Need a consultation? Call now:
Talk to our experts:
- Business Plan for Investors
- Bank/SBA Business Plan
- Operational/Strategic Planning
- E1 Treaty Trader Visa
- E2 Treaty Investor Visa
- Innovator Founder Visa
- UK Start-Up Visa
- UK Expansion Worker Visa
- Manitoba MPNP Visa
- Start-Up Visa
- Nova Scotia NSNP Visa
- British Columbia BC PNP Visa
- Self-Employed Visa
- OINP Entrepreneur Stream
- LMIA Owner Operator
- ICT Work Permit
- LMIA Mobility Program – C11 Entrepreneur
- USMCA (ex-NAFTA)
- Franchise Business Planning
- Landlord Business Plan
- Nonprofit Start-Up Business Plan
- USDA Business Plan
- Online Boutique
- Mobile Application
- Food Delivery
- Real Estate
- Business Continuity Plan
- Buy Side Due Diligence Services
- ICO whitepaper
- ICO consulting services
- Confidential Information Memorandum
- Private Placement Memorandum
- Feasibility study
- Fractional CFO
- Business Valuation
- How it works
- Business Plan Templates

Medical Device Business Plan
Published Nov.06, 2023
Updated Sep.14, 2024
By: Brandi Marcene
Average rating 5 / 5. Vote count: 4
No votes so far! Be the first to rate this post.

Table of Content
Medical Device Business Plan Sample
A medical device business plan is a document that outlines how to start and run a successful company that produces and sells products that diagnose, treat, or prevent diseases or injuries. Navigating the vast and expanding medical device sector presents thrilling opportunities alongside complex hurdles. A well-crafted business plan illuminates the route to success. Articulate your vision, milestones, tactics, and budgetary forecasts.
A business plan should also demonstrate how you will stand out from the crowd, satisfy users, adhere to regulations, and uphold ethical standards. A medical billing business plan is a specific type of medical device business plan that focuses on how to provide billing and coding services for healthcare providers.
In this article, we will provide you with a medical device business plan sample that you can use as a template or a reference for your business plan. We will cover the following sections:
- Executive Summary
- Company Overview
- Industry Analysis
- Customer Analysis
- Competitive Analysis
- Marketing Plan
- Operations Plan
Management Team
- Financial Plan
Executive Summary Section of Our Medical Device Business Plan
Business overview.
Medix is a medical device company that develops and sells innovative and affordable devices for diabetes management. We aim to enhance the well-being and health results of those managing diabetes. We aim to offer user-friendly and dependable products that assist in tracking and regulating blood sugar levels.
Products and Services
Medix offers two main products:
- Medix Glucometer – A smart glucose meter that connects to a mobile app via Bluetooth and provides accurate and instant readings of blood glucose levels.
- Medix Patch – A wearable patch that continuously measures blood glucose levels through the skin without needing finger pricks or test strips.
Customer Focus
Medix focuses on serving people with diabetes, seeking convenient and affordable solutions to manage their condition. According to the IDF Diabetes Atlas 10th edition report , 537 million adults (20-79 years) live with diabetes – 1 in 10. Experts predict that this number will rise to 643 million by 2030 and 783 million by 2045. Therefore, there is a huge demand for effective and accessible diabetes care products.
Leo Clark and Aria Bennett, two experienced entrepreneurs with biomedical engineering and business administration backgrounds, founded Medix. Leo is the CEO and head of product development, while Aria is the COO and head of marketing and sales. A team of qualified engineers, designers, developers, marketers, salespeople, and advisors supports them.
Success Factors
Medix has several competitive advantages that will enable it to succeed in the medical device industry:
- Innovation with cutting-edge technology to create novel devices
- High standards of quality and safety in every aspect of devices
- Customer satisfaction by providing user-friendly devices
- Social impact by addressing a major health problem globally
Financial Highlights
Medix seeks $5 million in seed funding to launch its products and scale its operations. The company projects to generate $1.2 million in revenue in the first year, $3.6 million in the second year, and $10.8 million in the third year, with a gross margin of 60% and a net profit margin of 20%. The company expects to break even in the second year and reach a valuation of $50 million by the end of the third year.
Company Overview Section of Our Medical Device Sales Business Plan

Who is Medix Medical Supply?
Medix dedicates itself to developing and selling innovative, affordable, and reliable devices for diabetes management. Our products help people with diabetes to monitor and control their blood glucose levels with ease and effectiveness, leading to better health outcomes and an improved quality of life.
Medix Medical Supply History
Medix is a company that provides innovative solutions for diabetes care. It was founded by Leo Clark and Aria Bennett in 2023, who both personally experienced the challenges and frustrations of living with diabetes. These challenges included frequent finger pricks, expensive test strips, inaccurate readings, and complicated insulin injections.
They started Medix with their personal funds and an incubator grant to address these issues. Medix developed two products – the Medix Glucometer and the Medix Patch – to make diabetes monitoring and treatment easier, more accurate, and more affordable.
The Medix products have received regulatory approvals from the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). They are now ready for launch in the US and European markets. For more information, please refer to our dentistry business plan .
Legal Structure
Medix, an LLC registered in Delaware, USA, has obtained ownership by Leo Clark (60%) and Aria Bennett (40%). Additionally, the company has applied for a patent for its products in the US Patent and Trademark Office (USPTO).
Industry Analysis Section of Our Medical Device Business Plan
The medical device industry is one of the world’s most innovative and dynamic sectors. Fortune Business Insights reported that the global medical device market was valued at $512.29 billion in 2022 and can grow from $536.12 billion in 2023 to $799.67 billion by 2030, at a CAGR of 5.9%.
The medical device industry is driven by several factors, such as:
- The increasing prevalence of diseases and the aging population
- The rising demand for minimally invasive and personalized treatments
- The advancement of technology and digitalization
- The emergence of new markets and segments
Customer Analysis Section of Our Medical Supply Business Plan
Demographic profile of target market.
Medix’s target market is the US market, which ranks third for the highest number of people with diabetes. We target diabetic people looking for convenient, affordable solutions to manage their condition.
According to the National Diabetes Statistics Report by CDC, here are some interesting stats about why the US market is best for Medix:
- 37.3 million people have diabetes (11.3% of the US population)
- 28.7 million people are diagnosed, including 28.5 million adults
- 8.5 million people are undiagnosed (23.0% of adults)
- 96 million people aged 18 years or older have prediabetes (38.0% of the adult US population)
- 26.4 million people aged 65 years or older (48.8%) have prediabetes
The demographic profile of our target market is as follows:
- Age – We target all ages, mainly the young and middle-aged, who are tech-savvy and have more money to spend. A CDC report says 34.1 million adults aged 18 years or older—or 13.0% of all US adults—have diabetes.
- Gender – We target both males and females, as diabetes does not discriminate by gender. A NIDDK (NIH) report says a higher percentage of men (41%) than women (32%) have prediabetes.
- Income – We target all income levels, mainly the low and middle-income who need better healthcare solutions. An NCBI (NIH) report says 80% of the adults worldwide with diabetes live in low- and middle-income countries (LMICs).
Customer Segmentation
Based on our market research and customer feedback, we have identified four main customer segments for our products:
- Segment A – Tech-savvy innovators who value quality, performance, and convenience. They share their views online.
- Segment B – Cost-conscious buyers who seek affordable and effective products. They trust their peers’ recommendations.
- Segment C – Health-conscious improvers who want products that motivate and support them. They join online health communities.
- Segment D – Compliance-driven users need products that ensure safety, security, and simplicity. They depend on their health providers and caregivers.
The table below summarizes our findings:
Based on the table, we have decided to target segments A and B as our primary segments, and segments C and D as our secondary segments.
Competitive Analysis Section of Our Medical Equipment Producer Business Plan
Direct and indirect competitors.
Our direct competitors are other medical device companies that offer similar or substitute surgical medical equipment for diabetes management. Some of the major players in this category are:
1. Abbott – A global healthcare company that offers a range of products for diabetes care with mobile apps for real-time data and insights.
- Strong brand recognition
- Global presence
- Innovation capabilities
- Customer loyalty
Weaknesses:
- Limited availability
- Technical issues
2. Dexcom – A medical device company specializing in CGMs for diabetes management. These devices use sensors to record and transmit data to a receiver or a smartphone.
- High accuracy
- Reliability
- Convenience
- Customer satisfaction
- Short sensor lifespan
- Skin irritation
3. Medtronic – A medical technology company that offers a range of durable medical equipment for diabetes care, such as insulin pumps, CGMs, and APSs. The system connects to a mobile app to monitor and control settings.
- Leadership position
- Advanced technology
- Clinical evidence
- Customer support
- Safety concerns
- Regulatory hurdles
- Competition
Our indirect competitors are other healthcare providers or solutions that offer alternative or complementary ways to manage diabetes, such as medications, diet plans, exercise programs, coaching services, etc. Refer to our hospital business plan to learn more.
Competitive Advantage
Medix’s unique value proposition and competitive advantage over its competitors are:
- Medix is more innovative
- Medix is more convenient
- Medix is more versatile
- Medix is more affordable
- Medix is more user-friendly
Marketing Plan Section of Our Medical Device Business Plan
Promotions strategy.
We will promote our products using online and offline channels to attract and retain customers. Our promotional mix consists of:
- Advertising – Online platforms (e.g., Google Ads, Facebook Ads) and offline media (e.g., newspapers, billboards) to deliver relevant and engaging messages.
- Public Relations – Press releases, media interviews, podcasts, webinars, etc., to generate positive publicity and exposure. Social media platforms (e.g., Facebook, Twitter) to interact and communicate with customers and stakeholders.
- Sales Promotion – Discounts, coupons, free samples, free trials, referrals, loyalty programs, etc., to stimulate sales and repeat purchases. Contests, sweepstakes, giveaways, etc., to create excitement and buzz.
- Personal Selling – Direct sales, telemarketing, email marketing, etc., to contact and persuade customers to buy our products. Online platforms (e.g., Amazon, eBay, Shopify) to sell our products directly.
We will use a value-based pricing strategy that reflects the value and benefits of our products and our competitive advantage. We will also offer competitive pricing that matches or undercuts our competitors’ prices.
We will charge $100 for each Medix Glucometer and $50 for each Medix Patch. We will also generate recurring revenue from the sales of test strips ($0.5 each) and insulin cartridges ($10 each). We estimate that each customer will use an average of 100 test strips and 12 insulin cartridges per year.
Operations Plan Section of Our Medical Device Business Plan
Operation functions.
We do these core activities to offer our products and services to our customers:
- Product Development – We research, design, test, and improve our products using agile methods, customer feedback, market trends, and tools like GitHub, Jira, Figma, etc.
- Manufacturing – We produce our products on a large scale and high quality by outsourcing to a reliable contract manufacturer.
- Distribution – We deliver our products to our customers quickly and cheaply using direct and indirect channels in different regions or countries.
- Customer Service – We support and assist our customers before, during, and after their purchase using various channels and methods.
Milestones and Timeline
We have these specific goals and objectives to track our progress and success in our operation functions:
- June 2024: Complete R&D, testing, prototyping of products
- September 2024: Obtain regulatory approvals and certifications
- December 2024: Launch marketing campaign and product launch in the US
- March 2025: Market research for Europe entry
- December 2025: Launch Europe marketing, market entry
- March 2026: Invest in production capacity
- June 2026: Expand manufacturing workforce
- December 2026: Evaluate production, increase to 100k units/month
Management Team Section of Our Medical Device Business Plan
Founders and co-founders.
Leo Clark, a biomedical engineer with type 1 diabetes, and Aria Bennett, the daughter of a type 2 diabetic and a business administrator, founded Medix. Leo is responsible for the product development function, while Aria leads the marketing and sales function. Both have several years of experience working in their respective fields and personal and professional experience with diabetes.
Other Key Team Members
- Alice Lee – Our chief engineer
- Bob Chen – Our chief developer
- Carol Wang – Our chief designer
- Dave Jones – Our chief marketer
- Emma Smith – Our chief salesperson
Financial Plan Section of Our Medical Device Business Plan
Key revenue and costs.
Medix’s main sources of revenue, along with pricing, are:
- Medix Glucometer – $100 for each Glucometer
- Medix Patch – $50 for each Patch
- Test Strips – $0.5 for each test strip
- Insulin Cartridge – $10 for each cartridge
We estimate that each customer will use an average of 100 test strips and 12 insulin cartridges per year.
Medix’s main categories of expenses are:
- Cost of Goods Sold (COGS) – Our main cost of goods sold is the cost of materials, components, parts, and additional supplies. We estimate that the COGS per unit is $40 for the Medix Glucometer, $20 for the Medix Patch, $0.1 for the test strip, and $2 for the insulin cartridge.
- Operating Expenses (OPEX) – Our main operating expenses are the costs we incur for running and operating our business, such as salaries, rent, utilities, marketing, advertising, R&D, etc. Our OPEX will be 40% of our revenue in the first year, 35% in the second year, and 30% in the third year.
Funding Requirements and Use of Funds
Funding Requirements – We seek $5 million in seed funding to launch our products and scale our operations. We have already raised $500,000 from our savings and a small grant from a local incubator. We need an additional $4.5 million to cover our expenses for the next 18 months until we reach the break-even point.
Use of Funds – We will use the funds for the following purposes as highlighted in the below chart:
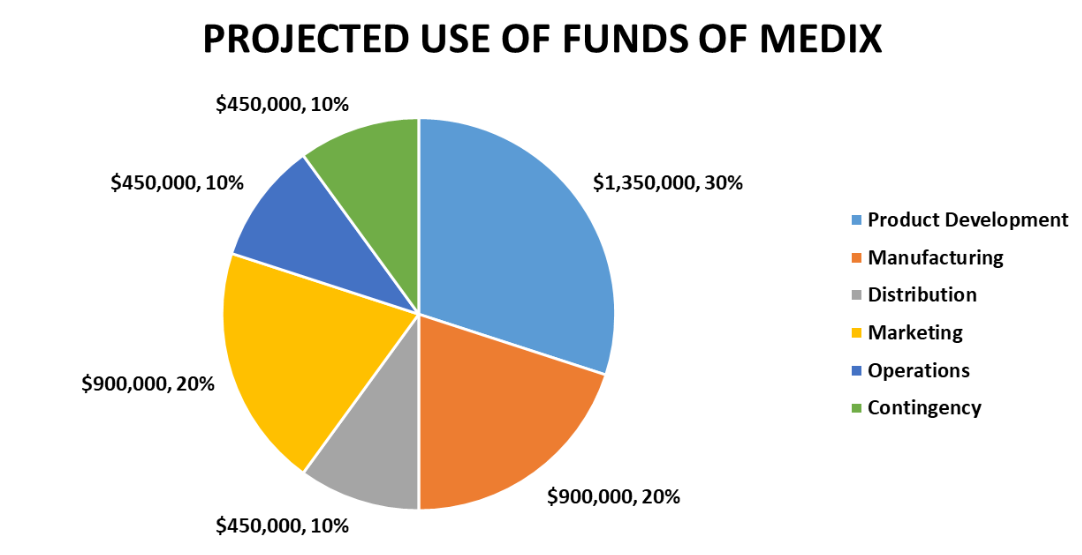
Key Assumptions
- Market size for our products is 10% of the total number of people with diabetes in the US and Europe
- Market share is projected to grow from 107,000 customers in 2024 to 444,000 customers in 2026
- Sales volume is projected to grow from 321,000 units in 2024 to 1.33 million units in 2026
- Gross margin is projected to be 60% in all three years
- Net margin is projected to grow from 20% in 2024 to 30% in 2026
Financial Projections
Based on the above assumptions, we have prepared the following financial projections for the next three years:
Income Statement
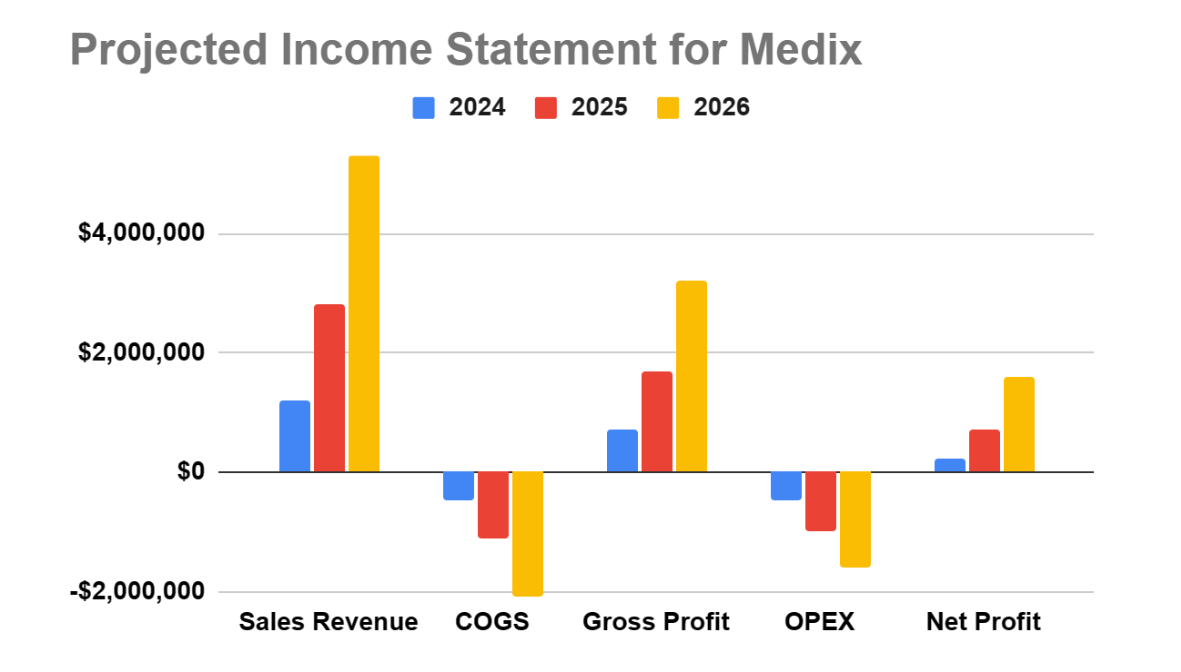
OGSCapital – Your Partner for Medical Device Startup Success
With over a decade of experience, at OGSCapital, we have helped various entrepreneurs craft winning business plans. Our consultants provide end-to-end support – from market research and competitor analysis to realistic profitability forecasts. We understand the medical device industry inside-out, including regulations, manufacturing, and distribution.
Whether you need help with your hospital feasibility study , medical equipment manufacturing business plan, or medical supply store business plan, we tailor our approach to your specific product and goals. Partner with us to launch your startup on the path to profitability and rapid growth.
Frequently Asked Questions
How to start a medical device business.
A strategic business plan is a key ingredient in a startup medical device company. But that alone won’t cut it – the company also requires a talented group of professionals, structured product development procedures, a plan for meeting regulatory guidelines, and effective marketing tactics. A distributor or a medical equipment supplier can help distribute the devices.
How profitable are medical devices?
The medical equipment industry is booming with high growth potential. The average operating margin for medical equipment and supplies companies averages 2.87%. The medical device market will grow at a CAGR of 5.5% to 5.9% from 2022 to 2030.
How do I market my medical device?
As highlighted in our Medical Clinic Business Plan , some popular marketing channels to market a medical device include online platforms, social media, trade shows, conferences, webinars, publications, referrals, and testimonials. A medical equipment rental company can also help market the device.
OGSCapital’s team has assisted thousands of entrepreneurs with top-rated document, consultancy and analysis. They’ve helped thousands of SME owners secure more than $1.5 billion in funding, and they can do the same for you.

