An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings

Trending Articles
- The multi-stage plasticity in the aggression circuit underlying the winner effect. Yan R, et al. Cell. 2024. PMID: 39406242
- Multiscale drug screening for cardiac fibrosis identifies MD2 as a therapeutic target. Zhang H, et al. Cell. 2024. PMID: 39413786
- IRE1α silences dsRNA to prevent taxane-induced pyroptosis in triple-negative breast cancer. Xu L, et al. Cell. 2024. PMID: 39419025
- Breast Cancer in Users of Levonorgestrel-Releasing Intrauterine Systems. Mørch LS, et al. JAMA. 2024. PMID: 39412770
- Acupuncture vs Sham Acupuncture for Chronic Sciatica From Herniated Disk: A Randomized Clinical Trial. Tu JF, et al. JAMA Intern Med. 2024. PMID: 39401008
Latest Literature
- J Biol Chem (2)
- J Immunol (2)
- PLoS One (111)
- Pediatrics (2)
- Proc Natl Acad Sci U S A (16)
- Science (37)
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Accelerating Biomedical Discovery and Data-Powered Health
Citations for biomedical literature
MedlinePlus
Reliable, up-to-date health information for you
An experimental multimedia search engine
Medical Subject Headings
ClinicalTrials.gov
A database of clinical studies, worldwide
Basic Local Alignment Search Tool
News and Highlights

NLM News, Events, and Updates

Musings from the Mezzanine

NCBI Insights

Circulating Now

NIH MedlinePlus Magazine

Technical Bulletin

NIH Virtual Tour: National Library of Medicine
NLM is the world's largest biomedical library and a national resource for health professionals, scientists, and the public.
News Spotlight
Discovery resource for women’s health research now available.

Partnering with the National Library of Medicine (NLM), the Office of Research on Women's Health (ORWH) launched the first phase of a novel discovery resource for women’s health research (WHR), called DiscoverWHR.
DiscoverWHR is a centralized resource for women’s health research and information from NIH that supports NIH-wide efforts to close the gaps in women’s health across the life course.
This innovative resource simplifies the finding women’s health information by patients, caregivers, medical professionals, researchers, and the public.
Users can explore the following research areas:
- Polycystic Ovary Syndrome (PCOS)
- Rheumatoid Arthritis
- Scleroderma
Learn more about DiscoverWHR , the NIH Discovery Portal for Women’s Health Research.
Research at NLM
Nlm division of intramural research.
Intramural research at NLM consists of the development and application of computational approaches to a broad range of problems in biomedicine, molecular biology, and health. READ RESEARCH HIGHLIGHTS | MEET OUR PRINCIPAL INVESTIGATORS | EXPLORE TRAINING OPPORTUNITIES
Historical Collections at NLM
Biomedical and clinical informatics at nlm, health it and health data standards.

Efficient health care information exchange in the US and worldwide is made possible by NLM’s work with IT Data Standards.
Learn about NLM’s contributions to Health IT
Unified Medical Language System (UMLS) Terminology Services
This set of tooling services brings together many health and biomedical vocabularies and standards to enable interoperability between computer systems.
Explore UMLS
Biomedical Informatics Training Program

This training program provides biomedical and clinical informatics training and research opportunities for individuals at various stages in their career.
Investigate training opportunities

The Library started as a shelf of books in the Surgeon General’s office in 1836 but has grown to a collection of millions of print and electronic resources.
Explore our past
Organization
The diverse centers, divisions, advisory bodies and other organizational units that make up NLM contribute in myriad ways to the Library’s mission.
Explore the Library
Strategic Plan

This ten year plan outlines NLM's role in a future where data and information transform and accelerate biomedical discovery and improve health and health care.
VIEW OUR STRATEGIC PLAN
Sterile fluids information; Florida patients: Hurricane Milton updates
Sterile fluid supply: Like many medical facilities across the nation, our supply chain is feeling the effects of Hurricane Helene’s aftermath. Johns Hopkins Medicine currently has a sufficient sterile fluid supply to meet treatment, surgical and emergency needs. However, we have put proactive conservation measures into place to ensure normal operations, always with patient safety as our first priority. Examples of sterile fluids include intravenous (IV), irrigation and dialysis fluids. Learn more .
Hurricane Milton: Get updates about Hurricane Milton’s impact on Johns Hopkins All Children’s Hospital and our outpatient centers in Florida.
Masks Strongly Recommended but Not Required in Maryland
Respiratory viruses continue to circulate in Maryland, so masking remains strongly recommended when you visit Johns Hopkins Medicine clinical locations in Maryland. To protect your loved one, please do not visit if you are sick or have a COVID-19 positive test result. Get more resources on masking and COVID-19 precautions .
- Vaccines
- Masking Guidelines
- Visitor Guidelines
Understanding Clinical Trials
Clinical research: what is it.

Your doctor may have said that you are eligible for a clinical trial, or you may have seen an ad for a clinical research study. What is clinical research, and is it right for you?
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn which new ideas may help people.
Every drug, device, tool, diagnostic test, technique and technology used in medicine today was once tested in volunteers who took part in clinical research studies.
At Johns Hopkins Medicine, we believe that clinical research is key to improve care for people in our community and around the world. Once you understand more about clinical research, you may appreciate why it’s important to participate — for yourself and the community.
What Are the Types of Clinical Research?
There are two main kinds of clinical research:
Observational Studies
Observational studies are studies that aim to identify and analyze patterns in medical data or in biological samples, such as tissue or blood provided by study participants.
Clinical Trials
Clinical trials, which are also called interventional studies, test the safety and effectiveness of medical interventions — such as medications, procedures and tools — in living people.
Clinical research studies need people of every age, health status, race, gender, ethnicity and cultural background to participate. This will increase the chances that scientists and clinicians will develop treatments and procedures that are likely to be safe and work well in all people. Potential volunteers are carefully screened to ensure that they meet all of the requirements for any study before they begin. Most of the reasons people are not included in studies is because of concerns about safety.
Both healthy people and those with diagnosed medical conditions can take part in clinical research. Participation is always completely voluntary, and participants can leave a study at any time for any reason.
“The only way medical advancements can be made is if people volunteer to participate in clinical research. The research participant is just as necessary as the researcher in this partnership to advance health care.” Liz Martinez, Johns Hopkins Medicine Research Participant Advocate
Types of Research Studies
Within the two main kinds of clinical research, there are many types of studies. They vary based on the study goals, participants and other factors.
Biospecimen studies
Healthy volunteer studies.

Goals of Clinical Trials
Because every clinical trial is designed to answer one or more medical questions, different trials have different goals. Those goals include:
Treatment trials
Prevention trials, screening trials, phases of a clinical trial.
In general, a new drug needs to go through a series of four types of clinical trials. This helps researchers show that the medication is safe and effective. As a study moves through each phase, researchers learn more about a medication, including its risks and benefits.
Is the medication safe and what is the right dose? Phase one trials involve small numbers of participants, often normal volunteers.
Does the new medication work and what are the side effects? Phase two trials test the treatment or procedure on a larger number of participants. These participants usually have the condition or disease that the treatment is intended to remedy.
Is the new medication more effective than existing treatments? Phase three trials have even more people enrolled. Some may get a placebo (a substance that has no medical effect) or an already approved treatment, so that the new medication can be compared to that treatment.
Is the new medication effective and safe over the long term? Phase four happens after the treatment or procedure has been approved. Information about patients who are receiving the treatment is gathered and studied to see if any new information is seen when given to a large number of patients.
“Johns Hopkins has a comprehensive system overseeing research that is audited by the FDA and the Association for Accreditation of Human Research Protection Programs to make certain all research participants voluntarily agreed to join a study and their safety was maximized.” Gail Daumit, M.D., M.H.S., Vice Dean for Clinical Investigation, Johns Hopkins University School of Medicine
Is It Safe to Participate in Clinical Research?
There are several steps in place to protect volunteers who take part in clinical research studies. Clinical Research is regulated by the federal government. In addition, the institutional review board (IRB) and Human Subjects Research Protection Program at each study location have many safeguards built in to each study to protect the safety and privacy of participants.
Clinical researchers are required by law to follow the safety rules outlined by each study's protocol. A protocol is a detailed plan of what researchers will do in during the study.
In the U.S., every study site's IRB — which is made up of both medical experts and members of the general public — must approve all clinical research. IRB members also review plans for all clinical studies. And, they make sure that research participants are protected from as much risk as possible.
Earning Your Trust
This was not always the case. Many people of color are wary of joining clinical research because of previous poor treatment of underrepresented minorities throughout the U.S. This includes medical research performed on enslaved people without their consent, or not giving treatment to Black men who participated in the Tuskegee Study of Untreated Syphilis in the Negro Male. Since the 1970s, numerous regulations have been in place to protect the rights of study participants.
Many clinical research studies are also supervised by a data and safety monitoring committee. This is a group made up of experts in the area being studied. These biomedical professionals regularly monitor clinical studies as they progress. If they discover or suspect any problems with a study, they immediately stop the trial. In addition, Johns Hopkins Medicine’s Research Participant Advocacy Group focuses on improving the experience of people who participate in clinical research.
Clinical research participants with concerns about anything related to the study they are taking part in should contact Johns Hopkins Medicine’s IRB or our Research Participant Advocacy Group .
Learn More About Clinical Research at Johns Hopkins Medicine
For information about clinical trial opportunities at Johns Hopkins Medicine, visit our trials site.
Video Clinical Research for a Healthier Tomorrow: A Family Shares Their Story
Clinical Research for a Healthier Tomorrow: A Family Shares Their Story

- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español

In Memoriam
NIH mourns the loss of John I. Gallin, M.D., whose 50 year career at NIH leaves a lasting legacy for clinician-scientists everywhere.
Learn more »

Nobel Laureates
David Baker receives the 2024 Nobel Prize in Chemistry for research supported by NIH. He shares the prize with Demis Hassabis and John Jumper.

What is CRISPR?
Test your knowledge with this new Kahoot! quiz from NIGMS.

October Is Liver Awareness Month
Learn about the liver's role, including digesting food and processing and distributing nutrients.

October is SIDS Awareness Month
Learn how to reduce baby's risk of Sudden Infant Death Syndrome (SIDS) from Safe to Sleep®.
In the News

Mapping Neural Connections
The first complete map of the fruit fly brain will help us understand larger brains.

West Nile Virus
Read about the latest research on this mosquito-borne disease.

Opioid Use Disorder
Higher doses of buprenorphine may improve treatment outcomes.

Cancer Incidence Rates
Researchers did not see the ‘rebound’ they expected following the pandemic.
NIH at a Glance
Virtual-tour-screenshot-square.jpg.

Take the Virtual Tour
Explore the Bethesda campus and how NIH turns discovery into health.
dr-monica-bertagnolli-thumbnail.jpg

The NIH Director
Monica M. Bertagnolli, M.D., is the NIH Director and provides leadership for the 27 Institutes and Centers that make up NIH.
nih-at-a-glance-funding.jpg
Funding for Research
NIH is the largest source of funding for medical research in the world, creating hundreds of thousands of high-quality jobs.
nih-at-a-glance-labs.jpg

Labs at NIH
Scientists conduct research on NIH campuses across the U.S., as part of our Intramural Research Program.
improving-health-collage.jpg

Impact of NIH Research
NIH-supported research has had a major positive impact on nearly all of our lives.
researcher-holding-petri-dish.jpg

