Talk to our experts
1800-120-456-456

NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules

Biomolecules Class 12 NCERT Solutions FREE PDF Download
Biomolecules class 12 NCERT solutions offering comprehensive insights and guidance. Explore the intricate structures and functions of biomolecules essential for life, from carbohydrates and proteins to nucleic acids and lipids. Access FREE PDF downloads of Biomolecules Class 12 to unravel the mysteries of biochemical processes and enhance your understanding of this crucial aspect of biology. Master concepts, crack exams, and embark on a journey through the molecular foundations of life with these invaluable resources.

Download the FREE PDF of Class 12 NCERT Solutions for Biomolecules prepared by master teachers. These NCERT Solutions are created according to the updated NCERT Syllabus for Class 12 Chemistry .
Quick Insights of Chapter 10 Biomolecules: Class 12 NCERT Solutions
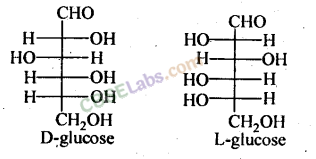
Biomolecules class 12 chemistry NCERT solutions will give you insights about the General Introduction: Carbohydrates - Classification (aldoses and ketoses), monosaccharides, D-L configuration oligosaccharides, polysaccharides, and the importance of carbohydrates.
Biomolecules class 12 chemistry NCERT solutions section will give you learnings on Proteins - Elementary idea of amino acids, peptide bonds, polypeptides, proteins, the structure of proteins - primary, secondary, tertiary structure and quaternary structures.
Detailed information on the denaturation of proteins and enzymes. Hormones - Elementary idea excluding structure, Vitamins - Classification and functions, Nucleic Acids: DNA and RNA.
NCERT solutions of Biomolecules class exercise solutions can help students analyse their level of preparation and understanding of concepts.
NCERT solutions biomolecules class 12 topics are included according to the revised syllabus for the academic year 2024-25.
Access NCERT Solutions for Class 12 Chemistry Chapter 10 - Biomolecules
Intext questions.
1. Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six-membered ring compounds) are insoluble in water. Explain.
Ans: The presence of H – bonding shows the dissolving property (solubility) of any compound. The glucose (5 -OH groups) and sucrose (8 -OH groups) can easily form H – bonding with water and thus, are soluble. Whereas, cyclohexane and benzene are not soluble in water due to the absence of -OH groups within them.
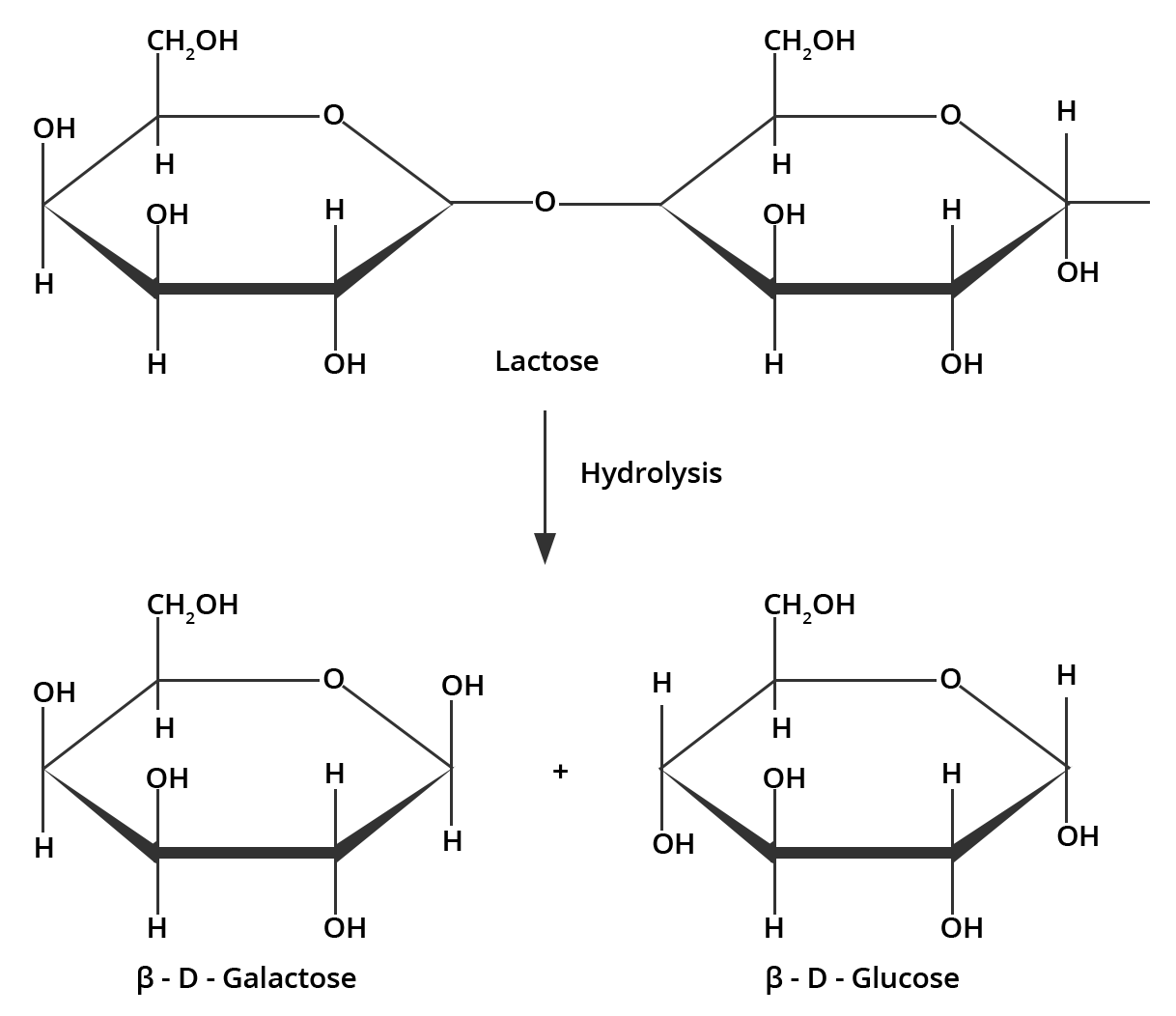
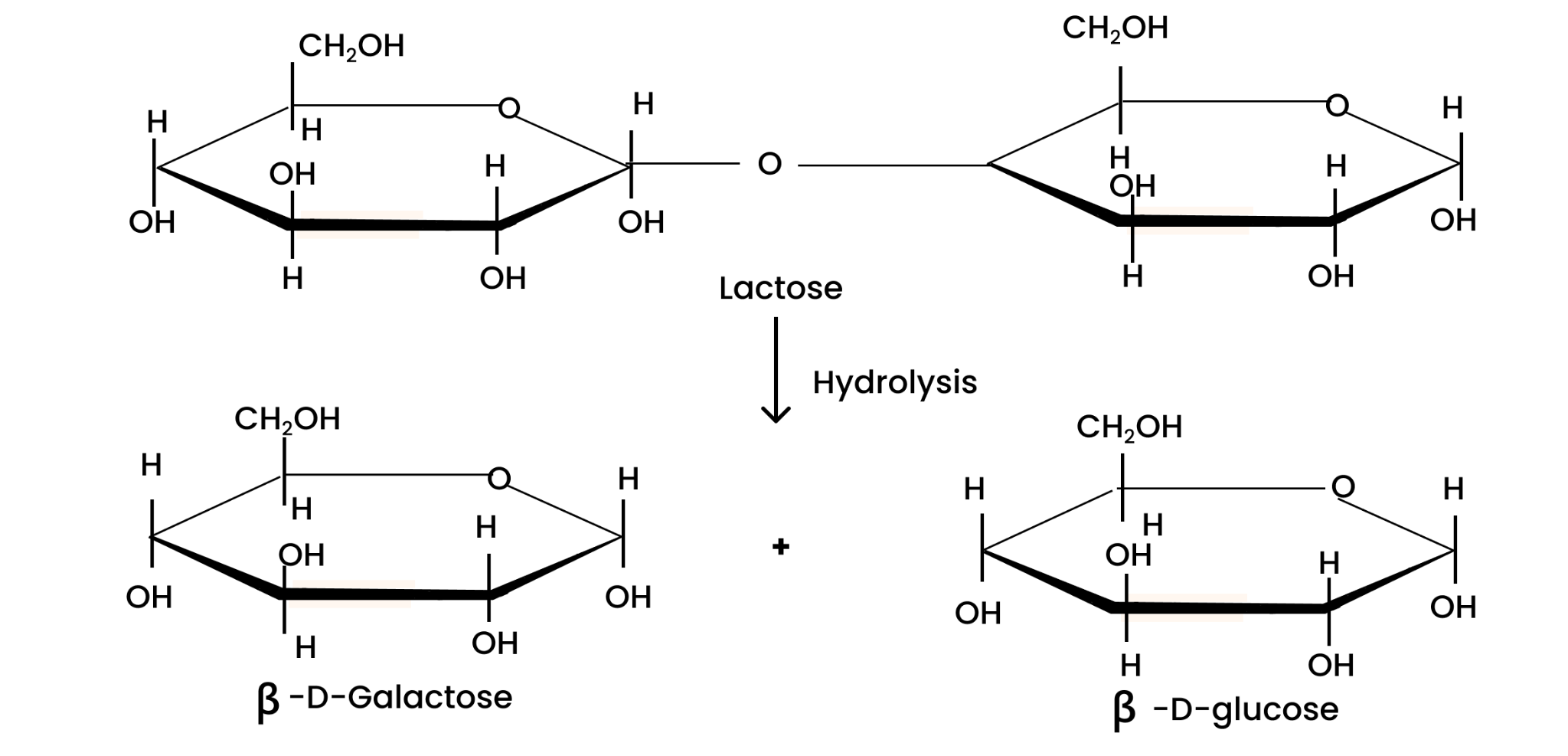
2. What are the expected products of hydrolysis of lactose?
Ans: Lactose is made up of $\beta $-D-galactose and $\beta $-D-glucose which on hydrolysis gives the same compounds. This can be illustrated by;
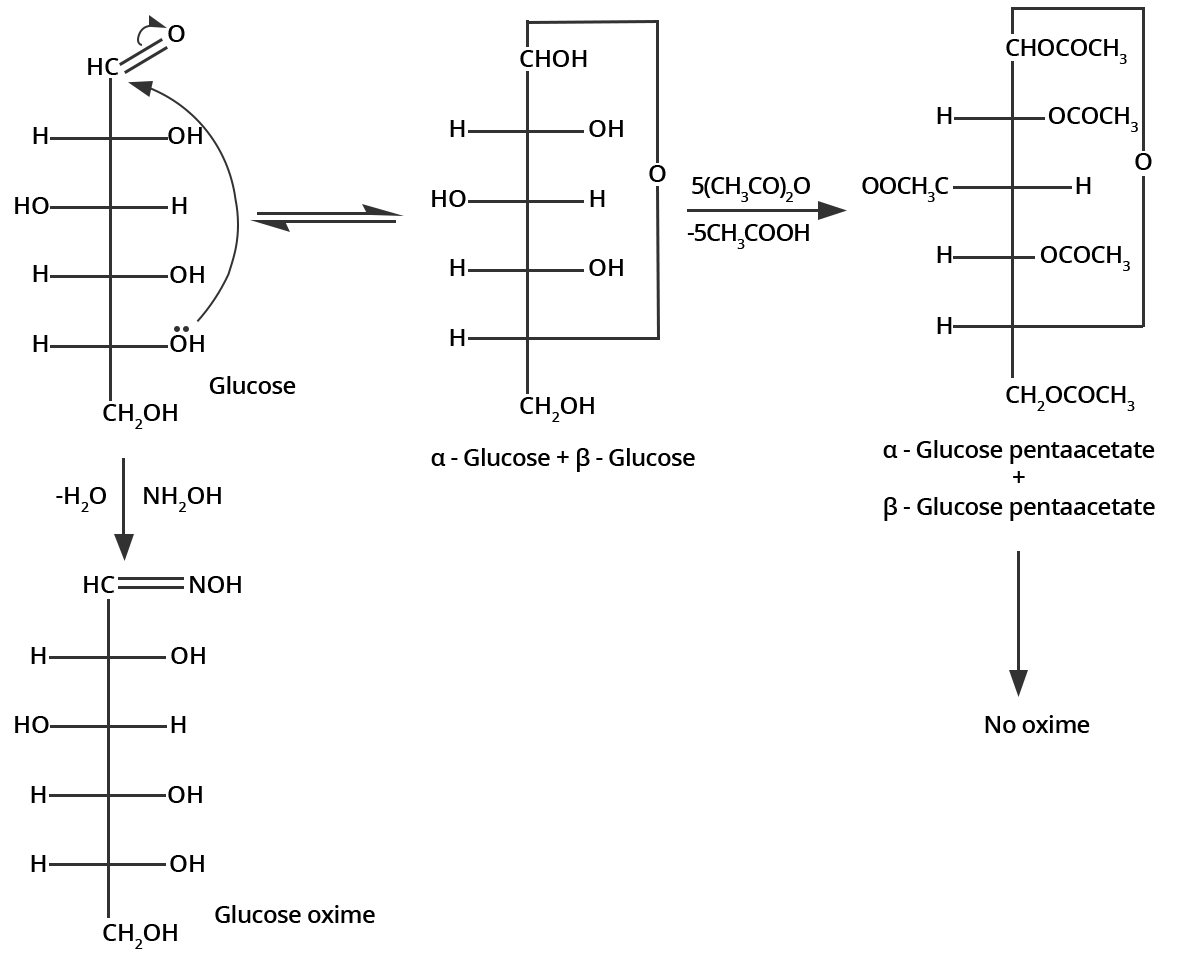
3. How do you explain the absence of an aldehyde group in the pentaacetate of D-glucose?
The open structure of D-glucose reacts with hydroxylamine $\left( N{{H}_{2}}OH \right)$ to form an oxime because of the presence of an aldehyde group in the structure. Whereas the pentaacetate of D-glucose is not an open structure and thus, it does not react with hydroxylamine. This shows the absence of an aldehydic group on pentaacetate of D-glucose.
This can be illustrated as follows;
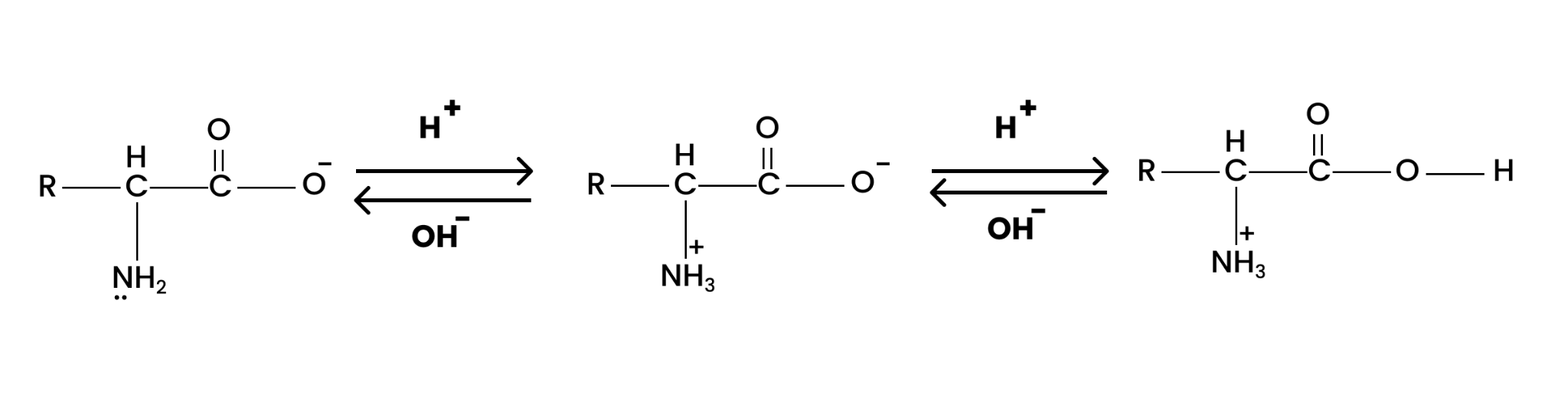
4. The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
The molecules of amino acid contain both acidic (carboxyl) and basic (amino) groups within them. Thus, they show dipolar behaviour when dissolved in water giving rise to zwitterion. Whereas, halo acids do not show the same behaviour.
The zwitterion is formed when the carboxyl group loses a proton and the amino group accepts the same. This can be illustrated by;
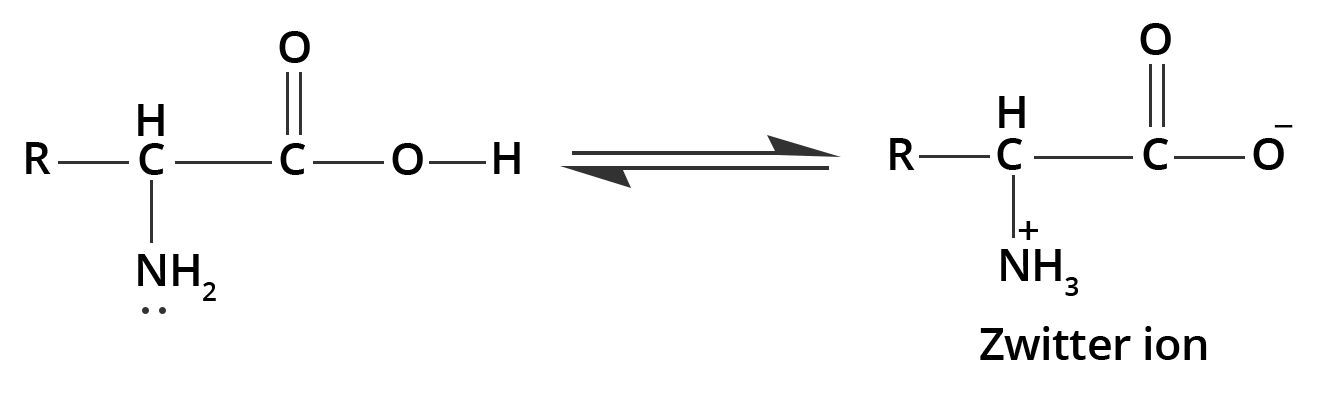
Hence, the melting points and the solubility of amino acids in water are higher than those of the corresponding halo-acids.
5. Where does the water present in the egg go after boiling the egg?
When we boil the egg, the proteins present within them get denatured and thus, go under coagulation. The excess water present is then absorbed by the coagulated protein through H – bonding.
6. Why cannot vitamin C be stored in our body?
The water-soluble compounds cannot retain in the human body due to constant excretion through urine. Vitamin C is a water-soluble component in our body and thus, cannot be stored.
7. What products would be formed when a nucleotide from DNA containing thymine is hydrolyzed?
The hydrolysis of a nucleotide of DNA having thymine as its base gives thymine $\beta $ -D-2 deoxyribose and phosphoric acid as products.
8. When RNA is hydrolyzed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Consider a DNA molecule; it has a double-stranded structure in which adenine always pairs up with thymine and cytosine always pairs up with guanine through H – bonding. Thus, when hydrolyzed the quantity of adenine produced is equal to that of thymine and similarly, the quantity of cytosine is equal to that of guanine.
But when RNA is hydrolyzed, there is no such relationship between the products obtained. Thus, this proves the single-stranded structure of RNA.
Text Solution
1. What are monosaccharides?
Monosaccharides are the most basic units of biomolecules. They cannot be hydrolyzed further to give simpler units. They are then classified on the basis of;
A number of C atoms: trioses, tetroses, pentoses, hexoses, and heptoses.
Functional groups: aldoses (aldehyde) and ketoses (ketone).
Now, if a monosaccharide having 5 C atoms and ketone as a functional group then it is named – ketopentose.
2. What are reducing sugars?
The carbohydrates that reduce Fehling’s solution and Tollen’s reagent are known as reducing sugars. All the monosaccharides and disaccharides are reducing sugars, except for sucrose.
3. Write two main functions of carbohydrates in plants.
The two main functions of carbohydrates (polysaccharides) in plants are:
Starch serves as storage molecules.
Cellulose is used to build the cell wall.
4. Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
The classification is given as;
Monosaccharides: Ribose, 2-deoxyribose, galactose and fructose.
Disaccharides: Maltose and lactose.
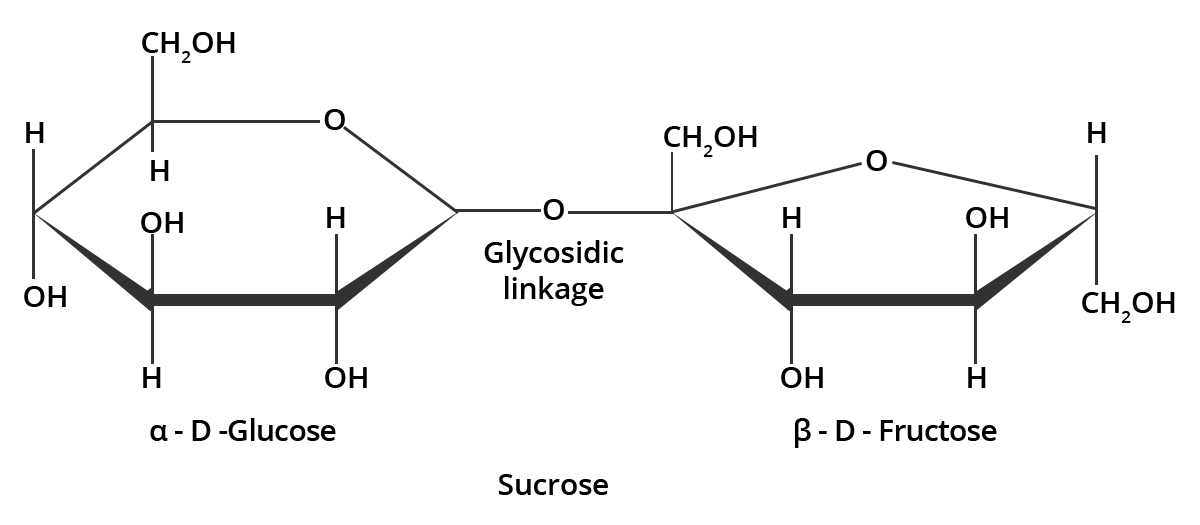
5. What do you understand by the term glycosidic linkage?
The linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule is known as glycosidic linkage.
For example:
Sucrose molecule has a glycosidic linkage which links $\alpha $ -D-glucose and $\beta $ -D-fructose. This can be illustrated as;
6. What is glycogen? How is it different from starch?
In animals, carbohydrates are stored in the form of glycogen which itself is a complex carbohydrate.
Starch is a carbohydrate consisting of two components i.e., amylose (nearly 15 – 20%) and amylopectin (nearly 80 – 85%) in nature. Whereas, glycogen consists of just one component which is similar to the structure of amylopectin but more branched than actual amylopectin.
7. What are the hydrolysis products of
(i) sucrose and
When hydrolyzed, sucrose gives molecules of $\alpha $ -D-glucose and $\beta $ -D-fructose, each. This can be illustrated as;