Any questions? Get in Touch!
We have been mentioned in the press:
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Search the site:
Medical Device Business Plan Template
Written by Dave Lavinsky
Medical Device Business Plan
You’ve come to the right place to create your Medical Device business plan.
We have helped over 1,000 entrepreneurs and business owners create business plans and many have used them to start or grow their Medical Device businesses.
Below is a template to help you create each section of your Medical Device business plan.
Executive Summary
Business overview.
MediTech LLC is a medical device company that sells Class I medical devices to hospitals, clinics, and other establishments in the medical industry. We manufacture a long list of devices including surgical instruments, syringes, and bandages. We know that patients can’t receive quality care if medical professionals don’t have good tools. Therefore, our mission is to provide the best medical devices in the industry so that all hospitals and clinics can provide the best care possible.
MediTech LLC is founded by Sarah Nelson. Sarah has considerable experience as a surgeon and used hundreds of medical devices throughout her career. She knows exactly what it takes to make high quality medical products and has made it her mission to create the best medical devices in the industry. Her expertise and knowledge of the industry will give us a considerable advantage over the competition.
Product Offering
MediTech LLC sells a long list of Class I medical devices. Class I medical devices are low risk devices and are unlikely to cause any harm to users. These include bandages, surgical tools, bedpans, gloves, and surgical masks. Our product list will grow and change depending on which devices are in high demand.
Customer Focus
MediTech LLC will primarily serve hospitals, clinics, and other medical organizations. Some products will be sold in stores to the public, including bandages, gloves, and face masks.
Management Team
MediTech LLC was founded by Sarah Nelson, a licensed and experienced surgeon. While working in the medical industry, she was frustrated by the quality of the medical devices she used. Her hospital routinely purchases low quality devices to save costs and this would affect the quality of her care. She researched what it would take to make higher quality versions of these products and decided to start a company that provides better quality devices for an affordable cost.
Success Factors
MediTech LLC will be able to achieve success by offering the following competitive advantages:
- We will provide the best quality medical devices in the industry. Our devices will help improve the quality of care that our clients give their patients.
- MediTech will price all of its products moderately so all of our clients and customers can afford them.
- Our founder has years of experience as a surgeon in the medical industry, bringing a vast amount of medical knowledge to the table. This will help us create perfect medical devices and products that all medical professionals will be eager to use.
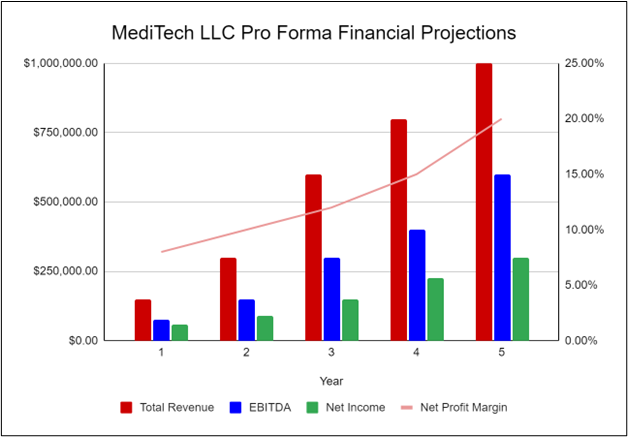
Financial Highlights
MediTech LLC is currently seeking $1,400,000 to launch. The funding will be dedicated to the facility build out, purchase of initial equipment, working capital, marketing costs, and startup overhead expenses. The breakout of the funding is below:
- Facility design/build: $500,000
- Equipment: $200,000
- Six months of overhead expenses (payroll, rent, utilities): $400,00
- Initial supplies and inventory: $100,000
- Marketing and advertising: $100,000
- Working capital: $100,000
The following graph below outlines the pro forma financial projections for MediTech LLC.
Company Overview
Who is meditech llc.
MediTech LLC sells high-quality Class I medical devices to the medical industry. Our management team knows from experience that patients can’t receive the best care possible if physicians aren’t using the best tools. However, many medical organizations order lower quality devices in order to save on costs. At MediTech LLC, we are committed to making the best medical devices in the industry that are more affordable than the competition.
MediTech LLC produces a long list of medical devices for the medical industry. These include bandages, surgical masks, gloves, surgical instruments, and bedpans. All of our products are Class I devices, meaning they present a low risk to the user.
MediTech LLC is founded by Sarah Nelson. Sarah has considerable experience as a surgeon and used hundreds of medical devices throughout her career. She knows exactly what it takes to make high quality medical products and has made it her mission to create the best medical devices in the industry. Her expertise and connections in the industry will ensure that MediTech LLC achieves its mission.
MediTech LLC History
Sarah Nelson founded and incorporated MediTech LLC as an LLC in June 2023. Though the company is currently running out of a small rented office, it will move to a large warehouse once the lease is finalized.
Since incorporation, MediTech LLC has achieved the following milestones:
- Developed the company’s name, logo, and website
- Determined equipment and fixture requirements
- Identified and established relationships with potential clients and suppliers
- Begun recruiting key employees
MediTech LLC Services
MediTech LLC manufactures and sells Class I medical devices. These include (but are not limited to) the following:
- Surgical instruments
- Non-electric wheelchairs
- Stethoscopes
- Surgical masks
Industry Analysis
The medical industry is dependent on the access to high-quality medical devices and products. From gloves and masks to EKG machines, every device used in the care of patients needs to be high quality and always in working order. Devices that are poor quality or don’t work properly can cause significant problems when being used to care for patients.
Medical devices are categorized into three classes. Class I devices are devices that pose very little risk to the user. These items include bandages, surgical instruments, and gloves. Class II devices are intermediate risk devices. These include intravenous pumps and CT machines. Class III devices are high risk and require a great amount of regulation. These devices are also critical to sustaining life. These include pacemakers and brain stimulators.
According to Fortune Business Insights, the medical device industry is valued at $539 billion and is expected to grow at a CAGR of 5.9%. Medical devices are constantly in high demand and are essential for the success of the medical industry. Therefore, now is a great time to start a new medical device company.
Customer Analysis
Demographic profile of target market, customer segmentation.
The company will primarily target the following customer segments:
- Medical clinics
Competitive Analysis
Direct and indirect competitors.
MediTech LLC will face competition from other companies with similar business profiles. A description of each competitor company is below.
Smith & Smith
Smith & Smith is a large corporation that sells thousands of products, including cosmetics, hygiene products, and certain medical devices. The medical devices they primarily produce include bandages, ointments, and low risk surgical and physician instruments. They sell many of their products to the general public (such as simple wound care devices) but also sell some devices to the medical industry. They will be a major competitor since they sell primarily Class I devices. However, they currently do not produce as many medical devices as MediTech LLC plans to produce, which gives us an advantage in the market.
MedMonitor is a medical device company that manufactures Class III medical devices. Some of their products include breast implants, pacemakers, implanted prosthetics, and defibrillators. They do sell some Class I and Class II products, such as gloves, wound care items, and surgical masks, but they are not a major manufacturer of these products. As such, we expect that MedMonitor will only be a minor competitor in the market.
MedSource is the source for most of the medical industry’s Class II medical devices. They produce a long list of devices including syringes, testing kits, contact lenses, and blood pressure cuffs. They do produce some products that can be categorized as Class I devices, but their product list does not overlap too much with ours. As such, we expect that MedSource will only be a minor competitor.
Competitive Advantage
MediTech LLC enjoys several advantages over its competitors. These advantages include the following:
- Management : Sarah Nelson has been extremely successful working in the medical industry and will be able to use her previous experience to design and manufacture the best medical devices in the industry.
- Relationships : Sarah knows many of the local leaders, business managers, and other influencers in the medical industry. These relationships will help her have access to quality materials and create an initial clientbase.
- Affordability : Thanks to Sarah’s connections within the industry, we are able to access high-quality materials for our products for an affordable cost. As a result, we can price all our products more moderately than the competition.
Marketing Plan
Brand & value proposition.
The MediTech LLC brand will focus on the company’s unique value proposition:
- High quality medical devices
- Affordable pricing
- Client-focused service
Promotions Strategy
The promotions strategy for MediTech LLC is as follows:
Social Media Marketing
Social media is one of the most cost-effective and practical marketing methods for improving brand visibility. MediTech LLC will use social media to develop engaging content in terms of the company’s product offerings. Engaging with prospective consumers and businesses on social media platforms like Facebook, Instagram, Twitter, and LinkedIn will also help understand changing customer needs.
Website/SEO
MediTech LLC will invest in developing a professional website that displays all of the products offered by the company. It will also invest in SEO so that the company’s website will appear at the top of search engine results.
Direct Mail
MediTech LLC will blanket businesses with direct mail pieces. These pieces will provide general information on MediTech LLC, offer discounts, and/or provide other incentives for companies to buy our products.
Advertisement
Advertisements in print publications like newspapers, magazines, etc., are an excellent way for businesses to connect with their audience. MediTech LLC will advertise its products in popular magazines and news dailies. Obtaining relevant placements in industry magazines and journals will also help in increasing brand visibility.
MediTech LLC’s pricing will be moderate, so clients feel they receive great value when purchasing our products.
Operations Plan
The following will be the operations plan for MediTech LLC. Operation Functions:
- Sarah Nelson will be the CEO of MediTech LLC. She will oversee the general operations and executive aspects of the business.
- Sarah is joined by Rebecca Smith who will act as the warehouse manager. She will train and manage the staff as well as oversee general production of our products.
- Sarah will hire an Administrative Assistant, Marketing Manager, and Accountant, to handle the administrative, marketing, and bookkeeping functions of the company.
- Sarah will also hire several employees to manufacture our products and maintain the equipment and machinery.
Milestones:
MediTech LLC will have the following milestones completed in the next six months.
- 02/202X Finalize lease agreement
- 03/202X Design and build out MediTech LLC
- 04/202X Hire and train initial staff
- 05/202X Kickoff of promotional campaign
- 06/202X Launch MediTech LLC
- 07/202X Reach break-even
Sarah Nelson is a former surgeon who is familiar with the most popular medical devices in the industry. She knows better than anyone that low quality products means low quality care for patients. As a surgeon, she was often disappointed with the quality of the medical devices she used. Her hospital would routinely choose the cheapest options to save costs. This resulted in more problems and low quality care being delivered to her patients. She is now passionate about starting her own company that provides high quality medical devices for an affordable cost.
Though Sarah has never run a business of her own, she has worked in the medical industry long enough to gain an in-depth knowledge of the operations (e.g., running day-to-day operations) and the business (e.g., staffing, marketing, etc.) sides of the industry. She will also hire several professionals to help her run other aspects of the business she is unfamiliar with.
Financial Plan
Key revenue & costs.
The key revenues for MediTech LLC will come from the sale of our medical devices and products.
The major cost drivers for the company will include manufacturing costs, overhead expenses, labor expenses, and marketing costs.
Funding Requirements and Use of Funds
- Six months of overhead expenses (payroll, rent, utilities): $400,000
Key Assumptions
The following outlines the key assumptions required in order to achieve the revenue and cost numbers in the financials and pay off the startup business loan.
- Number of wholesale contracts:
- Year 5: 100
- Average order value: $5,000
Financial Projections
Income statement, balance sheet, cash flow statement, medical device business plan faqs, what is a medical device business plan.
A medical device business plan is a plan to start and/or grow your medical device business. Among other things, it outlines your business concept, identifies your target customers, presents your marketing plan and details your financial projections.
You can easily complete your Medical Device business plan using our Medical Device Business Plan Template here .
What are the Main Types of Medical Device Businesses?
There are a number of different kinds of medical device businesses , some examples include: Class 1 medical device, Class 2 medical device, and Class 3 medical device.
How Do You Get Funding for Your Medical Device Business Plan?
Medical Device businesses are often funded through small business loans. Personal savings, credit card financing and angel investors are also popular forms of funding.
What are the Steps To Start a Medical Device Business?
Starting a medical device business can be an exciting endeavor. Having a clear roadmap of the steps to start a business will help you stay focused on your goals and get started faster.
1. Develop A Medical Device Business Plan - The first step in starting a business is to create a detailed medical device business plan that outlines all aspects of the venture. This should include potential market size and target customers, the services or products you will offer, pricing strategies and a detailed financial forecast.
2. Choose Your Legal Structure - It's important to select an appropriate legal entity for your medical device business. This could be a limited liability company (LLC), corporation, partnership, or sole proprietorship. Each type has its own benefits and drawbacks so it’s important to do research and choose wisely so that your medical device business is in compliance with local laws.
3. Register Your Medical Device Business - Once you have chosen a legal structure, the next step is to register your medical device business with the government or state where you’re operating from. This includes obtaining licenses and permits as required by federal, state, and local laws.
4. Identify Financing Options - It’s likely that you’ll need some capital to start your medical device business, so take some time to identify what financing options are available such as bank loans, investor funding, grants, or crowdfunding platforms.
5. Choose a Location - Whether you plan on operating out of a physical location or not, you should always have an idea of where you’ll be based should it become necessary in the future as well as what kind of space would be suitable for your operations.
6. Hire Employees - There are several ways to find qualified employees including job boards like LinkedIn or Indeed as well as hiring agencies if needed – depending on what type of employees you need it might also be more effective to reach out directly through networking events.
7. Acquire Necessary Medical Device Equipment & Supplies - In order to start your medical device business, you'll need to purchase all of the necessary equipment and supplies to run a successful operation.
8. Market & Promote Your Business - Once you have all the necessary pieces in place, it’s time to start promoting and marketing your medical device business. This includes creating a website, utilizing social media platforms like Facebook or Twitter, and having an effective Search Engine Optimization (SEO) strategy. You should also consider traditional marketing techniques such as radio or print advertising.
Learn more about how to start a successful medical device business:
- How to Start a Medical Device Company

