Congressionally Directed Medical Research Programs -- Joint Warfighter Medical Research Program -- Military Medical Research and Development Award
The U.S. Army Medical Research Acquisition Activity (USAMRAA) is soliciting applications/proposals to the fiscal year 2024 (FY24) Joint Warfighter Medical Research Program (JWMRP) using delegated authority provided by 10 USC 4001. The Congressionally Directed Medical Research Programs (CDMRP) at the U.S. Army Medical Research and Development Command (USAMRDC) is the program management agent for this funding opportunity. Congress initiated the JWMRP in 2012 to augment and accelerate high-priority Department of Defense (DOD) and Service medical requirements and to support the logical continuation of DOD-funded research and development initiatives that are close to achieving their objectives and yielding a benefit to military medicine. The ultimate goal of the program is to expedite the delivery of highly impactful and effective medical solutions to Service Members and Military Health System (MHS) beneficiaries; thus the Service advanced product development communities are critical partners in executing the JWMRP. Appropriations for the JWMRP from FY12 through FY23 totaled $595 million (M). The FY24 appropriation is $20M.
The MMRDA mechanism is intended to fund the logical continuation of previously DOD-funded research or development efforts relevant to the FY24 JWMRP Focus Areas with the highest potential to augment and accelerate medical product development and health care solutions for active-duty Service Members, their Families, Veterans, and/or the American public. Collaboration with DOD organizations is encouraged when this alliance would contribute to the success of the research effort, and any funds designated for DOD laboratories or activities should be identified in the application/proposal through submission of a “Suggested Intragovernmental/Intramural Budget Form,” Attachment 15. Applications/proposals from small businesses and/or partnerships with industry are also encouraged.
The MMRDA mechanism supports a wide range of research projects, spanning late-stage preclinical studies, late-state technology development efforts, technology demonstration, and translational research.
• Required Pre-Application Deadline: June 3, 2024 • Invitation to Submit an Application: July 2024 • Application Submission Deadline: August 29, 2024
The JWMRP Programmatic Panel identified the following Focus Areas as the highest priorities for FY24 JWMRP funding to meet critical research and development gaps and Service medical requirements. To meet the intent of the funding opportunity, applications/proposals to the FY24 JWMRP must address at least one of the Focus Areas listed below. • Broad spectrum and/or pathogen agnostic approaches to prevent and/or treat endemic or emerging infectious diseases of high operational impact. • Preventative capabilities to promote the Warfighter’s physiological and cognitive (1) performance and readiness and (2) injury prevention. • Solutions for semi-autonomous or autonomous medical care from point of injury across the continuum of care, including support of triage, prolonged patient care, and transport in contested environments. • Virtual/telehealth and decision support with artificial intelligence solutions to provide combat casualty care/prolonged care. • Hemorrhage control and resuscitation solutions, including blood and blood products, and anti-shock therapeutics, to enable delivery of life-saving care across the continuum of care.
Independent investigators at all academic levels (or equivalent) are eligible to be named by the organization as the PI in the application. There are no limitations on the number of applications for which an investigator may be named as a PI. An eligible PI, regardless of ethnicity, nationality, or citizenship status, must be employed by, or affiliated with, an eligible organization.
The anticipated total costs budgeted for the entire period of performance for an FY24 MMRDA]should not exceed $2,000,000, or $3,400,000 for the MMRDA–CRTO. Refer to Section II.D.5, Funding Restrictions, for detailed funding information.

U.S. Government Accountability Office

Biomedical Research: Observations on DOD's Management of Congressionally Directed Medical Research Programs
DOD awards funds to biomedical researchers for projects on topics identified by Congress. The projects contribute to the development of new drugs, vaccines, and medical devices.
DOD uses a cyclical, routine process to prioritize investments from these funds. In FYs 2015-19, it distributed nearly 100% of its $4.46 billion in funding.
DOD coordinates with the Department of Veterans Affairs and the National Institutes of Health, which also sponsor research. While we found a few DOD projects were similar in topic and methods to VA and NIH projects, their use of a shared database has helped identify and prevent overlap and duplication.

What GAO Found
The Department of Defense (DOD) awards funds from Congressionally Directed Medical Research Programs (CDMRP) appropriations to researchers for projects that focus on advancements in military medicine and public health benefits for areas such as cancers and substance abuse. A typical award ranges from less than $100,000 for a project with a new focus to millions of dollars for a clinical trial. Based on DOD data, for fiscal years 2015 through 2019, DOD obligated—committed government funds—nearly 100 percent of approximately $4.46 billion in available CDMRP appropriations. About 1.3 percent ($59.252 million) was unobligated for the period.
DOD uses a cyclical, routine process to prioritize and assess investments from CDMRP appropriations (see figure).
