library at Yale University outline how to build search strategies for a systematic review:
- Systematic Searches #4: Building Search Strategies (Part I)
- Systematic Searches #5: Building Search Strategies (Part II)
- Systematic Searches #6: Building Search Strategies (Part III)
- Systematic Searches #7: Building Search Strategies (Part IV)
- Systematic Searches #8: Building Search Strategies (Part V)
- Systematic Searches #9: Using Filters and Hedges
Need More Help? Book a consultation with a Learning and Research Librarian or contact [email protected] .
- << Previous: Citation Searching
- Next: 1. Formulate the Research Question >>
- Last Updated: Sep 3, 2024 10:18 AM
- URL: https://utas.libguides.com/SystematicReviews

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Constructing a search strategy and searching for evidence. A guide to the literature search for a systematic review
Affiliation.
- 1 Edoardo Aromataris is the director of synthesis science at the Joanna Briggs Institute in the School of Translational Health Science, University of Adelaide, South Australia, where Dagmara Riitano is a research officer. Contact author: Edoardo Aromataris, [email protected]. The authors have disclosed no potential conflicts of interest, financial or otherwise. The Joanna Briggs Institute aims to inform health care decision making globally through the use of research evidence. It has developed innovative methods for appraising and synthesizing evidence; facilitating the transfer of evidence to health systems, health care professionals, and consumers; and creating tools to evaluate the impact of research on outcomes. For more on the institute's approach to weighing the evidence for practice, go to http://joannabriggs.org/jbi-approach.html.
- PMID: 24759479
- DOI: 10.1097/01.NAJ.0000446779.99522.f6
This article is the third in a new series on the systematic review from the Joanna Briggs Institute, an international collaborative supporting evidence-based practice in nursing, medicine, and allied health fields. The purpose of the series is to show nurses how to conduct a systematic review-one step at a time. This article details the major considerations surrounding search strategies and presents an example of a search using the PubMed platform (pubmed.gov).
PubMed Disclaimer
Similar articles
- The systematic review: an overview. Aromataris E, Pearson A. Aromataris E, et al. Am J Nurs. 2014 Mar;114(3):53-8. doi: 10.1097/01.NAJ.0000444496.24228.2c. Am J Nurs. 2014. PMID: 24572533
- [Systematic literature search in PubMed : A short introduction]. Blümle A, Lagrèze WA, Motschall E. Blümle A, et al. Z Rheumatol. 2019 Mar;78(2):155-172. doi: 10.1007/s00393-019-0603-1. Z Rheumatol. 2019. PMID: 30756138 German.
- [Systematic literature search]. Guba B. Guba B. Wien Med Wochenschr. 2008;158(1-2):62-9. doi: 10.1007/s10354-007-0500-0. Wien Med Wochenschr. 2008. PMID: 18286251 German.
- Developing the review question and inclusion criteria. Stern C, Jordan Z, McArthur A. Stern C, et al. Am J Nurs. 2014 Apr;114(4):53-6. doi: 10.1097/01.NAJ.0000445689.67800.86. Am J Nurs. 2014. PMID: 24681476 Review.
- Presenting and interpreting findings. Robertson-Malt S. Robertson-Malt S. Am J Nurs. 2014 Aug;114(8):49-54. doi: 10.1097/01.NAJ.0000453044.01124.59. Am J Nurs. 2014. PMID: 25075699 Review.
- Evidence for motivational interviewing in educational settings among medical schools: a scoping review. Lei LYC, Chew KS, Chai CS, Chen YY. Lei LYC, et al. BMC Med Educ. 2024 Aug 8;24(1):856. doi: 10.1186/s12909-024-05845-w. BMC Med Educ. 2024. PMID: 39118104 Free PMC article. Review.
- A systematic review of AI literacy scales. Lintner T. Lintner T. NPJ Sci Learn. 2024 Aug 6;9(1):50. doi: 10.1038/s41539-024-00264-4. NPJ Sci Learn. 2024. PMID: 39107327 Free PMC article.
- Mapping the evidence on factors related to postpartum contraception among sub-Saharan African immigrant and refugee women in the United States of America: A scoping review protocol. Olorunsaiye CZ, Badru MA, Osborne A, Degge HM, Yaya S. Olorunsaiye CZ, et al. PLoS One. 2024 May 29;19(5):e0304222. doi: 10.1371/journal.pone.0304222. eCollection 2024. PLoS One. 2024. PMID: 38809899 Free PMC article. Review.
- Comparing Resection and Primary Anastomosis versus Hartmann's Stoma on the Mortality and Morbidity of Gangrenous Sigmoid Volvulus: Systematic Review and Meta-Analysis. Awedew AF, Asefa Z, Enkoye BD. Awedew AF, et al. Ethiop J Health Sci. 2023 Nov;33(6):1087-1096. doi: 10.4314/ejhs.v33i6.19. Ethiop J Health Sci. 2023. PMID: 38784481 Free PMC article. Review.
- Local people's perceptions of international clinical placements in low- and middle-income countries: A literature review. Beck K. Beck K. Int J Nurs Stud Adv. 2023 Nov 5;5:100165. doi: 10.1016/j.ijnsa.2023.100165. eCollection 2023 Dec. Int J Nurs Stud Adv. 2023. PMID: 38746555 Free PMC article. Review.
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- Wolters Kluwer
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Systematic Reviews: Search Strategy
- Methods & Resources
- Protocol & Registration
- Search Strategy
- Where to Search
- Study selection and appraisal
- Data Extraction, Study Characteristics, Results
- Reporting the quality/risk of bias
- PRISMA Reporting Items
- Manage citations using RefWorks This link opens in a new window
- Covidence Guide This link opens in a new window
Development of search strategy: PRISMA Item 7
1. Begin by defining your research question. You should identify who and what are the population, interventions, comparisons, outcomes (PICO) as well as the study design and study characteristics you are interested in.
2. Write down your keyword concepts from your PICO question. Identify the MEDLINE MeSH headings used for your keywords. Your initial search strategy may change as you grow as find new or related additional keywords and concepts.
What is PICO?
The PICO model can help you formulate a good clinical question. Sometimes it's referred to as PICO-T, containing an optional 5th factor.
| - Patient, Population, or Problem | What are the most important characteristics of the patient? How would you describe a group of patients similar to yours? |
| - Intervention, Exposure, Prognostic Factor | What main intervention, prognostic factor, or exposure are you considering? What do you want to do for the patient (prescribe a drug, order a test, etc.)? |
| - Comparison | What is the main alternative to compare with the intervention? |
| - Outcome | What do you hope to accomplish, measure, improve, or affect? |
| - Time Factor, Type of Study (optional) | How would you categorize this question? What would be the best study design to answer this question? |
Alternative Frameworks
Although PICO is the most widely used framework for generating a question, there are some situations in which you might find that a different framework is more appropriate. Here are a few alternatives.
- PEO: P opulation, E xposure, O utcome (Useful for public health and qualitative studies)
- SPICE: S etting, P erspective, I ntervention, C omparison, O utcome (Useful for qualitative studies and evaluating projects)
- SPIDER: S ample, P henomenon of I nterest, D esign, E valuation, R esearch Type (Useful for topics focused on samples rather than populations)
- PIPOH: P opulation, I ntervention, P rofession, O utcome, H ealth Care Setting (Useful for screening and creating practice guidelines)
- PICo: P opulation, I ntervention, Co ntext
- PIT or PICTO: P opulation, I ndex test, ( C omparator,) T arget condition, ( O utcome) (Used for diagnostic test accuracy)
- ECLIPSe: E xpectation, C lient group, L ocation, I mpact, P rofessionals, Se rvice (Useful for studying outcomes of policies or services)
PICO Resources
- Finding the Evidence 1 - Using PICO to formulate a search question Centre for Evidence-Based Medicine (3-minute YouTube video)
- How to ask a clinical question in PICO format PhysiotherapyEvidenceDatabase.PEDro (4-minute YouTube video)
- PICO & Search Query Worksheet Ebling Library, University of Wisconsin-Madison (1-page PDF about defining questions, types of scenarios, types of studies, and generating a search strategy)
- PICO Tutorials Marymount University (Two 5-minute videos, with transcripts, about formulating and researching PICO questions)
- Question Templates for PICOT Sonoma State University (3-page Word document that has templates for various question types and includes examples)
- EBM 101: Asking Answerable Clinical Questions—The PICO Model University of Iowa Health Care (2-minute YouTube video)
Reporting your Search Strategy
PRISMA Items 6-7 are:
- "Specify all databases, registers, websites, organizations, reference lists and other source searched or consulted to identify studies. Specify the date when each source was last searched or consulted."
- "Present the full search strategies for all databases, registers and websites, including any filters and limits used."
The PRISMA-Search checklist provides further guidance on how to report the search strategies you use.
Search Tips
- Use Boolean operators (AND, OR, NOT) to connect keywords.
- Use truncation: If you place an asterisk * (called a wildcard) at the end of a series of letters, the database will search for all words that begin with that series of letters. So the keyword adolescen* will search for adolescence, adolescent, and adolescents. This is useful for capturing the singular and plural versions of words.
- You can add field codes to narrow down your results. For example, in PubMed, searching nursing[tiab] tells the database to search for the keyword "nursing" but only in the titles and abstracts of articles, rather than the full text.
Sometimes, you don't need to reinvent the wheel. You might find a previous systematic review on your topic that shares the search strategy used.
- PubMed lists >4,500 reviews that have followed the PRISMA format and in general these will include an example of at least one database search.
- Another source for search strategies is the PROSPERO database of review protocols, type keywords into the search box to see if there are any reviews similar to your topic.
- Search the Dissertations and Theses Online database for completed Doctoral research. The peer-review process for a dissertation is different than for a published journal article, and may not have been subjected to independent scrutiny, however a good PhD dissertation or thesis should always include a literature review and this can be a good source for ideas of resources to search.
- Search MEDLINE or PubMed and use the "Publication type" limit for systematic review to limit the results to just this type of review article. Or search PubMed Clinical Queries using simple keywords and look in the center column of results for a list of recent systematic reviews.
You might also look for a validated "search hedge":
- The websites of the McMaster University Health Information Research Unit , and the University of York Centre for Reviews and Dissemination , may list a pre-existing evidence-based database search filter that can identify studies on your topic.
- PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement McGowan, J., Sampson, M., Salzwedel, D. M., Cogo, E., Foerster, V., & Lefebvre, C. (2016). Journal of clinical epidemiology, 75, 40–46. https://doi.org/10.1016/j.jclinepi.2016.01.021
- Searching clinical trials registers: guide for systematic reviewers Hunter, K. E., Webster, A. C., Page, M. J., et al. (2022). BMJ (Clinical research ed.), 377, e068791. https://doi.org/10.1136/bmj-2021-068791
- Identification of problems in search strategies in Cochrane Reviews Franco, J. V. A., Garrote, V. L., Escobar Liquitay, C. M., & Vietto, V. (2018). Research synthesis methods, 9(3), 408–416. https://doi.org/10.1002/jrsm.1302
Clinical Question Types and Study Design
What types of studies will you be looking for? Certain study designs are better for answering particular question types. The Appropriate Study Designs column lists the study designs best suited to each question type, in order of utility.
| Questions about the effectiveness of treatment in order to achieve an outcome (drugs, surgical intervention, exercise, counseling, etc.) | Randomized Controlled Trial (RCT) | PICO PEO | |
| Questions about identification of a disorder in a patient presenting with specific symptoms | RCT > Cohort Study | PICO PIT / PICTO | |
| Questions about the progression of a disease or outcome of a patient with a particular condition | Cohort Study > Case Control Studies > Case Series | PICO SPICE SPIDER | |
| Questions about the negative impact from an intervention or other procedure | Cohort Study > Case Control Studies > Case Series | PICO / PICo PIPOH ECLIPSe |
- << Previous: Protocol & Registration
- Next: Where to Search >>

- Last Updated: Aug 13, 2024 12:37 PM
- URL: https://guides.himmelfarb.gwu.edu/systematic_review

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2962
- [email protected]
- https://himmelfarb.gwu.edu
- University of Michigan Library
- Research Guides
Evidence Syntheses (Scoping, systematic, & other types of reviews)
- Search Strategy
- Types of Reviews
- Should You Do a Systematic Review?
- Work with a Search Expert
- Covidence Review Software
- Evidence in an Evidence Synthesis
- Information Sources
Developing an Answerable Question
Creating a search strategy, identifying synonyms & related terms, keywords vs. index terms, combining search terms using boolean operators, a sr search strategy, search limits.
- Managing Records
- Selection Process
- Data Collection Process
- Study Risk of Bias Assessment
- Reporting Results
- For Search Professionals
Validated Search Filters
Depending on your topic, you may be able to save time in constructing your search by using specific search filters (also called "hedges") developed & validated by researchers. Validated filters include:
- PubMed’s Clinical Queries & Health Services Research Queries pages
- Ovid Medline’s Clinical Queries filters (also documented by McMaster Health Information Research Unit)
- EBSCOhost’s main search page for CINAHL (Clinical Queries category)
- American U of Beirut, esp. for " humans" filters .
- Countway Library of Medicine methodology filters
- InterTASC Information Specialists' Sub-Group (ISSG) Search Filter Resource
- SIGN (Scottish Intercollegiate Guidelines Network) filters page
Why Create a Sensitive Search?
In many literature reviews, you try to balance the sensitivity of the search (how many potentially relevant articles you find) and specificit y (how many definitely relevant articles you find ), realizing that you will miss some. In an evidence synthesis, you want a very sensitive search: you are trying to find all potentially relevant articles. An evidence synthesis search will:
- contain many synonyms & variants of search terms
- use care in adding search filters
- search multiple resources, databases & grey literature, such as reports & clinical trials.
PICO is a good framework to help clarify your systematic review question.
P - Patient, Population or Problem: What are the important characteristics of the patients &/or problem?
I - Intervention: What you plan to do for the patient or problem?
C - Comparison: What, if anything, is the alternative to the intervention?
O - Outcome: What is the outcome that you would like to measure?
Beyond PICO: the SPIDER tool for qualitative evidence synthesis.
5-SPICE: the application of an original framework for community health worker program design, quality improvement and research agenda setting.
A well constructed search strategy is the core of your evidence synthesis and will be reported on in the methods section of your paper. The search strategy retrieves the majority of the studies you will assess for eligibility & inclusion. The quality of the search strategy also affects what items may have been missed. Informationists can be partners in this process.
For an evidence synthesis, it is important to broaden your search to maximize the retrieval of relevant results.
Use keywords: How other people might describe a topic?
Identify the appropriate index terms (subject headings) for your topic.
- Index terms differ by database (MeSH, or Medical Subject Headings , Emtree terms, Subject headings) are assigned by experts based on the article's content.
- Check the indexing of sentinel articles (3-6 articles that are fundamental to your topic). Sentinel articles can also be used to test your search results.
Include spelling variations (e.g., behavior , behaviour).
Both types of search terms are useful & both should be used in your search.
Keywords help to broaden your results. They will be searched for at least in journal titles, author names, article titles, & article abstracts. They can also be tagged to search all text.
Index/subject terms help to focus your search appropriately, looking for items that have had a specific term applied by an indexer.
Boolean operators let you combine search terms in specific ways to broaden or narrow your results.

An example of a search string for one concept in a systematic review.

In this example from a PubMed search, [mh] = MeSH & [tiab] = Title/Abstract, a more focused version of a keyword search.
A typical database search limit allows you to narrow results so that you retrieve articles that are most relevant to your research question. Limit types vary by database & include:
- Article/publication type
- Publication dates
In an evidence synthesis search, you should use care when applying limits, as you may lose articles inadvertently. For more information, see, Chapter 4: Searching for and selecting studies of the Cochrane Handbook particularly regarding language & format limits in Section 4.4.5 .

Systematic Literature Review
- What is a Systematic Literature Review?
- Doing a Systematic Literature Review
- Developing a Search Strategy
- Useful Links and Resources
- Using AI tools for Desk-based Research & Assignments
Searching for information
The Library Catalogue is good place to start looking for information.
Many of the resources described here can be found in the Library. Follow the link below to begin your search
TU Dublin Library Catalogue
Developing a search strategy
- Search Strategy
- Types of information resources
- Where to find your information
- Getting the best out of your search
- Subject headings
Researchers conducting a systematic literature review need to perform comprehensive searches to ensure they have retrieved all of the relevant information. Below is an overview of the steps involved in conducting a search for literature. For further information on conducting a comprehensive search, please see the Cochrane handbook .
Scope the topic. this will help to contextualise your topic within the broader context of your subject and also indicate the volume of literature available on your subject. it also gives an opportunity to refine your search strategy to ensure an effective and accurate search..
Inclusion/Exclusion criteria is used to identify the specific attributes of material that you want to include in the review. For example, the type of study or population, etc.
Identify the concepts in your research question. You can use a framework to help with this, such as PICO , SPIDER or another suitable method.
Identify keywords, synonyms and alternative keywords. You can develop your synonyms and alternative keywords using relevant articles or papers that you are already aware of. You will also need to use subject headings to ensure you do a comprehensive search (see subject headings tab).
Combine searches using Boolean operators, OR and AND. Use OR to combine the keywords and subject headings, and use AND to retrieve material that includes all the concepts in your research question. See the getting the best out of your searching tab for further details on truncation, phrased searching, etc. The idea of a search strategy is that it can be used across multiple sources, however, you may need to re-map your keywords to the subject headings of different databases.
Apply search filters. Many databases allow researchers to limit search results through the use of filters or hedges. For example results can be limited by publication type, study type, etc. However, these in-built filters may exclude relevant studies. For this reason, and to maintain consistency across databases, researchers conducting systematic literature reviews use standard filters that can be added to searches using the AND operator. The filters are re-useable and shared widely.
- The InterTASC Information Specialists' Sub Group Search Filter Resources (ISSG)
- BMJ Clinical Evidence Study Design Search Filters
Sensitivity versus precision involves balancing your strategy between retrieving a large number of documents that might include some irrelevant material, against are a more precise strategy that might miss relevant material.
Review your strategy. Make sure that your results are relevant, identify any keyword/concepts from your strategy that don't appear in your results. Check retrieved material for any relevant keywords or subject headings that you may have omitted.
Document your search. You need to record the details of your searches to ensure transparency and consistency. You can use a table or spreadsheet to do this. Include;
- search terms
- database/catalogue searched
- refinements or limitations (if used)
- date searched
- basic/advanced search
- number of records (hits)
- articles retained
- articles you can't access
Manage your results. Use bibliographic management software (Endnote, Mendeley or Zotero) to remove duplicate records.
Select material. Use the agreed criteria to select material. This is done in stages, initially by screening the title, then the abstract and finally by full text. This should be done by more than one person, to help reduce bias in the process. Having more than one person involved also means that it can be easier to resolve queries regarding selection of individual papers, should a query arise.
Move on to the data extraction phase. For further information see chapter 5 of the Cochrane handbook .
For further information on conducting searches for SLR, please see the following useful links:
- Search Strategy for SLR (NUI Galway)
- Search Strategy for SLR (UCD)
There are many different types of information resources. Students and researchers can use a combination of resources when completing assignments or research. Some of the most widely used sources are described below.
Textbooks that you find in the library are usually written by experts. Textbooks are structured for ease of use; they begin with simpler aspects of a topic before progressing towards more complicated aspects. Books often have useful glossaries and an index of subjects and authors. They also include references and bibliographies, which can help to expand your search.
Conference Proceedings can be very important for researchers because often, the first time research is published, is at a conference. Proceedings provide access to specialist and focused information.
Journals are published at regular intervals, such as monthly, bi-monthly or even annually. This means that journals can be a good source of up to date information. They can be written by either academics, specialist researchers or professional practitioners. Some journals are peer-reviewed, this means any material published in the journal has undergone an evaluation process to ensure the quality and validity of the information. Journals can be highly focused on a specific subject and also include references.
Official Publications are published by governments and government departments all over the world. They can be a useful source of information on areas such as legal, education, finance, science, health and social policies.
Reference Material includes subject directories, almanacs and encyclopedia. They can provide useful definitions and can be a good sources of primary data (statistics, speeches, diaries). There is no evaluation or interpretation of the material provided, which allows the student or researcher to use the data for their own purpose and to draw their conclusions.
Standards are an agreed set of procedures, processes or technical specifications that provide guidance across multiple disciplines and industries.
Social media, such as blogs and twitter feeds can highlight key topics and discussions that are current and fast moving. Social media has the potential to help researchers to stay up to date with developments in different disciplines.
Theses are final year research projects submitted by students completing degree and doctorate level qualifications. Theses are a valuable information source for researchers as they are highly focused on a particular topic, They will also contain a detailed literature review section, and have references and a bibliography.
Grey literature describes material and research that is not published by academic or commercial publishers. It is often produced by professional bodies or organisations. For example, theses, annual reports, technical guidelines, conference posters or government papers.
Other resources
It is important to be aware of the many different sources of information that exist, so you can select the most suitable ones for your project or research. Below is a list of some of the most popular sources.
Library Catalogues provide access to a combination of print and digital material and often cover multiple disciplines. Most library catalogues can be searched by anyone, although access to material in other institutions is subject to certain permissions.
Institutional repositories provide access to the research outputs of individual academic institutions. This includes PhD and other research theses. OpenDOAR allows users to find repositories. RIAN allows user to search all Irish repositories, including Arrow@TuDublin .
Databases contain many different types of information resources, such as journal articles, books, conference proceedings, newspapers and reports. The databases that are available through the library provide access to scholarly, and frequently, peer-reviewed material. Academic databases are designed to help researchers, so many have advanced search features to facilitate accurate and focused searches.
Search Engines are useful resources discovery tools. They contain links to and information on a wide variety of subjects in various formats. However, there is very little, if any, oversight or review of the material. So any information taken from search engines must be thoroughly evaluated. Although results may be largely relevance-based, some search engines manipulate how results are displayed for commercial purpose, so there is a chance that you are not seeing the most relevant information at the top of the results page. N.B. If you are doing a systematic literature review using Google, be sure to turn off personalisation by deleting your browser history, logging out of your account and removing all Google services through the "My Activity" page. Otherwise, your results may be influenced by the search history and this may impact the relevance of your results. Consider using other specialist search engines, such as BASE .
Subject Directories provide access to curated, and sometimes annotated, subject resources. Sites included in the directory are usually selected by an administrator according to set criteria. The directory is navigated by clicking through options rather than a search box. Directories contain less information than a search engine, but they can be useful for specific subject areas. See the subject guide in your area for further information.
OpenGrey is the system for information on grey literature in Europe and provides access to 700,000 bibliographical references of grey literature produced in Europe.
LENUS provides access to the research output of many healthcare organisations in the Republic of Ireland.
Other Sources available to researchers include government & EU websites, NGOs and other voluntary and professional organisations.
- Phrased searching
The default Boolean term for a database or search engine (unless otherwise stated) is AND. For example, a search for -information technology- will be interpreted as information AND technology. However, if you want to search for information on the topic - information technology, put inverted commas around both words, "information technology". Phrased searching should decrease the number of results you retrieve but increase the relevance of those results.
- Wildcard & Truncation
Wildcards (?/#) allow you to search for the American and British spellings of words. For example, a search for the word behavio?r, will include both behaviour and behavior. You can also use # to include plurals of words like man or men by using m#n.
Truncation (*) allows you to include variations of a word. For example, teach*, will include teachers, teacher, teaching and teach.
- Subject descriptors/headings
- Snowballing
- Citation searching
- Hand-searching
Involves searching for relevant material that may have been missed by databases. For further guidelines on hand-searching see the Cochrane handbook .
Subject headings are assigned by an administrator when a document is added to the database. The headings provide a consistent description of the subject content of the document. They are also known as descriptors or categories. They are similar to a hashtag but are added by the publisher, not the user. Subject headings are selected from a controlled vocabulary (a list of agreed or standard terms), which is maintained and updated by an administrator.
There are different ways of locating subject headings. Some databases allow access through a list of subject headings, descriptors, categories or provide a searchable thesaurus. The thesaurus will allow you to enter your keyword into the search box. The list of results should provide the subject heading closest to your keyword, and in some databases, you will be shown broader and narrower terms associated with your topic. Alternatively, you could do a standard keyword search and identify the subject headings or descriptors assigned to one or two relevant documents.
One of the most well-known lists of subject headings is the Medical Subject Headings or MeSH , used to search MEDLINE/PubMed or the National Library of Medicine.
- << Previous: Doing a Systematic Literature Review
- Next: Useful Links and Resources >>
- Last Updated: Aug 9, 2024 9:02 AM
- URL: https://tudublin.libguides.com/systematicliteraturereview
Searching for Systematic Reviews & Evidence Synthesis: Drawing up your search strategy
- Define your search question
- Searching Databases
- Drawing up your search strategy
- Advanced search techniques
- Using Filters
- Grey Literature
- Recording your search strategy and results
- Managing References & Software Tools
- Further information
- Library Workshops, Drop ins and 1-2-1s
- AI tools in evidence synthesis
Subject Headings
- Fixed list of terms arranged hierarchically with broader and narrower terms.
- Indexers classify the article by tagging it with subject headings that relate to the content.
- Some tags represent the main focus of the article and some refer to secondary aspects of the work.
- Can allow you to search more effectively and avoid missing relevant articles.
- Can retrieve relevant articles where the term does not occur in title or abstract.
- In these databases you need to rely on just keyword searching, ensuring that you use as many synonyms, and alternate terms as possible.
- This means that you cannot use the same subject headings from one database in another but will need to research for each concept in order to locate the relevant subject heading (if it exists) and add that to your search.
- On other database platforms you may need to select the subject heading you wish to use. You may also be presented with a list of subject headings to select on databases on the OvidSP platform if there is no exact match. Remember that the subject heading you select should be the one for the concept you are searching for - sometimes those subject headings suggested may be relevant to your search question but not the concept you are searching for at that point.
- If you are uncertain what explode and focus mean look at the explanation on this page.
- Use the literature search template (available from the Define your search question tab ) to remind you about breaking your concept down and combining it accurately with AND and OR.
Types of research design
Cochrane systematic reviews and those systematic reviews considering interventions often include a filter to restrict the results to studies reporting Randomised Controlled Trials (RCTs). Consider whether you wish to include a filter for a particular type of study design as part of your search strategy. These exist for RCTs but also for other study types including Observational Studies and Patient Issues. For more information about where to source standardised, pre-tested search filter search strategies optimised for different databases which you can copy and paste and add to your own search strategy see the Using Filters tab of this guide.
Free-text keyword searching
- article title
Combining your search - OR and AND
| Antidepressant drugs OR antidepressive agents | Eating disorders AND cognitive therapy |
AND and OR are Boolean operators.
OR is used to combine synonyms, abbreviations and all related terms on a similar concept. You can OR together subject headings for a particular concept with relevant keyword searches. 'OR is more'. Your result set will get larger as you OR together more terms.
AND is used (normally at the end of the search) to combine together different concepts and to retrieve results where all the concepts are present. AND narrows down your results and makes your search more specific. AND is sometimes automatic for two or more search terms depending on the database.
Explode and Focus - Subject Heading searching
Explode:
- The indexers will select the most specific subject heading possible to tag an article so by choosing explode you can select a high level subject heading (e.g. antibiotic agent is used in Embase for the concept antibiotic) and by exploding the subject heading you will automatically include the narrower, more specific terms, e.g. named antibiotics.
- To view the more specific subject headings which would be included click on the subject heading itself to view it in the thesaurus. The structure of the thesaurus may be displayed in different ways depending on the database, e.g. in Embase on the Ovid platform these are listed under Narrower Terms; in Medline narrower terms are indented in.
- In general it is good practice to explode the Subject Headings in your search. If you feel that too much irrelevant material is being retrieved then explore the thesaurus to see whether you need to pick a high level subject heading (not exploded) and also only some of the more specific subject headings which fall beneath it in the thesaurus.
Focus (in the Ovid databases):
- If you select Focus you will restrict your results to only those articles which the indexer feels the subject heading you have selected to search is key to what the article is about.
- In other databases Focus may be known as something else, e.g. Major Concept in CINAHL on the Ebsco platform.
- On the Ovid platform if you view the Complete Reference for an article you will see all the subject headings which have been assigned to an article, and the ones which are key (which would be retrieved by a focused search) are marked by an asterisk before the subject heading. In CINAHL if you view the Detailed Record you will see them listed under Major Subjects.
- Use Focus with care in a systematic review as it will dramatically reduce the number of results retrieved. Initially it would be a good idea to see what the results are without focusing after you have combined your terms. You may find it useful to use Focus for very peripheral aspects of your topic which you wish to include without being inundated with results.
It is possible to both Explode and Focus a subject heading search.
- By applying both Explode and Focus you will retrieve articles tagged with your subject heading and the narrower subject heading terms that fall underneath it in the thesaurus (E xplode) , but also limit (reduce) the results to where either the top level subject heading or any of its narrower terms have been identified by the indexers as being key/the focus/the major concept which the article is about.
A search on Embase on the Ovid platform showing the initial Subject Heading selection screen. Explode and Focus are available to select:

By clicking on a specific Subject Heading in the list, e.g. antibiotic agent, you enter the thesaurus and can see what the term is used for, the broader terms and narrower terms. By selecting Explode for antibiotic agent in Embase you will automatically retrieve articles which have also been tagged with all of the narrower more specific named antibiotics.

Below you can see the Full Record for a particular article (in Embase on the Ovid platform) with the Subject Headings listed which have been used to tag this particular article. You can see that 'penicillin allergy' is one of the subject headings assigned to this article and the asterisk * before the subject heading indicates that this was deemed to be key to what the article was about when it was added to the database and subject headings assigned. A normal subject heading search for 'penicillin allergy' or its exploded broader term would retrieve this article but so would a focussed search *penicillin allergy/. An exploded and focused search for the broader term, e.g. drug hypersensitivity, would also retrieve this article - exp *drug hypersensitivity/.

Using search strategies from published systematic reviews
It is always worth checking to see whether any systematic reviews which have a concept in common with your search question have published their search strategy. If they have then this will act as a useful starting point for you to use for your search. Remember it is not necessary for all the concepts of the systematic review to be the same as you should be able to isolate the specific lines of the search strategy relevant to you. So for example if I am undertaking a systematic review on the 'effectiveness of phototherapy for neonatal jaundice' I may find that part of a search strategy for a published review on 'Phototherapy for treating pressure ulcers' is very relevant for me. I will then need to either create a search strategy element for my other concept of 'neonatal jaundice' or locate another systematic review which may have already created a search strategy including an element for this concept, e.g. one on 'Early intravenous nutrition for the prevention of neonatal jaundice'.
Below is an excerpt from a published Cochrane systematic review on ' Phototherapy for treating pressure ulcers' showing the search lines for the phototherapy concept (optimised for Ovid Medline) which could then be used in a different search and combined with other concepts.

Chen C, Hou WH, Chan ESY, Yeh ML, Lo HLD. Phototherapy for treating pressure ulcers. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No.: CD009224. DOI: 10.1002/14651858.CD009224.pub2.
Systematic reviews in the Cochrane Library should all publish their search strategies and in many cases will show the search strategy they used for each database they searched (optimised for each database) - this normally appears in the Appendix of the full text for a Cochrane review.
The PRISMA 2020 checklist states for '#7 Search strategy' that you should "Present the full search strategies for all databases, registers and websites, including any filters and limits used". The 2021 PRISMA searching extension increases that to "Include the search strategies for each database and information source, copied and pasted exactly as run". However, unfortunately you may find that older systematic reviews and even some current ones may not do this. Note too that in some cases the full search strategy may appear as a supporting document on a journal site as opposed to forming part of the pdf of the article.
Pay attention when looking to use a published search strategy to both the database and the platform it is hosted on and for which the search strategy has been optimised. If you are searching the same database on the same platform then you should simply be able to copy and paste the search strategy in to generate results. If you are uncertain about what the lines of the search strategy mean and what they are searching, e.g. exp, adj or .tw then see the information on the Advanced search techniques tab.
Remember when using a search strategy from another systematic review that you should assess it for quality rather than simply copying it in. Are there any other search terms that should be included, have they located all appropriate subject headings, and so on? See the information about PRESS: Peer Review of Electronic Search Strategies on the Advanced search techniques tab. The age of the source systematic review is also important as subject headings change to reflect changes in medical science. As such if you are working in an area of rapid development and the strategy is a few years old you may wish to just use it as a starting point rather than directly copying.
Finally if you do use all or part of another's search strategy then do remember to acknowledge the source.
- << Previous: Searching Databases
- Next: Advanced search techniques >>
- Last Updated: Sep 5, 2024 6:28 PM
- URL: https://libguides.kcl.ac.uk/systematicreview
Systematic Review
- Systematic reviews
Being systematic
Search terms, choosing databases, finding additional resources.
- Search techniques
- Systematically search databases
- Appraisal & synthesis
- Reporting findings
- Systematic review tools
Searching literature systematically is useful for all types of literature reviews!
However, if you are writing a systematic literature review the search needs to be particularly well planned and structured to ensure it is:
- comprehensive
- transparent
These help ensure bias is eliminated and the review is methodologically sound.
To achieve the above goals, you will need to:
- create a search strategy and ensure it is reviewed by your research group
- document each stage of your literature searching
- report each stage of quality appraisal
Identify the key concepts in your research question
The first step in developing your search strategy is identifying the key concepts your research question covers.
- A preliminary search is often done to understand the topic and to refine your research question.
Identify search terms
Use an iterative process to identify useful search terms for conducting your search.
- Brainstorm keywords and phrases that can describe each concept you have identified in your research question.
- Create a table to record these keywords
- Select your keywords carefully
- Check against inclusion/exclusion criteria
- Repeated testing is required to create a robust search strategy for a systematic review
- Run your search on your primary database and evaluate the first page of records to see how suitable your search is
- Identify reasons for irrelevant results and adjust your keywords accordingly
- Consider whether it would be useful to use broader or narrower terms for your concepts
- Identify keywords in relevant results that you could add to your search to retrieve more relevant resources
Using a concept map or a mind map may help you clarify concepts and the relationships between or within concepts. Watch these YouTube videos for some ideas:
- How to make a concept map (by Lucidchart)
- Make sense of this mess world - mind maps (by Sheng Huang)
Example keywords table:
Research question: What is the relationship between adverse childhood experiences and depression in mothers during the perinatal period?
|
adverse childhood experiences |
|
perinatal depression |
|
mothers |
| ACE | postpartum depression | women | ||
| childhood trauma | postnatal depression | |||
| maternal mental health | ||||
| maternal psychological distress | ||||
Revise your strategy/search terms until :
- the results match your research question
- you are confident you will find all the relevant literature on your topic
See Creating search strings for information on how to enter your search terms into databases.
Example search string (using Scopus's Advanced search option) for the terms in the above table:
(TITLE-ABS-KEY("advserse childhood experienc*" OR ACE OR "childhood trauma") AND TITLE-ABS-KEY("perinatal depress*" OR "postpartum depress*" OR "postnatal depress*" OR "maternal mental health" OR "maternal psychological distress") AND TITLE-ABS-KEY(mother* OR women*))
See Subject headings for information on including these database specific terms to your search terms.
Systematic reviewers usually use several databases to search for literature. This ensures that the searching is comprehensive and biases are minimised.
Use both subject-specific and multidisciplinary databases to find resources relevant to your research question:
- Subject-specific databases: in-depth coverage of literature specific to a research field.
- Multi-disciplinary databases: literature from many research fields - help you find resources from disciplines you may not have considered.
Check for databases in your subject area via the Databases tab > Find by subject on the library homepage .
Find the key databases that are often used for systematic reviews in this guide.
Test searches to determine database usefulness. You can consult your Liaison Librarians to finalise the list of databases for your review.
Recommendations:
For all systematic reviews we recommend using Scopus , a high-quality, multidisciplinary database:
- Scopus is an abstract and citation database with links to full text on publisher websites or in other databases.
- Scopus indexes a curated collection of high quality journals along with books and conference proceedings.
- Research outputs are across a range of fields - science, technology, medicine, social science, arts and humanities.
For systematic reviews within the health/biomedical field, we recommend including Medline as one of the databases for your review:
MEDLINE (via Ebsco, via Ovid, via PubMed)
- Medline is the National Library of Medicine’s (NLM) article citation database.
- Medline is hosted individually on a variety of platforms (EBSCO, OVID) and comprises the majority of PubMed.
- Articles in Medline are indexed using MeSH headings. See Subject headings for more information on MeSH.
Note: PubMed contains all of Medline and additional citations, e.g. books, manuscripts, citations that predate Medline.
To ensure your search is comprehensive you may need to search beyond academic databases when conducting a systematic review, particularly to find grey literature (literature not published commercially and outside traditional academic sources such as journals).
Google Scholar
Google Scholar contains academic resources across disciplines and sources types. These come from academic publishers, professional societies, online repositories, universities and web sites.
Use Google Scholar
- as an additional tool to locate relevant publications not included in high-level academic databases
- for finding grey literature such as postgraduate theses and conference proceedings
You can limit your search to the type of websites by using site:ac . nz; site:edu
Note that Google Scholar searches are not as replicable or transparent as academic database searches, and may find large numbers of results.
Other sources of grey literature
- Grey literature checklist (health related grey literature)
- OpenGrey
- Public health Ontario guide to appraising grey literature
- Institutional Repository for Information Sharing (IRIS)
- Google search: use it for finding government reports, policies, theses, etc. You can limit your search to a particular type of websites by including site : govt.nz, site: . gov, site: . ac . nz, site: . edu, in your search
Watch our Finding grey literature video (3.49 mins) online.
- << Previous: Planning
- Next: Search techniques >>
- Last Updated: Aug 8, 2024 4:20 PM
- URL: https://aut.ac.nz.libguides.com/systematic_reviews
- University of Wisconsin–Madison
- University of Wisconsin-Madison
- Research Guides
- Evidence Synthesis, Systematic Review Services
- Write a Search Strategy
Evidence Synthesis, Systematic Review Services : Write a Search Strategy
- Literature Review Types, Taxonomies
- Develop a Protocol
- Develop Your Research Question
- Select Databases
- Select Gray Literature Sources
- Manage Your Search Process
- Register Your Protocol
- Citation Management
- Article Screening
- Risk of Bias Assessment
- Synthesize, Map, or Describe the Results
- Find Guidance by Discipline
- Manage Your Research Data
- Browse Evidence Portals by Discipline
- Automate the Process, Tools & Technologies
- Adapting Systematic Review Methods
- Additional Resources
Searching Strategies, Search Syntax
While constructing a precise and productive search for the literature will take time and testing (or scoping), here are some useful tips on the mechanics for constructing search expressions that can be interpreted by database search engines. Search terms (keywords), operators, wildcards, and more!
- Be sure to visit the database "Help" files to learn about the special features, operators and wildcard characters that a database may uniquely use.
See also sections 3 and 4 from the CEE Guidelines for Authors. ( Guidelines and Standards for Environmental Synthesis in Environmental Management , Version 5.1, 2022) Section 3: Planning the conduct of an Evidence Synthesis Section 4: Conducting a Search
A few fundamentals and tips:
Make a list of your search terms (keywords) and the alternative, like or similar concepts. You may find it helpful to consult a thesaurus (for example, see NAL's Thesaurus and Glossary )
- As you run a search, review "subject headings" or "descriptors" to identify essential vocabulary.
Use AND to combine search terms; retrieve records with those terms, in any order: friends AND communication
Use OR to combine similar search terms or synonyms: friends OR peers
Use quotation marks ( " ") to search a required phrase: "interpersonal communication"
- Note: In many instances, the adjacency of your search terms may be sufficient to retrieve a phrase. Unless specific phrasing is necessary for meaning (or to assist if retrieving too many erroneous results), enclosing your search phrases within quotation marks may be too limiting.
Use an asterisk ( * ) to retrieve singular and plural forms or suffixes for search terms: friend* = friend, friend s , friend ly or friend ship
- Consult the Help files provided within the database to determine the correct symbol used for this function.
Apply field limits or filters to your results, if necessary (by date, document type, or other).
Using the examples, above, and putting it all together as a simple search expression, you would have: (friend* OR peer*) AND "interpersonal communication"
Tools for Translating Your Search Syntax
- Database Syntax Guide (PDF), Cochrane Effective Practice and Organisation of Care Chart of commonly used syntax (operators, wildcard characters, field codes) and their behavior across several database products.
- Medline Transpose (project) Translate a search expression between Ovid MEDLINE and PubMed.
- Polyglot Search, SR-Accelerator Tool for translating search syntax across several database products.
Why Perform an Exploratory Search
In preparing for your review, you will begin with an exploratory or preliminary search of the literature.
This search will help you:
- Identify existing reviews (so as not to duplicate work),
- Assess the quantity and quality of relevant, primary research studies (so as to be sufficiently abundant and productive for your work), and
- Identify key (benchmark) articles/publications with which to inform your subsequent searching.
Hedges, Search Filters
- AVIS Search Strategies Working Group Hedges Free archive of search hedges that can be used in animal and veterinary searches. Created by the Animal and Veterinary Information Specialists (AVIS) caucus of the Medical Library Association.
- ISSG Search Filter Resource (InterTASC Information Specialists' Sub-Group) Collaborative venture to identify, assess and test search filters designed to retrieve research by study design or focus.
- searchRxiv Resource created to support researchers by reporting, storing and sharing their searches to review and re-use.
- Elsevier 2023 Sustainable Development Goals (SDGs) Mapping Scopus search strategies developed to retrieve articles for each of the 17 United Nations Sustainable Development Goals (SDGs). Review the 17 goals: https://sdgs.un.org/goals
How to Work with Sensitive Search Terms
- Addressing antiquated, non-standard, exclusionary, and potentially offensive terms in evidence syntheses and systematic searches Suggested wording provided is intended to be a template only, and should be adapted as appropriate for a given topic or project.
- AGROVOC, Multilingual Thesaurus, FAO
- CABI/CAB Thesaurus Stand-alone edition. Thesaurus can also be accessed from within the CAB Abstracts database.
- NAL Thesaurus (National Agricultural Library)
- << Previous: Select Gray Literature Sources
- Next: Manage Your Search Process >>
- Last Updated: Aug 29, 2024 3:55 PM
- URL: https://researchguides.library.wisc.edu/literature_review
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Med Res Methodol