Jobs at NIH
The central recruitment point of access to all NIH jobs and training opportunities
Featured Resources & Initiatives
A new science agency proposed by President Joseph Biden as part of NIH to drive biomedical breakthroughs and provide transformative solutions for all patients.
Anti-Sexual Harassment
NIH does not tolerate pervasive or severe harassment of any kind, including sexual harassment.
Ending Structural Racism
Learn more about NIH’s efforts to end structural racism in biomedical research through the UNITE initiative.
All of Us Research Program
A research effort to revolutionize how we improve health and treat disease.
NIH HEAL Initiative
Trans-agency effort to speed scientific solutions to stem the national opioid crisis.
Clinical Trials
Learn about participating in clinical trials and where to find them.
Accelerating Medicines Partnership
A bold venture to help identify new treatments and cures for diseases.
Medical Research Initiatives
Important initiatives aimed at improving medical research.
Training at NIH
NIH provides training opportunities internally, as well as at universities and other institutions across the U.S.
A new research initiative to understand, prevent, and treat the long-term effects of COVID-19.
COVID-19 Research information from NIH
NIH supports research in COVID-19 testing, treatments, and vaccines. También disponible en español.
Climate Change and Health Initiative
Research to reduce health threats from climate change.
Connect with Us
- More Social Media from NIH
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
How to write and publish a scientific manuscript.
Martin R. Huecker ; Jacob Shreffler .
Affiliations
Last Update: October 31, 2022 .
- Definition/Introduction
A clinician should continuously strive to increase knowledge by reviewing and critiquing research papers and thoughtfully considering how to integrate new data into practice. This is the essence of evidence-based medicine (EBM). [1] When new clinical queries arise, one should seek answers in the published literature. The ability to read a scientific or medical manuscript remains vitally important throughout a clinician's career. When gaps exist in the literature, clinicians should consider researching these questions. Though typically performed by academic doctors or physician-scientists, medical research is open to all clinicians in informal and formal methods. Anyone who treats patients can collect data on outcomes to assess the quality of care delivered (quality improvement is research). [2] Though beyond the scope of this chapter, many resources provide instruction for clinicians on conducting research and contributing to medical science. [3] [4] [5] Additionally, a clinician integrating a new practice can study its effects on patient outcomes retroactively or prospectively. Continuous practice improvement need not be shared with the larger population of treating providers. Still, dissemination to the entire scientific community allows widespread adoption, criticism, or further testing for replication of findings.
- Issues of Concern
Clinicians who seek to conduct retrospective chart reviews, prospective studies, or even randomized, controlled clinical trials should access the many resources to ensure quality methodology. [5] Once you have followed the appropriate steps to conduct a study (Table 1), you should complete the process by writing a manuscript to describe your findings and share it with other clinicians and researchers. Other resources detail the steps in writing a review article, but this StatPearls chapter focuses on writing a scientific manuscript for original research. See also the StatPearls chapter for the different types of research manuscripts. [6]
- Clinical Significance
Steps to Conducting Research
- Develop a research question
- Perform a literature search
- Identify a gap in the literature
- Design a study protocol (including personnel)
- Submit to an institutional review board for approval
- Collect, responsibly store, and then analyze data
- Write a manuscript to interpret and describe your research.
After conducting a quality investigation or a study, one should assemble an abstract and manuscript to share results. Researchers can write an abstract in a short amount of time, though the abstract evolves as the full manuscript moves to completion. Many published and presented abstracts do not reach full manuscript publication. [7] [8] Although journals and conferences often publish abstracts, studies with important results should be published in full manuscript form to ensure dissemination and allow attempts at replication. [9] IRB protocols, study design, and data collection and aggregation require a team effort. Those involved in the research should discuss who contributes to the full manuscript (ie, qualify as an author) and, thus, the planned order of authorship to reduce complications at the time of manuscript submission. The author who devotes the most effort to the paper is typically the first and corresponding author. In contrast, the last author is often the team's most senior member and often the study's principal investigator. All individuals listed as authors should contribute to the manuscript and overall project in some fashion. [10] The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist is perhaps the most valuable tool for preparing a manuscript for submission [11] . Original research manuscripts have the following sections (in chronologic order):
- Introduction (Background and Objectives)
- Methods (Design, Setting, Participants, Variables, Statistics)
- Results (Participants, Descriptives, Outcomes, Subgroups)
- Tables and Figures
- Discussion (Key findings, Limitations, Interpretations)
- Conflict of Interest (COI), Author affiliations, Acknowledgments, Funding
- References [11]
Individuals involved in the IRB submission (before data collection) can write the introduction and methods of the manuscript before and during the data collection and analysis process. This head start on writing makes the full manuscript composition task less formidable. The content of the introduction and methods should be well-known to the study group before data collection and analysis. The introduction should be organized into a “problem/gap/hook” order: what problem this study addresses, the precise gap in the literature, and the study's objectives (in addressing the gap). [12] The methods should provide enough detail so readers who want to replicate the study can do so.
Once data is collected and analyzed, authors can write an abstract to organize the major themes of the research, understanding that the abstract undergoes edits once the manuscript is complete. Similarly, the title can change with revisions as authors determine the most salient trends in the data. Most readers only read the title +/- abstract. Thus, these are the most important sections of the paper. The title should be concise and directly describe the trial result, which also helps generate more citations. The abstract must convey the crucial findings of the paper, ideally divided into sections for easier reading (unless the desired journal does not allow this). [13] With the larger picture in mind, authors should create tables and figures that visually convey the themes of the data analysis. Working with statisticians or data experts, authors should devote much time to this manuscript component. Some general concepts: [14]
- Only include tables/figures that you believe are necessary.
- Make sure tables/figures are high-quality, simple, clear, and have concise captions.
- Do not repeat language in results that appear in tables/figures; the tables/figures should stand alone.
- Consider how the figure looks in grayscale (in case the journal is not in color)
As with the abstract and title, the tables and figures likely undergo further edits before the manuscript's completion. The abstract and tables/figures should intuitively evolve together to convey the story of the research project. Now, refer back to the introduction and methods composed during data collection. Make revisions as necessary to reflect the overall narrative of the project. Ensure you have adhered to the originally determined objectives or hypotheses. Next, focus on the results and discussion. The results should contain only objective data with no interpretation of significance. Describe salient results that have not been explained in the figures and tables. The discussion section begins with a lead paragraph highlighting the most important findings from the study. Then, the discussion interprets the current results in light of prior published literature. Ensure citation of keystone papers on this topic, including new papers published since embarking on the current project. Frame your results, describing how this study adds to the literature. The discussion section usually includes study limitations. Attempt to anticipate criticisms of the methodology, the results, the organization of the manuscript itself, and the (ability to draw) conclusions. A stronger limitations section preempts journal reviewer feedback, potentially simplifying the revision/resubmission process.
The conclusion section should be concise, conveying the main take-home points from your study. You can make recommendations for current clinical practice and for future research endeavors. Finally, consider using citation management software such as Endnote or Mendeley. Though initially cumbersome, these software platforms drastically improve revision efforts and allow easy reference reformatting. All authors should review the manuscript multiple times, potentially sharing it with other uninvolved colleagues for objective feedback. Consider who should receive acknowledgment for supporting the project and prepare to disclose conflicts of interest and funding. Although authors should have an initial idea of which journal to submit to, this decision is more straightforward once the manuscript is near completion. Journal rankings are beyond the scope of this StatPearls chapter. Still, generally, one should devise a list of the journals within a specialty in order of highest to lowest impact factor (some sites categorize into tiers). High-quality prospective research and clinical trials have a higher likelihood of acceptance into the more prestigious journals within a specialty or to the high-quality general science or medicine journals. Although many journals have an option for open access publication, and numerous legitimate, open access journals now exist, beware of ‘predatory journals’ that charge a fee to publish and may not be indexed in Pubmed or other databases. [12]
Journals have diverse guidelines for formatting and submission, and the manuscript submission process can be tedious. Before submission, review Bordage’s paper on reasons for manuscript rejection. [15] Most journals require a title page and cover letter, representing an opportunity to lobby for your paper’s importance. When (not if) you experience manuscript rejections, take reviewer comments and recommendations seriously. Use this valuable feedback for resubmission to the original journal (when invited) or subsequent submission to other journals. When submitting a requested revision, compose a point-by-point response to the reviewers and attach a new manuscript with tracked changes. Attempt to resubmit manuscripts as promptly as possible, keeping your work in the hands of journals (allowing you to work on other research). [14]
- Nursing, Allied Health, and Interprofessional Team Interventions
The above logistic steps differ for review articles, case reports, editorials, and other submissions. [16] However, the organization, precise methods, and adherence to journal guidelines remain important. See work by Provenzale on principles to increase the likelihood of acceptance for original and revised manuscripts. After submission, revision, resubmission, and proofing, you may experience the fulfillment of an official publication. Academics should promote their scientific work, enhancing the dissemination of research to the wider scientific community. [17] [18] [17] [19]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Martin Huecker declares no relevant financial relationships with ineligible companies.
Disclosure: Jacob Shreffler declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Huecker MR, Shreffler J. How To Write And Publish A Scientific Manuscript. [Updated 2022 Oct 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- The future of Cochrane Neonatal. [Early Hum Dev. 2020] The future of Cochrane Neonatal. Soll RF, Ovelman C, McGuire W. Early Hum Dev. 2020 Nov; 150:105191. Epub 2020 Sep 12.
- Rules to be adopted for publishing a scientific paper. [Ann Ital Chir. 2016] Rules to be adopted for publishing a scientific paper. Picardi N. Ann Ital Chir. 2016; 87:1-3.
- Telemedicine for the Medicare population: pediatric, obstetric, and clinician-indirect home interventions. [Evid Rep Technol Assess (Summ)...] Telemedicine for the Medicare population: pediatric, obstetric, and clinician-indirect home interventions. Hersh WR, Wallace JA, Patterson PK, Shapiro SE, Kraemer DF, Eilers GM, Chan BK, Greenlick MR, Helfand M. Evid Rep Technol Assess (Summ). 2001 Aug; (24 Suppl):1-32.
- Review Evidence Brief: The Quality of Care Provided by Advanced Practice Nurses [ 2014] Review Evidence Brief: The Quality of Care Provided by Advanced Practice Nurses McCleery E, Christensen V, Peterson K, Humphrey L, Helfand M. 2014 Sep
- Review Culture of Care: Organizational Responsibilities. [Management of Animal Care and ...] Review Culture of Care: Organizational Responsibilities. Brown MJ, Symonowicz C, Medina LV, Bratcher NA, Buckmaster CA, Klein H, Anderson LC. Management of Animal Care and Use Programs in Research, Education, and Testing. 2018
Recent Activity
- How To Write And Publish A Scientific Manuscript - StatPearls How To Write And Publish A Scientific Manuscript - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Medical research articles within Nature
News | 18 October 2024
How does the brain react to birth control? A researcher scanned herself 75 times to find out
Extensive scans reveal rhythmic changes in the brain throughout the menstrual cycle and while on the pill.
Spotlight | 16 October 2024
Clinical-trial reporting is on the rise
Although some countries have made marked strides in reporting trial results, others still face a long road ahead.
- Inga Vesper
Article 16 October 2024 | Open Access
Spatial proteomics identifies JAKi as treatment for a lethal skin disease
Cell-type-resolved spatial proteomics of the skin from patients with toxic epidermal necrolysis reveals that it is driven by JAK/STAT signaling, leading to successful treatment of this potentially fatal condition in patients using JAK inhibitors.
- Thierry M. Nordmann
- , Holly Anderton
- & Matthias Mann
News | 14 October 2024
No bandage needed: electrical impulses to major nerve help stop bleeding
A ‘neural tourniquet’ increases blood-clot stability in human tests.
Obituary | 11 October 2024
Francisco Lopera obituary: neurologist who traced genetic origin of early-onset Alzheimer’s
Clinician whose family-centred approach upended work on mid-life memory loss.
- Kenneth S. Kosik
Book Review | 07 October 2024
How to keep a president healthy — tips from a former White House doctor
From quitting smoking to dealing with anthrax, a fascinating memoir looks behind the scenes at presidential health care.
- Henna Hundal
News | 04 October 2024
World-first therapy using donor cells sends autoimmune diseases into remission
The treatment’s success in three people raises hopes for mass production of cutting-edge CAR-T therapies.
- Smriti Mallapaty
Nature Index | 02 October 2024
The global imbalance of neurological conditions
Neurological conditions affect more than 40% of the population, and some countries are struggling to cope.
Neurotechnology race ramps up, but fundamental questions remain
The United States is setting the pace, but other nations are following their own paths.
United States sets the pace for implantable brain–computer interfaces
As development of the technology accelerates, countries are weighing the costs and benefits of how they regulate it.
Ones to watch: young neuroscientists on the rise
Using creative approaches and next-generation tools, these researchers hope to shed light on long-standing mysteries in neuroscience.
- Felicity Nelson
- & Benjamin Plackett
Will the big neuroscience brainstorm pay off?
Investment in large neuroscience initiatives is starting to show results, but questions remain over whether they can solve the most fundamental questions about cognition.
- James Mitchell Crow
How long COVID could lift the fog on neurocognitive disorders
Insights from a new critical mass of patients are proving invaluable.
- Michael J. Peluso
- & E. Wesley Ely
News Feature | 02 October 2024
Can flashing lights stall Alzheimer’s? What the science shows
Pulses of light and sound seem to have beneficial effects. But some argue it is too soon to market experimental devices.
- Elie Dolgin
News | 27 September 2024
Revolutionary drug for schizophrenia wins US approval
The medication is the first in decades to have a different mode of action from those of current drugs, achieving better symptom relief with fewer side effects.
Outlook | 25 September 2024
A drug-free prescription for pain
Non-invasive, non-pharmacological approaches such as virtual-reality therapy and yoga can help to relieve chronic pain.
- Carolyn Brown
How a ‘pain-o-meter’ could improve treatments
Pain is defined subjectively, but an objective measure of the experience promises to transform its management
The gut microbiome and chronic pain
Pain from conditions such as endometriosis could be alleviated if the gut’s resident bacteria can be understood and tamed.
- Clare Watson
News | 25 September 2024
Data integrity concerns flagged in 130 women’s health papers — all by one co-author
Some of the studies listed in a peer-reviewed paper as potentially problematic have been included in analyses that could inform clinical practice.
- Mariana Lenharo
Perspective | 25 September 2024
On human-in-the-loop optimization of human–robot interaction
A new approach to designing robotic systems that interact closely with people, called human-in-the-loop optimization, can improve human–robot interaction, but many important research questions remain before it can reach its full potential.
- Patrick Slade
- , Christopher Atkeson
- & Steven H. Collins
News | 19 September 2024
Obesity-drug pioneers win prestigious Lasker Award for medical science
Three scientists are honoured for developing a class of blockbuster weight-loss drugs. Is a Nobel prize on the way?
Research Highlight | 18 September 2024
Thalidomide-like drug staunches bleeding from genetic disease
Severe nosebleeds caused by hereditary hemorrhagic telangiectasia dwindled in people who took a drug used to treat cancer.
News & Views | 18 September 2024
Gut microbes fend off harmful bacteria by depriving them of nutrients
Microorganisms that normally reside in the gut can help to block microbial infection. A group of bacteria that lives in the human gut has been identified that can consume a nutrient needed by pathogenic bacteria.
- Eric G. Pamer
News Explainer | 17 September 2024
Should young kids take the new anti-obesity drugs? What the research says
Evidence shows that blockbuster weight-loss medications can reduce obesity even in children aged 6–11 years, but their long-term effects on growing bodies are unknown.
- Julian Nowogrodzki
News Feature | 17 September 2024
Doctors cured her sickle-cell disease. So why is she still in pain?
Gene and cell therapies bring fresh hope to people with genetic disorders, but recovery can be complex and long-term support remains sparse.
- Heidi Ledford
News & Views | 11 September 2024
Long-lasting heart-failure treatment could be a game-changer
Boosting the heart’s natural countermeasures against poor function is one way to treat heart failure, but existing therapies need to be given frequently. An antibody with enduring effects could offer a solution.
- John C. Burnett Jr
Lipid recycling by macrophage cells drives the growth of brain cancer
In brain tumours, immune cells called macrophages scavenge lipid debris from the myelin sheath of neurons. These lipid-laden macrophages cause immunosuppression, and their transfer of lipids to tumours fuels cancer growth.
- Lisa Sevenich
News | 11 September 2024
Why some women enter menopause early — and how that could affect their cancer risk
Genomic analysis reveals a host of genetic variants that affect how quickly fertility ends, among them one that reduces reproductive span by six years.
Nature Podcast | 11 September 2024
Ancient DNA debunks Rapa Nui ‘ecological suicide’ theory
Study refutes claim that mismanagement of natural resources led to population crash — plus a tiny wasp that’s been found in an unexpected place.
- Benjamin Thompson
- & Nick Petrić Howe
Correspondence | 10 September 2024
Artificial intelligence can help to make animal research redundant
- Calvin Drakos
- , Vineesha Manimangalam
- & Ozlem Equils
Update regulator guidance to show that animal research really is no longer king
- Lindsay Marshall
- , Rebecca Simmons
- & Greg Sower
The pharmaceutical industry must embrace synthetic alternatives to horseshoe-crab blood
- & Lawrence Niles
World View | 10 September 2024
Europe sidelines Alzheimer’s drug: lessons must be learnt
If the European Medicines Agency takes an overly cautious approach to selecting specialists to advise on new medicines, people could be left without treatments.
- Henrik Zetterberg
News | 09 September 2024
World’s first whole-eye transplant: the innovations that made it possible
The first face transplant to also include an eyeball was a surgical coup — but restoring vision remains a challenge.
News | 05 September 2024
Transparent mice made with light-absorbing dye reveal organs at work
A method that renders skin temporarily see-through could offer researchers a non-invasive way to look inside the bodies of live mice.
- Jude Coleman
News & Views | 04 September 2024
Breast cancer blocked by multiple natural lines of defence
Cancer-promoting mutations are common in healthy tissue but rarely lead to tumour formation. A study of the mouse mammary gland reveals three protective mechanisms that limit the ability of cells to give rise to cancer.
- Biancastella Cereser
The immune system of trans men reveals how hormones shape immunity
What drives sex biases in the immune system? Examining how gender-affirming therapy affects the molecular profiles of immune cells of trans men reveals how hormones underpin immune responses.
- Margaret M. McCarthy
Article 04 September 2024 | Open Access
Immune system adaptation during gender-affirming testosterone treatment
Examination of immunological changes in transgender individuals undergoing gender-affirming testosterone treatment reveals sex hormone-regulated pathways in humans and explains sex-divergent responses in cisgender individuals.
- Tadepally Lakshmikanth
- , Camila Consiglio
- & Petter Brodin
World View | 03 September 2024
African scientists must not be priced out of mental-health research
Under-representation of African populations in mental-health studies perpetuates inequities — change is needed.
- Vivien Chebii
News & Views | 03 September 2024
Skull bones harbour immune cells that are poised to target brain tumours
The human brain is usually considered to be beyond the reach of most immune cells. However, analysis of people who have brain tumours has revealed tumour-targeting T cells of the immune system in skull bones near the cancer site.
- & Justin D. Lathia
News | 03 September 2024
How rival weight-loss drugs fare at treating obesity, diabetes and more
Wegovy, Zepbound and similar medications all lead to metabolic improvements, but scientists are starting to unpick the differences between them.
News & Views | 28 August 2024
People who lack the immune protein TNF can still fight infection
The immune-signalling protein TNF has an essential role in inflammatory responses. Two people who were found to have no functional TNF are surprisingly healthy and able to fend off most infections, but are susceptible to tuberculosis.
- Charlie J. Pyle
- & David M. Tobin
Article 28 August 2024 | Open Access
Fate induction in CD8 CAR T cells through asymmetric cell division
We show that target-induced proximity labelling enables isolation of first-division CD8 chimeric antigen receptor T cells that asymmetrically distribute their surface proteome and transcriptome, resulting in distinct phenotypic, metabolic and functional profiles in proximal and distal daughter cells.
- Casey S. Lee
- , Sisi Chen
- & Christoph T. Ellebrecht
Correspondence | 27 August 2024
Urgently clarify how AI can be used in medicine under new EU law
- Thomas J. Hwang
- & Prokar Dasgupta
News | 22 August 2024

