Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 August 2018

The neuropathology of bipolar disorder: systematic review and meta-analysis
- Paul J. Harrison ORCID: orcid.org/0000-0002-6719-1126 1 , 2 ,
- Lucy Colbourne 1 , 2 &
- Charlotte H. Harrison ORCID: orcid.org/0000-0003-3772-219X 3
Molecular Psychiatry volume 25 , pages 1787–1808 ( 2020 ) Cite this article
12k Accesses
35 Citations
5 Altmetric
Metrics details
- Bipolar disorder
- Neuroscience
Various neuropathological findings have been reported in bipolar disorder (BD). However, it is unclear which findings are well established. To address this gap, we carried out a systematic review of the literature. We searched over 5000 publications, identifying 103 data papers, of which 81 were eligible for inclusion. Our main findings can be summarised as follows. First, most studies have relied on a limited number of brain collections, and have used relatively small sample sizes (averaging 12 BD cases and 15 controls). Second, surprisingly few studies have attempted to replicate closely a previous one, precluding substantial meta-analyses, such that the latter were all limited to two studies each, and comprising 16–36 BD cases and 16–74 controls. As such, no neuropathological findings can be considered to have been established beyond reasonable doubt. Nevertheless, there are several replicated positive findings in BD, including decreased cortical thickness and glial density in subgenual anterior cingulate cortex, reduced neuronal density in some amygdalar nuclei, and decreased calbindin-positive neuron density in prefrontal cortex. Many other positive findings have also been reported, but with limited or contradictory evidence. As an important negative result, it can be concluded that gliosis is not a feature of BD; neither is there neuropathological evidence for an inflammatory process.
Similar content being viewed by others
Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review
Individuals at increased risk for development of bipolar disorder display structural alterations similar to people with manifest disease
Mania-related effects on structural brain changes in bipolar disorder – a narrative review of the evidence
Introduction.
Like other ‘functional’ psychiatric disorders, bipolar disorder (BD) lacks any diagnostic neuropathology of the kind which characterises and defines the dementias, but this does not mean that BD has no morphological correlates. Magnetic resonance imaging (MRI) studies show small but robust differences in the volumes of some brain structures, notably decreases in hippocampus, amygdala and thalamus, and reduced cortical thickness [ 1 , 2 ]. There is also increasing evidence for white matter decrements [ 3 , 4 ] with anatomical and functional dysconnectivity of specific pathways and circuits [ 5 , 6 ]. Presumably these neuroimaging findings are reflected in alterations at the histological and cellular level. However, a review covering the period up to 1999 noted the remarkable lack of data [ 7 ]. By the definition of neuropathology adopted here (see Methods and Materials), the literature at that time comprised only nine publications. Vawter and colleagues [ 7 ] drew attention to some preliminary findings, notably a report of decreased glial density in the subgenual anterior cingulate cortex (sgACC) in BD and major depressive disorder [ 8 ], and a pilot study describing decreased interneuron density in the hippocampal CA2 subfield [ 9 ].
Öngür et al. (1998) [ 8 ] remains by some distance the most cited paper on the neuropathology of BD (Supplementary Table 1 ), and it was soon followed by a series of other morphometric studies. Many took advantage of the brain series collected by the Stanley Foundation (subsequently the Stanley Medical Research Institute), called the Stanley Neuropathology Consortium [ 10 ]. For the first time, this provided brain tissue specifically designed to allow comparison of BD, schizophrenia and major depressive disorder with healthy comparison subjects ( n = 15 in each group) [ 10 ]. Moreover, tissue was provided blind to diagnosis such that researchers had to include BD, even if their primary interest lay in one of the other diagnoses.
An updated narrative review of the neuropathology of mood disorder was reported by Harrison (2002) [ 11 ], by which time the BD literature extended to 27 papers. More recently, Price and Drevets (2010) [ 12 ] reviewed mood disorder neuropathology in the context of neuroimaging findings and normative brain connectivity, and Savitz et al. (2014) [ 13 ] focused on the prefrontal cortex. To our knowledge, there has been no substantive review covering BD neuropathology since then, and there has never been a systematic review. Here, we report the latter, accompanied by meta-analyses where possible. The review was registered on the PROSPERO international prospective register of systematic reviews (CRD42018089740).
Methods and Materials
Scope of systematic review.
We adopted a pragmatic definition of neuropathology to comprise studies which measured ‘visible’ parameters such the density, number, size or shape of cells (neurons, glia or subpopulations thereof), cellular constituents (e.g. synapses, dendrites and mitochondria), or cytopathological elements (e.g. neurofibrillary tangles, amyloid plaques), in patients with BD compared to control subjects. We included studies which identified neuronal and glial populations using antigens generally recognised for this purpose (e.g. parvalbumin [PV], glial fibrillary acidic protein [GFAP], ionised calcium-binding adaptor molecule 1 [Iba-1]). We also included studies which measured the size of a brain structure (e.g. cortical thickness, hippocampal volume). We excluded studies which used messenger RNAs to delineate cell populations or which used proteins as proxy markers of sub-cellular compartments (e.g. synaptophysin as a marker of presynaptic terminals, or spinophilin for dendritic spines). We also excluded studies using brain homogenates.
Literature search and data extraction
We identified publications by searching the Web of Science Core Collection (1945 to 8th June 2018) and MEDLINE (1950 to 8 th June 2018) using the following search terms: (‘bipolar disorder’ or ‘bipolar affective’ or ‘bipolar illness’ or ‘bipolar disease’ or ‘manic-depressi*’ or ‘manic depressi*’) and (‘neuropatholog*’ or ‘morphometr*’ or ‘neuron*’ or ‘glia*’ or ‘pyramidal’ or ‘oligodendro*’ or ‘astrocyt*’ or ‘astrogli*’ or ‘microgli*’ or ‘*gliosis’). We also searched papers’ reference lists and PJH’s reprint collection to identify additional studies meeting our criteria. We did not consider papers including less than three BD cases, conference abstracts, non-peer reviewed publications (e.g. book chapters), nor data papers not published in English. Two authors (PJH, with either LC or CHH) independently conducted the searches and the data extraction, and all authors met to resolve any divergent results. Graphical data were extracted using Webplotter ( https://apps.automeris.io/wpd/ ).
We decided a priori to meta-analyse studies where at least two datasets were available, and which had measured the same parameter in the same brain region. In practice, this required judgement about what constituted meta-analysable data. Only data presented in the form of group means together with a measure of variance were considered for meta-analysis; this excluded several studies (e.g. which used medians and interquartile ranges). Meta-analyses were conducted with a fixed effects model, using Review Manager (RevMan) 5.3. Standardised mean differences (SMD) were used except where stated. Where necessary, standard errors were converted to standard deviations. If subgroups needed to be combined for meta-analysis (e.g. if a paper analysed males and females separately, or presented data for both right and left hemisphere), we used the formulae for weighted means and standard deviations in the Cochrane Handbook [ 14 ]. For studies reporting data for individual cortical layers or hippocampal subfields, a summary statistic for an overall diagnostic effect across all layers/subfields was also computed by RevMan; this statistic uses the number of observations not the number of subjects to calculate significance, and so should be interpreted with caution.
The literature search found 5388 publications meeting our criteria, and an additional 15 papers were found from other sources. Of the 5403 papers, 103 were selected for detailed inspection based on the abstract. 81 proved to have eligible original data [ 8 , 9 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 ], and form the focus of the systematic review. The 22 excluded papers are listed in Supplementary Table 2 together with the reason for their omission. The PRISMA diagram is shown as Supplementary Figure 1 .
Characteristics of included studies
The basic demographics of the 81 studies are shown in Table 1 .
Most studies utilised tissue from recognised brain collections, notably the Stanley Neuropathology Consortium ( n = 35 studies), Harvard/McLean brain bank ( n = 17), and Magdeburg brain collection ( n = 11). The remainder came from a range of other sources; some studies used more than one series. Most studies used diagnostic criteria for BD: DSM-IV ( n = 59), DSM-III-R ( n = 13), and ICD-10 ( n = 4); the remaining studies used other criteria or did not specify. Reflecting the fact that the brain collections mentioned above also include schizophrenia and/or major depressive disorder, many studies also included one or both diagnostic groups (schizophrenia [ n = 58], major depressive disorder [ n = 57]). In terms of anatomical focus, a range of brain regions have been studied: prefrontal cortex (PFC; n = 19), especially dorsolateral (DLPFC; n = 15); ACC, including sgACC ( n = 17); other neocortical regions ( n = 10); hippocampus ( n = 13); amygdala ( n = 8); entorhinal cortex ( n = 7); white matter ( n = 10); thalamus ( n = 6); other ( n = 18). Fourteen studies included more than one region. We rated 28 studies as adhering broadly to stereological principles (e.g. random sampling, 3D counting), 24 did so partially, and 29 either did not or could not be rated. Sixty five studies were carried out blind to diagnosis; no information about blinding was given for 16 studies.
Results are discussed region by region, and summarised in Tables 2 – 6 and Supplementary Tables 3 and 4 . The Tables highlight, for each study, the main results which the authors reported as being statistically significant, as well as important negative findings. Studies which we meta-analysed and their findings are described in the text, summarised in Supplementary Table 5 , and illustrated in Figs. 1 – 4 and Supplementary Figures 2 – 7 . In the event, only two datasets for any given parameter in any one brain region were amenable to meta-analysis.
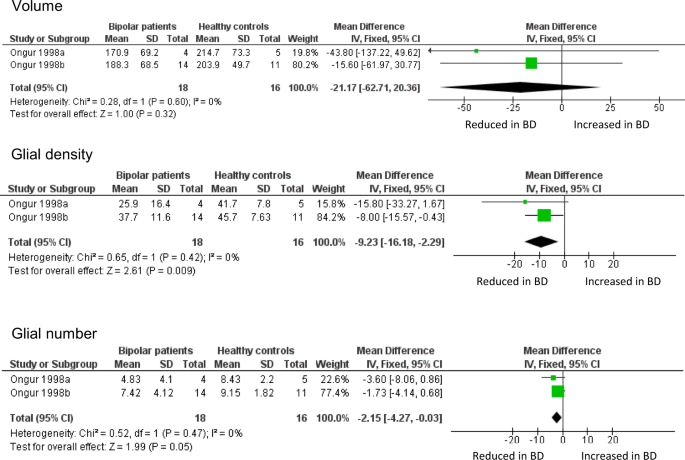
Meta-analysis of volume, glial density, and glial number in subgenual ACC. Data are taken from the two cohorts included in Öngür et al. [ 8 ]. ‘Ongur 1998a’ refers to their pilot study; ‘Ongur 1998b’ refers to the main study, which used brains from the Stanley Foundation. Results are presented as mean differences. Glial density (cells/mm 3 x 10 3 ) is reduced, with a borderline reduction in glial number (x10 6 ), but no difference in sgACC volume (mm 3 )
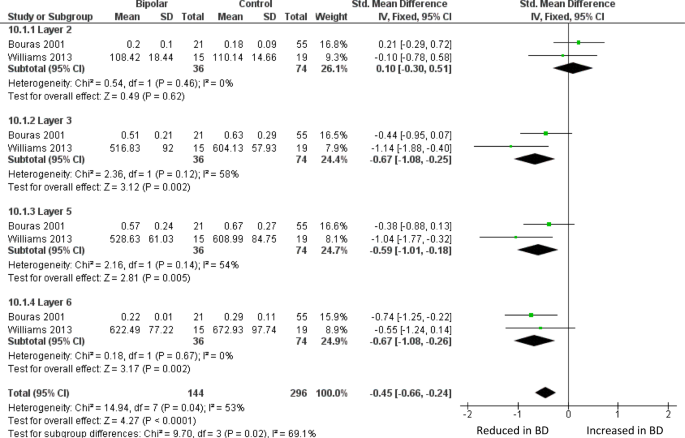
Meta-analysis of layer thickness in subgenual ACC. Meta-analysis of Bouras et al. [ 26 ] and Williams et al. [ 82 ] reveals decreased thickness in BD of sgACC layer III (SMD −0.67, p = 0.002), layer 5 (SMD −0.59, p = 0.005) and layer VI (SMD −0.61, p = 0.004). Thickness is also decreased across all four layers considered together (SMD −0.45, p < 0.0001). The data in ref. 82 were originally analysed separately for men and women; they are combined here as described in the text
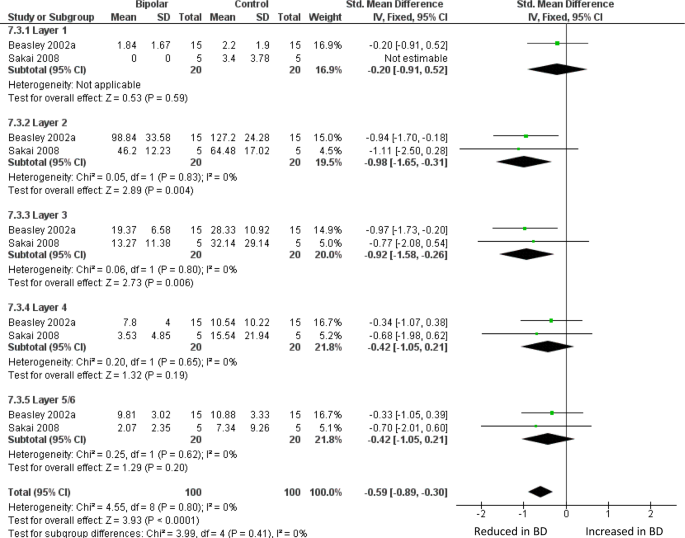
Meta-analysis of calbindin-immunoreactive neuron density in DLPFC. Meta-analysis of Beasley et al. [ 32 ] and Sakai et al. [ 62 ]. We used the data for the ‘medium’ size class of neuron reported in ref. 62 . Density of CB-positive neurons is reduced in BD in layer II (SMD −0.98, p = 0.004) and layer III (SMD −0.92, p = 0.006), and with an overall significant reduction if all 5 layers are considered together (SMD −0.59 p < 0.0001)
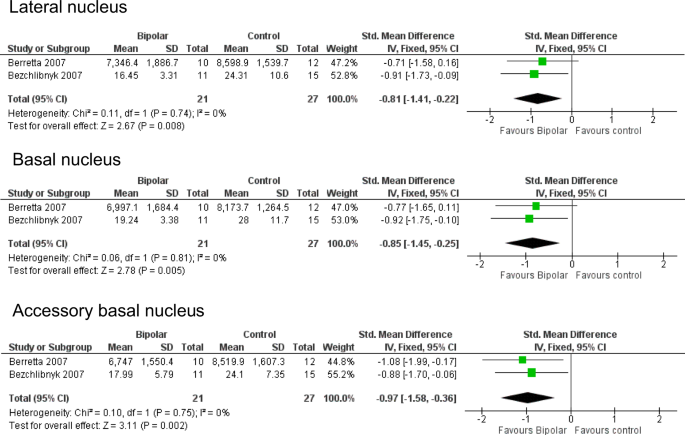
Meta-analysis of neuronal density in lateral, basal and accessory basal nuclei of the amygdala. Meta-analysis of Berretta et al. [ 53 ] and Bezchlibnyk et al. [ 54 ]. For the accessory basal nucleus, we combined data from parvocellular and magnocellular subnuclei reported in [ 54 ]. Neuronal density is reduced in BD in the lateral nucleus (SMD −0.81, p = 0.008), basal nucleus (SMD −0.85, p = 0.005) and accessory basal nucleus (SMD −0.97, p = 0.002). SMDs should be interpreted taking into account the fact that ref. 53 used 3D counting (neurons per unit volume) whereas ref. 54 used 2D counting (neurons per unit area)
Anterior cingulate cortex (ACC)
The ACC is an anatomically and functionally heterogeneous structure at the interface of cognition, emotion and behaviour [ 94 , 95 , 96 ]. It became of great interest in mood disorders after a study showing a focal decreased volume, and reduced metabolism, in the sgACC [ 97 ]. The same group then sought an anatomical correlate of these findings. They reported (in a pilot study and in a second, larger cohort) a reduction of glial density, using unbiased stereological methods on Nissl-stained sections, present in BD and in subjects with major depressive disorder [ 8 ]. Neuronal density and number were unchanged, and sgACC volume non-significantly reduced. Glial density was unaltered in the parietal cortex, suggesting a degree of anatomical localisation, and glial density was unchanged in the sgACC in schizophrenia, indicating a degree of diagnostic specificity.
Öngür et al. [ 8 ] was arguably the first significant neuropathological study of BD, and the first to use contemporary methods. It has been followed by 16 further studies of ACC neuropathology in BD (Table 2 ); 4 include sgACC, 13 examined other parts of the ACC. However, there have been no direct replications of the design or methods used by Öngür et al., precluding any meta-analysis of their data beyond simply combining the two datasets in their original paper. This confirms the reduction in glial density in BD in sgACC, and also shows a borderline significant decrease in glial number, but no difference in sgACC volume (Fig. 1 ). The findings strengthen the conclusions drawn by Öngür and colleagues, not least since their paper used one-tailed tests for some analyses.
The largest BD ACC study is by Bouras et al. [ 26 ], who reported reduced neuronal density in layers III, V, and VI of sgACC. These laminae were also thinner than in controls, as was the grey matter as a whole. The changes were similar but less pronounced in schizophrenia, and not seen in major depression. They state that no differences between BD and controls were seen in occipital cortex, but do not present the data. Glia were not measured. In a much smaller study of sgACC, Sinka et al. [ 78 ], who only measured layers III and V, found a non-significant trend towards decreased thickness of layer III, but no difference in neuronal density in BD in either lamina. Again, glia were not measured. Williams et al. [ 82 ] found a thinner grey matter in the sgACC crown in BD, with a reduced layer V thickness in the right hemisphere. Meta-analysis of sgACC layer thickness using data from refs. 26 and 82 shows a decrease in layers III, V, and VI in BD (Fig. 2 ). In a companion paper, Williams et al. (2013b) [ 83 ] counted sgACC oligodendrocytes using a Nissl stain, and astrocytes using GFAP immunohistochemistry, and found no differences in BD.
Studies of the non-subgenual parts of ACC provide a mixed picture (Table 2 ). Bouras et al. [ 26 ] measured the same parameters as noted above in sgACC, and found no differences between BD and controls in the dorsal ACC. However, when their layer thickness data were meta-analysed together with Benes et al. [ 25 ], the trend reduction seen in both studies for a thinner layer V became significant (Supplementary Figure 2 ).
The main finding of Benes et al. [ 25 ], who studied the rostral ACC, was a marked reduction in the density of non-pyramidal neurons in layer II, which remained significant after Abercrombie correction for cell size. The latter point is relevant since the neurons were larger in BD than in controls. The authors found no differences in pyramidal neuron density, or in glial density, in BD. The same group later used a different (three-dimensional, stereological) counting method in a subset of the same brains, and found modest but significant reductions in the density of non-pyramidal and pyramidal neurons, and glia, in BD, mostly in layer V [ 50 ]. In contrast, Cotter et al. [ 27 ] found no differences in glial density, neuronal density, or neuronal size in BD in the supra-callosal ACC (Brodmann area [BA] 24b) using a stereological approach. This group later used an adjacent part of the supragenual ACC (BA24c) of the same subjects for a two-dimensional study of density, size, and clustering of neurons and glia; in BD, neuronal size was decreased in layer V, and neuronal density increased in layer VI [ 40 ]. Neither study could be included in a meta-analysis because data were presented as medians [ 27 ] or as means but without any measure of variance [ 40 ].
Connor et al. [ 64 ] studied the white matter below the caudal ACC to study the density of white matter neurons (stained by NeuN) since altered distribution of these neurons in schizophrenia had been reported, and viewed as indicative of disordered neurodevelopment. In this relatively large study, they reported an increased density of ACC white matter neurons in BD, with a similar finding in PFC white matter.
In summary, there have been intriguing findings in the sgACC in BD, notably the glial deficits identified by Öngür et al. [ 8 ] and a thinning of the cortex found in two independent studies [ 26 , 82 ]. In other regions of the ACC, the findings are less prominent and not well replicated for either glial or neuronal alterations, though there is moderate evidence for a thinner layer V.
Prefrontal cortex (PFC)
The PFC has been the most studied brain region in BD. Virtually all studies have been carried out in the DLPFC (BA9 and 46). This neuropathological focus can be traced in part to the prominence of this region in studies of schizophrenia at the time when the Stanley Neuropathology Consortium tissue was being made available (see Introduction) [ 98 , 99 ]. There had also been emerging interest in cognitive aspects of BD which suggested potential involvement of the PFC [ 100 , 101 ].
Neuropathological studies of PFC in BD are summarised in Table 3 . The first report was by Guidotti et al. (2000) [ 23 ]. Amongst other parameters, they reported a marked decrease in the density of reelin-positive neurons in layer I (wherein most such cells are located) of BA9, with no change in overall neuronal density. The first dedicated, three-dimensional counting study of PFC was by Rajkowska et al. [ 24 ], again in BA9. Neuronal density was reduced in layer III, and pyramidal neuron density reduced in layers III and V. Glial density was decreased in layer III, with glial size increases. These authors noted the similarity of the glial findings to those of Öngür et al. [ 8 ], and their own findings in major depressive disorder in BA9, and contrasted them with the gliosis which would have been expected were BD a neurodegenerative disorder. Cotter et al. [ 36 ], using a two-dimensional counting method, did not replicate the findings of Rajkowska et al. [ 24 ], with no differences in neuronal density and only a trend reduction in glial density, limited to layer VI; Cotter et al. [ 36 ] did find a significant reduction in neuronal size in layers V and VI. Using the same tissue, Uranova et al. (2004) counted putative oligodendrocytes, and reported that density of these glial cells was decreased in layer VI [ 44 ]; the same group later described a reduction of perineuronal oligodendrocytes in layer III [ 58 ]. In the only study of its kind in BD, Golgi staining was used to quantify dendritic parameters of deep layer III pyramidal neurons, and a reduction in dendritic spine number and density, and dendritic length, were identified [ 85 ].
Two studies have counted sub-populations of DLPFC interneurons defined by immunoreactivity for the calcium binding proteins calbindin (CB), calretinin (CR) or PV [ 32 , 62 ]. Beasley et al. [ 32 ] found a reduced density of CB-immunoreactive neurons in layers II and III, with no significant differences in CR- or PV-positive neuron. When these data were meta-analysed together with findings from a much smaller study [ 62 ], there was a reduction in BD of CB-positive neurons, significant in layers II and III, and overall if all five layers are considered together (Fig. 3 ). Meta-analysis of these papers also showed a reduction of PV-positive neurons across all layers, but with no significant difference in any one layer (Supplementary Figure 3 ); CR-positive neurons were unaffected (Supplementary Figure 4 ). We also meta-analysed studies of DLPFC grey matter thickness (Supplementary Figure 5 ) and the density of oligodendrocytes in DLPFC white matter (Supplementary Figure 6 ): neither showed alterations in BD.
In summary, a range of alterations in neuronal and glial morphometry have been reported in DLPFC in BD, but apart from a decrease in density of CB-positive neurons, no specific finding has been replicated.
The central role of the amygdala in arousal and affect [ 102 , 103 ], its strong connections with the prefrontal cortex [ 5 ], and imaging data in BD [ 1 , 5 ], has made it a structure of neuropathological interest in the disorder [ 5 , 12 , 102 , 103 ]. Table 4 summarises the eight studies to date; three considered the amygdala as a single structure, whilst the others focused on one or more amygdaloid nuclei or groupings thereof [ 104 ].
The first study, by Bowley et al. in 2002 [ 34 ], was conducted to determine whether the amygdala shared the glial reductions seen in ACC and DLPFC. The result was negative, as were subsequent counts of glial subpopulations [ 43 , 54 , 65 , 70 ]. Berretta et al. [ 53 ] reported a decreased volume of the lateral nucleus, which was confirmed by Pantazopoulos et al. [ 92 ] in an expanded sample, and who also described a decreased volume of the cortical nucleus. Accompanying the decreased size of the lateral nucleus, reduced neuronal size [ 54 ], and a lower neuronal number and density [ 53 ] was found. The neuronal reduction in the lateral nucleus is in part due to a loss of somatostatin (SS)-positive neurons [ 92 ], whereas in the cortical nucleus, neuropeptide Y-immunoreactive neurons were decreased [ 92 ]. The number and density of PV-immunoreactive neurons were not changed in either nucleus [ 70 ]. We were able to meta-analyse the two independent studies which measured neuronal density in lateral, basal and accessory basal nuclei [ 53 , 54 ]. This revealed significant reductions in all three nuclei in BD (Fig. 4 ). However, this conclusion is weakened by the fact that Altshuler et al. [ 65 ], studying the same material as ref. 53 but with a different delineation of the ‘basolateral’ amygdala, found no diagnostic effect.
In summary, studies of the amygdala are consistent in showing no alterations in glia in BD, with moderate evidence for reduced density of neurons in lateral, basal and accessory basal nuclei.
Hippocampus
Neuropathological studies of the hippocampus (including the subicular complex) in BD are summarised in Table 5 .
Like PFC, the hippocampus was studied in BD in part because it had been a major focus in schizophrenia [ 105 , 106 ]. Indeed, the first such study in BD, by Benes et al. [ 9 ], primarily reported schizophrenia data but also included four BD subjects, and found a reduction of neuronal density (in both disorders), selective to CA2 subfield, and affecting non-pyramidal neurons (i.e. interneurons) but not pyramidal neurons. Non-pyramidal neurons in BD were also slightly smaller. Zhang and Reynolds [ 39 ] found a markedly reduced density of PV-positive interneurons in all subfields, and a reduced size of these neurons. The next substantive report was in 2011, when Konradi and colleagues carried out a larger, stereological study to measure hippocampal volume and the number and size of neurons, including interneurons labelled by PV or SS [ 73 ]. They found a selective reduction in the volume, and the somal volume, of the non-pyramidal sector of CA2/3, The numbers of both neuropeptide-delineated interneurons were reduced in BD, across all CA fields; pyramidal neurons were unaffected. In a related study, Wang et al. [ 74 ] reported PV and SS neuron reductions in the parasubiculum. In a stereological study of the posterior hippocampus, Malchow et al. [ 88 ] found no differences in hippocampal subfield volumes in BD (including CA2/3), and an increased number and density of neurons in CA1 and in subiculum; they did not distinguish pyramidal from non-pyramidal neurons. In CA1, oligodendrocyte number was also increased. Meta-analysing the two studies of hippocampal neuron number [ 73 , 88 ] revealed no differences in any subfield in BD (Supplementary Figure 7 ; see also Supplementary Table 5 ).
In summary, there is consistent albeit inconclusive evidence in the hippocampus for reductions of non-pyramidal neurons, especially of PV-positive neurons, although differences in methodology and subfields measured precluded meta-analysis. Evidence for involvement of other cell types, or for an altered hippocampal volume, is not compelling.
Other brain regions
Seven neuropathological studies have examined the entorhinal cortex in BD (Supplementary Table 3 ). The only replicated positive finding is that the density of PV-immunoreactive neurons is reduced [ 57 , 74 ]. An unchanged density of GFAP-positive astrocytes has been reported in two studies [ 28 , 70 ], complementing a lack of alteration in overall glial density [ 34 ].
Neuropathological investigations of other neocortical regions, sometimes included to determine the anatomical selectivity of changes found in ACC or DLPFC, are essentially negative, as summarised in Supplementary Table 4 . This includes a lack of alterations in the density of neurons or glia; the only exception is Brauch et al. (2007) who reported a modest increase in neuronal density in an unspecified region of temporal cortex [ 51 ].
In addition to the amygdala, several other subcortical nuclei and regions have been investigated in BD (Table 6 ). Various differences have been reported, particularly in the hypothalamus [ 18 , 47 , 49 , 79 ], but no specific finding has been replicated.
The existence and nature of the neuropathology of psychiatric disorders has been debated for well over a century. Contemporary studies began in earnest in the 1980s, with a focus on schizophrenia, with findings in that disorder informing and encouraging equivalent investigations of other psychiatric disorders, including BD. The latter literature now extends to over 100 empirical studies, of which 81 met our criteria for inclusion in this systematic review. Our findings can be summarised as follows. First, although this is a significant body of work, most studies have relied on a limited number of brain collections, and have used relatively small sample sizes (Table 1 ) and are thus vulnerable to both type I and type II errors. Second, surprisingly few studies have attempted to replicate closely a previous one, precluding substantial meta-analyses; such that the latter were all limited to analysis of two studies each, comprising 16–36 BD cases and 16–74 controls (summarised in Supplementary Table 5 ). Hence, no neuropathological findings in BD can be considered to have been unequivocally established. Nevertheless, several findings are significant after meta-analysis of the available data, and merit brief discussion. We also consider the evidence against the presence of gliosis.
Key positive findings
The sgACC remains of interest, with the findings of glial deficits (Fig. 1 ) and a thinning of grey matter (Fig. 2 ) being significant after meta-analysis. Indeed, the latter finding is probably the most robust positive result, given that it is based on two independent studies [ 26 , 82 ], both using relatively large samples (and comprising the largest combined sample), coupled with a third study showing a similar albeit non-significant trend [ 78 ], and the absence of any contradictory reports. The results mean that further neuropathological investigations of the sgACC are warranted, especially given the continuing focus on this region for the pathophysiology and therapeutics of mood disorder [ 107 , 108 ]. Whether a similar pathology is seen in other parts of the ACC is unclear, since whilst there is some evidence for a thinner layer V (Supplementary Figure 2 ), glial and neuronal density data are inconsistent, as discussed earlier.
The finding of a reduced density of CB-positive neurons in some layers of DLPFC is significant by meta-analysis (Fig. 3 ), albeit based on a modest sample size. Caution is also needed when interpreting the summary statistic which arises from considering the cortical layers together, such as the finding of reduced PV-positive neuron density (Supplementary Figure 6 ), especially given the lack of significant reduction of PV neurons in any one layer. On the other hand, these preliminary indications that interneurons may be affected in BD are supported by findings of a decreased density or number of interneurons, defined by a range of markers, in several brain regions [ 22 , 23 , 39 , 57 , 74 , 92 , 93 ]. No firm conclusions can be drawn regarding these disparate observations, but they do merit further investigation, and complement the well-established involvement of some interneuron populations in schizophrenia [ 109 , 110 , 111 , 112 ].
Reduced neuronal density has been identified in three nuclei of the amygdala (lateral, basal and accessory basal), arising from two reasonably sized samples by independent investigators using different methodologies ([ 53 , 54 ]; Fig. 4 ). The findings support an involvement of the amygdala in the key circuits of BD [ 5 , 12 ]. Data in other nuclei are insufficient to determine whether the findings reflect a broader distribution of amygdala changes, although the unaltered neuronal density seen in the amygdala as a whole [ 34 ] suggests that connections and functions of the laterobasal group of the amygdala may be particularly involved in BD [ 113 , 114 , 115 , 116 ]. However, as noted earlier, Altshuler et al. [ 65 ], using the same brains as Bezchilbnyk et al. [ 54 ], found no difference in neuronal density in BD in the basolateral nucleus. They do not define this structure, but the term conventionally refers to the lateral subdivision of the basal nucleus [ 104 ], and hence would have been subsumed within the latter region as measured in refs. 53 and 54 .
Absence of gliosis
Set against these positive findings, all of which remain to be confirmed beyond doubt, it is worth noting perhaps the clearest conclusion from this systematic review. That is, gliosis (an increase in the density, number or size of glia, especially astrocytes) is not a feature of BD. As summarised in Supplementary Table 6a , no increase in overall glial density has been reported in any of the 19 studies which have measured this parameter (and 4 of them reported reductions). Similarly, of the 12 studies which counted astrocytes (either as identified on Nissl stains, or using immunostaining), 10 reported no differences in BD, and 2 found a reduction (Supplementary Table 6b ). Data for oligodendrocytes and microglia are fewer, but again show no consistent pattern of alteration (Supplementary Table 6c and 6d ). Since astrocytic gliosis is usually considered to be indicative of a neurodegenerative process [ 117 , 118 ], this negative profile of results suggests strongly that BD is not a disorder of that kind. Similarly, the unchanged density of microglia provides no support for the presence of an underlying neuroinflammatory process. When drawing these conclusions, it should be noted that psychiatric brain banks usually exclude subjects if formal neuropathological examination revealed specific abnormalities, because they are viewed as coincidental and confounding findings. For example, the Stanley Foundation brain collection, used in almost half the studies included here, screened brains ‘to rule out Alzheimer’s disease and other cerebral pathology’ [ 10 ]. Nevertheless, as noted by others, the cumulative evidence is strong that BD, like other major psychiatric disorders, is not neurodegenerative in nature [ 7 , 11 , 24 , 105 , 117 , 119 ]. By default, these disorders are often viewed as being neurodevelopmental in origin, although the positive evidence in favour of that conclusion comes primarily from epidemiology and functional genomics rather than from neuropathology [ 120 , 121 , 122 , 123 , 124 ].