Defining the process to literature searching in systematic reviews: a literature review of guidance and supporting studies
Chris cooper.
1 Institute of Health Research, University of Exeter Medical School, Exeter, UK
Andrew Booth
2 HEDS, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK
Jo Varley-Campbell
Nicky britten.
3 Institute of Health Research, University of Exeter Medical School, Exeter, UK
Ruth Garside
4 European Centre for Environment and Human Health, University of Exeter Medical School, Truro, UK
Associated Data
Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving readers clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence.
Information specialists and review teams appear to work from a shared and tacit model of the literature search process. How this tacit model has developed and evolved is unclear, and it has not been explicitly examined before.
The purpose of this review is to determine if a shared model of the literature searching process can be detected across systematic review guidance documents and, if so, how this process is reported in the guidance and supported by published studies.
A literature review.
Two types of literature were reviewed: guidance and published studies. Nine guidance documents were identified, including: The Cochrane and Campbell Handbooks. Published studies were identified through ‘pearl growing’, citation chasing, a search of PubMed using the systematic review methods filter, and the authors’ topic knowledge.
The relevant sections within each guidance document were then read and re-read, with the aim of determining key methodological stages. Methodological stages were identified and defined. This data was reviewed to identify agreements and areas of unique guidance between guidance documents. Consensus across multiple guidance documents was used to inform selection of ‘key stages’ in the process of literature searching.
Eight key stages were determined relating specifically to literature searching in systematic reviews. They were: who should literature search, aims and purpose of literature searching, preparation, the search strategy, searching databases, supplementary searching, managing references and reporting the search process.
Conclusions
Eight key stages to the process of literature searching in systematic reviews were identified. These key stages are consistently reported in the nine guidance documents, suggesting consensus on the key stages of literature searching, and therefore the process of literature searching as a whole, in systematic reviews. Further research to determine the suitability of using the same process of literature searching for all types of systematic review is indicated.
Electronic supplementary material
The online version of this article (10.1186/s12874-018-0545-3) contains supplementary material, which is available to authorized users.
Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving review stakeholders clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence.
Information specialists and review teams appear to work from a shared and tacit model of the literature search process. How this tacit model has developed and evolved is unclear, and it has not been explicitly examined before. This is in contrast to the information science literature, which has developed information processing models as an explicit basis for dialogue and empirical testing. Without an explicit model, research in the process of systematic literature searching will remain immature and potentially uneven, and the development of shared information models will be assumed but never articulated.
One way of developing such a conceptual model is by formally examining the implicit “programme theory” as embodied in key methodological texts. The aim of this review is therefore to determine if a shared model of the literature searching process in systematic reviews can be detected across guidance documents and, if so, how this process is reported and supported.
Identifying guidance
Key texts (henceforth referred to as “guidance”) were identified based upon their accessibility to, and prominence within, United Kingdom systematic reviewing practice. The United Kingdom occupies a prominent position in the science of health information retrieval, as quantified by such objective measures as the authorship of papers, the number of Cochrane groups based in the UK, membership and leadership of groups such as the Cochrane Information Retrieval Methods Group, the HTA-I Information Specialists’ Group and historic association with such centres as the UK Cochrane Centre, the NHS Centre for Reviews and Dissemination, the Centre for Evidence Based Medicine and the National Institute for Clinical Excellence (NICE). Coupled with the linguistic dominance of English within medical and health science and the science of systematic reviews more generally, this offers a justification for a purposive sample that favours UK, European and Australian guidance documents.
Nine guidance documents were identified. These documents provide guidance for different types of reviews, namely: reviews of interventions, reviews of health technologies, reviews of qualitative research studies, reviews of social science topics, and reviews to inform guidance.
Whilst these guidance documents occasionally offer additional guidance on other types of systematic reviews, we have focused on the core and stated aims of these documents as they relate to literature searching. Table 1 sets out: the guidance document, the version audited, their core stated focus, and a bibliographical pointer to the main guidance relating to literature searching.
Guidance documents audited for this literature review
| Guidance documents | Version: Year | Core focus | Where the guidance is reported |
|---|---|---|---|
| Systematic Reviews: CRD’s guidance for undertaking reviews in health care [ ]. | 2009 | Systematic reviews of health care interventions | 1.3 Pages 16–22 |
| The Cochrane Handbook [ ]. | Version 5.1: June 2017 | Systematic reviews of interventions | Chapter 6: Searching for studies |
| Collaboration for environmental evidence: Guidelines for systematic reviews in environmental management [ ]. | Version 4.2 March 2013 | Systematic reviews of environmental evidence | Section “ ” (pages 36–41) |
| Joanna Briggs Institute Reviewers’ Manual [ ]. | 2014 edition | Systematic reviews of qualitative studies | Chapter 7 Information Retrieval (pages 28–31) |
| Institute for Quality and Efficiency in Health Care (IQWiG): IQWiG [ ]. | 2014 | Systematic reviews of health care interventions | Chapter 7: Information retrieval |
| Systematic Reviews in the Social Sciences: A Practical Guide [ ]. | 2006 | Systematic reviews of social science topics | Chapter 4. How to find the studies: the literature search (pages 81–124) |
| Process of information retrieval for systematic reviews and health technology assessments on clinical effectiveness. Eunethta [ ]. | Version 1.1 December 2016. | Systematic reviews of health care interventions | Standalone guideline on literature searching |
| The Campbell Handbook: Searching for studies: a guide to information retrieval for Campbell systematic reviews [ ]. | Version 1.1. February 2017. | Systematic reviews of interventions in social science topics | Standalone guideline on literature searching |
| Developing NICE guidelines: the manual [ ]. | 2014 | Systematic reviews to inform health care guidelines | Chapter 5. Identifying the evidence: literature searching and evidence submission. |
Once a list of key guidance documents was determined, it was checked by six senior information professionals based in the UK for relevance to current literature searching in systematic reviews.
Identifying supporting studies
In addition to identifying guidance, the authors sought to populate an evidence base of supporting studies (henceforth referred to as “studies”) that contribute to existing search practice. Studies were first identified by the authors from their knowledge on this topic area and, subsequently, through systematic citation chasing key studies (‘pearls’ [ 1 ]) located within each key stage of the search process. These studies are identified in Additional file 1 : Appendix Table 1. Citation chasing was conducted by analysing the bibliography of references for each study (backwards citation chasing) and through Google Scholar (forward citation chasing). A search of PubMed using the systematic review methods filter was undertaken in August 2017 (see Additional file 1 ). The search terms used were: (literature search*[Title/Abstract]) AND sysrev_methods[sb] and 586 results were returned. These results were sifted for relevance to the key stages in Fig. 1 by CC.

The key stages of literature search guidance as identified from nine key texts
Extracting the data
To reveal the implicit process of literature searching within each guidance document, the relevant sections (chapters) on literature searching were read and re-read, with the aim of determining key methodological stages. We defined a key methodological stage as a distinct step in the overall process for which specific guidance is reported, and action is taken, that collectively would result in a completed literature search.
The chapter or section sub-heading for each methodological stage was extracted into a table using the exact language as reported in each guidance document. The lead author (CC) then read and re-read these data, and the paragraphs of the document to which the headings referred, summarising section details. This table was then reviewed, using comparison and contrast to identify agreements and areas of unique guidance. Consensus across multiple guidelines was used to inform selection of ‘key stages’ in the process of literature searching.
Having determined the key stages to literature searching, we then read and re-read the sections relating to literature searching again, extracting specific detail relating to the methodological process of literature searching within each key stage. Again, the guidance was then read and re-read, first on a document-by-document-basis and, secondly, across all the documents above, to identify both commonalities and areas of unique guidance.
Results and discussion
Our findings.
We were able to identify consensus across the guidance on literature searching for systematic reviews suggesting a shared implicit model within the information retrieval community. Whilst the structure of the guidance varies between documents, the same key stages are reported, even where the core focus of each document is different. We were able to identify specific areas of unique guidance, where a document reported guidance not summarised in other documents, together with areas of consensus across guidance.
Unique guidance
Only one document provided guidance on the topic of when to stop searching [ 2 ]. This guidance from 2005 anticipates a topic of increasing importance with the current interest in time-limited (i.e. “rapid”) reviews. Quality assurance (or peer review) of literature searches was only covered in two guidance documents [ 3 , 4 ]. This topic has emerged as increasingly important as indicated by the development of the PRESS instrument [ 5 ]. Text mining was discussed in four guidance documents [ 4 , 6 – 8 ] where the automation of some manual review work may offer efficiencies in literature searching [ 8 ].
Agreement between guidance: Defining the key stages of literature searching
Where there was agreement on the process, we determined that this constituted a key stage in the process of literature searching to inform systematic reviews.
From the guidance, we determined eight key stages that relate specifically to literature searching in systematic reviews. These are summarised at Fig. Fig.1. 1 . The data extraction table to inform Fig. Fig.1 1 is reported in Table 2 . Table Table2 2 reports the areas of common agreement and it demonstrates that the language used to describe key stages and processes varies significantly between guidance documents.
The order of literature search methods as presented in the guidance documents
| Step | The CRD Handbook | The Cochrane Handbook | Collaboration for environmental evidence | Joanna Briggs Institute reviewers manual | IQWiG Methods Resources | Systematic reviews in the social sciences: a practical guide | Eunethta | Campbell Handbook | Developing NICE guidelines: the manual |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Searching electronic databases | Searching bibliographic databases | Searching online literature databases and catalogues | Databases (development of search strategies, phase one) | Bibliographic databases (1.search for primary literature. 2. search for SRs) | Databases | Bibliographic databases | Bibliographic databases (1. subject databases. 2. general databases) | No list of search methods but guidance distinguishes between database searching (first) and supplementary searching (second) |
| 2 | Scanning references lists of relevant studies | Handsearching | Searching websites of organisations and professional networks | Database searching (phase two) | Search in trial registries | Grey literature | Study registries | Conference proceedings and meeting abstracts | |
| 3 | Handsearching of key journals | Conference abstracts or proceedings | Searching the world-wide web | Review reference lists | Clinical practice guideline databases and providers | identifying on-going research | Searching for unpublished company documents | Existing review and publication reference lists | |
| 4 | Searching trials registers | Other reviews | Searching bibliographies of key articles/ reviews | Handsearching | Requests to manufacturers | Theses | Regulatory documents | Web searching | |
| 5 | Contacting experts and manufactures | Web-searching | Contacting key individuals who work in the area | Other data sources | Conference proceedings | Queries to authors | Unpublished studies | ||
| 6 | Searching relevant internet resources | Unpublished and on-going studies (inc. author contact) | Citation searches for key papers/ included papers | Citation searching | Further search techniques | On-going studies | |||
| 7 | Citation searching | Searching the web | Institutional repositories | ||||||
| 8 | Using a project website to canvas for studies | contact with experts | handsearching | ||||||
| 9 | Trials registers |
For each key stage, we set out the specific guidance, followed by discussion on how this guidance is situated within the wider literature.
Key stage one: Deciding who should undertake the literature search
The guidance.
Eight documents provided guidance on who should undertake literature searching in systematic reviews [ 2 , 4 , 6 – 11 ]. The guidance affirms that people with relevant expertise of literature searching should ‘ideally’ be included within the review team [ 6 ]. Information specialists (or information scientists), librarians or trial search co-ordinators (TSCs) are indicated as appropriate researchers in six guidance documents [ 2 , 7 – 11 ].
How the guidance corresponds to the published studies
The guidance is consistent with studies that call for the involvement of information specialists and librarians in systematic reviews [ 12 – 26 ] and which demonstrate how their training as ‘expert searchers’ and ‘analysers and organisers of data’ can be put to good use [ 13 ] in a variety of roles [ 12 , 16 , 20 , 21 , 24 – 26 ]. These arguments make sense in the context of the aims and purposes of literature searching in systematic reviews, explored below. The need for ‘thorough’ and ‘replicable’ literature searches was fundamental to the guidance and recurs in key stage two. Studies have found poor reporting, and a lack of replicable literature searches, to be a weakness in systematic reviews [ 17 , 18 , 27 , 28 ] and they argue that involvement of information specialists/ librarians would be associated with better reporting and better quality literature searching. Indeed, Meert et al. [ 29 ] demonstrated that involving a librarian as a co-author to a systematic review correlated with a higher score in the literature searching component of a systematic review [ 29 ]. As ‘new styles’ of rapid and scoping reviews emerge, where decisions on how to search are more iterative and creative, a clear role is made here too [ 30 ].
Knowing where to search for studies was noted as important in the guidance, with no agreement as to the appropriate number of databases to be searched [ 2 , 6 ]. Database (and resource selection more broadly) is acknowledged as a relevant key skill of information specialists and librarians [ 12 , 15 , 16 , 31 ].
Whilst arguments for including information specialists and librarians in the process of systematic review might be considered self-evident, Koffel and Rethlefsen [ 31 ] have questioned if the necessary involvement is actually happening [ 31 ].
Key stage two: Determining the aim and purpose of a literature search
The aim: Five of the nine guidance documents use adjectives such as ‘thorough’, ‘comprehensive’, ‘transparent’ and ‘reproducible’ to define the aim of literature searching [ 6 – 10 ]. Analogous phrases were present in a further three guidance documents, namely: ‘to identify the best available evidence’ [ 4 ] or ‘the aim of the literature search is not to retrieve everything. It is to retrieve everything of relevance’ [ 2 ] or ‘A systematic literature search aims to identify all publications relevant to the particular research question’ [ 3 ]. The Joanna Briggs Institute reviewers’ manual was the only guidance document where a clear statement on the aim of literature searching could not be identified. The purpose of literature searching was defined in three guidance documents, namely to minimise bias in the resultant review [ 6 , 8 , 10 ]. Accordingly, eight of nine documents clearly asserted that thorough and comprehensive literature searches are required as a potential mechanism for minimising bias.
The need for thorough and comprehensive literature searches appears as uniform within the eight guidance documents that describe approaches to literature searching in systematic reviews of effectiveness. Reviews of effectiveness (of intervention or cost), accuracy and prognosis, require thorough and comprehensive literature searches to transparently produce a reliable estimate of intervention effect. The belief that all relevant studies have been ‘comprehensively’ identified, and that this process has been ‘transparently’ reported, increases confidence in the estimate of effect and the conclusions that can be drawn [ 32 ]. The supporting literature exploring the need for comprehensive literature searches focuses almost exclusively on reviews of intervention effectiveness and meta-analysis. Different ‘styles’ of review may have different standards however; the alternative, offered by purposive sampling, has been suggested in the specific context of qualitative evidence syntheses [ 33 ].
What is a comprehensive literature search?
Whilst the guidance calls for thorough and comprehensive literature searches, it lacks clarity on what constitutes a thorough and comprehensive literature search, beyond the implication that all of the literature search methods in Table Table2 2 should be used to identify studies. Egger et al. [ 34 ], in an empirical study evaluating the importance of comprehensive literature searches for trials in systematic reviews, defined a comprehensive search for trials as:
- a search not restricted to English language;
- where Cochrane CENTRAL or at least two other electronic databases had been searched (such as MEDLINE or EMBASE); and
- at least one of the following search methods has been used to identify unpublished trials: searches for (I) conference abstracts, (ii) theses, (iii) trials registers; and (iv) contacts with experts in the field [ 34 ].
Tricco et al. (2008) used a similar threshold of bibliographic database searching AND a supplementary search method in a review when examining the risk of bias in systematic reviews. Their criteria were: one database (limited using the Cochrane Highly Sensitive Search Strategy (HSSS)) and handsearching [ 35 ].
Together with the guidance, this would suggest that comprehensive literature searching requires the use of BOTH bibliographic database searching AND supplementary search methods.
Comprehensiveness in literature searching, in the sense of how much searching should be undertaken, remains unclear. Egger et al. recommend that ‘investigators should consider the type of literature search and degree of comprehension that is appropriate for the review in question, taking into account budget and time constraints’ [ 34 ]. This view tallies with the Cochrane Handbook, which stipulates clearly, that study identification should be undertaken ‘within resource limits’ [ 9 ]. This would suggest that the limitations to comprehension are recognised but it raises questions on how this is decided and reported [ 36 ].
What is the point of comprehensive literature searching?
The purpose of thorough and comprehensive literature searches is to avoid missing key studies and to minimize bias [ 6 , 8 , 10 , 34 , 37 – 39 ] since a systematic review based only on published (or easily accessible) studies may have an exaggerated effect size [ 35 ]. Felson (1992) sets out potential biases that could affect the estimate of effect in a meta-analysis [ 40 ] and Tricco et al. summarize the evidence concerning bias and confounding in systematic reviews [ 35 ]. Egger et al. point to non-publication of studies, publication bias, language bias and MEDLINE bias, as key biases [ 34 , 35 , 40 – 46 ]. Comprehensive searches are not the sole factor to mitigate these biases but their contribution is thought to be significant [ 2 , 32 , 34 ]. Fehrmann (2011) suggests that ‘the search process being described in detail’ and that, where standard comprehensive search techniques have been applied, increases confidence in the search results [ 32 ].
Does comprehensive literature searching work?
Egger et al., and other study authors, have demonstrated a change in the estimate of intervention effectiveness where relevant studies were excluded from meta-analysis [ 34 , 47 ]. This would suggest that missing studies in literature searching alters the reliability of effectiveness estimates. This is an argument for comprehensive literature searching. Conversely, Egger et al. found that ‘comprehensive’ searches still missed studies and that comprehensive searches could, in fact, introduce bias into a review rather than preventing it, through the identification of low quality studies then being included in the meta-analysis [ 34 ]. Studies query if identifying and including low quality or grey literature studies changes the estimate of effect [ 43 , 48 ] and question if time is better invested updating systematic reviews rather than searching for unpublished studies [ 49 ], or mapping studies for review as opposed to aiming for high sensitivity in literature searching [ 50 ].
Aim and purpose beyond reviews of effectiveness
The need for comprehensive literature searches is less certain in reviews of qualitative studies, and for reviews where a comprehensive identification of studies is difficult to achieve (for example, in Public health) [ 33 , 51 – 55 ]. Literature searching for qualitative studies, and in public health topics, typically generates a greater number of studies to sift than in reviews of effectiveness [ 39 ] and demonstrating the ‘value’ of studies identified or missed is harder [ 56 ], since the study data do not typically support meta-analysis. Nussbaumer-Streit et al. (2016) have registered a review protocol to assess whether abbreviated literature searches (as opposed to comprehensive literature searches) has an impact on conclusions across multiple bodies of evidence, not only on effect estimates [ 57 ] which may develop this understanding. It may be that decision makers and users of systematic reviews are willing to trade the certainty from a comprehensive literature search and systematic review in exchange for different approaches to evidence synthesis [ 58 ], and that comprehensive literature searches are not necessarily a marker of literature search quality, as previously thought [ 36 ]. Different approaches to literature searching [ 37 , 38 , 59 – 62 ] and developing the concept of when to stop searching are important areas for further study [ 36 , 59 ].
The study by Nussbaumer-Streit et al. has been published since the submission of this literature review [ 63 ]. Nussbaumer-Streit et al. (2018) conclude that abbreviated literature searches are viable options for rapid evidence syntheses, if decision-makers are willing to trade the certainty from a comprehensive literature search and systematic review, but that decision-making which demands detailed scrutiny should still be based on comprehensive literature searches [ 63 ].
Key stage three: Preparing for the literature search
Six documents provided guidance on preparing for a literature search [ 2 , 3 , 6 , 7 , 9 , 10 ]. The Cochrane Handbook clearly stated that Cochrane authors (i.e. researchers) should seek advice from a trial search co-ordinator (i.e. a person with specific skills in literature searching) ‘before’ starting a literature search [ 9 ].
Two key tasks were perceptible in preparing for a literature searching [ 2 , 6 , 7 , 10 , 11 ]. First, to determine if there are any existing or on-going reviews, or if a new review is justified [ 6 , 11 ]; and, secondly, to develop an initial literature search strategy to estimate the volume of relevant literature (and quality of a small sample of relevant studies [ 10 ]) and indicate the resources required for literature searching and the review of the studies that follows [ 7 , 10 ].
Three documents summarised guidance on where to search to determine if a new review was justified [ 2 , 6 , 11 ]. These focused on searching databases of systematic reviews (The Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE)), institutional registries (including PROSPERO), and MEDLINE [ 6 , 11 ]. It is worth noting, however, that as of 2015, DARE (and NHS EEDs) are no longer being updated and so the relevance of this (these) resource(s) will diminish over-time [ 64 ]. One guidance document, ‘Systematic reviews in the Social Sciences’, noted, however, that databases are not the only source of information and unpublished reports, conference proceeding and grey literature may also be required, depending on the nature of the review question [ 2 ].
Two documents reported clearly that this preparation (or ‘scoping’) exercise should be undertaken before the actual search strategy is developed [ 7 , 10 ]).
The guidance offers the best available source on preparing the literature search with the published studies not typically reporting how their scoping informed the development of their search strategies nor how their search approaches were developed. Text mining has been proposed as a technique to develop search strategies in the scoping stages of a review although this work is still exploratory [ 65 ]. ‘Clustering documents’ and word frequency analysis have also been tested to identify search terms and studies for review [ 66 , 67 ]. Preparing for literature searches and scoping constitutes an area for future research.
Key stage four: Designing the search strategy
The Population, Intervention, Comparator, Outcome (PICO) structure was the commonly reported structure promoted to design a literature search strategy. Five documents suggested that the eligibility criteria or review question will determine which concepts of PICO will be populated to develop the search strategy [ 1 , 4 , 7 – 9 ]. The NICE handbook promoted multiple structures, namely PICO, SPICE (Setting, Perspective, Intervention, Comparison, Evaluation) and multi-stranded approaches [ 4 ].
With the exclusion of The Joanna Briggs Institute reviewers’ manual, the guidance offered detail on selecting key search terms, synonyms, Boolean language, selecting database indexing terms and combining search terms. The CEE handbook suggested that ‘search terms may be compiled with the help of the commissioning organisation and stakeholders’ [ 10 ].
The use of limits, such as language or date limits, were discussed in all documents [ 2 – 4 , 6 – 11 ].
Search strategy structure
The guidance typically relates to reviews of intervention effectiveness so PICO – with its focus on intervention and comparator - is the dominant model used to structure literature search strategies [ 68 ]. PICOs – where the S denotes study design - is also commonly used in effectiveness reviews [ 6 , 68 ]. As the NICE handbook notes, alternative models to structure literature search strategies have been developed and tested. Booth provides an overview on formulating questions for evidence based practice [ 69 ] and has developed a number of alternatives to the PICO structure, namely: BeHEMoTh (Behaviour of interest; Health context; Exclusions; Models or Theories) for use when systematically identifying theory [ 55 ]; SPICE (Setting, Perspective, Intervention, Comparison, Evaluation) for identification of social science and evaluation studies [ 69 ] and, working with Cooke and colleagues, SPIDER (Sample, Phenomenon of Interest, Design, Evaluation, Research type) [ 70 ]. SPIDER has been compared to PICO and PICOs in a study by Methley et al. [ 68 ].
The NICE handbook also suggests the use of multi-stranded approaches to developing literature search strategies [ 4 ]. Glanville developed this idea in a study by Whitting et al. [ 71 ] and a worked example of this approach is included in the development of a search filter by Cooper et al. [ 72 ].
Writing search strategies: Conceptual and objective approaches
Hausner et al. [ 73 ] provide guidance on writing literature search strategies, delineating between conceptually and objectively derived approaches. The conceptual approach, advocated by and explained in the guidance documents, relies on the expertise of the literature searcher to identify key search terms and then develop key terms to include synonyms and controlled syntax. Hausner and colleagues set out the objective approach [ 73 ] and describe what may be done to validate it [ 74 ].
The use of limits
The guidance documents offer direction on the use of limits within a literature search. Limits can be used to focus literature searching to specific study designs or by other markers (such as by date) which limits the number of studies returned by a literature search. The use of limits should be described and the implications explored [ 34 ] since limiting literature searching can introduce bias (explored above). Craven et al. have suggested the use of a supporting narrative to explain decisions made in the process of developing literature searches and this advice would usefully capture decisions on the use of search limits [ 75 ].
Key stage five: Determining the process of literature searching and deciding where to search (bibliographic database searching)
Table Table2 2 summarises the process of literature searching as reported in each guidance document. Searching bibliographic databases was consistently reported as the ‘first step’ to literature searching in all nine guidance documents.
Three documents reported specific guidance on where to search, in each case specific to the type of review their guidance informed, and as a minimum requirement [ 4 , 9 , 11 ]. Seven of the key guidance documents suggest that the selection of bibliographic databases depends on the topic of review [ 2 – 4 , 6 – 8 , 10 ], with two documents noting the absence of an agreed standard on what constitutes an acceptable number of databases searched [ 2 , 6 ].
The guidance documents summarise ‘how to’ search bibliographic databases in detail and this guidance is further contextualised above in terms of developing the search strategy. The documents provide guidance of selecting bibliographic databases, in some cases stating acceptable minima (i.e. The Cochrane Handbook states Cochrane CENTRAL, MEDLINE and EMBASE), and in other cases simply listing bibliographic database available to search. Studies have explored the value in searching specific bibliographic databases, with Wright et al. (2015) noting the contribution of CINAHL in identifying qualitative studies [ 76 ], Beckles et al. (2013) questioning the contribution of CINAHL to identifying clinical studies for guideline development [ 77 ], and Cooper et al. (2015) exploring the role of UK-focused bibliographic databases to identify UK-relevant studies [ 78 ]. The host of the database (e.g. OVID or ProQuest) has been shown to alter the search returns offered. Younger and Boddy [ 79 ] report differing search returns from the same database (AMED) but where the ‘host’ was different [ 79 ].
The average number of bibliographic database searched in systematic reviews has risen in the period 1994–2014 (from 1 to 4) [ 80 ] but there remains (as attested to by the guidance) no consensus on what constitutes an acceptable number of databases searched [ 48 ]. This is perhaps because thinking about the number of databases searched is the wrong question, researchers should be focused on which databases were searched and why, and which databases were not searched and why. The discussion should re-orientate to the differential value of sources but researchers need to think about how to report this in studies to allow findings to be generalised. Bethel (2017) has proposed ‘search summaries’, completed by the literature searcher, to record where included studies were identified, whether from database (and which databases specifically) or supplementary search methods [ 81 ]. Search summaries document both yield and accuracy of searches, which could prospectively inform resource use and decisions to search or not to search specific databases in topic areas. The prospective use of such data presupposes, however, that past searches are a potential predictor of future search performance (i.e. that each topic is to be considered representative and not unique). In offering a body of practice, this data would be of greater practicable use than current studies which are considered as little more than individual case studies [ 82 – 90 ].
When to database search is another question posed in the literature. Beyer et al. [ 91 ] report that databases can be prioritised for literature searching which, whilst not addressing the question of which databases to search, may at least bring clarity as to which databases to search first [ 91 ]. Paradoxically, this links to studies that suggest PubMed should be searched in addition to MEDLINE (OVID interface) since this improves the currency of systematic reviews [ 92 , 93 ]. Cooper et al. (2017) have tested the idea of database searching not as a primary search method (as suggested in the guidance) but as a supplementary search method in order to manage the volume of studies identified for an environmental effectiveness systematic review. Their case study compared the effectiveness of database searching versus a protocol using supplementary search methods and found that the latter identified more relevant studies for review than searching bibliographic databases [ 94 ].
Key stage six: Determining the process of literature searching and deciding where to search (supplementary search methods)
Table Table2 2 also summaries the process of literature searching which follows bibliographic database searching. As Table Table2 2 sets out, guidance that supplementary literature search methods should be used in systematic reviews recurs across documents, but the order in which these methods are used, and the extent to which they are used, varies. We noted inconsistency in the labelling of supplementary search methods between guidance documents.
Rather than focus on the guidance on how to use the methods (which has been summarised in a recent review [ 95 ]), we focus on the aim or purpose of supplementary search methods.
The Cochrane Handbook reported that ‘efforts’ to identify unpublished studies should be made [ 9 ]. Four guidance documents [ 2 , 3 , 6 , 9 ] acknowledged that searching beyond bibliographic databases was necessary since ‘databases are not the only source of literature’ [ 2 ]. Only one document reported any guidance on determining when to use supplementary methods. The IQWiG handbook reported that the use of handsearching (in their example) could be determined on a ‘case-by-case basis’ which implies that the use of these methods is optional rather than mandatory. This is in contrast to the guidance (above) on bibliographic database searching.
The issue for supplementary search methods is similar in many ways to the issue of searching bibliographic databases: demonstrating value. The purpose and contribution of supplementary search methods in systematic reviews is increasingly acknowledged [ 37 , 61 , 62 , 96 – 101 ] but understanding the value of the search methods to identify studies and data is unclear. In a recently published review, Cooper et al. (2017) reviewed the literature on supplementary search methods looking to determine the advantages, disadvantages and resource implications of using supplementary search methods [ 95 ]. This review also summarises the key guidance and empirical studies and seeks to address the question on when to use these search methods and when not to [ 95 ]. The guidance is limited in this regard and, as Table Table2 2 demonstrates, offers conflicting advice on the order of searching, and the extent to which these search methods should be used in systematic reviews.

Key stage seven: Managing the references
Five of the documents provided guidance on managing references, for example downloading, de-duplicating and managing the output of literature searches [ 2 , 4 , 6 , 8 , 10 ]. This guidance typically itemised available bibliographic management tools rather than offering guidance on how to use them specifically [ 2 , 4 , 6 , 8 ]. The CEE handbook provided guidance on importing data where no direct export option is available (e.g. web-searching) [ 10 ].
The literature on using bibliographic management tools is not large relative to the number of ‘how to’ videos on platforms such as YouTube (see for example [ 102 ]). These YouTube videos confirm the overall lack of ‘how to’ guidance identified in this study and offer useful instruction on managing references. Bramer et al. set out methods for de-duplicating data and reviewing references in Endnote [ 103 , 104 ] and Gall tests the direct search function within Endnote to access databases such as PubMed, finding a number of limitations [ 105 ]. Coar et al. and Ahmed et al. consider the role of the free-source tool, Zotero [ 106 , 107 ]. Managing references is a key administrative function in the process of review particularly for documenting searches in PRISMA guidance.
Key stage eight: Documenting the search
The Cochrane Handbook was the only guidance document to recommend a specific reporting guideline: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 9 ]. Six documents provided guidance on reporting the process of literature searching with specific criteria to report [ 3 , 4 , 6 , 8 – 10 ]. There was consensus on reporting: the databases searched (and the host searched by), the search strategies used, and any use of limits (e.g. date, language, search filters (The CRD handbook called for these limits to be justified [ 6 ])). Three guidance documents reported that the number of studies identified should be recorded [ 3 , 6 , 10 ]. The number of duplicates identified [ 10 ], the screening decisions [ 3 ], a comprehensive list of grey literature sources searched (and full detail for other supplementary search methods) [ 8 ], and an annotation of search terms tested but not used [ 4 ] were identified as unique items in four documents.
The Cochrane Handbook was the only guidance document to note that the full search strategies for each database should be included in the Additional file 1 of the review [ 9 ].
All guidance documents should ultimately deliver completed systematic reviews that fulfil the requirements of the PRISMA reporting guidelines [ 108 ]. The guidance broadly requires the reporting of data that corresponds with the requirements of the PRISMA statement although documents typically ask for diverse and additional items [ 108 ]. In 2008, Sampson et al. observed a lack of consensus on reporting search methods in systematic reviews [ 109 ] and this remains the case as of 2017, as evidenced in the guidance documents, and in spite of the publication of the PRISMA guidelines in 2009 [ 110 ]. It is unclear why the collective guidance does not more explicitly endorse adherence to the PRISMA guidance.
Reporting of literature searching is a key area in systematic reviews since it sets out clearly what was done and how the conclusions of the review can be believed [ 52 , 109 ]. Despite strong endorsement in the guidance documents, specifically supported in PRISMA guidance, and other related reporting standards too (such as ENTREQ for qualitative evidence synthesis, STROBE for reviews of observational studies), authors still highlight the prevalence of poor standards of literature search reporting [ 31 , 110 – 119 ]. To explore issues experienced by authors in reporting literature searches, and look at uptake of PRISMA, Radar et al. [ 120 ] surveyed over 260 review authors to determine common problems and their work summaries the practical aspects of reporting literature searching [ 120 ]. Atkinson et al. [ 121 ] have also analysed reporting standards for literature searching, summarising recommendations and gaps for reporting search strategies [ 121 ].
One area that is less well covered by the guidance, but nevertheless appears in this literature, is the quality appraisal or peer review of literature search strategies. The PRESS checklist is the most prominent and it aims to develop evidence-based guidelines to peer review of electronic search strategies [ 5 , 122 , 123 ]. A corresponding guideline for documentation of supplementary search methods does not yet exist although this idea is currently being explored.
How the reporting of the literature searching process corresponds to critical appraisal tools is an area for further research. In the survey undertaken by Radar et al. (2014), 86% of survey respondents (153/178) identified a need for further guidance on what aspects of the literature search process to report [ 120 ]. The PRISMA statement offers a brief summary of what to report but little practical guidance on how to report it [ 108 ]. Critical appraisal tools for systematic reviews, such as AMSTAR 2 (Shea et al. [ 124 ]) and ROBIS (Whiting et al. [ 125 ]), can usefully be read alongside PRISMA guidance, since they offer greater detail on how the reporting of the literature search will be appraised and, therefore, they offer a proxy on what to report [ 124 , 125 ]. Further research in the form of a study which undertakes a comparison between PRISMA and quality appraisal checklists for systematic reviews would seem to begin addressing the call, identified by Radar et al., for further guidance on what to report [ 120 ].
Limitations
Other handbooks exist.
A potential limitation of this literature review is the focus on guidance produced in Europe (the UK specifically) and Australia. We justify the decision for our selection of the nine guidance documents reviewed in this literature review in section “ Identifying guidance ”. In brief, these nine guidance documents were selected as the most relevant health care guidance that inform UK systematic reviewing practice, given that the UK occupies a prominent position in the science of health information retrieval. We acknowledge the existence of other guidance documents, such as those from North America (e.g. the Agency for Healthcare Research and Quality (AHRQ) [ 126 ], The Institute of Medicine [ 127 ] and the guidance and resources produced by the Canadian Agency for Drugs and Technologies in Health (CADTH) [ 128 ]). We comment further on this directly below.
The handbooks are potentially linked to one another
What is not clear is the extent to which the guidance documents inter-relate or provide guidance uniquely. The Cochrane Handbook, first published in 1994, is notably a key source of reference in guidance and systematic reviews beyond Cochrane reviews. It is not clear to what extent broadening the sample of guidance handbooks to include North American handbooks, and guidance handbooks from other relevant countries too, would alter the findings of this literature review or develop further support for the process model. Since we cannot be clear, we raise this as a potential limitation of this literature review. On our initial review of a sample of North American, and other, guidance documents (before selecting the guidance documents considered in this review), however, we do not consider that the inclusion of these further handbooks would alter significantly the findings of this literature review.
This is a literature review
A further limitation of this review was that the review of published studies is not a systematic review of the evidence for each key stage. It is possible that other relevant studies could help contribute to the exploration and development of the key stages identified in this review.
This literature review would appear to demonstrate the existence of a shared model of the literature searching process in systematic reviews. We call this model ‘the conventional approach’, since it appears to be common convention in nine different guidance documents.
The findings reported above reveal eight key stages in the process of literature searching for systematic reviews. These key stages are consistently reported in the nine guidance documents which suggests consensus on the key stages of literature searching, and therefore the process of literature searching as a whole, in systematic reviews.
In Table Table2, 2 , we demonstrate consensus regarding the application of literature search methods. All guidance documents distinguish between primary and supplementary search methods. Bibliographic database searching is consistently the first method of literature searching referenced in each guidance document. Whilst the guidance uniformly supports the use of supplementary search methods, there is little evidence for a consistent process with diverse guidance across documents. This may reflect differences in the core focus across each document, linked to differences in identifying effectiveness studies or qualitative studies, for instance.
Eight of the nine guidance documents reported on the aims of literature searching. The shared understanding was that literature searching should be thorough and comprehensive in its aim and that this process should be reported transparently so that that it could be reproduced. Whilst only three documents explicitly link this understanding to minimising bias, it is clear that comprehensive literature searching is implicitly linked to ‘not missing relevant studies’ which is approximately the same point.
Defining the key stages in this review helps categorise the scholarship available, and it prioritises areas for development or further study. The supporting studies on preparing for literature searching (key stage three, ‘preparation’) were, for example, comparatively few, and yet this key stage represents a decisive moment in literature searching for systematic reviews. It is where search strategy structure is determined, search terms are chosen or discarded, and the resources to be searched are selected. Information specialists, librarians and researchers, are well placed to develop these and other areas within the key stages we identify.
This review calls for further research to determine the suitability of using the conventional approach. The publication dates of the guidance documents which underpin the conventional approach may raise questions as to whether the process which they each report remains valid for current systematic literature searching. In addition, it may be useful to test whether it is desirable to use the same process model of literature searching for qualitative evidence synthesis as that for reviews of intervention effectiveness, which this literature review demonstrates is presently recommended best practice.
Additional file
Appendix tables and PubMed search strategy. Key studies used for pearl growing per key stage, working data extraction tables and the PubMed search strategy. (DOCX 30 kb)
Acknowledgements
CC acknowledges the supervision offered by Professor Chris Hyde.
This publication forms a part of CC’s PhD. CC’s PhD was funded through the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (Project Number 16/54/11). The open access fee for this publication was paid for by Exeter Medical School.
RG and NB were partially supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula.
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Abbreviations
| BeHEMoTh | Behaviour of interest; Health context; Exclusions; Models or Theories |
| CDSR | Cochrane Database of Systematic Reviews |
| Cochrane CENTRAL | The Cochrane Central Register of Controlled Trials |
| DARE | Database of Abstracts of Reviews of Effects |
| ENTREQ | Enhancing transparency in reporting the synthesis of qualitative research |
| IQWiG | Institute for Quality and Efficiency in Healthcare |
| NICE | National Institute for Clinical Excellence |
| PICO | Population, Intervention, Comparator, Outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SPICE | Setting, Perspective, Intervention, Comparison, Evaluation |
| SPIDER | Sample, Phenomenon of Interest, Design, Evaluation, Research type |
| STROBE | STrengthening the Reporting of OBservational studies in Epidemiology |
| TSC | Trial Search Co-ordinators |
Authors’ contributions
CC conceived the idea for this study and wrote the first draft of the manuscript. CC discussed this publication in PhD supervision with AB and separately with JVC. CC revised the publication with input and comments from AB, JVC, RG and NB. All authors revised the manuscript prior to submission. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chris Cooper, Email: [email protected] .
Andrew Booth, Email: [email protected] .
Jo Varley-Campbell, Email: [email protected] .
Nicky Britten, Email: [email protected] .
Ruth Garside, Email: [email protected] .