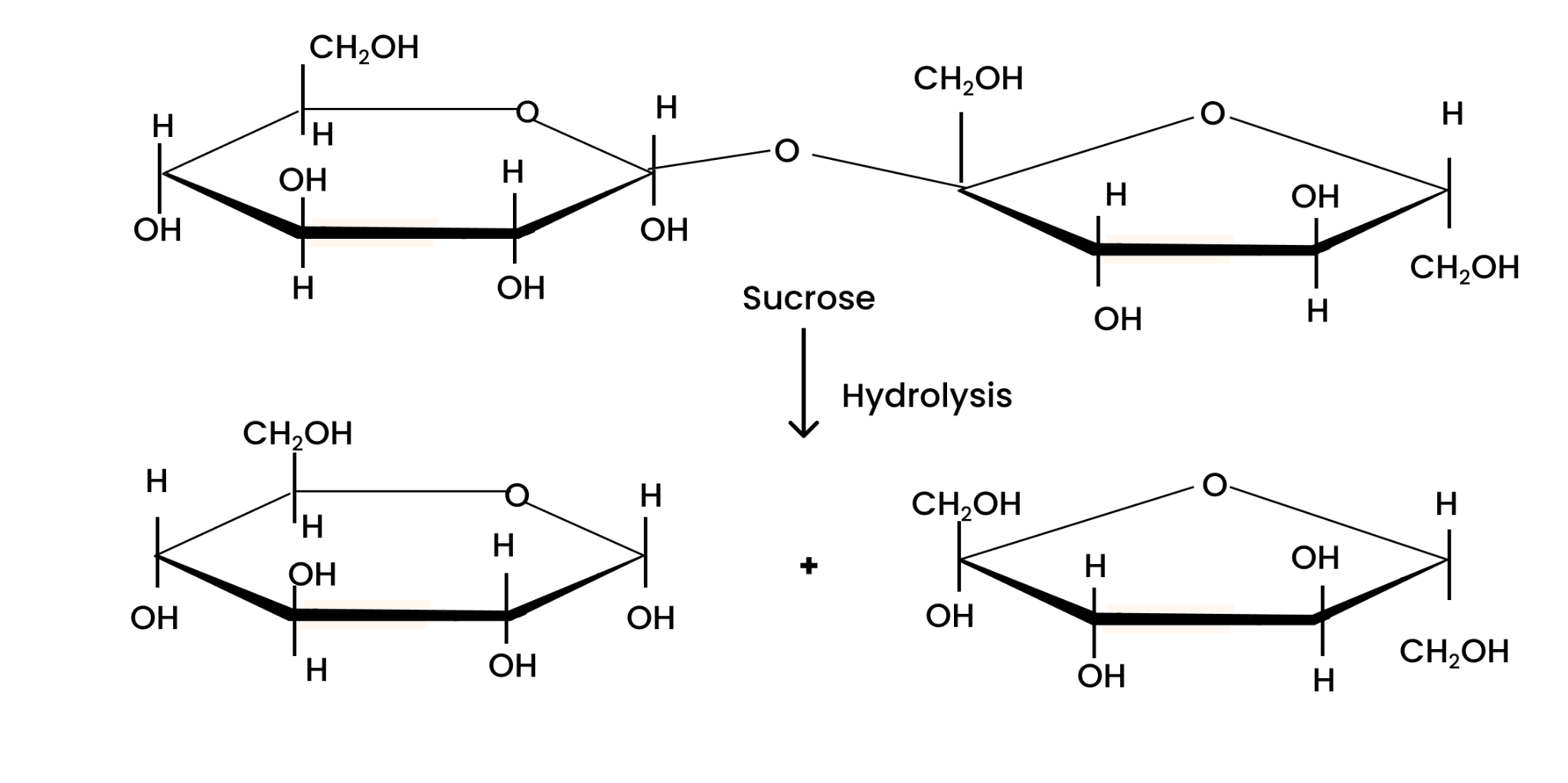
(ii) lactose?
When hydrolyzed, lactose gives molecules of $\beta $-D-galactose and $\beta $-D-glucose each. This can be illustrated as;
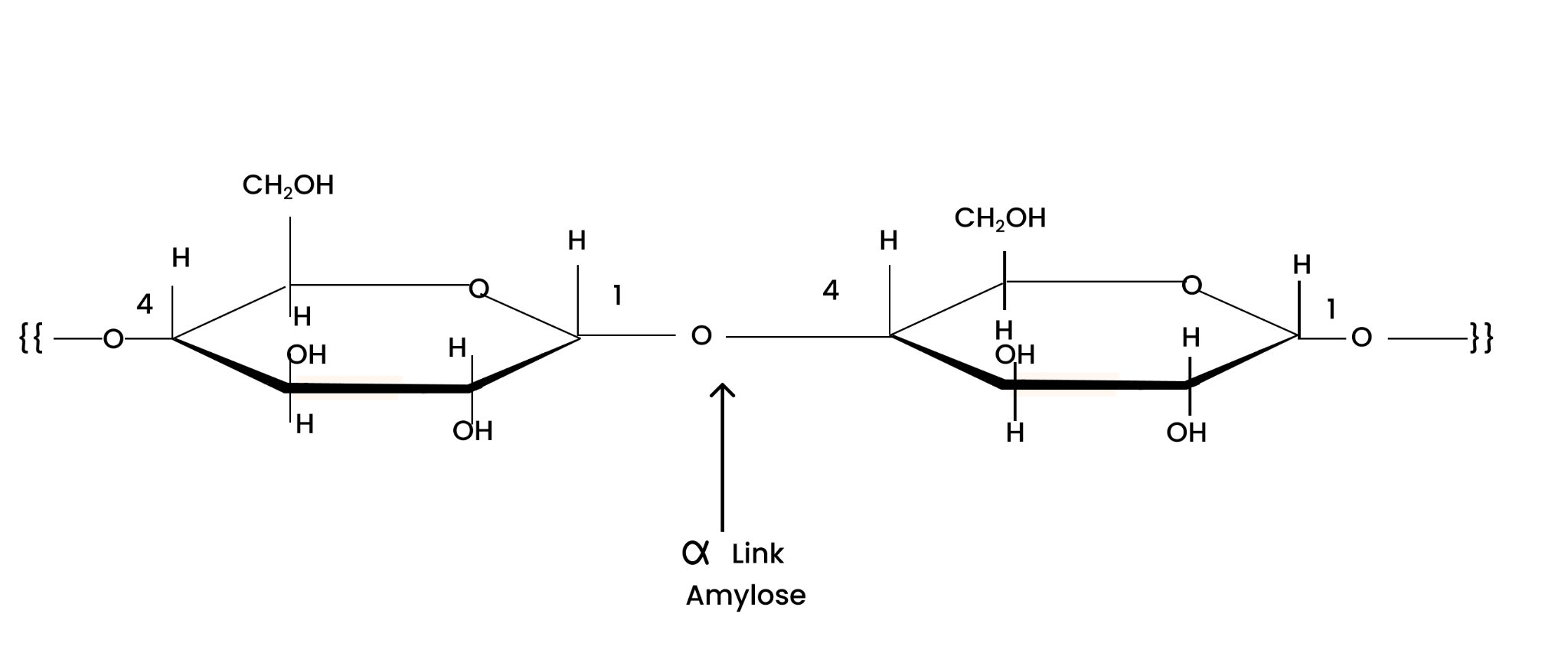
8. What is the basic structural difference between starch and cellulose?
It consists of two components i.e. amylose and amylopectin.
Amylose is a long linear chain of $\alpha $ -D-glucose units linked by a glycosidic linkage at positions 1 and 4 i.e., C1 – C4 linkage.
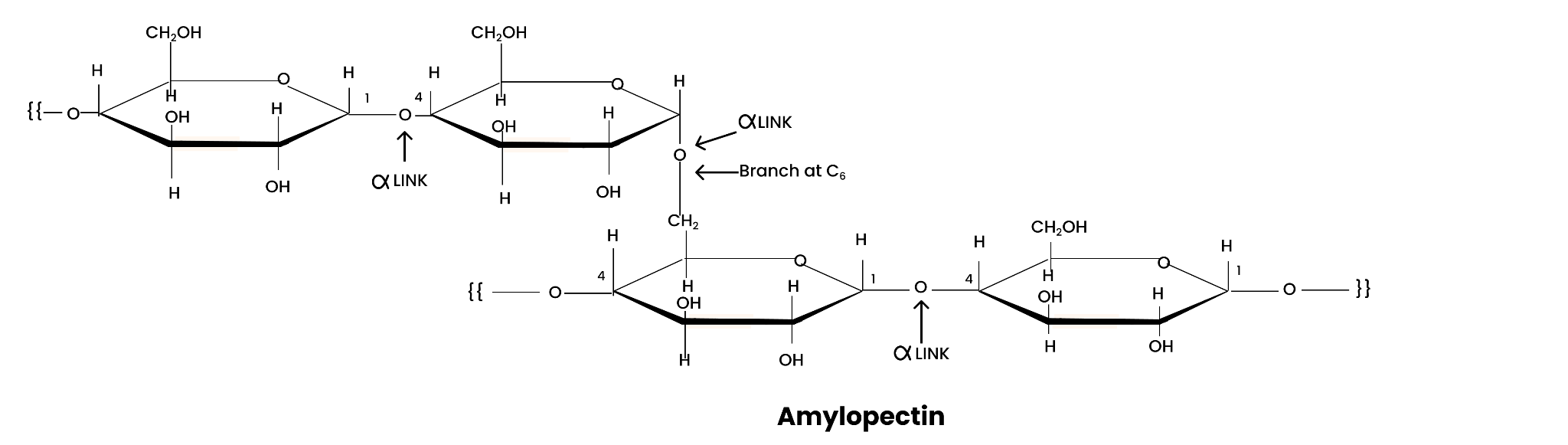
Amylopectin is a branched-chain polymer of $\alpha $ -D-glucose units. The chain is formed by C1 – C4 glycosidic linkage and branching occur at C1 – C4 position.
It is a straight-chain polysaccharide of $\beta $-D-glucose units linked by a glycosidic linkage at positions 1 and 4 i.e., C1 – C4 linkage.
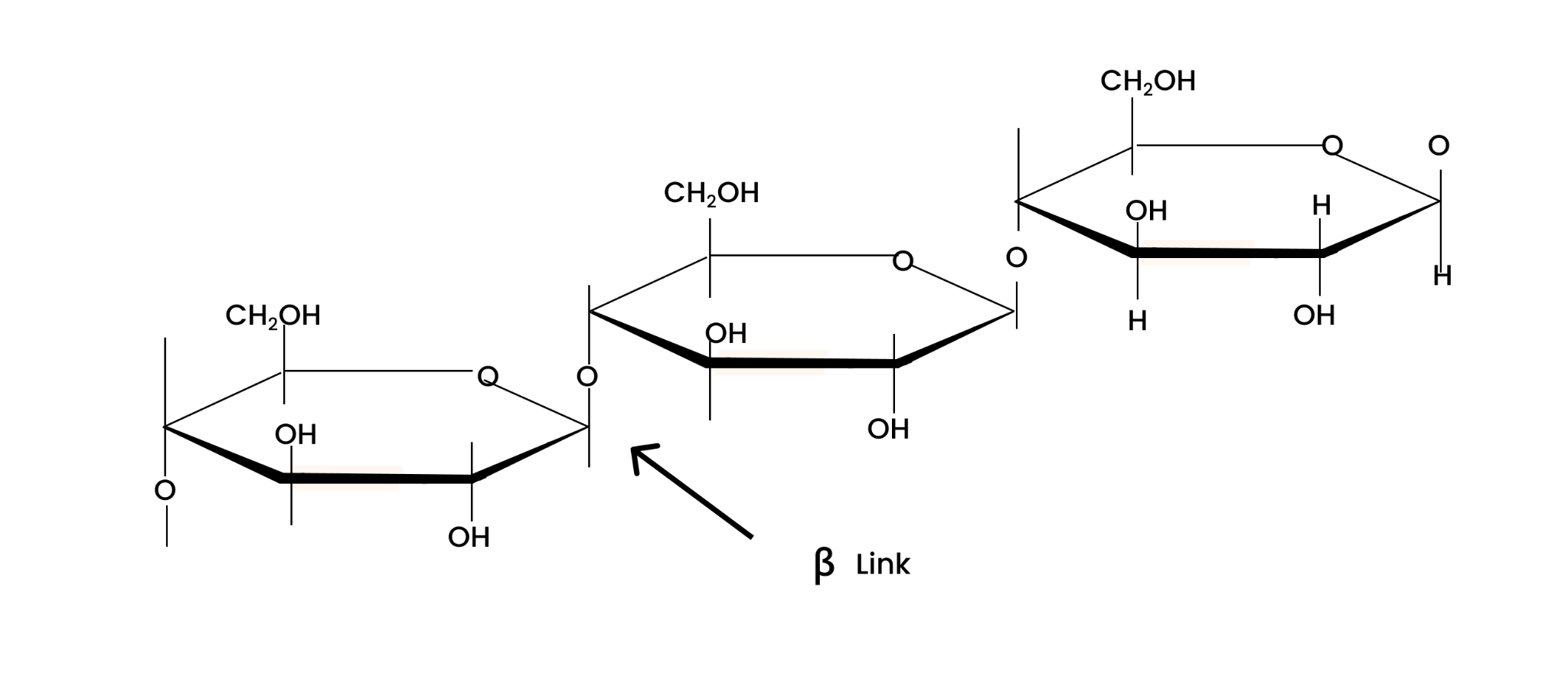
9. What happens when D-glucose is treated with the following reagents?
(i) HI
When heated with HI for a long time, D-glucose forms n-hexane as;
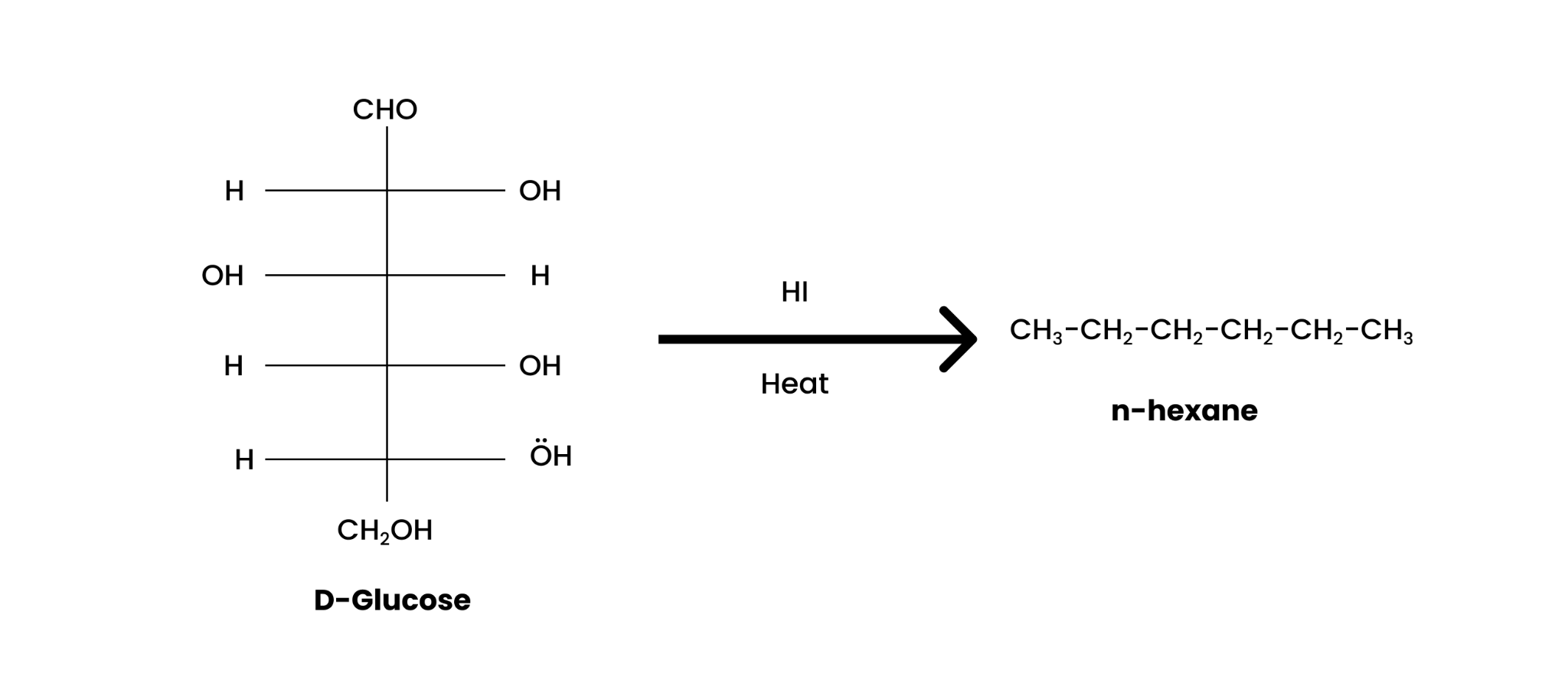
(ii) Bromine water
When treated with bromine water, D-glucose produces gluconic acid as;
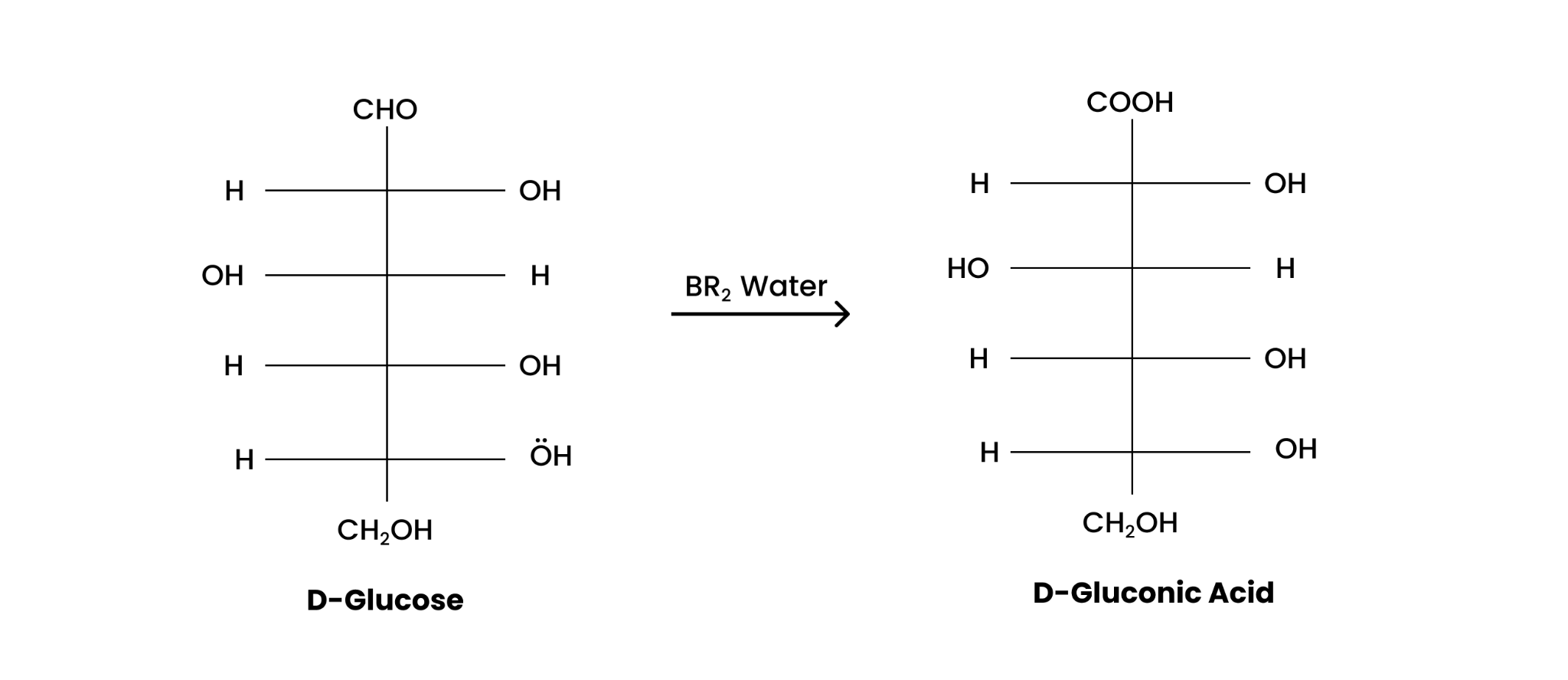
(iii) $HN{{O}_{3}}$
D-glucose when treated with $HN{{O}_{3}}$, gets oxidized to give saccharic acid as;
10. Enumerate the reactions of D-glucose which cannot be explained by its open-chain structure.
The reactions of D-glucose which cannot be explained by its open structure are;
2, 4-DNP test, Schiff’s test and reaction with $NaHS{{O}_{4}}$ to form hydrogen sulphite as an additional product. Whereas, aldehydes give all of them.
The pentaacetate of glucose does not react with hydroxylamine due to the absence of the free -CHO group.
Glucose exists in two crystalline forms i.e., $\alpha $ and $\beta $ which show differences in their respective melting points. The same behaviour cannot be executed by an open structure.
11. What are essential and non-essential amino acids? Give two examples of each type.
There are two types of amino acids in the human body:
Essential amino acids:
They are required by the body but cannot be synthesized within.
These must be taken up via food.
For example, leucine and valine.
Non-essential amino acids:
They too are required by the body but can be synthesized within.
For example, alanine and glycine.
12. Define the following as related to proteins
(i)Peptide linkage
The peptide linkage is formed when the -COOH group of one amino acid is attached to the $-N{{H}_{2}}$ group of another amino acid by the elimination of water molecules.
This can be easily illustrated as;
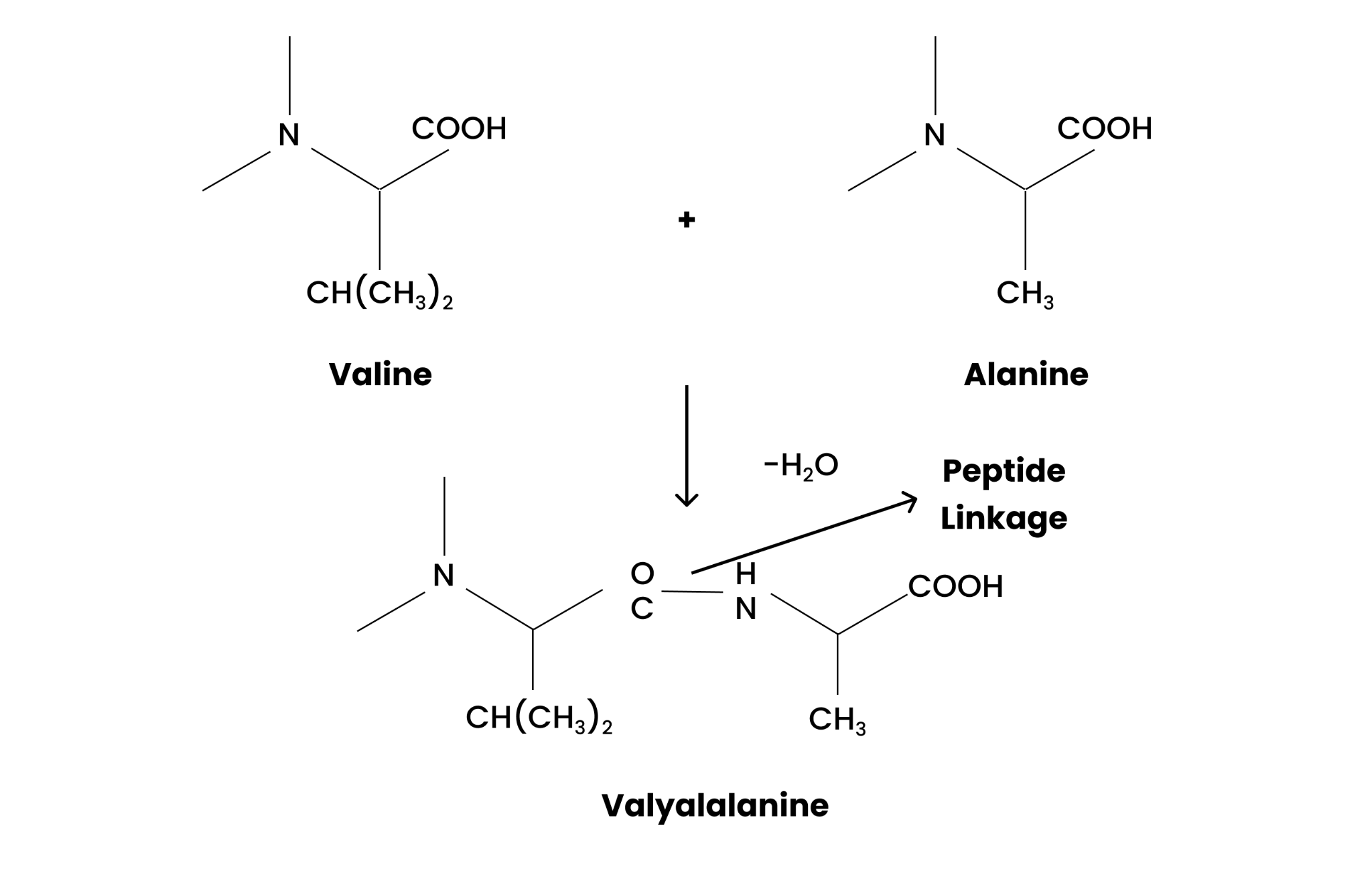
(ii) Primary structure
The specific sequence (the sequence of linkages between amino acids in a polypeptide chain) in which various amino acids are present is called the primary structure of the protein. The slight change in this sequence creates a new protein.
(iii) Denaturation.
A protein has a unique 3D structure and thus, specific biological activity in living systems. When such proteins are subjected to some changes i.e., change in temperature (physical) or change in pH (chemical), the H-bonds are disturbed. These disturbances unfold the globules and uncoil the helix which results in loss of biological activity by that protein molecule. This is known as the denaturation of protein.
Denaturation only destroys the secondary and tertiary structures of protein whereas the primary structure remains unaltered.
13. What are the common types of secondary structures of proteins?
The two common types of the secondary structure of the protein are;
$\alpha $ - helix structure:
Here, the $-N{{H}_{2}}$ group of amino acid residue forms an H – bond with the -COOH group of adjacent turns in the right direction (right-handed screw).
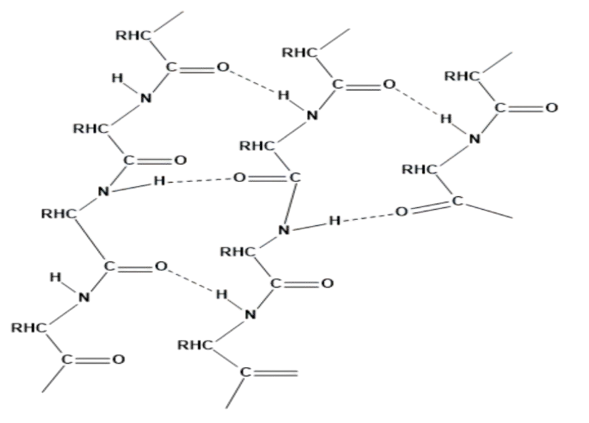
$\beta $ - pleated sheet structure:
It looks like pleated folds of drapery hence, the name $\beta $ - pleated sheet structure. Here, all the peptide chains are stretched out to nearly the maximum extension and then laid side by side. They are held together by intermolecular hydrogen bonds.
14. What type of bonding helps in stabilizing the $\alpha -$ helix structure of proteins?
The H - bonding formed between the $-N{{H}_{2}}$ group of amino acid and the -COOH group of adjacent amino acids helps in the stabilization of $\alpha $ -helix.
15. Differentiate between globular and fibrous proteins.
Ans: The difference between globular and fibrous proteins are:
Fibrous protein | Globular protein |
It is a fibre-like structure formed by the polypeptide chain. They are held together by strong hydrogen and disulphide bond. | The polypeptide chain in this protein is folded around itself, which gives rise to a spherical structure. |
Insoluble in water. | Soluble in water. |
Used for structural purposes. For example, keratin is present in nails and hair; collagen is present in tendons, and myosin is present in muscles. | All enzymes along with some hormones such as insulin are globular proteins. |
16. How do you explain the amphoteric behaviour of amino acids?
The amino acids when mixed with water, shows the dipolar behaviour i.e., the carboxyl group of an amino acid loses a proton and the amino group of another can accept the same proton to give a zwitterion. This can be shown as;
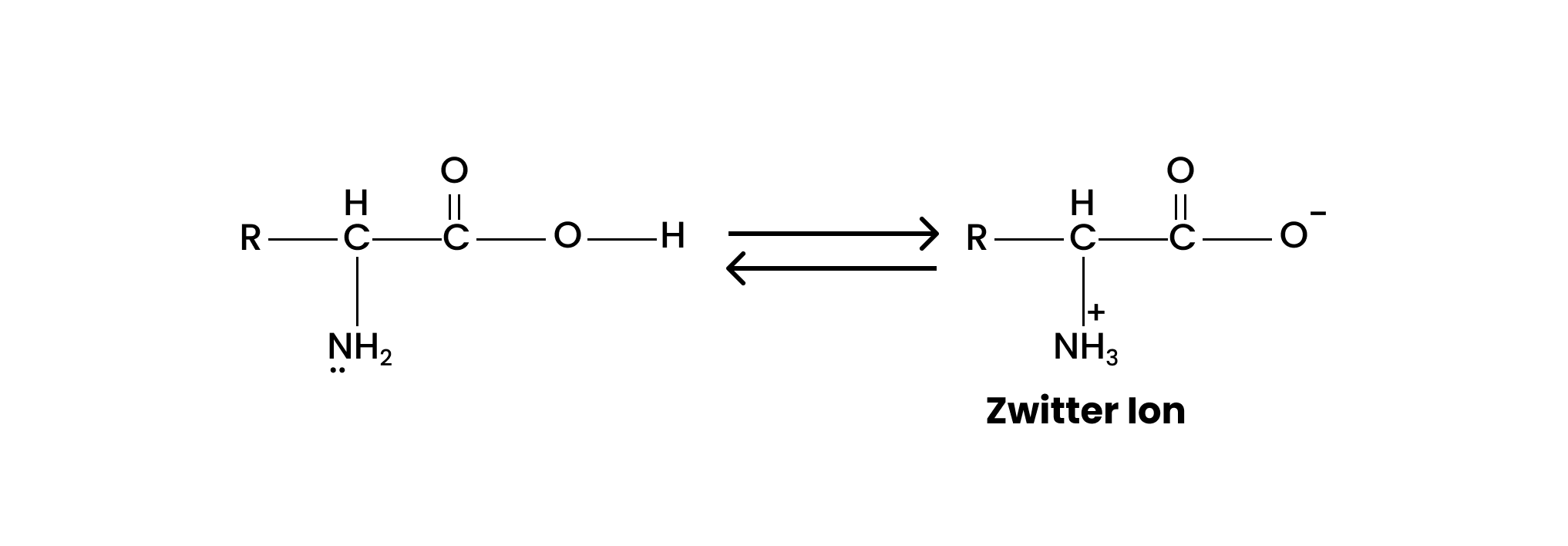
In this zwitterionic form, the amino acid acts as both acids as well as base showing its amphoteric behaviour.
17. What are enzymes?
The proteins that catalyze the biological reactions or biological catalysts are known as enzymes. They are very specific in nature and catalyze only a particular reaction for a particular substrate.
They are named according to the particular substrate or specific reaction taking place and always ends with ‘-ase’.
For example,
Maltase: The enzyme used to catalyze the hydrolysis of maltose into glucose.
Oxidoreductase: the enzymes used to catalyze the oxidation of one substrate with the simultaneous reduction of another substrate.
18. What is the effect of denaturation on the structure of proteins?
Ans:
Denaturation results in the unfolding of globules and the uncoiling of helixes. During this process, secondary and tertiary proteins are destroyed but the primary ones remain unaltered. Sometimes, secondary and tertiary proteins get converted to primarily structured proteins during denaturation.
19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Vitamins are classified on the basis of their solubility in water or fat as follows;
Vitamins such as A, D, E and K are soluble in fats and oils but not in water.
B group vitamins (${{B}_{1}},{{B}_{2}},{{B}_{6}},{{B}_{12}}$, etc.) and vitamin C are water soluble vitamins.
The exceptional cases are of biotin or vitamin H, as they are neither soluble in water nor in fat.
The vitamin responsible for the coagulation of blood is Vitamin K.
20. Why are vitamin A and vitamin C essential to us? Give their important sources.
Both vitamin A and C are essential to us as their deficiency causes some serious health problems. The deficiency of vitamin A leads to xerophthalmia (hardening of the cornea of the eye) and night blindness whereas deficiency of vitamin C leads to scurvy (bleeding gums).
