
2/22 nd Morawskiego Street 60-239 Poznań
Podole 76 KRAKOW, Malopolskie, 30-394 Poland Ardigen, a world-leading AI CRO, stands at the forefront of enabling AI transformation for the biotech and pharmaceutical sectors. Our objective is to increase the probability of success in the drug development process. Positioned at the intersection of biology and computational science, we utilize our advanced platforms and deep expertise to turn vast amounts of data into pivotal, ready-to-use scientific insights. This proficiency aids with tasks such as finding the right therapeutic targets, optimizing small molecules, and advancing biologics. Leveraging our expertise, we also enhance the infrastructure that allows our clients to integrate and utilize these capabilities seamlessly within their organizations. Our specialized solutions and services focus on data engineering such as digital transformation, data processing, and data management, to fully support the needs of our partners. By empowering companies with both essential knowledge and advanced tech solutions, Ardigen is committed to shaping the future of an AI-driven biopharma landscape.
ul. Chmielna 2/31 Warsaw, Mazowieckie, 00-020 Poland At Appsilon, we are driven by using Data Science at the forefront of business, leveraging the potential of the ever-increasing amount of data. Our team has proven their skill set many times over, winning the Global Management Challenge with AI support or World Port Hackathon in Rotterdam. Some industries we work with include but are not limited to Finance, Healthcare, Pharmaceuticals, Maritime, Retail and Real Estate. Appsilon delivers high-quality data analysis and technical support. If you have a difficult problem that requires strong analytical skills or think that your data is being underutilised, then we're the right people!
Masovian Voivodeship, 05 Poland BIOTON offers insulin products, clinically important medicinal products, biotechnological drugs and contract manufacturing.
Macierzysz, ul. Poznańska 12 05-850 Ożarów Mazowiecki Poland BIOTON offers insulin products, clinically important medicinal products, biotechnological drugs and contract manufacturing.
5 Vetterów Lublin, lubelskie, 20-001 Poland BioMaxima is a Polish company acting in widely understood scope of biotechnology on the market of laboratory diagnostics. It is one of the two Polish manufacturers of reagents for in vitro diagnostics. All company's products are CE marked and meet the provisions of European In Vitro Diagnostics Directive. BioMaxima also distributes products of the well-known diagnostic companies such as: Nova Biomedical, Mitsubishi Chemical. We supply almost 1800 laboratories in Poland and export to the markets of Lithuania, Latvia, Romania and France.
5 Vetterów Str. 20-277 Lublin Poland BioMaxima is a Polish company acting in widely understood scope of biotechnology on the market of laboratory diagnostics. It is one of the two Polish manufacturers of reagents for in vitro diagnostics. All company's products are CE marked and meet the provisions of European In Vitro Diagnostics Directive. BioMaxima also distributes products of the well-known diagnostic companies such as: Nova Biomedical, Mitsubishi Chemical. We supply almost 1800 laboratories in Poland and export to the markets of Lithuania, Latvia, Romania and France.
91-342 Łódź ul.Pojezierska 99 Poland BIOGENED is a pharmaceutical company that offers products in diet supplements, dermocosmetics and medical products.
BioCentrum Sp. z o.o. ul. Bobrzyńskiego 14 30-348 Krakow, Poland Biocentrum offers ADME and behavioral studies, protein chemistry services, custom chemical synthesis and X-ray crystallography services.
Trzy Lipy 3/1.38 Gdansk, Pomorskie, 80-172 Poland Founded in 1994, QIAGEN Gdańsk formerly BLIRT S.A. is a primary European manufacturer of recombinant enzymes for the Life Science industry. With several years of experience in recombinant enzymes production (downstream and upstream) with the use of bacterial and yeast expression systems, succeeded in over 300 projects done for numerous pharma and biotech clients, including long-term Big Pharma collaboration for non-GMP proteins for R&D use.
ul. Trzy Lipy 3 lok. 2.58 Gdańsk, 80-172 Poland Founded in 1994, QIAGEN Gdańsk formerly BLIRT S.A. is a primary European manufacturer of recombinant enzymes for the Life Science industry. With several years of experience in recombinant enzymes production (downstream and upstream) with the use of bacterial and yeast expression systems, succeeded in over 300 projects done for numerous pharma and biotech clients, including long-term Big Pharma collaboration for non-GMP proteins for R&D use.
Trzy Lipy 3/1.38 Gdańsk, 80-172 Poland Founded in 1994, QIAGEN Gdańsk formerly BLIRT S.A. is a primary European manufacturer of recombinant enzymes for the Life Science industry. With several years of experience in recombinant enzymes production (downstream and upstream) with the use of bacterial and yeast expression systems, succeeded in over 300 projects done for numerous pharma and biotech clients, including long-term Big Pharma collaboration for non-GMP proteins for R&D use.
Trzy Lipy 3/2.58 Gdańsk, 80-172 Poland Founded in 1994, QIAGEN Gdańsk formerly BLIRT S.A. is a primary European manufacturer of recombinant enzymes for the Life Science industry. With several years of experience in recombinant enzymes production (downstream and upstream) with the use of bacterial and yeast expression systems, succeeded in over 300 projects done for numerous pharma and biotech clients, including long-term Big Pharma collaboration for non-GMP proteins for R&D use.
ul. Biala 3 office 321 00-895 Warszawa - Poland Chiesi farmaceutici offers drug development, drug discovery, and clinical trials.
Oddział w Polsce, Ul. Jagiello¨½ska 103/616, 85-027 Bydgoszcz, Poland Cogtest provides the latest developments in cognitive neuroscience to the measurement of cognitive function in clinical trials.
Wadowicka 8a, Krakow, 30-415 Poland We are a consulting firm specialised in the fields of Global HEOR, Access and Pricing, informing Access and HTA strategy from early to late stage product development. For launch preparation, we provide integrated team of experts who develop all necessary core deliverables and strategic guidance for successful HTA submissions.
ul. Trzy Lipy 3/1.38 Gdańsk, 80-172 Poland DNA Gdańsk offers PCR products and bacterial and fungal strains genotyping services.
165 Wiertnicza Street 02-952 Warszawa Poland The Danish Technological Institute is a self-owned and not-for-profit institution. We develop, apply and disseminate research- and technologically-based knowledge for the Danish and International business sectors. As such, we participate in development projects, which are of use to society in close collaboration with leading research and educational institutions both in Denmark and abroad. On top of this, we carry out consultancy and standardisation services, which contribute to a dynamic and harmonious development of society.
Panska 73 Str./804 Warsaw, 00-834 Poland EastHORN Clinical Services is one of the leading CROs in Europe. EastHORN offers phase I-IV clinical research services specialized in rescue studies. Our services also include regulatory affairs, medical writing, pharmacovigilance, and data management.
Goliany 43, 05-620 Błędów Poland e are working in an agricultural research field. The main topic of our experiments is testing the efficacy of plant protection products. We also conduct field experiments with growth regulators, herbicides, pheromones, adjuvants and during fruit storage. Our trials are performed in accordance with the principles of GEP (Good Experimental Practice) and EPPO standards. We have the authorization of Plant Health and Seed Inspection Main Inspector. Fertico – a trustworthy partner in your research Fertico is a company founded by a Polish family Barański. The company employs professional research team composed of people with a university degree and years of experience in agricultural experiments. We have our own laboratory facility and own orchards and plantations . We have a database of proven locations for many pests and long-term cooperation with the owners of this plantations. Each year we prepare hundreds of experiments mostly for large, international chemical companies wishing to introduce their pesticides to the Polish market. We also cooperate with smaller companies that are outsourcing only single experiments. We have a group of regular customers which proves the quality of our service.
73-110 Stargard Szczeciński ul. Usługowa 1 POLAND Grizzly Medical offers assembling, manufacturing, polishing and filling services to medical devices.
Sp. z o.o. PL2040002710 Ulica Ogarna 15/19B m2 80-826 GDANSK Poland Gentaur is a supplier of life science research products.
72A Perkuna Warszawa, mazowieckie Poland Global Clinical Trials (GCT) is a premium contract-research organization (CRO) that offers support for the preclinical and clinical Phase I-IV studies as well as post-marketing activities to Big Pharma, small and medium-sized biotech enterprises, groups and funds, as well as large universities and institutions. Headquartered in Princeton, USA, GCT offers both local and global support through 8 operational offices in Bulgaria, Czech Republic, Hungary, India, Poland, Romania, Russia, Slovakia, Ukraine, USA and is a founding partner of a network of reliable partners in Scandinavia and Western Europe. GCT team, that now consists of over 60 full-time clinical research professionals, gives individualized attention to each project and provides full range of services following the latest GCP, EMA and FDA guidelines at every stage of product development path. Our monitors and project managers are all certified clinicians experienced in GCP clinical research. Our experience, competent staff, close contact with the KOLs and the immense enrollment potential of the covered region ensure fast study start-up and compliance with the set timelines. GCT owns 400 sq.m. (4300 ft2) Drug Storage and Distribution facility in St. Petersburg, Russia. It is fully equipped, disaster protected and has multiple modes of storage (ambient, climate controlled, refrigerated, frozen). High standards of work and client satisfaction are proved by the fact that more than 80% of the business come from repeat clients. Meanwhile, in the recent years the number of our clients worldwide increased dramatically. We actively participate in specialized industry events worldwide. Since 2006 GCT is also an Exclusive Partner of “The White Nights” International ophthalmology congress in Russia.
ul. Żmigrodzka 242E Wrocław, 51-131 Poland Hasco-Lek specializes in manufacturing of soft gelatin capsules and offers formulation development services in support of clinical manufacturing.
J.S. Hamilton Poland S.A. Chwaszczyńska 180 81-571 Gdynia The rapid industrialization, globalization and development of the world trade require professional evaluation and analysis of products and processes aimed at confirmation of their quality but also more frequently than ever at consumers safety and our environment. J.S. Hamilton Poland S.A. is an unique example of professional organization which combines a wide spectrum of chemical and physical analysis carried out by own laboratories with independent inspection services.
Obozowa 82 A (pavilion 1) 01-434 Warszawa POLAND Specialized Testing Laboratory ITA-TEST is a private, independent and non-sponsored company operating since April 1996. We are one of the largest and most dynamic companies in Poland providing wide range of testing and research services of: cosmetics, cleaning products, water, hygienic and sanitary products (like diapers, toilet paper, etc.) We are testing standard products, custom designed products, household and industrial products. Our staff consists of professional experts who are fully engaged in every step of the testing process making sure that all your requirements are satisfied. All work is performed according to Good Laboratory Practice (GLP) and as a proof of our attention to quality we were awarded with ISO 9001:2008 Certificate in June 2006. The scope of the certification includes: testing of influence of cosmetics and household products on people's safety and health, testing of properties and conforming to standards, microbiological testing, development of technical specifications, water testing. We offer high quality and timely services performed by over 20 very well qualified experts in areas of chemistry, microbiology, pharmacy, and dermatology. We have over 800 volunteers participating in testing of your products.
Konarskiego 42/13 Krakow, Malopolska, 30-046 Poland Intelliseq is a genome informatics company. Venture established in 2014 by a group of scientists fascinated with pharmacogenomics and transcriptomics. We are focused on the development of novel algorithms and bioinformatic tools devoted to the interpretation of the human DNA sequence. We aim to support whole-genome scientific research and the genetic diagnosis process. At Intelliseq, we understand that in-depth data analysis and interpretation lies at the very heart of successful genome-wide research. The company consists of an interdisciplinary team of experts in the field of genomics, molecular biology, bioinformatics, mathematics, neuropsychology, and software development.
ul. Bobrzyńskiego 14 Krakow, malopolskie, 30-348 Poland IntoDNA is a research service provider aiming at delivering its proprietary technology STRIDE for super-sensitive DNA damage detection combined with a set of comprehensive solutions and expertise to support customers and projects within areas of life science research, drug discovery and development, diagnostics, genome editing, as well as genome safety and toxicity testing.
Regus Park Avenue 2nd floor Ul. Wspolna 70 00-687 Warsaw , . Poland JSS Medical Research offers clinical trials, study design and development, data analysis, and quality assurance services.
6 Postepu, str. KCR offers clinical trials, quality assurance, clinical monitoring, site management, patient recruitment services.
LabConnect Europe|Synevo – Al. Rzeczpospolitej 33, 80-463 Gdansk, Poland LabConnect is the preeminent provider of central laboratory support services for logistically and analytically complex studies. LabConnect offers clinical chemistry, DNA sequencing, nucleic acid extraction, genotyping, flow cytometry, pharmacology, and toxicology services.
Al. Krakowska, 110/114, 02-256 Lambda Therapeutic Research provides bioanalytical services, clinical trials, pharmacovigilance, bioequivalence studies, and quality assurance.
CNBCh UW Żwirki i Wigury 101 Warsaw , Mazowieckie , 02-089 Poland Leaderna Biostructures provides services and consultancy in the field of structural biology including unique expertise in the crystallization of nucleic acid-based drugs with proteins. With the significant experience gained from 2015 as IIMCB Structural Biology Center, we are able to support both the biotech and pharmaceutical industry in structure-based drug design (SBDD) projects with the emphasis on early and preclinical stages of drug discovery process. We offer a flexible approach to the most advanced research which comprises: 1. Preparation of expression constructs and recombinant protein production; 2. X-ray crystallography of protein or protein-ligand complexes including unique expertise in the crystallization of protein-nucleic acid complexes; 3. Single-particle cryo-electron microscopy (cryo-EM) with the goal of determining high-resolution structures of macromolecules with a molecular weight ≥100 kDa; 4. Coupled cryo-EM/X-ray crystallography approach which links and integrates the favorable features of both methods; EXPERIENCE Over the years of supporting lead optimization process in drug discovery, we have established a well-grounded position in the field of structure-based drug design (SBDD) projects. In the course of cooperation with leading pharmaceutical companies from Europe (e.g., UbiQ Bio, Selvita, Celon Pharma, OncoArendi Therapeutics, Adamed) and from the United States (Ionis Pharmaceuticals), our services have supported drug discovery efforts for such diseases as cancer, asthma, and depression. Leaderna solved a crystal structure of OncoArendi’s OATD-01 compound with a target protein. The compound is undergoing clinical trials and in the case of positive results will be the first sarcoidosis drug on the market, with a new mechanism of action (first in class). Over time of extensive collaboration with Ionis Pharmaceuticals, a world leader in RNA-targeted therapeutics, we expanded our expertise in structure determination of nucleic acid-based drugs with proteins. EMINENT SPECIALISTS The success of Leaderna derives from the highest level of expertise in science, advanced skills, and the excellent quality of services that are rendered. With the support of renowned experts with distinguished scientific experience in the field of structural biology, we are able to run challenging projects. We closely collaborate with Polish science centers, among others, the Biological and Chemical Research Centre, University of Warsaw.
