

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
| 2023-07-14 | |
| 2020-01-17 | |
| 2020-01-17 | |
| 2020-01-17 | |
| 2023-04-10 | |
| 2023-06-27 | |
| 2023-04-10 | |
| The following documents are samples. IRBIS does NOT generate these documents with application-specific information. | |
| 2017-10-30 | |
| 2024-08-09 | |
| 2017-04-17 | |
| 2018-04-19 | |
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
- Privacy Policy

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.
Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Research Findings – Types Examples and Writing...

Purpose of Research – Objectives and Applications

Research Paper Introduction – Writing Guide and...

Dissertation – Format, Example and Template

Critical Analysis – Types, Examples and Writing...

Data Verification – Process, Types and Examples
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
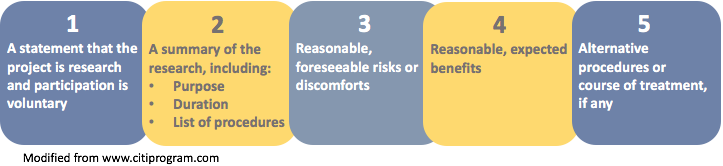
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 04/10/2024.
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects. Last updated 4/17/24
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 04/10/2024
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools. Last updated 4/10/24
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
- Child assent ages 3-6
- Child assent 7-11
- Parent permission
- Brief protocol for exempt research including data management and security questionnaire
- Child assent 12-14
- Introductory psychology subject pool general consent template
- Introductory psychology subject pool exempt consent template
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections

Information-consent samples
Guide to Creating an Information Letter and Consent Form | Information Letter Samples | Consent Form Samples | Resources
An information-consent letter is used most often to inform a potential participant about a research study and to document a participant's agreement to take part in the study.
Guide to Creating an Information Letter and Consent Form
The guide to creating an information letter and consent form (docx) is intended to provide researchers with the information needed to develop their information letters and consent documents. TCPS 2, Article 3.2 indicates that “ Researchers shall provide to participants, or authorized third parties, full disclosure of all information necessary for making an informed decision to participate in the research project.”
The guide to creating an information letter and consent form (docx) was developed as a resource for researchers to use when developing their materials.
Information Letter Samples
The samples below are intended to be examples of information letters. When developing your information letter refer to the guide and use the samples as a starting place. All information letters should be accompanied by a consent material documenting how consent is obtained.
| Paper-pencil surveys | (docx) (docx) |
| Anonymous online surveys | (docx) |
| In-lab procedures (e.g., fMRI, electromyography, other physiological procedures) | (docx) (docx) (docx) |
| In-lab procedures (e.g., surveys, computer tasks) | (docx) (docx) |
| Clinical trials involving exercise program | (docx) |
| Interviews | (docx) |
| Focus groups | (docx) |
| Studies with children as participants | (docx) (docx) (docx) (docx) |
| Course project | (docx) (docx) |
| Deception studies (Note: ) | (docx) (docx) (docx) |
Consent and Permission Form Samples
Consent and permission forms are generally used with an information letter. These forms document a participant’s agreement to take part in the study. Depending on the study methods and context, permission may also be needed to use quotations, take audio or video recordings, take photographs/digital images, or to store and use data for future research.
While consent is typically obtained in written form, researchers may obtain consent in other ways such as orally or verbally. Consent is to be document regardless of the method chosen ( TCPS 2, Article 3.12 ).
| Written | (docx) |
| Verbal | (docx) |
| Online (e.g., online surveys) | (docx) |
| Parental consent form for research with child | (docx) (docx) |
| Post de-briefing (e.g., for studies using deception) | (docx) |
Waterloo finance policy on remuneration to research participants
Waterloo guidelines for using plain language in participant materials
Waterloo guidelines for recruiting people to surveys using crowdsourcing
Waterloo guidelines for storing data and information
Updated June 2023
- IRB-SBS Home
- Contact IRB-SBS
- IRB SBS Staff Directory
- IRB SBS Board Members
- About the IRB-SBS
- CITI Training
- Education Events
- Virginia IRB Consortium
- IRB-SBS Learning Shots
- HRPP Education & Training
- Student Support
- Access iProtocol
- Getting Started
- iProtocol Question Guide
- iProtocol Management
- Protocol Review Process
- Certificate of Confidentiality
- Deception and/or Withholding Information from a Participant
- Ethnographic Research
- IRB-SBS 101
- IRB-SBS Glossary
- Participant Pools
- Paying Participants
- Research in an Educational Setting
- Research in an International Setting and/or Location
- Risk-Sensitive Populations
- Student Researchers and Faculty Sponsors
- Study Funding and the IRB
- Understanding Risk in Research
- Vulnerable Participants
- IRB-SBS PAM & Ed
- Federal Regulations
- Ethical Principals
- Partner Offices
- Determining Human Subjects Research
- Determining HSR or SBS