- Hirsh Health Sciences
- Lilly Music
- Webster Veterinary
- Hirsh Health Sciences Library
Systematic Reviews
- Search Strategies
- Controlled Vocabularies
- Keyword Searching
- Clinical Trials
- Grey Literature
- Citation Management
- Review Managment
- Library Assistance
On this page
- Generating a Question
- Creating a Searchable Question
- Creating Your Search Terms
- Saving Searches
- Tracking Your Searches and Results
Search Hedges
Generating a question.
Do I have to form a question?
It is highly recommended to frame your research in the form of a question. This helps you stay focused in your searching and only gather the information you need to accurately and thoroughly answer the research question.
Creating and searching a question is an iterative and exploratory process. You may have an idea of what you want to research, but it takes some time to determine a precise, searchable question.
- Do your backgorund research to inform yourself of what's already been published in the literature
- Formulate a basic question based on your background knowledge
- Was my topic too broad? Too narrow?
- What terms and phrases are used in the published literature?
- What aspects of the research am I truly interested in?
After doing a preliminary search of the literature and honing your interests, you can then form a specific, searchable question.
Creating a searchable question
Framing your search in the form of a precise question allows you to clarify the criteria needed for selecting relevant studies. An example question for research might be: Have laws limiting soda sales decreased rates of obesity in children?
In clinical medicine, one technique for creating a searchable question is to put it in the form of a PICO question.
- P: Population or Problem
- I: Intervention
- C: Comparison
We could form a PICO from our previous question about childhood obesity and soda laws:
- P: Children
- I: Laws limiting soda
Not all questions are comparing two treatments or interventions, so it is possible to follow a PICO and not include a comparison.
Once you execute a search, it may be that you do not find anything that answers your question. This can lead to either thinking of new or more terms to represent your PICO elements, or reframing your search question entirely. Research -whether bench, clinical or literature - is an iterative process.
Create a list of search terms
PICO may not specifically apply to your research question, but the principle of breaking your question into searchable chunks still applies.
Different databases require different search strategies. Some have controlled vocabularies , while others are keyword-based ; but all databases are more easily searched if you think of the terms that represent each idea in yourquestion prior to searching.
In a systematic review, you are required to search databases that use both controlled vocabularies and keywords. Setting up a simple chart like the pone below can help you stay organized in your searching. Create a column to represent each idea and two rows beneath. One row is to generate the controlled vocabulary terms that describe the topic, while the other are all of the synonyms and phrases to express the idea in a keyword search. terms within a column will typically be combined with OR, while different columns will be combined with AND.
Multiple charts might be made throughout a Systematic Review; perhaps one for each database searched, or a new iteration if terms were added or removed from a different iteration of the search later in the study.
Tracking your searches and results
It is important to keep track of your searches and results every step of the way in a Systematic Review. If you've read the PRISMA Guidelines, you know that there is a flow chart that should be included with your review which maps the results found, excludes vs. includes, reasons for decisions and dates. A spreadsheet in Excel can help you keep track of your searching workflow. Columns include:
- An optional ID to uniquely identify a search
- Date you ran the search
- A copy of the complete search string
- The database searched
- The publication dates searched
- Any filters you may have used
- The number of results retrived from the search
- The number of results immediately discarded
- The number discarded after chaecked against inclusion/exclusion criteria
- The # of results leftover for inclusion
It is important to keep in mind that the length of time required to do a Systematic Review means you will likely have to rerun searches you did a year ago in order to update any information that may have been recently published.
The purpose of hedges are to have a pre-made search strategy to find either a study type or a common medical topic. They can either be validated or non-validated. It's important to note that if you change a validated hedge, it is no longer considered validated.
- McMaster University Hedges Team The focus of the Hedges Project, which is funded by the National Library of Medicine, is to investigate ways to develop and harness search strategies ("hedges") that will improve retrieval of scientifically sound and clinically relevant study reports from large, general purpose, biomedical research bibliographic databases including MEDLINE, EMBASE, PsycINFO, and CINAHL.
- PubMed Search Strategies Blog "This blog has been created to share PubMed search strategies. Search strategies posted here are not perfect. They are posted in the hope that others will benefit from the work already put into their creation and/or will offer suggestions for improvements."
- InterTASC Search Filters Resource a collaborative venture to identify, assess and test search filters designed to retrieve research by study design or focus. The Search Filters Resource aims to provide easy access to published and unpublished search filters. It also provides information and guidance on how to critically appraise search filters, study design filters in progress and information on the development and use of search filters.
- SIGN Search Filters The search filters used by SIGN are developed in-house or are created by other research organisations and adapted to meet SIGN information needs. SIGN's filters may provide less sensitive searches than used by other systematic reviewers such as The Cochrane Collaboration, but enable the retrieval of medical studies that are most likely to match SIGN's methodological criteria.
- PubMed Clinical Queries Research Methodology Filters Table
- PubMed Systematic Review Filter Search Strategy
Saving searches
Many databases allow you to create personal user accounts and save searches in order to run updates later.
Two popular services are MyNCBI for PubMed and personal accounts in Web of Knowledge.
MyNCBI If you do not have a MyNCBI account, it is free to register. Simply go to PubMed, c lick on the "Sign in to NCBI" link in the upper right hand corner of the page and follow the instructions for registration.
If you are searching PubMed while signed into your MyNCBI account, you have the option to save a search and send email updates to yourself. You can also skip the email updates and just save the search in order to run it at a later time of your choosing.
Watch a brief video tutorial HERE .
NLM/NIH provides a guide to using MyNCBI HERE .
- << Previous: Intro
- Next: Controlled Vocabularies >>
- Literature Search Tracking Log
- Evidence Analysis Log

Desk Hours: M-F 7:45am-5pm
- Last Updated: Feb 9, 2024 3:19 PM
- URL: https://researchguides.library.tufts.edu/SystematicReviews
- Subscriptions
- Advanced search

Advanced Search
A systematic literature review of the clinical and socioeconomic burden of bronchiectasis
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- ORCID record for Marcus A. Mall
- ORCID record for Michal Shteinberg
- ORCID record for Sanjay H. Chotirmall
- Figures & Data
- Info & Metrics
Background The overall burden of bronchiectasis on patients and healthcare systems has not been comprehensively described. Here, we present the findings of a systematic literature review that assessed the clinical and socioeconomic burden of bronchiectasis with subanalyses by aetiology (PROSPERO registration: CRD42023404162).
Methods Embase, MEDLINE and the Cochrane Library were searched for publications relating to bronchiectasis disease burden (December 2017–December 2022). Journal articles and congress abstracts reporting on observational studies, randomised controlled trials and registry studies were included. Editorials, narrative reviews and systematic literature reviews were included to identify primary studies. PRISMA guidelines were followed.
Results 1585 unique publications were identified, of which 587 full texts were screened and 149 were included. A further 189 citations were included from reference lists of editorials and reviews, resulting in 338 total publications. Commonly reported symptoms and complications included dyspnoea, cough, wheezing, sputum production, haemoptysis and exacerbations. Disease severity across several indices and increased mortality compared with the general population was reported. Bronchiectasis impacted quality of life across several patient-reported outcomes, with patients experiencing fatigue, anxiety and depression. Healthcare resource utilisation was considerable and substantial medical costs related to hospitalisations, treatments and emergency department and outpatient visits were accrued. Indirect costs included sick pay and lost income.
Conclusions Bronchiectasis causes significant clinical and socioeconomic burden. Disease-modifying therapies that reduce symptoms, improve quality of life and reduce both healthcare resource utilisation and overall costs are needed. Further systematic analyses of specific aetiologies and paediatric disease may provide more insight into unmet therapeutic needs.
- Shareable abstract
Bronchiectasis imposes a significant clinical and socioeconomic burden on patients, their families and employers, and on healthcare systems. Therapies that reduce symptoms, improve quality of life and reduce resource use and overall costs are needed. https://bit.ly/4bPCHlp
- Introduction
Bronchiectasis is a heterogeneous chronic respiratory disease clinically characterised by chronic cough, excessive sputum production and recurrent pulmonary exacerbations [ 1 ], and radiologically characterised by the abnormal widening of the bronchi [ 2 ]. Bronchiectasis is associated with several genetic, autoimmune, airway and infectious disorders [ 3 ]. Regardless of the underlying cause, the defining features of bronchiectasis are chronic airway inflammation and infection, regionally impaired mucociliary clearance, mucus hypersecretion and mucus obstruction, as well as progressive structural lung damage [ 4 , 5 ]. These features perpetuate one another in a “vicious vortex” leading to a decline in lung function, pulmonary exacerbations and associated morbidity, mortality and worsened quality of life [ 4 , 5 ]. Bronchiectasis can be further categorised into several infective and inflammatory endotypes and is associated with multiple comorbidities and underlying aetiologies [ 6 ].
Bronchiectasis has been described as an emerging global epidemic [ 7 ], with prevalence and incidence rates increasing worldwide [ 8 – 12 ]. The prevalence of bronchiectasis, as well as of the individual aetiologies, varies widely across geographic regions [ 13 ]. In Europe, the reported prevalence ranges from 39.1 (females) and 33.3 (males) cases per 100 000 inhabitants in Spain and 68 (females) and 65 (males) cases per 100 000 inhabitants in Germany, to as high as 566 cases (females) and 486 cases (males) per 100 000 inhabitants in the UK [ 10 – 12 ]. In the US, the average overall prevalence was reported to be 139 cases per 100 000 [ 14 ], in Israel, the prevalence was reported to be 234 cases per 100 000 [ 15 ], and in China the prevalence was reported to be 174 per 100 000 [ 8 ]. Studies show that bronchiectasis prevalence increases with age [ 14 ]. This may increase the socioeconomic impact of bronchiectasis on countries with disproportionately higher number of older citizens. Large registry studies in patients with bronchiectasis have been published from the US (Bronchiectasis Research Registry) [ 16 ], Europe and Israel (European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC)); the largest and most comprehensive report available to date) [ 17 ], India (EMBARC-India) [ 18 , 19 ], Korea (Korean Multicentre Bronchiectasis Audit and Research Collaboration) [ 20 ] and Australia (Australian Bronchiectasis Registry) [ 21 ].
Although there are currently no approved disease-modifying therapies for bronchiectasis [ 4 ], comprehensive clinical care recommendations for the management of patients with bronchiectasis have been published [ 22 , 23 ]. However, the burden that bronchiectasis imposes on patients and their families, as well as on healthcare systems, payers and employers, remains poorly understood. No review to date has used a systematic method to evaluate the overall disease burden of bronchiectasis. This is the first systematic literature review aimed at investigating and synthesising the clinical and socioeconomic burden of bronchiectasis. A better understanding of the overarching burden of bronchiectasis, both overall and by individual aetiologies and associated diseases, will highlight the need for new therapies and assist healthcare systems in planning care and required resources.
The protocol of this systematic review was registered on PROSPERO (reference number: CRD42023404162).
Search strategy
This systematic literature review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [ 24 ]. Embase, MEDLINE and the Cochrane Library were searched for studies related to the clinical and socioeconomic burden of bronchiectasis (noncystic fibrosis bronchiectasis (NCFBE) and cystic fibrosis bronchiectasis (CFBE)) using the search terms available in supplementary table S1 . Articles written in English and published over a 5-year period (December 2017–December 2022) were included.
Selection criteria
The following article types reporting on prospective and retrospective observational studies, registry studies and randomised controlled trials (only baseline data extracted) were included: journal articles, preprints, research letters, conference proceedings, conference papers, conference abstracts, meeting abstracts and meeting posters. Reviews, literature reviews, systematic reviews and meta-analyses, as well as editorials, commentaries, letters and letters to the editor, were included for the purpose of identifying primary studies. A manual search of references cited in selected articles was performed and references were only included if they were published within the 5 years prior to the primary article being published.
Screening and data extraction
A reviewer screened all titles and abstracts to identify publications for full-text review. These publications then underwent full-text screening by the same reviewer for potential inclusion. A second reviewer independently verified the results of both the title/abstract screen and the full-text screen. Any discrepancies were resolved by a third independent reviewer. Data relating to aetiology, symptoms, disease severity, exacerbations, lung function, infection, comorbidities, patient-reported outcomes (PROs), exercise capacity, mortality, impact on family and caregivers, healthcare resource utilisation (HCRU), treatment burden, medical costs, and indirect impacts and costs, as well as data relating to the patient population, study design, sample size and country/countries of origin, were extracted from the final set of publications into a standardised Excel spreadsheet by one reviewer. Studies were grouped based on the burden measure, and aggregate data (range of reported values) were summarised in table or figure format. For the economic burden section, costs extracted from studies reporting in currencies other than the euros were converted to euros based on the average exchange rate for the year in which the study was conducted.
Data from patients with specific bronchiectasis aetiologies and in children (age limits varied from study to study and included upper age limits of 15, 18, 19 and 20 years) were reported separately, where available. As literature relating to NCFBE and CFBE is generally distinct, any data related to CFBE are reported separately in the tables and text. We conducted subanalyses of key disease burden indicators, in which we extracted data from multicentre studies or those with a sample size >1000 subjects, to try to identify estimates from the most representative datasets. These data from larger and multicentre studies are reported in square brackets in tables 1 – 3 and supplementary tables S2–S7 , where available.
- View inline
Prevalence and severity of bronchiectasis symptoms overall, in children, during exacerbations and in individual bronchiectasis aetiologies
Patient-reported outcome scores in patients with bronchiectasis overall and in individual bronchiectasis aetiologies
Healthcare resource utilisation (HCRU) in patients with bronchiectasis overall and in individual bronchiectasis aetiologies
Given the nature of the data included in this systematic literature review (that is, a broad range of patient clinical and socioeconomic characteristics rather than the outcome(s) of an intervention), in addition to the broad range of study types included, meta-analyses to statistically combine data of similar studies were not deemed appropriate and therefore not performed.
Summary of included studies
A total of 1834 citations were retrieved from the Embase, MEDLINE and Cochrane Library databases, of which 1585 unique citations were identified. Abstract/title screening led to the inclusion of 587 citations for full-text screening. Following full-text screening, 149 primary citations and 110 literature reviews, systematic reviews and meta-analyses as well as editorials and letters to the editor remained. From the reference lists of these 110 citations, a further 189 primary citations were identified. These articles were only included if 1) the primary articles contained data relating to the burden of bronchiectasis and 2) the primary articles were published within the 5 years prior to the original article's publication date. In total, 338 publications were considered eligible and included in this review ( supplementary figure S1 ). This included 279 journal articles, 46 congress abstracts and 13 letters to the editor or scientific/research letters. The results are summarised in the sections below. For the results from individual studies, including a description of the patient population, study design, sample size and country/countries of origin, please see the supplemental Excel file .
The most frequently reported aetiologies included post-infectious, genetic (primary ciliary dyskinesia (PCD), alpha-1 antitrypsin deficiency (AATD) and cystic fibrosis (CF)), airway diseases (COPD and asthma), allergic bronchopulmonary aspergillosis (ABPA), aspiration and reflux-related, immunodeficiency and autoimmune aetiologies ( supplementary figure S2 ). However, in up to 80.7% of adult cases and 53.3% of paediatric cases, the aetiology was not determined (referred to as “idiopathic bronchiectasis”) ( supplementary figure S2 ). When limited to larger or multicentre studies, the frequency of idiopathic bronchiectasis ranged from 11.5 to 66.0% in adults and from 16.5 to 29.4% in children. Further details and additional aetiologies can be seen in the supplemental Excel file .
Clinical burden
Symptom burden and severity.
Commonly reported symptoms in patients with bronchiectasis included cough, sputum production, dyspnoea, wheezing and haemoptysis, with these symptoms more prevalent in adults compared with children ( table 1 ). Other reported symptoms included chest discomfort, pain or tightness (both generally and during an exacerbation), fever and weight loss in both adults and children, and fatigue, tiredness or asthenia, appetite loss, and sweating in adults. In children, respiratory distress, hypoxia during an exacerbation, sneezing, nasal and ear discharge, thriving poorly including poor growth and weight loss, exercise intolerance, malaise, night sweats, abdominal pain, recurrent vomiting, and diarrhoea were reported ( supplemental Excel file ). Classic bronchiectasis symptoms such as sputum production (range of patients reporting sputum production across all studies: 22.0–92.7%) and cough (range of patients reporting cough across all studies: 24.0–98.5%) were not universally reported ( table 1 ).
In a study comparing bronchiectasis (excluding CFBE) in different age groups (younger adults (18–65 years), older adults (66–75 years) and elderly adults (≥76 years) [ 63 ]), no significant differences across age groups were reported for the presence of cough (younger adults: 73.9%; older adults: 72.8%; elderly adults: 72.9%; p=0.90), sputum production (younger adults: 57.8%; older adults: 63.8%; elderly adults: 6.0%; p=0.16) or haemoptysis (younger adults: 16.5%; older adults: 19.3%; elderly adults: 16.3%; p=0.47).
Disease severity
Disease severity was reported according to several measures including the bronchiectasis severity index (BSI), the forced expiratory volume in 1 s (FEV 1 ), Age, Chronic Colonisation, Extension, Dyspnoea (FACED) score and the Exacerbations-FACED (E-FACED) score, all of which are known to be associated with future exacerbations, hospitalisations and mortality ( supplementary table S2 and the supplemental Excel file ). Up to 78.7, 41.8 and 40.8% of patients with bronchiectasis reported severe disease according to the BSI, FACED score and E-FACED score, respectively ( supplementary table S2 ). In most studies, severity scores were greater among people with bronchiectasis secondary to COPD or post-tuberculosis (TB) than idiopathic bronchiectasis ( supplementary table S2 ). No data relating to disease severity were reported for CFBE specifically.
Exacerbations
The number of exacerbations experienced by patients with bronchiectasis in the previous year, per year and during follow-up are presented in figure 1 . For further details, please see the supplemental Excel file . Two studies reported exacerbation length in patients with bronchiectasis; this ranged from 11 to 16 days (both small studies; sample sizes of 191 and 32, respectively) [ 25 , 64 ]. A study in children with NCFBE reported a median of one exacerbation in the previous year. Additionally, the same study reported that 31.1% of children with bronchiectasis experienced ≥3 exacerbations per year [ 65 ].
- Download figure
- Open in new tab
- Download powerpoint
Range of bronchiectasis exacerbations in the previous year, per year and in the first and second years of follow-up. # : Two studies reported significant differences in the number of exacerbations experienced in the previous year across individual aetiologies. Study 1 [ 90 ]: Patients with idiopathic bronchiectasis had significantly fewer exacerbations in the previous year compared with other aetiologies (primary ciliary dyskinesia (PCD), COPD and post-infectious) (p<0.021). Study 2 [ 33 ]: significant difference between post-tuberculosis (TB) bronchiectasis (mean: 2.8) and other aetiologies excluding idiopathic bronchiectasis (mean: 1.7) (p<0.05).
Lung function
Reduced lung function was reported across several different measures in adults and children with bronchiectasis overall, including FEV 1 (absolute values and % predicted), forced vital capacity (FVC; absolute values and % pred) and lung clearance index (adults only) ( supplementary table S3 and the supplemental Excel file ). In most studies, lung function was lowest among people with post-TB bronchiectasis and bronchiectasis secondary to COPD or PCD ( supplementary table S2 ). Additional measures of lung function are detailed in the supplemental Excel file . Lung clearance index, considered more sensitive than spirometry to early airway damage, was elevated in two studies in adults with bronchiectasis, with a range of 9.0–12.8 (normal: 6–7 or less) [ 66 , 67 ].
In a study comparing bronchiectasis (people with CFBE excluded) in different age groups, elderly adults (≥76 years) had significantly lower FEV 1 % pred (median: 67) compared with both younger (18–65 years; median: 78) and older adults (66–75 years; median: 75) (p<0.017 for both comparisons) [ 63 ]. FVC % pred was found to be significantly lower in elderly adults (mean: 65) compared with both younger adults (median: 78) and older adults (median: 75) (p<0.017 for both comparisons) [ 63 ].
Chronic infection with at least one pathogen was reported in 22.3–79.6% of patients with bronchiectasis, although each study defined chronic infection differently (number of studies: 20). When limited to larger or multicentre studies, chronic infection with at least one pathogen was reported in 10.7–54.5% of patients with bronchiectasis (number of studies: 12). In two studies in NCFBE, significant differences in the proportion of patients chronically infected with at least one pathogen were reported across aetiologies (p<0.001 for both studies) [ 68 , 69 ]. Patients with post-infectious (other than TB) bronchiectasis (34.9%) [ 68 ] and patients with PCD-related bronchiectasis (68.3%) [ 69 ] had the highest prevalence of chronic infection.
The most commonly reported bacterial and fungal pathogens are shown in supplementary table S4 . The two most common bacterial pathogens were Pseudomonas ( P .) aeruginosa and Haemophilus ( H. ) influenzae . In several studies, more patients with PCD, TB and COPD as the aetiology of their bronchiectasis reported infection with P. aeruginosa . Additionally, in one study, significantly more children with CFBE had P. aeruginosa infection compared with children with NCFBE [ 70 ]. Further details and additional pathogens are reported in the supplemental Excel file .
Diversity of the sputum microbiome was assessed in two studies. In the first study in people with bronchiectasis (people with CFBE excluded), reduced microbiome alpha diversity (defined as the relative abundance of microbial species within a sample), particularly associated with Pseudomonas or Proteobacteria dominance, was associated with greater disease severity, increased frequency and severity of exacerbations, and a higher risk of mortality [ 71 ]. In the second study (unknown whether people with CFBE were excluded), a lower Shannon–Wiener diversity index (a measure of species diversity, with lower scores indicating lower diversity) score was associated with multiple markers of disease severity, including a higher BSI score (p=0.0003) and more frequent exacerbations (p=0.008) [ 72 ].
In a study comparing bronchiectasis (people with CFBE excluded) in different age groups (younger adults: 18–65 years; older adults: 66–75 years; elderly adults: ≥76 years) [ 63 ], chronic infection with H. influenzae was reported in 18.3% of younger adults, 12.8% of older adults and 8.8% of elderly adults, and chronic infection with Streptococcus ( Str. ) pneumoniae was reported in 5.3% of younger adults, 2.8% of older adults and 1.3% of elderly adults. For both of the above, the prevalence was significantly higher in younger adults compared with elderly adults (p<0.017 for both comparisons). However, no significant differences across age groups were reported for P. aeruginosa , Moraxella catarrhalis or Staphylococcus ( Sta .) aureus chronic infection.
P. aeruginosa infection was significantly associated with reduced FEV 1 [ 73 ], more severe disease [ 74 ], more frequent exacerbations [ 35 , 49 , 75 , 76 ], increased hospital admissions, reduced quality of life based on St. George's Respiratory Questionnaire (SGRQ) and increased and 4-year mortality [ 49 , 76 ]. Additionally, in a study reporting healthcare use and costs in the US between 2007–2013, healthcare costs and hospitalisation costs were found to be increased in patients infected with P. aeruginosa ($56 499 and $41 972 more than patients not infected with P. aeruginosa , respectively) [ 77 ]. In the same study, HCRU was also higher in patients infected with P. aeruginosa (fivefold increase in the number of hospitalisations and 84% more emergency department (ED) visits compared with patients not infected with P. aeruginosa ) [ 77 ].
Comorbidities
The most frequently reported comorbidities included cardiovascular (including heart failure, cerebrovascular disease and hypertension), respiratory (including asthma, COPD and sinusitis), metabolic (including diabetes and dyslipidaemia), malignancy (including haematological and solid malignancies), bone and joint-related (including osteoporosis and rheumatological disease), neurological (including anxiety and depression), renal, hepatic, and gastrointestinal comorbidities ( supplementary table S5 ). No data relating to comorbidities were reported for CFBE specifically. For further details and additional comorbidities, please see the supplemental Excel file .
In a study comparing bronchiectasis (people with CFBE excluded) in different age groups (younger adults: 18–65 years; older adults: 66–75 years; elderly adults: ≥76 years), younger adults had a significantly lower prevalence of diabetes compared with older adults, a significantly lower prevalence of stroke compared with elderly adults and a significantly lower prevalence of heart failure, solid tumours and renal failure compared with both older and elderly adults (p<0.0017 for all comparisons). Additionally, the prevalence of COPD was significantly lower in both younger and older adults compared with elderly adults (p<0.017) [ 63 ]. In studies reporting in children with bronchiectasis, the prevalence of comorbid asthma ranged from 22.2 to 25.8% [ 65 , 78 ] and the prevalence of sinusitis was reported to be 12.7% in a single study [ 79 ].
Charlson comorbidity index (CCI)
CCI scores can range from 0 to 37, with higher scores indicating a decreased estimate of 10-year survival. In this review, CCI scores ranged from 0.7 to 6.6 in studies reporting means (number of studies: 7). In one study, adults with bronchiectasis (people with CFBE excluded) who experienced ≥2 exacerbations per year were found to have significantly higher CCI scores (3.3) compared with patients who experienced less than two exacerbations per year (2.2) (p=0.001) [ 35 ]. In another study in adults with bronchiectasis (people with CFBE excluded), CCI scores increased significantly with increasing disease severity, with patients with mild (FACED score of 0–2), moderate (FACED score of 3–4) and severe (FACED score of 5–7) bronchiectasis reporting mean CCI scores of 3.9, 5.7 and 6.3, respectively [ 80 ]. No CCI scores were reported for CFBE specifically.
Prevalence of comorbidities in patients with bronchiectasis compared with control individuals
Several studies reported a higher prevalence of cardiovascular comorbidities. such as heart failure [ 81 ], stroke [ 82 , 83 ] and hypertension [ 82 – 84 ] in patients with bronchiectasis compared with a matched general population or healthy controls. Conversely, several additional studies reported no significant differences [ 81 , 85 , 86 ]. Two large studies reported an increased prevalence of diabetes in patients with bronchiectasis compared with nonbronchiectasis control groups [ 83 , 84 ]; however, three additional smaller studies reported no significant differences [ 81 , 82 , 86 ]. The prevalence of gastro–oesophageal reflux disease was found to be significantly higher in patients with bronchiectasis compared with matched nonbronchiectasis controls in one study [ 87 ], but no significant difference was reported in a second study [ 85 ]. Both anxiety and depression were found to be significantly more prevalent in patients with bronchiectasis compared with matched healthy controls in one study [ 55 ]. Lastly, two large studies reported an increased prevalence of asthma [ 84 , 87 ] and five studies reported a significantly higher prevalence of COPD [ 81 , 82 , 84 , 85 , 87 ] in patients with bronchiectasis compared with matched nonbronchiectasis controls or the general population. A smaller study reported conflicting evidence whereby no significant difference in the prevalence of asthma in patients with bronchiectasis compared with matched controls was reported [ 85 ].
Socioeconomic burden
Patient-reported outcomes.
Health-related quality of life (HRQoL), fatigue, anxiety and depression were reported across several PRO measures and domains. The most frequently reported PROs are discussed in further detail in the sections below ( table 2 ). Further details and additional PROs can be seen in the supplemental Excel file .
In a study comparing bronchiectasis (people with CFBE excluded) in different age groups (younger adults: 18–65 years; older adults: 66–75 years; elderly adults: ≥76 years), the median SGRQ total score was significantly higher in elderly adults (50.8) compared with younger adults (36.1), indicating a higher degree of limitation (p=0.017) [ 63 ].
In a study that reported Leicester Cough Questionnaire (LCQ) scores in men and women with bronchiectasis (people with CFBE excluded) separately, women had significantly lower LCQ total scores (14.9) when compared with men (17.5) (p=0.006), indicating worse quality of life [ 88 ]. Additionally, women had significantly lower scores across all three LCQ domains (p=0.014, p=0.005 and p=0.011 for physical, psychological and social domains, respectively) [ 88 ].
Exercise capacity
Exercise capacity in patients with bronchiectasis was reported using walking tests namely the 6-minute walk test (6MWT) and the incremental shuttle walk test (ISWT) ( supplementary table S6 ). The 6MWT data from patients with bronchiectasis generally fell within the normal range for healthy people; however, the ISWT data was below the normal range for healthy people ( supplementary table S6 ). Studies also reported on daily physical activity, daily sedentary time and number of steps per day in patients with bronchiectasis, and in children specifically ( supplementary table S6 ). No data relating to disease severity were reported for CFBE specifically. Further details can be seen in the supplemental Excel file .
Exercise capacity in patients with bronchiectasis compared with control individuals
In one study, the ISWT distance was reported to be significantly lower in patients with NCFBE compared with healthy controls (592.6 m versus 882.9 m; difference of ∼290 m; p<0.001) [ 89 ]. Additionally, patients with bronchiectasis spent significantly less time on activities of moderate and vigorous intensity compared with healthy controls (p=0.030 and 0.044, respectively) [ 89 ]. Lastly, a study reported that patients with NCFBE had a significantly lower step count per day compared with healthy controls (p<0.001) [ 89 ].
Mortality rate during study period
Mortality ranged from 0.24 to 67.6%; however, it should be noted that the study duration differed across studies. When limited to larger or multicentre studies, the mortality rate ranged from 0.24 to 28.1%. One study reported more deaths in patients with NCFBE (9.1%; 5.9-year mean follow-up period) compared with patients without bronchiectasis (0.8%; 5.4-year mean follow-up period) [ 84 ]. In one study, significantly more patients with COPD-related bronchiectasis died (37.5%) compared with other aetiologies (19.0%) (3.4-year mean follow-up period; p<0.001). After adjusting for several factors, multivariate analysis showed that the diagnosis of COPD as the primary cause of bronchiectasis increased the risk of death by 1.77 compared with the patients with other aetiologies [ 41 ]. Similarly, in another study, COPD-associated bronchiectasis was associated with higher mortality (55%) in multivariate analysis as compared with other aetiologies (rheumatic disease: 20%; post-infectious: 16%; idiopathic: 14%; ABPA: 13%; immunodeficiency: 11%) (hazard ratio 2.12, 95% CI 1.04–4.30; p=0.038; 5.2-year median follow-up period) [ 90 ].
Mortality rates by year
The 1-, 2-, 3-, 4- and 5-year mortality rates in patients with bronchiectasis (people with CFBE excluded, unless unspecified) ranged from 0.0 to 12.3%, 0.0 to 13.0%, 0.0 to 21.0%, 5.5 to 39.1% and 12.4 to 53.0%, respectively (number of studies: 9, 4, 7, 1 and 4, respectively). When limited to larger or multicentre studies, the 1-, 2-, 3- and 5-year mortality rates ranges were 0.4–7.9%, 3.9–13.0%, 3.7–21.0% and 12.4–53.0% (no 4-year mortality data from larger or multicentre studies). No data relating to mortality rates were reported for CFBE specifically.
Two studies reported mortality rate by bronchiectasis aetiology (people with CFBE excluded). In the first study, no significant difference in the 4-year mortality rate was reported across aetiologies (p=0.7; inflammatory bowel disease: 14.3%; post-TB: 13.4%; rheumatoid arthritis: 11.4%; idiopathic or post-infectious: 10.1%; ABPA: 6.1%; other aetiologies: 6.1%) [ 49 ]. In the second study, patients with post-TB bronchiectasis had a significantly higher 5-year mortality rate (30.0%) compared with patients with idiopathic bronchiectasis (18.0%) and other aetiologies (10.0%) (p<0.05 for both comparisons) [ 32 ].
In-hospital and intensive care unit mortality
In-hospital mortality ranged from 2.9 to 59.3% in patients with bronchiectasis (people with CFBE excluded, unless unspecified) hospitalised for an exacerbation or for other reasons (number of studies: 7). When limited to larger or multicentre studies, in-hospital mortality rate was reported in only one study (33.0%). One study reported mortality in bronchiectasis patients admitted to a tertiary care centre according to aetiology; in-hospital mortality was highest in patients with post-pneumonia bronchiectasis (15.8%), followed by patients with idiopathic (7.1%) and post-TB (2.6%) bronchiectasis. No deaths were reported in patients with COPD, ABPA or PCD aetiologies [ 42 ]. Intensive care unit mortality was reported in two studies and ranged from 24.6 to 36.1% [ 62 , 91 ]. No data relating to mortality rates were reported for CFBE specifically.
Impact on family and caregivers
Only two studies discussed the impact that having a child with bronchiectasis has on parents/caregivers. In the first study, parents of children with bronchiectasis (not specified whether children with CFBE were excluded) were more anxious and more depressed according to both the Hospital Anxiety and Depression Scale (HADS) and the Centre of Epidemiological Studies depression scale, compared with parents of children without any respiratory conditions (both p<0.001; sample size of 29 participants) [ 53 ]. In the second study, parents or carers of children with bronchiectasis (multicentre study with a sample size of 141 participants; children with CFBE excluded) were asked to vote for their top five greatest concerns or worries; the most common worries or concerns that were voted for by over 15% of parents were “impact on his/her adult life in the future, long-term effects, normal life” (29.8%), “ongoing declining health” (25.5%), “the cough” (24.8%), “impact on his/her life now as a child (play, development)” (24.1%), “lack of sleep/being tired” (24.1%), “concerns over aspects of antibiotic use” (22.7%), “missing school or daycare” (17.7%) and “breathing difficulties/shortness of breath” (16.3%) [ 92 ].
HCRU in terms of hospitalisations, ED visits, outpatient visits and length of stay overall and by bronchiectasis aetiology are reported in table 3 . No data relating to HCRU were reported for CFBE specifically.
In a study in children with bronchiectasis (children with CFBE excluded), 30.0% of children were hospitalised at least once in the previous year [ 65 ]. The median number of hospitalisations per year was 0 (interquartile range: 0–1) [ 65 ]. In another study, the mean length of hospital stay for children with bronchiectasis was 6.7 days (standard deviation: 4.8 days) [ 93 ]. In a study comparing bronchiectasis (people with CFBE excluded) in different age groups, significantly more elderly adults (≥76 years; 26.0%) were hospitalised at least once during the first year of follow-up compared with younger adults (18–65 years; 17.0%) and older adults (66–75 years; 17.0%) (p<0.017 for both comparisons) [ 63 ]. Additionally, length of stay was found to be significantly longer in male patients (mean: 17.6 days) compared with female patients (mean: 12.5 days) (p=0.03) [ 94 ].
HCRU in patients with bronchiectasis compared with control individuals
Length of stay was found to be 38% higher in patients with bronchiectasis (mean: 15.4 days; people with CFBE excluded) compared with patients with any other respiratory illness (mean: 9.6 days) (p<0.001) [ 94 ]. In a study reporting on HCRU in patients with bronchiectasis (people with CFBE excluded) over a 3-year period (Germany; 2012–2015) [ 85 ], a mean of 24.7 outpatient appointments per patient were reported; there was no significant difference in the number of outpatient appointments between patients with bronchiectasis and matched controls (patients without bronchiectasis matched by age, sex and distribution, and level of comorbidities) (mean: 23.4) (p=0.12). When assessing specific outpatient appointments over the 3-year period, patients with bronchiectasis attended a mean of 9.2 general practitioner appointments, 2.9 radiology appointments, 2.5 chest physician appointments and 0.8 cardiologist appointments. Patients with bronchiectasis had significantly fewer general practitioner appointments compared with matched controls (mean: 9.8) (p=0.002); however, they had significantly more radiology appointments (mean for matched controls: 2.3) and chest physician appointments (mean for matched controls: 1.4) compared with matched controls (p<0.001 for both comparisons).
Hospital admission rates
In England, Wales and Northern Ireland, the crude hospital admission rate in 2013 was 88.4 (95% CI 74.0–105.6) per 100 000 person-years [ 91 ]. In New Zealand (2008–2013), the crude and adjusted hospital admission rates were 25.7 and 20.4 per 100 000 population, respectively [ 95 ]. Lastly, in Australia and New Zealand (2004–2008) the hospital admission rate ranged from 0.7 to 2.9 per person-year [ 96 ]. In all of the abovementioned studies, people with CFBE were excluded.
Treatment burden
In two studies, the percentage of patients with bronchiectasis receiving any respiratory medication at baseline ranged from 60.8 to 85.7% [ 97 , 98 ]. Additionally, in a study comparing healthcare costs in patients with bronchiectasis before and after confirmation of P. aeruginosa infection, mean pharmacy visits in the year preceding diagnosis were reported to be 23.2; this increased significantly by 56.5% to 36.2 in the year post-diagnosis (p<0.0001) [ 99 ]. In another study, patients with bronchiectasis were prescribed a mean of 12 medications for bronchiectasis and other comorbidities [ 100 ]. In all of the abovementioned studies, people with CFBE were excluded. The most frequently reported respiratory treatments can be seen in supplementary table S7 . These included antibiotics (including macrolides), corticosteroids, bronchodilators, mucolytics and oxygen. No treatment data were reported for CFBE specifically. Other respiratory treatments included saline, anticholinergics and leukotriene receptor antagonists ( supplemental Excel file ).
In studies reporting in children with bronchiectasis, 23.9% of children were receiving any bronchodilator at baseline [ 101 ], 9.0–21.7% of children were receiving inhaled corticosteroids (ICS) at baseline [ 101 , 102 ], 4.3% of children were receiving oral corticosteroids at baseline [ 101 ] and 12.1% of children were receiving long-term oxygen therapy [ 103 ].
Medical and nonmedical indirect impacts and costs
Medical costs for bronchiectasis included overall costs, hospitalisation costs, ED visits and outpatient visit costs and costs of treatment; indirect impacts and costs included sick leave and sick pay, missed work and income loss for caregivers, and missed school or childcare for children ( table 4 and the supplemental Excel file ). People with CFBE were excluded from all of the studies in table 4 below. In studies reporting in currencies other than the €, costs were converted to € based on the average exchange rate for the year in which the study was conducted.
Bronchiectasis-related medical costs and indirect impacts and costs (individual studies)
No review to date has systematically evaluated the overall disease burden of bronchiectasis. Here, we present the first systematic literature review that comprehensively describes the clinical and socioeconomic burden of bronchiectasis overall and across individual aetiologies and associated diseases. A total of 338 publications were included in the final analysis. Together, the results indicate that the burden of clinically significant bronchiectasis on patients and their families, as well as on healthcare systems, is substantial, highlighting the urgent need for new disease-modifying therapies for bronchiectasis.
Bronchiectasis is associated with genetic, autoimmune, airway and infectious disorders. However, in many patients with bronchiectasis, an underlying aetiology cannot be identified (idiopathic bronchiectasis) [ 1 , 3 , 4 ]. This is supported by the results of this systematic literature review, in which up to 80.7% of patients were reported to have idiopathic bronchiectasis. The results are in line with those reported in a systematic literature review of bronchiectasis aetiology conducted by G ao et al. [ 13 ] (studies from Asia, Europe, North and South America, Africa and Oceania included) in which an idiopathic aetiology was reported in approximately 45% of patients with bronchiectasis, with a range of 5–82%. The maximum of 80.7% of patients with idiopathic bronchiectasis identified by this systematic literature review is much higher than in the recent report on the disease characteristics of the EMBARC where idiopathic bronchiectasis was the most common aetiology and reported in only ∼38% of patients with bronchiectasis [ 17 ]. This highlights the importance of sample size and geographic variation (80.7% reported from a single-country study with a small sample size versus ∼38% reported from a continent-wide study with a large sample size). Nevertheless, identifying the underlying aetiology is a recommendation of bronchiectasis guidelines as this can considerably alter the clinical management and prognosis [ 23 , 110 ]. Specific therapeutic interventions may be required for specific aetiologies, such as ICS for people with asthma-related bronchiectasis, antifungal treatment for those with ABPA-associated bronchiectasis and immunoglobulin replacement therapy for those with common variable immunodeficiency-related bronchiectasis [ 23 , 111 ]. Indeed, an observational study has shown that identification of the underlying aetiology affected management in 37% of people with bronchiectasis [ 112 ]. Future studies to determine the impact of identifying the underlying aetiology on management and prognosis are needed to fully understand its importance.
Patients with bronchiectasis experienced a significant symptom burden, with dyspnoea, cough, wheezing, sputum production and haemoptysis reported most commonly. These symptoms were also reported in children with bronchiectasis at slightly lower frequencies. Dealing with bronchiectasis symptoms are some of the greatest concerns from a patient's perspective. In a study assessing the aspects of bronchiectasis that patients found most difficult to deal with, sputum, dyspnoea and cough were the first, fifth and sixth most common answers, respectively [ 113 ]. Some aetiologies were reported to have a higher prevalence of certain symptoms. For example, in single studies, patients with PCD-related bronchiectasis were found to have a significantly higher prevalence of cough and wheezing [ 39 ], patients with COPD-related bronchiectasis were found to have a significantly higher prevalence of sputum production [ 41 ], and patients with post-TB bronchiectasis were found to have a higher prevalence of haemoptysis [ 30 ] compared with other aetiologies. Together, these results highlight the need for novel treatments that reduce the symptom burden of bronchiectasis. They also highlight the importance of teaching patients to perform and adhere to regular nonpharmacological interventions, such as airway clearance using physiotherapy techniques, which have been shown to improve cough-related health status and chronic sputum production [ 110 ]. Future studies assessing when airway clearance techniques should be started, and which ones are the most effective, are a research priority [ 113 ].
The burden of exacerbations in patients with bronchiectasis was high, with patients experiencing three or more exacerbations in the previous year (up to 73.6%), per year (up to 55.6%) or in the first year of follow-up (up to 32.4%). Few studies reported significant differences between aetiologies. Importantly, exacerbations are the second-most concerning aspect of bronchiectasis from the patient's perspective [ 113 ]. Patients with frequent exacerbations have more frequent hospitalisations and increased 5-year mortality [ 114 ] and exacerbations are also associated with poorer quality of life [ 114 , 115 ]. Therefore, prevention of exacerbations is of great importance in the management of bronchiectasis [ 116 ]. The exact cause of exacerbations in bronchiectasis (believed to be multifactorial) is not fully understood due a lack of mechanistic studies [ 116 ]. Future studies into the causes and risk factors for exacerbations [ 113 ] may lead to improvements in their prevention.
Many patients with bronchiectasis, including children, experienced chronic infections with bacterial pathogens such as P. aeruginosa , H. influenzae , Sta. aureus and Str. pneumoniae as well as non-tuberculous mycobacteria. Importantly, P. aeruginosa infection was significantly associated with more severe disease, reduced lung function and quality of life, and increased exacerbations, hospital admission, morality, HCRU and healthcare costs. Due to the clear and consistent association between P. aeruginosa and poor outcomes, patients with chronic P. aeruginosa colonisation should be considered to be at a higher risk of bronchiectasis-related complications [ 110 ]. Additionally, regular sputum microbiology screening should be performed in people with clinically significant bronchiectasis to detect new isolation of P. aeruginosa [ 110 ]; in which case, patients should be offered eradication antibiotic treatment [ 23 ]. Eradication of P. aeruginosa is not only of clinical importance, but also of economic importance due to the associated HCRU and healthcare costs. As such, a better understanding of the key factors leading to P. aeruginosa infection is a priority for future research [ 113 ].
Bronchiectasis markedly impacted HRQoL across several PROs including the SGRQ, Quality of Life–Bronchiectasis score, LCQ, COPD Assessment Test and Bronchiectasis Health Questionnaire. In children with bronchiectasis, significantly lower quality of life (according to the Paediatric Quality of Life Inventory score) compared with age-matched controls was reported [ 53 ]. The majority of studies reporting HRQoL in individual aetiologies and associated diseases either reported in a single aetiology, did not perform any statistical analyses to compare aetiologies, or reported no significant differences across aetiologies. Patients also experienced mild-to-moderate anxiety and depression according to the HADS-Anxiety, HADS-Depression and 9-question Patient Health Questionnaire scores, with very limited data reported in individual aetiologies. When compared with healthy controls, anxiety and depression were found to be significantly more prevalent in patients with bronchiectasis [ 55 ]. Additionally, exercise capacity was reduced, with patients with bronchiectasis reported to spend significantly less time on activities of moderate and vigorous intensity and have a significantly lower step count per day compared with healthy controls [ 89 ]. Improvements in anxiety, depression and exercise capacity are important priorities for people with bronchiectasis; in a study assessing the aspects of bronchiectasis that patients found most difficult to manage, “not feeling fit for daily activities”, anxiety and depression were the fourth, eighth and ninth most common answers, respectively [ 113 ].
The studies relating to HCRU and costs in this review were heterogeneous in terms of methodology, time period, country and currency, making them challenging to compare. Nevertheless, this study found that HCRU was substantial, with patients reporting a maximum of 1.3 hospitalisation, 1.3 ED and 21.0 outpatient visits per year. Length of stay was found to be significantly longer in patients with bronchiectasis compared with patients with any other respiratory illness in one study [ 91 ]. In another study, patients with bronchiectasis reported significantly more specialist appointments (radiologist appointments and chest physician appointments) compared with matched controls [ 85 ]. Patients with bronchiectasis also experienced a significant treatment burden, with up to 36.4, 58.0 and 83.0% of patients receiving long-term inhaled antibiotics, oral antibiotics and macrolides, respectively, up to 80.4% receiving long-term ICS and up to 61.7% and 81.4% receiving long-term long-acting muscarinic antagonists and long-acting beta agonists, respectively. Wide ranges of treatment use were reported in this study, which may reflect geographic variation in treatment patterns. Heterogeneous treatment patterns across Europe were observed in the EMBARC registry data with generally higher medication use in the UK and Northern/Western Europe and lower medication use in Eastern Europe (inhaled antibiotics: 1.8–8.9%; macrolides: 0.9–24.4%; ICS: 37.2–58.5%; long-acting beta agonists: 42.7–52.8%; long-acting muscarinic antagonists: 26.5–29.8%) [ 17 ]. Similarly, data from the Indian bronchiectasis registry indicate that the treatment of bronchiectasis in India is also diverse [ 19 ]. Furthermore, in a comparison of the European and Indian registry data, both long-term oral and inhaled antibiotics were more commonly used in Europe compared with India [ 19 ].
Cost varied widely across studies. However, patients, payers and healthcare systems generally accrued substantial medical costs due to hospitalisations, ED visits, outpatient visits, hospital-in-the-home and treatment-related costs. Other medical costs incurred included physiotherapy and outpatient remedies (including breathing or drainage techniques), outpatient medical aids (including nebulisers and respiration therapy equipment) and the cost of attending convalescence centres. Only one study compared the medical costs in patients with bronchiectasis and matched controls (age, sex and comorbidities) and found that patients with bronchiectasis had significantly higher total direct medical expenditure, hospitalisation costs, treatment costs for certain medications and costs associated with outpatient remedies and medical aids [ 85 ]. Bronchiectasis was also associated with indirect impacts and costs, including sick leave, sick pay and income lost due to absenteeism and missed work, and lost wages for caregivers of patients with bronchiectasis. Children with bronchiectasis also reported absenteeism from school or childcare.
Our findings regarding HRCU and costs in bronchiectasis are mirrored by a recent systematic literature review by R oberts et al . [ 117 ] estimating the annual economic burden of bronchiectasis in adults and children over the 2001–2022 time period. R oberts et al . [ 117 ] found that annual total healthcare costs per adult patient ranged from €3027 to €69 817 (costs were converted from USD to € based on the average exchange rate in 2021), predominantly driven by hospitalisation costs. Likewise, we report annual costs per patient ranging from €218 to €51 033, with annual hospital costs ranging from €1215 to €27 612 (adults and children included) ( table 4 ). Further, R oberts et al . [ 117 ] reports a mean annual hospitalisation rate ranging from 0.11 to 2.9, which is similar to our finding of 0.03–1.3 hospitalisations per year ( table 3 ). With regard to outpatient visits, R oberts et al . [ 117 ] reports a mean annual outpatient respiratory physician attendance ranging from 0.83 to 6.8 visits, whereas we report a maximum of 21 visits per year ( table 3 ). It should be noted, however, that our value is not restricted to visits to a respiratory physician. With regard to indirect annual costs per adult patient, R oberts et al . [ 117 ] reports a loss of income because of illness of €1109–€2451 (costs were converted from USD to € based on the average exchange rate in 2021), whereas we report a figure of ∼€1410 ( table 4 ). Finally, burden on children is similarly reported by us and R oberts et al . [ 117 ], with children missing 12 days of school per year per child ( table 4 ).
Limitations of this review and the existing literature
Due to the nature of this systematic literature review, no formal statistical analyses or formal risk of bias assessments were performed.
Several limitations within the existing literature were identified. Firstly, the vast majority of studies reported patients with NCFBE overall, with limited availability of literature reporting on individual aetiologies and associated disease. Furthermore, where this literature was available, it was limited to a handful of individual aetiologies and associated diseases, and in many of these studies, no statistical analyses to compare different aetiologies and associated disease were performed. Additionally, the methods used to determine aetiologies within individual studies may have differed. Literature on NCFBE and CFBE has traditionally been very distinct; as such, most of the studies included in this review have excluded people with CF. As the general term “CF lung disease” was not included in our search string in order to limit the number of hits, limited data on CFBE are included in this review. Bronchiectasis remains largely under-recognised and underdiagnosed, thus limiting the availability of literature. There is a particular knowledge gap with respect to paediatric NCFBE; however, initiatives such as the Children's Bronchiectasis Education Advocacy and Research Network (Child-BEAR-Net) ( www.improvebe.org ) are aiming to create multinational registries for paediatric bronchiectasis.
There were variations in the amount of literature available for the individual burdens. While there was more literature available on the clinical burden of bronchiectasis, economic data (related to both medical costs and indirect costs) and data on the impact of bronchiectasis on families and caregivers, were limited. Additionally, cost comparisons across studies and populations were difficult due to differences in cost definitions, currencies and healthcare systems.
Sample sizes of the studies included in this systematic literature review varied greatly, with the majority of studies reporting on a small number of participants. Furthermore, many of the studies were single-centre studies, thus limiting the ability to make generalisations about the larger bronchiectasis population, and cross-sectional, thus limiting the ability to assess the clinical and socioeconomic burden of bronchiectasis over a patient's lifetime. Furthermore, there may be potential sex/gender bias in reporting that has not been considered in this systematic literature review.
Finally, for many of the reported outcomes, data varied greatly across studies, with wide estimates for the frequency of different aetiologies and comorbidities as well as disease characteristics such as exacerbations and healthcare costs noted. This reflects the heterogeneity of both the study designs (including sample size and inclusion and exclusion criteria) and the study populations themselves. Additionally, the use of non-standardised terms across articles posed a limitation for data synthesis. Systematic collection of standardised data across multiple centres, with standardised inclusion and exclusion criteria such as that being applied in international registries, is likely to provide more accurate estimates than those derived from small single-centre studies.
- Conclusions
Collectively, the evidence identified and presented in this systematic literature review show that bronchiectasis imposes a significant clinical and socioeconomic burden on patients and their families and employers, as well as on healthcare systems. Disease-modifying therapies that reduce symptoms, improve quality of life, and reduce both HCRU and overall costs are urgently needed. Further systematic analyses of the disease burden of specific bronchiectasis aetiologies and associated disease (particularly PCD-, COPD- and post-TB-associated bronchiectasis, which appear to impose a greater burden in some aspects) and paediatric bronchiectasis (the majority of data included in this study were obtained from adults) may provide more insight into the unmet therapeutic needs for these specific patient populations.
Questions for future research
Further research into the clinical and socioeconomic burden of bronchiectasis for individual aetiologies and associated diseases is required.
- Supplementary material
Supplementary Material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figures and tables ERR-0049-2024.SUPPLEMENT
Supplementary Excel file ERR-0049-2024.SUPPLEMENT
- Acknowledgements
Laura Cottino, PhD, of Nucleus Global, provided writing, editorial support, and formatting assistance, which was contracted and funded by Boehringer Ingelheim.
Provenance: Submitted article, peer reviewed.
Conflict of interest: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). J.D. Chalmers has received research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Grifols, Novartis, Insmed and Trudell, and received consultancy or speaker fees from Antabio, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Insmed, Janssen, Novartis, Pfizer, Trudell and Zambon. M.A. Mall reports research grants paid to their institution from the German Research Foundation (DFG), German Ministry for Education and Research (BMBF), German Innovation Fund, Vertex Pharmaceuticals and Boehringer Ingelheim; consultancy fees from AbbVie, Antabio, Arrowhead, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Prieris, Recode, Santhera, Splisense and Vertex Pharmaceuticals; speaker fees from Vertex Pharmaceuticals; and travel support from Boehringer Ingelheim and Vertex Pharmaceuticals. M.A. Mall also reports advisory board participation for AbbVie, Antabio, Arrowhead, Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotec, Pari and Vertex Pharmaceuticals and is a fellow of ERS (unpaid). P.J. McShane is an advisory board member for Boehringer Ingelheim's Airleaf trial and Insmed's Aspen trial. P.J. McShane is also a principal investigator for clinical trials with the following pharmaceutical companies: Insmed: Aspen, 416; Boehringer Ingelheim: Airleaf; Paratek: oral omadacycline; AN2 Therapeutics: epetraborole; Renovian: ARINA-1; Redhill; Spero; and Armata. K.G. Nielsen reports advisory board membership for Boehringer Ingelheim. M. Shteinberg reports having received research grants from Novartis, Trudell Pharma and GlaxoSmithKline; travel grants from Novartis, Actelion, Boehringer Ingelheim, GlaxoSmithKline and Rafa; speaker fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Insmed, Teva, Novartis, Kamada and Sanofi; and advisory fees (including steering committee membership) from GlaxoSmithKline, Boehringer Ingelheim, Kamada, Syncrony Medical, Zambon and Vertex Pharmaceuticals. M. Shteinberg also reports data and safety monitoring board participation for Bonus Therapeutics, Israel and is an ERS Task Force member on bronchiectasis guideline development. S.D. Sullivan has participated in advisory boards for Boehringer Ingelheim and has research grants from Pfizer, Bayer and GlaxoSmithKline. S.H. Chotirmall is on advisory boards for CSL Behring, Boehringer Ingelheim and Pneumagen Ltd, served on a data and safety monitoring board for Inovio Pharmaceuticals Inc., and has received personal fees from AstraZeneca and Chiesi Farmaceutici.
Support statement: This systematic literature review was funded by Boehringer Ingelheim International GmbH. The authors did not receive payment related to the development of the manuscript. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. Funding information for this article has been deposited with the Crossref Funder Registry .
- Received March 8, 2024.
- Accepted June 4, 2024.
- Copyright ©The authors 2024
This version is distributed under the terms of the Creative Commons Attribution Licence 4.0.
- Murray MP ,
- Chalmers JD ,
- Aliberti S ,
- McShane PJ ,
- Naureckas ET ,
- Tino G , et al.
- Martins M ,
- Chalmers JD
- Chotirmall SH ,
- Sun X , et al.
- Curtis JR , et al.
- Monteagudo M ,
- Rodríguez-Blanco T ,
- Barrecheguren M , et al.
- Millett ERC ,
- Joshi M , et al.
- Ringshausen FC ,
- de Roux A ,
- Diel R , et al.
- Liu S-X , et al.
- Weycker D ,
- Hansen GL ,
- Shteinberg M ,
- Adir Y , et al.
- Aksamit TR ,
- O'Donnell AE ,
- Barker A , et al.
- Polverino E ,
- Crichton ML , et al.
- Talwar D , et al.
- Chalmers JD , et al.
- Visser SK ,
- Fox GJ , et al.
- Sullivan AL ,
- Goeminne PC ,
- McDonnell MJ , et al.
- Liberati A ,
- Altman DG ,
- Tetzlaff J , et al.
- Scioscia G ,
- Alcaraz-Serrano V , et al.
- Bilotta M ,
- Bartoli ML , et al.
- Rosales-Mayor E ,
- Benegas M , et al.
- Mackay IM ,
- Sloots TP , et al.
- Alcaraz-Serrano V ,
- Gimeno-Santos E ,
- Scioscia G , et al.
- Al-Harbi A ,
- Al-Ghamdi M ,
- Khan M , et al.
- de Gracia J ,
- Giron R , et al.
- Sunjaya A ,
- Reddel H , et al.
- Raguer L , et al.
- Martinez-Garcia MÁ ,
- Athanazio R ,
- Gramblicka G , et al.
- Ailiyaer Y ,
- Zhang Y , et al.
- Stockley R ,
- De Soyza A ,
- Gunawardena K , et al.
- de la Rosa Carrillo D ,
- Navarro Rolon A ,
- Girón Moreno RM , et al.
- de la Rosa D ,
- Martínez-Garcia M-A ,
- Giron RM , et al.
- Sharif S , et al.
- Pottier H ,
- Marquette CH , et al.
- Nagelschmitz J ,
- Kirsten A , et al.
- Artaraz A ,
- Crichton ML ,
- Finch S , et al.
- Aksamit T ,
- Bandel TJ , et al.
- Liu R , et al.
- Olveira C ,
- Olveira G ,
- Gaspar I , et al.
- Goeminne P ,
- Aliberti S , et al.
- Chalmers J ,
- Dimakou K , et al.
- Mitchelmore P ,
- Rademacher J , et al.
- Loebinger M ,
- Menendez R , et al.
- Bennett K , et al.
- Barker RE , et al.
- Zhu YN , et al.
- Yong SJ , et al.
- Inal-Ince D ,
- Cakmak A , et al.
- Araújo AS ,
- Figueiredo MR ,
- Lomonaco I , et al.
- Navas-Bueno B ,
- Casas-Maldonado F ,
- Padilla-Galo A , et al.
- Li T , et al.
- Gatheral T ,
- Sansom B , et al.
- Leem AY , et al.
- Bellelli G ,
- Sotgiu G , et al.
- Patel ARC ,
- Singh R , et al.
- Stroil-Salama E ,
- Morgan L , et al.
- Bradley JM ,
- Bradbury I , et al.
- Lo CY , et al.
- Padilla A ,
- Martínez-García M-Á , et al.
- Dicker AJ ,
- Lonergan M ,
- Keir HR , et al.
- Crichton M ,
- Cassidy A , et al.
- de Boer S ,
- Fergusson W , et al.
- Goeminne PC , et al.
- Suarez-Cuartin G ,
- Rodrigo-Troyano A , et al.
- Abo-Leyah H , et al.
- Blanchette CM ,
- Stone G , et al.
- Grimwood K ,
- Ware RS , et al.
- Kim HY , et al.
- Martínez-Garcia MA ,
- Olveira C , et al.
- Lin CS , et al.
- Navaratnam V ,
- Millett ER ,
- Hurst JR , et al.
- Kim JM , et al.
- Rabe KF , et al.
- Wang LY , et al.
- Schwartz BS ,
- Al-Sayouri SA ,
- Pollak JS , et al.
- Girón Moreno RM ,
- Sánchez Azofra A ,
- Aldave Orzaiz B , et al.
- Sonbahar-Ulu H , et al.
- Nawrot TS ,
- Ruttens D , et al.
- Muirhead CR ,
- Hubbard RB , et al.
- Marchant JM ,
- Roberts J , et al.
- Lovie-Toon YG ,
- Byrnes CA , et al.
- Costa JdC ,
- Blackall SR ,
- King P , et al.
- Jiang N , et al.
- Jayaram L ,
- Karalus N , et al.
- McCullough AR ,
- Tunney MM ,
- Stuart Elborn J , et al.
- Joschtel B ,
- Gomersall SR ,
- Tweedy S , et al.
- Pizzutto SJ ,
- Bauert P , et al.
- Nam H , et al.
- Navarro-Rolon A ,
- Rosa-Carrillo D ,
- Esquinas C , et al.
- Seifer FD ,
- Ji Y , et al.
- McPhail SM ,
- Hurley F , et al.
- McCallum GB ,
- Singleton RJ ,
- Redding GJ , et al.
- Contarini M ,
- Shoemark A ,
- Ozerovitch L ,
- Masefield S ,
- Polverino E , et al.
- Filonenko A , et al.
- Xu G , et al.
- Roberts JM ,
- Kularatna S , et al.