Medical Device Business Plan Template [Updated 2024]
Medical Device Business Plan Template
If you want to start a medical device business or expand your current medical device business, you need a business plan.
The following Medical Device business plan template gives you the key elements to include in a winning Medical Device business plan.
You can download our Business Plan Template (including a full, customizable financial model) to your computer here.
Medical Device Business Plan Example
I. executive summary, business overview.
[Company Name] is a brand new medical device designer and manufacturer, established in 2022 as a C corporation by two college friends. The friends, [Founder’s Name] and [Founder’s Name], developed a cutting edge device during graduate school that has since been improved and FDA approved.
This technology helps solve medication compliance challenges observed and experienced by [Founder’s Name] and [Founder’s Name]. It works by automating the dispensing and refill of medications, as well as providing automatic reminders when it is time for a dose. The device will be available in three versions, intended for the consumer, elder care, and travel markets. Products are customized to meet the needs of each of the three markets.
Helping patients with medication compliance is the bottom line goal of [Founder’s Name] and [Founder’s Name] at [Company Name].
Products Offered
[Company Name] produces three versions of its medication dispenser. These devices have various features specific to their respective markets, and all dispensers feature smart connections. These devices have been approved by the U.S. Food and Drug Administration.
Customer Focus
[Company Name] serves consumers of all ages. The demographics of current and potential clients and customers of our clients are as follows:
- 131 million people in the U.S. use prescription drugs
- 53% of all Americans aged 18 to 34
- 62% of those aged 35 to 49
- 75% of those aged 50 to 64
- 87% of those aged 65 to 79
- 91% of Americans aged 80 or older
[Company Name] recently entered into a contract to supply a 25-location regional pharmacy chain.
Management Team
[Founder’s Name] and [Founder’s Name] comprise the management team of [Company Name], and are in the process of securing additional management and staff personnel.
[Founder’s Name] holds a graduate degree from Florida State University in engineering. She is the CEO of the C corporation and her primary occupation is to present the vision of [Company Name], while encouraging major corporate relationships with retail pharmacies across the United States. She is a former regional sales manager for a major medical device corporation, with a track record of 10 years in that role, garnering over 25M in sales.
[Founder’s Name] holds an MBA degree from University of Alabama, with a focus on corporate strategic management. He is a former sales representative for a major medical device corporation, with a track record of 15 years in that role, garnering over 20M in sales.
Success Factors
[Company Name] is positioned in several ways to become a well-established corporation for the following reasons:
- Founders are highly-experienced in the medical device industry
- Founders have a contract to supply 25 retail pharmacies, which have a combined total of more than 100K customers
- Founders have patented these medical devices
- [Company Name] has a track record of continuous improvement to the original product, as technology progresses
- Founders have staff with experience in medical device manufacturing
Financial Highlights
[Company Name] is currently seeking $430,000 to launch. Specifically, these funds will be used to build on property, pay salaries, market services, and pay for research and development, including prototyping and concept modeling, as follows:
- Manufacturing center design/build: $150,000
- Working capital: $120,000 to pay for salaries, and overhead costs until [Company Name] reaches break-even
- Concept modeling, prototyping, and CAD-drawn sampling: $80,000
- Patenting and Trademarking: $50,000
- Marketing, advertising, customer presentations: $30,000
Top line projections over the next five years are as follows:
II. Company Overview
Who is [company name].
[Company Name] is a brand new medical device designer and manufacturer, registered in 2022 as a C corporation by two college friends. The friends, [Founder’s Name] and [Founder’s Name], developed a cutting edge device during graduate school that has since been improved and FDA approved. The device uses technology that helps solve medication compliance challenges.
In an informal survey of physicians within the greater Los Angeles area, [Founder’s Name] and [Founder’s Name] found that 56% of patients report some issue with medication compliance. In most cases, these patients rely on their memory to take medications as prescribed. Because memories can be faulty, physicians are eager to find a solution which will easily increase medication compliance.
Both [Founder’s Name] and [Founder’s Name] have personally experienced problems with medication compliance. Both have taken prescription medications since college, and quickly learned how easy it is to forget a dose or two. They soon discovered that this is a common experience, and the two of them embarked on a solution-finding mission: that of creating a device which minimized or entirely eliminated medication non-compliance.
The search led to an array of potential solutions, and with the help of their college professors and mentors, they prototyped several until they arrived at the first iteration of the device they build today. While they were still in school, the pair were awarded a patent for the device. Some years later, when their dreams began to coalesce into a solid plan, the founders took steps to ensure the device was approved by the FDA.
[Company Name]’s History
[Company Name] was formed as a C corporation. The history of the device is substantial. Extensive experience within the medical device industry provides the backbone of this corporation’s history, with high projections for profitability and multi-stage growth in the years to come.
[Company Name]’s Products
The devices produced by [Company Name] are designed to dispense, refill, and remind patients to take their prescription medications. This device offers a simple solution to medication compliance issues.
Significant advances in the process of concept and discovery, prototyping and production will be patented and trademarked as appropriate. The patents and/or trademarks will add to the value of the medical devices produced.
III. Industry Analysis
The U.S. medical device industry is projected to grow a healthy 3.3% over the next five years, with an increasing demand as time continues. The industry is in a growth cycle, and exhibits high profitability. An aging population and rising community expectations will drive demand for medical devices over the coming years. With the rise of chronic conditions such as diabetes, heart disease, cancer, AIDS, and hepatitis, prescription sales increased an estimated six times faster than population growth, resulting in a dramatic increase in the average number of retail prescriptions per capita. This represents a significant opportunity for [company name].
IV. Customer Analysis
Demographic profile of target market.
[Company Name] will serve the entire United States, targeting several customer segments within that market.
The largest market is retail pharmacies. There are 828,738 pharmacies in the U.S., as of 2022. Pharmacy and drug store staff members typically assist in recommending products to potential customers, as well.
The end consumers are the patients who take prescription medications. They include patients who take medication for chronic conditions, as well as those with acute conditions.
Customer Segmentation
The customers of [Company Name] can be segmented into primary and secondary segments.
Primary Customers:
- Chain Pharmacies
- Independent Pharmacies
- Hospital Pharmacies
- Online Medical Product Retailers
Secondary Customers:
- End Consumers
- Home Health Care Providers
- Families of Elderly and Disabled Patients