CNBCh UW Żwirki i Wigury 101 Warsaw, Mazowieckie, 02-089 Poland Leaderna Biostructures provides services and consultancy in the field of structural biology including unique expertise in the crystallization of nucleic acid-based drugs with proteins. With the significant experience gained from 2015 as IIMCB Structural Biology Center, we are able to support both the biotech and pharmaceutical industry in structure-based drug design (SBDD) projects with the emphasis on early and preclinical stages of drug discovery process. We offer a flexible approach to the most advanced research which comprises: 1. Preparation of expression constructs and recombinant protein production; 2. X-ray crystallography of protein or protein-ligand complexes including unique expertise in the crystallization of protein-nucleic acid complexes; 3. Single-particle cryo-electron microscopy (cryo-EM) with the goal of determining high-resolution structures of macromolecules with a molecular weight ≥100 kDa; 4. Coupled cryo-EM/X-ray crystallography approach which links and integrates the favorable features of both methods; EXPERIENCE Over the years of supporting lead optimization process in drug discovery, we have established a well-grounded position in the field of structure-based drug design (SBDD) projects. In the course of cooperation with leading pharmaceutical companies from Europe (e.g., UbiQ Bio, Selvita, Celon Pharma, OncoArendi Therapeutics, Adamed) and from the United States (Ionis Pharmaceuticals), our services have supported drug discovery efforts for such diseases as cancer, asthma, and depression. Leaderna solved a crystal structure of OncoArendi’s OATD-01 compound with a target protein. The compound is undergoing clinical trials and in the case of positive results will be the first sarcoidosis drug on the market, with a new mechanism of action (first in class). Over time of extensive collaboration with Ionis Pharmaceuticals, a world leader in RNA-targeted therapeutics, we expanded our expertise in structure determination of nucleic acid-based drugs with proteins. EMINENT SPECIALISTS The success of Leaderna derives from the highest level of expertise in science, advanced skills, and the excellent quality of services that are rendered. With the support of renowned experts with distinguished scientific experience in the field of structural biology, we are able to run challenging projects. We closely collaborate with Polish science centers, among others, the Biological and Chemical Research Centre, University of Warsaw.
26-600 Radom, ul. Kozienicka 97, Poland Medicofarma manufactures pharmaceuticals and nutraceuticals.
00-854 Warszawa, al. Jana Pawła II 23, Poland Medicofarma manufactures pharmaceuticals and nutraceuticals.
ul. Władysława Łokietka 10 98-200 SIERADZ Poland Medana offers production of pediatric drugs and vitamin preparation in modern forms.
Józefów 9 Street 99-300 Kutno, Poland Mabion provides antibody humanization, cell line development, and GMP stability studies.
Kutno, Łódź Voivodeship Poland Mabion provides antibody humanization, cell line development, and GMP stability studies.
Gedymina 28a Medical Network offers clinical research services including study monitoring, data management, study design, and regulatory submission.
Warsaw, Masovian Voivodeship Poland Medical Network offers clinical research services including study monitoring, data management, study design, and regulatory submission.
26 Iłżecka St., 02-135 Warsaw, Poland Medpace is a Full Service CRO and offers clinical development services including medical writings, biometrics, regulatory affairs and quality assurance. Also offered are Central Lab Services, Bio-Analytical, Biomarker Testing, BioSample Storage and Management, Long-term BioRepository, Core Lab Services, Imaging, and a Phase I Safety Clinic.
Ul.Pl. Pilsudskiego 3 00-078 Warsaw Nexus Oncology offers data management, site selection, project management, and drug development consultancy to support studies in oncology.
ul. Andrzeja Sołtana 7 Otwock 05-400 POLAND National Centre for Nuclear Research (NCBJ) Radioisotope Centre POLATOM is manufacturer and distributor of the isotopic goods applied in medicine, research and development, industry and environment protection. Activity of the company contributes to development of nuclear medicine, biochemistry, radiochemistry, molecular biology and other fields, which apply radioactive isotopes.
7 Andrzeja Sołtana Otwock-Świerk, mazowieckie, 05-402 Poland National Centre for Nuclear Research (NCBJ) Radioisotope Centre POLATOM is manufacturer and distributor of the isotopic goods applied in medicine, research and development, industry and environment protection. Activity of the company contributes to development of nuclear medicine, biochemistry, radiochemistry, molecular biology and other fields, which apply radioactive isotopes.
Plot No. 5B, Natural Remedies offers in vitro bioassay (enzyme and cell based) services along with in vitro ADME studies. Natural Remedies also provides analytical chemistry services and offers phytochemicals for purchase.
ul. Belwederska 26/30, 00-585 Warsaw, Poland Neox is a privately owned full services clinical CRO. We conduct clinical trials for pharmaceutical, biotechnology, medical device companies and other CRO’s throughout Europe and US. Our services are offered for Phase I-IV. DRUG DEVELOPMENT - Medical Writing, Site Contracting, Clinical Monitoring, Project Management, Pharmacovigilance, Regulatory, Medical Monitoring, Outsourcing. MEDICAL DEVICE DEVELOPMENT PHARMACOVIGILANCE & REGULATORY REAL WORLD EVIDENCE/DATA & HEOR DATA MANAGEMENT & BIOSTATISTICS We offer our services as a full service package or individually as a tailored solution to your specific needs. Years of clinical research experience, understanding local requirements and established relationships with investigators ensures that your trial will always be performed in an optimal setting for high patient enrollment. Neox offices: Czechia, California, Germany, Poland, Hungary, Bulgaria, Slovakia, Romania. Neox also operates in: Austria, Belgium, Estonia, France, Finland, Italy, Latvia, Lithuania, Netherlands, Spain, United Kingdom and others.
2 Gronostajowa Kraków, malopolskie, 30-387 Poland Recepton is a newly founded biotech company focused on cancer immunotherapy, the process of mobilizing the immune system to kill cancer. In particular, we have designed: 1) a novel service platform composed of a broad portfolio of the recombinant immune checkpoint receptors. The platform can be used for rapid typing and testing of new types of inhibitors for the immunoreceptors, especially for the signaling axis of the programmed cell death protein 1 (PD-1) and its ligand, protein PD-L1. 2) chemistry synthetic and biophysical methods of rational drug design, cell-based assays, preclinical studies that aim at developing new types of small-molecule inhibitors for the immune checkpoint blockade (ICB).
3 Trzy Lipy Gdańsk , pomorskie, 80-172 Poland Recepton is a newly founded biotech company focused on cancer immunotherapy, the process of mobilizing the immune system to kill cancer. In particular, we have designed: 1) a novel service platform composed of a broad portfolio of the recombinant immune checkpoint receptors. The platform can be used for rapid typing and testing of new types of inhibitors for the immunoreceptors, especially for the signaling axis of the programmed cell death protein 1 (PD-1) and its ligand, protein PD-L1. 2) chemistry synthetic and biophysical methods of rational drug design, cell-based assays, preclinical studies that aim at developing new types of small-molecule inhibitors for the immune checkpoint blockade (ICB).
3 Trzy Lipy Gdańsk, pomorskie, 80-172 Poland Recepton is a newly founded biotech company focused on cancer immunotherapy, the process of mobilizing the immune system to kill cancer. In particular, we have designed: 1) a novel service platform composed of a broad portfolio of the recombinant immune checkpoint receptors. The platform can be used for rapid typing and testing of new types of inhibitors for the immunoreceptors, especially for the signaling axis of the programmed cell death protein 1 (PD-1) and its ligand, protein PD-L1. 2) chemistry synthetic and biophysical methods of rational drug design, cell-based assays, preclinical studies that aim at developing new types of small-molecule inhibitors for the immune checkpoint blockade (ICB).
Tenisowa 10/5 Phage Consultants provides phage manufacturing, phage purification, and consulting services.
Gdańsk, Pomeranian Voivodeship Poland Phage Consultants provides phage manufacturing, phage purification, and consulting services.
Poland Phytopharm Klęka offers natural extract and raw materials synthesis and analysis services.
Pelplińska 19 Polpharma manufactures generic drugs and pharmaceutical substances with specializations in preparations used in cardiology, gastroenterology and neurology.
Legnicka 48E building E Wrocław, 54-202 Poland Pure Biologics S.A. is one of the largest Polish biotech companies. Founded in 2010, it is listed on the the Warsaw Stock Exchange. Pure Biologics’ major R&D activity focuses on bispecific and fusion antibodies, as well as aptamers to be developed into viable candidates for therapeutics in immunooncology (colorectal, breast and lung cancers), and in the treatment of neurological diseases (neuromyelitis optica, myasthenia gravis). To this end, Pure Biologics has created highly efficient platforms to generate specific antibodies and modified aptamers, supported by state-of-the-art assays and analytics. Moreover, Pure Biologics utilizes its broad experience in antibodies, aptamers, cell and molecular biology, protein and antigen expression and purification, custom assay development, and ligand-binding analysis, offering complex solutions not only as a contract research organization and service provider, but primarily as a professional consultant and R&D partner. Its customers represent a wide variety of environments, ranging from academic collaborations to pharma and biotech companies. Pure Biologics aims to out-license its early-stage assets and to connect with partners in need of complex biotechnological solutions.
Duńska 11 Wrocław, 54-427 Poland Pure Biologics S.A. is one of the largest Polish biotech companies. Founded in 2010, it is listed on the the Warsaw Stock Exchange. Pure Biologics’ major R&D activity focuses on bispecific and fusion antibodies, as well as aptamers to be developed into viable candidates for therapeutics in immunooncology (colorectal, breast and lung cancers), and in the treatment of neurological diseases (neuromyelitis optica, myasthenia gravis). To this end, Pure Biologics has created highly efficient platforms to generate specific antibodies and modified aptamers, supported by state-of-the-art assays and analytics. Moreover, Pure Biologics utilizes its broad experience in antibodies, aptamers, cell and molecular biology, protein and antigen expression and purification, custom assay development, and ligand-binding analysis, offering complex solutions not only as a contract research organization and service provider, but primarily as a professional consultant and R&D partner. Its customers represent a wide variety of environments, ranging from academic collaborations to pharma and biotech companies. Pure Biologics aims to out-license its early-stage assets and to connect with partners in need of complex biotechnological solutions.
Marconich str. 6 Parexel provides bioequivalence studies as well as clinical services across Phase I to Phase III of drug development including dose escalation, drug-drug interaction, biomarkers, first-in-man studies, ADME, imaging, and clinical research services.
49 Domaniewska Street Trinity Park III; 1st floor 02-672 Warsaw Poland PPD offers central lab services, bioanalytical services, biomarker discovery services, assay development services, cGMP analytical services, CMC consulting, and pharmacology services.
ul. Piłsudkiego 74/51A Wroclaw, 50-020 Poland Physiolution GmbH is a highly innovative company focused on investigations of the dissolution behaviour of solid oral dosage forms under bio-relevant test conditions. The analytical services of Physiolution GmbH include pharmacopoeial dissolution testing and other pharmacopoeial investigations of solid oral dosage forms and their mechanical stability as well as development and validation of analytical methods and procedures, biochemical characterisation of biomolecules and formulation of peptide drugs. Please see our publications: https://scholar.google.com/citations?user=LzX9m0QAAAAJ&hl=pl&oi=ao
prof. Michala Bobrzynskiego 14 Krakow, Malopolskie, 30-348 Poland Providers of LifeGel products, unique ready to use customizable hydrogels for 3-D culturing of diverse cell types, and personalised services to help define optimal LifeGel physical parameters for desired cell growth characteristics. Free from endogenous growth factors, LifeGels are highly repeatable media in which to create conditions tailored to specific cell morphologies often not achievable with other hydrogels. Our experience has shown that small differences in the design of our hydrogels can make a big difference in the ability to grow cells with the desired morphologies. This is achieved by varying the elasticity and porosity of our LifeGel. We primarily offer a hydrogel optimisation service for researchers with specific cell growth needs but can also supply LifeGel containing plates directly in 48, 96 and 384-well formats.
Wolnosc 7 lok. 15, 01-018, Warszawa Randox Laboratories, through its business units, offer diagnostic services, life science reagents, biomarker testing services, antibody/drug assay development, and toxicology services.
22/1 Sulkowskiego St, 01-602 Warsaw, Poland Smerud Medical Research offers clinical research services including trial management, project funding, data management, regulatory affairs, and quality assurance.
Kraków, Lesser Poland Voivodeship Poland Selvita offers integrated drug discovery and development services spanning from medicinal chemistry, process chemistry, assay development and screening, recombinant protein production, structural studies, ADME/DMPK, in vitro and in vivo pharmacology, translational research and toxicology. Our major expertise is in oncology, inflammation, fibrosis, respiratory diseases, infectives and neuroscience. In the field of drug development we offer analytical support for small molecules and biologics, including methods development and validation, QC, stability studies, GMP batch release, impurities identification and testing, cell-based assays and biophysical methods. The services are performed according to GMP or GLP certification.
ul. Bobrzynskiego 14 30-348 Kraków Poland Selvita offers integrated drug discovery and development services spanning from medicinal chemistry, process chemistry, assay development and screening, recombinant protein production, structural studies, ADME/DMPK, in vitro and in vivo pharmacology, translational research and toxicology. Our major expertise is in oncology, inflammation, fibrosis, respiratory diseases, infectives and neuroscience. In the field of drug development we offer analytical support for small molecules and biologics, including methods development and validation, QC, stability studies, GMP batch release, impurities identification and testing, cell-based assays and biophysical methods. The services are performed according to GMP or GLP certification.
Podole 79 Krakow, 30-394 Poland Selvita offers integrated drug discovery and development services spanning from medicinal chemistry, process chemistry, assay development and screening, recombinant protein production, structural studies, ADME/DMPK, in vitro and in vivo pharmacology, translational research and toxicology. Our major expertise is in oncology, inflammation, fibrosis, respiratory diseases, infectives and neuroscience. In the field of drug development we offer analytical support for small molecules and biologics, including methods development and validation, QC, stability studies, GMP batch release, impurities identification and testing, cell-based assays and biophysical methods. The services are performed according to GMP or GLP certification.
ul. Malczewskiego 47 02-622 Warsaw Poland TopPharm Consulting provides consulting for regulatory affairs, scientific dossier preparation and liasion with authorities.
Warsaw, Masovian Voivodeship Poland TopPharm Consulting provides consulting for regulatory affairs, scientific dossier preparation and liasion with authorities.
Nieklanska 35 03-924 Warsaw, Poland Theorem Clinical Research offers full service clinical research solutions including DM, biostatistics, programming including CDISC, PM, clinical operations, and regulatory affairs.
University of Warmia & Mazury in Olsztyn ul. Michała Oczapowskiego 13 Olsztyn, Warmia & Mazury , 10-957 Poland Transpharmation provides translational pre-clinical and clinical pharmacology services to the pharmaceutical and biotechnology industry, with the aim of better understanding potential new medicines as they transition into the clinic.
Krzemowa 1, Złotniki 62-002 Suchy Las Poland VitaInSilica offers a wide range of bioinformatics services including genomic analysis, proteomics, drug design, and systems biology.
Suchy Las, Greater Poland Voivodeship Poland VitaInSilica offers a wide range of bioinformatics services including genomic analysis, proteomics, drug design, and systems biology.
32 Prosta ul. Prosta 32 Warszawa, mazowieckie, 00-838 Poland Worldwide Clinical Trials employs more than 1,600 professionals around the world, with offices in North and South America, Eastern and Western Europe, Russia and Asia. Founded by physicians committed to advancing medical science, Worldwide is out to change how the world experiences CROs – in the best possible way. From Early Phase and Bioanalytical Sciences through Late Phase and post approval, we provide world-class, full-service drug development services. With infrastructure and talent spanning 60 countries, we execute predictable, successful studies with operational excellence. We never compromise on science or safety. We’re never satisfied with the status quo. We’re the Cure for the Common CRO.
Al. Jana Pawla II 15 Ilmet building 00-828 Warsaw Poland Accovion provides regulatory consulting, medical services, clinical management, and pharmacovigilance.
Aleksandrowska 24/13 Lodz 91-120 Poland Analytics offers polymorph screening, NMR, and API services.
Pienków 149 05-152 Czosnów near Warsaw, Poland Adamed provides prescription drugs, OTCs and dermocosmetics.