Department of Defense Cyclical, Routine Process for Prioritizing and Assessing CDMRP Investments
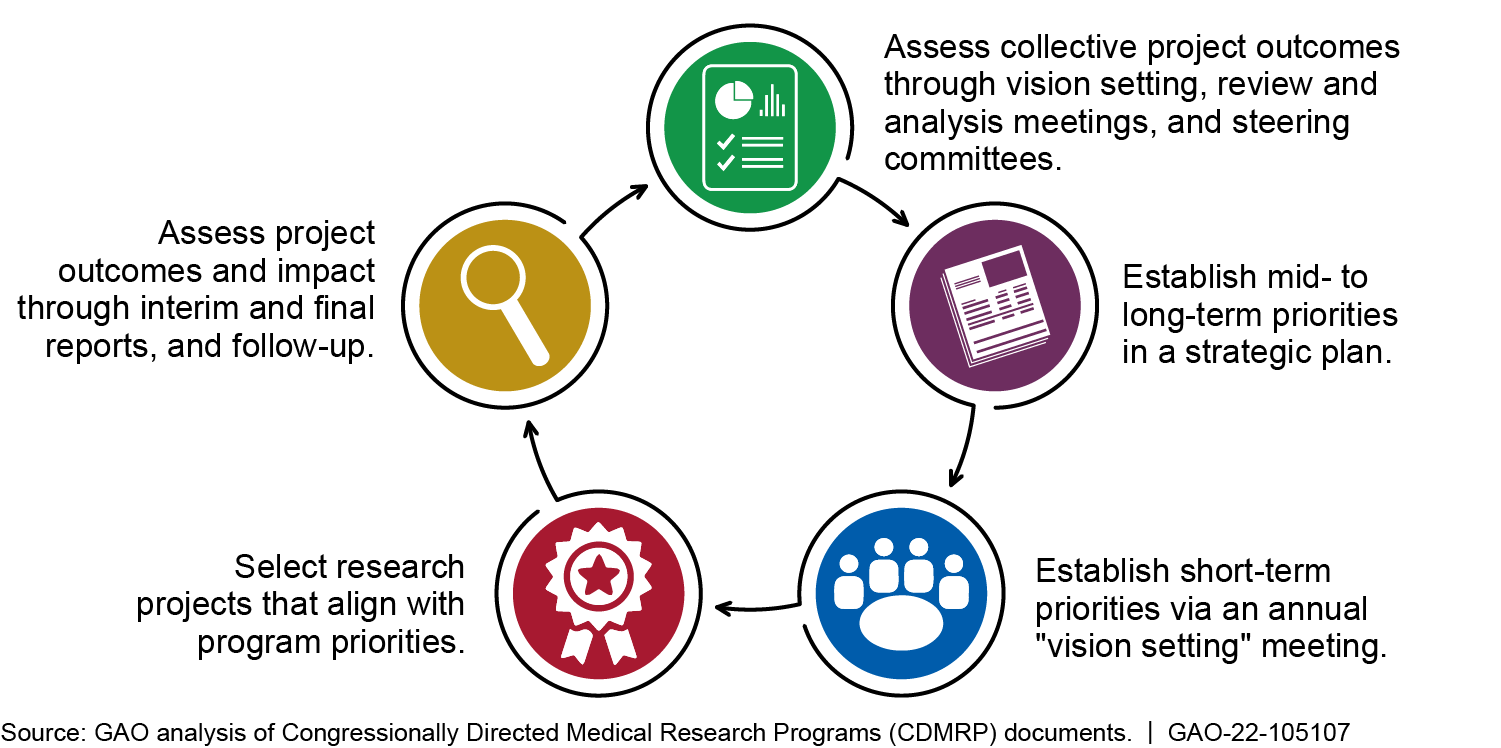
For example, each year following receipt of appropriations, DOD convenes a panel of experts for each program to help CDMRP staff agree on a strategy and priorities for the mid- and long-term and the upcoming year. These panels also review research applications and recommend ones to award funds based on their alignment with program priorities and expected impact, according to DOD officials. GAO's analysis of documents for 25 selected projects and the respective research programs found that each applicant clearly stated the relationship between the project and one or more program priorities.
The National Institutes of Health (NIH) and the Department of Veterans Affairs (VA) are significant federal sponsors of biomedical research. DOD coordinates with NIH and VA for CDMRP planning and project selection throughout the process shown in the above figure. For program planning during annual "vision setting," panels discuss research sponsored by other organizations and help ensure CDMRP investments are complementary. The panels generally include officials from NIH, VA, or both.
CDMRP also coordinates with NIH and VA by leveraging shared data to identify and mitigate project overlap. In response to a 2012 GAO report, DOD and NIH implemented an electronic interface between their research administration systems through the NIH Query View Report system, which includes VA data. CDMRP's full implementation of the system began in fiscal year 2019, when staff used it during application reviews to identify potential overlap with NIH and VA research. According to DOD officials, using shared data has helped them identify overlap and duplication in research projects, which led them to take mitigation steps and avoid cost for the federal government. In comparing 25 selected CDMRP projects with NIH- and VA- projects funded during the same period, GAO found that some projects were similar in topic and methods. GAO identified two projects (one from CDMRP and one from NIH) that contained verifiable overlap, which program managers had identified and addressed. In this instance, CDMRP and the researcher reduced the project's scope and budget. Through these and other coordination activities that are consistent with leading practices for collaboration, CDMRP is able to avoid and mitigate overlap and duplication of biomedical research efforts across DOD, NIH, and VA.
Why GAO Did This Study
DOD is among the United States' largest federal sponsors of biomedical research. For fiscal year 2021, DOD's appropriations included about $1.5 billion for 36 research programs known collectively as CDMRP. This represents a significant increase from CDMRP's initial appropriation in 1992 of $210 million for a breast cancer program.
The Joint Explanatory Statement accompanying the Consolidated Appropriations Act, 2021 includes a provision for GAO to review DOD’s CDMRP. This report provides information on CDMRP’s (1) execution of annual appropriations; (2) efforts to prioritize and assess biomedical research programs and investments; and (3) coordination of biomedical research with NIH and VA.
To address these objectives, GAO:
- analyzed DOD budget data for each CDMRP program for fiscal years 2015 through 2019 appropriation years;
- reviewed planning documents and research outcomes for a nongeneralizable sample of five programs for fiscal years 2018 through 2020 selected on the basis of size, subject, and research organizations awarded;
- analyzed records for 25 projects selected randomly from each selected program;
- reviewed reports and memorandums on research partnerships and collaboration;
- compared CDMRP research abstracts for selected projects with abstracts for NIH- and VA-funded projects to identify similarities and understand coordination steps; and
- interviewed officials from DOD, NIH, and VA.
For more information, contact Elizabeth Field at (202) 512-2775 or [email protected] .
Full Report
Gao contacts.