V. Competitive Analysis
Direct & indirect competitors.
MedTime – direct competitor Established in 2018, MedTime is a privately held business that has developed the first and only in-home medication manager that intuitively sorts and dispenses medication. The MedTime device has audible and digital reminders; automates refills; and provides real-time as well as historical adherence data. MedTime operates on a subscription fee and offers a free 90 Days Risk-Free trial. Each subscription comes with a MedTime smart dispenser. Additionally, the subscription gives access to a medication management app that gives pill-time reminders and missed-dose alerts, tracks what was taken and when, and gives remote caregiver monitoring for safety and medication schedule management with HIPAA-compliant security. The subscription also has automatic refills with free delivery and access to a 24/7 live support.
MedMonitor – direct competitor MedMonitor is a privately held business that provides services that simplify medication management and improve medication adherence. MedMonitor offers an automatic pill dispenser that reminds users when it is time to take their medication through flashes, beeps, text messages, and phone calls. The dispenser also provides the user’s caregivers, family members, or pharmacists with notifications about dosage activity, which can be monitored remotely through a patient profile’s on MedMonitor’s website. The subscription comes with a dispense device that is filled for several weeks, depending on the number of dosages per day, optional Locking, refill trays, an optional Medical Alert that has a two-way voice channel with the medical alert professionals, and an interactive screen that can be personalized. Additionally, the subscription comes with access to a secure website, where users and caregivers can easily program their unit’s medication schedule remotely, set up preferred notifications and review reports.
MediBox – direct competitor MediBox is a privately held business that developed a medication dispenser designed for people who have a hard time remembering to take their medicine. It reminds patients to take their medications, automatically dispenses a 90-day supply of up to 16 different medications, and sends messages to family and caregivers if they miss a dose. The MediBox is easy to setup, provides refill alerts and fast refills, provides a picture of the pills for each dose given, has a tilt sensor alerts for possible tampering or incorrect dosing/spillage, is lockable, and is the only medication dispenser tested to significantly improve adherence in an FDA approved clinical trial.
Competitive Advantage
[Company Name] has several competitive advantages over competitors, listed as follows:
- Founders have experience and in-depth understanding of the target markets, as well as direct contact with over 200 decision-makers in this industry
- Founders have several years of experience in sales and marketing of medical devices throughout the U.S.
- Patented and trademarked medical devices will increase value of the corporation
VI. Marketing Plan
The [company name] brand.
There are many medical device providers within the U.S. industry; there is only one, [Company Name], which delivers an affordable device proven to increase medication compliance.
Promotions Strategy
[Company Name] will promote its devices via the following methods:
Public Relations [Company Name] will make a series of press releases as the company continues in phase one of growth. Each announcement will present current notes of interest, such as medical device innovations, key company personnel additions, patents received, or trademarks confirmed. Each press release will contain forward-looking statements. Press releases will be sent to newspapers, industry newsletters and social media outlets.
Social Media [Company Name] will build activity on Instagram, Facebook, and, in particular, LinkedIn. These media markets will help drive traffic to the website and serve as a source for new hires and competitor information.
Website The website for [Company Name] will be primarily an introduction to the corporation, with successive pages demonstrating pictorial examples of its device improvements, concept models and CAD-drawn samples. A “From the Bench” article written by one of the founders shall be dropped monthly to add to reader interest.
Partnerships [Company Name] will partner with various philanthropic organizations in giving to charitable causes, including those that support the medical device industry. This effort will create potential target markets, as well as serve as a way of giving back to the customers served.
Medical Device Trade Shows [Company Name] shall be represented at booths at national industry trade shows, where the company will be highlighted via video presentations and examples of its devices.
Medical Conferences [Founder’s Name] and [Founder’s Name] shall both serve as company representatives on expert panels or in seminars. They will represent [Company Name] as industry leaders and as experts within the medical device industry.
Pricing Strategy
The pricing strategy for [Company Name] will be based on models that conform to the pharmacies, physicians’ offices, caregivers and patients who purchase the devices.
VII. Operations Plan
Functional roles.
[Company Name] contains the following functional roles:
Research & Development Role
- Direct responsibility for technological advances relating to the device
- Responsible for production of prototyping, 3D printing, and CAD-drawn samples
- Oversight of staff members who work with every phase of production
FDA Administration Role
- Direct responsibility for timely FDA review and approval process
- Oversight of timelines for customization process; in tandem with R & D
Administrative & Human Resources Role
- Direct responsibility for all office functions and administrative procedures, including financial accounting and tax processes
- Customer relations responsibility
- HR responsibility (until future HR Director hired)
- Oversight of maintenance staff and inventory, stock, and supplies
- Marketing & Sales Manager
- Responsible for all marketing and sales promotions and presentations
- Responsible for representation of the company at trade shows and in seminars or other industry events
- Oversight of sales representatives
VIII. Management Team
Management team members.
[Company Name] was founded by [Founder’s Name] and [Founder’s Name]. The corporation shall be under the direction of the founders who share equal shares of the corporation. Each founder will be responsible for a portion of the functional roles within the corporation and those who operate it.
Given the history of the founders within the medical device industry, building a staff of teams will be done with purposeful and serious thought and with an understanding of which team member will perform best in each role.
Hiring Plan
[Company Name] will hire the following personnel as soon as possible in phase one of growth:
- Research & Development Director
- FDA Administrative Officer
- Administrative & Human Resources Director
- Administrative staff members (2)
IX. Financial Plan
Revenue and cost drivers.
The revenue for [Company Name] will be generated by sales of medical devices offered to pharmacies, physicians, caregivers, and patients.
[Company Name] will produce the three current versions of its medication dispenser, as well as developing continuous improvements to the devices offered by [Company Name].
The major costs for [Company Name] will include overhead (including employees, building and material costs), costs for research and development, prototyping, 3D printing, and costs related to FDA approval processes.
Capital Requirements and Use of Funds
[Company Name] is currently seeking $430,000 to launch and take the company through phase one of the growth cycle. Specifically, these funds will be used to build on property, pay salaries, market products, and for research and development, including prototyping and concept modeling, as follows:
Key Assumptions
5 Year Annual Income Statement
Comments are closed.


Medical and Health Business Plans and Resources
Written by Dave Lavinsky

In the medical and healthcare industry, where the balance between patient care and operational efficiency is paramount, a comprehensive business plan stands as a critical element for success. It serves as a strategic guide, vital for navigating the sector’s regulatory complexities, technological advancements, and evolving patient needs.
Below you will find an extensive array of business plan examples for a variety of medical and healthcare businesses, including private clinics, medical device startups, telemedicine services, and healthcare consulting firms. Each plan is meticulously crafted to address crucial aspects such as market research, compliance with healthcare regulations, service delivery models, and financial management. These plans are invaluable for professionals and entrepreneurs in the healthcare sector, providing a structured approach to developing services that are not only medically effective but also financially viable and operationally sustainable. They underscore the importance of strategic planning in creating a healthcare business capable of delivering high-quality patient care while navigating the challenges of this highly regulated and competitive industry.
Medical Practice Business Plans and Resources
Acupuncture Business Plan PDF Chiropractic Business Plan Template Chiropractic Business Plan PDF Counseling Private Practice Business Plan Template How to Open a Counseling Private Practice Business Dental Business Plan Template Medical Practice Business Plan Template Sample Medical Practice Business Plan How to Open a Medical Practice Business Medical Clinic Business Plan PDF Mental Health Private Practice Business Plan Template Mental Health Private Practice Business Plan PDF Mobile Health Clinic Business Plan Template Optometrist Business Plan Template
Nursing Home, Elderly Care and Home Health Business Plans and Resources
Assisted Living Business Plan Template Sample Assisted Living Business Plan How to Start an Assisted Living Business Assisted Living Business Profit Margins Monthly Expenses of Assisted Living Business How Much Does an Assisted Living Business Makes Assisted Living Business Startup Costs Assisted Living Business Plan PDF Elderly Daycare Business Plan Template Group Home Business Plan Template Group Home Business Plan PDF Home Health Care Business Plan Template How to Start a Home Health Care Business Home Health Care Business Plan PDF Non Medical Home Care Business Plan Template How to Start a Non Medical Home Care Business Non Medical Home Care Business Plan PDF Nursing Home Business Plan Template How to Start a Nursing Home Business
Other Medical and Health Business Plans and Resources
Apothecary Business Plan Template Drug Rehabilitation Business Plan Template Health Coaching Business Plan Template IV Hydration Business Plan Template How to Start an IV Hydration Business IV Hydration Business Plan PDF Medical Billing Business Plan Template How to Start a Medical Billing Business Medical Device Business Plan Template How to Start a Medical Device Company Medical Device Business Plan PDF Non Emergency Medical Transportation Business Plan Template Mobile Phlebotomy Business Plan Template Pharmaceutical Business Plan Template Pharmacy Business Plan Template Sample Pharmacy Business Plan How to Start a Pharmacy Pharmacy Business Plan PDF Physical Therapy Business Plan Template Physical Therapy Business Plan PDF Sober Living Business Plan Template Transitional Housing Business Plan Template How to Start a Transitional Housing Business Urgent Care Business Plan Template Medical Transportation Business Plan Template Medical Lab Business Plan Template


Medical Business Plan

You cannot start and run a successful medical business without writing and implementing a comprehensive business plan. A business plan gives you a complete framework for researching and analyzing the market, identifying possible opportunities, financing the project, and scaling it all together. Here are some of the best examples of medical business plans worth having a look.
Medical Practice Business Plan Template

- Google Docs
Size: A4, US
Medical Laboratory Business Plan Template

Dispensary Business Plan Template

Fertility Clinic Business Plan Template

Fertility Clinic Marketing Plan Template

Pharma or Drug Sales Plan Template

Medical Clinic Sales Plan Template

Medical Device Sales Plan Template

Executive Medical Reimbursement Plan Template

Best Medical Business Plan Examples & Templates
medical business plan example.