Interpreting the neuropathological findings
MRI studies show reductions in grey matter thickness in several cortical regions in BD, including ACC [ 2 , 125 ]. As noted, there is also good neuropathological evidence for a thinning of sgACC (Fig. 2 ). However, in all other cortical areas examined, post mortem studies show minimal or no difference in grey matter thickness in BD [ 24 , 25 , 26 , 32 , 40 , 42 , 48 , 66 ], in contrast to the anatomically widespread MRI findings. There is also a divergence between the robust MRI evidence for decreased volumes of the hippocampus [ 1 ] and most of its constituent subfields [ 126 ], and the neuropathological studies which are divided, with two reporting reduced hippocampal size [ 47 , 73 ] and two which do not [ 9 , 88 ]. When reconciling observations from the two modalities, it should be borne in mind that the imaging data are based on findings from over 1700 BD patients and 2500 controls, and the differences between BD and controls for each parameter are only 1–2% [ 1 , 2 ]. Hence the neuropathological studies (which are about two orders of magnitude smaller; Table 1 ) are grossly underpowered to detect such differences. It is also possible that the group differences seen on neuroimaging are not exclusively reflective of brain structure, but have other potential interpretations and confounders [ 127 ], including the effects of lithium [ 128 , 129 ].
The diagnostic status of BD and its relationships with schizophrenia and major depressive disorder continue to be under active debate clinically and genetically. This issue also has a neuropathological dimension. As noted earlier, the majority of BD studies also include one or both of these other disorders. Although it is beyond the scope of this systematic review to perform a comparative analysis, it is apparent that there is no consistent pattern of similarities or differences between these disorders (with the exception of the absence of gliosis, which is a common observation). Thus, some reported positive findings are specific to BD (e.g. [ 54 ]), some are common to all three disorders (e.g. [ 40 , 44 ]), some affect BD and schizophrenia (e.g [ 26 ]), and others are shared by BD and major depressive disorder (e.g. [ 48 ]). Equally, other parameters are altered in schizophrenia and/or major depressive disorder but not in BD (e.g. [ 27 , 63 ]). Overall, therefore, the neuropathological data are in line with the view, supported strongly by genomic findings [ 130 , 131 ], that BD, schizophrenia and major depressive disorder are not distinct disorders, but have many features in common as well as some which distinguish them. It is also possible that some of the heterogeneity in the neuropathological data reflects the fact that there are morphological correlates of the genetic predisposition to BD as well as to the syndrome itself [ 132 , 133 ].
This latter point relates to perhaps the most fundamental interpretational issue. The nature of the findings – modest reductions in volume, and in the content of neurons or glia, in certain brain regions – cannot be assumed to be pathological in the sense that lesions such as neurofibrillary tangles or infarcts are. They might instead reflect pre-existing (and partly genetically-mediated) differences in brain structure and connectivity which render the person vulnerable to BD. Or, the extant morphometric findings could be secondary to the illness in some way, e.g. cell loss or atrophy secondary to chronic stress, reduced neurotrophic factor support, etc. These issues are impossible to disentangle using post mortem studies alone, and require triangulation of neuropathological data with other findings, such as neuroimaging and relevant model systems.
Limitations
In addition to the diagnostic issues and power considerations mentioned earlier, the literature has several other limitations to consider. The first concerns clinical phenotyping. For most studies, there is sparse information available, for example regarding the age of onset and main features of BD; the mood state at death; the presence of comorbid disorders, etc. In any event, the small sample sizes preclude any meaningful attempts at subdividing BD or correlating clinical or demographic variables with neuropathological parameters. Even the clinical diagnosis of BD itself is not straightforward when made on retrospective review of case notes or interview with relatives: Deep-Soboslay et al. [ 134 ] found that only about half of cases referred to their brain bank as BD met (DSM-IV) diagnostic criteria, with the remainder having inadequate documentation and/or substantial comorbid substance abuse. Other variables such as family history, brain hemisphere and sex could also influence neuropathological findings (e.g., refs. [ 8 , 82 , 88 ]), but have not been reported consistently or in sufficient detail to allow us to examine these factors. There may also be confounding by medications used in BD, since mood stabilisers, antipsychotics and antidepressants can all impact on neuronal and glial indices; such effects may either contribute to, or mitigate, the reported alterations [ 135 , 136 , 137 , 138 , 139 ]. Finally, the neuropathological studies of BD have been of variable methodological quality. For example, only the minority unequivocally meet stereological criteria; the remainder are subject to the limitations and potential biases of studies which do not adhere to these principles [ 140 , 141 ]. Also, studies differ in the statistical approaches taken, such as whether significance values were adjusted for multiple comparisons (e.g. for the number of cortical layers examined).
Conclusions
There remain no neuropathological correlates of BD of sufficient robustness, magnitude, and specificity, to be of clinical or diagnostic value. Clearly, this does not rule out the possibility, but it is unlikely that a neuropathology - in the conventional sense of the term - exists and which has avoided discovery. Nevertheless, the key findings of this systematic review do merit further study to either confirm or refute them. This would require research of a much larger scale and scope than has occurred to date, to ensure the results are conclusive, and to allow assessment of potential clinico-pathological correlates and subgroupings. This would be a challenging undertaking, but transcriptomic and other molecular studies of psychiatric disorders, including BD, now routinely include many hundreds of brains (e.g. refs. [ 123 , 142 , 143 ]). Neuropathological research should have similar aspirations.
Hibar DP, Westlye LT, van Erp TG, Rasmussen J, Leonardo CD, Faskowitz J, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry. 2016;12:1710–1716.
Google Scholar
Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;4:932–942.
Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. 2017;22:1455–1463.
CAS PubMed Google Scholar
Pezzoli S, Emsell L, Yip SW, Dima D, Giannakopoulos P, Zarei M, et al. Meta-analysis of regional white matter volume in bipolar disorder with replication in an independent sample using coordinates, T-maps, and individual MRI data. Neurosci Biobehav Rev. 2018;84:162–170.
PubMed PubMed Central Google Scholar
Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;8:829–843.
Wise T, Radua J, Nortje G, Cleare AJ, Young AH, Arnone D. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry. 2016;4:293–302.
Vawter MP, Freed WJ, Kleinman JE. Neuropathology of bipolar disorder. Biol Psychiatry. 2000;6:486–504.
Öngür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295.
PubMed Google Scholar
Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97.
Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The Stanley Foundation brain collection and neuropathology consortium. Schizophr Res. 2000;2:151–155.
Harrison PJ. The neuropathology of primary mood disorder. Brain. 2002;125:1428–1449.
Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216.
Savitz JB, Price JL, Drevets WC. Neuropathological and neurormorphometric abnormalities in bipolar disorder: view from the prefrontal cortical network. Neurosci Biobehav Rev. 2014;42:132–147.
Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available from www.cochrane-handbook.org .
Elvidge AR, Reed GE. Biopsy studies of cerebral pathologic changes in schizophrenia and manic-depressive psychosis. Arch Neurol Psychiatry. 1938;40:227–268.
Nasrallah HA, McCalley-Whitters M, Bigelow LB, Rauscher FP. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res. 1983;8:251–260.
Beckmann H, Jakob H. Prenatal disturbances of nerve cell migration in the entorhinal region: a common vulnerability factor in the functional psychoses? J Neural Transm Gen Sect. 1991;84:155–164.
Purba JS, Hoogendijk WJG, Hofman MA, Swaab DF. Increased numbers of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143.
Baumann B, Danos P, Krell D, Diekmann S, Wurthmann C, Bielau H, et al. Unipolar-bipolar dichotomy of mood disorders is supported by noradrenergic brainstem system morpholoy. J Affect Dis. 1999a;54:217–224.
Baumann B, Danos P, Diekmann S, Krell D, Bielau H, Geretsegger C, et al. Tyrosine hydroxylase immunoreactivity in the locus ceruleus is reduced in depressed non-suicidal patients but normal in depressed suicide patients. Eur Arch Psychiatry Clin Neurosci. 1999b;249:212–219.
Helmkamp CE, Bigelow LB, Paltan-Ortiz JD, Torrey EF, Kleinman JE, Herman MM. Evaluation of vermal Purkinje cell placement in mental illness. Biol Psychiatry. 1999;45:1370–1375.
Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;6:654–663.
Guidotti A, Auta J, Davis JM, DiGiorgi Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic aciddecarboylase67 (GAD67) expression in schizophrenia and bipolar disorder. A postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069.
Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752.
Benes FM, Vincent SL, Todtenkopf MS. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenc and bipolar subjects. Biol Psychiatry. 2001;50:395–406.
Bouras C, Kövari E, Hof PR, Riederer BM, Giannokopoulos P. Anterior cingulate pathology in schizophrenia and bipolar disorder. Acta Neuropathol. 2001;102:373–379.
Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553.
Damadzic R, Bigelow LB, Krimer LS, Goldenson DA, Saunders RC, Kleinman JE, et al. A quantitative immunohistochemical study of astrocytes in the entorhinal cortex in schizophrenia, bipolar disorder and major depression: Absence of significant astrocytosis. Brain Res Bull. 2001;55:611–618.
Uranova N, Orlovskaya D, Vikhereva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610.
Webster MJ, Knable MB, Johnston-Wilson N, Nagata K, Inagaki M, Yolken RH. Immuno-histochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immun. 2001;15:388–401.
Baumann B, Bielau H, Krell D, Agelnik MW, Diekmann S, Wurthmann C, et al. Circumscribed numerical deficit of dorsal raphe neurons in mood disorders. Psychol Med. 2002;32:93–103.
Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002a;52:708–715.
Beasley CL, Cotter DR, Everall IP. Density and distribution of white matter neurons in schizophrenia, bipolar disorder and major depressive disorder: no evidence for abnormalities of neuronal migration. Mol Psychiatry. 2002b;7:564–570.
Bowley MP, Drevets WC, Öngür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412.
Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, et al. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002a;51:377–386.
Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002b;12:386–394.
Damadzic R, Shuangshoti S, Giblen G, Herman MM. Neuritic pathology is lacking in the entorhinal cortex, subiculum and hippocampus in middle-aged adults with schizophrenia, bipolar disorder or unipolar depression. Acta Neuropathol. 2002;103:488–494.
Gilmore JH, Bouldin T. Analysis of ependymal abnormalities in subjects with schizophrenia, bipolar disorder, and depression. Schizophr Res. 2002;57:267–271.
Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10.
Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal sized and increased neuronal density. Biol Psychiatry. 2003;53:108–1098.
Law AJ, Harrison PJ. The distribution and morphology of prefrontal cortex pyramidal neurons identified using anti-neurofilament antibodies SMI32, N200 and FNP7. Normative data and a comparison in subjects with schizophrenia, bipolar disorder or major depression. J Psychiatr Res. 2003;37:487–499.
Cotter D, Mackay D, Frangou S, Hudson L, Landau S. Cell density and cortical thickness in Heschl’s gyrus in schizophrenia, major depression and bipolar disorder. Br J Psychiatry. 2004;185:258–259.
Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569.
Uranova N, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275.
Young KA, Holcomb LA, Yazdani U, Hicks PB, German DC. Elevated neuron number in the limbic thalamus in major depression. Am J Psychiatry. 2004;161:1270–1277.
Beasley CL, Chana G, Honavar M, Landau S, Everall IP, Cotter D. Evidence for altered neuronal organisation within the planum temporale in major psychiatric disorders. Schizophr Res. 2005;73:69–78.
Bielau H, Trübner K, Krell D, Agelnik MW, Bernstein H-G, Stauch R, et al. Volume deficits of subcortical nuclei in mood disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255:401–412.
Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005;7:358–369.
Manaye KF, Lei D-L, Tizabi Y, Davila-Garcia MI, Mouton PR, Kelly PH. Selective neuron loss in the paraventricular nucleus of hypothalamus in patients suffering from major depression and bipolar disorder. J Neuropathol Exp Neurol. 2005;64:224–229.
Todtenkopf MS, Vincent SL, Benes FM. A cross-study meta-analysis and three-dimensional comparison of cell counting in the anterior cingulate cortex of schizophrenic and bipolar brain. Schizophr Res. 2005;73:79–89.
Brauch RA, El-Masri MA, Parker JC Jr, El-Mallakh RS. Glial cell number and neuron/glial cell ratios in postmortem brains of bipolar individuals. J Affect Disord. 2007;91:87–90.
Toro CT, Hallak JEC, Dunham JS, Deakin JFW. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and bipolar disorder. Neurosci Lett. 2006;404:276–281.
Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62:884–893.
Bezchlibnyk YB, Sun X, Wang J-F, MacQueen GM, McEwen BS, Young LT. Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. J Psychiatry Neurosci. 2007;32:203–210.
Bielau H, Steiner J, Mawrin C, Trübner K, Brisch R, Meyer-Lotz G, et al. Dysregulation of GABAergic neurotransmission in mood disorders, a postmortem study. Ann N Y Acad Sci. 2007;1096:157–169.
Liu L, Schulz C, Lee S, Reutimann TJ, Fatemi SH. Hippocampal CA1 pyramidal cell size is reduced in bipolar disorder. Cell Mol Neurobiol. 2007;27:351–358.
Pantazopoulos H, Lange N, Baldessarini RJ, Berretta S. Parvalbumin neurons in the entorhinal cortex of subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;61:640–652.
Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in schizophrenia and mood disorders. Schizophr Res. 2007;94:273–280.
Young KA, Holcomb LA, Bonkale WL, Hicks PB, Yazdani U, German DC. 5HTTPLR polymorphism and enlargement of the pulvinar: unlocking the backdoor to the limbic system. Biol Psychiatry. 2007;61:813–818.
Byne W, Tatusov A, Yiannoulos G, Vong GS, Marcus S. Effects of mental illness and aging in two thalamic nuclei. Schizophr Res. 2008;106:172–181.
Pennington K, Dicker P, Hudson L, Cotter DR. Evidence for reduced neuronal somal size within the insular cortex in schizophrenia, but not in affective disorders. Schizophr Res. 2008;106:164–171.
Sakai T, Oshima A, Nozaki Y, Ida I, Haga C, Akiyama H, et al. Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology. 2008;28:143–150.
Beasley CL, Honavar M, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the superior temporal cortex white matter in schizophrenia, major depressive disorder and bipolar disorder. Schizophr Res. 2009;115:156–162.
Connor CM, Guo Y, Akbarian S. Cingulate white matter neurons in schizophrenia and bipolar disorder. Biol Psychiatry. 2009;66:486–493.
Altshuler LL, Abulseoud OA, Foland-Ross L, Bartzokis G, Chang S, Mintz J, et al. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 2010;12:541–549.
Brüne M, Schöbel A, Karu R, Benali A, Fasutmann PM, Juckel G, et al. Von Economo neuron density in the anterior cingulate cortex is reduced in early onset schizophrenia. Acta Neuropathol. 2010;119:771–778.
Cataldo AM, McPhie DL, Lange NT, Punzell S, Elmiligy S, Ye NZ, et al. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol. 2010;177:575–585.
Hercher C, Canetti L, Turecki G, Mechawar N. Anterior cingulate pyramidal neurons display altered dendritic branching in depressed suicides. J Psychiatr Res. 2010;44:286–93.
Maloku E, Covelo IR, Hanbauer I, Guidotti A, Kadriu B, Hu Q, et al. Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci USA. 2010;107:4407–4411.
Pantazopoulos H, Woo T-UW, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–166.
Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein H-G. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med. 2010;40:557–567.
Brisch R, Bernstein H-G, Dobrowolny H, Krell D, Stauch R, Trubner K, et al. A morphometric analysis of the septal nuclei in schizophrenia and affective disorders: reduced neuronal density in the lateral septal nucleus in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261:47–58.
Konradi C, Zimmerman EI, Yang K, Lohmann KM, Gresch P, Pantazopoulos H, et al. Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry. 2011;68:340–350.
Wang AY, Lohmann KM, Yang CK, Zimmerman EI, Pantazopoulos H, Herring N, et al. Bipolar disorder type I and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathol. 2011;122:615–626.
CAS PubMed PubMed Central Google Scholar
Bernstein H-G, Klix M, Dobrowolny H, Brisch R, Steiner J, Bielau H, et al. A postmortem assessment of mammillary body volume, neuronal number and densities, and fornix volume in subjects with mood disorders. Eur Arch Psychiatry Neurosci. 2012;262:637–646.
Comte I, Kotagiri P, Szele FG. Regional differences in human ependymal and subventricular zone cytoarchitecture are unchanged in neuropsychiatric disease. Dev Neurosci. 2012;34:299–309.
Matthews PR, Harrison PJ. A morphometric, immunohistochemical, and in situ hybridization study of the dorsal raphe nucleus in major depression, bipolar disorder, schizophrenia, and suicide. J Affect Disord. 2012;137:125–134.
Sinka L, Kovari E, Santos M, Herrmann FR, Gold G, Hof PR, et al. Microvascular changes in late-life schizophrenia and mood disorders: stereological assessment of capillary diameters in anterior cingulate cortex. Neuropathol Appl Neurobiol. 2012;38:696–709.
Gao S-F, Klomp A, Wu J-L, Swaab DF, Bao A-M. Reduced GAD 65/67 immunoreactivity in the hypothalamic paraventricular nucleus in depresison: a postmortem study. J Affect Disord. 2013;143:422–425.
Gos T, Schroeter ML, Lessel W, Bernstein H-G, Dobrowolny H, Schiltz K, et al. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. J Psychiatr Res. 2013;47:1694–1699.
Mosebach J, Keilhoff G, Gos T, Schiltz K, Schoeneck L, Dobrowolny H, et al. Increased nuclear Olig1-expression in the pregenual anterior cingulate white matter of patients with major depression: a regenerative attempt to compensate oligodendrocyte loss? J Psychiatr Res. 2013;47:1069–1079.
Williams MR, Chaudhry R, Perera S, Pearce RKB, Hirsch SR, Ansorge O, et al. Changes in cortical thickness in the frontal lobes in schizophrenia are a result of thinning of pyramidal cell layers. Eur Arch Psychiatry Clin Neurosci. 2013a;263:25–39.
Williams MR, Hampton T, Pearce RKB, Hisrch SR, Ansorge O, Thom M, et al. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013b;263:41–52.
Hercher C, Chopra V, Beasley CL. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci. 2014;39:376–385.
Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71:1323–1331.
Williams MR, Pearce RKB, Hirsch SR, Ansorge O, Thom M, Maier M. Fibrillary astrocytes are decreased in the subgenual cingulate in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264:357–362.
Bernstein H-G, Meyer-Lotz G, Dobrowolny H, Bannier J, Steiner J, Walter M, et al. Reduced density of glutamine synthetase immunoreactive astrocytes in different cortical areas in major depression but not in bipolar I disorder. Front Cell Neurosci. 2015;9:273.
Malchow B, Strocka S, Frank F, Bernstein H-G, Steiner J, Schneider-Axmann T, et al. Stereological investigation of the posterior hippocampus in affective disorders. J Neural Transm. 2015;122:1019–1023.
Shioya A, Saito Y, Arima K, Kakuta Y, Yuzuriha T, Tanaka N, et al. Neurodegenerative changes in patients with clinical history of bipolar disorders. Neuropathol. 2015;35:245–253.
Brisch R, Steiner J, Mawrin C, Krzyzanowska M, Jankowski Z, Gos T. Microglia in the dorsal raphe nucleus plays a potential role in both suicide facilitation and prevention in affective disorders. Eur Arch Psychiatry Clin Neurosci. 2017;267:403–415.
Krause M, Theiss C, Brüne M. Ultrastructural alterations of von Economo neurons in the anterior cingulate cortex in schizophrenia. Anat Rec. 2017;300:2017–2024.
Pantazopoulos H, Wiseman JT, Markota M, Ehrenfeld L, Berretta S. Decreased numbers of somatostatin-expressing neurons in the amygdala of subjects with bipolar disorder or schizophrenia: relationship to circadian rhythms. Biol Psychiatry. 2017;81:536–547.
Steullet P, Cabungcal J-H, Bukhari SA, Ardelt MI, Pantazaopoulos H, Hamati F, et al. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry (AOL 28 November 2017; https://doi.org/10.1038/mp.2017.230
Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306.
Bush G, Liu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222.
Gittins R, Harrison PJ. Neuronal density, size and shape in the human anterior cingulate cortex: a comparison of Nissl and NeuN staining. Brain Res Bull. 2004;63:155–60.
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature . 1997;386:824–7.
Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669.
Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull. 1997;23:437–58.
Ferrier IN, Stanton BR, Kelly TP, Scott J. Neuropsychological function in euthymic patients with bipolar disorder. Br J Psychiatry. 1999;175:246–51.
Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–84.
Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87.
Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–83.
Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl). 2005;210:343–52.
CAS Google Scholar
Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624.
Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl). 2004;174:151–62.
Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2011;72:595–603.
Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76:963–969.
Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacol. 2001;25:1–27.
Harrison PJ, Lewis DA, Kleinman JE. Neuropathology of schizophrenia. In: Weinberger DR, Harrison PJ, editors. Schizophrenia, 3rd edition. Oxford: Wiley-Blackwell, pp 372–392.
Gonzalez-Burgos G, Cho Y, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–1040.
Schmidt MT, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Biol Psychiatry. 2015;40:190–206.
Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380.
Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2013;34:3247–3266.
Kerestes R, Chase HW, Phillips ML, Ladouceur CD, Eickhoff SB. Multimodal evaluation of the amygdala’s functional connectivity. Neuroimage. 2015;148:219–229.
Hortensius R, Terburg D, Morgan B, Stein DJ, van Honk J, de Gelder B. The role of the basolateral amygdala in the perception of faces in natural contexts. Philos Trans R Soc B Biol Sci. 2016;371:20150376.
Harrison PJ. On the neuropathology of schizophrenia and its dementia: neurodevelopmental, neurodegenerative, or both? Neurodegeneration. 1995;4:1–12.
Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248.
Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: Th evidence and implications. Brain Res Bull. 2001;55:585–595.
de la Torre-Ubieta L, Won H, Stein JL, Geschwind DH. Advancing the understanding of autism disease mechanisms through genetics. Nat Med. 2016;22:345–61.
Marín O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med. 2016;22:1229–1238.
Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18:727–740.
Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359:693–697.
Mühleisen TW, Reinbold CS, Forstner AJ, Abramova LI, Alda M, Babadjanova G, et al. Gene set enrichment analysis and expression pattern exploration implicate an involvement of neurodevelopmental processes in bipolar disorder. J Affect Disord. 2018;228:20–25.
Hanford LC, Nazarov A, Hall GB, Sassi RB. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 2016;18:4–18.
Haukvik UK, Westlye LT, Mørch-Johnsen L, Jørgensen KN, Lange EH, Dale AM. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2015;77:581–8.
Weinberger DR, Radulescu E. Finding the elusive psychiatric “lesion” with 21st-Century neuroanatomy: a note of caution. Am J Psychiatry. 2016;173:27–33.
Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16.
Cousins DA, Aribisala B, Nicol Ferrier I, Blamire AM. Lithium, gray matter, and magnetic resonance imaging signal. Biol Psychiatry. 2013;73:652–7.
Owen MJ. New approaches to psychiatric diagnostic classification. Neuron. 2014;84:564–71.
Lawrie SM, O’Donovan MC, Saks E, Burns T, Lieberman JA. Towards diagnostic markers for the psychoses. Lancet Psychiatry. 2016;3:375–85.
Kleinman JE, Law AJ, Lipska BK, Hyde TM, Ellis JK, Harrison PJ, et al. Genetic neuropathology of schizophrenia: new approaches to an old question and new uses for postmortem human brains. Biol Psychiatry. 2011;69:140–5.
Jaffe AE. Postmortem human brain genomics in neuropsychiatric disorders--how far can we go? Curr Opin Neurobiol. 2016;36:107–11.
Deep-Soboslay A, Iglesias B, Hyde TM, Bigelow LB, Imamovic V, Herman MM, et al. Evaluation of tissue collection for postmortem studies of bipolar disorder. Bipolar Disord. 2008;10:822–8.
Harrison PJ. The neuropathological effects of antipsychotic drugs. Schizophr Res. 1999;40:87–99.
Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, et al. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759–765.
Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, Mann JJ, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacol. 2009;34:2376–2389.
Cotel MC, Lenartowicz EM, Natesaon S, Modo MM, Cooper JD, Williams SCR, et al. Microglial activation in the rat brain following chronic antipsychotic treatment at clinically relevant doses. Eur Neuropsychopharmacol. 2015;25:2098–2107.
Rajkowska G, Clarke G, Mahajan G, Licht CMM, van de Werd H, Yuan P, et al. Differential effect of lithium on cell number in the hippocampus and prefrontal cortex in adult mice: a stereological study. Bipolar Disord. 2016;18:41–51.
Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24:11–7.
Dorph-Petersen KA, Lewis DA. Stereological approaches to identifying neuropathology in psychosis. Biol Psychiatry. 2011;69:113–26.
Tao R, Cousijn H, Jaffe AE, Burnet PW, Edwards F, Eastwood SL, et al. Expression of ZNF804A in human brain and alterations in schizophrenia, bipolar disorder, and major depressive disorder: a novel transcript fetally regulated by the psychosis risk variantrs1344706. JAMA Psychiatry. 2014;71:1112–20.
Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–84.
Download references
Acknowledgements
We thank Andrea Cipriani for expert advice and assistance. LC is funded by the Wellcome Trust Oxford Clinical Doctoral Fellowship Programme. PJH’s research is supported by the Wellcome Trust, Medical Research Council, and Oxford Health National Institute for Health Research (NIHR) Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR or the Department of Health.
Author information
Authors and affiliations.
Department of Psychiatry, University of Oxford, Warneford Hospital, Oxford, OX3 7JX, UK
Paul J. Harrison & Lucy Colbourne
Oxford Health NHS Foundation Trust, Warneford Hospital, Oxford, UK
Faculty of Medicine, University of Southampton, Southampton General Hospital, Southampton, UK
Charlotte H. Harrison
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Paul J. Harrison .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary tables, supplementary figures, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Harrison, P.J., Colbourne, L. & Harrison, C.H. The neuropathology of bipolar disorder: systematic review and meta-analysis. Mol Psychiatry 25 , 1787–1808 (2020). https://doi.org/10.1038/s41380-018-0213-3
Download citation
Received : 30 April 2018
Revised : 16 July 2018
Accepted : 24 July 2018
Published : 20 August 2018
Issue Date : August 2020
DOI : https://doi.org/10.1038/s41380-018-0213-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Comparing stem cells, transdifferentiation and brain organoids as tools for psychiatric research.
- Alfredo Bellon
Translational Psychiatry (2024)
Astrocytes in human central nervous system diseases: a frontier for new therapies
- Alexei Verkhratsky
- Arthur Butt
- Michael V. Sofroniew
Signal Transduction and Targeted Therapy (2023)
Functional connectivity gradients of the cingulate cortex
- Huanhuan Cai
Communications Biology (2023)
Brain Inflammatory Marker Abnormalities in Major Psychiatric Diseases: a Systematic Review of Postmortem Brain Studies
- Yang-wen Ai
Molecular Neurobiology (2023)
Phenotypes, mechanisms and therapeutics: insights from bipolar disorder GWAS findings
Molecular Psychiatry (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Change Password
Your password must have 6 characters or more:.
- a lower case character,
- an upper case character,
- a special character
Password Changed Successfully
Your password has been changed
Create your account
Forget yout password.
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password
Forgot your Username?
Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username

- Spring 2024 | VOL. 22, NO. 2 Autism Across the Lifespan CURRENT ISSUE pp.147-262
- Winter 2024 | VOL. 22, NO. 1 Reproductive Psychiatry: Postpartum Depression is Only the Tip of the Iceberg pp.1-142
The American Psychiatric Association (APA) has updated its Privacy Policy and Terms of Use , including with new information specifically addressed to individuals in the European Economic Area. As described in the Privacy Policy and Terms of Use, this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences.
Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.
Psychotherapy for Bipolar Disorder in Adults: A Review of the Evidence
- Holly A. Swartz , M.D. , and
- Joshua Swanson
Search for more papers by this author
Although pharmacotherapy is the mainstay of treatment for bipolar disorder, medication offers only partial relief for patients. Treatment with pharmacologic interventions alone is associated with disappointingly low rates of remission, high rates of recurrence, residual symptoms, and psychosocial impairment. Bipolar-specific therapy is increasingly recommended as an essential component of illness management. This review summarizes the available data on psychotherapy for adults with bipolar disorder. We conducted a search of the literature for outcome studies published between 1995 and 2013 and identified 35 reports of 28 randomized controlled trials testing individual or group psychosocial interventions for adults with bipolar disorder. These reports include systematic trials investigating the efficacy and effectiveness of individual psychoeducation, group psychoeducation, individual cognitive-behavioral therapy, group cognitive-behavioral therapy, family therapy, interpersonal and social rhythm therapy, and integrated care management. The evidence demonstrates that bipolar disorder-specific psychotherapies, when added to medication for the treatment of bipolar disorder, consistently show advantages over medication alone on measures of symptom burden and risk of relapse. Whether delivered in a group or individual format, those who receive bipolar disorder-specific psychotherapy fare better than those who do not. Psychotherapeutic strategies common to most bipolar disorder-specific interventions are identified.
Recurrent affective illnesses characterized by both depression and mania have been described since the time of Hippocrates. The modern era of bipolar disorder, however, began in 1949 with the introduction of lithium. John Cade’s discovery of a chemical compound that effectively and specifically treats bipolar disorder ( 1 ) revolutionized not only the management of bipolar disorder but also altered how the illness was viewed by researchers and clinicians. What was once conceptualized as an illness caused by unbalanced humours ( 2 ) became recognized as a biologic illness amenable to biochemical intervention. Because many with bipolar disorder responded so well to lithium (at least compared with previous interventions), attention turned toward finding and optimizing pharmacologic treatments for bipolar disorder. Positive experiences with major and minor tranquilizers led further credence to this approach. For the next 30 years, little attention was paid to psychosocial treatments for bipolar disorder ( 3 ) as it was considered a “problem solved” (E. Frank, personal communication, 2013).
Although rarely the focus of systematic inquiry, psychotherapy was routinely offered to patients suffering from bipolar disorder during the 20th century. Most of these treatments were based on the prevailing psychotherapeutic paradigm of the era (i.e., psychoanalysis), which relied on transference and development of insight to bring about change. Not surprisingly, psychoanalysis had little to offer manic patients who, by definition, suffer from marked impairments in insight ( 4 ). Although psychodynamic psychotherapy played a greater role in the management of bipolar depression and bipolar spectrum disorders, early practitioners of psychotherapy for bipolar disorder concluded that “[w]hereas it appeared to work with the schizophrenic, it was not generally successful with the manic-depressive” ( 5 ) (p.158). These treatment failures further reinforced the concept that bipolar disorder was best treated primarily–if not exclusively–with pharmacotherapy.
Toward the end of the 20th century, it became increasingly apparent that medication offered only partial relief from bipolar disorder. Treatment with pharmacologic interventions alone was associated with disappointingly low rates of remission ( 6 , 7 ), high rates of recurrence ( 8 , 9 ), residual symptoms ( 10 ), and psychosocial impairment ( 11 ). Gradually, the field moved from conceptualizing bipolar disorder as a disorder requiring only medication to an illness that, like many chronic disorders, is best treated using a combination of pharmacotherapy and psychotherapy ( 12 , 13 ).
At face value, psychotherapy for bipolar disorder makes a lot of sense. Bipolar disorder is characterized by a high degree of psychosocial impairment ( 11 , 14 – 16 ), low rates of medication adherence ( 17 – 19 ), interpersonal dysfunction ( 11 , 20 – 22 ), and cognitive impairment ( 23 – 25 ). Each of these domains is reasonably addressed by psychotherapeutic interventions—especially when delivered in combination with pharmacotherapy. Indeed, beginning in the 1990s, a series of clinical trials demonstrating the efficacy and effectiveness of bipolar-specific psychotherapies for the treatment of bipolar disorder appeared in the literature. Unlike the psychodynamic therapies of the previous decades that focused on intrapsychic conflicts and acquisition of insight, contemporary bipolar-specific psychotherapies utilize more directive and symptom-focused strategies such as encouragement of medication adherence, provision of psychoeducation, involvement of family members, development of strategies for relapse prevention, exploration of the reciprocal relationship between mood and either cognitions or interpersonal relationships, and establishment of regular sleep-wake cycles. The goal of this review is to examine the evidence supporting psychotherapy as an efficacious approach to treating bipolar disorder and to summarize the strategies for illness management that have been proven to be helpful for individuals with bipolar disorder.