- Table of Contents
- Index by author
Thank you for your interest in spreading the word on European Respiratory Society .
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- CF and non-CF bronchiectasis
- Tweet Widget
- Facebook Like
- Google Plus One
More in this TOC Section
- Adherence-enhancing interventions for pharmacological and oxygen therapy in COPD patients
- PM 2.5 and microbial pathogenesis in the respiratory tract
Related Articles

Systematic Reviews
- The Research Question
- Inclusion and Exclusion Criteria
- Original Studies
- Translating
- Deduplication
- Project Management Tools
- Useful Resources
- What is not a systematic review?
Cochrane resources
Cochrane Handbook for Systematic Reviews of Interventions
- Introduction to Systematic Reviews
Here is a PowerPoint presentation that provides a brief overview of Systematic Reviews Texas Medical Center Library
What is a systematic review?
A systematic review attempts to collate all empirical evidence that fits pre-specified eligibility criteria in order to answer a research question. 1 Systematic Reviews are research projects that provide new insight on a topic and are designed to minimize bias. The project creates accessible research that examines relevant literature, which aids decision makers by aggregating information in a systematic way. Methodological transparency, along with its systematic approach and project reproducibility, are key elements of a systematic review.
1. Taken from Lasserson TJ, Thomas J, Higgins JPT. Chapter 1: Starting a review. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook
Components of a Systematic Review
Key elements of a systematic review include :.
- A specific and well-formulated question
- A reproducible methodology intended to avoid bias
- Multiple databases searched for the review's data
- Specified and predefined inclusion and exclusion criteria
- Multiple reviewers of the literature
- Study assessments conducted in a standardized way with definitive methodology
- Adherence to a standardized reporting guideline, such as PRISMA
Systematic reviews can have an impact on the development of public health policies and on resource allocation decisions. They can inform clinical practices and implement evidence-based interventions for diseases and illnesses. Moreover, systematic reviews can compare benefits and harms of treatment options.
The systematic review process has been developed to minimize bias and ensure transparency. Methods should be adequately documented so that they can be replicated. The integrity of a systematic review is based on its transparency and reproducibility of the methods used for the review.
There are many resources on how to conduct, organize, and publish a systematic review. This guide is by no means exhaustive; its aim is to provide a starting place for understanding the core of what a systematic review is and how to conduct one.
What does it take to do a systematic review?
Time : On average, systematic reviews can require up to 18 months of preparation.
A team: A systematic review can't be done alone! You need to work with subject experts to clarify issues related to the topic; librarians to develop comprehensive search strategies and identify appropriate databases; reviewers to screen abstracts and read the full text; a statistician who can assist with data analysis; and a project leader to coordinate the team and movement of data.
A clearly defined question : A clearly defined research question can help clarify the key concepts of a systematic review and explain the rationale for the review. It is recommended to use a framework (e.g. the PICO framework) to identify key concepts of the question.
A written protocol : The protocol should outline the study methodology. The protocol should include the rationale for the systematic review; the research question broken into PICO components; explicit inclusion/exclusion criteria; relevant known literature on the research question; preliminary search terms and databases to be used; intended data abstraction/data management tools; and other components that may be unique to register the protocol.
A registered protocol : A few recommendations are PROSPERO , an International Prospective Register of Systematic Reviews; Cochrane; and the Agency for Healthcare Research and Quality. Registering a protocol is important because it reduces duplication of effort and promotes transparency.
Inclusion/exclusion criteria: Inclusion/exclusion criteria can help researchers define the terms of the investigation. These will include the predefined question; study types; study-analysis criteria (i.e. criteria for reporting bias within studies); and quantitative methods to be used for any statistical analysis.
Comprehensive literature searches : Identify appropriate databases and conduct comprehensive and detailed literature searches that can be documented and duplicated. Cochrane recommends that 3+ different databases be used to conduct the searches. A strategy must be developed and then translated across the multiple pre-specified databases, preferably by an information specialist.
Citation management: You should have working knowledge of EndNote or another citation management system that will be accessible to the research team to help manage citations retrieved from literature searches.
Follow reporting guidelines : Use appropriate guidelines for reporting your review for publication.
Time - requires about 18 months of preparation .
The suggested timeline for a Cochrane review is:
- Preparation of protocol 1 – 2 months
- Searches for published and unpublished studies 3-8 months
- Pilot test of eligibility criteria 2-3 months
- Inclusion assessments 3-8 months
- Pilot test of ‘Risk of bias’ assessment 3 months
- Validity assessments 3-10 months
- Pilot test of data collection 3 months
- Data collection 3-10 months
- Data entry 3-10 months
- Follow up of missing information 5-11 months
- Analysis 8-10 months
- Preparation of review report 1-11 months
- Keeping the review up-to-date 12 months
- Next: Services >>
- Last Updated: Jul 29, 2024 2:41 PM
- URL: https://libguides.sph.uth.tmc.edu/SystematicReviews
- Open access
- Published: 04 September 2024
Palatal groove associated with periodontal lesions: a systematic review illustrated by a decisional tree for management
- Yvan Gaudex 1 , 2 ,
- Vianney Gandillot 1 , 2 , 7 ,
- Isabelle Fontanille 3 ,
- Philippe Bouchard 1 , 2 ,
- Stephane Kerner 1 , 2 , 4 , 5 &
- Maria Clotilde Carra 1 , 2 , 6
BMC Oral Health volume 24 , Article number: 1037 ( 2024 ) Cite this article
Metrics details
Palatal groove represents a relatively uncommon developmental root anomaly, usually found on the palatal aspect of maxillary incisors. While its origin is controversial, its presence predisposes to severe periodontal defects.
This study aimed to provide a systematic review of the literature focusing on the varied diagnostic techniques and treatment modalities for periodontal lesions arising from the presence of palatal groove. Based on the existing evidence and knowledge, the study also provides a comprehensive decisional tree, guiding clinicians in the challenging decision-making process face to a palatal groove.
The literature search was conducted on Medline and Cochrane databases by two independent reviewers, who also performed the screening and selection process, looking for English written articles reporting on diagnosis and management (all treatment approaches) of periodontal lesion(s) associated with a palatal groove. Based on this literature, a comprehensive decisional tree, including a standardized palatal groove evaluation and tailored treatment approaches, is proposed. Moreover, a clinical case is described to demonstrate the practical application of the developed decisional tree.
Over a total of 451 articles initially identified, 34 were selected, describing 40 patients with 40 periodontal lesions associated with palatal grooves. The case report illustrates a deep, large, circumferential intra-bony defect on the palatal side of the tooth #22 associated with a shallow, moderately long palatal groove in an 18-year-old male patient. Following reevaluation, a single flap surgery was deemed necessary, combined with a regenerative procedure. At 2 years post-treatment, the tooth #22 is healthy, in a functional and esthetic position. The decision-making process, based on local and systemic patient’s conditions, should allow an early and precise diagnosis to prevent further complications and undertake an adequate treatment.
Palatal grooves are relatively rare; however, they are frequently associated with severe periodontal defects. The identification, diagnosis, prompt, and tailored management of the associated lesion is essential to mitigate potential periodontal and endodontic complications related to the presence of palatal groove.
Systematic Review Registration
[ https://www.crd.york.ac.uk/prospero/ ], identifier [C CRD42022363194].
Peer Review reports
Introduction
Palatal groove (PG) is defined as an anatomic anomaly characterized by the presence of a developmental groove on a dental root that, when present, is usually found on the palatal aspect of maxillary incisors [ 1 ]. Over the years, several terms have been used to describe this anomaly, including palatal or palate-gingival groove [ 2 , 3 ], developmental radicular anomaly [ 4 ], distolingual groove [ 5 ], radicular lingual groove [ 6 , 7 ], palatoradicular groove [ 8 , 9 ], radicular groove [ 10 ], and cinguloradicular groove [ 11 ].
The origin of the PG is controversial, but it is assumed to be related to the infolding of the enamel organ or Hertwig epithelial root sheath during the tooth development [ 12 ]. Additional hypogenetic root formation [ 13 , 14 ] as well as an altered genetic mechanism [ 15 ] have also been suggested.
PG is relatively rare. Everett et al. [ 5 ] reported a prevalence of PG on 2.8% of lateral incisors whereas Withers et al. [ 16 ] observed a PG on 2.3% of maxillary incisors (4.4% of maxillary laterals and 0.28% of maxillary centrals). Kogon et al. [ 8 ] examined 3168 extracted maxillary central and lateral incisors and found PG on 4.6% of them (3.4% of maxillary centrals and 5.6% of maxillary lateral incisors), with over half of the PG extending more than 5 mm apical to the cementoenamel junction leading to a localized periodontal lesion. The most recent study by Mazzi-Chevez et al. [ 17 ] observed 150 maxillary central incisors, lateral incisors, and canines with a micro-CT and found that PG affected 2% of central incisors and 4% of lateral incisors. In 100% of cases, the PG originated in the enamel.
As the term implies, PG is formed around the cingulum of the tooth and continues apically down from the cementoenamel junction, terminating at various depths and length along the root [ 18 ]. In contrast to maxillary bicuspids, incisors generally display a U-shaped groove.
This anatomic anomaly is frequently associated with a breakdown of the periodontal attachment involving the groove; a self-sustaining localized periodontal pocket can develop [ 4 ], where the PG itself provides a site for bacterial accumulation. The subsequent progressive inflammation along the PG and its apical portion may lead to periodontal and endodontic pathologic conditions [ 19 ]. Furthermore, there may be communication between the pulp canal system and the periodontium through the pulp cavity and/or accessory canals, which may also lead to combined endodontic-periodontal lesions [ 20 ]. According to the 2017 classification of periodontal and peri-implant diseases and conditions [ 21 ], PG can be classified as a localized tooth-related factor that modifies or predisposes to plaque-induced gingival diseases/periodontitis [ 22 ], and can be associated with periodontal abscess in non-periodontitis patients.
The prognosis for teeth with PG extending apically is often poor [ 12 ], highlighting the critical need for prompt and accurate diagnosis to avert further periodontal and endodontic complications, ultimately preventing tooth extraction. This study is fundamentally motivated by the scarcity of consolidated guidelines for managing such complex dental conditions. Hence, the objective of this study was to conduct a systematic review of the existing literature, focusing on the diagnosis and management of periodontal lesions linked to PG. Based on this review, the goal was to develop a comprehensive decisional tree, thereby proposing a standardized treatment protocol to aid in the clinical decision-making. This study also includes a clinical case report to demonstrate the practical application of the developed decisional tree, reinforcing its clinical relevance and utility.
Material and methods
Development of the systematic review protocol.
A protocol covering all aspects of the systematic review methodology was developed before starting the review. The protocol included the definition of: a focused question; the literature search strategy; the study selection criteria; the outcome measures; the screening methods; the data extraction; and the data synthesis. The protocol was registered in PROSPERO (CRD42022363194).
Defining the focused question
The research question was formulated according to the PICOS (Population, Intervention, Comparison, Outcome, Study) strategy, which identify the search and selection criteria as follows:
P: Patients with periodontal lesion(s) associated with a PG
I: PG identification (diagnosis) and management. All treatment approaches (non-surgical, surgical, with or without the adjunctive use of potentially regenerative materials, i.e. barrier membranes, grafting materials, growth factors/proteins and combinations thereof) were considered.
C: alternative treatment approach or no comparison.
O: periodontal parameters, including clinical attachment level (CAL, measure in mm), probing pocket depth (PPD, measured in mm), recession (REC, measured in mm), plaque index (PI, any validated clinical score), bleeding on probing (BOP) or other inflammatory indexes, radiographic bone loss.
S: Any type of human studies including case reports, with a minimum of 6 weeks follow-up after treatment. Only studies published in English were considered. Studies written in languages other than English, review articles, cell and/or animal studies, letters, editorials, conference summaries, commentaries, and studies considering PG with only an endodontic involvement or that used self-report assessment of treatment outcomes were not considered.
So, the focused question was formulated as follows: what is the efficacy of treatments for periodontal lesions associated with PG?
Search strategy
The literature was searched for articles published up to June 2022 on MEDLINE and Cochrane databases. Multiple combinations of pertinent search terms were employed (Supplemental Table 1). The reference lists of the included studies were also evaluated in order to identify additional articles. To ensure its reproducibility, the PRISMA guidelines were followed [ 23 ], and the PRISMA flowchart was filled [ 24 ] (Fig. 1 ).

PRISMA flow diagram on the selection process of the studies included in the systematic review
Literature screening and data extraction
The titles and abstracts of the initially identified studies were screened by two independent reviewers (Y.G. and V.G.). Then, the pre-selected studies underwent a full text evaluation to assess the final inclusion or not. All records for which inclusion was obtained “uncertain” for on reviewer, disagreement was solved by discussion between authors. Whenever needed, the authors of the selected studies were contacted to provide missing data.
Study screening and selection was carried out by using the Rayyan online software [ 25 ], which assisted the reviewers in the different step of the literature review process. Duplicate references were removed automatically using Mendeley software. Data extraction was carried out on a dedicated excel spreadsheet. The risk of bias assessment was carried out by using the Joanna Briggs Institute (JBI) scale [ 26 , 27 ].
The literature search resulted in 451 potentially relevant publications (Fig. 1 ). After the first selection step, based upon the title and abstract, 88 articles were pre-selected. After full-text evaluation, 34 articles were included and analyzed. All of them were case series and case reports. A total of 40 patients were described, of which 23 women (57.5%). The characteristics of the selected studies are presented in Table 1 . Their quality assessment is reported in Table 2 .
Qualitative synthesis of the literature
Among those 40 clinical cases, 12 cases report failed to provide a clinical description of the PG. Four studies described the PG depth alone, 17 studies described the PG length alone, and 7 studies provided a combined description of depth and length of the PG. From a periodontal point of view, the periodontal lesion morphology was correctly described (depth and width) in only 4 cases, 2 of which also reported the number of bony walls. Among the 22 cases reporting a diagnosis, 17 (77.3%) described combined endo-periodontal lesions, whereas 5 were purely periodontal lesions.
Endodontic involvement was present in 29 cases: 22 cases presented with a pulp necrosis, and 7 cases with an endodontic treatment. Pulp vitality was present in 10 cases and 1 case failed to report the endodontic status.
The endodontic treatment consisted in either a temporary filling (calcium hydroxide) later replaced by a definitive filling (gutta percha), or directly with a definitive filling (gutta percha) when indicated. Among those 29 endodontically treated teeth, 9 underwent an apicoectomy (using mineral trioxyde aggregate) at the surgical phase.
PG sealing was performed in 16 cases using mainly glass-ionomer cement but also mineral trioxide aggregate (MTA), tricalcium silicate cement, composite flow and amalgam. In 5 cases, an extra-oral filling of the groove was performed before the tooth reimplantation. In all cases, radiculoplasty was performed either for groove removal when it was shallow or by saucerization to allow a proper filling when grooves were deep.
To treat the PG associated periodontal defect, several different intervention types were described, using: allogenic bone, xenogeneic bone, alloplastic materials, barriers, growth factors and biological factors (and combinations thereof). These surgical regenerative procedures were reported in 25 cases. Only 2 cases [ 3 , 40 ], justified the use of biomaterials and flap designs in relation to the analysis of the associated periodontal lesion after PG management.
All cases reported clinical healing except for 2 cases of failures following tooth reimplantation due to external root resorption leading to tooth removal after 36 months [ 33 ] and 2 failures after 6 months following a surgery without regeneration or root filling [ 29 ]. The case with the longest follow-up (324 months) indicated that following an endodontic treatment with a periodontal regeneration and an orthodontic treatment, a recurrent periodontal breakdown occurred 11 years, leading to tooth extraction and implant placement [ 35 ].
Case-report
We describe the case of an 18-year-old male patient referred to the periodontics department of the Rothschild Hospital (AP-HP) in Paris. Written informed consent was obtained for the publication of clinical data and images included in this article. The patient was experiencing pain due to the inflammation on the palatal side of tooth #22 with intermittent suppuration. The clinical examination revealed a central, shallow, and of moderate length (up to 70% of the root length) PG on the tooth #22, with a probing pocket depth of 12 mm on the palatal side associated with a tooth mobility 3 (Mühlemann 1951). The tooth responded positively to electrical test. At the radiographic evaluation, bone loss could be noted mesially and distally of #22 (Fig. 2 ).

Case report. Clinical and radiographical initial situation of the tooth #22 presenting with a palatal groove. The periodontal charting showed deep periodontal pockets on the palatal probing sites associated with bleeding and plaque accumulation
A slight bony bridge could be distinguished between #21 and #22 in the coronal portion. Thus, a localized periodontal defect due to the presence of subgingival PG was diagnosed.
The periodontal treatment first consisted in a non-surgical debridement performed in one session. Tooth splinting was performed from #21 to #23 to minimize mobility (Fig. 3 ).

Root planning and flattening of PG on tooth #22: initial occlusal view of #22 ( a ); Manual scaling 22 ( b ); flattening of PG 22 in the coronal part ( c )
At the re-evaluation 8 weeks later, the tooth presented no superficial inflammation, but a persistent periodontal pocket of 12 mm deep on the palatal side. Surgery was indicated due to the presence of a large, deep, 3-wall intra-bony defect around tooth #22 (Fig. 4 ).

Regenerative therapy: view at the periodontal re-evaluation, 2-months after the initial treatment ( a ); large and deep 3-walls intra-bony defect ( b ); application of EMD ( c ); application of DBBM (soft tissue support, osteoconductive) ( d ); sutures ( e ); radiographic image at the 2-month follow-up ( f )
A SFA (Single Flap Approach) was designed with a surgical access limited on the palatal side for esthetic reason and optimal visualization. A full periosteal flap was raised, and the granulation tissue was removed. The aberrant local anatomy was corrected up to the most apical part and a regenerative procedure combining enamel matrix derivates with a bone substitute was applied to avoid soft tissue shrinkage and collapse. Sutures with a non-resorbable monofilament 6/0 were made using U-crossed and single points. A postoperative radiograph was taken (Fig. 4 f). An antibiotic therapy with amoxicillin (1 g twice a day for 7 days) was administered. Paracetamol was prescribed as a painkiller and a mouthwash containing 0.12% chlorhexidine gluconate were prescribed for 2 weeks postoperatively. Healing was uneventful and sutures were removed 10 days postoperatively.
At the 6 months reevaluation, the periodontal pocket was no deeper than 4 mm on the palatal side with no bleeding on probing. A recession of 1 mm was observed. Radiographically, a mineralized tissue could be observed up to both bony peaks mesially and distally to #22 (Fig. 5 ).

Re-evaluation at 6 months ( a ); 18 months ( b ) and 30 months ( c )
At the 1-year follow-up, periodontal health was maintained and an orthodontic treatment was undertaken. After 2 years of treatment, tooth #22 is still healthy with a CAL gain of 7 mm, a functional and esthetic position resulting in the patient’s satisfaction. These results support that periodontal regeneration can be effectively carried out also for deep intra-bony defect associated with PG, once the local risk factor has been adequately managed.
The results of the present systematic review indicate that PG are relatively uncommon root anomaly, but they are frequently associated with periodontal lesion that require treatment. The selected studies showed that PG can be managed concomitantly with periodontal regeneration, with or without associated endodontic treatment. It must be noted that the presence of a PG may play a significant role in exacerbating periodontal lesions. This could be explained, at least partly, by the mediation role of inflammatory factors like the TGF-B1, which is involved in the regulation of the inflammatory response and in the remodeling of periodontal tissues, as highlighted by recent studies [ 58 , 59 ]. These findings necessitate a nuanced and well-defined diagnostic and therapeutic approach, which should consider not only on the anatomical challenges linked to the presence of a PG but also on the underlying inflammatory mechanisms, in order to ensure an effective treatment and prevent potential endodontic complications.
A variety of treatments approaches has been described in case reports and case series and summarized in the present review. The appreciation of the morphology and origin of PG on maxillary incisors may be challenging and thus delay the diagnosis and treatment planning. Therefore, developing a standardized approach based on the available literature is advisable.
A PG can be classified according to its location, length along the root, and depth of the groove towards the pulp cavity [ 60 ]. The analysis of the associated periodontal lesion is also a key parameter to consider. Based on the work of Kim et al. [ 60 ], a simplified version including the groove description and the periodontal parameters has been suggested. Such a classification (Table 3 ) would provide the clinician with precise criteria to justify the therapeutic approach.
Groove location was disregarded in most cases, only one case [ 40 ] reported a distal location of the PG. It can be explained by the fact that this parameter will not affect the prognosis or the treatment sequence. In the latest study done on extracted teeth, PG appeared to originate in the distal area of the cingulum margin in most cases (65%), followed by the central fossa (25%), and the mesial area of the cingulum margin (10%) [ 61 ].
In terms of depth, only 7 cases reported a shallow PG (50%) and 7 cases reported a deep PG (50%) and no closed tube has been described. This finding is in accordance with Kogon’s study [ 8 ] where 44% percent of the PG were described as shallow depressions, 42% as deep depressions, and 4% as closed tubes.
Considering the groove length, 4 cases reported an extension in the cervical third of the groove (17%), 6 in the middle third (25%) and 14 cases in the apical third (58%). According to Pinheiro’s study [ 61 ], those grooves extended rarely only to the cervical third (5%), followed by the middle thirds (45%) and the apical thirds of the root in most cases (50%). It is of paramount importance for clinicians to understand the combination of both variations of groove depth along with their length to adapt an adequate treatment considering the fact that PG with deeper grooves and greater degree of extension are the determinants and predictors of poor prognosis periodontally and endodontically wise [ 5 , 31 , 42 ].
Considering the groove description in the selected studies, most of them failed to adequately report it. Only 7 of the 40 cases described the depth and length of the PG. This lack of analysis might result in an inadequate treatment highlighting the need for a classification.
Considering the periodontal approach of the associated intra-bony defect, the selection of the regenerative biologic principle (or material) to use with the soft tissue surgical approach dependeds on the morphology of the intra- bony defect (width, depth, and number of residual bony walls) and on the amount (and quality) of the soft tissues available to cover it [ 62 ]. As a general rule, deep and wide defects with only one residual bony wall require a mechanical stabilizer of the blood coagulum (membrane and/or bone filler), whereas in defects with lower defect angles and a greater number of bony walls, biologic mediators of the healing process (e.g. enamel matrix derivates) are indicated [ 62 ]. In the present study, only 2 cases [ 3 , 40 ] succeeded in justifying the use of their regenerative procedure based on the description and analysis of the associated intra-bony lesion. As for PG anatomy, this lack of description of the associated periodontal lesion morphology could mislead the diagnosis and result in a non-optimal treatment. The PG issue had mostly been a concern for endodontist based on those case reports coming from endodontic journals, which might explain the few periodontal parameters reported and the lack of a clear description of the intra-bony defect associated to justify the different management of the periodontal defect. Moreover, the selected case reports do not cover all potentially applicable regenerative techniques, which continue to evolve [ 63 , 64 , 65 ] and should be further investigated in the particular context, from the microbiological and inflammatory perspectives, of PG-associate lesions.
Based on the presented literature review and in order to guide clinicians towards a comprehensive and complete evaluation of PG associated lesion, we suggested a decisional tree (Fig. 6 ) that introduces the periodontal parameter in the PG assessment, after evaluating the endodontic status. Indeed, the successful management of a tooth with a PG is firstly dependent on endodontic status, which should be systematically assessed. In cases of negative pulp response and periapical lesions, an endodontic treatment has to be undertaken in the first place [ 66 ]. But, the periodontal evaluation is also cardinal to obtain a successful and long-lasting management of PG.