Elizabeth Field Director [email protected] (202) 512-4300
Office of Public Affairs
Sarah Kaczmarek Acting Managing Director [email protected] (202) 512-4800
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on the Health of Select Populations; Committee on the Evaluation of Research Management by DoD Congressionally Directed Medical Research Programs (CDMRP). Evaluation of the Congressionally Directed Medical Research Programs Review Process. Washington (DC): National Academies Press (US); 2016 Dec 19.

Evaluation of the Congressionally Directed Medical Research Programs Review Process.
- Hardcopy Version at National Academies Press
2 Overview of the Congressionally Directed Medical Research Programs
The Department of Defense's (DoD's) Congressionally Directed Medical Research Programs (CDMRP) has a well-established process for managing the review and selection of funding applications that it receives for its medical research programs. This chapter presents an overview of that process and provides a brief overview of CDMRP's organization and structure. The current functions of the program office, how it is funded, and its place within the health care hierarchy of DoD are described. The chapter also includes a brief summary of CDMPR's program processes from program initiation and goal setting through application solicitation and review to award negotiation. This chapter is intended to provide general information about CDMRP in order to set the stage for the subsequent chapters that detail the specific steps in the review process.
- THE CURRENT CDMRP
CDMRP views its role in the medical research community as a leader in advancing medical and scientific research and filling research gaps “by funding high impact, high risk and high gain projects that other agencies may not venture to fund” ( CDMRP, 2016a ). The vision and mission statements for CDMRP can be found in Box 2-1 . CDMRP currently manages 29 research programs (see Box 2-2 ), including several programs on behalf of other DoD offices, in particular, the Defense Health Agency (DHA). Each research program has its own specific vision and mission statement.
CDMRP Vision and Mission.
2016 CDMRP Research Programs.
CDMRP has emphasized that its research programs and funded applications need to be relevant to the health of service members, veterans, and their families. The committee is aware that the military health care system (TRICARE) is one of the largest health care systems in the world and cares not only for active duty and retired service members, but also for their families ( TRICARE, 2016 ). Thus, health-related issues that affect service members or their dependents would fall within the purview of DoD medical research. The scope of research performed by DoD has been a topic of debate in the U.S. Senate. In June 2016, the Senate voted that it would not put restrictions on the DoD money used for medical research so that it may continue to benefit military members and their families and also the general public ( Tritten, 2016 ).
CDMRP is located within the U.S. Army Medical Research and Materiel Command (USAMRMC), headquartered at Fort Detrick, Maryland (see Figure 2-1 ). The USAMRMC established the CDMRP program office in response to congressional funding for research initiatives; it decides how CDMRP is staffed and how it functions. Although CDMRP is based within the Department of the Army, the program office also has involvement from both the Department of the Navy and the Department of the Air Force. Over the years, CDMRP leadership has come from all three branches, with representatives from each service having served as CDMRP director.
CDMRP organizational chart. NOTE: DHA = Defense Health Agency; RDA = Research, Development, and Acquisition; USAMRAA = U.S. Army Medical Research Acquisition Activity. SOURCE: Adapted from Salzer, 2016d.
As noted in Box 2-2 , CDMRP provides management support for several research programs on behalf of the DHA's Research, Development, and Acquisition Directorate. DHA receives core funds 1 from Congress for these research programs, which have a strong military focus. Each of those programs is aligned with one or more joint program committees (JPCs), but the level of involvement in a program is at the discretion of the JPC. JPC membership consists of both DoD and non-DoD medical and military technical experts and representatives from the Department of Veterans Affairs and the Department of Health and Human Services. The largest DHA research program for which CDMRP provides management support is the Defense Medical Research and Development Program (DMRDP), which is overseen by the following six 2 JPCs:
- medical training and health information services (JPC-1),
- military infectious diseases (JPC-2),
- military operational medicine (JPC-5),
- combat casualty care (JPC-6),
- radiation health effects (JPC-7), and
- clinical and rehabilitative medicine (JPC-8).
JPCs support DMRDP and other CDMRP programs with a strong active-duty military focus by providing guidance, through programmatic review of applications and by making funding recommendations ( Resnik et al., 2013 ). CDMRP states that JPCs also engage in strategic planning activities that may feed into the CDMRP vision setting process ( Salzer, 2016a ). CDMRP staff and contractors work closely with the JPCs to provide program and award management support for JPC research initiatives ( CDMRP, 2016b ).
Although all CDMRP research programs are aligned with at least one JPC, programs that do not have a strong military health focus such as the Breast Cancer Research Program, the Autism Research Program, and the Parkinson's Research Program do not necessarily have substantial input from the affiliated JPC. The strongly JPC-aligned programs are an integral part of CDMRP, but they require different chain-of-command procedures and approvals from those that CDMRP generally uses to manage its other research programs ( Salzer, 2016a ; Santullo, 2016 ).
- CDMRP FUNDING
Funding for CDMRP research programs is appropriated on an annual basis. Congress, in response to advocacy groups and other interested parties, selects which programs will be funded and at what level each year. Language in the appropriations bills can range from very specific instructions on how to spend the money for a program to quite vague statements or just the line item; this can result in considerable variation in program funding and focus. Figure 2-2 provides an example of line items for CDMRP research programs as well as an example of accompanying congressional language from the Department of Defense Appropriations Act, 2016.
Examples of language in the Department of Defense Appropriations Act, 2016, funding CDMRP programs: (A) excerpt of sample line items in the budget; (B) text to note specific instructions for the Peer Reviewed Cancer Research Programs. SOURCE: Senate Report (more...)