Size: 8.86 MB
If writing a medical business plan were easy, every business aspirant would do it with ease. To be clear, a comprehensive business plan for a new healthcare facility takes time to put together. But, what if you are green in this in the first place? The best thing to do is to look at a sample plan to get a clear picture of what a comprehensive strategy for a health facility looks like. This PDF file is a unique sample of a well-written business strategy for a healthcare facility. The plan covers everything you need to know about establishing a healthcare center, from business objectives and guiding principles to demographic analysis and their access to care.
Printable Medical Business Plan

Size: 780 KB
It doesn’t make sense to start a medical business without a strategy in place. You need to identify your audience, sturdy the market, conduct a SWOT analysis , and develop a marketing technique. This calls for a comprehensive business plan and here is an example that you can download and use as a guide to writing your own strategy. This sample will help you to understand a few things. First, you’ll learn how to write a clear summary of the proposed medical business. Second, you will see what a detailed business description looks like. Third, you will dive deep into learning and understanding the competition. Then, you will learn about financial strategy, which is important for starting and running a healthcare business at scale.
Free Medical Business Plan Example

Size: 88 KB
It is important to understand that a business plan does not have to be complicated. It doesn’t have to be dozens or hundreds of pages either. In fact, you can set up and run a successful medical business with a very simple business plan . This PDF document is an example of a very simple business strategy, which a clear indication that even lean business plans can help you take a step in the right direction. This plan covers a number of sections, including an executive summary for the proposed business, proposed services, market evaluation, and marketing approach. Click the link above to download the PDF file.
Medical Practice Business Plan Example

Size: 237 KB
This PDF document is one of the most comprehensive medical business plans that you can read to understand what a professionally written business strategy looks likes. The 37-page file features a unique outline, which makes the entire document easy to scan. This file has a lot of information. From the executive summary and market sturdy to SWOT and market analysis , there is quite a lot to learn. The author uses a simple language throughout the document. So, whether you are green to writing a business proposal or you just need a simple example for reference, this is a good template to download.
Medical Startup Business Plan Example

Size: 219 KB
You cannot start and run a successful business without a plan. In fact, many businesses that begin to operate without a strategy often close within the first six months. This happens across various industries, even in medical business. Now that you have made up your mind to start a medical business, you first need to write a business plan. If you have never written one before, don’t worry. You can just download this PDF file, read the entire strategy example, and then use the same knowledge that you pick from it to write a comprehensive business plan of your own. Click the link above to download this template.
Medical Business Plan Example for Clinic

Size: 262 KB
Ask every serious entrepreneur what it takes to start and run a successful business and they’ll tell you that the first thing you need is a business plan. This tells you that a successful business depends on a comprehensive strategy . Now that you have made up your mind that it is time for you and your stakeholders to start a clinic in your locale, it’s important to write a business plan before investing money in the project. The plan will enable you to determine whether the business is feasible to pursue. By finding, connecting with, and studying the target market, it becomes easy to understand your business even before starting it. Download this medical plan for the clinic to learn more.
Private Hospital Business Plan Example

Size: 2.15 MB
Even if you have enough funding to start a private hospital, you’ll need to do in-depth research and then come up with a solid business plan that will help you set up the enterprise. At the end of the day, your goal is to run a successful business . This is something you can’t do if you start with a proper strategy. Remember, the success of your private medical startup will depend on the structure of the plan. The success of the upcoming business will depend on the structure of the plan. In other words, a business plan is a must-have.
Simple Medical Business Plan Example

Size: 141 KB
You already know that it is difficult to run a successful business without a plan. So, if you want to set up a new medical facility in your area, first start by writing a business plan for the foreseen startup. The PDF file above is a unique example of a business plan that you can use for reference. This guide is important because it focuses on the most important elements that make up a comprehensive business plan.
Comprehensive Business Plan for Medical Facility

You can use this sample template to write a comprehensive business plan for a medical facility. Some of the highlights include identifying challenges and determining their respective opportunities. This gives you a clear understanding of the market that you would like to target so that you can align your medical services to their needs once you start operation.
Short Medical Business Plan Example

Size: 264 KB
If you are the kind of a medical business enthusiast who prefers to write a short business plan, this example is suitable for you. The content of the file includes an executive summary, market analysis, business growth, marketing strategy, and financial projection.
Text prompt
- Instructive
- Professional
Create a study plan for final exams in high school
Develop a project timeline for a middle school science fair.
Don't bother with copy and paste.
Get this complete sample business plan as a free text document.
Medical Equipment Developer Business Plan
Start your own medical equipment developer business plan
Medquip, Inc.
Executive summary executive summary is a brief introduction to your business plan. it describes your business, the problem that it solves, your target market, and financial highlights.">.
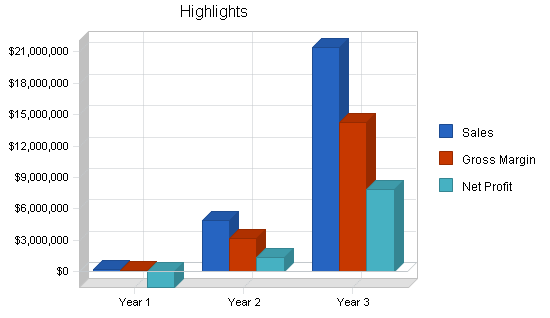
Medquip, Inc. is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche markets. Three devices have already been designed with the participation of leading physicians and surgeons in gastroenterology. Seven patents are initially incorporated. The company projects $16 million in sales in year three. The company expects to have $50 million in revenue by year five. Patent applications on its first three market entries have already been accomplished using a top patent law firm.
The market segments are clearly defined and all are subject to a high growth trend. One market is projected to exceed $160 million in the next three years. That is the endoscopic variceal ligation market. One of the founders of Medquip participated in the design of the current market leader in that field and has improved upon the product significantly. Another market addresses a well-defined and unanswered need in endoscopic surgery: the clearing of fundal pools of blood and tissue during surgical procedures. A new and innovative design has been created to answer the needs of surgeons.
This market should begin at $20 million but could expand to several hundred million as soon as approvals are obtained for many varied surgical procedures. Medquip intends to license this technology to a larger company. The company becomes mature in year three. The company is potentially profitable in year one only if a proposed licensing agreement can be closed.
The mission of Medquip, Inc. is to design, develop, and market new patented technologies in the medical device field. The technologies will fill market niches that each account for a minimum of $20 million dollars in potential sales. Each technology will fill a current need in medical procedure by improving upon an existing technology or device, or by designing a device to serve a need that is clearly defined and acknowledged by medical professionals. Each product shall be priced to appeal to a managed-care market that stresses lowest cost of total treatment parameters.
Keys to Success
The keys to success for Medquip, Inc. are as follows:
- Initial capitalization obtained.
- All patent applications filed.
- The ability to generate early revenue from non-regulated markets in Europe.
- Licensing at least one technology and application to a major medical device corporation.
- Getting low interest loans and/or grants to fully fund product development and prototype manufacture.
- Recruiting top-notch CEO prior to second round financing and market roll-out.
- Successful 510k approval from FDA to market Visi-Band in the U.S.
- Successful implementation of sales and marketing plan to U.S. managed care market to obtain a minimum 10% market share in the second full year to generate $16 million in revenue.
- Increased product development and continued market share gains to produce a $50 million revenue company by year five.
The principal objectives of Medquip, Inc. are as follows:
- To achieve a 10% market penetration in the endoscopic variceal ligation market by year three.
- To achieve $16 million in revenue by year three.
- To raise $1 million in private seed capital in the first six months.
- To win low interest loans and grants from the government of Puerto Rico totaling $1.2 million in year one.
- To license its technology for the obliteration/suction/irrigation market for $1 million dollars in year one.
Company Summary company overview ) is an overview of the most important points about your company—your history, management team, location, mission statement and legal structure.">
Medquip, Inc. will develop and market endoscopic medical devices through multiple distribution channels both foreign and domestic. The company is currently developing its patent-applied technologies to final product and approval stage. It is also seeking to establish its corporate identity in the medical products field. Growth strategy calls for one joint venture license as well as the following objectives:
- Complete the patent process.
- Establish corporate identity, brand names, trademarks.
- Establish a medical advisory board.
- Build staff, infrastructure, and retain consultants for trial and compliance issues.
- Conduct animal trials.
- Prepare for FDA clinical trials.
- Continue R & D and product development.
- Explore options for 2nd round financing (venture capital, corporate alliance, licensing, public offering) to maximize value to shareholders.
Note: Management believes that accelerated FDA approval process will be available on the band ligation device since it involves only modifications on an existing, approved device. There is past precedent in 510k approvals (in an average of 3 months) in documented cases.
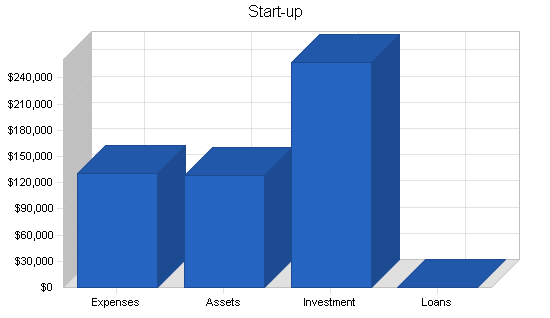
Start-up Summary
The key elements in the Start-up plan for Medquip, Inc. are:
- The legal expense for filing all patent applications.
- The establishment of Corporate Identity.
- The location and place of doing business.
- Funding of additional capital raising alternatives.
- Salary for the two key managers and founders.
- Formulation of Strategic Plan. Costs of raising capital through private placement.
$215,000 was raised from the initial two investors for these purposes. This funding came in in early 1998 and these tasks have either been completed successfully or are in the final process of completion. These are treated purely as start-up expenses by this plan. $128,000 is treated as cash-on-hand as of the start of this plan on January 1, 1998. The remainder of the start-up capital required as well as capital required for the continuation of operations in the first six months will be provided by selling the shares in the private placement. The capital obtained from these sales is expected to total an additional $850,000 and the plan calls for this cash to be infused in May and June, 1998.
Company Ownership
Medquip, Inc. is a South State “C” corporation.
Its founding shareholders are:
Eric Smith (2,545,000 shares) Timothy Jones (500,000 shares)
At the date of this plan, two additional shareholders are of record:
Arthur C. Clark (50,000 shares) Genesis Corp. (14,000 shares)
Company Locations and Facilities
Medquip, Inc. business offices are at 1234 Main Street, Anytown, U.S.A. Phone is …. Fax is …. These offices are leased month-to-month on a temporary basis. This business plan calls for the establishment of corporate offices, R&D facilities, and prototype and small-run manufacturing facilities. These facilities are to be located in Puerto Rico with 10,000 sq. ft. initially expandable to 30,000 sq.ft. Rental costs in Puerto Rico range from $1.75 to $4.00 per sq. ft. Currently available space in Puerto Rico may also be used on a joint-venture basis to be negotiated.
Medquip, Inc. will initially market three distinct products.
- The Visi-Band, a disposable device that is used in endoscopic variceal ligation procedures.
- The Visi-Gator, a partially disposable device that is used to remove blood clots during various endoscopic surgical procedures.
- The Visi-Lyser, a suction/irrigation device for laparoscopic procedures.
The technology used in these products is the subject of seven patents in the application process.
These three product areas may be more generally defined as follows:
- Endoscopy Devices–used for esophageal variceal ligation, hemorrhoidal ligation . The Visi-Band: Consisting of ligating bands with greater stretchability and grip (Super-Elastic bands incorporated into a multi-band dispensing device).
- Endoscopy Devices–used for lysis (tissue dissolving). The Visi-Gator: Consisting of a rotary cutting tool to clear fundal pools of blood in the stomach.
- Endoscopy Devices–used for suction/irrigation and tissue removal. The Visi-Lyser: Consisting of a suction/irrigation tool to remove tissue effectively.
Product Description