We conducted a literature search of outcome studies for psychosocial interventions for adults with bipolar disorder published between 1995 and 2013. One study published in 2014 was included in this review because it was published online in 2012 ( 26 ). We searched major databases: PubMed (U.S. National Library of Medicine and National Institutes of Health), Psych INFO (American Psychological Association), and OvidSP (Wolters Kluwer). We also examined the bibliographies of major review articles on the topic to search for missing references. Search terms included combinations of bipolar disorder, mania, depression, psychotherapy, adult, and psychosocial. We restricted our review to randomized controlled trials (RCTs), treatments for adults, and manuscripts published in English. We excluded RCTs of interventions directed primarily at caregivers of individuals with bipolar disorder because the focus of this review is psychotherapy for adult patients.
Table 1 summarizes the published RCTs of psychotherapies for the treatment of adults with bipolar disorder by type of psychotherapy. The trials are listed once in Table 1 , even if they test psychotherapies from multiple categories. When trials compare psychotherapies of different modalities, it is described under the first category listed that summarizes information about at least one type of psychotherapy included in the trial. (e.g., an RCT comparing individual cognitive-behavioral therapy and group psychoeducation appears under the group psychoeducation heading only). We identified 35 reports of 28 RCTs testing individual or group psychosocial interventions for individuals with bipolar disorder. In all but one trial ( 27 ), psychotherapy was administered as an adjunct to pharmacologic treatments. In most cases, the addition of psychotherapy to pharmacotherapy was associated with improved outcomes for bipolar disorder compared with the control condition [but for exceptions, see, Scott et al. ( 28 ), Meyer and Hautzinger ( 29 ), Parikh et al. ( 30 ), Crowe et al. ( 31 ), and de Barros Pellegrinelli et al. ( 32 )]. Below we briefly describe individual and group models of psychotherapy tested for bipolar disorder and the evidence supporting their efficacy/effectiveness. (See Table 2 for brief descriptions of each type of psychotherapy.)
BPNOS=bipolar disorder not otherwise specified; CBT: cognitive-behavioral therapy; CBGT: cognitive-behavioral group therapy; CD: cyclothymic disorder; CESD: Center for Epidemiological Studies for Depression Scale; FFT: family-focused therapy; HPI: health-promoting intervention; HAM-D: Hamilton Depression Rating Scale; ICM: integrated care management; IPSRT: interpersonal and social rhythm therapy; MBCT: mindfulness-based cognitive therapy; MSI: mood sensitivity index; PE: psychoeducation; SCM: systematic care management; SUD: substance use disorder; TAU: treatment as usual; WLC: wait-list control; YMRS: Young Mania Rating Scale.
a Includes exercises for memory, executive functions, and functioning in daily routines.
b Based on a cognitive behavioral relapse prevention model with focus on interaction of bipolar disorder and substance abuse.
c Developed using the collaborative therapy framework and integrates strategies for monitoring mood, assessing prodromes, preventing relapse, and setting goals (Castle et al., “Pilot of group intervention for bipolar disorder.” [Int J Psychiatry Clin Pract 2007; 11:279–284]).
Individual Psychoeducation
Individual psychoeducation (PE) typically consists of 6–21 structured sessions that focus on the provision of information about illness etiology, treatments, course and outcomes, strategies to identify prodromes/early warning signs of relapse, and illness-coping strategies ( 33 – 35 ).
Perry and colleagues conducted the first trial of individual PE, compared with treatment as usual (TAU), for individuals with bipolar I and II disorder with two or more relapses in the preceding 12 months (N=69). Those assigned to individual PE had significant reductions in time to manic relapses (log rank 7.04, df=1, p=0.008) and number of manic relapses over 18 months (median difference of 30%). Although the difference did not reach any statistical significance, there was a trend toward fewer depressive episodes over time in the PE group (p=0.05) ( 34 ).
Zaretsky and colleagues compared seven sessions of individual PE to seven sessions of PE followed by 13 additional sessions of cognitive-behavioral therapy (CBT) as treatments for euthymic or minimally symptomatic individuals with bipolar disorder. Among the randomized subjects (N=79), those who received CBT plus PE had significantly greater decreases in Hamilton Depression Rating Scale (HAM-D) scores from baseline to posttreatment than those assigned to PE alone (F 1,44 =3.87, p=0.055); but there was no difference between groups in the number of hospitalizations or psychosocial functioning ( 36 ).
Rea and colleagues compared 21 sessions of individual PE to family-focused therapy (FFT) in 53 patients with bipolar disorder who were recently hospitalized for mania and partially stabilized on pharmacotherapy. Over 2 years, those assigned to FFT had fewer relapses than those in individual PE (χ 2 =5.04, p<0.05) and fewer hospitalizations (χ 2 =3.87, p<0.05) but did not differ on likelihood of a first relapse (χ 2 =0.50, p>0.10) ( 37 ).
Collectively, these studies suggest that individual PE alone offers a benefit over TAU, especially as a strategy for preventing manic episodes, but that more intensive interventions such as CBT and FFT confer additional advantages over and above PE alone.
Group Psychoeducation
Colom and Vieta developed a 21-session group PE intervention that focuses on 1) awareness of the disorder (symptoms, classification, etiologies, course, and prognosis), 2) medication adherence (information about classes of medications, alternative therapies, withdrawal syndromes, and risks of nonadherence), 3) avoiding substance abuse, 4) early detection of new episodes (detection of prodromes, warning signs of relapse, and relapse prevention planning), and 5) stress management (regularity of habits and problem-solving) ( 38 ).
Colom and colleagues in Barcelona randomized euthymic individuals with bipolar disorder (N=120) to 21 sessions of either the PE group or an unstructured support group. Over a 2-year period, an assignment to group PE was associated with a longer time to any mood episode (log rank=9.3, p<0.003), lower hospitalization rates (Mann-Whitney U=−2.69, p<0.01), and lower cumulative mean number of hospitalizations (U=−2.23, p<0.05) compared with the unstructured support group ( 39 ). At a 5-year follow-up, recurrence rates were lower among those assigned to the PE group compared with the unstructured group (log rank=10.0, p<0.01) ( 40 ).
Candini and colleagues ( 41 ) compared 21 sessions of the PE group, using the Colom and Vieta model, to TAU in a sample of euthymic patients receiving care in Italy. The results from this trial (N=102) compared favorably to the Barcelona trial, with fewer hospitalizations (U=934, p=0.001) and longer time to hospitalization (log rank=13.5, p<0.001) over a 1-year period among those assigned to group PE compared with TAU ( 41 ).
In contrast to these earlier trials, de Barros Pellegrinelli and colleagues ( 32 ) in Brazil found no advantage of the PE group (administered as a 16- rather than 21-session intervention) compared with TAU over a 1-year period. They found that on the HAM-D, both groups had increased depressive symptoms at endpoint (p=0.014) with no differences between groups. Only one subject was hospitalized, and there were no differences between groups on the number of depressive or manic relapses ( 32 ). Although the Brazilian trial failed to confirm the positive findings of the Spanish and Italian trials, their sample size was much smaller (N=55 versus 120 and 102, respectively), raising the possibility of type II error (i.e., failure to reject the null hypothesis because of inadequate sample size).
Parikh and colleagues ( 30 ) compared a six-session PE group to 20 sessions of individual cognitive-behavioral therapy (CBT) (N=204) for adults with bipolar disorder who were either euthymic or had minimal symptoms. Over 72 weeks, both groups had significant reductions in mood symptoms as measured by the Longitudinal Interval Follow-Up Evaluation (LIFE) (p<0.01 for both groups) with no significant differences between the groups. The time to manic or depressive recurrence was not significantly different between groups ( 30 ).
Torrent and colleagues ( 42 ) randomly assigned 239 euthymic individuals with bipolar disorder to a PE group (N=82), functional remediation (FR) (N=77), or TAU (N=80) for 21 weeks. The PE group was based on the model of Colom and Vieta (see above). FR consists of 21 weekly 90-minute group sessions that use written and oral exercises to address neurocognitive issues such as attention, memory, and executive functions as well as functioning in daily routines. The primary outcome measure was global psychosocial functioning, as measured by change in the Functioning Assessment Short Test from baseline to endpoint. Analyses revealed significant functional improvement over the 21 weeks of treatment (last observation carried forward) in all groups and as well as differences between groups (Pillai’s Trace=0.065, F=6.51, df=2, p=0.002). Those assigned to FR had significantly greater improvement in functioning than those assigned to TAU (p=0.001), but fell short of statistical significance when compared with the PE group (p=0.056). Effect sizes within groups showed a large effect for FR (d=0.93), a moderate effect size for PE (d=0.41), and a small effect size for TAU (d=0.22) ( 42 ), suggesting that FR was a more potent treatment for impaired functioning than either PE or TAU, but that PE was somewhat helpful.
In many respects group PE is an ideal “base” treatment for bipolar disorder. Groups are more cost-effective and resource-friendly than individual treatments. Knowledge about bipolar disorder is essential to illness management, and a manualized PE group is well-suited to standardized transmission of this information. However, PE, although necessary for individuals with bipolar disorder, may not be sufficient. Indeed, these studies demonstrate that treatment with a PE group (both 21- and six-session formats) confer benefits for those with bipolar disorder including longer time to recurrence, decreased rates of hospitalization, and improved symptoms over time. The advantages are less apparent, however, when a PE group is compared with a more active comparator than TAU. For instance, outcomes with six-session PE did not differ from 20-session individual CBT. Similarly, both 21-session PE and FR groups were associated with improvement in global functioning, although those assigned to FR fared even better than those assigned to PE. These studies raise the possibility that a stepped care approach to bipolar disorder may be indicated, i.e., treat patients with the less costly/burdensome group PE prior to adding CBT or functional remediation for those who do not achieve an adequate benefit with PE alone.
Individual Cognitive or Cognitive-Behavioral Therapy
Cognitive-behavioral therapy (CBT) and cognitive therapy (CT) for bipolar disorder build on Beck's CT for depression ( 43 ), a skills-based treatment that helps individuals recognize and modify the link between maladaptive thoughts and moods. Through the use of thought records, mood diaries, and activity scheduling, patients learn to modify automatic negative thoughts, remove distorted thinking, and interrupt cycles of mania and depression. As described by Lam and colleagues, CT for bipolar disorder adds additional modules of psychoeducation, strategies for coping with prodromes, activities for regulating sleep and routines, and approaches to managing long-term sequelae of the illness ( 44 ). Several groups have elaborated variants of CBT and CT for working with bipolar disorder including Basco and Rush ( 45 ), Scott ( 46 ), Otto et al. ( 47 ), and Lam et al. ( 48 ).
Lam and colleagues randomized 103 patients with histories of frequent relapses (at least two mood episodes in the prior 2 years or three episodes in the prior 5 years) to individual CT or TAU. CT was administered as 12–18 sessions over 6 months followed by two booster sessions. Over 12 months, the risk for relapse was significantly lower in the CT group compared with TAU (0.40 with 95% confidence interval 0.21–0.74; p=0.004) ( 49 ). Over 30 months, those assigned to CT also had lower rates of relapse compared with TAU (64% versus 84%) and a longer time to depressive relapse than TAU (hazard ratio=0.38 with 95% confidence interval=0.19–0.75; p<0.006); however, the difference was not significant for manic/hypomanic episodes ( 50 ). The addition of CT to TAU was deemed to be cost-effective because of associated reductions in service use ( 51 ).
Ball and colleagues ( 52 ) randomized 52 patients with histories of frequent relapses (at least one mood episode over the prior 18 months) to individual CT or TAU. After 6 months, those assigned to CT had significantly greater improvement on the Beck Depression Inventory (BDI) than those assigned to TAU (t=2.71, p=0.009). In regression models, there was a significant time effect for BDI over 12 months (F=5.14, df=7.3, p<0.00) but there were no time × group interactions, meaning that both groups’ depression symptoms improved but there were no differences between groups. There were no significant differences in groups on time to relapse, although there was a trend toward a difference in time to depressive relapse with those assigned to CT remaining well longer (hazard ratio=0.38, 95% confidence interval=0.14 to 1.03; p=0.057) ( 52 ).
Scott and colleagues ( 28 ) randomly assigned 253 individuals with bipolar disorder to 22 sessions of individual CBT or TAU in a multisite pragmatic trial. There were no significant differences in groups on time to relapse, although among those with fewer than 12 previous episodes, assignment to CBT was associated with significantly fewer relapses than TAU (p=0.04) ( 28 ).
The systematic treatment enhancement program for bipolar disorder (STEP-BD), is a multisite trial, compared the effects of three intensive psychosocial treatments—CBT, Interpersonal and Social Rhythm Therapy (IPSRT), or family-focused therapy (FFT)—with a brief (three session) psychoeducational control condition (CC) for the acute treatment of bipolar depression (N=293). Patients who received one of the three intensive psychotherapies had significantly higher rates of recovery (64% versus 52%) and recovered more rapidly (median=113 days ±78.2 versus 146 days ±80.0) than patients in the control condition, after controlling for site, family involvement, and bipolar disorder I versus bipolar disorder II ( 53 ). Compared with controls, individuals receiving intensive psychotherapy showed a significantly greater improvement in functioning as well as symptoms over time ( 54 ).
Meyer and Hautzinger ( 29 ) randomly assigned 76 individuals with bipolar disorder to 20 sessions of either CBT or supportive psychotherapy (ST). According to the investigators, “both treatment conditions included information (e.g., symptoms, etiology, and medication) and mood monitoring in the form of a mood diary. The difference between therapies was such that in the ST condition, therapists adopted a client-centerd focus, meaning that whatever problems the patient presented were dealt with by providing emotional support and general advice. Participants were followed for 33 months, and there were no differences between groups in relapse rates or mood symptoms over time ( 29 ). (See also trials by Zaretsky et al. ( 36 ) and Parikh et al. ( 30 ) described above.)
Individual CBT or CT has been evaluated in seven acute and maintenance trials (see Table 1 ). In general, CBT out-performs TAU but is comparable to more intensive psychotherapies. When compared with TAU ( 49 , 52 ), CBT/CT was associated with lower relapse rates and lower depression severity over time. When tested against a very brief control condition (“collaborative care,” which consisted of three sessions of PE) in STEP-BD, CBT was again shown to be the “better” treatment on measures of symptoms ( 55 ) and functioning ( 54 ). However, CBT appears to have comparable effects to other manualized therapies of adequate duration. Meyer and Hautzinger compared CBT to supportive psychotherapy, finding no differences in relapse rates or mood symptoms over 2-year follow-up ( 29 ). Similarly, as discussed above, Parikh and colleagues found no differences between CBT and six-session group PE ( 30 ). And, in STEP-BD, the active therapies did not separate from one another ( 55 ). An exception to this observation, Zaretsky and colleagues found that CBT plus PE was associated with fewer days depressed and fewer medication changes over 1 year than PE alone ( 36 ). Thus, CBT is clearly better than TAU, but it appears to be equivalent to other bipolar disorder-specific treatments.
The Scott et al. trial ( 28 ) raises somewhat different issues. This was an effectiveness study, conducted in routine practice settings. As interventions move from academic to real world settings, they typically appear to lose efficacy, resulting in an “efficacy-effectiveness gap” ( 56 ). Perhaps not surprisingly, under pragmatic conditions, outcomes with CBT did not differ from TAU—although treatment with CBT was associated with increased time to recurrence among those with fewer than 12 prior mood episodes.
Group Cognitive and Cognitive-Behavioral Therapy
Williams and colleagues compared an eight-session group Mindfulness-Based Cognitive Therapy (MBCT) to wait list control (WLC) for individuals with both bipolar disorder (N=17) and unipolar disorder (N=53) ( 57 ). An assignment to MBCT was associated with greater reductions in depressive symptoms than WLC: for BDI scores, there was a main effect for time (F 1,41 =6.07; p=0.018) and a significant time × condition interaction (F 1,41 =8.05; p=0.007) with those receiving MBCT having greater reductions in scores compared with WLC. On the Beck Anxiety Inventory, there was a significant three-way time × group × condition interaction (F 1,41 =7.55; p=0.009) such that those with bipolar disorder who received MBCT had fewer anxiety symptoms posttreatment compared with both WLC and those with unipolar depression ( 57 ).
Costa and colleagues ( 58 ) randomly assigned euthymic or mildly symptomatic individuals with bipolar disorder to either cognitive-behavioral group therapy (CBGT; N=27) or TAU (N=14), and followed them for 6 months. CBGT consisted of 14 2-hour sessions focusing on both psychoeducation and cognitive and behavioral skills for illness management. An assignment to CBGT was associated with significantly greater declines in depression scores on the BDI compared with TAU (R 2 =0.909, p=0.002) but no differences in Young Mania Rating Scale (YMRS) scores over time ( 58 ). Those who received CBGT also experienced significantly greater improvements in quality of life as measured by the Medical Outcomes Study 36-item Short-Form Health Survey (all subscales except functional capacity) from baseline to week 14 ( 59 ).
Gomes and colleagues ( 60 ) randomly assigned euthymic individuals with bipolar disorder (N=50) to either 18 sessions of CBGT or TAU. Over 12 months, there were no differences between groups on time until relapse or number of relapses (64% in CBGT versus 56% in TAU). The median time to first relapse was longer for patients treated with CBGT compared with TAU (66 weeks versus 31 weeks; Mann-Whitney=−2.554; p=0.011) ( 60 ).
Gonzalez Isasi and colleagues ( 26 ) randomly assigned patients with refractory bipolar disorder (frequent relapses, rapid cycling, suicide attempts, or persistent symptoms despite pharmacotherapy) to either 20 one-and-a-half hour sessions of CBGT (N=20) or TAU (N=20) and followed them for 5 years. Those assigned to CBGT had fewer hospitalizations than TAU at the 12-month evaluation (t=2.71, p=0.015). The CBGT group had lower depression and anxiety scores on the BDI and State Trait Anxiety Inventory, respectively, at the 6-month (p=0.006; p=0.019), 12-month (p=0.001; p<0.001), and 5-year (p<0.001, p<0.001) evaluation time points. At 5-year follow-up, 89% of patients in the TAU group and 20% of patients in the CBGT group showed persistent affective symptoms (BDI>7, YMRS>6) and/or difficulties in social-occupational functioning (χ 2 =18.03, p=0.001) ( 26 ).
CBGT, although not as widely tested as individual CBT, has demonstrated efficacy for bipolar disorder. Each of the four studies summarized above showed superior outcomes to TAU on measures of depressive symptoms ( 26 , 60 ), anxiety symptoms ( 26 , 57 ), functioning ( 57 , 59 ), and time to relapse ( 60 ). These studies suggest that CBGT is a useful adjunct to pharmacotherapy for the management of bipolar disorder. It will be interesting to see how CBGT fares when tested against more intensive psychotherapies.
Family Therapy
Family-focused therapy (FFT), as described by Miklowitz and colleagues, is a 21-session intervention that is conducted conjointly with a patient and family member (parent, sibling). Treatment focuses on psychoeducation, communication enhancement training, and problem-solving skills training ( 61 ). Miklowitz and colleagues compared FFT to three-session crisis management (CM) in 101 adults who had been recently hospitalized for bipolar disorder and partially stabilized on pharmacotherapy. Over a 2-year follow-up period, those assigned to FFT had longer delays prior to relapse (χ 2 =8.71, p=0.003). A hazard ratio of 0.38 reflects a threefold higher rate of survival without a mood episode in the FFT group over 2 years (52%) than the CM group (17%). Affective (both manic and depressive symptoms) symptoms were derived from the Schedule for Affective Disorders and Schizophrenia–Change Version (SADS-C). A repeated measure mixed model analysis of variance revealed a significant time × treatment interaction favoring FFT on the SADS–C-derived mood symptoms (F 7,549 =2.81; p=0.007), showing that those assigned to FFT had greater reductions in mood symptoms than those assigned to CM ( 61 ).
Solomon, Miller, and colleagues randomly assigned individuals with bipolar disorder (N=92), currently in a mood episode, to individual family therapy plus pharmacotherapy, multifamily group therapy plus pharmacotherapy, or pharmacotherapy alone. Individual family therapy involved one therapist meeting with a single patient and the patient’s family members, with the content of each session and number of sessions determined by the therapist and family. Multifamily group psychotherapy involved two therapists meeting together for six sessions with multiple patients and their respective family members, with the content of each session preset. Fifty-eight percent of subjects (53/92) recovered from their intake mood episode, and there were no differences between groups on the proportion recovering or the time to recovery. However, there were significant treatment conditions by family impairment interactions (p<0.05): in patients from families with high levels of impairment, the addition of a family intervention (family therapy or multifamily group) resulted in a significantly improved course of illness, particularly with respect to the number of depressive episodes (p<0.01) and the proportion of time spent in a depressive episode (p<0.01). Patients receiving either family intervention had approximately half the number of depressive episodes and spent half as much time depressed as those receiving pharmacotherapy alone ( 62 ). Of those suffering a recurrence over 28 months, a significantly smaller proportion of patients assigned to adjunctive multifamily group therapy required hospitalization, compared with those receiving adjunctive individual family therapy or pharmacotherapy alone (χ 2 =6.53, df=2, p<0.04) ( 63 ). (Also see studies of Rea and colleagues ( 37 ) and STEP-BD ( 55 ) described above.)
For patients with family members who are willing and able to participate in treatment, family therapy is an excellent option. Compared with psychoeducation, FFT hastens recovery ( 55 ) and confers additional protection against recurrence ( 37 , 55 ). Families with greater levels of impairment may derive additional benefit from family therapy when delivered either as individual or multifamily group therapy ( 63 ).
Interpersonal and Social Rhythm Therapy
Interpersonal and Social Rhythm Therapy (IPSRT) was developed by Frank and colleagues to help patients with bipolar disorder address interpersonal problems and regulate their social rhythms ( 64 ). Social rhythms are those daily activities that help to set (or disrupt) underlying biologic rhythms. Examples of social rhythms include sleep and wake schedules, mealtimes, start time for work/school, and daily exercise. IPSRT postulates that improved regularity of social rhythms will help to entrain/regulate underlying biologic rhythms, thereby addressing the putative link between circadian rhythm disruption and genesis of mood episodes in bipolar disorder ( 65 , 66 ). IPSRT combines the well-established principles of interpersonal psychotherapy (IPT) for unipolar depression ( 67 ) with a behavioral strategy designed to regularize daily routines (social rhythm therapy), and psychoeducation to enhance adherence to medication regimens. IPSRT focuses on: 1) the identification and management of affective symptoms; 2) the link between mood and life events; 3) the maintenance of regular daily rhythms as elucidated by the Social Rhythm Metric ( 68 ); 4) the identification and management of potential precipitants of rhythm dysregulation, with special attention to interpersonal triggers; and 5) the facilitation of mourning the lost healthy self ( 69 ).
Frank and colleagues ( 70 ) evaluated IPSRT as a maintenance treatment for individuals with bipolar disorder (N=175). Acutely ill patients were treated with medication and randomly assigned to either IPSRT or intensive clinical management (CM). Once stabilized [defined as 4 weeks of symptom scores averaging ≤7 on the HAM-D and ≤7 on the Bech-Rafaelsen Mania Scale ( 70 ) while on a stable medication regimen], patients were reassigned to either IPSRT or CM (in conjunction with the medication regimen that led to stabilization) for 2 years of monthly maintenance treatment. Those assigned to IPSRT in acute treatment survived significantly longer without a new affective episode during the 2-year maintenance phase than those assigned to CM (p=0.01; hazard ratio=0.35) ( 71 ). An assignment to IPSRT was also associated with more rapid improvement in occupational functioning ( 72 ).
Swartz and colleagues ( 27 ) conducted a small RCT comparing IPSRT (N=14) to quetiapine (N=11), flexibly dosed from 25–300 mg daily, in unmedicated individuals meeting DSM-IV criteria for bipolar disorder II disorder, currently depressed. Over 12 weeks, both groups showed significant declines in the 25-item HAM-D (F 1,21 = 44, p<0.0001) and YMRS (F 1,21 =20, p=0.0002) scores over time but no differences between groups were detected ( 27 ). (Also see description of STEP-BD study ( 55 ) above.)
IPSRT has demonstrated efficacy as both an acute ( 55 ) and maintenance treatment for bipolar disorder ( 71 ). Interestingly, it appears that administering IPSRT during the acute phase of treatment confers the greatest advantage to patients ( 55 ). IPSRT also shows promise as monotherapy (i.e., without medication) for bipolar disorder II depression ( 27 ). Although IPSRT is typically administered as an individual psychotherapy, it has been implemented in routine practice settings as a group intervention as well ( 73 ).
Integrated Care Management
Integrated Care Management (ICM) utilizes the strategies of case management in conjunction with psychotherapy to optimize outcomes for individuals with bipolar disorder. Using a chronic care model of disease management, ICM is defined by Bauer and colleagues as “an organization of care that emphasizes the patient’s development of illness management skills and supports provider capability and availability in order to engage patients in timely, joint decision making about their illness” ( 74 ). Thus, ICM encompasses both patient- and provider-level interventions.
Bauer and colleagues randomized 306 individuals recently discharged from the hospital and receiving care at 11 U.S. Department of Veterans Affairs (VA) Hospitals to either TAU or collaborative care. Those assigned to collaborative care participated in weekly group psychoeducation using the Life Goals Program ( 75 ), which focuses on the identification of personal symptom profiles, early warning symptoms, and triggers of illness relapse. The patients also had access to evidence-based pharmacotherapy delivered by a psychiatrist with expertise in bipolar disorder and care management from a nurse specialist who coordinated services. Primary clinical outcome was weeks in any episode. Those assigned to collaborative care had 6.2 fewer weeks in any affective episode over 3 years of follow-up compared with TAU (95% CI, −0.3 to −12.5, p<0.05). Over all social role dysfunction as measured by the Social Adjustment Scale decreased significantly more over 3 years in the collaborative care group relative to TAU (p=0.003) ( 74 , 76 ).
Simon and colleagues ( 77 ) tested an integrated care intervention in individuals with bipolar disorder enrolled in four group-model behavioral health clinics of a managed care organization, Group Health Cooperative. Patients hospitalized in the prior 12 months (N=441) were randomly assigned to either ICM or TAU and followed for 1 year. ICM consisted of a group psychoeducation program, monthly telephone monitoring of symptoms and medications, and feedback to the mental health treatment team. Those assigned to ICM had significantly lower mean mania rating scores on the Longitudinal Interval Follow-up Evaluation’s (LIFE) 6-point Psychiatric Status Rating over time (Z=2.24, p=0.025), but there was no difference between groups on change in LIFE mania scores over time. The intervention group showed a significantly greater decline in depression ratings over time compared with TAU (Z=1.98, p=0.048) ( 77 ).
Crowe and colleagues ( 31 ) conducted a pragmatic trial of nurse-led specialist supportive care (SSC), delivered in a community mental health clinic. SSC is designed to promote and maintain a therapeutic relationship between patient and clinician, improve adherence to pharmacotherapy, and develop problem-solving tools to help them better manage bipolar disorder. It includes psychoeducation and strategies to promote self-esteem and self-efficacy. SSC was delivered weekly for 2 months and then every 2 weeks for seven additional months. Patients with bipolar disorder, currently in a mood episode, were randomly assigned to either SSP (N=36) or TAU (N=42). Forty-two percent of those assigned to SSP (15/36) declined the intervention. Depression and mania symptoms were assessed using the SCL-90, and there were no differences in changes in these measures over time between the two groups. Notably, this study was powered to detect relatively large effect sizes (>0.7), which suggests the possibility of type II error ( 31 ).
Large effectiveness trials of integrated care interventions in both private (Group Health Cooperative) and public (VA) were associated with decreased time in episodes ( 74 , 76 ) and decline in depressive symptoms ( 77 ). These studies show that psychosocial treatments delivered in the context of a larger set of interventions designed to improve medication adherence and facilitate communication with care providers leads to reduced illness burden. A smaller trial of SSC was probably underpowered to detect the modest effects that occur in routine practice settings.
Other Group Interventions
Below we briefly describe trials of group interventions that cannot be categorized as one of the types of psychotherapies previously discussed.
Weiss and colleagues ( 78 ) compared integrated group therapy (N=31) to group drug counseling (N=31) as adjunctive treatments for individuals with comorbid bipolar disorder and substance dependence. Integrated group therapy consists of 20 weekly sessions utilizing a cognitive-behavioral approach to relapse prevention focusing on the similarities between bipolar disorder and substance dependence. Group drug counseling is also a 20-week program but focuses only on substance use-related problems and goals. Those assigned to integrated group therapy had half as many days of substance use compared with group drug counseling (5.3 [SD=6.6] versus 10.0 [SD=9.1] days/month) with significantly shorter time from treatment initiation to first abstinent month (hazard ratio=2.12, z=2.23, p<0.03). In contrast to the positive effects on substance use, there were no group differences in proportion of weeks in a mood episode, and those receiving integrated group therapy had more depressive and (hypo)manic symptoms than those in group drug counseling (p<0.001) ( 78 ). A follow-up study evaluating a shorter (12 session) version of integrated group therapy (N=31) versus group drug counseling (N=30) showed trends favoring integrated group therapy, with greater reductions in substance use during follow-up and a greater decline in risk of mood episodes during treatment ( 79 ).
Castle and colleagues ( 80 ) randomized euthymic individuals with bipolar disorder (N=84) to either a manualized MAPS group (described in the following sentence) intervention or to TAU. The MAPS acronym reflects the strategies employed in treatment: monitoring and mood activities (M), assessing prodromes (A), preventing relapse (P), and setting specific, measurable, achievable, realistic, time-framed (SMART) goals (S). The MAPS group intervention consists of 12 weekly sessions followed by monthly booster sessions. Over 9 months, there was a significantly decreased risk of relapse in those participating in the MAPS group compared with TAU [log rank χ 2 (1)=4.31, p=0.04] ( 80 ).
Van Dijk and colleagues ( 81 ) randomly assigned a small number of individuals with bipolar disorder (N=26) to treatment with a dialectical behavior therapy (DBT)-based PE group or TAU. The group intervention consisted of 12 weekly 90-minute sessions, which taught DBT skills, mindfulness techniques, and general psychoeducation. Individuals assigned to group intervention demonstrated a trend toward reduced depressive symptoms posttreatment (F=3.43, p=0.078), and significant improvement in measures of mindfulness (F=9.41, p=0.006). Furthermore, group attendees had fewer emergency room visits and mental health-related admissions in the 6 months following treatment than those assigned to TAU (t=4.6, p<0.0001) ( 81 ).
Weiss and colleagues’ groups show that an integrated treatment group is better than a group focused on addiction only for individuals with bipolar disorder and co-occurring substance use disorders—although the benefit of treatment appears to impact substance use rather than mood symptoms ( 78 , 79 ). Both MAPS and DBT groups utilize strategies from both PE and CBT. Derived from treatments that have demonstrated efficacy for bipolar disorder, it is not surprising that they also show a favorable impact on bipolar disorder when compared with TAU ( 80 , 81 )
Psychotherapy, when added to medication for the treatment of bipolar disorder, consistently shows advantages over medication alone as a treatment for bipolar disorder. Whether delivered in a group or individual format, those who receive bipolar disorder-specific psychotherapy fare better than those who do not. Meta-analyses of psychotherapy for bipolar disorder confirm these findings ( 82 , 83 ). Psychotherapy hastens the recovery from depressive episodes and prevents new mood episodes. It also helps to improve functioning and quality of life. Given the relatively modest risks associated with psychotherapy (i.e., loss of confidentiality) and robust benefits, psychosocial treatments should be considered an important component of bipolar disorder illness management.
We have previously argued that psychotherapies for bipolar disorder may have a more robust impact on depression compared with mania ( 84 ). In general, psychotherapy trials show a bigger impact on depressive symptoms than manic symptoms. This may be related to the fact that many bipolar disorder psychotherapies, originally developed for treatment of unipolar depression and later adapted for management of bipolar disorder (for example, CBT and IPT), have a bigger impact on depression. It may also be explained at least in part by the fact that depressive symptoms are much more prevalent than [hypo]manic symptoms ( 85 , 86 ), and, unless patients are specifically recruited on the basis of elevated mania symptoms, there is likely to be a “ceiling effect” on the change in mania scores. It makes sense, for instance, that in acute depression trials, studies show more of an effect on the depressive than manic pole of the illness. However, even when subjects are recruited in a euthymic state, some studies show more of an impact on depressive symptoms [compare ( 36 )]. Exceptions to this observation include an ICM trial that found reduced time in manic or hypomanic episodes in those receiving ICM and having a high symptom burden at baseline, but no impact on depressive symptoms ( 87 ), and an individual PE study finding reductions in the time to mania but not depression ( 34 ). These studies suggest the possibility that more intensive interventions targeting more severely ill patients may have preferential effects on mania.
