An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Dementia-friendly interventions to improve the care of people living with dementia admitted to hospitals: a realist review
Melanie handley, frances bunn, claire goodman.
- Author information
- Article notes
- Copyright and License information
Correspondence to Melanie Handley; [email protected]
Received 2016 Nov 20; Revised 2017 May 2; Accepted 2017 May 4; Collection date 2017.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
To identify features of programmes and approaches to make healthcare delivery in secondary healthcare settings more dementia-friendly, providing a context-relevant understanding of how interventions achieve outcomes for people living with dementia.
A realist review conducted in three phases: (1) stakeholder interviews and scoping of the literature to develop an initial programme theory for providing effective dementia care; (2) structured retrieval and extraction of evidence; and (3) analysis and synthesis to build and refine the programme theory.
Data sources
PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Library, NHS Evidence, Scopus and grey literature.
Eligibility criteria
Studies reporting interventions and approaches to make hospital environments more dementia-friendly. Studies not reporting patient outcomes or contributing to the programme theory were excluded.
Phase 1 combined findings from 15 stakeholder interviews and 22 publications to develop candidate programme theories. Phases 2 and 3 identified and synthesised evidence from 28 publications. Prominent context–mechanism–outcome configurations were identified to explain what supported dementia-friendly healthcare in acute settings. Staff capacity to understand the behaviours of people living with dementia as communication of an unmet need, combined with a recognition and valuing of their role in their care, prompted changes to care practices. Endorsement from senior management gave staff confidence and permission to adapt working practices to provide good dementia care. Key contextual factors were the availability of staff and an alignment of ward priorities to value person-centred care approaches. A preoccupation with risk generated responses that werelikely to restrict patient choice and increase their distress.
Conclusions
This review suggests that strategies such as dementia awareness training alone will not improve dementia care or outcomes for patients with dementia. Instead, how staff are supported to implement learning and resources by senior team members with dementia expertise is a key component for improving care practices and patient outcomes.
Trial registration number
CRD42015017562.
Keywords: People living with dementia, hospitals, dementia, realist review, dementia friendly
Strengths and limitations of this study.
Applying realist methods enabled a theory-driven explanation of how dementia-friendly healthcare can be supported in hospital settings.
The process of the review facilitated the development of a new programme theory, which can be used to inform future initiatives that support people living with dementia in hospital environments.
The involvement of stakeholders from the outset ensured the plausibility and relevance of the findings for hospital environments.
The extent of evidence to support some elements of the programme theory was limited, especially where interventions lacked specificity about process and patient outcomes.
Introduction
There is increasing recognition that hospital staff and services need to understand the complexity of caring for and treating people living with dementia. 1 At any one time, 25% of hospital beds are used by people living with dementia, rising to a higher proportion on some wards. 2 Comorbidities are common and many people are admitted to hospital for reasons not directly related to their dementia. 3–5 Healthcare outcomes for people living with dementia are variable across the country and are inequitable when compared with outcomes for people without cognitive impairments. 5 Adverse incidents occurring during admissions, such as falls, poor nutrition and hydration, infections, and the onset of delirium, contribute to longer stays and reduced functional abilities, which may result in admission to a care home. 6–8
A number of factors may impact on the disparity of health outcomes for people living with dementia, including a lack of focus and leadership for dementia in hospitals 5 ; healthcare staff who have inadequate knowledge and training in dementia and dementia care 9 10 ; difficulties faced by healthcare professionals when assessing the risk and benefits of treatment options 11 ; widespread use of care practices that are detrimental to people living with dementia, such as the use of antipsychotics for behavioural management 12 ; stigma and discrimination towards people living with dementia 13 14 ; and confusing, unsafe environments. 15 The National Dementia Strategy 16 aimed to improve the quality of care for people living with dementia in general hospitals through leadership that addresses quality improvements in dementia care, defined care pathways and the use of liaison mental health teams. It also highlighted the importance of education and training to break down the stigma associated with dementia and to develop dementia awareness within the healthcare workforce. To address these ambitions, interventions have been designed and implemented with the aim of creating dementia-friendly healthcare in hospitals. 17 18
Dementia-friendly
The concept of dementia-friendly developed from initiatives to promote age-friendly communities. 19 It was first used to describe physical and social environments that promoted inclusion, acceptance and accessibility for people living with dementia, 20 21 and includes initiatives supporting the independence and safety of people living with dementia. 22 In the UK, this includes the Dementia Friends initiative 23 and the Dementia Engagement and Empowerment Project. 24
At the patient level, dementia-friendly healthcare is the practice and organisation of care that is aware of the impact dementia has on a person’s ability to engage with services and manage their health. It promotes the inclusion of people living with dementia and their carer in treatments, care decisions and discussions, with the aim of improving outcomes for the patient and carer. 16 17 25–27
Interventions to promote dementia-friendly healthcare environments have been diverse in terms of their design and application in practice. 27–29 This review of the evidence acknowledges that the effectiveness of programmes to address the known problems of being a patient with dementia is contingent on multiple factors, such as staff knowledge and skills in dementia care, the care environment, and the competing demands on staff time and attention. The review objectives were the following:
to identify how dementia-friendly interventions in hospital settings are thought to achieve the desired patient and carer outcomes
to develop evidence-based explanations to understand what it is about dementia-friendly interventions in hospitals that works for people living with dementia and their carers, in what circumstances and why.
Realist methodology
Realist review is a theory-led method that applies the principles of realism to evidence review. 30 31 In realism, change is not directly achieved by an intervention, rather change is generated through the influence of intervention resources and contextual factors on human reasoning. A realist approach seeks to explain how the relationship between these elements (context and mechanism) leads to particular outcomes ( box 1 ). 30
Box 1. Glossary of realist terms.
Context: refers to factors, including but not limited to, personal, social, organisational or policy aspects that influence the way resources are engaged with to generate outcomes. For example, staff’s professional focus may influence how they use information about a person’s social, rather than medical, history, or an organisation’s expectations for dementia care may affect how staff prioritise their work with patients with dementia.
Mechanism: includes the resource the intervention provides (such as training, assessments of pain or access to biographical information about the patient) and the reasoning of the subjects, in this case the reasoning of staff (such as recognising the benefit of working differently). 32
Demi-regularity: a semipredictable pattern of outcomes. For example, the provision of meaningful activities for patients with dementia will reduce their boredom and distress in hospital, leading to a reduction in the onset of behaviours that are challenging for staff.
Outcome: the intended (or unintended) result. Patient outcomes of interest included patient well-being, medication use (specifically analgesic and antipsychotic), access to assessments, evidence of inclusion in care decisions, reduced distress, adverse incidents (such as falls or hospital-acquired infection), length of stay, reduction in the onset of behaviours that challenge, and maintenance of functions (such as activities of daily living).
Context–mechanism–outcome configuration: specifies the relationship between the features (context, mechanism and outcome). It is the unit of analysis that supports synthesis across studies to build and refine the programme theory.
Realist review was appropriate for this study as the evidence base for dementia-friendly interventions is in its early stages. As such, theory building derives from a variety of sources and study types. Complexity is inherent in both design and implementation of the interventions: they are multicomponent and rely on human agency that is influenced by individual, service and organisational pressures. Realist enquiry acknowledges these features and incorporates them to develop an explanatory account of how different aspects influence reasoning and outcomes. 33
Realist review methods were used to develop a theoretical understanding of what supports effective dementia care in hospital settings. There were three overlapping, iterative phases: (1) defining the scope of the review informed from key literature and stakeholder interviews; (2) structured searches, screening and data extraction; and (3) analysis and synthesis leading to refinement of the programme theory. A fuller account of the review protocol is available in Handley et al . 34
The phases did not follow a linear format, but informed and refined understanding throughout the review, leading to new interpretations and building of evidence. Sources were identified and revisited, new evidence was incorporated, and inclusion criteria were reconsidered as new theoretical understanding developed. The RAMESES (Realist and Meta-narrative Evidence Syntheses: Evolving standards) publication standards informed the preparation of this report and has been vetted against RAMESES criteria (see online supplementary files 1 and 2 ).
Changes to the review process
One change was made to the review process subsequent to the published review protocol. 34 The expert steering group workshop was not held. However, emerging findings and the refined programme theory were shared with the with Alzheimer’s Society research network monitors (RP, JW, PM) who were volunteer representatives with experience of caring for family members living with dementia. They commented on the resonance and relevance of the inferences that contributed to the developing theory throughout the review process. Review findings were presented and discussed at a seminar on dementia-friendly healthcare with 75 participants, 19 of whom worked in hospitals. The findings are being taken forward for testing in a realist evaluation.
Phases of the realist review
Phase 1: defining the scope of the review — concept mining and theory development.
Evidence from interviews with stakeholders and a scoping of the literature were used to (1) identify the range of dementia-friendly interventions in healthcare settings both in the UK and internationally, (2) determine possible theoretical assumptions about how and why interventions were thought to work (or not), and (3) clarify what were understood to be the significant mechanisms for change. Stakeholders, defined as people with experience in designing, implementing and using dementia-friendly interventions, were identified from knowledge within the team, internet searches and dementia-specific conference abstracts. They were purposively sampled from a range of settings (academia, healthcare, commissioning, social work, the community) and backgrounds (nursing, education, physiotherapy, research, person living with dementia). 34 Stakeholders were not further involved in the development of the emerging context–mechanism–outcome configurations (CMOCs) or building the programme theory. Ethical approval for the interviews was secured from the University of Hertfordshire Ethics Committee (HSK/PG/UH/00339).
Data from interviews and the literature were coded using framework analysis, 35 with emerging themes and competing accounts discussed and debated among the authors (MH, FB, CG) and with the Alzheimer’s Society research network monitors (RP, JW, PM). Mapping this evidence demonstrated limited understanding at the point of staff interaction with patients and how this influenced patient outcomes. A decision was made to focus the review on how interventions led to patient outcomes. Data from the interviews and literature were scrutinised for demi-regularities (see box 1 , glossary of realist terms) and informed hypotheses set out in the form of ‘If… then statements’. These statements were used to define the conditions thought to be necessary to achieve (1) staff outcomes, such as taking action to investigate the cause of patient behaviours and applying best practice with people living with dementia; and (2) patient outcomes, such as reduced distress, reduction in adverse incidents and improved well-being. Discussions among the authors based on these statements led to the development of a conceptual framework. 30 Three overlapping theoretical propositions were generated to explain what supports the implementation and uptake of interventions that promote dementia-friendly healthcare within a ward based environment.
Phase 2: retrieval and review
Searching for relevant studies.
Informed by the theoretical propositions derived from the work in phase 1, search terms were revised. The inclusion/exclusion criteria were refined to focus on studies that reported patient outcomes and provided information about the characteristics and role of change agents (staff who supported the implementation and uptake of interventions).
Searches were limited to 2000–2016 to reflect the impact of the work of Kitwood and Bredin 36 on dementia care practices that recognise the importance of person-centred care and the promotion of personhood. In addition to the electronic database searches ( box 2 ), we undertook extensive lateral searching, including forward and backward citations, and contact with experts. Additional searches were performed as emerging themes around the management of pain and behaviours that challenge became apparent. These were purposive searches that applied the same inclusion criteria. Theory development continued until theoretical saturation was achieved 37 38 ( box 2 ).
Box 2 Phase 2 search terms and search strategy.
Searches initially run September 2015, search alerts scanned to February 2016, language restricted to English, date restricted 2000–2016
Search terms:
(dementia AND (friendly OR appropriate OR awareness OR champion OR liaison OR ward OR environment OR education OR training OR nurse specialist OR lead* OR person centred care) AND (hospital OR acute care OR secondary care))

Additional search terms developed from work in phase 1:
dementia AND (change agent OR champion OR knowledge transfer OR knowledge translation OR opinion leader)
Additional search terms reflecting emerging themes in phase 2:
Searches ran January 2016, search alerts scanned to February 2016(dementia AND (pain) AND (hospital OR acute care OR secondary care))(dementia AND (behaviour* OR BPSD) AND (hospital OR acute care OR secondary care))
Cochrane Library (including CENTRAL, CDSR, DARE, HTA) (244), CINAHL (610), PubMed (4253), NHS Evidence (819) and Scopus (410)
Study screening and data extraction
Search results were downloaded into EndNote bibliographical software and duplicates were deleted. One author (MH) screened the titles and abstracts identified by the electronic search and applied the selection criteria to potentially relevant papers. Full texts of potentially relevant manuscripts were screened for relevance (whether the study has contributed to specific propositions relevant to the theory building) and rigour (whether they were of sufficient quality to provide credible evidence to help refine specific components of the proposition). 30 31 Appraisal of the contributions and reliability of evidence from papers continued throughout the synthesis through discussion with the other authors.
Data were extracted by one author (MH) using a bespoke data extraction form organised to establish contributions and challenges to the theories, and strengths and weaknesses of the studies. Study characteristics such as design, setting, participants and sample size were also recorded. 31 The data extraction form was piloted by MH and shared with the team for comment (see online supplementary file 3 ). To reduce the potential for bias during data extraction, a sample of the papers and their completed data extraction forms (6/28) were shared with FB and CG to appraise the extraction process and identified data. Information about the role and work of the change agent, the resources provided by the interventions, the contextual features of the settings (eg, workforce, knowledge of dementia), explicit and implicit theories for how interventions were anticipated to work, and patient and carer outcomes were extracted. Coded data from all the papers and their contribution to theory development were shared with FB and CG. Challenges to interpretations were discussed to test credibility. Evidence from the studies was first mapped to capture the complete range of possibilities of how different approaches and resources triggered different responses from patients, family and staff. After discussion among the authors, data were organised into tables to reflect the theoretical propositions they addressed (see online supplementary file 4 ) and to assist comparison of data across studies.
bmjopen-2016-015257supp003.pdf (374.2KB, pdf)
bmjopen-2016-015257supp004.pdf (247.3KB, pdf)
Phase 3: analysis and synthesis
Data synthesis was led by MH and emerging findings were discussed with the team (CG and FB) and the research network monitors (RP, JW, PM). Deliberations assisted the refinement of propositions, ensuring that emerging theories were plausible and clear. Discussions of papers included the key characteristics of members of staff who support the implementation and uptake of interventions, resources, and new ways of working with people living with dementia (change agents); resources from interventions and how they were thought to influence staff reasoning; the impact of context; and possible undesired outcomes (such as stigmatising practices and broad application of strategies at the expense of individual needs). The focus was on understanding how patient outcomes were achieved through the actions of staff and what had supported the staff to behave in particular ways. Recurring patterns in context and outcome (demi-regularities) detectable across studies were explained by explicit or implicit mechanisms. This led to the development of CMOCs designed to explain what it is about an intervention that works, for whom and in what circumstances. The configurations were used to refine components of the initial theoretical propositions against the evidence.
Evidence from 15 stakeholders was combined with literature on interventions aimed at improving healthcare for people living with dementia (22 papers) to generate three initial propositions for developing dementia-friendly hospital environments. Interventions described in the literature can be seen in table 1 .
Papers included in phase 1
A key contextual factor to emerge from phase 1 related to the role of change agents, although there were competing accounts of how a change agent might work and the responses they might trigger in staff. There appeared to be three distinct roles for change agents’ activities that could lead to improved outcomes, and these were the following:
to support staff awareness and learning
to possess the authority to institute and sustain changes
to be a resource for staff as a clinical expert.
Change agent characteristics (eg, supportive peer facilitator, organisational authority, clinical expertise) were considered to differently influence how staff engaged with interventions, and this in turn would impact on patient outcomes ( table 2 ).
Initial theoretical propositions developed from phase 1
Evidence from 28 papers, 12 of which were identified and included in phase 1 of the review (see online supplementary file 5 ), led to the development of six CMOCs that explored the components of the three theoretical propositions developed in phase 1 (an overview of the selection process can be seen in figure 1 ). These configurations are interconnected, representing key elements from the theories and how they relate to other factors ( table 3 ). The CMOCs and supporting evidence are discussed below. Illustrative examples of evidence from the literature that guided CMOC development are supplied in online supplementary file 6 .
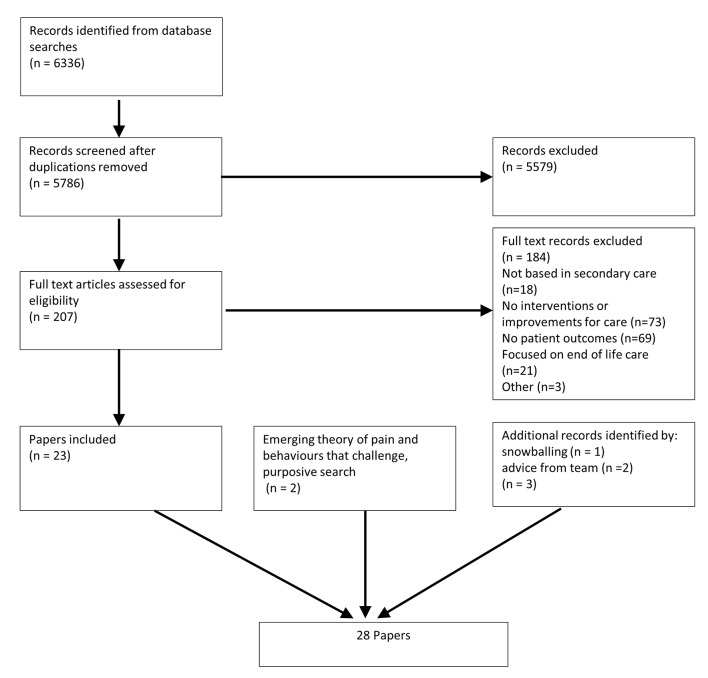
Flow diagram of searches and evidence retrieval.
Context–mechanism–outcome configurations and supporting evidence
bmjopen-2016-015257supp001.pdf (754.5KB, pdf)
CMOC 1: understanding behaviour as communication to improve staff’s ability to respond
Studies frequently reported that where staff understood behaviour that challenged as communication of an unmet need, they were more likely to investigate the underlying cause rather than attempting to control and restrict the behaviour. 15 39–44 By addressing the unmet need, staff reduced patient distress 44–50 and maintained independence, for example by supporting mobility and toileting needs. 51–54 Inappropriate and negative staff responses arose from lack of understanding and misinterpretation of behaviours that challenge, for example, interpreting the patient as being deliberately difficult. 55 56
Strategies employed to reframe staff understanding of behaviours included training in dementia 10 15 46–48 ; the use of biographical tools, completed in partnership with informal carers 39 41 44 51 57 ; assessments of cognition, pain and psychological needs 42 45 52 58 ; and access to experts in dementia care. 39 40 44 45 52 59 Common to these interventions was that they supported staff to consider potential causes of behaviours and provided strategies to address the unmet need, such as the development of individualised care plans 57 59 and personalised strategies for reducing distress. 44 51 Training to recognise behaviours as the expression of an unmet need 47 60 and knowledge of a patient gained through continuity in their care 46 48 60 helped staff become aware that particular care practices were unsuitable and to adapt their work in a way that benefited the individual. However, personalisation of practices appeared to occur in pockets of activity rather than as an ethos of care provision. Even when staff understood behaviours that challenged as communication of an unmet need and were supported to work well with people living with dementia, staffs' ability and willingness to address psychological needs was limited. Conflicting work demands, staff fatigue, long shifts and difficulty in identifying and resolving patient issues resulted in staff responding to behaviours by ignoring and disengaging from the patient. 45 50
CMOC 2: the role of experiential learning and creating empathy to encourage reflection for responsibilities of care
Staff training that improved awareness of the impact of dementia and that addressed negative concepts was found to be a prerequisite for supporting good dementia care. While the literature suggested training had a positive impact on knowledge and confidence for working with people living with dementia, more work is needed to understand how this works in practice. 10 39 47 51
Training strategies that employed experiential learning techniques and cultivated empathy in staff for people living with dementia prompted reflection on current practices. Evidence suggested these training sessions produced ‘lightbulb moments’ for staff where they gained a sudden realisation of the problems faced by people living with dementia. 39 47 53 This appreciation for the importance to adapt care practices prompted staff to work in ways that would better support the patient, and improved staff satisfaction with their work. 51 61
Furthermore, one study reported how staff associated the portrayals of people living with dementia in training materials to their own relatives. This encouraged staff to see people living with dementia as individuals and motivated them to take responsibility to put their learning into practice. 47
The use of reflection and examples of good care practices in recognisable situations gave staff a framework for working well with people living with dementia and demonstrated the benefit to their own work. 47 53 However, these practices were often referred to by staff as ‘going the extra mile’ or being additional to their workload rather than being an expectation of their role. Staff needed to be confident additional time spent with patients would not be viewed negatively by colleagues or impact on the requirements to manage the ward effectively, to support adaptations to care practices. 46 47
CMOC 3: clinical experts who legitimise priorities for care
Change agents influenced staff working practices through clinical expertise and organisational authority. 39 40 44 45 48 49 52 59 62 Experts in dementia care supported staff in the use of assessment tools and person-centred care planning, 52 62 role-modelled appropriate behaviour and communication for working with people living with dementia, 39 45 and provided professional advice for complex situations, such as decisions around best interests. 40 59 Access to experts in dementia care was suggested to reassure and encourage staff to provide good care for people living with dementia. Endorsement of these practices was communicated by clinical experts with organisational authority at the ward level 46–48 52 63 and across the organisation. 41 47 49 They addressed staff apprehensions to adaptations to care practices that previously prioritised medical and physical needs, ward routines, task-focused ways of working, and organisational expectations for the completion of documentation and risk reduction. 41 48 49 52 63 Our review found that when change agents in authority communicated new expectations for standards of care and changes to procedures, they validated the priorities for care and legitimised staff’s adaptation of care practices accordingly. 41 47 52 63 However, the impact of changes to staff’s work needed to be recognised and supported. 41 44 45 48 52 54 63 For example, studies reported there was reduced capacity to work with previous levels of patient allocation, 41 48 54 and changes to risk management strategies, such as encouraging mobility in a frail patient population at risk of falls, required staff training. 52 63
There was limited evidence that new practices were adopted by staff and embedded into everyday practice directly through their contact with dementia experts. Instead, it appeared that the experts maintained responsibility for dementia care, either personally or by providing direction. The use of experts alone could potentially concentrate responsibility for dementia care in a small staff group rather than create a culture where all staff are responsible. Evidence from one paper 45 suggested that even when ward staff as a whole were better able to work with people living with dementia, they would defer issues unrelated to physical or medical healthcare to dementia experts.
CMOC 4: staff with confidence to adapt working practices and routines to individualise care
The ability of staff to organise their work around the needs of people living with dementia rather than being restricted to the ward routine was linked to the provision of person-centred care. 45 46 48 50 54 60 Where staff could incorporate getting to know the person, or recognise and respond directly to expressions of distress and unmet needs, patient well-being reportedly improved, evidenced through observations of positive mood. 46 48 50 60 Clarity in staff’s responsibility for patient care was an important resource for improving their autonomy and encouraging them to respond in timely, creative ways to meet individual needs. 46 48 60
Flexibility in working practices was suggested to be a factor in improving functional outcomes for people living with dementia. One study 54 attributed gains in mobility after hip surgery to therapy staff using their professional judgement to recognise optimal times that a person living with dementia would engage with a physiotherapy session, rather than risk the session being rejected. Additional factors that supported therapy staff to work flexibly included training in dementia care, reduced patient lists and treatment rooms located on the ward. 64
CMOC 5: staff with responsibility to focus on psychosocial needs
Time constraints and staffing resources limited staff capacity to provide good dementia care. This was often addressed by employing staff with a specific role prioritising psychological, emotional and social needs through the use of cognitive and psychosocial assessments, therapeutic activities, supervising mealtimes and managing risk. 10 45 48 50 52 55 56 The use of these staff and the activities they provided improved patient experience, 48 assisted orientation to time and place, 50 reduced distress 45 48 50 and reduced the onset of behaviours that challenged staff. 45 Studies reported how activities were sometimes deliberately scheduled to cover known times of high need within the patient population, such as during the afternoon when ‘sun-downing’ might occur 52 or when staffing levels were stretched, such as during mealtimes. For example, activities coordinators offered social dining opportunities where they could support conversations and prompt patients to eat. 44 45 48 50 Although studies reported improved nutritional intake, this was not formally evaluated.
Patients with more severe physical illness or cognitive impairment may not be able to participate in activities, 45 55 although it is possible they may have benefited indirectly as healthcare staff had more time to address their physical and medical needs. While this was referred to in two of the interviews, this was not explored in any of the papers.
Ward-wide staffing levels and skill mix impacted on staff ability to prioritise emotional, psychological and social needs. 45 48 At times of staff shortages, ward management prioritised safety and managing risk over other non-medical needs. 45 48 Risk management techniques, such as the use of ‘specials’, could be applied in a way that also addressed psychosocial needs. Two studies 45 48 described how staff allocated to monitor patients at risk of falls engaged the patients in games, activities and conversations. However, this was not always the case as staff assigned as ‘specials’ were often junior team members, had not received training in dementia care and were unclear of the purpose of the role beyond monitoring the patient. This resulted in a lack of interaction with the patient and increased patient distress. 56
CMOC 6: building staff confidence to provide person-centred risk management
We found evidence that addressing risk in a way that supported a person’s abilities, choices and independence improved mobility, 52 53 reduced adverse incidents 44 and improved patient and carer satisfaction. 41 45 57 Training, for example, on new skills and procedures for managing risk from change agents with clinical expertise and organisational authority ensured staff understood the benefits to patients and had confidence to implement approved working practices. 52 57 63 Structural factors influenced the way risk was addressed. For example wards with locked door access meant patients could be monitored from a distance without restricting their movement around the ward, 41 43 45 52 and could help staff to perceive 'wandering' behaviours as positive rather than challenging.
In open wards, alternative methods were developed to easily identify patients considered at risk of leaving the ward, such as the use of wrist bands and different coloured hospital clothing, allowing staff to monitor them from a distance and intervene as necessary. 10 47 53 Identification methods were supported by staff training t the appropriate way to encourage patients to return to their ward. 10 53
Refined programme theory
From data in phase 1 we hypothesised that the existence of a change agent was important for improving hospital care for people living with dementia. However, work in phase 2 suggested that a reliance on single initiatives, such as a change agent, was insufficient to change staff behaviour. Additional contextual factors were also necessary in order for staff to make use of the resources interventions provided and use them in their practice with people living with dementia. The six CMOCs have been incorporated into a refined programme theory to suggest what needs to be in place to encourage best practice for dementia care in hospitals ( figure 2 ). Figure 2 presents the programme theory. The preliminary CMOC suggests that resources that promote dementia awareness and an understanding of what constitutes ‘good’ dementia care are often initially implemented in situations where staff have limited understanding of how to provide care that addresses the needs of people living with dementia. These resources support staff to recognise the benefit of working well with patients with dementia and provide them with a common understanding of what good care looks like. This preliminary outcome then becomes part of the new context. Contextual factors, such as organisational endorsement of dementia care practices and clarity in staff responsibilities to patients with dementia, encourage staff to value resources, reinforcing improvements to care provision. It is anticipated that this will lead to improved patient outcomes, although evidence on outcomes was limited.
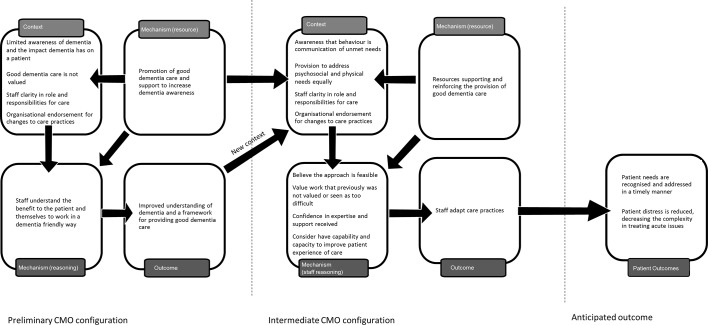
Refined programme theory: context–mechanism–outcome configuration (CMOC) for best practice for care of people living with dementia admitted to the hospital.
Our review demonstrates how consideration of different contextual components in hospitals, hospital staff and patients was fundamental to how the resources of an intervention might influence staff reasoning to adopt good dementia care practices. These changes in care practices may then lead to improved healthcare outcomes for people living with dementia. Developing an understanding in staff of the difficulties dementia presents for people with the condition helped them to recognise the need to approach care differently. Previous reviews of dementia care in hospital settings have identified training as an important strategy to improve staff knowledge of dementia and confidence to work well with people living with dementia, but have provided limited evidence for how this affects patient outcomes. 29 65 66 Findings from this review would suggest that training as a single strategy is not enough to influence staff to adapt the care they provide for people living with dementia. The culture of care within an organisation needs to support staff to provide good care for people living with dementia, legitimising practices so they are valued by staff. This means organisations need to recognise the impact this has on staff workload and roles and the changes that are necessary to ensure care provision can be adaptive to the needs of the patient. Staff needed to have a clear understanding of the expectation for care standards, and be confident that these changes are accepted by colleagues and senior staff if they are to improve the way care is provided for people living with dementia. Managerial endorsement for staff to work flexibly within their role, using practices and resources that enable them to get to know the person, will help staff to recognise and address signs of distress and implement best practice in dementia care.
Turner et al 65 suggest that to achieve the type of culture where person-centred care is valued, training in dementia should be aimed at a managerial level. Findings from this review would support their opinion; in the included studies, change agents in senior positions, who understood dementia and the associated impact on patient experience and care of the patient, were reportedly able to positively influence the culture of care. 41 44 46 47 49 52 61 They communicated their vision for good dementia care, addressed processes within and between departments, provided resources that supported staff’s work and considered the impact of changes to roles and responsibilities. However, even with this endorsement, there were still times, such as concerns for managing risk and resource shortages, where staff responsibilities were reorganised to prioritise physical over psychological well-being.
Limited time and resources and a preoccupation with managing risk are commonly cited factors that impacted on the ability of staff and organisations to sustain dementia-friendly hospital environments. 29 56 65 67 68 Employing staff who have a responsibility for the psychosocial needs of the patient can potentially improve patient experience of care while also making time available for nursing and medical staff to focus on the physical and medical care needs of the patient. However, it is essential that contextual factors, such as staff awareness in dementia and dementia care, and staff clarification of their role and responsibilities, are addressed before staffing resources are implemented into the setting. Moyle et al 56 demonstrated how the use of ‘specials’ without training in dementia care, a clear understanding of their role and a prioritisation of risk management over addressing psychosocial needs resulted in poor outcomes for patients, such as increased agitation and reduced autonomy. A review on special observation 69 underlined the importance of clarity in the purpose of the role and adequately trained staff to optimise the role’s therapeutic potential. Where responsibilities for care are assigned solely by the patient’s symptoms, this can lead to a narrow reactive approach to dementia care. Staff will still need to work as a team, rather than creating new tasks to focus on.
The initial aim of the review was to develop, test and refine a programme theory for how dementia-friendly interventions influence outcomes for people living with dementia during hospital admissions. However, testing the theory was problematic; evidence was limited, much was descriptive, and there were few evaluations of interventions and approaches, and limited descriptions of setting and component parts of the interventions which impacted on the development of CMOC. Moreover, most studies included in the review reported little information around patient characteristics (eg, type and severity of dementia), which meant we were unable to establish how the characteristics of people living with dementia interacted with the components of the interventions to influence outcomes. With these considerations, it is recognised that the proposed CMOCs were constrained by the evidence that was available and the inferences that could be made from the data; further development is needed.
Available evidence clustered around the training for staff and organisational support for changes to care practices. There was less evidence on how the introduction of staff providing activity and therapy for people living with dementia impacted on the practices of other staff. This review does, however, provide a programme theory that can be used as the basis for future evaluations. Our review also highlights the importance of focusing on patient-related outcomes. It was clear from the initial interviews that while there was a shared understanding of the importance of dementia-friendly care, less attention has been paid to how different approaches enhanced patient outcomes. By focusing on outcomes as the basis for inclusion, this review addresses a knowledge gap about how different resources and approaches for dementia-friendly healthcare are effective for patients.
The programme theory that has emerged from this review has the potential to improve how interventions to support dementia-friendly care in hospitals are designed and evaluated. The review highlights what needs to be in place to maximise the impact of training and the key characteristics for staff acting as change agents to influence colleagues to practise good dementia care. Specifically, the elements of interventions need to be relevant to provide ward staff with the awareness, authority and resources to provide personalised care with support from staff with the relevant expertise. Educational interventions should focus on how staff can identify with the experience of being a patient living with dementia, combined with opportunities for staff to share their experiences of addressing behaviours they find challenging and accommodating person-centred practices within ward routines and priorities. This review provides a timely contribution and challenges the assumption that dementia awareness initiatives in acute care settings alone are sufficient to improve patient care.
bmjopen-2016-015257supp002.pdf (191KB, pdf)
bmjopen-2016-015257supp005.pdf (153.9KB, pdf)
bmjopen-2016-015257supp006.pdf (224.2KB, pdf)
Supplementary Material
Acknowledgments.
We would like to thank the stakeholders who gave their time to participate in the interviews; Paul Millac, Rosemary Phillips and Jackie Whitting, research network monitors for Alzheimer’s Society, who provided opinion and competing interpretations of emerging themes; and Diane Munday and Marion Cowe, Public Involvement in Research Group (PIRg), University of Hertfordshire, who contributed to the design of the funding application and review.
Contributors: MH led the design and prepared the review as part of her PhD (University of Hertfordshire, Hertfordshire, UK) and led the manuscript preparation. FB and CG wrote the original funding application, supervised the review development and critically reviewed manuscript drafts. All authors contributed to the debate and interpretation of data, read and approved the final manuscript.
Competing interests: None declared.
Ethics approval: Ethical approval for the interviews was secured from the University of Hertfordshire Ethics Committee (HSK/PG/UH/00339).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All evidence cited in this review is available in the public domain. Data from the stakeholder interviews are not available to protect individuals’ anonymity.
- 1. Society Alzheimer's. Fix Dementia Care: hospitals. London: Alzheimer's Society, 2016. [ Google Scholar ]
- 2. Society Alzheimer's. Counting the cost: caring for people with dementia on hospital wards. Alzheimer's Society London, 2009. [ Google Scholar ]
- 3. Poblador-Plou B, Calderón-Larrañaga A, Marta-Moreno J, et al. . Comorbidity of dementia: a cross-sectional study of primary care older patients. BMC Psychiatry 2014;14:84 10.1186/1471-244X-14-84 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Bunn F, Burn AM, Goodman C, et al. . Comorbidity and dementia: a scoping review of the literature. BMC Med 2014;12:192. 10.1186/s12916-014-0192-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Royal College of Psychiatrists. 2013. Report of the National Audit of Dementia Care in General Hospitals 2011: Healthcare Improvement Quality Partnership.
- 6. Bunn F, Dickinson A, Simpson C, et al. . Preventing falls among older people with mental health problems: a systematic review. BMC Nurs 2014;13:4. 10.1186/1472-6955-13-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Orsitto G, Fulvio F, Tria D, et al. . Nutritional status in hospitalized elderly patients with mild cognitive impairment. Clin Nutr 2009;28:100–2. 10.1016/j.clnu.2008.12.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Bail K, Goss J, Draper B, et al. . The cost of hospital-acquired complications for older people with and without dementia; a retrospective cohort study. BMC Health Serv Res 2015;15:91. 10.1186/s12913-015-0743-1 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Elvish R, Burrow S, Cawley R, et al. . 'Getting to Know Me': the development and evaluation of a training programme for enhancing skills in the care of people with dementia in general hospital settings. Aging Ment Health 2014;18:481–8. 10.1080/13607863.2013.856860 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Galvin JE, Kuntemeier B, Al-Hammadi N, et al. . "Dementia-friendly hospitals: care not crisis": an educational program designed to improve the care of the hospitalized patient with dementia. Alzheimer Dis Assoc Disord 2010;24:372. 10.1097/WAD.0b013e3181e9f829 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Bunn F, Goodman C, Brayne C, et al. . Comorbidity and dementia: improving healthcare for people with dementia. www. nets.nihr. ac. Uk/projects/hsdr/11101707. NIHR, HS & DR 2012. [ Google Scholar ]
- 12. White N, Leurent B, Lord K, et al. . The management of behavioural and psychological symptoms of dementia in the acute general medical hospital: a longitudinal cohort study. Int J Geriatr Psychiatry 2017;32 10.1002/gps.4463 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Benbow SM, Jolley D. Dementia: stigma and its effects. Neurodegener Dis Manag 2012;2:165–72. 10.2217/nmt.12.7 [ DOI ] [ Google Scholar ]
- 14. Swaffer K. Dementia: stigma, language, and dementia-friendly. Dementia 2014;13:709–16. 10.1177/1471301214548143 [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Waller S, Masterson A. Designing dementia-friendly hospital environments. Future Hospital Journal 2015;2:63–8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Department of Health. Do H, Living Well with Dementia: a national dementia strategy. London, 2009. [ Google Scholar ]
- 17. Department of Health. Do H, Prime Minister’s challenge on dementia 2020. London, 2015. [ Google Scholar ]
- 18. England HE. Dementia: improving dementia care through effective basic training and continuous professional and vocational development. secondary dementia: improving dementia care through effective basic training and continuous professional and vocational development. 2016. https://www.hee.nhs.uk/our-work/hospitals-primary-community-care/mental-health-learning-disability/mental-health/dementia
- 19. World Health Organization. Global age-friendly cities: a guide: world Health Organization, 2007. [ Google Scholar ]
- 20. Crampton J, Dean J, Eley R, AESOP Consortium. Creating a dementia-friendly York. Joseph Rowntree Foundation York, 2012. [ Google Scholar ]
- 21. Mitchell L, Burton E, Raman S. Dementia‐friendly cities: designing intelligible neighbourhoods for life. Journal of Urban Design 2004;9:89–101. 10.1080/1357480042000187721 [ DOI ] [ Google Scholar ]
- 22. Mitchell L, Burton E. Designing Dementia‐friendly Neighbourhoods: helping people with dementia to get out and about. J Integr Care 2010;18:11–18. 10.5042/jic.2010.0647 [ DOI ] [ Google Scholar ]
- 23. Dementia Friends. https://www.dementiafriends.org.uk/
- 24. Joseph Rowntree Foundation. Developing a national user movement for people with dementia - learning from the Dementia engagement and Empowerment Project (DEEP), 2015. [ Google Scholar ]
- 25. Royal College of Nursing. Dementia: commitment to the care of people with dementia in hospital settings. London, 2013. [ Google Scholar ]
- 26. Department of Health. Do H, Prime Minister's challenge on dementia: Delivering major improvements in dementia care and research by 2015. London, 2012. [ Google Scholar ]
- 27. Mayrhofer A, Goodman C, Holman C. Establishing a community of practice for dementia champions (innovative practice). Dementia 2015;14:259–66. 10.1177/1471301214542534 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Mayrhofer A, Goodman C, Sharpe R, et al. . Dementia Education in Hertfordshire and Bedfordshire: an organisational audit commissioned by Health Education East of England Centre for Research in primary and Community Care (CRIPACC), University of Hertfordshire. Hatfield: University of Hertfordshire, 2014. [ Google Scholar ]
- 29. Dewing J, Dijk S. What is the current state of care for older people with dementia in general hospitals? A literature review. Dementia 2016;15:1471301213520172 10.1177/1471301213520172 [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Pawson R. Evidence-based policy: a realist perspective: Sage publications, 2006. [ Google Scholar ]
- 31. Wong G, Greenhalgh T, Westhorp G, et al. . RAMESES publication standards: realist syntheses. BMC Med 2013;11:21. 10.1186/1741-7015-11-21 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Dalkin SM, Greenhalgh J, Jones D, et al. . What's in a mechanism? development of a key concept in realist evaluation. Implement Sci 2015;10:49. 10.1186/s13012-015-0237-x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Pawson and Tilley. Realistic evaluation: Sage, 1997. [ Google Scholar ]
- 34. Handley M, Bunn F, Goodman C. Interventions that support the creation of dementia friendly environments in health care: protocol for a realist review. Syst Rev 2015;4:1. 10.1186/s13643-015-0168-2 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Spencer. Ra. Qualitative data analysis for applied policy research The qualitative researcher’s companion, 2002:305–29. [ Google Scholar ]
- 36. Kitwood T, Bredin K. Evaluating dementia care the DCM method. Bradford, England: Bradford Dementia Research Group, Bradford University, 1997. [ Google Scholar ]
- 37. Wong G, Greenhalgh T, Pawson R. Internet-based medical education: a realist review of what works, for whom and in what circumstances. BMC Med Educ 2010;10:12. 10.1186/1472-6920-10-12 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Pawson R, Greenhalgh T, Harvey G, et al. . Realist review--a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10(suppl 1):21–34. 10.1258/1355819054308530 [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Banks P, Waugh A, Henderson J, Sharp J.;, et al. . Enriching the care of patients with dementia in acute settings? the Dementia Champions Programme in Scotland. Dementia 2014;13:717–36. 10.1177/1471301213485084 [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Ellison S, Watt G, Christie I. Evaluating the impact of the alzheimer Scotland Dementia nurse Consultants/Specialists & Dementia Champions in bringing about improvements to dementia care in acute general hospitals, 2014. [ Google Scholar ]
- 41. Nichols JN, Heller KS. Windows to the heart: creating an acute care dementia unit. J Palliat Med 2002;5:181–92. 10.1089/10966210252785187 [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Dowding D, Lichtner V, Allcock N, et al. . Using sense-making theory to aid understanding of the recognition, assessment and management of pain in patients with dementia in acute hospital settings. Int J Nurs Stud 2016;53:152–62. 10.1016/j.ijnurstu.2015.08.009 [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Gonski PN, Moon I. Outcomes of a behavioral unit in an acute aged care service. Arch Gerontol Geriatr 2012;55:60–5. 10.1016/j.archger.2011.06.013 [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Upton. An evaluation of quality and cost effectiveness of a newly defined suite of care interventions for patients with dementia and their carers in the acute hospital setting developed by the Royal Wolverhampton Hospitals NHS Trust REPORT PHASE 2 – Volume 1: University of Worcester. 2012.
- 45. Goldberg SE, Whittamore KH, Pollock K, et al. . Caring for cognitively impaired older patients in the general hospital: a qualitative analysis of similarities and differences between a specialist Medical and Mental Health Unit and standard care wards. Int J Nurs Stud 2014;51:1332–43. 10.1016/j.ijnurstu.2014.02.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Scerri A, Innes A, Scerri C. Discovering what works well: exploring quality dementia care in hospital wards using an appreciative inquiry approach. J Clin Nurs 2015;24:1916–25. 10.1111/jocn.12822 [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Baillie L. An evaluation of Barbara's Story: Final Report. London: Guy's and St Thomas' NHS Foundation Trust, London South Bank University, 2015. [ Google Scholar ]
- 48. Bray J, Evans S, Bruce M, et al. . Improving activity and engagement for patients with dementia. Nurs Older People 2015;27:22–6. 10.7748/nop.27.8.22.e700 [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Brooker DM, Evans Sarah;, Carter Simon;, et al. . RCN Development Programme: transforming Dementia Care in Hospitals evaluation Report, 2014. [ Google Scholar ]
- 50. Edvardsson D, Sandman PO, Rasmussen B. Forecasting the ward climate: a study from a dementia care unit. J Clin Nurs 2012;21:1136–114. 10.1111/j.1365-2702.2011.03720.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Williams R. Hospital programme for dementia-specific care: using detailed observations of patients’ experiences, Barbara Hodkinson developed the Butterfly Scheme to identify people with memory impairment and improve their care. She tells Ruth Williams how it works. Nursing older people 2011;23:14–17. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Zieschang T, Dutzi I, Müller E, et al. . Improving care for patients with dementia hospitalized for acute somatic illness in a specialized care unit: a feasibility study. Int Psychogeriatr 2010;22:139–46. 10.1017/S1041610209990494 [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Duffin C. Raising awareness to support people with dementia in hospital: a film produced to train staff at one trust has had such a positive effect that it is to be used nationwide. christian Duffin reports on this and other measures to improve care. Nursing older people 2013;25:14–17. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Rösler A, von Renteln-Kruse W, Mühlhan C, et al. . Treatment of dementia patients with fracture of the proximal femur in a specialized geriatric care unit compared to conventional geriatric care. Z Gerontol Geriatr 2012;45:400–3. 10.1007/s00391-012-0299-1 [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Spencer K, Foster P, Whittamore KH, et al. . Delivering dementia care differently--evaluating the differences and similarities between a specialist medical and mental health unit and standard acute care wards: a qualitative study of family carers' perceptions of quality of care. BMJ Open 2013;3:e004198 10.1136/bmjopen-2013-004198 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Moyle W, Borbasi S, Wallis M, et al. . Acute care management of older people with dementia: a qualitative perspective. J Clin Nurs 2011;20:420–8. 10.1111/j.1365-2702.2010.03521.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Luxford K, Axam A, Hasnip F, et al. . Improving clinician-carer communication for safer hospital care: a study of the 'TOP 5' strategy in patients with dementia. Int J Qual Health Care 2015;27:175–82. 10.1093/intqhc/mzv026 [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Goldberg SE, Bradshaw LE, Kearney FC, et al. . Care in specialist medical and mental health unit compared with standard care for older people with cognitive impairment admitted to general hospital: randomised controlled trial (NIHR TEAM trial). BMJ 2013;347:f4132. 10.1136/bmj.f4132 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Elliot R, Adams J. The creation of a Dementia nurse specialist role in an acute general hospital. J Psychiatr Ment Health Nurs 2011;18:648–52. 10.1111/j.1365-2850.2011.01771.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Schneider J, Scales J, Bailey J, et al. . Challenging care: the role and experience of health care assistants in dementia wards. National Institute for Health Research Service delivery and Organisation programme. http://panicoa.org.uk/sites/assets/challenging_care.pdf (accessed August 2010).
- 61. Harwood RH PD, King N, Edwards G, et al. . Development of a Specialist Medical and Mental Health Unit for Older people in an Acute General Hospital. MedicalCrises in Older people Discussion Paper Series. Issue 2010;5:2010. [ Google Scholar ]
- 62. Baldwin R, Pratt H, Goring H, et al. . Does a nurse-led mental health liaison service for older people reduce psychiatric morbidity in acute general medical wards? A randomised controlled trial. Age Ageing 2004;33:472–8. 10.1093/ageing/afh154 [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Enns E, Rhemtulla R, Ewa V, et al. . A controlled quality improvement trial to reduce the use of physical restraints in older hospitalized adults. J Am Geriatr Soc 2014;62:541–5. 10.1111/jgs.12710 [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. von Renteln-Kruse W, Neumann L, Klugmann B, et al. . Geriatric patients with cognitive impairment: patient characteristics and treatment results on a Specialized Ward. Deutsches Ärzteblatt International 2015;112:103. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Turner A, Eccles FJ, Elvish R, et al. . The experience of caring for patients with dementia within a general hospital setting: a meta-synthesis of the qualitative literature. Aging Ment Health 2017;21:1–11. 10.1080/13607863.2015.1109057 [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Digby R, Lee S, Williams A. Interviewing people with dementia in hospital: recommendations for researchers. J Clin Nurs 2016;25:1156–65. 10.1111/jocn.13141 [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Digby R, Lee S, Williams A. The experience of people with dementia and nurses in hospital: an integrative review. Journal of clinical nursing 2016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Borbasi S, Jones J, Lockwood C, et al. . Health professionals' perspectives of providing care to people with dementia in the acute setting: toward better practice. Geriatr Nurs 2006;27:300–8. 10.1016/j.gerinurse.2006.08.013 [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Dewing J. Special observation and older persons with dementia/delirium: a disappointing literature review. Int J Older People Nurs 2013;8:19–28. 10.1111/j.1748-3743.2011.00304.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Crabtree J, Mack J. Developing champions to enhance the care of people with dementia in general hospitals. Nurs Times 2010;106:13–14. [ PubMed ] [ Google Scholar ]
- 71. Waugh A, Marland G, Henderson J, et al. . Improving the care of people with dementia in hospital. Nurs Stand 2011;25:44–9. 10.7748/ns.25.32.44.s50 [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Wilkinson I, Coates A, Merrick S, et al. . Junior doctor dementia champions in a district general hospital (innovative practice). Dementia 2016;15 10.1177/1471301215584083 [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Griffiths P, Bridges J, Sheldon H, et al. . The role of the dementia specialist nurse in acute care: a scoping review. J Clin Nurs 2015;24 10.1111/jocn.12717 [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Holmes J, Montaňa C, Powell G, et al. . Liaison Mental Health Services for older people: a literature review, service mapping and in-depth evaluation of service models. Produced for the National Institute for Health Research Service Delivery and Organisation programme 2010. [ Google Scholar ]
- 75. Waller S. Redesigning wards to support people with dementia in hospital: sarah Waller describes a programme that is bringing staff and patients together to create more welcoming and inspiring therapeutic environments. Nursing older people 2012;24:16–21. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Waugh A, Marland G, Henderson J, et al. . Improving the care of people with dementia in hospital. Nurs Stand 2011;25:44–9. 10.7748/ns.25.32.44.s50 [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Mayrhofer A, Goodman C, Holman C. Establishing a community of practice for dementia champions (innovative practice). Dementia 2015;14:259–66. 10.1177/1471301214542534 [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Griffiths A, Knight A, Harwood R, et al. . Preparation to care for confused older patients in general hospitals: a study of UK health professionals. Age Ageing 2014;43:aft171 10.1093/ageing/aft171 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (651.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
The qualitative component of this review will consider studies that investigate the experience of being involved in nursing interventions to improve the care of people with dementia, and may include perspectives of people with dementia, caregivers, or nurses.
The current study utilizes in-depth qualitative interviews of nurses and social workers in a major academic hospital system to understand barriers and facilitators to quality dementia care for patients with BPSD from a multidisciplinary perspective.
Evidence from 15 stakeholders was combined with literature on interventions aimed at improving healthcare for people living with dementia (22 papers) to generate three initial propositions for developing dementia-friendly hospital environments. Interventions described in the literature can be seen in table 1.
These pieces of research addressed dementia nursing care and treatments in different healthcare facilities (e.g. residential aged care facilities, nursing homes and hospital settings), and some considered nurses’ emotions, feelings and challenges in caring for dementia patients.
This study demonstrates factors that can enable and challenge nursing care of persons with dementia who are acutely unwell and experience responsive behaviours in acute hospitals.
Some progress, however, has been made in understanding symptoms and progression of dementias. Recent studies have also shed light on potentially modifiable risk factors, identified new methods of diagnosis, and explored ways to slow disease progression and improve patients' quality of life.