Consent Templates
The templates below were created to help you create the documents you will need to communicate to participants what they will do in the study. The documents you provide participants will range from recruitment materials to post-debrief consent forms, and you need to submit everything that you provide to a participant to our Board for review. For more information about the consent process see Consent .
- General Consent Template : This form covers all of the basic elements that are required for a consent document. Even if you don't plan to use this exact document, refer to it to ensure that you have all of the appropriate elements in place in your consent procedure.
- Electronic Consent Template : This form is modeled after the General Consent Template with some modifications that make it more appropriate for an online format. For more information about this template, see Electronic Consent .
- Parent Consent : If you are including minors in your study, you will need to provide a consent form for parents and an age appropriate assent form for minors. This form is a guide for creating a parent informed consent document. This form can also be used as a guide for surrogate consent procedures.
- Minor Assent (for ages 13-17) : This template provides the basic elements required for older minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity.
- Minor Assent (for ages 7-12) : This template provides the basic elements required for younger minors to provide assent and could also be used as a model for higher functioning individuals with diminished mental capacity. For children younger than 7, assent forms are not required but include information in the consent section regarding what you will say to them about the study (where appropriate).
- Capacity to Consent Template : For some participant populations, it may be necessary to determine if a participant is able to provide consent; if not, a surrogate can be used (you will also need a surrogate consent form and participant assent form, similar to the parent/child consent/assent forms).
- GDPR Informed Consent Addendum : If you are collecting data from citizens of the European Union or the United Kingdom, you will need to provide additional information to your participants, per the GDPR. For more information, see the Research in an International Setting and/or Location and International Research Data Source .
- Study Information Sheet : While many studies do not require researchers to collected signed consent forms, we generally require that participants receive a Study Information Sheet to provide them with information about the study. This information can be provided as a paper document at the beginning of a survey.
- Electronic Study Information Page : This template is similar to the Study Information Sheet with modification for an electronic delivery. For more information about this template, see Electronic Consent .
- Parent Notification Template : Typically used for studies in an educational setting (particularly where the study is exempt but parent notification is still required), this template is a guide for creating a notification letter to send home to parents.
- Oral Consent Card : Typically used in anthropology studies where the participant may be uncomfortable with a form and/or unable to use it, the Oral Consent Card provides all of the elements required for consent in a bullet format so that the researcher can refer to each point as he or she is obtaining consent from the participant.
- Oral Consent Template : This form is also used in situations where the participant is uncomfortable with a form and/or unable to use it. It is more suited to non-anthropology research (though anthropologists are welcome to refer to it as well).
- Sample Debriefing Form : A debriefing form is a summary of the study given to a participant in a deception study and/or a study that includes students from a participant pool. The purpose is to educate participants about the study and to provide them with resources, particularly if the study is upsetting.
- Advertising Flyer Template : Recruitment materials are part of the consent process and it is important that participants are accurately informed about the study throughout the process. You are not required to use this flyer template (it is a model appropriate for a flyer posted around campus), but it is important that you follow the guide provided in Recruitment .
- ResearchMatch Advertising Template : The NIH funds a free and secure recruitment tool called ResearchMatch that helps to connect researchers with volunteers that are interested in participating in studies. If you are interested in using ResearchMatch to help advertise for your study, complete this ResearchMatch Advertising Template and upload.
- Materials Release Form : The data you collect from your participants may be useful in other spheres, such as an educational tool and/or library archive. Using data in this manner is beyond the scope of the study and you should seek additional permission to use the participant's data in this way. This form allows a participant to declare how they would like their materials to be used by the researcher if the researcher wants to use the materials in situations beyond the study.