Site Information
The Contract Research Map is owned and maintained by Scientist.com. It was created to help researchers in the life sciences identify and connect with contract research organizations (CROs) based on geography. Updated nightly, this map features all of the available CROs within our network, so you can order services with a few clicks. Click on a specific country, scroll on the map itself or type into the search bar at the top—there are many ways to find the location and suppliers that you’re looking for. From Argentina to New Zealand, use this map to connect with a CRO near you.
We believe that every researcher across the world should be able to connect with the thousands of global CROs that exist and have the opportunity to work together. Like many industries,the life science supply chain has been disrupted over the last year. But there are many other circumstances such as international customs regulations or sensitive shipping times that create limitations around which countries are feasible to partner with. Sometimes, finding a CRO based in a country that best suits your research needs is imperative. We hope this contract research map allows you to find the right partner in the right place at the right time.
Have questions or feedback? We’d love to help. You can find our FAQs and contact information on the Learn more page.
Interested in connecting with one or more of the contract research organizations listed on this map? By clicking on the company’s name, you will be directed to their supplier profile on the Scientist.com marketplace. Once you set up a marketplace account you can start the ordering process immediately.
What is Scientist.com?
Scientist.com is the world's largest enterprise marketplace for outsourced R&D services. It saves time and money and provides access to innovation while maintaining compliance with an organization’s procurement policies.
How can I use Scientist.com?
Scientist.com has built private, enterprise marketplaces from 24 of the 30 largest pharmaceutical companies, 80+ biotech companies, the US National Institutes of Health (NIH) and numerous other pharma and biotech companies. If you are employed by one of these organizations, you can log in to get started today. If you are unsure about how to get started, you can email our team at [email protected] or go to our website www.scientist.com to speak to someone via our live chat.
Why should I place my order through Scientist.com?
Scientist.com is a highly efficient enterprise-wide outsourcing marketplace that makes it possible for research organizations to save time and money, access innovation and ensure compliance. It utilizes a universal legal agreement and AI technologies to enable research like never before. See how comparing proposals and getting 1-on-1 support from our Research Concierge® team will enable you to place more research today.
How do I get my organization listed on the map?
If your CRO isn’t showing up on the map, then please be sure your company profile is up to date in Scientist.com’s Backoffice. After logging in, click the Your Company button in the navigation at the top, and then select the Locations tab.
Information about my organization is incorrect, how do I fix it?
Head over to backoffice.scientist.com to update your supplier profile and information. It may take up to two business days for the updates to be reflected on the map.