Although appropriations for individual research programs (and thus CDMRP) in general can (and occasionally do) vary from year-to-year, in most cases funding for the individual programs has stayed relatively consistent since their inception; however, a few programs have been discontinued because of a lack of congressional funding (e.g., defense women's health, osteoporosis, genetic studies of food allergies). Table 2-1 shows the funding history for each program and the number of programs funded by year, and Figure 2-3 summarizes the growth of CDMRP since 1992. Funding for CDMRP as a whole increased by $433.3 million in 2014 from $557.7 million to $991.0 million, and increased again in 2015 by $33.4 million to a total of $1.024 billion. The initial program, the Breast Cancer Research Program, has been funded since 1992 for a total of more than $3 billion, and the Prostate Cancer Research Program (begun in 1997) and the Peer Reviewed Medical Research Program (begun in 1999) have both received more than $1 billion. The Psychological Health/Traumatic Brain Injury Research Program, a program that the CDRMP administers on behalf of three JPCs, is the fourth largest program at just over $800 million, and has been funded for the past 8 years.
CDMRP Funding History (in Millions) for Each Program by Year.
The growth of CDMRP programs and funding since 1992 as shown in Table 2-1.
- OVERVIEW OF THE CDMRP REVIEW PROCESS
All CDMRP research programs follow the same general multi-step process for soliciting, reviewing, and making funding decisions for applications (see Figure 2-4 ). These steps are conducted by two panels: the programmatic panel and the peer review panel. The application review cycle (the left side of Figure 2-4 in blue) spans approximately 12 months, beginning with the annual congressional appropriation of funds and ending with a list of funding recommendations being submitted to USAMRMC for approval. Award negotiation and implementation may take an additional year.
The CDMRP review process. Note that the programmatic panel conducts several steps in the review process: vision setting (Step 1; see Chapter 4); the development of the investment strategy, which results in the release of program announcements (Step 2; (more...)
After CDMRP receives its appropriations, it has 2 years by law to obligate the money; thus, each CDMRP award is fully funded up front. However, even though each award is fully funded, principal investigators do not necessarily receive all their funding at once; rather, milestones are established and must be met for the release of further funds. Program announcements (see Chapter 4 ) specify the maximum length of the award over which money may be allocated; the length of the award may not exceed 5 years ( IOM, 2004 ).
The second part of the process is the award management cycle (the right side of Figure 2-4 in red), in which recommended awards are selected and approved for funding, negotiations are undertaken to finalize the awards, and the progress of the awards is then monitored from initiation through to closeout. In this report, the committee evaluates the CDMRP review process only up to the point of the funding recommendations (Step 5).
The five major steps in the application review process considered by the committee (in yellow in Figure 2-4 ) are as follows ( CDMRP, 2015a ):
Vision Setting ( Chapter 4 ): When the congressional appropriation process is complete, CDMRP receives funds for each research program.
For existing programs, the first step in the process is the vision setting meeting held by the programmatic panel. Vision setting occurs in months 1–4 of the program cycle to identify research gaps and to define an investment strategy for that year (that is, to choose award mechanisms and topics) to address those gaps. The annual investment strategy identifies the award mechanisms to be used for each research program; the award mechanisms result in program announcements that drive the application process.
If the appropriation is for a new research program, a one-time stakeholders meeting is held before the first vision setting meeting to help scope out the Congressional intent for the program as well as research needs.
Program Announcement Release ( Chapter 4 ): Following the vision setting meeting, a program announcement for each award mechanism is developed by the CDMRP program manager and released by the CDMRP contracting office within 2–5 months of the meeting. In some cases, a pre-announcement will be released to alert interested parties to an upcoming program announcement. Interested researchers can sign up to be electronically notified when new program announcements are released or can find the program announcements online. Program announcements notify the research community that new funding opportunities are available for that program and delineate the information that must be submitted to CDMRP for each application.
The electronic biomedical research application portal, eBRAP, is used for a variety of program activities, including application submission, notification of availability of program announcements, and communications with applicants. Although government owned, it is maintained by the peer review contractor.
Pre-Application Screening, Review, and Invitation to Submit Full Application ( Chapter 4 ): Applications must be submitted individually in response to a specific program announcement; no other applications are accepted. Most programs use a pre-application step to reduce the number of full applications to be reviewed. Pre-application submission requirements and screening criteria are specified in the program announcement. Some program announcements for specific award mechanisms, such as the Clinical Consortium Research Site Award, use letters of intent as the pre-application and do not have screening criteria. Pre-applications are typically submitted and screened by the programmatic panel during months 5–8. Applications that meet the pre-application screening criteria, as determined by the programmatic panel, are then invited to submit a full application.
Peer Review ( Chapter 5 ): Between months 7 and 11, full applications are received and undergo review for scientific and technical merit by the peer review panel; this represents the first tier of CDMRP's two-tier review process. The results of the peer review (both numeric scores and narrative summaries) are submitted to the programmatic panel.
Programmatic Review ( Chapter 6 ): Following peer review, applications are reviewed by the same programmatic panel as in steps 1 and 3 to determine programmatic relevance and portfolio balance, in addition to considering the scores and summaries from peer review. Programmatic review (months 9–14) serves as the second tier of the two-tier review process and results in a list of applications that are recommended for funding, along with a list of alternate applications that may be considered for funding if sufficient funds become available.
The committee notes that CDMRP established an Inquiry Review Panel to address questions and appeals by applicants regarding either peer or programmatic review of their applications. The committee was informed that less than 0.5% of applications have re-review requests (Colonel Wanda Salzer, CDMRP director, personal communication, October 16, 2015).
Once the list of funding recommendations has been completed, it must be approved or concurred with by the commanding general, USAMRMC, and/or the DHA's Research, Development and Acquisition Directorate.
Awards that have been approved for funding move into the award-management phase (the right side of Figure 2-4 in red). Award management begins with award negotiations, which can take more than 1 year to complete (months 11–24). Finally, once an award is in place, it is actively managed and monitored by CDMRP throughout its duration. Award management includes, but is not limited to, progress reports from the research institution and financial reporting. When an award's end date approaches, preparations are made to close the award, and the closure is finalized approximately 6 months after the period of performance has expired.