- Data Release Form : This form is similar to the Post-Debrief Consent Form; it is used when a participant has been recorded or photographed without their knowledge.
- Post-Debrief Consent Form : This form is used in a deception study after the deception is revealed to the participant. The participant is given an opportunity to decide if they still want to participate after the true purpose of the study is revealed.
- The title of protocol must match the title on all consent forms. The title must be relevant, appropriate, and easy to understand. Include the project title on all pages of the consent form.
- List the page numbers on all pages of the consent form in the standard format: Page 1.
- Delete all colored text from the final copy of your form. The colored text is for explanation purposes only.
- Make sure that the form matches the descriptions in the protocol and vice versa.
- Include all relevant information in the consent form rather than referring to previous verbal explanations. The consent form should provide a complete explanation of what the participant is agreeing to do in the study.
- Be aware of the needs of the participant. Avoid using jargon and acronyms that the participant may not understand; make sure the reading comprehension level is appropriate.
- Do not use statements that make implicit demands on participants to participate , e.g., "You will enjoy and benefit from participating in this study."
- Prepare the consent forms in the standard format provided in the template, with all headings addressed. Use the standard language provided on the template where appropriate.
- Please proofread the consent forms for grammar and spelling errors.
- Do not use language that revokes a participant’s legal rights . A consent form is not a legal document.
- Do not require the participants to sign consent to long statements written in first person , e.g., “I agree to participate in this research study. I understand that the risks are minimal and that I will receive no benefits. I know how to withdraw from this study. I will receive $X in payment for participating. I understand that if I withdraw from the study before my participation is complete, I will receive prorated payment according to the following schedule . . . I agree not to hold the researchers liable for any injuries resulting from participation in this study. . .” DO ask participants to sign consent to a simple agreement statement at the end of the consent form: “I agree to participate in the research study described above.”
Human Subjects Division
- [email protected]
- 206.543.0098
Consent Examples
About this page.
To assist UW researchers with designing subject-focused consent, the UW IRB provides example consent forms. Many of these examples are actual UW IRB approved consent forms designed by UW researchers. Some of the examples were created using one of our consent templates . The use of our template is not required and some of the examples deviate significantly from our templates.
We encourages researchers to use the Designing the Consent Process guidance and the examples below to create consent forms and processes that: (1) are written from the perspective of the subject population being enrolled, emphasizing the Key Information that is mostly likely to assist those subjects with deciding whether to enroll; and (2) are designed and presented in a way that facilitates comprehension and understanding.
- Exempt Research Example Consents
- Expedited and Full Board Research Example Consents
- Key Information Examples
University of Washington Office of Research
Or support offices.
- Human Subjects Division (HSD)
- Office of Animal Welfare (OAW)
- Office of Research (OR)
- Office of Research Information Services (ORIS)
- Office of Sponsored Programs (OSP)
OR Research Units
- Applied Physics Laboratory (APL-UW)
- WA National Primate Research Center (WaNPRC)
Research Partner Offices
- Corporate and Foundation Relations (CFR)
- Enivronmental Health and Safety (EH&S)
- Grant and Contract Accounting (GCA)
- Institute of Translational Health Sciences (ITHS)
- Management Accounting and Analysis (MAA)
- Post Award Fiscal Compliance (PAFC)
Collaboration
- Centers and Institutes
- Collaborative Proposal Development Resources
- Research Fact Sheet
- Research Annual Report
- Stats and Rankings
- Honors and Awards
- Office of Research
© 2024 University of Washington | Seattle, WA
- Eviction Notice Forms
- Power of Attorney Forms Forms
- Bill of Sale (Purchase Agreement) Forms
- Lease Agreement Forms
- Rental Application Forms
- Living Will Forms Forms
- Recommendation Letters Forms
- Resignation Letters Forms
- Release of Liability Agreement Forms
- Promissory Note Forms
- LLC Operating Agreement Forms
- Deed of Sale Forms
- Consent Form Forms
- Support Affidavit Forms
- Paternity Affidavit Forms
- Marital Affidavit Forms
- Financial Affidavit Forms
- Residential Affidavit Forms
- Affidavit of Identity Forms
- Affidavit of Title Forms
- Employment Affidavit Forms
- Affidavit of Loss Forms
- Gift Affidavit Forms
- Small Estate Affidavit Forms
- Service Affidavit Forms
- Heirship Affidavit Forms
- Survivorship Affidavit Forms
- Desistance Affidavit Forms
- Discrepancy Affidavit Forms
- Guardianship Affidavit Forms
- Undertaking Affidavit Forms
- General Affidavit Forms
- Affidavit of Death Forms
Letter of Consent