The sources of vitamin A are fish liver oil, carrots, butter, and milk; whereas, that of vitamin C are citrus fruits and green leafy vegetables.
21. What are nucleic acids? Mention their two important functions.
The biomolecules found in nuclei of living cells (as one of the important constituents of chromosomes) are known as nucleic acids. There are mainly two types of nucleic acids i.e., deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
DNA is responsible for the transmission of inherent characters from one generation to another. This is known as heredity.
DNA and RNA, both are responsible for the protein synthesis in the cell.
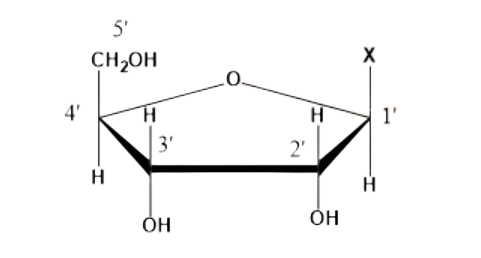
22. What is the difference between a nucleoside and a nucleotide?
Nucleoside:
It is formed by the attachment of a base to the 1’ position of the sugar. Its generalized formulation and structure are given as;
Nucleoside = sugar + base (X).
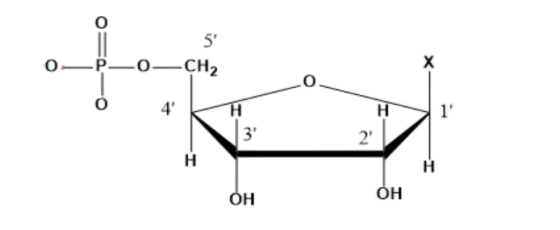
Nucleotide:
It is formed by all the three components of nucleic acids i.e., base, sugar and phosphoric acid. Its generalized formulation and structure are given as;
Nucleotide = sugar + base (X) + phosphoric acid.
23. The two strands in DNA are not identical but are complementary. Explain.
DNA has a double-stranded helical structure where those strands are held together by H – bonds between the specific base pairs. Cytosine forms H – bonds with guanine and Adenine forms H – bonds with thymine. Thus, the two strands are complementary to each other.
24. Write the important structural and functional differences between DNA and RNA.
Ans: The structural and functional difference between DNA and RNA is:
Structural differences:
DNA | RNA |
The sugar present here is $\beta $-D-2- deoxyribose. | The sugar present here is $\beta $-D-ribose. |
It contains thymine (T). | It contains uracil (U). |
The helical structure is double-stranded. | The helical structure is single-stranded. |
Functional differences:
DNA | RNA |
It is the chemical basis of heredity. | It is not responsible for heredity. |
They do not synthesize proteins but transfer coded messages for the synthesis of proteins in the cells. | Proteins are synthesized by RNA molecules in the cells. |
25. What are the different types of RNA found in the cell?
Types of RNA found in cells are;
Messenger RNA (mRNA)
Ribosomal RNA (rRNA)
Transfer RNA (t-RNA).
Class 12 Chemistry Chapter 10 Biomolecules Quick Overview of Topics
Chemistry class 12 chapter 10 NCERT Solutions - Quick Overview of Detailed Structure of Topics and Subtopics Covered.
Topic | Subtopics |
Carbohydrates | - Monosaccharides |
- Disaccharides | |
- Polysaccharides | |
- Structure and functions in living organisms | |
Proteins | - Amino acids |
- Peptides and polypeptides | |
- Structure and functions in living organisms | |
Enzymes | - Structure and function |
- Mechanism of enzyme action | |
Vitamins | - Classification and functions |
- Deficiency diseases | |
Nucleic Acids | - DNA and RNA |
- Structure and functions in living organisms | |
Hormones | - Types and functions |
- Role in biochemical processes |
Some Important Concepts for Class 12 Chemistry Chapter 10 Biomolecules
Class 12 NCERT solutions help the students to go through the Concepts easily. Here, find the important topics in Chapter 10 - Biomolecules - to crack your exams.
Classification of Biomolecules: Biomolecules are classified into four main categories based on their chemical nature and functions:
Carbohydrates: Sugars, starches, cellulose, etc.
Proteins: Polymers of amino acids.
Lipids: Fats, oils, phospholipids, etc.
Nucleic Acids: DNA, RNA, ATP, etc.
Primary Structure of Proteins: The primary structure of a protein refers to the sequence of amino acids in the polypeptide chain. It is determined by the order of amino acids linked by peptide bonds.
Carbohydrate Chemistry: Key concepts in carbohydrate chemistry include:
Monosaccharides: Simple sugars such as glucose, fructose, and galactose.
Disaccharides: Two monosaccharides linked by a glycosidic bond, such as sucrose, lactose, and maltose.
Polysaccharides: Complex carbohydrates formed by the polymerisation of monosaccharide units, such as starch, glycogen, and cellulose.
Enzyme Kinetics: Enzymes are biological catalysts that increase the rate of biochemical reactions.
Nucleic Acid Structure: Nucleic acids are polymers of nucleotides and include DNA and RNA. Key concepts include:
DNA structure: Double helix structure composed of two complementary strands of nucleotides held together by hydrogen bonds.
RNA structure: Single-stranded molecule involved in protein synthesis and gene expression.
Benefits of Class 12 NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules
The Vedantu’s class 12 NCERT Solutions of Biomolecules provided here in PDFs offer various benefits, including:
Detailed explanations and step-by-step solutions for all topics in Biomolecules.
Solutions curated by experienced educators to ensure accuracy and clarity.
Covers important concepts like Carbohydrates - Classification (aldoses and ketoses), monosaccharides, D-L configuration oligosaccharides, polysaccharides, Importance of carbohydrates.
Clear and concise explanations using precise chemical terminology.
In-depth analysis of key concepts and their applications in real-life scenarios.
A detailed explanation of Biomolecules in the class 12 chemistry NCERT solutions section will give you learnings on Proteins -Elementary idea of amino acids, peptide bonds, polypeptides, proteins, the structure of proteins - primary, secondary, tertiary structure and quaternary structures.
Solutions to a variety of problems to strengthen analytical and problem-solving abilities.
Step-by-step solutions for numerical problems and reaction mechanisms.
Related Study Materials for Class 12 Chemistry Chapter 10 NCERT Solutions
Students can access extra study materials on biomolecules. These resources are available for download and offer additional support for your studies.
S. No | Study Material Links for Chemistry Chapter 10 - Biomolecules |
1. |
|
2. |
|
3. |
|
NCERT solutions of Biomolecules in class 12 play a crucial role in Class 12 exam prep. Start by thoroughly reading the textbook chapter. After that, solve the NCERT questions for Class 12, Chapter 10 - Biomolecules. You can find detailed solutions on Vedantu that align with CBSE guidelines. Download the free NCERT Solutions for Class 12 Chapter 10 - Biomolecules to guide your exam preparation with expert-reviewed answers. Vedantu has provided complete resources including chapter notes, important questions and exemplar solutions to help you score more.
NCERT Solutions Class 12 Chemistry | Chapter-wise Links
Access Vedantu’s chapter-wise NCERT Chemistry Class 12 Solutions PDFs below for all other chapters.
NCERT Solutions Class 12 Chemistry Chapter-wise Links |
|
|
|
|
|
|
|
|
NCERT Solutions Class 12 Chemistry - Related Links
S. No | Important Resources Links for Class 12 Chemistry |
1 |
|
2 |
|
3 |
|
4 |
|