Interestingly, there is considerable overlap among the bipolar disorder-specific psychotherapies. Just as nonspecific factors contribute to a large percentage of the variance in outcomes for all psychotherapies (see Markowitz’ supportive psychotherapy article in the current issue of FOCUS [88] for a discussion of this topic) there are several core strategies that are common to most, if not all, of the efficacious treatments for bipolar disorder. These core strategies include psychoeducation and self-rated mood charts. (See Table 3 for a description of strategies used across psychotherapeutic approaches.)
CBT: cognitive behavioral therapy; FT: family therapy; IPSRT: interpersonal and social rhythm therapy; PE: psychoeducation; ICM: integrated care management.
How then, should a patient decide which bipolar disorder-specific psychotherapy is best for him or her? Although active treatments consistently separate from TAU, most RCTs that compare two evidence-based psychotherapies show little difference between them, suggesting that any of the bipolar disorder-specific psychotherapies will help. Unfortunately, the availability of evidence-based psychotherapy in routine practice settings has not kept pace with the increasing demand for these services ( 89 ). Thus, the choice of treatment may be primarily driven by the availability of trained therapists and preference for individual versus group treatment. Stepped psychotherapy care for bipolar disorder, i.e., administering brief interventions that deliver core components of psychotherapy followed by longer treatments of increasing specificity for those who do not benefit fully from shorter treatments, may help the field to allocate efficiently relatively scarce psychotherapy resources, improve outcomes, and ensure that as many people as possible have access to bipolar disorder-specific psychotherapies. Studies of this approach, however, are needed.
Author Information and Disclosure
Holly A. Swartz, M.D., Associate Professor of Psychiatry, Department of Psychiatry, University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinic, 3811 O’Hara St., Pittsburgh, PA 15213
Joshua Swanson, Undergraduate Student, Carnegie Mellon University, Pittsburgh, PA
The authors report no financial relationships with commercial interests.
Dr. Swartz is supported by National Institute of Mental Health grant MH-84831.
Acknowledgments
This article includes material from a previously published chapter: Swartz HA, Frank E, Kupfer DJ: Psychotherapy for bipolar disorder, in American Psychiatric Publishing Textbook of Mood Disorders. Edited by Stein DJ, Kupfer DJ, Schatzberg AF. Washington, DC, American Psychiatric Publishing, 2006, pp 405–420. Copyright © 2006 American Psychiatric Publishing. Used with permission.
1 Cade JFL : Lithium salts in the treatment of psychotic excitement . Med J Aust 1949 ; 2:349–352 Crossref , Google Scholar
2 Goodwin FK, Jamison KR : Manic-Depressive Illness . New York, Oxford University Press, Inc., 2007 Google Scholar
3 Benson R : The forgotten treatment modality in bipolar illness: psychotherapy . Dis Nerv Syst 1975 ; 36:634–638 Google Scholar
4 McEvoy JP, Wilkinson ML : The role of insight in the treatment and outcome of bipolar disorder . Psychiatr Ann 2000 ; 30:406–408 Crossref , Google Scholar
5 Fromm-Reichmann F : Intensive psychotherapy of manic-depressives . Confin Neurol 1949 ; 9:158–165 Crossref , Google Scholar
6 Prien RF, Himmelhoch JM, Kupfer DJ : Treatment of mixed mania . J Affect Disord 1988 ; 15:9–15 Crossref , Google Scholar
7 Yatham LN, Kusumakar V, Parikh SV, Haslam DRS, Matte R, Sharma V, Kennedy S : Bipolar depression: treatment options . Can J Psychiatry 1997 ; 42(Suppl 2):87S–91S Google Scholar
8 Markar HR, Mander AJ : Efficacy of lithium prophylaxis in clinical practice . Br J Psychiatry 1989 ; 155:496–500 Crossref , Google Scholar
9 Prien RF : NIMH report. Five-center study clarifies use of lithium, imipramine for recurrent affective disorders . Hosp Community Psychiatry 1984 ; 35:1097–1098 Google Scholar
10 Goldberg JF, Harrow M, Grossman LS : Course and outcome in bipolar affective disorder: a longitudinal follow-up study . Am J Psychiatry 1995 ; 152:379–384 Crossref , Google Scholar
11 Coryell W, Scheftner W, Keller M, Endicott J, Maser J, Klerman GL : The enduring psychosocial consequences of mania and depression . Am J Psychiatry 1993 ; 150:720–727 Crossref , Google Scholar
12 United States Department of Veteran Affairs and Department of Defense: Clinical Practice Guideline for Management of Bipolar Disorder in Adults. 2010; http://www.healthquality.va.gov/bipolar/bd_305_full.pdf ; accessed Jan 29, 2014 Google Scholar
13 Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, O’Donovan C, Macqueen G, McIntyre RS, Sharma V, Ravindran A, Young LT, Milev R, Bond DJ, Frey BN, Goldstein BI, Lafer B, Birmaher B, Ha K, Nolen WA, Berk M : Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013 . Bipolar Disord 2013 ; 15:1–44 Crossref , Google Scholar
14 Merikangas KR, Jin R, He J-P, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z : Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative . Arch Gen Psychiatry 2011 ; 68:241–251 Crossref , Google Scholar
15 Nierenberg AA, Akiskal HS, Angst J, Hirschfeld RM, Merikangas KR, Petukhova M, Kessler RC : Bipolar disorder with frequent mood episodes in the national comorbidity survey replication (NCS-R) . Mol Psychiatry 2010 ; 15:1075–1087 Crossref , Google Scholar
16 Ruggero CJ, Chelminski I, Young D, Zimmerman M : Psychosocial impairment associated with bipolar II disorder . J Affect Disord 2007 ; 104:53–60 Crossref , Google Scholar
17 Sajatovic M, Biswas K, Kilbourne AK, Fenn H, Williford W, Bauer MS : Factors associated with prospective long-term treatment adherence among individuals with bipolar disorder . Psychiatr Serv 2008 ; 59:753–759 Crossref , Google Scholar
18 Sajatovic M, Valenstein M, Blow F, Ganoczy D, Ignacio R : Treatment adherence with lithium and anticonvulsant medications among patients with bipolar disorder . Psychiatr Serv 2007 ; 58:855–863 Crossref , Google Scholar
19 Scott J, Pope M : Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization . Am J Psychiatry 2002 ; 159:1927–1929 Crossref , Google Scholar
20 Beyer JL, Kuchibhatla M, Looney C, Engstrom E, Cassidy F, Krishnan KR : Social support in elderly patients with bipolar disorder . Bipolar Disord 2003 ; 5:22–27 Crossref , Google Scholar
21 Johnson SL, Winett CA, Meyer B, Greenhouse WJ, Miller I : Social support and the course of bipolar disorder . J Abnorm Psychol 1999 ; 108:558–566 Crossref , Google Scholar
22 Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC : Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication . Arch Gen Psychiatry 2007 ; 64:543–552 Crossref , Google Scholar
23 Bora E, Yücel M, Pantelis C, Berk M : Meta-analytic review of neurocognition in bipolar II disorder . Acta Psychiatr Scand 2011 ; 123:165–174 Crossref , Google Scholar
24 Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Hansen CF, Jónsdóttir H, Ringen PA, Opjordsmoen S, Friis S, Andreassen OA : Neurocognitive profiles in bipolar I and bipolar II disorder: differences in pattern and magnitude of dysfunction . Bipolar Disord 2008 ; 10:245–255 Crossref , Google Scholar
25 Sole B, Bonnin CM, Torrent C, Martinez-Aran A, Popovic D, Tabarés-Seisdedos R, Vieta E : Neurocognitive impairment across the bipolar spectrum . CNS Neurosci Ther 2012 ; 18:194–200 Crossref , Google Scholar
26 González Isasi A, Echeburúa E, Limiñana JM, González-Pinto A : Psychoeducation and cognitive-behavioral therapy for patients with refractory bipolar disorder: a 5-year controlled clinical trial . Eur Psychiatry 2014 ; 29:134–141 Crossref , Google Scholar
27 Swartz HA, Frank E, Cheng Y : A randomized pilot study of psychotherapy and quetiapine for the acute treatment of bipolar II depression . Bipolar Disord 2012 ; 14:211–216 Crossref , Google Scholar
28 Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, Abbott R, Hayhurst H : Cognitive-behavioural therapy for severe and recurrent bipolar disorders: randomised controlled trial . Br J Psychiatry 2006 ; 188:313–320 Crossref , Google Scholar
29 Meyer TD, Hautzinger M : Cognitive behaviour therapy and supportive therapy for bipolar disorders: relapse rates for treatment period and 2-year follow-up . Psychol Med 2012 ; 42:1429–1439 Crossref , Google Scholar
30 Parikh SV, Zaretsky A, Beaulieu S, Yatham LN, Young LT, Patelis-Siotis I, Macqueen GM, Levitt A, Arenovich T, Cervantes P, Velyvis V, Kennedy SH, Streiner DL : A randomized controlled trial of psychoeducation or cognitive-behavioral therapy in bipolar disorder: a Canadian Network for Mood and Anxiety treatments (CANMAT) study [CME] . (CME) J Clin Psychiatry 2012 ; 73:803–810 Crossref , Google Scholar
31 Crowe M, Inder M, Carlyle D, Wilson L, Whitehead L, Panckhurst A, O’Brien T, Frampton C, Joyce P : Nurse-led delivery of specialist supportive care for bipolar disorder: a randomized controlled trial . J Psychiatr Ment Health Nurs 2012 ; 19:446–454 Crossref , Google Scholar
32 de Barros Pellegrinelli K, de O Costa LF, Silval KI, Dias VV, Roso MC, Bandeira M, Colom F, Moreno RA : Efficacy of psychoeducation on symptomatic and functional recovery in bipolar disorder . Acta Psychiatr Scand 2013 ; 127:153–158 Crossref , Google Scholar
33 Perry A, Tarrier N, Morriss R : Identification of prodromal signs and symptoms and early intervention in manic depressive psychosis patients: a case example . Behav Cogn Psychother 1995 ; 23:399–409 Crossref , Google Scholar
34 Perry A, Tarrier N, Morriss R, McCarthy E, Limb K : Randomised controlled trial of efficacy of teaching patients with bipolar disorder to identify early symptoms of relapse and obtain treatment . BMJ 1999 ; 318:149–153 Crossref , Google Scholar
35 Soares JJF, Stintzing CP, Jackson C, Skoldin B : Psychoeducation for patients with bipolar disorder - An exploratory study . Nord J Psychiatry 1997 ; 51:439–446 Crossref , Google Scholar
36 Zaretsky A, Lancee W, Miller C, Harris A, Parikh SV : Is cognitive-behavioural therapy more effective than psychoeducation in bipolar disorder? Can J Psychiatry 2008 ; 53:441–448 Crossref , Google Scholar
37 Rea MM, Tompson MC, Miklowitz DJ, Goldstein MJ, Hwang S, Mintz J : Family-focused treatment versus individual treatment for bipolar disorder: results of a randomized clinical trial . J Consult Clin Psychol 2003 ; 71:482–492 Crossref , Google Scholar
38 Colom F, Vieta E : Psychoeducation Manual for Bipolar Disorder . Cambridge, Cambridge University Press, 2006 Crossref , Google Scholar
39 Colom F, Vieta E, Martinez-Aran A, Reinares M, Goikolea JM, Benabarre A, Torrent C, Comes M, Corbella B, Parramon G, Corominas J : A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission . Arch Gen Psychiatry 2003 ; 60:402–407 Crossref , Google Scholar
40 Colom F, Vieta E, Sanchez-Moreno J, Palomino-Otiniano R, Reinares M, Goikolea JM, et al. Group psychoeducation for stabilised bipolar disorders: 5-year outcome of a randomised clinical trial . Br J Psychiatry 2009 ; 194(3):260–265. Google Scholar
41 Candini V, Buizza C, Ferrari C, Caldera MT, Ermentini R, Ghilardi A, Nobili G, Pioli R, Sabaudo M, Sacchetti E, Saviotti FM, Seggioli G, Zanini A, de Girolamo G : Is structured group psychoeducation for bipolar patients effective in ordinary mental health services? A controlled trial in Italy . J Affect Disord 2013 ; 151:149–155 Crossref , Google Scholar
42 Torrent C, Bonnin CdelM, Martínez-Arán A, Valle J, Amann BL, González-Pinto A, Crespo JM, Ibáñez Á, Garcia-Portilla MP, Tabarés-Seisdedos R, Arango C, Colom F, Solé B, Pacchiarotti I, Rosa AR, Ayuso-Mateos JL, Anaya C, Fernández P, Landín-Romero R, Alonso-Lana S, Ortiz-Gil J, Segura B, Barbeito S, Vega P, Fernández M, Ugarte A, Subirà M, Cerrillo E, Custal N, Menchón JM, Saiz-Ruiz J, Rodao JM, Isella S, Alegría A, Al-Halabi S, Bobes J, Galván G, Saiz PA, Balanzá-Martínez V, Selva G, Fuentes-Durá I, Correa P, Mayoral M, Chiclana G, Merchan-Naranjo J, Rapado-Castro M, Salamero M, Vieta E : Efficacy of functional remediation in bipolar disorder: a multicenter randomized controlled study . Am J Psychiatry 2013 ; 170:852–859 Crossref , Google Scholar
43 Beck AT, Rush AJ, Shaw BF, Gary E : Cognitive therapy of depression . New York, Guilford Press, 1979 Google Scholar
44 Lam DH, Bright J, Jones S, Hayward P, Schuck N, Chisholm D, et al. : Cognitive therapy for bipolar illness–a pilot study of relapse prevention . Cognit Ther Res 2000 ; 24:503–520 Crossref , Google Scholar
45 Basco MR, Rush AJ : Cognitive-behavioral therapy for bipolar disorder . New York, NY, Guilford Press, 1996 Google Scholar
46 Scott J : Cognitive therapy for clients with bipolar disorder . Cognit Behav Pract 1996 ; 3:29–51 Crossref , Google Scholar
47 Otto MW, Reilly-Harrington NA, Kogan J, Henin A : Managing bipolar disorder: A cognitive behavior treatment program therapist guide . New York, Oxford University Press, Inc., 2008 Crossref , Google Scholar
48 Lam DH, Jones SH, Hayward P : Cognitive therapy for bipolar disorder: a therapist’s guide to concepts, methods, and practice . Chichester, U.K, John Wiley & Sons Ltd., 2010 Crossref , Google Scholar
49 Lam DH, Watkins ER, Hayward P, Bright J, Wright K, Kerr N, Parr-Davis G, Sham P : A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: outcome of the first year . Arch Gen Psychiatry 2003 ; 60:145–152 Crossref , Google Scholar
50 Lam DH, Hayward P, Watkins ER, Wright K, Sham P : Relapse prevention in patients with bipolar disorder: cognitive therapy outcome after 2 years . Am J Psychiatry 2005 ; 162:324–329 Crossref , Google Scholar
51 Lam DH, McCrone P, Wright K, Kerr N . Cost-effectiveness of relapse-prevention cognitive therapy for bipolar disorder: 30-month study . Br J Psychiatry 2005 ; 186:500–506. Google Scholar
52 Ball JR, Mitchell PB, Corry JC, Skillecorn A, Smith M, Malhi GS : A randomized controlled trial of cognitive therapy for bipolar disorder: focus on long-term change . J Clin Psychiatry 2006 ; 67:277–286 Crossref , Google Scholar
53 Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF : Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) . Biol Psychiatry 2003 ; 53:1028–1042 Crossref , Google Scholar
54 Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Kogan JN, Sachs GS, Thase ME, Calabrese JR, Marangell LB, Ostacher MJ, Patel J, Thomas MR, Araga M, Gonzalez JM, Wisniewski SR : Intensive psychosocial intervention enhances functioning in patients with bipolar depression: results from a 9-month randomized controlled trial . Am J Psychiatry 2007 ; 164:1340–1347 Crossref , Google Scholar
55 Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Wisniewski SR, Kogan JN, Nierenberg AA, Calabrese JR, Marangell LB, Gyulai L, Araga M, Gonzalez JM, Shirley ER, Thase ME, Sachs GS : Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program . Arch Gen Psychiatry 2007 ; 64:419–426 Crossref , Google Scholar
56 Bauer MS, Williford WO, Dawson EE, Akiskal HS, Altshuler L, Fye C, Gelenberg A, Glick H, Kinosian B, Sajatovic M : Principles of effectiveness trials and their implementation in VA Cooperative Study #430: ‘Reducing the efficacy-effectiveness gap in bipolar disorder.’ J Affect Disord 2001 ; 67:61–78 Crossref , Google Scholar
57 Williams JM, Alatiq Y, Crane C, Barnhofer T, Fennell MJ, Duggan DS, Hepburn S, Goodwin GM : Mindfulness-based Cognitive Therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning . J Affect Disord 2008 ; 107:275–279 Crossref , Google Scholar
58 Costa RT, Cheniaux E, Rosaes PA, Carvalho MR, Freire RC, Versiani M, Rangé BP, Nardi AE : The effectiveness of cognitive behavioral group therapy in treating bipolar disorder: a randomized controlled study . Rev Bras Psiquiatr 2011 ; 33:144–149 Crossref , Google Scholar
59 Costa RT, Cheniaux E, Rangé BP, Versiani M, Nardi AE : Group cognitive behavior therapy for bipolar disorder can improve the quality of life . Braz J Med Biol Res 2012 ; 45:862–868 Crossref , Google Scholar
60 Gomes BC, Abreu LN, Brietzke E, Caetano SC, Kleinman A, Nery FG, Lafer B : A randomized controlled trial of cognitive behavioral group therapy for bipolar disorder . Psychother Psychosom 2011 ; 80:144–150 Crossref , Google Scholar
61 Miklowitz DJ, George EL, Richards JA, Simoneau TL, Suddath RL : A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder . Arch Gen Psychiatry 2003 ; 60:904–912 Crossref , Google Scholar
62 Miller IW, Keitner GI, Ryan CE, Uebelacker LA, Johnson SL, Solomon DA : Family treatment for bipolar disorder: family impairment by treatment interactions . J Clin Psychiatry 2008 ; 69:732–740 Crossref , Google Scholar
63 Solomon DA, Keitner GI, Ryan CE, Kelley J, Miller IW : Preventing recurrence of bipolar I mood episodes and hospitalizations: family psychotherapy plus pharmacotherapy versus pharmacotherapy alone . Bipolar Disord 2008 ; 10:798–805 Crossref , Google Scholar
64 Frank E : Treating bipolar disorder: A clinician’s guide to interpersonal and social rhythm therapy . New York, NY, Guilford Press, 2005 Google Scholar
65 Harvey AG : Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation . Am J Psychiatry 2008 ; 165:820–829 Crossref , Google Scholar
66 McClung CA : Circadian genes, rhythms and the biology of mood disorders . Pharmacol Ther 2007 ; 114:222–232 Crossref , Google Scholar
67 Weissman MM, Markowitz JC, Klerman GL : Comprehensive guide to interpersonal psychotherapy . New York, NY, Basic Books, 2000 Google Scholar
68 Monk TH, Flaherty JF, Frank E, Hoskinson K, Kupfer DJ : The Social Rhythm Metric. An instrument to quantify the daily rhythms of life . J Nerv Ment Dis 1990 ; 178:120–126 Crossref , Google Scholar
69 Frank E, Swartz HA, Kupfer DJ : Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder . Biol Psychiatry 2000 ; 48:593–604 Crossref , Google Scholar
70 Bech P, Bolwig TG, Kramp P, Rafaelsen OJ : The Bech-Rafaelsen Mania Scale and the Hamilton Depression Scale . Acta Psychiatr Scand 1979 ; 59:420–430 Crossref , Google Scholar
71 Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, Grochocinski V, Houck P, Scott J, Thompson W, Monk T : Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder . Arch Gen Psychiatry 2005 ; 62:996–1004 Crossref , Google Scholar
72 Frank E, Soreca I, Swartz HA, Fagiolini AM, Mallinger AG, Thase ME, Grochocinski VJ, Houck PR, Kupfer DJ : The role of interpersonal and social rhythm therapy in improving occupational functioning in patients with bipolar I disorder . Am J Psychiatry 2008 ; 165:1559–1565 Crossref , Google Scholar
73 Swartz HA, Frank E, O’Toole K, Newman N, Kiderman H, Carlson S, Fink JW, Cheng Y, Maihoefer CC, Wells KF, Houck PR, Painter T, Ortenzio SH, Simon SL, Henschke P, Ghinassi F : Implementing interpersonal and social rhythm therapy for mood disorders across a continuum of care . Psychiatr Serv 2011 ; 62:1377–1380 Crossref , Google Scholar
74 Bauer MS, McBride L, Williford WO, Glick H, Kinosian B, Altshuler L, Beresford T, Kilbourne AM, Sajatovic M ; Cooperative Studies Program 430 Study Team : Collaborative care for bipolar disorder: part I. Intervention and implementation in a randomized effectiveness trial . Psychiatr Serv 2006 ; 57:927–936 Crossref , Google Scholar
75 Bauer MS, McBride L : Structured group psychotherapy for bipolar disorder: the life goals program . New York, NY, Springer Publishing Co, Inc, 1996 Google Scholar
76 Bauer MS, McBride L, Williford WO, Glick H, Kinosian B, Altshuler L, Beresford T, Kilbourne AM, Sajatovic M ; Cooperative Studies Program 430 Study Team : Collaborative care for bipolar disorder: Part II. Impact on clinical outcome, function, and costs . Psychiatr Serv 2006 ; 57:937–945 Crossref , Google Scholar
77 Simon GE, Ludman EJ, Unützer J, Bauer MS, Operskalski B, Rutter C : Randomized trial of a population-based care program for people with bipolar disorder . Psychol Med 2005 ; 35:13–24 Crossref , Google Scholar
78 Weiss RD, Griffin ML, Kolodziej ME, Greenfield SF, Najavits LM, Daley DC, Doreau HR, Hennen JA : A randomized trial of integrated group therapy versus group drug counseling for patients with bipolar disorder and substance dependence . Am J Psychiatry 2007 ; 164:100–107 Crossref , Google Scholar
79 Weiss RD, Griffin ML, Jaffee WB, Bender RE, Graff FS, Gallop RJ, Fitzmaurice GM : A “community-friendly” version of integrated group therapy for patients with bipolar disorder and substance dependence: a randomized controlled trial . Drug Alcohol Depend 2009 ; 104:212–219 Crossref , Google Scholar
80 Castle D, White C, Chamberlain J, Berk M, Berk L, Lauder S, et al. Group-based psychosocial intervention for bipolar disorder: randomised controlled trial . Br J Psychiatry 2010 ; 196(5):383–388. Google Scholar
81 Van Dijk S, Jeffrey J, Katz MR : A randomized, controlled, pilot study of dialectical behavior therapy skills in a psychoeducational group for individuals with bipolar disorder . J Affect Disord 2013 ; 145:386–393 Crossref , Google Scholar
82 Scott J, Colom F, Vieta E : A meta-analysis of relapse rates with adjunctive psychological therapies compared to usual psychiatric treatment for bipolar disorders . Int J Neuropsychopharmacol 2007 ; 10:123–129 Crossref , Google Scholar
83 Szentagotai A, David D : The efficacy of cognitive-behavioral therapy in bipolar disorder: a quantitative meta-analysis . J Clin Psychiatry 2010 ; 71:66–72 Crossref , Google Scholar
84 Swartz HA, Frank E : Psychotherapy for bipolar depression: a phase-specific treatment strategy? Bipolar Disord 2001 ; 3:11–22 Crossref , Google Scholar
85 Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB : A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder . Arch Gen Psychiatry 2003 ; 60:261–269 Crossref , Google Scholar
86 Judd LL, Schettler PJ, Akiskal HS, Maser J, Coryell W, Solomon D, Endicott J, Keller M : Long-term symptomatic status of bipolar I vs. bipolar II disorders . Int J Neuropsychopharmacol 2003 ; 6:127–137 Crossref , Google Scholar
87 Simon GE, Ludman EJ, Bauer MS, Unützer J, Operskalski B : Long-term effectiveness and cost of a systematic care program for bipolar disorder . Arch Gen Psychiatry 2006 ; 63:500–508 Crossref , Google Scholar
88 Markowitz JC : What is supportive psychotherapy ? Focus 2014 ; 12:285–289 Link , Google Scholar
89 National Mental Health Advisory Council’s Workgroup on Services and Clinical Epidemiology Research : The Road Ahead: Research Partnerships to Transform Services . Washington, D.C., National Institute of Mental Health, 2006 Google Scholar
- Psychotherapies for the Treatment of Bipolar Disorder 30 June 2024 | Psikiyatride Güncel Yaklaşımlar, Vol. 16, No. 2
- Trial‐based economic evaluation of mindfulness‐based cognitive therapy compared to treatment as usual for bipolar disorder 12 September 2023 | International Journal of Methods in Psychiatric Research, Vol. 33, No. 1
- Mindfulness and cognitive training interventions that address intersecting cognitive and aging needs of older adults 16 October 2023 | Journal of Social Work, Vol. 24, No. 1
- Caitlin Hasser , M.D. ,
- Maithri Ameresekere , M.D., M.Sc. ,
- Christina Girgis , M.D. ,
- Jacquelyn Knapp , M.D. ,
- Riva Shah , M.D.
- Psychosocial Interventions: A Key Component in an Evidence-Based Treatment Approach to Bipolar Disorder 26 December 2023 | Journal of Contemporary Psychotherapy, Vol. 185
- EMDR therapy vs. supportive therapy as adjunctive treatment in trauma-exposed bipolar patients: A randomised controlled trial Spanish Journal of Psychiatry and Mental Health, Vol. 4
- Brain stimulation treatment for bipolar disorder 21 December 2022 | Bipolar Disorders, Vol. 25, No. 1
- Therapeutic Application of Lithium in Bipolar Disorders: A Brief Review Cureus, Vol. 41
- Mathematical Model of Interaction of Therapist and Patients with Bipolar Disorder: A Systematic Literature Review 7 September 2022 | Journal of Personalized Medicine, Vol. 12, No. 9
- Compassion Focused Group Therapy for People With a Diagnosis of Bipolar Affective Disorder: A Feasibility Study 20 July 2022 | Frontiers in Psychology, Vol. 13
- Bipolar Disorder 28 May 2022
- An Overview of Group Cognitive Behavioral Therapy: Science and Practice
- Treating Bipolar Disorder in Primary Care: Diagnosis, Pharmacology, and Management 1 November 2022 | International Journal of General Medicine, Vol. Volume 15
- Schema Therapy for Patients with Bipolar Disorder: Theoretical Framework and Application 1 January 2022 | Neuropsychiatric Disease and Treatment, Vol. Volume 18
- Perceived criticism and family attitudes as predictors of recurrence in bipolar disorder 31 March 2022 | Clinical Psychology in Europe, Vol. 4, No. 1
- Contemporary Supportive Therapy: A Review of History, Theory, and Evidence Psychodynamic Psychiatry, Vol. 49, No. 4
- Advancing health through research: A scoping review of and model for adjunctive psychosocial interventions to improve outcomes for perinatal women with bipolar disorder Journal of Affective Disorders, Vol. 294
- Application of a Manualized Cognitive-Behavioral Treatment Protocol to an Individual with Bipolar Disorder in a Private Practice Setting 4 May 2021
- The Treatment of Bipolar Depression: Current Status and Future Perspectives 15 January 2020 | Current Behavioral Neuroscience Reports, Vol. 7, No. 1
- Cognitive Behavioral Therapy in Late Life
- A positive psychology intervention for patients with bipolar depression: a randomized pilot trial 26 October 2018 | Journal of Mental Health, Vol. 29, No. 1
- Repetitive transcranial magnetic stimulation in the treatment of bipolar disorder 18 November 2020 | Therapeutic Advances in Psychopharmacology, Vol. 10
- Danielle M. Novick , Ph.D. , and
- Holly A. Swartz , M.D.
- Frontiers in Psychiatry, Vol. 10
- Bipolar Disorder in Primary Care: Considerations in Management 12 December 2018 | Current Treatment Options in Psychiatry, Vol. 5, No. 4
- Hold on Tight: Coping Strategies of Persons With Bipolar Disorder and Their Partners 27 June 2018 | Family Relations, Vol. 67, No. 5
- World Journal of Psychiatry, Vol. 8, No. 5
- Systemic challenges in bipolar disorder management: A patient‐centered approach 13 September 2017 | Bipolar Disorders, Vol. 19, No. 8
- Brief psychoeducation for bipolar disorder: Evaluation of trophic factors serum levels in young adults Psychiatry Research, Vol. 257
- Psychotherapy and Psychosocial Interventions, Family Psychoeducation, and Support for Older Age Bipolar Disorder 6 January 2017
- Migraine headache and bipolar disorders: Common comorbidities 1 April 2016 | Scandinavian Journal of Pain, Vol. 11, No. 1

Precursors of bipolar disorders: a systematic literature review of prospective studies
Affiliation.
- 1 245 East 50th St, Ste 2A, New York, NY 10022 [email protected].
- PMID: 26035191
- DOI: 10.4088/JCP.13r08900
Objective: To evaluate the presence of affective signs and symptoms as precursors of bipolar disorder in prospective studies, including assessment of their prevalence, duration, and predictive value.
Data sources: We followed PRISMA guidelines to search PubMed, CINAHL, PsycINFO, EMBASE, SCOPUS, and ISI Web of Science databases to May 31, 2013, using the terms bipolar disorder AND (antecedent* OR predict* OR prodrom* OR prospect*) AND (diagnosis OR development). Hand searching of identified reports led to additional relevant references.
Study selection: We included only English-language articles containing (1) prospective, longitudinal studies with at least 2 structured clinical assessments (intake and follow-up); (2) no previous DSM-III or DSM-IV diagnoses of bipolar I or bipolar II; and (3) diagnostic outcome of bipolar I or bipolar II. Studies of subjects at familial risk of bipolar disorder were excluded, as these have been reviewed elsewhere.
Data extraction: We tabulated details of study design, outcomes, precursors, and predictive value. Only studies reporting a positive predictive association were included.
Results: In 26 published reports meeting selection criteria, methods varied widely in terms of design, duration of follow-up, ages, and populations investigated. Despite such heterogeneity in methods, findings were notably consistent. Precursors of bipolar disorder include mood lability, subsyndromal and major depression, subsyndromal hypomanic symptoms with or without major depression, cyclothymia and bipolar not otherwise specified, major depression with psychotic features, and other psychotic disorders. Bipolar disorder was also predicted by juvenile onset of major depression as well as frequency and loading of hypomanic or depressive symptoms.
Conclusions: Despite the limitations of published reports, prospectively identified precursors of bipolar disorder typically arose years prior to syndromal onset, often with significant early morbidity and disability. Prospectively identified precursors of bipolar disorder are generally consistent with findings in retrospective and family-risk studies. Combining precursors and other risk factors may increase predictive value, support earlier diagnosis, improve treatment, and limit disability in bipolar disorder.
© Copyright 2015 Physicians Postgraduate Press, Inc.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Systematic Review
- Bipolar Disorder / diagnosis
- Bipolar Disorder / epidemiology*
- Prodromal Symptoms*
Grants and funding
- MH-47370/MH/NIMH NIH HHS/United States
- MH-73049/MH/NIMH NIH HHS/United States
- RC1 MH089743/MH/NIMH NIH HHS/United States
- Open access
- Published: 22 April 2024
HERV-W upregulation expression in bipolar disorder and schizophrenia: unraveling potential links to systemic immune/inflammation status
- Sara Coelho Rangel 1 na1 ,
- Michelly Damasceno da Silva 1 na1 ,
- Décio Gilberto Natrielli Filho 2 ,
- Samuel Nascimento Santos 1 ,
- Jonatas Bussador do Amaral 3 ,
- Jefferson Russo Victor 1 ,
- Kevin Cezar Nascimento Silva 1 ,
- Izabela Dorota Tuleta 4 ,
- Carolina Nunes França 1 ,
- Marina Tiemi Shio 1 ,
- Lucas Melo Neves 1 ,
- André Luis Lacerda Bachi 1 &
- Luiz Henrique da Silva Nali 1
Retrovirology volume 21 , Article number: 7 ( 2024 ) Cite this article
290 Accesses
10 Altmetric
Metrics details
Bipolar disorder (BD) and schizophrenia (SZ) are the two main mental disorders with unknown etiology that significantly impact individuals’ quality of life. The potential pro-inflammatory role in their pathogenesis is postulated and Human Endogenous Retrovirus W (HERV-W) is an emerging candidate to modulate this pathogenic finding. HERVs, ancient retroviruses in the human genome, may play roles in inflammation and disease pathogenesis. Despite HERVs’ involvement in autoimmune diseases, their influence on mental disorders remains underexplored. Therefore, the aim of this study was to assess the level of HERV-W-env expression and the systemic inflammatory profile through the concentration of IL-2, IL-4, IL-6, IL-10, TNF-α and INF-γ cytokines in BD and SZ patients.