CLINICAL RESEARCH OPERATIONS SERVICES
Sites and Investigators Network was developed to have a unique approach for feasibility and site selection process both on the country and site level. We know that every Sponsor demands accelerated timelines, measurable results, and reliable data.
CLINICAL DEVELOPMENT SOLUTIONS
Project management.
- Project set up and Delivery from Feasibility to Close out visit
- Risk Management
- Vendor Management
- Project coordination
CLINICAL MONITORING
Patients’ recruitment.
- Study sites coordination
- Site recruitment
- Cooperation with recruitment companies
- Retention Plans
Our experience and cooperation with physicians, clinics and societies makes us a unique full service CRO.

THERAPEUTIC AREAS
Our fields of work:.
- Endocrinology
- Dermatology
- Orthopaedics
- Rheumatology
- Healthy patients
- Women’s health
- Infection disease
OUR PARTNERS

- Company's profile
Polish Contract Research Organization
Monitoring of phase i - iv clinical trials, trainings: ich-gcp, brillance academy.

Brillance Sp. z o.o. is an entirely independent and private Polish CRO (Contract Research Organization) – a company providing clinical trials management and monitoring services for pharmaceutical industry.
Our activities are focused on research and development services for medical, pharmaceutical and biotechnology industries. Comprehensive range of professional services offered by Brillance includes monitoring and management of phase I – IV clinical trials and concerns both local and big international complex projects in many therapeutic areas. We provide preparation and extensive training for Polish sites (hospitals, outpatient units) in order to encourage and train them to conduct local and international clinical trial projects. For our clients, we have a wide range offer of additional services, such as study feasibility (site and investigators selection), staff outsourcing for CROs and medical/pharmaceutical companies, and data management services. We are proud to implement GCP principles in medical and pharmaceutical environment. Our clients are famous Polish and international leading medical, pharmaceutical and biotechnology companies.
Brillance was founded and started its activities in year 2004, covering Poland, Czech Republic and Slovak Republic.
Research and development activity is one of the main factors which decides about company's success and development on the medical, pharmaceutical and biotechnology market. Clinical trials are conducted in order to assess efficiency and safety of pharmacological products and to register and introduce them to the pharmaceutical market. Progressive, safe and effective medical products are registered and brought into the market thanks to the clinical research process and allow to extend life of patients and improve its quality.
Brillance sponsoring 2018 BIO CEO & Investor Conference

Brillance attends Partnerships in Clinical Trials Europe 2017

Brillance awarded in the Forbes Diamonds 2017 ranking

Clinical Trials
Monitoring of clinical trials.