FAQs on NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules
1. What are biomolecules? What are the 4 main types of biomolecules?
The organic molecules that are essential for the various metabolic processes such as digestion, cell repair, growth, etc are called biomolecules. The biomolecules, in a way, support all the life processes required for our survival.
The 4 main types of biomolecules are proteins, lipids, carbohydrates, and nucleic acids. Apart from these, there are various other biomolecules that are involved in carrying out the metabolic activities.
2. What are monosaccharides?
Those carbohydrate molecules that cannot be broken down (hydrolyzed) further into ketones or units of polyhydroxy aldehyde are called monosaccharides. These monosaccharides can be further classified based on their functional group and the number of carbon atoms present in them.
Classification Based on Functional Groups:
The monosaccharides that contain a keto- group are classified as ketoses, whereas, those containing aldehyde groups are called aldoses.
Classification Based on the Number of Carbon Atoms:
Based on the number of carbon atoms present in monosaccharides, they can be classified as trioses, pentoses, tetroses, and heptoses.
The monosaccharides are named according to the above two classifications. For example, an aldose containing 4 carbon atoms is called aldotetrose while a ketose containing 4 carbon atoms is called ketotetrose.
3. What is the difference between glycogen and starch?
Carbohydrates are stored in animals in the form of glycogen. Glycogen is a polysaccharide. Whereas, starch is made up of two components, amylopectin, and amylose. Though both glycogen and starch are forms of carbohydrates, yet, unlike starch, glycogen is made up of only one component. The structure of glycogen is far more branched as compared to amylopectin.
4. Are the Biomolecules class 12 NCERT solutions on Vedantu reliable study resources?
Yes, the Biomolecules class 12 chemistry NCERT solutions on Vedantu make very reliable study resources. These NCERT Solutions are prepared by our subject-matter experts, in accordance with the CBSE guidelines for Class 12. You can find the relevant chemical formulae and structures for every question, in these NCERT Solutions. By going through these Biomolecules class 12 chemistry NCERT solutions , you will be able to revise all the key points covered in this chapter. The in-text questions are also solved in this PDF, thereby making it a comprehensive study resource for your board examination. So, download this PDF for free and refer to the best study guide for Biomolecules for your 12th board Chemistry examination.
5. What are some of the materials that are obtained from cellulose?
Several materials are obtained from cellulose, some of which are -
Mercerised Cotton: When the cellulose is treated with a cone, the sodium hydroxide solution develops a silky lustre that is called the mercerised cotton.
Gun Cotton: This is nitrated cellulose that is highly explosive in nature. Its main use is the manufacturing of smokeless gun powder also known as blasting gelatine.
Cellulose Acetate: This is used in the manufacturing of acetone rayon and making motion pictures film.
6. What are some of the properties of glucose?
Some properties of glucose are :
When glucose undergoes acetylation with acetic anhydride, it produces pentaacetate which confirms the presence of hydroxyl groups in glucose.
In reaction with hydroxylamine, glucose gives monoxime and goes on to add with the molecule of hydrogen cyanide, producing cyanohydrin. This confirms that glucose has in them the carbonyl group.
When reacted with mild oxidising agents such as bromine water, glucose gets oxidised to form gluconic acid. On the other hand, saccharic acid is produced, on oxidation with nitric acid.
7. What are essential and non-essential amino acids?
The ten amino acids that the human body synthesizes are called the non-essential amino acids. The other ten amino acids that are required for protein synthesis but are not synthesized by the human body are called essential amino acids.
These essential amino acids are:
Phenylalanine
Tryptophane
8. What are the structures of protein?
Primary Structure - It has the function of simply presenting the sequence of the amino acids.
S econdary Structure - The secondary structure presents the α-helix structure presented by the hydrogen bonds or β-pleated sheet structure showcased by the hydrogen bond when the R is a small group.
Tertiary Structure - This presents the folding and the superimposing of the polypeptide chains that form a globular shape. This structure is termed the tertiary structure and is stabilized by the covalent, ionic, hydrogen and disulphide bonds.
9. How to prepare with NCERT solutions Biomolecules class 12?
The reading of the chapter is essential to understand what this chapter has to offer. Students must mark all the important areas while reading the chapter so that it gets easier for them to access these during the examination. The student should practice the various names, formulas, and functions regularly to be able to solve any question that might be asked in the question paper from this chapter. Apart from this, it is also mandatory for the student to refer to the NCERT Solutions and practice all the exercises that are present in them.
10. Is Class 12 Chemistry Chapter 10, Biomolecules, difficult?
Biomolecules NCERT solutions chapter difficulty varies with NCERT solutions for Class 12, understanding improves, making it manageable for students.
11. What are the main points of Chapter 10 Biomolecules NCERT?
Class 12 biomolecules NCERT solutions' Main points of biomolecules include carbohydrates, proteins, lipids, and nucleic acids. NCERT solutions clarify these, aiding comprehension for students.
12. How much time will it take to complete Biomolecules class 12?
Biomolecules class 12 chemistry NCERT solutions Completion time for biomolecules chapter varies per student; with structured NCERT solutions, efficient learning typically spans a few study sessions. Download Class 12 Biomolecules NCERT solutions For better preparations to ace your exams.
13. Which biomolecule is the most important?
Class 12 Chemistry Biomolecules NCERT solutions involve Carbohydrates, proteins, lipids, and nucleic acids that are vital. Understanding their roles and structures in NCERT solutions for Class 12 elucidates their importance.
14. What are the four essential biomolecules?
Biomolecules complexity varies, yet with Class 12 Chemistry Biomolecules NCERT solutions, understanding the four essential biomolecules becomes clearer for students. These solutions offer clarity and insight, making the understanding of the four essential biomolecules - carbohydrates, proteins, lipids, and nucleic acids - clearer and more accessible to students.
NCERT Solutions Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry chapter 14-Biomolecules
- Active page
NCERT Solutions for Class 12 Chemistry
NCERT Solutions for class-12 Chemistry Chapter 14 Biomolecules is prepared by our senior and renowned teachers of Physics Wallah primary focus while solving these questions of class-12 in NCERT textbook, also do read theory of this Chapter 14 Biomolecules while going before solving the NCERT questions. Our Physics Wallah team Prepared Other Subjects NCERT Solutions for class 12.
Checkout Class 12th Books from PW Store
Chapter 14 Biomolecules
Answer the following Questions.
Question 1. What are monosaccharides?
Solution : Monosaccharides are carbohydrates that cannot be hydrolysed further to give simpler units of polyhydroxy aldehyde or ketone.
Monosaccharides are classified on the bases of number of carbon atoms and the functional group present in them. Monosaccharides containing an aldehyde group are known as aldoses and those containing a keto group are known as ketoses. Monosaccharides are further classified as trioses, tetroses, pentoses, hexoses, and heptoses according to the number of carbon atoms they contain. For example, a ketose containing 3 carbon atoms is called ketotriose and an aldose containing 3 carbon atoms is called aldotriose.
Question 2. What are reducing sugars?
Solution : Reducing sugars are carbohydrates that reduce Fehling’s solution and Tollen’s reagent. All monosaccharides and disaccharides, excluding sucrose, are reducing sugars.
Question 3. Write two main functions of carbohydrates in plants.
Solution : Two main functions of carbohydrates in plants are:
(i) Polysaccharides such as starch serve as storage molecules.
(ii) Cellulose, a polysaccharide, is used to build the cell wall.
Question 4. Classify the following into monosaccharides and disaccharides.
Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose
Solution : Monosaccharides:
Ribose, 2-deoxyribose, galactose, fructose
Disaccharides:
Maltose, lactose
Checkout Class 12th Sample Papers from PW Store
Question 5. What do you understand by the term glycosidic linkage?
Solution : Glycosidic linkage refers to the linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule.
For example, in a sucrose molecule, two monosaccharide units, ∝-glucose and β-fructose, are joined together by a glycosidic linkage.