All participants showed HERV-W-env expression, but its expression was higher in mental disorder patients ( p < 0.01) than in control. When separated, SZ individuals exhibited higher HERV-W expression than the control group ( p < 0.01). Higher serum levels of TNF-α and IL-10 were found in BD ( p = 0.0001 and p = 0.001, respectively) and SZ ( p = 0.01) and p = 0.01, respectively) than in the control group, while SZ showed decreased levels IFN-γ and IL-2 as compared to controls ( p = 0.05) and BD patients ( p = 0.05), respectively. Higher TNF-α/IL-4 and TNF-α/IL-10 ratios, and lower IFN-γ/IL-10 were observed in BD and SZ patients than controls. Significant negative correlation between HERV-W-env expression and IL-10 ( r =-0.47 p < 0.05), as well as positive correlations between HERV-W-env expression and TNF-α/IL-10 or IFN-γ/IL-10 ratios ( r = 0.48 p < 0.05 and r = 0.46 p < 0.05, respectively) were found in BD patients.
These findings suggest not only a potential link between HERV-W-env expression both in BD and SZ, but also a possible involvement of systemic inflammatory status in BD patients.
Bipolar disorder (BD) and Schizophrenia (SZ) are two main frequent and severe mental disorders that significantly compromise the quality of life and the social conditions of affected individuals [ 1 ]. Both disorders do not present any specific etiology, but genetic and environmental factors may be associated with the disease onset and clinical evolution of the disease [ 2 , 3 ]. Despite the unknown etiology of the disease, it is utmost to emphasize the possible involvement of systemic pro-inflammatory status in the context of the disease’s pathogenesis [ 4 , 5 ].
However, triggering this inflammatory response profile and sustaining this condition in both these diseases is still poorly understood. In this sense, Human Endogenous Retroviruses (HERV) may be highlighted not only as a potential candidate for promoting inflammatory response [ 6 , 7 ], but might also be associated with these disease’s pathogenesis [ 8 , 9 ].
HERVs are ancient retroviruses that have become integral components of the human genome, which originated from infections in our ancestor’s germline cells millions of years ago [ 10 , 11 , 12 ]. These retroviruses have been transmitted and perpetuated initially by horizontal transmission [ 13 ] and later inherited by Mendelian way [ 14 ], and today we know that HERVs compose 8% of the human genome [ 10 , 11 , 12 , 15 ]. Over generations, retrotransposition events have contributed to the genomic diversity of these elements within the genome. However, HERVs have undergone mutations, resulting in their silencing by the presence of stop codon within the coding region, isolated genes/Long Terminal Repeats (LTRs) and incomplete sequences, which resulted in their inability to replicate [ 16 ]. Nevertheless, HERVs can still exhibit expression, and the virions may be formed through the combination of retroviral genes from various loci within the genome [ 13 , 17 , 18 , 19 ].
Notably, these retroviruses play crucial roles in human physiology, e.g. the HERV-W-env protein, also known as Syncytin-1, assumes pivotal participation in human placentation by mediating the fusion of Syncytiotrophoblast during early nidation and placenta formation [ 20 ]. Additionally, the LTRs of HERVs may serve as promoters for human genes [ 21 , 22 ]. Unfortunately, HERVs are also linked to the development of autoimmune diseases, such as Multiple Sclerosis, as it has been described in the past 30 years [ 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 ]. Interestingly, HERVs may promote an inflammatory response, and some pieces of evidence also point out that the inflammatory response may trigger HERV expression [ 6 , 7 ], suggesting a looping between them.
HERVs have been postulated to play a role in BD and SZ diseases since there was a higher level of HERVs expression in brains Cerebrospinal fluid (CSF) and Peripheral Blood Mononuclear cells (PBMC) from patients with these diseases [ 31 , 32 ]. Furthermore, higher concentration of some pro-inflammatory cytokines associated with HERV-W antigenemia [ 33 ] and direct role of HERV in the pathogenic reduction of neuronal density in the hippocampus region and expressive changes of dendritic morphologic were found in SZ patients [ 34 ]. Therefore, this evidence indicates possible and dynamic mechanisms for the role of HERVs, especially HERV-W, in BD and SZ’s pathogenesis.
Regarding systemic immune/inflammatory status in mental diseases, it was documented that the balance between some pro-inflammatory cytokines, such as TNF-α and IFN-γ, and anti-inflammatory cytokines, such as IL-4 and IL-10, which are involved in the T-helper type 1 (Th1) and T-helper type 2 (Th2) profiles, plays a corollary role in this mental disorders as BD [ 35 ] and SZ [ 36 ].
Although compelling evidence points out that HERVs might be associated with the pathogenesis of mental disorders, such as BD and SZ, the interplay between HERV-W-env expression in those patients and its systemic inflammatory status is still poorly understood. Based on this, here, we aimed to investigate both the HERV-W-env expression and the systemic inflammatory status in BD and SZ patients.
Study population
Patients with mental disorders (MD) ( n = 48) were separated into two groups according to their diagnosis in Schizophrenia (SZ, n = 24) and Bipolar Disorder (BD, n = 24) groups. Another group with healthy individuals, paired by age without any previous report of autoimmune disease and mental disorders in the family, was included as a control group ( n = 46). Sociodemographic data were collected from all volunteers through questionnaires. MD patients had previous diagnosis and were followed in the psychiatric outpatient clinic of Universidade Santo Amaro and in the Centro de Apoio de Atenção Psicossocial (CAPS), both located in the south region of São Paulo city, Brazil. Mini International Neuropsychiatric Interview (MINI) [ 37 , 38 ] was used to confirm the diagnosis. Only patients out of the mania phase were included in the BD group. Symptoms of depression were assessed by Montgomery–Åsberg Depression Rating Scale [ 39 ] and symptoms of mania by Young Mania Rating Scale [ 40 ]. For the SZ group we recruited only patients who were not in a psychosis event. The study was approved by the ethical committee of Universidade Santo Amaro under protocol # 5.469.700. It is worth mentioning that the study was performed in agreement with the Declaration of Helsinki.
Blood sample collection and preparation
Blood samples were collected in tubes containing the anticoagulant EDTA for peripheral blood mononuclear cells (PBMCs) obtention, which was used to perform the molecular analysis, and in gel-barrier dry tubes for serum obtention, which was used to determine the systemic cytokine concentration.
PBMCs were obtained by Ficoll-HyPaque protocol and RNA was extracted by Trizol-chloroform Method. Briefly, Ficoll-Hypaque was added into whole blood in 1:1 proportion and centrifuged at 800 g for 20 min. Afterward, the upper solution (PBMC) was collected and washed repeatedly with sterile PBS and centrifugation until the removal of residual erythrocytes was removed entirely. Finally, cell pellet containing exclusively the PBMCs undergoing RNA extraction as follows: 1mL of Trizol was added into each of cell pellet samples and up-down was performed until complete homogenization, then 200µL of chloroform was added and samples were centrifuged at 10.000 g at -4 o C. The upper phase was completely removed (around 600 µL) and then RNA was precipitated with Isopropanol 100% and washed twice with 70% Ethanol. RNA was resuspended in 40 µL H 2 O-Nuclease free. Rigorous decontamination of genomic DNA was performed with DNA-free turbo (Ambion). The absence of contaminant genomic DNA was confirmed by Real-Time PCR with primers complementary to the GAPDH gene with the absence of Reverse Transcriptase. RNA was quantified and then stored at -80 o C. Around 150ng of RNA was used to synthesize the cDNA with a High-Capacity Reverse Transcription Kit.
HERV-W detection and relative quantification analysis
We used primers complementary to HERV-W-env [ 41 ] and GAPDH as housekeeping genes [ 42 ]. The RT-PCR mix included 0.1 µM of each primer and 1x of PCR Master Mix Sybr-Green one step (Merck). The cycling conditions for HERV-W were: 50oC for 2 min, 95oC for 10 min followed by 40 cycles of 95oC for 1 min, 50oC for 45 s and 60oC for 1 min. For GAPDH assay the cycling conditions were: 50oC for 2 min, 95oC for 10 min followed by 40 cycles of 95oC for 1 min and 60oC for 1 min. In both assays a previous step was added of 37oC for 30 min for cDNA synthesis and a final cycle to determine the melting temperature of the samples (55oC to 95oC). HERV activity expression was evaluated qualitatively (absence/presence) and quantitatively (level of expression). The level of expression was determined by the 2-ΔΔCt method where ΔCt = (HERV Ct- GAPDH Endogenous Control Ct)– (Average of ΔCt of all controls), and the results were represented as fold changes. In all cases, samples were considered positive for HERVs if the melting curve was the same or ± 0.3oC distinct from the control samples and therefore included in the relative quantification analysis.
Determination of systemic cytokine concentration
Serum concentrations of the cytokines IL-2, IL-4, IL-6, IL-10, TNF-α and INF-γ were determined by using the ELISA commercial kits (ThermoFisher), following the manufacturer’s instructions. The concentration of each cytokine was calculated through an appropriate standard curve that presented a correlation coefficient from 0.95 to 0.99, with coefficients of variance intra-assay varying from 2,5 to 4% and from 8 to 10% in inter-assay.
Statistical analysis
The sample size was collected by convenience. Descriptive data was obtained, and for the comparison of scores between different groups, both parametric and non-parametric tests were employed according to the normality distribution of the data. The tests used were as follows: The normality test was performed using the Shapiro-Wilk test, and the homogeneity of variance was evaluated using the Levene test. Mann-Whitney test was used to analyze HERV-W-env expression between MD and control groups, whereas the Kruskal-Wallis test was used to assess the differences between the BD, SZ, and control groups. In addition, Spearman’s rank coefficient correlation test was also applied. All tests were conducted under the assumption of a first-type error probability (alpha) of 5% ( p < 0.05).
Clinical and demographic findings of the volunteers
Table 1 summarizes the demographic characteristics of the volunteers included in the present study.
In addition to the demographic findings, it is noteworthy to mention that individuals in the BD group received a confirmed diagnosis at an average age of 26 ± 11.3 years, and the SZ group received a confirmed diagnosis at a similar average age (27 ± 10.9 years). In addition, both groups have been diagnosed for more than 15 years. It is essential to mention that most of the patients enrolled did not exhibit any other comorbidities, with only 8 (33%) in the BD group and 7 (25%) in the SZ group presenting additional inflammatory disorders, such as hypertension and dyslipidemia.
HERV-W-envexpression is upregulated in SZ patients
All participants of the study showed expression of HERV-W-env. However, the expression in MD patients was 3.3-fold higher on average than the control group ( p < 0.01, Fig. 1 A). When analyzing the HERV-W-env expression levels in the SZ and BD groups separately, the SZ group presented 3.3-fold higher on average than the control group ( p < 0.01, Fig. 1 B), whilst the BD group did not show significant differences in the HERV-W-env expression as compared to the control group ( p = 0.54, Fig. 1 B). In addition we have performed the analysis of HERV-W expression according to the therapeutic scheme, however no significant difference was found in any analysis.
HERV-W env relative expression in the individuals enrolled in the study. Figure 1 A: MD patients presented higher HERV-W expression * p < 0.01; Fig. 1B: HERV-W expression levels were assessed in MD individuals according to each group. SZ patients showed significantly higher levels of HERV-W expression than CG * p < 0.01, Mann-Whitney`s test
BD and SZ patients present higher concentration of some systemic cytokines
Figure 2 shows the systemic cytokine levels of IL-2 (Fig. 2 A), IL-4 (Fig. 2 B), IL-6 (Fig. 2 C), IL-10 (Fig. 2 D), TNF-α (Fig. 2 E), and IFN-γ (Fig. 2 F) in the volunteer groups. Both BD and SZ groups presented higher serum concentrations of IL-10 ( p = 0.001 and p = 0.01, respectively) and TNF-α ( p = 0.0001 and p = 0.01, respectively) than those observed in the control group. In addition, the SZ group showed lower circulating levels of IL-2 and IFN-γ than the BD group ( p = 0.05) and the control group ( p = 0.05), respectively.
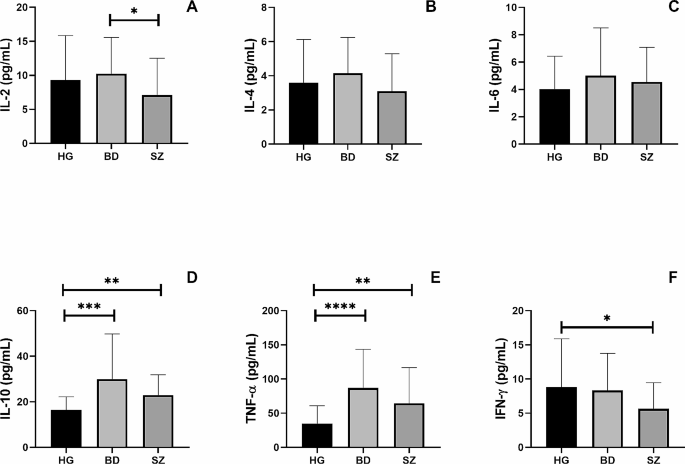
Overall pro and anti-inflammatory cytokines concentration analysis in the serum of individuals enrolled in the study. Legend: BD = Bipolar Disorder Group, SZ = Schizophrenia Group, HG = Healthy individuals Group *= p < 0.05, **= p < 0.01, ***= p < 0.001, **** p < 0.0001
Additionally, Fig. 3 shows the ratio between the circulating levels of IL-6/IL-10 (Fig. 3 A), TNF-α/IL-10 (Fig. 3 B), IFN-γ/IL-10 (Fig. 3 C), TNF-α/IL-4 (Fig. 3 D), and IFN-γ/IL-4 (Fig. 4 E) was also assessed. Higher TNF-α/IL-4 ( p = 0.0001) and TNF-α/IL-10 ( p = 0.001) ratios, as well as lower IFN-γ/IL-10 ratio ( p = 0.05), were found in BD and SZ groups than in the control group. In addition, a lower IFN-γ/IL-4 ratio was observed in the BD group than in the control group ( p = 0.05).
The ratio between pro-inflammatory and anti-inflammatory cytokines in HG, BD and SZ groups. The ratio between the concentrations of the pro-inflammatory cytokines IL-6 (A) , TNF-α (B) , and IFN-γ (C) and the anti-inflammatory cytokine IL-10 and the ratio between the concentrations of the pro-inflammatory cytokines TNF-α (D) , IFN-γ (E) and the anti-inflammatory cytokine IL-4 in the plasma of patients enrolled in the study. Legend: BD = Bipolar Disorder Group, SZ = Schizophrenia Group, HG = Healthy individuals Group *= p < 0.05, **= p < 0.01, ***= p < 0.001, **** p < 0.0001
HERV-W-env expression correlates with the pro-inflammatory profile in BD patients
Figure 4 shows the significant results obtained in the Spearman coefficient correlation analysis. Exclusively in the BD group, it was observed a significant negative correlation ( r =-0.4724) between the levels of HERV-W-env expression and serum IL-10 (Fig. 4 A), as well as significant positive correlations between the HERV-W-env expression levels and TNF-α/IL-10 ( r = 0.4868) (Fig. 4 B) or IFN-γ/IL-10 ( r = 0.4650) (Fig. 4 C) ratios.
Correlation between HERV-W expression and cytokines to BD group. Correlation between IL-10 and HERV-W (A) , Correlation between the ratio of TNF-α/IL-10 with HERV-W (B) , Correlation between the ratio of IFN- γ /IL-10 with HERV-W (C)
Here, we have described a higher HERV-W-env expression in PBMCs obtained from MD patients compared to healthy individuals, and, specifically, the SZ group showed that this increase was 3.3-fold higher than HG ( p < 0.01). This finding is not only in touch with previous studies that described higher levels of HERV-W expression in SZ patients [ 43 ], but can also provide additional evidence supporting the involvement of altered HERV-W-env activity in SZ patients. Moreover, and in an exciting way, SZ patients presented higher serum TNF-α concentrations than the control group, despite this finding not being positively correlated with HERV-W expression nor the pro- and anti-inflammatory cytokines ratios. This result not only corroborates the suggestion that the systemic inflammatory status has an essential role in SZ, but also might indicate that this condition was not closely associated with the HERV-W expression, even though its expression was also increased in those patients. Based on these facts, we can putatively suggest that systemic inflammatory status and HERV-W expression components may show distinct pathogenic roles in this disease. Previous reports in the literature have pointed out a direct involvement of neuronal pathological modifications with HERV-W-env expression and, particularly, some of these findings should be highlighted: neuronal apoptosis induction [ 44 ], structural and functional abnormalities in dopaminergic neurons that stimulates substantially the production of dopamine through Dopamine Receptor D2 (DRD2) and leads to alteration in sodium and calcium influx, which suggests a pivotal role of HERV-W not only in neuronal pathophysiology [ 45 ] but also in the reduction of hippocampal neurons density and the alteration of its dendritic and perikaryon morphology [ 46 ]. Although these previous data are very interesting in the context of HER-W expression and the nervous system, it is noteworthy to cite that our results were associated with the HERV-W-env expression in PBMC and not specifically concerning the central nervous system (CNS).
In a different way to SZ patients, the BD patients did not show significant differences in the HERV-W-env expression in PBMC as compared to the control group. This finding is in contrast with previous reports in the literature since it has been demonstrated higher levels of HERV-W expression both systemically [ 43 ] and in the brain [ 47 ] of BD patients. Even though we cannot affirm, some hypotheses can help us to understand this lack of a significant difference in HERV-W-env expression between BD and control groups found here: (i) since the HERV-W-env expression levels could putatively be impacted by different clinical manifestations in BD, it would be expected that BD patients in euthymic conditions could present a reduction on the HERV-W-env expression levels, however, it is paramount to mention that, until now, the dynamics of HERV expression in BD patients was not fully understood, thus a remarkable variation in their expression can also be presented in euthymic condition; and: (ii) despite no significant difference was found, a tendency for it could be observed ( p = 0.054) and maybe this lack of statistically significant difference could be related to the number of BD patients enrolled in the study.
Previous findings report that both SZ and BD patients are associated with the deregulation of the systemic cytokine’s concentration. The main hypothesis is that the inflammatory condition may interfere specially in the blood and brain barrier permeability [ 48 , 49 ]. It is supposed that in both BD and SZ, a pro-inflammatory profile is present and, chronically, could promote pathological modification in the CNS way before the disease’s onset. In this sense, it is known that not only genetic background added to the fundamental environmental factors is necessary to deregulate the balance of the immune-inflammatory responses [ 50 ] but also, as previously cited, that HERVs may elicit the inflammation in both physiological and pathological conditions [ 7 , 51 , 52 ]. Taken together, these pieces of information can corroborate our findings in which BD patients presented not only higher circulating TNF-α levels, a well-known pro-inflammatory cytokine associated with Th1 immune profile, than the control group but also a significant negative correlation between the levels of HERV-W-env expression and IL-10, a classical anti-inflammatory cytokine, besides significant positive correlations with TNF-α/IL-10 and IFN-γ/IL-10 ratios. Based on these data, it is clear that BD patients presented a prominent systemic pro-inflammatory status, which agrees with previous reports [ 53 , 54 , 55 ]. At this point, it is paramount to highlight that the ratio assessment has been considered as an accurate measure concerning the balance of pro- and anti-inflammatory cytokines in different contexts, including in MD patients [ 56 ]. Moreover, it is worth also mentioning that, although the pro-inflammatory status is present in BD patients, the triggers involved in this condition are yet to be found [ 57 ]. Thus, our findings can putatively suggest that HERV-W-env could be a potential player in this context. However, this hypothesis requires further investigation.
Beyond these findings, it is also of utmost importance to highlight that, regardless of the association with HERV-W-env expression, both BP and SZ groups showed a higher TNF-α/IL-4 ratio, which together with the higher TNF-α/IL-10 ratio, than the control group, which allows us to suggest that, in general, the T-help immune response was towards to Th1 profile, since there is a consensus that both TNF-α and IFN-γ are related to the Th1 immune profile, whereas the IL-4 is a classical cytokine of Th2 immune profile and IL-10 is closely associated with T regulatory (T-reg) immune profile [ 58 ]. In fact, according to the literature, increased TNF-α/IL-4 and IFN-γ/IL-4 ratios were verified in BP patients during manic episodes as compared with normal controls [ 59 ], and a relative predominance of the Th2 immune profile was evidenced in patients with acute exacerbation of schizophrenia [ 36 ]. Despite an imbalance in the Th1/Th2/Treg immune profile could be related to some worse outcomes both in BP and SZ patients, the results observed here allow us to putatively suggest that a “regulated” Th1 immune profile was present in the BP and SZ individuals enrolled in the present study since they showed not only higher circulating IL-10 levels but also lower IFN-γ/IL-10 ratio than the control group. In addition, the BP group also showed a significant reduction in the IFN-γ/IL-4 ratio compared to the control group, which corroborates our suggestion that although the Th1 immune response was predominant, this profile was not exacerbated at this point.
Among other limitations of the study formerly cited, our study presents another important limitation that should be mentioned since none of the patients enrolled in the study were under clinical episodes of BD and SZ in the sampling time, which might underestimate the level of HERV-W-env expression and cytokine concentration in thisstudy. Also, we were not able to gather enough patients to properly compare the levels of HERV-W-env expression and the systemic cytokine concentration according to the therapeutic scheme.
In summary, we have described high levels of HERV-W-env expression in SZ patients, which generally did not show a close association with the systemic cytokine levels. Therefore, in those patients, whereas the HERV-W-env may be related to SZ pathogenesis, their interplay with systemic inflammatory status was not evidenced, which allows us to suppose that HERV could act directly in a pathological manner, causing cellular changes capable of interfering with neuronal functioning, and not necessarily immune-inflammatory mediated. On the other hand, BD seems to present a distinct profile since a close association with the systemic inflammatory status was evidenced in those patients. Even though the HERV-W-env expression was not significantly higher in BD patients ( p = 0.054), significant positive correlations between HERV-W-env expression and TNF-α/IL-10 and IFN-γ/IL-10 ratios, as well as significant negative correlation observed between the level of HERV-W-env expression and circulating IL-10, were found. Based on these findings, it is reasonable to consider that HERV-W-env may be modulating the inflammatory conditions of BD patients, which might propose a distinct pathogenic mechanism for this disease in contrast to SZ patients.
Finally, although significant findings have continually been described, subsequent studies should focus on understanding the natural role of HERVs in the systemic inflammatory profile in these diseases in order not only to identify possible interplay pathways between them in this context as well as to investigate whether HERV-W-env could represent a possible target candidate for treatment of both diseases. Additionally, and equally important, a comprehensive analysis of distinct HERV families upregulated in MD patients should also be a priority to improve the understanding of the dynamics of these retroelements’ expression in the MD pathogenesis.
Data availability
No datasets were generated or analysed during the current study.
Wang PS, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Borges G, Bromet EJ et al. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet (London, England) [Internet]. 2007 [cited 2023 Nov 27];370:841–50. https://pubmed.ncbi.nlm.nih.gov/17826169/ .
Solmi M, Cortese S, Vita G, De Prisco M, Radua J, Dragioti E et al. An umbrella review of candidate predictors of response, remission, recovery, and relapse across mental disorders. Mol Psychiatry [Internet]. 2023 [cited 2023 Nov 27]; https://pubmed.ncbi.nlm.nih.gov/37957292/ .
Fryar-Williams S, Tucker G, Strobel J, Huang Y, Clements P. Molecular Mechanism Biomarkers Predict Diagnosis in Schizophrenia and Schizoaffective Psychosis, with Implications for Treatment. Int J Mol Sci [Internet]. 2023 [cited 2023 Nov 27];24. https://pubmed.ncbi.nlm.nih.gov/37958826/ .
Schmitt Junior AA, Primo de Carvalho Alves L, Padilha BL, da Rocha NS. Serum cytokine variations among inpatients with major depression, bipolar disorder, and schizophrenia versus healthy controls: a prospective true-to-life study. Ther Adv Psychopharmacol [Internet]. 2023 [cited 2023 Nov 27];13. https://pubmed.ncbi.nlm.nih.gov/36814596/ .
Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry [Internet]. 2016 [cited 2023 Nov 27];21:1696–709. https://pubmed.ncbi.nlm.nih.gov/26903267/ .
Helmy M, Selvarajoo K. Systems Biology to Understand and Regulate Human Retroviral Proinflammatory Response. Front Immunol [Internet]. 2021 [cited 2022 Aug 10];12. https://pubmed.ncbi.nlm.nih.gov/34867957/ .
Rangel SC, da Silva MD, da Silva AL, dos Santos J, de MB, Neves LM, Pedrosa A et al. Human endogenous retroviruses and the inflammatory response: A vicious circle associated with health and illness [Internet]. Front. Immunol. 2022. https://www.frontiersin.org/articles/ https://doi.org/10.3389/fimmu.2022.1057791 .
Dolei A. Endogenous retroviruses and human disease. Expert Rev Clin Immunol [Internet]. 2006/01/01. 2006;2:149–67. http://www.ncbi.nlm.nih.gov/pubmed/20477095 .
Wang X, Wu X, Huang J, Li H, Yan Q, Zhu F. Human endogenous retrovirus W family envelope protein (HERV-W env) facilitates the production of TNF-α and IL-10 by inhibiting MyD88s in glial cells. Arch Virol [Internet]. 2021 [cited 2022 Aug 10];166:1035–45. https://pubmed.ncbi.nlm.nih.gov/33438105/ .
Weiss RA. The discovery of endogenous retroviruses. Retrovirology [Internet]. 2006 [cited 2015 Jun 23];3:67. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1617120&tool=pmcentrez&rendertype=abstract
Griffiths DJ. Endogenous retroviruses in the human genome sequence. Genome Biol [Internet]. 2001 [cited 2015 Jun 10];2:REVIEWS1017. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=138943&tool=pmcentrez&rendertype=abstract
Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG et al. The Sequence of the Human Genome. Science (80-) [Internet]. 2001 [cited 2017 Oct 24];291:1304–51. http://www.ncbi.nlm.nih.gov/pubmed/11181995 .
Belshaw R, Pereira V, Katzourakis A, Talbot G, Paces J, Burt A et al. Long-term reinfection of the human genome by endogenous retroviruses. Proc Natl Acad Sci U S A [Internet]. 2004 [cited 2015 Aug 3];101:4894–9. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=387345&tool=pmcentrez&rendertype=abstract
Weiss RA. The discovery of endogenous retroviruses. Retrovirology [Internet]. 2006 [cited 2017 Sep 28];3:67. http://www.ncbi.nlm.nih.gov/pubmed/17018135 .
Patience C, Wilkinson DA, Weiss RA. Our retroviral heritage. Trends Genet [Internet]. 1997 [cited 2015 Aug 3];13:116–20. http://www.ncbi.nlm.nih.gov/pubmed/9066271 .
Mager DL, Goodchild NL. Homologous recombination between the LTRs of a human retrovirus-like element causes a 5-kb deletion in two siblings. Am J Hum Genet [Internet]. 1989 [cited 2017 Oct 24];45:848–54. http://www.ncbi.nlm.nih.gov/pubmed/2573998 .
Perron H, Perin JP, Rieger F, Alliel PM. Particle-associated retroviral RNA and tandem RGH/HERV-W copies on human chromosome 7q: possible components of a chain-reaction triggered by infectious agents in multiple sclerosis? J Neurovirol [Internet]. 2000 [cited 2015 Mar 10];6 Suppl 2:S67-75. http://www.ncbi.nlm.nih.gov/pubmed/10871789 .
Contreras-Galindo R, Kaplan MH, Markovitz DM, Lorenzo E, Yamamura Y. Detection of HERV-K(HML-2) viral RNA in plasma of HIV type 1-infected individuals. AIDS Res Hum Retroviruses [Internet]. 2006 [cited 2016 Apr 8];22:979–84. http://www.ncbi.nlm.nih.gov/pubmed/17067267 .
Ruprecht K, Ferreira H, Flockerzi A, Wahl S, Sauter M, Mayer J et al. Human Endogenous Retrovirus Family HERV-K(HML-2) RNA Transcripts Are Selectively Packaged into Retroviral Particles Produced by the Human Germ Cell Tumor Line Tera-1 and Originate Mainly from a Provirus on Chromosome 22q11.21. J Virol [Internet]. 2008 [cited 2017 Oct 24];82:10008–16. http://www.ncbi.nlm.nih.gov/pubmed/18684837 .
Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature [Internet]. 2000 [cited 2015 Jun 8];403:785–9. http://www.ncbi.nlm.nih.gov/pubmed/10693809 .
Medstrand P, Landry JR, Mager DL. Long terminal repeats are used as alternative promoters for the endothelin B receptor and apolipoprotein C-I genes in humans. J Biol Chem [Internet]. 2001 [cited 2015 Jun 8];276:1896–903. http://www.ncbi.nlm.nih.gov/pubmed/11054415 .
Ting CN, Rosenberg MP, Snow CM, Samuelson LC, Meisler MH. Endogenous retroviral sequences are required for tissue-specific expression of a human salivary amylase gene. Genes Dev [Internet]. 1992 [cited 2015 Jun 24];6:1457–65. http://www.ncbi.nlm.nih.gov/pubmed/1379564 .
Voisset C, Blancher A, Perron H, Mandrand B, Mallet F, Paranhos-Baccala G. Phylogeny of a Novel Family of Human Endogenous Retrovirus Sequences, HERV-W, in Humans and Other Primates. AIDS Res Hum Retroviruses [Internet]. 1999 [cited 2017 Oct 25];15:1529–33. http://www.ncbi.nlm.nih.gov/pubmed/10580403 .
Perron H, Geny C, Laurent A, Mouriquand C, Pellat J, Perret J et al. Leptomeningeal cell line from multiple sclerosis with reverse transcriptase activity and viral particles. Res Virol [Internet]. 1989/11/01. 1989;140:551–61. http://www.ncbi.nlm.nih.gov/pubmed/2482522 .
do Olival GS, Faria TS, Nali LHS, de Oliveira ACP, Casseb J, Vidal JE et al. Genomic analysis of ERVWE2 locus in patients with multiple sclerosis: absence of genetic association but potential role of human endogenous retrovirus type W elements in molecular mimicry with myelin antigen. Front Microbiol [Internet]. 2013 [cited 2015 Jun 22];4:172. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3693062&tool=pmcentrez&rendertype=abstract
Nali LH, Olival GS, Montenegro H, da Silva IT, Dias-Neto E, Naya H et al. Human endogenous retrovirus and multiple sclerosis: A review and transcriptome findings. Mult Scler Relat Disord [Internet]. 2022 [cited 2022 Mar 15];57. https://pubmed.ncbi.nlm.nih.gov/34922254/ .
Kremer D, Gruchot J, Weyers V, Oldemeier L, Göttle P, Healy L et al. PHERV-W envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis. Proc Natl Acad Sci U S A [Internet]. 2019 [cited 2021 Sep 4];116:15216–25. https://pubmed.ncbi.nlm.nih.gov/31213545/ .
Mameli G, Poddighe L, Astone V, Delogu G, Arru G, Sotgiu S et al. Novel reliable real-time PCR for differential detection of MSRVenv and syncytin-1 in RNA and DNA from patients with multiple sclerosis. J Virol Methods [Internet]. 2009 [cited 2015 Jun 22];161:98–106. http://www.ncbi.nlm.nih.gov/pubmed/19505508 .
Mameli G, Madeddu G, Mei A, Uleri E, Poddighe L, Delogu LG et al. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein-Barr virus latency: The missing link with multiple sclerosis? PLoS One [Internet]. 2013 [cited 2021 Sep 24];8. https://pubmed.ncbi.nlm.nih.gov/24236019/ .