Country: Poland
Staszów, poland • velocity clinical research.
11 listopada 78, Staszów, Poland 28-200
Zamość, Poland • Velocity Clinical Research
Peowiaków 1, Zamość, Poland 22-400
Lublin, Poland • Velocity Clinical Research
Tetmajera 21, Lublin, Poland 20-362
Puławy, Poland • Velocity Clinical Research
Sieroszewskiego 34, Puławy, Poland 24-100
Biała Podlaska, Poland • Velocity Clinical Research
Terebelska 57-65, Biała Podlaska, Poland 21-500
Skierniewice, Poland • Velocity Clinical Research
Ogrodowa 21/23 1 piętro 96-100, Skierniewice, Poland

Velocity Clinical Research Expands to Poland
Velocity Clinical Research, the leading multi-specialty clinical sites business, announced it has expanded into Poland, acquiring its first site, ClinMedica Research . Located in Skierniewice, which is halfway between Warsaw and Lodz, the site marks the first in a series of key acquisitions targeted by the company in Poland by year’s end.
In addition to being one of Poland’s top recruiting sites in cardiology trials, ClinMedica also recruits patients across a broad range of therapeutic areas involving ambulatory medicine. Led by Grzegorz Kania, MD , the site has an excellent reputation for quality recruitment and exceptional patient care. The site is recognized as one of the best research facilities in the country by Sponsors and contract research organizations (CROs).
Dominic Clavell, European General Manager for Velocity, commented, “Velocity began its European expansion in July 2022 and is showing no signs of slowing down. Poland is a strategic market for us since it is recognized by the biopharmaceutical companies and CROs as a top recruiting country, supported by a government that understands the benefits commercial clinical trials bring to communities.
“The acquisition of ClinMedica is the first step to building a network of high quality dedicated research sites in Poland. It also increases our regional footprint and is another point in our journey to reach critical mass in the U.K. and Europe, meaning Velocity will be able to conduct entire studies on our own with geographical diversity.”
Agnieszka Gackowska, MD , who was previously Global Head Site Solutions at Parexel and is a qualified physician, has joined Velocity as Vice President and Country Head, Poland, to lead the company’s expansion.
Dr. Gackowska said, “Velocity’s expansion into Poland allows us to think globally but act locally when it comes to clinical research. Not enough people in smaller cities have the opportunity or access to clinical research, which could be their only lifeline to innovative treatments.
“Importantly, the presence of a global company that puts patient care at the heart of everything it does, means there are now more career opportunities for local doctors so we do not lose Polish talent to larger markets.”
Velocity operates an integrated site network of more than 80 locations across four countries, including the U.S., U.K., Germany, and Poland, and has a tech hub in Hyderabad, India. The Polish site joins five locations in the U.K. and four in Germany.
Velocity has access to more than 220 principal investigators across a range of therapeutic areas and a database of more than one million patients. Its sites are fully integrated via a centralized infrastructure and common technology backbone, allowing for superior patient enrollment and consistent, high-quality data delivery. As a result, Sponsors and CROs can benefit from simplified access to international clinical research capabilities.
About Velocity Clinical Research
Velocity is the leading integrated site organization for clinical trials. With 80 sites and more than 220 investigators, Velocity partners with pharmaceutical and biotechnology companies to research new drugs, medical devices, diagnostics, and combination products that could improve human health and wellbeing. Velocity offers unified research site solutions to efficiently provide the right patients, investigators, and research staff for clinical trials across the U.S. and Europe.
The company also operates a technology hub in India, where it is unlocking a new era in clinical research by developing innovative systems to leverage expansive site, patient, and historical performance data. To learn more about how Velocity delivers high-quality data, exemplary patient care, and unprecedented efficiency for clinical trials at any scale, visit VelocityClinical.com .
About ClinMedica Research
ClinMedica Research provides clinical research and specialized medical care. ClinMedica began conducting clinical research in 2000 and is fueled by the passion and willingness to search for innovative therapeutic solutions and the latest treatments for patients. It has completed over 350 studies and currently treats more than 18,000 patients as part of its primary health care services and approximately 25,000 thousand patients as outpatient specialist care. Conducting clinical research across a range of therapeutic areas, it is focused on expanding its research capabilities in ophthalmology and oncology. For more information, visit clinmedicaresearch.com .
About Agnieszka Gackowska, MD
Agnieszka Gackowska, MD is a senior leader and physician with nearly three decades experience working in the pharmaceuticals industry, including global CRO and pharmaceutical companies in both regional and global positions. Dr. Gackowska has led international teams dedicated to clinical trials management, product launch, market access, marketing, and sales. She currently consults on research sites models and is a postgraduate lecturer for studies dedicated to clinical trials at several medical universities in Poland.
About Grzegorz Kania, MD
Grzegorz Kania, MD specializes in cardiology as well as internal and family medicine. In 2000, he founded Centrum Medyczne Ogrodowa, which is the largest outpatient clinic in Skierniewice, Poland, before he founded ClinMedica Research in 2016 as a dedicated research center. He has been a co-investigator and principal investigator in approximately 300 clinical trials in the field of cardiology and internal diseases.
Published in several medical journals, his work has contributed to some of the most recent academic thinking on cardiovascular treatments. He holds a medical degree from the Medical University of Lodz and did postgraduate studies in clinical research management at the Medical University of Lublin. His wife and two daughters are also in the medical field.

Recent posts
- VISIOIN Reaches $2 Million in Patient Stipends Paid Globally August 22, 2024
- Kris Kowdley, MD, Supported Research Leading to Accelerated FDA Approval of Gilead’s Livdelzi® for Primary Biliary Cholangitis August 15, 2024
- An interview with Dr. Robert Cupelo on Alzheimer’s Treatment and the Legacy of Lecanemab August 8, 2024
- Velocity appoints ICON exec Rachael Buck Ph.D. as Head of U.K. August 7, 2024
- Velocity Sites and Investigators Supported Research for Nine Products That Have Received FDA Approval in 2024 July 30, 2024
Quality. Continuity. Velocity.

ABOUT CLINMARK
We turn concepts, ideas and plans into final deliverables..

FAST&FLEXIBLE
Unique business model and structure allows us to be the most flexible CRO worldwide.
Thoughtful organization and internal processes plus profound research knowledge makes us fast in decision making and action.

Any project, any country worldwide.
Anywhere you do your project we can effectively assist in your needs
We understand regional specificity and conditions of clinical research in America, Asia, Europe, Africa.

Science and ethics is in our blood.
We are widely recognized as a quality driven and “brain” CRO.
Having best experts in class on board covering all areas allows us to find best solutions and implement them in your projects for best results.
25 000+ ENROLLED SUBJECTS
450+ RESEARCH CONSULTING PROJECTS WORLDWIDE
400+ CLINICAL TRIALS EXECUTED WORLDWIDE
75+ COUNTRIES WORLDWIDE
YEARS ON GLOBAL CLINICAL RESEARCH MARKET
Our clients, our services, clinical research, audits & quality, medical devices, get in touch.

- Privacy Overview
- Strictly Necessary Cookies
This website uses cookies to improve your experience while you navigate through the website. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. We also use third-party cookies that help us analyze and understand how you use this website. These cookies will be stored in your browser only with your consent. You also have the option to opt-out of these cookies. But opting out of some of these cookies may have an effect on your browsing experience.
Privacy Policy
Any cookies that may not be particularly necessary for the website to function and is used specifically to collect user personal data via analytics, ads, other embedded contents are termed as non-necessary cookies. It is mandatory to procure user consent prior to running these cookies on your website.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.

Contract research organization in Poland
Interested in becoming a vendor.
REACH OUT TO [email protected] FOR MORE INFORMATION.
Participate in a study
For questions or assistance please email [email protected]
PLEASE CONTACT HR SUPPORT FOR ALL CAREER RELATED INQUIRIES.
Interested in becoming an investigator?
PLEASE CLICK HERE TO SUBMIT INQUIRY.
" * " indicates required fields
Your form has been successfully submitted!
We will respond to your inquiry as soon as possible.
Warsaw location
Prosta Tower 13th floor ul. Prosta 32 00-838 Warsaw, Poland
If you wish to participate in our clinical trials, please call +1 210-635-1515 or visit here .
Visit Careers to view and apply for all open jobs here.
View all Worldwide offices on our Global Reach page.
Contract research services in Warsaw
Worldwide Clinical Trials employs more than 2,000+ professionals around the world, with offices in North and South America, Eastern and Western Europe, Russia, and Asia. Founded by physicians committed to advancing medical science, Worldwide is out to change how the world experiences CROs – in the best possible way.
From early phase and bioanalytical sciences through late phase, post-approval and real-world evidence, we provide world-class, full-service drug development services. With infrastructure and talent spanning 60 countries, we execute predictable, successful studies with operational excellence across a range of therapeutic areas, including central nervous system, cardiovascular, metabolic, general medicine, oncology and rare diseases. We never compromise on science or safety. We’re never satisfied with the status quo.
Cookies on the Worldwide Clinical Trials website
We use cookies to improve your browsing experience and help us improve our websites. We have carefully selected third parties that use cookies to achieve purposes illustrated in the cookie policy. For more information. Read more about online privacy statement . By closing this banner or continuing to browse our website, you agree to our use of such cookies.
- At a glance
- Mission and Vision
- Founders and Leadership
- Quality and reliability
- Testimonials
- Czech Republic
CRO in Poland, cultural insight, market overview, medical system, and more

GCT in Poland

Poland is located in Central Europe, has population of 37.9 million people and is the fifth-most populous member state of the European Union. Poland remains to be one of the largest clinical trial markets in CEE, having a share close to 20% in terms of the number of clinical trials held.
An EU member since 2004, Poland has its medical and pharmaceutical legislation harmonized with the EU. The typical timeline for receiving authorization from the regulatory authorities to conduct medicinal or device trials is 60 days.
Similarly to other countries within the EU, Poland requires Regulatory Agency/Competent Authority (RA) and Ethics Committee (EC) approvals before the drug or device trial can start.
Regulatory Agency/Competent Authority (RA) approval
The Clinical Trial Application (CTA) should be submitted to the RA by the clinical trial sponsor or its representative. To apply for the trial approval in the EU, sponsor who do not have a registered office within the territory of the EEA has to assign EU legal representative to act as primary contact for the Authorities on behalf of the Sponsor.
Official links
https://www.gov.pl/web/zdrowie/
Pharmaceutical Industry & Clinical Trials Market
The clinical trials market in Poland is well organized, mature, and presents a valuable and acknowledged potential. Clinical trials enrollment in Poland is typically faster than in many other countries. This is due to a large population and specialized medical centers located around main cities. There are more and more new centers that specialize only in clinical trials. The investigators and site staff are highly qualified and interested in participation, as this creates opportunities for hands-on testing of new treatments, scientific knowledge exchange, publications co-authoring, and financial benefits. The investigators are familiar with ICH-GCP, modern treatment standards and experienced in clinical trials, capable of delivering high-quality data.
Another key driving force for the domestic clinical trials’ market is the population size of 38 million. The country can facilitate an average of 40,000 patients a year taking part in clinical trials.
Medical System in Poland
Therapeutic areas.
Find out what is required for your study in Poland here [email protected]
I have read, understood and agreed to Terms of Service and Privacy Policy. I agree to be contacted by a GCT representative.
Please leave this field empty.
Investigators registration
Let’s keep in touch.
Get the latest news about GCT and the projects we support in a monthly email. View the mailing archive
By clicking subscribe, you agree to receive this newsletter. We use a third-party provider, Mailchimp, to deliver our newsletters. For information about how we handle your data, please read our Privacy Policy. You can unsubscribe at any time using the links in the emails that you receive.
- ICH GCP (De)
- ICH GCP (En)
- ICH GCP (Es)
- ICH GCP (Fr)
- ICH GCP (It)
- ICH GCP (Pt)
- ICH GCP (Ru)
- AUSTRALIA (NHMRC)
- JAPAN (PMDA)
- US Clinical Trials Registry
- EU Clinical Trials Registry
- Pharmaceutical Companies
- Clinical Research Labs
- Service Companies
- Clinical Research Events
- Publications
- Researchers
List of Contract Research Organizations in Poland starting with "s
Featured cros.