Cover Letter For Internship
Teacher recommendation letter, cooking recommendation letter.
Download Letter of Consent Bundle - PDF
What is a Letter of Consent?
A Letter of Consent is a formal document that grants permission or authorizes another party to take specific actions on behalf of the signer. This letter can cover a wide range of scenarios, from parental consent for a child’s travel to authorizing medical treatment or financial transactions. It’s an essential tool for clearly communicating consent in a verifiable manner, ensuring all parties understand the permissions granted.
Letter of Consent Format
[Your Full Name] [Your Address] City, State, Zip Code [Your Email Address] [Your Phone Number]
[Date][Recipient’s Name or Title]
[Recipient’s Address] City, State, Zip CodeDear [Recipient’s Name or Title],
Introduction:
Begin with a statement of intent, clearly stating that you are writing to give your consent. Mention your relationship to the parties involved or your authority to give consent.
Body of the Letter:
Specific Details of Consent: Clearly outline what you are giving consent for. Include specific details such as names, dates, locations, and any relevant identification numbers or documents.
Conditions or Limitations: If your consent is subject to certain conditions or limitations, specify them clearly. This ensures that the terms of your consent are well understood.
Duration: If applicable, mention the period for which your consent is valid. Include start and end dates to avoid any ambiguity.
Conclusion: Reiterate your consent for the action to proceed, and express your willingness to provide further information if required. Offer a way for the recipient to contact you, such as a phone number or email address, for any questions or clarifications.
Closing: Close the letter with a polite sign-off, such as “Sincerely,” or “Best regards,” followed by your signature (if sending a hard copy) and typed name.
Attachments: List any documents you are attaching with the letter that support or are required for the consent process.
[Handwritten Signature (for a hard copy)] [Typed Name]
Letter of Consent to Travel