Dolei A, Uleri E, Ibba G, Caocci M, Piu C, Serra C. The aliens inside human DNA: HERV-W/MSRV/syncytin-1 endogenous retroviruses and neurodegeneration. J Infect Dev Ctries [Internet]. 2015 [cited 2016 Apr 2];9:577–87. http://www.ncbi.nlm.nih.gov/pubmed/26142666 .
Huang W, Li S, Hu Y, Yu H, Luo F, Zhang Q et al. Implication of the env gene of the human endogenous retrovirus W family in the expression of BDNF and DRD3 and development of recent-onset schizophrenia. Schizophr Bull [Internet]. 2011 [cited 2023 Nov 30];37:988–1000. https://pubmed.ncbi.nlm.nih.gov/20100784/ .
Karlsson H, Bachmann S, Schröder J, McArthur J, Torrey EF, Yolken RH. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proc Natl Acad Sci U S A [Internet]. 2001 [cited 2023 Nov 30];98:4634–9. https://pubmed.ncbi.nlm.nih.gov/11296294/ .
Tamouza R, Meyer U, Foiselle M, Richard JR, Lu C, lieng, Boukouaci W et al. Identification of inflammatory subgroups of schizophrenia and bipolar disorder patients with HERV-W ENV antigenemia by unsupervised cluster analysis. Transl Psychiatry [Internet]. 2021 [cited 2023 Nov 28];11. https://pubmed.ncbi.nlm.nih.gov/34230451/ .
Nagase H, Yao S, Ikeda S. Acute and chronic effects of exercise on mRNA expression in the skeletal muscle of two mouse models of peripheral artery disease. PLoS One [Internet]. 2017 [cited 2023 Aug 22];12. https://pubmed.ncbi.nlm.nih.gov/28771574/ .
Kim YK, Myint AM, Lee BH, Han CS, Lee SW, Leonard BE et al. T-helper types 1, 2, and 3 cytokine interactions in symptomatic manic patients. Psychiatry Res [Internet]. 2004 [cited 2023 Dec 5];129:267–72. https://pubmed.ncbi.nlm.nih.gov/15661320/ .
Avgustin Bojana, Wraber Branka TR. Increased Th1 and Th2 immune reactivity with relative Th2 dominance in patients with acute exacerbation of schizophrenia - PubMed. Croat Med J [Internet]. 2005 [cited 2023 Dec 5];46:268–74. https://pubmed.ncbi.nlm.nih.gov/15849849/ .
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J Clin Psychiatry [Internet]. 1998 [cited 2024 Mar 28];59:11980. https://www.psychiatrist.com/jcp/mini-international-neuropsychiatric-interview-mini .
Pettersson A, Modin S, Wahlström R, Af Winklerfelt Hammarberg S, Krakau I. The Mini-International Neuropsychiatric Interview is useful and well accepted as part of the clinical assessment for depression and anxiety in primary care: a mixed-methods study. BMC Fam Pract [Internet]. 2018 [cited 2024 Mar 28];19. https://pubmed.ncbi.nlm.nih.gov/29368585/ .
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry [Internet]. 1979 [cited 2024 Mar 28];134:382–9. https://pubmed.ncbi.nlm.nih.gov/444788/ .
Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry [Internet]. 1978 [cited 2024 Mar 28];133:429–35. https://pubmed.ncbi.nlm.nih.gov/728692/ .
Nellåker C, Yao Y, Jones-Brando L, Mallet F, Yolken RH, Karlsson H. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology [Internet]. 2006 [cited 2015 Jul 2];3:44. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1539011&tool=pmcentrez&rendertype=abstract
de Jonge HJM, Fehrmann RSN, de Bont ESJM, Hofstra RMW, Gerbens F, Kamps WA et al. Evidence based selection of housekeeping genes. PLoS One [Internet]. 2007 [cited 2016 Jan 22];2:e898. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1976390&tool=pmcentrez&rendertype=abstract
Perron H, Hamdani N, Faucard R, Lajnef M, Jamain S, Daban-Huard C et al. Molecular characteristics of Human Endogenous Retrovirus type-W in schizophrenia and bipolar disorder. Transl Psychiatry [Internet]. 2012 [cited 2023 Nov 30];2. https://pubmed.ncbi.nlm.nih.gov/23212585/ .
Li X, Wu X, Li W, Yan Q, Zhou P, Xia Y et al. HERV-W ENV Induces Innate Immune Activation and Neuronal Apoptosis via linc01930/cGAS Axis in Recent-Onset Schizophrenia. Int J Mol Sci [Internet]. 2023 [cited 2023 Nov 30];24. https://pubmed.ncbi.nlm.nih.gov/36769337/ .
Yan Q, Wu X, Zhou P, Zhou Y, Li X, Liu Z et al. HERV-W Envelope Triggers Abnormal Dopaminergic Neuron Process through DRD2/PP2A/AKT1/GSK3 for Schizophrenia Risk. Viruses [Internet]. 2022 [cited 2023 Nov 30];14. https://pubmed.ncbi.nlm.nih.gov/35062349/ .
Yao W, Zhou P, Yan Q, Wu X, Xia Y, Li W et al. ERVWE1 Reduces Hippocampal Neuron Density and Impairs Dendritic Spine Morphology through Inhibiting Wnt/JNK Non-Canonical Pathway via miR-141-3p in Schizophrenia. Viruses [Internet]. 2023 [cited 2023 Nov 28];15. https://pubmed.ncbi.nlm.nih.gov/36680208/ .
Li F, Sabunciyan S, Yolken RH, Lee D, Kim S, Karlsson H. Transcription of human endogenous retroviruses in human brain by RNA-seq analysis. PLoS One [Internet]. 2019 [cited 2023 Nov 30];14. https://pubmed.ncbi.nlm.nih.gov/30605476/ .
Altamura AC, Buoli M, Pozzoli S. Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: comparison with schizophrenia. Psychiatry Clin Neurosci [Internet]. 2014 [cited 2023 Dec 1];68:21–36. https://pubmed.ncbi.nlm.nih.gov/24102953/ .
Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci [Internet]. 2010 [cited 2023 Dec 1];64:217–30. https://pubmed.ncbi.nlm.nih.gov/20602722/ .
Giacomo F, Di, Strippoli M-PF, Castelao E, Amoussou JR, Gholam M, Ranjbar S et al. Risk factors for mood disorders among offspring of parents with bipolar disorder: Findings from a discordant-sibling study. Psychiatry Res [Internet]. 2023 [cited 2023 Dec 5];330:115615. https://pubmed.ncbi.nlm.nih.gov/38007982/ .
Buzdin AA, Prassolov V, Garazha AV. Friends-Enemies: endogenous retroviruses are major transcriptional regulators of human DNA. Front Chem. 2017;5:35.
Article PubMed PubMed Central Google Scholar
Perron H, Germi R, Bernard C, Garcia-Montojo M, Deluen C, Farinelli L et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler [Internet]. 2012 [cited 2015 Jun 22];18:1721–36. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3573672&tool=pmcentrez&rendertype=abstract
Rosenblat JD, McIntyre RS. Bipolar Disorder and Inflammation. Psychiatr Clin North Am [Internet]. 2016 [cited 2023 Dec 1];39:125–37. https://pubmed.ncbi.nlm.nih.gov/26876323/ .
Saccaro LF, Crokaert J, Perroud N, Piguet C. Structural and functional MRI correlates of inflammation in bipolar disorder: A systematic review. J Affect Disord [Internet]. 2023 [cited 2023 Dec 1];325:83–92. https://pubmed.ncbi.nlm.nih.gov/36621677/ .
Quidé Y, Bortolasci CC, Spolding B, Kidnapillai S, Watkeys OJ, Cohen-Woods S et al. Systemic inflammation and grey matter volume in schizophrenia and bipolar disorder: Moderation by childhood trauma severity. Prog Neuropsychopharmacol Biol Psychiatry [Internet]. 2021 [cited 2023 Dec 1];105. https://pubmed.ncbi.nlm.nih.gov/32540496/ .
Maes M, Carvalho AF. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol Neurobiol [Internet]. 2018 [cited 2023 Dec 5];55:8885–903. https://pubmed.ncbi.nlm.nih.gov/29611101/ .
Freund N, Juckel G. Bipolar Disorder: Its Etiology and How to Model in Rodents. Methods Mol Biol [Internet]. 2019 [cited 2023 Dec 1];2011:61–77. https://pubmed.ncbi.nlm.nih.gov/31273693/ .
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF et al. The Role of Aberrations in the Immune-Inflammatory Response System (IRS) and the Compensatory Immune-Regulatory Reflex System (CIRS) in Different Phenotypes of Schizophrenia: the IRS-CIRS Theory of Schizophrenia. Mol Neurobiol [Internet]. 2020 [cited 2023 Dec 5];57:778–97. https://pubmed.ncbi.nlm.nih.gov/31473906/ .
Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord [Internet]. 2007 [cited 2023 Dec 5];104:91–5. https://pubmed.ncbi.nlm.nih.gov/17434599/ .
Download references

Acknowledgements
Not applicable.
Fundação de Amparo à Pesquisa do Estado de São Paulo Grants #2023/08773-0 and #2022/07567-5.
Author information
Sara Coelho Rangel and Michelly Damasceno da Silva contributed equally to this work.
Authors and Affiliations
Post-graduation Program in Health Sciences, Santo Amaro University, Rua Isabel Schmitt, 540, São Paulo, Brazil
Sara Coelho Rangel, Michelly Damasceno da Silva, Samuel Nascimento Santos, Jefferson Russo Victor, Kevin Cezar Nascimento Silva, Carolina Nunes França, Marina Tiemi Shio, Lucas Melo Neves, André Luis Lacerda Bachi & Luiz Henrique da Silva Nali
Hospital Escola Wladimir Arruda– Departamento de Psiquiatria– Santo Amaro University, Rua Prof. Enéas de Siqueira Neto, 340, São Paulo, Brazil
Décio Gilberto Natrielli Filho
Ent Research Lab, Department of Otorhinolaryngology-Head and Neck Surgery, Federal University of Sao Paulo, São Paulo, Brazil
Jonatas Bussador do Amaral
Department of Medicine-Cardiology, Albert Einstein College of Medicine, New York, EUA, USA
Izabela Dorota Tuleta
You can also search for this author in PubMed Google Scholar
Contributions
SCR and MDS performed data collection, experimental analysis and data analysis and paper writing DGNF performed clinical analysis of the patients and applied the inclusion and exclusion in order to select the volunteersSNS performed data collection and experimental analysisJBA performed the inflammatory status analysis JRV performed the critical review of the manuscript KCNS performed the inflammatory status analysisIDT critical review of the manuscript and paper writingCNF performed the critical review of the manuscript, paper writing and study designMTS coordinated the molecular assays and data analysisLMN performed the clinical analysis of the patients and ALLB performed the study design, funding acquisition, paper writing and reviewingLHSN performed the study design, funding acquisition, paper writing, data analysis, paper reviewing.All authors have read and approved the final version of the manuscript.
Corresponding author
Correspondence to Luiz Henrique da Silva Nali .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the ethical committee of Universidade Santo Amaro under protocol # 5.469.700. It is worth mentioning that the study was performed in agreement with the Declaration of Helsinki.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Rangel, S.C., da Silva, M.D., Natrielli Filho, D.G. et al. HERV-W upregulation expression in bipolar disorder and schizophrenia: unraveling potential links to systemic immune/inflammation status. Retrovirology 21 , 7 (2024). https://doi.org/10.1186/s12977-024-00640-3
Download citation
Received : 06 December 2023
Accepted : 01 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s12977-024-00640-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bipolar Disorder
- Schizophrenia
- Human Endogenous Retrovirus
- Inflammation
Retrovirology
ISSN: 1742-4690
- Submission enquiries: [email protected]
- Warning Signs and Symptoms
- Mental Health Conditions
- Common with Mental Illness
- Mental Health By the Numbers
- Individuals with Mental Illness
- Family Members and Caregivers
- Kids, Teens and Young Adults
- Veterans & Active Duty
- Identity and Cultural Dimensions
- Frontline Professionals
- Mental Health Education
- Support Groups
- NAMI HelpLine
- Publications & Reports
- Podcasts and Webinars
- Video Resource Library
- Justice Library
- Find Your Local NAMI
- Find a NAMIWalks
- Attend the NAMI National Convention
- Fundraise Your Way
- Create a Memorial Fundraiser
- Pledge to Be StigmaFree
- Awareness Events
- Share Your Story
- Partner with Us
- Advocate for Change
- Policy Priorities
- NAMI Advocacy Actions
- Policy Platform
- Crisis Intervention
- State Fact Sheets
- Public Policy Reports
- About Mental Illness
Bipolar Disorder
In this 2-part podcast series, NAMI Chief Medical Officer Dr. Ken Duckworth guides discussions on bipolar disorder that offer insights from individuals, family members and mental health professionals. Read the transcript . Note: Content includes discussions on topics such as suicide attempts and may be triggering.
Bipolar disorder is a mental illness that causes dramatic shifts in a person’s mood, energy and ability to think clearly. People with bipolar experience high and low moods—known as mania and depression—which differ from the typical ups-and-downs most people experience.
The average age-of-onset is about 25, but it can occur in the teens, or more uncommonly, in childhood. The condition affects men and women equally, with about 2.8% of the U.S. population diagnosed with bipolar disorder and nearly 83% of cases classified as severe.
If left untreated, bipolar disorder usually worsens. However, with a good treatment plan including psychotherapy, medications, a healthy lifestyle, a regular schedule and early identification of symptoms, many people live well with the condition.
Symptoms and their severity can vary. A person with bipolar disorder may have distinct manic or depressed states but may also have extended periods—sometimes years—without symptoms. A person can also experience both extremes simultaneously or in rapid sequence.
Severe bipolar episodes of mania or depression may include psychotic symptoms such as hallucinations or delusions. Usually, these psychotic symptoms mirror a person’s extreme mood. People with bipolar disorder who have psychotic symptoms can be wrongly diagnosed as having schizophrenia .
Mania. To be diagnosed with bipolar disorder, a person must have experienced at least one episode of mania or hypomania. Hypomania is a milder form of mania that doesn’t include psychotic episodes. People with hypomania can often function well in social situations or at work. Some people with bipolar disorder will have episodes of mania or hypomania many times throughout their life; others may experience them only rarely.
Although someone with bipolar may find an elevated mood of mania appealing—especially if it occurs after depression—the “high” does not stop at a comfortable or controllable level. Moods can rapidly become more irritable, behavior more unpredictable and judgment more impaired. During periods of mania, people frequently behave impulsively, make reckless decisions and take unusual risks.
Most of the time, people in manic states are unaware of the negative consequences of their actions. With bipolar disorder, suicide is an ever-present danger because some people become suicidal even in manic states. Learning from prior episodes what kinds of behavior signals “red flags” of manic behavior can help manage the symptoms of the illness.
Depression . The lows of bipolar depression are often so debilitating that people may be unable to get out of bed. Typically, people experiencing a depressive episode have difficulty falling and staying asleep, while others sleep far more than usual. When people are depressed, even minor decisions such as what to eat for dinner can be overwhelming. They may become obsessed with feelings of loss, personal failure, guilt or helplessness; this negative thinking can lead to thoughts of suicide.
The depressive symptoms that obstruct a person’s ability to function must be present nearly every day for a period of at least two weeks for a diagnosis. Depression associated with bipolar disorder may be more difficult to treat and require a customized treatment plan.
Scientists have not yet discovered a single cause of bipolar disorder. Currently, they believe several factors may contribute, including:
- Genetics. The chances of developing bipolar disorder are increased if a child’s parents or siblings have the disorder. But the role of genetics is not absolute: A child from a family with a history of bipolar disorder may never develop the disorder. Studies of identical twins have found that, even if one twin develops the disorder, the other may not.
- Stress . A stressful event such as a death in the family, an illness, a difficult relationship, divorce or financial problems can trigger a manic or depressive episode. Thus, a person’s handling of stress may also play a role in the development of the illness.
- Brain structure and function . Brain scans cannot diagnose bipolar disorder, yet researchers have identified subtle differences in the average size or activation of some brain structures in people with bipolar disorder.
To diagnose bipolar disorder, a doctor may perform a physical examination, conduct an interview and order lab tests. While bipolar disorder cannot be seen on a blood test or body scan, these tests can help rule out other illnesses that can resemble the disorder, such as hyperthyroidism. If no other illnesses (or medicines such as steroids) are causing the symptoms, the doctor may recommend mental health care.
To be diagnosed with bipolar disorder, a person must have experienced at least one episode of mania or hypomania. Mental health care professionals use the Diagnostic and Statistical Manual of Mental Disorders (DSM) to diagnose the “type” of bipolar disorder a person may be experiencing. To determine what type of bipolar disorder a person has, mental health care professionals assess the pattern of symptoms and how impaired the person is during their most severe episodes.
Four Types Of Bipolar Disorder
- Bipolar I Disorder is an illness in which people have experienced one or more episodes of mania. Most people diagnosed with bipolar I will have episodes of both mania and depression, though an episode of depression is not necessary for a diagnosis. To be diagnosed with bipolar I, a person’s manic episodes must last at least seven days or be so severe that hospitalization is required.
- Bipolar II Disorder is a subset of bipolar disorder in which people experience depressive episodes shifting back and forth with hypomanic episodes, but never a “full” manic episode.
- Cyclothymic Disorder or Cyclothymia is a chronically unstable mood state in which people experience hypomania and mild depression for at least two years. People with cyclothymia may have brief periods of normal mood, but these periods last less than eight weeks.
- Bipolar Disorder, “other specified” and “unspecified” is when a person does not meet the criteria for bipolar I, II or cyclothymia but has still experienced periods of clinically significant abnormal mood elevation.
Bipolar disorder is treated and managed in several ways:
- Psychotherapy , such as cognitive behavioral therapy and family-focused therapy.
- Medications , such as mood stabilizers, antipsychotic medications and, to a lesser extent, antidepressants.
- Self-management strategies , like education and recognition of an episode’s early symptoms.
- Complementary health approaches , such as aerobic exercise meditation, faith and prayer can support, but not replace, treatment.
The largest research project to assess what treatment methods work for people with bipolar disorder is the Systematic Treatment Enhancement for Bipolar Disorder , otherwise known as Step-BD. Step-BD followed over 4,000 people diagnosed with bipolar disorder over time with different treatments.
Related Conditions
People with bipolar disorder can also experience:
- Attention-deficit hyperactivity disorder ( ADHD )
- Posttraumatic stress disorder ( PTSD )
- Substance use disorders/ dual diagnosis
People with bipolar disorder and psychotic symptoms can be wrongly diagnosed with schizophrenia . Bipolar disorder can be also misdiagnosed as Borderline Personality Disorder ( BPD ).
These other illnesses and misdiagnoses can make it hard to treat bipolar disorder. For example, the antidepressants used to treat OCD and the stimulants used to treat ADHD may worsen symptoms of bipolar disorder and may even trigger a manic episode. If you have more than one condition (called co-occurring disorders), be sure to get a treatment plan that works for you.
Reviewed August 2017
Proper treatment helps most people living with bipolar disorder control their mood swings and other symptoms. Because bipolar disorder is a chronic illness, treatment must be ongoing. If left untreated, the symptoms of bipolar disorder get worse, so diagnosing it and beginning treatment early is important.
Treating bipolar disorder may include medication, psychotherapy, education, self-management strategies and external supports such as family, friends and support groups. There is no one approach to treating bipolar disorder.
Psychotherapy
Psychotherapy, support groups and psychoeducation about the illness are essential to treating bipolar disorder:
- Cognitive behavioral therapy (CBT) helps change the negative thinking and behavior associated with depression. The goal of this therapy is to recognize negative thoughts and to teach coping strategies.
- Family-focused therapy helps people with bipolar disorder learn about the illness and carry out a treatment plan.
- Psychotherapy focused on self-care and stress regulation, and helps a person improve self-care, recognize patterns of the onset of the symptoms and to manage stress.
An NIMH clinical trial, the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) showed that patients taking medications to treat bipolar disorder are more likely to get well faster and stay well if they receive a combination of several intensive psychotherapy interventions. Individuals in the study received three types of psychotherapy, which focused on cognitive strategies, family involvement and stress regulation.
Medications
With the prescribing doctor, work together to review the options for medication. Different types of bipolar disorder may respond better to a particular type. The side effects can vary between medications and it may take time to discover the best medicine.
Lithium (Lithobid, Eskalith) is effective at stabilizing mood and preventing the extreme highs and lows of bipolar disorder. Periodic blood tests are required because lithium can cause thyroid and kidney problems. Common side effects include restlessness, dry mouth and digestive issues. Lithium levels should be monitored carefully to ensure the best dosage and watch for toxicity.
Lithium is used for continued treatment of bipolar depression and for preventing relapse. There is evidence that lithium can lower the risk of suicide but the FDA has not granted approval specifically for this purpose.
Anticonvulsants
Many medications used to treat seizures are also used as mood stabilizers . They are often recommended for treating bipolar disorder. Common side effects include weight gain, dizziness and drowsiness. But sometimes, certain anticonvulsants cause more serious problems, such as skin rashes, blood disorders or liver problems.
Valproic acid and carbamazepine are used to treat mania. These drugs, also used to treat epilepsy, were found to be as effective as lithium for treating acute mania. They may be better than lithium in treating the more complex bipolar subtypes of rapid cycling and dysphoric mania.
Lamotrigine is used to delay occurrences of bipolar I disorder. Lamotrigine does not have FDA approval for treatment of the acute episodes of depression or mania. Studies of lamotrigine for treatment of acute bipolar depression have produced inconsistent results.
Second-Generation Antipsychotics (SGAs)
SGAs are commonly used to treat the symptoms of bipolar disorder and are often paired with other medications, including mood stabilizers. They are generally used for treating manic or mixed episodes.
SGAs are often prescribed to help control acute episodes of mania or depression. Finding the right medication is not an exact science; it is specific to each person. Currently, only quetiapine and the combination of olanzepine and fluoxetine (Symbax) are approved for treating bipolar depression. Regularly check with your doctor and the FDA website, as side effects can change over time.
Standard Antidepressants
Antidepressants present special concerns when used in treating bipolar disorder, as they can trigger mania in some people. A National Institute of Mental Health study showed that taking an antidepressant also to a mood stabilizer is no more effective that using a mood stabilizer alone for bipolar I. This is an essential area to review treatment risks and benefits.
Other Treatments
Electroconvulsive therapy (ect).
In rare instances, ECT can be considered as an intervention for severe mania or depression. ECT involves transmitting short electrical impulses into the brain. Although ECT is a highly effective treatment for severe depression, mania or mixed episodes, it is reserved for specific situations and for symptoms that have not responded to other treatments.
Treatment Considerations For Women And For Children
Women. Women with bipolar disorder who are of childbearing age, or who are considering getting pregnant, need special attention. A complex risk-benefit discussion needs to occur to look at the treatment options available. Some medicines can have risk to the developing fetus and to children in breast milk. However, there is also evidence that being off of all medications increases the likelihood of bipolar symptoms, which itself creates risks to both mother and fetus or baby. Planning ahead and getting good information from your health care team based on your individual circumstances improves your chance of a best outcome.
Children. The diagnosis of bipolar disorder in children has been controversial. Before receiving any psychiatric diagnosis, children must have a comprehensive evaluation of their physical and mental health. Children with bipolar disorder may also have other conditions including attention-deficit hyperactivity disorder, early childhood psychosis, posttraumatic stress disorder, learning disabilities or substance abuse problems. Each of these co-occurring conditions requires a thoughtful and individualized treatment plan. Children with bipolar disorder usually receive psychotherapy and psychosocial interventions before medications are considered.
The identification of a new mental health condition, Disruptive Mood Dysregulation Disorder (DMDD), could affect how bipolar disorder is diagnosed in children. DMDD better describes children who are intensely irritable, have temper tantrums, but do not have classic symptoms of mania. Early evidence suggests children with DMDD do not have an increased risk of developing bipolar disorder as adults, but they may have other co-occurring illnesses like depression.
Coping with the ups and downs of bipolar disorder isn’t easy. But if you or a family member or friend is struggling, there is help. NAMI and NAMI Affiliates are there to provide you with support for you and your family and information about community resources.
Contact the NAMI HelpLine at 1-800-950-NAMI (6264) or [email protected] if you have any questions about bipolar disorder or finding support and resources.
Helping Yourself
If you have bipolar disorder, the condition can exert control over your thoughts, interfere with relationships and if not treated, lead to a crisis. Here are some ways to help manage your illness.
Pinpoint your stressors and triggers. Are there specific times when you find yourself stressed? People, places, jobs and even holidays can play a big role in your mood stability. Symptoms of mania and depression may start slow, but addressing them early can prevent a serious episode. Feelings of mania may feel good at first, but they can spiral into dangerous behavior such as reckless driving, violence or hypersexuality. Depression may begin with feeling tired and being unable to sleep.
Avoid drugs and alcohol . These substances can disturb emotional balance and interact with medications. Both depression and mania make drugs and alcohol attractive options to help you “slow down” or “perk up,” but the potential damage can block your recovery.
Establish a routine. Committing to a routine can help you take control and help prevent depression and mania from taking control. For example, to keep the energy changes caused by depression and mania in check, commit to being in bed only eight hours a night and up and moving the rest of the time. Aerobic exercise is a good strategy for regulating body rhythm.
Learn from past episodes. Pattern recognition is essential to spotting the early symptoms of an impending manic episode. Accepting support from family members or friends who can recognize early symptoms is important. Symptoms often follow very specific patterns, and this can be learned and planned for. 2 nights of a small sleep change or the even the repeated use of a certain phrase can be examples of early warning signs.
Form healthy relationships. Relationships can help stabilize your moods. An outgoing friend might encourage you to get involved with social activities and lift your mood. A more relaxed friend may provide you with a steady calm that can help keep feelings of mania under control.
If you live with a mental health condition, learn more about managing your mental health and finding the support you need .
Helping A Family Member Or Friend
Recognize early symptoms. You may be able to prevent a serious episode of the illness before it happens. Symptoms of mania and depression often have warning signs. The beginnings of mania typically feel good and that means your family member may not want to seek help. Identify signals such as lack of sleep and speaking quickly that signal impending mania. A deep depression often only begins with a low mood, feeling fatigued or having trouble sleeping.
Communicate. Not everyone enjoys confronting problems head on, but doing so is critical to healthy communication. Make time to talk about problems. But know that not just any time is right. For example, if your family member has bipolar II and becomes angry, it might be safe to try and talk through the situation. But if your friend with bipolar I becomes angry, your reaction may need to be different. It’s more likely that this anger will turn to rage and become dangerous, including physical violence.
React calmly and rationally. Even in situations where your family member or friend may “go off,” ranting at you or others, it’s important to remain calm. Listen to them and make them feel understood, then try to work toward a positive outcome.
Find out more about taking care of your family member or friend and yourself.
- Discovering Self-Love and Acceptance after Tragedy and Mental Illness
- Transcending the Self-Stigma From my Youth

Know the warning signs of mental illness

Learn more about common mental health conditions
NAMI HelpLine is available M-F, 10 a.m. – 10 p.m. ET. Call 800-950-6264 , text “helpline” to 62640 , or chat online. In a crisis, call or text 988 (24/7).
SYSTEMATIC REVIEW article
Prevalence and risk factors for depression in factitious disorder: a systematic review.

- 1 Unit of Psychiatry, Department of Medicine (DMED), University of Udine, Udine, Italy
- 2 School of Nursing, Department of Medicine (DMED), University of Udine, Udine, Italy
- 3 Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom
Objective: Factitious disorder is characterized by a pattern of abnormal behavior in which patients deliberately produce, falsify, or exaggerate physical and/or psychological symptoms that have no, or little, organic basis, to assume the sick role. In the context of a factitious disorder, depression can be both a feigned disease and an associated comorbidity. We performed a systematic review to provide an overview of the relationship between factitious disorder and depression, describe the prevalence of depression in factitious disorder, and identify factors that can contribute to the development of depression in patients suffering from factitious disorder.
Methods: A literature search was performed using the electronic databases PubMed, EMBASE and Cochrane Library following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies were eligible for inclusion in this review if they investigated factitious disorder or Munchausen Syndrome with comorbid depression.
Results: Depression was found to be highly prevalent in factitious disorder, affecting around 30% of the samples. Risk factors for depression in factitious disorder included having suffered from childhood and adulthood traumatic experiences and having a history of psychosocial problems.
Conclusion: The treatment of factitious disorder is challenging and requires a multidisciplinary team approach. Given the high levels of depression in patients with factitious disorder, we recommend to always screen for depression once a factitious disorder is diagnosed.
Introduction
Factitious disorder is characterized by a pattern of abnormal behavior in which patients deliberately produce, falsify, or exaggerate physical and/or psychological symptoms that have no, or little, organic basis, to assume the sick role ( 1 ). Factitious disorder can be misdiagnosed as conversion disorder, but in conversion disorder the production of physical and/or psychological symptoms is unconscious, whereas in factitious disorder this production is conscious. Factitious disorder can also be imposed on other people, when the perpetrator actively harms his victims in order to make them ill. In such a case, the disorder is also called Munchausen Syndrome by proxy. Factitious disorder imposed on another can involve a dependent adult, an elderly person, or a child as a victim and, in the latter case, it is a form of childhood abuse ( 2 , 3 ). Factitious disorder was first described by the British psychiatrist Asher in 1951, and named after Baron Hieronymous Karl Friedrich von Münchausen (1720–1791), a German officer who was known for telling invented and unbelievable stories about himself and his life ( 4 ) The American Psychiatric Association first included factitious disorder in Diagnostic and Statistical Manual of Mental Disorders (DSM) III: Diagnostic and Statistical Manual of Mental Disorders in 1980 ( 5 ). However, despite decades have passed since the inclusion of factitious disorder in the DSM manual, its incidence remains controversial ( 6 , 7 ). According to DSM-5, factitious disorder in hospital settings is estimated to be present in 1% of individuals ( 1 ). Skin alteration (i.e., ulcers, dermatitis artefacta, hyperkeratosis) is the most common presentation of factitious disorder ( 8 , 9 ), but factitious disorder appears to be common also in neurological settings, where it represents up to 30% of neurologist consultations ( 10 ).
In the context of factitious disorder, depression can be both a feigned disease and an associated comorbidity. However, since most patients with factitious disorder reject psychiatric consultation, its real prevalence is likely underestimated ( 6 , 11 ). As a consequence, only a few patients with factitious disorder that also present with depression receive an adequate psychiatric diagnosis ( 12 ). Moreover, it is important to note that not all patients with factitious disorder suffer from depression, and literature on risk factors for depression development in factitious disorder patients is scarce and has never been put into a congruent frame. Based on these premises, the present study aimed to: 1. Provide an overview of the relationship between factitious disorder and depression; 2. Identify the prevalence of depression in factitious disorder; 3. Identify factors that can contribute to the development of depression in patients suffering from factitious disorder.
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines ( 13 ). A literature search was performed using the electronic databases PubMed, EMBASE and Cochrane Library, using a combination of the following MESH terms: “factitious disorder”, “Munchausen Syndrome”, “depression”, “depressive disorder” and “depressive episode. The search was conducted on December 9 th , 2022. Studies were eligible for inclusion in this review if they investigated factitious disorder imposed on self or imposed on another with comorbid depression. Only original papers published in English, French or Italian in peer-reviewed journals were accepted for inclusion. No predefined time window for the study search was adopted, to be the most inclusive as possible. By using a three-step screening approach, articles were screened through title, abstract, and full-text reading, if needed. Studies were excluded if they (i) reported on children and adolescents; (ii) provided mainly commentary or proposed guidelines; (iii) did not assess depression in factitious disorder; or (iv) reported on factitious depression. The screening and data extraction was done manually. Publication data screening and extraction were performed following a 2-step selection process (conventional double-screening) conducted by two reviewers independently of each other (CC and DMM). In the rare instances of discrepant screening, a consensus was reached through discussion with a third senior clinical researcher (MC). Further research evidence, gathered outside of the search or identified through manual search of the reference section of the included articles, was reported if considered appropriate by researchers. By applying a flexible approach, other articles that were deemed to cover prominent related topics were also searched by accessing grey literature and/or screening the reference lists of the eligible studies, to provide a more comprehensive overview (See Figure 1 – flow chart). The following information was extracted from the included studies: Study ID (including authors, year of publication and country in which the study was conducted), study design characteristics (including study type, number of patients and patients’ sex and age), brief description of symptoms presentation, diagnostic tools used and risk factors for depression.