Core funds are part of the annual DoD budget set by Congress and the president (known as the President's Budget). The CDMRP appropriations are not considered to be DoD core funds.
There is no JPC-3 or JPC-4 committee.
- Cite this Page National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on the Health of Select Populations; Committee on the Evaluation of Research Management by DoD Congressionally Directed Medical Research Programs (CDMRP). Evaluation of the Congressionally Directed Medical Research Programs Review Process. Washington (DC): National Academies Press (US); 2016 Dec 19. 2, Overview of the Congressionally Directed Medical Research Programs.
- PDF version of this title (3.3M)
In this Page
Recent activity.
- Overview of the Congressionally Directed Medical Research Programs - Evaluation ... Overview of the Congressionally Directed Medical Research Programs - Evaluation of the Congressionally Directed Medical Research Programs Review Process
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Customize Your Path
Filters Applied
Customize Your Experience.
Utilize the "Customize Your Path" feature to refine the information displayed in myRESEARCHpath based on your role, project inclusions, sponsor or funding, and management center.
DoD funding programs
Need assistance with DoD CDMRP proposals?

Get help with DoD CDMRP proposals.
Contact the Office of Campus Research Development (Provost Area or University Campus):
- Email: [email protected]
Contact the Research Navigators (School of Medicine or School of Nursing):
- Email: [email protected]
Utilize this page to access guidance and toolkits for various DoD funding programs. For consultations with a Research Development Professional about planning a DoD proposal contact:
- Sohini Sengupta (Provost Area or Campus Schools)
- Christine Erlien (School of Medicine)
- Lauren Anderson (Division of Surgical Sciences)
- Shila Nordone (School of Nursing)
Congressionally Directed Medical Research Program (CDMRP) grants
When applying for a CDMRP grant, follow the funding opportunity announcement’s guidelines in combination with the General Application Instructions (GAI) guide for that research program. General formatting guidelines are highlighted in the checklists provided in each of the program folders linked below as well as Appendix 4 of any of GAI PDFs.
Access the Review and submit the proposal page for guidance on submission of the proposal, the grant manager should be contacted with questions (e.g., what documents transfer from SPS versus what is uploaded into grants.duke).
Note: the preproposal stage is submitted through eBRAP (CDMRP’s system), while the full proposal is submitted through grants.duke
- Research Classification Codes
- Letter of intent
- Solicitations with preproposals also typically require biosketches for key personnel (NIH or DoD format), uploaded as a single combined file.
- Project narrative (if applicable) – see Program folders below for preproposal project narrative templates tailored to solicitation requirements
- Solicitations with required preproposals typically request the following documents, uploaded as individual files: References Cited and List of Abbreviations, Acronyms, and Symbols .
Templates for documents common across solicitations are linked directly below. See specific Program folders in the following accordion for additional documents as applicable (e.g., Technical Abstract, Lay Abstract, Impact Statement, Innovation Statement, Military Relevance, Partnership Statement)
- Project narrative - see Program folders below for project narrative tailored to solicitation requirements
- References Cited
- List of Abbreviations, Acronyms, and Symbols
- Facilities and Resources Repository (access boilerplate language and contacts for facilities and resources to potentially support your project)
- Publications and/or Patents
- Letter(s) of Organizational Support
- Data and Research Resources Sharing Plan ; policy on data resource sharing
- Letters of Collaboration
- Use of DoD Resources (if applicable)
- Use of VA Resources (if applicable)
- Intellectual and Material Property Plan (if applicable; see template for contact info for Office of Translation and Commercialization)
- Commercialization Strategy (if applicable; see template for contact info for Office of Translation and Commercialization)
- Lay and Technical Abstracts: see Program folders below
- Statement of Work (fill out the one requested by your solicitation): Generic , Clinical
- Suggested Intragovernmental/Intramural Budget Form (if applicable)
- Biosketches: NIH format (allowed), with example ; DOD format
- Previous/Current/Pending Support
- Budget Justification
- Project/Performance Site Location(s) Form
- Data Management Plan
Explore solicitations that were recently pursued by Duke faculty using the links below. Included are the solicitation and general application instructions, a checklist for preapplication and full application documents, the programmatic panel roster, and templates tailored to the submission.
CDMRP program toolkits:
- Alcohol and Substance Abuse Disorders Research Program
- ALS Research Program
- Autism Research Program
- Bone Marrow Failure Research Program
- Breast Cancer Research Program
- Chronic Pain Management Research Program
- Combat Casualty Care Research Program
- Combat Readiness Medical Research Program
- Duchenne Muscular Dystrophy Research Program
- Epilepsy Research Program
- Gulf War Illness Research Program
- Hearing Restoration Research Program
- Lung Cancer Research Program
- Lupus Research Program
- Kidney Cancer Research Program
- Melanoma Research Program
- Military Burn Research Program
- Multiple Sclerosis Research Program
- Neurofibromatosis Research Program
- Neurotoxin Exposure Treatment Parkinson’s
- Orthotics and Prosthetics Outcomes Research Program
- Ovarian Cancer Research Program
- Pancreatic Cancer Research Program
- Parkinson’s Research Program
- Peer Reviewed Alzheimer’s Research Program
- Peer Reviewed Cancer Research Program
- Peer Reviewed Medical Research Program
- Peer Reviewed Orthopaedic Research Program
- Prostate Cancer Research Program
- Rare Cancers Research Program Reconstructive Transplant Research Program
- Scleroderma Research Program
- Spinal Cord Injury Research Program
- Tick-Borne Disease Research Program
- Toxic Exposures Research Program
- Traumatic Brain Injury and Psychological Health Research Program
- Tuberous Sclerosis Complex Research Program
- Vision Research Program
Other programs
Air Force Office of Scientific Research FY24 Young Investigator Program (AFOSR YIP) (FOA-AFRLAFOSR20240004) supports early stage, tenure-track scientists and engineers who are in a full-time tenure-track appointment and show exceptional ability and promise for conducting basic research in areas of interest identified in the BAA “ Research Interests of the Air Force Office of Scientific Research ”.