SanaClis is a full-service Global CRO with a fully integrated clinical supply chain, thereby offering a comprehensive range of end-to-end services for clinical trials. SanaClis was founded in 2000 by seasoned industry experts, all of whom have had ex...

IMS Health and Quintiles are now IQVIA, a world leader in using data, technology, advanced analytics and expertise to help customers drive healthcare - and human health - forward. Together with the companies we serve, we are enabling a more modern, m...
Global Contract Research Organizations in Poland starting with "s"
SanaClis is a full-service Global CRO with a fully integrated clinical supply chain, thereby offering a comprehensive range of end-to-end services for clinical trials. SanaClis was founded in 2000 by seasoned industry experts, all of whom have had ex... View full profile
- Bosnia & Herzegovina
- Czech Republic
- Netherlands
- United Kingdom
- United States
Life sciences services from SGS – optimize your development timelines to get medicines and medical devices to market quickly and safely. There is no other area of business that is more heavily regulated than the development, testing and distribution... View full profile
- Burkina Faso
- Congo, DR of
- Cote d'Ivoire
- Dominican Republic
- El Salvador
- Equatorial Guinea
- New Zealand
- Papua New Guinea
- Philippines
- Saint Lucia
- Saudi Arabia
- South Africa
- South Korea
- Switzerland
- Trinidad & Tobago
- Turkmenistan
The Smerud Medical Research Group (SMERUD) is a full-service clinical Contract Research Organisation operating in the Northern European area with head office in Norway and subsidiary offices in Denmark, Finland, Sweden, United Kingdom, Germany, Austr... View full profile
Syneos Health (Nasdaq:SYNH) is the only fully integrated biopharmaceutical solutions organization purpose-built to accelerate customer success. We lead with a product development mindset, strategically blending clinical development, medical affairs a... View full profile
Synexus is a company dedicated to conducting clinical studies and have been investigating the effectiveness of new medicines and treatments for more than 20 years. We provide a friendly, relaxed environment where you have the chance to help shape the... View full profile
List of CROs by location
- United Arab Emirates
- Why Cromos Pharma
- Company facts
- Countries of operation
- Core strengths
- Quality philosophy
- No Patients — No Payment
- Patients’ portal
- Patient recruitment
- Clinical research services
- Regulatory support
- EU legal representation
- Medical writing
- Biostatistics and data-management
- CTM logistics
- Independent audits
- Therapeutic Areas
- Publications
- Company videos
- Privacy policy
- Testimonials
CROMOS PHARMA IN POLAND
Poland is the largest clinical trials market in Central and Eastern Europe. Cromos Pharma has been managing clinical research in Poland since 2015 and opened a permanent office in Warsaw in February 2020.
WHY POLAND?
- Strong track record, over 20 years, of producing high quality data.
- Large population (38.15 million) in comparison with neighboring countries offers great potential for patient recruitment.
- Large proportion of treatment-naïve patients in a wide range of therapeutic areas.
- Patients are eager to participate in clinical trials as a means of accessing novel therapies.
- EU member state since 2004.
- Highly skilled, qualified, experienced and motivated investigators and site staff.
- Large network of specialized medical facilities located around major urban centers.
- Lower costs – on average 30% less than US – due in part to efficiencies in patient recruitment and comparatively lower salaries and fees.
- Poland is a country located in Central Europe with a population of 38.151 million.
- It is the sixth most populous member state of the European Union, and its largest city and capital is Warsaw.
- It is however once of the EU economic least affected by the pandemic and the World Bank predicts a return to growth in 2021.
- Poland has a well-diversified economy with a strong clinical research sector and attracts a significant number of International Sponsors to conduct trials there.
- Overall, Poland is perceived as a good place to carry out clinical research due to several advantages including a large population (38+ million) with a significant naïve patient population, in a diverse range of clinical areas.
- Its membership in the EU, advanced economy, and high standard of medical care, add to the positive perceptions about conducting trials there. It also has a strong track record, across several decades, for producing high quality data corroborated by regulatory authorities, FDA and EMA.
SKILLED CLINICAL PROFESSIONALS AND STRONG HEALTHCARE INFRASTRUCTURE
Having a well-educated clinical workforce with experience in ICH GCP-compliant clinical trials allows for efficient recruitment of skilled investigators. Most public hospitals are well disposed towards taking part in trials and have established protocols for working with International Sponsors. Poland also boasts excellent access to sophisticated diagnostic tools and laboratory evaluations often required in the conduct of global trials.
POSITIVE PATIENT ATTITUDES TOWARDS TRIALS
In general, Polish investigators and their patients are favorably inclined to participate in trials as a way of accessing novel therapies not yet available through their national health system.
GOVERNMENTAL SUPPORT FOR THE SECTOR
Polish authorities are actively seeking to grow its clinical research sector e.g., to make site contracting, regulatory approvals and other processes more streamlined, and to encourage patients to take part in global trials.
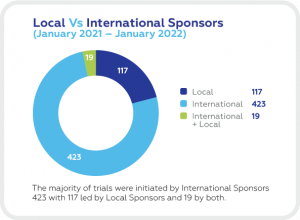
A SNAPSHOT OF CLINICAL TRIALS IN POLAND
Clinical trials in poland.
With high levels of patient recruitment, an established framework for conducting clinical trials, a large population of skilled clinical professionals and a reputation for producing high quality data, Poland remains a key location for International Sponsors. Cromos Pharma has opened its permanent office in Poland (February 2020) to further develop and expand its operations in the country.
Cromos Pharma provides tailored and effective clinical trial services to support the development of drugs that transform healthcare. It is an international CRO offering fully integrated services with expertise in delivering all aspects of clinical trials, in all clinical phases, throughout a wide range of therapeutic areas. Cromos Pharma delivers rapid recruitment and excellent patient retention, as well as expert study design and management. Cromos Pharma has strong regional experience in Central and Eastern Europe. Its international HQ is situated in Dublin, Ireland and its US base is in Portland, Oregon.
Read our PDF here .
If you would like to find out more about how Cromos Pharma can help you with your clinical trials in Poland email: [email protected]
To arrange an introductory meeting and find out how our experience can benefit your next clinical project.
OUR PUBLICATIONS

New Research Alert: Alarming Trends in Men’s Cancer Incidence & Projections to 2050

Poland: Europe’s New Hub for Clinical Research

Clinical Research Focus. 31st Edition

Revolutionary HIV Breakthrough: Is Lenacapavir the Ultimate Game-Changer?

Unleashing Biotech Success: Why Smaller CROs Are the Key to Big Wins

PMBoK, ISO 9001, and ICH GCP: Three Pillars of Quality in Clinical Research

Clinical Research Focus. 30th Edition

Major Enhancements Set to Transform Clinical Trial Practices with ICH GCP E6 R3

Digital Twins in Clinical Research: Revolutionizing Study Design

FDA’s Comprehensive New Guidelines Impacting Clinical Trials and Gene Therapy

Key Takeaways From The 2024 ASCO Annual Meeting

Clinical Research Focus. 29th Edition

Celebrating Clinical Trials Day: Highlighting the Outside-the-Box Studies that Push the Boundaries

Clinical Research Focus. 28th Edition

Ukraine Strives to Remain a Hub for Clinical Trials Despite Conflict

Anticipating ASCO: Key Trends in Oncology Clinical Research

Rezdiffra Approval Sheds Light on the Challenges of NASH Diagnosis and Fibrosis Identification

Clinical Research Focus. 27th Edition

Navigating the Challenges: The Impact of US-China Tensions on Biopharmaceutical Innovation

Exploring Clinical Trial Opportunities: Hungary’s 2024 Country Profile

World Kidney Day 2024

Clinical Research Focus. 26th Edition


Celebrating Rare Disease Day 2024

Clinical Research Focus. 25th Edition

Navigating the Future of Gene Therapy

Türkiye’s Emerging Role in Clinical Research: Regulatory Updates and Simplified Processes

J.P. Morgan’s Healthcare Conference: A Glimpse into the Optimistic Pulse of 2024

Pragmatic Clinical Trials in 2024

Clinical Research Focus. 24th Edition

Expanding Horizons: “Syngene – Cromos Pharma” Alliance Transforms Clinical Research

Happy Bastille Day from Cromos Pharma!

Happy Independence Day 2024

Successful Partnership Between MAIA Biotechnology and Cromos Pharma

Cromos Pharma’s Agnieszka Milewska-Kranc Leads the Way in EU’s Rare Disease Advocacy

We are at the BIO International Convention 2024

Cromos Pharma’s Country Head Speaks at GCP Training Event in Istanbul

Cromos Pharma’s Strategic Meeting in Miami

Happy Passover 2024

Cromos Pharma’s Strategic Meeting in Warsaw

Happy Easter 2024

Revolutionizing Diabetes Management: FDA Approves First Direct-to-Consumer Glucose Monitor

Saint Patrick’s Day 2024

Cromos Pharma’s new Country Head for Poland

Happy Valentine’s Day!

Cromos Pharma’s Team at SCOPE 2024

Happy Lunar New Year of the Dragon!

Cromos Pharma’s new Country Head for Türkiye

Cromos Pharma’s umbrellas are a hit

Happy Hanukkah 2023

Happy Thanksgiving Day 2023

New Weight Loss Drug Zepbound Receives FDA Approval Amid Calls for Comprehensive Coverage of Obesity Treatments

Happy Halloween 2023

FDA Initiates Formation of a New Advisory Committee for Digital Health Technologies

Cromos Pharma’s Free Multicountry Feasibility Report

Cancer research challenges in Ukraine

Happy Labor Day 2023

Cromos Pharma commences a pivotal multicountry clinical trial of OT-101 in patients with advanced pancreatic cancer

Empowering Patient Recruitment with Cromos Pharma: The Invaluable Role of Explainer Videos
Hemochromatosis Screening And Awareness Month

- Mission & Values
- Corporate & Governance
- Certifications
Ophthalmology
Rare Diseases
Cardiovascular Diseases
Respiratory Diseases
Advanced Therapy Medicinal Products
Infectious Diseases
Medical Devices
- Nutraceutis and FSMPs
- View others
- Study Management
- Medical Affairs
- Safety Vigilance
- Quality Assurance
- Data Management
- Statistical Analysis and Consultancy
- Clinical.net
- Collaborations
- OPIS Career
As data-driven and quality-focused clinical CRO , OPIS collaborates with numerous international Sponsors on multi-country interventional as well as non-interventional trials. OPIS teams have sound experience in a wide range of therapeutic areas , new-generation drugs, advanced therapeutic procedures and biologic treatment options.
Medical and scientific expertise in combination with technological and operational know-how have earned OPIS established partnerships with numerous pharma and biotechnology and medical device clients.
Targeted / Immunotherapies
OPIS collaborates with expert consultants in the industry and a team of UNI EN ISO 14155 trained staff to assist clients through challenging clinical compliance issues.
OPIS provides full-service , 360° clinical trial support from study concept creation and protocol development to full project execution, study data handling and up to study closure, analysis and reporting.
OPIS operates internationally with flexible business models and fully, ICH-GCP -trained operational staff . Our systems are ISO 9001:2015, ISO 27001:2014 and FDA 21 CFR Part 11 compliant.
Over 21-years of experience in clinical trial management and monitoring by proficient and well-trained international clinical operational staff.
An OPIS team of experts assist clients with regulatory aspects related to clinical studies submissions (Phase I-IV, observational), medical device and nutraceutical products authorization processes.
OPIS provides clients with sound scientific advice, medical and methodological considerations of the highest standards and assists clients to overcome regulatory and/or pharmacological obstacles.
OPIS Medical Affairs Unit has been created with the aim of enhancing the medical-scientific oversight of research activities carried out across the Company.
OPIS offers a comprehensive and flexible suite of safety monitoring services such as pharmacovigilance, phytovigilance and Medical Device Vigilance and Surveillance, for drugs, nutraceuticals and medical devices.
To ensure quality of performance and data OPIS has established a Quality Management System based on OPIS´ standard operating procedures (SOPs) and OPIS Policies.
Data Management & Biometry OPIS provides study data handling tools to guarantee scientifically sound study objectives.
More than just EDC. Fully customizable modular framework completely developed by OPIS.
Visit this area to read our latest news and to find out how OPIS can assist you in your clinical drug development programmes.
To see how OPIS delivers excellence clinical trials, please click here to find out some recent case studies.
OPIS’ Clients
"OPIS was the excellent choice to transition a critical project to a qualified team that delivered on their promises and obtained great results." - Mid-Size Pharma Company -
"The close collaboration with OPIS was instrumental to guarantee high methodological standards, fundamental requirements for any new clinical development and the international registration of new drugs." - Mid-Size Pharma Company -
"We would like to recognize the skill and hard work of each member of OPIS team who worked on this important clinical trial. Thank you for your support in providing site recruitment, initiation and management, timely subject enrollment, monitoring and project management." - Large Pharma Company -
Contact Us!
Whether you are Sponsor, a Clinical Research Professional or an Investigator contact us to learn more about how we can help.

- News & Events
- Physician Privacy Policy
- Privacy Policy
- Cookie Policy
- Terms and Conditions
- Legal Notes
WORK WITH US
Work with us
Candidate Privacy Policy
Strategies for commercialising oncology treatments for young adults
Icon innovation.
Our experience, knowledge and technological advancements converge to shape a better future for healthcare.
Applying AI to manage the risks and costs of postmarketing requirements
Translating intent to impact.
The triad of trust: Navigating real-world healthcare data integration
Uncover how reshaping data and integration strategies can empower your organisation.
Ensuring the validity of clinical outcomes assessment data
Important considerations and best practices for effective COA implementation.
Advancements in Artificial Intelligence for site selection
Using human-enabled AI to enhance decision-making and minimise risk.
A multifaceted risk factor: Addressing obesity's impact across the disease spectrum
Considerations for clinical trial design focused on obesity treatments.
Featured Solutions
Blended solutions
Bespoke, seamless solutions to meet unique sponsor challenges.
Digital Health Technologies
ICON acquires HumanFirst, a cloud-based technology company for life sciences supporting precision measurement in patient centred clinical research.
ICON provides full service outsourcing and flexible support for biotech specific needs such as due diligence and asset valuation.
Cardiac Safety Solutions
End-to-end cardiac safety solutions, including ECG, event monitoring, BPM, long-term Holter monitoring, ECHO and MUGA studies.
Early Clinical and Bioanalytical Solutions
Innovative early clinical solutions that will advance your drug development strategy.
Site & Patient Solutions
Transforming recruitment through patient-centric trials and real-world, real-time data.
Market Access
Expertise in mission-critical pricing, market access, and reimbursement.
Specialty Laboratory Solutions
Supporting precision medicine programs across all phases of drug diagnostic co-development.
Considerations for Oligonucleotide therapeutics in CMC
4 September 2024. Register today.
Regulatory submission writing for NDA success
10 September 2024. Register today.
Singlicate ADA analysis
11 September 2024. Register today.
ICON Insights
- 19 Aug 2024
Enhancing diversity in clinical trials with AI and human expertise
- 14 Aug 2024
The rise of precision outcome measures
Understanding China's 2024 NRDL: Key changes and their impact
The race to develop combination vaccines – and the importance of indirect effects
Unlocking quality in machine translation: The impact of ISO 18587 certification on clinical research
- 02 Aug 2024
Outcome measures: Driving precision health strategies forward
Digital endpoints widely adopted in pharmaceutical and biotech-sponsored clinical trials
- 24 Jul 2024
The impact of AI on the evolution of machine translation
- 19 Jul 2024
Streamlining early phase clinical trial submissions under the EU CTR
What’s happening in icon, how can we help.
- Cell and Gene Therapies
- Early Clinical
- Medical Device
- Rare & Orphan Diseases
- Real World Evidence
- Site & Patient Recruitment
- Strategic Solutions
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Guselkumab in Patients With Moderately to Severely Active Ulcerative Colitis: QUASAR Phase 2b Induction Study
Collaborators.
- QUASAR Study Group : Orest Abrahamovych 13 , Halyna Afanasieva 14 , Lilia Aitova 15 , Engin Altintas 16 , Romain Altwegg 17 , Pavel Andreev 18 , Kazuki Aomatsu 19 , Monika Augustyn 20 , Paola Balestrieri 21 , Jakob Begun 22 , Luciana Brunatto 23 , Diego Bulgheroni 23 , Elena Bunkova 24 , Mercedes Cabello 25 , Qian Cao 26 , Flavio Caprioli 27 , Rute Cerqueira 28 , Baili Chen 29 , Chou-Chen Chen 30 , Chou-Pin Chen 30 , Cheng-Tang Chiu 31 , Chang Hwan Choi 32 , Michele Cicala 21 , Olena Datsenko 33 , Pieter Dewint 34 , Eugeni Domenech 35 , Joris Dutré 36 , George Duvall 37 , Juan Fernandez 38 , Rafal Filip 39 , Ronald Fogel 40 , Sharyle Fowler 41 , Toshimitsu Fujii 42 , Masayuki Fukata 43 , Yohei Furumoto 44 , Antonio Gasbarrini 45 , Beata Gawdis-Wojnarska 46 , Cyrielle Gilletta 47 , Paolo Gionchetti 48 , Eran Goldin 49 , Oleksandr Golovchenko 50 , Maciej Gonciarz 51 , Can Gonen 52 , Gaston Gonzalez Segura 53 , Oleksii Gridnyev 54 , Tibor Gyokeres 55 , Xavier Hébuterne 56 , Charlotte Hedin 57 , Per Hellström 58 , Ida Normiha Hilmi 59 , Ivo Horný 60 , Gyula Horvat 61 , Namiko Hoshi 62 , Ludek Hrdlicka 63 , Shunji Ishihara 64 , Olha Ivanishyn 65 , Byung Ik Jang 66 , Odery Junior 67 , Takashi Kagaya 68 , Shuji Kanmura 69 , Marina Karakina 70 , Nakai Katsuhiko 71 , Jaroslaw Kierkus 72 , Hyo Jong Kim 73 , Tae-Oh Kim 74 , Young-Ho Kim 75 , Gyula G Kiss 76 , Jochen Klaus 77 , Dariusz Kleczkowski 78 , Maria Klopocka 79 , Taku Kobayashi 80 , Iwona Kobielusz-Gembala 81 , Ja Seol Koo 82 , Adam Kopon 83 , Tetiana Kravchenko 84 , Masatoshi Kudo 85 , Kwang An Kwon 86 , Paula Lago 87 , David Laharie 88 , Ian Lawrance 89 , Jaroslaw Leszczyszyn 90 , Yan Li 91 , Milan Lukas 92 , Christian Maaser 93 , Atsuo Maemoto 94 , Hiroyuki Marusawa 95 , Matthew McBride 96 , Shoba Mendu 97 , Pal Miheller 98 , Hideharu Miyabayashi 99 , Wolfgang Mohl 100 , Gregory Moore 101 , Satoshi Motoya 102 , Narayanachar Murali 103 , Mohammed Naem 104 , Koichi Nakajima 105 , Yasunari Nakamoto 106 , Stéphane Nancey 107 , Joaquim Neto 108 , Michio Onizawa 109 , Yohei Ono 110 , Yohei Ono 111 , Taro Osada 112 , Marina Osipenko 113 , Danuta Owczarek 114 , Bhaktasharan Patel 115 , Kamal Patel 116 , Elina Petrova 117 , Elena Poroshina 118 , Francisco Portela 119 , Lyudmyla Prystupa 120 , Monserrat Rivero 121 , Xavier Roblin 122 , Jacek Romatowski 123 , Grazyna Rydzewska 124 , Simone Saibeni 125 , Hirotake Sakuraba 126 , Mark Samaan 127 , Michael Schultz 128 , Joerg Schulze 129 , Shahriar Sedghi 130 , Ursula Seidler 131 , Sung Jae Shin 132 , Mykola Stanislavchuk 133 , David Stokesberry 96 , Takayoshi Suzuki 134 , Hiroki Taguchi 110 , Lyudmila Tankova 135 , Lena Thin 136 , Alexander Tkachev 137 , Leyanira Torrealba 138 , Nataliia Tsarynna 139 , Zsolt Tulassay 140 , Tetsuya Ueo 141 , Ekaterina Valuyskikh 142 , Olga Vasilevskaya 143 , Manuel Viamonte 144 , Shu-Chen Wei 145 , Roni Weisshof 146 , Katarzyna Wojcik 147 , Byong Duk Ye 141 , Hsu-Heng Yen 142 , Hyuk Yoon 143 , Kosuke Yoshida 144 , Andriy Yurkiv 145 , Osamu Zaha 146 , Qiang Zhan 147
Affiliations
- 1 Department of Gastroenterology, Nancy University Hospital, F-54500 Vandœuvre-lès-Nancy, France; INSERM, NGERE, University of Lorraine, F-54000 Nancy, France; INFINY Institute, Nancy University Hospital, F-54500 Vandœuvre-lès-Nancy, France; FHU-CURE, Nancy University Hospital, F-54500 Vandœuvre-lès-Nancy, France; Groupe Hospitalier privé Ambroise Paré-Hartmann, Paris IBD Center, 92200 Neuilly sur Seine, France; Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, Quebec, Canada.
- 2 Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts.
- 3 University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, Illinois.
- 4 University of British Columbia, Vancouver, British Columbia, Canada.
- 5 Janssen Research & Development, LLC, Spring House, Pennsylvania.
- 6 Western University, London, Ontario, Canada.
- 7 University of California San Diego, La Jolla, California.
- 8 Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain.
- 9 Kyorin University, Tokyo, Japan.
- 10 University of Pennsylvania Health System, The Raymond and Ruth Perelman School of Medicine of the University of Pennsylvania, Gastroenterology Division, Philadelphia, Pennsylvania.
- 11 Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, New York.
- 12 Department of Medicine I, Agaplesion Markus Hospital, Goethe University, Frankfurt, Germany. Electronic address: [email protected].
- 13 Communal Nonprofit Enterprise of Lviv Regional Council "Lviv Regional Clinical Hospital." Lviv, Ukraine.
- 14 Municipal Institution "Kherson City Clinical Hospital n.a. Y.Y.Karabelesh," Kherson, Ukraine.
- 15 City Clinical Hospital # 21, Ufa, Bashkortostan, Respublika, Russian Federation.
- 16 Mersin University Medical Faculty Hospital, Mersin, Turkey.
- 17 Hopital Saint Eloi, Montpellier, France.
- 18 NUZ Railway Clinical Hospital on Samara station of LLC "Russian Railways," Samara, Samarskaya oblast, Russian Federation.
- 19 Izumiotsu Municipal Hospital, Osaka, Japan.
- 20 Centrum Medyczne Plejady, Krakow, Małopolskie, Poland.
- 21 Università Campus Biomedico di Roma, Roma, Roma, Italy.
- 22 Sanatorio Duarte Quiroz, Cordoba, Argentina.
- 23 Medical University Reaviz, Multidisciplinary clinic, Samara, Samarskaya oblast, Russian Federation.
- 24 Hospital NTRA. SRA. de Valme, Sevilla, Spain.
- 25 Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
- 26 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano, Italy.
- 27 The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China.
- 28 Taichung Veterans General Hospital, Taichung, Taiwan, Province of China.
- 29 Chang-Gung Memorial Hospital, LinKou Branch, Taoyuan, Taiwan, Province of China.
- 30 Chung-Ang University Hospital, Seoul, Dongjak-gu, Republic of Korea.
- 31 Communal Nonprofit Enterprise "City Clinical Hospital # 2 N.A. Prof. O.O. Shalimov," Kharkiv, Ukraine.
- 32 AZ Maria Middelares, Gent, Oost-Vlaanderen, Belgium.
- 33 Hosp. Univ. Germans Trias I Pujol, Badalona, Catalonia, Spain.
- 34 Algemeen Ziekenhuis Jan Palfijn Merksem, Merksem, Belgium.
- 35 Tyler Research Institute, LLC, Tyler, Texas.
- 36 Harmony Medical Research Institute, Inc., Hialeah, Florida.
- 37 Centrum Medyczne Medyk, Rzeszow, Poland.
- 38 Clinical Research Institute of Michigan, LLC, Chesterfield, Michigan.
- 39 Royal University Hospital, Saskatoon, Saskatchewan, Canada.
- 40 Tokyo Medical and Dental University Hospital, Bunkyo-Ku, Tokyo, Japan.
- 41 Tokyo Yamate Medical Center, Shinjuku-ku, Tokyo, Japan.
- 42 Tokyo Metropolitan Bokutoh Hospital, Sumida-ku, Tokyo, Japan.
- 43 Fondazione Policlinico Gemelli Università Cattolica, Roma, Italy.
- 44 Twoja Przychodnia - Szczecinskie Centrum Medyczne, Szczecin, Poland.
- 45 CHU Rangueil, Toulouse, France.
- 46 Policlinico Sant'Orsola Malpighi, Bologna, Italy.
- 47 Shaare Zedek Medical Center, Jerusalem, Israel.
- 48 Wojskowy Instytut Medyczny, Warszawa, Poland.
- 49 Acibadem Kozyatagi Hospital, Istanbul, Turkey.
- 50 Centro Médico Dra. De Salvo, Caba, Buenos Aires, Argentina.
- 51 SI "L.T. Maloyi National Institute of Therapy of National Academy of Medical Sciences of Ukraine," Kharkiv, Ukraine.
- 52 Magyar Honvedseg Egeszsegugyi Kozpont, Budapest, Hungary.
- 53 CHU de Nice Hopital de l'Archet, Nice Cedex 3, France.
- 54 Karolinska Universitetssjukhuset, Stockholm, Sweden.
- 55 Uppsala University, Uppsala, Sweden.
- 56 University Malaya Medical Centre, Kuala Lumpur, Malaysia.
- 57 Nemocnice Strakonice, a.s., Strakonice, Czechia.
- 58 Bugat Pal Korhaz, Gyongyos, Hungary.
- 59 ResTrial GastroEndo, s.r.o, Praha 4, Czechia.
- 60 Shimane University Hospital, Izumo, Japan.
- 61 Lviv Clinical Hospital on Railway Transport of Affiliate Healthcare Center of JSC Ukrainian Railway, Lviv, Ukraine.
- 62 Yeungnam University Hospital, Daegu, Daegu Gwang'yeogsi, Republic of Korea.
- 63 National Hospital Organization Kanazawa Medical Center, Kanazawa, Ishikawa, Japan.
- 64 Kagoshima University Hospital, Kagoshima City, Kagoshima, Japan.
- 65 Medical Center Meditsinskie Tekhnologii, Ekaterinburg, Russian Federation.
- 66 Matsuda Hospital, Shizuoka, Japan.
- 67 WIP Warsaw IBD Point Profesor Kierkus, Warszawa, Poland.
- 68 KyungHee University Hospital, Seoul, Republic of Korea.
- 69 Inje University Haeundae Paik Hospital, Busan, Republic of Korea.
- 70 Samsung Medical Center, Seoul, Republic of Korea.
- 71 Vasutegeszsegugyi Nonprofit Kozhasznu Kft Debreceni Kozpont, Debrecen, Hajdú-Bihar, Hungary.
- 72 Universitaetsklinikum Ulm, Ulm, Baden-Württemberg, Germany.
- 73 Endoskopia Sp z o.o., Sopot, Pomorskie, Poland.
- 74 Szpital Uniwersytecki nr 2 im. dr. Jana Biziela w Bydgoszczy, Bydgoszcz, Poland.
- 75 Kitasato University Kitasato Institute Hospital, Minato-ku, Tokyo, Japan.
- 76 Medicome Sp z o.o., Oswiecim, Poland.
- 77 Korea University Ansan Hospital, Gyeonggi-do, Republic of Korea.
- 78 GASTROMED Kopon, Zmudzinski i wspolnicy SP.j., Specjalistyczne Centrum Gastrologii i Endoskopii, Torun, Poland.
- 79 Kyiv City Clinical Hospital #18, Kyiv, Ukraine.
- 80 Kindai University Hospital, Osaka-Sayama, Ôsaka, Japan.
- 81 Gachon University Gil Medical Center, Incheon, Incheon Gwang'yeogsi, Republic of Korea.
- 82 Hopital Haut-Leveque, CMC Magellan, Pessac, France.
- 83 St John of God Subiaco Hospital, Subiaco, WA, Australia.
- 84 Melita Medical Sp. z o.o., Wroclaw, Dolnośląskie, Poland.
- 85 Shengjing Hospital of China Medical University, Shenyang, Liaoning, China.
- 86 ISCARE a.s., Praha 7, Czechia.
- 87 Staedtisches Klinikum Lueneburg, Lueneburg, Germany.
- 88 Sapporo Higashi Tokushukai Hospital, Sapporo, Japan.
- 89 Japanese Red Cross Osaka Hospital, Osaka, Japan.
- 90 Digestive Disease Specialists Inc, Oklahoma City, Oklahoma.
- 91 Gastroenterology Associates of Tidewater, Chesapeake, Virginia.
- 92 Semmelweis Egyetem, Budapest, Hungary.
- 93 National Hospital Organization Matsumoto Medical Center, Matsumoto, Nagano, Japan.
- 94 Zentrum für Gastroenterologie Saar MVZ GmbH, Saarbrücken, Germany.
- 95 Monash Health, Clayton, VIC, Australia.
- 96 Hokkaido P.W.F.A.C. Sapporo-Kosei General Hospital, Sapporo, Japan.
- 97 Gastroenterology Associates of Orangeburg, Orangeburg, South Carolina.
- 98 Northshore Gastroenterology Research, LLC, Westlake, Ohio.
- 99 Matsushima Clinic, Kanagawa, Japan.
- 100 University of Fukui Hospital, Yoshida, Fukui, Japan.
- 101 Centre Hospitalier Lyon Sud, Pierre-Bénite, France.
- 102 Sociedade Campineira de Educacao e Instrucao-Hospital e Maternidade Celso Pierro, Campinas, São Paulo, Brazil.
- 103 Fukushima Medical University Hospital, Fukushima, Japan.
- 104 Imamura General Hospital, Kagoshima, Japan.
- 105 Kagoshima IBD Gastroenterology Clinic, Kagoshima, Japan.
- 106 Juntendo University Hospital Urayasu, Chiba, Japan.
- 107 Medical Center SibNovoMed LLC, Novosibirsk, Russian Federation.
- 108 Centrum Medyczne Promed, Krakow, Poland.
- 109 Peak Gastroenterology Associates, Colorado Springs, Colorado.
- 110 St George's Hospital, London, United Kingdom and Northern Ireland.
- 111 OOO MO New Hospital, Ekaterinburg, Russian Federation.
- 112 Eco-safety Ltd, Saint-Petersburg, Russian Federation.
- 113 Centro Hospitalar e Universitário de Coimbra, EPE, Coimbra, Portugal.
- 114 Sumy State University, Sumy Regional Clinical Hospital, Sumy, Ukraine.
- 115 Hosp. Univ. Marques De Valdecilla, Santander, Cantabria, Spain.
- 116 CHU Saint-Etienne-Hôpital Nord, Saint-Priest en Jarez, France.
- 117 Gastromed Kralisz Romatowski Stachurska Sp. j., Bialystok, Poland.
- 118 Centralny Szpital Kliniczny Mswia, Warszawa, Mazowieckie, Poland.
- 119 Azienda Ospedaliera G. Salvini Ospedale di Rho, Rho, Milan, Italy.
- 120 Hirosaki University Hospital, Hirosaki, Japan.
- 121 Guy's and St Thomas' Hospital, London, United Kingdom and Northern Ireland.
- 122 Dunedin Hospital, Dunedin, Otago, New Zealand.
- 123 Praxisgemeinschaft Jerichow, Jerichow, Germany.
- 124 Gastroenterolgy Associates of Central GA, Macon, Georgia.
- 125 Medizinische Hochschule Hannover, Hannover, Niedersachsen, Germany.
- 126 Ajou University Hospital, Suwon, Gyeonggi-do, Republic of Korea.
- 127 Vinnitsia Regional Clinical Hospital n.a. M. I. Pyrogov, Vinnytsya, Ukraine.
- 128 Tokai University Hachioji Hospital, Hachioji, Tokyo, Japan.
- 129 DCC Convex EOOD, Sofia, Bulgaria.
- 130 Fiona Stanley Hospital, Murdoch, WA, Australia.
- 131 Rostov State Medical University, Rostov-on-Don, Russian Federation.
- 132 Hosp. Univ. Dr. Josep Trueta, Girona, Spain.
- 133 Medical Center "Ok Clinic" of LLC "International Institute of Clinical Studies," Kyiv, Ukraine.
- 134 Semmelweis Egyetem, Ii. Belgyogyaszati Klinika, Budapest, Hungary.
- 135 Oita Red Cross Hospital, Oita, Japan.
- 136 LLC "Novosibirsk Gastrocentr," Novosibirsk, Russian Federation.
- 137 Regional Clinical Hospital, Yaroslavl, Russian Federation.
- 138 Columbus Clinical Services LLC, Miami, Florida.
- 139 National Taiwan University Hospital, Taipei, Taiwan, Province of China.
- 140 ETG Zamosc, Zamosc, Poland.
- 141 Asan Medical Center, Seoul, Seoul Teugbyeolsi, Republic of Korea.
- 142 Chang-Hua Christian Hospital, Changhua, Taiwan, Province of China.
- 143 Seoul National University Bundang Hospital, Gyeonggi-do, Republic of Korea.
- 144 National Hospital Organization Tokyo Medical Center, Tokyo, Japan.
- 145 Municipal Non-profit Enterprise "Odesa Regional Clinical Hospital" Odesa Regional Council, Odesa, Ukraine.
- 146 Nakagami Hospital, Okinawa, Japan.
- 147 Wuxi People's Hospital, Wuxi, Jiangsu, China.
- PMID: 37659673
- DOI: 10.1053/j.gastro.2023.08.038
Background & aims: The QUASAR Phase 2b Induction Study evaluated the efficacy and safety of guselkumab, an interleukin-23p19 subunit antagonist, in patients with moderately to severely active ulcerative colitis (UC) with prior inadequate response and/or intolerance to corticosteroids, immunosuppressants, and/or advanced therapy.
Methods: In this double-blind, placebo-controlled, dose-ranging, induction study, patients were randomized (1:1:1) to receive intravenous guselkumab 200 or 400 mg or placebo at weeks 0/4/8. The primary endpoint was clinical response (compared with baseline, modified Mayo score decrease ≥30% and ≥2 points, rectal bleeding subscore ≥1-point decrease or subscore of 0/1) at week 12. Guselkumab and placebo week-12 clinical nonresponders received subcutaneous or intravenous guselkumab 200 mg, respectively, at weeks 12/16/20 (uncontrolled study period).
Results: The primary analysis population included patients with baseline modified Mayo scores ≥5 and ≤9 (intravenous guselkumab 200 mg, n = 101; 400 mg, n = 107; placebo, n = 105). Week-12 clinical response percentage was greater with guselkumab 200 mg (61.4%) and 400 mg (60.7%) vs placebo (27.6%; both P < .001). Greater proportions of guselkumab-treated vs placebo-treated patients achieved all major secondary endpoints (clinical remission, symptomatic remission, endoscopic improvement, histo-endoscopic mucosal improvement, and endoscopic normalization) at week 12. Among guselkumab week-12 clinical nonresponders, 54.3% and 50.0% of patients in the 200- and 400-mg groups, respectively, achieved clinical response at week 24. Safety was similar among guselkumab and placebo groups.
Conclusions: Guselkumab intravenous induction was effective vs placebo in patients with moderately to severely active UC. Guselkumab was safe, and efficacy and safety were similar between guselkumab dose groups.
Clinicaltrials: gov number: NCT04033445 .
Keywords: Advanced Therapy; Interleukin-23p19 Subunit Antagonist; QUASAR; Ulcerative Colitis.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
PubMed Disclaimer
Similar articles
- Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): a phase 3, randomised, controlled trial. Peyrin-Biroulet L, Hart A, Bossuyt P, Long M, Allez M, Juillerat P, Armuzzi A, Loftus EV Jr, Ostad-Saffari E, Scalori A, Oh YS, Tole S, Chai A, Pulley J, Lacey S, Sandborn WJ; HICKORY Study Group. Peyrin-Biroulet L, et al. Lancet Gastroenterol Hepatol. 2022 Feb;7(2):128-140. doi: 10.1016/S2468-1253(21)00298-3. Epub 2021 Nov 17. Lancet Gastroenterol Hepatol. 2022. PMID: 34798039 Clinical Trial.
- Etrolizumab for maintenance therapy in patients with moderately to severely active ulcerative colitis (LAUREL): a randomised, placebo-controlled, double-blind, phase 3 study. Vermeire S, Lakatos PL, Ritter T, Hanauer S, Bressler B, Khanna R, Isaacs K, Shah S, Kadva A, Tyrrell H, Oh YS, Tole S, Chai A, Pulley J, Eden C, Zhang W, Feagan BG; LAUREL Study Group. Vermeire S, et al. Lancet Gastroenterol Hepatol. 2022 Jan;7(1):28-37. doi: 10.1016/S2468-1253(21)00295-8. Epub 2021 Nov 17. Lancet Gastroenterol Hepatol. 2022. PMID: 34798037 Clinical Trial.
- Etrolizumab versus adalimumab or placebo as induction therapy for moderately to severely active ulcerative colitis (HIBISCUS): two phase 3 randomised, controlled trials. Rubin DT, Dotan I, DuVall A, Bouhnik Y, Radford-Smith G, Higgins PDR, Mishkin DS, Arrisi P, Scalori A, Oh YS, Tole S, Chai A, Chamberlain-James K, Lacey S, McBride J, Panés J; HIBISCUS Study Group. Rubin DT, et al. Lancet Gastroenterol Hepatol. 2022 Jan;7(1):17-27. doi: 10.1016/S2468-1253(21)00338-1. Epub 2021 Nov 17. Lancet Gastroenterol Hepatol. 2022. PMID: 34798036 Clinical Trial.
- Etrolizumab for induction of remission in ulcerative colitis. Rosenfeld G, Parker CE, MacDonald JK, Bressler B. Rosenfeld G, et al. Cochrane Database Syst Rev. 2015 Dec 2;2015(12):CD011661. doi: 10.1002/14651858.CD011661.pub2. Cochrane Database Syst Rev. 2015. PMID: 26630451 Free PMC article. Review.
- Methotrexate for induction of remission in ulcerative colitis. Chande N, Wang Y, MacDonald JK, McDonald JW. Chande N, et al. Cochrane Database Syst Rev. 2014 Aug 27;2014(8):CD006618. doi: 10.1002/14651858.CD006618.pub3. Cochrane Database Syst Rev. 2014. PMID: 25162749 Free PMC article. Review.
- Anti-IL23/12 agents and JAK inhibitors for inflammatory bowel disease. Tian Z, Zhao Q, Teng X. Tian Z, et al. Front Immunol. 2024 Jul 17;15:1393463. doi: 10.3389/fimmu.2024.1393463. eCollection 2024. Front Immunol. 2024. PMID: 39086483 Free PMC article. Review.
- Immunity in digestive diseases: new drugs for inflammatory bowel disease treatment-insights from Phase II and III trials. Massironi S, Furfaro F, Bencardino S, Allocca M, Danese S. Massironi S, et al. J Gastroenterol. 2024 Sep;59(9):761-787. doi: 10.1007/s00535-024-02130-x. Epub 2024 Jul 9. J Gastroenterol. 2024. PMID: 38980426 Free PMC article. Review.
- IL-23 past, present, and future: a roadmap to advancing IL-23 science and therapy. Krueger JG, Eyerich K, Kuchroo VK, Ritchlin CT, Abreu MT, Elloso MM, Fourie A, Fakharzadeh S, Sherlock JP, Yang YW, Cua DJ, McInnes IB. Krueger JG, et al. Front Immunol. 2024 Apr 15;15:1331217. doi: 10.3389/fimmu.2024.1331217. eCollection 2024. Front Immunol. 2024. PMID: 38686385 Free PMC article. Review.
- Early Symptomatic Improvement With Guselkumab Induction Treatment in Moderately to Severely Active Ulcerative Colitis: Results From the Phase 3 QUASAR Study. [No authors listed] [No authors listed] Gastroenterol Hepatol (N Y). 2023 Dec;19(12 Suppl 9):2-3. Gastroenterol Hepatol (N Y). 2023. PMID: 38445181 Free PMC article. No abstract available.
- IL-23. [No authors listed] [No authors listed] Gastroenterol Hepatol (N Y). 2024 Jan;20(1 Suppl 1):7-9. Gastroenterol Hepatol (N Y). 2024. PMID: 38444832 Free PMC article. No abstract available.
Publication types
- Search in MeSH
Associated data
- Search in ClinicalTrials.gov
Related information
Linkout - more resources, full text sources.
- Elsevier Science
Other Literature Sources
- The Lens - Patent Citations
- Genetic Alliance
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

IMAGES
COMMENTS
dicentra. Address: 603-7 St Thomas Street Toronto, ON M5S 2B7, Canadadicentra is a full-service Contract Research Organization (CRO) and professional consulting firm that specializes in addressing all matters related to safety, quality, and compliance for all... View full profile. View locations. Dokumeds.
MTZ Clinical Research powered by Pratia. Company founded in 2002 in Warsaw, Poland, focuses on phase I, early clinical development, bioequivalence, and phase III studies. In January 2021 MTZ Clinical Research joined Pratia. Pratia is a network of clinical research sites in Europe and is part of the Neuca Group. Services offered:
11 listopada 78, Staszów, Poland 28-200. Phone: Our Vision. ... Velocity Clinical Research is the world's leading organization of fully integrated research sites. The company partners with pharmaceutical and biotechnology companies to research new drugs, medical devices, and diagnostics that could improve human health and wellbeing. ...
Panska 73 Str./804 Warsaw, 00-834 Poland EastHORN Clinical Services is one of the leading CROs in Europe. EastHORN offers phase I-IV clinical research services specialized in rescue studies. Our services also include regulatory affairs, medical writing, pharmacovigilance, and data management.
CROS is specialized in site identification, Protocol Feasibility, Startup, contracting, and clinical research operations from phase I to IV. Also, we support the Investigator Payments and Investigator Budget Development. We provide comprehensive clinical trial execution services for pharmaceutical, biotechnology, and medical device companies.
Cromos Pharma has been conducting clinical research in Poland since 2015 and opened a permanent office in Warsaw in early in 2020. Poland Quick Facts. 2.3. doctors (2017) per 1 000 population. 6.1. ... where it initially conducted phase 3 oncology trials, but since has broadened its scope of services. The company opened a regional office in ...
Pharmaxi LLC is a client-focused, adaptable, inventive, and cost-effective company that specializes in clinical trials (CRO). We provide clinical services for medicinal products, medical devices, and dietary supplements. We conduct clinical trials in Poland, Ukraine, and Eastern Europe. We understand that each project is unique, and as such ...
Brillance was founded and started its activities in year 2004, covering Poland, Czech Republic and Slovak Republic. Research and development activity is one of the main factors which decides about company's success and development on the medical, pharmaceutical and biotechnology market. Clinical trials are conducted in order to assess ...
The company partners with pharmaceutical and biotechnology companies to research new drugs, medical devices, and diagnostics that could improve human health and wellbeing. Velocity's unified research site solutions deliver the right patients, investigators, and research staff for Phase 1-4 clinical trials across Europe and the United States.
Velocity Clinical Research, the leading multi-specialty clinical sites business, announced it has expanded into Poland, acquiring its first site, ClinMedica Research.Located in Skierniewice, which is halfway between Warsaw and Lodz, the site marks the first in a series of key acquisitions targeted by the company in Poland by year's end.
Thoughtful organization and internal processes plus profound research knowledge makes us fast in decision making and action. SMART GLOBAL. Any project, any country worldwide. Anywhere you do your project we can effectively assist in your needs. We understand regional specificity and conditions of clinical research in America, Asia, Europe, Africa.
Warsaw location. Prosta Tower. 13th floor. ul. Prosta 32. 00-838 Warsaw, Poland. If you wish to participate in our clinical trials, please call +1 210-635-1515 or visit here. Visit Careers to view and apply for all open jobs here. View all Worldwide offices on our Global Reach page.
The Polish pharmaceutical industry is projected to record a steady compound annual growth rate of 3.2%, according to a report by Global Data. The report forecasts the industry to grow from $9.4bn last year to $11bn by 2021. Health care in Poland is delivered through a publicly funded health care system, but the private sector is steadily ...
Clinical Research Service Companies in Poland in alphabetical order. Avantor. Avantor®, a Fortune 500 company, is a leading global provider of mission-critical products and services to customers in the biopharma, healthcare, education & government, and advanced technologies & applied materials industries. Our portfolio...
Global Contract Research Organizations in Poland starting with "s" SanaClis. SanaClis is a full-service Global CRO with a fully integrated clinical supply chain, thereby offering a comprehensive range of end-to-end services for clinical trials. SanaClis was founded in 2000 by seasoned industry experts, all of whom have had ex... View full profile
A total of 559 clinical trials were initiated between 1 January 2021 and 1 January 2022. The majority of trials initiated in this period were Phase 3 (203) with a significant number of Phase 2 (144) studies. Using clinicaltrials.gov our team analyzed the trends in the clinical trials market in Poland between 1 January 2021 and 1 January 2022.
OPIS provides full-service, 360° clinical trial support from study concept creation and protocol development to full project execution, study data handling and up to study closure, analysis and reporting. OPIS operates internationally with flexible business models and fully, ICH-GCP -trained operational staff. Our systems are ISO 9001:2015 ...
ICON is the world's leading clinical research organisation, providing outsourced clincal development and commercialisation services to the pharmaceutical, ... ICON acquires HumanFirst, a cloud-based technology company for life sciences supporting precision measurement in patient centred clinical research.
Apply for CTM - FSP - Poland job with Thermo Fisher Scientific in Remote, Poland. Clinical Research jobs at Thermo Fisher Scientific
11 Clinical Research Center, Instituto de Oncologia do Parana (IOP), Curitiba, Brazil. ... Hungary, Israel, Italy, Poland, Russia, Spain, Ukraine, the UK, and the USA), we recruited patients aged 18 years and older with BRCA1-mutated or BRCA2-mutated ovarian carcinoma, with an Eastern Cooperative Oncology Group performance status of 0 or 1, and ...
Wroclaw, Poland (M Pazgan-Simon MD); Department of Liver and Pancreas Gland Oncosurgery, Regional Centre of Oncology, Kharkiv, Ukraine (M Pisetska MD); Research in context. Evidence before this study. We searched PubMed for clinical trials published in English . between Jan 1, 2017, and Nov 30, 2022, using the terms
Adams Clinical, a Watertown, Mass.-based research company focused on late-phase psychiatric and neurologic clinical trials, signed a long-term lease with Simone Development for a 7,236-square-foot ...
Background & aims: The QUASAR Phase 2b Induction Study evaluated the efficacy and safety of guselkumab, an interleukin-23p19 subunit antagonist, in patients with moderately to severely active ulcerative colitis (UC) with prior inadequate response and/or intolerance to corticosteroids, immunosuppressants, and/or advanced therapy. Methods: In this double-blind, placebo-controlled, dose-ranging ...
Ufa, Republic of Bashkortostan, Russian Federation . Search. Location