Decisional tree. This graph proposes a decision-making process for the management of PG-associated lesions that takes into account the endodontic status, the characteristics of the palatal groove, and the presence of intra-bony defect
The recognition and management of PG for tooth survival has been reported in details in a study done by Kim et al. [ 60 ] in 2017. In the rest of the considered literature, half of the treatments described were made without a clear initial diagnosis or proper description of the associated lesions to justify the type of regenerative strategy and flap design approached. Another interesting observation made in this review is that in the case of intentional replantation, among the 5 reported cases, 2 resulted in a failure necessitating the tooth removal [ 33 ]. This suggests that replantation strategy should be used as a the latest resort for complex cases involving a PG to the apex with a deep groove.
It must be acknowledged that the available literature and thus the present systematic review present several limitations. Firstly, as mentioned above, there is a lack of standardization in the diagnostic and treatment processes, with a high heterogeneity among the selected articles, most of the times case reports or case series. Secondly, the follow-up time was mostly set between 6 and 24 months, which may be too short to assess treatment outcomes or observed complications and relapse. Indeed, after a 36 months follow-up, failures have been reported [ 33 ] and after 10 years, a periodontal breakdown occurred on a treated tooth [ 35 ] and both resulted in the tooth removal. No re-entry surgery and/or histologic evaluations were described and no prospective longitudinal studies evaluating the stability of the clinical and radiographic parameters and the absence of the recurrence of disease were found. Thus, any conclusion about the success achieved with the treatments described in the present review should be drawn with caution as the long-term prognosis of the treatment of PG-associated lesions of teeth remains to be determined. Updates of case series and case reports that could describe results after 5, 10 and 15 years from the initial PG diagnosis are advocated. Finally, the level of the body of evidence on PG is considered as low. Although the nature of PG as rare condition may explain why mainly case reports or case series are published, future clinical and comparative studies should be designed to investigate PG management and treatment success at long term. Nonetheless, based on the currently available literature, a decisional tree (Fig. 6 ) has been proposed to guide clinicians and create a reference for PG management to respond to a patient’s health condition. This should be periodontally updated as new evidence emerges but in the meantime, it can be useful to provide a clinical guidance as well as a model for the standardization of the diagnostic and treatment processes in clinical cases dealing with PG management.
Teeth with PG represent a challenge for clinicians. Despite their rarity (2% of maxillary lateral incisors), the complexities associated with PG, such as diverse anatomical features and clinical scenarios, underscore the necessity for accurate diagnosis and tailored treatment approaches. This study provides a systematic review of pertinent literature, consisting mainly in case reports, and culminates in the proposal of a decision tree, which aims to assist clinicians in the decision-making process through a structured evaluation of the PG characteristics guiding the treatment approach. The ultimate goal is to mitigate potential periodontal and endodontic complications of PG while providing a successful management. In parallel, the present study highlights the need of future research on this topic, particularly with clinical studies with a sufficiently long follow-up to monitor the treatment outcomes and their stability over time. Indeed, further evidence is needed to develop standardized diagnostic and treatment protocols for PG.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Glossary of Endodontic Terms. American Association of Endodontics. 10th ed. 2020.
Google Scholar
Bacic M, Karakas Z, Kaiét Z, Sutalot J. The association between palatal grooves in upper incisors and periodontal complications. J Periodontol. 1990;2:197–9.
Article Google Scholar
Schwartz SA, Koch MA, Deas DE, Powell CA. Combined endodontic-periodontic treatment of a palatal Groove : a case report. J Endod. 2006;32(6):573–8.
Article PubMed Google Scholar
Simon J. Predictable endodontic and periodontic failures as a result of radicular anomalies. J Oral Maxillofac Surger Oral Surg. 1971;42:823–6.
Everett FG. The Disto-lingual Groove in the Maxillary Lateral Incisor; a periodontal hazard. A Periodontal Hazard J Periodontol. 1972;43(6):352–61.
CAS PubMed Google Scholar
August D. The radicular lingual groove: an overlooked differential diagnosis. J Am Dent Assoc. 1978;96:1037–9.
Article CAS PubMed Google Scholar
Meister F. Successful treatment of a radicular lingual groove: case report. J Endod. 1983;9:561–4.
Kogon S. The prevalence, location and conformation of palato- radicular Grooves in Maxillary Incisors*. J Periodontol. 1985;57:231–4.
Hou GL. Relationship between palato-radicular grooves and localized periodontitis. J Clin Periodontol. 1993;20:678–82.
Pécora J. Study of the incidence of radicular grooves in maxillary incisors. Braz Dent J. 1992;3:11–6.
PubMed Google Scholar
Assaf M. The cingulo-radicular groove: its significance and management: two case reports. Compendium. 1992;13:94–8.
Gound TG MGI. Treatment options for the radicular lingual groove: a review and discussion. Pract Periodontics Aesthet Dent. 1998;10:369–75.
Ennes JP, Lara VS. Comparative morphological analysis of the root developmental groove with the palato-gingival groove. Oral Dis. 2004;10:378–82.
Goon WWY, Carpenter WM, Brace NM, Ahlfeld RJ. Complex facial radicular Groove in a maxillary lateral incisor. J Endod. 1991;17(5):244–8.
Mittal M, Vashisth P, Arora R, Dwivedi S. Combined endodontic therapy and periapical surgery with MTA and bone graft in treating palatogingival groove. BMJ Case Rep. 2013:1–4.
Withers JA, Brunsvold MA, William J, Rahe AJ. The relationship of palato-gingival grooves localized periodontal disease. J Periodontol. 1981;52(1):41–4.
Mazzi-chaves JF. Influence of anatomical features in the endodontic treatment planning of maxillary anterior teeth. Braz Dent J. 2022;36:1–15.
Lee KW. Palato-gingival grooves in maxillary incisors. A possible predisposing factor to localised periodontal disease. Br Dent J. 1968;124:14–8.
Sharma S. Palatogingival groove : recognizing and managing the hidden tract in a maxillary incisor : A case report. J Int Oral Health. 2015;7(6):110–4.
PubMed PubMed Central Google Scholar
Gao Z, Shi J, Wang Y, Gu F. Scanning electron microscopic investigation of maxillary lateral incisors with a radicular lingual groove. Oral Surg Oral Med Oral Pathol. 1989;68:462–6.
Herrera D, Alonso B, Feres M. Acute periodontal lesions ( periodontal abscesses and necrotizing periodontal diseases ) and endo-periodontal lesions. J Periodontol. 2017;89(1):85–102.
Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89(1):S237–48.
Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann C, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews systematic reviews and meta-analyses. BMJ. 2021;372(71):1–9.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. 2015. p. 1–9.
Ouzzani M. Rayyan — a web and mobile app for systematic reviews. Syst Rev. 2017;5:1–10.
Munn A, et al. introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–33.
Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Implement. 2021;19:3–10.
Hungund S, Kumar M. Palato-radicular groove and localized periodontitis : a series of case reports. J Contemp Dent Pract. 2010;11(5):1–8.
Mayne JR, Martini IG. The palatal radicular groove. Two case reports. Aust Dent J. 1990;35(3):277–81.
Corbella S, Alberti A, Zotti B, Francetti L. Case report periodontal regenerative treatment of intrabony defects associated with palatal grooves : a report of two cases. Case Rep Dent. 2019;2019:1–7.
Cho YD, Lee JE, Chung Y, Lee WC, Seol YJ, Lee YM. Collaborative management of combined periodontal-endodontic lesions with a palatogingival groove: a case series. J Endod. 2017;43(2):332–7.
Karunakaran JV, Fenn SM, Jayaprakash N, Ragavendran N. Successful surgical management of palatogingival groove using platelet-rich fibrin and guided tissue regeneration: a novel approach. J Pharm Bioall Sci. 2017;9:268–73.
Han B, Liu YY, Liu KN, Gao M, Wang ZH, Wang XY. Is intentional replantation appropriate for treatment of extensive endodontic-periodontal lesions related to palatogingival groove? Chin J Dent Res. 2020;23(3):205–14.
Hans M. Management of lateral incisor with palatal radicular groove. Indian J Dent Res. 2010;21(2):306–8.
Mathews D. Interdisciplinary management of a maxillary central incisor with a palato-radicular groove: a case report with 27 years follow-up. J Esthet Restor Dent. 2021;33:1077–83.
Kishan KV. Management of palato radicular groove in a. J Nat Sci. 2014;5(1):178–81.
CAS Google Scholar
Friedman S. The radicular palatal groove - a therapeutic modality. Endod Dent Traumatol. 1988;4:282–6.
Sooratgar A, Tabrizizade M, Nourelahi M, Asadi Y, Sooratgar H. Management of an endodontic-periodontal lesion in a maxillary lateral incisor with palatal radicular groove: a case report. Iran Endod J. 2016;11(2):142–5.
Schäfer E. Malformations in maxillary incisors : case report of radicular palatal groove. Endod Dent Traumatol. 2000;16:132–7.
Zucchelli G, Mele M, Checchi L. The Papilla Amplification Flap for the treatment of a localized periodontal defect associated with a palatal groove. J Periodontol. 2006;77(10):1788–96.
Rankow HJ, Krasner PR. Endodontic applications of guided tissue regeneration in endodontic surgery. J Endod. 1996;22(1):34–43.
Castelo-Baz P, Ramos-Barbosa I, Bel A. Combined endodontic-periodontal treatment of a palatogingival groove. J Endod. 2015;41(11):1918–22.
Hasan A, Ali J. Combined endodontic and surgical management of twin rooted maxillary lateral incisor with a palatogingival groove. Iran Endod J. 2018;13(3):413–9.
Garrido I. Combined endodontic therapy and intentional replantation for the treatment of palatogingival groove. J Endod. 2016;42(2):324–8.
Sucheta A. Treatment of an intrabony osseous lesion associated with a palatoradicular groove. Contemp Clin Dent. 2012;3:260–3.
Ferreira ZA, Pilatti ML. Treatment of a palatal groove-related periodontal bone defect. Quintessence Int. 2000;31(5):342–5.
Andreana S. A combined approach for treatment of developmental groove associated periodontal defect. A Case Report*. J Periodontol. 1998;69:601–7.
Al-hezaimi K, Frcd C, Naghshbandi J. Successful treatment of a radicular groove by intentional replantation and Emdogain therapy : four years follow-up. YMOE. 2009;107(3):e82–5.
Forero-López J, Gamboa-Martínez L, Pico-Porras L, Niño-Barrera JL. Surgical management with intentional replantation on a tooth with palato-radicular groove. Restor Dent Endod. 2015;7658:166–71.
Guruprasad CN, Pradeep AR, Agarwal E. Use of platelet-rich plasma combined with hydroxyapatite in the management of a periodontal endosseous defect associated with a palato-radicular groove : a case report. Clin Adv Periodontics. 2012;2(1):28–33.
Jeng JH, Lu HKJ. Treatment of an osseous lesion associated with a severe palato- radicular groove : a case report. J Periodontol. 1992;63:708–12.
Kerezoudis NP, Siskos GJ, Tsatsas V. Bilateral buccal radicular groove in maxillary incisors: case report. Int Endod J. 2003;36:898–906.
Kozlovsky A. Facial radicular groove in maxillary central incisor: a case report. J Periodontol. 1988;46:615–22.
Ling DH, Shi WP, Wang YH, Lai DP, Zhang YZ. Management of the palato-radicular groove with a periodontal regenerative procedure and prosthodontic treatment: a case report. World J Clin Cases. 2022;10(17):5732–41.
Article PubMed PubMed Central Google Scholar
Narmatha V. The complex radicular groove: interdisciplinary management with mineral trioxide aggregate and bone substitute. J Contemp Dent Pract. 2014;15(6):792–6.
Wei PC, Geivelis M, Chan CP, Ju YR. Successful treatment of pulpal-periodontal combined lesion in a birooted maxillary lateral incisor with concomitant palato-radicular groove. J Periodontol. 1999;70(12):1540–6.
Gandhi A. Endodontic-periodontal management of a maxillary lateral incisor with an associated radicular lingual groove and severe periapical osseous destruction - a case report. J Ir Dent Assoc. 2012;58(2):95–100.
Ramadan DE, Hariyani N, Indrawati R, Ridwan RD, Diyatri I. Cytokines and chemokines in periodontitis. Eur J Dent. 2020;14(3):483–95.
Xu Y, Qiu J, Sun Q, Yan S, Wang W, Yang P, et al. One-year results evaluating the effects of concentrated growth factors on the healing of intrabony defects treated with or without bone substitute in chronic periodontitis. Med Sci Monit. 2019;12(25):4384–9.
Kim HJ, Choi Y, Yu K, Lee W, Min KS. Recognition and management of palatogingival groove for tooth survival: a literature review. Restor Dent Endod. 2017;42(2):77–86.
Pinheiro TN, Consolaro A, Cintra LTA, Azuma MM, Benetti F, Silva CC. Palatogingival groove and root canal instrumentation. Int Endod J. 2020;53:660–70.
Cortellini P. Clinical concepts for regenerative therapy in intrabony defects. Periodontol. 2000;2015(68):282–307.
Bhati A, Fageeh H, Ibraheem W, Fageeh H, Chopra H, Panda S. Role of hyaluronic acid in periodontal therapy (Review). Biomed Rep. 2022;17(5):91.
Article CAS PubMed PubMed Central Google Scholar
Wulandari P, Amalia M, Budi, Simanjuntak R, Satria D. Hyaluronic acid and its role in periodontal healing: Asam Hialuronat dan Peranannya Dalam Penyembuhan Periodontal. Dentika Dental J. 2022;25(1):22–7.
El-Bana AM, El-Shinnawi UM, Attia IM. The effect of hyaluronic acid in combination With β- tri-calcium phosphate in regeneration of periodontal vertical bone defect in periodontitis patients. Mansoura J Dent. 2020;7(27):80–7.
Attam K, Tiwary R, Talwar S, Lamba AK. Palatogingival groove : endodontic-periodontal. J Endod. 2010;36(10):1717–20.
Download references
Acknowledgements
Author information, authors and affiliations.
Service of Odontology, Rothschild Hospital (AP-HP), 5 Rue Santerre, Paris, 75012, France
Yvan Gaudex, Vianney Gandillot, Philippe Bouchard, Stephane Kerner & Maria Clotilde Carra
Department of Periodontology, UFR of Odontology, Université Paris Cité, 5 Rue Garanciere, Paris, 75006, France
Service of Odontology, CH Eure Seine Hospital, Evreux, France
Isabelle Fontanille
Cordeliers Research Centre, Laboratory of Molecular Oral Physiopathology, Paris, France
Stephane Kerner
Department of Periodontology, Loma Linda University School of Dentistry, Loma Linda, CA, USA
INSERM- Sorbonne Paris Cité Epidemiology and Statistics Research Centre, Paris, France
Maria Clotilde Carra
Institution Nationale Des Invalides, Paris, France
Vianney Gandillot
You can also search for this author in PubMed Google Scholar
Contributions
Y.G. and V.G. drafted the manuscript text, and were involved in the literature review, data acquisition, analysis, and interpretation. Y.G. and V.G. prepared Tables 1 and 2 . Y.G., P.B. and I.F. Contributed the case report and Figs. 2 , 3 , 4 and 5 M.C.C and S.K. prepared Table 3 and Fig. 6 . M.C.C., P.B. and S.K revised the draft of the manuscript and contributed to the general criticism. All authors reviewed and approved the manuscript.
Authors’ information
Corresponding author.
Correspondence to Maria Clotilde Carra .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Gaudex, Y., Gandillot, V., Fontanille, I. et al. Palatal groove associated with periodontal lesions: a systematic review illustrated by a decisional tree for management. BMC Oral Health 24 , 1037 (2024). https://doi.org/10.1186/s12903-024-04771-z
Download citation
Received : 20 August 2023
Accepted : 19 August 2024
Published : 04 September 2024
DOI : https://doi.org/10.1186/s12903-024-04771-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Palatal groove
- Palatal radicular groove
- Tooth developmental anomaly
- Periodontal lesion
- Decisional tree
BMC Oral Health
ISSN: 1472-6831
- General enquiries: [email protected]
A systematic literature review of deep learning-based text summarization: : Techniques, input representation, training strategies, mechanisms, datasets, evaluation, and challenges
New citation alert added.
This alert has been successfully added and will be sent to:
You will be notified whenever a record that you have chosen has been cited.
To manage your alert preferences, click on the button below.
New Citation Alert!
Please log in to your account
Information & Contributors
Bibliometrics & citations, view options, recommendations, optimal features set for extractive automatic text summarization.
The goal of text summarization is to reduce the size of the text while preserving its important information and overall meaning. With the availability of internet, data is growing leaps and bounds and it is practically impossible summarizing all this ...
RankSum—An unsupervised extractive text summarization based on rank fusion
In this paper, we propose Ranksum, an approach for extractive text summarization of single documents based on the rank fusion of four multi-dimensional sentence features extracted for each sentence: topic information, semantic content, ...
Display Omitted
- A unified summarization framework with multi-dimensional sentence features.
An Experimental Investigation on Unsupervised Text Summarization for Customer Reviews
People generally turn to websites such as Yelp to find reviews for the food/restaurant before trying it first-hand. However, some reviews are so long and ambiguous that users get confused about whether the review appreciates or disparages the ...
Information
Published in.
Pergamon Press, Inc.
United States
Publication History
Author tags.
- Deep learning
- Text summarization
- Abstractive
- Review-article
Contributors
Other metrics, bibliometrics, article metrics.
- 0 Total Citations
- 0 Total Downloads
- Downloads (Last 12 months) 0
- Downloads (Last 6 weeks) 0
View options
Login options.
Check if you have access through your login credentials or your institution to get full access on this article.
Full Access
Share this publication link.
Copying failed.
Share on social media
Affiliations, export citations.
- Please download or close your previous search result export first before starting a new bulk export. Preview is not available. By clicking download, a status dialog will open to start the export process. The process may take a few minutes but once it finishes a file will be downloadable from your browser. You may continue to browse the DL while the export process is in progress. Download
- Download citation
- Copy citation
We are preparing your search results for download ...
We will inform you here when the file is ready.
Your file of search results citations is now ready.
Your search export query has expired. Please try again.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Blockchain forensics: a systematic literature review of techniques, applications, challenges, and future directions.