Upcoming deadlines: White paper (required): 4/22/2024; Full application: 6/21/2024 (This toolkit is updated annually.)
Defense Advanced Research Projects Agency Young Faculty Award (DARPA YFA) (DARPARA2401) supports innovative research in one of the specific topic areas that are of interest to DARPA’s six technical offices. Eligibility: tenure-track and tenured faculty within 3 years of their Tenure date.
Upcoming deadlines: Executive summary (strongly encouraged): December 2024, full proposal: February 2025 (This toolkit is updated annually.)
Defense University Research Instrumentation Program (DURIP) (FOAAFRLAFOSR20240000) provides funds for the acquisition of major research equipment or instrumentation to augment current, or develop new, research capabilities in support of research areas important to the DoD. DURIP is sponsored by ONR, ARO, and AFOSR.
Upcoming deadline: February 2025 (This toolkit is updated annually.)
Multidisciplinary Research Program of The University Research Initiative (MURI) (ONR: N0001424SF002; ARO: W911NF24S0006; AFOSR: FOAAFRLAFOSR20240003) supports multidisciplinary, high-risk basic research in science and engineering that is of interest to DoD. MURI is sponsored by ARO/AMC, ONR, and AFOSR. White papers (optional but strongly recommended) are due 5/17/2024.
Upcoming full application deadline: 9/6/2024 (This toolkit is updated annually.)
Office of Naval Research Young Investigator Program (ONR YIP) (N0001424SF004) supports early stage, tenure-track scientists and engineers who are in their first or second full-time tenure-track appointment and show exceptional promise for doing research in 1 or more of the ONR Technology and Research areas.
Upcoming deadline: Passed for 2024 (This toolkit is updated annually.)
DoD Amyotrophic Lateral Sclerosis Research Program
Therapeutic development award.
Amount of funding: Direct costs budgeted for the entire period of performance will not exceed $1.5M. Purpose: The FY23 ALSRP Therapeutic Development Award supports research ranging from preclinical validation of therapeutic leads through U.S. Food and Drug Administration (FDA) Investigational New Drug (IND)-enabling studies. The proposed studies are expected to be empirical in nature and product driven. Applicants with limited ALS experience are strongly encouraged to include collaborators with substantial experience in the relevant ALS model systems, endpoints, and pathophysiology. Eligibility: Independent investigators - Faculty with PI eligibility and CE faculty (with an approved CE faculty PI waiver obtained through their RPM in RMG prior to the pre-application/letter of intent) may be the PI. Not eligible : Instructors, Clinical Instructors, Academic staff-research (i.e., research associates), and postdocs are not eligible for this RFP because Stanford does not consider them to be independent positions. Timeline: REQUIRED Pre-Application (Pre-Proposal) Deadline: April 13, 2023 via eBrap Please include your RPM’s name as business official in the pre-application. Institutional representative (RPM/RMG or CGO/OSR) Deadline: July 6, 2023 Application Submission Deadline: July 13, 2023 via grants.gov Guidelines: https://cdmrp.army.mil/funding/alsrp
Therapeutic Idea Award
Amount of funding: Direct costs budgeted for the entire period of performance will not exceed $600,000. Direct costs for a Therapeutic Idea Award – Biomarker Option should not exceed $750,000. Purpose: The FY23 ALSRP Therapeutic Idea Award supports the initial exploration of innovative, highrisk, high-gain ideas aimed at Amyotrophic Lateral Sclerosis (ALS) drug or treatment discovery. The studies supported by this award mechanism are expected to be hypothesis-driven and generate preliminary data for future avenues of therapeutic investigation. All research projects should include a well-formulated, testable hypothesis based on strong scientific rationale that holds translational potential to improve ALS treatment and/or advance a novel treatment modality. Applications may demonstrate the ability to achieve interpretable results in the absence of preliminary data supporting the hypothesis. While the inclusion of preliminary data is not prohibited, the strength of the application should not rely on preliminary data, but on the innovative approach. Eligibility: Independent investigators - Faculty with PI eligibility and CE faculty (with an approved CE faculty PI waiver obtained through their RPM in RMG prior to the pre-application/letter of intent) may be PI. Early career investigators (defined as independent, non-mentored investigators within 10 years of their last training position e.g., postdoctoral fellowship, medical residency, clinical fellowship as of the application submission deadline) are encouraged to apply. Not eligible : Instructors, Clinical Instructors, Academic staff-research (i.e., research associates), and postdocs are not eligible for this RFP because Stanford does not consider them to be independent positions. Timeline: REQUIRED Pre-Application (Pre-Proposal) Deadline: April 13, 2023 via eBrap Please include your RPM’s name as business official in the pre-application. Institutional representative (RPM/RMG or CGO/OSR) Deadline: July 6, 2023 Application Submission Deadline: July 13, 2023 via grants.gov Guidelines: https://cdmrp.army.mil/funding/alsrp
Pilot Clinical Trial Award
Amount of funding: Direct costs budgeted for the entire period of performance will not exceed $2M for the standard award or $1M for the clinical care tier award. Purpose: The FY23 ALSRP Pilot Clinical Trial Award supports the rapid implementation of clinical trials with the potential to have a significant impact on the treatment or management of Amyotrophic Lateral Sclerosis (ALS). Projects may range from phase 1 to small-scale phase 2 trials and should aim to de-risk and inform the design of more advanced trials by investigating safety, feasibility, biomarker application, and therapeutic efficacy in relevant patient populations. Clinical trials may be designed to evaluate promising drugs, biologics, or devices with anticipated therapeutic impact that is supported by strong scientific rationale and existing preliminary studies and/or preclinical data. Eligibility: Independent investigators - Faculty with PI eligibility and CE faculty (with an approved CE faculty PI waiver obtained through their RPM in RMG prior to the pre-application/letter of intent) may be PI. Not eligible : Instructors, Clinical Instructors, Academic staff-research (i.e., research associates), and postdocs are not eligible for this RFP because Stanford does not consider them to be independent positions. Timeline: REQUIRED Pre-Application (Pre-Proposal) Deadline: April 13, 2023 via eBrap Please include your RPM’s name as business official in the pre-application. Institutional representative (RPM/RMG or CGO/OSR) Deadline: July 6, 2023 Application Submission Deadline: July 13, 2023 via grants.gov Guidelines: https://cdmrp.army.mil/funding/alsrp