PDF Word Google Docs
Essential for travelers, this letter combines a Photography Consent Form and Questionnaire Consent Form to authorize travel or participation in activities, ensuring legal and ethical compliance.
Letter of Consent for Consent

A Letter of Consent for Consent authorizes healthcare procedures, leveraging a Patient Consent Form , Piercing Consent Form , and Psychology Consent Form to safeguard patient rights and outline the scope of consent.
Sample Letter of Consent to Travel with One Parent PDF

This letter facilitates minors traveling with one parent, requiring a Parent Consent Form , Parental Consent Form , and Photo Consent Form to authorize travel and ensure child safety across borders.
Letter of Consent for Research

Crafting a Letter of Consent for Research is crucial for ethical studies, aligning with a Model Consent Form , Participant Consent Form , Privacy Consent Form , and Research Consent Form to protect participants’ rights and privacy.
Letter of Consent for Passport
A Letter of Consent for Passport is essential for minors traveling abroad, incorporating elements of a Medical Consent Form , Minor Travel Consent Form , Passport Consent Form , and Photo Release Consent Form to ensure a smooth and secure travel process.
More Letter of Consent Samples
Free school consent letter template.

- Google Docs
Free Email To Employee For Consent To Reduce Salary Letter

Drug Testing Consent Agreement Letter Template

- Apple Pages
Letter of Consent for Travel of a Minor Child Sample Form

Size: 53 KB
Parental Consent Letter Sample Form Document

Size: 41 KB
Notarized Marriage Consent Letter Sample

Size: 23 KB
Approval Letter of Consent To Passport Application Template

Size: 28 KB
Property Landlord Letter of Consent Template

Size: 129 KB
Letter of Consent from the Bank Employee Template

Size: 441 KB
Simple Format of a Consent Letter in PDF Template

Basic Letter of Consent for Business Research in Word

Size: 10 KB
Printable Parent Consent Letter for School

Size: 34 KB
Free Informed Authorization Letter of Consent

Size: 144 KB
Automobile Letter of Consent with Permission

Size: 234 KB
These were only a few of the many and varied situations where permission from one person has to be granted to the other in concrete and written form instead of a verbal “Go ahead.”
When done right, it is a beneficial practice for all concerned rather than a limitation on freedom. A successful letter of consent leaves all addressed parties informed and in agreement. This will encourage rational behavior and lessen chances for conflicts and miscommunication over the matter.
If you happen to be looking for more examples of Consent Letters, feel free to check out our Child Travel Consent Forms , to have an idea on how to go about granting your child permission to be accompanied by a designated reliable guardian.
What is a Letter of Consent Form?
A letter of consent form is a document that provides official permission for a specific action or decision. It is often used to grant authority to an individual or organization to carry out activities that require explicit permission from someone with legal rights or powers over the situation.
Here are some common scenarios where a letter of consent form is used:
- Parental Consent: Parents or guardians give permission for their child to participate in an activity, such as a school trip, or to receive medical care.
- Research Consent: Participants in a study agree to the terms of the research and acknowledge that they understand the purpose, procedures, and potential risks involved.
- Property Consent: A property owner allows another party to use their property for a specific purpose, such as filming or construction.
- Travel Consent: Parents authorize their child to travel with an adult guardian, often required for international travel or when one parent is traveling alone with the child.
What are Consent Form Letters for Parents?
Consent form letters for parents are documents that schools, medical facilities, or organizations use to obtain permission from a parent or guardian for their child to participate in certain activities or receive specific services. These forms are designed to inform parents about the details of the activity or service, including any potential risks, and to document their consent or refusal. They are often used for field trips, medical treatments, counseling services, participation in sports, or any other situation where a minor’s involvement requires parental approval. The forms ensure that parents are making an informed decision and that the organization has legal authorization to proceed with the child’s participation. You also browse our Fillable Forms .
How do I write a Consent Letter?
Writing a consent letter involves expressing your permission for something specific in a clear and formal manner. Start by addressing the recipient directly, then state your relationship to the parties involved and explicitly express your consent. Include relevant details such as dates, locations, and any conditions of your consent. Conclude with your contact information, signature, and date. For added clarity and legality, consider having the letter notarized, especially for significant matters like child travel or medical decisions.
What is the Purpose of a Consent Letter?
A consent letter serves to formally document one’s permission or agreement for certain actions or decisions involving another party. This type of letter is crucial in various situations, including but not limited to, authorizing medical treatment, allowing children to travel with one parent or another guardian, or granting permission to use intellectual property. It provides a written record that protects the rights and intentions of all parties involved, ensuring clarity and preventing potential legal disputes.
10 Tips for Letter of Consent