Figure 1 Flow diagram of the screening process according to PRISMA ( 13 ).
A total of 2288 articles were identified and cross-checked by two researchers. By using a three-step screening approach, titles, abstracts, or full texts of all records were screened against the inclusion and exclusion criteria. A total of 22 articles were included, consisting of n = 18 case reports ( 2 , 6 , 12 , 14 – 28 ) and n = 4 cohort studies ( 9 , 11 , 29 , 30 ).
The studies were conducted in 13 countries, with 33% of them being performed in the United States (US), 33% in the European Union (EU), and 33% in Turkey, Morocco, Canada, and India, by involving from 1 to 60 patients, mainly female. The most common presentation of factitious disorder was skin lesions ( n = 11) and hypoglycemia ( n = 2). Beyond this, there was a wide range of presentations, such as factitious mourning, Acquired immune deficiency syndrome (AIDS), cancer, Cushing syndrome, vomiting, and anaphylaxis.
Diagnostic procedures
In all studies included the diagnosis of factitious disorder was made after exclusion of any medical condition, prolonged clinical examination, and detailed history collection. To define the presence of depression, we adhered to the criteria adopted in the individual studies. Depression was diagnosed after clinical examination ( n = 13) or instrumental assessment ( n = 6). Two studies did not specify how depression was diagnosed. Among diagnostic tools, Minnesota Multiphasic Personality Inventory (MMPI) was used in n = 2 studies; Beck Depression Inventory (BDI) and Hospital and Anxiety Depression Scale (HADS) were used in n = 1 study; projective tests such as the Rorschach test ( 31 ), the Rosenzweig Picture-Frustration Test ( 32 ) and the Rotter Sentence Completion Test ( 33 ) were used in n = 2 studies. One study reported generic “psychological test” without any further explanation. The Rosenzweig Picture-Frustration Test is a projective technique for the assessment of frustration tolerance and of how a person reacts to conflict situations; The Rotter Sentence Completion Test is a sentence completion test intended to detect psychological maladjustment. Intelligence Quotient (IQ) was investigated in n = 2 studies (see Table 1 ). Factitious disorder imposed on another was diagnosed in n = 2 studies and involved mothers in their post-partum ( 2 , 22 ).
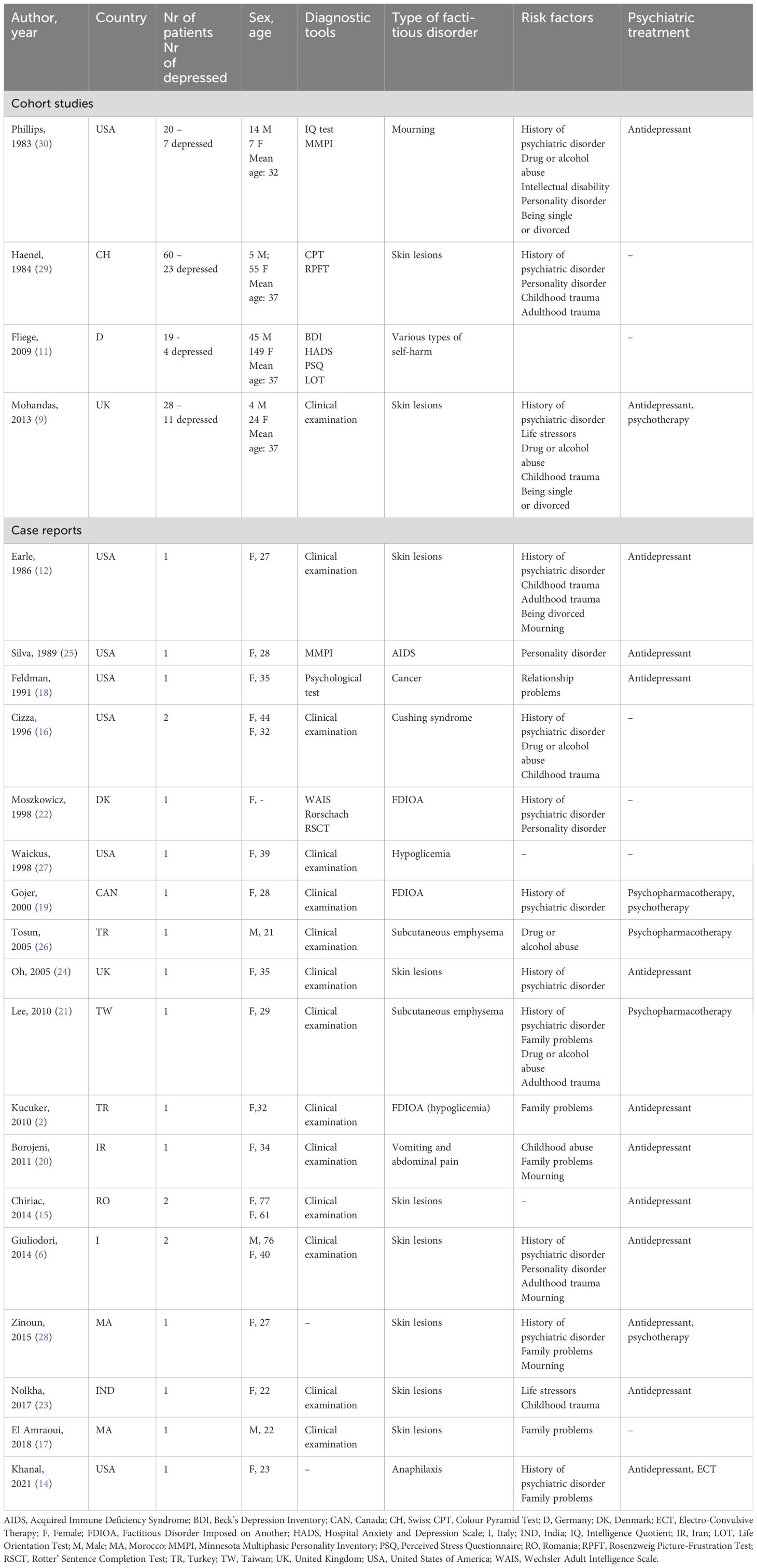
Table 1 Characteristics of the included studies.
Prevalence of depression in factitious disorder was reported for all the four cohort studies. Phillips ( 30 ) reported on 20 patients with factitious mourning (defined as the falsely reported death of loved ones), 7 of which (35%) were later diagnosed as having depression. Haenel ( 29 ) analyzed 60 cases of factitious dermatitis, 23 of which (38%) showed symptoms of depression. Fliege ( 11 ) analyzed 19 cases of factitious disorder referring to a department of psychosomatic medicine and found a 15.8% prevalence of depression and a 26.3% prevalence of anxiety. Finally, Mohandas ( 9 ) found that 10 patients out of a cohort of 28 patients with factitious dermatitis were suffering from depression (36%).
Factitious disorder and depression
Patients with factitious disorder and depression displayed the classic features of depression: tendency to weep, feelings of guilt, loss of interest in daily activities, and loss of concentration ( 25 , 28 ). Also, they reported insomnia, suicidal thoughts, loss of appetite, and fatigue ( 15 , 17 , 19 , 25 , 28 ).
Depression was treated with antidepressant in n = 16 studies. Add-on treatment included psychotherapy ( n = 2) and electroconvulsive therapy ( n = 1). In all cases, antidepressant treatment led to an improvement of both factitious and depressive symptoms (See Table 1 ).
Risk factors
Risk factors for factitious disorder comorbid with depression were reported in n = 20 studies. They included: history of childhood trauma, mourning, recent divorce or severe family problems, specific psychological traits such as high levels of psychological tension and scarce tolerance to frustration, history of psychiatric disorders, drug abuse and intellectual disability (see Table 1 ).
This review found depression to be highly prevalent in factitious disorder, affecting around 30% of the samples. This result provides support for an association between factitious disorder and mood disturbance ( 34 ). According to evidence gathered in this review, signs and symptoms of depression in factitious disorder are identical to those expressed by patients with depression who do not have a factitious disorder. For this reason, depression among factitious disorder patients is expected to be easily identified by an expert psychiatrist. Diagnostic tools for depression can be used, as well as projective tests. Projective tests can be especially helpful when factitious depression is suspected. The theoretical basis for use of projective measures is that in the absence of specific instruction or highly directive stimuli, people will have only their own internal resources available for managing the demands of the test. Thus, they will project their own internal psychological functioning onto the test stimuli ( 31 ).
Results of our review suggest that patients with factitious disorder and comorbid depression who receive antidepressant treatment improve both in factitious and depressive symptoms. Therefore, early detection and treatment of depressive symptoms in this population appears to be crucial also for the management of the factitious disorder. Factitious disorder diagnosis is often problematic due to the nature of the disorder that leads clinicians to focus more on somatic symptoms rather than on psychological problems, at least at first. The exaggerated, atypical, and contradictory presentation of factitious disorder symptoms is likely to lead clinicians to perform unnecessary diagnostic tests and invasive diagnostic procedures and to prescribe unnecessary treatments and needless hospitalization, also to avoid exposure to malpractice litigation ( 35 , 36 ). Patients’ seeking attitude towards procedures and treatment goes along with clinicians’ fears of litigation, which can result in important delays in factitious disorder diagnosis. Moreover, the focus on somatic complains and the waiting for procedure results may lead clinicians to underestimate levels of emotional distress in patients with factitious disorder ( 6 ). Indeed, it is likely that there is a significant window of time in which depression in factitious disorder goes undetected. Importantly, delays in depression treatment in patients with factitious disorder have been related to poor prognosis and high risk of chronicity of both depressive and factious symptoms ( 37 ).
Reported risk factors for depression in factitious disorder overlap with risk factors for factitious disorder with regard to childhood trauma and history of psychiatric disorder. The association between childhood adversities and the subsequent development of depression in adult life has been extensively studied ( 38 ). Childhood abuse involves experiences of being rejected, degraded, terrorized, isolated or teased. When childhood abuse is perpetrated by caregivers, it affects secure attachment, which can lead to the development of distorted and negative internal working models of the self and the others ( 39 ). Childhood abuse and insecure attachment are also linked to alexithymia, which is the inability to express and regulate emotions. Alexithymia is often found across several mental disorders, including depression. Further, such inability to recognize others’ emotions and to properly express one’s own emotions can lead to the development of somatic symptoms. The production of somatic symptoms, either conscious or unconscious, may thus be functional to avoid trauma-related symptoms. People exposed to childhood traumatic experiences are more likely to develop emotional distress compared to non-exposed. Emotional distress may lead to emotional fragility, feelings of insecurity, social isolation, low self-esteem, and loneliness intolerance, that also continue into adult life, leading to the development of a factitious disorder with comorbid depression ( 6 , 28 ). With regard to having a history of psychiatric disorder, it is known that some psychiatric disorders, such as bipolar and personality disorders, are at high risk to present with depressive episodes. For this reason, it is likely that having a factitious disorder and a history of psychiatric disorder increases the odds of developing depression. Instead, specific risk factors for depression in factitious disorder seem to include having suffered from adulthood traumatic experiences, especially mourning. Under this perspective, factitious disorder symptoms may act as a try to avoid the foster of complex situations related to traumatic experiences underlying depression.
Strengths and limitations
To our knowledge, this is the first review exploring the interplay between depression and factitious disorder. Following PRISMA guidelines, our systematic review described characteristics of depression in factitious disorder. We found that depression is highly prevalent in factitious disorder, affecting 1:3 patients and we identified and discussed specific risk factors for depression and factitious disorder. However, the main limitation of the study is that most evidence came from case reports, whose methodology is not always robust. Although in the hierarchy of evidence-based medicine, case reports do not top the list, the hypothesis generated from them may be appealing, leading to physiological studies and clinical trials ( 40 ). In addition, there was high heterogeneity in terms of screening tools for depression and assessment procedures. Also, even though the systematic review followed the PRISMA statement, no review protocol was registered. Lastly, a significant proportion of articles included in the present systematic review are older than 5 years. This could reflect a progressive reduction of interest in the topic, possibly accelerated by the switch towards psychological themes related to the Coronavirus Disease 2019 (COVID-19) pandemic in recent years.
Conclusions
Given the high prevalence of depression in factitious disorder, a multidisciplinary team approach in cooperation with mental health professionals appears to be essential for the management of these patients. However, this field of research is still sparse and mainly based upon case-report studies, suggesting the need to increase research in this area with more robust investigations.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author contributions
CC: Conceptualization, Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. DMM: Conceptualization, Data curation, Investigation, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. RB: Conceptualization, Data curation, Investigation, Resources, Validation, Visualization, Writing – review & editing. AP: Conceptualization, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. MB: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. MC: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
MC has been a consultant/advisor to GW Pharma Limited, GW Pharma Italy SRL, and F. Hoffmann-La Roche Limited outside of this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Psychiatric Association. DSM-5 Diagnostic and Statistical Manual of Mental Disorders . 5th. Washington, DC: American Psychiatric Publishing (2013). doi: 10.1176/appi.books.9780890425596
CrossRef Full Text | Google Scholar
2. Kucuker H, Demir T, Oral R. Pediatric condition falsification (Munchausen syndrome by Proxy) as a continuum of maternal factitious disorder (Munchausen syndrome). Pediatr Diabetes . (2010) 11:572–8. doi: 10.1111/pdi.2010.11.issue-8
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Comacchio C, Lasalvia A, Ruggeri M. Current evidence of childhood traumatic experiences in psychosis - focus on gender differences. Psychiatry Res . (2019) 281:112507. doi: 10.1016/j.psychres.2019.112507
4. Ozmen S, Ozmen OA, Yilmaz T. Clear otorrhea: a case of Munchausen syndrome in a pediatric patient. Eur Arch Otorhinolaryngol . (2008) 265:837–8. doi: 10.1007/s00405-007-0527-2
5. American Psychiatric Association. DSM III. Manuale diagnostico e statistico dei disturbi mentali, tr. it . Milano: Masson (1980).
Google Scholar
6. Giuliodori K, Campanati A, Rosa L, Marconi B, Cellini A, Brandozzi G, et al. Factitious disorders in adults: two cases of unusual skin ulcers. Acta Dermatovenerol Alp Pannonica Adriat . (2014) 23:13–5.
PubMed Abstract | Google Scholar
7. Poloni N, Vender S, Bolla E, Bortolaso P, Costantini C, Callegari C. Gluten encephalopathy with psychiatric onset: case report. Clin Pract Epidemiol Ment Health . (2009) 5:16. doi: 10.1186/1745-0179-5-16
8. Green S, Hicks A, Hilsendager C, Bauer M, Frank GKW. An adolescent girl with signs and symptoms of anaphylaxis and negative immunologic workup: a case report. J Med Case Rep . (2020) 14:49. doi: 10.1186/s13256-020-02371-3
9. Mohandas P, Bewley A, Taylor R. Dermatitis artefacta and artefactual skin disease: the need for a psychodermatology multidisciplinary team to treat a difficult condition. Br J Dermatol . (2013) 169:600–6. doi: 10.1111/bjd.2013.169.issue-3
10. Galli S, Tatu L, Bogousslavsky J, Aybek S. Conversion, factitious disorder and Malingering: A distinct pattern or a continuum? Front Neurol Neurosci . (2018) 42:72–80. doi: 10.1159/000475699
11. Fliege H, Lee JR, Grimm A, Fydrich T, Klapp BF. Axis I comorbidity and psychopathologic correlates of autodestructive syndromes. Compr Psychiatry . (2009) 50:327–34. doi: 10.1016/j.comppsych.2008.09.008
12. Earle JR, Folks DG. Factitious disorder and coexisting depression: a report of successful psychiatric consultation and case management. Gen Hosp Psychiatry . (1986) 8:448–50. doi: 10.1016/0163-8343(86)90029-0
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ . (2021) 372:n71. doi: 10.1136/bmj.n71
14. Khanal R, Sendil S, Oli S, Bhandari B, Atrash A. Factitious disorder masquerading as a life-threatening anaphylaxis. J Investig Med High Impact Case Rep . (2021) 9:23247096211006248. doi: 10.1177/23247096211006248
15. Chiriac A, Foia L, Birsan C, Goriuc A, Solovan C. Cutaneous factitia in elderly patients: alarm signal for psychiatric disorders. Clin Interv Aging . (2014) 9:421–4. doi: 10.2147/CIA
16. Cizza G, Nieman LK, Doppman JL, Passaro MD, Czerwiec FS, Chrousos GP, et al. Factitious cushing syndrome. J Clin Endocrinol Metab . (1996) 81:3573–7. doi: 10.1210/jcem.81.10.8855803
17. El Amraoui M, Hassam B. Self-induced dermatosis on the shoulder. Pan Afr Med J . (2018) 30:149. doi: 10.11604/pamj.2018.30.149.14708
18. Feldman MD, Escalona R. The longing for nurturance. A case of factitious cancer. Psychosomatics . (1991) 32:226–8. doi: 10.1016/S0033-3182(91)72096-3
19. Gojer J, Berman T. Postpartum depression and factitious disorder: a new presentation. Int J Psychiatry Med . (2000) 30:287–93. doi: 10.2190/FU9U-JPW1-YR3R-Q819
20. Hemmatian Borojeni B, Zaheriany SM, Hemmatian Borojeni N, Bidaki R. Münchausen’s syndrome in the form of factitious vomiting in a young female. Iran J Psychiatry Behav Sci . (2011) 5:146–9.
21. Lee YP, Lee JY. Recurrent factitious subcutaneous emphysema: report of a complex case in a young woman and a literature review. Kaohsiung J Med Sci . (2010) 26:377–83. doi: 10.1016/S1607-551X(10)70062-0
22. Moszkowicz M, Bjørnholm KI. Factitious illness by proxy presenting as anorexia and polydipsia by proxy. Acta Paediatr . (1998) 87:601–2. doi: 10.1080/08035259850158380
23. Nolkha N, Wakhlu A, Tandon V, Bansal N. Factitious lobular panniculitis presenting as recurrent breast ulcers. Int J Rheum Dis . (2017) 20:1778–80. doi: 10.1111/1756-185X.12657
24. Oh CC, McKenna DB, McLaren KM, Tidman MJ. Factitious panniculitis masquerading as pyoderma gangrenosum. Clin Exp Dermatol . (2005) 30:253–5. doi: 10.1111/j.1365-2230.2004.01713.x
25. Silva JA, Leong GB, Weinstock R, Ready DJ. Factitious AIDS in a psychiatric inpatient. Can J Psychiatry . (1989) 34:320–2. doi: 10.1177/070674378903400410
26. Tosun F, Ozer C, Akcam T, Gerek M, Yetiser S. A patient of severe cervicofacial subcutaneous emphysema associated with Munchausen’s syndrome. J Craniofac Surg . (2005) 16:661–4. doi: 10.1097/01.scs.0000168772.71356.2b
27. Waickus CM, de Bustros A, Shakil A. Recognizing factitious hypoglycemia in the family practice setting. J Am Board Fam Pract . (1999) 12:133–6. doi: 10.3122/jabfm.12.2.133
28. Zinoun M, Chiheb S, Marnissi F, Kadiri N, Benchikhi H. Unilateral bullous and purpuric lesions: skin pathomimicry. Pan Afr Med J . (2015) 20:301. doi: 10.11604/pamj.2015.20.301.5048
29. Haenel T, Rauchfleisch U, Schuppli R, Battegay R. The psychiatric significance of dermatitis artefacta. Eur Arch Psychiatry Neurol Sci . (1984) 234:38–41. doi: 10.1007/BF00432881
30. Phillips MR, Ward NG, Ries RK. Factitious mourning: painless patienthood. Am J Psychiatry . (1983) 140:420–5. doi: 10.1176/ajp.140.4.420
31. Rorschach H. Psychodiagnostics: A diagnostic test based on perception . Bern, Switzerland: Verlag (1942).
32. Rosenzweig S. The picture-association method and its application in a study of reactions to frustration. J Pers . (1945) 14:3–23. doi: 10.1111/j.1467-6494.1945.tb01036.x
33. Rotter JB, Rafferty JE. Rotter Incomplete Sentences Blank manual . San Antonio, TX: Psychological Corporation (1992). doi: 10.1037/t15153-000
34. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry . (2016) 41:20–8. doi: 10.1016/j.genhosppsych.2016.05.002
35. Rodriguez RM, Anglin D, Hankin A, Hayden SR, Phelps M, McCollough L, et al. A longitudinal study of emergency medicine residents’ malpractice fear and defensive medicine. Acad Emerg Med . (2007) 14:569–73. doi: 10.1111/j.1553-2712.2007.tb01834.x
36. Sekhar MS, Vyas N. Defensive medicine: a bane to healthcare. Ann Med Health Sci Res . (2013) 3:295–6. doi: 10.4103/2141-9248.113688
37. Del Casale A, Ferracuti S, Rapinesi C, Serata D, Simonetti A, Caloro M, et al. Factitious disorder comorbid with bipolar I disorder. A case report. Forensic Sci Int . (2012) 219:e37–40. doi: 10.1016/j.forsciint.2012.01.011
38. Bifulco A, Schimmenti A, Moran P, Jacobs C, Bunn A, Rusu AC. Problem parental care and teenage deliberate self-harm in young community adults. Bull Menn Clin . (2014) 78:95–114. doi: 10.1521/bumc.2014.78.2.95
39. Bowlby J. Attachment: Attachment and Loss Volume One . 2nd. New York: Basic Books (1982).
40. Nayak BK. The significance of case reports in biomedical publication. Indian J Ophthalmol . (2010) 58:363–4. doi: 10.4103/0301-4738.67038
Keywords: factitious disorder, depression, prevalence, risk factors, comorbidity
Citation: Comacchio C, Misca DM, Bortoletto R, Palese A, Balestrieri M and Colizzi M (2024) Prevalence and risk factors for depression in factitious disorder: a systematic review. Front. Psychiatry 15:1355243. doi: 10.3389/fpsyt.2024.1355243
Received: 13 December 2023; Accepted: 15 April 2024; Published: 26 April 2024.
Reviewed by:
Copyright © 2024 Comacchio, Misca, Bortoletto, Palese, Balestrieri and Colizzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Comacchio, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clinicoecon Outcomes Res
The Economic Burden of Bipolar Disorder in the United States: A Systematic Literature Review
Leona bessonova.
1 Health Economics and Outcomes Research, Alkermes, Inc., Waltham, MA, USA
Kristine Ogden
2 Evidence, Worldwide Clinical Trials, Morrisville, NC, USA
Michael J Doane
Amy k o’sullivan, mauricio tohen.
3 Department of Psychiatry and Behavioral Sciences, University of New Mexico, Albuquerque, NM, USA
Bipolar disorder (BD) is a mood disorder with subtypes characterized by episodes of mania, hypomania, and/or depression. BD is associated with substantial economic burden, and the bipolar I disorder (BD-I) subtype is associated with high medical costs. This review further evaluated the economic burden of BD and BD-I in the United States (US), describing health-care resource utilization (HCRU) and sources of direct medical and indirect costs. Data were obtained from systematic searches of MEDLINE ® , EMBASE ® , and National Health Service Economic Evaluation Database. Citations were screened to identify primary research studies (published 2008–2018) on the economic burden of BD/BD-I or its treatment in real-world settings. Reported costs were converted to 2018 US dollars. Of identified abstracts (N=4111), 56 studies were included. The estimated total annual national economic burden of BD/BD-I was more than $195 billion, with approximately 25% attributed to direct medical costs. Individuals with BD/BD-I used health-care services more frequently and had higher direct medical costs than matched individuals without the disease. Drivers of higher direct costs included frequent psychiatric interventions, presence of comorbid medical/psychiatric conditions, and both suboptimal medication adherence and clinical management. Indirect costs (eg, unemployment, lost work productivity for patients/caregivers) accounted for 72–80% of the national economic burden of BD/BD-I. Different definitions for study populations and cost categories limited comparisons of economic outcomes. This review builds on existing literature describing the economic burden of BD and confirmed cost drivers of BD/BD-I. Improved clinical management of BD/BD-I and associated comorbidities, together with better medication adherence, may reduce health-care costs and improve patient outcomes.
Introduction
Bipolar disorder (BD) is a severe and complex mental health disorder composed of different subtypes that present variably. The disorder is characterized by shifts in mood (ie, alternating periods of elation, irritability, and depression), energy, and behavior. 1 , 2 The lifetime prevalence of BD is estimated to be 4.4% in the United States (US), with most cases emerging during adolescence or early adulthood. 3 BD is a leading cause of disability among young people, 2 , 4 and is associated with impairments that negatively impact personal, social, and occupational functioning, and reduce quality of life. 1 , 2 , 5 - 8
Prior reviews of cost of illness studies have found a substantial economic burden associated with BD, and that cost estimates for the disorder vary considerably across studies. For example, one analysis estimated the per-person total lifetime costs of BD in the US ranged from $11,720 for a single manic episode to $624,785 for a disease course marked by nonresponsive/chronic episodes (1998 US dollars [USD]). 9 Sources of direct health-care costs for individuals with BD include medical expenses associated with psychiatric care (both inpatient and outpatient), treatment (pharmacological and non-pharmacological), and emergency room (ER) visits. 6 , 9 - 11 Persons with BD tend to have higher rates of comorbid medical (eg, metabolic syndrome, hypertension) and psychiatric (eg, substance use disorder, anxiety) conditions, which contribute to higher utilization of general medical services compared to the general population. 1 , 12 - 15 Fewer studies have examined indirect costs (eg, expenditures associated with reduced work productivity, use of caregivers) for those with BD; 6 , 10 , 16 yet, their impact is sizable with losses in work productivity previously estimated to represent 20% to 94% of the total societal cost of BD. 6
Bipolar I disorder (BD-I) is a subtype of BD in which individuals experience one or more manic episodes, and accounts for approximately one-quarter of all cases of BD. 3 , 17 The disease course for BD-I is typically chronic and is associated with significant functional disability and premature mortality. 3 , 5 , 18 - 20 Some evidence suggests that BD-I may also be associated with higher direct medical costs compared to other subtypes of BD; however, the reasons for this are poorly understood. 6 Few studies have elucidated the different drivers that may contribute to greater cost burden for those living with BD, in general, and those with BD-I, specifically.
The objective of this systematic review is to provide an updated report of the economic burden of BD in the US, including a broader spectrum of cost and/or health-care resource use (HCRU) estimates compared with previous reviews. 6 , 10 Direct and indirect costs of the disorder are summarized, and drivers of these costs are identified. Where specific data existed for BD-I, these estimates are reported separately from those for BD overall. While BD-I has been associated with higher direct medical costs compared with other BD subtypes, 6 this review examines broader cost outcomes and drivers of these costs specifically for patients with BD-I.
Materials and Methods
MEDLINE ® , MEDLINE ® in-process, EMBASE ® , and National Health Service Economic Evaluation Database (NHS EED) databases were searched for primary research studies published between 1 January 2008 and 9 July 2018 on the economic burden of BD and BD-I in the US. Search strategies combined terms related to disease and outcomes and were limited to English-language publications only. The full search strategies and search terms are available in the electronic supplementary materials Tables 1 and 2 .
The start year (2008) was selected because it captured a decade of published literature at the time the review was conducted. From 2008 to 2018, several new medications became available for the treatment of BD/BD-I 21 and multiple international guidelines, including a major North American clinical guideline, were revised. 22 – 28 In addition, two federal laws were passed in 2008 and 2010 (Mental Health Parity and Addictions Equity Act [MHPAEA] and Affordable Care Act [ACA], respectively) that substantially changed the insurance landscape and availability of mental health benefits in the US. 29 Given these collective events, conducting an updated review to understand the contemporary economic burden of BD/BD-I in the US was warranted.
Publications were included if the population of interest was adults with BD (generally or not otherwise specified) or BD-I, and the economic burden of the disorder or its treatment in the US was reported or could be derived. Economic burden was defined broadly; studies that discussed patterns of HCRU without cost estimates and papers that described other economic impacts associated with BD or BD-I (eg, workplace productivity, disability) were included in this review. Inclusion was restricted to studies conducted in a real-world setting (ie, not randomized controlled trials) and studies that included cohorts of at least 100 patients. Studies that focused on bipolar depression only, or on subtypes other than BD-I, were excluded, as were case reports and cost-effectiveness analyses and similar economic evaluations of specific medications. Reviews were not included but their bibliographies were screened for relevant studies.
Citations from all database searches were combined; duplicates and excluded publication types (eg, randomized controlled trials, case reports) were flagged electronically and removed. Titles and abstracts for the remaining articles were screened by one reviewer, with independent review by a second reviewer if inclusion/exclusion was unclear. Inclusion was confirmed by review of full-text publications by one reviewer, with queries resolved by discussion with a second reviewer. Data from included studies were extracted into a structured spreadsheet by two reviewers, and disagreements were resolved by consensus. Data specific to BD-I were extracted separately wherever possible. The extraction spreadsheet was organized to capture discrete categories of economic outcomes to facilitate descriptive summary of the findings for this review. The methodological characteristics of included cost of illness studies were assessed with the checklist utilized by Kleine-Budde et al, 10 and these results are included in the electronic supplementary materials Tables 4 and 5 .
Costs were converted to a common year currency (2018 USD) using the Consumer Price Index (CPI) for Medical Care. 30 If cost-year was not reported in a study, it was assumed to be the last year of the observation period mentioned in the source publication.
Literature Search Results
A total of 4111 abstracts were identified. Following screening, 99 articles were selected for full-text review. After inclusion and exclusion criteria were applied, 56 articles were included in the review. Thirteen studies (23.2%) reported data specific to BD-I, whereas the other 43 studies (76.8%) reported data on BD (generally or not otherwise specified). The study selection process is shown in Figure 1 .

PRISMA diagram showing the literature search process.
Notes: The 2529 records excluded prior to title/abstract review were eliminated electronically by identifying duplicate citations (eg, records with duplicate identifiers or citation data fields) and publications indexed for excluded types of publications (eg, randomized controlled trials, case reports). The step “articles retrieved from other sources” refers to papers identified from bibliographic review and other known, relevant research papers.
Abbreviations: BOI, burden of illness; NHS EED, National Health Service Economic Evaluation Database; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Of the 56 included studies, 30 studies (54%) reported cost data. The assessment of methodological characteristics of these studies found that most reported their data sources and analysis perspective; however, only 15 studies (50%) reported the monetary value of all HCRU and 13 studies (23%) provided separate information about the number of services (eg, health-care) and costs for the cost categories described. Inclusion of sensitivity analyses in these cost studies was uncommon. Of the papers not reporting cost data, 4 studies (7%) reported on HCRU, and 22 studies (39%) described other topics associated with economic burden (eg, work productivity, caregiver burden).
The electronic supplementary materials Tables 3 – 5 provide a list of all studies included in this review and the assessment of methodological characteristics for the cost studies identified.
Total National Economic Burden
Two studies estimated national costs using prevalence data for BD-I and bipolar II disorder (BD-II) in the US population. Cloutier et al estimated the total annual costs of BD-I in the US at $219.1 billion, corresponding to an average of $88,443 per person with BD-I per year. This figure included $50.9 billion in direct health-care costs (ie, medical and pharmacy); $9.7 billion in direct non-healthcare costs (eg, BD-related substance use disorder, criminal justice involvement for those who commit or are victims of crime, prevention/research costs); and $158.5 billion in indirect costs (eg, loss of work productivity or premature mortality). The excess costs of BD-I (the difference between costs incurred by individuals with and without BD-I) were reported to be $129.9 billion annually, an average of $52,413 per person with BD-I per year. Total costs for individuals with BD-I were 2.46 times greater than for controls without BD-I. 31
A second study estimated the total annual cost burden of BD-I and BD-II at $194.8 billion, including direct costs of $39.6 billion and indirect costs of $155.2 billion. However, the author explicitly acknowledged that these cost estimates were likely to be substantially underestimated, due to certain assumptions on which the analysis was based (eg, prevalence figures that did not include all subtypes of BD; direct and indirect cost estimates sourced from a 20-year-old cost analysis; the assumption that BD-I and BD-II are equally costly disorders; pharmacy costs that only included lithium). 32
Direct Health-Care Costs
Seventeen studies (two for BD-I, 15 for BD) reported on direct all-cause and/or mental health-related costs ( Table 1 ). Some cost estimates for cohorts with BD were higher and spanned a wider range than those reported for patients with BD-I. Several factors may contribute to this variation, including methodological differences between studies, consideration of different cost components (eg, inclusion of emergency room or other costs), and differences in clinical management and available treatments during the periods studied (2004 to 2007 for BD-I and 1998 to 2014 for BD, respectively).