Clinical Biomarker Development Award
Amount of funding: Direct costs budgeted for the entire period of performance will not exceed $750,000. Purpose: The intent of the FY23 ALSRP Clinical Biomarker Development Award is to support the development of biomarkers to enrich clinical trials in Amyotrophic Lateral Sclerosis (ALS). Biomarker development projects can be relevant to a specific therapy, a class of therapeutics, or to a specific ALS subtype (such as a particular genetic mutation) and do not have to broadly apply to all patients. A description of the biomarker category and intended context of use (COU) in ALS therapeutic development, including regulatory considerations for use in ALS clinical trials or clinical practice, is an important component. Eligibility: Independent investigators - Faculty with PI eligibility and CE faculty (with an approved CE faculty PI waiver obtained through their RPM in RMG prior to the pre-application/letter of intent) may be PI. Early-career investigators and/or early-career physician scientists (defined as independent, nonmentored investigators within 10 years of their last training position e.g., postdoctoral fellowship, medical residency, clinical fellowship as of the application submission deadline) are encouraged to apply. Not eligible: Instructors, Clinical Instructors, Academic staff-research (i.e., research associates), and postdocs are not eligible for this RFP because Stanford does not consider them to be independent positions. Timeline: REQUIRED Pre-Application (Pre-Proposal) Deadline: April 13, 2023 via eBrap Please include your RPM’s name as business official in the pre-application. Institutional representative (RPM/RMG or CGO/OSR) Deadline: July 6, 2023 Application Submission Deadline: July 13, 2023 via grants.gov Guidelines : https://cdmrp.army.mil/funding/alsrp
Additional Information
Please include your institutional official's name (RPM/RMG or CGO/OSR) as business official in the pre-application/LOI.
eBRAP Funding Opportunities and Forms (including the General Application Instructions).
On this page:
Advertisement
Supported by
Fact-Checking Biden’s and Trump’s Claims on Domestic Policy
We scrutinized the presidential candidates’ recent claims on abortion, health care, crime and climate change ahead of the debate.
- Share full article

By Linda Qiu
Reporting from Washington
President Biden and former President Donald J. Trump will face off Thursday night for the first time in four years, giving each ample opportunity to fling accusations about the other’s positions. Some will hew close to the facts, but there will most likely be ample exaggeration or statements lacking adequate nuance.
The two candidates have not been shy in their critiques and attacks on each other on the campaign trail.
The presumptive nominees have sparred over immigration policy and the state of the economy . Mr. Trump has portrayed the country, hyperbolically, under Mr. Biden as lawless. Mr. Biden has sometimes omitted context while describing Mr. Trump’s views on abortion rights and the Affordable Care Act.
Here’s a fact check of some of their recent claims on domestic issues.
Mr. Trump misrepresented abortion laws and crime levels across the country.
What Was Said
“It’s hard to believe they have some states passing legislation where you can execute the baby after birth.” — Mr. Trump in an interview on Fox News in June
False. No state has passed a law allowing the execution of a baby after it is born, which is infanticide.
Since the Supreme Court overturned the constitutional right to abortion, about 20 states have enacted protections enshrining the right in state constitutions and shielding those seeking or providing abortions in the state from restrictions in other states. None of these new laws allow for “executing” the baby after birth.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .

COMMENTS
Additional Supported DOD Programs/Projects. Defense Medical Research and Development. Medical Simulation and Information Sciences Research Program (JPC-1) Military Infectious Diseases Research Program (JPC-2) Military Operational Medicine Research Program (JPC-5) Combat Casualty Care Research Program (JPC-6)
Currently Funded Research Programs. Currently Has Open Funding Opportunities. Alcohol and Substance Use Disorders Amyotrophic Lateral Sclerosis Arthritis Autism Bone Marrow Failure Breast Cancer Chronic Pain Management Combat Readiness-Medical Duchenne Muscular Dystrophy Epilepsy Glioblastoma Gulf War Illness Hearing Restoration Joint ...
Fiscal Year 2024 (FY24) Joint Warfighter Medical Research Program (JWMRP) is currently accepting only invited applications, or if a letter of Intent or preproposal has been submitted as required by the award mechanism. Full details for submission: JWMRP Funding Opportunities | Synopsis of JWMRP Award Mechanisms.
Amount of funding: Direct Costs budgeted for the entire period of performance will not exceed $7.2M. Purpose: The FY23 PRMRP Focused Program Award (FPA) is intended to optimize research and accelerate solutions to a critical question related to one of the congressionally directed FY23 PRMRP Topic Areas and one of the FY23 PRMRP Strategic Goals through a synergistic, multidisciplinary research ...
The Military Health System Research Program is encouraging researchers to submit grant applications for studies on areas that will directly benefit force readiness, service members, retirees, and their families. Military and civilian researchers are welcome to submit applications. The funding prioritizes research projects that focus on the ...
Combat Casualty Care Research Program (August Pre-app deadline) Chronic Pain Management Research Program (August Pre-app deadline) Toxic Exposures Research Program (September Pre-app deadlines) More programs to be posted soon. The most up to date information may be found on the DoD Funding Opportunities webpage.