Question 6. What is glycogen? How is it different from starch?
Solution : Glycogen is a carbohydrate (polysaccharide). In animals, carbohydrates are stored as glycogen.
Starch is a carbohydrate consisting of two components − amylose (15 − 20%) and amylopectin (80 − 85%).
However, glycogen consists of only one component whose structure is similar to amylopectin. Also, glycogen is more branched than amylopectin.
Question 7. What are the hydrolysis products of (i) sucrose and (ii) lactose?
Solution : (i) On hydrolysis, sucrose gives one molecule of ∝-D glucose and one molecule of β- D-fructose.

(ii) The hydrolysis of lactose gives β-D-galactose and β-D-glucose.

Question 8. What is the basic structural difference between starch and cellulose?
Solution : Starch consists of two components − amylose and amylopectin. Amylose is a long linear chain of ∝−D−(+)−glucose units joined by C1−C4 glycosidic linkage (∝-link).

Amylopectin is a branched-chain polymer of ∝-D-glucose units, in which the chain is formed by C1−C4 glycosidic linkage and the branching occurs by C1−C6 glycosidic linkage.

On the other hand, cellulose is a straight-chain polysaccharide of β-D-glucose units joined by C1−C4 glycosidic linkage (β-link).

Question 9. What happens when D-glucose is treated with the following reagents?
(i) HI (ii) Bromine water (iii) HNO 3
Solution : (i) When D-glucose is heated with HI for a long time, n-hexane is formed.

(ii) When D-glucose is treated with Br 2 water, D- gluconic acid is produced.

(iii) On being treated with HNO 3 , D-glucose get oxidised to give saccharic acid.

Question 10. Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Solution : (1) Aldehydes give 2, 4-DNP test, Schiff’s test, and react with NaHSO 4 to form the hydrogen sulphite addition product. However, glucose does not undergo these reactions.
(2) The pentaacetate of glucose does not react with hydroxylamine. This indicates that a free −CHO group is absent from glucose.
(3) Glucose exists in two crystalline forms − ∝ andβ. The ∝-form (m.p. = 419 K) crystallises from a concentrated solution of glucose at 303 K and the β-form (m.p = 423 K) crystallises from a hot and saturated aqueous solution at 371 K. This behaviour cannot be explained by the open chain structure of glucose.
Question 11. What are essential and non-essential amino acids? Give two examples of each type.
Solution : Essential amino acids are required by the human body, but they cannot be synthesised in the body. They must be taken through food. For example: valine and leucine
Non-essential amino acids are also required by the human body, but they can be synthesised in the body. For example: glycine, and alanine
Question 12. Define the following as related to proteins
(i) Peptide linkage (ii) Primary structure (iii) Denaturation.
Solution : (i) Peptide linkage:
The amide formed between −COOH group of one molecule of an amino acid and −NH 2 group of another molecule of the amino acid by the elimination of a water molecule is called a peptide linkage.

(ii) Primary structure:
The primary structure of protein refers to the specific sequence in which various amino acids are present in it, i.e., the sequence of linkages between amino acids in a polypeptide chain. The sequence in which amino acids are arranged is different in each protein. A change in the sequence creates a different protein.
(iii) Denaturation:
In a biological system, a protein is found to have a unique 3-dimensional structure and a unique biological activity. In such a situation, the protein is called native protein. However, when the native protein is subjected to physical changes such as change in temperature or chemical changes such as change in pH, its H-bonds are disturbed. This disturbance unfolds the globules and uncoils the helix. As a result, the protein loses its biological activity. This loss of biological activity by the protein is called denaturation. During denaturation, the secondary and the tertiary structures of the protein get destroyed, but the primary structure remains unaltered.
One of the examples of denaturation of proteins is the coagulation of egg white when an egg is boiled.
Question 13. What are the common types of secondary structure of proteins?
Solution : There are two common types of secondary structure of proteins:
(i) ∝-helix structure
(ii) β-pleated sheet structure
∝- Helix structure:

β-pleated sheet structure:
This structure is called so because it looks like the pleated folds of drapery. In this structure, all the peptide chains are stretched out to nearly the maximum extension and then laid side by side. These peptide chains are held together by intermolecular hydrogen bonds.

Question 14. What type of bonding helps in stabilising the ∝-helix structure of proteins?
Solution : The H-bonds formed between the −NH group of each amino acid residue and
Question 15. Differentiate between globular and fibrous proteins.
|
|
| ||
| 1. | It is a fibre-like structure formed by the polypeptide chain. These proteins are held together by strong hydrogen and disulphide bonds. | 1. | The polypeptide chain in this protein is folded around itself, giving rise to a spherical structure. |
| 2. | It is usually insoluble in water. | 2. | It is usually soluble in water. |
| 3. | Fibrous proteins are usually used for structural purposes. For example, keratin is present in nails and hair; collagen in tendons; and myosin in muscles. | 3. | All enzymes are globular proteins. Some hormones such as insulin are also globular proteins. |
Question 16. How do you explain the amphoteric behaviour of amino acids?
Solution : In aqueous solution, the carboxyl group of an amino acid can lose a proton and the amino group can accept a proton to give a dipolar ion known as zwitter ion.

Therefore, in zwitter ionic form, the amino acid can act both as an acid and as a base.

Thus, amino acids show amphoteric behaviour.
Question 17. What are enzymes?
Solution : Enzymes are proteins that catalyse biological reactions. They are very specific in nature and catalyse only a particular reaction for a particular substrate. Enzymes are usually named after the particular substrate or class of substrate and some times after the particular reaction.
For example, the enzyme used to catalyse the hydrolysis of maltose into glucose is named as maltase.

Again, the enzymes used to catalyse the oxidation of one substrate with the simultaneous reduction of another substrate are named as oxidoreductase enzymes.
The name of an enzyme ends with ‘− ase’.
Question 18. What is the effect of denaturation on the structure of proteins?
Solution : As a result of denaturation, globules get unfolded and helixes get uncoiled. Secondary and tertiary structures of protein are destroyed, but the primary structures remain unaltered. It can be said that during denaturation, secondary and tertiary-structured proteins get converted into primary-structured proteins. Also, as the secondary and tertiary structures of a protein are destroyed, the enzyme loses its activity.
Question 19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Solution : On the basis of their solubility in water or fat, vitamins are classified into two groups.
(i) Fat-soluble vitamins: Vitamins that are soluble in fat and oils, but not in water, belong to this group. For example: Vitamins A, D, E, and K
(ii) Water-soluble vitamins: Vitamins that are soluble in water belong to this group. For example: B group vitamins (B 1 , B 2 , B 6 , B 12 , etc.) and vitamin C
However, biotin or vitamin H is neither soluble in water nor in fat.
Vitamin K is responsible for the coagulation of blood.
Question 20. Why are vitamin A and vitamin C essential to us? Give their important sources.
Solution : The deficiency of vitamin A leads to xerophthalmia (hardening of the cornea of the eye) and night blindness. The deficiency of vitamin C leads to scurvy (bleeding gums).
The sources of vitamin A are fish liver oil, carrots, butter, and milk. The sources of vitamin C are citrus fruits, amla, and green leafy vegetables.
Question 21. What are nucleic acids? Mention their two important functions.
Solution : Nucleic acids are biomolecules found in the nuclei of all living cells, as one of the constituents of chromosomes. There are mainly two types of nucleic acids − deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are also known as polynucleotides as they are long-chain polymers of nucleotides.
Two main functions of nucleic acids are:
- DNA is responsible for the transmission of inherent characters from one generation to the next. This process of transmission is called heredity.
- Nucleic acids (both DNA and RNA) are responsible for protein synthesis in a cell. Even though the proteins are actually synthesised by the various RNA molecules in a cell, the message for the synthesis of a particular protein is present in DNA.
Question 22. What is the difference between a nucleoside and a nucleotide?

Nucleoside = Sugar + Base

On the other hand, all the three basic components of nucleic acids (i.e., pentose sugar, phosphoric acid, and base) are present in a nucleotide.
Nucleotide = Sugar + Base + Phosphoric acid

Question 23. The two strands in DNA are not identical but are complementary. Explain.
Solution : In the helical structure of DNA, the two strands are held together by hydrogen bonds between specific pairs of bases. Cytosine forms hydrogen bond with guanine, while adenine forms hydrogen bond with thymine. As a result, the two strands are complementary to each other.
Question 24. Write the important structural and functional differences between DNA and RNA.
Solution : The structural differences between DNA and RNA are as follows:
|
|
| ||
| 1. | The sugar moiety in DNA molecules is β-D-2 deoxyribose. | 1. | The sugar moiety in RNA molecules is β-D-ribose. |
| 2. | DNA contains thymine (T). It does not contain uracil (U). | 2. | RNA contains uracil (U). It does not contain thymine (T). |
| 3. | The helical structure of DNA is double-stranded. | 3. | The helical structure of RNA is single-stranded. |
The functional differences between DNA and RNA are as follows:
|
|
| ||
| 1 | DNA is the chemical basis of heredity. | 1 | RNA is not responsible for heredity. |
| 2 | DNA molecules do not synthesise proteins, but transfer coded message for the synthesis of proteins in the cells. | 2 | Proteins are synthesised by RNA molecules in the cells. |
Question 25. What are the different types of RNA found in the cell?
Solution : (i) Messenger RNA (m-RNA)
(ii) Ribosomal RNA (r-RNA)
(iii) Transfer RNA (t-RNA)
Question 26. Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
Solution : A glucose molecule contains five −OH groups while a sucrose molecule contains eight −OH groups. Thus, glucose and sucrose undergo extensive H-bonding with water.
Hence, these are soluble in water.
But cyclohexane and benzene do not contain −OH groups. Hence, they cannot undergo H-bonding with water and as a result, are insoluble in water.
Question 27. What are the expected products of hydrolysis of lactose?
Solution : Lactose is composed of β-D galactose and β-D glucose. Thus, on hydrolysis, it gives β-D galactose and β-D glucose.

Question 28. How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
Solution : D-glucose reacts with hydroxylamine (NH 2 OH) to form an oxime because of the presence of aldehydic (−CHO) group or carbonyl carbon. This happens as the cyclic structure of glucose forms an open chain structure in an aqueous medium, which then reacts with NH 2 OH to give an oxime.

But pentaacetate of D-glucose does not react with NH 2 OH. This is because pentaacetate does not form an open chain structure.

Question 29. The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
Solution : Both acidic (carboxyl) as well as basic (amino) groups are present in the same molecule of amino acids. In aqueous solutions, the carboxyl group can lose a proton and the amino group can accept a proton, thus giving rise to a dipolar ion known as a zwitter ion.

Due to this dipolar behaviour, they have strong electrostatic interactions within them and with water. But halo-acids do not exhibit such dipolar behaviour.
For this reason, the melting points and the solubility of amino acids in water is higher than those of the corresponding halo-acids.
Question 30. Where does the water present in the egg go after boiling the egg?
Solution : When an egg is boiled, the proteins present inside the egg get denatured and coagulate. After boiling the egg, the water present in it is absorbed by the coagulated protein through H-bonding.
Question 31. Why cannot vitamin C be stored in our body?
Solution : Vitamin C cannot be stored in our body because it is water soluble. As a result, it is readily excreted in the urine.
Question 32. What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Solution : When a nucleotide from the DNA containing thymine is hydrolyzed, thymine β-D-2-deoxyribose and phosphoric acid are obtained as products.
Question 33. When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Solution : A DNA molecule is double-stranded in which the pairing of bases occurs. Adenine always pairs with thymine, while cytosine always pairs with guanine. Therefore, on hydrolysis of DNA, the quantity of adenine produced is equal to that of thymine and similarly, the quantity of cytosine is equal to that of guanine.
But when RNA is hydrolyzed, there is no relationship among the quantities of the different bases obtained. Hence, RNA is single-stranded.
NCERT Solutions For Class 12 Chemistry Chapter Wise.
Chapter 1 The Solid States
Chapter 2 Solutions
Chapter 3 Electrochemistry
Chapter 4 Chemical Kinetics
Chapter 5 Surface Chemistry
Chapter 6 General Priciples and Processes of Isolation of Elements
Chapter 7 The P Block Elements
Chapter 8 The D and F Elements
Chapter 9 Coordination Compounds
Chapter 10 Haloalkanes and Haloarenes
Chapter 11 Alcohols, Phenols and Ethers
Chapter 12 Aldehydes, ketones and Carboxylics Acids
Chapter 13 Amines
Chapter 15 Polymers
Chapter 16 Chemistry In Everyday Life
Related Chapters
- chapter 14-Biomolecules
- chapter 15-Polymers
- chapter 16-Chemistry In Everyday Life

Recent Concepts

Talk to Our counsellor

Biomolecules Class 12 Notes and Mind map

- Download file
Welcome to our comprehensive guide on Biomolecules for Class 12 students. Whether you're studying for your CBSE exams or simply looking to deepen your understanding of this fascinating subject, you're in the right place. In this article, we have compiled detailed class notes, mind maps, and scoring questions to help you master the concepts of Biomolecules. Biomolecules are the building blocks of life, playing critical roles in the functioning of living organisms.
From carbohydrates to lipids, proteins, and nucleic acids, each biomolecule has its own unique structure and function. Understanding these molecules is essential for grasping fundamental concepts in biology, biochemistry, and genetics. Our notes provide a clear and concise overview of each biomolecule, highlighting their structures, properties, and important functions.
The mind maps offer a visual representation of the interconnections between biomolecules, aiding in memory retention and concept mapping. Lastly, our scoring questions are designed to test and reinforce your understanding, allowing you to assess your knowledge and identify areas for improvement. So, dive in and explore our Biomolecules Class 12 Notes, Mind map, and Scoring Questions to ace your exams and enhance your knowledge of this fascinating subject.
Class 12 Chemistry Chapter 10, dedicated to Biomolecules, offers an extensive exploration into the fascinating world of biological molecules that are crucial for life processes. This chapter is a key part of the Class 12 curriculum, providing students with vital insights into the structure, function, and importance of biomolecules. The Class 12 Chemistry Biomolecules notes encompass comprehensive details about various biomolecules such as carbohydrates, proteins, nucleic acids, and vitamins, essential for students in their academic journey in chemistry.
For Class 12th students, understanding biomolecules is not just important for exams but also for a foundational knowledge in biochemistry, which has applications in fields like medicine, nutrition, and biotechnology. The biomolecules class 12 chemistry notes are crafted to cover every aspect of the topic, including the classification of biomolecules, their physical and chemical properties, and their roles in biological systems.
These notes also include biomolecules class 12th MCQs, which are crucial for students preparing for competitive exams as well as their board exams. The biomolecules class 12 chemistry important questions focus on key areas that are often emphasized in exams, helping students to solidify their understanding and prepare effectively.
For a more visual approach to learning, the biomolecules class 12 mind map provides a quick and efficient way to review the entire chapter, making it easier for students to recall important information during their exams. Additionally, the biomolecules class 12 chemistry notes PDF offers a convenient way for students to access study materials anytime, enhancing their learning experience.
In conclusion, the Chapter 10 of Class 12 Chemistry is a comprehensive guide for understanding biomolecules. With detailed notes, mind maps, MCQs, and important questions, students can effectively prepare for their exams and gain a thorough understanding of this vital topic in the field of chemistry.
Classification of Biomolecules
Biomolecules in living organisms are classified into several major categories based on their structure and function. These include carbohydrates, lipids, proteins, nucleic acids, and enzymes. Each class of biomolecules plays a crucial role in the structure and functioning of cells and organisms.
Carbohydrates
Carbohydrates are organic compounds made up of carbon, hydrogen, and oxygen, often following the formula (CH₂O)n. They are classified into monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Carbohydrates serve as the primary source of energy and as structural components in organisms.
Lipids are a diverse group of hydrophobic molecules, including fats, oils, waxes, phospholipids, and steroids. They are essential for storing energy, forming cell membranes, and acting as signaling molecules.
Proteins are large biomolecules made of amino acids linked by peptide bonds. They are crucial for various functions such as catalysis of biochemical reactions, structural support, transport, communication, and immune response.
Nucleic Acids
Nucleic acids, namely DNA and RNA, are the molecules responsible for storing and transmitting genetic information. DNA serves as the blueprint for protein synthesis, while RNA plays a role in translating this genetic code into proteins.
Enzymes and Their Functions
Enzymes are specialized proteins that act as catalysts in biochemical reactions. They speed up reactions by lowering the activation energy, thus playing a vital role in metabolism and other cellular processes.
Importance of Biomolecules in Living Organisms
Biomolecules are fundamental to life, as they constitute the cells and perform a range of functions necessary for the survival and growth of living organisms. They are involved in everything from energy production and storage to genetic information transfer and cellular structure maintenance.
Scoring Questions for Class 12 CBSE Exams
In Class 12 CBSE exams, scoring questions on biomolecules often involve understanding their structures, functions, and roles in biological systems. Questions may include explaining the properties of different types of biomolecules, their classification, and the significance of enzymes in metabolism. Additionally, questions might cover the application of biomolecules in health and disease, making a thorough understanding of this chapter crucial for success in exams.
- Biomolecules class 12 notes
- All CBSE notes
- All CHEMISTRY notes
- All 12 SCIENCE notes
You may like these also


The Tiny Teacher Question Answers

Chitthiyon Mein Europe Question Answer

Fractions Class 6 Extra Questions With Answers & Mind map
Heat Class 7 Notes, Mind map & Revision Questions
Teaching resources, test generator, worksheet generator, elearning for students, practice question paper, mock test series, happy parenting, elearning for child, worksheet for child, mock test series for child, witknowlearn, privacy policy, terms and condition, refund/cancellation policy.