Debate rages over Alzheimer’s drug lecanemab as UK limits approval
The medicine is being assessed by agencies including the European Union regulator, but the community is divided on its efficacy and safety.
Book Review | 21 August 2024
Forever young: what science can and can’t tell us about cheating ageing
High-profile advances, such as anti-ageing drugs called senolytics, have sparked hope that old age and death could be postponed considerably, and have even fostered fantasies of eternal life. But the reality is more nuanced.
- Jan H. J. Hoeijmakers
News Feature | 21 August 2024
The testing of AI in medicine is a mess. Here’s how it should be done
Hundreds of medical algorithms have been approved on basis of limited clinical data. Scientists are debating who should test these tools and how best to do it.
Career Feature | 20 August 2024
Whistleblowing in science: this physician faced ostracization after standing up to pharma
Physician scientist Nancy Olivieri describes hard-won lessons from decades of fighting for scientific integrity.
- Sara Reardon
Research Highlight | 16 August 2024
Child with ultra-rare disease gets a treatment just for her
Therapy designed for one seems to have improved a young girl’s quality of life.
News | 16 August 2024
Hopes dashed for drug aimed at monkeypox virus spreading in Africa
Early results from clinical trial show that the antiviral drug tecovirimat is no better than placebo against the clade I virus type.
Browse broader subjects
- Health sciences
Browse narrower subjects
- Drug development
- Epidemiology
- Experimental models of disease
- Genetics research
- Outcomes research
- Paediatric research
- Preclinical research
- Stem-cell research
- Clinical trial design
- Translational research
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Medical Research
Scientists and physicians in academic medicine conduct groundbreaking biomedical research that improves our knowledge of human health and promotes the development of treatments from bench to bedside to community.

Through policy and advocacy initiatives, data and research projects, professional learning and networking opportunities, and the development of tools and resources, the AAMC supports the entire spectrum of medical research from basic discovery and translational science to clinical and population health research, research policies and regulations that promote robust and ethical science and minimize administrative burden, a diverse biomedical research workforce and an inclusive and equitable research environment across all career stages.
On this page:
Science policy updates .
AAMC updates on federal science policy and regulatory topics impacting institutions and researchers can be found below.
The AAMC submitted a letter to FDA and HHS on draft guidance.
- May 3, 2024
AAMC submitted comments to the Department of Justice regarding access to bulk sensitive personal data by countries of concern.
- April 26, 2024
AAMC submitted comments to the National Institutes of Health (NIH) on the updated NIH Strategic Plan for Data Science, 2023-2028
- March 15, 2024
For more on the latest legislative and regulatory activities affecting academic medicine, check out Washington Highlights . For the latest news, current trends, and ongoing conversations about the most important topics in academic medicine, visit AAMCNews .
Meet our New Chief Scientific Officer

As chief scientific officer, Elena Fuentes-Afflick, MD, MPH, leads AAMC programs that support medical research and the training of physician-scientists and researchers in academic medicine. In this role, she provides leadership and vision for addressing research and science policy and other related critical issues facing academic medicine, medical schools, teaching health systems, and teaching hospitals. Learn more about Dr. Fuentes-Afflick .
Connect with Colleagues
The AAMC convenes several affinity groups focused on connecting individuals who work in biomedical research or support researchers at their institution. Affinity groups include councils, professional development groups, and other organizations that provide individuals at member institutions access to professional growth, leadership development, networking, and collaboration opportunities.
Find information about how to join each group on the pages below.
FOCI provides a national forum for leadership who oversee and manage conflict of interest related to research, medical education, and clinical decision-making.
GRAND convenes research leaders in discussion of issues critical to the research enterprise and linking research advancements with improvements to health.
GREAT provides a forum for discussion of the research training enterprise with leadership of biomedical graduate, postdoctoral, and MD-PhD programs.
CFAS represents the collective interests of medical school faculty and academic societies on a range of cross-cutting issues.
The GDI unites expertise, experience, and innovation to guide the advancement of diversity, equity, and inclusion throughout medicine and biomedical sciences.
The research subgroup of COF promotes compliance and ethical conduct in academic medicine with a specific focus on research and laboratory issues.
Attend an Event
The AAMC is committed to providing professional learning opportunities for biomedical researchers in every stage of their career. Find information about the AAMC’s webinars, meetings, and more below.
Related AAMC Initiatives
The AAMC engages in other work that may be of interest to researchers and scientists:
The Center sparks community-centered, multi-sector research, collaboration, and action to make the case for policies and practices that ensure all communities have an equal opportunity to thrive.
An optimal research environment that drives impactful biomedical discovery is supportive, diverse, equitable, and inclusive.
The Medical Science Liaison: A Critical Link Between Research and Clinical Practice

Anna Naim, PharmD
Oct 15, 2024
12 minutes read
What is the role of a MSL?
In the evolving field of the pharmaceutical industry, the role of a Medical Science Liaison (MSL) has become increasingly vital. The MSL role is a specialized role within Medical Affairs that focuses on cultivating relationships with Key Opinion Leaders (KOLs) (may also be termed External Experts or Thought Leaders) and facilitating the exchange of scientific information acting as a linker between the organization and the health care professionals. These individuals act as the bridge between clinical practice, research, and commercial interests linking the internal environment–such as organizationals dynamics and resources within a pharmaceutical company– to the external environment, which includes healthcare professionals, researchers, and patient advocacy groups. The key role of the MSL is to ensure that scientific insights translate to meaningful patient outcomes by ensuring that healthcare professionals have a deep understanding of the therapeutic area as well as the specific products.
The Interview Process:
Candidates typically meet with various members of the Medical Affairs team and the Medical Science Liaison manager or director. At the end of the interview process , applicants are often asked to present on a clinical or scientific topic of their choice to assess their ability to articulate and communicate information effectively. Typically the interview process has 3 steps. The first step is a phone screen by the recruiter. Step two involves talking with the hiring manager by phone. Step 3 is the final step in the process where you usually interview with a panel of internal stakeholders and are asked to do a clinical presentation. Step 3 can be virtual or in person.
MSL Compensation/Salary:
The starting base salary of a MSL can range from $125,000 to $130,000 and go up to over $200,000. The starting base salary is dependent on the experience and degree of the individual. MDs will typically have a higher starting base salary compared to a PharmD or PhD. Additionally, MSLs may receive a bonus of 15 to 25% of the base salary, stocks, and sometimes a company car or car stipend depending on the company, although these benefits can change depending on location, company size, and specific employer, making this a very lucrative role from a financial standpoint.
Choosing Your Company
The choice between the size or type of the company may be difficult as both have their advantages and disadvantages.
Big Pharma: Large pharmaceutical companies are well known and have established processes with more job security.However, the financial stability and pipeline of the particular company you work for might have a significant impact on your ability to grow in the field.
Biotech Companies: These are mid sized companies making it easier for individuals to enter due to less competition. There is a potential for rapid growth and significant stock value increases if the company is acquired by big pharma. At the same token, there is a higher risk due to the possibility of disbanding the medical affairs team if products do not get approval, essentially offering less job security compared to big pharma.
Travel and Territory Sizes: Some companies have very large territories that cover several states. This would require several overnight trips per month. Some companies have very small territories where very little overnight travel is required.
Day to Day of a MSL
The primary goal for the day to day activities for a MSL is to be in the field every day meeting with healthcare professionals. These interactions can include live meetings, virtual meetings, phone calls, and/or emails - depending on the HCP. Striving to maximize daily interactions is crucial. MSLs are tasked with several critical responsibilities that are composed of three main buckets - education, support for medical meetings, and clinical research in addition to other key responsibilities. Furthermore, MSLs devote an incredible amount of time to keeping up with the most current research in their therapeutic area. This frequently entails reading scientific literature, attending conferences, and participating in webinars to stay informed on clinical knowledge.
Education: MSLs provide training and education to healthcare professionals ensuring that they are well informed about the latest advancements, new therapies, clinical data and the best practices to be used. MSLs provide product training to healthcare professionals about the mechanism, efficacy, and safety of the company's products. MSLs provide disease education offering insights into disease state management and up to date guidelines and medical advancements in a balanced and timely manner to keep healthcare professionals up to date. MSLs can also be responsible for internal training to the sales and marketing teams on the scientific aspects of the products and therapeutic area(s). Although common, this is not the case for all organizations. MSLs are responsible for communicating and educating complex scientific and clinical information to healthcare professionals. With this, MSLs should be prepared to respond to questions from healthcare providers about the company’s products. This extensive knowledge allows the MSL to answer unsolicited requests for a broad range of information. Delivering presentations at medical conferences and meetings can also be expected to educate a broader crowd.
Support: This includes one of the primary responsibilities of an MSL which is to engage with key opinion leaders to build collaborative, non-promotional relationships in the medical community and facilitate scientific discussions. MSLs build and maintain relationships with KOLs to gather insights on clinical practices and/or unmet medical needs as well as providing updates on new research findings and ongoing clinical trials. Liaisons also gather cutting edge knowledge from researchers and thought leaders and bring insights back to the company.
Clinical Research: This role entails assisting in the design and execution of clinical trials by providing scientific insights and ensuring the clinical relevance of the study. MSLs have the ability to nominate investigators sites which have the appropriate patient population for the trial. MSLs also build and maintain relationships with the clinical trial investigators since they assist in the identification and initiation of clinical trial sites. While MSLs are not responsible for data collection and analysis, clinical research is crucial for data collection and analysis since gathering and analyzing the data from these ongoing trials will provide insights to the clinical development team. MSLs assist in clinical trial support, site and investigator nominations, support site initiation visits, educate sites and communicate trial results based on approved content.

Skills and Qualifications of a MSL
To be effective, MSLs must possess a unique set of skills and qualifications. A Medical Science Liaison at minimum should have a doctoral degree whether that be a PhD, PharmD, MD, OD, etc. While a doctorate degree is essential to facilitate peer to peer discussions (doctor to doctor), not all companies require it. MSLs should build a strong background in the relevant therapeutic area that they cover. It is important to recognize that specifications can be learned within the company, but real life clinical and research experience in the relevant therapeutic field is more beneficial. The real life clinical and research experience are what make the conversations with the health care professionals more impactful and relatable.
Communication: Communication skills are critical as the role depends on the ability to convey complex scientific information clearly and effectively to diverse audiences. MSLs should be able to relay this information in a clear manner and possess strong verbal skills with an ability to tailor messages depending on the audience.
Relationship building and Interpersonal skills: Relationship building and interpersonal skills is a pivotal requirement for the MSL role. Strong interpersonal skills are required to establish and maintain relationships. This skill is especially important to the MSL role as MSLs are responsible for data dissemination. Establishing a level of trust and credibility with KOLs, HCPs, and other stakeholders ensures that they are receptive to the information shared by MSLs.
Build relationships with your clinical background leveraging your clinical expertise to establish more credibility when engaging with KOLs
Relationships with KOLs can be built by seeking introductions from existing contacts rather than making cold calls
Most MSL roles involve taking over territories with pre-existing relationships and companies typically provide this list of KOLs
Use technology platforms to identify and engage with KOLs. This can be started with an email and using cross-functional relationships to compliantly collaborate and get introductions to form new relationships
Analytical skills: Analytical skills may be significant to analyze clinical data and derive insights to inform strategic decisions.
Independence and Time Management: The role of a MSL involves being able to work independently and manage one’s own territory. A MSL is responsible for all activities within their assigned therapeutic area and structuring their own schedule.
Attitude and Emotional Intelligence: These skills are essential as individuals are typically hired for the medical science liaison role based on attitude. While skills can be taught, a positive attitude and emotional intelligence are crucial.
Adaptability: As the healthcare field is constantly evolving, MSLs should be adaptable and open to continuous learning to stay ahead and updated on the latest scientific advancements and regulatory changes. MSLs should have a passion for lifelong learning as that is what the role entails.
Resilience and Patience: In the scientific community, the role of the MSL can often be misunderstood by other healthcare professionals. It is imperative to communicate the role and expertise a MSL has and establish oneself as a scientific expert.
Gathering and Utilizing Insights to Drive Medical Strategy
Gathering Insights begins with identifying the gaps in knowledge and guidelines, ensuring diversity within clinical trials, staying on top of new technology and advancements and addressing roadblocks that may arise.
Identify Knowledge Gaps: Identify the areas where more education is needed
Diversity in Trials: Ensure diversity and inclusion within clinical trials.
Gaps within Guidelines: Educate around clinical guidelines and identify unmet needs
New Advancements: Stay informed and up to date about compounds and new research
Roadblocks: Identify and address institutional roadblocks that may arise. Ask what can your company do to assist in overcoming roadblocks
Utilizing Insights: enter insights into the CRM and other platforms and these insights should drive medical strategies to help meet the needs of your specific area