The US Army Medical Research and Development Command (USAMRDC) Military Operational Medicine Research Program (MOMRP) manages research funding on behalf of the Department of the Army and the DoD Defense Health Program (DHP). This funding supports research at DoD laboratories and myriad extramural research organizations from small colleges to ...
solicited by the U.S. Army Medical Research Acquisition Activity (USAMRAA) using delegated authority provided by United States Code, Title 10, Section 4001 (10 USC 4001). The execution management agent for this program announcement is the Congressionally Directed Medical Research Programs (CDMRP) at the U.S. Army Medical Research and Development
Career Development Award - Fellow Option. Amount of funding: Direct costs budgeted for the entire period of performance will not exceed $400,000 or $200,000 for a CDA-RO Award Research Level 1. Purpose: The PRCRP is seeking to advance cancer research through development of early-career investigators. Depending on the career path of the ...
Medical Research Program (PRMRP). For FY2022, $370 million was appropriated for the PRMRP. PRMRP funding supports grants for medical research on a number of conditions or treatment. modalities that are of "clear scientific merit and direct relevance to military health." Congress specifies an annual list of eligible conditions or treatments.
Medical Research Program (PRMRP). For FY2024, $370 million was appropriated for the PRMRP. PRMRP funding supports grants for medical research on a number of conditions or treatment. modalities that are of "clear scientific merit and direct relevance to military health." Congress specifies an annual list of eligible conditions or treatments.
The MIDRP's role continues in importance as bacterial diarrheal diseases, viral diseases, combat wound infections and emerging infectious diseases continue to adversely impact readiness, military operations and the health of the Warfighter and their families. More than 500 M.D., Ph.D., and DVM level Army, Navy, Air Force, Government Service ...
The MMRDA mechanism is intended to fund the logical continuation of previously DOD-funded research or development efforts relevant to the FY24 JWMRP Focus Areas with the highest potential to augment and accelerate medical product development and health care solutions for active-duty Service Members, their Families, Veterans, and/or the American ...
DOD awards funds to biomedical researchers for projects on topics identified by Congress. The projects contribute to the development of new drugs, vaccines, and medical devices. DOD uses a cyclical, routine process to prioritize investments from these funds. In FYs 2015-19, it distributed nearly 100% of its $4.46 billion in funding.
The Department of Defense's (DoD's) Congressionally Directed Medical Research Programs (CDMRP) has a well-established process for managing the review and selection of funding applications that it receives for its medical research programs. This chapter presents an overview of that process and provides a brief overview of CDMRP's organization and structure. The current functions of the program ...
National Security Education Program The David L. Boren National Security Education Act of 1991 mandated that the Secretary of Defense create and sustain a program to award scholarships to U.S. undergraduate students, fellowships to U.S. graduate students, and grants to U.S. institutions of higher education. These awards are for study or program ...
The U.S. Army Medical Research and Development Command's (USAMRDC) Joint Warfighter Medical Research Program (JWMRP) Military Medical Research and Development Award (MMRDA) BAA can be found by searching Grants.gov (https://www.grants.gov) using the funding opportunity number HT9425-23-S-JWMRP or the Assistance Listing (formerly,
The pre-application submission deadline for the following PRMRP funding opportunities has passed. Submission of full applications to these funding opportunities will only be accepted if you received an invitation to submit an application or if a Letter of Intent was required and it was submitted by the pre-application due date deadline.
1. Search results include only those projects assigned to CDMRP for management. 2. All projects within a given program may not be available until the award has been finalized. 3. Analysis of data presented on this site may be limited based on searching capability, timing and availability of appropriate research codes. 4.
The Congressionally Directed Medical Research Program (CDMRP) aims to fill research gaps by funding high impact, high risk and high gain projects that other agencies may not venture to fund. While individual programs are unique in their focus, all of the programs managed by the CDMRP share the common goal of advancing paradigm shifting research ...
Extramural grants draw on sources outside of Walter Reed National Military Medical Center. We can help researchers apply for grants, but also encourage using our foundation partners to pursue opportunities. The Business Office offers grant writing to all research investigators at the medical center. Our staff can also help investigators locate ...
Amount of funding: Direct costs budgeted for the entire period of performance will not exceed $600,000.Direct costs for a Therapeutic Idea Award - Biomarker Option should not exceed $750,000. Purpose: The FY23 ALSRP Therapeutic Idea Award supports the initial exploration of innovative, highrisk, high-gain ideas aimed at Amyotrophic Lateral Sclerosis (ALS) drug or treatment discovery.
CHRISTUS Health provides equal employment opportunities to all employees and applicants. All hiring and selections for job opportunities will be based on CHRISTUS Health's business needs and the qualifications, skills, relative abilities and performance of the candidates being considered, without regard to an individual's race, color, religion, sex, sexual orientation, pregnancy, national ...
Vision - To advance mission readiness of U.S. military members affected by cancer. Since Fiscal Year 2009, the Peer Reviewed Cancer Research Program (PRCRP) has been charged by U.S. Congress to fund innovative basic, applied, and translational cancer research to support Service members, their families, and the American public.
CHICAGO - June 25, 2024 — The American Board of Medical Specialties (ABMS) Research and Education Foundation (REF) has announced the recipients of its inaugural research grants program. Awarded in two categories, the two-year grants represent $550,000 in funding and a significant commitment to research by the ABMS REF.
Defense Medical Research and Development Program (DMRDP) ... It is the responsibility of the applicant to select the funding option that is most appropriate for the proposed research project. Funding Level 1 is intended for preclinical research studies supported by substantial preliminary or published data. Clinical research and clinical trials ...
The Health Care Cost Institute, a research nonprofit, estimated that per person spending on insulin doubled from 2012 to 2016, to $5,705 a year, or about $475 a month. But that is an estimate for ...