Or login with Google
Notification
Upgrade to better learning opportunities.
Case Study Based Questions on Biomolecules – Chapter 14 Class 12
Case Study Based Questions on Biomolecules
1. Read the given passage and answer the questions that follow Monosaccharides containing an aldehyde group are called aldoses while those containing a keto group are called ketoses. All monosaccharides containing five and six carbon atoms have cyclic structures, furanose (five-membered) and pyranose (six-membered). During ring formation, C 1 aldoses and C 2 in ketoses becomes chiral and hence all these monosaccharides exist in two forms called the α-anomer and β-anomer while C 1 and C2 are called glycosidic or anomeric carbon atoms. In contrast, stereoisomers, which differ in configuration at any other chiral carbon other than the glycosidic carbon are called epimers. Two molecules of the same or different monosaccharides combine together through glycosidic linkage to form disaccharides Que 1. Which of the following compounds show furanose structures?

2. Read the given passage and answer the questions that follow The sequence of bases in mRNA is read in serial order in groups of three at a time. Each triplet of nucleotides (having a specific sequence of bases) is known as codon. Each codon specifies one amino acid. Many amino acids have more than one codons. The amino acids are brought to mRNA by another type of RNA called tRNA each amino acid has at least one corresponding tRNA. At one end of the tRNA is a trinucleotide base sequence on mRNA. Que 1. Which of the following nitrogen bases is not present in RNA? a) Thymine b) Adenine c) Guanine d) Cytosine Ans 1. (a) Que 2. Each triplet of nucleotides is called a) Anticodon b) Codon c) mRNA d) tRNA Ans 2. (b) Que 3. Each codon specifies a) 1 amino acid b) 2 amino acids c) 3 amino acids d) None of these Ans 3. (a)
Case Study based questions on Biomolecules
3. Read the given passage and answer the questions that follow Proteins are the most abundant biomolecules of the living system. The chief sources of proteins are milk, cheese, pulses, fish, meat, peanuts, etc. They are found in every part of the body and form a fundamental basis of the structure and functions of life. These are also required for the growth and maintenance of the body. The word protein is derived from the Greek word, ‘proteios’ meaning ‘primary’ or of ‘prime importance’. Chemically, proteins are the polymers in which the monomeric units are the α-amino acids. Amino acids contain an amino (-NH 2 ) and carboxylic (-COOH) functional groups. Depending upon the relative position of the amino group with respect to the carboxylic group, the amino acids can be classified as α, β, and γ-amino acids. Amino acids which are synthesised by the body are called non-essential amino acids. On the other hand, those amino acids which cannot be synthesized in the human body and are supplied in the form of diet (because they are required for proper health and growth) are called essential amino acids. Que 1. Amino acids show amphoteric behavior. Why? a) They have an amino group b) They have a carboxylic group c) Both (a) and (b) d) none of the above Ans 1. (c) Que 2. The name of linkage joining two amino acids a) Hydrogen bonding b) Peptide linkage c) Amino linkage d) Imino joints Ans 2. (b) Que 3. What are polypeptides? a) 10 < α-amino acids joined together b) amino acids joined together c) 20 < β-amino acids joined together d) None of the above Ans 3. (a)
Que 4. What type of bonding helps in stabilizing the α-helix structure of proteins? a) Peptide linkage b) Hydrogen bonding c) Amino linkage d) Van der waals force Ans 4. (b)
4. Read the given passage and answer the questions that follow
Biomolecules are complex molecules that build up living organisms and are required for their growth, maintenance, and ability to reproduce. Carbohydrates are polyhydroxy aldehydes and ketones which are major sources of energy. Monosaccharides are simple sugars that cannot be hydrolyzed. Oligosaccharides, on hydrolysis, give 2 to 10 molecules of monosaccharides. Polysaccharides like starch and cellulose on hydrolysis give a large number of molecules of glucose a-glucose and b-glucose (Anomers). Proteins are complex nitrogenous polymers of amino acids connected through peptide bonds. The sequence in which amino acids are linked is called Primary structure. Secondary structures are of 2 types a-helix in globular proteins and b-pleated structure in fibrous proteins involving H-bonds. The tertiary structure has H-bonds, disulphide linkage, ionic bonding, and van der Waals’ forces. Insulin is a hormone for the metabolism of glucose, has a quarternary structure. Denaturation of protein destroys a secondary and tertiary structure, loss of biological activity but primary structure remaining the same. Enzymes are highly specific, work at specific pH, moderate temperature, and catalyze biochemical reactions. Hormones perform specific functions and are secreted by endocrine glands. Vitamins are essential for a healthy body. A, D, E, K are fat-soluble vitamins. Vitamin C and B1, B2, B6 are water-soluble. B 12 is neither water nor fat-soluble. Nucleic acids are polymers of nucleotides. RNA consists of m-RNA, t-RNA, r-RNA. RNA has Adenine, Cytosine, Uracil, and Guanine. It helps in protein synthesis. It cannot replicate. DNA contains deoxyribose, A, C, G, and Thymine. It transfers genetic characteristics. DNA has a double helix structure and undergoes replication. Que 1. Name a disaccharide which on hydrolysis give glucose and galactose. Ans 1. Lactose. Que 2. What type of protein is albumin? Ans 2. Globular protein. Que 3. Name one non-reducing sugar. Ans 3. Sucrose Que 4. Which one is the complementary base of cytosine in one strand of DNA to that in another strand of DNA? Ans 4. Guanine. Que 5. Which linkage by which nucleotides are joined together between 5′ and 3′ atoms of pentose sugar? Ans 5. Phosphodiester linkage. Que 6. Which vitamin helps in the coagulation of blood? Ans 6. Vitamin K. Que 7. Which enzyme can dissolve blood clots to prevent a heart attack? Ans 7. Streptokinase
Related Posts

CBSE Sample Paper Session 2022-23 (Chemistry) PDF

Haloalkanes and Haloarenes Class 12 – Questions & Answers

CBSE Important Questions Electrochemistry
Save my name, email, and website in this browser for the next time I comment.
Type above and press Enter to search. Press Esc to cancel.
- Chemistry Worksheets
- Class 12 Chemistry Worksheets
- Class 12 Chemistry Worksheet on Chapter 14 Biomolecules – Set 1
Chemistry Worksheets Class 12 on Chapter 14 Biomolecules with Answers - Set 1
Biomolecules are the most essential organic molecules, which are involved in the maintenance and metabolic processes of living organisms. These non-living molecules are the actual foot-soldiers of the battle of sustenance of life. They range from small molecules such as primary and secondary metabolites and hormones to large macromolecules like proteins, nucleic acids, carbohydrates, lipids etc.
There are four major classes of Biomolecules – Carbohydrates, Proteins, Nucleic acids and Lipids.
Download Chemistry Worksheet Class 12 Chapter 14 Biomolecules PDF – Set 1
Download PDF

CBSE Class 12 Chemistry Worksheet Chapter 14 Biomolecules – Set 1
Q1. Carbohydrates obtaining more than 10 simple units of sugar are called:
a.) Monosaccharides
b.) Disaccharides
c.) Trisaccharides
d.) Polysaccharides
Q2. Which of the following is a protein?
a.) Glycogen
b.) Amylopectin
c.) Keratin
d.) Lecithin
Q3. Night blindness may be caused by the deficiency of vitamin-
Q4. Which of the following gives maximum energy in metabolic process?
a.) Proteins
b.) Vitamins
d.) Carbohydrates
Q5. The relation between nucleotide triplets and the amino acids is called:
b.) Genetic code
c.) Replication
d.) Enzymes
Q6. How do anomers differ from epimers?
Q7. What are reducing and non reducing sugars? What is the structural feature characterising reducing sugars?
Q8. Write the formula of a tripeptide alanylglycyl phenylalanine.
Q9. Define the following terms:
a.) Anomers
b.) Peptide bond
Q10. Describe the terms D– and L– configurations used for amino acids with example.
Q11. Coagulation of egg white on boiling is an example of denaturation of protein. Explain in term of structural changes.
Q12. What are neutral, acidic and basic amino acids? Which vitamin deficiency leads to scurvy? Mention one function of Vitamin C.
Q13. What type of forces are responsible for the formation of :
a.) Cross linking of polypeptide chains
b.) ⍺-helix formation
c.) β-sheet structure
Q14. a.) What is denaturation of proteins?
b.) What type of bonds hold a DNA double helix together?
c.) Which enzyme is present in saliva? What is the function?
Q15. Differentiate between globular and fibrous proteins.
Q16. Name a disease that is caused due to deficiency of the following vitamins:
a.) Thiamine
b.) Riboflavin
Q17. Write reactions to show how glucose separately reacts with
i.) NH 2 OH
iii.) ammoniacal AgNO 3
b.) What do you understand by renaturation of proteins?
Q18. What are enzymes? How do enzymes differ from ordinary chemical catalysts? Comment on the specificity of enzyme action. What is the most important reason for their specificity?
Q19. The two strands in DNA are not identical but are complementary. Explain.
Q20 . a.) What is glycogen? How is it different from starch?
b.) How is starch structurally different from cellulose?
c.) Explain what is meant by the following:
Peptide linkage and pyranose structure of glucose.
Download PDF to access answers of Chemistry Worksheet for Class 12 Chemistry Chapter 14 Biomolecules set -1. Download PDF
- NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules
- NCERT Exemplar Class 12 Chemistry Solutions for Chapter 14 – Biomolecules
- Class 12 Chemistry Chapter 14 Biomolecules MCQs
- Important Questions for Class 12 Chemistry Chapter 14 – Biomolecules
| CHEMISTRY Related Links | |
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Study Abroad Get upto 50% discount on Visa Fees
- Top Universities & Colleges
- Abroad Exams
- Top Courses
- Read College Reviews
- Admission Alerts 2024
- Education Loan
- Institute (Counselling, Coaching and More)
- Ask a Question
- College Predictor
- Test Series
- Practice Questions
- Course Finder
- Scholarship
- All Courses
- B.Sc (Nursing)
CBSE Class 12 Chemistry Notes Chapter 14 Biomolecules

Jasmine Grover
Content Strategy Manager
Biomolecules are important organic molecules that fuse together to form complex organic compounds and govern the metabolism of living organisms. Proteins, carbohydrates, nucleic acids, enzymes and lipids form the living system of biomolecules .
- Carbohydrates are essential polyhydroxy aldehydes or ketones , forming the building blocks of energy in living organisms.
- They encompass monosaccharides, disaccharides, and polysaccharides, with glucose being a vital energy source derived from starch digestion.
- Proteins are composed of amino acids linked by peptide bonds.
- Denaturation of proteins, disrupting protein structure, occurs with changes in pH or temperature.
- Enzymes , primarily proteins, accelerate biochemical reactions with specificity.
- Vitamins are crucial for health and are classified into fat-soluble (A, D, E, K) and water-soluble (B, C) types.
- Nucleic acids , polymers of nucleotides, store genetic information as DNA and RNA.
- DNA, with deoxyribose sugar, encodes hereditary data, while RNA, containing ribose, aids in protein synthesis via mRNA, rRNA, and tRNA.
According to the CBSE Class 12 Chemistry Syllabus 2023-24, this chapter has been renumbered as Chapter 10. Biomolecules Class 12 Notes are important for revision as the chapter holds a weightage of 7 marks in the Class 12 Chemistry examination 2024.
Biomolecules Notes
Carbohydrates
- Carbohydrates are organic compounds primarily produced by plants.
- Examples include cane sugar, glucose, and starch.
- They are characterized by a general formula, Cx(H 2 O)y, but not all compounds fitting this formula are classified as carbohydrates.
- Carbohydrates are defined as optically active polyhydroxy aldehydes or ketones , or compounds that produce such units upon hydrolysis .
Classification of Carbohydrates
Carbohydrates are classified into three groups based on their behavior on hydrolysis.
Monosaccharides
Monosaccharides cannot be further hydrolyzed to simpler units. Examples include glucose, fructose, and ribose.
- Monosaccharides are classified into aldoses and ketoses based on their functional groups and the number of carbon atoms they contain.
- Glucose is obtained from sucrose through hydrolysis with dilute acids or from starch via hydrolysis with dilute sulfuric acid under pressure and elevated temperature.
- Its structure comprises six carbon atoms arranged in a straight chain, featuring functional groups such as aldehyde and hydroxyl.
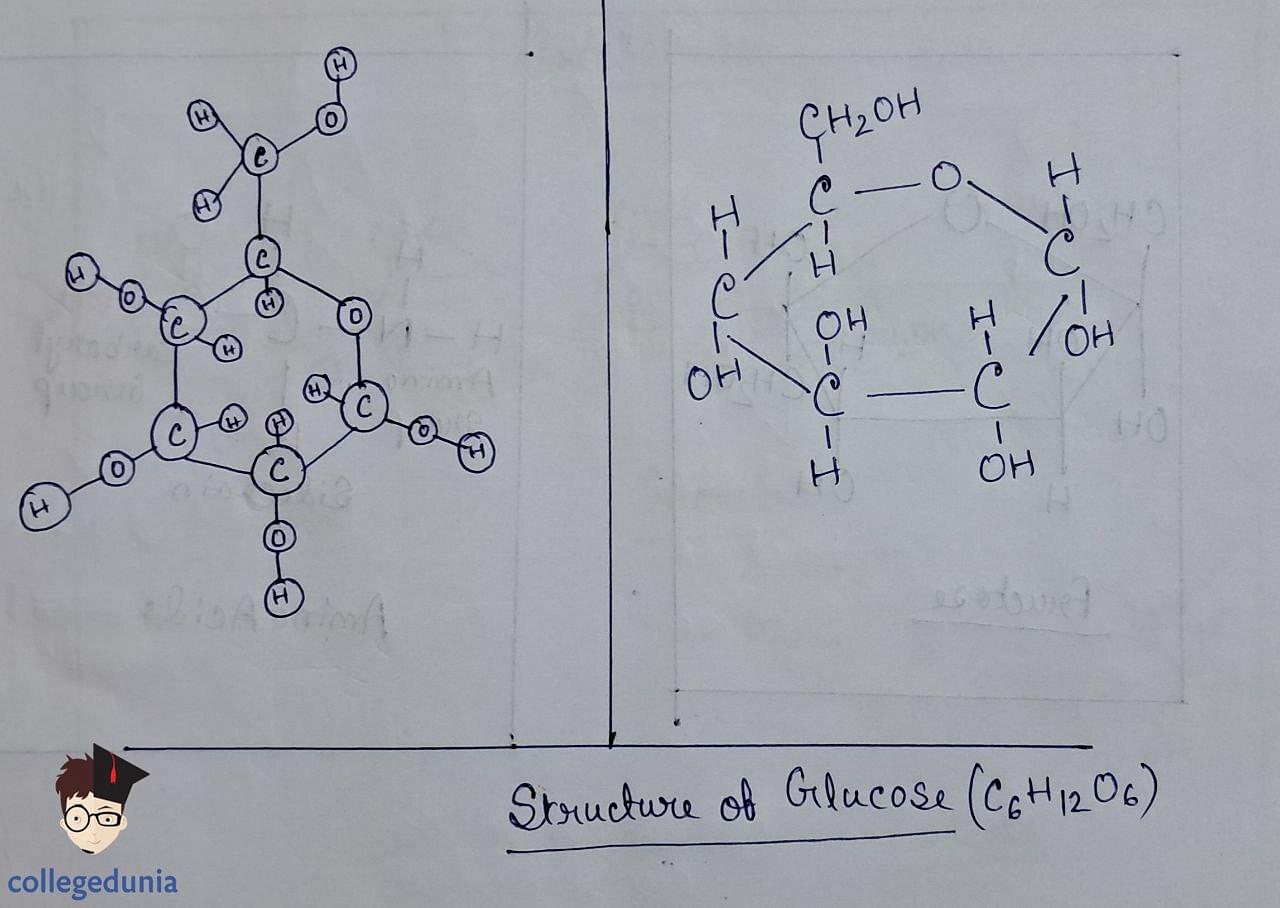
- Glucose, an aldohexose, is also known as dextrose and exists as the monomer of larger carbohydrates like starch and cellulose.
- It forms two cyclic hemiacetal structures (a and b forms) known as anomers, with the configuration determined by the hydroxyl group at C1.
- The cyclic structure is depicted using the Haworth projection, representing the pyranose structure.
- Fructose, a ketohexose, is derived from sucrose hydrolysis and is naturally present in fruits, honey, and vegetables, serving as a natural sweetener.
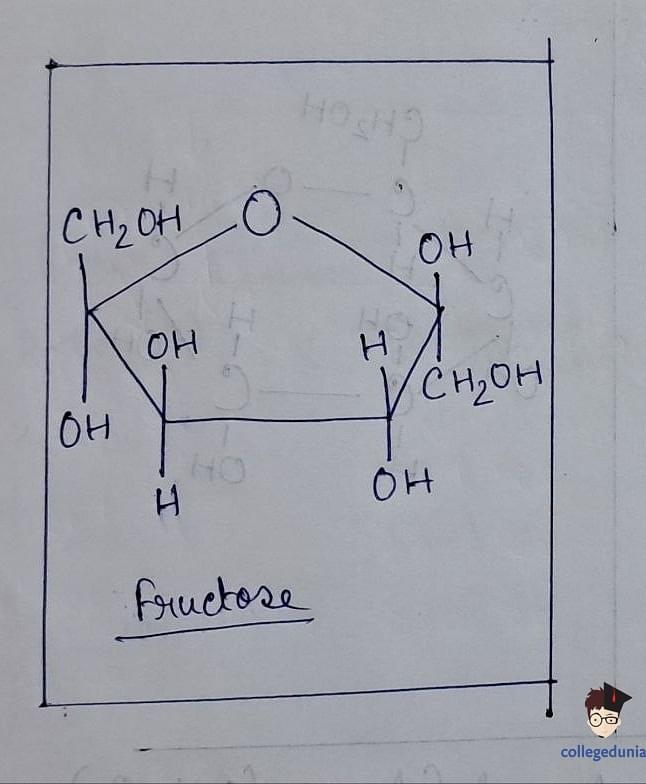
- With the molecular formula C 6 H 12 O 6 , fructose contains a ketonic functional group at carbon number 2 and belongs to the D-series, exhibiting laevorotatory properties.
- Fructose exists in both open-chain and cyclic forms, with the cyclic form forming a five-membered ring called furanose upon the addition of a hydroxyl group at C5.
Disaccharides
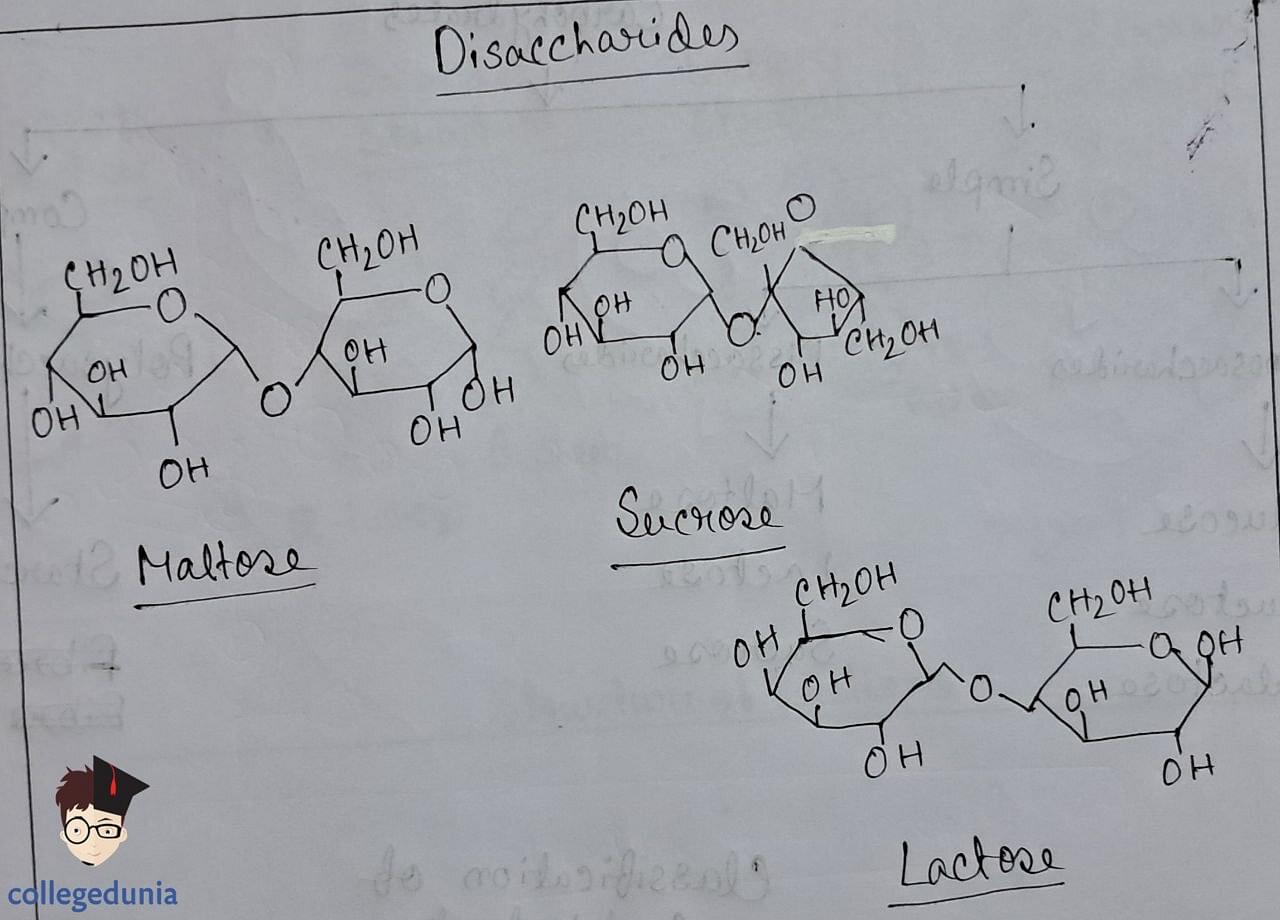
- Disaccharides form via glycosidic linkage between two monosaccharide units, resulting from the loss of water.
- Sucrose yields invert sugar upon hydrolysis and exhibits laevorotatory properties.
- Maltose comprises two glucose units linked by a glycosidic linkage between C1 and C4.
- Lactose, found in milk, consists of galactose and glucose units linked between C1 and C4, and is a reducing sugar .
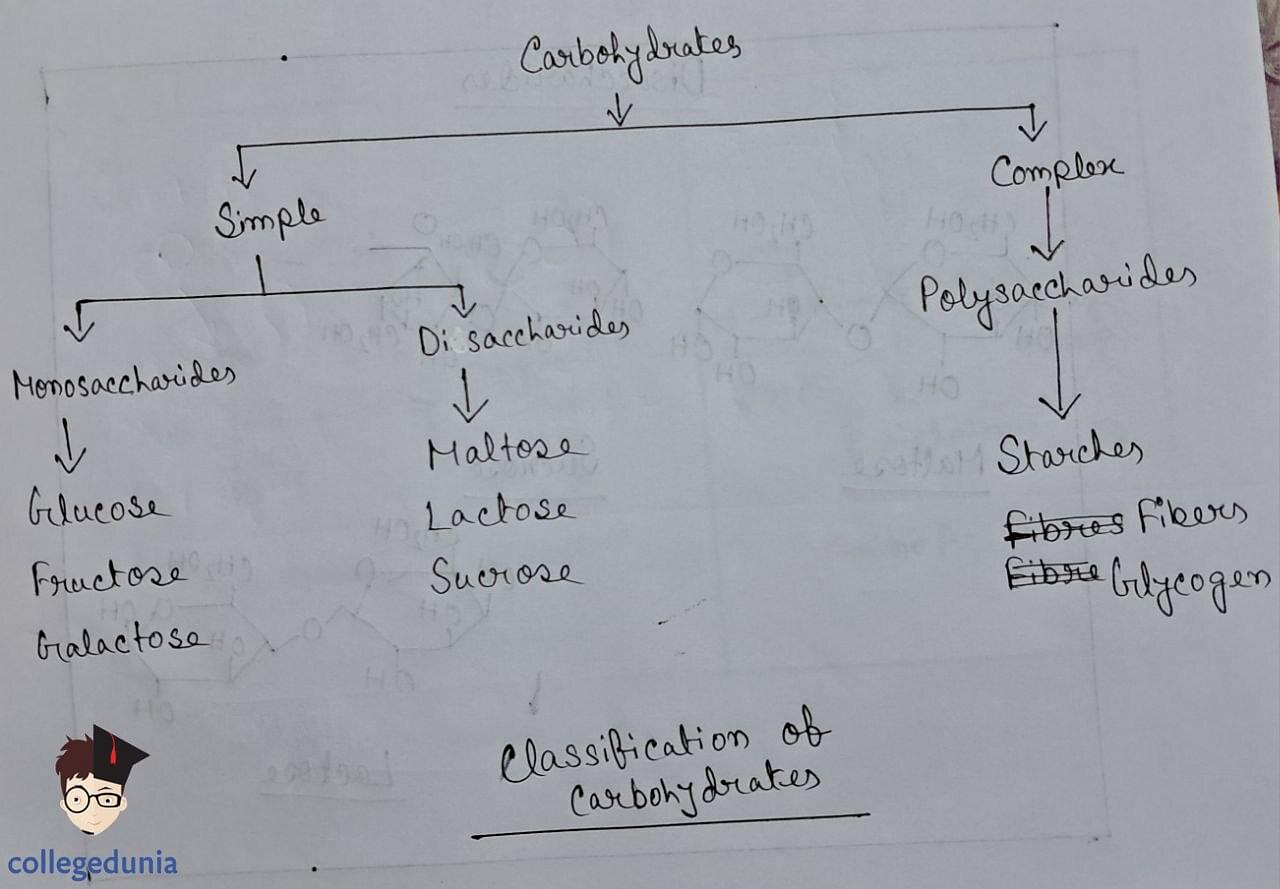
Polysaccharides
- Polysaccharides , composed of multiple monosaccharide units, function as vital food storage and structural materials in living organisms.
- Starch, a primary plant storage polysaccharide, contains amylose and amylopectin.
- Amylose is water-soluble and unbranched, while amylopectin is insoluble and branched.
- Cellulose , predominant in plant cell walls, comprises straight chains of β-D-glucose units linked by glycosidic bonds between C1 and C4.
- Glycogen , akin to animal starch, is stored in the liver, muscles, and brain, characterized by high branching and serving as a glucose reserve.
Importance of carbohydrates
- Carbohydrates provide a significant portion of the diet of plants and animals, serving as vital energy sources.
- Starch in plants and glycogen in animals function as storage forms of carbohydrates, offering easily accessible energy reserves.
- Cellulose constitutes the cell walls of bacteria and plants, with industrial applications including wood and cotton fiber products.
- Carbohydrates are crucial raw materials in industries like textiles, paper, lacquers, and breweries.
- Aldopentoses like D-ribose and 2-deoxy-D-ribose are key components of nucleic acids, underscoring their importance in biological systems.
- Proteins are abundant biomolecules in living organisms, vital for biological processes.
- Classification of proteins is done based on the shape, constituents and nature of molecules.
- Composed of α-amino acids, proteins are sourced from foods like milk, cheese, pulses, peanuts, fish, and meat.
- They contribute to growth, maintenance, and bodily functions.
Amino Acids
- Amino acids contain amino (–NH 2 ) and carboxyl (–COOH) functional groups.
- They are categorized as acidic, basic, or neutral depending on the relative number of amino and carboxyl groups in their molecule.
- Essential amino acids must be obtained through diet, while non-essential amino acids can be synthesized in the body.
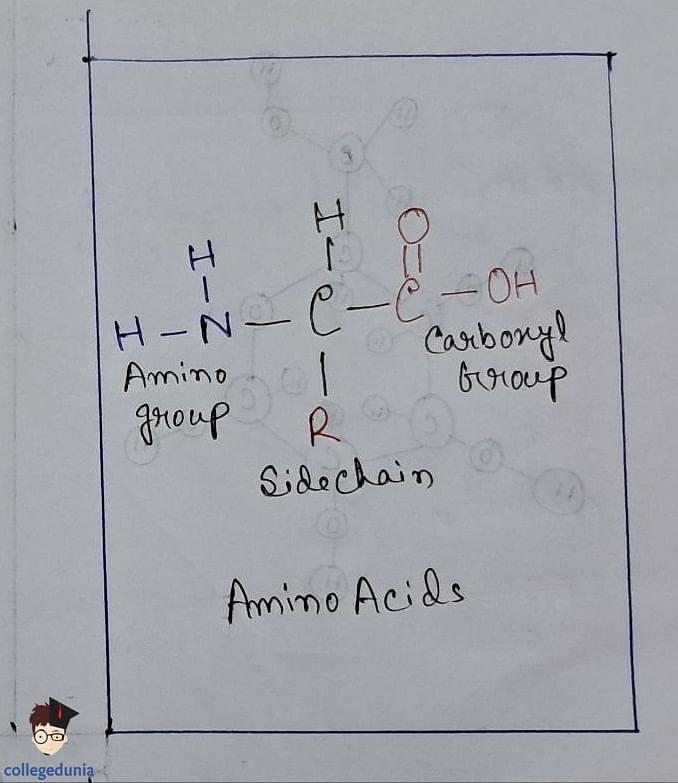
Structure of proteins
Proteins are composed of amino acids linked by peptide bonds , formed by the condensation of amino and carboxyl groups.
- Dipeptides, tripeptides, and polypeptides result from the combination of amino acids, with proteins containing more than a hundred amino acid residues.
- Proteins are classified into fibrous and globular types based on their molecular shape.
- Fibrous proteins have parallel polypeptide chains held by hydrogen and disulfide bonds, while globular proteins have coiled polypeptide chains, giving them a spherical shape.
- Primary (amino acid sequence)
- Secondary (a-helix, b-pleated sheet)
- Tertiary (overall folding)
- Quaternary (spatial arrangement of subunits).
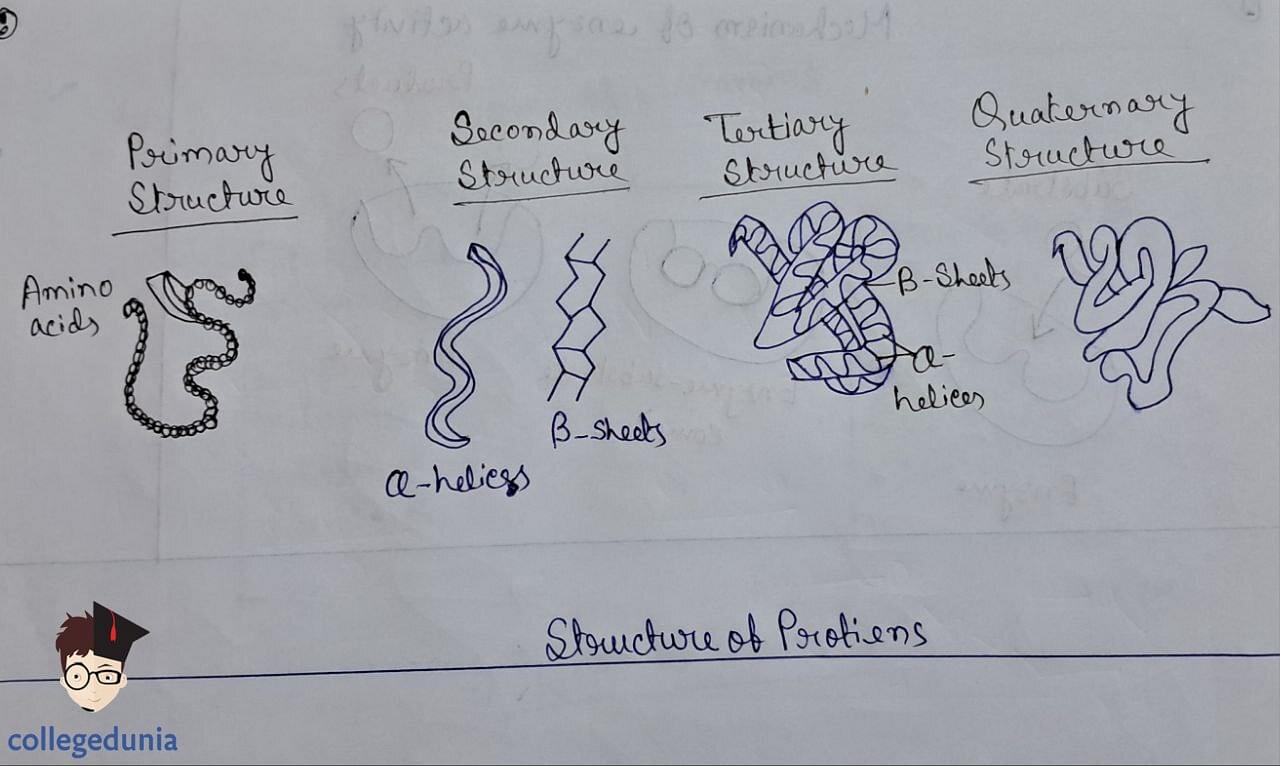
Structure of Proteins
Denaturation of Proteins
- Proteins in biological systems with unique three-dimensional structures and biological activity are termed native proteins.
- Denaturation occurs when native proteins undergo physical or chemical changes such as temperature fluctuations or pH alterations.
- It disrupts hydrogen bonds, causing globules to unfold and helices to uncoil, resulting in the loss of the protein's biological activity.
- During denaturation, secondary and tertiary protein structures are destroyed, while the primary structure remains intact.
- Common examples include the coagulation of egg white upon boiling and the curdling of milk due to lactic acid formation by bacteria.
- Enzymes facilitate chemical reactions vital for life, such as food digestion and energy production.
- It acts as biocatalysts and are mostly globular proteins, enabling reactions to occur under mild conditions in the body.
- The compounds are highly specific for particular reactions and substrates, often named after the compounds they act upon or the reactions they catalyze.
- It reduces the activation energy required for reactions, similar to chemical catalysts, allowing reactions to proceed efficiently.
- Enzymes typically end with "-ase" and may be named based on the reaction they catalyze or the substrate they act upon.
- For instance, maltase catalyzes the hydrolysis of maltose to glucose.

- Vitamins are essential organic compounds required in small amounts in the diet to maintain optimum growth and health.
- Most of these compounds cannot be synthesized in the human body but are abundant in various foods.
- Plants can synthesize most vitamins.
- They are classified into fat-soluble (A, D, E, K) and water-soluble (B complex, C) groups based on their solubility properties.
- While these compounds are essential, excess intake can be harmful.
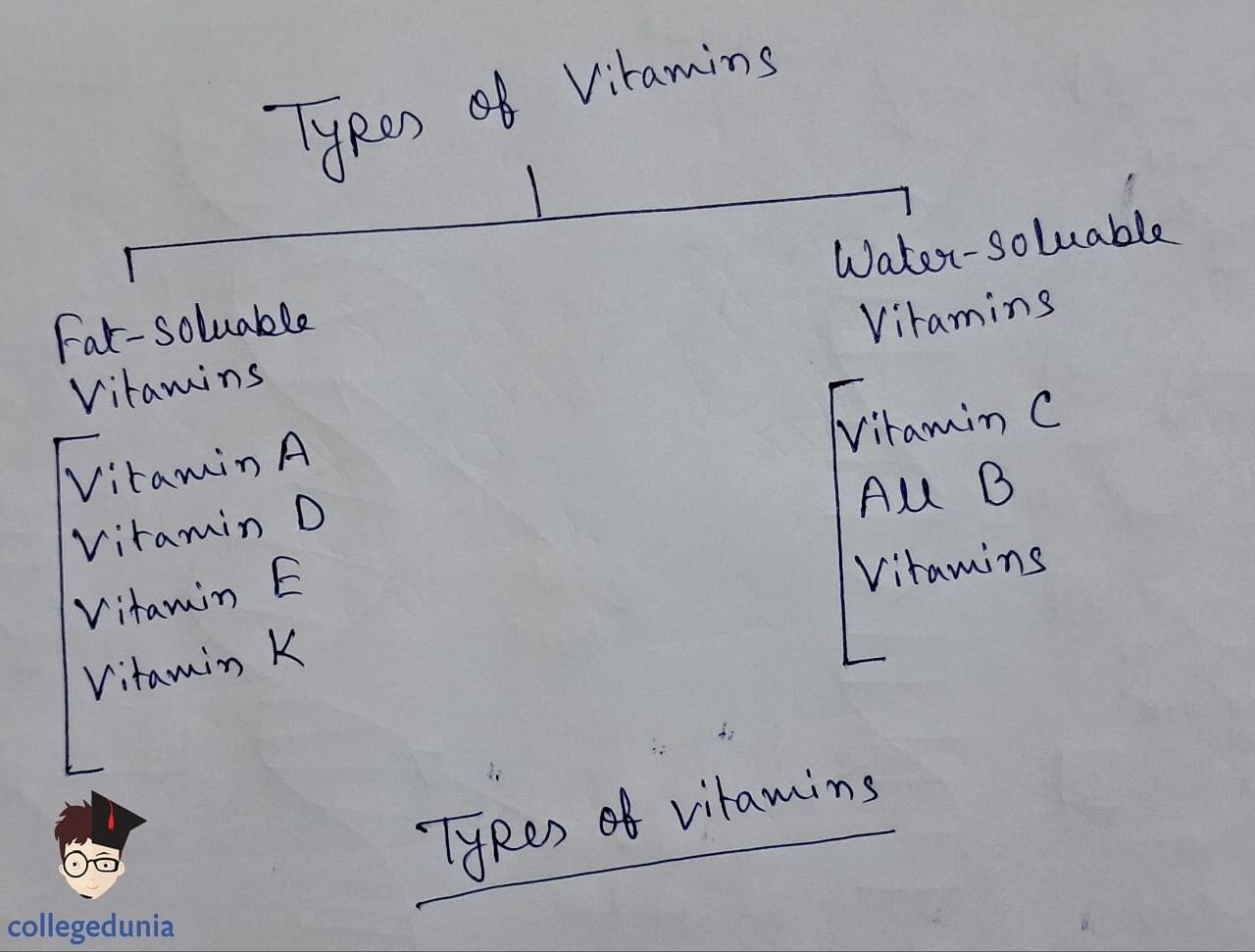
Types of vitamins
Nucleic acids
- Nucleic acids are polymers of nucleotides, comprising a-pentose sugar, phosphoric acid , and nitrogen-containing bases.
- In the nucleus of living cells, characteristics are transmitted via chromosomes , composed of proteins and nucleic acids.
- DNA and RNA are the two main types of nucleic acids.
- They are responsible for the transmission of characteristics from one generation to another.
- Nucleotides are joined by phosphodiester linkages between the 5' and 3' carbon atoms of the pentose sugar.
- DNA forms a double-stranded helix held together by hydrogen bonds between specific base pairs.
- RNA , categorized into messenger RNA (m-RNA), ribosomal RNA (r-RNA), and transfer RNA (t-RNA), plays distinct roles in protein synthesis.
- DNA serves as the repository of genetic information and is responsible for species identity maintenance.
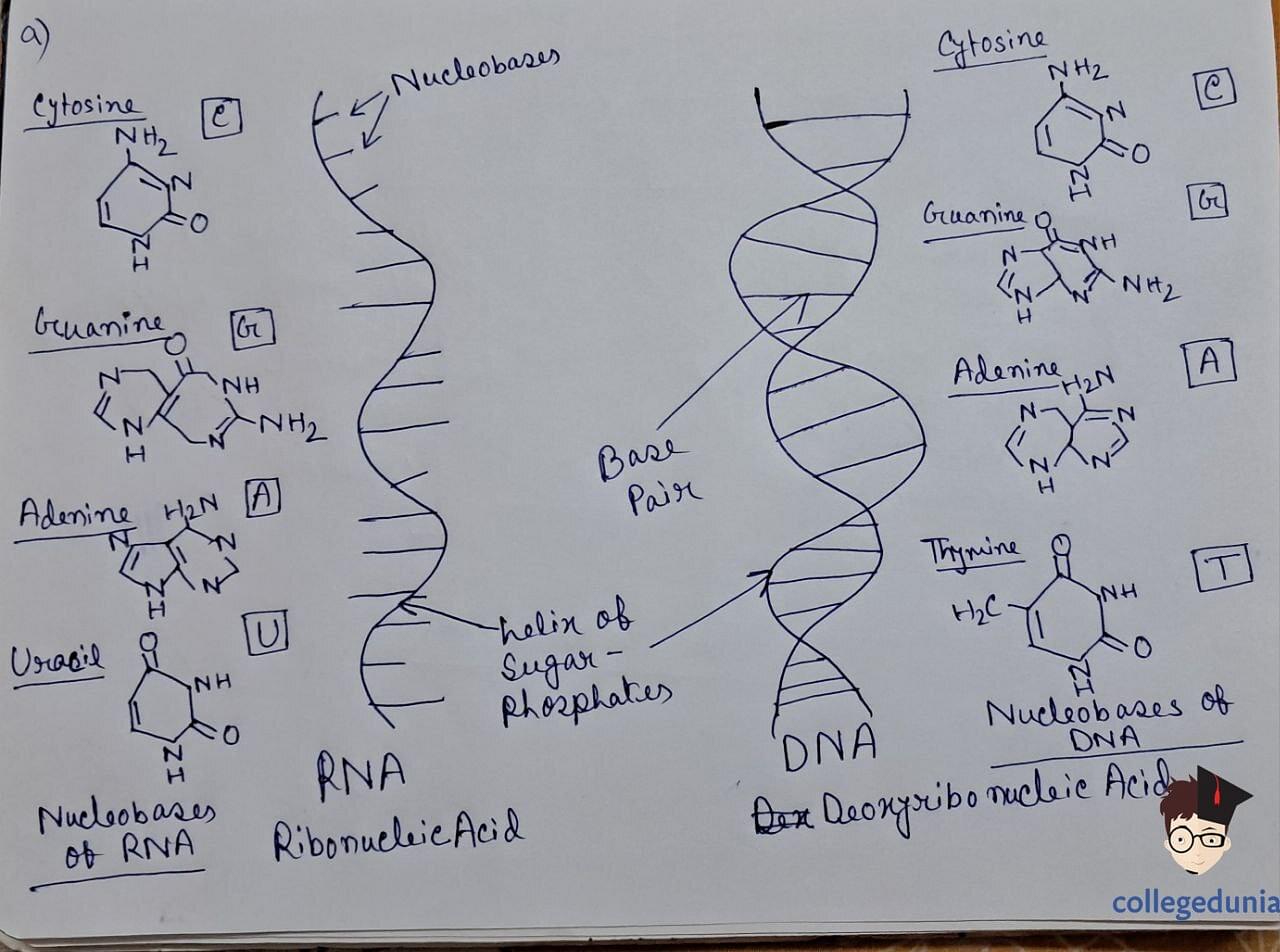
Nucleic Acid
- Hormones , produced by endocrine glands , act as intracellular messengers in the body.
- They include steroids (e.g., estrogens, androgens), polypeptides (e.g., insulin, endorphins), and amino acid derivatives (e.g., epinephrine, norepinephrine).
- Peptide and steroid hormones are two types of hormones.
- They maintain biological balance, such as insulin regulating blood glucose levels, and glucagon increasing glucose levels.
- Thyroxine, produced by the thyroid gland , regulates metabolism, with abnormal levels leading to conditions like hypothyroidism or hyperthyroidism.
- Steroid hormones from adrenal cortex and gonads (testes, ovaries) regulate various body functions, such as carbohydrate metabolism and secondary sex characteristics.
- An imbalance in hormone levels can lead to disorders like Addison's disease (adrenal cortex dysfunction) or menstrual irregularities.
There are Some important List Of Top Chemistry Questions On Biomolecules Asked In CBSE CLASS XII
CBSE CLASS XII Related Questions
1. in the button cells widely used in watches and other devices the following reaction takes place: zn(s) + ag 2 o(s) + h 2 o(l) \(\rightarrow\) zn 2+ (aq) + 2ag(s) + 2oh - (aq) determine \(\triangle _rg^\ominus\) and \(e^\ominus\) for the reaction., 2. name the oxometal anions of the first series of the transition metals in which the metal exhibits the oxidation state equal to its group number., 3. indicate the steps in the preparation of: k 2 cr 2 o 7 from chromite ore. kmno 4 from pyrolusite ore., 4. azeotropic mixture of water and hcl boils at 381.5 k. by distilling the mixture it is possible to obtain.
- Pure HCl only
- Pure water only
- Neither water nor HCl
- Both water and HCl in pure state
5. The rate constant for the decomposition of hydrocarbons is 2.418 x 10 -5 s -1 at 546 K. If the energy of activation is 179.9 kJ/mol, what will be the value of pre-exponential factor.
6. give the iupac names of the following compounds: (i)ch 3 ch(cl)ch(br)ch 3 (ii)chf 2 cbrclf (iii)clch 2 c≡cch 2 br (iv)(ccl3) 3 ccl (v)ch 3 c(p-clc 6 h 4 ) 2 ch(br)ch 3 (vi)(ch 3 ) 3 cch=cclc 6 h 4 i-p, similar chemistry concepts.
Exam Pattern
Paper Analysis
Physics Syllabus
Chemistry Syllabus
Mathematics Syllabus
Biology Syllabus
English Syllabus
Physical Education Syllabus
Computer Science Syllabus
Economics Syllabus
Business Studies Syllabus
Political Science Syllabus
Physics Practical
Chemistry Practical
History Syllabus
Accountancy Syllabus
Chapter Wise Weightage
Geography Syllabus
Biology Practical
SUBSCRIBE TO OUR NEWS LETTER

NCERT Solutions for Class 6, 7, 8, 9, 10, 11 and 12
Biomolecules Class 12 Notes Chemistry Chapter 14
January 9, 2024 by Sastry CBSE
Class 12 Chemistry Notes
Free resources.
NCERT Solutions
Quick Resources

IMAGES
VIDEO
COMMENTS
CBSE Class 12 Chemistry - Biomolecules Assignment - Free download as PDF File (.pdf), Text File (.txt) or read online for free. This document contains questions about biomolecules at different levels of difficulty. The level one questions cover basic information about monosaccharides, disaccharides, and polysaccharides. Example questions ask about the monosaccharide in milk (lactose ...
CBSE Class 12 Chemistry - Biomolecules Assignment - Free download as PDF File (.pdf), Text File (.txt) or read online for free. This document contains a chapter on biomolecules from a study guide, including three levels of questions. It begins with 15 multiple choice questions testing basic knowledge of carbohydrates such as lactose, glucose, and fructose.
Also working on Class 12 Chemistry Chapter 14 Biomolecules NCERT Solutions will be most helpful to the students to solve their Homeworks and Assignments on time. Students can also download NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules PDF to access them even in offline mode.
The NCERT Solutions for Class 12 Chemistry is developed by expert subject teachers according to the latest CBSE Syllabus 2023-24 and its guidelines. These solutions are written in simple language for ease of comprehension. Get the free PDF of NCERT Solutions for Class 12 Chemistry Chapter 14 from the link below.
Biomolecules Class 12 Notes Chapter 14. According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 10. Carbohydrates are classified into three groups viz monosaccharides, disaccharides, and polysaccharides. Glucose is obtained by the digestion of starch.
The organic molecules that are essential for the various metabolic processes such as digestion, cell repair, growth, etc are called biomolecules. The biomolecules, in a way, support all the life processes required for our survival. The 4 main types of biomolecules are proteins, lipids, carbohydrates, and nucleic acids.
NCERT Solutions for Class 12 Chemistry. NCERT Solutions for class-12 Chemistry Chapter 14 Biomolecules is prepared by our senior and renowned teachers of Physics Wallah primary focus while solving these questions of class-12 in NCERT textbook, also do read theory of this Chapter 14 Biomolecules while going before solving the NCERT questions.
The biomolecules class 12 chemistry important questions focus on key areas that are often emphasized in exams, helping students to solidify their understanding and prepare effectively. For a more visual approach to learning, the biomolecules class 12 mind map provides a quick and efficient way to review the entire chapter, making it easier for ...
NCERT Exemplar Class 12 Chemistry Chapter 14 Biomolecules. Multiple Choice Questions. Single Correct Answer Type. Question 1. Glycogen is a branched chain polymer of a-D-glucose units in which chain is formed by C1-C4 glycosidic linkage whereas branching occurs by the formation of C1-C6 glycosidic linkage. Structure of glycogen is similar to.
The assignment then covers various types of biomolecules including micromolecules like amino acids, sugars, lipids and nucleotides. It also discusses macromolecules including polysaccharides, nucleic acids and proteins. Each biomolecule type is defined in detail with examples and structures provided. The assignment was completed by Amarendra P ...
Ans 3. (a) Case Study based questions on Biomolecules. 3. Read the given passage and answer the questions that follow. Proteins are the most abundant biomolecules of the living system. The chief sources of proteins are milk, cheese, pulses, fish, meat, peanuts, etc. They are found in every part of the body and form a fundamental basis of the ...
Important Questions for Class 12 Chemistry Chapter 14 Biomolecules Class 12 Important Questions Biomolecules Class 12 Important Questions Very Short Answer Type Question 1. What is meant by 'reducing sugars'? (All India 2010) Answer: Reducing sugar contains aldehydic or ketonic group in the hemiacetal and hemiketal forms and can reduce Tollen's reagent or Fehlmg's solution. […]
Chemistry Worksheets Class 12 on Chapter 14 Biomolecules with Answers - Set 1. Biomolecules are the most essential organic molecules, which are involved in the maintenance and metabolic processes of living organisms. These non-living molecules are the actual foot-soldiers of the battle of sustenance of life. They range from small molecules such ...
14.1. Glucose or sucrose are soluble in water but cyclohexane and benzene (simple six membred ring compounds) are insoluble in water Explain. Ans: The .solubility of a solute in a given solvent follows the rule ' Like dissolves like'.Glucose contains five and sucrose contains eight -OH groups. These -OH groups form H-bonds with water.
CBSE Class 12 Chemistry Notes Chapter 14 Biomolecules. Jasmine Grover. Senior Content Specialist. Biomolecules are important organic molecules that fuse together to form complex organic compounds and govern the metabolism of living organisms. Proteins, carbohydrates, nucleic acids, enzymes and lipids form the living system of biomolecules.
The Selfstudys website provides the Biomolecules class 12 notes in a PDF so that students can easily access it. All students should have easy and free access to the class 12 Chemistry notes so that they don't need to search for here and there. The Biomolecules notes can be very helpful for those students who are struggling to manage their ...
12. Cellulose (C6H10O5)n is a linear polymer of β-D-glucose in which the β-D- glucose units are joined by β-D-glucosidic linkage. 13. Proteins are the most abundant biomolecules of the living system. They are required for growth and maintenance of body. All proteins are polymers of α-amino acids. 14.
283 Biomolecules H+ C H O H O C H O + C H O12 22 11 2+ → 6 12 6 6 12 6 Sucrose Glucose Fructose 2. From starch: Commercially glucose is obtained by hydrolysis of starch by boiling it with dilute H2SO4 at 393 K under pressure. H+ (C H O ) + nH O6 10 5 n 2 →393K; 2-3 atm nC H O6 12 6 Starch or cellulose Glucose
The document discusses various types of biomolecules including carbohydrates, proteins, nucleic acids, and their structures and functions. It provides detailed information about monomers like monosaccharides and how they combine to form polysaccharides. It also describes the primary, secondary, tertiary, and quaternary structure of proteins and discusses the double helix structure of DNA and ...