- Be Specific : Clearly define the scope of the consent, including specific activities, duration, and any limitations. Precision helps avoid misunderstandings and legal issues.
- Use Simple Language : Write in plain English to ensure that all parties fully understand the terms and conditions of the consent given.
- Include Relevant Details : Always mention pertinent information such as names, dates, and identification details of all parties involved to personalize and legitimize the consent.
- State the Validity Period : Clearly specify the start and end dates of the consent’s validity to avoid any ambiguity regarding the time frame of the authorization.
- Incorporate Witness Signatures : If possible, include witness signatures to add an extra layer of authenticity and verification to the consent.
- Keep It Formal but Friendly : Maintain a professional tone throughout the letter while ensuring it’s approachable and easy to comprehend.
- Provide Contact Information : Include contact details for all parties involved, offering a straightforward way to clarify any doubts or concerns.
- Mention Revocation Terms : Outline the conditions under which the consent can be revoked, giving the grantor control over their authorization.
- Attach Supporting Documents : If necessary, attach any relevant documents that support the consent provided, such as legal documents or additional agreements.
- Review and Update Regularly : Consent conditions may change over time, so it’s essential to review and, if necessary, update the letter to reflect current circumstances accurately.
By following these tips, you can create a Letter of Consent that is effective in obtaining informed and voluntary consent, while also protecting the rights of all parties involved. Our Printable Forms is also worth a look at
How long is a Letter of Consent Valid for?
A Letter of Consent’s validity varies, often outlined in the document itself, like a Sublease Consent Form , typically ranging from months to years based on its purpose.
What should a Letter of Consent look like?
A Letter of Consent should be formal and clear, containing specific details of the agreement, similar to a Surgical Consent Form , with signatures from all parties involved.
Do I need a Consent Letter?
Yes, a Consent Letter is needed for activities requiring formal permission, such as surveys or tattoos, indicated in Survey Consent Form and Tattoo Consent Form .
What Should be Written in a Consent Form?
A Consent Form should include detailed information on the activity, risks, benefits, and participant rights, akin to a Sexual Consent Form , ensuring informed consent.
What is a Letter of Consent from Parents?
A Letter of Consent from Parents authorizes minors to partake in activities or services, requiring a Service Consent Form and Student Consent Form for validation.
In conclusion, a Letter of Consent is a powerful tool in the authorization process, providing a clear, legal basis for actions taken by others on your behalf. From drafting with the help of Sample Letters and Sample Forms to understanding its implications, this guide ensures you’re well-prepared to grant permission confidently and securely. Remember, the key to effective consent is clarity, specificity, and legal validity.
Related Posts
Free 8+ recommendation letter samples in ms word | pdf, free 6+ law school recommendation letters in pdf, free 5+ debt collection letters in pdf | ms word, free 8+ sample termination letters in pdf | ms word, free 6+ law school recommendation letters in pdf | ms word, free 5+ internship recommendation letters in pdf, free 7+ sample reference letters in pdf | ms word, free 9+ sample resignation letters in pdf | ms word, free 6+ scholarship letter of recommendation in pdf, free 7+ sample job offer letters in ms word | pdf, free 6+sample thank you letter for donation in ms word | pdf, free 8+ sample college recommendation letters in pdf | ms word, free 4+ character reference (for immigration) recommendation letters in pdf, free 8+ sample resign letters in pdf | ms word, free 7+ rent increase letters in pdf | ms word, free 7+ sample teacher appreciation letters in pdf | ms word, free 9+ letter of recommendation samples in ms word | pdf, free 4+ 1 week’s notice resignation letters in pdf, free 9+ sample termination letters in ms word | pdf.