Multiple Ligating Band Dispenser: The Visi-Band
Application is endoscopic variceal ligation which is a rapidly growing surgical procedure quickly replacing sclerotherapy for the removal of polyps in both upper and lower gastro-intestinal exploration.
Scope: This innovation applies to the internal technology of ligating bands independent of the dispenser or delivery system. Visi-Band is a pre-loaded delivery device for applying multiple ligating bands remotely from the distal tip of an endoscope. (The leading current product in this category is the Speedband made by Boston Scientific).
Clinical Advantages: A perceived clinical advantage of these bands is ligation of a greater range of tissue sizes with a single band. These ligating bands stretch easily over the largest tissue to be ligated and yet will grip securely even the tiniest tissue to be removed. These bands can have an inner diameter near zero so that even tiny varices are gripped firmly.
Current State of the Art Technology: Market-leading bands today are molded of homogeneous rubber materials. Material properties of elasticity have limited the stretch of conventional ligating bands to a range of about seven-fold. A typical market-leading band for esophageal variceal ligation has an inner diameter of 1.8mm. This band can stretch to a maximum inner diameter of 12.4 mm to ligate a varix. This maximum size roughly corresponds to the endoscope diameter. A varix of 1.8 mm would not be ligated because the band would be loose around the tissue.
Medquip Technology: The Super-Elastic Band innovation effectively engineers the band material stresses in a way that increases the apparent stretchability of the band many times. We have created bands with proximate zero inner diameters, which can be stretched at least as large as conventional bands with large inner diameters. Bands created with this technology can also hold their elasticity for longer periods of time. The basis of our technology is an internal compressive pre-stress at the band inner diameter. This can be achieved in at least five practical ways covered in our patent documentation. The true zero inner diameter band is a result of the compressive forces creating small scale creasing or wrinkling which fill the band interior. Bands made to date exhibit an effective elasticity of 20 times and more versus the seven times stretch in the market-leading band.
Medquip ligation devices should have an unprecedented and superior range of application to meet ligation requirements. Super-Elastic Bands can mean fewer special sized devices to manufacture, purchase, specify, and stock. This fits well in a managed-care environment through lower costs with inherent clinical advantages. Medquip and the health care system could benefit from higher volumes of a smaller number of different products.
Further Clinical Advantages:
- Visi-Band is designed for multiple band ligations with a single scope insertion.
- Visi-Band delivers maximum visibility with zero “tunnel vision” during insertion and exploration, a limitation of all competitors.
- Visi-Band delivers maximum mobility by being nearly flush with the distal end of the endoscope during insertion and exploration.
- Visi-Band should be significantly faster to install to the endoscope by having many fewer assembly parts and steps than the competition.
- Visi-Band is smaller in diameter than Speedband for patient acceptance and comfort.
- Visi-Band patient entry is smoother and protected at all times from miss-fires by a smooth, transparently clear outer shield.
- Visi-Band can ligate smaller varices with the super-elastic bands as described above.
- Visi-Band can be supplied in a single configuration with multiple bands, seven or eight, at a cost similar to the competition’s three or six band unit.
Note: Today’s multiple ligating band dispensers release bands off a tube at the distal endoscope end with typically two filaments for each band. This tube is at least as long as the endoscope diameter and creates severe “tunnel vision” because the bands typically cover the outside surface of the clear plastic tube. This added length also reduces the mobility of the distal tip. Each filament must be precisely assembled and triggered for each band. Bands exposed on the outside of the tube are prone to miss-fire as evidenced by a clear shield with instruction to remove just prior to insertion into the patient. A conically tapered dispenser is typical and necessary to help bands roll off the distal end. This taper increases the diameter of the ligating unit. Installation is a complex, multiple step process often involving a separate ligating unit, handle unit, a trip wire, scope fastener, and irrigation catheter. Actuating a competitive unit can cause the distal tip to move from the tension of the trip wire.
Medquip Technology: The Medquip Visi-Band multiple band ligator is planned to have two components, the ligating unit and handle. The Ligating Unit comprises a clear tubular base with bands stretched around the distal end, a clear sleeve outside of and concentric with the base, and a trip tube which passes through the biopsy channel of the scope. The ligating unit mounts in a retracted position around and substantially flush with the distal tip of the endoscope. The ligating unit is placed on the distal tip of the scope by pushing the trip tube through the biopsy channel. The handle is then snapped on the free end of the trip tube. The unit is extended distally prior to ligation, and the sleeve is moved axially to load then deliver a single band at a time. No individual filaments are required for each band so manufacturing cost is low. The unit is “digitally” actuated by repeated axial motion of the sleeve rather than small incremental displacements. The tip of the scope should not move because a compressive force in the tube cancels the tension in the trip wire internal to the trip tube.
Summary of advantages over currently available products:
- Better visibility due to side-mounting of band dispensing device.
- Smaller band, more stretch, enabling banding of smaller varices.
- Internal loading of bands, enabling protection (bands are not dislodged).
- Capable of carrying more bands (8 vs. 6).
- Significant reduction in manufacturing and assembly costs.
Lysis (tissue dissolving) Rotary Cutting/Suction Tool: The Visi-Gator
Applications include the removal of blood clot and stray tissue during suction irrigation in laparoscopic surgery, examination of bleeding ulcers in the stomach, hematoma, clot in the fallopian tube, or cerebral aneurysm. Current solutions employ crude tubes that incorporate a few holes for anti-clogging combined with drug treatments which are largely ineffective.
Scope: An uncleared fundal pool of retained blood in the stomach precludes complete visualization of the stomach in 5.6% of cases of acute upper gastrointestinal bleeding. According to an October 1997 article in Gastrointestinal Endoscopy: “In conclusion, the results of this study provide evidence that the inability to clear a fundal pool of blood at the time of emergent upper endoscopy for acute UGI bleeding is associated with substantial morbidity and mortality and … Aggressive mechanical and/or pharmacological measures to clear the fundus of blood are warranted in patients undergoing urgent endoscopy for acute UGI bleeding.”
Clinical Advantages: The Visi-Gator tool may be inserted from the outside into the biopsy channel of the endoscope as needed. The scope does not need to be withdrawn to install and use the Visi-Gator.
Medquip Technology: Visi-Gator is a thin, ultra-flexible, spring-like device that is introduced through the biopsy channel to the distal end of the endoscope. High-speed rotations of one or more of many concentric spiral elements both drive rotating lysing filaments and positively pump solids and liquids out. Centripetal force expands and stiffens the filaments from a stored position during insertion to a generally planar and circular path. Vacuum may also be applied. A hollow central channel may also be provided for irrigation, access, or other therapy.
- Visi-Gator clot dissolving aggressiveness varies from mild to intense and is proportional to the adjustable input speed control.
- Visi-Gator actively draws fluid and solids up through the biopsy channel with a positive pumping action.
- Visi-Gator continues to dissolve solids even as they pass through the biopsy channel.
- Visi-Gator will tend to be non-clogging and self-cleaning.
- Visi-Gator does not rub high-speed surfaces against the biopsy channel and cause high rates of wear.
- Visi-Gator provides a central channel for clear water irrigation, additional suction or other therapy.
- Visi-Gator motor driver is envisioned to be reusable. The fluid path and lysis head is likely to be disposable.
- Visi-Gator dissolving action and lysing head is transparent. One can see through the scope real time in the zone of clot lysis continuously as the lysing progresses.
- Visi-Gator appears to be capable of rapid clot lysis yet is gentle to tissue.
- Visi-Gator tip is soft and pliant to flex over any non-clot tissues.
- Visi-Gator lysis zone is generally a defined planar disc perpendicular to the axis of the distal end of the scope.
- Visi-Gator allows lysis not to penetrate beyond the disc boundaries.
Suction Irrigation Device for General Laparoscopic Surgery: (incorporated into Visi-Gator): The Visi-Lyser
Scope: Visi-Lyser suction irrigation device includes a self contained, stand-alone suction/irrigation device to aid the surgeon during laparoscopic surgical procedures. Clinical Advantages: Lysis of the clot or tissue at the entry point, which will tend to be clog resistant. A likely disposable fluid path and reusable power head.
Current State of the Art Technology: Conventional suction irrigation devices are prone to clog when presented with a clot during surgery.
Medquip Technology: Similar to the Visi-Gator tool with the lysing filaments contained within a perforated suction tube.
Note: The Visi-Lyser is designed to work with the Visi-Gator in laparoscopic procedures but may also be used by itself in distinct procedures.
One embodiment inserts into the working channel of an endoscope as an accessory. A flexible spiral spring-like element rotates at several thousand RPM inside of a close spiral spring tube of opposite hand wind. A tiny motor on the outside proximal end of the endoscope spins the spiral element. Liquid or solid material inside the spiral element windings is drawn from distal to proximal positions by the interaction of the rotating and stationary spiral geometry. Suction is preferably applied at the proximal end of the spiral element before the motor. At the distal end of the spiral element, a generally spherical ball tip covers the spiral end to protect soft tissues from the spiral screwing into tissue and causing trauma. Projecting from the tip is a single or multiple of filaments, which spin at the high speed of the spiral element. The filaments lyse the unwanted clot or soft tissue fragments, depending on speed of rotation, diameter, material, and construction details. The filaments may optionally be extended or retracted from the outside to lyse differing diameters or body cavity sections. Rotation speed of the motor is a direct way to control aggressiveness of lysis dynamically from the outside. A flexible tube may be placed at the center of the rotating spiral element to carry water or saline to flush the cavity being lysed. A single or multiple of apertures near the distal end of the water tube may spray a jet sideways to clear potential clogs. The water center tube is preferentially not rotating.
In a second embodiment, designed for suction irrigation of a surgical site, the rotating filaments and spiral are placed in a tube with small apertures. The filaments rotating inside server to lyse clot and tissue which would normally clog the apertures. A rotating jet of water inside could also serve to clear any clogs. The spiral element could also be present inside.
Advantages over currently available products: No Products Currently Available.
Note: A variety of medical journal articles and research studies are available that cover both potential product areas.
Patent searches and filings are under way with Sidley and Austin. Opinion is that as many as seven distinct patents may be available and obtainable on the three devices cumulatively. Mr. Smith has assigned these patents (as obtained) and any future issued patents in the medical device arena to Medquip, Inc.
Competitive Comparison
The leading product currently available in the endoscopic variceal ligation market is the Speedband made by Boston Scientific. Other product entries are from C.R. Bard and Wilson-Cook. The Speedband from the Microvasive division of Boston Scientific is far and away the market leader with an estimated 60% market share. The Six Shooter from Wilson-Cook has a 20% market share. Rapid-Fire made by C.R. Bard has a 10% market share. All of the devices are disposable (single patient use).
Eric Smith, a founder of Medquip and the developer of Medquip’s patented technologies had significant participation in the design of the Speedband. He is aware of both its strengths and shortcomings. The Visi-Band is a much improved product in a rapidly growing market application. Many of the product advantages were highlighted in the previous section of this plan. To summarize the key advantages:
- Band itself enables smaller varices to be banded.
- Smaller hole plus more stretch in band.
- Device has better field of view.
- Device has better mobility.
- Bands are fired internally vs. externally.
- Significant cost reductions in mfg.
The Visi-Gator and the Visi-Lyser represent an entirely new application with no current competition. Their use can potentially range from stomach procedures to heart procedures and a variety of other surgical procedures. Together they solve the well documented and acknowledged problem of lack of visibility in endoscopic procedures where blood clots are involved. The Visi-Lyser also allows an improved and more efficient means of removing fundal pools of clotted blood and tissue.
Sales Literature
Sales literature for Medquip, Inc. remains to be developed.
Primary raw materials needed for Medquip products are as follows:
- Molded plastic parts.
- The tooling and molds (capital expenditure).
- Band material (polymer).
- Small electric motor.
All of these components are easily sourced and multiple suppliers have been identified. In addition, injection molders have been identified to manufacture the molded components for Medquip products. There are multiple potential sources.
A potential source for additional research and design help and compliance is Valnet in Puerto Rico.
Medquip will perform final assembly and distribution from its own facility in Puerto Rico or utilizing contract manufacturing depending on the extent of financial support from the government of Puerto Rico.
The principal areas (general descriptions) of the patents applied for are as follows:
- The ligating band itself.
- The movable dispenser.
- The dispenser itself.
- Two additional alternative dispensers.
- The tissue dissolving device.
- The transporting catheter.
- The dispenser control device.
Care has been taken to take into account all potential claims of the inventions as well as to protect them from possible competition from other technologies (including inferior ones). All patent application documents are available for examination by potential investors. The first document is entitled “A ligating structure having greater stretchability, greater shelf life, and greater ligating characteristics and method of manufacture.” It lists more than 40 independent claims.
Also available is the assignment of all patents in the medical device field (above listed and future developed) by Eric Smith to Medquip, Inc. The first four patents listed above relate to the Visi-Band. The last two relate to the Visi-Gator.
Trademark application has been filed on the name Medquip. Trademark applications are in process on the names Visi-Band, Visi-Gator, and Visi-Lyser. No conflicts or other use of these names has been found in an initial search.
Future Products
Plans for future development by Medquip include additional ideas and technologies to be created by Eric Smith as VP of R&D for Medquip. In addition Medquip may seek to acquire technologies developed by others once it attains sufficient capitalization to do so. It is the objective of Medquip to both innovate and market its products. Once an industry reputation has been achieved and marketing channels opened expansion into other medical device areas becomes potentially rewarding.
A recent article in Red Herring indicates the bio-tech field in general is a current hotbed of activity and most of the companies involved are early-stage development companies.
Market Analysis Summary how to do a market analysis for your business plan.">
The two key factors influencing discussion of Medquip Inc.’s market are the medical procedures and product usage statistics and the customer or chain of distribution considerations.
In both cases the trends are upwards in the favor of Medquip. Banding is growing rapidly replacing sclerotherapy and managed care stresses lowest cost of total treatment. The following sections explain how both offer great market potential to Medquip.
Market Segmentation
The potential customers of Medquip, Inc. are both domestic and foreign.
Domestic customers include managed care groups, hospital buying groups, physician groups, independent physicians, and other (catalogues) and medical supply houses. The market is dominated by managed care groups. More than 50% of all purchases of medical devices are made by these groups and that is forecast to reach 75% by the year 2000.
The foreign market includes many of the above segments but also includes key distributors. For example, only four distributors are required to penetrate the European, Middle Eastern, African, Central and South American and Japanese markets. These distributors have already been identified.
The following chart illustrates the approximate total number of these buying groups that exist. But initial concentration may be more defined by targeting the largest 50 customers in each segment. This data is clearly definable and available.
Industry Analysis