Challenges within the MSL Role
Regulatory and Compliance Challenges: MSLs engage in non-promotional scientific discussion. Ensuring that communication between healthcare professionals and key opinion leaders are compliant and non promotional requires a deep understanding of regulatory requirements and being able to adapt to strategies accordingly. MSLs may potentially have conflicts of interest, especially when talking about off-label usage or communicating with medical professionals who are also involved in clinical trials.
Rapidly changing environment: The rapid pace of scientific advancements can pose a challenge for MSLs as they may not be able to keep up the pace with the most recent scientific advancements. MSLs must stay current with the latest research and clinical developments to provide accurate and up to date information to KOLs, HCPs, and other stakeholders.
Balance: The role of a MSL requires them to be present for multiple stakeholders. MSLs should balance the needs and expectations of various KOLs, HCPs, and other stakeholders as well as their own organization. The different stakeholders may have different priorities, concerns, wants and needs which makes it especially important for MSLs to be able to create this balance.
Tools for Success for MSLs
A rapidly changing environment is a challenge that MSLs face on a day to day basis in their role. To be successful, it is important for them to stay updated on therapeutic areas, especially their assigned therapeutic area.
Training Programs: Most companies provide robust programs and training tailored to the specific therapeutic area a MSL works in. This ensures that MSLs have a solid foundation of knowledge in their tailored therapeutic area
Skill development: Continuous development of training skills to consistently stay proficient and effective in communicating scientific information
Attending Conferences: Participating in major regional and CMA conferences provides valuable opportunities to learn about the latest advancements
Identifying Gaps in Therapy: To be successful, MSLs should have an understanding of the current gaps in therapeutic areas and staying informed about upcoming products and treatments in the company's pipelines as well as the products of competitors
Patient Insights: MSLs primarily interact with healthcare professionals who communicate patient experiences. Listening to how treatments have positively impacted patients can offer a unique perspective that can enhance a MSL’s understanding of the product to a new level
Continuous Learning: Engaging in ongoing learning through peer interaction, webinars, educational sessions and company programs can ensure that MSL’s stay at the forefront of their field and effectively communicate to their team
The Future of the MSL Role
Digital Technology: The future of the role will heavily be based on integration of digital technology since digital tools can enhance data collection, improve engagement and provide new platforms for scientific communication. Furthermore, in order to stay connected and keep up with technology changes, MSLs may have to acquire new skills including advanced data analysis and efficient use of digital communication platforms. This is because the demand for real-world evidence is only growing. For MSLs to continue to be successful in their profession, these abilities must continue to develop.
Real World Evidence: The demand for real world evidence grows daily which requires MSLs to gather and interpret real world data to support safety and efficacy of new and current therapies. This will require a closer collaboration with healthcare providers to collect meaningful data and insights
Patient Centered Focus: There has been a shift toward patient centered care that continues to shape the role of a MSL. MSLs will need to engage more closely with healthcare professionals and patient advocacy groups to incorporate patient perspectives into their strategies.
Evolving Skill Sets: Future MSLS will most likely need to be proficient in digital and AI technology, pharmacogenomics, bioinformatics and have a diverse set of knowledge in healthcare information. The role will evolve to be more involved in virtual engagements and MSLs will be required to act as the first line of information for healthcare providers. The future of the MSL role will most likely take away from an individual being an expert in one therapeutic area and having a more broader and diverse understanding
Advice for Aspiring Medical Science Liaisons
Set Your Goal: MSL role is widely regarded as one of the most rewarding positions in the pharmaceutical industry. Aim to secure a role as a MSL
Interview Proactively: Apply for every available MSL position regardless of your perceived qualifications. You might possess qualities or experiences that apply to employers even if they are not immediately obvious to you. The MSL role is more than what is on your CV/resume. Engaging in as many interview as possible is key to gain experience and improve your chances of landing the role of a MSL
Networking: Networking is crucial. Network internally within your organization and externally. Connect with current MSLs and seek opportunities to shadow current MSLs. This will give you valuable insight into the role and help you understand the specific requirements and skills that MSLs possess. Connect with colleagues and industry professionals and reach out to learn from their experiences. Many successful MSLs have benefited from the advice and insights shared by other MSLs in the field
Contract MSL Role: If you lack direct experience, consider starting with a contract MSL position. The contract MSL position can carve a pathway into a permanent role in the future
Self Reflection: Reflect on whether the MSL role suits your lifestyle and preferences. The MSL position often involves travel, requires adaptability and independence that comes with an extensive amount of responsibilities. If you prefer a predictable schedule this role may not be the best for you
The Medical Science Liaison role is a dynamic position that is critical to the success of Medical Affairs. By fostering relationships with KOLs, facilitating scientific exchange and supporting clinical development these individuals ensure that the latest scientific insights are effectively translated into improved patient care.
References:
Dixson, K., Kelly, T., Mastanduono, J. Everything you need to know about being a medical science liaison (MSL) . Jul 2024. YouTube. https://www.youtube.com/watch?v=Tm6xJLwZaPE&t=1s&ab_channel=AccreditationCouncilforMedicalAffairs
Solimon, W. Medical science liaison role| insider secrets . Dec 2022. YouTube. https://www.youtube.com/watch?v=BtIL03M4Grc&ab_channel=WilliamSoliman
Excel your medical affairs career with BCMAS
Recognized Globally as the Badge of Excellence for Medical Science Liaisons & Medical Affairs Professionals
Keep up with medical affairs trends
Sign up for our newsletter (no spam)
Similar Blogs

Career Development
Medical Science Liaison Salary Insights: How much do MSLs m...
Medical science liaisons earn highly competitive salaries influenced by factors like region, therapeutic area, and performance met...

Kiana Dixson, PharmD
Dec 13, 2023

Top 7 Reasons to Become a Medical Science Liaison and How B...
"Unlock a rewarding MSL career with BCMAS certification: Competitive salary, autonomy, ethical impact, and diverse opportunities i...

Mar 28, 2024

Medical Affairs Essentials: A Day in the Life of a Medical ...
Explore a day in the life of an MSL with insights from Monica Saleh, PharmD. Discover the varied tasks and challenges faced by Med...

Monica Saleh
Feb 1, 2021
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Medical Honey for Wound Care—Still the ‘Latest Resort’?
Kirsten traynor, gisela blaser, peter molan.
- Author information
- Article notes
- Copyright and License information
For reprints and all correspondence: Dr med. Arne Simon, Paediatric Haematology and Oncology, Children's Hospital Medical Centre, University of Bonn, Adenauerallee 119, 53113 Bonn, Germany. Tel: 0049 (0) 2282873-3254/3255/3305; Fax: 0049 (0) 2282873-3301; E-mail: [email protected]
Corresponding author.
Received 2007 Jul 19; Accepted 2007 Nov 5; Issue date 2009 Jun.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( http://creativecommons.org/licenses/by-nc/2.0/uk/ ) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
While the ancient Egyptians and Greeks used honey for wound care, and a broad spectrum of wounds are treated all over the world with natural unprocessed honeys from different sources, Medihoney™ has been one of the first medically certified honeys licensed as a medical product for professional wound care in Europe and Australia. Our experience with medical honey in wound care refers only to this product. In this review, we put our clinical experience into a broader perspective to comment on the use of medical honey in wound care. More prospective randomized studies on a wider range of types of wounds are needed to confirm the safety and efficacy of medical honey in wound care. Nonetheless, the current evidence confirming the antibacterial properties and additional beneficial effects of medical honey on wound healing should encourage other wound care professionals to use CE-certified honey dressings with standardized antibacterial activity, such as Medihoney™ products, as an alternative treatment approach in wounds of different natures.
Keywords: Medical honey, wound care, MRSA
Introduction
In our paediatric oncology department at the Children's Hospital Medical Centre, University of Bonn, Germany most patients suffer from a profoundly suppressed immune system, due to their underlying disease (i.e. leukemia) ( 1 ) and the chemotherapy they undergo. This frequently results in wound healing problems ( 2 , 3 ), leaving the patient susceptible to wound infections. Unfortunately these infections spread easily in immunocompromized patients and can cause secondary potentially life-threatening bloodstream infections ( 4 ).
Five years ago a 12-year old patient was submitted to our unit. Doctors at another hospital had removed an abdominal lymphoma, leaving an open drainage site on his abdomen. On admission, his wound was infected with methicillin-resistant Staphylococcus aureus (MRSA). In order to avoid nosocomial spread, the patient was immediately isolated, a difficult situation for the child to comprehend with significant additional costs from the perspective of the hospital. Although the patient was scheduled to receive chemotherapy, treatment could not commence until the infection cleared. The wound was treated with a local antiseptic (octenidin) for 12 days. Since no improvement occurred, we decided to use an Australian medical honey (Medihoney™), which contains leptospermum honey, a type with excellent in vitro activity against MRSA ( 5–7 ). The wound was free of bacteria two days later, and the chemotherapy against the underlying illness could be started.
While the ancient Egyptians and Greeks used honey for wound care, a broad spectrum of wounds are treated all over the world with natural unprocessed honeys from different sources ( 8–13 ), Medihoney™ has been one of the first medically certified honeys licensed as medical product for professional wound care in Europe and Australia ( 14 , 15 ). Our experience with medical honey in wound care refers only to this product. We were asked by one of the editors of eCAM to put our clinical experience ( 2 , 16 ) into a broader perspective and to comment on the use of medical honey in wound care (Table 1).
Key information from this review
| What makes medical honey effective? | Medical honey is hygroscopic, meaning it draws moisture out of the environment and thus dehydrates bacteria. The enzyme glucose oxidase, produces gluconic acid and minute amounts of hydrogen peroxide when in contact with the wound surface. In addition, each charge contains light- and heat-stable, confirmed antibacterial properties from spp. honeys. The pH level of honey is low (mean 4.4). Bacterial colonization or infection and recalcitrant wound healing situations are often accompanied by high pH values in wound exudates and lowering the pH speeds healing. |
| Why is medical honey irradiated? | Honey may contain spores from , which are inactivated. The ready to use product is delivered sterile. |
| What is Medihoney™? | Medihoney is a mixture of two honeys derived from Australia and New Zealand containing glucose oxidase and compounds which contribute to its antibacterial activity. |
| Legal background | Medihoney™ is licensed for wound care in Australia, Europe and the USA. In Europe a CE-certification exists declaring Medihoney™ as medical product. |
| Practical advances | Medical honey dressings are easy to change without pain and without harm to the regenerating tissue. Malodour from recalcitrant wounds as a result of critical colonization and partial tissue necrosis is successfully abandoned with medical honey due to its antibacterial, anti-inflammatory and debriding effects. Medical honey can be used in all different stages of wound healing, in many different types of wounds and even in patients with diabetes. Medical honey is well accepted by most patients and their families. |
| Drawbacks | Some patients (5 out of 100) experience pain after the application of medical honey to the wound. In some of them, treatment with medical honey has to be stopped. Few patients with pre-existing atopic diseases show local atopic reactions. No severe systemic atopic reaction due to medical honey has been reported in the literature. Medical honey has to be kept in the wound for 12–24 h a day. Thus, it is combined with particular dressings like calcium alginates of hydrofiber dressings, which add substantially to the overall cost of treatment. Depending on the amount of exudate, the dressing with medical honey has to be changed up to 2 times a day in acute inflammatory wounds. The most often practised change interval for medical honey dressings is every 24–48 h. |
| Scientific evidence | For Medihoney™: one prospectively randomized controlled study considering the prevention of catheter-related bacteremias in patients with dialysis catheters. Many studies and animal studies confirming the antibacterial properties and the positive effects on wound healing. Growing body of clinical experiences and observational studies derived from independent wound care facilities all over the world. |
#For the medical use of honey in general please refer to the outstanding review of Peter Molan from 2006 ( 15 )
What Makes Medical Honey Effective?
Honey works differently from antibiotics, which attack the bacteria's cell wall or inhibit intracellular metabolic pathways. Honey is hygroscopic, meaning it draws moisture out of the environment and thus dehydrates bacteria. Its sugar content is also high enough to hinder the growth of microbes, but the sugar content alone is not the sole reason for honey's antibacterial properties.
When honey is diluted with water, reducing its high sugar content, it still inhibits the growth of many different bacterial species that cause wound infections ( 14 , 15 , 17–20 ).
Leptospermum spp. are known by a number of common names in Australia and New Zealand including Tea Tree, Manuka, Goo Bush and Jelly Bush. A least 79 species of Leptospermum have been described in Australia and New Zealand ( 21 ). The medical honey, our clinical experience refers to (Medihoney™), consists of standard mixture of different Leptospermum spp. honeys ( Fig. 1 ), and exhibits a standard antibacterial activity as confirmed by appropriate in vitro testing methods ( 22 ).