IMAGES
COMMENTS
se this template if your research is NOT. derally-sponsore. A. D participants are adults.Avoid Common Problems with Consent Forms. Read these tips!1. ustomize this template to reflect the specifics of your study and participan. population.Text in [brackets] represents study-specific information that must be added.A ba.
2023-04-10. The following documents are samples. IRBIS does NOT generate these documents with application-specific information. Exempt Research Information Sheet. 2017-10-30. Addendum to provide additional information to subject after original consent. 2024-08-09. SSN Collection for Medicare Recipients. 2017-04-17.
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. General Consent Form Templates Social and Behavioral Research Projects (last updated 03/16/2023)
Contact. Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
If you like, a summary of the results of the study will be sent to you. If you have any other concerns about your rights as a research participant that have not been answered by the investigators, you may contact the Smith College Institutional Review Board at [email protected] or (413) 585-3562.
SAMPLE LETTER OF CONSENT. (Place on Department or Faculty Letterhead) (Insert Date) Dear (Insert Research Participant's Name): You are being invited to participate in a research study on motor development in infants. In particular, we are interested in the motor development of skilled limb movements and corresponding neural development.
Informed Consent in Research. Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of ...
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent ...
The following is a sample consent form for a research project. It is a research project on faculty life on campus, carried out by the principle investigator (PI) of this project from the fake-named Century University. The interviewer (the investigator) should have the interviewee read this form carefully and ask any questions the interviewee ...
Before doing so, however, the IRB must make findings regarding the research justification for different procedures (i.e. a waiver of some of the informed consent requirements must be necessary for the research is to be "practicably carried out.") The IRB must also find that the research involves "no more than minimal risk to the subjects."
The Research Ethics Board must approve any changes to the consent form before the research begins. Changes to an approved study and its documents are done via an Amendment. Your application will be sent back, and approval delayed, if a complete consent form or consent document is not submitted with your application.
An information-consent letter is used most often to inform a potential participant about a research study and to document a participant's agreement to take part in the study. Guide to Creating an Information Letter and Consent Form. The (docx) is intended to provide researchers with the information needed to develop their information letters ...
Letter of Introduction and Informed Consent Form . Study Title: Researchers: Before agreeing to participate in this research, we strongly encourage you to read the following explanation of this study. This statement describes the purpose and procedures of the study. Also described is your right to withdraw from the study at any time.
A Consent Form is read by the participant, signed and handed back to the researcher and should include the following features: 1. Use University of Wollongong/AHS letterhead. 2. Provide the title of the research project, the researcher(s) name, supervisor's name (for student research), the Unit in which the researcher is based and the name of the
In research with children, considerations regarding consent — both process and documentation — become more complex than with adult subjects. See IRB guidance on Children and Minors in Research for information about the documentation needed for consenting children and parents, and use the Sample Consent and Assent Forms for examples of how ...
Prepare the consent forms in the standard format provided in the template, with all headings addressed. Use the standard language provided on the template where appropriate. Do not require the participants to sign consent to long statements written in first person, e.g., "I agree to participate in this research study.
Version. Consent Addendum This is a consent addendum to allow already enrolled participants to agree to additional study procedures not disclosed in the initial consent form. This form supplements the consent and HIPAA authorization the subject already provided for a research study. Consent Addendum. 3-11-2020.
Your specific consent form should include information pertinent to your specific research project, and may need to be considerably different from this sample. The highlighted sections are to be filled in by you.) My name is (name of person doing project), and I am a (student/professor, etc.) at Union College. I am inviting you to participate in ...
Many of these examples are actual UW IRB approved consent forms designed by UW researchers. Some of the examples were created using one of our consent templates. The use of our template is not required and some of the examples deviate significantly from our templates. We encourages researchers to use the Designing the Consent Process guidance ...
2. This resource provides points to consider and sample language for informed consent documents of research studies which plan to store and share data and/ or biospecimens for future use. 3. This resource does not address collection, storage, or sharing of data or biospecimens
Letter of Informed Consent. This research is being conducted by (Insert your full name) who is a student in the College of Business at Westcliff University, Irvine working on a dissertation. This study is a requirement to fulfill my degree and will not be used for decision-making by any organization. This study is for research purposes only.
1. Please note that this is a template developed by the Research Ethics Review Office to assist research proponents in the design of their informed consent forms (ICF). Researchers are encouraged to use this when creating their informed consent forms to best suit the design of their study. Use of alternative wording or format is allowed. 2.
Consent for participation in research interview [name of the project] funded by [name of the sponsor] agree to participate in a research project conducted by Prof. [Name of the Principal Investigator] from the European University Institute (EUI) in Florence, Italy. I have received sufficient information about this research project and ...
A Letter of Consent is a formal document that grants permission or authorizes another party to take specific actions on behalf of the signer. This letter can cover a wide range of scenarios, from parental consent for a child's travel to authorizing medical treatment or financial transactions. It's an essential tool for clearly communicating ...