1. Introduction
- Investigating and reviewing recent and state-of-the-art studies on blockchain forensics by highlighting the merits and limitations of each study.
- Identifying diverse digital forensic investigation frameworks and methodologies used in blockchain forensics.
- Determining common applications of blockchain-based digital forensic investigation frameworks across various domains.
- Identifying legal and regulatory challenges encountered in conducting forensic investigations on blockchain systems.
- Presenting open issues and future research directions of blockchain forensics.
2. An Overview of Blockchain Forensics
2.1. blockchain technology, 2.2. digital forensics.
- Identification: This initial phase involves recognizing and determining the specific types of digital evidence relevant to the investigation. It requires a detailed understanding of the case to identify relevant digital artefacts, which may include files, metadata, logs, or communication records.
- Collection: Once the evidence has been identified, the collection phase focuses on the systematic gathering of digital evidence from the crime scene or relevant sources. This involves securing and documenting the devices or media, such as computers, smartphones, or servers, to ensure that no data are altered or lost during the process. Proper procedures, including using write-blockers and ensuring chain-of-custody documentation, are crucial to maintaining the integrity of the evidence.
- Extraction: During the extraction phase, the digital investigator retrieves the data from the identified devices. This may involve creating forensic images or copies of hard drives, memory cards, or other storage media. The aim is to extract relevant data while preserving the original evidence intact. Extraction often requires specialized tools and techniques to handle encrypted or damaged files and to ensure that all potentially relevant information is obtained.
- Analysis: In the analysis phase, the extracted data are examined in detail to uncover meaningful information. This involves interpreting file structures, recovering deleted files, analysing log entries, and correlating data across different sources. The goal is to identify patterns, connections, and anomalies that can support or disprove the claims made in the investigation. This phase often requires deep technical expertise and may involve reconstructing events or understanding complex data relationships.
- Examination: The examination phase is where the investigator carefully scrutinizes the features of the digital evidence. This involves verifying the authenticity of the data, validating findings through repeated tests, and ensuring that all aspects of the evidence are thoroughly explored. The examination phase aims to provide a detailed and accurate representation of the evidence, ensuring that all relevant details are considered.
- Report: The final phase involves compiling and presenting the findings in a comprehensive report. This report summarizes the investigative process, methodologies employed, and the conclusions drawn from the analysis and examination. It must be clear, detailed, and structured in a way that is understandable to non-technical audiences, including legal professionals and court personnel. The report plays a critical role in legal proceedings, providing evidence that is both admissible and persuasive in court.
2.3. Blockchain Forensics
- Identification: The first step in a blockchain forensic investigation is identifying the relevant data that needs to be examined. This involves determining the specific blockchain platform involved (e.g., Bitcoin and Ethereum), identifying relevant addresses, transactions, and smart contracts, and understanding the nature of the suspected illegal activity. The goal is to identify the exact data on the blockchain that is relevant to the investigation. For instance, studies have shown the importance of identifying specific addresses and transactions linked to criminal activities such as money laundering or ransomware payments.
- Collection: In the collection phase, investigators gather the identified data from the blockchain. This includes downloading the entire blockchain or extracting specific blocks, transactions, or addresses of interest. Given the public nature of most blockchains, these data are typically accessible without a warrant. However, the process must ensure that data are collected in a manner that preserves its integrity and authenticity. Advanced tools and techniques, such as blockchain explorers and forensic software, are often used to facilitate this process.
- Preservation: Preservation involves maintaining the integrity of the collected data to ensure they remain unchanged and reliable throughout the investigation. This includes creating cryptographic hashes of the data and securely storing them in a manner that prevents tampering. Blockchain’s inherent immutability aids in this process, but proper handling and documentation are still essential to uphold evidentiary standards in legal contexts.
- Analysis: The analysis phase is where investigators explore the collected data to uncover meaningful patterns, relationships, and anomalies. This may involve tracking the flow of cryptocurrencies, analysing transaction histories, and identifying links between blockchain addresses and real-world identities. Sophisticated analytical tools and techniques, such as clustering algorithms and graph analysis, are employed to make sense of the complex and often pseudonymous data on the blockchain.
- Examination: During this phase, investigators contextualize their findings within the broader scope of the investigation. This includes correlating blockchain data with external sources of information, such as IP logs, email records, or traditional financial records. The goal is to build a coherent narrative that explains how the blockchain data fit into the overall case and supports the allegations being investigated.
- Report: The final phase involves compiling the analysis and interpretation into a comprehensive report that can be presented in legal or regulatory settings. This report must clearly explain the methods used, the findings, and their significance, making it understandable for non-technical stakeholders such as lawyers, judges, and juries. Proper documentation and expert testimony are often required to validate the findings and ensure their admissibility in court.
3. Research Methodology
3.1. research questions.
- RQ1: What are the state-of-the-art studies related to blockchain forensics and blockchain-based solutions for digital forensics?
- RQ2: How can blockchain technology enhance digital forensic investigations?
- RQ3: What are the digital forensic frameworks and methodologies used in blockchain forensics?
- RQ4: What are the common applications of blockchain-based digital forensic investigation frameworks?
- RQ5: What are the legal and regulatory challenges in conducting a forensic investigation on blockchain systems?
3.2. Inclusion and Exclusion Criteria
- Peer-reviewed journals and conference articles to ensure high-quality and credible sources;
- Relevant to the specific research questions;
- Topic mainly on blockchain forensics and blockchain-based forensic solutions;
- Full and available articles to allow for a comprehensive review of the content;
- English-language articles to maintain consistency in analysis.
- Articles concerning all other security aspects of blockchain apart from digital forensic investigations;
- Articles not focused on blockchain forensics or significantly deviating from the primary research questions;
- Unpublished articles, non-peer-reviewed articles, and editorial articles to ensure credibility;
- Articles that are not fully available;
- Non-English articles to avoid translation issues and maintain analysis consistency;
- Duplicates of already included articles to avoid redundancy.
3.3. Data Sources
- IEEE Xplore;
- Elsevier ScienceDirect;
- Google Scholar;
- ACM Digital Library;
- SpringerLink.
- Blockchain forensic investigation;
- Blockchain forensics;
- Digital forensics in blockchain;
- Cryptocurrency forensics;
- Forensic techniques in blockchain;
- Investigating blockchain transactions;
- Blockchain tracing;
- Blockchain evidence collection;
- Forensic challenges in blockchain;
- Legal aspects of blockchain forensics;
- Blockchain forensic tools;
- Cryptocurrency crime investigation;
- Blockchain fraud detection.
3.4. Selection of Relevant Articles
- Phase 1: Publications found during the search and those already in the collection were sorted using the inclusion and exclusion criteria. The scope of the search was narrowed to include only articles published recently and consider the topic of blockchain forensics.
- Phase 2: The titles and abstracts of the articles collected from several digital libraries were reviewed to determine how well they addressed the topic and the questions posed in this research work.
- Phase 3: During this stage, we focused on eliminating duplicates among the six digital libraries used for our publication collection.
4. Analysis of Results
5. results and discussion.
- IoT Forensics
6. Open Issues and Future Directions
6.1. lack of standardization, 6.2. regulatory and legal issues, 6.3. scalability challenges, 6.4. applicability of blockchain forensic frameworks, 6.5. integration of ai and ml in blockchain forensics, 6.6. education and training, 7. conclusions, author contributions, data availability statement, conflicts of interest.
- Cebe, M.; Erdin, E.; Akkaya, K.; Aksu, H.; Uluagac, S. Block4Forensic: An Integrated Lightweight Blockchain Framework for Forensics Applications of Connected Vehicles. IEEE Commun. Mag. 2018 , 56 , 50–57. [ Google Scholar ] [ CrossRef ]
- Akanfe, O.; Lawong, D.; Rao, H.R. Blockchain technology and privacy regulation: Reviewing frictions and synthesizing opportunities. Int. J. Inf. Manag. 2024 , 76 , 102753. [ Google Scholar ] [ CrossRef ]
- Conti, M.; Kumar, G.; Lal, C.; Saha, R. Blockchain-Based Distributed and Secure Digital Forensic Investigation Systems. In Blockchains: A Handbook on Fundamentals, Platforms and Applications ; Ruj, S., Kanhere, S.S., Conti, M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 337–362. ISBN 978-3-031-32146-7. [ Google Scholar ]
- Atlam, H.F.; Wills, G.B. Technical aspects of blockchain and IoT. In Advances in Computers ; Kim, S., Deka, G.C., Zhang, P., Eds.; Role of Blockchain Technology in IoT Applications; Elsevier: Amsterdam, The Netherlands, 2019; Volume 115, pp. 1–39. [ Google Scholar ]
- Mercan, S.; Cebe, M.; Aygun, R.S.; Akkaya, K.; Toussaint, E.; Danko, D. Blockchain-based video forensics and integrity verification framework for wireless Internet-of-Things devices. Secur. Priv. 2021 , 4 , e143. [ Google Scholar ] [ CrossRef ]
- Xiao, N.; Wang, Z.; Sun, X.; Miao, J. A novel blockchain-based digital forensics framework for preserving evidence and enabling investigation in industrial Internet of Things. Alex. Eng. J. 2024 , 86 , 631–643. [ Google Scholar ] [ CrossRef ]
- Li, S.; Qin, T.; Min, G. Blockchain-Based Digital Forensics Investigation Framework in the Internet of Things and Social Systems. IEEE Trans. Comput. Soc. Syst. 2019 , 6 , 1433–1441. [ Google Scholar ] [ CrossRef ]
- Dasaklis, T.K.; Casino, F.; Patsakis, C. SoK: Blockchain Solutions for Forensics. arXiv 2020 , arXiv:2005.12640. [ Google Scholar ]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021 , 372 , n71. [ Google Scholar ] [ CrossRef ]
- Jena, S.K.; Barik, R.C.; Priyadarshini, R. A systematic state-of-art review on digital identity challenges with solutions using conjugation of IOT and blockchain in healthcare. Internet Things 2024 , 25 , 101111. [ Google Scholar ] [ CrossRef ]
- Atlam, H.F.; Azad, M.A.; Alzahrani, A.G.; Wills, G. A Review of Blockchain in Internet of Things and AI. Big Data Cogn. Comput. 2020 , 4 , 28. [ Google Scholar ] [ CrossRef ]
- Atlam, H.F.; Alenezi, A.; Alassafi, M.O.; Wills, G.B. Blockchain with Internet of Things: Benefits, Challenges, and Future Directions. Int. J. Intell. Syst. Appl. 2018 , 10 , 40. [ Google Scholar ] [ CrossRef ]
- Atlam, H.F.; Wills, G.B. Intersections between IoT and distributed ledger. In Advances in Computers ; Kim, S., Deka, G.C., Zhang, P., Eds.; Role of Blockchain Technology in IoT Applications; Elsevier: Amsterdam, The Netherlands, 2019; Volume 115, pp. 73–113. [ Google Scholar ]
- Indrason, N.; Saha, G. Exploring Blockchain-driven security in SDN-based IoT networks. J. Netw. Comput. Appl. 2024 , 224 , 103838. [ Google Scholar ] [ CrossRef ]
- Choi, W.; Woo, J.; Hong, J.W. Fractional non-fungible tokens: Overview, evaluation, marketplaces, and challenges. Int. J. Netw. Manag. 2024 , 34 , e2260. [ Google Scholar ] [ CrossRef ]
- Garfinkel, S.L. Digital forensics research: The next 10 years. Digit. Investig. 2010 , 7 , S64–S73. [ Google Scholar ] [ CrossRef ]
- Atlam, H.F.; Alenezi, A.; Alassafi, M.O.; Alshdadi, A.A.; Wills, G.B. Security, Cybercrime and Digital Forensics for IoT. In Principles of Internet of Things (IoT) Ecosystem: Insight Paradigm ; Peng, S.-L., Pal, S., Huang, L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 551–577. ISBN 978-3-030-33596-0. [ Google Scholar ]
- Atlam, H.F.; El-Din Hemdan, E.; Alenezi, A.; Alassafi, M.O.; Wills, G.B. Internet of Things Forensics: A Review. Internet Things 2020 , 11 , 100220. [ Google Scholar ] [ CrossRef ]
- Kumar, G.; Saha, R.; Lal, C.; Conti, M. Internet-of-Forensic (IoF): A blockchain based digital forensics framework for IoT applications. Future Gener. Comput. Syst. 2021 , 120 , 13–25. [ Google Scholar ] [ CrossRef ]
- Casino, F.; Dasaklis, T.K.; Patsakis, C. A systematic literature review of blockchain-based applications: Current status, classification and open issues. Telemat. Inform. 2019 , 36 , 55–81. [ Google Scholar ] [ CrossRef ]
- Aswal, P. Blockchain Nodes-Blockchain Council. Available online: https://www.blockchain-council.org/blockchain/blockchain-nodes/ (accessed on 26 August 2024).
- Haque, E.U.; Shah, A.; Iqbal, J.; Ullah, S.S.; Alroobaea, R.; Hussain, S. A scalable blockchain based framework for efficient IoT data management using lightweight consensus. Sci. Rep. 2024 , 14 , 7841. [ Google Scholar ] [ CrossRef ]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009 , 339 , b2700. [ Google Scholar ] [ CrossRef ]
- Akinbi, A.; MacDermott, Á.; Ismael, A.M. A systematic literature review of blockchain-based Internet of Things (IoT) forensic investigation process models. Forensic Sci. Int. Digit. Investig. 2022 , 42–43 , 301470. [ Google Scholar ] [ CrossRef ]
- Atlam, H.F.; Oluwatimilehin, O. Business Email Compromise Phishing Detection Based on Machine Learning: A Systematic Literature Review. Electronics 2023 , 12 , 42. [ Google Scholar ] [ CrossRef ]
- Atlam, H.F.; Azad, M.A.; Alassafi, M.O.; Alshdadi, A.A.; Alenezi, A. Risk-Based Access Control Model: A Systematic Literature Review. Future Internet 2020 , 12 , 103. [ Google Scholar ] [ CrossRef ]
- Förstl, N.; Adler, I.; Süß, F.; Dendorfer, S. Technologies for Evaluation of Pelvic Floor Functionality: A Systematic Review. Sensors 2024 , 24 , 4001. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ahmad, L.; Khanji, S.; Iqbal, F.; Kamoun, F. Blockchain-based chain of custody: Towards real-time tamper-proof evidence management. In Proceedings of the 15th International Conference on Availability, Reliability and Security, Virtual Event, Ireland, 25–28 August 2020; pp. 1–8. [ Google Scholar ]
- Siaam, I.B.S.; Mahmud, N.; Titas, A.R. Securing Digital Evidence with Blockchain ; Islamic University of Technology: Gazipur, Bangladesh, 2022. [ Google Scholar ]
- Billard, D. Weighted Forensics Evidence Using Blockchain. In Proceedings of the 2018 International Conference on Computing and Data Engineering, Shanghai, China, 4–6 May 2018; pp. 57–61. [ Google Scholar ] [ CrossRef ]
- Chopade, M.; Khan, S.; Shaikh, U.; Pawar, R. Digital Forensics: Maintaining Chain of Custody Using Blockchain. In Proceedings of the 2019 Third International conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC), Palladam, India, 12–14 December 2019; pp. 744–747. [ Google Scholar ]
- Florid, M.I.; Mutaqin, H.; Purnamasari, P. Analyze the Application of Blockchain Technology in Digital Forensics and Hunt for Threats Lurking in Security. Asian J. Manag. Entrep. Soc. Sci. 2024 , 4 , 1407-1017. [ Google Scholar ]
- Fröwis, M.; Gottschalk, T.; Haslhofer, B.; Rückert, C.; Pesch, P. Safeguarding the evidential value of forensic cryptocurrency investigations. Forensic Sci. Int. Digit. Investig. 2020 , 33 , 200902. [ Google Scholar ] [ CrossRef ]
- Hsu, C.-L.; Chen, W.-X.; Le, T.-V. An Autonomous Log Storage Management Protocol with Blockchain Mechanism and Access Control for the Internet of Things. Sensors 2020 , 20 , 6471. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jin, P.; Kim, N.; Lee, S.; Jeong, D. Forensic investigation of the dark web on the Tor network: Pathway toward the surface web. Int. J. Inf. Secur. 2024 , 23 , 331–346. [ Google Scholar ] [ CrossRef ]
- Khan, A.A.; Uddin, M.; Shaikh, A.A.; Laghari, A.A.; Rajput, A.E. MF-Ledger: Blockchain Hyperledger Sawtooth-Enabled Novel and Secure Multimedia Chain of Custody Forensic Investigation Architecture. IEEE Access 2021 , 9 , 103637–103650. [ Google Scholar ] [ CrossRef ]
- Khanji, S.; Alfandi, O.; Ahmad, L.; Kakkengal, L.; Al-kfairy, M. A systematic analysis on the readiness of Blockchain integration in IoT forensics. Forensic Sci. Int. Digit. Investig. 2022 , 42 , 301472. [ Google Scholar ] [ CrossRef ]
- Li, M.; Lal, C.; Conti, M.; Hu, D. LEChain: A blockchain-based lawful evidence management scheme for digital forensics. Future Gener. Comput. Syst. 2021 , 115 , 406–420. [ Google Scholar ] [ CrossRef ]
- Mahrous, W.A.; Farouk, M.; Darwish, S.M. An Enhanced Blockchain-Based IoT Digital Forensics Architecture Using Fuzzy Hash. IEEE Access 2021 , 9 , 151327–151336. [ Google Scholar ] [ CrossRef ]
- Muyambo, E.; Baror, S. Systematic Review to Propose a Blockchain-based Digital Forensic Ready Internet Voting System. Int. Conf. Cyber Warf. Secur. 2024 , 19 , 219–230. [ Google Scholar ] [ CrossRef ]
- Patil, H.; Kohli, R.K.; Puri, S.; Puri, P. Potential applicability of blockchain technology in the maintenance of chain of custody in forensic casework. Egypt. J. Forensic Sci. 2024 , 14 , 12. [ Google Scholar ] [ CrossRef ]
- Ryu, J.H.; Sharma, P.K.; Jo, J.H.; Park, J.H. A blockchain-based decentralized efficient investigation framework for IoT digital forensics. J. Supercomput. 2019 , 75 , 4372–4387. [ Google Scholar ] [ CrossRef ]
- Sheelvant, Y. An Implementation of Blockchain Technology in Forensic Evidence Management system. Int. Res. J. Mod. Eng. Technol. Sci. (IRJMETS) 2023 , 5 , 194–198. [ Google Scholar ]
- Zarpala, L.; Casino, F. A blockchain-based Forensic Model for Financial Crime Investigation: The Embezzlement Scenario. Digit. Finance 2021 , 3 , 301–332. [ Google Scholar ] [ CrossRef ]
- Sakshi; Malik, A.; Sharma, A.K. A survey on blockchain based IoT forensic evidence preservation: Research trends and current challenges. Multimed. Tools Appl. 2023 , 83 , 42413–42458. [ Google Scholar ] [ CrossRef ]
- Alqahtany, S.S.; Syed, T.A. ForensicTransMonitor: A Comprehensive Blockchain Approach to Reinvent Digital Forensics and Evidence Management. Information 2024 , 15 , 109. [ Google Scholar ] [ CrossRef ]
- Onyeashie, B.I.; Leimich, P.; McKeown, S.; Russell, G. A Bibliometric Analysis and Systematic Review of a Blockchain-Based Chain of Custody for Digital Evidence. In Big Data Technologies and Applications ; Tan, Z., Wu, Y., Xu, M., Eds.; Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Springer Nature: Cham, Switzerland, 2024; Volume 555, pp. 112–131. ISBN 978-3-031-52264-2. [ Google Scholar ]
- Goyal, R. Blockchain Technology in Forensic Science. A Bibliometric Review. In Proceedings of the 2021 3rd International Conference on Advances in Computing, Communication Control and Networking (ICAC3N), Greater Noida, India, 17–18 December 2021. [ Google Scholar ]
- Jacob, J.; Kumar, S. A Framework for Digital Forensics Using Blockchain to Secure Digital Data. In Proceedings of the 2022 IEEE World Conference on Applied Intelligence and Computing (AIC), Sonbhadra, India, 17–19 June 2022; pp. 899–904. [ Google Scholar ]
- Akbarfam, A.J.; Heidaripour, M.; Maleki, H.; Dorai, G.; Agrawal, G. ForensiBlock: A Provenance-Driven Blockchain Framework for Data Forensics and Auditability. arXiv 2023 , arXiv:2308.03927. [ Google Scholar ]
- Mas’ud, M.Z.; Hassan, A.; Shah, W.M.; Abdul-Latip, S.F.; Ahmad, R.; Ariffin, A.; Yunos, Z. A Review of Digital Forensics Framework for Blockchain in Cryptocurrency Technology. In Proceedings of the 2021 3rd International Cyber Resilience Conference (CRC), Langkawi Island, Malaysia, 29–31 January 2021; pp. 1–6. [ Google Scholar ]
- Almutairi, W.; Moulahi, T. Joining Federated Learning to Blockchain for Digital Forensics in IoT. Computers 2023 , 12 , 157. [ Google Scholar ] [ CrossRef ]
- Cong, L.; Grauer, K.; Rabetti, D.; Updegrave, H. Blockchain Forensics and Crypto-Related Cybercrimes. SSRN J. 2023 , 1–115. [ Google Scholar ] [ CrossRef ]
- Alqahtany, S.S.; Syed, T.A. Integrating Blockchain and Deep Learning for Enhanced Mobile VPN Forensics: A Comprehensive Framework. Appl. Sci. 2024 , 14 , 4421. [ Google Scholar ] [ CrossRef ]
- Srivasthav, D.P.; Maddali, L.P.; Vigneswaran, R. Study of Blockchain Forensics and Analytics tools. In Proceedings of the 2021 3rd Conference on Blockchain Research & Applications for Innovative Networks and Services (BRAINS), Paris, France, 27–30 September 2021; pp. 39–40. [ Google Scholar ]
- Khan, A.A.; Shaikh, A.A.; Laghari, A.A. IoT with Multimedia Investigation: A Secure Process of Digital Forensics Chain-of-Custody using Blockchain Hyperledger Sawtooth. Arab. J. Sci. Eng. 2023 , 48 , 10173–10188. [ Google Scholar ] [ CrossRef ]
- Al-Khateeb, H.; Epiphaniou, G.; Daly, H. Blockchain for Modern Digital Forensics: The Chain-of-Custody as a Distributed Ledger. In Blockchain and Clinical Trial ; Jahankhani, H., Kendzierskyj, S., Jamal, A., Epiphaniou, G., Al-Khateeb, H., Eds.; Advanced Sciences and Technologies for Security Applications; Springer International Publishing: Cham, Switzerland, 2019; pp. 149–168. ISBN 978-3-030-11288-2. [ Google Scholar ]
- Ragu, G.; Ramamoorthy, S. A blockchain-based cloud forensics architecture for privacy leakage prediction with cloud. Healthc. Anal. 2023 , 4 , 100220. [ Google Scholar ] [ CrossRef ]
- Brotsis, S.; Grammatikakis, K.P.; Kavallieros, D.; Mazilu, A.I.; Kolokotronis, N.; Limniotis, K.; Vassilakis, C. Blockchain meets Internet of Things (IoT) forensics: A unified framework for IoT ecosystems. Internet Things 2023 , 24 , 100968. [ Google Scholar ] [ CrossRef ]
- Bonomi, S.; Casini, M.; Ciccotelli, C. B-CoC: A Blockchain-Based Chain of Custody for Evidences Management in Digital Forensics. Open Access Ser. Inform. (OASIcs) 2020 , 71 , 12:1–12:15. [ Google Scholar ] [ CrossRef ]
- Tian, Z.; Li, M.; Qiu, M.; Sun, Y.; Su, S. Block-DEF: A secure digital evidence framework using blockchain. Inf. Sci. 2019 , 491 , 151–165. [ Google Scholar ] [ CrossRef ]
- Lusetti, M.; Salsi, L.; Dallatana, A. A blockchain based solution for the custody of digital files in forensic medicine. Forensic Sci. Int. Digit. Investig. 2020 , 35 , 301017. [ Google Scholar ] [ CrossRef ]
- Verma, A.; Bhattacharya, P.; Saraswat, D.; Tanwar, S. NyaYa: Blockchain-based electronic law record management scheme for judicial investigations. J. Inf. Secur. Appl. 2021 , 63 , 103025. [ Google Scholar ] [ CrossRef ]
- Chen, S.; Zhao, C.; Huang, L.; Yuan, J.; Liu, M. Study and implementation on the application of blockchain in electronic evidence generation. Forensic Sci. Int. Digit. Investig. 2020 , 35 , 301001. [ Google Scholar ] [ CrossRef ]
- Awuson-David, K.; Al-Hadhrami, T.; Alazab, M.; Shah, N.; Shalaginov, A. BCFL logging: An approach to acquire and preserve admissible digital forensics evidence in cloud ecosystem. Future Gener. Comput. Syst. 2021 , 122 , 1–13. [ Google Scholar ] [ CrossRef ]
- Olukoya, O. Distilling blockchain requirements for digital investigation platforms. J. Inf. Secur. Appl. 2021 , 62 , 102969. [ Google Scholar ] [ CrossRef ]
- Burri, X.; Casey, E.; Bollé, T.; Jaquet-Chiffelle, D.-O. Chronological independently verifiable electronic chain of custody ledger using blockchain technology. Forensic Sci. Int. Digit. Investig. 2020 , 33 , 300976. [ Google Scholar ] [ CrossRef ]
- Naqvi, S. Challenges of Cryptocurrencies Forensics: A Case Study of Investigating, Evidencing and Prosecuting Organised Cybercriminals. In Proceedings of the 13th International Conference on Availability, Reliability and Security, Association for Computing Machinery, New York, NY, USA, 27–30 August 2018; pp. 1–5. [ Google Scholar ]
- Rana, S.K.; Rana, A.K.; Rana, S.K.; Sharma, V.; Lilhore, U.K.; Khalaf, O.I.; Galletta, A. Decentralized Model to Protect Digital Evidence via Smart Contracts Using Layer 2 Polygon Blockchain. IEEE Access 2023 , 11 , 83289–83300. [ Google Scholar ] [ CrossRef ]
- Agarwal, U.; Rishiwal, V.; Tanwar, S.; Yadav, M. Blockchain and crypto forensics: Investigating crypto frauds. Int. J. Netw. Manag. 2024 , 34 , e2255. [ Google Scholar ] [ CrossRef ]
- Ellul, J.; Galea, J.; Ganado, M.; Mccarthy, S.; Pace, G.J. Regulating Blockchain, DLT and Smart Contracts: A technology regulator’s perspective. ERA Forum 2020 , 21 , 209–220. [ Google Scholar ] [ CrossRef ]
- Batista, D.; Mangeth, A.L.; Frajhof, I.; Alves, P.H.; Nasser, R.; Robichez, G.; Silva, G.M.; Miranda, F.P. de Exploring Blockchain Technology for Chain of Custody Control in Physical Evidence: A Systematic Literature Review. J. Risk Financ. Manag. 2023 , 16 , 360. [ Google Scholar ] [ CrossRef ]
- Rožman, N.; Corn, M.; Škulj, G.; Berlec, T.; Diaci, J.; Podržaj, P. Exploring the Effects of Blockchain Scalability Limitations on Performance and User Behavior in Blockchain-Based Shared Manufacturing Systems: An Experimental Approach. Appl. Sci. 2023 , 13 , 4251. [ Google Scholar ] [ CrossRef ]
- Zbrog, M. Digital Forensics in Blockchain: How Investigators Track Crypto. Forensics Colleges. Available online: https://www.forensicscolleges.com/blog/blockchain-forensics (accessed on 20 July 2024).
- Atlam, H.F.; Azad, M.A.; Altamimi, M.; Fadhel, N. Role of Blockchain and AI in Security and Privacy of 6G. In AI and Blockchain Technology in 6G Wireless Network ; Dutta Borah, M., Singh, P., Deka, G.C., Eds.; Springer Nature: Singapore, 2022; pp. 93–115. ISBN 978-981-19286-8-0. [ Google Scholar ]
- Mani, N.; Parab, S.S.; Manaswini, S.; Philip, S.; Hari, P.B.; Singh, N. Forensic Block Chain and it’s linkage with Artificial Intelligence: A new Approach. In Proceedings of the 2021 2nd International Conference on Computation, Automation and Knowledge Management (ICCAKM), Dubai, United Arab Emirates, 19–21 January 2021; pp. 70–74. [ Google Scholar ]
- BIG Investigations. The New Era Must-Have Blockchain Investigator Training. Blockchain Intelligence Group. 2024. Available online: https://blockchaingroup.io/the-new-era-must-have-blockchain-investigator-training/ (accessed on 20 July 2024).
Click here to enlarge figure
| Data Source | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
| Google Scholar | 421 | 27 | 18 |
| IEEE explore | 153 | 12 | 8 |
| PubMed | 6 | 2 | 1 |
| Elsevier ScienceDirect | 27 | 13 | 9 |
| ACM Digital Library | 31 | 9 | 5 |
| SpringerLink | 34 | 8 | 5 |
| Total | 672 | 71 | 46 |
| Citation | Summary of Contribution | Limitations |
|---|---|---|
| Ahmad et al. [ ] | This paper proposes a blockchain-based chain of custody framework to ensure tamper-proof evidence management. The framework uses a private Ethereum blockchain to securely record evidence metadata while storing physical evidence in a reliable medium locked with smart locks. This approach aims to provide authenticated access, maintaining evidence integrity and admissibility among multiple stakeholders. | The scalability issues inherent in private blockchains and the need for smooth integration with existing digital evidence systems. Also, it does not discuss or address the challenges of managing large volumes of evidence and their associated costs. |
| Akinbi et al. [ ] | This paper presents a comprehensive review of blockchain-based IoT forensic investigation models. It systematically reviews how blockchain is used to securely improve forensic investigations and discusses the efficiency of these models. This paper highlights the challenges, open issues, and future research directions of blockchain in IoT forensic investigations. | This paper does not provide a detailed analysis of the different techniques and methodologies used, and it does not discuss the legal and ethical implications of using blockchain in IoT forensic investigations. |
| Siaam et al. [ ] | This paper proposes Probe-IoT, a forensic investigation framework for IoT systems that uses a public digital ledger to identify facts in IoT crime cases. It addresses the challenges of evidence spoliation and lack of transparency in IoT environments by recording interactions between IoT devices, users, and cloud services on a blockchain. The framework allows investigators to trace the flow of data and identify potential perpetrators. | This paper lacks a detailed implementation and evaluation of the proposed framework. In addition, the dependence on a public blockchain could raise privacy and legal concerns for users, as their interactions and communications are publicly accessible. |
| Billard [ ] | This paper proposes a framework for building a fact-based confidence rating of digital evidence. It uses a blockchain-based Digital Evidence Inventory (DEI) to ensure immutability and traceability, categorizes digital evidence into data types with associated confidence ratings, and creates a Global Digital Timeline (GDT) to order evidence through time. | The confidence rating system needs to be refined by incorporating error rate probabilities and relevance measures. This paper also relies on expert’s judgment for data categorization and rating, which adds subjectivity to the process. |
| Cebe et al. [ ] | This paper proposes a blockchain-based framework called Block4Forensic (B4F) for vehicular forensics. B4F provides a secure, trustworthy, and comprehensive platform for collecting and analysing vehicle data. It integrates vehicular public key infrastructure for membership establishment and privacy and utilizes a fragmented ledger to store detailed vehicle information. B4F enables trustless, traceable, and privacy-aware post-accident analysis, facilitating dispute resolution and identifying faulty parties. | This paper lacks implementation details and performance evaluation. This paper also does not address the practical challenges of integrating B4F with existing vehicular systems. Also, this paper does not discuss potential security vulnerabilities of the proposed framework. |
| Chopade et al. [ ] | This paper proposes a blockchain-based model for maintaining the chain of custody in digital forensics. The model utilizes a distributed ledger to record and track the transfer of digital evidence between various participants in an investigation to ensure its integrity and authenticity. The model employs Base64 encryption to generate a hash of the evidence, which is then transferred instead of the original data, preventing tampering and providing a verifiable record of ownership. | This paper lacks implementation and evaluation of the proposed model. It also does not discuss integrating the model with existing digital forensic frameworks and does not consider potential security vulnerabilities of using Base64 for evidence hashing, which could be vulnerable to certain attacks. |
| Dasaklis et al. [ ] | This paper provides a comprehensive overview and classification of blockchain-based digital forensic tools to analyse their main features, benefits, and challenges. It examines the potential of blockchain to enhance digital forensics by addressing issues including evidence immutability, transparency, and auditability. | This paper does not discuss the legal, regulatory, and ethical implications of using blockchain in digital forensic investigations, which are crucial considerations for real-world applications. |
| Floride et al. [ ] | This paper explores the application of blockchain in digital forensics, particularly focusing on its use in threat hunting and evidence management. It highlights the benefits of blockchain in ensuring evidence integrity, traceability, and immutability. This paper also examines the use of deep learning models for detecting vulnerabilities in smart contracts on the Ethereum blockchain. | This paper does not consider the practical challenges of implementing blockchain-based digital forensic systems in real-world applications. This paper also lacks empirical research to validate the effectiveness of the framework. |
| Frowis et al. [ ] | This paper investigates the legal and technical aspects of forensic cryptocurrency investigations. It identifies key legal requirements for safeguarding the evidential value of such investigations, including lawfulness, authenticity, reliability, qualification, verifiability, chain of evidence, and the right to inspect records. This paper then translates these requirements into a data-sharing framework for law enforcement agencies to promote efficient and effective investigations while protecting individuals’ privacy. | This paper lacks an in-depth analysis of blockchain forensic tools and techniques and does not provide a complete evaluation of the effectiveness of the proposed technique. This paper also does not discuss the complex legal implications of processing publicly available data for law enforcement purposes. |
| Hsu et al. [ ] | This paper proposes an autonomous log storage management protocol for IoT environments that incorporates blockchain mechanisms and access control. Integrating blockchain and a novel “signature chain” concept provides robust identity verification, data integrity, non-repudiation, tamper resistance, and evidence legality, making it suitable for digital forensic investigations. | The performance of the proposed protocol in large-scale IoT deployments with high data volumes needs to be discussed. This paper does not discuss potential scalability issues associated with blockchain, particularly in terms of transaction throughput and latency. |
| Jin et al. [ ] | This paper proposes a methodology for tracing operators of illegal dark websites through cryptocurrency transactions. It highlights the importance of tracking the flow of funds on the blockchain to link Bitcoin addresses to real-world bank accounts and use it in digital forensic investigations. This paper provides valuable insights into identifying perpetrators by analysing cryptocurrency transactions, despite the anonymity provided by cryptocurrencies. | This paper focuses only on publicly available information, neglecting the complexities of cryptocurrency and dynamic Bitcoin addresses. Also, this paper relies on POW consensus, which introduces latency and energy inefficiency, impacting real-time forensic analysis. |
| Khan et al. [ ] | This paper proposes MF-Ledger, a blockchain-based architecture for multimedia digital forensic investigations using Hyperledger Sawtooth. MF-Ledger provides secure evidence integrity, preservation, transparency, and resistance to tampering by leveraging a permissioned blockchain network. It addresses the challenges of traditional digital forensics by offering a secure and transparent process for collecting, storing, analysing, and interpreting digital evidence. The architecture utilizes smart contracts to manage the chain of custody events and ensures privacy protection for evidence stored in an encrypted ledger. | This paper does not consider the challenges of implementing the proposed method in the real world. The proposed architecture is only simulated using sequence diagrams; however, it lacks validation and evaluation in a real-world forensic environment. Furthermore, this paper does not address the legal and regulatory challenges associated with using blockchain in forensic investigations. |
| Khanji et al. [ ] | This paper presents a systematic review of the readiness of blockchain integration in IoT forensics. It analyses the literature to review the deployment of Blockchain to resolve various challenges presented in IoT forensics. | This paper does not provide a detailed analysis of the efficiency of the different models and frameworks reviewed in the literature. |
| Li et al. [ ] | This paper proposes LEChain, a blockchain-based lawful evidence management scheme for digital forensics that addresses the entire lifecycle of evidence, from collection to court trial and sentencing. LEChain utilizes short randomizable signatures for anonymous witness authentication, fine-grained access control based on CP-ABE for evidence access, and secure voting to protect juror privacy. The system is built on a consortium blockchain to ensure transparency, immutability, and auditability of evidence transactions. | The proposed method was implemented on a consortium blockchain, which may not be suitable for all digital forensic scenarios. In addition, the evaluation of the proposed technique is based on a local Ethereum test network, which may not accurately reflect the performance of the system in a real-world setting. |
| Li et al. [ ] | This paper proposes a blockchain-based digital forensic framework for the IoT, called IoT Forensic-Chain (IoTFC). IoTFC records all examination operations, including evidence identification, preservation, analysis, and presentation, in a chain of blocks. | This paper does not discuss potential privacy concerns associated with storing sensitive evidence on a public blockchain. |
| Mahrous et al. [ ] | This paper proposes a blockchain-based IoT digital forensic architecture that incorporates fuzzy hashing into the blockchain’s Merkle tree. This approach enhances the ability to identify potentially incriminating evidence that may have undergone benign or malicious alterations, which traditional hashing methods struggle to detect. By comparing blocks/files to all nodes in the blockchain network using fuzzy hash similarity, digital forensic investigators can verify their authenticity. | This paper does not discuss the challenges of integrating fuzzy hashing into existing blockchain platforms or discuss the potential performance overhead associated with fuzzy hash computations. Also, this paper lacks a detailed analysis of the security implications of using fuzzy hashing in a blockchain context. |
| Muyambo et al. [ ] | This paper presents a systematic review of blockchain-based digital forensics in Internet voting systems. This paper also proposes a blockchain-based digital forensic-ready internet voting system called DFRMIV, which addresses issues of transparency, privacy, integrity, confidentiality, and auditability in online voting systems. | This paper does not discuss detailed information and technical details on how the proposed DFRMIV system would work in practice and how it would address challenges related to blockchain forensics. |
| Patil et al. [ ] | This paper explores the potential of blockchain to improve the chain of custody in forensic investigations. It highlights how blockchain’s decentralized, immutable, and transparent nature can address challenges like evidence tampering, excessive paperwork, and difficulty in tracking evidence interactions. The authors also propose a framework where evidence details are recorded on a blockchain, creating a tamper-proof and auditable record. | This paper lacks real implementation details and analysis of the practical challenges of blockchain in the chain of custody. This paper also lacks a detailed discussion on the legal and ethical implications of using blockchain in forensic investigations. |
| Ryu et al. [ ] | This paper proposes a blockchain-based framework for digital forensics in the IoT. The framework utilizes blockchain to store all communications of IoT devices as transactions to ensure data integrity and simplify the chain of custody process. This decentralized approach enhances security, transparency, and reliability. | This paper does not discuss the technical details of implementing the proposed framework, such as the specific blockchain platform used or the methods for verifying digital signatures. |
| Sheelvanth et al. [ ] | This paper proposes a blockchain-based forensic evidence management system to address vulnerabilities in traditional systems. It utilizes blockchain’s decentralized and immutable nature to ensure data integrity, automate the chain of custody, and enhance transparency and accountability. | This paper only focuses on the conceptual design and lacks a detailed technical implementation, such as the specific blockchain platform used or the cryptographic algorithms employed. |
| Xiao et al. [ ] | This paper proposes a blockchain-based digital forensic framework for IIoT environments. It utilizes a decentralized blockchain storage mechanism to ensure tamper-proof and permanent storage of digital evidence. The framework utilizes smart contracts for efficient evidence retrieval and tracing, and a token mechanism for access control. | This paper does not discuss the potential privacy risks associated with storing sensitive IIoT data on a public blockchain. Also, this paper does not explore the scalability of the proposed framework for handling large volumes of data generated by IIoT systems. |
| Zarpala and Casino [ ] | This paper proposes a blockchain-based forensic model for financial crime investigations. The model uses blockchain’s immutability and verifiability to create a tamper-proof audit trail to ensure the integrity of evidence and facilitate the chain of custody. | This paper focuses only on the embezzlement scenario, which limits its generalizability to other financial crimes. |
| Sakshi et al. [ ] | This paper provides a review of research trends and challenges related to blockchain-based IoT forensic evidence preservation. It analyses the integration of blockchain with IoT forensics and discusses various blockchain platforms and tools. | This paper lacks technical details on implementing blockchain solutions for evidence preservation. It also does not discuss legal and regulatory aspects. |
| Alqahtany and Syed [ ] | This paper proposes a framework for integrating blockchain technology into digital forensics, encompassing data preservation, acquisition, analysis, and documentation. The framework utilizes smart contracts and APIs to record every forensic transaction on the blockchain to ensure transparency, immutability, and authenticity of the evidence. | This paper focuses only on the conceptual design and theoretical aspects of the framework. It lacks detailed implementation and evaluation of the proposed solution on a real-world blockchain platform. |
| Onyeashie et al. [ ] | This paper provides a systematic review of blockchain applications in the chain of custody. It examines how blockchain can strengthen the evidential chain of custody and interoperate with actual evidence storage. This paper highlights the benefits of blockchain in providing an immutable and decentralized structure for documenting and auditing evidence trails. | This paper does not discuss the implementation details of the system and real-world applications. This paper also does not discuss the technical challenges of integrating blockchain with existing forensic tools. |
| Kumar et al. [ ] | This paper proposes a blockchain-based digital forensics framework called Internet-of-Forensics (IoF) for the IoT. IoF addresses the lack of transparency and heterogeneity in IoT using a consortium blockchain to manage evidence and ensure the chain of custody. It uses lattice-based cryptography for low complexity and post-quantum security, making it suitable for resource-constrained devices. | This paper does not discuss the practical challenges of integrating the proposed framework with existing forensic tools. Also, this paper does not evaluate the performance of the proposed framework in real-world scenarios. |
| Goyal [ ] | This paper provides a review of blockchain in forensic science by highlighting the potential of blockchain to enhance privacy, authenticity, reliability, and evidence management in forensic investigations. | This paper does not discuss technical details, novel forensic frameworks, or considerations and evaluation related to real-world implementation. |
| Jacob and Kumar [ ] | This paper proposes a framework for digital forensics using blockchain to secure digital data. The framework uses blockchain’s immutability and transparency to ensure the integrity and authenticity of digital evidence. | The proposed framework is only conceptual and does not address practical challenges such as scalability, interoperability, and legal considerations. |
| Akbarfam et al. [ ] | This paper presents ForensiBlock, a private blockchain framework designed for digital forensics provenance. ForensiBlock ensures secure data access, traces data origins, preserves records, and expedites provenance extraction, offering a secure, efficient, and reliable solution for handling digital forensic data. | This paper does not provide a detailed analysis of the performance of ForensiBlock in real-world scenarios. It also does not discuss the scalability of the proposed framework. |
| Masud et al. [ ] | This paper reviews existing research on digital forensics frameworks for blockchain and cryptocurrency. It highlights the challenges and opportunities in applying digital forensic techniques to the unique characteristics of blockchain. | This paper does not discuss methods for evidence preservation in blockchain, which is a critical aspect for ensuring the admissibility of digital evidence. |
| Almutairi and Moulahi [ ] | This paper proposes a framework for digital forensics in IoT that combines blockchain and federated learning. The blockchain is used to store the trained models from the federated learning process to ensure data integrity and traceability. The federated learning is used to address privacy concerns associated with data sharing. | This paper does not discuss the potential for blockchain attacks, such as 51% attacks, which could compromise the integrity of the evidence stored on the blockchain. |
| Cong et al. [ ] | This paper explores various criminal activities related to cryptocurrencies, including investment scams, Ponzi schemes, rug pulls, ransomware attacks, money laundering, and darknet markets. It discusses how blockchain forensic techniques can be used to investigate and limit some of these cybercrimes. | This paper lacks a detailed technical analysis and implementation of blockchain forensic techniques and methods and their application in real-world investigations. |
| Alqahtany and Syed [ ] | This paper proposes a framework for mobile VPN forensics by integrating blockchain with deep learning models. The blockchain acts as a secure and tamper-proof ledger for recording VPN transactions to enhance the integrity and admissibility of forensic evidence. | This paper does not discuss potential challenges related to blockchain scalability, transaction costs, or privacy concerns associated with storing sensitive VPN data on a public blockchain. |
| Srivasthav et al. [ ] | This paper provides a survey of blockchain forensics and analytics tools, categorizing them based on their key features and comparing them across three practical parameters: cryptocurrency support, feature availability, and ease of access. | This paper focuses on only a limited number of tools and does not consider the rapidly evolving landscape of blockchain forensics. |
| Khan et al. [ ] | This paper proposes an IoT-blockchain architecture for multimedia forensics investigations. The proposed system utilizes a private permissioned network to facilitate secure collaboration among stakeholders, including the exchange of video surveillance data and chain-of-custody details. Smart contracts automate ledger verification and validation, ensuring immutability and transparency in the investigation process. | This paper lacks a detailed analysis of the performance impact of smart contracts on the blockchain network. In addition, this paper does not discuss the scalability challenges of the proposed system when handling a large volume of multimedia data. |
| Al-Khateeb et al. [ ] | This paper surveys the potential of blockchain to enhance digital forensics and incident response. It argues that blockchain can improve the implementation of digital investigation models by automating the identification and preservation phases. | This paper lacks technical details and implementation strategies for integrating blockchain into existing digital investigation frameworks. |
| Ragu and S. [ ] | This paper proposes a blockchain-based cloud forensics architecture for privacy leakage prediction using SDN and blockchain to address the challenges of evidence integrity and centralized evidence collection in cloud environments. | This paper focuses only on the conceptual design and lacks technical details and evaluation of the proposed system in a real-world scenario. |
| Brotsis et al. [ ] | This paper reviews recent blockchain-enabled forensics frameworks and extracts best practices for integrating blockchain into the process. It then presents a novel blockchain-enabled platform for IoT forensics, implemented with Hyperledger Fabric and evaluated on a virtualized testbed. | This paper focuses only on a specific blockchain platform (Hyperledger Fabric) and a limited number of attack scenarios. It also does not discuss the privacy implications of the proposed system. |
| Bonomi et al. [ ] | This paper proposes a blockchain-based chain of custody (B-CoC) for managing digital evidence in digital forensics. B-CoC utilizes a private permissioned blockchain to ensure the integrity, traceability, authentication, and verifiability of digital evidence throughout its lifecycle. | This paper does not discuss the legal and practical implications of the proposed system. This paper also does not discuss the potential challenges of integrating B-CoC with existing legal frameworks. |
| Tian et al. [ ] | This paper proposes a secure digital evidence framework using blockchain (Block-DEF) for blockchain forensics. Block-DEF employs a mixed block structure and a name-based consensus mechanism to address blockchain scalability issues. | This paper does not discuss the security implications of the Block-DEF and does not discuss the challenges of integrating Block-DEF with existing frameworks. |
| Lusetti et al. [ ] | This paper proposes a blockchain-based solution called Custody Chain (CC) for the secure storage and sharing of digital forensic medical evidence. CC uses a hybrid platform that encrypts digital evidence and stores it in a redundant online file storage system, while using a private Hyperledger Fabric blockchain to record file properties, access history, and user permissions. | The proposed solution is mainly based on a private and permissioned blockchain, which limits the potential for wider adoption and interoperability with other forensic systems. |
| Verma et al. [ ] | This paper proposes a blockchain-based electronic law record management scheme called NyaYa, which utilizes a public blockchain with off-chain storage in IPFS to maintain ELRs to ensure scalability and security. It also incorporates smart contracts for case closure and financial settlements. | This paper does not provide a detailed analysis of the security of the proposed scheme against existing blockchain forensic attacks. |
| Chen et al. [ ] | This paper reviews the application of blockchain in generating electronic evidence for judicial proceedings, specifically focusing on its benefits in ensuring immutability, traceability, and independence of evidence. This paper proposes a consortium blockchain-based system for electronic evidence generation, enabling judicial bodies to verify evidence legitimacy and improve the reliability of evidence. | This paper lacks a discussion of specific forensic techniques and tools used for evidence analysis on the blockchain. This paper focuses on a single case study, which limits its generalizability to other types of cases and blockchain platforms. |
| Awuson-David et al. [ ] | This paper proposes a Blockchain Cloud Forensic Logging (BCFL) framework that uses a permissioned blockchain to maintain tamper-proof logs within the cloud ecosystem. BCFL integrates a permissioned blockchain into the cloud, enabling evidence acquisition that enhances GDPR compliance and maintains a secured chain of custody. | This paper focuses only on a single case study, which may not be generalizable to other cloud environments. This paper also does not discuss potential scalability issues of the BCFL framework. |
| Olukoya et al. [ ] | This paper proposes a framework for distilling blockchain requirements for security incident response platforms (SIRPs) to enhance auditability and integrity. The framework extracts actions, audit records, and relevant metadata from the SIRP, then designs payloads for these actions and defines a blockchain structure for storing the transactions. | This paper lacks a comprehensive evaluation of the proposed framework’s performance and scalability. This paper also does not address the potential challenges of integrating the proposed blockchain system with existing SIRPs. |
| Burri et al. [ ] | This paper proposes a blockchain-based solution for maintaining a chronological and independently verifiable electronic chain of custody (e-CoC) ledger for digital evidence using a private blockchain managed by a trusted entity, with periodic updates to a public blockchain for enhanced security. | The proposed solution relies on the integrity of the trusted entity and does not fully address the decentralized nature of blockchain technology. |
| Citation | Evidence Integrity | Chain of Custody | Transparency | Auditability | Security | Scalability |
|---|---|---|---|---|---|---|
| Ahmad et al. [ ] | ✓ | × | × | × | ✓ | × |
| Siaam et al. [ ] | × | × | ✓ | × | × | × |
| Billard [ ] | × | ✓ | × | × | × | × |
| Cebe et al. [ ] | × | ✓ | ✓ | ✓ | × | × |
| Chopade et al. [ ] | ✓ | × | × | ✓ | × | × |
| Hsu et al. [ ] | × | × | × | × | ✓ | × |
| Khan et al. [ ] | × | ✓ | ✓ | × | × | × |
| Li et al. [ ] | ✓ | × | ✓ | ✓ | × | × |
| Li et al. [ ] | × | ✓ | ✓ | × | × | × |
| Mahrous et al. [ ] | ✓ | × | × | × | × | × |
| Muyambo et al. [ ] | × | ✓ | ✓ | × | × | × |
| Ryu et al. [ ] | × | × | × | ✓ | × | × |
| Sheelvanth et al. [ ] | × | ✓ | × | × | × | × |
| Xiao et al. [ ] | × | ✓ | × | × | ✓ | × |
| Zarpala and Casino [ ] | ✓ | × | × | ✓ | × | × |
| Alqahtany and Syed [ ] | × | × | ✓ | ✓ | × | × |
| Kumar et al. [ ] | × | ✓ | ✓ | × | ✓ | × |
| Jacob and Kumar [ ] | ✓ | × | ✓ | × | × | × |
| Akbarfam et al. [ ] | ✓ | ✓ | × | × | × | × |
| Almutairi and Moulahi [ ] | × | ✓ | ✓ | × | × | ✓ |
| Alqahtany and Syed [ ] | ✓ | × | × | × | × | × |
| Khan et al. [ ] | ✓ | × | ✓ | ✓ | ✓ | × |
| Ragu and S. [ ] | ✓ | × | × | ✓ | × | × |
| Bonomi et al. [ ] | ✓ | ✓ | × | × | × | × |
| Tian et al. [ ] | × | × | ✓ | × | ✓ | ✓ |
| Lusetti et al. [ ] | × | × | × | ✓ | × | ✓ |
| Verma et al. [ ] | × | × | ✓ | × | ✓ | ✓ |
| Chen at al. [ ] | ✓ | ✓ | ✓ | × | × | × |
| Awuson-David et al. [ ] | ✓ | × | × | × | ✓ | × |
| Olukoya et al. [ ] | × | ✓ | ✓ | × | × | × |
| Burri et al. [ ] | ✓ | ✓ | ✓ | × | × | × |
| Citation | Digital Forensic Frameworks and Methodologies |
|---|---|
| Ahmad et al. [ ] | The proposed framework consists of three layers: an evidence layer with smart locks for secure evidence storage, a blockchain layer using a private Ethereum for tamper-proof metadata recording, and a network layer enabling communication among authorized parties. |
| Siaam et al. [ ] | The proposed IoT probe framework involves four key components: Transaction Creation, Insertion into Blockchain Ledgers, Escrow Service, and Investigation Analysis. |
| Billard [ ] | This paper proposes a digital forensic framework consisting of three key components: the DEI, the Forensics Confidence Rating (FCR), and the GDT for timeline reconstruction and presentation. |
| Cebe et al. [ ] | The proposed Block4Forensic (B4F) consists of a forensic daemon, a permissioned blockchain, and various stakeholders. B4F’s forensic daemon mirrors collection, the blockchain acts as secure storage, and stakeholder interactions represent analysis and reporting. |
| Chopade et al. [ ] | The proposed blockchain-based framework includes evidence creation, evidence hash transfer, and evidence display. This framework enhances the reliability and security of digital evidence throughout the investigation lifecycle. |
| Hsu et al. [ ] | The proposed blockchain-based framework for IoT includes components for the acquisition of sensor logs, analysis of log data, and presentation of evidence in a tamper-proof and legally defensible manner. The framework also utilizes a signature chain to ensure data integrity and non-repudiation. |
| Khan et al. [ ] | The proposed MF-Ledger framework consists of a private, permissioned network where stakeholders securely interact using smart contracts to record and manage evidence. This ensures transparency, immutability, and secure storage of the evidence chain of custody. |
| Li et al. [ ] | The proposed LEChain framework manages evidence from its collection by victims, witnesses, and monitoring devices, through analysis by crime scene analysts, to its upload and access via the blockchain, closing in a court trial. |
| Li et al. [ ] | The proposed IoTFC framework consists of users and IoT devices, Merkle tree, blocks, and smart contracts. The output of the framework includes a comprehensive view of evidence items, continuous integrity, immutability and audibility, tamper-proof environment, full provenance, and traceability. |
| Mahrous et al. [ ] | The proposed framework consists of evidence acquisition, a forensic-chain framework, and blockchain-based evidence management. It involves acquisition for uploading fingerprinted records to the blockchain, analysis to verify the authenticity of the evidence items, and reporting to generate a report for the investigation |
| Muyambo et al. [ ] | The proposed DFRMIV framework consists of four main layers: the acquisition layer to gather evidence from the blockchain network, the preservation layer to use cryptographic techniques and secure storage methods to preserve the integrity of the evidence, the analysis layer to analyse the preserved data to identify any anomalies or evidence of tampering, and the reporting layer to report the findings. |
| Ryu et al. [ ] | The proposed framework consists of three layers: the IoT device layer for gathering the evidence, the blockchain layer to utilize a block structure with a block header and transaction data, where each transaction includes a transaction ID, digital signature, and PUF IDs of the sender and receiver devices, and the participants’ layer where analysis and reporting of evidence occur. |
| Sheelvanth et al. [ ] | The proposed framework supports evidence acquisition through secure storage on the blockchain, analysis by providing access to authorized personnel and reporting through transparent and auditable records. |
| Xiao et al. [ ] | The proposed framework comprises a decentralized blockchain storage mechanism, smart contract mechanisms for evidence retrieval and tracing, a token mechanism for access control, and an efficient batch consensus algorithm. |
| Zarpala and Casino [ ] | The proposed framework consists of a smart contract deployed on the Ethereum blockchain that records all actions performed during the investigation. The framework also includes a mechanism for evidence custody changes and destruction, ensuring a complete and auditable trail of events. |
| Alqahtany and Syed [ ] | The proposed framework consists of data preservation, where a forensic image of the evidence is created; data acquisition, where the evidence is collected and analysed; and finally reporting, where the findings are documented. |
| Kumar et al. [ ] | The proposed IoF consists of four layers: Edge-IoF, Fog-IoF, Consortium-IoF, and Cloud Storage. Edge-IoF gathers evidence from heterogeneous devices, Fog-IoF performs forensic analysis and maintains the chain of custody, Consortium-IoF facilitates collaboration among various stakeholders, and Cloud Storage stores the evidence. |
| Jacob and Kumar [ ] | The proposed framework involves collecting digital evidence, hashing it, and storing it in the blockchain using a hash directory to prevent duplicate data. The evidence stored on the blockchain is then analysed, and the findings are documented. |
| Akbarfam et al. [ ] | The proposed ForensiBlock framework consists of three main components: blockchain, user nodes, and off-chain storage. The blockchain serves as a decentralized ledger for recording transactions and data changes. User nodes represent authorized individuals involved in investigations, while off-chain storage securely stores digital forensic data and maintains provenance records. |
| Almutairi and Moulahi [ ] | The proposed framework uses federated learning for privacy-preserving model training on IoT devices, followed by model aggregation on a lightweight blockchain. This process involves the acquisition of data from IoT devices, analysing them through federated learning, preservation of model parameters on the blockchain, and reporting results based on the aggregated models. |
| Alqahtany and Syed [ ] | The proposed framework consists of data collection, VPN traffic analysis using CNN and GNN models, and secure logging on a blockchain. The output of the framework is a comprehensive forensic report that includes the identification of the VPN protocol, the classification of VPN traffic, and the secure storage of the evidence on the blockchain. |
| Khan et al. [ ] | The proposed framework utilizes blockchain forensics tools to collect blockchain data, analyse transactions and addresses, and identify suspicious activities. The output is a report that includes identified high-risk activities, real-time analysis, and a strong audit trail. |
| Ragu and S. [ ] | The proposed framework consists of six stages: identification, preservation, collection, examination, analysis, and presentation. Blockchain is integrated into the framework to automate the acquisition and preservation phases, improving efficiency and reliability while providing continuous fraud detection and forensic readiness. |
| Bonomi et al. [ ] | The proposed B-CoC framework consists of seven phases: investigation initiation, incident reporting, preparation and planning, evidence identification, acquisition, preservation, analysis, presentation, and investigation closure. Blockchain is used to address issues related to evidence integrity, chain of custody, and data privacy. |
| Tian et al. [ ] | The proposed Block-DEF framework consists of three layers: a service layer for evidence submission and retrieval, a blockchain layer for consensus and storage of evidence information, and a network layer for communication. The evidence stored in the blockchain is then analysed, and the findings will be documented. |
| Lusetti et al. [ ] | The proposed CC framework combines a secure online file storage system with a private implementation of the Hyperledger FabricTM blockchain. The framework encompasses encryption, file hashing, and a robust chain-of-custody mechanism. The framework includes the acquisition of digital files, processing through encryption and hashing, analysis of file properties, and reporting of access logs and evidence in a secure and verifiable manner. |
| Verma et al. [ ] | The proposed NyaYa framework comprises four phases: registration of judicial stakeholders on the BC, case registration with meta-hash keys in the public BC to reference external off-chain interplanetary file storage, chronological updates of investigative findings among law enforcement agencies on the BC and IPFS, and case hearing and settlement through smart contracts. |
| Chen et al. [ ] | The proposed framework consists of data acquisition (screenshots and source code), data preservation (Factom blockchain), and data analysis (verification of SHA256 hash values, blockchain queries, and analysis of Bitcoin storage content). |
| Awuson-David et al. [ ] | The proposed BCFL framework consists of four key components: blockchain distributed ledger technology (DLT), smart contracts, data validation, and immutability. These components are used to acquire, preserve, analyse, and report digital evidence in the cloud ecosystem. |
| Olukoya et al. [ ] | The proposed framework utilizes Parnassus to record and manage forensic actions throughout the investigation lifecycle. This framework encompasses four key operations: acquisition of evidence using Parnassus to store evidence details, preservation of evidence integrity through blockchain immutability, analysis of evidence using tools integrated with Parnassus, and reporting of findings. |
| Burri et al. [ ] | The proposed e-CoC framework includes a secure ledger managed by a trusted entity, with blocks linked by hash values. The e-CoC ledger is periodically secured to a public blockchain for tamper-proof verification. Digital evidence is hashed, and the hash values are timestamped and stored in the e-CoC ledger. The evidence stored in the blockchain is then analysed, and the findings will be documented. |
| Citation | IoT Forensics | Cloud Forensics | Vehicular Forensics | Mobile Forensics | Internet Voting | Dark Web | Multimedia Forensics |
|---|---|---|---|---|---|---|---|
| Akinbi et al. [ ] | ✓ | × | × | × | × | × | × |
| Siaam et al. [ ] | ✓ | × | × | × | × | × | × |
| Cebe et al. [ ] | × | × | ✓ | × | × | × | × |
| Hsu et al. [ ] | ✓ | × | × | × | × | × | × |
| Jin et al. [ ] | × | × | × | × | × | ✓ | × |
| Khan et al. [ ] | × | × | × | × | × | × | ✓ |
| Khanji et al. [ ] | ✓ | × | × | × | × | × | × |
| Li et al. [ ] | ✓ | × | × | × | × | × | × |
| Mahrous et al. [ ] | ✓ | × | × | × | × | × | × |
| Muyambo et al. [ ] | × | × | × | × | ✓ | × | × |
| Ryu et al. [ ] | ✓ | × | × | × | × | × | × |
| Xiao et al. [ ] | ✓ | × | × | × | × | × | × |
| Sakshi et al. [ ] | ✓ | × | × | × | × | × | × |
| Alqahtany and Syed [ ] | × | ✓ | × | × | × | × | × |
| Kumar et al. [ ] | ✓ | × | × | × | × | × | × |
| Almutairi and Moulahi [ ] | ✓ | × | × | × | × | × | × |
| Alqahtany and Syed [ ] | × | × | × | ✓ | × | × | × |
| Khan et al. [ ] | ✓ | × | × | × | × | × | × |
| Ragu and S. [ ] | × | ✓ | × | × | × | × | × |
| Brotsis et al. [ ] | ✓ | × | × | × | × | × | × |
| Awuson-David et al. [ ] | × | ✓ | × | × | × | × | × |
| Burri et al. [ ] | × | ✓ | × | × | × | × | × |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Atlam, H.F.; Ekuri, N.; Azad, M.A.; Lallie, H.S. Blockchain Forensics: A Systematic Literature Review of Techniques, Applications, Challenges, and Future Directions. Electronics 2024 , 13 , 3568. https://doi.org/10.3390/electronics13173568
Atlam HF, Ekuri N, Azad MA, Lallie HS. Blockchain Forensics: A Systematic Literature Review of Techniques, Applications, Challenges, and Future Directions. Electronics . 2024; 13(17):3568. https://doi.org/10.3390/electronics13173568
Atlam, Hany F., Ndifon Ekuri, Muhammad Ajmal Azad, and Harjinder Singh Lallie. 2024. "Blockchain Forensics: A Systematic Literature Review of Techniques, Applications, Challenges, and Future Directions" Electronics 13, no. 17: 3568. https://doi.org/10.3390/electronics13173568
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Open access
- Published: 04 September 2024
Urinary CXCL-10, a prognostic biomarker for kidney graft injuries: a systematic review and meta-analysis
- Sahar Janfeshan 1 ,
- Afsoon Afshari 1 ,
- Ramin Yaghobi 2 &
- Jamshid Roozbeh 1
BMC Nephrology volume 25 , Article number: 292 ( 2024 ) Cite this article
Metrics details
The challenges of long-term graft survival and the side effects of current immunosuppressive therapies in kidney transplantation highlight the need for improved drugs with fewer adverse effects. Biomarkers play a crucial role in quickly detecting post-transplant complications, with new biomarkers showing promise for ongoing monitoring of disease and potentially reducing the need for unnecessary invasive biopsies. The chemokines such as C-X-C motif chemokine ligand 10 (CXCL10), are particularly promising protein biomarkers for acute renal rejection, with urine samples being a desirable source for biomarkers. The aim of this review is to analyze the literature on the potential role of urinary CXCL10 protein in predicting kidney graft injuries. The results of this study demonstrate that evaluating urinary CXCL10 levels is more successful in identifying post-transplant injuries compared to assessing the CXCL10/Cr ratio.
Peer Review reports
Introduction
Kidney allograft transplantation is the best treatment for end-stage renal disease (ESRD), but long-term graft survival and adverse effects of current immunosuppressive regimens remain significant challenges [ 1 , 2 ]. Laboratory tests, including serum urea, creatinine, and proteinuria, play a crucial role in monitoring kidney transplants. While histopathological analysis of graft biopsies remains the gold standard for diagnosing post-transplant injuries, it has limitations due to its invasive nature, potential sampling errors, and high costs. Consequently, this method is impractical for continuous graft monitoring over time [ 3 , 4 ]. Therefore, exploring novel biomarkers for early detection of post-transplant injuries holds promise for reducing unnecessary biopsies [ 4 , 5 ]. Considering these facts, urine samples, which directly reflect allograft function and are minimally affected by systemic inflammation, represent an optimal source for biomarkers in this context [ 5 ].
Based on the search results provided, the primary new biomarkers being studied for the detection of kidney transplant complications are urinary C-X-C motif chemokine ligand 9 (CXCL9) and 10 (CXCL10) [ 6 ]. Additionally, the analysis of urinary mRNA transcripts such as CD3 + , perforin, granzyme B, CD103, and CXCR3, has been conducted [ 6 , 7 ]. Urinary perforin and granzyme B and CD103, can serve as screening tools for acute rejection. Moreover, elevated levels of donor-derived cell-free DNA (dd-cf DNA) in the blood or urine may indicate acute rejection, while declining levels could signal recovery from rejection [ 6 , 7 , 8 , 9 , 10 ]. In conclusion, urinary and blood transcriptomics are emerging as promising biomarkers in the field of kidney transplantation, offering valuable insights into the status of kidney allografts. The identification of specific mRNAs and microRNAs associated with rejection episodes has proven beneficial in detecting T cell-mediated rejection (TCMR) and antibody-mediated rejection (ABMR), thereby enhancing our understanding of the immunological processes involved in kidney transplant rejection [ 11 ]. Some of mentioned biomarkers such as the CXCL9 and CXCL10, seems to be more promising for kidney graft problems early detection and ongoing disease monitoring. These chemokines recruit T cells to inflammatory sites and have shown potential as biomarkers for diagnosing rejection [ 6 ]. A multicenter prospective study found that urinary CXCL9 and CXCL10 protein levels were significantly higher in patients with acute rejection compared to stable graft conditions, and low urinary CXCL9 protein levels could be used to rule out acute rejection with a high negative predictive value (NPV). Furthermore, CXCL10 levels have been shown to increase up to 30 days before the biopsy, which can help identify patients at risk for acute rejection [ 6 ]. CXCL9 is also a promising biomarker, but CXCL10 has a more robust and consistent association with graft complications, making it a more reliable indicator [ 12 ]. CXCL10 is a member of the CXC chemokine family also known as IFNγ-induced protein 10 (IP-10). It is an 8.7 kDa protein encoded by the CXCL10 gene located on human chromosome 4q21. The CXCL10 gene consists of 4 exons and 3 introns and elicits its effects by binding to the cell surface chemokine receptor CXCR3. This chemokine secreted by monocytes, endothelial cells and fibroblasts in response to IFNγ and recruit immune cells to sites of inflammation. It also plays roles in anti-tumor activity, adhesion of T cells, and inhibition of angiogenesis and bone marrow colony formation [ 13 , 14 ]. Studies have demonstrated that CXCL10 is directly involved in the development of kidney conditions through its chemoattractant properties and effects on cell proliferation [ 14 ]. CXCL10 or its ratio to creatinine has been more extensively studied and validated as a biomarker for kidney allograft rejection and can predict early rejection risk and longer-term graft survival [ 5 , 6 , 15 , 16 , 17 , 18 , 19 , 20 ]. This chemokine, is detected as a urinary biomarker for both TCMR and ABMR. Low levels of CXCL10 are associated with immunological quietness, making it ideal for ruling out rejection and identifying transplant recipients at low immunological risk [ 21 ].
Integrating the urinary CXCL10 biomarker with clinical indicators such as serum urea, creatinine, and proteinuria holds promise for reducing unnecessary invasive biopsies and improving patient outcomes [ 22 ].
Aim of this review
The aim of this systematic review and meta-analysis is to examine the existing literature to determine the potential role of urinary CXCL10 protein in predicting kidney graft injuries.
Materials and methods
Literature search.
This review is conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [ 23 ]. The objectives of the study, the search strategy, inclusion and exclusion criteria, and study evaluation method were carefully designed, refined, and unanimously approved by all contributing authors well in advance. Pubmed, Scopus, Web of Sciences, EMBASE electric databases were searched from 6 March 2022 to 2 October 2023. The medical subject headings (MeSH) and their entry terms used in the literature search included: ((“Renal Transplantation*”) or (“Kidney Transplantation*”) or (“Kidney Graft*”) or (“Renal Graft*”)) and ((“Chemokine CXCL10”) or (“Interferon-Inducible Protein 10”) or (“Small Inducible Cytokine B10”) or (“IFN-gamma-Inducible Protein, 10 kDa”)). We applied no restrictions regarding the language or the publication date of the sources. Furthermore, a manual search of references in review articles was conducted to uncover additional relevant studies.
Selection process and data extraction
In the preliminary phase of the eligibility assessment, the screening of titles and abstracts was conducted independently by two investigators (A.A., S.J.). This initial review aimed to identify original research articles that investigated the utility of urinary CXCL10, either singularly or in combination with creatinine, in the prediction of kidney injuries post-transplantation.
In the course of our study, we excluded abstracts, reviews, and research focusing on CXCL10 derived from sources other than urine, such as blood samples or histological stains. Studies that primarily evaluated different outcomes, including infections or allograft survival, were also excluded. Subsequently, in the inclusion phase, the selected articles underwent an independent full-text review by two researchers (A.A., S.J.), ensuring a comprehensive and rigorous evaluation of the relevant literature. Discrepancies in the assessments made by the two investigators were deliberated upon and resolved through consultative discussions involving the entire authorial team. Data extraction from the included studies was carried out using a pre-defined spreadsheet and an extraction table, collaboratively developed and refined by all authors. The extracted data encompassed a range of variables, including, authors, years of enrollment, exposure, protein cut-off, outcome, sensitivity, specificity, and measures of diagnostic accuracy such as True Positive (TP), False Negative (FN), False Positive (FP), True Negative (TN), and the Area Under the Receiver Operating Characteristic Curve (AUC) for protein. These variables were systematically tabulated for descriptive analysis. Two independent investigators conducted a thorough review of eligible publications, meticulously extracting relevant data into a standardized format. This rigorous selection process involved an initial review of titles and abstracts, followed by a detailed examination of the full texts. Any discrepancies encountered during this process were effectively resolved through consultation with a third reviewer.
Quality assessment
In the present meta-analysis, the integrity and robustness of the included studies were rigorously assessed using the Newcastle Ottawa Scale (NOS), a bespoke tool for the evaluation of nonrandomized studies. The NOS framework awards up to nine points, distributed across three critical dimensions: the selection process of study participants (maximum of 4 points), the comparability of the study groups (maximum of 2 points), and the accuracy and reliability of outcome assessment (maximum of 3 points). Studies achieving a score of 7 to 9 are classified as high quality, indicating lower risk of bias. Those garnering 4 to 6 points are categorized as having a high risk of bias, while a score of 0 to 3 suggests a very high risk of bias, potentially impacting the reliability of their findings.
Statistical analysis
In the initial phase of our analysis, we computed the natural logarithms (Ln) of the Relative Risks (RRs) along with their 95% Confidence Intervals (CIs) to derive the summary Effect Size (ES). To assess the comparative impact of the highest versus lowest categories, we implemented a random-effects model, specifically chosen to adequately account for the variability among studies (between-study heterogeneity). Furthermore, this model was instrumental in calculating I² values, which serve as quantitative measures of heterogeneity. We considered I² values exceeding 50% as a threshold for significant between-study heterogeneity. Upon encountering substantial heterogeneity, our analytical strategy included conducting subgroup analyses, with a particular focus on differing outcomes, to elucidate potential sources of variability among the included studies.
Summary of searches and study selection process
Upon conducting a comprehensive search of the relevant database, a total of 878 articles were initially retrieved. Rigorous deduplication procedures resulted in removing 224 articles. A cursory examination of titles and abstracts facilitated the exclusion of 587 articles, which primarily consisted of review articles, conference proceedings, and additional publications by the same author. A more in-depth evaluation led to the further exclusion of 23, 31 and 4 articles in three steps due to reasons including the assay techniques, the use of non-urinary sample types, and the inability to extract complete data sets. Consequently, a final selection of 9 articles [ 18 , 20 , 24 , 25 , 26 , 27 , 28 , 29 , 30 ], representing 10 studies (with one article encompassing two studies), was made [ 27 ] (Fig. 1 ).
Flow diagram for screening related articles; a total of 878 articles were initially retrieved and finally after removing deduplication (224), incompatibility of titles and abstracts )587(, further exclusion of 23, 31 and 4 articles due to the assay techniques, use of non-urinary sample types, and the inability of extracting data, resulted in 9 articles
The basic characteristics of literatures
The fundamental characteristics of the selected publications are systematically cataloged in Table-S1. From each study, we meticulously extracted key data components, encompassing the first author’s name, country of research, publication date, exposure details, protein cut-off values, and the primary diagnostic metrics, including the number of true positives, false positives, false negatives, and true negatives.
Quality evaluation results
The result of quality assessment using NOS is shown in Fig. 2 . The quality assessment of the 9 selected articles was performed using Stata14 (metareg), as illustrated in Fig. 3 . Overall, the quality of the included literature was deemed satisfactory. Each study adopted a case-control design, with the unanimous gold standard for diagnosis across all experiments being the pathological results post-transplantation. An analysis of quality scores in relation to sensitivity revealed that the study quality did not significantly influence the sensitivity outcomes. The pertinent data and findings from this analysis are detailed in Table 1 .

The quality assessment of included studies using the Newcastle-Ottawa Scale (NOS); The NOS evaluates studies on their selection of groups, comparability of groups, and ascertainment of exposure or outcomes, represented in the star ratings displayed in X-axis

The metareg plot evaluating the quality of included studies in the meta-analysis; This plot visually displays the relationship between the study quality scores based on sensitivity
Statistical analysis results
In this meta-analysis, Stata 14 was utilized as the primary statistical software. Due to significant heterogeneity observed in the initial results, indicated by an I2 value exceeding 50%, comprehensive subgroup analyses and meta-regression were undertaken to delve deeper into the data. The subgroup analysis was specifically tailored based on the study outcomes, with a particular focus on the methodologies used for CXCL10 quantification (isolated CXCL10 measurement versus the CXCL10/Creatinine ratio). The heterogeneity analysis revealed that the I 2 values for sensitivity and specificity, when utilizing the CXCL10 only measurement approach, were 63.4% ( p = 0.027) and 80.4% ( p = 0.000) respectively, as illustrated in Fig. 4 . In contrast, the I 2 values for sensitivity and specificity with the CXCL10/Creatinine ratio method were 0% ( p = 0.454) and 97.6% ( p = 0.000) respectively, detailed in Fig. 5 .

Forest plots; show the 5 included studies heterogeneity and estimated exposures, sensitivity (left) and specificity (right), with urinary CXCL10 as outcome

Forest plots; show the 5 included studies heterogeneity and estimated exposures, sensitivity (left) and specificity (right), with urinary CXCL10/Cr as outcome
Publication bias test
Funnel plots are commonly used in meta-analysis to assess publication bias and small-study effects. Asymmetric funnel plots can indicate the presence of such biases, but they can also be caused by other factors such as the choice of the plotted effect size, the presence of a moderator correlated with the study effect and size, or chance. These plots add contours of statistical significance to the funnel plot to aid interpretation. Stata 14 was used to draw funnel plot. As displayed in Fig. 6 , it had a moderate asymmetry.

The funnel plot; used to assess the potential for publication bias among the included studies for sensitivity as the exposure
Previous research underscores the significance of CXCL10 as a biomarker in the prognosis of kidney graft injuries (acute rejection (AR), TCMR, ABMR), examining its presence in both serum and urinary assays. Investigations have not only focused on CXCL10 as an isolated marker but have also encompassed its correlative studies with serum creatinine levels.
These studies propose that CXCL10 may serve as an integral biomarker, offering predictive insights into the functional status of renal transplants [ 5 , 6 , 15 , 16 , 17 , 20 ]. Rabant et al. suggested that low levels of urinary CXCL10 could predict immunological quiescence, or a low risk of acute rejection, as early as one month into stable graft conditions [ 31 ]. The study by Mühlbacher J et al. highlighted that the association of urinary CXCL10/Cr ratio with donor-specific antibodies (DSA) significantly improved the identification of ABMR and the prediction of graft loss. Their finding emphasizes the potential of CXCL10 as a biomarker in transplant medicine [ 19 ]. Earlier research, demonstrated that measuring the serum level of CXCL10 before kidney transplantation could be a predictor of acute rejection which suggest that CXCL10 levels could serve as an important indicator for preemptive measures in transplant recipients [ 32 ]. Finally, Jackson et al. concluded that CXCL10 levels don’t seem to distinguish between AR and BK virus infection. They both show elevated levels of this chemokine, although diagnostic certainty is still possible when combined with other tests like a creatinine assay [ 33 ].This study presents a comprehensive systematic review and meta-analysis that focuses on the clinical validation and comparison of CXCL10 and CXCL10/Cr urinary levels in the detection of post-kidney transplantation injuries. The analysis encompasses data from 10 studies (9 articles) involving a total of 3035 kidney transplant recipients. The findings indicate that CXCL10 protein level demonstrated a sensitivity of 0.78 (0.69–0.89) and a specificity of 0.82 (0.72–0.94), while CXCL10/Cr level exhibited a sensitivity of 0.77 (0.72–0.81) and a specificity of 0.73 (0.60–0.90). These results indicate that assessing the sensitivity and specificity of CXCL10, as opposed to CXCL10/Cr, may offer greater efficacy in predicting injuries in kidney transplant recipients. It may be related to notable variations in urinary creatinine excretion rates (uCER) among kidney transplant recipients. For instance, those with delayed graft function may have values below 300 mg/day, while patients showing prompt graft function can exceed 2,100 mg/day [ 34 ]. These differences can be influenced by several factors, including age, sex, race [ 35 ], daily changes in creatinine production, levels of physical activity, dietary habits, emotional stress, muscle mass, and overall health condition [ 36 ]. Research indicates that urinary creatinine can fluctuate significantly even within an individual, with intraindividual coefficients of variation (CVs) reported to be between 10.5% and 14.4%. Additionally, creatinine excretion may vary throughout the day and across different days [ 37 ]. Some studies have found that normalizing urinary biomarker values to creatinine, such as in the case of neutrophil gelatinase-associated lipocalin (NGAL), can help lower these intraindividual CVs [ 38 , 39 ]. Waikar et al. noted that kidney injury molecule-1(KIM-1) excretion and uCER have different responses during acute disease states [ 34 ], suggesting that normalizing to creatinine is not always appropriate. Therefore, the appropriateness of normalizing urinary creatinine depends significantly on the specific research objectives, the biomarker involved, and the clinical context of the patients being studied [ 40 ].
This review encompassed nine articles [ 18 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 ], five of which examined urinary CXCL10 protein levels, [ 25 , 26 , 27 , 29 , 30 ] while one included two groups [ 27 ]. The first group exclusively evaluated urinary CXCL10 protein levels, while the second group measured urinary CXCL10 to serum Cr ratio. The other four articles in the review explored urinary CXCL10 to urinary Cr ratio as a biomarker under study [ 18 , 24 , 28 , 31 ].
Moreover, Matz et al. conducted a study involving two groups, acute cellular rejection and borderline rejection (BR), which were assessed at three different time points (2/3, 4/5, and 6/7 days) prior to rejection. The study reported varying sensitivities of 0.47, 0.62, and 0.71, with a consistent specificity of 0.95 for all time points, focusing on early post-transplant urinary CXCL10 protein levels after kidney transplantation. These data were subsequently aggregated for inclusion in the final evaluation, yielding a combined sensitivity and specificity of 0.63 and 0.95, respectively [ 29 ].
The other study that was pooled is Rabant et al. that examined urinary samples collected at three time points post-kidney transplantation 10 days, 1 month, and 3 months. The study focused on measuring the CXCL10/Cr ratio in recipients with ABMR, TCMR, and mixed rejection. The reported sensitivities for these time points were 0.57, 0.83, and 0.53, with specificities of 0.52, 0.51, and 0.76, respectively. Upon combining the data from these time points, the resulting sensitivity and specificity were 0.74 and 0.66, respectively [ 31 ].
The last study that was pooled for including in meta-analysis is Van loon et al. [ 24 ] who assessed the CXCL10/Cr protein level through an automated immunoassay method at three distinct thresholds (5%, 16%, and 25%) derived from a 5-parametere model for the non-invasive detection of acute rejection. The sensitivities reported at theses thresholds were 0.882, 0.392, and 0.248, with corresponding specificities of 0.314, 0.9, and 0.96. When the data of these threshold were combined the resulting sensitivity and specificity were 0.8 and 0.93, respectively.
The detection methods used for urinary CXCL10 in the included studies were based on assessing protein expression levels. All studies detected this urinary protein using ELISA, except for Hu [ 30 ] and Van loon [ 24 ] who used luminex assay and automated immunoassay, respectively. Across all included studies, an increased level of urinary CXCL10 was associated with a type of kidney graft injury. All urinary samples were collected post-transplantation and almost always before biopsy procedures (Table S1 ). Finally, it is worth noting that measuring urinary protein levels based on antibody-using tests such as ELISA is currently one of the most reliable and accurate existing methods.
The injuries related to the included studies in this review encompassed 14 different types of dysfunctions of kidney grafts. These included AR, TCMR, ABMR, mixed rejection (MR), BR, subclinical rejection (SR), clinical rejection (CLR), acute vascular rejection (AVR), BK virus nephropathy (BKVN), acute tubular necrosis (ATN), chronic rejection (CHR), late clinical rejection, graft functional decline, and graft loss. This review discusses the potential role of urinary CXCL10 assessment before performing an invasive biopsy procedure in identifying high-risk kidney transplant recipients who were developing at least one of the 14 different types of dysfunctions. Therefore, serial urinary CXCL10 monitoring in the weeks and months following transplantation may help accelerate clinical diagnosis of recipients at risk for rejection or graft loss which might help in reducing the number of biopsies. Accordingly, CXCL10 shows promise as a marker for identifying post-kidney transplant injuries, particularly rejection, further comprehensive studies are essential. These studies should focus on standardizing factors such as study design, sample type, evaluation methods, types of post-transplant injuries, and patient monitoring for a minimum of 6 months before and after transplantation. Moreover, combining the clinical data of CXCL10 with indicators like serum urea, creatinine, and proteinuria could lead to more precise models for predicting various potential injuries following kidney transplantation.
This manuscript is subject to several limitations. Firstly, numerous articles were excluded from the analysis due to insufficient information for calculating effect size. Additionally, a standardized method for grouping patients was not available, resulting in the comparison of studies with vastly different study groups, making it impossible to merge their results. Moreover, the articles employed two distinct approaches - studying CXCL10 levels and CXCL10/Cr ratios - which are not compatible for combination. These factors led to a significant decrease in the number of studies included in the meta-analysis.
The identification of specific and sensitive biomarkers could potentially reduce unnecessary biopsies, leading to more individualized treatment plans and improved health outcomes. While urinary CXCL10 levels have been studied as an important inflammatory chemokine in kidney post-transplant outcomes, relying solely on CXCL10 is insufficient for determining graft complications. Therefore, considering other critical clinical parameters alongside CXCL10 may facilitate early detection and intervention in graft-related complications. Notably, urinary CXCL10 assessment appears more effective in detecting post-transplant injuries than measuring the CXCL10/Cr ratio.
Data availability
“All data generated or analyzed during this study are included in this published article and its supplementary information file.”
Szumilas K, Wilk A, Wiśniewski P, Gimpel A, Dziedziejko V, Kipp M et al. Current Status regarding immunosuppressive treatment in patients after renal transplantation. Int J Mol Sci. 2023;24(12).
Wojciechowski D, Wiseman A. Long-term immunosuppression management opportunities and uncertainties. Clin J Am Soc Nephrol. 2021;16(8):1264–71.
Article CAS PubMed PubMed Central Google Scholar
Han HS, Lubetzky ML. Immune monitoring of allograft status in kidney transplant recipients. Front Nephrol. 2023;3(November):1–7.
Google Scholar
Guzzi F, Cirillo L, Buti E, Becherucci F, Errichiello C, Roperto RM, et al. Urinary biomarkers for diagnosis and prediction of acute kidney allograft rejection: a systematic review. Int J Mol Sci. 2020;21(18):1–21.
Article Google Scholar
Quaglia M, Merlotti G, Guglielmetti G, Castellano G, Cantaluppi V. Recent advances on biomarkers of early and late kidney graft dysfunction. Int J Mol Sci. 2020;21(15):1–34.
Eikmans M, Gielis EM, Ledeganck KJ, Yang J, Abramowicz D, Claas FFJ. Non-invasive biomarkers of acute rejection in kidney transplantation: Novel targets and strategies. Front Med. 2019;6(JAN).
Loga L, Dican L, Matei HV, Mărunțelu I, Constantinescu I. Relevant biomarkers of kidney allograft rejection. J Med Life. 2022;15(11):1330–3.
Article PubMed PubMed Central Google Scholar
Han S, Zhao W, Wang C, Wang Y, Song R, Haller H, et al. Preliminary investigation of the biomarkers of Acute renal transplant rejection using Integrated Proteomics studies, Gene expression Omnibus datasets, and RNA sequencing. Front Med. 2022;9(May):1–10.
CAS Google Scholar
Sharaby I, Alksas A, Abou El-Ghar M, Eldeeb M, Ghazal M, Gondim D, et al. Biomarkers for kidney-transplant rejection: a short review study. Biomedicines. 2023;11(9):1–30.
Salvadori M. Role of biomarkers in detecting Acute rejection in kidney transplantation. Transplantology. 2023;4(1):18–21.
Dooley BJ, Verma A, Ding R, Yang H, Muthukumar T, Lubetzky M, et al. Urinary cell transcriptome profiling and identification of ITM2A, SLAMF6, and IKZF3 as biomarkers of Acute rejection in human kidney allografts. Transpl Direct. 2020;6(8):1–11.
Hirt-Minkowski P, Handschin J, Stampf S, Hopfer H, Menter T, Senn L, et al. Randomized Trial to assess the clinical utility of renal allograft monitoring by urine CXCL10 chemokine. J Am Soc Nephrol. 2023;34(8):1456–69.
Dabiri S, Kariminik A, Kennedy D. The role of CXCR3 and its ligands in renal transplant outcome. Eur Cytokine Netw. 2016;27(2):16–22.
Gao J, Wu L, Wang S, Chen X. Role of Chemokine (C-X-C Motif) Ligand 10 (CXCL10) in Renal Diseases. Mediators Inflamm. 2020;2020.
Etta P, Rao M. Renal allograft dysfunction: an update on immunological graft injury. Indian J Transpl. 2019;13(2):69.
Schaub S, Nickerson P, Rush D, Mayr M, Hess C, Golian M et al. Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am J Transpl. 2009;9(6).
Jamshaid F, Froghi S, Di Cocco P, Dor FJMF. Novel non-invasive biomarkers diagnostic of acute rejection in renal transplant recipients: a systematic review. Int J Clin Pract. 2018;72(8):1–12.
Hirt-Minkowski P, Rush DN, Gao A, Hopfer H, Wiebe C, Nickerson PW, et al. Six-month urinary CCL2 and CXCL10 levels predict long-term renal allograft outcome. Transplantation. 2016;100(9):1988–96.
Article CAS PubMed Google Scholar
Mühlbacher J, Doberer K, Kozakowski N, Regele H, Camovic S, Haindl S, et al. Non-invasive chemokine detection: improved prediction of antibody-mediated rejection in Donor-specific antibody-positive renal allograft recipients. Front Med. 2020;7(April):1–10.
Rabant M, Amrouche L, Lebreton X, Aulagnon F, Benon A, Sauvaget V, et al. Urinary C-X-C motif chemokine 10 independently improves the Noninvasive diagnosis of antibody-mediated kidney allograft rejection. J Am Soc Nephrol [Internet]. 2015;26:2840–51. Available from: www.jasn.org.
Salvadori M, Tsalouchos A. Biomarkers in renal transplantation: an updated review. World J Transpl. 2017;7(3):161.
Singh N, Samant H, Hawxby A, Samaniego MD. Biomarkers of rejection in kidney transplantation. Curr Opin Organ Transpl. 2019;24(1):103–10.
Article CAS Google Scholar
Moher D, Liberati A, Tetzlaff J, Altman DG, Antes G, Atkins D et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 6, PLoS Med. 2009.
Van Loon E, Tinel C, de Loor H, Bossuyt X, Callemeyn J, Coemans M et al. Automated Urinary Chemokine Assays for Noninvasive Detection of Kidney Transplant Rejection: A Prospective Cohort Study. Am J Kidney Dis [Internet]. 2023;1–10. https://doi.org/10.1053/j.ajkd.2023.07.022
Oktay SB, Akbaş SH, Yilmaz VT, Küçükçetin IÖ, Toru HS, Yücel SG. Association between Graft Function and urine CXCL10 and acylcarnitines levels in kidney transplant recipients. Lab Med. 2022;53(1):78–84.
Raza A, Firasat S, Khaliq S, Aziz T, Mubarak M, Naqvi SAA, et al. The association of urinary interferon-gamma inducible protein-10 (IP10/CXCL10) levels with kidney allograft rejection. Inflamm Res. 2017;66(5):425–32.
Millán O, Budde K, Sommerer C, Aliart I, Rissling O, Bardaji B, et al. Urinary mir-155-5p and CXCL10 as prognostic and predictive biomarkers of rejection, graft outcome and treatment response in kidney transplantation. Br J Clin Pharmacol. 2017;83(12):2636–50.
Ho J, Rush DN, Karpinski M, Storsley L, Gibson IW, Bestland J, et al. Validation of urinary CXCL10 as a marker of borderline, subclinical, and clinical tubulitis. Transplantation. 2011;92(8):878–82.
Matz M, Beyer J, Wunsch D, Mashreghi MF, Seiler M, Pratschke J, et al. Early post-transplant urinary IP-10 expression after kidney transplantation is predictive of short- and long-term graft function. Kidney Int. 2006;69(9):1683–90.
Hu H, Aizenstein BD, Puchalski A, Burmania JA, Hamawy MM, Knechtle SJ. Elevation of CXCR3-Binding chemokines in urine indicates Acute renal-allograft dysfunction. Am J Transpl. 2004;4(3):432–7.
Rabant M, Amrouche L, Morin L, Bonifay R, Lebreton X, Aouni L et al. Early low urinary CXCL9 and CXCL10 might predict immunological quiescence in clinically and histologically stable kidney recipients. Am J Transpl. 2016;16(6).
Lazzeri E, Rotondi M, Mazzinghi B, Lasagni L, Buonamano A, Rosati A et al. High CXCL10 expression in rejected kidneys and predictive role of pretransplant serum CXCL10 for acute rejection and chronic allograft nephropathy. In: Transplantation. 2005.
Jackson JA, Kim EJ, Begley B, Cheeseman J, Harden T, Perez SD, et al. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am J Transpl. 2011;11(10):2228–34.
Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78(5):486–94.
Mattix HJ, Hsu CY, Shaykevich SCG. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13(4):1034–9.
Mitch WE, Collier VU, Walser M. Creatinine metabolism in chronic renal failure. Clin Sci. 1980;58(4):327–35.
GREENBLATT DJ, RANSIL BJ, HARMATZ JS, SMITH TW, DUHME DW, KOCH-WESER J. Variability of 24‐Hour urinary creatinine excretion by normal subjects. J Clin Pharmacol. 1976;16(7):321–8.
Delanaye P, Rozet E, Krzesinski JM, Cavalier E. Urinary NGAL measurement: Biological variation and ratio to creatinine. Clin Chim Acta [Internet]. 2011;412(3–4):390. https://doi.org/10.1016/j.cca.2010.10.011
Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJBJ. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010;77(8):708–14.
Tang KWA, Toh QC, Teo BW. Normalisation of urinary biomarkers to creatinine for clinical practice and research – when and why. Singap Med J. 2015;56(1):7–10.
Download references
Acknowledgements
The authors wish to thank Shiraz University of Medical Sciences for their corporation in data gathering.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Shiraz Nephro-Urology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Sahar Janfeshan, Afsoon Afshari & Jamshid Roozbeh
Shiraz Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Ramin Yaghobi
You can also search for this author in PubMed Google Scholar
Contributions
“The study concept and design, data acquisition and analysis, drafting of manuscript and critical revision of the manuscript were done by S. J., A. A., R.Y., and J. R.”
Corresponding author
Correspondence to Afsoon Afshari .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Janfeshan, S., Afshari, A., Yaghobi, R. et al. Urinary CXCL-10, a prognostic biomarker for kidney graft injuries: a systematic review and meta-analysis. BMC Nephrol 25 , 292 (2024). https://doi.org/10.1186/s12882-024-03728-2
Download citation
Received : 18 December 2023
Accepted : 26 August 2024
Published : 04 September 2024
DOI : https://doi.org/10.1186/s12882-024-03728-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Kidney graft
- Meta-analysis
BMC Nephrology
ISSN: 1471-2369
- General enquiries: [email protected]
- Open access
- Published: 31 August 2024
Impaired glucose metabolism and the risk of vascular events and mortality after ischemic stroke: A systematic review and meta-analysis
- Nurcennet Kaynak ORCID: orcid.org/0000-0002-0637-8421 1 , 2 , 3 , 4 , 5 ,
- Valentin Kennel ORCID: orcid.org/0009-0000-0354-4167 1 , 2 ,
- Torsten Rackoll ORCID: orcid.org/0000-0003-2170-5803 2 , 6 ,
- Daniel Schulze ORCID: orcid.org/0000-0001-9415-2555 7 ,
- Matthias Endres ORCID: orcid.org/0000-0001-6520-3720 1 , 2 , 4 , 5 , 8 &
- Alexander H. Nave ORCID: orcid.org/0000-0002-0101-4557 1 , 2 , 3 , 5
Cardiovascular Diabetology volume 23 , Article number: 323 ( 2024 ) Cite this article
1 Altmetric
Metrics details
Diabetes mellitus (DM), prediabetes, and insulin resistance are highly prevalent in patients with ischemic stroke (IS). DM is associated with higher risk for poor outcomes after IS.
Investigate the risk of recurrent vascular events and mortality associated with impaired glucose metabolism compared to normoglycemia in patients with IS and transient ischemic attack (TIA).
Systematic literature search was performed in PubMed, Embase, Cochrane Library on 21st March 2024 and via citation searching. Studies that comprised IS or TIA patients and exposures of impaired glucose metabolism were eligible. Study Quality Assessment Tool was used for risk of bias assessment. Covariate adjusted outcomes were pooled using random-effects meta-analysis.
Main outcomes
Recurrent stroke, cardiac events, cardiovascular and all-cause mortality and composite of vascular outcomes.
Of 10,974 identified studies 159 were eligible. 67% had low risk of bias. DM was associated with an increased risk for composite events (pooled HR (pHR) including 445,808 patients: 1.58, 95% CI 1.34–1.85, I 2 = 88%), recurrent stroke (pHR including 1.161.527 patients: 1.42 (1.29–1.56, I 2 = 92%), cardiac events (pHR including 443,863 patients: 1.55, 1.50–1.61, I 2 = 0%), and all-cause mortality (pHR including 1.031.472 patients: 1.56, 1.34–1.82, I 2 = 99%). Prediabetes was associated with an increased risk for composite events (pHR including 8,262 patients: 1.50, 1.15–1.96, I 2 = 0%) and recurrent stroke (pHR including 10,429 patients: 1.50, 1.18–1.91, I 2 = 0), however, not with mortality (pHR including 9,378 patients, 1.82, 0.73–4.57, I 2 = 78%). Insulin resistance was associated with recurrent stroke (pHR including 21,363 patients: 1.56, 1.19–2.05, I 2 = 55%), but not with mortality (pHR including 21,363 patients: 1.31, 0.66–2.59, I 2 = 85%).
DM is associated with a 56% increased relative risk of death after IS and TIA. Risk estimates regarding recurrent events are similarly high between prediabetes and DM, indicating high cardiovascular risk burden already in precursor stages of DM. There was a high heterogeneity across most outcomes.
Introduction
Ischemic stroke (IS) is associated with high mortality and high risk of recurrent vascular events worldwide [ 1 , 2 , 3 ]. Despite adequate secondary prevention, about 11% of patients suffer a recurrent stroke within the first year [ 4 ]. Diabetes mellitus (DM) is a highly prevalent cardiovascular risk factor and is present in about one-third of IS patients [ 5 , 6 ]. Stroke prevention guidelines recommend screening for unrecognized DM after IS [ 7 ]. Besides DM, other forms of impaired glucose metabolism (IGM), such as prediabetes and insulin resistance (IR) have been gaining importance over the last decades in terms of their association with increased cardiovascular risk [ 8 ]. Prediabetes, comprising impaired fasting glucose and impaired glucose tolerance, represents a hyperglycemic condition of patients not yet within the diabetic range [ 9 ]. In comparison, IR constitutes a pathophysiological mechanism, which usually precedes and coexists with both DM and prediabetes [ 10 ]. Observational studies report that 70% of the patients with IS have either DM (46%) or prediabetes (24%), and 50% of those who have no DM at baseline have IR [ 11 , 12 ].
Considering that the majority of patients with stroke have some form of IGM, it represents an important aspect of secondary stroke prevention. Numerous studies, including systematic reviews, have shown the association between DM and prediabetes and stroke recurrence [ 13 , 14 , 15 ]. However, only few studies have looked at composite vascular events as an outcome. Furthermore, mortality risk associated with DM after stroke has not been addressed in previous meta-analyses. A comprehensive systematic approach is needed to identify and compare risks associated with composite vascular events and mortality after IS and TIA between different forms of IGM.
Stroke prevention guidelines recommend the use of new generation antidiabetics based on the finding that these agents demonstrated cardiovascular protective effects in patients with previous cardiovascular disease including stroke [ 7 ]. However, only the minority of patients had a history of stroke and subgroup analyses of patients with a previous IS or TIA remained mostly inconclusive [ 16 , 17 ]. In contrast, in the IRIS Trial only patients with IR and a recent IS or TIA were included [ 18 ]. Despite the lower risk of cardiovascular events associated with pioglitazone, the high risk of adverse events restricted the clinical implication of the drug. Currently, it remains unclear which pharmacological treatments are beneficial in terms of secondary stroke prevention in patients with acute or subacute IS or TIA and different forms of IGM.
Identifying increased cardiovascular risk not only in DM but also other forms of IGM would capture a greater population at risk and eventually prompt implementation of secondary preventive measures. We conducted a systematic literature review and meta-analysis to extend our knowledge on the burden of IGM in patients with IS and TIA in the context of cardiovascular events and mortality.
This manuscript adheres to the PRISMA guideline [ 19 ]. Study protocol was pre-registered in open science framework in 2021 [ 20 ].
Information sources
We conducted a systematic literature search on Medline via Pubmed, Ovid via Embase, and Cochrane Library that was last updated on March 21, 2024. Search terms included “diabetes”, “prediabetes”, “insulin resistance”, “stroke” and “transient ischemic attack”, restricted to English language. See full search strategy in supplementary material methods. Reference lists of previous systematic reviews and of studies included in our review were searched manually.
Study selection and data extraction
Screening was performed by two reviewers independently (NK and VK) and consensus was reached with two additional reviewers (TR and AHN) in case of disagreement. Eligible studies were observational studies that included patients within 3 months after an IS or TIA and reported at least one of the following outcomes: composite vascular events, recurrent stroke, cardiovascular and all-cause mortality, cardiac events including but not limited to myocardial infarction, all regardless of follow-up duration (see supplementary Table 1 for the eligibility criteria). Composite events comprised at least stroke, cardiac events, and cardiovascular death. Studies were required to report hazard ratios (HR), odds ratios (OR), or risk ratios using a multivariable model. Exposures of interest were DM, prediabetes and IR, which were included independently of the definition used in the respective study. Additionally, we screened for studies that compared the use of an antidiabetic therapy to placebo or another antidiabetic therapy within the same population and outcomes mentioned above, regardless of study design.
Data extraction and assessment of risk of bias were performed by one reviewer (NK) and the internal validity was checked with a second reviewer (VK) for a random sample of 10% of studies. Interrater reliability was calculated. Authors were contacted via email if substantial outcome data were lacking, unclear or discrepant. Risk of bias assessment was made using the Study Quality Assessment Tool of National Heart, Lung, and Blood Institute [ 21 ]. A detailed methodological description can be found in the methods section of the supplementary material.
Data synthesis
We performed random effects meta-analyses with the restricted maximum likelihood estimator method after grouping studies into outcome measures HR for each study outcome. OR were pooled using meta-regression with follow-up duration as moderator and with random effects meta-analysis if moderator showed no significant effect (p < 0.05). Studies used different sets of covariates that included sociodemographic and clinical characteristics. We included the effect size from the models with the most adjusting factors available. We calculated the 95% confidence interval (CI) and prediction intervals. Prediction intervals describe the expected range of future study results, while confidence intervals relate to the precision of the aggregated effect. Multi-level meta-analysis was performed if multiple subgroups from a single study were included in the analysis. Furthermore, we performed meta-analyses of absolute risks derived from event numbers for each outcome and exposure group, whenever such data were reported. Heterogeneity was assessed using Cochran’s Q and I 2 and was assumed present when p < 0.05 or I 2 > 50% [ 22 ]. Results of meta-analyses were visualized using forest plots. Subgroup analyses were conducted based on history of previous stroke (first-ever event, yes/no) and type of ischemic event (IS/TIA/both). Subgroup analyses based on sex were not conducted because the studies included both sexes in their analyses, and individual patient data were not available. As a sensitivity analysis, we conducted meta-analyses using unadjusted odds ratios. Publication bias was assessed by funnel plots and Egger´s regression. Statistical calculations were performed using the Software R Version 4.0.2 with the package “Metafor” [ 23 ]. Studies investigating the association between antidiabetic therapies and recurrent cardiovascular events after IS or TIA were summarized narratively.
Systematic literature search
The systematic literature search yielded 10,974 records. After screening titles and abstracts, 8,219 records were excluded, and 1,717 records were further screened based on full texts (Fig. 1 ). Finally, 159 studies met the eligibility criteria (supplementary references). Of those, 26 reported data for composite outcome, 71 for recurrent stroke, 10 for cardiac events, 104 for all-cause mortality, and five for cardiovascular mortality (Table 1 ). During data extraction an inter-rater reliability of 90% was reached. Authors of twenty-six studies were contacted for missing information, and seven of them provided the requested data. Most studies were observational studies (n = 146), and others were post-hoc analyses of randomized trials (n = 13). Follow-up duration ranged from end-of-hospital-stay to longer than 20 years. The diagnostic criteria used for DM varied highly including based on medical records or medication history only (n = 61), laboratory biomarkers only (n = 14) and both (n = 50). Twenty-one studies did not report the definition used. Prediabetes was defined either according to American Diabetes Association [ 24 ] or World Health Organization criteria [ 25 ], whereas one study defined prediabetes as a non-fasting glucose level of 140–198 mg/dL. IR was quantified using: HOMA-IR, Triglyceride-Glucose Index, Matsuda Insulin Sensitivity Index, Glucose/Insulin Ratio, QUICKI Index, and estimated glucose disposal rate. Overall, 67% (n = 107) of the included studies were rated as having good quality of evidence, 27% (n = 43) as fair and 6% (n = 9) as poor (supplementary Fig. 1). Study characteristics are presented in supplementary Table 2.

Flowchart of the screening and selection process of the systematic review
Association of IGM with cardiovascular events
Composite vascular events.
Twenty-four studies were eligible for the exposure DM, three studies for prediabetes and two studies for IR. Five studies reporting data from the same cohort were excluded, resulting in 19 eligible studies for the exposure DM (16 reported HR, three reported OR; see supplementary Table 3). Except for one study reporting a 3-month follow-up period, all studies reported at least 1-year follow-up. One study that assessed incident DM during follow-up opposed to pre-existing DM as an exposure was not included in the analysis [ 26 ].
Presence of DM was statistically significantly associated with an increased risk of composite vascular events with a pooled HR (pHR) of 1.58 (95% confidence interval (CI) 1.34 to 1.85, I 2 = 88%) including 445,808 patients (Fig. 2 A) and a pooled OR (pOR) of 1.87 (95% CI 0.76 to 4.60, I 2 = 64%) including 1,609 patients. No publication bias was observed (supplementary Fig. 2). The meta-analysis of absolute risks reported in seven studies revealed that during a mean follow-up of three years, 43% (95% CI 23% to 64%) of stroke patients with DM reached a composite endpoint of a recurrent cardiovascular event or death. This rate was 17% (95% CI 3% to 31%) in patients without DM (supplementary Table 4).

a Forest plot for the meta-analysis of studies that reported the association of diabetes with composite outcome. b Forest plot for the meta-analysis of studies that reported the association of prediabetes with composite outcome
Meta-analysis of two studies showed an increased risk of composite events associated with prediabetes with a pHR of 1.50 (95% CI 1.15 to 1.96, I 2 = 0%; Fig. 2 B) in 8,262 patients. An absolute risk of 31% (95% CI 12% to 50%) and 7% (95% CI 5% to 10%) was observed in the group of patients with and without prediabetes, respectively. IR was reported in two studies, which were derived from the same cohort. One of the studies demonstrated no association between high IR and composite vascular events [ 27 ]. In the other study, which only encompassed patients without DM, increased IR based on HOMA-IR was statistically significantly associated with an increased risk for vascular events [ 28 ].
Recurrent stroke
Sixty-three studies reported recurrent stroke outcome data in patients with DM, see supplementary Table 5. Follow-up duration ranged from discharge from hospital to a mean follow-up time of 12.3 years. Studies encompassing the same population were excluded from the analysis. Finally, 40 studies reporting HR and 12 studies reporting OR were eligible for analysis, respectively. The pHR was 1.42 (95% CI 1.29 to 1.56, I 2 = 92%; Fig. 3 A) involving 1.161.527 patients. There was evidence for possible publication bias (supplementary Fig. 3). Studies that reported OR involving 47,629 patients showed a similar increase of risk (pOR 1.33, 95% CI 1.13 to 1.56, I 2 = 48%; supplementary Fig. 4). Follow-up duration was not a statistically significant moderator for the outcome (p = 0.40). Neither the type of baseline event (IS or TIA), nor previous stroke was a statistically significant moderator (p = 0.08 and p = 0.90, respectively, see supplementary Fig. 5) in subgroup analyses. Baujat plots revealed that the studies contributing most to heterogeneity had a design of post-hoc analysis of randomized trials. Meta-analysis of absolute risks extracted from 23 studies resulted in 13% (95% CI 10% to 16%) for patients with diabetes vs. 9% (95% CI 6% to 11%) without, within a follow-up period of more than a year.
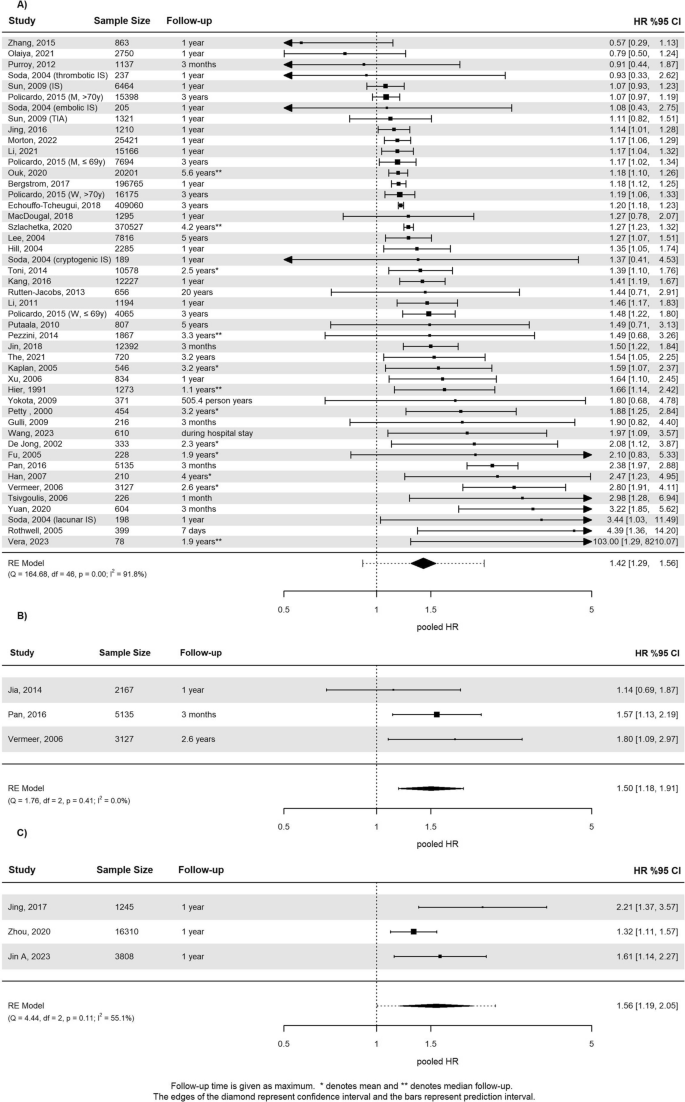
a Forest plot for the meta-analysis of studies that reported the association of diabetes with recurrent stroke. b Forest plot for the meta-analysis of studies that reported the association of prediabetes with recurrent stroke. c Forest plot for the meta-analysis of studies that reported the association of insulin resistance with recurrent stroke
Patients with prediabetes had an increased risk for recurrent stroke compared to patients with normoglycemia (pHR in 10,429 patients 1.50, 95% CI 1.18 to 1.91, I 2 = 0%, see Fig. 3 B). This was also the case in terms of absolute risk 10% (95% CI 8% to 12%) and 7% (95% CI 7% to 8%), respectively. Of five studies eligible for IR, only three could be included in the meta-analysis, because multiple studies were conducted in the same cohort. The pHR for recurrent stroke associated with IR in 21,363 patients was 1.56, 95% CI 1.19 to 2.05, I 2 = 55% (Fig. 3 C). Absolute risks associated with IR during 10.4 months follow-up was 10% (95% CI 5% to 15%) vs. 7% (95% CI 6% to 7%) in patients without increased IR.
Cardiac events
All studies eligible for cardiac events comprised DM as the exposure, see supplementary Table 6. The shortest follow-up time was three months, all other studies followed patients for at least one year. One study that investigated new DM during follow-up was not included in the meta-analysis [ 26 ]. Presence of DM was associated with an increased risk of cardiac events with a pHR of 1.55 (95% CI 1.50 to 1.61, I 2 = 0%) involving 443,863 patients. The pOR of two studies with 839,029 patients was 1.47 (95% CI 0.48 to 4.44), I 2 = 89% (supplementary Fig. 6). Meta-analysis of three studies reporting data revealed an absolute risk of 5% (95% CI − 1% to 11%) in patients with DM and 3% (95% CI 0% to 6%) without DM. One study that investigated prediabetes reported a HR of 2.0 (95% CI 1.30 to 3.20) for cardiac events. No study reported IR as an exposure.
Association between IGM and mortality
Cardiovascular mortality.
Five studies reported data of cardiovascular mortality in patients with DM (supplementary Table 7). Meta-analysis involving 127,445 patients showed a statistically significant association between DM and cardiovascular mortality (pHR 1.65, 95% CI 1.41 to 1.93, I 2 = 50%, see supplementary Fig. 7). Pooling available data of absolute risks from three studies, resulted in a pooled risk of 18% (95% CI −10% to 47%) in patients with DM vs. 16% (95% CI −9% to 41%) in patients without DM, during 1 year of follow-up.
All-cause mortality
Ninety-four studies investigated associations between all-cause mortality and DM, see supplementary Table 8. Studies that included patients from the same population were excluded from the analysis (n = 10). Presence of DM was associated with an increased risk for all-cause mortality (pHR 1.56, 95% CI 1.34 to 1.82, I 2 = 99%, see Fig. 4 A) summarizing 42 studies including 1.031.472 patients. Subgroup analyses based on follow-up duration resulted in a pHR of 1.10 (95% CI 0.72 to1.68) during hospitalization (n = 3 studies), pHR of 1.35 (95% CI 1.18 to 1.56) up to one year (n = 12 studies), and pHR of 1.74 (95% CI 1.40 to 2.17) longer than one year (n = 27 studies). However, follow-up duration was not revealed as a statistically significant moderator (p = 0.15, see supplementary Fig. 8). The Galbraith plot revealed the most influential studies to be the subgroups of the study from Zamir et al. (supplementary Fig. 9). The meta-analysis of forty-two studies involving 3.290.353 patients reporting OR showed a risk estimate of 1.30 (95% CI 1.21 to 1.41, see supplementary Fig. 10). Subgroup analyses based on first-ever vs. recurrent event at baseline and the type of ischemic event revealed no statistically significant difference between groups. Funnel plots suggested existence of publication bias (supplementary Fig. 11). During a mean follow-up of 1.8 months, the absolute risk of all-cause mortality was 23% (95% CI 14% to 31%) for patients with DM vs. 17% (95% CI 11% to 23%) without DM.

a Forest plot for the meta-analysis of studies that reported the association of diabetes with all-cause mortality. b Forest plot for the meta-analysis of studies that reported the association of prediabetes with all-cause mortality. c Forest plot for the meta-analysis of studies that reported the association of insulin resistance with all-cause mortality
Six studies were eligible for prediabetes and all-cause mortality (3 HR, 3 OR). Prediabetes was not statistically significantly associated with an increased risk for mortality after IS (pHR 1.82, 95% CI 0.73 to 4.57, I 2 = 78% in 9,378 patients, and pOR 1.37, 95% CI 0.54 to 3.43, I 2 = 71% in 1,969 patients, see Fig. 4 B & supplementary Fig. 12). Meta-analysis of absolute risks during a mean follow-up of seven months was 8% (95% CI 2% to 15%) for patients with prediabetes vs. 9% (95% CI 0% to 18%) with normoglycemia.
Nine studies reported IR as an exposure. The meta-analyses could not demonstrate an association between increased IR and mortality (pHR 1.31, 95% CI 0.66 to 2.59, I 2 = 85%, including 21,363 patients across three studies and pOR 1.05, 95% CI 0.76 to 1.45, I 2 = 16%, including 6,434 patients across 2 studies). Absolute risks were 6% (95% CI -1% to 12%) for patients with increased IR and 4% (95% CI 2% to 6%) without.
Sensitivity analyses with crude odds ratios
Sensitivity analyses using unadjusted odds ratios, to accommodate the variation in adjustment factors used across studies, revealed similar risk estimates, though often slightly higher than the respective adjusted pooled outcomes (supplementary Fig. 13 and 14).
Antidiabetic therapy and recurrent vascular events
Nine observational studies investigated the association between antidiabetic therapies and cardiovascular events after an IS or TIA in the preceding three months, see Table 2 . The drug classes investigated were metformin, sulfonylurea, thiazolidinedione, and incretin-mimetics. We did not identify and studies with SGLT-2 Inhibitors or alfa glucosidase inhibitors. Due to the differences in the exposure and comparator groups, we did not perform a meta-analysis. Studies showed a risk reduction for recurrent stroke, mortality and composite vascular events associated with the use of pioglitazone and lobeglitazone as well as a lower risk of mortality associated with metformin use [ 29 , 30 , 31 , 32 ]. There were no clear benefits in terms of decreased risk of cardiovascular events associated with sulfonylurea or incretin-mimetics [ 33 , 34 , 35 , 36 , 37 ].
In this systematic review and meta-analysis, we provide a comprehensive and up-to-date summary of previous studies investigating the association between IGM and residual cardiovascular risk following IS and TIA. To our knowledge, this is the first meta-analysis to investigate the risk of composite vascular events associated with IGM as well as the risk of mortality associated with DM in this population. The results of the presented meta-analysis indicate that (1) patients with DM have an approximately 1.6-fold (60%) increased risk of both death and recurrent vascular events after IS and TIA, (2) the risk of recurrent vascular events after stroke is already increased in the prediabetic stage and appears just as high as in patients with DM, and (3) presence of IR is associated with recurrent stroke risk. In contrast, this meta-analysis was unable to demonstrate an increased mortality risk after stroke associated with prediabetes or IR. Overall, there were significantly fewer eligible studies on prediabetes and IR compared to DM (Table 1 ).
DM is a well-known risk factor for cardiovascular disease. The results of our study confirm a robust association between DM and risk of composite recurrent vascular events after IS and TIA. We could confirm the risk of recurrent stroke associated with DM that was previously reported in a meta-analysis by Zhang et al . [ 14 ] The risk of mortality in patients with DM is observed to be 56% higher compared to patients without DM. Although mortality risk estimates were greater for diabetic patients with increasing mean follow-up durations of studies, we could not observe a statistically significant interaction between mortality risk and follow-up duration. This could be due to the fact that there were only a few studies with short-term follow-up in studies that reported HR (supplementary Fig. 8) and only a few studies with long-term follow-up in studies that reported OR (supplementary Fig. 10). Still, inferring from this finding, DM likely remains a relevant risk factor over time and an important target for secondary prevention strategies, given the high prevalence of DM in this population [ 6 ].
Our analyses demonstrated a positive relationship between prediabetes and recurrent vascular events as well as between IR and stroke recurrence. However, there was no association detected between the two conditions and mortality. This difference could have several reasons: First, patients with prediabetes or IR are less likely to have been exposed to deleterious effects of a dysregulated glucose metabolism for a longer time, compared to patients with DM. Second, the shorter follow-up duration of studies investigating prediabetes and IR generally limits the probability to detect difference in mortality risk. The risk associated with prediabetes and recurrent stroke is in line with a previous meta-analysis conducted by Pan et al . in 2019 [ 15 ]. Despite substantial methodological differences such as avoiding pooling ORs and HRs together and excluding studies with hemorrhagic stroke in our study, also having identified two more studies, similar to Pan et al ., we also could not demonstrate a relationship between prediabetes and mortality.
Contrary to DM, prediabetes has rather recently been regarded as a cardiovascular risk factor [ 39 ]. The meta-analysis conducted by Cai et al. showed a risk increase in all-cause mortality and vascular events associated with prediabetes in population-based cohorts as well as in patients with previous atherosclerotic disease [ 40 ]. Further, a recent analysis of the UK Biobank cohort including more than 400 thousand individuals confirmed the excess risk for any cardiovascular disease in patients with IGM compared to normoglycemia [ 41 ]. The risk was higher for DM than for prediabetes. Still, after accounting for obesity and use of antihypertensive and statins both risks were attenuated, lending support to the modifiability of the excess risk. Together with these previous findings, our results strongly support considering prediabetes as a continuous entity with DM on the spectrum of IGM, with a relevant increase in cardiovascular and mortality risk.
There was a statistically significant association between increased IR and stroke recurrence. However, it should be noted that, there were only three studies eligible for the analysis and the parameters used to define an increased IR as well as the timing of measurement after stroke (7 days and 14 days) was heterogeneous between studies. IR can be increased during the acute phase of the stroke due to the stress reaction and show changes during this time [ 42 ]. The increased relative risk for recurrent stroke observed in patients with IR compared to patients without IR was higher than the relative risk in diabetics compared to non-diabetics. This might be explained by the differences in the patient groups. Patients with DM are more likely to receive antidiabetic treatment and have a higher risk of dying before suffering a recurrent stroke. Another difference could be in the comparator groups, namely that the patients without IR could be generally healthier than patients without DM.
Despite the association between increased IR and stroke recurrence, we could not identify many studies with other cardiovascular outcomes. Furthermore, we encountered different parameters and criteria to define IR across studies. Thus, prognostic value of increased IR in terms of composite cardiovascular risk as well as the best biomarker to predict the said risk remains speculative in patients with IS or TIA. Further research is needed to investigate this conundrum.
We observed a significant research gap in the number of large studies with congruent definitions of prediabetes and IR. Uncertainty remains about the different diagnostic criteria for both prediabetes and IR [ 24 , 25 , 43 , 44 ], leading to the lack of adequate implementation of preventive strategies [ 45 ]. As the prevalence of prediabetes expected to rise, the whole spectrum of IGM rather than DM alone is assumed to gain more significance in terms of primary and secondary stroke prevention [ 46 ]. Consistent diagnostic criteria would facilitate a reliable data synthesis and the development of prevention strategies.
Until the advent of the GLP1 and SGLT2 therapies, no antidiabetic therapy has improved cardiovascular risk or death despite improvements in glucose control [ 47 ]. Both classes of drugs revolutionized the field after randomized controlled trials showed cardiovascular risk reduction in patients with DM [ 48 , 49 , 50 , 51 ]. However, until now, it is unclear if these drugs are equally effective at reducing cardiovascular risk in patients with IS [ 33 , 34 , 52 ]. As our systematic review indicates, to date, only few studies exist that investigated the effectiveness of antidiabetic therapy in preventing recurrent vascular events after an acute or subacute IS. Even though the promising results related to pioglitazone use in patients with IR from the IRIS trial unfortunately faced a limitation due to side effects [ 18 ], recent cohort studies shown beneficial effects associated with thiazolidinediones [ 29 , 30 ]. Clinical trial investigating secondary stroke prevention in patients with prediabetes are yet to been undertaken.
Strengths and limitations
The most important strength of our study lies in the comprehensiveness, encompassing over 10.000 records and having included more than seven million patients over all exposures and outcomes. This enabled us to investigate all three entities of IGM together. Another strength constitutes the methodology. We included studies with both outcome measures HR and OR, which led us to identify more studies. We also used multi-level meta-analysis to account for multiple subgroups of the same cohorts and used meta-regression to account for moderators.
There are limitations to this study. Firstly, as in every meta-analysis, the quality of synthesized evidence depends on the quality of evidence of the individual studies. We assessed the risk of bias of the included studies and could not identify an influence of studies with high risk of bias on the effect estimates. Secondly, we encountered high heterogeneity between studies. As this systematic review included observational studies, the high variability across study populations and diagnostic criteria used was expected. Further, the fact that studies used different adjustment factors in their multivariable analyses most likely contributed substantially to the high heterogeneity. To alleviate the difference in the adjustment factors, we have conducted sensitivity analyses. Both crude odds ratios and absolute risks indicated a similar change of risk estimates to the per protocol analyses, strengthening our primary results. Another factor contributing to heterogeneity could be methodological differences between studies, such as how competing events were treated. This could not be taken into consideration when determining eligibility, since the information was mostly not available. Finally, severity and duration of DM could not be taken into consideration.
Different types of IGM are associated with increased cardiovascular risk and mortality after IS and TIA. The entities of IGM should be considered as a continuous spectrum with increased cardiovascular risk that represent an important target for early cardiovascular prevention programs.
Availability of data and materials
The extracted data from the involved studies in this systematic review have been made available in supplementary material.
Abbreviations
- Ischemic stroke
Diabetes mellitus
Impaired glucose metabolism
- Insulin resistance
Hazard ratios
Odds ratios
Confidence interval
GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. https://doi.org/10.1016/S1474-4422(21)00252-0 .
Article Google Scholar
Chen Y, Wright N, Guo Y, et al. Mortality and recurrent vascular events after first incident stroke: a 9-year community-based study of 0·5 million Chinese adults. Lancet Glob Health. 2020;8(4):e580–90. https://doi.org/10.1016/S2214-109X(20)30069-3 .
Article PubMed PubMed Central Google Scholar
Carlsson A, Irewall AL, Graipe A, Ulvenstam A, Mooe T, Ögren J. Long-term risk of major adverse cardiovascular events following ischemic stroke or TIA. Sci Rep. 2023;13(1):8333. https://doi.org/10.1038/s41598-023-35601-x .
Article PubMed PubMed Central CAS Google Scholar
Mohan KM, Wolfe CDA, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42(5):1489–94. https://doi.org/10.1161/STROKEAHA.110.602615 .
Article PubMed Google Scholar
Sarwar N, Gao P, Kondapally Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. The Lancet. 2010;375(9733):2215–22. https://doi.org/10.1016/S0140-6736(10)60484-9 .
Article CAS Google Scholar
Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J Diabetes Investig. 2019;10(3):780–92. https://doi.org/10.1111/jdi.12932 .
Kleindorfer DO, Towfighi A, Chaturvedi S, et al. Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021. https://doi.org/10.1161/str.0000000000000375 .
Schlesinger S, Neuenschwander M, Barbaresko J, et al. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65(2):275–85. https://doi.org/10.1007/s00125-021-05592-3 .
Article PubMed CAS Google Scholar
Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–9. https://doi.org/10.2337/dc07-9920 .
Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–9. https://doi.org/10.2337/dc05-2179 .
Jia Q, Zheng H, Zhao X, et al. Abnormal glucose regulation in patients with acute stroke across China: prevalence and baseline patient characteristics. Stroke. 2012;43(3):650–7. https://doi.org/10.1161/STROKEAHA.111.633784 .
Kernan WN, Inzucchi SE, Viscoli CM, et al. Impaired insulin sensitivity among nondiabetic patients with a recent TIA or ischemic stroke. Neurology. 2003;60(9):1447–51. https://doi.org/10.1212/01.WNL.0000063318.66140.A3 .
Echouffo-Tcheugui JB, Xu H, Matsouaka RA, et al. Diabetes and long-term outcomes of ischaemic stroke: findings from Get With The Guidelines-Stroke. Eur Heart J. 2018;39(25):2376–86. https://doi.org/10.1093/eurheartj/ehy036 .
Zhang L, Li X, Wolfe CDA, O’Connell MDL, Wang Y. Diabetes as an independent risk factor for stroke recurrence in ischemic stroke patients: an updated meta-analysis. Neuroepidemiology. 2021;55(6):427–35. https://doi.org/10.1159/000519327 .
Pan Y, Chen W, Wang Y. Prediabetes and outcome of ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2019;28(3):683–92. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.11.008 .
Strain WD, Frenkel O, James MA, et al. Effects of semaglutide on stroke subtypes in type 2 diabetes: post hoc analysis of the randomized SUSTAIN 6 and PIONEER 6. Stroke. 2022;53(9):2749–57. https://doi.org/10.1161/STROKEAHA.121.037775 .
Zhou Z, Lindley RI, Rådholm K, et al. Canagliflozin and stroke in type 2 diabetes mellitus. Stroke. 2019;50(2):396–404. https://doi.org/10.1161/STROKEAHA.118.023009 .
Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–31. https://doi.org/10.1056/NEJMoa1506930 .
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. https://doi.org/10.1136/bmj.n71 .
Kaynak N, Rackoll T, Endres M, Nave AH. The residual risk of impaired glucose metabolism on vascular events and mortality after ischemic stroke and the effect of antidiabetic therapy on reducing this risk: A systematic review and Meta-analysis. 2021. https://doi.org/10.17605/OSF.IO/JVYHW .
National Heart Lung and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2013. Accessed December 18, 2020. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools .
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557 .
Viechtbauer W. metafor: Meta-analysis package for R. R package version 2.4–0. R package version 24–0. 2020;(1):1–275.
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(January):S14-S31. https://doi.org/10.2337/dc20-S002 .
Geneva: World Health Organization. Classification of Diabetes Mellitus. 2019.
Rutten-Jacobs LCA, Keurlings PAJ, Arntz RM, et al. High incidence of diabetes after stroke in young adults and risk of recurrent vascular events: The FUTURE study. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0087171 .
Lu Z, Xiong Y, Feng X, et al. Insulin resistance estimated by estimated glucose disposal rate predicts outcomes in acute ischemic stroke patients. Cardiovasc Diabetol. 2023;22(1):225. https://doi.org/10.1186/s12933-023-01925-1 .
Jin A, Wang S, Li J, et al. Mediation of systemic inflammation on insulin resistance and prognosis of nondiabetic patients with ischemic stroke. Stroke. 2023;54(3):759–69. https://doi.org/10.1161/STROKEAHA.122.039542 .
Yoo J, Jeon J, Baik M, Kim J. Lobeglitazone, a novel thiazolidinedione, for secondary prevention in patients with ischemic stroke: a nationwide nested case-control study. Cardiovasc Diabetol. 2023;22(1):106. https://doi.org/10.1186/s12933-023-01841-4 .
Woo MH, Lee HS, Kim J. Effect of pioglitazone in acute ischemic stroke patients with diabetes mellitus: a nested case-control study. Cardiovasc Diabetol. 2019;18(1):67. https://doi.org/10.1186/s12933-019-0874-5 .
Morgan CL, Inzucchi SE, Puelles J, Jenkins-Jones S, Currie CJ. Impact of treatment with pioglitazone on stroke outcomes: a real-world database analysis. Diabetes Obes Metab. 2018;20(9):2140–7. https://doi.org/10.1111/dom.13344 .
Tu WJ, Liu Z, Chao BH, et al. Metformin use is associated with low risk of case fatality and disability rates in first-ever stroke patients with type 2 diabetes. Ther Adv Chronic Dis. 2022;13:20406223221076896. https://doi.org/10.1177/20406223221076894 .
Chen DY, Wang SH, Mao CT, et al. Sitagliptin after ischemic stroke in type 2 diabetic patients: a nationwide cohort study. Medicine. 2015;94(28): e1128. https://doi.org/10.1097/MD.0000000000001128 .
Chen DY, Li YR, Mao CT, et al. Cardiovascular outcomes of vildagliptin in patients with type 2 diabetes mellitus after acute coronary syndrome or acute ischemic stroke. J Diabetes Investig. 2020;11(1):110–24. https://doi.org/10.1111/jdi.13078 .
Li YR, Tsai SS, Chen DY, et al. Linagliptin and cardiovascular outcomes in type 2 diabetes after acute coronary syndrome or acute ischemic stroke. Cardiovasc Diabetol. 2018;17(1):2. https://doi.org/10.1186/s12933-017-0655-y .
Favilla CG, Mullen MT, Ali M, Higgins P, Kasner SE. Sulfonylurea use before stroke does not influence outcome. Stroke. 2011;42(3):710–5. https://doi.org/10.1161/STROKEAHA.110.599274 .
Tsivgoulis G, Goyal N, Iftikhar S, et al. Sulfonylurea Pretreatment and In-Hospital Use Does Not Impact Acute Ischemic Strokes (AIS) Outcomes Following Intravenous Thrombolysis. J Stroke Cerebrovasc Dis. 2017;26(4):795–800. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.10.019 .
Horsdal HT, Mehnert F, Rungby J, Johnsen SP. Type of preadmission antidiabetic treatment and outcome among patients with ischemic stroke: a nationwide follow-up study. J Stroke Cerebrovasc Dis. 2012;21(8):717–25. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.03.007 .
Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10(10): CD012661. https://doi.org/10.1002/14651858.CD012661.pub2 .
Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370: m2297. https://doi.org/10.1136/bmj.m2297 .
Rentsch CT, Garfield V, Mathur R, et al. Sex-specific risks for cardiovascular disease across the glycaemic spectrum: a population-based cohort study using the UK Biobank. The Lancet Regional Health - Europe. 2023;32:1–14. https://doi.org/10.1016/j.lanepe.2023.100693 .
Huff TA, Lebovitz HE, Heyman A, Davis L. Serial changes in glucose utilization and insulin and growth hormone secretion in acute cerebrovascular disease. Stroke. 1972;3(5):543–52. https://doi.org/10.1161/01.STR.3.5.543 .
Cleeman JI. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J Am Med Assoc. 2001;285(19):2486–97. https://doi.org/10.1001/jama.285.19.2486 .
Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289–304. https://doi.org/10.2337/diacare.28.9.2289 .
Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2020;42:59–77. https://doi.org/10.1146/annurev-publhealth-090419-102644 .
Lee M, Saver JL, Hong KS, Song S, Chang KH, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ. 2012;344: e3564. https://doi.org/10.1136/bmj.e3564 .
Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. https://doi.org/10.1056/NEJMoa1607141 .
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720 .
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. https://doi.org/10.1056/NEJMoa1611925 .
Dawson J, Béjot Y, Christensen LM, et al. European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack. Eur Stroke J. 2022;7(3):I–II. https://doi.org/10.1177/23969873221100032 .
Gerstein HC, Hart R, Colhoun HM, et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes Endocrinol. 2020;8(2):106–14. https://doi.org/10.1016/S2213-8587(19)30423-1 .
Download references
This study was partially funded by the Corona Foundation. Protocol https://osf.io/jvyhw . The funder had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Center for Stroke Research Berlin (CSB), Charité– Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Berlin, Germany
Nurcennet Kaynak, Valentin Kennel, Matthias Endres & Alexander H. Nave
Department of Neurology with Experimental Neurology, Charité– Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Charitéplatz 1, 10117, Berlin, Germany
Nurcennet Kaynak, Valentin Kennel, Torsten Rackoll, Matthias Endres & Alexander H. Nave
Berlin Institute of Health at Charité, Charité– Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Berlin, Germany
Nurcennet Kaynak & Alexander H. Nave
German Center for Neurodegenerative Diseases (DZNE), partner site Berlin, Berlin, Germany
Nurcennet Kaynak & Matthias Endres
German Center for Cardiovascular Research (DZHK), partner site Berlin, Berlin, Germany
Nurcennet Kaynak, Matthias Endres & Alexander H. Nave
Berlin Institute of Health (BIH) QUEST Center for Responsible Research, Charité– Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt Universität zu Berlin, Berlin, Germany
Torsten Rackoll
Department of Biometry and Clinical Epidemiology, Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin und Humboldt-Universität zu Berlin, Berlin, Germany
Daniel Schulze
German Center for Mental Health (DZPG), partner site Berlin, Berlin, Germany
Matthias Endres
You can also search for this author in PubMed Google Scholar
Contributions
NK had full access to study data and is the guarantor of the study, taking full responsibility for the conduct of the study. NK, AHN, TR and ME conceived the study design and contributed to study protocol. NK, VK, and TR acquired data and performed the analysis. DS contributed to statistical methods and analyses. NK drafted the manuscript and all authors contributed to interpretation of the data and critical appraisal of the final work. AHN supervised the study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Correspondence to Alexander H. Nave .
Ethics declarations
Ethics approval.
Ethics approval was not required.
Consent for publication
Not applicable.
Competing interests
NK, VK, DS report no conflicts of interest. ME reports grants from Bayer and fees paid to the Charité from Amgen, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, BMS, Daiichi Sankyo, Sanofi, Pfizer, all outside the submitted work. AHN receives funding from the Corona foundation and the German Center for cardiovascular research (DZHK), no conflict of interest. TR receives funding from the European Commission, no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1 (docx 3212 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kaynak, N., Kennel, V., Rackoll, T. et al. Impaired glucose metabolism and the risk of vascular events and mortality after ischemic stroke: A systematic review and meta-analysis. Cardiovasc Diabetol 23 , 323 (2024). https://doi.org/10.1186/s12933-024-02413-w
Download citation
Received : 30 June 2024
Accepted : 19 August 2024
Published : 31 August 2024
DOI : https://doi.org/10.1186/s12933-024-02413-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Prediabetes
- Vascular events
Cardiovascular Diabetology
ISSN: 1475-2840
- Submission enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
A systematic approach to searching: an efficient and ...
INTRODUCTION. A key factor affecting the quality of evidence syntheses, such as systematic reviews (SRs), is the inclusion of a comprehensive, reproducible, and well-conducted systematic search strategy [].An inadequate or poorly implemented search can miss relevant studies (i.e., poor recall) and impact the findings of the SR [].It may also increase the number of irrelevant articles that need ...
The authors have established a method that describes step by step the process of developing a systematic search strategy as needed in the systematic review. This method describes how single-line search strategies can be prepared in a text document by typing search syntax (such as field codes, parentheses, and Boolean operators) before copying ...
A systematic review differs from other types of literature review in several major ways. It requires a transparent, reproducible methodology which indicates how studies were identified and the criteria upon which they were included or excluded. ... Search strategy. The search strategy for systematic reviews is the main method of collecting the ...
How to write a search strategy for your systematic review
The systematic literature review, widely regarded as the gold standard for determining evidence-based practice, is increasingly used to guide policy decisions and the direction of future research. ... DEVELOPING THE SEARCH STRATEGY. A review protocol with a clearly defined review question and inclusion criteria will provide the foundation for ...
Search strategy formulation for systematic reviews: Issues ...
Developing a Search Strategy - Systematic Review
For the protocol-driven archetypes systematic literature reviews and systematic reviews a search strategy based on keywords, controlled vocabulary and databases is the standard; see Section 5.4. However, for the latter search strategy, normally complementary search strategies are necessary as discussed in Section 5.6.
Defining the process to literature searching in systematic ...
A systematic review aims to review as much of the literature as possible to minimize bias. As such, it is recommended that at least 3 databases are searched for a systematic review. As PubMed/MEDLINE excludes newer journals and health sciences disciplines, additional databases must be used to minimize publication bias.
Steps of Building Search Strategies. These are the steps required when developing a comprehensive search strategy for a systematic review: 1. Formulate the research question. 2. Identify the key concepts. 3. Develop search terms - free-text terms. 4. Develop search terms - controlled vocabulary terms. 5. Search fields. 6.
A guide to the literature search for a systematic review Am J Nurs. 2014 May;114(5):49-56. doi: 10.1097/01.NAJ.0000446779.99522.f6. ... This article details the major considerations surrounding search strategies and presents an example of a search using the PubMed platform (pubmed.gov).
PRISMA Items 6-7 are: "Specify all databases, registers, websites, organizations, reference lists and other source searched or consulted to identify studies. Specify the date when each source was last searched or consulted." "Present the full search strategies for all databases, registers and websites, including any filters and limits used."
Search Strategy - Systematic Reviews - Research Guides
Researchers conducting a systematic literature review need to perform comprehensive searches to ensure they have retrieved all of the relevant information. Below is an overview of the steps involved in conducting a search for literature. For further information on conducting a comprehensive search, please see the Cochrane handbook. Scope the topic.
Drawing up your search strategy - Searching for Systematic ...
Developing a search strategy - Systematic Review
In preparing for your review, you will begin with an exploratory or preliminary search of the literature. This search will help you: Identify existing reviews (so as not to duplicate work), Assess the quantity and quality of relevant, primary research studies (so as to be sufficiently abundant and productive for your work), and
Background. Systematic literature searching is recognised as a critical component of the systematic review process. It involves a systematic search for studies and aims for a transparent report of study identification, leaving readers clear about what was done to identify studies, and how the findings of the review are situated in the relevant evidence.
It is important to keep track of your searches and results every step of the way in a Systematic Review. If you've read the PRISMA Guidelines, you know that there is a flow chart that should be included with your review which maps the results found, excludes vs. includes, reasons for decisions and dates. A spreadsheet in Excel can help you keep ...
Search strategy. This systematic literature review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [].Embase, MEDLINE and the Cochrane Library were searched for studies related to the clinical and socioeconomic burden of bronchiectasis (noncystic fibrosis bronchiectasis (NCFBE) and cystic fibrosis bronchiectasis (CFBE)) using ...
Time: On average, systematic reviews can require up to 18 months of preparation.. A team: A systematic review can't be done alone! You need to work with subject experts to clarify issues related to the topic; librarians to develop comprehensive search strategies and identify appropriate databases; reviewers to screen abstracts and read the full text; a statistician who can assist with data ...
Literature Search Strategy. A systematic literature review following the PRISMA review guidelines was conducted in the databases Scopus, ERIC, Academic Research Complete, ProQuest, APA PsycArticles, SPORTDiscus, Web of Science, and Humanities International Complete. Google Scholar was also searched for meta-analyses focused on formative ...
A protocol covering all aspects of the systematic review methodology was developed before starting the review. The protocol included the definition of: a focused question; the literature search strategy; the study selection criteria; the outcome measures; the screening methods; the data extraction; and the data synthesis.
The full search strategy including all variant terms used is available in Appendix A. No language limits were applied. ... This systematic review synthesised available evidence of strategies to address hesitancy and increase COVID-19 vaccination uptake among First Nations peoples. To our knowledge, this is the only systematic review examining ...
Therefore, this paper provides a Systematic Literature Review (SLR) of deep learning-based text summarization in both types (extractive and abstractive) between 2014 and 2023. To the best of our knowledge, this is the first SLR that offers a comprehensive overview of extractive and abstractive text summarization techniques based on Deep ...
Based on the selected search strategy, 46 articles (out of 672) were chosen for closer examination. The contributions of these articles were discussed and summarized, highlighting their strengths and limitations. ... Hence, this paper provides a systematic literature review and examination of state-of-the-art studies in blockchain forensics to ...
Literature search. This review is conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [].The objectives of the study, the search strategy, inclusion and exclusion criteria, and study evaluation method were carefully designed, refined, and unanimously approved by all contributing authors well in advance.
Search terms included "diabetes", "prediabetes", "insulin resistance", "stroke" and "transient ischemic attack", restricted to English language. See full search strategy in supplementary material methods. Reference lists of previous systematic reviews and of studies included in our review were searched manually.