The health care industry in the United States has been dominated by managed care and hospital buying groups. Lowest total cost treatment has been the evolution of the pricing model. Medquip is ideally positioned to capitalize on this trend. There has been a rapid trend to endoscopic variceal ligation from the previous norm of sclerotherapy for the following reasons:
- Fewer post-op complications.
- Better control of bleeding.
- Lower risk of re-bleeding.
- Reduction of over-all treatment cost.
- Positioned ideally for managed care.
- Current research studies available.
The growth of banding has been as high as 30% per quarter according to IMI. The top 25 to 50 customers in each market category may account for as much as 70% of the potential business, making it easy to target customers with a multi-channel tiered strategy. The foreign markets may be penetrated initially with as few as four key distributors.
Competition and Buying Patterns
Large companies with established brand names and distribution patterns have a distinct advantage in the medical device arena. But new small companies are succeeding on a regular basis dependent on their technology and its over-all cost-of-treatment advantages. Product cost in and of itself is not paramount but education and training are. The product must deliver performance as promised in order to do a procedure more effectively with the fewest complications.
Time saving and effectiveness are the key economic parameters. Medquip will succeed based upon the capability of its products. They are already competitively priced…except they are more effective. After initial market resistance to any new product, Medquip’s products can grow to dominate a market segment…in this case distinct surgical applications.
Variceal ligation is a huge and growing market. The market for the band dispenser is a currently existing one with accurate, up-to-date data from IMI. This clearly defined market represents one of the fastest growing segments in the medical device industry. The reason is that doctors are transitioning rapidly from the old and traditional sclerotherapy surgical protocol to banding procedures.
The entire endoscopic market is a $1 billion annual market. Nationally, upper GI endoscopy accounts for more than 1.2 million procedures per year according to MDI. The trend from endoscopic sclerotherapy (ES) to endoscopic variceal ligation (EVL) has been rapid.
In dollars the Endoscopy–Misc. Supplies category has been growing by 30% per quarter. The most recent qtr. ending June ‘97 was $18.8 million (IMI). The market segment exceeds $70 million in annual sales. This growth can be pegged almost exclusively to the market share gained by EVL at the expense of ES. Best estimates indicate that the percent of EVL procedures to total procedures is still in the 30% to 40% range. Thus, the potential market for EVL could be as high as $180 million.
Main Competitors
The most important competitor to be considered is Microvasive (Boston Scientific). Its strengths are its reputation, current market position, and its entrenched loyalty among physicians using its products. Its weakness is that it is not particularly innovative, normally depending on other companies to develop and perfect its technologies. This makes it vulnerable to a new, improved entry.
Eric Smith did significant design work for the company that perfected the Speedband for Microvasive. He is well aware of the performance gaps in the product.
Industry Participants
The major companies in the endoscopic device field directly competitive with Medquip proposed products are Microvasive (Boston Scientific), Bard, and Wilson-Cook. No manufacturer has a product competitive with the Visi-Gator although a company like Johnson and Johnson could be a likely joint venture partner or licensee.
Managed care providers such as Humana, Kaiser, Blue Cross, etc. are easily identified. Hospital groups are also easily identified as are physician groups.
However, buying methods are diverse, and there is significant overlap of decision making between these segments. That is why it is imperative that Medquip have an experienced CEO and an experienced Sales and Marketing Manager to effectively attack these channels.
Distribution Patterns
Distribution patterns in the health care industry are such that the large buying groups dictate what products are used for certain procedures throughout their sphere of influence. Thus, our products could be mandated or forced out for thousands of patients due to their health plan or hospital group. Others recommend several alternatives which require physician education and intervention, similar to pharmaceuticals.
Distributors are key for foreign markets.
Strategy and Implementation Summary
Medquip, Inc. will pursue specific, definable, market segments with a multi-tiered, multi-channel approach. We will leverage our technologies with a licensing agreement in one key area and a direct sales and distribution strategy in the other using established distributors.
We will look to foreign markets first with established distributors for initial revenue. Domestic revenue will follow. Large groups and plans will be targeted first.
Marketing Strategy
Marketing will follow from industry and trade and physician awareness campaigns to specific executions directed at specific customer segments. The top tier of 20 to 30 customers in each segment will be attacked first. Only a few sales hits in these top tiers will enable achievement of targeted forecasts. Medquip will achieve its initial sales goals from direct and distributed sales of the Visi-Band. This product is targeted first since it is an existing, well-defined market and 510k approval is anticipated. Worldwide sales through distributors will provide needed cash flow.
Promotion Strategy
Public relations, industry media, will help in over-all industry awareness plans. Feature articles and product reviews will help launch awareness. Direct mail to buying groups and ads in trade publications will help with buyer impressions. Finally, all will be integrated with physician materials and training video tapes once approval has been obtained to increase point-of-surgery usage.
Medquip has already worked closely with physicians to design its products. The importance of working with physicians is well known. As an outgrowth of our Physician Advisory Board, Medquip will actively recruit allied physicians with sponsored events and seminars. Every major market area will be targeted. An annual event will also be sponsored.
Pricing Strategy
Pricing for the Visi-Band is $250 per unit. Terms are 2% 10 days, net 30. All collections in this plan for cash flow purposes are based on an average 45 day collection span. Our 80% gross margin allows for factoring receivables if that should become viable or necessary.
A 30% discount will be offered to distributors. Quantity discounts are not included but remain possible in negotiations with major buying groups.
The Visi-Gator is priced in two components:
- The motorized unit at $1,000.
- The cutting/irrigation/suction component at $200 (disposable).
Note: These are suggested retail prices. They represent ten times our estimated cost of manufacture. However, the strategy is to license these products for a 10% royalty. Pricing would be negotiated with the licensee.
Sales Strategy
Medquip’s sales strategy is to open foreign markets on a limited basis at the end of ’98. To fully exploit them in ’99 along with initial penetration of the US market with the Visi-Band (assuming 510k approval). Then to grow both markets in 2000 up to a 10% penetration.
Additionally, the strategy is to license the Visi-Gator to a major company such as J&J due to its increased regulatory requirements and initial marketing costs due to the fact that it is a new segment entry. The objective for the license is a $500,000 fee, $500,000 in advance royalties and a 10% royalty level per unit. Royalty projections are included in ’99 and ’00 sales forecasts.
Sales Forecast
This sales forecast includes small unit sales into the international market. The product is the Visi-Band. Unit sales are at $250 US. Product cost of direct sales is 20% while product cost through distributors is 50%.
It is important to note that $500k license fee and $500k advance royalties are the target figures for a license on the Visi-Gator. If these figures are not attainable or turn out to be a lesser number (but still acceptable to the Medquip board) then they should not be considered for cash flow purposes.
Investment money sought for Medquip will still be based on a sales forecast that does not include a successful license sale.
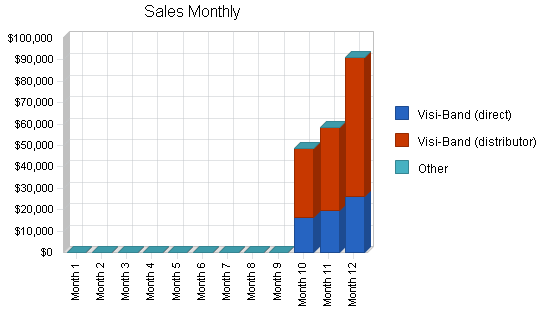
Sales Programs
Sales programs include direct wholesale sales to international distributors. Sales materials, video training tapes, and support materials will be produced. Physician materials will be included.
Direct sales will be by personal contact, direct mail, public relations, and media directed at key industry segments.
In addition electronic marketing will be deployed whenever it fits with the buying patterns of a key group.
A website and electronic commerce site will be utilized to cultivate direct sales to key industry groups.
The following are the key milestones for the first year of operations.
- All patents will be applied for by the May 1st date. The total legal fees are expected to be less than the $50k allocated.
- Start-up capital was successfully raised.
- The business plan has been completed.
- The government of Puerto Rico has been presented on April 8, 1998.
- All other first year milestones are currently on target time wise and budget wise.
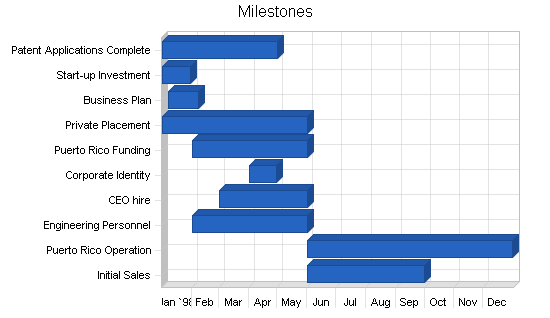
Management Summary management summary will include information about who's on your team and why they're the right people for the job, as well as your future hiring plans.">
The founders of Medquip, Inc. are Eric Smith and Timothy R. Jones. Eric will serve the company as Vice-President of Research & Development. Tim will serve as Vice-President of Corporate Development. Their biographies follow in the Management Team section.
Several key people are actively being sought. These are summarized in Management Team Gaps.
Organizational Structure
Medquip, Inc. will have a CEO to be recruited (see Management Team Gaps) who will have Eric Smith reporting to him as well as Timothy R. Jones and a V.P. of Sales and Marketing (see Management Team Gaps).
Eric will handle responsibility for R&D, design, compliance, and initial manufacturing and sourcing.
Tim will handle strategic growth plans, capitalization, and serve as CFO initially.
Reporting to Eric will be additional design engineers and compliance documentation personnel. Some of these tasks can also be handled by outside consultants in the early going. The ramp-up of essential personnel and tasks are included in the Personnel Plan that follows.
Management Team
Eric Smith (40)
BS, Mechanical Engineering, M.I.T., 1980
Eric Smith began his training in mechanical engineering at MIT and has a broad background in leading edge medical, semiconductor, biotechnology, and cleanroom product design and management. Creatively integrating technologies from diverse fields and transforming these elements into world class product solutions is his specialty. He has spent the past two years developing two medical devices, a full visibility ligating band dispenser and a revolutionary tissue dissolving lysis technology. Recent accomplishments include innovations for the Boston Scientific Speedband multiple ligating band dispenser. He was also responsible for the mechanism design of the Boston Scientific Corp. Alliance esophageal balloon inflation device through their supplier, ACT Medical Corp. Both devices have rapidly attained significantly greater than a 50% world market share for Boston Scientific Corp. Smith pioneered the application of high-energy particle beams to titanium hip and knee implants to increase their life by a factor of ten and for this work received an Industrial Research and Development IR100 award for one of the 100 best technology products. He initiated the development of a peritoneal dialysis system for Millipore Corporation, which incorporated ultra pure water and sterile connection technologies. Earlier research and development on the first Nitinol blood clot filter at Harvard Medical School was in cooperation with Dr. Morris Simon. Mr. Smith also was a founder of ABS, Inc., in 1995, a bio remediation products company currently manufacturing and cross licensing technology sold worldwide.
Ten United States Patents have been issued to Smith to date. Several more have also been granted internationally. A total of sixty-four subsequent United States patents granted to corporations including IBM, Medtronics, Northrop Grumman, Applied Materials, Eaton, Hitachi, and others cite these ten patents granted to Smith, a strong indication of the strategic significance of his diverse body of work. A number of biotechnology patent applications are currently being prosecuted in the United States and in several other countries. Patents issued to Smith include a medical X-ray system, clean room laminar airflow systems, chemical and thermal transfer processes, and robotic mechanisms for semiconductor and medical particle accelerators used worldwide by leading corporations such as Intel, IBM, Hitachi, Fujitsu, Motorola. A detailed list is below.
Mr. Smith has led new product developments for the past 17 years as Director of Engineering and Technology and various Research and Development positions. He led the systems design team for a $3 million production particle accelerator with a $50 million product development budget as Mechanical Systems Manager for Varian Associates, a large multinational technology corporation. He has managed an international staff of engineers and successfully transferred leading edge technology from Far East joint venture partners. His responsibilities have also included manufacturing management for a supplier of critical production equipment to IBM, Intel, and Eastman Kodak CD. Systems plagued by months of installation, rework, and errors performed flawlessly the first time and every time at Intel wafer fabrication cleanrooms after Smith re-designed the product and assumed full manufacturing responsibility.
An invited speaker at a worldwide technical conference in Kyoto, Japan, publications include a recent cover story for “Cleanrooms” magazine, and numerous technical articles related to high purity manufacturing, robotics, heat transfer, and mechanism designs.
United States Patent Numbers and Titles Currently Issued to Eric Smith as inventor:
5040484 Apparatus for retaining wafers [Semiconductor]. 4997606 Methods and apparatus for fabricating a high purity thermally-conductive polymer. 4927438 Horizontal laminar air flow work station [Semiconductor and medical applications]. 4899059 Disk scanning apparatus for batch ion implanters. 4836733 Wafer transfer system [Robot]. 4832781 Methods and apparatus for thermal transfer with a semiconductor wafer in a vacuum. 4817556 Apparatus for retaining wafers. 4580058 Scanning treatment apparatus [Application: human hip and knee implants]. 4531821 Item transporting [medical X-ray].
Timothy R. Jones (50)
BBA, Marketing, U. of Notre Dame, 1970 MBA, Finance, Executive Program, Loyola University, 1972
Mr. Jones has more than 27 years of business management experience. He is the founder of Plinth Capital in Ourtown, USA. He has successfully assisted early stage companies in capital formation, strategic planning, and business growth. His background includes management positions with two Fortune 500 companies, Lever Brothers Company, and the LCR Division of Squibb, Inc. Mr. Jones has previously been an executive in three start-up ventures and one turn-around.
His experience includes working with two investment banking firms, one that focused on privately held companies and the other on early stage publicly traded companies in development stage. Additionally, the management team of Medquip, Inc. includes a physician advisory board which is already being assembled. The Board of Directors is currently open.
Management Team Gaps
An experienced CEO is actively being sought. Timothy Jones is presently conducting the search. The desired profile is for a CEO experienced in the medical device arena, ideally who was part of a previous start-up venture that grew to exceed a minimum of $20 million in sales and had a successful exit strategy.
The CEO will help to identify and bring in a VP of Sales and Marketing.
Eric Smith is actively searching for design engineers and consultants. Several have been identified and are available.
Personnel Plan
The Personnel Plan chronicles the growth of the organization to 31 employees in the first three years. The third year could require a few additional people besides those indicated especially if sales reach or exceed $20 million. Production assembly people are grouped together at approx. $15k per person. Payroll costs and benefits are pegged at 22% although they could be lower in Puerto Rico.
Financial Plan investor-ready personnel plan .">
The value of the patents and the size of their potential markets enables several back-up plans of action if this plan doesn’t work as indicated. Venture funds are available early on and historically investments of $3 to $5 million are common for similar companies.
Even after successfully completing the start and seed stage as indicated, second round venture or mezzanine funding is potentially available in the $5 million range. We have planned for additional capital input in years two and three as a safety net for cash flow/cash balance.
However, cash flow achievement within the parameters of the indicated plan plus further funding on the senior debt side will lead to the best value for shareholders. Then, strategy can dictate the best valuation for ramp-up and roll-out.
Important Assumptions
The following are the key financial assumptions for this plan. However, it’s important to note that several of the assumptions could be considerably less than those indicated if the business is located in Puerto Rico. The personnel burden could go from 22% to 12%. The short term interest rate could go from 10% to 5% or less. The tax rate could go from 25% to less than 10%. So, all of the bottom line projections in this plan could improve appreciably.
However, the plan is still based on the following assumptions as if it were a U.S. based Georgia operation.
Key Financial Indicators
All of our benchmarks being attained will allow expansion strategies of merger, acquisition, roll-up or IPO. The following chart illustrates our planned performance in the most critical profit variables.