Pictures of different Leptospermum spp. plants from Australia.
Unlike glucose oxidase, the antibacterial properties from Leptospermum spp. honeys are light- and heat-stable. Neither type of activity is influenced by the final sterilizing procedure of gamma-irradiation ( 23 ). Over 100 substances are candidates for the particular antibacterial property of these honeys, but the active ingredient has not yet been identified. Even if the hydrogen peroxide activity is blocked and the osmotic effect of honey is circumvented by dilution, selected honeys from Leptospermum spp. still display significant antibacterial effects. Natural honey of other sources can vary as much as 100-fold in the potency of their antibacterial activity (which is due to hydrogen peroxide) ( 5–7 , 24–27 ).
In addition to its antibacterial properties, medical honey hastens the healing of wounds through its anti-inflammatory effects. The amount of wound exudate is related to the activity of the local inflammatory process, in particular in wounds, which are colonized or infected with bacteria. Thus, the anti-inflammatory action of honey reduces oedema and the amount of exudate by down regulating the inflammatory process. It also reduces pain, as the pain in wounds results from the nerve endings being sensitized by prostaglandins produced in the process of inflammation, as well from the pressure on tissues resulting from oedema.
Due to its high sugar content, honey also prevents pain on dressing changes as it keeps the wound surface moist by mobilizing the oedema from the surrounding tissues.
The anti-inflammatory action of honey has been extensively observed clinically ( 19 , 28 , 29 ) and in animal models ( 15 ). Animals do not demonstrate any placebo effects, as they are incapable of having attitudes influence their healing process, such as believing that natural products would be more effective, or from hearing via the news media of the effectiveness of honey in wound treatment ( 30 ). Thus, these observational and histological studies can be cited as convincing evidence for the positive results with honey not being due to a placebo effect. Similarly, this can be concluded also for the observations in wounds in animal models of honey stimulating the rate of angiogenesis, granulation and epithelialization ( 31 , 32 ), which would explain the findings in clinical trials that honey speeds up the healing process ( 15 ).
Although the mechanism(s) by which honey speeds up the healing process have not been determined, there are some findings, which provide possible explanations. One way in which honey may work is through its stimulation of an inflammatory response in leukocytes ( 28 , 29 , 33 ), as inflammation is what triggers the cascade of cellular events that give rise to the production of growth factors which control angiogenesis and proliferation of fibroblasts and epithelial cells. Very recently, Tonks et al . discovered a 5.8 kDA component of manuka honey which stimulates the production of TNF-α in macrophages via Toll-like receptor 4 ( 34 ).
Another mechanism may be related to the pH level of honey being low (3.4–5.5; mean 4.4) ( 35 , 36 ). Bacterial colonization or infection and recalcitrant wound healing situations are often accompanied by pH values >7.3 in wound exudates ( 37 , 38 ). It has been demonstrated that acidification of wounds speeds healing ( 39 ), this being attributed to the low pH increasing the amount of oxygen off-loaded from hemoglobin in the capillaries. More recently it has also been attributed to suppression of protease activity in wounds by getting away from the neutral pH that is the optimum for their activity ( 37 ). Excessive protease activity in a wound can slow or prevent healing by destroying growth factors (which are proteins) and destroying the protein fibres and fibronectin in the wound matrix, attachment to which activates fibroblasts and is necessary for the migration of these and of epithelial cells ( http://www.worldwidewounds.com/2005/august/Schultz/Extrace-Matric-Acute-Chronic-Wounds.html ). This protease activity results from excessive inflammation. The anti-inflammatory activity of honey would thus remove this impediment to healing, as would the antibacterial activity working through removing infecting bacteria stimulating the inflammatory response.
The remarkable debriding action of honey that has been noted by many would also assist in this by removing slough which is a rich source of bacteria to stimulate an inflammatory response ( 19 ).
Last but not least, medical honey successfully manages the problem of malodour from chronic colonized wounds, which results in severe discomfort and social isolation in these patients. We relate the experience of one female patient with relapsed breast cancer after chemotherapy and irradiation. Her husband informed us, that other members of the family would not enter her room, because her wound smelled like a ‘dead rabbit’. This malodour problem was successfully eliminated with the application of medical honey in 2 weeks.
Why is the Final Product Irradiated?
Clostridium botulinum spores pervade our environment, existing in the soil, air, dust and raw agricultural products. Due to the possibility of Clostridial contamination and many reports of infant botulism in the literature since the first description of this syndrome in 1976, it has been recommended by paediatricians, not to feed infants with honey and to place warning labels on packaging, as done in the United Kingdom and Norway ( 36 ).
In deep wound cavities the possibility exists of an anaerobic environment, where the spores could proliferate and produce botulinum toxin. Negative effects such as paralysis and cardiac arrhythmia have been described related to systemic effects of the toxin. To eliminate botulism spores with heat, honey must be heated to 120°C (248°F) for 10 min, which results in adverse changes to some of honeys’ beneficial properties. Since spores have occasionally been found in honey, each batch of Medihoney™ is gamma irradiated to inactivate spores such as those from Clostridium spp. This does not have a detrimental impact on the antibacterial activity of honey. On the other hand, irradiating honey is only a safety measure on the side of caution since we ( 40 ) could not detect a single case report in the literature of C. botulinum wound infection related to the use of non-irradiated honey in wound care ( 41 ).
Medihoney is not an Antiseptic
An ideal wound antiseptic would meet the following criteria ( 42 ):
Fast onset of bactericidal action and a remnant, broad spectrum effect against bacteria and fungi, even under the unfavorable conditions of exudating, colonized or infected wounds.
Enhancement and acceleration of the physiologic process of wound healing (debridement, granulation), even if applied for prolonged periods.
No adverse local or systemic effects (allergy, toxicity related to absorption).
Moderate cost even if applied two times daily.
Medihoney meets all of the above criteria except ‘a fast onset of activity’, as it does not seem to produce the desired reduction of bacteria (more than five log steps) and fungi in 1–10 min to be qualified as an antiseptic.
What About Conventional Wound Care Products in Paediatric Oncology?
Some of the parents in our unit have at first been sceptical about the benefits of honey in wound care. However, the scientific evidence for using conventional wound care products in paediatric oncology patients is non-existent, since no prospective randomized studies have been performed in this particular population and no research has been done on the long-term effects of modern conventional treatments such as silver dressings.
There has been a report of a Silver-coated dressing which caused raised liver enzymes and an argyria -like syndrome in an adolescent burn patient ( 43 ). Another common treatment is povidone iodine, which has the advantage of antiseptic properties and is well suited for skin disinfection prior to invasive procedures. However, the antiseptic activity of iodine products is hampered by interactions with the protein content of the wound exudate and severe adverse effects of systemic absorption of iodine on thyroid function must be considered in infants and toddlers as well as in adult patients with latent hyperthyreosis.
In principle, the same problem exists with alcohol-containing antiseptics, since they are almost completely absorbed and need to be metabolized by the children, who are treated concomitantly with many other drugs. In contrast, medical honey does not display the problem of systemic absorption and thus can even be utilized in patients with diabetes mellitus without adverse effects on blood glucose levels.
Medical Honey in Severely Immunocompromized Patients?
One of our patients with acute myeloic leukemia in relapse had a wound infection with methicillin-resistant coagulase-negative staphylococci after thoracic surgery for invasive Aspergillus infection of the lung, but was allocated to allogenic bone marrow transplantation. Using medical honey, the infection was eliminated.
The patient continued to receive medical honey applications during and after the transplantation ( Fig. 2 ), leading to a successful healing without further local or systemic complications.
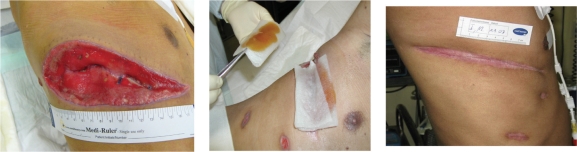
Thoracotomy wound of a patient with acute myeloic leukemia after surgical site infection (Medihoney™ was applied on a calcium-alginate dressing and left in the wound for 24 h).
Medical Honey for Recalcitrant Wounds
Many of the 150 wound care patients we have treated with medical honey had previously received specialist attention for recalcitrant wounds ( 2 , 3 , 16 , 44 ). When treatment with honey was commenced, their wound status changed to healing from non-healing ( 2 , 3 , 16 , 44 ). Similar findings have been reported by others ( 19 , 45 ).
Medihoney™ for the protection Of Catheter Entry Sites
The clinical usefulness of hemodialysis catheters is limited by increased infectious morbidity and mortality. Topical antiseptic agents, such as mupirocin, are effective at reducing this risk but have been reported to select for antibiotic-resistant strains. Johnson et al . ( 46 ) performed a randomized, controlled trial comparing the effect of thrice-weekly exit-site application of Medihoney™ versus mupirocin on infection rates in patients who were receiving hemodialysis via tunnelled, cuffed central venous catheters. A total of 101 patients were enrolled. The incidences of catheter-associated bacteremia in honey-treated ( n = 51) and mupirocin-treated ( n = 50) patients were comparable (0.97 versus 0.85 episodes per 1000 catheter-days, respectively; NS). No exit-site infections occurred. During the study period, 2% of staphylococcal isolates within the hospital were mupirocin resistant. Thrice-weekly application of standardized antibacterial honey to hemodialysis catheter exit sites was safe, cheap, and effective and resulted in a comparable rate of catheter-associated infection to that obtained with mupirocin (although the study was not adequately powered to assess therapeutic equivalence).
The effectiveness of honey against antibiotic-resistant microorganisms and its low likelihood of selecting for further resistant strains suggest that this agent may represent a satisfactory alternative means of chemoprophylaxis in patients with central venous catheters. In our paediatric oncology unit, all entry sites of tunnelled central venous catheters, which show any sign of inflammation or dehiscence, are covered with medical honey.
Honey Treatment for Prevention of Oral Mucositis and for Gingivitis
Many cancer patients suffer from mucositis, a side effect of chemotherapy that attacks the entire gastrointestinal tract from the mouth to the anus. The cancer treatment breaks down the epithelial cells lining the tract, leaving the patient prone to ulcerations and infections. These important and beneficial cells replicate and divide rapidly, which is why in a typical healthy individual wounds in the mouth heal quickly. Chemotherapy does not distinguish between healthy and malignant cells, attacking all that reproduce rapidly, including these epithelial cells.
Thus 20–40% of all cancer patients receiving intensive chemotherapy suffer from mucositis, the number climbs to 80% when chemotherapy and radiation are combined, and staggers even higher in patients receiving treatment for cancer in the head and neck area. Open sores in cancer patients suffering from mucositis leave them susceptible to infection. In a study conducted in 2003, Biswal et al . ( 47 ) investigated the use of honey in 40 adult patients with head and neck cancer. Patients consumed 20 ml (one and one-third teaspoon) of pure honey 15 min before, 15 min after and 6 h post-treatment. There was significant reduction in the symptomatic grade three-fourth mucositis (which describes the necessity for morphine infusion and parenteral nutrition) among honey-treated patients compared with controls; i.e. 20% versus 75% ( P < 0.001).
The compliance of the honey-treated group of patients was better than controls. A total of 55%patients treated with topical honey showed no change or a positive gain in body weight compared with only 25% in the control arm ( P = 0.05). According to a recent review, honey has potential for the treatment of periodontal disease, mouth ulcers and other problems of oral health ( 48 ), and a trial has demonstrated a statistically significant difference between chewing gelled honey and chewing chewing-gum in decreasing the number of bleeding sites on gums with gingivitis ( 49 ).
Use of Honey for Wound Care in Neonates
Due to the safeness of Medihoney™, we used this product for wound care in premature neonates. One infant patient had a recalcitrant wound after surgical intervention for meningomyelocele. The wound stagnated, and during intensive care treatment, it became colonized with multiresistant Klebsiella oxitoca . Before treatment with Medihoney™ was initiated, the child had received numerous intravenous antibiotics and many other conventional wound care treatments to clear the wound, spending the first 3 months of its life in the hospital. Medihoney™ dressings cleared the wound of the pathogen and allowed the patient to be discharged after 3 weeks. The wound healed completely without further surgical intervention. Viardi et al . ( 40 ) published an impressive case series including nine neonates with polymicrobial wound infection after surgery for congenital heart disease. All infants were considered as treatment failures, since chlorhexidine 0.05% plus fusidic acid ointment plus systemic antibiotics did not result in any improvement of the sternal wound infection. All infants showed marked clinical improvement after 5 days of treatment with topical application of 5–10 ml of fresh unprocessed (non-pasteurized, non-irradiated) honey twice daily. The wounds were closed, clean and sterile in all infants after 21 days of honey application. There were no adverse reactions to the treatment. In a recent review for Neonatal Network , Susan Bell reviewed the current literature dealing with the medical use of medical honey in neonatal wound care. Nurses in her unit are using medical honey for this purpose since 2005. She came to the conclusion that the scientific evidence regarding the use of honey for wound treatment in neonates and infants is interesting, but still not strong. The sample sizes in the available clinical studies were small; there were no comparison groups and no randomization. She stated that double-blinded randomized controlled clinical trials with sufficient power are needed to determine the efficacy of honey in both initial wound management and secondary treatment of infected and poorly healing wounds. It has to be questioned, how double or even single (on the side of the patent) blinding can be established in studies, which investigate medical honey for wound care and compare it with other conventional modes of treatment.
Honey Treatment Against Viral Disease
Al-Waili et al . published a cross over trial ( 9 ) in which 16 adult patients with a history of recurrent attacks of herpetic lesions, 8 labial and 8 genital, were treated by topical application of honey for one attack and acyclovir cream for another attack. For labial herpes, the mean duration of attacks and pain, occurrence of crusting and mean healing time with honey treatment were 35, 39, 28 and 43% shorter, respectively, than with acyclovir treatment. For genital herpes, the mean duration of attacks and pain, occurrence of crusting and mean healing time with honey treatment were 53, 50, 49 and 59% shorter, respectively, than with acyclovir. Two cases of labial herpes and one case of genital herpes remitted completely with the use of honey. No side effects were observed with repeated applications of honey, whereas three patients developed local itching with acyclovir. The authors came to the conclusion that topical honey application is safe and effective in the management of the signs and symptoms of recurrent lesions from labial and genital herpes ( 9 ). According to this experience, we treat children and adults with recurrent herpetic lesions on the lips with medical honey, as soon as a new lesion is developing. In addition, we use topical medical honey in addition to systemic acyclovir in immunocompromized patients with zoster to prevent secondary bacterial skin infection and to accelerate healing of the herpetic lesions.
Medical Honey in Chronic Ocular Surface Diseases
Albietz et al . ( 50 ) recently published the results of a study that assessed the effect of Medihoney™ antibacterial honey on the ocular flora in patients with dry eye caused by tear deficiency and/or Meibomian gland disease. In this prospective, open-label pilot study, bacteria isolated from the eyelid margin and conjunctiva were identified and quantified before and at 1 and 3 months after initiation of treatment with topical application of antibacterial honey thrice daily. The total colony-forming units (CFUs) isolated from each of the dry eye subgroups before antibacterial honey use was significantly greater than the total CFU isolated from the non-dry eye group.
Use of Medihoney™ Antibacterial honey significantly reduced total CFUs for the eyelids and the conjunctiva of dry eye subjects from baseline at month 1 and 3. At month 3, the total CFUs for all dry eye subgroups were not significantly different from those of the non-dry eye group. A review of the medical uses of honey ( 8 ) has cited several other reports of honey, an ancient treatment for sore eyes, being found to be successful in modern ophthalmology.
Comparing Different Types of Honey
A comparison of different types of honey, which is beyond the scope of this article, may be an important issue in future trials ( 24 ). In particular the results obtained by Prof. Descotte from France, presented during several international Apitherapy meetings, point into this direction; unfortunately they have not been published in a medline listed journal. Irish et al . ( 51 ) found Jarrah honey (derived from Eucalyptus marginata ), which contains higher amounts of glucose oxidase, to be significantly more effective against Candida spp. in vitro ; the activity of the other honeys including Medihoney™ against Candida spp. with minimal inhibitory concentrations above 40% (w/v) were not distinguishable from the activity of an artificial honey.
Minimal Requirements for Clinical Use
From a professional medical perspective honey used in wound care should have a proven antibacterial activity against the most important pathogens in wound infection ( S. aureus, Pseudomonas aeruginosa ) ( 16 , 52 ) measured with an appropriate microbiological in vitro method ( 5 , 25 , 27 , 51 , 52 ). In addition, it should be irradiated ( 23 ) in particular if it is used in deep or partially necrotic wounds ( 53 ) which possess a potential of anaerobic wound conditions. Surely, we do not criticize the emergency care use of raw honey directly derived from local bee keepers in countries with extremely limited medical resources ( 54 ) in which physicians or other health care workers do not have access to conventional wound care products ( 13 , 55–57 ).
Who Should Supervise the Use of Medical Honey in Clinical Practice?
For safety reasons, wound care with medical honey should always be supervised by a physician or an experienced wound care nurse in patients with chronic complicated wounds or with significant comorbidities. The ‘honey approach’ to wound care must be part of a comprehensive wound care concept. Any comorbidity which contributes to the problems in wound healing should be diagnosed and treated thoroughly with state of the art interventions (e.g. bypass surgery in patients with reduced arterial perfusion, compressive bandages in patients with chronic venous ulcers, sufficient control of blood glucose levels in patients with diabetic food syndrome) ( 58 ).
Honey Must be Kept in Contact to the Wound
Medical honey dressings should keep the honey in contact with the wound for at least 12 h, but preferably for 24 h. Some patients apply the wound dressing overnight, so as not to restrict their mobility during the day. If the dressing is inappropriate, the honey may be washed out of the wound by exudate. The clinical benefits of medical honey including antibacterial protection, wound cleaning and pH modulation may as a result be reduced. From our experience ( 2 ) and the experience of others ( 59 ), the best way to keep the honey in the wound is to soak medical honey into a calcium-alginate or hydrofiber dressing, which forms a gel with the honey as it absorbs the exudate. The various registered medical honey products available, some in the form of prepared honey-impregnated dressings, have been described ( 14 , 45 ).
When to Use Systemic Antibiotics Concomitantly
In case of an infected wound or in case of colonization with a multiresistant bacterial isolate, a hospital grade, licensed antiseptic (e.g. octenidine) is applied to the wound in addition to Medihoney™ on the first 2 days of treatment. In patients with neutropenia (neutrophil count <0.5 × 10 9 l −1 or leukocyte counts <1 × 10 9 l −1 and no differential count available) systemic antibiotics have to be administered concomitantly after a local wound swab has been taken if fever or local sign reveals soft tissue infection. With hospitals facing increasing problems of bacterial resistance, it is important to note that the use of medical honey has never been observed to foster bacterial resistance ( 20 ).
Frequency of Dressing Changes
The frequency of dressing changes depends on the amount of exudate. In early stages, fresh surgical wounds infected with pathogenic bacteria may necessitate a dressing change twice a day. In stable wound care situations, the Medihoney™ dressing has been left in place for up to 7 days ( 58 , 60 ). Prospective studies confirming the safety of such an extended dressing change interval are not yet available.
Compliance Issues
Dunford et al . ( 61 ) undertook a four-center feasibility study to determine whether Medihoney™ is an acceptable treatment for patients with leg ulcers in terms of pain relief, odour control and overall patient satisfaction. A total of 40 patients whose leg ulcers had not responded to 12 weeks of compression therapy were recruited. Medihoney dressings were applied on their ulcers for the 12-week study period. All other aspects of their care, including the use of compression bandaging, remained unchanged. Overall, ulcer pain and size decreased significantly and odorous wounds were deodorized promptly.
These results revealed a high patient acceptance for and concordance with this treatment. The same first author reported the care of an adolescent with multiple wounds, infected with resistant bacterial pathogens after meningococcal sepsis and limb amputation ( 62 ). Before the use of medical honey, each dressing change had to be performed under general anaesthesia to alleviate pain and anxiety. Shortly after the introduction of medical honey dressings, the wounds improved and dressing changes could be performed without any analgesic medication. We have had similar experiences with children in our clinic. If the medical honey dressing is completely moistened with sterile Ringer solution, it can easily and painlessly be removed without any negative attachment to the wound. In our unit and our wound care ambulance, parents or relatives are educated in the aseptic procedure of the dressing change and thus, they are enabled to perform it at home.
Adverse Effects
There are two important adverse effects related to the use of medical honey. We have observed in about 5% of our patients stinging pain after administration. This problem may be circumvented by the conditioning of the wound surface with a sterile anaesthetic cream. Unfortunately, local anaesthetics cause vasoconstriction. Thus, the application of an anaesthetic to the wound surface may result in an objectionable reduction of local perfusion. In some patients who experienced pain after administration, treatment with medical honey had to be stopped ( 16 ) or postponed to later phases of wound healing after the acute inflammatory process has been controlled by other approaches. Two of 150 patients from our patient population showed reproducible local atopic reactions to Medihoney™. Both children had an underlying atopic disposition. We are not aware of any severe systemic atopic reaction related to the use of medical honey from our experience and from the published literature.
Cost Issues
Ingle et al . ( 63 ) from South Africa performed a randomized controlled trail to investigated the use of natural honey (monofloral aloe honey) in shallow wounds, donor sites for skin grafting and partial thickness burns in 44 goldmine workers. They compared this group with another group of 43 workers whose wounds were treated with IntraSite™ Gel (Smith and Nephew Ltd). They did not find significant differences in healing time, adverse events (itching, pain) and satisfaction with treatment but the average cost of the use of honey was 4% that of IntraSite™ treatment. More prospective studies investigating the overall cost of treatment with medical honey compared with conventional wound care approaches are awaited ( 64 ) in particular in chronic, recalcitrant wounds. In clinical practice, the use of calcium alginate or hydrofiber gauzes and semipermeable dressings to keep the honey in the wound has to be added to the acquisition cost of the medical honey. To contain the demands on personnel resources, it would be of outstanding interest to develop ready to use medical honey dressings, which have to be changed only every 2–3 days even in acute wound care situations.
WoundViewer © Database
We recently developed a database for the standardized prospective collection of datasets considering the clinical use of medical honey products in wound care to foster the collection of clinical evidence to help design prospective comparative studies in the near future.
More prospective randomized studies on a wider range of types of wounds are needed to confirm the safety and efficacy of medical honey in wound care ( 65 ). Nonetheless, the current evidence ( 15 ) confirming the antibacterial properties and additional beneficial effects of medical honey on wound healing should encourage other wound care professionals to use CE-certified honey dressings with standardized antibacterial activity, such as Medihoney™ products, as an alternative treatment approach in wounds of different natures.
Acknowledgements
The research of K.T. was made possible by the generous support of the Humboldt Foundation through a German Chancellor Scholarship to the author. The development of the WoundvViewer© database for the prospective standardized documentation of wound care courses in patients treated with medical honey has been financially supported by Medihoney Inc. ( http:/www.medihoney.com ).
- 1. Lehrnbecher T, Foster C, Vazquez N, Mackall CL, Chanock SJ. Therapy-induced alterations in host defense in children receiving therapy for cancer. J Pediatr Hematol Oncol. 1997;19:399–417. doi: 10.1097/00043426-199709000-00001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Simon A, Sofka K, Wiszniewsky G, Blaser G, Bode U, Fleischhack G. Wound care with antibacterial honey (Medihoney) in pediatric hematology-oncology. Support Care Cancer. 2006;14:91–7. doi: 10.1007/s00520-005-0874-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Gaur AH, Liu T, Knapp KM, Daw NC, Rao BN, Neel MD, et al. Infections in children and young adults with bone malignancies undergoing limb-sparing surgery. Cancer. 2005;104:602–10. doi: 10.1002/cncr.21212. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Gaur AH, Flynn PM, Shenep JL. Optimum management of pediatric patients with fever and neutropenia. Indian J Pediatr. 2004;71:825–35. doi: 10.1007/BF02730723. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Lusby PE, Coombes AL, Wilkinson JM. Bactericidal activity of different honeys against pathogenic bacteria. Arch Med Res. 2005;36:464–7. doi: 10.1016/j.arcmed.2005.03.038. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Cooper RA, Molan PC, Harding KG. The sensitivity to honey of gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol. 2002;93:857–63. doi: 10.1046/j.1365-2672.2002.01761.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Cooper RA, Molan PC, Harding KG. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J R Soc Med. 1999;92:283–5. doi: 10.1177/014107689909200604. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Molan PC. Why honey is effective as a medicine. 1. Its use in modern medicine. Bee World. 1999;80:80–92. [ Google Scholar ]
- 9. Al-Waili NS. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med Sci Monit. 2004;10:MT94–8. [ PubMed ] [ Google Scholar ]
- 10. Al-Waili NS. Topical application of natural honey, beeswax and olive oil mixture for atopic dermatitis or psoriasis: partially controlled, single-blinded study. Complement Ther Med. 2003;11:226–34. doi: 10.1016/s0965-2299(03)00120-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Al-Waili NS. Therapeutic and prophylactic effects of crude honey on chronic seborrheic dermatitis and dandruff. Eur J Med Res. 2001;6:306–8. [ PubMed ] [ Google Scholar ]
- 12. Al-Waili NS. Clinical and mycological benefits of topical application of honey, olive oil and beeswax in diaper dermatitis. Clin Microbiol Infect. 2005;11:160–3. doi: 10.1111/j.1469-0691.2004.01013.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Al-Waili NS. Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. J Med Food. 2004;7:210–22. doi: 10.1089/1096620041224139. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Molan PC, Betts JA. Clinical usage of honey as a wound dressing: an update. J Wound Care. 2004;13:353–6. doi: 10.12968/jowc.2004.13.9.26708. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds. 2006;5:40–54. doi: 10.1177/1534734605286014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Blaser G, Santos K, Bode U, Vetter H, Simon A. Treatment of MRSA-colonized or infected wounds with Medihoney™ antibacterial honey products. J Wound Care. 2007;16:325–8. doi: 10.12968/jowc.2007.16.8.27851. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Molan PC. The role of honey in the management of wounds. J Wound Care. 1999;8:415–8. doi: 10.12968/jowc.1999.8.8.25904. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Molan PC. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol. 2001;2:13–9. doi: 10.2165/00128071-200102010-00003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Molan PC. Re-introducing honey in the management of wounds and ulcers - theory and practice. Ostomy Wound Manage. 2002;48:28–40. [ PubMed ] [ Google Scholar ]
- 20. Blair S, Carter D. The potential for honey in the management of wound and infection. J Australian Infection Control. 2005;10:24–31. [ Google Scholar ]
- 21. Thompson J. A revision of the genus Leptospermum (Myrtaceae). National herbarium of New South Wales, Royal Botanic Gardens, Sydney, Australia. Telopea (Syd.) 1988;3:301–449. [ Google Scholar ]
- 22. Bang LM, Buntting C, Molan P. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J Altern Complement Med. 2003;9:267–73. doi: 10.1089/10755530360623383. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Molan PC, Allen KL. The effect of gamma-irradiation on the antibacterial activity of honey. J Pharm Pharmacol. 1996;48:1206–9. doi: 10.1111/j.2042-7158.1996.tb03922.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Molan PC. The antibacterial activity of honey. 2. Variation in the potency of the antibacterial activity. Bee World. 1992;73:59–76. [ Google Scholar ]
- 25. French VM, Cooper RA, Molan PC. The antibacterial activity of honey against coagulase-negative staphylococci. J Antimicrob Chemother. 2005;56:228–31. doi: 10.1093/jac/dki193. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Cooper R, Molan P. The use of honey as an antiseptic in managing Pseudomonas infection. J Wound Care. 1999;8:161–4. doi: 10.12968/jowc.1999.8.4.25867. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Cooper RA, Halas E, Molan PC. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J Burn Care Rehabil. 2002;23:366–70. doi: 10.1097/00004630-200211000-00002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Tonks AJ, Cooper RA, Jones KP, Blair S, Parton J, Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine. 2003;21:242–7. doi: 10.1016/s1043-4666(03)00092-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Tonks A, Cooper RA, Price AJ, Molan PC, Jones KP. Stimulation of TNF-alpha release in monocytes by honey. Cytokine. 2001;14:240–2. doi: 10.1006/cyto.2001.0868. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Shmueli A, Shuval J. Are users of complementary and alternative medicine sicker than non-users? Evid Based Complement Alternat Med. 2007;4:251–5. doi: 10.1093/ecam/nel076. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Gupta S, Singh H, Varshney A, Prakash P. Therapeutic efficacy of honey in infected wounds in buffaloes. Indian J Anim Sci. 1992;62:521–3. [ Google Scholar ]
- 32. Bergman A, Yanai J, Weiss J, Bell D, David M. Acceleration of wound healing by topical application of honey. An animal model. Am J Surg. 1983;145:374–6. doi: 10.1016/0002-9610(83)90204-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Abuharfeil N, Al-Oran R, Abo-Shehada M. The effect of bee honey on proliferative activity of human B- and T-lymphocytes and the activity of phagocytes. Food Agri Immunol. 1999;11:169–77. [ Google Scholar ]
- 34. Tonks AJ, Dudley E, Porter NG, Parton J, Brazier J, Smith EL, et al. A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J Leukoc Biol. 2007;82:1147–55. doi: 10.1189/jlb.1106683. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Molan PC. The antibacterial activity of honey. 1. The nature of the antibacterial activity. Bee World. 1992;73:5–28. [ Google Scholar ]
- 36. Aureli P, Franciosa G, Fenicia L. Infant botulism and honey in Europe: a commentary. Pediatr Infect Dis J. 2002;21:866–8. doi: 10.1097/00006454-200209000-00016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Rushton I. Understanding the role of proteases and pH in wound healing. Nurs Stand. 2007;21:68–72. doi: 10.7748/ns2007.04.21.32.68.c4499. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res. 2007;298:413–20. doi: 10.1007/s00403-006-0713-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Rendel M, Mayer C, Weninger W, Tschachler E. Topically applied of lactic acid increases spontaneous secretion of vascular endothelial growth factor by human constructed epidermis. Br J Dermatol. 2001;145:3–9. doi: 10.1046/j.1365-2133.2001.04274.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Vardi A, Barzilay Z, Linder N, Cohen HA, Paret G, Barzilai A. Local application of honey for treatment of neonatal postoperative wound infection. Acta Paediatr. 1998;87:429–32. doi: 10.1080/08035259850157048. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Bell SG. The therapeutic use of honey. Neonatal Netw. 2007;26:247–51. doi: 10.1891/0730-0832.26.4.247. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Kramer A, Daeschlein G, Kammerlander G, Abdriessen A, Aspöck C, Bergemann R, et al. Consensus recommendation for the choice of antiseptic agents in wound care (Article in German) Hygiene und Medizin. 2004;29:147–57. [ Google Scholar ]
- 43. Trop M, Novak M, Rodl S, Hellbom B, Kroell W, Goessler W. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648–52. doi: 10.1097/01.ta.0000208126.22089.b6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Cooper RA, Molan PC, Krishnamoorthy L, Harding KG. Manuka honey used to heal a recalcitrant surgical wound. Eur J Clin Microbiol Infect Dis. 2001;20:758–9. doi: 10.1007/s100960100590. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Visavadia BG, Honeysett J, Danford MH. Manuka honey dressing: An effective treatment for chronic wound infections. Br J Oral Maxillofac Surg. 2006 doi: 10.1016/j.bjoms.2006.09.013. Electronically published on November 17, 2006, [Epub ahead of print] [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Johnson DW, van Eps C, Mudge DW, Wiggins KJ, Armstrong K, Hawley CM, et al. Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J Am Soc Nephrol. 2005;16:1456–62. doi: 10.1681/ASN.2004110997. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Biswal BM, Zakaria A, Ahmad NM. Topical application of honey in the management of radiation mucositis: a preliminary study. Support Care Cancer. 2003;11:242–8. doi: 10.1007/s00520-003-0443-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Molan PC. The potential of honey to promote oral wellness. Gen Dent. 2001;49:584–9. [ PubMed ] [ Google Scholar ]
- 49. English H, Pack A, Molan P. The effects of manuka honey on plaque and gingivitis: a pilot study. J Int Acad Periodontol. 2004;6:63–7. [ PubMed ] [ Google Scholar ]
- 50. Albietz JM, Lenton LM. Effect of antibacterial honey on the ocular flora in tear deficiency and Meibomian gland disease. Cornea. 2006;25:1012–9. doi: 10.1097/01.ico.0000225716.85382.7b. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Irish J, Carter DA, Shokohi T, Blair SE. Honey has an antifungal effect against Candida species. Med Mycol. 2006;44:289–91. doi: 10.1080/13693780500417037. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Mullai V, Menon T. Bactericidal activity of different types of honey against clinical and environmental isolates of Pseudomonas aeruginosa. J Altern Complement Med. 2007;13:439–41. doi: 10.1089/acm.2007.6366. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Yapucu Gunes U, Eser I. Effectiveness of a honey dressing for healing pressure ulcers. J Wound Ostomy Continence Nurs. 2007;34:184–90. doi: 10.1097/01.WON.0000264833.11108.35. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Mphande AN, Killowe C, Phalira S, Jones HW, Harrison WJ. Effects of honey and sugar dressings on wound healing. J Wound Care. 2007;16:317–9. doi: 10.12968/jowc.2007.16.7.27053. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Subrahmanyam M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns. 1998;24:157–61. doi: 10.1016/s0305-4179(97)00113-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Subrahmanyam M. Honey impregnated gauze versus polyurethane film (OpSite) in the treatment of burns—a prospective randomised study. Br J Plast Surg. 1993;46:322–3. doi: 10.1016/0007-1226(93)90012-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Subrahmanyam M. Honey-impregnated gauze versus amniotic membrane in the treatment of burns. Burns. 1994;20:331–3. doi: 10.1016/0305-4179(94)90061-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Gethin G, Cowman S. Case series of use of Manuka honey in leg ulceration. Int Wound J. 2005;2:10–5. doi: 10.1111/j.1742-4801.2005.00078.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. White R, Cooper R, Molan PC, editors. Honey: A Modern Wound Management Product. Aberdeen, UK: Wounds, UK; 2005. [ Google Scholar ]
- 60. Gethin G. Is there enough clinical evidence to use honey to manage wounds? J Wound Care. 2004;13:275–8. doi: 10.12968/jowc.2004.13.7.26637. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Dunford CE, Hanano R. Acceptability to patients of a honey dressing for non-healing venous leg ulcers. J Wound Care. 2004;13:193–7. doi: 10.12968/jowc.2004.13.5.26614. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Dunford C, Cooper R, Molan P. Using honey as a dressing for infected skin lesions. Nurs Times. 2000;96(Suppl 14):7–9. [ PubMed ] [ Google Scholar ]
- 63. Ingle R, Levin J, Polinder K. Wound healing with honey–a randomised controlled trial. S Afr Med J. 2006;96:831–5. [ PubMed ] [ Google Scholar ]
- 64. Canter PH, Coon JT, Ernst E. Cost-effectiveness of complementary therapies in the United Kingdom-a systematic review. Evid Based Complement Alternat Med. 2006;3:425–32. doi: 10.1093/ecam/nel044. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians’ attitudes toward complementary & alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3:495–501. doi: 10.1093/ecam/nel036. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (408.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
You're using Internet Explorer - therefore, some pages or features may not display properly. We recommend switching to a modern browser such as Chrome, Microsoft Edge, or Firefox for a smoother experience.
- Mayo Clinic Careers
- Diversity, Equity & Inclusion
- Employees with Disabilities
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Internal Medicine
- Lung Transplant
- Psychiatry & Psychology
- Med Surg RN
- Nurse Practitioner & Physician Assistant
- Ambulance Service
- Clinical Labs
- Radiology Imaging
- Clinical Research Coordinator
- Respiratory Care
- Senior Care
- Surgical Services
- Travel Surgical Tech
- Practice Operations
- Administrative Fellowship Program
- Administrative Internship Program
- Career Exploration
- Nurse Residency and Training Program
- Nursing Intern/Extern Programs
- Residencies & Fellowships (Allied Health)
- Residencies & Fellowships (Medical)
- SkillBridge Internship Program
- Training Programs & Internships
- Our Locations
- Upcoming Events
- United States Applicants
- United Kingdom Applicants
- United Arab Emirate Applicants
- Current Employees
Search life-changing careers.
Job title or keyword
City or region
Radius Radius 5 miles 15 miles 25 miles 35 miles 50 miles 75 miles 100 miles
Associate Clinical Research Coordinator
- Rochester, MN
Not ready to apply? Join our talent community
Are you interested in a position that provides career development and growth? How about a position that is multifaceted, rewarding, and where the learning never stops? If so, then Mayo Clinic invites you to consider a life-changing career as an Associate Clinical Research Coordinator. As an Associate Clinical Research Coordinator, you will have the opportunity to work with a variety of people, from patients to care teams, enabling you to be a part of the legacy that puts the needs of the patients first. We have several Associate Clinical Research Coordinator positions available in multiple locations and departments. In this role, you could join an area such as Gastroenterology, Neurology, Radiology, Surgery, Orthopedics, Endocrinology, Cardiovascular Medicine, Psychiatry & Psychology, the Office of Clinical Trials, and many others. We have multiple schedules and shifts dependent upon position availability to fit your individual needs. Positions may vary in FTE, from supplemental to full-time. Some positions may also be temporary (less than one year) or limited tenure status (1-2 years in duration) and may have the possibility of turning into a regular position. When you apply, we will take the time to assess your qualifications and assess your preferences to determine which of our current openings would be the best match and ensure your application is reviewed by those active hiring managers. As an Associate Clinical Research Coordinator, you will :
- Coordinate non-therapeutic (i.e. minimal risk, survey, chart review) clinical research protocols with direction from the principal investigator and/or supervisor in compliance with regulatory laws and institutional guidelines. May assist in complex studies with direction but does not have overall responsibility for these studies.
- Screen, enroll, and recruit research participants.
- Coordinate schedules and monitor research activities and subject participation.
- Recognize adverse events, protocol deviations, and other unanticipated problems and reports appropriately.
- Collect, abstract, and enter research data.
- Perform administrative and regulatory duties related to the study as assigned.
- Participate in other protocol development activities and executes other assignments as warranted and assigned.
**This position will require candidates to work on campus and live within driving distance of the campus. To learn more about Research at Mayo Clinic, visit our website here . **During the selection process you will participate in an OnDemand (pre-recorded) interview that you can complete at your convenience. During the OnDemand interview, a question will appear on your screen, and you will have time to consider each question before responding. You will have the opportunity to re-record your answer to each question – Mayo Clinic will only see the final recording. The complete interview will be reviewed by a Mayo Clinic staff member and you will be notified of next steps.**
Must meet one of the following:
- HS Diploma with at least 3 years of experience OR
- Associate's degree/college Diploma/Certificate Program with at least 1 year of experience, Associate’s in Clinical Research from an accredited academic institution without experience OR
- Bachelor's degree.
- Experience should be in the clinical setting or related experience.
Additional Qualifications
- Graduate or diploma from a study coordinator training program is preferred.
- One year of clinical research experience is preferred.
- Medical terminology course is preferred.
*Visa sponsorship is not available for this position. This position is not eligible for F-1 OPT STEM extension.
*Internal candidates are encouraged to attach their performance appraisals (1-3), if available.
About our location
Rochester, Minnesota
We would love to connect with you.
Click the button for a list of our upcoming events.
Join Our Talent Community
Sign up, stay connected and get opportunities that match your skills sent right to your inbox
Email Address
Phone Number
Upload Resume/CV (Must be under 1MB) Remove
Job Category* Select One Administrative Anesthesiology Business Cardiology CRNA Dermatology Dialysis Technician Education Emergency Department Engineering ER Nurse Executive Facilities Management Family Medicine Fellowships Gastroenterology Hospitalist Housekeeping ICU Nurse Information Technology Internship Laboratory Licensed Practical Nurse Medical Surgical New Grad Nurse Nurse Manager Nurse Practitioner Nursing Nursing LPN Nursing RN Nursing Support Nursing Surgical Tech / Assistant OB/GYN Oncology Ophthalmology OR Nurse Patient Care Pediatrics Pharmacy Phlebotomy Physician Physician Assistant Psychiatry Psychology Radiology Research Scientist Security Surgery Therapy Travel Nurse
Location Select Location Albert Lea, Minnesota Arcadia, Wisconsin Austin, Minnesota Barron, Wisconsin Belle Plaine, Minnesota Bloomer, Wisconsin Caledonia, Minnesota Cannon Falls, Minnesota Duluth, Minnesota Eau Claire, Wisconsin Fairmont, Minnesota Faribault, Minnesota Jacksonville, Florida La Crosse, Wisconsin Lake City, Minnesota London, England Mankato, Minnesota Menomonie, Wisconsin Minneapolis-St. Paul-Bloomington, Minnesota New Prague, Minnesota Onalaska, Wisconsin Osseo, Wisconsin Owatonna, Minnesota Phoenix, Arizona Red Wing, Minnesota Rice Lake, Wisconsin Rochester, Minnesota Saint Augustine, Florida Saint Cloud, Minnesota Saint James, Minnesota Saint Peter, Minnesota Scottsdale, Arizona Sparta, Wisconsin Waseca, Minnesota
- Research, Rochester, Minnesota, United States Remove
Confirm Email
Join our talent community.
Join our global talent community to receive alerts when new life-changing opportunities become available.
Jobs for you
- Top Matches for You
- Featured Jobs
- Recently Viewed Jobs
- Eau Claire, Wisconsin Phlebotomist I or II - Phlebotomy Phlebotomy
- New Prague, Minnesota Environmental Services Technician Housekeeping
- Owatonna, Minnesota Phlebotomist I or II - Phlebotomy Phlebotomy
You have no Recently Viewed Jobs. View all available opportunities.

If you want to know what it's really like at Mayo Clinic, just ask. You'll find that our pride–in where we work, and in what we do–is a common trait. You will also find a lot of inspiring stories about lives changed for the better.
Nurse Residency Program
The Nurse Residency Program (NRP) is for all nurses with less than 12 months of experience, to be completed within the first year. NRP provides a framework for a successful transtion to a professional nurse by promoting educational and personal advancement.

As your career evolves, our compensation and benefits packages are designed to change with you — meeting needs now, and anticipating what comes next. We know that when Mayo Clinic takes care of you, you can take better care of our patients.
Equal opportunity
All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, gender identity, sexual orientation, national origin, protected veteran status, or disability status. Learn more about "EEO is the Law." Mayo Clinic participates in E-Verify and may provide the Social Security Administration and, if necessary, the Department of Homeland Security with information from each new employee's Form I-9 to confirm work authorization.
Reasonable accommodations
Mayo Clinic provides reasonable accommodations to individuals with disabilities to increase opportunities and eliminate barriers to employment. If you need a reasonable accommodation in the application process; to access job postings, to apply for a job, for a job interview, for pre-employment testing, or with the onboarding process, please contact HR Connect at 507-266-0440 or 888-266-0440.
Job offers are contingent upon successful completion of a post offer placement assessment including a urine drug screen, immunization review and tuberculin (TB) skin testing, if applicable.
Recruitment Fraud
Learn more about recruitment fraud and job scams
Advertising
Mayo Clinic is a not-for-profit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
Advertising and sponsorship policy | Advertising and sponsorship opportunities
Reprint permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Any use of this site constitutes your agreement to the Terms and Conditions and Privacy Policy linked below.
Terms and Conditions | Privacy Policy | Notice of Privacy Practices | Notice of Nondiscrimination
© 1998-2024 Mayo Foundation for Medical Education and Research (MFMER). All rights reserved.
Medical Devices and Equipment Logistics Market Size & Share Analysis - Growth Trends & Forecasts (2024 - 2029)
The Medical Devices and Equipment Logistics Market Report is Segmented by Product Type (Medical Devices and Medical Equipment) and Geography (North America, Europe, Asia-Pacific, Latin America, and Middle East and Africa). The Report Offers Market Size and Forecasts for all the Above Segments in Value (USD).
Medical Devices and Equipment Logistics Market Size
Single User License
Team License
Corporate License

| Study Period | 2019 - 2029 |
| Market Size (2024) | USD 63.70 Billion |
| Market Size (2029) | USD 93.95 Billion |
| CAGR (2024 - 2029) | 7.81 % |
| Fastest Growing Market | Asia Pacific |
| Largest Market | Asia Pacific |
Need a report that reflects how COVID-19 has impacted this market and its growth?
Medical Devices and Equipment Logistics Market Analysis
The Medical Devices And Equipment Logistics Market size is estimated at USD 63.70 billion in 2024, and is expected to reach USD 93.95 billion by 2029, growing at a CAGR of 7.81% during the forecast period (2024-2029).
The medical devices and equipment logistics market is rapidly expanding, fueled by surging demand for advanced healthcare services and technological advancements in medical devices. Manufacturers and healthcare providers are increasingly streamlining their supply chain operations to ensure the timely global delivery of critical equipment. This efficiency drive is further empowered by automation, real-time tracking systems, and advanced inventory management techniques to boost transparency and cut costs.
In February 2024, GobalMed Logistix (GMLx) made headlines by unveiling a 200% expansion of its Atlanta campus, introducing a new 65,000-sq. ft facility. This move directly responds to the escalating need for medical device logistics and third-party services, marking a significant stride in the industry's logistics capabilities.
In collaboration with Wing, groundbreaking initiatives like Medtronic's drone delivery trials are revolutionizing last-mile healthcare logistics and promising swifter and more adaptable supply chain solutions. Moreover, the ongoing global expansion of healthcare infrastructure, especially in emerging economies, is creating a ripe environment for logistics providers to establish robust distribution networks and cater to mounting demands.
For instance, UK-based Apian partnered with Zipline in December 2023 to enhance NHS medical supply deliveries, underscoring the continuous wave of innovation in healthcare logistics.
Medical Devices and Equipment Logistics Market Trends
Medical devices manufacturer integrate cutting-edge logistics solutions.
Medtronic is a multinational producer of medical devices and therapies, such as insulin pumps, pacemakers, and diabetes therapies. Since 2022, Medtronic has embarked on a massive overhaul of its logistics and supply chain operations that will culminate in 2024. The company has focused on three key areas: global operations and supply chain (GOSC), core technology, and commercial strategies targeting large enterprise customers. It represents a significant shift in direction.
In July 2024, Medtronic was among the first companies in Ireland to use the drone delivery company Wing to deliver medical supplies and devices to hospitals. The two companies are teaming up with Blackrock Health and St. Vincent's Private Hospital in Dublin to launch a drone delivery trial to demonstrate how drones can be used in healthcare.
Medtronic's initiative with Wing underscores the growing importance of integrating cutting-edge logistics solutions in the medical devices market. Competitors and industry stakeholders may follow suit, accelerating the adoption of innovative technologies to enhance supply chain efficiency and customer service.

UK Medical Devices Manufacturing Propels the Logistics Industry
In 2022, the Office for National Statistics reported that the UK manufacturing industry for medical and dental instruments and supplies saw its gross value added (GVA) climb to around GBP 2.98 billion (USD 3.81 billion). This consistent growth highlights the industry’s economic significance and mirrors the escalating production and demand for medical instruments within the United Kingdom.
With the industry’s expansion, robust supply chains and distribution networks become paramount. These networks are vital for the efficient global transportation of medical products, ensuring they reach healthcare facilities promptly and comply with stringent regulations. The industry’s heightened manufacturing output also spurs investments in cutting-edge logistics technologies, including temperature-controlled storage, automated inventory systems, and specialized transportation solutions.
Recent humanitarian endeavors, like the country’s provision of medical supplies to civilians affected by the Russian invasion of Ukraine through UK-Med, further underscore the industry's global healthcare support role.
In essence, the escalating GVA in the UK medical and dental supplies manufacturing industry accentuates the indispensable role of logistics in broadening the accessibility and availability of crucial healthcare equipment, both domestically and internationally.

Medical Devices and Equipment Logistics Industry Overview
The medical devices and equipment logistics market boasts a competitive landscape, hosting a diverse array of players, from global logistics powerhouses to niche service providers. Logistics giants like UPS Healthcare, DHL Supply Chain, FedEx Healthcare Solutions, Kuehne + Nagel, and Ceva Logistics lead the charge. These industry giants leverage their expansive global networks, cutting-edge technology, and a robust suite of services. Conversely, specialized players such as Cardinal Health, Owens & Minor, and DB Schenker carve their niche by tailoring logistics solutions for the healthcare sector, encompassing warehousing, distribution, and inventory management.
Collaborations between logistics entities and healthcare stakeholders are rising to broaden market penetration and enrich service portfolios. Embracing technologies like IoT, AI, and blockchain is pivotal, as they bolster efficiency and transparency in the logistics chain. Moreover, given the stringent oversight in medical device logistics, a steadfast commitment to regulatory compliance and quality assurance remains paramount.
Medical Devices and Equipment Logistics Market Leaders
UPS Healthcare
DHL Supply Chain
FedEx Healthcare Solutions
Kuehne + Nagel
Ceva Logistics
*Disclaimer: Major Players sorted in no particular order

Medical Devices and Equipment Logistics Market News
- April 2024: UPS Healthcare channeled EUR 55 million (USD 60 million) into expanding its footprint in Italy. This investment sees the addition of 100,000 sq. m of warehouse space, split between Passo Corese, near Rome, and Somaglia in Lodi. With this financial commitment, UPS Healthcare aims to align the Passo Corese and Somaglia facilities with stringent distribution and manufacturing practices, ensuring the safe storage of medical and pharmaceutical products.
- February 2024: FedEx Express, a subsidiary of FedEx Corp., inaugurated the 'FedEx Life Science Center' in Mumbai, marking a milestone in India's and the global clinical trial supply chain. This new center is designed to cater to the storage and logistics needs of healthcare clients participating in clinical trials, both domestically in India and internationally shipping to the country.
Medical Devices and Equipment Logistics Market Report - Table of Contents
1. INTRODUCTION
1.1 Study Deliverables
1.2 Study Assumptions
1.3 Scope of the Study
2. RESEARCH METHODOLOGY
2.1 Analysis Methodology
2.2 Research Phases
3. EXECUTIVE SUMMARY
4. MARKET INSIGHTS
4.1 Current Market Scenario
4.2 Technological Trends
4.3 Insights on Supply Chain/Value Chain Analysis
4.4 Insights into Governement Regualtions in the Industry
4.5 Insights into Technological Advancements in the Industry
4.6 Impact of Geopolitics and Pandemics on the Market
5. MARKET DYNAMICS
5.1 Market Drivers
5.1.1 Global Expansion of Healthcare Services
5.1.2 Technological Advancements
5.2 Market Restraints
5.2.1 High Operational Costs
5.2.2 Regulatory Complexity
5.3 Market Opportunities
5.3.1 Expansion into Emerging Markets
5.3.2 Forging Strategic Partnerships and Collaborations With Healthcare Providers
5.4 Industry Attractiveness - Porter's Five Forces Analysis
5.4.1 Threat of New Entrants
5.4.2 Bargaining Power of Buyers/Consumers
5.4.3 Bargaining Power of Suppliers
5.4.4 Threat of Substitute Products
5.4.5 Intensity of Competitive Rivalry
6. MARKET SEGMENTATION
6.1 By Product Type
6.1.1 Medical Devices
6.1.2 Medical Equipment
6.2 By Geography
6.2.1 North America
6.2.1.1 United States
6.2.1.2 Canada
6.2.1.3 Mexico
6.2.2 Europe
6.2.2.1 Germany
6.2.2.2 United Kingdom
6.2.2.3 France
6.2.2.4 Italy
6.2.2.5 Spain
6.2.2.6 Rest of Europe
6.2.3 Asia-Pacific
6.2.3.1 China
6.2.3.2 Japan
6.2.3.3 India
6.2.3.4 Australia
6.2.3.5 South Korea
6.2.3.6 Rest of Asia-Pacific
6.2.4 Middle East and Africa
6.2.4.1 GCC
6.2.4.2 South Africa
6.2.4.3 Rest of Middle East and Africa
6.2.5 South America
6.2.5.1 Brazil
6.2.5.2 Argentina
6.2.5.3 Rest of South America
7. COMPETITIVE LANDSCAPE
7.1 Market Concentration Overview
7.2 Company Profiles
7.2.1 UPS Healthcare
7.2.2 DHL Supply Chain
7.2.3 FedEx Healthcare Solutions
7.2.4 Kuehne + Nagel
7.2.5 Ceva Logistics
7.2.6 Cardinal Health
7.2.7 Owens & Minor
7.2.8 DB Schenker
7.2.10 World Courier*
- *List Not Exhaustive
7.3 Other Companies
8. MARKET OPPORTUNITIES AND FUTURE TRENDS
9. APPENDIX
9.1 Macroeconomic Indicators
9.2 Insight Into Capital Flows (Investments In Transport and Storage Sector)
9.3 E-commerce and Consumer Spending-related Statistics
9.4 External Trade Statistics
Medical Devices and Equipment Logistics Industry Segmentation
The medical devices and equipment logistics industry involves transporting, storing, and distributing medical devices and equipment. It encompasses a spectrum of activities, services, and stakeholders committed to the efficient, safe, and compliant delivery of medical products from manufacturers to end-users, including hospitals, clinics, and patients. A complete background analysis of the medical devices and equipment logistics market, including the assessment of the economy and contribution of sectors in the economy, market overview, market size estimation for key segments, and emerging trends in the market segments, market dynamics, and geographical trends is covered in the report.
The medical devices and equipment logistics market report is segmented by product type (medical devices and medical equipment) and geography (North America, Europe, Asia-Pacific, Latin America, and Middle East and Africa). The report offers market size and forecasts for all the above segments in value (USD).
| By Product Type | |
| Medical Devices | |
| Medical Equipment |





















IMAGES
VIDEO
COMMENTS
PubMed® comprises more than 37 million citations for biomedical literature from MEDLINE, life science journals, and online books. Citations may include links to full text content from PubMed Central and publisher web sites.
The New England Journal of Medicine (NEJM) is a weekly general medical journal that publishes new medical research and review articles, and editorial opinion on a wide variety of topics of ...
The University of Florida Cancer and Genetics Research Complex is an integrated medical research facility. Medical research (or biomedical research), also known as health research, refers to the process of using scientific methods with the aim to produce knowledge about human diseases, the prevention and treatment of illness, and the promotion ...
DiscoverWHR is a centralized resource for women's health research and information from NIH that supports NIH-wide efforts to close the gaps in women's health across the life course. This innovative resource simplifies the finding women's health information by patients, caregivers, medical professionals, researchers, and the public.
Medical research involves research in a wide range of fields, such as biology, chemistry, pharmacology and toxicology with the goal of developing new medicines or medical procedures or improving ...
The planning and execution of clinical research is of vital importance for the advancement of medical science. The validity of clinical research findings depends on a variety of factors, such as study design, sampling techniques and statistical analysis. Choosing an appropriate study design requires detailed knowledge of the types of clinical ...
Significant problems in medical research undermine efficient scientific discovery and efforts to achieve improved outcomes for patients. Across scientific disciplines, a diverse range of issues has resulted in a situation where published research findings frequently cannot be replicated ('the reproducibility crisis'). ...
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
A medical researcher, also known as a medical scientist, studies diseases and conditions to help improve and protect public health. They design studies, perform research and collect and analyze data. The purpose of their studies may be to find ways to prevent or treat diseases or identify connections between certain conditions and illnesses.
10 careers in medical research. Here are 10 careers you can pursue in the field of medical research: 1. Clinical laboratory scientist. National average salary: $89,291 per year Primary duties: A clinical laboratory scientist is a scientist who specializes in using lab equipment to perform tests on biological specimens.
Official website of the National Institutes of Health (NIH). NIH is one of the world's foremost medical research centers. An agency of the U.S. Department of Health and Human Services, the NIH is the Federal focal point for health and medical research. The NIH website offers health information for the public, scientists, researchers, medical professionals, patients, educators,
A clinician should continuously strive to increase knowledge by reviewing and critiquing research papers and thoughtfully considering how to integrate new data into practice. This is the essence of evidence-based medicine (EBM).[1] When new clinical queries arise, one should seek answers in the published literature. The ability to read a scientific or medical manuscript remains vitally ...
Medical Research News. Health news on everything from cancer to nutrition. Updated daily.
Breast cancer blocked by multiple natural lines of defence. Cancer-promoting mutations are common in healthy tissue but rarely lead to tumour formation. A study of the mouse mammary gland reveals ...
Medical Research. Scientists and physicians in academic medicine conduct groundbreaking biomedical research that improves our knowledge of human health and promotes the development of treatments from bench to bedside to community. Through policy and advocacy initiatives, data and research projects, professional learning and networking ...
Clinical Research: This role entails assisting in the design and execution of clinical trials by providing scientific insights and ensuring the clinical relevance of the study. MSLs have the ability to nominate investigators sites which have the appropriate patient population for the trial. MSLs also build and maintain relationships with the ...
1 Children's Hospital Medical Centre, University of Bonn, Bonn, 2 Institute for Bee Research, Celle, Germany and 3 Department of Biological Sciences, University of Waikato, ... Medical honey dressings are easy to change without pain and without harm to the regenerating tissue. Malodour from recalcitrant wounds as a result of critical ...
2 years of clinical research experience and/or basic science research. Preferred Qualifications. Five (5) years of experience in late clinical development as part of a pharmaceutical organization. Five (5) years of clinical research experience and/or basic science research combined with clinical teaching and patient care activities
As an Associate Clinical Research Coordinator, you will have the opportunity to work with a variety of people, from patients to care teams, enabling you to be a part of the legacy that puts the needs of the patients first. We have several Associate Clinical Research Coordinator positions available in multiple locations and departments.
The Medical Devices And Equipment Logistics Market is expected to reach USD 63.70 billion in 2024 and grow at a CAGR of 7.81% to reach USD 93.95 billion by 2029. UPS Healthcare, DHL Supply Chain, FedEx Healthcare Solutions, Kuehne + Nagel and Ceva Logistics are the major companies operating in this market.
An understanding of medical terminology, experience in a large complex health care setting, ability to effectively communicate with staff and faculty of all levels, and knowledge of university policies and procedures is desirable. 1+ years of experience in clinical pain research; Has previously built forms in REDCap and Qualtrics
All NIH employees, staff, or groups of staff (which can also include contractors) performing research, administering clinical/scientific disciplines, or working in scientific research professions. Criteria. Exceptional display of leadership in promoting the advancement of the understanding or application of scientific phenomena, processes, or ...
Problem Analysis: Low Effectiveness of Cervical Cancer Preventive Measures in Yuzhno-Sakhalinsk City 02.01.2003 - 09.15.2003 Funded by ACCP. SAOG unites Ob/Gyns of Sakhalin Oblast SAOG Objectives: Slideshow 3568313 by lily
Yuzhno-Sakhalinsk began as a small Russian settlement called Vladimirovka, founded by convicts in 1882. [2] The Treaty of Portsmouth in 1905, which brought an end to the Russo-Japanese War of 1904-1905, awarded the southern half of the Sakhalin Island to Japan.Vladimirovka was renamed Toyohara (meaning "bountiful plain"), and was the prefect capital of the Japanese Karafuto Prefecture.
유즈노사할린스크에는 맥도날드, 버거킹, KFC, 헤스버거 [7] 등 유명 패스트푸드 브랜드가 없다. 대신 이곳만의 햄버거 체인이 있어서 간단히 식사하기가 어렵지는 않다. 주러시아 대한민국 대사관 의 주 (駐)유즈노사할린스크 출장소가 있다. [1] 따라서 토요하라 ...
Wikivoyage. Instance of. administrative territorial entity of Russia (1991-) city or town (1905-) big city (1969-) Location. Yuzhno-Sakhalinsk Urban Okrug, Sakhalin Oblast, Russia. Inception. 1882.