Direct Health-Care Costs (Annualized, per Person, 2018 USD)
Notes: *Includes deductibles and co-payments; **limited to those with resource use; ***Costs over a 2-year period; – denotes not reported. † Other services include the use of intermediate care or skilled nursing facility, home-based care, ambulance, and laboratory tests.
Abbreviations: BD, bipolar disorder; BD-I, bipolar I disorder; MCO, managed care organization; USD, US dollars.
All-Cause Health-Care Costs
Fourteen studies (two for BD-I, 12 for BD) provided estimates of annual all-cause direct health-care costs ( Table 1 ). Among cohorts with a BD-I diagnosis, annual all-cause direct health-care costs varied from $11,239 to $19,446 per-person-per-year (PPPY). 33 , 34 Estimates of annual all-cause direct costs reported for patients with BD spanned a wider range, from $11,051 to $46,971 PPPY. 35 , 36
A retrospective study of commercial health-care claims reported that PPPY all-cause health-care costs (ie, inpatient, outpatient, prescription medications) for individuals with BD were about four times higher than for matched individuals with no mental health disorders and no psychotropic medication use ($19,131 [BD] vs $4706 [no mental health disorders]). 12 A separate study reported that individuals in an employer-based health plan diagnosed with BD had higher all-cause, mean per-member-per-month health-care costs than those with diabetes, depression, asthma, or coronary artery disease. This was largely due to higher costs for medications and psychiatric care (inpatient and outpatient) among those with BD. Only individuals diagnosed with both diabetes and coronary artery disease had higher all-cause health-care costs than those with BD. Sixty-four percent of total costs for the BD group were incurred by a small subgroup (20%) of patients; who were more likely to be female, have frequent hospital stays, and have a higher number of comorbidities. 37
In a cohort of community-dwelling dual-eligible Medicare/Medicaid beneficiaries with a mental health disorder in 2005, individuals with a diagnosis of BD had 34% higher medical care costs, and 59% higher prescription drug expenditures than those without a diagnosis of BD. Among members of this group who used Medicaid-paid community-based long-term care services (eg, in-home services), a diagnosis of BD was associated with 5% higher medical care costs, 15% higher long-term care costs, and 55% higher prescription drug expenditures than those without a diagnosis of BD. The authors reported a similar pattern for individuals who resided in Medicaid-paid institutional (eg, nursing home) long-term care facilities, noting the increased medication costs relative to those with other mental health diagnoses were expected due to this population’s greater reliance on pharmacotherapy and having more comorbid conditions. 38
Costs Related to Mental Health Care and Psychiatric Hospitalization
Eleven studies (two for BD-I, nine for BD) evaluated mental health-related costs ( Table 1 ). These studies estimated that annual mental health-related costs totaled between $4521 and $9132 PPPY for individuals with BD-I 33 , 34 and between $6374 and $21,523 PPPY for individuals with BD. 35 , 39
Two studies examined the cost of psychiatric hospitalization. The first estimated the cost of a psychiatric hospitalization in patients with BD-I to be $9544. 40 This figure is within the range reported by Stensland et al, who reported that the average cost of community hospital-based inpatient psychiatric care for patients with BD was $1159 to $1262 per day, depending on payer, with an average length of stay between 5.5 days (uninsured) and 9.4 days (Medicare). 41
Health-Care Resource Utilization
HCRU was reported in four studies ( Table 2 ); however, no study reported data specific to patients with BD-I. A diagnosis of BD was associated with high use of outpatient, inpatient, emergency, pharmaceutical, medical, and mental health services (eg, psychotherapy, BD-related acute care).
Health-Care Utilization (Annualized) in Cohorts with BD
Abbreviations: BD, bipolar disorder; BD-I, bipolar I disorder; ER, emergency room; OR, odds ratio; SUD, substance use disorder.
One study found that having a BD diagnosis increased the odds of being a “high-use consumer” of health care by 70% relative to a diagnosis of depression (“high-use” was defined as using inpatient, mental health ER services, or crisis residential visits three or more times in a fiscal year). 42 Another study found individuals with a diagnosis of BD had greater HCRU compared to age- and gender-matched individuals with no mental health disorders or psychotropic medication use, with the greatest increases reported for acute care services. The percentage of patients in the BD cohort with an inpatient admission was four times higher (22.4% vs 5.0%) and the percentage of BD patients with ER visits was more than twice as high (37.0% vs 14.8%) than the matched cohort. Increased use of outpatient visits (99.9% vs 95.0%) and prescription drugs (97.5% vs 82.0%) was reported for those with BD relative to the matched cohort. 12 Two other studies providing descriptive data for annual HCRU among commercially insured patients with BD 43 , 44 and are summarized in Table 2 .
Drivers of Direct Health-Care Costs
Eleven studies reported factors that were associated with either increased or decreased direct health-care costs in individuals with BD-I or BD. Factors associated with increased direct health-care costs included having frequent psychiatric interventions (ie, hospitalization, ER visit), the presence of comorbid medical and psychiatric conditions, nonadherence to BD-related medication, approach to pharmacotherapy (eg, use of certain combination treatments), and suboptimal clinical management due to a misdiagnosis of unipolar depression following a BD diagnosis. 12 , 33 , 34 , 37 , 39 , 45 - 50
Frequent Psychiatric Intervention
Three studies (two for BD-I, one for BD) examined patients who had “frequent psychiatric intervention” (FPI) over a 12-month period (Year 1), evaluating their health-care costs over the subsequent 12-month period (Year 2), relative to patients without FPI. Two studies defined FPI as ≥2 ER visits or hospitalizations with a principal diagnosis of BD, addition of a new medication to the first observed treatment regimen, or ≥50% increase in BD medication dose, within a 12-month period. 33 , 34 The third study utilized a similar definition for FPI but specified a frequency of ≥4 such events within a 12-month period. 39 FPI was common, with a prevalence of 40% to 53% in BD-I cohorts 33 , 34 and 52.5% in a group with BD-I or BD-II. 39 Compared to those without FPI, individuals who had FPI incurred greater mental health-related and all-cause medical costs in the year following the FPI ( Table 3 ). They also had a 3.7-fold higher risk of subsequent mental health hospitalization and 3.1-fold higher risk of subsequent ER visits in the year following the FPI. 34
Notes: All three studies identified patients who had FPI over a 12-month period (Year 1) and examined health care costs for these patients over the subsequent 12-month period (Year 2) relative to patients without FPI. Costs adjusted to 2018 US dollars.
Abbreviations: BD, bipolar disorder; BD I, bipolar I disorder; BD II, bipolar II disorder; ER, emergency room; FPI, frequent psychiatric interventions; max, maximum; PPPY, per patient per year.
Comorbidities
Seven studies (two for BD-I, five for BD) reported on the economic burden of comorbidities among individuals with the disorder ( Table 4 ). Across studies, cardiometabolic comorbidities (eg, hyperglycemia or diabetes, cardiovascular disease, dyslipidemia, hypertension, and obesity) and psychiatric comorbidities (eg, substance/alcohol abuse, anxiety disorder) were associated with higher medical care costs and/or increased HCRU in patients with the disorder.
Economic Impact of Comorbidities in Persons with BD
Note: Costs adjusted to 2018 US dollars.
Abbreviations: BD, bipolar disorder; BD-I, bipolar I disorder; CCI, Charlson Comorbidity Index; ER, emergency room; FPI, frequent psychiatric intervention; HR, hazard ratio; max, maximum; MCO, managed care organization; PPPY, per person per year, RR, rate ratio.
Patients with FPI incurred significantly higher medical care costs and had significantly greater comorbidity burden compared to those without FPI. 33 , 34 , 39 Comorbidities with significantly higher prevalence rates in patients with FPI included anxiety disorder, substance use disorder, and depressive disorder. Additionally, in the two studies of BD-I populations, patients with FPI had significantly higher comorbidity scores and significantly greater rates of hypertension and dyslipidemia. 33 , 34 In a cost analysis by Durden et al, these three factors were associated with the higher total annual adjusted all-cause medical costs for patients with FPI relative to those without FPI. 34
Studies of BD in general also reported that comorbidities contribute significantly to the economic burden of BD. Guo et al estimated that 33% of PPPY direct health-care costs were related to the treatment of BD, while the remaining 67% were attributable to treatment of psychiatric (eg, substance/alcohol use disorders, personality disorder) and medical (eg, obesity, diabetes) comorbidities. 46 Similarly, analyses of health-care claims from an employer-based health plan found that health-care costs associated with BD were driven, in part, by patients’ comorbidity burden. 37
Another two studies reported associations between cardiometabolic comorbidity burden and increased acute care HCRU and costs for BD patients. In an evaluation of administrative hospital data for 30 days post-discharge, 60.5% of patients with an inpatient diagnosis of BD had at least one cardiometabolic comorbidity, and 33.4% had two or more. Those with one or more cardiometabolic conditions (vs none) had an increased likelihood of hospital readmission in the 30 days post-discharge, higher costs, longer lengths of stay, and higher in-hospital mortality. 50 Centorrino et al reported that individuals with BD had significantly more metabolic comorbidities than matched individuals from the general population (prevalence 37% vs 30%, p<0.0001). This was reflected in significantly higher medical service, particularly due to inpatient admissions, ER visits, and prescription costs for these conditions in the BD cohort. 12
Adherence to BD-Related Medication
Six studies (one for BD-I, five for BD) reported on economic aspects of adherence to BD-related medications, using various definitions of adherence. 40 , 45 , 51 - 54 Suboptimal adherence to BD medication was common. In a claims database study, only 35.3% of individuals with BD were adherent as measured by medication possession ratio (MPR) ≥0.80 over 12 months. 53 Individuals with lower antipsychotic adherence, as measured by MPR, had higher direct health-care costs in the form of inpatient and outpatient mental health-related HCRU and expenditures. 40 , 45 , 51 , 52 For example, one retrospective study reported that improved adherence to SGA therapy (ie, a 1-unit increase in MPR) was associated with lower quarterly mental health-related medical costs of $192 to $686 per patient. 45 Additionally, suboptimal adherence to BD-related medications resulted in higher indirect costs in the form of reduced workplace productivity ($427 - $1156 PPPY) 53 and reduced functional status, 53 , 54 compared to those who maintained higher levels of adherence.
Approach to Pharmacotherapy
Seven studies evaluated associations between medication regimens and health-care service use and/or costs among individuals with BD, predominately with antipsychotics. 46 , 47 , 55 - 59 One of them, a longitudinal cohort study, found that more than 8% of patients with BD receiving a second-generation antipsychotic (SGA) received combination treatment with more than one SGA concurrently; analyses found no association between disease severity and use of combination SGA treatment. Patients receiving a combination SGA regimen had greater rates of adverse events (eg, dry mouth, tremor, and sedation), nearly two- to three-times greater HCRU for medical and psychiatric services, respectively, and this regimen was associated with slightly worse global functioning relative to those treated with SGA monotherapy. 55 A second study of Medicaid claims for patients initiated on SGA therapy found only 58% were prescribed a clinically recommended dose of their index SGA. In this subset, there were no significant differences in annual medical and mental health-related costs across individual SGA treatment groups. 57 Other studies evaluated use of SGAs as a class compared to use of traditional mood stabilizers or in combination with traditional mood stabilizers 46 , 59 or examined costs differences for BD patients treated with different SGA agents alone or in combination with a mood stabilizer. 47 , 56 , 58 In general, studies that reported significant cost differences between treatment groups were driven by the risk or use of hospital services during the study period. 46 , 47 , 56
BD Patients with Subsequent Diagnoses of Unipolar Depression
Two studies estimated the occurrence of “incongruent diagnoses” among patients previously diagnosed with BD, defined as receipt of a diagnosis of unipolar depression 12 months following an initial BD diagnosis (17.5% to 27.5% of BD patients). 48 , 49 Unipolar depression diagnoses were considered “incongruent” as depressive episodes among BD patients would be expected to be treated as BD. The BD patients who received a subsequent diagnosis of unipolar depression had significantly higher average annual health-care costs (+$2676 PPPY), three times more psychiatric hospitalizations, and twice as many psychiatric ER visits than BD patients without a subsequent unipolar depression diagnosis. 48 A chart review for a subset of patients with incongruent diagnoses found different providers were documented for the initial BD diagnosis vs the subsequent unipolar depression diagnosis 76% of the time, suggesting gaps in continuity of care may have contributed to these patterns of “incongruent diagnoses”. 49
Direct Non-Health-Care Costs
Criminal justice system.
There were two studies (both in BD-I populations) that reported incremental costs of BD to the criminal justice system, such as costs of incarceration, policing, and legal costs. 60 , 61 In a patient survey, employees with BD-I were more likely to report having been involved in a crime than co-workers without a diagnosis of BD-I. 60 The second study examined public expenditures related to criminal justice, medical, mental health, and social welfare services for persons arrested in a large Florida county who had serious mental illnesses (SMIs). This analysis found psychiatric diagnosis influenced total expenditures; individuals with BD-I had the second-highest total quarterly costs ($2525) behind those with a psychotic disorder ($4209). 61
Indirect Costs
National burden.
The total annual indirect costs of BD-I in the US was estimated at $158.5 billion, constituting 72.3% of the total economic burden of the disorder. 31 About half (50.3%) of indirect costs were related to unemployment; the rest were attributed to caregiving productivity loss (34.1%); all-cause premature mortality among individuals with BD-I (8.6%); productivity loss among individuals with BD-I (6.4%); and direct health-care costs for caregivers (0.6%). The annual all-cause mortality rate for the BD-I population was found to be 3.4- to 11.4-times higher than for the US general population, depending on age group. Suicide was 10.3- to 16.2-times more common than for the general US population, and was responsible for an estimated 19% of the costs (measured as productivity loss) associated with premature deaths among those with BD-I. These findings were similar to another study that estimated total annual indirect costs for BD-I and BD-II of $155.2 billion (79.7% of the total cost burden). 32
Workplace Productivity
Seven studies (three for BD-I, four for BD) evaluated effects on workplace productivity or employment, and all found that the disorder had a negative economic impact for employed individuals and their employers ( Table 5 ).
Effects of BD on Workplace Productivity and Employment
Notes: *The EWPS is a 25-item instrument measuring absenteeism and presenteeism. Total EWPS scores range from zero (best) to 100 (worst). Costs adjusted to 2018 US dollars.
Abbreviations: BD, bipolar disorders; BD-I, bipolar I disorder; BD-II, bipolar II disorder; EWPS, Endicott Work Productivity Scale; MDE, major depressive episode; MEPS, Medical Expenditure Panel Survey; MPR, medication possession ratio; NA, not applicable; OR, odds ratio; PPPY, per person per year.
Individuals with BD or BD-I were more likely to be unemployed, miss work, have reduced work hours due to medical or mental health-related reasons, receive disability payments, or have been fired or laid off compared with those with no mood disorders. 60 , 62 Studies of employed persons with BD reported increased indirect costs due to work absence and disability, 53 as well as functional deficits that adversely affected work quality, work attendance, and ability to maintain employment. 63 – 65 Moreover, an increased number of lifetime mood episodes was associated with higher likelihood of permanent disability and unemployment. 64
Caregivers and Families
The economic burden of BD often extends to families and caregivers of these patients. In an analysis of the national burden of BD-I in the US, it was estimated that caregivers’ productivity loss and direct health-care costs accounted for more than a third of the total annual indirect costs of the disorder. These estimates were based on assumptions that caregivers devoted an average of almost 29 hours per week to caring for an individual with BD-I and that more than half (57.6%) of individuals with BD-I resided with family members. 31 A second study reported that total annual health-care costs were 239% higher for families containing a member with BD compared to matched families without a diagnosis of SMI. Specifically, families including a member with BD made more outpatient visits, had more inpatient hospital stays, and filled more prescription medications than the matched families. Notably, most of the total HCRU and costs related to conditions other than BD. The authors suggested that this may be related to the psychological stress of living with and/or caring for a family member with BD. Another possibility for the greater HCRU costs observed is that BD families may have more frequent interactions with the health-care system (on behalf of the member with BD), providing them with additional opportunities to discuss and/or pursue help with their own health concerns compared to families that do not include a member with a SMI. 66
This literature synthesis presents a comprehensive review of contemporary literature describing the direct and indirect costs associated with BD and BD-I in real-world settings in the US, and the drivers of those costs. It builds on the findings of prior reviews describing the disease burden of BD, which were more focused on methodologic differences among published studies that specifically described cost data related to the burden of BD. 6 , 10 This review included a broader collection of research than prior reviews, such as papers describing resource use or changes in work productivity without associated cost estimates, for additional perspective on the economic burden of BD/BD-I. Because BD encompasses multiple disease subtypes, this review reflected economic drivers that are applicable to both BD as a whole as well as the subset of patients who live with BD-I. While previous research identified differences in direct medical costs between BD-I and other BD subtypes, 6 this review allowed for identification of broader cost outcomes and drivers of these costs among patients with BD-I specifically.
National burden estimates for BD and BD-I in this review show the costs associated with this disorder are substantial. Two studies estimated total annual costs of $195 billion (BD-I and BD-II) and $219 billion for BD-I (both 2018 USD), in analyses that assumed a lifetime prevalence of 2.1% (BD-I and BD-II) and 1.0% (BD-I) of the adult population, respectively. 31 , 32 In contrast, the total annual cost of diagnosed diabetes was estimated at $333 billion (2018 USD) for a disease that affects 9.7% of the adult population. 67 For both BD and BD-I, the majority of total economic burden (72% to 80%) was attributed to indirect costs, such as losses in work productivity (eg, unemployment, absences associated with morbidity) and caregiving. These population-level findings were aligned with other studies in this review that reported reduced work attendance, functioning, and ability to maintain employment for individuals with BD or BD-I 53 , 60 , 62 , 65 as well as increased HCRU and worsening health status for family members of affected individuals. 31 , 66 , 68 Given the degree to which indirect costs impact the total costs of BD and BD-I, this topic should remain a research priority.
Thirteen studies in this review reported data specific to BD-I populations, summarizing considerable indirect and direct medical costs, with many of the cost drivers reported similar to those in studies of BD as a whole. Functional impairments among individuals with BD-I were associated with increased risk of unemployment or becoming disabled. 31 , 60 , 64 This risk was higher in persons who experienced recurrent mood episodes. 64 High direct medical costs, particularly for acute care, were reported for patients with BD-I specifically. 31 , 33 , 34 , 40 Among those with greater HCRU, nonadherence to pharmacotherapy and presence of comorbid conditions (eg, substance use disorder, hypertension) contributed to higher cost burden. 33 , 34 , 40 These data did not clarify if the BD-I subtype is a more costly form of the disease; thus, additional research into real-world indirect and direct costs associated with the clinical management and treatment of BD-I are needed to help inform key stakeholders and public policy decisions for this population.
Taken together, these observations underscore the need to improve patient outcomes and reduce overall economic burden by implementing strategies of disease and medication management. Treatment guidelines recommend patients receive long-term pharmacotherapy to reduce the recurrence of mood episodes and improve the stability of patients’ psychiatric and general health, their general functioning, and quality of life; however, this is a population in which medication adherence is typically poor. 22 , 28 , 69 Most currently prescribed mood stabilizing agents have undesirable side effects (eg, changes in cognitive function, tremor) that are poorly tolerated by patients 70 , 71 and also have the potential to induce or exacerbate comorbid conditions that may require intervention. 22 , 28 , 69 Choice of medication for BD is complex, balancing patient needs, symptoms, and treatment preferences with the risks of available therapies.
Interventions aimed at optimizing care delivery, such as integrated health-care programs combining primary care with specialists (eg, psychiatrists, pharmacists), have promise for improving the clinical management of BD and its comorbidities. 72 – 75 These teams work collaboratively to tailor treatment choices to patients’ psychiatric and medical care needs and to efficiently intervene to address factors that may be barriers to medication adherence. 72 , 75 Collaborative care of this kind has the potential to reduce the need for acute, intensive, or emergency health-care interventions by providing better continuity of care, while simultaneously reducing the indirect costs of BD and improving patients’ lives. 75
Our review should be considered in context of its limitations. Only studies that were published between 2008 and 2018 were included. Importantly, many of the studies characterized costs and burden using definitions of BD that predate the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Because the DSM-5 criteria broaden the definition of BD, 76 cost estimates reported from studies using earlier DSM criteria may not be representative of contemporary clinical experience. Included studies did not shed light on cost differences between persons with BD-I relative to other BD subtypes; however, results from Cloutier et al’s recent analysis of the national burden of BD-I in the US suggest that the cost burden of BD-I is similar to figures reported for BD generally when costs were adjusted to a common year. 31 The assessment of methodological characteristics of included cost studies found that most sufficiently reported the components in the quality checklist; however, only 4 studies provided results of sensitivity analyses, which may increase the level of uncertainty around some of these estimates. Categorization of non-medical costs was inconsistent in the literature and limits comparability; for example, costs of criminal justice involvement were categorized differently across studies where it was included in the analysis. In addition, aggregate cost estimates were bundled in ways that made it challenging to reliably separate component costs. Therefore, the CPI for Medical Care as the standard for converting costs to common-year currency (2018) may not accurately reflect BD-related non-health-care costs.
Other limitations inherent to the literature summarized included methodological variability in approaches to costing of data sources, selection of cost items and comorbidities, statistical methods used, and patient selection. Similar to prior reviews, 6 , 10 which discussed the methodological and quality issues in cost studies of BD in greater detail, this review found that greater transparency and specificity in study methods is needed to improve the comparability of results across studies. Many included studies relied on administrative claims data, thus limiting their analyses to those costs for which a payer is responsible. Other relevant costs, such as out-of-pocket payments or expenses carried by other payers (eg, rehabilitation paid from pension funds), were rarely included. Some studies focused on general cost categories (inpatient or outpatient care), while others also included the services of supporting departments such as ER, laboratory, and social work. Some studies matched their samples according to individuals’ age and gender; others used statistical methods to adjust for sociodemographic characteristics. Most studies recruited from special populations (eg, privately insured individuals; recent hospital discharges; employed persons) in which reported costs may not be representative of the general BD population. Some cost studies focused on newly available treatments, which may have resulted in higher reported costs compared to studies that included a broader selection of treatments due to increased pharmacy costs. Additionally, inconsistency in the way that “indirect” costs were defined or apportioned across publications led to heterogeneous definitions of indirect cost categories, resulting in widely differing estimates. This variability in costing methodologies and definitions limited comparisons across and between studies. However, this review summarized a broad range of studies to provide a comprehensive picture of the economic burden of BD and BD-I.
There is clear evidence from the published literature that BD (including BD-I specifically) and its comorbidities exert a large economic burden in the US on patients, caregivers, families, employers, and society. This burden encompasses direct health-care utilization and costs, loss of workplace productivity, caregiving, and other indirect costs. While estimates of indirect costs associated with BD and BD-I are substantial, they are infrequently quantified in the literature and warrant further study. Interventions that target better disease management and medication adherence may reduce the direct and indirect cost burden of BD and BD-I and improve patient outcomes.
Acknowledgments
The authors thank Nancy Neil, PhD (Worldwide Clinical Trials, Inc., USA), who assisted in the planning and conduct of the literature searches and analyses. Medical writing and editorial support for the preparation of the manuscript, under the direction of the authors, was provided by Jo Whelan, MSc (Textpharm Limited, UK) and was funded by Alkermes, Inc.
Funding Statement
Funding for the planning and conduct of the systematic literature review and the preparation of this manuscript was provided by Alkermes, Inc. In May of 2019, data included in this manuscript were presented as a poster at the annual meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) in New Orleans, Louisiana. The poster’s abstract was published in “Abstracts” in Value in Health, Vol 22: https://doi.org/10.1016/j.jval.2019.04.1065 .
Abbreviations
BD, bipolar disorder; BD-I, bipolar I disorder; BD-II, bipolar II disorder; CPI, Consumer Price Index; DSM, Diagnostic and Statistical Manual of Mental Disorders; FPI, frequent psychiatric intervention; HCRU, health-care resource utilization; MPR, medication possession ratio; NHS EED, National Health Service Economic Evaluation Database; PPPY, per person per year; SGA, second-generation antipsychotic; SMI, serious mental illness; US, United States; USD, United States Dollars.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Leona Bessonova, Michael J. Doane, and Amy K. O’Sullivan are employees of Alkermes, Inc. and may own stock/options in the company. Kristine Ogden is an employee of Worldwide Clinical Trials, Inc., which has received consulting fees from Alkermes, Inc. for conducting this study. Mauricio Tohen was an employee of Lilly (1997 to 2008) and has received honoraria from or consulted for Abbott, AstraZeneca, Alkermes, Allergan, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Johnson & Johnson, Otsuka, Merck, Gedeon Richter Plc, Sunovion, Forest, Roche, Elan, Lundbeck, Teva, Pamlab, Minerva, Neurocrine, Pfizer, Wyeth and Wiley Publishing. The authors report no other conflicts of interest in this work.

IMAGES
VIDEO
COMMENTS
Introduction. Bipolar spectrum disorders are a major public health problem, with estimates of lifetime prevalence in the general population of the United States at 3.9 percent, 1 with a range from 1.5 to 6.0 percent. 2 Bipolar disorder is also associated with significant mortality risk, with approximately 25 percent of patients attempting suicide and 11 percent of patients completing. 3 ...
Most of the patients were adults (apart from one adolescent and two cases with missing data), female (62%), and their symptoms met the DSM-5 criteria for bipolar I disorder (62%; followed by bipolar II disorder, 24%). Most of the patients were treated in private practice (52 vs. 24% in public health service, with the remaining unknown).
Overview of bipolar disorder. Bipolar disorder is a chronic and complex mood disorder that is characterized by an admixture of manic (bipolar mania), hypomanic and depressive (bipolar depression) episodes, with significant subsyndromal symptoms that commonly present between major mood episodes Citation 1.Ranked among the leading causes of worldwide disability Citation 2, bipolar I disorder has ...
Abstract. Various neuropathological findings have been reported in bipolar disorder (BD). However, it is unclear which findings are well established. To address this gap, we carried out a ...
For instance, a systematic review and meta-analysis showed a 0.11% lifetime prevalence rate for bipolar disorder in China. 11 The prevalence of bipolar I disorder is similar for males and females ...
A literature review exploring bipolar disorder's impact goals and aspirations, and the striving that the person engages in toward destabilizing and obstructing nature of bipolar disorder to the ...
Epigenetics in bipolar disorder: a critical review of the literature Psychiatr Genet. 2021 Feb 1 ... Bipolar disorder (BD) is a chronic, disabling disease characterised by alternate mood episodes, switching through depressive and manic/hypomanic phases. Mood stabilizers, in particular lithium salts, constitute the cornerstone of the treatment ...
Despite the increasing number of studies examining the effects of mindfulness interventions on symptoms associated with Bipolar Disorder (BD), the effectiveness of this type of interventions remains unclear. ... the aim of the present systematic literature review was to (i) critically review all available evidence on MBCT in BD, (ii) discuss ...
This review summarizes the available data on psychotherapy for adults with bipolar disorder. We conducted a search of the literature for outcome studies published between 1995 and 2013 and identified 35 reports of 28 randomized controlled trials testing individual or group psychosocial interventions for adults with bipolar disorder.
10 Namjoshi MA, Buesching DP: A review of the health-related quality of life literature in bipolar disorder. Qual Life Res 2001, 10:105-115.Crossref, Google Scholar. 11 Evenson RC, Vieweg BW: Using a quality of life measure to investigate outcome in outpatient treatment of severely impaired psychiatric clients. Compr Psychiatry 1998, 39:57-62.
Bipolar disorder (BD) is a chronic, disabling disease characterised by alternate mood episodes, switching through depressive and manic/hypomanic phases. Mood stabilizers, in particular lithium salts, constitute the cornerstone of the treatment in the acute phase as well as for the prevention of recurrences.
Bipolar-specific therapy is increasingly recommended as an essential component of illness management. This review summarizes the available data on psychotherapy for adults with bipolar disorder. We conducted a search of the literature for outcome studies published between 1995 and 2013 and identified 35 reports of 28 randomized controlled ...
1 INTRODUCTION. A meta-analysis of 25 studies conducted in 15 countries estimated a pooled lifetime prevalence of 1.06% for bipolar I disorder (BP-I) and 1.57% for bipolar II disorder (BP-II). 1, 2 However, US epidemiologic studies suggest that approximately 60% of patients with BP-I receive mental health treatment, a statistic that is similar to what has been reported for other countries. 3 ...
Discussion. This article systematically reviews the literature examining stigma toward bipolar disorder (BD). A considerable literature has addressed the issue, with a diversity of perspectives and approaches. In general, the literature shows that stigma is a major concern for individuals with BD and their family members.
This article reviews the research examining stigma towards bipolar disorder (BD) with a view to guiding the development of stigma reduction initiatives and ongoing research. Methods: PsychInfo, Medline, and Embase databases were searched for peer-reviewed studies addressing stigma in BD. Results: Stigma is a serious concern for individuals with ...
We aimed to perform a review of studies addressing the assessment of generic and health-related QoL in patients with bipolar disorder. A literature search was conducted in a comprehensive selection of databases including MEDLINE up to November 2004. Key words included: bipolar disorder or manic-depression, mania, bipolar depression, bipolar ...
Prescribed psychostimulants and other pro-cognitive medications in bipolar disorder: A systematic review and meta-analysis of recurrence of manic symptoms. Andreea Chiorean, Andreea Chiorean. Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada ... The gray literature search was limited to Clinicaltrials.gov and ...
Our review found that there is growing interest in characterizing QoL in bipolar disorder populations, and determining the impact of treatment interventions upon life quality. The scientific quality of research in this field has been variable, but increasing numbers of studies of good design are now being conducted.
The review of the literature describes the clinical features of bipolar affective disorder (BAD). Difficulties in diagnosing BAD due to the complex clinical picture that cause untimely diagnosis and the formation of an unfavorable course of the disease are examined in detail. The data on mental disorders it is necessary to differentiate BAD from are presented. The characteristics of the course ...
Factors associated with stigma among caregivers of patients with bipolar disorder in the STEP-BD study. Mental illness stigma was found to be prevalent among caregivers of persons with bipolar disorder who have active symptoms as well as for caregivers of those who have remitted symptoms. Expand.
Abstract and Figures. Bipolar disorder is a mental illness that causes dramatic shifts in a person's mood, energy and ability to think clearly. People with bipolar experience high and low moods ...
Precursors of bipolar disorder include mood lability, subsyndromal and major depression, subsyndromal hypomanic symptoms with or without major depression, cyclothymia and bipolar not otherwise specified, major depression with psychotic features, and other psychotic disorders. Bipolar disorder was also predicted by juvenile onset of major ...
Bipolar disorder (BD) and schizophrenia (SZ) are the two main mental disorders with unknown etiology that significantly impact individuals' quality of life. The potential pro-inflammatory role in their pathogenesis is postulated and Human Endogenous Retrovirus W (HERV-W) is an emerging candidate to modulate this pathogenic finding. HERVs, ancient retroviruses in the human genome, may play ...
Bipolar II Disorder is a subset of bipolar disorder in which people experience depressive episodes shifting back and forth with hypomanic episodes, but never a "full" manic episode. Cyclothymic Disorder or Cyclothymia is a chronically unstable mood state in which people experience hypomania and mild depression for at least two years. People ...
Results. Six articles were included in the final review. All included articles assessed populations aged 17 years or older. The literature indicates that BP disorder was addressed in telepsychiatry services at a similar rate as in‐person services, reliable diagnoses can be made using remote interviews, satisfaction rates are comparable to in‐person services, telepsychiatry services are ...
Objective: To investigate the prevalence and correlates of eating disorder symptoms in adolescents with bipolar I disorder (BP I). Methods: We retrospectively collected a DSM-IV-TR-based diagnostic assessment of 179 adolescents with BP I and evaluated clinical variables in those with and without eating disorder symptoms. For comparison, we retrospectively evaluated eating disorder symptoms in ...
We performed a systematic review to provide an overview of the relationship between factitious disorder and depression, describe the prevalence of depression in factitious disorder, and identify factors that can contribute to the development of depression in patients suffering from factitious disorder. Methods: A literature search was performed ...
Bipolar disorder (BD) is a mood disorder with subtypes characterized by episodes of mania, hypomania, and/or depression. ... This review builds on existing literature describing the economic burden of BD and confirmed cost drivers of BD/BD-I. Improved clinical management of BD/BD-I and associated comorbidities, together with better medication ...
The studies available have found that 32% of people with bipolar disorder also have delayed sleep-wake phase disorder, meaning they go to sleep and wake up later than average. During manic phases ...