Break-even Analysis
Medquip, Inc. has calculated a break-even maintenance point for sales once full management staffing and facility costs are reached. Included are payroll and rent considerations.
The break-even target can sustain Medquip, Inc. in operation in late ’98 and throughout ’99 even if expansion and capitalization plans are late in materializing. It is anticipated that direct sales can produce these numbers and more in Europe, Middle Eastern, and African markets since those markets are not as dominated by managed care, there is more direct purchasing. A distributor has been identified for those markets as well as a distributor for Latin and South America, and a distributor for Japan. No Japanese sales are included, however, since regulatory barriers are more pronounced there.
The break-even analysis is restricted to this late ’98 and early ’99 time frame since the early ramp-up phase in business development is characteristic of most cash-flow shortages that represent exposure to early stage investors.

Projected Profit and Loss
The profit in each of the first two years of operation is expected to be minimal. However that includes $1 million in revenue in the first year from the sale of a license. If that does not occur, then over $800k will be burned in year one. That must come from investment infusion. The third year profit reflects the performance of a mature company. Over-all gross margins are excellent.

Projected Cash Flow
We began the year with $128,000 in cash from initial sales of shares to investors. This provided our start-up capital. The private placement to 13 or fewer investors is expected to bring in another $450,000 in May and $400,000 in June. We are targeting an additional equity investment from Puerto Rico and long term loans.
Thus, our cash flow will be sufficient in year one even if we can’t conclude a licensing agreement.
Second round financing will include venture, mezzanine, or IPO options. If sales and profits hit targets then further investment needs will be limited to higher value options to roll-up a national level and world-wide company.

Projected Balance Sheet
The highlights of the balance sheets are a solid cash position and net worth at the end of year three.
Business Ratios
By year three the return on equity should be very attractive to early investors. All ratios are in good shape for traditional borrowing to fund further growth. Industry profile ratios based on the Standard Industrial Classification (SIC) code 3841, Surgical and Medical Instruments, are shown for comparison.
The quickest way to turn a business idea into a business plan
Fill-in-the-blanks and automatic financials make it easy.
No thanks, I prefer writing 40-page documents.
Discover the world’s #1 plan building software

IMAGES
VIDEO
COMMENTS
Industry Analysis Section of Our Medical Device Business Plan. The medical device industry is one of the world's most innovative and dynamic sectors. Fortune Business Insights reported that the global medical device market was valued at $512.29 billion in 2022 and can grow from $536.12 billion in 2023 to $799.67 billion by 2030, at a CAGR of ...
Medical Device Business Plan. Over the past 20+ years, we have helped over 500 entrepreneurs and business owners create business plans to start and grow their medical device companies. If you're unfamiliar with creating a medical device business plan, you may think creating one will be a time-consuming and frustrating process.
Business planning that's simpler and faster than you think Creating a business plan using Upmetrics to start and grow a business is literally the easiest thing in the World. Simply read the instructions and fill in the blanks. It's as simple as that. Upmetrics has everything you need to create a comprehensive business plan.
Learn how to write a medical equipment manufacturing business plan with a sample and a template. Find out the market, products, competition, and financial projections for your business.
Download a customizable template to create your own medical device business plan. Learn how to write an executive summary, product offering, financial highlights, and more for your medical device company.
Download a free medical equipment business plan template that includes pre-written examples for every section to help you write your own plan. Business Planning. ... Download as PDF Finish your business plan with confidence. Step-by-step guidance and world-class support from the #1 business planning software. Get 50% off LivePlan Now ...
The U.S. medical device industry is projected to grow a healthy 3.3% over the next five years, with an increasing demand as time continues. The industry is in a growth cycle, and exhibits high profitability.
Explore a real-world medical equipment business plan example and download a free template with this information to start writing your own business plan. ... MedNexis, Inc. (the company) is a medical device development company that has designed and patented medical devices which it plans to produce and market. A magnetic muscle stimulator/field ...
MedNexis, Inc. is a start-up medical device company that has designed and patented devices to aid in atrophy treatment/prevention. Medical Equipment Developer Business Plan Medquip, Inc. is a start-up business that will develop and market endoscopic medical devices through multiple distribution channels, both foreign and domestic.
Florence Medical developed medical devices for the diagnosis and treatment of coronary and renal artery disease through novel interventional applications of computational fluid dynamics and related advanced principles. Cayenne prepared the business plan and investor pitch deck for this medical device company.. Florence Medical Business Plan
This business plan has been developed to present our company to prospective supplier partners, employers, and investors. Zenergy Medical Industries is a start-up company focused initially on distribution of leading brands of therapeutic systems for use by residents of Homecare and Assisted Living facilities at risk of complications from X disease.
Below you will find an extensive array of business plan examples for a variety of medical and healthcare businesses, including private clinics, medical device startups, telemedicine services, and healthcare consulting firms.
Find various templates and examples of medical business plans in PDF format for different types of healthcare facilities and services. Learn how to write a comprehensive and effective strategy for your medical startup or expansion.
MedNexis, Inc. is a start-up medical device company that has designed and patented devices to aid in atrophy treatment/prevention. Medical Equipment Developer Business Plan Medquip, Inc. is a start-up business that will develop and market endoscopic medical devices through multiple distribution channels, both foreign and domestic.
Business Plan for an Innovative Feminine Medical Device III ABSTRACT The gynecological area related to vaginal infections is not very explored from the practical point of view of the patient. The range of options of self-testing kits is limited and little publicized, so women tend to go to the doctor whenever they feel any discomfort, or they
PDF | A presentation around business model building for small businesses in the area of medical devices with a small focus on the India market. | Find, read and cite all the research you need on ...
This business plan is part of our regular business planning process. We revise this plan every quarter and it is placed under change control. In the next two years we intend to develop two new products and to improve revenues. Our keys to success and critical factors for the next year are, in order of importance: Product approval: CE mark.
Explore a real-world medical equipment developer business plan example and download a free template with this information to start writing your own business plan. Don't bother with copy and paste. ... Medquip, Inc. is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical ...