Center for Healthy Eating and Activity Research


Understanding Childhood Obesity and Weight Stigma: Breaking Down Barriers for Better Health
- Post published: October 25, 2024
At (CHEAR), we are deeply committed to helping children and their families navigate the complex journey of managing obesity in children. It’s a conversation many of us might shy away from, but it’s one we need to have—because the more we understand about obesity in children and the stigma that often accompanies it, the better we can support our kids.
Childhood obesity is not just a physical issue; it’s intertwined with emotional well-being, mental health, and societal perceptions. In this post, we’ll break down the complexities of obesity in children, dive into the damaging effects of weight stigma, and highlight why early intervention is crucial. We’ll also explore treatment options that work and how we can create a more supportive, stigma-free environment for children.
Pediatric Obesity – More Than Just Weight
Understanding pediatric obesity.
Pediatric obesity is often misunderstood. It’s not simply about a child carrying a few extra pounds; it’s a chronic condition that can deeply affect a child’s overall well-being. At , we recognize that this condition, like other chronic illnesses, requires thoughtful, consistent care. But here’s the thing: children with obesity is rarely the result of one single cause. It’s often a combination of factors—genetics, environmental influences, emotional health, and lifestyle behaviors—that contribute to the development and persistence of obesity. Excess weight gain can result from a combination of genetic, environmental, and lifestyle factors.
Parents often find themselves worrying about their children’s health, whether it’s about eating habits, activity levels, or how they’re coping emotionally. If you’re reading this because you’re concerned about your child’s weight, know that you’re not alone. Childhood obesity is far more common than many people realize, and it can carry health risks related to body weight well into adulthood if not addressed early. That’s why, at CHEAR, we emphasize the importance of early, compassionate intervention.
Definition and Causes of Childhood Obesity
Childhood obesity is a complex condition that affects children and adolescents, characterized by an excessive accumulation of body fat that can negatively impact their physical and emotional health. It’s important to understand that obesity in children is not just about carrying extra weight; it’s a multifactorial issue involving a combination of genetic, environmental, and lifestyle factors.
Genetic factors, such as a family history of obesity, can increase a child’s risk of developing obesity. However, environmental factors play a significant role as well. For instance, a sedentary lifestyle and a diet high in processed foods and sugar can contribute to weight gain. Additionally, socioeconomic status, cultural background, and access to healthy food and physical activity opportunities are crucial elements that influence a child’s risk of having obesity.
The World Health Organization (WHO) defines obesity in children as a body mass index (BMI) above the 95th percentile for age and sex. While BMI is a useful tool, it doesn’t account for muscle mass or body composition. Therefore, other measures like waist circumference and skinfold thickness can also be used to assess body fatness in children. Understanding these factors can help us address obesity in children more effectively, focusing on both emotional health and physical activity.
The Complexity of Pediatric Obesity
It’s common to hear phrases like, “They’ll grow out of it” or “It’s just baby fat,” but children with obesity is far more complex than these statements suggest. Yes, genetics can play a role in a child’s predisposition to gain weight, but the environment is also significant. What kind of food is accessible at home or at school? Are there safe spaces for children to be active, or are they spending more time indoors due to lack of safe outdoor environments?
Societal perceptions and biases about excess weight can significantly impact children’s health and well-being, leading to negative outcomes and reinforcing harmful stereotypes.
Moreover, emotional health is often a critical factor. Children sometimes turn to food as a way to cope with stress, anxiety, or boredom—a behavior known as emotional eating. This isn’t about willpower; it’s about understanding the deeper issues that may be driving their behaviors. At CHEAR, we’re dedicated to exploring these emotional triggers and helping families implement healthier coping strategies.Effects of Childhood Obesity
Effects of Childhood Obesity
Childhood obesity can have serious consequences for a child’s physical and emotional health, both in the short and long term. Some of the effects of obesity in children include:
- Increased risk of developing type 2 diabetes, high blood pressure, and high cholesterol.
- Higher likelihood of cardiovascular disease, including heart attacks and strokes.
- Elevated risk of certain types of cancer, such as breast, colon, and kidney cancer.
- Greater chance of experiencing mental health problems, such as depression and anxiety.
- Decreased self-esteem and body image issues.
- Reduced physical activity and mobility.
- Increased risk of sleep apnea and other respiratory problems.
- Higher likelihood of joint problems and osteoarthritis.
These health issues can persist into adulthood, leading to a higher risk of developing obesity-related health problems later in life. Addressing obesity in children early is crucial to mitigate these risks and improve both the physical and emotional health of children.

Weight Stigma: A Hidden Barrier
What is weight stigma.
Weight stigma is one of the most damaging aspects of obesity in children, yet it often goes unaddressed. Weight stigma refers to the negative attitudes, judgments, and discrimination children may face due to their size. At CHEAR, we believe it’s crucial to bring attention to this issue. Kids face weight stigma in many places—in the classroom, on the playground, even in healthcare settings.
Weight discrimination manifests in attitudes and behavior towards children with obesity, affecting their access to services and overall wellbeing.
If your child has ever come home feeling hurt or embarrassed because someone made a comment about their weight, you’ve seen firsthand the emotional toll weight stigma can take. Unfortunately, this can lead to feelings of shame, social isolation, and a reluctance to engage in healthy behaviors like physical activity.
Consequences of Weight Stigma
Weight stigma doesn’t just hurt feelings; it has real, long-term consequences. When children are judged or teased because of their weight, it can lead to emotional eating, avoidance of physical activity, and even depression. Some kids may develop unhealthy relationships with food as a way to cope, while others might feel too embarrassed to seek help from a healthcare provider.
Negative health outcomes associated with weight stigma include both physical and mental health problems, such as increased risk of cardiovascular disease, diabetes, anxiety, and depression.
At CHEAR, we know that the emotional and mental health of children is just as important as their physical health. That’s why our approach emphasizes empathy, education, and creating environments where children feel safe and supported. By reducing weight stigma, we can help children feel empowered to take control of their health without fear of judgment.
Why Early Intervention and Intensive Treatment Matters
Why “wait and see” doesn’t work.
In the past, healthcare providers often took a “wait and see” approach, assuming children would grow out of their weight issues. However, research has shown that delaying treatment can have serious long-term consequences. Delaying treatment can lead to excess weight gain and associated health risks, including type 2 diabetes and cardiovascular disease. At CHEAR, we advocate for early intervention as soon as children with obesity is identified. The earlier we can start addressing the underlying issues—whether they’re related to genetics, environment, or emotional health—the better the outcome for the child.
By addressing obesity in children early, we can reduce the risk of developing related health conditions like type 2 diabetes, cardiovascular disease, and orthopedic problems. More importantly, early intervention helps children develop healthy habits that can improve their overall quality of life—physically, emotionally, and socially.
A Full Range of Treatment Options
When it comes to obesity in children, there’s no one-size-fits-all solution. At CHEAR, we tailor treatment plans to each child’s unique situation. These plans often include behavior and lifestyle therapy, and emotional support. Every child is different, and their treatment should be personalized to reflect their specific needs.
Weight management presents significant challenges, including psychological and social aspects such as stigma and bias, which can impact health outcomes and patient care. Personalized treatment plans are crucial in addressing these challenges effectively.
We recognize that tackling obesity is a family effort. At CHEAR, we work closely with parents and caregivers to help them create home environments that support healthier choices. This holistic approach is key to achieving long-term success.
Treatment That Works: A Breakdown
Behavior therapy and lifestyle therapy.
At CHEAR, we know that behavior and lifestyle therapy is key to helping children develop healthier habits. This form of therapy focuses on guiding families to make small, practical changes, like swapping out sugary drinks for water or getting more physical activity in fun and accessible ways. These changes may seem minor, but they can make a huge difference in a child’s physical and emotional well-being over time.
Helping kids learn how to make healthier food choices, stay active, and create supportive home environments is at the heart of what we do at CHEAR. We also understand that some families may need additional support, which is where our no-cost studies or clinic services come in.

CHEAR’s No-Cost Child and Adolescent Studies
At CHEAR, we’re proud to offer several no-cost studies that focus on helping children and adolescents who are struggling with obesity or unhealthy eating patterns. These studies give families access to evidence-based treatments that can support lasting, positive changes. Here are some of the child and adolescent studies currently available.
FRESH-FR (Family, Responsibility, Education, Support & Health for Food Responsiveness) : The FRESH-FR program is a no-cost weight loss program for children ages 7-12 with obesity & their parent who tend to think and talk about food a lot and overeat. The FRESH-FR program examines 4 different treatments that may help children reduce their overeating and lose weight.
FRESH-A (Family, Responsibility, Education, Support & Health for Autism) : The FRESH-A study is a telehealth program for parents of children ages 6-12 with autism and overweight or obesity that focuses on developing healthier eating and activity behaviors. The no-cost treatment program is 6-months and only parents attend weekly groups.
FRESH-LC (Family, Responsibility, Education, Support and Health for Latino Caregivers): The FRESH-LC study is a 6-month, no-cost telehealth program designed for Latino families with a child who has overweight or obesity. Both a parent and an additional caregiver will participate in the treatment. The FRESH-LC program examines two different group treatments for parents, which may help children lose weight. All participation is conducted remotely, and treatment is offered in both Spanish and English to two caregivers per family.
These studies provide an incredible opportunity for families to access expert care at no cost, while also contributing to valuable research that helps shape the future of children with obesity treatment.
For more information about these studies and to see if your child qualifies, please visit our Center for Healthy Eating and Activity Research Current Studies page . For children who aren’t eligible for research studies or are unable to participate, they may still be able to receive care at the clinic .
Promoting Positive Body Image and Self-Esteem
Promoting positive body image and self-esteem is essential for children and adolescents, especially those with overweight or obesity. A positive body image can help children develop a healthy relationship with food and physical activity, reducing the risk of developing eating disorders and other mental health problems.
Here are some strategies for promoting positive body image and self-esteem in children and adolescents:
- Encourage children to focus on their strengths and abilities, rather than their weight or appearance.
- Foster a positive and supportive family environment where children feel valued and respected.
- Motivate children to engage in physical activity and sports, which can help build confidence and self-esteem.
- Educate children about the importance of healthy eating and nutrition, and encourage them to make healthy food choices.
- Avoid criticizing or teasing children about their weight or appearance, as this can negatively impact their self-esteem and body image.
- Support children in expressing their feelings and emotions, providing guidance and understanding when needed.
By promoting positive body image and self-esteem, we can help children and adolescents develop a healthy and positive relationship with their bodies, reducing the risk of obesity-related health problems and enhancing their overall well-being.

Building a Healthier Future: Let’s Support Kids with Empathy and Care
At CHEAR, we believe that obesity in children is not something to blame children or their families for. It’s a complex health condition that needs to be approached with understanding, compassion, and support. Our goal is not to “fix” children, but to help them live their healthiest lives—physically, emotionally, and mentally.
Supportive environments play a crucial role in improving health outcomes for children with obesity.
A key part of our mission at CHEAR is to create environments that encourage healthy choices and reduce the stigma surrounding obesity. Whether it’s at home, in schools, or within healthcare settings, children need to feel safe and supported. By spreading awareness, reducing weight stigma, and providing the care and tools children need to succeed, we can help them build a healthier future.
Let’s work together to empower children and their families to make positive changes. At CHEAR, we’re here to support you every step of the way. Together, we can make a difference—one step at a time, with empathy, care, and understanding.
You Might Also Like

Nourishing Spaces: The Art of Organizing Your Home for Healthy Eating Success
Celebrating thanksgiving the healthy way: tips and tasty recipes.

Staying Active at Home (Plus Free Online Workouts)
REVIEW article
Childhood and adolescent obesity: a review.

- 1 Division of Endocrinology, Diabetes and Metabolism, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, United States
- 2 Division of Adolescent Medicine, Department of Pediatrics, Medical College of Wisconsin Affiliated Hospitals, Milwaukee, WI, United States
- 3 Division of Adolescent Medicine, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, United States
Obesity is a complex condition that interweaves biological, developmental, environmental, behavioral, and genetic factors; it is a significant public health problem. The most common cause of obesity throughout childhood and adolescence is an inequity in energy balance; that is, excess caloric intake without appropriate caloric expenditure. Adiposity rebound (AR) in early childhood is a risk factor for obesity in adolescence and adulthood. The increasing prevalence of childhood and adolescent obesity is associated with a rise in comorbidities previously identified in the adult population, such as Type 2 Diabetes Mellitus, Hypertension, Non-alcoholic Fatty Liver disease (NAFLD), Obstructive Sleep Apnea (OSA), and Dyslipidemia. Due to the lack of a single treatment option to address obesity, clinicians have generally relied on counseling dietary changes and exercise. Due to psychosocial issues that may accompany adolescence regarding body habitus, this approach can have negative results. Teens can develop unhealthy eating habits that result in Bulimia Nervosa (BN), Binge- Eating Disorder (BED), or Night eating syndrome (NES). Others can develop Anorexia Nervosa (AN) as they attempt to restrict their diet and overshoot their goal of “being healthy.” To date, lifestyle interventions have shown only modest effects on weight loss. Emerging findings from basic science as well as interventional drug trials utilizing GLP-1 agonists have demonstrated success in effective weight loss in obese adults, adolescents, and pediatric patients. However, there is limited data on the efficacy and safety of other weight-loss medications in children and adolescents. Nearly 6% of adolescents in the United States are severely obese and bariatric surgery as a treatment consideration will be discussed. In summary, this paper will overview the pathophysiology, clinical, and psychological implications, and treatment options available for obese pediatric and adolescent patients.
Introduction
Obesity is a complex issue that affects children across all age groups ( 1 – 3 ). One-third of children and adolescents in the United States are classified as either overweight or obese. There is no single element causing this epidemic, but obesity is due to complex interactions between biological, developmental, behavioral, genetic, and environmental factors ( 4 ). The role of epigenetics and the gut microbiome, as well as intrauterine and intergenerational effects, have recently emerged as contributing factors to the obesity epidemic ( 5 , 6 ). Other factors including small for gestational age (SGA) status at birth, formula rather than breast feeding in infancy, and early introduction of protein in infant's dietary intake have been reportedly associated with weight gain that can persist later in life ( 6 – 8 ). The rising prevalence of childhood obesity poses a significant public health challenge by increasing the burden of chronic non-communicable diseases ( 1 , 9 ).
Obesity increases the risk of developing early puberty in children ( 10 ), menstrual irregularities in adolescent girls ( 1 , 11 ), sleep disorders such as obstructive sleep apnea (OSA) ( 1 , 12 ), cardiovascular risk factors that include Prediabetes, Type 2 Diabetes, High Cholesterol levels, Hypertension, NAFLD, and Metabolic syndrome ( 1 , 2 ). Additionally, obese children and adolescents can suffer from psychological issues such as depression, anxiety, poor self-esteem, body image and peer relationships, and eating disorders ( 13 , 14 ).
So far, interventions for overweight/obesity prevention have mainly focused on behavioral changes in an individual such as increasing daily physical exercise or improving quality of diet with restricting excess calorie intake ( 1 , 15 , 16 ). However, these efforts have had limited results. In addition to behavioral and dietary recommendations, changes in the community-based environment such as promotion of healthy food choices by taxing unhealthy foods ( 17 ), improving lunch food quality and increasing daily physical activity at school and childcare centers, are extra measures that are needed ( 16 ). These interventions may include a ban on unhealthy food advertisements aimed at children as well as access to playgrounds and green spaces where families can feel their children can safely recreate. Also, this will limit screen time for adolescents as well as younger children.
However, even with the above changes, pharmacotherapy and/or bariatric surgery will likely remain a necessary option for those youth with morbid obesity ( 1 ). This review summarizes our current understanding of the factors associated with obesity, the physiological and psychological effects of obesity on children and adolescents, and intervention strategies that may prevent future concomitant issues.
Definition of Childhood Obesity
Body mass index (BMI) is an inexpensive method to assess body fat and is derived from a formula derived from height and weight in children over 2 years of age ( 1 , 18 , 19 ). Although more sophisticated methods exist that can determine body fat directly, they are costly and not readily available. These methods include measuring skinfold thickness with a caliper, Bioelectrical impedance, Hydro densitometry, Dual-energy X-ray Absorptiometry (DEXA), and Air Displacement Plethysmography ( 2 ).
BMI provides a reasonable estimate of body fat indirectly in the healthy pediatric population and studies have shown that BMI correlates with body fat and future health risks ( 18 ). Unlike in adults, Z-scores or percentiles are used to represent BMI in children and vary with the age and sex of the child. BMI Z-score cut off points of >1.0, >2.0, and >3.0 are recommended by the World Health Organization (WHO) to define at risk of overweight, overweight and obesity, respectively ( 19 ). However, in terms of percentiles, overweight is applied when BMI is >85th percentile <95th percentile, whereas obesity is BMI > 95th percentile ( 20 – 22 ). Although BMI Z-scores can be converted to BMI percentiles, the percentiles need to be rounded and can misclassify some normal-weight children in the under or overweight category ( 19 ). Therefore, to prevent these inaccuracies and for easier understanding, it is recommended that the BMI Z-scores in children should be used in research whereas BMI percentiles are best used in the clinical settings ( 20 ).
As BMI does not directly measure body fat, it is an excellent screening method, but should not be used solely for diagnostic purposes ( 23 ). Using 85th percentile as a cut off point for healthy weight may miss an opportunity to obtain crucial information on diet, physical activity, and family history. Once this information is obtained, it may allow the provider an opportunity to offer appropriate anticipatory guidance to the families.
Pathophysiology of Obesity
The pathophysiology of obesity is complex that results from a combination of individual and societal factors. At the individual level, biological, and physiological factors in the presence of ones' own genetic risk influence eating behaviors and tendency to gain weight ( 1 ). Societal factors include influence of the family, community and socio-economic resources that further shape these behaviors ( Figure 1 ) ( 3 , 24 ).
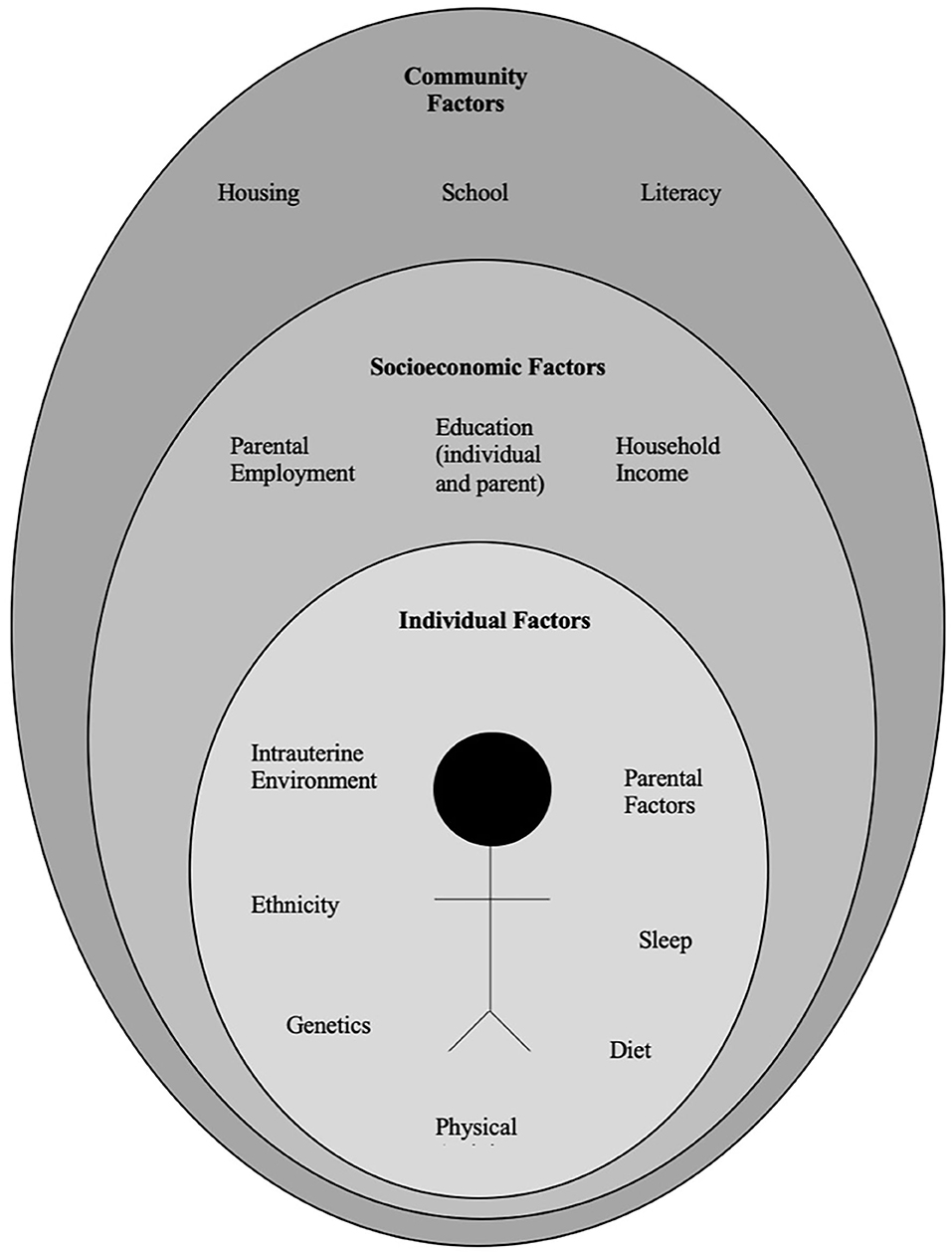
Figure 1 . Multidimensional factors contributing to child and adolescent obesity.
Biological Factors
There is a complex architecture of neural and hormonal regulatory control, the Gut-Brain axis, which plays a significant role in hunger and satiety ( Figure 2 ). Sensory stimulation (smell, sight, and taste), gastrointestinal signals (peptides, neural signals), and circulating hormones further contribute to food intake ( 25 – 27 ).
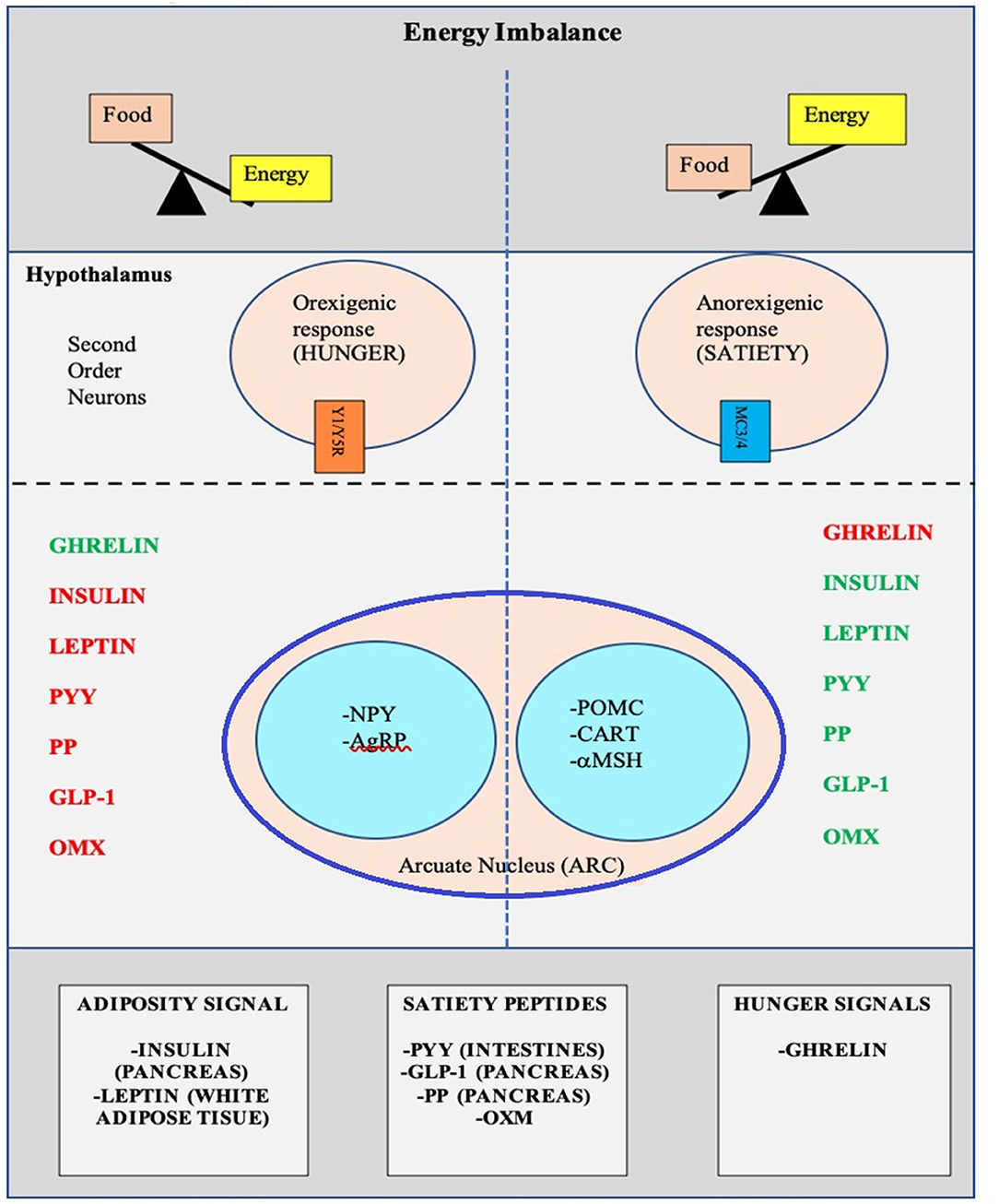
Figure 2 . Pictorial representation of the Hunger-Satiety pathway a and the various hormones b involved in the pathway. a, Y1/Y5R and MC3/4 are second order neuro receptors which are responsible in either the hunger or satiety pathway. Neurons in the ARC include: NPY, Neuropeptide Y; AgRP, Agouti-Related Peptide; POMC, Pro-Opiomelanocortin; CART, Cocaine-and Amphetamine-regulated Transcript; α-MSH, α-Melanocyte Stimulating Hormone. b, PYY, Peptide YY; PP, Pancreatic Polypeptide; GLP-1, Glucagon-Like Peptide- I; OMX, Oxyntomodulin.
The hypothalamus is the crucial region in the brain that regulates appetite and is controlled by key hormones. Ghrelin, a hunger-stimulating (orexigenic) hormone, is mainly released from the stomach. On the other hand, leptin is primarily secreted from adipose tissue and serves as a signal for the brain regarding the body's energy stores and functions as an appetite -suppressing (anorexigenic) hormone. Several other appetite-suppressing (anorexigenic) hormones are released from the pancreas and gut in response to food intake and reach the hypothalamus through the brain-blood barrier (BBB) ( 28 – 32 ). These anorexigenic and orexigenic hormones regulate energy balance by stimulating hunger and satiety by expression of various signaling pathways in the arcuate nucleus (ARC) of the hypothalamus ( Figure 2 ) ( 28 , 33 ). Dysregulation of appetite due to blunted suppression or loss of caloric sensing signals can result in obesity and its morbidities ( 34 ).
Emotional dysfunction due to psychiatric disorders can cause stress and an abnormal sleep-wake cycles. These modifications in biological rhythms can result in increased appetite, mainly due to ghrelin, and can contribute to emotional eating ( 35 ).
Recently, the role of changes in the gut microbiome with increased weight gain through several pathways has been described in literature ( 36 , 37 ). The human gut serves as a host to trillions of microorganisms, referred to as gut microbiota. The dominant gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with Firmicutes and Bacteroidetes representing 90% of human gut microbiota ( 5 , 38 ). The microbes in the gut have a symbiotic relationship within their human host and provide a nutrient-rich environment. Gut microbiota can be affected by various factors that include gestational age at birth, mode of infant delivery, type of neonatal and infant feeding, introduction of solid food, feeding practices and external factors like antibiotic use ( 5 , 38 ). Also, the maturation of the bacterial phyla that occurs from birth to adulthood ( 39 ), is influenced by genetics, environment, diet, lifestyle, and gut physiology and stabilizes in adulthood ( 5 , 39 , 40 ). Gut microbiota is unique to each individual and plays a specific role in maintaining structural integrity, and the mucosal barrier of the gut, nutrient metabolism, immune response, and protection against pathogens ( 5 , 37 , 38 ). In addition, the microbiota ferments the indigestible food and synthesizes other essential micronutrients as well as short chain fatty acids (SCFAs') ( 40 , 41 ). Dysbiosis or imbalance of the gut microbiota, in particularly the role of SCFA has been linked with the patho-physiology of obesity ( 36 , 38 , 41 , 42 ). SCFAs' are produced by anaerobic fermentation of dietary fiber and indigestible starch and play a role in mammalian energy metabolism by influencing gut-brain communication axis. Emerging evidence has shown that increased ratio of Firmicutes to Bacteroidetes causes increased energy extraction of calories from diets and is evidenced by increased production of short chain fatty acids (SCFAs') ( 43 – 45 ). However, this relationship is not affirmed yet, as a negative relationship between SCFA levels and obesity has also been reported ( 46 ). Due to the conflicting data, additional randomized control trials are needed to clarify the role of SCFA's in obese and non-obese individuals.
The gut microbiota also has a bidirectional interaction with the liver, and various additional factors such as diet, genetics, and the environment play a key role in this relationship. The Gut- Liver Axis is interconnected at various levels that include the mucus barrier, epithelial barrier, and gut microbiome and are essential to maintain normal homeostasis ( 47 ). Increased intestinal mucosal permeability can disrupt the gut-liver axis, which releases various inflammatory markers, activates an innate immune response in the liver, and results in a spectrum of liver diseases that include hepatic steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC) ( 48 , 49 ).
Other medical conditions, including type 2 Diabetes Mellitus, Metabolic Syndrome, eating disorders as well as psychological conditions such as anxiety and depression are associated with the gut microbiome ( 50 – 53 ).
Genetic Factors
Genetic causes of obesity can either be monogenic or polygenic types. Monogenic obesity is rare, mainly due to mutations in genes within the leptin/melanocortin pathway in the hypothalamus that is essential for the regulation of food intake/satiety, body weight, and energy metabolism ( 54 ). Leptin regulates eating behaviors, the onset of puberty, and T-cell immunity ( 55 ). About 3% of obese children have mutations in the leptin ( LEP ) gene and the leptin receptor (LEPR) and can also present with delayed puberty and immune dysfunction ( 55 , 56 ). Obesity caused by other genetic mutations in the leptin-melanocortin pathway include proopiomelanocortin (POMC) and melanocortin receptor 4 (MC4R), brain-derived neurotrophic factor (BDNF), and the tyrosine kinase receptor B (NTRK2) genes ( 57 , 58 ). Patients with monogenic forms generally present during early childhood (by 2 years old) with severe obesity and abnormal feeding behaviors ( 59 ). Other genetic causes of severe obesity are Prader Willi Syndrome (PWS), Alström syndrome, Bardet Biedl syndrome. Patients with these syndromes present with additional characteristics, including cognitive impairment, dysmorphic features, and organ-specific developmental abnormalities ( 60 ). Individuals who present with obesity, developmental delay, dysmorphic features, and organ dysfunction should receive a genetics referral for further evaluation.
Polygenic obesity is the more common form of obesity, caused by the combined effect of multiple genetic variants. It is the result of the interplay between genetic susceptibility and the environment, also known as the Gene-Environment Interaction (GEI) ( 61 – 64 ). Genome-wide association studies (GWAS) have identified gene variants [single nucleotide polymorphism (SNPs)] for body mass index (BMI) that likely act synergistically to affect body weight ( 65 ). Studies have identified genetic variants in several genes that may contribute to excessive weight gain by increasing hunger and food intake ( 66 – 68 ). When the genotype of an individual confers risk for obesity, exposure to an obesogenic environment may promote a state of energy imbalance due to behaviors that contribute to conserving rather than expending energy ( 69 , 70 ). Research studies have shown that obese individuals have a genetic variation that can influence their actions, such as increased food intake, lack of physical activity, a decreased metabolism, as well as an increased tendency to store body fat ( 63 , 66 , 67 , 69 , 70 ).
Recently the role of epigenetic factors in the development of obesity has emerged ( 71 ). The epigenetic phenomenon may alter gene expression without changing the underlying DNA sequence. In effect, epigenetic changes may result in the addition of chemical tags known as methyl groups, to the individual's chromosomes. This alteration can result in a phenomenon where critical genes are primed to on and off regulate. Complex physiological and psychological adjustment occur during infancy and can thereafter set the stage for health vs. disease. Developmental origins of health and disease (DOHaD) shows that early life environment can impact the risk of chronic diseases later in life due to fetal programming secondary to epigenetic changes ( 72 ). Maternal nutrition during the prenatal or early postnatal period may trigger these epigenetic changes and increase the risk for chronic conditions such as obesity, metabolic and cardiovascular disease due to epigenetic modifications that may persist and cause intergenerational effect on the health children and adults ( 58 , 73 , 74 ). Similarly, adverse childhood experiences (ACE) have been linked to a broad range of negative outcomes through epigenetic mechanisms ( 75 ) and promote unhealthy eating behaviors ( 76 , 77 ). Other factors such as diet, physical activity, environmental and psychosocial stressors can cause epigenetic changes and place an individual at risk for weight gain ( 78 ).
Developmental Factors
Eating behaviors evolve over the first few years of life. Young children learn to eat through their direct experience with food and observing others eating around them ( 79 ). During infancy, feeding defines the relationship of security and trust between a child and the parent. Early childhood eating behaviors shift to more self-directed control due to rapid physical, cognitive, communicative, and social development ( 80 ). Parents or caregivers determine the type of food that is made available to the infant and young child. However, due to economic limitations and parents having decreased time to prepare nutritious meals, consumption of processed and cheaper energy-dense foods have occurred in Western countries. Additionally, feeding practices often include providing large or super-sized portions of palatable foods and encouraging children to finish the complete meal (clean their plate even if they do not choose to), as seen across many cultures ( 81 , 82 ). Also, a segment of parents are overly concerned with dietary intake and may pressurize their child to eat what they perceive as a healthy diet, which can lead to unintended consequences ( 83 ). Parents' excessive restriction of food choices may result in poor self-regulation of energy intake by their child or adolescent. This action may inadvertently promote overconsumption of highly palatable restricted foods when available to the child or adolescent outside of parental control with resultant excessive weight gain ( 84 , 85 ).
During middle childhood, children start achieving greater independence, experience broader social networks, and expand their ability to develop more control over their food choices. Changes that occur in the setting of a new environment such as daycare or school allow exposure to different food options, limited physical activity, and often increased sedentary behaviors associated with school schedules ( 24 ). As the transition to adolescence occurs, physical and psychosocial development significantly affect food choices and eating patterns ( 25 ). During the teenage years, more independence and interaction with peers can impact the selection of fast foods that are calorically dense. Moreover, during the adolescent years, more sedentary behaviors such as video and computer use can limit physical exercise. Adolescence is also a period in development with an enhanced focus on appearance, body weight, and other psychological concerns ( 86 , 87 ).
Environmental Factors
Environmental changes within the past few decades, particularly easy access to high-calorie fast foods, increased consumption of sugary beverages, and sedentary lifestyles, are linked with rising obesity ( 88 ). The easy availability of high caloric fast foods, and super-sized portions, are increasingly common choices as individuals prefer these highly palatable and often less expensive foods over fruits and vegetables ( 89 ). The quality of lunches and snacks served in schools and childcare centers has been an area of debate and concern. Children and adolescents consume one-third to one-half of meals in the above settings. Despite policies in place at schools, encouraging foods, beverages, and snacks that are deemed healthier options, the effectiveness of these policies in improving children's dietary habits or change in obesity rate has not yet been seen ( 90 ). This is likely due to the fact that such policies primarily focus on improving dietary quality but not quantity which can impact the overweight or obese youth ( 91 ). Policies to implement taxes on sugary beverages are in effect in a few states in the US ( 92 ) as sugar and sugary beverages are associated with increased weight gain ( 2 , 3 ). This has resulted in reduction in sales of sugary drinks in these states, but the sales of these types of drinks has risen in neighboring states that did not implement the tax ( 93 ). Due to advancements in technology, children are spending increased time on electronic devices, limiting exercise options. Technology advancement is also disrupting the sleep-wake cycle, causing poor sleeping habits, and altered eating patterns ( 94 ). A study published on Canadian children showed that the access to and night-time use of electronic devices causes decreased sleep duration, resulting in excess body weight, inferior diet quality, and lower physical activity levels ( 95 ).
Infant nutrition has gained significant popularity in relation to causing overweight/obesity and other diseases later in life. Breast feeding is frequently discussed as providing protection against developing overweight/obesity in children ( 8 ). Considerable heterogeneity has been observed in studies and conducting randomized clinical trials between breast feeding vs. formula feeding is not feasible ( 8 ). Children fed with a low protein formula like breast milk are shown to have normal weight gain in early childhood as compared to those that are fed formulas with a high protein load ( 96 ). A recent Canadian childbirth cohort study showed that breast feeding within first year of life was inversely associated with weight gain and increased BMI ( 97 ). The effect was stronger if the child was exclusively breast fed directly vs. expressed breast milk or addition of formula or solid food ( 97 ). Also, due to the concern of poor growth in preterm or SGA infants, additional calories are often given for nutritional support in the form of macronutrient supplements. Most of these infants demonstrate “catch up growth.” In fact, there have been reports that in some children the extra nutritional support can increase the risk for overweight/obesity later in life. The association, however, is inconsistent. Recently a systemic review done on randomized controlled trials comparing the studies done in preterm and SGA infants with feeds with and without macronutrient supplements showed that macronutrient supplements may increase weight and length in toddlers but did not show a significant increase in the BMI during childhood ( 98 ). Increased growth velocity due to early introduction of formula milk and protein in infants' diet, may influence the obesity pathways, and can impact fetal programming for metabolic disease later in life ( 99 ).
General pediatricians caring for children with overweight/obesity, generally recommend endocrine testing as parents often believe that there may be an underlying cause for this condition and urge their primary providers to check for conditions such as thyroid abnormalities. Endocrine etiologies for obesity are rarely identified and patients with underlying endocrine disorders causing excessive weight gain usually are accompanied by attenuated growth patterns, such that a patient continues to gain weight with a decline in linear height ( 100 ). Various endocrine etiologies that one could consider in a patient with excessive weight gain in the setting of slow linear growth: severe hypothyroidism, growth hormone deficiency, and Cushing's disease/syndrome ( 58 , 100 ).
Clinical-Physiology of Pediatric Obesity
It is a well-known fact that early AR(increased BMI) before the age of 5 years is a risk factor for adult obesity, obesity-related comorbidities, and metabolic syndrome ( 101 – 103 ). Typically, body mass index (BMI) declines to a minimum in children before it starts increasing again into adulthood, also known as AR. Usually, AR happens between 5 and 7 years of age, but if it occurs before the age of 5 years is considered early AR. Early AR is a marker for higher risk for obesity-related comorbidities. These obesity-related health comorbidities include cardiovascular risk factors (hypertension, dyslipidemia, prediabetes, and type 2 diabetes), hormonal issues, orthopedic problems, sleep apnea, asthma, and fatty liver disease ( Figure 3 ) ( 9 ).
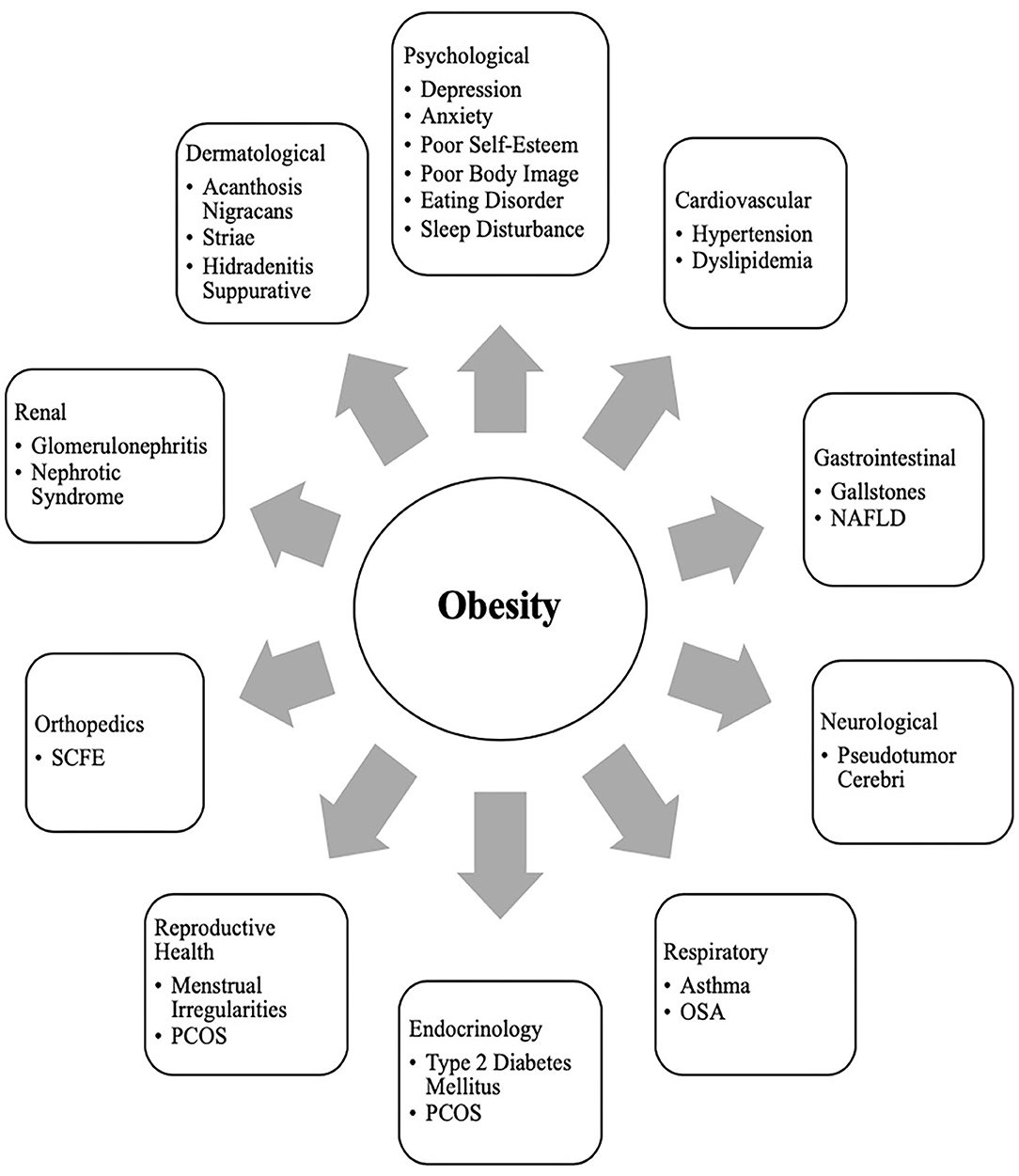
Figure 3 . Obesity related co-morbidities a in children and adolescents. a, NAFLD, Non-Alcoholic Fatty Liver Disease; SCFE, Slipped Capital Femoral Epiphysis; PCOS, Polycystic Ovary Syndrome; OSA, Obstructive Sleep Apnea.
Clinical Comorbidities of Obesity in Children
Growth and puberty.
Excess weight gain in children can influence growth and pubertal development ( 10 ). Childhood obesity can cause prepubertal acceleration of linear growth velocity and advanced bone age in boys and girls ( 104 ). Hyperinsulinemia is a normal physiological state during puberty, but children with obesity can have abnormally high insulin levels ( 105 ). Leptin resistance also occurs in obese individuals who have higher leptin levels produced by their adipose tissue ( 55 , 106 ). The insulin and leptin levels can act on receptors that impact the growth plates with a resultant bone age advancement ( 55 ).
Adequate nutrition is essential for the typical timing and tempo of pubertal onset. Excessive weight gain can initiate early puberty, due to altered hormonal parameters ( 10 ). Obese children may present with premature adrenarche, thelarche, or precocious puberty (PP) ( 107 ). The association of early pubertal changes with obesity is consistent in girls, and is well-reported; however, data is sparse in boys ( 108 ). One US study conducted in racially diverse boys showed obese boys had delayed puberty, whereas overweight boys had early puberty as compared to normal-weight boys ( 109 ). Obese girls with PP have high leptin levels ( 110 , 111 ). Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) is a cross-sectional study and suggested an indirect relationship between elevated leptin levels, early puberty, and cardiometabolic and inflammatory markers in obese girls ( 112 ). Additionally, obese girls with premature adrenarche carry a higher risk for developing polycystic ovary syndrome (PCOS) in the future ( 113 , 114 ).
Sleep Disorders
Obesity is an independent risk factor for obstructive sleep apnea (OSA) in children and adolescents ( 12 , 115 ). Children with OSA have less deleterious consequences in terms of cardiovascular stress of metabolic syndrome when compared to adolescents and adults ( 116 , 117 ). In children, abnormal behaviors and neurocognitive dysfunction are the most critical and frequent end-organ morbidities associated with OSA ( 12 ). However, in adolescents, obesity and OSA can independently cause oxidative systemic stress and inflammation ( 118 , 119 ), and when this occurs concurrently, it can result in more severe metabolic dysfunction and cardiovascular outcomes later in life ( 120 ).
Other Comorbidities
Obesity is related to a clinical spectrum of liver abnormalities such as NAFLD ( 121 ); the most important cause of liver disease in children ( 122 – 124 ). NAFLD includes steatosis (increased liver fat without inflammation) and NASH (increased liver fat with inflammation and hepatic injury). While in some adults NAFLD can progress to an end-stage liver disease requiring liver transplant ( 125 , 126 ), the risk of progression during childhood is less well-defined ( 127 ). NAFLD is closely associated with metabolic syndrome including central obesity, insulin resistance, type 2 diabetes, dyslipidemia, and hypertension ( 128 ).
Obese children are also at risk for slipped capital femoral epiphysis (SCFE) ( 129 ), and sedentary lifestyle behaviors may have a negative influence on the brain structure and executive functioning, although the direction of causality is not clear ( 130 , 131 ).
Clinical Comorbidities of Obesity in Adolescents
Menstrual irregularities and pcos.
At the onset of puberty, physiologically, sex steroids can cause appropriate weight gain and body composition changes that should not affect normal menstruation ( 132 , 133 ). However, excessive weight gain in adolescent girls can result in irregular menstrual cycles and puts them at risk for PCOS due to increased androgen levels. Additionally, they can have excessive body hair (hirsutism), polycystic ovaries, and can suffer from distorted body images ( 134 , 135 ). Adolescent girls with PCOS also have an inherent risk for insulin resistance irrespective of their weight. However, weight gain further exacerbates their existing state of insulin resistance and increases the risk for obesity-related comorbidities such as metabolic syndrome, and type 2 diabetes. Although the diagnosis of PCOS can be challenging at this age due to an overlap with predictable pubertal changes, early intervention (appropriate weight loss and use of hormonal methods) can help restore menstrual cyclicity and future concerns related to childbearing ( 11 ).
Metabolic Syndrome and Sleep Disorders
Metabolic syndrome (MS) is a group of cardiovascular risk factors characterized by acanthosis nigricans, prediabetes, hypertension, dyslipidemia, and non-alcoholic steatohepatitis (NASH), that occurs from insulin resistance caused by obesity ( 136 ). Diagnosis of MS in adults requires at least three out of the five risk factors: increased central adiposity, hypertension, hyperglycemia, hypertriglyceridemia, or low HDL level. Definitions to diagnose MS are controversial in younger age groups, and many definitions have been proposed ( 136 ). This is due to the complex physiology of growth and development during puberty, which causes significant overlap between MS and features of normal growth. However, childhood obesity is associated with an inflammatory state even before puberty ( 137 ). In obese children and adolescents, hyperinsulinemia during puberty ( 138 , 139 ) and unhealthy sleep behaviors increase MS's risk and severity ( 140 ). Even though there is no consensus on diagnosis regarding MS in this age group, when dealing with obese children and adolescents, clinicians should screen them for MS risk factors and sleep behaviors and provide recommendations for weight management.
Social Psychology of Pediatric Obesity in Children and Adolescents
Obese children and adolescents may experience psychosocial sequelae, including depression, bullying, social isolation, diminished self-esteem, behavioral problems, dissatisfaction with body image, and reduced quality of life ( 13 , 141 ). Compared with normal-weight counterparts, overweight/obesity is one of the most common reasons children and adolescents are bullied at school ( 142 ). The consequence of stigma, bullying, and teasing related to childhood obesity are pervasive and can have severe implications for emotional and physical health and performance that can persist later in life ( 13 ).
In adolescents, psychological outcomes associated with obesity are multifactorial and have a bidirectional relationship ( Figure 4 ). Obese adolescents due to their physique may have a higher likelihood of psychosocial health issues, including depression, body image/dissatisfaction, lower self-esteem, peer victimization/bullying, and interpersonal relationship difficulties. They may also demonstrate reduced resilience to challenging situations compared to their non-obese/overweight counterparts ( 9 , 143 – 146 ). Body image dissatisfaction has been associated with further weight gain but can also be related to the development of a mental health disorder or an eating disorder (ED) or disorder eating habits (DEH). Mental health disorders such as depression are associated with poor eating habits, a sedentary lifestyle, and altered sleep patterns. ED or DEH that include anorexia nervosa (AN), bulimia nervosa (BN), binge-eating disorder (BED) or night eating syndrome (NES) may be related to an individual's overvaluation of their body shape and weight or can result during the treatment for obesity ( 147 – 150 ). The management of obesity can place a patient at risk of AN if there is a rigid focus on caloric intake or if a patient overcorrects and initiates obsessive self-directed dieting. Healthcare providers who primarily care for obese patients, usually give the advice to diet to lose weight and then maintain it. However, strict dieting (hypocaloric diet), which some patients may later engage in can lead to an eating disorder such as anorexia nervosa ( 151 ). This behavior leads to a poor relationship with food, and therefore, adolescents perseverate on their weight and numbers ( 152 ).
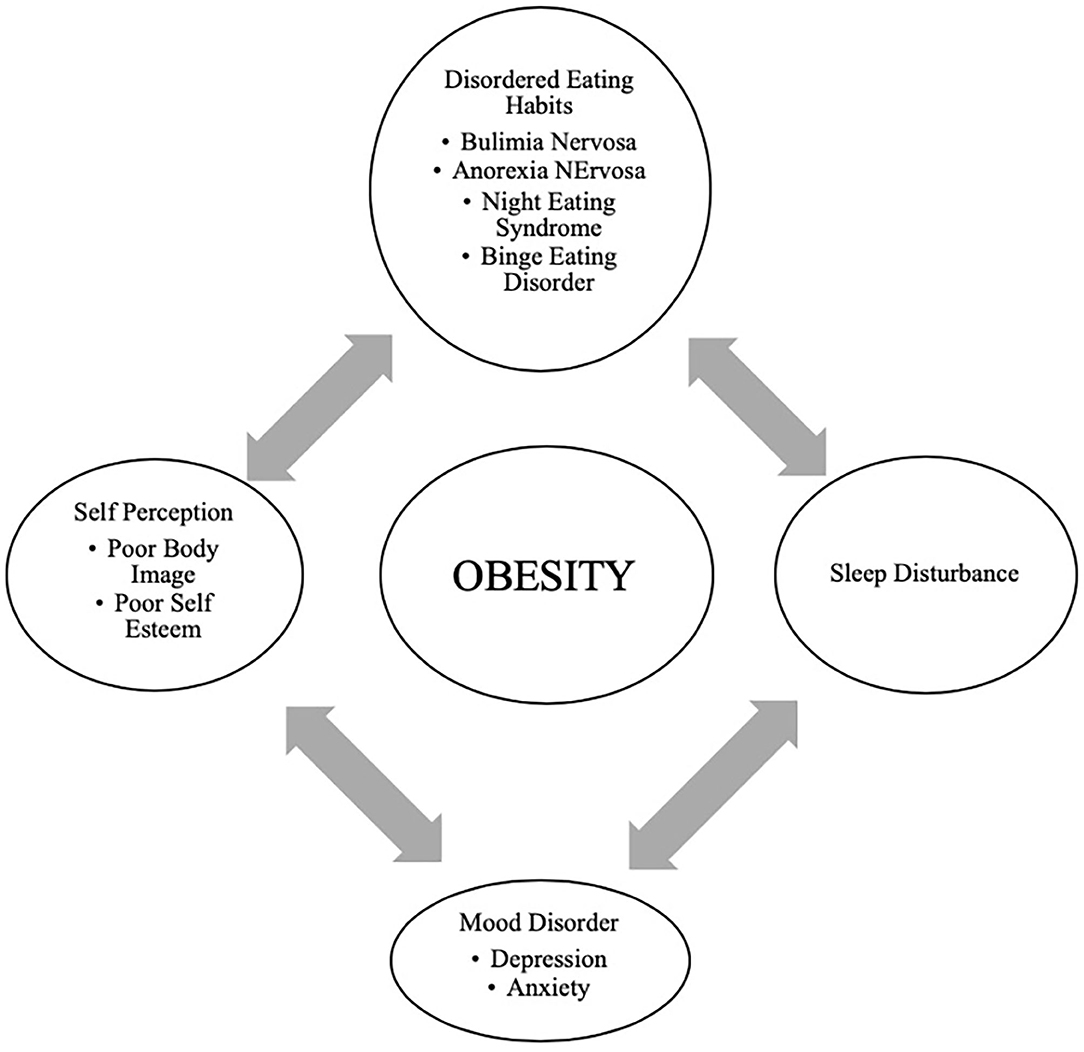
Figure 4 . Bidirectional relationship of different psychological outcomes of obesity.
Providers may not recognize DEHs when a morbidly obese patient loses the same weight as a healthy weight individual ( 149 ). It may appear as a positive result with families and others praising the individual without realizing that this youth may be engaging in destructive behaviors related to weight control. Therefore, it is essential to screen regarding the process of how weight loss was achieved ( 144 , 150 ).
Support and attention to underlying psychological concerns can positively affect treatment, overall well-being, and reduce the risk of adult obesity ( 150 ). The diagram above represents the complexity of the different psychological issues which can impact the clinical care of the obese adolescent.
Eating family meals together can improve overall dietary intake due to enhanced food choices mirrored by parents. It has also may serve as a support to individuals with DEHs if there is less attention to weight and a greater focus on appropriate, sustainable eating habits ( 148 ).
Prevention and Anticipatory Guidance
It is essential to recognize and provide preventive measures for obesity during early childhood and adolescence ( 100 , 153 , 154 ). It is well-established that early AR is a risk factor for adult obesity ( 66 – 68 ). Therefore, health care providers caring for the pediatric population need to focus on measures such as BMI but provide anticipatory guidance regarding nutritional counseling without stigmatizing or judging parents for their children's overweight/obesity ( 155 ). Although health care providers continue to pursue effective strategies to address the obesity epidemic; ironically, they frequently exhibit weight bias and stigmatizing behaviors. Research has demonstrated that the language that health care providers use when discussing a patient's body weight can reinforce stigma, reduce motivation for weight loss, and potentially cause avoidance of routine preventive care ( 155 ). In adolescents, rather than motivating positive changes, stigmatizing language regarding weight may negatively impact a teen and result in binge eating, decreased physical activity, social isolation, avoidance of health care services, and increased weight gain ( 156 , 157 ). Effective provider-patient communication using motivational interviewing techniques are useful to encourage positive behavior changes ( 155 , 158 ).
Anticipatory guidance includes educating the families on healthy eating habits and identifying unhealthy eating practices, encouraging increased activity, limiting sedentary activities such as screen time. Lifestyle behaviors in children and adolescents are influenced by many sectors of our society, including the family ( Figure 1 ) ( 3 , 24 ). Therefore, rather than treating obesity in isolation as an individual problem, it is crucial to approach this problem by focusing on the family unit. Family-based multi-component weight loss behavioral treatment is the gold standard for treating childhood obesity, and it is having been found useful in those between 2 and 6 years old ( 150 , 159 ). Additionally, empowering the parents to play an equal role in developing and implementing an intervention for weight management has shown promising results in improving the rate of obesity by decreasing screen time, promoting healthy eating, and increasing support for children's physical activity ( 160 , 161 ).
When dietary/lifestyle modifications have failed, the next option is a structured weight -management program with a multidisciplinary approach ( 15 ). The best outcomes are associated with an interdisciplinary team comprising a physician, dietician, and psychologist generally 1–2 times a week ( 15 , 162 ). However, this treatment approach is not effective in patients with severe obesity ( 122 ). Although healthier lifestyle recommendations for weight loss are the current cornerstone for obesity management, they often fail. As clinicians can attest, these behavioral and dietary changes are hard to achieve, and all too often is not effective in patients with severe obesity. Failure to maintain substantial weight loss over the long term is due to poor adherence to the prescribed lifestyle changes as well as physiological responses that resist weight loss ( 163 ). American TV hosts a reality show called “The Biggest Loser” that centers on overweight and obese contestants attempting to lose weight for a cash prize. Contestants from “The Biggest Loser” competition, had metabolic adaptation (MA) after drastic weight loss, regained more than they lost weight after 6 years due to a significant slow resting metabolic rate ( 164 ). MA is a physiological response which is a reduced basal metabolic rate seen in individuals who are losing or have lost weight. In MA, the body alters how efficient it is at turning the food eaten into energy; it is a natural defense mechanism against starvation and is a response to caloric restriction. Plasma leptin levels decrease substantially during caloric restriction, suggesting a role of this hormone in the drop of energy expenditure ( 165 ).
Pharmacological Management
The role of pharmacological therapy in the treatment of obesity in children and adolescents is limited.
Orlistat is the only FDA approved medication for weight loss in 12-18-year-olds but has unpleasant side effects ( 166 ). Another medicine, Metformin, has been used in children with signs of insulin resistance, may have some impact on weight, but is not FDA approved ( 167 ). The combination of phentermine/topiramate (Qsymia) has been FDA approved for weight loss in obese individuals 18 years and older. In studies, there has been about 9–10% weight loss over 2 years. However, caution must be taken in females as it can lead to congenital disabilities, especially with use in the first trimester of pregnancy ( 167 ).
GLP-1 agonists have demonstrated great success in effective weight loss and are approved by the FDA for adult obesity ( 168 – 170 ). A randomized control clinical trial recently published showed a significant weight loss in those using liraglutide (3.0 mg)/day plus lifestyle therapy group compared to placebo plus lifestyle therapy in children between the ages of 12–18 years ( 171 ).
Recently during the EASL conference, academic researchers and industry partners presented novel interventions targeting different gut- liver axis levels that include intestinal content, intestinal microbiome, intestinal mucosa, and peritoneal cavity ( 47 ). The focus for these therapeutic interventions within the gut-liver axis was broad and ranged anywhere from newer drugs protecting the intestinal mucus lining, restoring the intestinal barriers and improvement in the gut microbiome. One of the treatment options was Hydrogel technology which was shown to be effective toward weight loss in patients with metabolic syndrome. Hydrogel technology include fibers and high viscosity polysaccharides that absorb water in the stomach and increasing the volume, thereby improving satiety ( 47 ). Also, a clinical trial done in obese pregnant mothers using Docosahexaenoic acid (DHA) showed that the mothers' who got DHA had children with lower adiposity at 2 and 4 years of age ( 172 ). Recently the role of probiotics in combating obesity has emerged. Probiotics are shown to alter the gut microbiome that improves intestinal digestive and absorptive functions of the nutrients. Intervention including probiotics may be a possible solution to manage pediatric obesity ( 173 , 174 ). Additionally, the role of Vitamin E for treating the comorbidities of obesity such as diabetes, hyperlipidemia, NASH, and cardiovascular risk, has been recently described ( 175 , 176 ). Vitamin E is a lipid- soluble compound and contains both tocopherols and tocotrienols. Tocopherols have lipid-soluble antioxidants properties that interact with cellular lipids and protects them from oxidation damage ( 177 ). In metabolic disease, certain crucial pathways are influenced by Vitamin E and some studies have summarized the role of Vitamin E regarding the treatment of obesity, metabolic, and cardiovascular disease ( 178 ). Hence, adequate supplementation of Vitamin E as an appropriate strategy to help in the treatment of the prevention of obesity and its associated comorbidities has been suggested. Nonetheless, some clinical trials have shown contradictory results with Vitamin E supplementation ( 177 ). Although Vitamin E has been recognized as an antioxidant that protects from oxidative damage, however, a full understanding of its mechanism of action is still lacking.
Bariatric Surgery
Bariatric surgery has gained popularity since the early 2000s in the management of severe obesity. If performed earlier, there are better outcomes for reducing weight and resolving obesity-related comorbidities in adults ( 179 – 182 ). Currently, the indication for bariatric in adolescents; those who have a BMI >35 with at least one severe comorbidity (Type 2 Diabetes, severe OSA, pseudotumor cerebri or severe steatohepatitis); or BMI of 40 or more with other comorbidities (hypertension, hyperlipidemia, mild OSA, insulin resistance or glucose intolerance or impaired quality of life due to weight). Before considering bariatric surgery, these patients must have completed most of their linear growth and participated in a structured weight-loss program for 6 months ( 159 , 181 , 183 ). The American Society for Metabolic and Bariatric Surgery (AMBS) outlines the multidisciplinary approach that must be taken before a patient undergoing bariatric surgery. In addition to a qualified bariatric surgeon, the patient must have a pediatrician or provider specialized in adolescent medicine, endocrinology, gastroenterology and nutrition, registered dietician, mental health provider, and exercise specialist ( 181 ). A mental health provider is essential as those with depression due to obesity or vice versa may have persistent mental health needs even after weight loss surgery ( 184 ).
Roux-en-Y Gastric Bypass (RYGB), laparoscopic Sleeve Gastrectomy (LSG), and Gastric Banding are the options available. RYGB and LSG currently approved for children under 18 years of age ( 166 , 181 , 185 ). At present, gastric banding is not an FDA recommended procedure in the US for those under 18y/o. One study showed some improvements in BMI and severity of comorbidities but had multiple repeat surgeries and did not believe a suitable option for obese adolescents ( 186 ).
Compared to LSG, RYGB has better outcomes for excess weight loss and resolution of obesity-related comorbidities as shown in studies and clinical trials ( 183 , 184 , 187 ). Overall, LSG is a safer choice and may be advocated for more often ( 179 – 181 ). The effect on the Gut-Brain axis after Bariatric surgery is still inconclusive, especially in adolescents, as the number of procedures performed is lower than in adults. Those who underwent RYGB had increased fasting and post-prandial PYY and GLP-1, which could have contributed to the rapid weight loss ( 185 ); this effect was seen less often in patients with gastric banding ( 185 ). Another study in adult patients showed higher bile acid (BA) subtype levels and suggested a possible BA's role in the surgical weight loss response after LSG ( 188 ). Adolescents have lower surgical complication rates than their adult counterparts, hence considering bariatric surgery earlier rather than waiting until adulthood has been entertained ( 180 ). Complications after surgery include nutritional imbalance in iron, calcium, Vitamin D, and B12 and should be monitored closely ( 180 , 181 , 185 ). Although 5-year data for gastric bypass in very obese teens is promising, lifetime outcome is still unknown, and the psychosocial factors associated with adolescent adherence post-surgery are also challenging and uncertain.
Obesity in childhood and adolescence is not amenable to a single easily modified factor. Biological, cultural, and environmental factors such as readily available high-density food choices impact youth eating behaviors. Media devices and associated screen time make physical activity a less optimal choice for children and adolescents. This review serves as a reminder that the time for action is now. The need for interventions to change the obesogenic environment by instituting policies around the food industry and in the schools needs to be clarified. In clinical trials GLP-1 agonists are shown to be effective in weight loss in children but are not yet FDA approved. Discovery of therapies to modify the gut microbiota as treatment for overweigh/obesity through use of probiotics or fecal transplantation would be revolutionary. For the present, ongoing clinical research efforts in concert with pharmacotherapeutic and multidisciplinary lifestyle programs hold promise.
Author Contributions
AK, SL, and MJ contributed to the conception and design of the study. All authors contributed to the manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Gurnani M, Birken C, Hamilton. J. Childhood obesity: causes, consequences, and management. Pediatr Clin North Am. (2015) 62:821–40. doi: 10.1016/j.pcl.2015.04.001
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria. AS. Childhood obesity: causes and consequences. J Family Med Prim Care. (2015) 4:187–92. doi: 10.4103/2249-4863.154628
3. Brown CL, Halvorson EE, Cohen GM, Lazorick S, Skelton JA. Addressing childhood obesity: opportunities for prevention. Pediatr Clin North Am. (2015) 62:1241–61. doi: 10.1016/j.pcl.2015.05.013
4. Qasim A, Turcotte M, de Souza RJ, Samaan MC, Champredon D, Dushoff J, et al. On the origin of obesity: identifying the biological, environmental, and cultural drivers of genetic risk among human populations. Obes Rev. (2018) 19:121–49. doi: 10.1111/obr.12625
5. Rinninella E, Raoul P, Cintoni M, Fransceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms. (2019) 7:14. doi: 10.3390/microorganisms7010014
6. Indrio F, Martini S, Francavilla R, Corvaglia L, Cristofori F, Mastrolia SA, et al. Epigenetic matters: the link between early nutrition, microbiome, and long-term health development. Front Pediatr. (2017) 5:178. doi: 10.3389/fped.2017.00178
7. Marcovecchio ML, Gorman S, Watson LPE, Dunger DB, Beardsall K. Catch-up growth in children born small for gestational age related to body composition and metabolic risk at six years of age in the UK. Horm Res Paediatr. (2020) 93:119–27. doi: 10.1159/000508974
8. Koletzko B, Fishbein M, Lee WS, Moreno L, Mouane N, Mouzaki M, et al. Prevention of childhood obesity: a position paper of the global federation of international societies of paediatric gastroenterology, hepatology nutrition (FISPGHAN). J Pediatr Gastroenterol Nutr. (2020) 70:702–10. doi: 10.1097/MPG.0000000000002708
9. Pulgarón ER. Childhood obesity: a review of increased risk for physical and psychological comorbidities. Clin Ther. (2013) 35:A18–32. doi: 10.1016/j.clinthera.2012.12.014
10. De Leonibus C, Marcovecchio ML, Chiarelli F. Update on statural growth and pubertal development in obese children. Pediatr Rep. (2012) 4:e35. doi: 10.4081/pr.2012.e35
11. Witchel SF, Burghard AC, Tao RH, Oberfield SE. The diagnosis and treatment of PCOS in adolescents. Curr Opin Pediatr . (2019) 31:562–9. doi: 10.1097/MOP.0000000000000778
12. Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics . (2012) 130:e714–55. doi: 10.1542/peds.2012-1672
CrossRef Full Text | Google Scholar
13. Rankin J, Matthews L, Cobley S, Han A, Sanders R, Wiltshire HD, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther . (2016) 7:125–46. doi: 10.2147/AHMT.S101631
14. Topçu S, Orhon FS, Tayfun M, Uçaktürk SA, Demirel F. Anxiety, depression, and self-esteem levels in obese children: a case-control study. J Pediatr Endocrinol Metabol. (2016) 29:357–61. doi: 10.1515/jpem-2015-0254
15. Katzmarzyk PT, Barlow S, Bouchard C, Catalano PM, Hsia DS, Inge TH, et al. An evolving scientific basis for the prevention and treatment of pediatric obesity. Int J Obes. (2014) 38:887–905. doi: 10.1038/ijo.2014.49
16. Brown T, Moore TH, Hooper L, Gao Y, Zayegh A, Ijaz S, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev . (2019) 7:CD001871. doi: 10.1002/14651858.CD001871.pub4
17. Smith E, Scarborough P, Rayner M, Briggs ADM. Should we tax unhealthy food and drink? Proc Nutr Soc. (2019) 77:314–20. doi: 10.1017/S0029665117004165
18. Adab P, Pallan M, Whincup PH. Is BMI the best measure of obesity? BMJ. (2018) 360:k 1274. doi: 10.1136/bmj.k1274
19. Anderson LN, Carsley S, Lebovic G, Borkhoff CM, Maguire JL, Parkin PC, et al. Misclassification of child body mass index from cut-points defined by rounded percentiles instead of Z-scores. BMC Res Notes. (2017) 10:639. doi: 10.1186/s13104-017-2983-0
20. Must A, Anderson SE. Body mass index in children and adolescents: consideration for population-based applications. Int J Obes. (2006) 30:590–4. doi: 10.1038/sj.ijo.0803300
21. Flegal KM, Wei R, Ogden C. Weight-for-stature compared with body mass index-for-age growth charts for the United States from the centers for disease control and prevention. Am J Clin Nutr. (2002) 75:761–6.22. doi: 10.1093/ajcn/75.4.761
22. Himes JH, Dietz WH. Guidelines for overweight in adolescent preventive services: recommendations from an expert committee. The expert committee on clinical guidelines for overweight in adolescent preventive services. Am J Clin Nutr. (1994) 59:307–16. doi: 10.1093/ajcn/59.2.307
23. Lazarus R, Baur L, Webb K, Blyth F. Body mass index in screening for adiposity in children and adolescents: systematic evaluation using receiver operating characteristic curves. Am J Clin Nutr. (1996) 63:500–6. doi: 10.1093/ajcn/63.4.500
24. McGinnis JM, Gootman JA. Food Marketing to Children and Youth: Threat or Opportunity? Institute of Medicine of the National Academies. Washington, DC: The National Academies Press. (2006).
Google Scholar
25. Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol. (2008) 70:239–55. doi: 10.1146/annurev.physiol.70.113006.100506
26. Scaglioni S, De Cosmi V, Ciappolino V, Parazzini F, Brambilla P, Agostoni C. Factors influencing children's eating behaviours. Nutrients. (2018) 10:706. doi: 10.3390/nu10060706
27. Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. (2008) 37:811–23. doi: 10.1016/j.ecl.2008.08.005
28. Niswender KD, Baskin DG, Schwartz MW. Review insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab. (2004) 15:362–9. doi: 10.1016/j.tem.2004.07.009
29. Niswender KD, Schwartz MW. Review insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. (2003) 24:1–10. doi: 10.1016/S0091-3022(02)00105-X
30. Amitani M, Asakawa A, Amitani H, Inui. A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. (2013) 7:51. doi: 10.3389/fnins.2013.00051
31. Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. (2003) 37:649–61. doi: 10.1016/S0896-6273(03)00063-1
32. Buhmann H, le Roux CW, Bueter M. The gut–brain axis in obesity. Best Prac Res Clin Gastroenterol. (2014) 28:559–71. doi: 10.1016/j.bpg.2014.07.003
33. Cone RD. Review anatomy and regulation of the central melanocortin system. Nat Neurosci. (2005) 8:571–8. doi: 10.1038/nn1455
34. Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. (2017) 10:679–89. doi: 10.1242/dmm.026609
35. Labarthe A, Fiquet O, Hassouna R, Zizzari P, Lanfumey L, Ramoz N, et al. Ghrelin-derived peptides: a link between appetite/reward, gh axis, and psychiatric disorders? Front Endocrinol. (2014) 5:163. doi: 10.3389/fendo.2014.00163
36. Hills R. D Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. (2019) 11:1613. doi: 10.3390/nu11071613
37. Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. (2017) 2:747–56. doi: 10.1016/S2468-1253(17)30147-4
38. Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. (2016) 73:147–62. doi: 10.1007/s00018-015-2061-5
39. Derrien M, Alvarez AS, de Vos WM. The gut microbiota in the first decade of life. Trends Microbiol. (2019) 27:997–1010.40. doi: 10.1016/j.tim.2019.08.001
40. Dao MC, Clément K. Gut microbiota and obesity: concepts relevant to clinical care. Eur J Intern Med . (2018) 48:18–24.41. doi: 10.1016/j.ejim.2017.10.005
41. Kim KN, Yao Y., Ju SY. Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. (2019) 11:2512. doi: 10.3390/nu11102512
42. Castaner O, Goday A, Park YM, Lee SH, Magkos F, Shiow STE, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. (2018) 2018:4095789. doi: 10.1155/2018/4095789
43. Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in firmicutes populations. Enviroin Microbiol. (2017) 19:95–105. doi: 10.1111/1462-2920.13463
44. Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TMS, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes . (2014) 4:e121. doi: 10.1038/nutd.2014.23
45. Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TMS. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes . (2014) 38:1525–31. doi: 10.1038/ijo.2014.46
46. Barczyńska R, Litwin M, Slizewska K, Szalecki M, Berdowska A, Bandurska K, et al. Bacterial microbiota fatty acids in the faeces of overweight obese children. Pol. J. Microbiol. (2018) 67:339–45. doi: 10.21307/pjm-2018-041
47. Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
48. Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J Pediatr. (2019) 207:64–70. doi: 10.1016/j.jpeds.2018.11.021
49. Ranucci G, Spagnuolo MI, Iorio R. Obese children with fatty liver: Between reality and disease mongering. World J Gastroenterol. (2017) 23:8277–82. doi: 10.3748/wjg.v23.i47.8277
50. Cox AJ, West NP, Cripps A. W. Obesity, inflammation, and the gut microbiota. Lancet Diabet Endocrinol. (2015) 3:207–15. doi: 10.1016/S2213-8587(14)70134-2
51. Seitz J, Trinh S, Herpertz-Dahlmann B. The microbiome and eating disorders. Psychiatr Clin North Am. . (2019) 42:93–103. doi: 10.1016/j.psc.2018.10.004
52. Deans E. Microbiome and mental health in the modern environment. J Physiol Anthropol. (2016) 36:1. doi: 10.1186/s40101-016-0101-y
53. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res . (2019) 97:1223–41. doi: 10.1002/jnr.24476
54. Ranadive SA, Vaisse C. Lessons from extreme human obesity: monogenic disorders. Endocrinol Metab Clin North Am. (2008) 37:733–51. doi: 10.1016/j.ecl.2008.07.003
55. Soliman AT, Yasin M, Kassem A. Leptin in pediatrics: a hormone from adipocyte that wheels several functions in children. Indian J Endocrinol Metab . (2012) 16(Suppl. 3):S577–87. doi: 10.4103/2230-8210.105575
56. Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. (2007) 356:237–47. doi: 10.1056/NEJMoa063988
57. Mutch DM, Clément K. Unraveling the genetics of human obesity. PLoS Genet. (2006) 2:e188. doi: 10.1371/journal.pgen.0020188
58. Crocker MK, Yanovski JA. Pediatric obesity: etiology and treatment. Endocrinol Metab Clin North Am. (2009) 38:525–48. doi: 10.1016/j.ecl.2009.06.007
59. Huvenne H, Dubern B, Clément K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts. (2016) 9:158–73. doi: 10.1159/000445061
60. Stefan M, Nicholls RD. What have rare genetic syndromes taught us about the pathophysiology of the common forms of obesity? Curr Diab Rep. (2004) 4:143–50. doi: 10.1007/s11892-004-0070-0
61. Hetherington MM, Cecil JE. Gene-Environment interactions in obesity. Forum Nutr. (2009) 63:195–203. doi: 10.1159/000264407
62. Reddon H, Guéant JL, Meyre D. The importance of gene-environment interactions in human obesity. Clin Sci. (2016) 130:1571–97. doi: 10.1042/CS20160221
63. Castillo JJ, Orlando RA, Garver WS. Gene-nutrient interactions and susceptibility to human obesity. Genes Nutr. (2017) 12:29. doi: 10.1186/s12263-017-0581-3
64. Heianza Y, Qi L. Gene-Diet interaction and precision nutrition in obesity. Int J Mol Sci. (2017) 18:787. doi: 10.3390/ijms18040787
65. Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. (2018) 6:223–36. . doi: 10.1016/S2213-8587(17)30200-0
66. Bouchard L, Drapeau V, Provencher V, Lemieux S, Chagnon Y, Rice T, et al. Neuromedin beta: a strong candidate gene linking eating behaviors and susceptibility to obesity. Am J Clin Nutr. (2004) 80:1478–86. . doi: 10.1093/ajcn/80.6.1478
67. Grimm ER, Steinle NI. Genetics of eating behavior: established and emerging concepts. Nutr Rev. (2011) 69:52–60. . doi: 10.1111/j.1753-4887.2010.00361.x
68. van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell. (2015) 161:119–32. . doi: 10.1016/j.cell.2015.03.008
69. Martinez JA. Bodyweight regulation causes of obesity. Proc Nutr Soc. (2000) 59:337–45. Review. doi: 10.1017/S0029665100000380
70. Rask-Andersen M, Karlsson T, Ek WE, Johansson Å. Gene-environment interaction study for BMI reveals interactions between genetic factors and physical activity, alcohol consumption and socioeconomic status. PLoS Genet. (2017) 5:1. doi: 10.1371/journal.pgen.1006977
71. Xulong S, Pengzhou L, Xiangwu Y, Weizheng L, Xianjie Q, Shaihong Z, et al. From genetics and epigenetics to the future of precision treatment for obesity. Gastroenterol Rep. (2017) 5:266–70. doi: 10.1093/gastro/gox033
72. Bianco-Miotto T, Craig JM, Gasser YP, van dijk SJ, Ozanne SE. Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis. (2017) 8:513–9. doi: 10.1017/S2040174417000733
73. van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS, Members of EpiSCOPE. Epigenetics and human obesity. Int J Obes . (2015) 39:85–97. doi: 10.1038/ijo.2014.34
74. Li Y. Epigenetic mechanisms link maternal diets and gut microbiome to obesity in the offspring. Front Genet . (2018) 9:342. doi: 10.3389/fgene.2018.00342
75. Kaufman J, Montalvo-Ortiz JL, Holbrook H, O'Loughlin K, Orr C, Kearney C, et al. Adverse childhood experiences, epigenetic measures, and obesity in youth. J Pediatr. (2018) 202:150–6.76. doi: 10.1016/j.jpeds.2018.06.051
76. May Gardner R, Feely A, Layte R, Williams J, McGavock J. Adverse childhood experiences are associated with an increased risk of obesity in early adolescence: a population-based prospective cohort study. Pediatr Res. (2019) 86:522–28. doi: 10.1038/s41390-019-0414-8
77. Cheon BK„ Hong YY. Mere experience of low subjective socioeconomic status stimulates appetite food intake. Proc Natl Acad Sci USA . (2017) 114:72–7. doi: 10.1073/pnas.1607330114
78. Alegría-Torres JA, Baccarelli A, Bollati V. Epigenetics lifestyle. Epigenomics . (2011) 3:267-77. doi: 10.2217/epi.11.22
79. Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics . (2011) 101:539–49.
PubMed Abstract | Google Scholar
80. Birch L, Savage JS, Ventura A. Influences on the development of children's eating behaviours: from infancy to adolescence. Can J Diet Pract Res. (2007) 68:s1–s56.
81. Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977- 1998. JAMA. (2003) 289:450–53. . doi: 10.1001/jama.289.4.450
82. Munoz KA, Krebs-Smith SM, Ballard-Barbash R, Cleveland LE. Food intakes of US children and adolescents compared with recommendations. Pediatrics. (1997) 100:323–29. doi: 10.1542/peds.100.3.323
83. Fisher JO, Birch LL. Restricting access to palatable foods affects children's behavioral response, food selection, and intake. Am J Clin Nutr. (1999) 69:1264–72. doi: 10.1093/ajcn/69.6.1264
84. Faith MS, Scanlon KS, Birch LL, Francis LA, Sherry B. Parent-child feeding strategies and their relationships to child eating and weight status. Obes Res. (2004) 12:1711–22. . doi: 10.1038/oby.2004.212
85. Smith AD, Sanchez N, Reynolds C, Casamassima M, Verros M, Annameier SK, et al. Associations of parental feeding practices and food reward responsiveness with adolescent stress-eating. Appetite. (2020) 152:104715. doi: 10.1016/j.appet.2020.104715
86. Lowe CJ, Morton JB, Reichelt AC. Adolescent obesity and dietary decision making-a brain-health perspective. Lancet Child Adolesc Health. (2020) 4:388–96. doi: 10.1016/S2352-4642(19)30404-3
87. Goran MI, Treuth MS. Energy expenditure, physical activity, and obesity in children. Pediatr Clin North Am. (2001) 48:931–53. doi: 10.1016/S0031-3955(05)70349-7
88. Romieu I, Dossus L, Barquera S, Blottière HM, Franks PW, Gunter M, et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. (2017) 28:247–58. doi: 10.1007/s10552-017-0869-z
89. Mattes R, Foster GD. Food environment and obesity. Obesity. (2014) 22:2459–61. doi: 10.1002/oby.20922
90. Ickovics JR, O'Connor Duffany K, Shebl FM, Peters SM, Read MS, Gilstad-Hayden KR, et al. Implementing school-based policies to prevent obesity: cluster randomized trial. Am J Prev Med. (2019) 56:e1–11. doi: 10.1016/j.amepre.2018.08.026
91. Micha R, Karageorgou D, Bakogianni I, Trichia E, Whitsel LP, Story M, et al. Effectiveness of school food environment policies on children's dietary behaviors: A systematic review and meta-analysis. PLoS ONE. ( 2018 ) 13:e0194555. doi: 10.1371/journal.pone.0194555
92. Cawley J, Frisvold D, Hill A, Jones DJ. The impact of the philadelphia beverage tax on purchases and consumption by adults and children. Health Econ. (2019) 67:102225. doi: 10.1016/j.jhealeco.2019.102225
93. John Cawley J, Thow AM, Wen K, Frisvold D. The economics of taxes on sugar-sweetened beverages: a review of the effects on prices, sales, cross-border shopping, and consumption. Annu Rev Nutr. (2019) 39:317–38. doi: 10.1146/annurev-nutr-082018-124603
94. Fuller C, Lehman E, Hicks S, Novick MB. Bedtime use of technology and associated sleep problems in children. Glob Pediatr Health. (2017) 4:2333794X17736972. doi: 10.1177/2333794X17736972
95. Chahal H, Fung C, Kuhle S, Veugelers PJ. Availability and night-time use of electronic entertainment and communication devices are associated with short sleep duration and obesity among Canadian children. Pediatr Obes. (2012) 8:42–51. doi: 10.1111/j.2047-6310.2012.00085.x
96. Minghua T. Protein intake during the first two years of life and its association with growth and risk of overweight. Int J Environ Res Public Health. ( 2018 ) 15:1742. doi: 10.3390/ijerph15081742
97. Azad MB, Vehling L, Chan D, Klopp A, Nickel NC, McGavock JM, et al. Infant feeding and weight gain: separating breast milk from breastfeeding and formula from food. Pediatrics. (2018) 142:e20181092. doi: 10.1542/peds.2018-1092
98. Lin L, Amissah E, Gamble GD, Crowther CA, Harding JE. Impact of macronutrient supplements on later growth of children born preterm or small for gestational age: a systematic review and meta-analysis of randomised and quasirandomised controlled trials. PLoS Med. (2020) 17:e1003122. . doi: 10.1371/journal.pmed.1003122
99. Rzehak P, Oddy WH, Mearin ML, Grote V, Mori TA, Szajewska H, et al. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am J Clin Nutr. (2017) 106:568–80. doi: 10.3945/ajcn.116.140962
100. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2017) 102:709–57. doi: 10.1210/jc.2016-2573
101. Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics . (1998) 101:E5. doi: 10.1542/peds.101.3.e5
102. Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. (2018) 379:1303–12. doi: 10.1056/NEJMoa1803527
103. Jabakhanji SB, Boland F, Ward M, Biesma RJ. Body mass index changes in early childhood. Pediatrics. (2018) 202:106–14. doi: 10.1016/j.jpeds.2018.06.049
104. Chung S. Growth and puberty in obese children and implications of body composition. J Obes Metab Syndr. (2017) 26:243–50. doi: 10.7570/jomes.2017.26.4.243
105. Tagi VM, Giannini C, Chiarelli F. Insulin resistance in children. Front Endocrinol. (2019) 10:342. doi: 10.3389/fendo.2019.00342
106. Kelesidis I, Mantzoros CS. Leptin and its emerging role in children and adolescents. Clin Pediatr Endocrinol . (2006) 15:1–14. doi: 10.1297/cpe.15.1
107. Burt Solorzano CM, McCartney CR, Obesity and the pubertal transition in girls and boys. Reproduction . (2010) 140:399–410. doi: 10.1530/REP-10-0119
108. Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health. (2017) 14:1266. doi: 10.3390/ijerph14101266
109. Lee JM, Wasserman R, Kaciroti N, Gebremariam A, Steffes J, Dowshen S, et al. Timing of puberty in overweight vs. obese boys. Pediatrics. (2016) 137:e20150164. doi: 10.1542/peds.2015-0164
110. He J, Kang Y, Zheng L. Serum levels of LH, IGF-1 and leptin in girls with idiopathic central precocious puberty (ICPP) and the correlations with the development of ICPP. Minerva Pediatr . (2018). doi: 10.23736/S0026-4946.18.05069-7
111. Kang MJ, Oh YJ, Shim YS, Baek JW, Yang S, Hwang IT. The usefulness of circulating levels of leptin, kisspeptin, and neurokinin B in obese girls with precocious puberty. Gynecol Endocrinol. (2018) 34:627–30. doi: 10.1080/09513590.2017.1423467
112. Rendo-Urteaga T, Ferreira de Moraes AC, Torres-Leal FL, Manios Y, Gottand F, Sjöström M, et al. Leptin and adiposity as mediators on the association between early puberty and several biomarkers in European adolescents: the helena study. J Pediatr Endocrinol Metab. (2018) 31:1221–29. doi: 10.1515/jpem-2018-0120
113. Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab. (2002) 16:263–72. doi: 10.1053/beem.2002.0203
114. Franks S. Polycystic ovary syndrome in adolescents. Int J Obes. (2008) 32:1035–41. doi: 10.1038/ijo.2008.61
115. Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers K, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. (2017) 1:00019.
116. Patinkin ZW, Feinn R, Santos M. Metabolic consequences of obstructive sleep apnea in adolescents with obesity: a systematic literature review and meta-analysis. Childhood Obes. (2017) 13:102–10. doi: 10.1089/chi.2016.0248
117. Kaditis A. From obstructive sleep apnea in childhood to cardiovascular disease in adulthood: what is the evidence? Sleep. (2010) 33:1279–80. doi: 10.1093/sleep/33.10.1279
118. Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rose G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci . (2014) 16:378–400. doi: 10.3390/ijms16010378
119. Eisele HJ, Markart P, Schulz R. Obstructive sleep apnea, oxidative stress, and cardiovascular disease: evidence from human studies. Oxid Med Cell Longev . (2015) 2015:608438. doi: 10.1155/2015/608438
120. Hui W, Slorach C, Guerra V, Parekh RS, Hamilton J, Messiha S, et al. Effect of obstructive sleep apnea on cardiovascular function in obese youth. Am J Cardiol. (2019) 123:341–7. doi: 10.1016/j.amjcard.2018.09.038
121. Matteoni CA, Younossi Z .m., Gramlich T, Boparai N, Liu YC, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. (1999) 1999:116:1413. doi: 10.1016/S0016-5085(99)70506-8
122. Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. ( 2004 ) 8:549. doi: 10.1016/j.cld.2004.04.010
123. Huang JS, Barlow SE, Quiros-Tejeira RE, Scheimann A, Skelton J, Suskind D, et al. Childhood obesity for pediatric gastroenterologists. J Pediatr Gastroenterol Nutr. (2013) 2013:56:99. doi: 10.1097/MPG.0b013e31826d3c62
124. Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS ONE. ( 2015 ) 10:e0140908. doi: 10.1371/journal.pone.0140908
125. Nobili V, Alisi A, Newton KP, Schwimmer JB. Comparison of the phenotype and approach to pediatric vs adult patients with nonalcoholic fatty liver disease. Gastroenterology. (2016) 150:1798–810. doi: 10.1053/j.gastro.2016.03.009
126. Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. (2009) 7:234–38. doi: 10.1016/j.cgh.2008.11.005
127. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angula P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. (2009) 58:1538. doi: 10.1136/gut.2008.171280
128. Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation . (2008) 118:277. doi: 10.1161/CIRCULATIONAHA.107.739920
129. Perry DC, Metcalfe D, Lane S, Turner S. Childhood obesity and slipped capital femoral epiphysis. Pediatrics. (2018) 142:e20181067. doi: 10.1542/peds.2018-1067
130. Zavala-Crichton JP, Esteban-Cornejo I, Solis-Urra P, Mora-Gonzalez J, Cadenas-Sanchez C, Rodriguez-Ayllon M, et al. Association of sedentary behavior with brain structure and intelligence in children with overweight or obesity: Active Brains Project . (2020) 9:1101. doi: 10.3390/jcm9041101
131. Ronan L, Alexander-Bloch A, Fletcher PC. Childhood obesity, cortical structure, and executive function in healthy children. Cereb Cortex. (2019) 30:2519–28. doi: 10.1093/cercor/bhz257
132. Baker ER. Body weight and the initiation of puberty. Clin Obstetr Gynecol. (1985) 28:573–9. doi: 10.1097/00003081-198528030-00013
133. Siervogel RM, Demerath EW, Schubert C, Remsberg KE, Chumlea WM, Sun S, et al. Puberty and body composition. Horm Res. (2003) 60:36–45. doi: 10.1159/000071224
134. Sadeeqa S, Mustafa T, Latif S. Polycystic ovarian syndrome- related depression in adolescent girls. J Pharm Bioallied Sci. (2018) 10:55–9. doi: 10.4103/JPBS.JPBS_1_18
135. Himelein MJ, Thatcher SS. Depression and body image among women with polycystic ovary syndrome. J Health Psychol . (2006) 11:613–25. doi: 10.1177/1359105306065021
136. Magge SN, Goodman E, Armstrong SC. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. (2017) 140:e20171603. doi: 10.1542/peds.2017-1603
137. Mauras N, Delgiorno C, Kollman C, Bird K, Morgan M, Sweeten S, et al. Obesity without established comorbidities of the metabolic syndrome is associated with a proinflammatory and prothrombotic state, even before the onset of puberty in children. J Clin Endocrinol Metab. (2010) 95:1060–8. doi: 10.1210/jc.2009-1887
138. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. (2004) 350:2362–74. doi: 10.1056/NEJMoa031049
139. Erdmann J, Kallabis B, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am J Physiol Endocrinol Metabol. (2008) 294:e568–75. . doi: 10.1152/ajpendo.00560.2007
140. Pulido-Arjona L, Correa-Bautista JE, Agostinis-Sobrinho C, Mota J, Santos R, Correa-Rodrigues M, et al. Role of sleep duration and sleep- related problems in the metabolic syndrome among children and adolescents. Ital J Pediatr. (2018) 44:9. doi: 10.1186/s13052-018-0451-7
141. Harriger JA, Thompson JK. Psychological consequences of obesity: weight bias and body image in overweight and obese youth. Int Rev Psychiatry. (2012) 24:247–53. . doi: 10.3109/09540261.2012.678817
142. Bacchini D, Licenziati MR, Garrasi A, Corciulo N, Driul D, Tanas R, et al. Bullying and victimization in overweight and obese outpatient children and adolescents: an italian multicentric study. PLoS ONE. (2015) 10:e0142715. doi: 10.1371/journal.pone.0142715
143. Loth KA, Watts AW, Berg PVD, Neumark-Sztainer D. Does body satisfaction help or harm overweight teens? A 10-year longitudinal study of the relationship between body satisfaction and body mass index. J Adolesc Health. (2015) 57:559–61. doi: 10.1016/j.jadohealth.2015.07.008
144. Gowey MA, Lim CS, Clifford LM, Janicke DM. Disordered eating and health-related quality of life in overweight and obese children. J Pediatr Psychol. (2014) 39:552–61. doi: 10.1093/jpepsy/jsu012
145. Mannan M, Mamun A, Doi S, Clavarino A. Prospective associations between depression and obesity for adolescent males and females- a systematic review and meta-analysis of longitudinal studies. PLoS ONE. (2016) 11:e0157240. doi: 10.1371/journal.pone.0157240
146. Ruiz LD, Zuelch ML, Dimitratos SM, Scherr RE. Adolescent obesity: diet quality, psychosocial health, and cardiometabolic risk factors. Nutrients. (2019) 12:43. doi: 10.3390/nu12010043
147. Goldschmidt AB, Aspen VP, Sinton MM, Tanofsky-Kraff M, Wilfley DE. Disordered eating attitudes and behaviors in overweight youth. Obesity. (2008) 16:257–64. doi: 10.1038/oby.2007.48
148. Golden NH, Schneider M, Wood C. Preventing obesity and eating disorders in adolescents. Pediatrics. (2016) 138:e1–e12. doi: 10.1542/peds.2016-1649
149. Rastogi R, Rome ES. Restrictive eating disorders in previously overweight adolescents and young adults. Cleve Clin J Med. (2020) 87:165–71. doi: 10.3949/ccjm.87a.19034
150. Hayes JF, Fitzsimmons-Craft EE, Karam AM, Jakubiak JL, Brown ME, Wilfley D. Disordered eating attitudes and behaviors in youth with overweight and obesity: implications for treatment. Curr Obes Rep. (2018) 7:235. doi: 10.1007/s13679-018-0316-9
151. Goldschmidt AB, Wall MM, Loth KA, Neumark-Sztainer D. Risk factors for disordered eating in overweight adolescents and young adults: Table I. J Pediatr Psychol. (2015) 40:1048–55. doi: 10.1093/jpepsy/jsv053
152. Follansbee-Junger K, Janicke DM, Sallinen BJ. The influence of a behavioral weight management program on disordered eating attitudes and behaviors in children with overweight. J Am Diet Assoc. (2010) 110:653–9. doi: 10.1016/j.jada.2010.08.005
153. Blake-Lamb TL, Locks LM, Perkins ME, Woo Baidal JA, Cheng ER, Taveras EM. Interventions for childhood obesity in the first 1,000 days a systematic review. Am J Prev Med. (2016) 50:780–9. doi: 10.1016/j.amepre.2015.11.010
154. McGuire S. Institute of Medicine (IOM). Early childhood obesity prevention policies. Washington, DC: The National Academies Press. Adv Nutr . (2011) 3:56–7. doi: 10.3945/an.111.001347
155. Pont SJ, Puhl R, Cook SR, Slusser W. Stigma experienced by children and adolescents with obesity. Pediatrics. (2017) 140:e20173034. doi: 10.1542/peds.2017-3034
156. Puhl R, Suh Y. Health consequences of weight stigma: implications for obesity prevention and treatment. Curr Obes Rep. (2015) 4:182–90. doi: 10.1007/s13679-015-0153-z
157. Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. (2003) 289:1813–9. doi: 10.1001/jama.289.14.1813
158. Carcone AI, Jacques-Tiura AJ, Brogan Hartlieb KE, Albrecht T, Martin T. Effective patient-provider communication in pediatric obesity. Pediatr Clin North Am. (2016) 63:525–38. doi: 10.1016/j.pcl.2016.02.002
159. Coppock JH, Ridolfi DR, Hayes JF, Paul MS, Wilfley DE. Current approaches to the management of pediatric overweight and obesity. Curr Treat Options Cardiovasc Med. (2014) 16:343. doi: 10.1007/s11936-014-0343-0
160. Davison KK, Jurkowski JM, Li K, Kranz S, Lawson HA. A childhood obesity intervention developed by families for families: results from a pilot study. Int J Behav Nutr Phys Act. (2013) 10:3. doi: 10.1186/1479-5868-10-3
161. Krystia O, Ambrose T, Darlington G, Ma DWL, Buchholz AC, Haines J. A randomized home- based childhood obesity prevention pilot intervention has favourable effects on parental body composition: preliminary evidence from the guelph family health study. BMC Obes. (2019) 6:10. doi: 10.1186/s40608-019-0231-y
162. Skjåkødegård HF, Danielsen YS, Morken M, Linde SRF, Kolko RP, Balantekin KN, et al. Study protocol: a randomized controlled trial evaluating the effect of family-based behavioral treatment of childhood and adolescent obesity–The FABO-study. BMC Public Health. (2016) 16:1106. doi: 10.1186/s12889-016-3755-9
163. Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. (2018) 102:183–97. doi: 10.1016/j.mcna.2017.08.012
164. Hall KD. Diet vs. exercise in “the biggest loser” weight loss competition. Obesity. (2013) 21:957–9. doi: 10.1002/oby.20065
165. Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metabol. (2011) 96:E1512–E516. doi: 10.1210/jc.2011-1286
166. Kaur KK, Allahbadia G, Singh M. Childhood obesity: a comprehensive review of epidemiology, aetiopathogenesis and management of this global threat of the 21st century. Acta Sci Paediatr. (2019) 2:56–66. doi: 10.31080/ASPE.2019.02.0132
167. Crimmins NA, Xanthakos SA. Obesity. in Neinstein's Adolescent and Young Adult Health , Guide. Philadelphia, PA: Wolters Kluwer (2016). p. 295–300.
168. Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomized, double-blind, placebo-controlled study. Lancet. (2009) 374:1606–16. doi: 10.1016/S0140-6736(09)61375-1
169. Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. (2012) 2012:672658. doi: 10.1155/2012/672658
170. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. (2015) 373:11–22 . doi: 10.1056/NEJMoa1411892
171. Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. (2020) 382:2117–28. doi: 10.1056/NEJMoa1916038
172. Foster BA, Escaname E, Powell T, Larsen B, Siddiqui SK, Menchaca J, et al. Randomized controlled trial of DHA supplementation during pregnancy: child adiposity outcomes. Nutrients . (2017) 9:566. doi: 10.3390/nu9060566
173. Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-Rad J, et al. Gut microbiota and obesity: a role for probiotics. Nutrients. (2019) 11:2690. doi: 10.3390/nu11112690
174. Vajro P, Mandato C, Veropalumbo C, De Micco I. Probiotics: a possible role in treatment of adult and pediatric nonalcoholic fatty liver disease. Ann Hepatol. (2013) 12:161–63. doi: 10.1016/S1665-2681(19)31401-2
175. Zhao L, Fang X, Marshall M, Chung S. Regulation of obesity and metabolic complications by gamma and delta tocotrienols. Molecules. (2016) 21:344. doi: 10.3390/molecules21030344
176. Wong SK, Chin K-Y, Suhaimi FH, Ahmad F, Ima-Nirwana S. Vitamin E as a potential interventional treatment for metabolic syndrome: evidence from animal and human studies. Front Pharmacol. (2017) 8:444. doi: 10.3389/fphar.2017.00444
177. Galli F, Azzi A, Birringer A, Cook-Mills JM, Eggersdorfer M, Frank J, et al. Vitamin E: Emerging aspects and new directions. Free Radic Biol Med. (2017) 102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017
178. Galmés S, Serra F, Palou A. Vitamin E metabolic effects and genetic variants: a challenge for precision nutrition in obesity and associated disturbances. Nutrients . (2018) 10:1919. doi: 10.3390/nu10121919
179. Ahn SM. Current issues in bariatric surgery for adolescents with severe obesity: durability, complications, and timing of intervention. J. Obes Metabol Syndrome. (2020) 29:4–11. doi: 10.7570/jomes19073
180. Lamoshi A, Chernoguz A, Harmon CM, Helmrath M. Complications of bariatric surgery in adolescents. Semin Pediatr Surg. (2020) 29:150888. doi: 10.1016/j.sempedsurg.2020.150888
181. Weiss AL, Mooney A, Gonzalvo JP. Bariatric surgery. Adv Pediatr. (2017) 6:269–83. doi: 10.1016/j.yapd.2017.03.005
182. Stanford FC, Mushannen T, Cortez P, Reyes KJC, Lee H, Gee DW, et al. Comparison of short and long-term outcomes of metabolic and bariatric surgery in adolescents and adults. Front Endocrinol. (2020) 11:157. doi: 10.3389/fendo.2020.00157
183. Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the teen-longitudinal assessment of bariatric surgery (Teen-LABS) study . JAMA Pediatr . (2014) 168:47–53. doi: 10.1001/jamapediatrics.2013.4296
184. Järvholm K, Bruze G, Peltonen M, Marcus C, Flodmark CE, Henfridsson P, et al. 5-year mental health and eating pattern outcomes following bariatric surgery in adolescents: a prospective cohort study. Lancet Child AdolescHealth . (2020) 4:210–9. doi: 10.1016/S2352-4642(20)30024-9
185. Xanthakos SA. Bariatric surgery for extreme adolescent obesity: indications, outcomes, and physiologic effects on the gut–brain axis. Pathophysiology. (2008) 15:135–46. doi: 10.1016/j.pathophys.2008.04.005
186. Zitsman JL, Digiorgi MF, Kopchinski JS, Sysko R, Lynch L, Devlin M, et al. Adolescent Gastric Banding: a five-year longitudinal study in 137 individuals. Surg Obes Relat Dis. (2018) 14. doi: 10.1016/j.soard.2018.09.030
187. Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+). A prospective follow-up analysis. Lancet Diabet Endocrinol . (2017) 5:165–73. doi: 10.1016/S2213-8587(16)30315-1
188. Kindel TL, Krause C, Helm MC, Mcbride CL, Oleynikov D, Thakare R, et al. Increased glycine-amidated hyocholic acid correlates to improved early weight loss after sleeve gastrectomy. Surg Endosc. (2017) 32:805–12. doi: 10.1007/s00464-017-5747-y
Keywords: obesity, childhood, review (article), behavior, adolescent
Citation: Kansra AR, Lakkunarajah S and Jay MS (2021) Childhood and Adolescent Obesity: A Review. Front. Pediatr. 8:581461. doi: 10.3389/fped.2020.581461
Received: 08 July 2020; Accepted: 23 November 2020; Published: 12 January 2021.
Reviewed by:
Copyright © 2021 Kansra, Lakkunarajah and Jay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alvina R. Kansra, akansra@mcw.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Patient Care & Health Information
- Diseases & Conditions
- Childhood obesity
Childhood obesity is a serious medical condition that affects children and adolescents. It's particularly troubling because the extra pounds often start children on the path to health problems that were once considered adult problems — diabetes, high blood pressure and high cholesterol. Childhood obesity can also lead to poor self-esteem and depression.
One of the best strategies to reduce childhood obesity is to improve the eating and exercise habits of your entire family. Treating and preventing childhood obesity helps protect your child's health now and in the future.
Not all children carrying extra pounds are overweight. Some children have larger than average body frames. And children normally carry different amounts of body fat at the various stages of development. So you might not know by how your child looks if weight is a health concern.
The body mass index (BMI), which provides a guideline of weight in relation to height, is the accepted measure of overweight and obesity. Your child's doctor can use growth charts, the BMI and, if necessary, other tests to help you figure out if your child's weight could pose health problems.
When to see a doctor
If you're worried that your child is putting on too much weight, talk to his or her doctor. The doctor will consider your child's history of growth and development, your family's weight-for-height history, and where your child lands on the growth charts. This can help determine if your child's weight is in an unhealthy range.
Lifestyle issues — too little activity and too many calories from food and drinks — are the main contributors to childhood obesity. But genetic and hormonal factors might play a role as well.
More Information
- Mayo Clinic Minute: Out of shape kids and diabetes
Risk factors
Many factors — usually working in combination — increase your child's risk of becoming overweight:
- Diet. Regularly eating high-calorie foods, such as fast foods, baked goods and vending machine snacks, can cause your child to gain weight. Candy and desserts also can cause weight gain, and more and more evidence points to sugary drinks, including fruit juices and sports drinks, as culprits in obesity in some people.
- Lack of exercise. Children who don't exercise much are more likely to gain weight because they don't burn as many calories. Too much time spent in sedentary activities, such as watching television or playing video games, also contributes to the problem. TV shows also often feature ads for unhealthy foods.
- Family factors. If your child comes from a family of overweight people, he or she may be more likely to put on weight. This is especially true in an environment where high-calorie foods are always available and physical activity isn't encouraged.
- Psychological factors. Personal, parental and family stress can increase a child's risk of obesity. Some children overeat to cope with problems or to deal with emotions, such as stress, or to fight boredom. Their parents might have similar tendencies.
- Socioeconomic factors. People in some communities have limited resources and limited access to supermarkets. As a result, they might buy convenience foods that don't spoil quickly, such as frozen meals, crackers and cookies. Also, people who live in lower income neighborhoods might not have access to a safe place to exercise.
- Certain medications. Some prescription drugs can increase the risk of developing obesity. They include prednisone, lithium, amitriptyline, paroxetine (Paxil), gabapentin (Neurontin, Gralise, Horizant) and propranolol (Inderal, Hemangeol).
Complications
Childhood obesity often causes complications in a child's physical, social and emotional well-being.
Physical complications
Physical complications of childhood obesity may include:
- Type 2 diabetes. This chronic condition affects the way your child's body uses sugar (glucose). Obesity and a sedentary lifestyle increase the risk of type 2 diabetes.
- High cholesterol and high blood pressure. A poor diet can cause your child to develop one or both of these conditions. These factors can contribute to the buildup of plaques in the arteries, which can cause arteries to narrow and harden, possibly leading to a heart attack or stroke later in life.
- Joint pain. Extra weight causes extra stress on hips and knees. Childhood obesity can cause pain and sometimes injuries in the hips, knees and back.
- Breathing problems. Asthma is more common in children who are overweight. These children are also more likely to develop obstructive sleep apnea, a potentially serious disorder in which a child's breathing repeatedly stops and starts during sleep.
- Nonalcoholic fatty liver disease (NAFLD). This disorder, which usually causes no symptoms, causes fatty deposits to build up in the liver. NAFLD can lead to scarring and liver damage.
Social and emotional complications
Children who have obesity may experience teasing or bullying by their peers. This can result in a loss of self-esteem and an increased risk of depression and anxiety.
To help prevent excess weight gain in your child, you can:
- Set a good example. Make healthy eating and regular physical activity a family affair. Everyone will benefit and no one will feel singled out.
- Have healthy snacks available. Options include air-popped popcorn without butter, fruits with low-fat yogurt, baby carrots with hummus, or whole-grain cereal with low-fat milk.
- Offer new foods multiple times. Don't be discouraged if your child doesn't immediately like a new food. It usually takes multiple exposures to a food to gain acceptance.
- Choose nonfood rewards. Promising candy for good behavior is a bad idea.
- Be sure your child gets enough sleep. Some studies indicate that too little sleep may increase the risk of obesity. Sleep deprivation can cause hormonal imbalances that lead to increased appetite.
Also, be sure your child sees the doctor for well-child checkups at least once a year. During this visit, the doctor measures your child's height and weight and calculates his or her BMI . A significant increase in your child's BMI percentile rank over one year may be a possible sign that your child is at risk of becoming overweight.
- Helping your child who is overweight. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/weight-management/helping-your-child-who-is-overweight. Oct. 14, 2020.
- Childhood obesity causes and consequences. Centers for Disease Control and Prevention. https://www.cdc.gov/obesity/childhood/causes.html. Accessed Oct. 14, 2020.
- Kliegman RM, et al. Overweight and obesity. In: Nelson Textbook of Pediatrics. 21st ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Oct. 14, 2020.
- Hay WW, et al., eds. Normal childhood nutrition and its disorders. In: Current Diagnosis & Treatment: Pediatrics. 25th ed. McGraw Hill; 2020. https://accessmedicine.mhmedical.com. Accessed Oct. 20, 2020.
- Skelton JA. Management of childhood obesity in the primary care setting. https://www.uptodate.com/contents/search. Accessed Oct. 14, 2020.
- Klish WJ, et al. Definition, epidemiology and etiology of obesity in children and adolescents. https://www.uptodate.com/contents/search. Accessed Oct. 14, 2020.
- Polfuss ML, et al. Childhood obesity: Evidence-based guidelines for clinical practice — Part one. Journal of Pediatric Health Care. 2020; doi:10.1016/j.pedhc.2019.12.003.
- Davis RL, et al. Childhood obesity: Evidence-based guidelines for clinical practice — Part two. Journal of Pediatric Health Care. 2020; doi:10.1016/j.pedhc.2020.07.011.
- Mayo Clinic Children's Center Pediatric Weight Management Clinic
- Mayo Clinic Minute: Weight loss surgery for kids
Associated Procedures
- Bariatric surgery
- Cholesterol test
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- NEW: Listen to Health Matters Podcast - Mayo Clinic Press NEW: Listen to Health Matters Podcast
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Thank a researcher today
Their crucial work saves lives every day. Let Mayo Clinic researchers know they’re appreciated with a quick message.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 18 May 2023
Child and adolescent obesity
- Natalie B. Lister ORCID: orcid.org/0000-0002-9148-8632 1 , 2 ,
- Louise A. Baur ORCID: orcid.org/0000-0002-4521-9482 1 , 3 , 4 ,
- Janine F. Felix 5 , 6 ,
- Andrew J. Hill ORCID: orcid.org/0000-0003-3192-0427 7 ,
- Claude Marcus ORCID: orcid.org/0000-0003-0890-2650 8 ,
- Thomas Reinehr ORCID: orcid.org/0000-0002-4351-1834 9 ,
- Carolyn Summerbell 10 &
- Martin Wabitsch ORCID: orcid.org/0000-0001-6795-8430 11
Nature Reviews Disease Primers volume 9 , Article number: 24 ( 2023 ) Cite this article
53k Accesses
107 Citations
326 Altmetric
Metrics details
- Paediatric research
The prevalence of child and adolescent obesity has plateaued at high levels in most high-income countries and is increasing in many low-income and middle-income countries. Obesity arises when a mix of genetic and epigenetic factors, behavioural risk patterns and broader environmental and sociocultural influences affect the two body weight regulation systems: energy homeostasis, including leptin and gastrointestinal tract signals, operating predominantly at an unconscious level, and cognitive–emotional control that is regulated by higher brain centres, operating at a conscious level. Health-related quality of life is reduced in those with obesity. Comorbidities of obesity, including type 2 diabetes mellitus, fatty liver disease and depression, are more likely in adolescents and in those with severe obesity. Treatment incorporates a respectful, stigma-free and family-based approach involving multiple components, and addresses dietary, physical activity, sedentary and sleep behaviours. In adolescents in particular, adjunctive therapies can be valuable, such as more intensive dietary therapies, pharmacotherapy and bariatric surgery. Prevention of obesity requires a whole-system approach and joined-up policy initiatives across government departments. Development and implementation of interventions to prevent paediatric obesity in children should focus on interventions that are feasible, effective and likely to reduce gaps in health inequalities.
Similar content being viewed by others

Management for children and adolescents with overweight and obesity: a recommendations mapping

Unraveling Complexity about Childhood Obesity and Nutritional Interventions: Modeling Interactions Among Psychological Factors

Pediatric weight management interventions improve prevalence of overeating behaviors
Introduction.
The prevalence of child and adolescent obesity remains high and continues to rise in low-income and middle-income countries (LMICs) at a time when these regions are also contending with under-nutrition in its various forms 1 , 2 . In addition, during the COVID-19 pandemic, children and adolescents with obesity have been more likely to have severe COVID-19 requiring hospitalization and mechanical ventilation 3 . At the same time, the pandemic was associated with rising levels of childhood obesity in many countries. These developments are concerning, considering that recognition is also growing that paediatric obesity is associated with a range of immediate and long-term negative health outcomes, a decreased quality of life 4 , 5 , an increased presentation to health services 6 and increased economic costs to individuals and society 7 .
Body weight is regulated by a range of energy homeostatic and cognitive–emotional processes and a multifactorial interplay of complex regulatory circuits 8 . Paediatric obesity arises when multiple environmental factors — covering preconception and prenatal exposures, as well as broader changes in the food and physical activity environments — disturb these regulatory processes; these influences are now widespread in most countries 9 .
The treatment of obesity includes management of obesity-associated complications, a developmentally sensitive approach, family engagement, and support for long-term behaviour changes in diet, physical activity, sedentary behaviours and sleep 10 . New evidence highlights the role, in adolescents with more severe obesity, of bariatric surgery 11 and pharmacotherapy, particularly the potential for glucagon-like peptide 1 (GLP1) receptor agonists 12 .
Obesity prevention requires a whole-system approach, with policies across all government and community sectors systematically taking health into account, avoiding harmful health impacts and decreasing inequity. Programmatic prevention interventions operating ‘downstream’ at the level of the child and family, as well as ‘upstream’ interventions at the level of the community and broader society, are required if a step change in tackling childhood obesity is to be realized 13 , 14 .
In this Primer, we provide an overview of the epidemiology, causes, pathophysiology and consequences of child and adolescent obesity. We discuss diagnostic considerations, as well as approaches to its prevention and management. Furthermore, we summarize effects of paediatric obesity on quality of life, and open research questions.
Epidemiology
Definition and prevalence.
The World Health Organization (WHO) defines obesity as “abnormal or excessive fat accumulation that presents a risk to health” 15 . Paediatric obesity is defined epidemiologically using BMI, which is adjusted for age and sex because of the physiological changes in BMI during growth 16 . Global prevalence of paediatric obesity has risen markedly over the past four decades, initially in high-income countries (HICs), but now also in many LMICs 1 .
Despite attempts to standardize the epidemiological classification, several definitions of paediatric obesity are in use; hence, care is needed when comparing prevalence rates. The 2006 WHO Child Growth Standard, for children aged 0 to 5 years, is based on longitudinal observations of multiethnic populations of children with optimal infant feeding and child-rearing conditions 17 . The 2007 WHO Growth Reference is used for the age group 5–19 years 18 , and the 2000 US Centers for Disease Control and Prevention (CDC) Growth Charts for the age group 2–20 years 19 . The WHO and CDC definitions based on BMI-for-age charts are widely used, including in clinical practice. By contrast, the International Obesity Task Force (IOTF) definition, developed from nationally representative BMI data for the age group 2–18 years from six countries, is used exclusively for epidemiological studies 20 .
For the age group 5–19 years, between 1975 and 2016, the global prevalence of obesity (BMI >2 standard deviations (SD) above the median of the WHO growth reference) increased around eightfold to 5.6% in girls and 7.8% in boys 1 . Rates have plateaued at high levels in many HICs but have accelerated in other regions, particularly in parts of Asia. For the age group 2–4 years, between 1980 and 2015, obesity prevalence (IOTF definition, equivalent to an adult BMI of ≥30 kg/m 2 ) increased from 3.9% to 7.2% in boys and from 3.7% to 6.4% in girls 21 . Obesity prevalence is highest in Polynesia and Micronesia, the Middle East and North Africa, the Caribbean and the USA (Fig. 1 ). Variations in prevalence probably reflect different background levels of obesogenic environments, or the sum total of the physical, economic, policy, social and cultural factors that promote obesity 22 . Obesogenic environments include those with decreased active transport options, a ubiquity of food marketing directed towards children, and reduced costs and increased availability of nutrient-poor, energy-dense foods. Particularly in LMICs, the growth of urbanization, new forms of technology and global trade have led to reduced physical activity at work and leisure, a shift towards Western diets, and the expansion of transnational food and beverage companies to shape local food systems 23 .
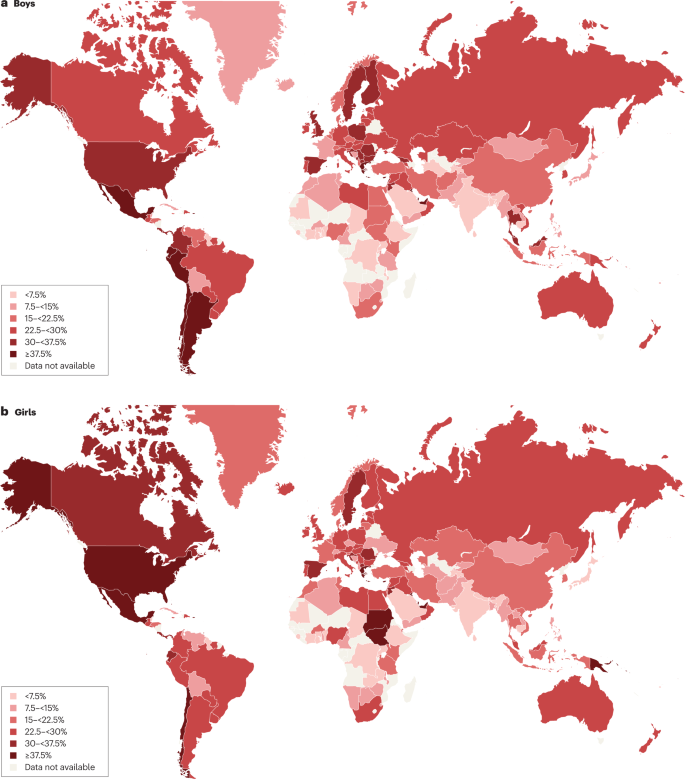
Maps showing the proportions of children and adolescents living with overweight or obesity (part a , boys; part b , girls) according to latest available data from the Global Obesity Observatory . Data might not be comparable between countries owing to differences in survey methodology.
The reasons for varying sex differences in prevalence in different countries are unclear but may relate to cultural variations in parental feeding practices for boys and girls and societal ideals of body size 24 . In 2016, obesity in the age group 5–19 years was more prevalent in girls than in boys in sub-Saharan Africa, Oceania and some middle-income countries in other regions, whereas it was more prevalent in boys than in girls in all HICs, and in East and South-East Asia 21 . Ethnic and racial differences in obesity prevalence within countries are often assumed to mirror variations in social deprivation and other social determinants of obesity. However, an independent effect of ethnicity even after adjustment for socioeconomic status has been documented in the UK, with Black and Asian boys in primary school having higher prevalence of obesity than white boys 25 .
Among individuals with obesity, very high BMI values have become more common in the past 15 years. The prevalence of severe obesity (BMI ≥120% of the 95th percentile (CDC definition), or ≥35 kg/m 2 at any age 26 , 27 ) has increased in many HICs, accounting for one-quarter to one-third of those with obesity 28 , 29 . Future health risks of paediatric obesity in adulthood are well documented. For example, in a data linkage prospective study in Israel with 2.3 million participants who had BMI measured at age 17 years, those with obesity (≥95th percentile BMI for age) had a much higher risk of death from coronary heart disease (HR 4.9, 95% CI 3.9–6.1), stroke (HR 2.6, 95% CI 1.7–4.1) and sudden death (HR 2.1, 95% CI 1.5–2.9) compared with those whose BMI fell between the 5th and 24th percentiles 30 .
Causes and risk factors
Early life is a critical period for childhood obesity development 9 , 31 , 32 , 33 . According to the Developmental Origins of Health and Disease framework, the early life environment may affect organ structure and function and influence health in later life 34 , 35 . Meta-analyses have shown that preconception and prenatal environmental exposures, including high maternal pre-pregnancy BMI and, to a lesser extent, gestational weight gain, as well as gestational diabetes and maternal smoking, are associated with childhood obesity, potentially through effects on the in utero environment 33 , 36 , 37 , 38 . Paternal obesity is also associated with childhood obesity 33 . Birthweight, reflecting fetal growth, is a proxy for in utero exposures. Both low and high birthweights are associated with later adiposity, with high birthweight linked to increased BMI and low birthweight to central obesity 33 , 39 .
Growth trajectories in early life are important determinants of later adiposity. Rapid weight gain in early childhood is associated with obesity in adolescence 32 . Also, later age and higher BMI at adiposity peak (the usual peak in BMI around 9 months of age), as well as earlier age at adiposity rebound (the lowest BMI reached between 4 and 7 years of age), are associated with increased adolescent and adult BMI 40 , 41 . Specific early life nutritional factors, including a lower protein content in formula food, are consistently associated with a lower risk of childhood obesity 42 , 43 . These also include longer breastfeeding duration, which is generally associated with a lower risk of childhood obesity 42 . However, some controversy exists, as these effects are affected by multiple sociodemographic confounding factors and their underlying mechanisms remain uncertain 44 . Some studies comparing higher and lower infant formula protein content have reported that the higher protein group have a greater risk of subsequent obesity, especially in early childhood 41 , 42 ; however, one study with a follow-up period until age 11 years found no significant difference in the risk of obesity, but an increased risk of overweight in the high protein group was still observed 42 , 43 , 45 . A high intake of sugar-sweetened beverages is associated with childhood obesity 33 , 46 .
Many other behavioural factors are associated with an increased risk of childhood obesity, including increased screen time, short sleep duration and poor sleep quality 33 , 47 , reductions in physical activity 48 and increased intake of energy-dense micronutrient-poor foods 49 . These have been influenced by multiple changes in the past few decades in the broader social, economic, political and physical environments, including the widespread marketing of food and beverages to children, the loss of walkable green spaces in many urban environments, the rise in motorized transport, rapid changes in the use of technology, and the move away from traditional foods to ultraprocessed foods.
Obesity prevalence is inextricably linked to relative social inequality, with data suggesting a shift in prevalence over time towards those living with socioeconomic disadvantage, and thus contributes to social inequalities. In HICs, being in lower social strata is associated with a higher risk of obesity, even in infants and young children 50 , whereas the opposite relationship occurs in middle-income countries 51 . In low-income countries, the relationship is variable, and the obesity burden seems to be across socioeconomic groups 52 , 53 .
Overall, many environmental, lifestyle, behavioural and social factors in early life are associated with childhood obesity. These factors cannot be seen in isolation but are part of a complex interplay of exposures that jointly contribute to increased obesity risk. In addition to multiple prenatal and postnatal environmental factors, genetic variants also have a role in the development of childhood obesity (see section Mechanisms/pathophysiology).
Comorbidities and complications
Childhood obesity is associated with a wide range of short-term comorbidities (Fig. 2 ). In addition, childhood obesity tracks into adolescence and adulthood and is associated with complications across the life course 32 , 41 , 54 , 55 .
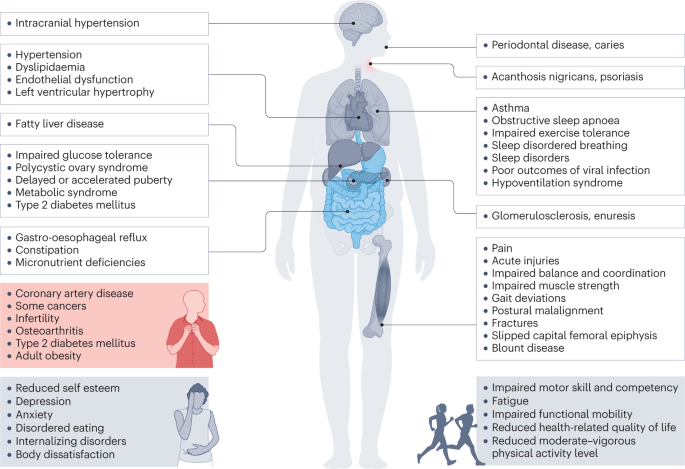
Obesity in children and adolescents can be accompanied by various other pathologies. In addition, childhood obesity is associated with complications and disorders that manifest in adulthood (red box).
Increased BMI, especially in adolescence, is linked to a higher risk of many health outcomes, including metabolic disorders, such as raised fasting glucose, impaired glucose tolerance, type 2 diabetes mellitus (T2DM), metabolic syndrome and fatty liver disease 56 , 57 , 58 , 59 . Other well-recognized obesity-associated complications include coronary heart disease, asthma, obstructive sleep apnoea syndrome (itself associated with metabolic dysfunction and inflammation) 60 , orthopaedic complications and a range of mental health outcomes including depression and low self-esteem 27 , 55 , 57 , 61 , 62 , 63 .
A 2019 systematic review showed that children and adolescents with obesity are 1.4 times more likely to have prediabetes, 1.7 times more likely to have asthma, 4.4 times more likely to have high blood pressure and 26.1 times more likely to have fatty liver disease than those with a healthy weight 64 . In 2016, it was estimated that, at a global level by 2025, childhood obesity would lead to 12 million children aged 5–17 years with glucose intolerance, 4 million with T2DM, 27 million with hypertension and 38 million with fatty liver disease 65 . These high prevalence rates have implications for both paediatric and adult health services.
Mechanisms/pathophysiology
Body weight regulation.
Body weight is regulated within narrow limits by homeostatic and cognitive–emotional processes and a multifactorial interplay of hormones and messenger substances in complex regulatory circuits (Fig. 3 ). When these regulatory circuits are disturbed, an imbalance between energy intake and expenditure leads to obesity or to poor weight gain. As weight loss is much harder to achieve than weight gain in the long term due to the regulation circuits discussed below, the development of obesity is encouraged by modern living conditions, which enable underlying predispositions for obesity to become manifest 8 , 66 .
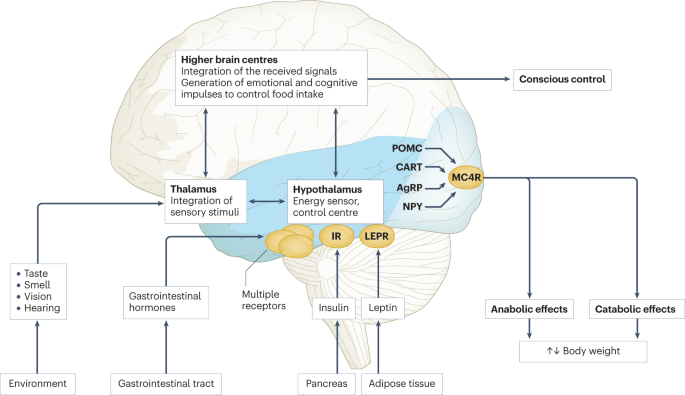
Body weight is predominantly regulated by two systems: energy homeostasis and cognitive–emotional control. Both homeostatic and non-homeostatic signals are processed in the brain, involving multiple hormone and receptor cascades 217 , 218 , 219 . This overview depicts the best-known regulatory pathways. The homeostatic system, which is mainly regulated by brain centres in the hypothalamus and brainstem, operates on an unconscious level. Both long-term signals from the energy store in adipose tissue (for example, leptin) and short-term hunger and satiety signals from the gastrointestinal tract signal the current nutrient status. During gastric distension or after the release of gastrointestinal hormones (multiple receptors are involved) and insulin, a temporary feeling of fullness is induced. The non-homeostatic or hedonic system is regulated by higher-level brain centres and operates at the conscious level. After integration in the thalamus, homeostatic signals are combined with stimuli from the environment, experiences and emotions; emotional and cognitive impulses are then induced to control food intake. Regulation of energy homeostasis in the hypothalamus involves two neuron types of the arcuate nucleus: neurons producing neuropeptide Y (NPY) and agouti-related peptide (AgRP) and neurons producing pro-opiomelanocortin (POMC). Leptin stimulates these neurons via specific leptin receptors (LEPR) inducing anabolic effects in case of decreasing leptin levels and catabolic effects in case of increasing leptin levels. Leptin inhibits the production of NPY and AgRP, whereas low leptin levels stimulate AgRP and NPY production resulting in the feeling of hunger. Leptin directly stimulates POMC production in POMC neurons. POMC is cleaved into different hormone polypeptides including α-melanocyte-stimulating hormone which in turn activates melanocortin 4 receptors (MC4R) of cells in the nucleus paraventricularis of the hypothalamus, leading to the feeling of satiety. CART, cocaine and amphetamine responsive transcript; IR, insulin receptor.
In principle, there are two main systems in the brain which regulate body weight 8 , 66 (Fig. 3 ): energy homeostasis and cognitive–emotional control. Energy homeostasis is predominantly regulated by brain centres in the hypothalamus and brainstem and operates at an unconscious level. Both long-term signals from the adipose tissue energy stores and short-term hunger and satiety signals from the gastrointestinal tract signal the current nutrient status 8 , 66 . For example, negative energy balance leading to reduced fat mass results in reduced leptin levels, a permanently reduced urge to exercise and an increased feeling of hunger. During gastric distension or after the release of gastrointestinal hormones and insulin, a temporary feeling of fullness is induced 8 , 66 . Cognitive–emotional control is regulated by higher brain centres and operates at a conscious level. Here, the homeostatic signals are combined with stimuli from the environment (sight, smell and taste of food), experiences and emotions 8 , 66 . Disorders at the level of cognitive–emotional control mechanisms include emotional eating as well as eating disorders. For example, the reward areas in the brain of people with overweight are more strongly activated by high-calorie foods than those in the brain of people with normal weight 67 . Both systems interact with each other, and the cognitive–emotional system is strongly influenced by the homeostatic control circuits.
Disturbances in the regulatory circuits of energy homeostasis can be genetically determined, can result from disease or injury to the regulatory centres involved, or can be caused by prenatal programming 8 , 66 . If the target value of body weight has been shifted, the organism tries by all means (hunger, drive) to reach the desired higher weight. These disturbed signals of the homeostatic system can have an imperative, irresistible character, so that a conscious influence on food intake is no longer effectively possible 8 , 66 . The most important disturbances of energy homeostasis are listed in Table 1 .
The leptin pathway
The peptide hormone leptin is primarily produced by fat cells. Its production depends on the amount of adipose tissue and the energy balance. A negative energy balance during fasting results in a reduction of circulating leptin levels by 50% after 24 h (ref. 68 ). In a state of weight loss, leptin production is reduced 69 . In the brain, leptin stimulates two neuron types of the arcuate nucleus in the hypothalamus via specific leptin receptors: neurons producing neuropeptide Y (NPY) and agouti-related peptide (AgRP) and neurons producing pro-opiomelanocortin (POMC). High leptin levels inhibit the production of NPY and AgRP, whereas low leptin levels stimulate AgRP and NPY production. By contrast, leptin directly stimulates POMC production in POMC neurons (Fig. 3 ). POMC is a hormone precursor that is cleaved into different hormone polypeptides by specific enzymes, such as prohormone convertase 1 (PCSK1). This releases α-melanocyte-stimulating hormone (α-MSH) which in turn activates melanocortin 4 receptors (MC4R) of cells in the nucleus paraventricularis of the hypothalamus, leading to the feeling of satiety. Rare, functionally relevant mutations in the genes for leptin and leptin receptor, POMC , PCSK1/3 or MC4R lead to extreme obesity in early childhood. These forms of obesity are potential indications for specific pharmacological treatments, for example setmelanotide 70 , 71 . MC4R mutations are the most common cause of monogenic obesity, as heterozygous mutations can be symptomatic depending on the functional impairment and with variable penetrance and expression. Other genes have been identified, in which rare heterozygous pathological variants are also associated with early onset obesity (Table 1 ).
Pathological changes in adipose tissue
Adipose tissue can be classified into two types, white and brown adipose tissue. White adipose tissue comprises unilocular fat cells and brown adipose tissue contains multilocular fat cells, which are rich in mitochondria 72 . A third type of adipocyte, beige adipocytes, within the white adipose tissue are induced by prolonged exposure to cold or adrenergic signalling, and show a brown adipocyte-like morphology 72 . White adipose tissue has a large potential to change its volume to store energy and meet the metabolic demands of the body. The storage capacity and metabolic function of adipose tissue depend on the anatomical location of the adipose tissue depot. Predominant enlargement of white adipose tissue in the visceral, intra-abdominal area (central obesity) is associated with insulin resistance and an increased risk of metabolic disease development before puberty. Accumulation of adipose tissue in the hips and flanks has no adverse effect and may be protective against metabolic syndrome. In those with obesity, adipose tissue is characterized by an increased number of adipocytes (hyperplasia), which originate from tissue-resident mesenchymal stem cells, and by enlarged adipocytes (hypertrophy) 73 . Adipocytes with a very large diameter reach the limit of the maximal oxygen diffusion distance, resulting in hypoxia, the development of an inflammatory expression profile (characterized by, for example, leptin, TNF and IL-6) and adipocyte necrosis, triggering the recruitment of leukocytes. Resident macrophages switch from the anti-inflammatory M2 phenotype to a pro-inflammatory M1 phenotype, which is associated with insulin resistance, further promoting local sterile inflammation and the development of fibrotic adipose tissue. This process limits the expandability of the adipose tissue for further storage of triglycerides. In the patient, the increase in fat mass in obesity is associated with insulin resistance and systemic low-grade inflammation characterized by elevated serum levels of C-reactive protein and pro-inflammatory cytokines. The limitation of adipose tissue expandability results in storage of triglycerides in other organs, such as the liver, muscle and pancreas 74 .
Genetics and epigenetics in the general population
Twin studies have found heritability estimates for BMI of up to 70% 75 , 76 . In contrast to rare monogenic forms of obesity, which are often caused by a single genetic defect with a large effect, the genetic background of childhood obesity in the general population is shaped by the joint effects of many common genetic variants, each of which individually makes a small contribution to the phenotype. For adult BMI, genome-wide association studies, which examine associations of millions of such variants across the genome at the same time, have identified around 1,000 genetic loci 77 . The largest genome-wide association studies in children, which include much smaller sample sizes of up to 60,000 children, have identified 25 genetic loci for childhood BMI and 18 for childhood obesity, the majority of which overlap 78 , 79 . There is also a clear overlap with genetic loci identified in adults, for example for FTO , MC4R and TMEM18 , but this overlap is not complete, some loci are specific to early life BMI, or have a relatively larger contribution in childhood 78 , 79 , 80 . These findings suggest that biological mechanisms underlying obesity in childhood are mostly similar to those in adulthood, but the relative influence of these mechanisms may differ at different phases of life.
The role of epigenetic processes in childhood and adolescent obesity has gained increasing attention. In children, several studies found associations between DNA methylation and BMI 81 , 82 , 83 , 84 , but a meta-analysis including data from >4,000 children identified only minimal associations 85 . Most studies support the hypothesis that DNA methylation changes are predominantly a consequence rather than a cause of obesity, which may explain the lower number of identified (up to 12) associations in children, in whom duration of exposure to a higher BMI is shorter than in adults, in whom associations with DNA methylation at hundreds of sites have been identified 85 , 86 , 87 . In addition to DNA methylation, some specific circulating microRNAs have been found to be associated with obesity in childhood 84 .
The field of epigenetic studies in childhood obesity is relatively young and evolving quickly. Future studies will need to focus on defining robust associations in blood as well as other tissues and on identifying cause-and-effect relationships. In addition, other omics, such as metabolomics and proteomics, are promising areas that may contribute to an improved aetiological understanding or may provide biological signatures that can be used as predictive or prognostic markers of childhood obesity and its comorbidities.
Parental obesity and childhood obesity
There is an established link between increased parental BMI and increased childhood BMI 88 , 89 . This link may be due to shared genetics, shared environment, a direct intrauterine effect of maternal BMI or a combination of these factors. In the case of shared genetics, the child inherits BMI-increasing genetic variants from one or both parents. Shared environmental factors, such as diet or lifestyle, may also contribute to an increased BMI in both parents and child. In addition, maternal obesity might create an intrauterine environment that programmes metabolic processes in the fetus, which increases the risk of childhood obesity. Some studies show larger effects of maternal than paternal BMI, indicating a potential causal intrauterine mechanism of maternal obesity, but evidence showing similar maternal and paternal effects is increasing. The data may indicate that there is only a limited direct intrauterine effect of maternal obesity on childhood obesity; rather, genetic effects inherited from the mother or father, or both, and/or shared environmental factors may contribute to childhood obesity risk 90 , 91 , 92 , 93 , 94 , 95 .
Diagnosis, screening and prevention
Diagnostic work-up.
The extent of overweight in clinical practice is estimated using BMI based on national charts 96 , 97 , 98 , 99 , 100 . Of note, the clinical classification of overweight or obesity differ depending on the BMI charts used and national recommendations; hence, local guidelines should be referred to. For example, the US CDC Growth Charts and several others use the 85th and 95th centile cut-points to denote overweight and obesity, respectively 19 . The WHO Growth Reference for children aged 5–19 years defines cut-points for overweight and obesity as a BMI-for-age greater than +1 and +2 SDs for BMI for age, respectively 18 . For children <5 years of age, overweight and obesity are defined as weight-for-height greater than +2 and +3 SDs, respectively, above the WHO Child Growth Standards median 17 . The IOTF and many countries in Europe use cut-points of 85th, 90th and 97th to define overweight, obesity and extreme obesity 26 .
BMI as an indirect measurement of body fat has some limitations; for example, pronounced muscle tissue leads to an increase in BMI, and BMI is not independent of height. In addition, people of different ethnicities may have different cut-points for obesity risk; for example, cardiometabolic risk occurs at lower BMI values in individuals with south Asian than in those with European ancestry 101 . Thus, BMI is best seen as a convenient screening tool that is supplemented by clinical assessment and investigations.
Other measures of body fat may help differentiate between fat mass and other tissues. Some of these tools are prone to low reliability, such as body impedance analyses (high day-to-day variation and dependent on level of fluid consumption) or skinfold thickness (high inter-observer variation), or are more expensive or invasive, such as MRI, CT or dual-energy X-ray absorptiometry, than simpler measures of body composition or BMI assessment.
Primary diseases rarely cause obesity in children and adolescents (<2%) 102 . However, treatable diseases should be excluded in those with obesity. A suggested diagnostic work-up is summarized in Fig. 4 . Routine measurement of thyroid-stimulating hormone (TSH) is not recommended 96 . Moderately elevated TSH levels (usually <10 IU/l) are frequently observed in obesity and are a consequence, and not a cause, of obesity 103 . In a growing child with normal height velocity, a normal BMI at the age of 2 years and normal cognitive development, no further diagnostic steps are necessary to exclude primary diseases 96 , 104 .
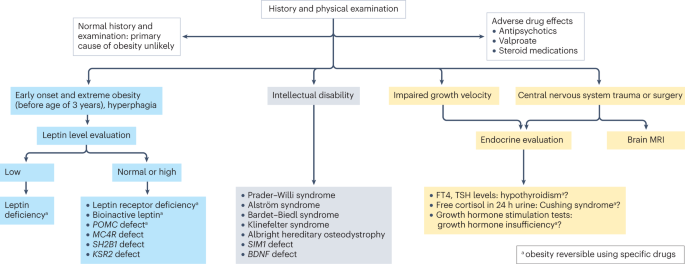
Concerning findings from a detailed medical history and physical examination will lead to further examinations. In individuals with early onset, extreme obesity (before age 3 years) and signs of hyperphagia, serum leptin level should be measured to rule out the extremely rare condition of congenital leptin deficiency. In individuals with normal or high leptin levels, genetic testing is indicated to search for monogenetic obesity. In individuals with intellectual disability, a syndromic disease may be present. Signs of impaired growth velocity or the history of central nervous system trauma or surgery will result in deeper endocrine evaluation and/or brain MRI. BDNF , brain-derived neurotropic factor; FT4, free thyroxin; KSR2 , kinase suppressor of ras 2; MC4R , melanocortin 4 receptor; POMC , pro-opiomelanocortin; SH2B1 , Src-homology 2 (SH2) B adapter protein 1; SIM1 , single-minded homologue 1; TSH, thyroid-stimulating hormone.
Clinical findings which need no further examination include pseudogynaecomastia (adipose tissue mimicking breast development; differentiated from breast tissue by ultrasonography), striae (caused by rapid weight increase) and a hidden penis in suprapubic adipose tissue (differentiated from micropenis by measurement of stretched penis length while pressing down on the suprapubic adipose tissue) 96 , 105 . Girls with obesity tend to have an earlier puberty onset (usually at around 8–9 years of age) and boys with severe obesity may have a delayed puberty onset (usually at around 13–14 years of age) 106 . Thus, if pubertal onset is slightly premature in girls or slightly delayed in boys, no further endocrine assessment is necessary.
Assessment of obesity-associated comorbidities
A waist to height ratio of >0.5 is a simple tool to identify central obesity 107 , 108 . Screening for cardiometabolic risk factors and fatty liver disease is recommended, especially in adolescents, and in those with more severe obesity or central adiposity, a strong family history of T2DM or premature heart disease, or relevant clinical symptoms, such as high blood pressure or acanthosis nigricans 96 , 97 , 98 , 99 , 109 . Investigations generally include fasting glucose levels, lipid profile, liver function and glycated haemoglobin, and might include an oral glucose tolerance test, polysomnography, and additional endocrine tests for polycystic ovary syndrome 96 , 97 , 98 , 99 .
T2DM in children and adolescents often occurs in the presence of a strong family history and may not be related to obesity severity 110 . T2DM onset usually occurs during puberty, a physiological state associated with increased insulin resistance 111 and, therefore, screening for T2DM should be considered in children and adolescents with obesity and at least one risk factor (family history of T2DM or features of metabolic syndrome) starting at pubertal onset 112 . As maturity-onset diabetes of the young (MODY) type II and type III are more frequent than T2DM in children and adolescents in many ethnicities, genetic screening for MODY may be appropriate 112 . Furthermore, type 1 diabetes mellitus (T1DM) should be excluded by measurement of autoantibodies in any individual with suspected diabetes with obesity. The differentiation of T2DM from MODY and T1DM is important as the diabetes treatment approaches differ 112 .
Several comorbidities of obesity should be considered if specific symptoms occur 96 , 109 . For polycystic ovary syndrome in hirsute adolescent girls with oligomenorrhoea or amenorrhoea, moderately increased testosterone levels and decreased sex hormone binding globulin levels are typical laboratory findings 113 . Obstructive sleep apnoea can occur in those with more severe obesity and who snore, have daytime somnolence or witnessed apnoeas. Diagnosis is made by polysomnography 114 . Minor orthopaedic disorders, such as flat feet and genu valgum, are frequent in children and adolescents with obesity and may cause pain. Major orthopaedic complications include slipped capital femoral epiphyses (acute and chronic), which manifest with hip and knee pain in young adolescents and are characterized by reduced range of hip rotation and waddling gait; and Blount disease (tibia vara), typically occurring in children aged 2–5 years 105 , 115 . In addition, children and adolescents with extreme obesity frequently have increased dyspnoea and decreased exercise capacity. A heightened demand for ventilation, elevated work of breathing, respiratory muscle inefficiency and diminished respiratory compliance are caused by increased truncal fat mass. This may result in a decreased functional residual capacity and expiratory reserve volume, ventilation to perfusion ratio abnormalities and hypoxaemia, especially when supine. However, conventional respiratory function tests are only mildly affected by obesity except in extreme cases 116 . Furthermore, gallstones should be suspected in the context of abdominal pain after rapid weight loss, which can be readily diagnosed via abdominal ultrasonography 105 . Finally, pseudotumor cerebri may present with chronic headache, and depression may present with flat affect, chronic fatigue and sleep problems 105 .
Obesity in adolescents can also be associated with disordered eating, eating disorders and other psychological disorders 117 , 118 . If suspected, assessment by a mental health professional is recommended.
A comprehensive approach
The 2016 report of the WHO Commission on Ending Childhood Obesity stated that progress in tackling childhood obesity has been slow and inconsistent, with obesity prevention requiring a whole-of-government approach in which policies across all sectors systematically take health into account, avoiding harmful health impacts and, therefore, improving population health and health equity 13 , 119 . The focus in developing and implementing interventions to prevent obesity in children should be on interventions that are feasible, effective and likely to reduce health inequalities 14 . Importantly, the voices of children and adolescents living with social disadvantage and those from minority groups must be heard if such interventions are to be effective and reduce inequalities 120 .
Figure 5 presents a system for the prevention of childhood obesity within different domains of the socioecological model 121 and highlights opportunities for interventions. These domains can be described on a continuum, from (most downstream) individual and interpersonal (including parents, peers and wider family) through to organizational (including health care and schools), community (including food, activity and environment), society (including media and finally cultural norms) and (most upstream) public policy (from local to national level). Interventions to prevent childhood obesity can be classified on the Nuffield intervention ladder 122 . This framework was proposed by the Nuffield Council on Bioethics in 2007 (ref. 122 ) and distributes interventions on the ladder steps depending on the degree of agency required by the individual to make the behavioural changes that are the aim of the intervention. The bottom step of the ladder includes interventions that provide information, which requires the highest agency and relies on a child, adolescent and/or family choosing (and their ability to choose) to act on that information and change behaviour. The next steps of the ladder are interventions that enable choice, guide choice through changing the default policy, guide choice through incentives, guide choice through disincentives, or restrict choice. On the top-most step of the ladder (lowest agency required) are interventions that eliminate choice.
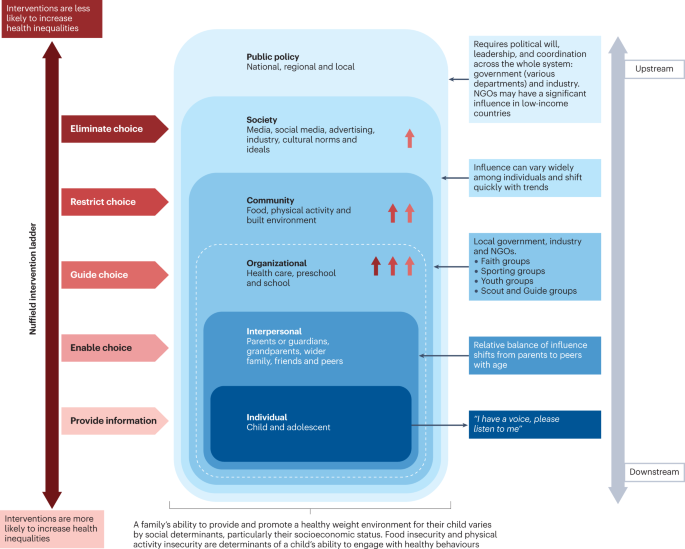
This schematic integrates interventions that were included in a Cochrane review 127 of 153 randomized controlled trials of interventions to prevent obesity in children and are high on the Nuffield intervention ladder 122 . The Nuffield intervention ladder distributes interventions depending on the degree of agency required for the behavioural changes that are the aim of the intervention. The socioecological model 121 comprises different domains (or levels) from the individual up to public policy. Interventions targeting the individual and interpersonal domains can be described as downstream interventions, and interventions within public policy can be described as the highest level of upstream interventions. Within each of these domains, arrow symbols with colours corresponding to the Nuffield intervention ladder category are used to show interventions that were both included in the Cochrane review 127 and that guide, restrict or eliminate choice as defined by the Nuffield intervention ladder 122 . Upstream interventions, and interventions on the top steps of the Nuffield ladder, are more likely to reduce inequalities. NGO, non-governmental organization.
Downstream and high-agency interventions (on the bottom steps of the Nuffield ladder) are more likely to result in intervention-generated inequalities 123 . This has been elegantly described and evidenced, with examples from the obesity prevention literature 124 , 125 . A particularly strong example is a systematic review of 38 interventions to promote healthy eating that showed that food price (an upstream and low-agency intervention) seemed to decrease inequalities, all interventions that combined taxes and subsidies consistently decreased inequalities, and downstream high-agency interventions, especially dietary counselling, seemed to increase inequalities 126 .
Effectiveness of prevention interventions
A 2019 Cochrane review of interventions to prevent obesity in children 127 included 153 randomized controlled trials (RCTs), mainly in HICs (12% were from middle-income countries). Of these RCTs, 56% tested interventions in children aged 6–12 years, 24% in children aged 0–5 years, and 20% in adolescents aged 13–18 years. The review showed that diet-only interventions to prevent obesity in children were generally ineffective across all ages. Interventions combining diet and physical activity resulted in modest benefits in children aged 0–12 years but not in adolescents. However, physical activity-only interventions to prevent obesity were effective in school-age children (aged 5–18 years). Whether the interventions were likely to work equitably in all children was investigated in 13 RCTs. These RCTs did not indicate that the strategies increased inequalities, although most of the 13 RCTs included relatively homogeneous groups of children from disadvantaged backgrounds.
The potential for negative unintended consequences of obesity prevention interventions has received much attention 128 . The Cochrane review 127 investigated whether children were harmed by any of the strategies; for example, by having injuries, losing too much weight or developing damaging views about themselves and their weight. Of the few RCTs that did monitor these outcomes, none found any harms in participants.
Intervention levels
Most interventions (58%) of RCTs in the Cochrane review aimed to change individual lifestyle factors via education-based approaches (that is, simply provide information) 129 . In relation to the socioecological model, only 11 RCTs were set in the food and physical activity environment domain, and child care, preschools and schools were the most common targets for interventions. Of note, no RCTs were conducted in a faith-based setting 130 . Table 2 highlights examples of upstream interventions that involve more than simply providing information and their classification on the Nuffield intervention ladder.
Different settings for interventions to prevent childhood obesity, including preschools and schools, primary health care, community settings and national policy, offer different opportunities for reach and effectiveness, and a reduction in inequalities.
Preschools and schools are key settings for public policy interventions for childhood obesity prevention, and mandatory and voluntary food standards and guidance on physical education are in place in many countries. Individual schools are tasked with translating and implementing these standards and guidance for their local context. Successful implementation of a whole-school approach, such as that used in the WHO Nutrition-Friendly Schools Initiative 131 , is a key factor in the effectiveness of interventions. Careful consideration should be given to how school culture can, and needs to, be shifted by working with schools to tailor the approach and manage possible staff capacity issues, and by building relationships within and outside the school gates to enhance sustainability 132 , 133 .
Primary health care offers opportunities for guidance for obesity prevention, especially from early childhood to puberty. Parent-targeted interventions conducted by clinicians in health-care or community settings have the strongest level of evidence for their effectiveness in reducing BMI z -score at age 2 years 134 . These interventions include group programmes, clinic nurse consultations, mobile phone text support or nurse home visiting, and focusing on healthy infant feeding, healthy childhood feeding behaviours and screen time.
A prospective individual participant data meta-analysis of four RCTs involving 2,196 mother–baby dyads, and involving nurse home visiting or group programmes, resulted in a small but significant reduction in BMI in infants in the intervention groups compared with control infants at age 18–24 months 134 . Improvements were also seen in television viewing time, breastfeeding duration and feeding practices. Interventions were more effective in settings with limited provision of maternal and child health services in the community. However, effectiveness diminished by age 5 years without further intervention, highlighting the need for ongoing interventions at each life stage 135 . Evidence exists that short-duration interventions targeting sleep in very early childhood may be more effective than nutrition-targeted interventions in influencing child BMI at age 5 years 136 .
Primary care clinicians can provide anticipatory guidance, as a form of primary prevention, to older children, adolescents and their families, aiming to support healthy weight and weight-related behaviours. Clinical guidelines recommend that clinicians monitor growth regularly, and provide guidance on healthy eating patterns, physical activity, sedentary behaviours and sleep patterns 97 , 100 . Very few paediatric trials have investigated whether this opportunistic screening and advice is effective in obesity prevention 100 . A 2021 review of registered RCTs for the prevention of obesity in infancy found 29 trials 137 , of which most were delivered, or were planned to be delivered, in community health-care settings, such as nurse-led clinics. At the time of publication, 11 trials had reported child weight-related outcomes, two of which showed a small but significant beneficial effect on BMI at age 2 years, and one found significant improvements in the prevalence of obesity but not BMI. Many of the trials showed improvements in practices, such as breastfeeding and screen time.
At the community level, local public policy should be mindful of the geography of the area (such as urban or rural) and population demographics. Adolescents usually have more freedom in food and beverage choices made outside the home than younger children. In addition, physical activity levels usually decline and sedentary behaviours rise during adolescence, particularly in girls 138 , 139 . These behavioural changes offer both opportunities and barriers for those developing community interventions. On a national societal level, public policies for interventions to prevent obesity in children include the control of advertising of foods and beverages high in fat, sugar and/or salt in some countries. Industry and the media, including social media, can have a considerable influence on the food and physical activity behaviours of children 13 , 119 .
Public policy may target interventions at all domains from the individual to the societal level. The main focus of interventions in most national public policies relies on the ability of individuals to make the behavioural changes that are the aim of the intervention (high-agency interventions) at the individual level (downstream interventions). An equal focus on low-agency and upstream interventions is required if a step change in tackling childhood obesity is to be realized 140 , 141 .
COVID-19 and obesity
Early indications in several countries show rising levels of childhood obesity, and an increase in inequalities in childhood obesity during the COVID-19 pandemic 142 . The substantial disruptions in nutrition and lifestyle habits of children during and since the pandemic include social isolation and addiction to screens 143 . Under-nutrition is expected to worsen in poor countries, but obesity rates could increase in middle-income countries and HICs, especially among vulnerable groups, widening the gap in health and social inequalities 143 . Public health approaches at national, regional and local levels should include strategies that not only prevent obesity and under-nutrition, but also reduce health inequalities.
In summary, although most trials of obesity prevention have occurred at the level of the individual, the immediate family, school or community, effective prevention of obesity will require greater investment in upstream, low-agency interventions.
Treatment goals
Treatment should be centred on the individual and stigma-free (Box 1 ) and may aim for a reduction in overweight and improvement in associated comorbidities and health behaviours. Clinical considerations when determining a treatment approach should include age, severity of overweight and the presence of associated complications 144 , 145 .
Box 1 Strategies for minimizing weight stigma in health care 220 , 221 , 222
Minimizing weight bias in the education of health-care professionals
Improved education of health professionals:
pay attention to the implicit and explicit communication of social norms
include coverage of the broader determinants of obesity
include discussion of harms caused by social and cultural norms and messages concerning body weight
provide opportunities to practise non-stigmatizing care throughout education
Provide causal information focusing on the genetic and/or socioenvironmental determinants of weight.
Provide empathy-invoking interventions, emphasizing size acceptance, respect and human dignity.
Provide a weight-inclusive approach, by emphasizing that all individuals, regardless of size, have the right to equal health care.
Addressing health facility infrastructure and processes
Provide appropriately sized chairs, blood pressure cuffs, weight scales, beds, toilets, showers and gowns.
Use non-stigmatizing language in signage, descriptions of clinical services and other documentation.
Providing clinical leadership and using appropriate language within health-care settings
Senior clinicians and managers should role-model supportive and non-biased behaviours towards people with obesity and indicate that they do not tolerate weight-based discrimination in any form.
Staff should identify the language that individuals prefer in referring to obesity.
Use person-first language, for example a ‘person with obesity’ rather than ‘an obese person’.
Treatment guidelines
Clinical guidelines advise that first-line management incorporates a family-based multicomponent approach that addresses dietary, physical activity, sedentary and sleep behaviours 97 , 99 , 109 , 146 . This approach is foundational, with adjunctive therapies, especially pharmacotherapy and bariatric surgery, indicated under specific circumstances, usually in adolescents with more severe obesity 144 , 145 . Guideline recommendations vary greatly among countries and are influenced by current evidence, and functionality and resourcing of local health systems. Hence, availability and feasibility of therapies differs internationally. In usual clinical practice, interventions may have poorer outcomes than is observed in original studies or anticipated in evidence-based guidelines 147 because implementation of guidelines is more challenging in resource-constrained environments 148 . In addition, clinical trials are less likely to include patients with specialized needs, such as children from culturally diverse populations, those living with social disadvantage, children with complex health problems, and those with severe obesity 149 , 150 .
Behavioural interventions
There are marked differences in individual responses to behavioural interventions, and overall weight change outcomes are often modest. In children aged 6–11 years, a 2017 Cochrane review 150 found that mean BMI z -scores were reduced in those involved in behaviour-changing interventions compared with those receiving usual care or no treatment by only 0.06 units (37 trials; 4,019 participants; low-quality evidence) at the latest follow-up (median 10 months after the end of active intervention). In adolescents aged 12–17 years, another 2017 Cochrane review 149 found that multicomponent behavioural interventions resulted in a mean reduction in weight of 3.67 kg (20 trials; 1,993 participants) and reduction in BMI of 1.18 kg/m 2 (28 trials; 2,774 participants). These effects were maintained at the 24-month follow-up. A 2012 systematic review found significant improvements in LDL cholesterol triglycerides and blood pressure up to 1 year from baseline following lifestyle interventions in children and adolescents 151 .
Family-based behavioural interventions are recommended in national level clinical practice guidelines 97 , 100 , 146 , 152 . They are an important element of intensive health behaviour and lifestyle treatments (IHBLTs) 109 . Family-based approaches use behavioural techniques, such as goal setting, parental monitoring or modelling, taught in family sessions or in individual sessions separately to children and care givers, depending on the child’s developmental level. The priority is to encourage the whole family to engage in healthier behaviours that result in dietary improvement, greater physical activity, and less sedentariness. This includes making changes to the family food environment and requires parental monitoring.
Family-based interventions differ in philosophy and implementation from those based on family systems theory and therapy 153 . All are intensive interventions that require multiple contact hours (26 or more) with trained specialists delivered over an extended period of time (6–12 months) 10 . Changing family lifestyle habits is challenging and expensive, and the therapeutic expertise is not widely available. Moving interventions to primary care settings, delivered by trained health coaches, and supplemented by remote contact (for example by phone), will improve access and equity 154 .
Very few interventions use single psychological approaches. Most effective IHBLTs are multicomponent and intensive (many sessions), and include face-to-face contact. There has been interest in motivational interviewing as an approach to delivery 155 . As client-centred counselling, this places the young person at the centre of their behaviour change. Fundamental to motivational interviewing is the practitioner partnership that helps the young person and/or parents to explore ambivalence to change, consolidate commitment to change, and develop a plan based on their own insights and expertise. Evidence reviews generally support the view that motivational interviewing reduces BMI. Longer interventions (>4 months), those that assess and report on intervention fidelity, and those that target both diet and physical activity are most effective 155 , 156 .
More intensive dietary interventions
Some individuals benefit from more intensive interventions 98 , 144 , 157 , 158 , which include very low-energy diets, very low-carbohydrate diets and intermittent energy restriction 159 . These interventions usually aim for weight loss and are only recommended for adolescents who have reached their final height. These diets are not recommended for long periods of time due to challenges in achieving nutritional adequacy 158 , 160 , and lack of long-term safety data 158 , 161 . However, intensive dietary interventions may be considered when conventional treatment is unsuccessful, or when adolescents with comorbidities or severe obesity require rapid or substantial weight loss 98 . A 2019 systematic review of very low-energy diets in children and adolescents found a mean reduction in body weight of −5.3 kg (seven studies) at the latest follow‐up, ranging from 5 to 14.5 months from baseline 161 .
Pharmacological treatment
Until the early 2020s the only drug approved in many jurisdictions for the treatment of obesity in adolescents was orlistat, a gastrointestinal lipase inhibitor resulting in reduced uptake of lipids and, thereby, a reduced total energy intake 162 . However, the modest effect on weight in combination with gastrointestinal adverse effects limit its usefulness overall 163 .
A new generation of drugs has been developed for the treatment of both T2DM and obesity. These drugs are based on gastrointestinal peptides with effects both locally and in the central nervous system. GLP1 is an incretin that reduces appetite and slows gastric motility. The GLP1 receptor agonist liraglutide is approved for the treatment of obesity in those aged 12 years and older both in the USA and Europe 164 , 165 . Liraglutide, delivered subcutaneously daily at a higher dose than used for T2DM resulted in a 5% better BMI reduction than placebo after 12 months 166 . A 2022 trial of semaglutide, another GLP1 receptor agonist, delivered subcutaneously weekly in adolescents demonstrated 16% weight loss after 68 weeks of treatment, with modest adverse events and a low drop-out rate 12 . Tirzepatide, an agonist of both GLP1 and glucose-dependent insulinotropic polypeptide (GIP), is approved by the FDA for the treatment of T2DM in adults 167 . Subcutaneous tirzepatide weekly in adults with obesity resulted in ~20% weight loss over 72 weeks 168 . Of note, GIP alone increases appetite, but the complex receptor–agonist interaction results in downregulation of the GIP receptors 169 , illustrating why slightly modified agonists exert different effects. A study of the use of tirzepatide in adolescents with T2DM has been initiated but results are not expected before 2027 (ref. 170 ). No trials of tirzepatide are currently underway in adolescents with obesity but without T2DM.
Hypothalamic obesity is difficult to treat. Setmelanotide is a MC4R agonist that reduces weight and improves quality of life in most people with LEPR and POMC mutations 71 . In trials of setmelanotide, 8 of 10 participants with POMC deficiency and 5 of 11 with LEPR deficiency had weight loss of at least 10% at ~1 year. The mean percentage change in most hunger score from baseline was −27.1% and −43.7% in those with POMC deficiency and leptin receptor deficiency, respectively 71 .
In the near future, effective new drugs with, hopefully, an acceptable safety profile will be available that will change the way we treat and set goals for paediatric obesity treatment 171 .
Bariatric surgery
Bariatric surgery is the most potent treatment for obesity in adolescents with severe obesity. The types of surgery most frequently used are sleeve gastrectomy and gastric bypass, both of which reduce appetite 172 . Mechanisms of action are complex, involving changes in gastrointestinal hormones, neural signalling, bile acid metabolism and gut microbiota 173 . Sleeve gastrectomy is a more straightforward procedure and the need for vitamin supplementation is lower than with gastric bypass. However, long-term weight loss may be greater after gastric bypass surgery 174 .
Prospective long-term studies demonstrate beneficial effects of both sleeve gastrectomy and gastric bypass on weight loss and comorbidities in adolescents with severe obesity 175 , 176 . In a 5-year follow-up period, in 161 participants in the US TEEN-LABS study who underwent gastric bypass, mean BMI declined from 50 to 37 kg/m 2 (ref. 11 ). In a Swedish prospective study in 81 adolescents who underwent gastric bypass, the mean decrease in BMI at 5 years was 13.1 kg/m 2 (baseline BMI 45.5 kg/m 2 ) compared with a BMI increase of 3.1 kg/m 2 in the control group 176 . Both studies showed marked inter-individual variations. Negative adverse effects, including gastrointestinal problems, vitamin deficits and reduction in lean body mass, are similar in adults and adolescents. Most surgical complications following bariatric surgery in the paediatric population are minor, occurring in the early postoperative time frame, but 8% of patients may have major perioperative complications 177 . Up to one-quarter of patients may require subsequent related procedures within 5 years 109 . However, many adolescents with severe obesity also have social and psychological problems, highlighting the need for routine and long-term monitoring 109 , 178 .
Recommendations for bariatric surgery in adolescents differ considerably among countries, with information on long-term outcomes emerging rapidly. In many countries, bariatric surgery is recommended only from Tanner pubertal stage 3–4 and beyond, and only in children with severe obesity and cardiometabolic comorbidities 177 . The 2023 American Academy of Pediatrics clinical practice guidelines recommend that bariatric surgery be considered in adolescents ≥13 years of age with a BMI of ≥35 kg/m 2 or 120% of the 95th percentile for age and sex, whichever is lower, as well as clinically significant disease, such as T2DM, non-alcoholic fatty liver disease, major orthopaedic complications, obstructive sleep apnoea, the presence of cardiometabolic risk, or depressed quality of life 109 . For those with a BMI of ≥40 kg/m 2 or 140% of the 95th percentile for age and sex, bariatric surgery is indicated regardless of the presence of comorbidities. Potential contraindications to surgery include correctable causes of obesity, pregnancy and ongoing substance use disorder. The guidelines comment that further evaluation, undertaken by multidisciplinary centres that offer bariatric surgery for adolescents, should determine the capacity of the patient and family to understand the risks and benefits of surgery and to adhere to the required lifestyle changes before and after surgery.
Long-term weight outcomes
Few paediatric studies have investigated long-term weight maintenance after the initial, more intensive, weight loss phase. A 2018 systematic review of 11 studies in children and adolescents showed that a diverse range of maintenance interventions, including support via face-to-face psychobehavioural therapies, individual physician consultations, or adjunctive therapeutic contact via newsletters, mobile phone text or e-mail, led to stabilization of BMI z -score compared with control participants, who had increases in BMI z -score 179 . Interventions that are web-based or use mobile devices may be particularly useful in young people 180 .
One concern is weight regain which occurs after bariatric surgery in general 181 but may be more prevalent in adolescents 176 . For example, in a Swedish prospective study, after 5 years, 25–30% of participants fulfilled the definitions of low surgical treatment effectiveness, which was associated with poorer metabolic outcomes 176 . As with adults, prevention of weight regain for most at-risk individuals might be possible with the combination of lifestyle support and pharmacological treatment 182 . Further weight maintenance strategies and long-term outcomes are discussed in the 2023 American Academy of Pediatrics clinical practice guidelines 109 . The appropriate role and timing of other therapies for long-term weight loss maintenance, such as anti-obesity medications, more intensive dietary interventions and bariatric surgery, are areas for future research.
In summary, management of obesity in childhood and adolescence requires intensive interventions. Emerging pharmacological therapies demonstrate greater short-term effectiveness than behavioural interventions; however, long-term outcomes at ≥2 years remain an important area for future research.
Quality of life
Weight bias describes the negative attitudes to, beliefs about and behaviour towards people with obesity 183 . It can lead to stigma causing exclusion, and discrimination in work, school and health care, and contributes to the inequities common in people with obesity 184 . Weight bias also affects social engagement and psychological well-being of children.
Children and adolescents with obesity score lower overall on health-related quality of life (HRQoL) 4 , 5 . In measures that assess domains of functioning, most score lower in physical functioning, physical/general health and psychosocial areas, such as appearance, and social acceptance and functioning. HRQoL is lowest in treatment-seeking children and in those with more extreme obesity 185 . Weight loss interventions generally increase HRQoL independent of the extent of weight loss 186 , especially in the domains most affected. However, changes in weight and HRQoL are often not strongly correlated. This may reflect a lag in the physical and/or psychosocial benefit from weight change, or the extent of change that is needed to drive change in a child’s self-perception.
Similar observations apply to the literature on self-esteem. Global self-worth is reduced in children and adolescents with obesity, as is satisfaction with physical appearance, athletic competence and social acceptance 187 . Data from intensive interventions suggest the psychological benefit of weight loss may be as dependent on some feature of the treatment environment or supportive social network as the weight loss itself 188 . This may include the daily company of others with obesity, making new friendships, and experienced improvements in newly prioritized competences.
There is a bidirectional relationship between HRQoL and obesity 189 , something also accepted in the relationship with mood disorder. Obesity increases the risk of depression and vice versa, albeit over a longer period of time and which may only become apparent in adulthood 190 . Obesity also presents an increased risk of anxiety 191 .
Structured and professionally delivered weight management interventions ameliorate mood disorder symptoms 192 and improve self-esteem 193 . Regular and extended support are important components beyond losing weight. Such interventions do not increase the risk of eating disorders 194 . This is despite a recognition that binge eating disorder is present in up to 5% of adolescents with overweight or obesity 195 . They are five times more likely to have binge eating symptoms than those with average weight. Importantly, adolescents who do not have access to professionally delivered weight management may be more likely to engage in self-directed dieting, which is implicated in eating disorder development 196 .
The literature linking childhood obesity with either attention deficit hyperactivity disorder or autism spectrum disorder is complex and the relationship is uncertain. The association seems to be clearer in adults but the mechanisms and their causal directions remain unclear 109 , 197 . Young children with obesity, especially boys, are more likely to be parent-rated as having behavioural problems 198 . This may be a response to the behaviour of others rather than reflect clinical diagnoses such as attention deficit hyperactivity disorder or autism spectrum disorder. Conduct and peer relationship problems co-occur in children, regardless of their weight.
Children with obesity experience more social rejection. They receive fewer friendship nominations and more peer rejections, most pronounced in those with severe obesity 199 . This continues through adolescence and beyond. Children with obesity are more likely to report being victimized 200 . Younger children may respond by being perpetrators themselves. While it is assumed that children are victimized because of their weight, very few studies have looked at the nature or reason behind victimization. A substantial proportion of children with obesity fail to identify themselves as being fat-teased 187 . Although the stigma associated with obesity should be anticipated in children, especially in those most overweight, it would be inappropriate to see all as victims. A better understanding of children’s resilience is needed.
Many gaps remain in basic, translational and clinical research in child and adolescent obesity. The mechanisms (genetic, epigenetic, environmental and social) behind the overwhelming association between parental obesity and child and adolescent obesity are still unclear given the paradoxically weak association in BMI between adopted children and their parents in combination with the modest effect size of known genetic loci associated with obesity 201 .
Early manifestation of extreme obesity in childhood suggests a strong biological basis for disturbances of homeostatic weight regulation. Deep genotyping (including next-generation sequencing) and epigenetic analyses in these patients will reveal new genetic causes and causal pathways as a basis for the development of mechanism-based treatments. Future work aiming to understand the mechanisms underlying the development of childhood obesity should consider the complex biopsychosocial interactions and take a systems approach to understanding causal pathways leading to childhood obesity to contribute to evidence-based prevention and treatment strategies.
Long-term outcome data to better determine the risks of eating disorders are required. Although symptoms improve during obesity treatment in most adolescents, screening and monitoring for disordered eating is recommended in those presenting for treatment 202 and effective tools for use in clinical practice are required. A limited number of tools are validated to identify binge eating disorder in youth with obesity 203 but further research is needed to screen appropriately for the full spectrum of eating disorder diagnoses in obesity treatment seeking youth 203 . Recent reviews provide additional detail regarding eating disorder risk in child and adolescent obesity 117 , 202 , 204 .
Most studies of paediatric obesity treatment have been undertaken in HICs and predominantly middle-class populations. However, research is needed to determine which strategies are best suited for those in LMICs and low-resource settings, for priority population groups including indigenous peoples, migrant populations and those living with social disadvantage, and for children with neurobehavioural and psychiatric disorders. We currently have a limited understanding of how best to target treatment pathways for different levels of genetic risk, age, developmental level, obesity severity, and cardiometabolic and psychological risk. Current outcomes for behavioural interventions are relatively modest and improved treatment outcomes are needed to address the potentially severe long-term health outcomes of paediatric obesity. Studies also need to include longer follow-up periods after an intervention, record all adverse events, incorporate cost-effectiveness analyses and have improved process evaluation.
Other areas in need of research include the role of new anti-obesity medications especially in adolescents, long-term outcomes following bariatric surgery and implementation of digital support systems to optimize outcomes and reduce costs of behavioural change interventions 205 . We must also better understand and tackle the barriers to implementation of treatment in real-life clinical settings, including the role of training of health professionals. Importantly, treatment studies of all kinds must engage people with lived experience — adolescents, parents and families — to understand what outcomes and elements of treatment are most valued.
Obesity prevention is challenging because it requires a multilevel, multisectoral approach that addresses inequity, involves many stakeholders and addresses both the upstream and the downstream factors influencing obesity risk. Some evidence exists of effectiveness of prevention interventions operating at the level of the child, family and school, but the very poor progress overall in modifying obesity prevalence globally highlights many areas in need of research and evidence implementation. Studies are needed especially in LMICs, particularly in the context of the nutrition transition and the double burden of malnutrition. A focus on intergenerational research, rather than the age-based focus of current work, is also needed. Systems research approaches should be used, addressing the broader food and physical activity environments, and links to climate change 206 . In all studies, strategies are needed that enable co-production with relevant communities, long-term follow-up, process evaluation and cost-effectiveness analyses. In the next few years, research and practice priorities must include a focus on intervention strategies in the earliest phases of life, including during pregnancy. The effects of COVID-19 and cost of living crises in many countries are leading to widening health inequalities 207 and this will further challenge obesity prevention interventions. Available resourcing for prevention interventions may become further constrained, requiring innovative solutions across agendas, with clear identification of co-benefits. For example, public health interventions for other diseases, such as dental caries or depression, or other societal concerns, such as urban congestion or climate change, may also act as obesity prevention strategies. Ultimately, to implement obesity prevention, societal changes are needed in terms of urban planning, social structures and health-care access.
Future high-quality paediatric obesity research can be enabled through strategies that support data sharing, which avoids research waste and bias, and enables new research questions to be addressed. Such approaches require leadership, careful engagement of multiple research teams, and resourcing. Four national or regional level paediatric weight registries exist 208 , 209 , 210 , 211 , which are all based in North America or Europe. Such registries should be established in other countries, especially in low-resource settings, even if challenging 208 . Another data-sharing approach is through individual participant data meta-analyses of intervention trials, which can include prospectively collected data 212 and are quite distinct from systematic reviews of aggregate data. Two recent examples are the Transforming Obesity Prevention in Childhood (TOPCHILD) Collaboration, which includes early interventions to prevent obesity in the first 2 years of life 213 , and the Eating Disorders in Weight-Related Therapy (EDIT) Collaboration, which aims to identify characteristics of individuals or trials that increase or protect against eating disorder risk following obesity treatment 214 . Formal data linkage studies, especially those joining up routine administrative datasets, enable longer-term and broader outcome measures to be assessed than is possible with standard clinical or public health intervention studies.
Collaborative research will also be enhanced through the use of agreed core outcome sets, supporting data harmonization. The Edmonton Obesity Staging System – Paediatric 215 is one option for paediatric obesity treatment. A core outcome set for early intervention trials to prevent obesity in childhood (COS-EPOCH) has been recently established 216 . These efforts incorporate a balance between wanting and needing to share data and adhering to privacy protection regulations. Objective end points are ideal, including directly measured physical activity and body composition.
Collaborative efforts and a systems approach are paramount to understand, prevent and manage child and adolescent obesity. Research funding and health policies should focus on feasible, effective and equitable interventions.
NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet https://doi.org/10.1016/S0140-6736(17)32129-3 (2017).
Article Google Scholar
Popkin, B. M., Corvalan, C. & Grummer-Strawn, L. M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 395 , 65–74 (2020).
Article PubMed Google Scholar
Kompaniyets, L. et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw. Open 4 , e2111182 (2021).
Article PubMed PubMed Central Google Scholar
Griffiths, L. J., Parsons, T. J. & Hill, A. J. Self‐esteem and quality of life in obese children and adolescents: a systematic review. Int. J. Pediatr. Obes. 5 , 282–304 (2010).
Buttitta, M., Iliescu, C., Rousseau, A. & Guerrien, A. Quality of life in overweight and obese children and adolescents: a literature review. Qual. Life Res. 23 , 1117–1139 (2014).
Hayes, A. et al. Early childhood obesity: association with healthcare expenditure in Australia. Obesity 24 , 1752–1758 (2016).
Marcus, C., Danielsson, P. & Hagman, E. Pediatric obesity – long-term consequences and effect of weight loss. J. Intern. Med. 292 , 870–891 (2022).
Berthoud, H. R., Morrison, C. D. & Münzberg, H. The obesity epidemic in the face of homeostatic body weight regulation: what went wrong and how can it be fixed? Physiol. Behav. 222 , 112959 (2020).
Article CAS PubMed PubMed Central Google Scholar
World Health Organization. Report of the commission on ending childhood obesity. WHO https://www.who.int/publications/i/item/9789241510066 (2016). This report from the WHO on approaches to childhood and adolescent obesity has six main recommendations for governments, covering food and physical activity, age-based settings and provision of weight management for those with obesity.
O’Connor, E. A. et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA 317 , 2427–2444 (2017).
Inge, T. H. et al. Five-year outcomes of gastric bypass in adolescents as compared with adults. N. Engl. J. Med. 380 , 2136–2145 (2019).
Weghuber, D. et al. Once-weekly semaglutide in adolescents with obesity. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2208601 (2022). To our knowledge, the first RCT of semaglutide 2.4 mg, administered weekly by subcutaneous injection, in adolescents with obesity.
World Health Organization. Report of the Commission on Ending Childhood Obesity: Implementation Plan: Executive Summary (WHO, 2017).
Hillier-Brown, F. C. et al. A systematic review of the effectiveness of individual, community and societal level interventions at reducing socioeconomic inequalities in obesity amongst children. BMC Public Health 14 , 834 (2014).
World Health Organization. Obesity. WHO https://www.who.int/health-topics/obesity#tab=tab_1 (2023).
Mei, Z. et al. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am. J. Clin. Nutr. 75 , 978–985 (2002).
Article CAS PubMed Google Scholar
World Health Organization. Child growth standards. WHO https://www.who.int/tools/child-growth-standards/standards (2006).
World Health Organization. Growth reference data for 5–19 years. WHO https://www.who.int/tools/growth-reference-data-for-5to19-years (2007).
National Center for Health Statistics. CDC growth charts. Centers for Disease Control and Prevention http://www.cdc.gov/growthcharts/ (2022).
Cole, T. J., Bellizzi, M. C., Flegal, K. M. & Dietz, W. H. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320 , 1240 (2000).
Di Cesare, M. et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 17 , 212 (2019).
Swinburn, B., Egger, G. & Raza, F. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev. Med. 29 , 563–570 (1999).
Ford, N. D., Patel, S. A. & Narayan, K. V. Obesity in low-and middle-income countries: burden, drivers, and emerging challenges. Annu. Rev. Public Health 38 , 145–164 (2017).
Shah, B., Tombeau Cost, K., Fuller, A., Birken, C. S. & Anderson, L. N. Sex and gender differences in childhood obesity: contributing to the research agenda. BMJ Nutr. Prev. Health 3 , 387–390 (2020).
Public Health England. Research and analysis: differences in child obesity by ethnic group. GOV.UK https://www.gov.uk/government/publications/differences-in-child-obesity-by-ethnic-group/differences-in-child-obesity-by-ethnic-group#data (2019).
Cole, T. J. & Lobstein, T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatr. Obes. 7 , 284–294 (2012).
Kelly, A. S. et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 128 , 1689–1712 (2013).
Garnett, S. P., Baur, L. A., Jones, A. M. & Hardy, L. L. Trends in the prevalence of morbid and severe obesity in Australian children aged 7–15 years, 1985-2012. PLoS ONE 11 , e0154879 (2016).
Spinelli, A. et al. Prevalence of severe obesity among primary school children in 21 European countries. Obes. Facts 12 , 244–258 (2019).
Twig, G. et al. Body-Mass Index in 2.3 million adolescents and cardiovascular death in adulthood. N. Engl. J. Med. 374 , 2430–2440 (2016).
González-Muniesa, P. et al. Obesity. Nat. Rev. Dis. Primers 3 , 17034 (2017).
Geserick, M. et al. Acceleration of BMI in early childhood and risk of sustained obesity. N. Engl. J. Med. 379 , 1303–1312 (2018).
Larqué, E. et al. From conception to infancy – early risk factors for childhood obesity. Nat. Rev. Endocrinol. 15 , 456–478 (2019).
Barker, D. J. Fetal origins of coronary heart disease. Br. Med. J. 311 , 171–174 (1995).
Article CAS Google Scholar
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359 , 61–73 (2008).
Philips, E. M. et al. Changes in parental smoking during pregnancy and risks of adverse birth outcomes and childhood overweight in Europe and North America: an individual participant data meta-analysis of 229,000 singleton births. PLoS Med. 17 , e1003182 (2020).
Voerman, E. et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med. 16 , e1002744 (2019). Individual participant data meta-analysis of >160,000 mothers and their children on the associations of maternal BMI and gestational weight gain and childhood overweight or obesity.
McIntyre, H. D. et al. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 5 , 47 (2019).
Oken, E. & Gillman, M. W. Fetal origins of obesity. Obes. Res. 11 , 496–506 (2003).
Hughes, A. R., Sherriff, A., Ness, A. R. & Reilly, J. J. Timing of adiposity rebound and adiposity in adolescence. Pediatrics 134 , e1354–e1361 (2014).
Rolland-Cachera, M. F. et al. Tracking the development of adiposity from one month of age to adulthood. Ann. Hum. Biol. 14 , 219–229 (1987).
Koletzko, B. et al. Prevention of childhood obesity: a position paper of the Global Federation of International Societies of Paediatric Gastroenterology, Hepatology and Nutrition (FISPGHAN). J. Pediatr. Gastroenterol. Nutr. 70 , 702–710 (2020).
Weber, M. et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am. J. Clin. Nutr. 99 , 1041–1051 (2014).
Cope, M. B. & Allison, D. B. Critical review of the World Health Organization’s (WHO) 2007 report on ‘evidence of the long‐term effects of breastfeeding: systematic reviews and meta‐analysis’ with respect to obesity. Obes. Rev. 9 , 594–605 (2008).
Totzauer, M. et al. Different protein intake in the first year and its effects on adiposity rebound and obesity throughout childhood: 11 years follow‐up of a randomized controlled trial. Pediatr. Obes. 17 , e12961 (2022).
Deren, K. et al. Consumption of sugar-sweetened beverages in paediatric age: a position paper of the European academy of paediatrics and the European Childhood Obesity Group. Ann. Nutr. Metab. 74 , 296–302 (2019).
Felső, R., Lohner, S., Hollódy, K., Erhardt, É. & Molnár, D. Relationship between sleep duration and childhood obesity: systematic review including the potential underlying mechanisms. Nutr. Metab. Cardiovasc. Dis. 27 , 751–761 (2017).
Farooq, A. et al. Longitudinal changes in moderate‐to‐vigorous‐intensity physical activity in children and adolescents: a systematic review and meta‐analysis. Obes. Rev. 21 , e12953 (2020).
Mahumud, R. A. et al. Association of dietary intake, physical activity, and sedentary behaviours with overweight and obesity among 282,213 adolescents in 89 low and middle income to high-income countries. Int. J. Obes. 45 , 2404–2418 (2021).
Ballon, M. et al. Socioeconomic inequalities in weight, height and body mass index from birth to 5 years. Int. J. Obes. 42 , 1671–1679 (2018).
Buoncristiano, M. et al. Socioeconomic inequalities in overweight and obesity among 6- to 9-year-old children in 24 countries from the World Health Organization European region. Obes. Rev. 22 , e13213 (2021).
Jiwani, S. S. et al. The shift of obesity burden by socioeconomic status between 1998 and 2017 in Latin America and the Caribbean: a cross-sectional series study. Lancet Glob. Health 7 , e1644–e1654 (2019).
Monteiro, C. A., Conde, W. L., Lu, B. & Popkin, B. M. Obesity and inequities in health in the developing world. Int. J. Obes. 28 , 1181–1186 (2004).
Guo, S. S. & Chumlea, W. C. Tracking of body mass index in children in relation to overweight in adulthood. Am. J. Clin. Nutr. 70 , 145S–148S (1999).
Aarestrup, J. et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int. J. Obes. 44 , 1546–1560 (2020).
Eslam, M. et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol. Hepatol. 6 , 864–873 (2021).
Daniels, S. R. et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation 111 , 1999–2012 (2005).
Cioana, M. et al. The prevalence of obesity among children with type 2 diabetes: a systematic review and meta-analysis. JAMA Netw. Open 5 , e2247186 (2022).
Gepstein, V. & Weiss, R. Obesity as the main risk factor for metabolic syndrome in children. Front. Endocrinol. 10 , 568 (2019).
Kuvat, N., Tanriverdi, H. & Armutcu, F. The relationship between obstructive sleep apnea syndrome and obesity: a new perspective on the pathogenesis in terms of organ crosstalk. Clin. Respir. J. 14 , 595–604 (2020).
Baker, J. L., Olsen, L. W. & Sorensen, T. I. Childhood body-mass index and the risk of coronary heart disease in adulthood. N. Engl. J. Med. 357 , 2329–2337 (2007).
Bjerregaard, L. G. et al. Change in overweight from childhood to early adulthood and risk of type 2 diabetes. N. Engl. J. Med. 378 , 1302–1312 (2018).
Kelsey, M. M., Zaepfel, A., Bjornstad, P. & Nadeau, K. J. Age-related consequences of childhood obesity. Gerontology 60 , 222–228 (2014).
Sharma, V. et al. A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes. Rev. 20 , 1341–1349 (2019).
Lobstein, T. & Jackson-Leach, R. Planning for the worst: estimates of obesity and comorbidities in school-age children in 2025. Pediatr. Obes. 11 , 321–325 (2016).
Berthoud, H. R., Münzberg, H. & Morrison, C. D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152 , 1728–1738 (2017).
Devoto, F. et al. Hungry brains: a meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci. Biobehav. Rev. 94 , 271–285 (2018).
Blum, W. F., Englaro, P., Attanasio, A. M., Kiess, W. & Rascher, W. Human and clinical perspectives on leptin. Proc. Nutr. Soc. 57 , 477–485 (1998).
Friedman, J. M. Leptin and the endocrine control of energy balance. Nat. Metab. 1 , 754–764 (2019).
Kühnen, P. et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 375 , 240–246 (2016).
Clément, K. et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 8 , 960–970 (2020).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156 , 20–44 (2014).
Fischer-Posovszky, P., Roos, J., Zoller, V. & Wabitsch, M. in Pediatric Obesity: Etiology, Pathogenesis and Treatment (ed. Freemark, M. S.) 81–93 (Springer, 2018).
Hammarstedt, A., Gogg, S., Hedjazifar, S., Nerstedt, A. & Smith, U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 98 , 1911–1941 (2018).
Silventoinen, K. et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the collaborative project of development of anthropometrical measures in twins (CODATwins) study. Am. J. Clin. Nutr. 104 , 371–379 (2016).
Silventoinen, K. et al. Differences in genetic and environmental variation in adult BMI by sex, age, time period, and region: an individual-based pooled analysis of 40 twin cohorts. Am. J. Clin. Nutr. 106 , 457–466 (2017).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum. Mol. Genet. 27 , 3641–3649 (2018).
Vogelezang, S. et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 16 , e1008718 (2020).
Bradfield, J. P. et al. A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet. 28 , 3327–3338 (2019). To our knowledge, currently the largest genome-wide association study meta-analysis on childhood obesity in >13,000 individuals with obesity and >15,500 controls.
Couto Alves, A. et al. GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci. Adv. 5 , eaaw3095 (2019).
Ding, X. et al. Genome-wide screen of DNA methylation identifies novel markers in childhood obesity. Gene 566 , 74–83 (2015).
Huang, R. C. et al. Genome-wide methylation analysis identifies differentially methylated CpG loci associated with severe obesity in childhood. Epigenetics 10 , 995–1005 (2015).
Rzehak, P. et al. DNA-methylation and body composition in preschool children: epigenome-wide-analysis in the European Childhood Obesity Project (CHOP)-Study. Sci. Rep. 7 , 14349 (2017).
Alfano, R. et al. Perspectives and challenges of epigenetic determinants of childhood obesity: a systematic review. Obes. Rev. 23 , e13389 (2022).
Vehmeijer, F. O. L. et al. DNA methylation and body mass index from birth to adolescence: meta-analyses of epigenome-wide association studies. Genome Med. 12 , 105 (2020). Meta-analysis of epigenome-wide association studies of childhood BMI in >4,000 children.
Richmond, R. C. et al. DNA methylation and BMI: investigating identified methylation sites at HIF3A in a causal framework. Diabetes 65 , 1231–1244 (2016).
Wahl, S. et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 541 , 81–86 (2017).
Kivimäki, M. et al. Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the Cardiovascular Risk in Young Finns Study. Am. J. Clin. Nutr. 86 , 1509–1514 (2007).
Whitaker, R. C., Wright, J. A., Pepe, M. S., Seidel, K. D. & Dietz, W. H. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 337 , 869–873 (1997).
Davey Smith, G., Steer, C., Leary, S. & Ness, A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC). Arch. Dis. Child. 92 , 876–880 (2007).
Fleten, C. et al. Parent-offspring body mass index associations in the Norwegian Mother and Child Cohort Study: a family-based approach to studying the role of the intrauterine environment in childhood adiposity. Am. J. Epidemiol. 176 , 83–92 (2012).
Gaillard, R. et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 63 , 683–691 (2014).
Lawlor, D. A. et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 5 , e33 (2008).
Patro, B. et al. Maternal and paternal body mass index and offspring obesity: a systematic review. Ann. Nutr. Metab. 63 , 32–41 (2013).
Sorensen, T. et al. Comparison of associations of maternal peri-pregnancy and paternal anthropometrics with child anthropometrics from birth through age 7 y assessed in the Danish National Birth Cohort. Am. J. Clin. Nutr. 104 , 389–396 (2016).
Styne, D. M. et al. Pediatric obesity – assessment, treatment, and prevention: an Endocrine Society Clinical Practice guideline. J. Clin. Endocrinol. Metab. 102 , 709–757 (2017).
National Institute for Health and Care Excellence. Obesity: identification, assessment and managenent: clinical guideline [CG189]. NICE https://www.nice.org.uk/guidance/cg189 (2022). A high quality clinical practice guideline for obesity management.
Barlow, S. E. Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120 (Suppl. 4), S164–S192 (2007).
Canadian Task Force on Preventive Health Care. Recommendations for growth monitoring, and prevention and management of overweight and obesity in children and youth in primary care. Can. Med. Assoc. J. 187 , 411–421 (2015).
US Preventive Services Task Force. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA 317 , 2417–2426 (2017).
McConnell-Nzunga, J. et al. Classification of obesity varies between body mass index and direct measures of body fat in boys and girls of Asian and European ancestry. Meas. Phys. Educ. Exerc. Sci. 22 , 154–166 (2018).
Reinehr, T. et al. Definable somatic disorders in overweight children and adolescents. J. Pediatr. 150 , 618–622.e5 (2007).
Reinehr, T. Thyroid function in the nutritionally obese child and adolescent. Curr. Opin. Pediatr. 23 , 415–420 (2011).
Kohlsdorf, K. et al. Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. Int. J. Obes. 42 , 1602–1609 (2018).
Armstrong, S. et al. Physical examination findings among children and adolescents with obesity: an evidence-based review. Pediatrics 137 , e20151766 (2016).
Reinehr, T. & Roth, C. L. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc. Health 3 , 44–54 (2019).
Garnett, S. P., Baur, L. A. & Cowell, C. T. Waist-to-height ratio: a simple option for determining excess central adiposity in young people. Int. J. Obes. 32 , 1028–1030 (2008).
Maffeis, C., Banzato, C., Talamini, G. & Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J. Pediatr. 152 , 207–213.e2 (2008).
Hampl, S. E. et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics 151 , e2022060640 (2023). A new, comprehensive clinical practice guideline outlining current recommendations on assessment and treatment of children and adolescents with obesity.
Reinehr, T. et al. Comparison of cardiovascular risk factors between children and adolescents with classes III and IV obesity: findings from the APV cohort. Int. J. Obes. 45 , 1061–1073 (2021).
Reinehr, T. Metabolic syndrome in children and adolescents: a critical approach considering the interaction between pubertal stage and insulin resistance. Curr. Diabetes Rep. 16 , 8 (2016).
Zeitler, P. et al. ISPAD Clinical Practice Consensus Guidelines 2018: type 2 diabetes mellitus in youth. Pediatr. Diabetes 19 , 28–46 (2018).
Ibáñez, L. et al. An International Consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm. Res. Paediatr. 88 , 371–395 (2017).
Brockmann, P. E., Schaefer, C., Poets, A., Poets, C. F. & Urschitz, M. S. Diagnosis of obstructive sleep apnea in children: a systematic review. Sleep Med. Rev. 17 , 331–340 (2013).
Taylor, E. D. et al. Orthopedic complications of overweight in children and adolescents. Pediatrics 117 , 2167–2174 (2006).
Winck, A. D. et al. Effects of obesity on lung volume and capacity in children and adolescents: a systematic review. Rev. Paul. Pediatr. 34 , 510–517 (2016).
PubMed PubMed Central Google Scholar
Jebeile, H., Lister, N., Baur, L., Garnett, S. & Paxton, S. J. Eating disorder risk in adolescents with obesity. Obes. Rev. 22 , e13173 (2021).
Quek, Y. H., Tam, W. W. S., Zhang, M. W. B. & Ho, R. C. M. Exploring the association between childhood and adolescent obesity and depression: a meta-analysis. Obes. Rev. 18 , 742–754 (2017).
World Health Organization. Consideration of the Evidence on Childhood Obesity for the Commission on Ending Childhood Obesity . Report of the Ad Hoc Working Group on Science and Evidence for Ending Childhood Obesity (WHO, 2016).
Pickett, K. et al. The Child of the North: building a fairer future after COVID-19. The Northern Health Science Alliance and N8 Research Partnership https://www.thenhsa.co.uk/app/uploads/2022/01/Child-of-the-North-Report-FINAL-1.pdf (2021).
Bronfenbrenner, U. Toward an experimental ecology of human development. Am. Psychol. 32 , 513–531 (1977).
Nuffield Council on Bioethics. Public Health: Ethical Issues (Nuffield Council on Bioethics, 2007).
Lorenc, T., Petticrew, M., Welch, V. & Tugwell, P. What types of interventions generate inequalities? Evidence from systematic reviews. J. Epidemiol. Community Health 67 , 190–193 (2013).
Adams, J., Mytton, O., White, M. & Monsivais, P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med. 13 , e1001990 (2016).
Backholer, K. et al. A framework for evaluating the impact of obesity prevention strategies on socioeconomic inequalities in weight. Am. J. Public Health 104 , e43–e50 (2014).
McGill, R. et al. Are interventions to promote healthy eating equally effective for all? Systematic review of socioeconomic inequalities in impact. BMC Public Health 15 , 457 (2015).
Brown, T. et al. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 7 , Cd001871 (2019). A Cochrane review involving 153 RCTs of diet and/or physical activity interventions to prevent obesity in children and adolescents, highlighting varying effectiveness of interventions in different age groups.
PubMed Google Scholar
Le, L. K.-D. et al. Prevention of high body mass index and eating disorders: a systematic review and meta-analysis. Eat. Weight Disord. 27 , 2989–3003 (2022).
Nobles, J., Summerbell, C., Brown, T., Jago, R. & Moore, T. A secondary analysis of the childhood obesity prevention Cochrane Review through a wider determinants of health lens: implications for research funders, researchers, policymakers and practitioners. Int. J. Behav. Nutr. Phys. Act. 18 , 22 (2021).
Rai, K. K., Dogra, S. A., Barber, S., Adab, P. & Summerbell, C. A scoping review and systematic mapping of health promotion interventions associated with obesity in Islamic religious settings in the UK. Obes. Rev. 20 , 1231–1261 (2019).
World Health Organization. Nutrition Action in Schools: A Review of Evidence Related to the Nutrition-Friendly Schools Initiative (WHO, 2021).
Daly-Smith, A. et al. Using a multi-stakeholder experience-based design process to co-develop the Creating Active Schools Framework. Int. J. Behav. Nutr. Phys. Act. 17 , 13 (2020).
Tibbitts, B. et al. Considerations for individual-level versus whole-school physical activity interventions: stakeholder perspectives. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph18147628 (2021).
Askie, L. M. et al. Interventions commenced by early infancy to prevent childhood obesity-The EPOCH Collaboration: an individual participant data prospective meta-analysis of four randomized controlled trials. Pediatr. Obes. 15 , e12618 (2020). To our knowledge, the first prospective individual participant data meta-analysis showing that interventions commencing in late pregnancy or very early childhood are associated with healthier BMI z -score at age 18–24 months.
Seidler, A. L. et al. Examining the sustainability of effects of early childhood obesity prevention interventions: follow-up of the EPOCH individual participant data prospective meta-analysis. Pediatr. Obes. 17 , e12919 (2022).
Taylor, R. W. et al. Sleep, nutrition, and physical activity interventions to prevent obesity in infancy: follow-up of the prevention of overweight in infancy (POI) randomized controlled trial at ages 3.5 and 5 y. Am. J. Clin. Nutr. 108 , 228–236 (2018).
Mihrshahi, S. et al. A review of registered randomized controlled trials for the prevention of obesity in infancy. Int. J. Environ. Res. Public Health 18 , 2444 (2021).
Farooq, M. A. et al. Timing of the decline in physical activity in childhood and adolescence: Gateshead Millennium Cohort Study. Br. J. Sports Med. 52 , 1002–1006 (2018).
van Sluijs, E. M. F. et al. Physical activity behaviours in adolescence: current evidence and opportunities for intervention. Lancet 398 , 429–442 (2021).
Griffin, N. et al. A critique of the English national policy from a social determinants of health perspective using a realist and problem representation approach: the ‘Childhood Obesity: a plan for action’ (2016, 2018, 2019). BMC Public Health 21 , 2284 (2021).
Knai, C., Lobstein, T., Petticrew, M., Rutter, H. & Savona, N. England’s childhood obesity action plan II. Br. Med. J. 362 , k3098 (2018).
World Health Organization. WHO European Regional Obesity Report 2022 (WHO, 2022).
Zemrani, B., Gehri, M., Masserey, E., Knob, C. & Pellaton, R. A hidden side of the COVID-19 pandemic in children: the double burden of undernutrition and overnutrition. Int. J. Equity Health 20 , 44 (2021).
Alman, K. L. et al. Dietetic management of obesity and severe obesity in children and adolescents: a scoping review of guidelines. Obes. Rev. https://doi.org/10.1111/obr.13132 (2020).
Pfeiffle, S. et al. Current recommendations for nutritional management of overweight and obesity in children and adolescents: a structured framework. Nutrients https://doi.org/10.3390/nu11020362 (2019).
Scottish Intercollegiate Guidelines Network. Management of obesity. A National Clinical Guideline . SIGN 115 (SIGN, 2010).
Reinehr, T. et al. Two-year follow-up in 21,784 overweight children and adolescents with lifestyle intervention. Obesity 17 , 1196–1199 (2009).
Ells, L. J. et al. Interventions for treating children and adolescents with overweight and obesity: an overview of Cochrane reviews. Int. J. Obes. 42 , 1823–1833 (2018).
Al‐Khudairy, L. et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst. Rev. 6 , CD012691 (2017). One of three Cochrane reviews looking at lifestyle treatment of paediatric obesity, in this case in adolescents, which identified 44 completed trials, finding low quality evidence of improvements in BMI and moderate quality evidence of improvements in weight.
Mead, E. et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst. Rev. 6 , CD012651 (2017). A Cochrane Review, involving 70 RCTs, showing that multicomponent behavioural interventions can lead to small, short-term reductions in BMI and related measures in children aged 6–11 years with obesity.
Ho, M. et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics 130 , e1647–e1671 (2012). To our knowledge, the first systematic review of lifestyle interventions in children and adolescents with obesity to show improvements in cardiometabolic outcomes (LDL cholesterol, triglycerides, fasting insulin and blood pressure), as well as weight.
Clinical Practice Guideline Panel. Clinical practice guideline for multicomponent behavioral treatment of obesity and overweight in children and adolescents: current state of the evidence and research needs. American Psychological Association https://www.apa.org/obesity-guideline/clinical-practice-guideline.pdf (2018).
Nowicka, P. & Flodmark, C. E. Family therapy as a model for treating childhood obesity: useful tools for clinicians. Clin. Child. Psychol. Psychiatry 16 , 129–145 (2011).
Wilfley, D. E. et al. Improving access and systems of care for evidence-based childhood obesity treatment: conference key findings and next steps. Obesity 25 , 16–29 (2017).
Amiri, P. et al. Does motivational interviewing improve the weight management process in adolescents? A systematic review and meta-analysis. Int. J. Behav. Med. 29 , 78–103 (2022).
Kao, T. A., Ling, J., Hawn, R. & Vu, C. The effects of motivational interviewing on children’s body mass index and fat distributions: a systematic review and meta-analysis. Obes. Rev. 22 , e13308 (2021).
Hassapidou, M. et al. European Association for the Study of Obesity (EASO) position statement on medical nutrition therapy for the management of overweight and obesity in children and adolescents developed in collaboration with the European Federation of the Associations of Dietitians (EFAD). Obes. Facts https://doi.org/10.1159/000527540 (2022).
Hoelscher, D. M., Kirk, S., Ritchie, L. & Cunningham-Sabo, L. Position of the Academy of Nutrition and Dietetics: interventions for the prevention and treatment of pediatric overweight and obesity. J. Acad. Nutr. Diet. 113 , 1375–1394 (2013).
Hoare, J. K., Jebeile, H., Garnett, S. P. & Lister, N. B. Novel dietary interventions for adolescents with obesity: a narrative review. Pediatr. Obes. 16 , e12798 (2021).
Lister, N. et al. Nutritional adequacy of diets for adolescents with overweight and obesity: considerations for dietetic practice. Eur. J. Clin. Nutr. 71 , 646–651 (2017).
Andela, S. et al. Efficacy of very low-energy diet programs for weight loss: a systematic review with meta-analysis of intervention studies in children and adolescents with obesity. Obes. Rev. 20 , 871–882 (2019).
Srivastava, G. & Apovian, C. M. Current pharmacotherapy for obesity. Nat. Rev. Endocrinol. 14 , 12–24 (2018).
Apperley, L. J. et al. Childhood obesity: a review of current and future management options. Clin. Endocrinol. 96 , 288–301 (2022).
European Medicines Agency. Saxenda. European Medicines Agency https://www.ema.europa.eu/en/medicines/human/EPAR/saxenda (2022).
US Food and Drug Administration. FDA approves weight management drug for patients aged 12 and older. FDA https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-weight-management-drug-patients-aged-12-and-older (2021).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382 , 2117–2128 (2020). To our knowledge, the first RCT of liraglutide, administered daily via subcutaneous injection, in adolescents with obesity.
US Food and Drug Administration. FDA approves novel, dual-targeted treatment for type 2 iabetes. FDA https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes (2022).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387 , 205–216 (2022).
Holst, J. J. & Rosenkilde, M. M. GIP as a therapeutic target in diabetes and obesity: insight from incretin co-agonists. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgaa327 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT05260021 (2023).
Müller, T. D., Blüher, M., Tschöp, M. H. & DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 21 , 201–223 (2022).
Chalklin, C. G., Ryan Harper, E. G. & Beamish, A. J. Metabolic and bariatric surgery in adolescents. Curr. Obes. Rep. 10 , 61–69 (2021).
Albaugh, V. L. et al. Regulation of body weight: lessons learned from bariatric surgery. Mol. Metab. https://doi.org/10.1016/j.molmet.2022.101517 (2022).
Uhe, I. et al. Roux-en-Y gastric bypass, sleeve gastrectomy, or one-anastomosis gastric bypass? A systematic review and meta-analysis of randomized-controlled trials. Obesity 30 , 614–627 (2022).
Inge, T. H. et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 5 , 165–173 (2017).
Olbers, T. et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. https://doi.org/10.1016/S2213-8587(16)30424-7 (2017).
Pratt, J. S. et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg. Obes. Relat. Dis. 14 , 882–901 (2018).
Jarvholm, K. et al. 5-year mental health and eating pattern outcomes following bariatric surgery in adolescents: a prospective cohort study. Lancet Child Adolesc. Health 4 , 210–219 (2020).
Van Der Heijden, L., Feskens, E. & Janse, A. Maintenance interventions for overweight or obesity in children: a systematic review and meta‐analysis. Obes. Rev. 19 , 798–809 (2018).
Park, J., Park, M.-J. & Seo, Y.-G. Effectiveness of information and communication technology on obesity in childhood and adolescence: systematic review and meta-analysis. J. Med. Internet Res. 23 , e29003 (2021).
Brissman, M., Beamish, A. J., Olbers, T. & Marcus, C. Prevalence of insufficient weight loss 5 years after Roux-en-Y gastric bypass: metabolic consequences and prediction estimates: a prospective registry study. BMJ Open 11 , e046407 (2021).
El Ansari, W. & Elhag, W. Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps – a scoping review. Obes. Surg. 31 , 1755–1766 (2021).
World Health Organization Regional Office for Europe. Weight Bias and Obesity Stigma: Considerations for the WHO European Region (WHO, 2017).
Puhl, R. M. & Latner, J. D. Stigma, obesity, and the health of the nation’s children. Psychol. Bull. 133 , 557–580 (2007).
Black, W. R. et al. Health-related quality of life across recent pediatric obesity classification recommendations. Children 8 , 303 (2021).
Finne, E., Reinehr, T., Schaefer, A., Winkel, K. & Kolip, P. Changes in self-reported and parent-reported health-related quality of life in overweight children and adolescents participating in an outpatient training: findings from a 12-month follow-up study. Health Qual. Life Outcomes 11 , 1 (2013).
Hill, A. J. Obesity in children and the ‘myth of psychological maladjustment’: self-esteem in the spotlight. Curr. Obes. Rep. 6 , 63–70 (2017).
McGregor, S., McKenna, J., Gately, P. & Hill, A. J. Self‐esteem outcomes over a summer camp for obese youth. Pediatr. Obes. 11 , 500–505 (2016).
Jansen, P., Mensah, F., Clifford, S., Nicholson, J. & Wake, M. Bidirectional associations between overweight and health-related quality of life from 4–11 years: longitudinal study of Australian children. Int. J. Obes. 37 , 1307–1313 (2013).
Mannan, M., Mamun, A., Doi, S. & Clavarino, A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J. Psychiatr. 21 , 51–66 (2016).
Lindberg, L., Hagman, E., Danielsson, P., Marcus, C. & Persson, M. Anxiety and depression in children and adolescents with obesity: a nationwide study in Sweden. BMC Med. 18 , 30 (2020).
Jebeile, H. et al. Association of pediatric obesity treatment, including a dietary component, with change in depression and anxiety: a systematic review and meta-analysis. JAMA Pediatr. 173 , e192841 (2019).
Gow, M. L. et al. Pediatric obesity treatment, self‐esteem, and body image: a systematic review with meta‐analysis. Pediatr. Obes. 15 , e12600 (2020).
Jebeile, H. et al. Treatment of obesity, with a dietary component, and eating disorder risk in children and adolescents: a systematic review with meta-analysis. Obes. Rev. 20 , 1287–1298 (2019). To our knowledge, the first systematic review to show that structured and professionally led weight management interventions in children and adolescents with obesity are associated with reductions in eating disorder risk and symptoms.
Kjeldbjerg, M. L. & Clausen, L. Prevalence of binge-eating disorder among children and adolescents: a systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry https://doi.org/10.1007/s00787-021-01850-2 (2021).
Patton, G. C., Selzer, R., Coffey, C., Carlin, J. B. & Wolfe, R. Onset of adolescent eating disorders: population based cohort study over 3 years. BMJ 318 , 765–768 (1999).
Cortese, S. The association between ADHD and obesity: intriguing, progressively more investigated, but still puzzling. Brain Sci. 9 , 256 (2019).
Griffiths, L. J., Dezateux, C. & Hill, A. Is obesity associated with emotional and behavioural problems in children? Findings from the Millennium Cohort Study. Int. J. Pediatr. Obes. 6 , e423–e432 (2011).
Harrist, A. W. et al. The social and emotional lives of overweight, obese, and severely obese children. Child. Dev. 87 , 1564–1580 (2016).
Van Geel, M., Vedder, P. & Tanilon, J. Are overweight and obese youths more often bullied by their peers? A meta-analysis on the relation between weight status and bullying. Int. J. Obes. 38 , 1263–1267 (2014).
Albuquerque, D., Nóbrega, C., Manco, L. & Padez, C. The contribution of genetics and environment to obesity. Br. Med. Bull. 123 , 159–173 (2017).
Rancourt, D. & McCullough, M. B. Overlap in eating disorders and obesity in adolescence. Curr. Diabetes Rep. 15 , 78 (2015).
House, E. T. et al. Identifying eating disorders in adolescents and adults with overweight or obesity: a systematic review of screening questionnaires. Int. J. Eat. Disord. 55 , 1171–1193 (2022).
Lister, N. B., Baur, L. A., Paxton, S. J. & Jebeile, H. Contextualising eating disorder concerns for paediatric obesity treatment. Curr. Obes. Rep. 10 , 322–331 (2021).
Hagman, E. et al. Effect of an interactive mobile health support system and daily weight measurements for pediatric obesity treatment, a 1-year pragmatical clinical trial. Int. J. Obes. 46 , 1527–1533 (2022).
Swinburn, B. A. et al. The global syndemic of obesity, undernutrition, and climate change: The Lancet commission report. Lancet 393 , 791–846 (2019).
Whitehead, M., Taylor-Robinson, D. & Barr, B. Poverty, health, and covid-19. Br. Med. J. 372 , n376 (2021).
Morrison, K. M. et al. The CANadian Pediatric Weight Management Registry (CANPWR): lessons learned from developing and initiating a national, multi-centre study embedded in pediatric clinical practice. BMC Pediatr. 18 , 237 (2018).
Kirk, S. et al. Establishment of the pediatric obesity weight evaluation registry: a national research collaborative for identifying the optimal assessment and treatment of pediatric obesity. Child. Obes. 13 , 9–17 (2017).
Hagman, E., Danielsson, P., Lindberg, L. & Marcus, C., BORIS Steering Committee. Paediatric obesity treatment during 14 years in Sweden: lessons from the Swedish Childhood Obesity Treatment Register – BORIS. Pediatr. Obes. 15 , e12626 (2020).
Bohn, B. et al. Changing characteristics of obese children and adolescents entering pediatric lifestyle intervention programs in Germany over the last 11 years: an adiposity patients registry multicenter analysis of 65,453 children and adolescents. Obes. Facts 10 , 517–530 (2017).
Seidler, A. L. et al. A guide to prospective meta-analysis. BMJ 367 , l5342 (2019).
Hunter, K. E. et al. Transforming obesity prevention for children (TOPCHILD) collaboration: protocol for a systematic review with individual participant data meta-analysis of behavioural interventions for the prevention of early childhood obesity. BMJ Open 12 , e048166 (2022).
Lister, N. B. et al. Eating disorders in weight-related therapy (EDIT) collaboration: rationale and study design. Nutr. Res. Rev. https://doi.org/10.1017/S0954422423000045 (2023).
Hadjiyannakis, S. et al. Obesity class versus the Edmonton Obesity Staging System for Pediatrics to define health risk in childhood obesity: results from the CANPWR cross-sectional study. Lancet Child Adolesc. Health 3 , 398–407 (2019).
Brown, V. et al. Core outcome set for early intervention trials to prevent obesity in childhood (COS-EPOCH): agreement on “what” to measure. Int. J. Obes. 46 , 1867–1874 (2022). A stakeholder-informed study that identified the minimum outcomes recommended for collecting and reporting in obesity prevention trials in early childhood.
Han, J. C., Lawlor, D. A. & Kimm, S. Y. Childhood obesity. Lancet 375 , 1737–1748 (2010).
Shin, A. C., Zheng, H. & Berthoud, H. R. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol. Behav. 97 , 572–580 (2009).
Lennerz, B., Wabitsch, M. & Eser, K. Ätiologie und genese [German]. Berufl. Rehabil. 1 , 14 (2014).
Google Scholar
Pont, S. J., Puhl, R., Cook, S. R. & Slusser, W. Stigma experienced by children and adolescents with obesity. Pediatrics 140 , e20173034 (2017).
Talumaa, B., Brown, A., Batterham, R. L. & Kalea, A. Z. Effective strategies in ending weight stigma in healthcare. Obes. Rev. 23 , e13494 (2022).
Rubino, F. et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 26 , 485–497 (2020).
Download references
Author information
Authors and affiliations.
Children’s Hospital Westmead Clinical School, Sydney Medical School, The University of Sydney, Sydney, New South Wales, Australia
Natalie B. Lister & Louise A. Baur
Institute of Endocrinology and Diabetes, The Children’s Hospital at Westmead, Sydney, New South Wales, Australia
Natalie B. Lister
Sydney School of Public Health, The University of Sydney, Sydney, New South Wales, Australia
Louise A. Baur
Weight Management Services, The Children’s Hospital at Westmead, Sydney, New South Wales, Australia
The Generation R Study Group, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
Janine F. Felix
Department of Paediatrics, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
Institute of Health Sciences, School of Medicine, University of Leeds, Leeds, UK
Andrew J. Hill
Division of Paediatrics, Department of Clinical Science Intervention and Technology, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden
Claude Marcus
Vestische Hospital for Children and Adolescents Datteln, University of Witten/Herdecke, Datteln, Germany
Thomas Reinehr
Department of Sport and Exercise Sciences, Durham University, Durham, UK
- Carolyn Summerbell
Division of Paediatric Endocrinology and Diabetes, Department of Paediatrics and Adolescent Medicine, Ulm University Medical Centre, Ulm, Germany
Martin Wabitsch
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (L.A.B., J.F.F. and N.B.L.); Epidemiology (L.A.B. and J.F.F.); Mechanisms/pathophysiology (L.A.B., J.F.F., T.R. and M.W.); Diagnosis, screening and prevention (L.A.B., N.B.L., T.R., C.S. and M.W.); Management (L.A.B., N.B.L., A.J.H., C.M. and T.R.); Quality of life (L.A.B., N.B.L. and A.J.H.); Outlook (L.A.B., N.B.L., J.F.F., A.J.H., C.M., T.R., C.S. and M.W.); Overview of the Primer (L.A.B. and N.B.L.).
Corresponding author
Correspondence to Louise A. Baur .
Ethics declarations
Competing interests.
A.J.H. reports receiving payment for consultancy advice for Slimming World (UK). L.A.B. reports receiving honoraria for speaking in forums organized by Novo Nordisk in relation to management of adolescent obesity and the ACTION-Teens study, which is sponsored by Novo Nordisk. L.A.B. is the Australian lead of the study. T.R. received funding from the German Federal Ministry of Education and Research (BMBF; 01GI1120A/B) as part of the German Competence Network Obesity (Consortium ‘Youth with Extreme Obesity’). T.R. receives payment for consultancy advice related to pharmacological treatment of obesity from Novo Nordisk and Lilly, as well as honoraria for lectures in symposia organized by Novo Nordisk, Novartis and Merck. C.M. receives payments for consultancy advice and advisory board participation from Novo Nordisk, Oriflame Wellness, DeFaire AB and Itrim AB. C.M. also receives honoraria for speaking at meetings organized by Novo Nordisk and Astra Zeneca. C.M. is a shareholder and founder of Evira AB, a company that develops and sells systems for digital support for weight loss, and receives grants from Novo Nordisk for epidemiological studies of the effects of weight loss on future heath. M.W. received funding from the German Federal Ministry of Education and Research (BMBF; 01GI1120A/B) as part of the German Competence Network Obesity (Consortium ‘Youth with Extreme Obesity’). M.W. receives payment for consultancy advice related to pharmacological treatment of obesity from Novo Nordisk, Regeneron, Boehringer Ingelheim and LG Chem, as well as honoraria for speaking in symposia organized by Novo Nordisk, Rhythm Pharmaceuticals and Infectopharm. M.W. is principal investigator in phase II and phase III studies of setmelanotide sponsored by Rhythm Pharmaceuticals. N.B.L., J.F.F. and C.S. declare no competing interests.

Peer review
Peer review information.
Nature Reviews Disease Primers thanks C. Maffeis, L. Moreno, R, Weiss and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Lister, N.B., Baur, L.A., Felix, J.F. et al. Child and adolescent obesity. Nat Rev Dis Primers 9 , 24 (2023). https://doi.org/10.1038/s41572-023-00435-4
Download citation
Accepted : 12 April 2023
Published : 18 May 2023
DOI : https://doi.org/10.1038/s41572-023-00435-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Relationship between 24-h activity behavior and body fat percentage in preschool children: based on compositional data and isotemporal substitution analysis.
BMC Public Health (2024)
Young people's experiences of physical activity insecurity: a qualitative study highlighting intersectional disadvantage in the UK
- Caroline Dodd-Reynolds
- Naomi Griffin
Enough with simplifying: “eat less and move more”: at what point are we with the treatment of excess weight in paediatrics?
- Giovanni Corsello
- Sergio Bernasconi
Italian Journal of Pediatrics (2024)
Association between watching eating broadcast “Mukbang and Cookbang” and body mass index status in South Korean adolescents stratified by gender
- Sang-yeon Park
- Jeongha Eom
- Eun-Cheol Park
Nutrition Journal (2024)
Effect of a carbohydrate-restricted diet on weight loss in overweight and obese pediatric population: a meta-analysis of randomized controlled trials
- Pejman Rohani
- Zahra Rasoulizadeh
- Nathalia Sernizon Guimarães
Diabetology & Metabolic Syndrome (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Perspective: Childhood Obesity Requires New Strategies for Prevention
Barbara j deal, mark d huffman, helen binns, neil j stone.
- Author information
- Article notes
- Copyright and License information
Address correspondence to BJD (e-mail: [email protected] )
Received 2020 Jan 10; Revised 2020 Mar 10; Accepted 2020 Mar 13; Collection date 2020 Sep.
This article is published and distributed under the terms of the Oxford University Press, Standard Journals Publication Model ( https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model )
The prevalence of obesity among youth in the USA is currently >18% with projections that more than half of today's children will be obese as adults. The growth trajectory of children more likely to become obese is determined by weight in earliest childhood, and childhood body mass index (BMI) tracks through adolescence and adulthood. Childhood consequences of obesity include increased risk of asthma, type 2 diabetes mellitus, orthopedic disorders, and reduced academic performance. Health implications of obesity in adulthood include premature coronary artery disease, hypertension, type 2 diabetes, stroke, and certain cancers, contributing to the leading causes of adult mortality. Early childhood obesity is influenced by prenatal exposure to maternal obesity and environmental obesogens, and is associated with poverty, food insecurity, and poor nutritional quality. New strategies for primordial prevention of early childhood obesity require focusing attention on growth parameters during the first 2 y of life, with support for increasing the duration of breastfeeding, and improvements in dietary quality and availability, particularly the reduced consumption of added sugars. Reducing the prevalence of obesity among adolescent females and reducing exposure to environmental obesogens may reduce the prevalence of transgenerational obesity. The reduction of early childhood obesity could improve population health, quality of life, and longevity throughout the life course.
Keywords: early childhood obesity, obesity/morbidity, body mass index, breastfeeding, population health
Introduction
Despite major national and state-level efforts, by 2016 the prevalence of obesity in the USA had increased to 39.8% among adults (compared with 33.7% in 2007–2008) and to 18.5% among youth <18 years of age (from 16.8% in 2007–2008) ( 1 , 2 ). Based on 2016 levels of childhood obesity in the USA, simulated growth trajectories predict 57% of today's children will be obese at the age of 35 y ( 3 ). The consequences of obesity contribute to the leading causes of death and disability among adults: cardiovascular diseases including premature coronary artery disease, hypertension, atrial fibrillation and stroke, cancer, osteoarthritis, type 2 diabetes, and chronic kidney and liver diseases ( 4 ). In contrast to adults, mortality related to obesity among youth is rare ( 5 ), contributing to complacency regarding the health implications and morbidity of childhood obesity, including early onset of type 2 diabetes and hypertension. Obesity-related conditions progress through adulthood affecting all areas of adult well-being and life expectancy ( 6 ). Thus, it is imperative that public policy interventions be implemented to alter the development of obesity in childhood.
Growth trajectories for obesity are established in infancy and early childhood, and track into adulthood ( 3 ). Previous publications have recommended initiating screening for childhood overweight and obesity at ages 6–12 y, however, this may miss an important window during which obesity may be developing in many younger children ( 7 – 9 ). By waiting until age 6 y, these positions fall into the category of primary prevention of obesity-related health morbidities. This review summarizes contemporary data on lifelong health consequences of pediatric obesity, pediatric obesity growth trajectories, causes of childhood obesity, and new strategies for the prevention and reduction of early childhood obesity, including population-level, primordial efforts to reduce the risk factors predisposing to obesity, and the life course health consequences and costs of childhood obesity ( 10 , 11 ) ( Box 1 ).
Box 1: Key points
Childhood obesity often begins in utero and early infancy. Maternal obesity, excessive weight gain during pregnancy, and rapid weight gain during the first 2 y of life are associated with childhood obesity.
Children who are obese by age 5 y are more likely to be obese as adolescents, and adolescents with obesity are highly likely to be obese as adults.
Obesity during childhood is associated with increased risk of asthma, type 2 diabetes, orthopedic disorders, and reduced academic performance.
Obesity during pregnancy is associated with increased risk of miscarriage, birth defects among newborns, and adult obesity among offspring.
Obesity during adulthood contributes to the major causes of adult mortality: premature coronary artery disease, hypertension, stroke, chronic kidney and liver disease, and many types of cancer.
New strategies for primordial prevention of early childhood obesity include achievement of healthy maternal weight prior to pregnancy, widespread adoption of breastfeeding for the first 6 mo of life, careful monitoring and intervention for excessive weight gain during the first 2 y of life, reducing consumption of added sugars, such as juices, among children, improving dietary quality and availability for children, and reducing exposures to environmental obesogens.
Current Status of Knowledge
Childhood obesity: c auses and growth trajectories.
Extensive data now exist to help understand the etiology of obesity as a multigenerational disease that begins during fetal life, with multiple contributing causes as summarized in Table 1 ( 12 ). Viewing obesity as a lifetime disease, with origins preconception, in utero, and during early infancy with intergenerational effects is essential to guide efforts to reduce obesity and cardiovascular disease of adulthood.
Risk factors for the development of childhood obesity during prenatal, neonatal/infancy, and childhood/adolescent periods
Prenatal causes
Preconception maternal and paternal dietary quality, weight status, assisted reproductive technology involving embryo culture, and environmental exposures alter the developmental plasticity of gametes, and subsequent fetal programming, resulting in postnatal cardiometabolic disease risk ( 12 , 13 ). Prenatal or early life exposure to endocrine disruptor chemicals (also known as obesogens), such as air pollutants or pesticides, at a critical time for differentiation of mesenchymal stem cells into either adipocytes or osteoblasts may result in enhanced adipocyte numbers, which is considered irreversible and may be transmitted across future generations ( 13 ).
A mother who begins pregnancy obese has a significantly higher risk of late childhood obesity in her offspring (OR = 4.47; 95% CI: 3.99, 5.23) ( 14 ). The prevalence of prepregnancy obesity in the USA in 2015 was ∼26%, a 2% absolute increase since 2011 ( 15 ). For the developing fetus in the setting of maternal obesity, exposure to increased concentrations of inflammatory cytokines, hypermethylation of DNA, and histone modification produce epigenetic changes associated with increased risk of obesity in both the child and subsequent generations ( 13 ). Estimates of the proportion of childhood overweight/obesity prevalence attributable to maternal overweight, obesity, and excessive gestational weight gain ranged from 10% to 22% in a recent meta-analysis ( 14 ).
Early childhood contributions to obesity
Methodologic issues complicate the assessment of the effect of feeding infants formula versus breast-feeding for the first 6 mo of life on childhood obesity. A 2013 meta-analysis suggests a longer duration of breastfeeding results in a 13% lower prevalence of childhood obesity ( 16 ). Exclusive breastfeeding for the first 6 mo of life is recommended with a Healthy People 2020 target in the USA of 60%, compared with the current level of 25% ( 17 , 18 ). Breastfeeding is lowest among families living under 200% of the USA poverty level, lower maternal educational attainment, younger maternal age, and among African Americans or Hispanics compared with Caucasians ( 19 ).
The health effects of a suboptimal diet and high BMI contributed to an estimated 11 million deaths among adults globally in 2017 ( 20 ). The rapid and fundamental changes in food and beverages has translated into the consumption of 80% of calories from packaged foods and beverages among Americans, with >70% considered ultraprocessed foods ( 21 ). The contribution of added sugars to poor cardiometabolic health, obesity, and type 2 diabetes mellitus (DM) is well-documented ( 22 ). Among US children ages 2 y and above, added sugars accounted for 14% of daily caloric intake in 2013–2018, versus the recommended intake <5–10% of total calories for children and adolescents ( 23 ). The consumption of sugar-sweetened beverages, accounting for more than half of dietary added sugars in the USA, begins in the first year of life, with 43% of infants and 72% of toddlers consuming ≥1 sugar-sweetened beverage or dessert daily in 2009 ( 24 ). Sugar-sweetened beverage consumption has been positively associated with higher BMI among children ( 24 – 26 ).
Socioeconomic factors and obesity
Poverty, food insecurity, social stressors, rural environments, and lower educational attainment of parents are important associates of early childhood obesity ( 27 – 29 ). Young children with obesity are more likely to live in poverty and in households with lower educational attainment ( 28 , 29 ). Food insecurity, defined as a lack of dependable, regular access to high-quality food, affects ≥12% of households and almost 17 million children in the USA who do not know when, or how adequate, their next meal will be ( 30 ). Obesity is present in 30% of Hispanic children living in households with food insecurity, with the highest rates of obesity (40%) among American Indians ( 31 , 32 ). Episodic household food shortages are associated with the consumption of more energy-dense/nutrient-poor foods when available, with the average added sugar intake over 70 g daily, compared with the recommended level of 25 g or less daily ( 33 ).
Pediatric obesity growth trajectories
The growth trajectory of children more likely to become obese is determined by weight in earliest childhood, and childhood BMI tracks through adolescence and adulthood ( 27 , 34 ). Infants and toddlers with rapid postnatal growth, as evidenced by crossing weight-for-length percentiles in the first 3–6 mo of life or accelerating BMI between the ages of 2–6 y, were more likely to be obese by age 12–14 y ( 34 , 35 ). Between 70 and 90% of children with obesity in kindergarten were obese through age 14 y, independent of sex, race, or socioeconomic backgrounds ( 34 , 35 ). Approximately 70–80% of adolescents with overweight or obesity will be obese as adults ( 36 , 37 ).
Clinical presentation and health implications of obesity in childhood
Health implications of obesity affect virtually every organ system, with some effects well-recognized in childhood, whereas other long-term effects manifest in adulthood.
Morbidity associated with obesity in childhood and adolescence
In addition to well-known comorbidities of childhood obesity as summarized in Table 2 , additional adverse health effects of obesity among children and adolescents include reactive airway disease and increasing prevalence of type 2 DM ( 38 , 39 ). The risk of asthma among youth with obesity is almost twice that of normal weight children ( 38 ). Prediabetes with abnormal fasting glucose and elevated hemoglobin A1c is prevalent among 17% of youth in the USA, and is highly associated with obesity ( 40 ). Type 2 DM, once diagnosed in middle-aged adults with obesity, is increasingly detected among adolescents by age 14 y. Type 2 DM increased to an incidence of 13.8/100,000 among US youth aged 10–19 y in 2014–2015, and is projected to quadruple between 2010 and 2050 ( 39 , 41 ). By adulthood, these youth have experienced years of chronic exposure to metabolic and atherogenic abnormalities, who have higher odds of diabetic-related complications and premature cardiovascular mortality compared with those with type 1 DM ( 42 ). Moreover, those with type 2 DM who develop acute myocardial infarction have increased complication rates and mortality ( 43 ).
Lifelong health consequences of obesity ( 6 , 49 , 50 , 51 )
Effects of obesity on reproductive health
For adolescent and young adult females, obesity is associated with polycystic ovarian syndrome, decreased fertility, and increased risks of complications of miscarriage, preterm delivery, or stillbirth with pregnancy ( 44 , 45 ). Offspring born to obese women are at increased risk of birth defects, including congenital heart disease, as well as elevated blood pressure and lipid abnormalities ( 46 , 47 ). The likelihood of obesity in young adulthood for offspring of obese parents has increased by 2- to almost 6-fold, depending on if 1 or both parents were obese ( 48 ).
Effects of obesity on adult-onset, chronic noncommunicable diseases
The life-long consequences of obesity from childhood into adulthood include increased risk of cardiovascular disease, cancer, disability, and shortened life expectancy ( 6 , 52 – 55 ). Obesity-related cardiovascular disease accounted for >4 million deaths and 120 million disability-adjusted life-years globally in 2015 ( 6 ). The economic burden of caring for obesity-related cardiovascular diseases is staggering: total costs are estimated to increase in the USA by $28 billion dollars annually between 2015 and 2035, from the current level of ≥$351 billion dollars ( 4 , 56 ).
The origins of atherosclerotic vascular disease associated with childhood obesity have been documented for several decades, although the clinical consequences of hypertension, premature ischemic heart disease, and stroke become clinically apparent in adulthood ( 36 , 52 , 54 , 57 ). The age at incident cardiovascular events is decreasing in some demographic groups and may be a principal driver of the plateau in national age-adjusted death rates due to cardiovascular diseases over the past decade ( 4 ).
The pathophysiology of adipose tissue promoting and accelerating cancer development shares a common pathway of proinflammatory changes with cardiometabolic disorders ( 58 ). By adulthood, excess weight is associated with a higher risk of ≥13 different types of cancer, accounting for 40% of all cancers diagnosed in 2014 ( 59 ). These cancers, particularly colon cancer, are being diagnosed with increasing frequency among young adults with obesity ( 60 ). Improved awareness of the role of obesity in the promotion of multiple forms of cancer should lend urgency to the need for prevention, treatment, and control of childhood obesity.
Strategies for the prevention of early childhood obesity
The US Healthy People 2020 goal aimed to lower the proportion of obesity among young children aged 2–5 y from 10.4% in 2008 to 9.4%; the 2030 goals are in progress ( 61 ). Comprehensive recommendations to address obesity in older children and adults focus on individual nutritional and behavioral changes, which demonstrate limited success ( 17 , 49 ). Primordial prevention of overweight and obesity in early childhood incorporating environmental, societal, and policy changes may have the largest impact and opportunity for lasting improvement in cardiovascular health across future generations, with strategies listed in Table 3 ( 10 , 11 , 17 , 62 , 63 ).
New strategies for primordial prevention of early childhood obesity
SNAP, Supplemental Nutritional Assistance Program.
Broaden support for early breastfeeding
Improving the rate of initiation and duration of exclusive breastfeeding for the first 6 mo of life could be expected to reduce childhood obesity by ≥13–30% ( 64 , 65 ). Governmental and health care policy support and changes in infant formula purchasing agreements are necessary to increase participation in the effective Baby-Friendly Hospitals Initiative for successful breastfeeding ( 66 ). A major barrier to continued breastfeeding includes the need to return to work soon after birth, which disproportionately affects lower income households ( 67 ). Extending paid maternity leave has been associated with increased rates and longer duration of breastfeeding; in turn, this can be expected to reduce obesity in later childhood and adulthood by ≥12–15% ( 64 , 68 ).
Focus attention on growth parameters in the first years of life
Identifying families and newborns at increased risk of obesity as listed in Table 2 could prompt early home health visits and more frequent weight and feeding monitoring ( 17 ). Avoidance of fruit juices in the first year of life, with efforts to make water the normative beverage after the age-appropriate consumption of milk is advocated ( 69 ).
Reduce the consumption of added sugars in children's diets
The implementation of taxation on sugar-sweetened beverages could reduce consumption by >25% and result in estimated reduced direct medical costs of $23 billion dollars annually by 2025, in addition to averting over 101,000 disease-attributable disability-adjusted life years ( 70 ). In January 2017, a 1.5 penny-per-ounce sugar-sweetened and artificially sweetened beverage tax was implemented in Philadelphia to support prekindergarten education, resulting in both a net 27% decline in sugar-sweetened and artificially sweetened beverage purchasing based on sales volume and the creation of new programming ( 71 ).
Improve dietary quality and availability
Specific policy strategies to modify the poor diet that is the leading cause of cardiovascular disease globally have been proposed ( 72 – 74 ). A combined approach of subsidies for fruits, vegetables, nuts, and whole grains of 15–30% with taxation of sugar and sugar-sweetened beverages was estimated to produce the greatest reduction in cardiovascular disease and reduction of disparities in disease burden ( 73 ). Marketing of calorie-dense, nutrient-poor products to children, supported by enormous federal subsidies, are demonstrated as important contributors to childhood and adolescent obesity ( 72 ).
Invest in nutritional support for populations at highest risk of obesity in childhood
Recommendations to modify the federal Supplemental Nutritional Assistance Program (SNAP) to benefit families at highest risk of suboptimal nutrition include restrictions to purchasing taxable products not consumed for nourishment and incentives to increase purchasing of healthy foods ( 75 ). An analysis of the impact of SNAP changes estimated that over 5 y, >11,900 cardiovascular deaths in adults would be averted, while achieving health care cost savings of >$5 billion dollars annually ( 75 ). By reducing food insecurity and improving dietary quality for children, the improvements in health quality and cost savings could be even greater ( 73 ).
Reduce obesity among adolescent females
Healthy People 2020 aims to reduce the proportion of obese adolescents to a target of 16%; currently, over 21% of adolescent females are obese. Reducing obesity in adolescent and young adult females could be expected to reduce childhood obesity by 10–22%, with ongoing effects for subsequent generations. Food banks and medically tailored meals (low-fat or low-glycemic index meals with reduced calories) may be successful in reducing obesity among adolescents at highest risk ( 76 ).
Increase research, governmental, and industry efforts to guide reduced exposure to environmental obesogens
Environmental chemical exposures contribute health care costs that may exceed 10% of the global domestic product ( 77 ). Obesogens are estimated to contribute ≥2% to 4% of obesity prevalence; however, this may be underestimated due to the irreversible, transgenerational effects of exposures in early childhood. To reduce environmental exposure to endocrine-disrupting chemicals which contribute to obesity, legislation is necessary ( 77 ).
Improve the economics of direct health care costs of obesity
Direct health care costs related to childhood and adult obesity are estimated at >$275 billion dollars annually ( 56 , 63 ). Medical costs related to children with obesity accounted for ≥$14 billion dollars annually in 2008 and childhood obesity has since increased by 10% ( 78 ). Small weight reductions of 1% in early childhood obesity among children aged 6 y, requiring expenditure of $103 million dollars, are projected to result in annual savings in adult medical expenditures of $845 million dollars annually ( 62 ). Paid maternity leave has been shown to increase breastfeeding duration, which would reduce childhood and adult obesity by ≥15%. Estimates of cost savings related to breastfeeding are impressive: if 90% of US families could comply with recommendations to breastfeed exclusively for the first 6 mo of life (a high goal compared with the 25% rate in 2018, and the Healthy People 2020 goal of 61%), then $13 billion dollars in annual health care costs would be saved ( 18 , 79 , 80 ).
Conclusions
Obesity is a severe, chronic disease associated with shortened life expectancy due to cardiovascular events, diabetes, chronic kidney disease, and several forms of cancer. Determinants of obesity in childhood are well established before the age of 5 y, with these children especially likely to become obese adults. New policies directed at reducing obesity at the earliest stages by targeting the nutritional environments and well-being of infants, toddlers, and preschool children could alter the trajectory of childhood and adult obesity and improve population health, longevity, and quality of life throughout the life course. Starting early isn't an option, it's essential.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—all authors: wrote, read and approved the final manuscript.
The authors reported no funding received for this work.
Author disclosures: The authors report no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition . Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: DM, diabetes mellitus; SNAP, Supplemental Nutritional Assistance Program.
Contributor Information
Barbara J Deal, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Mark D Huffman, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; The George Institute for Global Health, Sydney, Australia.
Helen Binns, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; Pediatrics, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, USA.
Neil J Stone, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
- 1. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319(16):1723–5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2015–2016. In: National Center for Health Statistics, editor Hyattsville (MD): Health E-Stats; 2018. [ Google Scholar ]
- 3. Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. 2017;377(22):2145–53. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR et al. . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68(6):1–77. [ PubMed ] [ Google Scholar ]
- 6. The Global Burden of Disease 2015 Obesity Collaborators Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF et al. . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Steinberger J, Daniels SR, Hagberg N, Isasi CR, Kelly AS, Lloyd-Jones D, Pate RR, Pratt C, Shay CM, Towbin JA et al. . Cardiovascular health promotion in children: challenges and opportunities for 2020 and beyond: a Scientific Statement from the American Heart Association. Circulation. 2016;134(12):e236–55. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Grossman DC, Bibbins-Domingo K, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kurth AE et al. . Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(23):2417–26. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Gillman MW, Ludwig DS. How early should obesity prevention start? N Engl J Med. 2013;369(23):2173–5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Van Horn L, Vincent E, Perak AM. Preserving cardiovascular health in young children: beginning healthier by starting earlier. Curr Atheroscler Rep. 2018;20(6):26. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, Barker M, Saffery R, Yajnik CS, Eckert JJ et al. . Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391(10132):1842–52. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Shafei AE, Nabih ES, Shehata KA, Abd Elfatah ESM, Sanad ABA, Marey MY, Hammouda A, Mohammed MMM, Mostafa R, Ali MA. Prenatal exposure to endocrine disruptors and reprogramming of adipogenesis: an early-life risk factor for childhood obesity. Child Obes. 2018;14(1):18–25. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Voerman E, Santos S, Patro Golab B, Amiano P, Ballester F, Barros H, Bergstrom A, Charles MA, Chatzi L, Chevrier C et al. . Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med. 2019;16(2):e1002744. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Deputy NP, Dub B, Sharma AJ. Prevalence and trends in prepregnancy normal weight—48 states, New York City, and District of Columbia, 2011–2015. MMWR. 2018;66(51–52):1402–7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Horta BL, Victora CG. Long term effects of breastfeeding: a systematic review. World Health Organization; 2013;74. [ Google Scholar ]
- 17. Institute of Medicine Early childhood obesity prevention policies goals, recommendations, and potential actions. Washington (DC):2011. [ Google Scholar ]
- 18. Centers for Disease Control and Prevention Breastfeeding Report Card: United States, 2018. Atlanta (GA): 2019. [ Google Scholar ]
- 19. Anstey AH, Chen J, Elam-Evans LD, Perrine CG. Racial and geographic differences in breastfeeding—United States, 2011–2015. MMWR. 2017;66(27):723–7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, Mullany EC, Abate KH, Abbafati C, Abebe Z et al. . Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet. 2019;393(10184):1958–72. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Baldridge AS, Huffman MD, Taylor F, Xavier D, Bright B, Van Horn LV, Neal B, Dunford E. The healthfulness of the US packaged food and beverage supply: a cross-sectional study. Nutrients. 2019;11(8):1704. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Singh GM, Micha R, Khatibzadeh S, Lim S, Ezzati M, Mozaffarian D; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group . Estimated global, regional, and national disease burdens related to sugar-sweetened beverage consumption in 2010. Circulation. 2015;132(8):639–66. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. US Department of Health and Human Services and US Department of Agriculture 2015–2020 Dietary Guidelines for Americans, 8th edition2015; [Internet] [accessed 2019 Sep 1]. Available from: https://health.gov/dietaryguidelines/2015/guidelines . [ Google Scholar ]
- 24. Saavedra JM, Deming D, Dattilo A, Reidy K. Lessons from the Feeding Infants and Toddlers study in North America: what children eat, and implications for obesity prevention. Ann Nutr Metab. 2013;62:(Suppl 3):27–36. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367(15):1397–406. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367(15):1407–16. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Smego A, Woo JG, Klein J, Suh C, Bansal D, Bliss S, Daniels SR, Bolling C, Crimmins NA. High body mass index in infancy may predict severe obesity in early childhood. J Pediatr. 2017;183:87–93. e1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Tester JM, Phan TT, Tucker JM, Leung CW, Dreyer Gillette ML, Sweeney BR, Kirk S, Tindall A, Olivo-Marston SE, Eneli IU. Characteristics of children 2 to 5 years of age with severe obesity. Pediatrics. 2018;141(3):e20173228. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Ogden CL, Fryar CD, Hales CM, Carroll MD, Aoki Y, Freedman DS. Differences in obesity prevalence by demographics and urbanization in US children and adolescents, 2013–2016. JAMA. 2018;319(23):2410–8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6–9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Papas MA, Trabulsi JC, Dahl A, Dominick G. Food insecurity increases the odds of obesity among young Hispanic children. J Immigr Minor Health. 2016;18(5):1046–52. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Adams AK, Tomayko EJ, Cronin KA, Prince RJ, Kim K, Carmichael L, Parker T. Predictors of overweight and obesity in American Indian families with young children. J Nutr Educ Behav. 2019;51(2):190–8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Au LE, Zhu SM, Nhan LA, Plank KR, Frongillo EA, Laraia BA, Gurzo K, Ritchie LD. Household food insecurity is associated with higher adiposity among US schoolchildren ages 10–15 years: the Healthy Communities study. J Nutr. 2019;149(9):1642–50. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370(5):403–11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, Pfaffle R, Kiess W, Korner A. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018;379(14):1303–12. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W et al. . Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–85. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Charakida M, Khan T, Johnson W, Finer N, Woodside J, Whincup PH, Sattar N, Kuh D, Hardy R, Deanfield J. Lifelong patterns of BMI and cardiovascular phenotype in individuals aged 60–64 years in the 1946 British Birth Cohort study: an epidemiological study. Lancet Diabetes Endocrinol. 2014;2(8):648–54. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Azizpour Y, Delpisheh A, Montazeri Z, Sayehmiri K, Darabi B. Effect of childhood BMI on asthma: a systematic review and meta-analysis of case-control studies. BMC Pediatr. 2018;18(1):143. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ et al. . Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419–29. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Casagrande SS, Menke A, Linder B, Osganian SK, Cowie CC. Cardiovascular risk factors in adolescents with prediabetes. Diabet Med. 2018:1202–9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Divers JM-DE, Lawrence JM, Isom S, Dabelea D, Dolan L, Imperatore G, Marcovina S, Pettitt DJ, Pihoker C, Hamman RF et al. . Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and Indian reservations, United States, 2002–2015. MMWR. 2020;69(6):161–5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Dabelea D, Stafford JM, Mayer-Davis EJ, D'Agostino R Jr, Dolan L, Imperatore G, Linder B, Lawrence JM, Marcovina SM, Mottl AK et al. . Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825–35. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Afanasiev SA, Garganeeva AA, Kuzheleva EA, Andriyanova AV, Kondratieva DS, Popov SV. The impact of type 2 diabetes mellitus on long-term prognosis in patients of different ages with myocardial infarction. J Diabetes Res. 2018;2018:1780683. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Mitanchez D, Chavatte-Palmer P. Review shows that maternal obesity induces serious adverse neonatal effects and is associated with childhood obesity in their offspring. Acta Paediatr. 2018;107(7):1156–65. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Tan HC, Roberts J, Catov J, Krishnamurthy R, Shypailo R, Bacha F. Mother's pre-pregnancy BMI is an important determinant of adverse cardiometabolic risk in childhood. Pediatr Diabetes. 2015;16(6):419–26. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Persson M, Cnattingius S, Villamor E, Soderling J, Pasternak B, Stephansson O, Neovius M. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ. 2017;357:j2563. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–73. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, Yanovski JA. Pediatric obesity-assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709–57. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Steele CB TC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, Puckett M, Richardson LC. Vital signs: trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. MMWR. 2017;66(39):1052–8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, Kushner RF, Daniels SR, Wadden TA, Tsai AG et al. . The science of obesity management: an Endocrine Society scientific statement. Endocr Rev. 2018;39(2):79–132. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–93. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, Ben-Ami Shor D, Tzur D, Afek A, Shamiss A et al. . Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016;374(25):2430–40. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN, Lloyd-Jones DM. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiology. 2018;3(4):280–7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3):e137–e47. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–25. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357(23):2329–37. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Weihrauch-Bluher S, Schwarz P, Klusmann JH. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism. 2019;92:147–52. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group . Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Berger NA. Young adult cancer: influence of the obesity pandemic. Obesity (Silver Spring, Md). 2018;26(4):641–50. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Wang YC, Orleans CT, Gortmaker SL. Reaching the Healthy People goals for reducing childhood obesity: closing the energy gap. Am J Prev Med. 2012;42(5):437–44. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Trasande L. How much should we invest in preventing childhood obesity?. Health Aff (Millwood). 2010;29(3):372–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Sonntag D. Why early prevention of childhood obesity is more than a medical concern: a health economic approach. Ann Nutr Metab. 2017;70(3):175–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):30–7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Tambalis KD, Mourtakos S, Panagiotakos DB, Sidossis LS. Association of exclusive breastfeeding with risk of obesity in childhood and early adulthood. Breastfeed Med. 2018. doi:10.1089/bfm.2018.0117. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Bass JL, Gartley T, Kleinman R. World Health Organization Baby-Friendly Hospital Initiative Guideline and 2018 implementation guidance. JAMA Pediatr. 2019;173(1):93–4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Kim JH, Shin JC, Donovan SM. Effectiveness of workplace lactation interventions on breastfeeding outcomes in the United States: an updated systematic review. J Hum Lact. 2019;35(1):100–13. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Chai Y, Nandi A, Heymann J. Does extending the duration of legislated paid maternity leave improve breastfeeding practices? Evidence from 38 low-income and middle-income countries. BMJ Glob Health. 2018;3(5):e001032. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Heyman MB, Abrams SA; Section On Gastroenterology, Hepatology, and Nutrition and Committee on Nutrition . Fruit juice in infants, children, and adolescents: current recommendations. Pediatrics. 2017;139(6):1–10, e20170967. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Long MW, Gortmaker SL, Ward ZJ, Resch SC, Moodie ML, Sacks G, Swinburn BA, Carter RC, Claire Wang Y. Cost effectiveness of a sugar-sweetened beverage excise tax in the U.S. Am J Prev Med. 2015;49(1):112–23. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Roberto CA, Lawman HG, LeVasseur MT, Mitra N, Peterhans A, Herring B, Bleich SN. Association of a beverage tax on sugar-sweetened and artificially sweetened beverages with changes in beverage prices and sales at chain retailers in a large urban setting. JAMA. 2019;321(18):1799–810. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Ludwig DS, Blumenthal SJ, Willett WC. Opportunities to reduce childhood hunger and obesity. JAMA. 2012;308(24):2567–8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Penalvo JL, Cudhea F, Micha R, Rehm CD, Afshin A, Whitsel L, Wilde P, Gaziano T, Pearson-Stuttard J, O'Flaherty M et al. . The potential impact of food taxes and subsidies on cardiovascular disease and diabetes burden and disparities in the United States. BMC Med. 2017;15(1):208. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Mozaffarian D, Angell SY, Lang T, Rivera JA. Role of government policy in nutrition—barriers to and opportunities for healthier eating. BMJ. 2018;361:k2426. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Mozaffarian D, Liu J, Sy S, Huang Y, Rehm C, Lee Y, Wilde P, Abrahams-Gessel S, de Souza Veiga Jardim T, Gaziano T et al. . Cost-effectiveness of financial incentives and disincentives for improving food purchases and health through the US Supplemental Nutrition Assistance Program (SNAP): a microsimulation study. PLoS Med. 2018;15(10):e1002661. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. de Ferranti SD, Milliren CE, Denhoff ER, Quinn N, Osganian SK, Feldman HA, Ebbeling CB, Ludwig DS. Providing food to treat adolescents at risk for cardiovascular disease. Obesity (Silver Spring). 2015;23(10):2109–17. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Attina TM, Hauser R, Sathyanarayana S, Hunt PA, Bourguignon JP, Myers JP, DiGangi J, Zoeller RT, Trasande L. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016;4(12):996–1003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Trasande L, Liu Y, Fryer G, Weitzman M. Effects of childhood obesity on hospital care and costs, 1999–2005. Health Aff (Millwood). 2009;28(4):w751–60. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Bartick M, Reinhold A. The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics. 2010;125(5):e1048–56. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Anstey EH, MacGowan CA, Allen JA. Five-year progress update on the Surgeon General's call to action to support breastfeeding, 2011. J Womens Health (Larchmt). 2016;25(8):768–76. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (214.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases & Disorders Education Program
- Publications and Resources
- Clinical Trials
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Ask a Scientist
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- < Back To Overweight and Obesity
- Childhood Obesity
- What Are Overweight and Obesity?
- Symptoms and Diagnosis
- Causes and Risk Factors
- Obesity and Women's Health
- Obesity Hypoventilation Syndrome
MORE INFORMATION
Overweight and Obesity Childhood Obesity
Language switcher.
Childhood obesity is an increasingly serious problem in the United States. Nearly 1 in 5 children have obesity. Children with obesity are more likely to develop other serious health problems, including heart disease and type 2 diabetes. They are also more likely to suffer from anxiety, depression, and low self-esteem.
Obesity affects children from different backgrounds differently. About 1 in 4 Hispanic and non-Hispanic Black children have obesity. This is a challenge for parents, because addressing their child’s weight often means making lifestyle changes for the whole family.
All children should visit a healthcare provider every year for wellness check-ups that include monitoring of weight and calculation of body mass index (BMI) percentiles. Some of the best ways to prevent childhood obesity are to:
- Choose and prepare healthy foods that are lower in fat and have less calories. Use this guide (PDF, 136 KB) to help your family make smart food choices.
- Get regular physical activity. Your children should get at least 60 minutes of daily physical activity. Learn more about helping them get active every day.
- Reduce screen time. Try to limit screen time at home to 2 hours or less each day.
- Get enough good-quality sleep. NHLBI research has shown a relationship between lack of sleep and obesity that begins as early as infancy. See the recommended hours for children at every age.
The We Can! program offers a free, printable guide for parents, called Eat! Play! Grow! (PDF, 31.3 MB), on how to accomplish these goals.
Risk Factors
Researchers agree that children inherit genes , the blueprints for our bodies, that make them more likely to have obesity. However, that genetic risk does not account for the increase in childhood obesity seen in recent years. A child’s community also has an impact on their weight, as the community can affect a family’s ability to make healthy choices. For example, fresh fruits and vegetables may be difficult to get, roads without sidewalks may make it unsafe to walk for exercise, or healthy meal choices in schools may be unavailable.
Most parents, however, do have some control over other risk factors that increase a child’s risk of having obesity. These include:
- Eating a high-calorie, low-nutrient diet
- Not getting enough good-quality sleep
- Too much screen time
- Too little physical activity
- Personal or family stress or trauma
BMI for children
BMI is used to determine whether your child’s weight fits the criteria for overweight or obesity. It is compared with growth charts for children who are the same age and sex as your child.
To learn your child’s percentile, use the Center for Disease Control and Prevention’s BMI percentile calculator for children and teens .
- Underweight is a BMI below the 5th percentile.
- Healthy weight is a BMI between the 5th to the 85th percentile.
- Overweight is a BMI between the 85th percentile and the 95th percentile.
- Obesity is a BMI in the 95th percentile or above.
Your child’s provider will monitor your child’s BMI and overall health during regular visits. They may talk to you about healthy lifestyle changes you can make as a family. If your child’s weight does not respond to those, your child’s provider may recommend medicine.
The good news for parents is that childhood obesity is reversible. Even small decreases in weight can have a positive impact on current health and future risk of health problems. The key is to learn the basics of maintaining a healthy weight, seek out resources in your community, and get both medical and mental health care for your child as needed.
Causes and Effects of Obesity Essay
Introduction, laziness as the main cause of obesity, social effects of obesity, effects of obesity: health complications.
Bibliography
Maintaining good body weight is highly recommended by medical doctors as a way of promoting a healthy status of the body. This is to say that there is allowed body weight, which a person is supposed to maintain. Extreme deviations from this weight expose a person to several health complications.
While being underweight is not encouraged, cases of people who are overweight and increasing effects of this condition have raised concerns over the need of addressing the issue of obesity in the society today, where statistics are rising day and night. What is obesity? This refers to a medical condition in which a person’s body has high accumulation of body fat to the level of being fatal or a cause of serious health complications. Additionally, obesity is highly associated with one’s body mass index, abbreviated as BMI.
This denotes the value obtained when a person’s weight in kilograms is divided by the square of their height in meters (Burniat 3). According to medical experts, obesity occurs when the BMI exceeds 30kg/m 2 . While this is the case, people who have a BMI of between 25 and 29 and considered to be overweight. Obesity has a wide-range of negative effects, which may be a threat to the life of a person.
The fist effect of obesity is that it encourages laziness in the society. It is doubtless that obese people find it hard and strenuous to move from one point to the other because of accumulated fats. As a result, most of these people lead a sedentary lifestyle, which is usually characterized by minimal or no movement. In such scenarios, victims prefer being helped doing basic activities, including moving from one point to another.
Moreover, laziness makes one to be inactive and unproductive. For example, a student who is obese may find it hard to attend to his or her homework and class assignments, thus affecting performance. With regard to physical exercises, obese people perceive exercises as punishment, which is not meant for them (Korbonits 265). As a result, they do not accept simple activities like jogging because of their inability to move.
In line with this, obese people cannot participate in games like soccer, athletics, and rugby among others. Based on this sedentary lifestyle, obese people spend a lot of their time watching television, movies, and playing video games, which worsen the situation.
The main effect of obesity is health complications. Research indicates that most of the killer diseases like diabetes, heart diseases, and high blood pressure are largely associated with obesity. In the United States, obesity-related complications cost the nation approximately 150 billion USD and result into 0.3 million premature deaths annually.
When there is increase in body fat, it means that the body requires more nutrients and oxygen to support body tissues (Burniat 223). Since these elements can only be transported by the blood to various parts of the body, the workload of the heart is increased.
This increase in the workload of the heart exerts pressure on blood vessels, leading to high blood pressure. An increase in the heart rate may also be dangerous due to the inability of the body to supply required blood to various parts. Moreover, obesity causes diabetes, especially among adults as the body may become resistant to insulin. This resistance may lead to a high level of blood sugar, which is fatal.
Besides health complications, obesity causes an array of psychological effects, including inferiority complex among victims. Obese people suffer from depression, emanating from negative self-esteem and societal rejection. In some cases, people who become obese lose their friends and may get disapproval from teachers and other personalities (Korbonits 265). This is mainly based on the assumption that people become obese due to lack of self-discipline. In extreme cases, obese people may not be considered for promotion at workplaces, because of the negative perception held against them.
Due to inferiority complex, obese people avoid being in public and prefer being alone. This is because they imagine how the world sees them and may also find it hard being involved in public activities because of their sizes.
This further makes them to consider themselves unattractive based on their deviation from what is considered as the normal body size and shape. Regardless of how obese people are treated, they always believe that they are being undermined because of their body size.
In summary, obesity is a major cause of premature deaths in the United States and around the world. This health condition occurs when there is excess accumulation of body fat, caused by unhealthy lifestyles. Obesity is largely associated with several killer diseases like high blood pressure, diabetes, and diseases of the heart.
These diseases drain world economies since most of them are fatal and expensive to manage. Additionally, obesity promotes sedentary life where victims minimize movement by adopting an inactive lifestyle. Moreover, obese victims suffer psychologically because of societal rejection. In general, obesity has a wide-range of negative effects, which may be a threat to the life of a person.
Burniat, Walter. Child and Adolescent Obesity: Causes and Consequences, Prevention and Management . United Kingdom: Cambridge University Press, 2002. Print.
Korbonits, Márta. Obesity and Metabolism . Switzerland: Karger Publishers, 2008. Print.
- Childhood Obesity: Causes/Solutions
- Why are poor people more likely to be obese?
- Teachings of Hebrew Wisdom
- Recreation Hub as a Way to Combat Sedentary Lifestyle
- Parental Education on Overweight and Obese Children
- Eating Disorders: Assessment & Misconceptions
- Human Digestion
- Definitions of Obesity and Criteria for Diagnosing It
- Obesity Could Be Catching
- White Wines vs. Red Wines
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2018, December 11). Causes and Effects of Obesity Essay. https://ivypanda.com/essays/effects-of-obesity/
"Causes and Effects of Obesity Essay." IvyPanda , 11 Dec. 2018, ivypanda.com/essays/effects-of-obesity/.
IvyPanda . (2018) 'Causes and Effects of Obesity Essay'. 11 December.
IvyPanda . 2018. "Causes and Effects of Obesity Essay." December 11, 2018. https://ivypanda.com/essays/effects-of-obesity/.
1. IvyPanda . "Causes and Effects of Obesity Essay." December 11, 2018. https://ivypanda.com/essays/effects-of-obesity/.
IvyPanda . "Causes and Effects of Obesity Essay." December 11, 2018. https://ivypanda.com/essays/effects-of-obesity/.
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
IvyPanda uses cookies and similar technologies to enhance your experience, enabling functionalities such as:
- Basic site functions
- Ensuring secure, safe transactions
- Secure account login
- Remembering account, browser, and regional preferences
- Remembering privacy and security settings
- Analyzing site traffic and usage
- Personalized search, content, and recommendations
- Displaying relevant, targeted ads on and off IvyPanda
Please refer to IvyPanda's Cookies Policy and Privacy Policy for detailed information.
Certain technologies we use are essential for critical functions such as security and site integrity, account authentication, security and privacy preferences, internal site usage and maintenance data, and ensuring the site operates correctly for browsing and transactions.
Cookies and similar technologies are used to enhance your experience by:
- Remembering general and regional preferences
- Personalizing content, search, recommendations, and offers
Some functions, such as personalized recommendations, account preferences, or localization, may not work correctly without these technologies. For more details, please refer to IvyPanda's Cookies Policy .
To enable personalized advertising (such as interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. These partners may have their own information collected about you. Turning off the personalized advertising setting won't stop you from seeing IvyPanda ads, but it may make the ads you see less relevant or more repetitive.
Personalized advertising may be considered a "sale" or "sharing" of the information under California and other state privacy laws, and you may have the right to opt out. Turning off personalized advertising allows you to exercise your right to opt out. Learn more in IvyPanda's Cookies Policy and Privacy Policy .
Childhood Obesity – Causes and Potential Long-Term Effects
How it works
- 2.1 Causes and Potential Long-Term Effects
- 2.2 Causes of Childhood Obesity
- 2.3 Long-Term Effects of Childhood Obesity
- 2.4 Potential Solutions
There is growing concern about the state of children’s health. Every year there is an increase in the number of overweight and obese children. What causes this and what does it mean for them long-term? There are many contributing factors to children’s weight issues. Some of these factors are limited access to healthy food, more time spent in front of a screen, and less physical activity. Long-term health affects include a rising risk of Type 2 diabetes, coronary heart disease, and some forms of cancer.
The implications are that children are becoming unhealthier every year which raise the cost of medical care and reduce their life expectancy.
Childhood Obesity:
Causes and potential long-term effects.
A stunning number of pre-school aged children are overweight or obese and those numbers are growing every year. One study, conducted over the course of 30 years, has shown childhood obesity rates have risen steadily and show no sign of stopping. “The percentage of children and adolescents affected by obesity has more than tripled since the 1970s” (CDC, 2017). How did the American and Global populations rise to the obesity rates that we are seeing today? Historically, weight issues only seemed to affect the upper echelons of society, those who could afford food in abundance. Yet today we are seeing rising numbers of obese children in every demographic. What causes childhood obesity and what are the long-term effects? This essay will cover four areas of interest in the growing epidemic that is childhood obesity. There are:
- The causes of childhood obesity
- The cost of medical treatment of childhood obesity and its attendant long-term effects
- The long-term effects of childhood obesity
- Potential solutions for the treatment and prevention of childhood obesity
With an understanding of these areas, we can hope to find solutions that will help current and future generations deal with this growing trend. Childhood obesity effects every area of that child’s life and will continue to affect that child as he or she grows to adulthood.
Causes of Childhood Obesity
There are several causes of childhood obesity from genetics, to a lifestyle of convenience, to ease of access in acquiring healthy foods, among others. While the causes of obesity based on genetics is still being studied, there is some evidence that genetics play a role in childhood obesity. According to Sahoo et al. (2015) “Some studies have found that BMI is 25-40% heritable.” Unfortunately, this means that some children are predisposed to having a higher BMI. They go on to say only 5% of childhood obesity cases can be attributed to this genetic factor and that “Females are more likely to be obese as compared to males, owing to inherent hormonal differences.” These hormonal differences can also be genetically passed down from mother to daughter and father to son.
Another area for concern, in terms of childhood obesity, is the lifestyle of convenience. It is far easier for people to find food in this day and age. Glenn Berall, in his article Obesity: A crisis of growing proportions states that “Never before in the history of civilization has a population had such plentiful food sources without interruption of famine” (2002). This may have led some children to develop a “thrift metabolism” states Rolland-Cachera et al. (2006). A thrifty metabolism is the theory that thrifty genes allow for the storage of food as fat that the body can then use during a famine to continue to give needed energy to the individual. This could explain some of the obesity epidemic as there is rarely ever a famine in this abundant food culture. The lifestyle of convenience goes past readily available to food to available food choices. During the times of hunter-gatherers, food was hard to come by and was considered, by today’s standards, to be healthy. There were no sugary, fatty foods available to these people. They ate what they could find, such as berries, roots, and meat that they caught or killed. Today the food choices are overwhelming. One can simply drive to McDonald’s on the way home to pick up dinner for the family rather than spending time at the grocery store buying healthier options then going home to prepare the meal. In the increasingly busy lifestyle convenience has become the norm, not the atypical go-to for meals.
However, it needs to be mentioned that healthy food is not always easily available to certain demographics. Ashlesha Datar (2017) states that disadvantaged families have higher obesity rates. According to Howlett, Davis, and Burton (2014), “the majority of food deserts in the U.S. are found in low-income neighborhoods.” They state that “food deserts” are areas without “immediate access to fresh, healthy, and affordable food.” There is a high percentage of convenience stores in these areas and a lack of grocery stores or supercenters that would allow for the purchase of healthy foods. Howlett, Davis, and Burton (2014) go on to say:
“only 5 to 10% of convenience stores had fresh produce. In addition, the top selling food items sold at convenience stores include energy-dense foods such as sweet snacks, candy/gum/mints, and salty snacks.”
They continue on to compare the cost of meals. They estimate that it costs roughly $18.16 per 1000 calories for low-calorie meals compared to the $1.76 per 1000 calories of high-calorie foods. This makes it more expensive for low income families to eat healthier and thus could contribute to the rising rates of childhood obesity.
Along with convenience, comes a sedentary lifestyle. Children spend more time in front of a screen, whether it be television, tablet, computer, or phone, than in the past. This leads to less physical activity. Every hour of television a day increases the risk of obesity by 2% (Sahoo et al. 2015). According to the CDC (2017), “Energy imbalance is a key factor behind the high rates of obesity seen in the United States and globally.” Children are consuming more calories today than ever before but are not getting the needed activity to burn those extra calories. This creates a positive energy balance which leads to the storage of excess energy as fat. Convenience extends beyond food. Some of these contributing trends are the increased use of vehicles, more hazards for cyclists and walkers, increased food and drink choices, and media promotion of energy-dense foods (Lobstein et al. 2004).
Rising Cost of Medical Care
Childhood obesity has contributed to the rising cost of health care. Statistically, children who are obese will remain obese in adulthood. This creates the need for long-term medical treatment for a variety of issues. Long-term medical costs for a single obese child come in at approximately $12,660 per year according to Finkelstein et al (2014). In their article, medical for males ranged from $9,640 to $38, 680 with a mean of $24,160. Medical for females ranged from $14,440 to $49,230 with a mean of $31,835. However, some of these numbers do not account for weight fluctuations over a lifetime. By accounting for the fluctuations, they came to an approximate amount of $12,660 a year for both sexes and across each demographic. What time means long-term is that children are obese will pay, on average, more medical expenses than a person who becomes obese as an adult.
Long-Term Effects of Childhood Obesity
There is some controversy over the long-term effects of childhood obesity. Some studies show very little long-term effects while others show an astounding number of effects. However, there are some undisputed long-term risks involved with childhood obesity. Park, Falconer, Viner, and Kinra (2012) have compiled a list of six potential long-term risks associated with childhood obesity. These risks are:
- Type 2 Diabetes
- Hypertension
- All-cause mortality
They state that the higher the BMI (body mass index) of the child the greater the risk of developing one or more of the above diseases. “There is a consistent body of evidence for associations between childhood overweight and cardiovascular outcomes and mortality in adulthood” (Park et al. 2012).
Other studies have listed even more potential health problems for obese children. Glenn Berall (2002) lists “sleep apnea, slipped capital femoral epiphyses, nonalcoholic steatohepatitis, polycystic ovarian disease, and metabolic syndrome” as potential problems for overweight children as the reach maturity. Sahoo et al. (2015) list vitamin deficiencies as a current and potential long-term problem for obese children.
What one must also consider is the emotional ramifications of childhood obesity. Sahoo et al. (2015) looked at the relationship between eating disturbances and psychological effects on obese children. They found that obese children are more likely to suffer from self-esteem issues, body dissatisfaction, depression and anxiety, and eating disorder symptoms. They continue on to say that “Childhood obesity effects children’s and adolescent’s social and emotional health.” Overweigth children are more likely to be bullied or teased due to their weight. They are also more likely to be discriminated against. “These negative social problems contribute to low self-esteem, low self-confidence, and a negative body image in children and can also affect academic performance” (Sahoo et al. 2015).
Potential Solutions
What can society do to help treat or prevent childhood obesity? There are several things that society can do including changing governmental policies on food distribution and promotion, promote healthier food choices at schools, and advocate for healthier food options in the media.
Governmental policies could create incentives for schools to promote healthier food options. If a school is willing to participate, there could be a financial advantage to that school so that they could help offset the cost of healthier food. The government could offer incentives to grocery stores to build in low-income neighbors to help provide better food options to low-income families. They could also create policies aimed at the media to promote healthy food over junk food.
Schools could help promote better eating by offering students fruits and vegetables rather than high-fat foods. They could also limit or ban the use of vending machines on the property which give students less access the energy-dense foods. Schools could also promote education on the long-term effects of obesity and increase the amount of time spent on physical activity.
The media could promote healthier food options and more realistic body ideals. This would help with the perception that all women must be skinny and all men must be buff which create an unrealistic ideal for children to live up and may contribute to increased eating.
There are several contributing factors to why childhood obesity rates have grown exponentially over the last 40 years. A sedentary and convenient lifestyle, increased access to all types of food, and genetics all play a role. These can lead to long-term habit creation, as in the lack of consistent physical activity and eating a high fat diet, as well as long-term health effects such as Type 2 diabetes, heart disease, and increased risk of cancer. Childhood obesity also leads to higher long-term medical and emotional costs. Change needs to become a priority. The government, media, and schools need to lead the charge in promoting healthier food and education on the long-term risk factors associated with childhood obesity. Change won’t happen overnight, but it can happen.
Cite this page
Childhood Obesity - Causes and Potential Long-Term Effects. (2019, Oct 30). Retrieved from https://papersowl.com/examples/childhood-obesity-causes-and-potential-long-term-effects/
"Childhood Obesity - Causes and Potential Long-Term Effects." PapersOwl.com , 30 Oct 2019, https://papersowl.com/examples/childhood-obesity-causes-and-potential-long-term-effects/
PapersOwl.com. (2019). Childhood Obesity - Causes and Potential Long-Term Effects . [Online]. Available at: https://papersowl.com/examples/childhood-obesity-causes-and-potential-long-term-effects/ [Accessed: 28 Oct. 2024]
"Childhood Obesity - Causes and Potential Long-Term Effects." PapersOwl.com, Oct 30, 2019. Accessed October 28, 2024. https://papersowl.com/examples/childhood-obesity-causes-and-potential-long-term-effects/
"Childhood Obesity - Causes and Potential Long-Term Effects," PapersOwl.com , 30-Oct-2019. [Online]. Available: https://papersowl.com/examples/childhood-obesity-causes-and-potential-long-term-effects/. [Accessed: 28-Oct-2024]
PapersOwl.com. (2019). Childhood Obesity - Causes and Potential Long-Term Effects . [Online]. Available at: https://papersowl.com/examples/childhood-obesity-causes-and-potential-long-term-effects/ [Accessed: 28-Oct-2024]
Don't let plagiarism ruin your grade
Hire a writer to get a unique paper crafted to your needs.

Our writers will help you fix any mistakes and get an A+!
Please check your inbox.
You can order an original essay written according to your instructions.
Trusted by over 1 million students worldwide
1. Tell Us Your Requirements
2. Pick your perfect writer
3. Get Your Paper and Pay
Hi! I'm Amy, your personal assistant!
Don't know where to start? Give me your paper requirements and I connect you to an academic expert.
short deadlines
100% Plagiarism-Free
Certified writers
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Preventing Childhood Obesity
- Health Care Strategies
- About Obesity
- What Can Be Done
- Obesity Data and Statistics
Related Topics:
- View All Home
- About Healthy Weight and Growth
- Body Mass Index (BMI)
- About Nutrition
- About Physical Activity
Preventing Childhood Obesity: 6 Things Families Can Do
Childhood obesity is a complex disease with many contributing factors, including genetics, eating patterns, physical activity levels, and sleep routines. Compared to children with healthy weight, children with obesity are at a higher risk for asthma, sleep apnea, bone and joint problems, type 2 diabetes, and other health issues. Although there is no one solution, there are many ways parents and caregivers can help children have a healthy weight and set up lifelong healthy habits.

Why it matters
About 1 in 5 American children have obesity. Compared to children with healthy weight, children with obesity are at a higher risk for asthma, sleep apnea, bone and joint problems, type 2 diabetes, and risk factors for heart disease such as high blood pressure.
Obesity also has an impact on medical costs. Compared to children with healthy weight, total medical expenditures for children with severe obesity are $909 higher each year.
Children with obesity are more likely to have obesity as adults. Adults with obesity have higher risks for stroke, many types of cancer , heart disease, type 2 diabetes, premature death, and mental illness, such as clinical depression and anxiety.
Factors that influence obesity include genetics, eating patterns, physical activity levels, access to health care, and sleep routines. Also, conditions where we live, learn, work, and play can make healthy eating and getting enough physical activity difficult.
Though there is no one solution to addressing obesity, there are many ways parents and caregivers can help children have a healthy weight and set up lifelong healthy habits at home.
Here are ways families can help prevent obesity.
1. model a healthy eating pattern.
Offer a variety of fruits and vegetables throughout the day. Frozen and canned fruits and vegetables are often less expensive than fresh and are still good for you. Look for low sodium or no salt added vegetables and fruits packed in 100% fruit juice.
Adopting healthy eating patterns as a family helps children reach and maintain a healthy weight as they age. Eating a variety of vegetables and fruits, whole grains, lean protein foods, and low-fat and fat-free dairy products follows nutrition guidelines and sets children and adults up for optimal health.
Help kids rethink their drink by replacing sugary drinks, such as soda, fruit drinks, and flavored milk, with water, 100% juice, or plain low-fat milk.
Hunger Hotline
For information about meal sites, food banks, and other services near you, call 1-866-3-HUNGRY (or 1-877-8-HAMBRE for Spanish).
Hours: 7 a.m. to 10 p.m. ET, Monday – Friday.
2. Move more as a family
Physically active youth have stronger muscles and bones, better cardiovascular fitness, and lower body fat than those who are inactive. Children aged 3–5 years should be physically active throughout the day. Children aged 6–17 years need at least 60 minutes of physical activity every day.
Help your children move more and meet the physical activity recommendations by making it a family affair. Walking the family pet before and after school, riding bikes, and having races in the yard all count toward physical activity. Active chores, such as washing the car, vacuuming a room, or raking leaves, also count.
3. Set consistent sleep routines
Good sleep helps prevent type 2 diabetes, obesity, injuries, and problems with attention and behavior. Children who don't get enough sleep are at risk for unhealthy weight gain. Researchers are still trying to learn how sleep is linked to weight gain. Some reasons might include that lack of sleep can cause a child to eat more or to be less physically active because of to lack of energy.
Preschoolers need 10–13 hours of sleep per day , including naps. Children 6–12 years old need 9–12 hours of uninterrupted sleep a night, and youth 13–17 need 8–10 hours. Staying with a consistent sleep schedule, including on weekends, can help children sleep better .
4. Replace screen time with family time
During childhood, too much screen time can lead to poor sleep, weight gain, lower grades in school , and poor mental health. Reducing screen time can free up time for family activities and can remove cues to eat unhealthy food.
Turning off screens an hour before bed and removing screens from children's bedrooms can help reduce screen time and improve sleep. The American Academy of Pediatrics recommends creating a family media plan with examples of ways to reduce screen time.
5. Support obesity prevention in Early Care and Education
About 3 in 5 children birth through age 5 who are not yet in kindergarten are in a nonparental care arrangement at least once a week. The number of children in Early care and education (ECE) settings makes them among the best places outside the home to help young children build a foundation for healthy living. High-quality ECE programming can have a positive impact on a child's social-emotional wellbeing, educational achievement, health, and socioeconomic outcomes later in life.
Look for ECE settings supporting healthy infant feeding, healthy eating, physical activity, and screen time limits . When looking for ECE programs for your child, ask about policies and practices related to breastfeeding and feeding breast milk to infants, nutrition standards for the food served, access to outdoor physical activity during the day, and how much time the child will spend daily in front of a screen.
6. Find a Family Healthy Weight Program
If you are concerned about your child's weight, talk with their health care provider. They can assess the health risks related to excess weight. If your child has overweight or obesity, your health care provider may refer you to a family healthy weight program (FHWP). FHWPs are comprehensive, family-based lifestyle change programs to help children who are overweight or who have obesity make progress toward a healthier weight through positive behavior changes.
- Tips to Support Healthy Routines for Children and Teens
- Good Nutrition Starts Early
- Water and Healthier Drinks
- Making Physical Activity Part of a Child's Life
- How Much Physical Activity do Children Need?
- Child and Teen BMI Calculator
- Adult BMI Calculator
CDC's obesity prevention efforts focus on policy and environmental strategies to make healthy eating and active living accessible for everyone.
For Everyone
Health care providers, public health.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Junk Food in Schools and Childhood Obesity
Ashlesha datar, nancy nicosia.
- Author information
- Copyright and License information
Corresponding author.
Despite limited empirical evidence, there is growing concern that junk food availability in schools has contributed to the childhood obesity epidemic. In this paper, we estimate the effects of junk food availability on BMI, obesity, and related outcomes among a national sample of fifth-graders. Unlike previous studies, we address the endogeneity of the school food environment by controlling for children’s BMI at school entry and estimating instrumental variables regressions that leverage variation in the school’s grade span. Our main finding is that junk food availability does not significantly increase BMI or obesity among this fifth grade cohort despite the increased likelihood of in-school junk food purchases. The results are robust to alternate measures of junk food availability including school administrator reports of sales during school hours, school administrator reports of competitive food outlets, and children’s reports of junk food availability. Moreover, the absence of any effects on overall food consumption and physical activity further support the null findings for BMI and obesity.
Keywords: Junk food, Competitive foods, Obesity
1. Introduction
The prevalence of childhood obesity in the US is at an all-time high with nearly one-third of all children and adolescents now considered overweight or obese ( Ogden et al 2008 ). Considerable attention has been focused on schools in an attempt to identify policy levers that will help reverse the obesity epidemic. In particular, the availability of “competitive foods”, defined as foods and beverages available or sold in schools outside of the school lunch and breakfast programs, has been a much debated issue. On the one hand, opponents question the nutritional value of competitive foods and consider them the primary source of “junk foods” in schools. Indeed, the available evidence suggests that these foods are higher in fat compared with foods sold as part of the school meal programs ( Gordon et al 2007b , Harnack et al 2000 , Wechsler et al 2000, Story, Hayes & Kalina 1996 ). On the other hand, supporters argue that revenues from these food sales provide much-needed funding for schools, especially in times of budgetary pressures ( Gordon et al 2007a ).
The debate draws from largely cross-sectional research that rarely addresses the potential endogeneity of the school food environment. Our paper advances the literature by attempting to isolate the causal effect of junk food availability on children’s food consumption and BMI. We use longitudinal data on BMI for a national sample of fifth graders from the Early Childhood Longitudinal Study – Kindergarten Class (ECLS-K) and an instrumental variables (IV) approach that leverages the well-documented fact that junk foods are significantly more prevalent in middle and high schools relative to elementary schools ( Finkelstein, Hill and Whitaker 2008 ). Plausibly exogenous variation in junk food availability across a cohort of fifth graders is identified using the grade structure in their schools. We argue that a fifth grader attending a combined (e.g. K-8, K-12) or middle school (e.g. 5–8) is more likely to be exposed to junk foods compared to a fifth grader in an elementary school (e.g. K-5, K-6), but that the school’s grade span has no direct effect on a child’s weight. First-stage regressions confirm that combined school attendance is a strong predictor of junk food availability. Further tests for instrument validity including an examination of sorting and peer effects support our use of the instrument.
We find that junk food availability has small positive associations with BMI and obesity in basic OLS models that only control for a limited set of covariates, but those associations become insignificant when controls for BMI at school entry and state fixed effects are added. Our IV models, which address potential bias in the OLS models, generate somewhat larger, albeit less precise, point estimates that are also not statistically significant. Even if the IV point estimates were statistically significant, they would still represent only minor increases in BMI and obesity, generally one-third of one percent. Moreover, reduced form estimates, which are more precisely estimated than IV estimates, provide further support because combined school attendance has no significant effects on 5 th graders’ BMI and obesity. These results are robust to alternative measures of junk food availability and sample restrictions. The models also produce the expected findings on various falsification tests.
While we acknowledge their limitations, ancillary analyses of children’s in-school junk food purchases, total consumption of healthy and unhealthy foods, and physical activity are consistent with our null findings for BMI and obesity. Our estimates suggest that the caloric contributions of in-school junk food purchases are likely to be small. Moreover, we find evidence consistent with substitution between in- and out-of-school consumption. Specifically, the total amount of soda and fast food consumed in- and out-of-school, is not significantly higher among those children with greater exposure to junk food in school (i.e. attending a combined school). And, finally, we find little support for the notion that children substitute calories from healthy foods or increase their physical activity to compensate for increased junk food intake.
The remainder of this paper is organized as follows. We first discuss junk food availability in schools and the findings from the existing literature in Section 2. Section 3 describes our data and relevant analysis variables. In Section 4, we describe our empirical strategy, which leverages longitudinal information on BMI and implements an instrumental variables approach to identify the causal impact of junk food availability. In Section 5, we first discuss our main results for children’s BMI and obesity and then support these findings with robustness checks and falsification tests. We also present supporting evidence from models of in-school purchases of junk food, total consumption of various healthy and unhealthy items, and physical activity. Finally, Section 6 concludes with the policy implications of our findings.
2. Background and Literature
Competitive foods are sold through a la carte lines, vending machines, school canteens/stores, and fundraisers and, in contrast to the federally-reimbursable school meal programs, are not subject to federal nutritional standards. As a result, competitive foods account for much of the variation in the food environment across schools. Competitive foods are available in a large share of schools, although the availability of these foods varies significantly across elementary, middle, and high schools. For example, as many as 97% of high schools and 82% of middle schools have vending machines compared to only 17% of elementary schools ( Gordon et al 2007a ). However, a la carte lines, which are the predominant source of competitive food sales, operate not only in most high (93%) and middle (92%) schools, but also in a large proportion of elementary schools (71%) ( Gordon et al 2007b ).
Sales of competitive foods have the potential to generate significant revenues for schools. During 2005–2006, middle and high schools earned an average of $10,850 and $15,233, respectively, from a la carte sales alone ( Gordon et al 2007a ). In addition, nearly a third of high schools and middle schools earned between $1,000–$9,999 during that same year from vending machines, another ten percent earned between $10,000–$50,000, and a small number earned in excess of $50,000 per year. These revenues may in turn be supplemented by on-site school stores and pouring contracts with beverage companies. While availability and revenues were less common in elementary schools, nearly half of elementary schools had pouring rights contracts, and competitive food sales from fundraising activities were also common.
The U.S. Department of Agriculture’s regulations on competitive foods in schools had been comprehensive, but in 1983, a successful lawsuit by the National Soft Drink Association limited the scope of these regulations to food service areas during meal hours ( Institute of Medicine 2007 ). In recent years, several states, districts, and schools have enacted competitive food policies that are more restrictive than federal regulations. And, between 2003 and 2005, approximately 200 pieces of legislation were introduced in US state legislatures to establish nutritional standards in schools or to address the availability or quality of competitive foods ( Boehmer et al 2007 ). At the federal level, legislation was passed in 2004 requiring local education agencies to develop a “wellness policy” by 2006 that included nutrition guidelines for all of the foods available in schools. More recently, there has been debate in the US Congress over enacting an amendment to the farm bill that would further restrict the sale of unhealthy foods and beverages in schools ( Black 2007 ). At the local level, two of the largest school districts in the nation, New York City Public School District and Los Angeles Unified School District, imposed a ban on soda vending in schools in 2003 and 2004, respectively.
Despite the growing support for competitive food regulation, it is hard to deny opponents’ claims that the evidence against competitive foods is limited. Existing research does show that competitive food availability is associated with a decline in nutritional quality of meals consumed at school ( Cullen et al 2000 , Cullen & Zakeri 2004 ; Templeton, Marlette & Panemangalore 2005 ). 1 However, less is known about the effects on overall diet quality (consumed both in and out of school) and children’s weight. The literature does provide some evidence of substitution of caloric intake across meals and locations among adults ( Anderson and Matsa 2011 ), but the evidence is less clear regarding children for whom parental oversight can also play a role. Only Kubik and colleagues have examined 24 hour dietary recall (2003) and BMI (2005) among children, however these studies are based on small cross-sectional samples and do not address the potential endogeneity of the school food environment. 2 , 3
The only effort to address endogeneity is in Anderson and Butcher (2006) , who use national data on adolescents aged 14–20 years to examine whether various school food policies influence BMI (based on self-reported height and weight data). In the absence of a single data source containing information on school food policies and BMI among adolescents, the authors use a two-sample IV approach that employs county, state, and regional characteristics as instruments to capture budgetary pressures on schools. They find that a 10 percentage point increase in the proportion of schools in the county that offer junk foods leads to a 1 percent increase in BMI. But this effect is primarily driven by adolescents with an overweight parent, which the authors interpret as a measure of family susceptibility. 4 Their IV approach constitutes an innovation over the literature, but the authors acknowledge that their results may be undermined by a weak first stage.
Our paper adds to the existing literature in its sample, methodology and scope. First, to our knowledge, ours is the only study that addresses the endogeneity of the school food environment among younger children. The focus on fifth graders is useful because junk food regulations are increasingly targeting elementary and middle schools. 5 And our national sample of children provides a larger and more representative sample with significant variation in school environments. Second, our data contain actual measurements of children’s height and weight, unlike the self-reports from other national datasets that have been used to examine this question previously. Third, our approach improves on the common cross-sectional designs by controlling for children’s BMI at school entry and state fixed-effects, and leveraging variation in schools’ grade spans to estimate IV models. Finally, unlike previous studies, we also provide evidence on the underlying mechanisms by examining effects on food consumption and physical activity.
The ECLS-K is a panel dataset on a nationally representative cohort of kindergarteners in the U.S. who entered school in fall 1998. In the fall and spring of kindergarten and the spring of the first, third, and fifth grades, the study collected information from the children and their parents, teachers, and schools on children′s cognitive, social, emotional, physical development (including BMI), and their home, classroom, and school environments. One limitation is that the information on the school food environment and children’s food consumption was collected only in the fifth grade. Our analysis sample includes the approximately 9,380 children attending the fifth grade in public and private schools in the 2003–04 school year. 6 In this section, we describe the key variables for our analyses.
3.1. Dependent Variables Measuring BMI, Food Consumption and Physical Activity
Body mass index (bmi).
A distinct advantage of the ECLS-K is that it collected height and weight measurements from children at kindergarten (school) entry and in the spring of kindergarten and first, third, and fifth grades. Measurements are superior to self- or parent-reported height and weight data that may introduce non-random measurement error. These measurements are used to compute BMI, defined as weight in kilograms divided by height in meters squared. The average BMI in our sample during the fifth grade is 20.4 ( Table 1 ). Approximately 20% of the ECLS-K sample is categorized as obese – this is nearly identical to prevalence rates among 6–11 years olds from the 2007–8 National Health and Nutrition Examination Survey ( Ogden et al 2010 ). 7
Descriptive Statistics in the Fifth Grade
Notes: N=9,380. Means are unweighted. Standard deviation in parentheses.
Junk Food Purchase in School
The food consumption questionnaire collected information on in-school junk food purchase during the fifth grade. These questions asked children about their purchases of sweets, salty snack foods, and sweetened beverages (hereafter, referred to as “soda”) during the previous week. 8 A substantial majority of the children did not purchase junk food in school during the reference week: 77% for sweets, 84% for salty snacks, and 88% for soda (see Appendix Table A1 ). But a large share of these children did not have junk food available in their schools (see Section 3.2). Conditional on availability, about half the sample purchased any of these unhealthy foods at least once a week in school. Among those who did purchase, the modal response was 1 to 2 purchases per week: 68 percent for sweets, 72 percent for salty snacks, and 70 percent for soda. 9
In-School and Total Food Consumption in Fifth Grade
Notes: N=9,380. Percentages are unweighted. Figures in the top panel are not conditional on availability in school.
Total Consumption of Selected Foods and Beverages
The child food consumption questionnaire asked about the frequency of overall consumption of specific food items during the past week. Children were asked to include foods they ate at home, at school, at restaurants, or anywhere else. We examine the consumption of two unhealthy items - soda and fast food, and six healthy food items – milk, green salad, potatoes 10 , carrots, other vegetables, and fruits. The percentage of children not consuming any soda or fast food during the previous week was 16 and 29 percent, respectively, with modal responses at 1 to 3 times per week (see Appendix Table A1 ). Among the healthy foods, green salad, carrots and potatoes were consumed most infrequently with nearly half of children reporting no consumption during the past week. The modal responses for the other healthy foods were 1 to 3 times during the past week.
3.2. Junk Food Availability
Detailed information on junk food availability in schools was collected from the school administrators and from children in the fifth grade. School administrators were asked whether students could purchase 17 individual food and beverage items, either from vending machines, school store, canteen, snack bar or a la carte items from the cafeteria during school hours. From these responses, we constructed an indicator variable of junk food availability in school that equals 1 if the administrator reports that students can purchase food and beverage items containing high sodium and/or sugar, including candy, chocolate, baked foods (e.g. cookies), salty snacks (e.g. potato chips), ice cream or frozen yogurt, or sweetened beverages during school hours, and zero otherwise. 11 Based on these school administrator reports, approximately 61 percent of the children had junk food availability in school. For robustness checks, we also considered two alternative measures of availability. The first is based on whether the modal child at each school reports that foods containing sugar, salty snacks, or sweetened beverages can be purchased at school. Based on this measure, about 75 percent of the children had junk foods available. And the second is based on whether the administrator reports any of the following competitive food outlets operate in the school: vending machines, school stores, canteens, snack bars, and a la carte lines. About 60 percent of the sample had at least one competitive food outlet. 12
4. Empirical Approach
4.1. econometric model.
The relationship between junk food availability and children’s BMI in fifth grade can be estimated cross-sectionally using the following linear regression model.
where, BMI iks , denotes fifth grade BMI for child i attending school k located in state s , JF k captures junk food availability in the child’s school, X i and S k are the vectors of individual/family (gender, age, age interacted with gender, race/ethnicity, mother’s education, household income) and school characteristics (private/public, percent minority, enrollment, urbanicity, state/region), respectively, and ε iks is the error term. The child’s baseline BMI (BBMI i ) is included to address potential heterogeneity that can bias OLS estimates such as student demand for junk foods, genetic susceptibility, and sorting. Because junk food availability is collected only in fifth grade, we do not know the length of exposure during prior school years. Therefore, BMI at school entry is the preferred baseline because it is measured prior to any exposure to the school food environment. Finally, since states differ markedly in terms of obesity prevalence in their populations as well as the policy environment geared towards combating obesity, we include state fixed effects (θ s ) to control for state-specific time-invariant unobserved heterogeneity that may be correlated with school food environments and children’s weight.
The parameter of interest in Equation (1) is β 1 . Obtaining an unbiased estimate of β 1 is challenging because the school food environment is not exogenous to the outcomes of interest. Schools that serve high-fat, energy-dense junk foods may differ on many observable and unobservable factors that are correlated with children’s weight and dietary behavior. In particular, the decision to offer junk foods in schools may be influenced by a variety of factors including budgetary pressures, demands of the student population, parental involvement, and state/district policies. These factors could independently influence children’s weight as well. For example, budgetary pressures may induce schools or districts to scale back or eliminate physical education programs, which might increase children’s weight. As a result, coefficient estimates from the ordinary least squares (OLS) estimation of Equation 1 would be biased.
4.2. Addressing Endogeneity of Junk Food Availability in Schools
We address the potential endogeneity of junk food availability using instrumental variables. Specifically, we estimate the model in Equations (2.1) and (2.2) using Two-Stage Least Squares.
Equation 2.1 represents the first-stage regression where junk food availability (JF k ) is regressed on the combined school attendance instrument (CS k ), individual (X i ) and school (S k ) characteristics, baseline BMI (BBMI i ), and state fixed effects (θ s ). Equation 2.2 represents the second stage where children’s BMI (or obesity) is regressed on the predicted availability of junk foods from the first stage (ĴF k ) in addition to the common covariates.
We also report results from the reduced form, which regresses BMI or obesity directly on the instrument ( Equation 3 ). These results have the advantage of being unbiased and providing evidence of whether a causal relationship exists in the regression of interest. 13
4.2.1. Instrument
Our sample consists of a single cohort of 5 th graders attending schools with a variety of grade spans. Given that junk food availability is significantly higher in middle and high schools compared to elementary schools, a potentially useful instrument for junk food availability is whether the 5 th grader attends a combined school (defined as the highest grade is seventh or higher) or whether the 5 th grader is in an elementary school (defined as highest grade is 5 th or 6 th ). Our instrument considers only this dichotomy of school type: elementary versus combined. Over 70 percent of our sample attends elementary schools while the remainder attends combined schools usually with grade spans of K-8, K-12 and 5–8 (see Appendix Table A2 ).
Variation in Grade Span in Fifth Grade
Notes: N=9380. “Combined” schools are defined as schools with highest grade equal to 7 or higher.
For combined school attendance to be a valid instrument, it must be the case that the school’s grade span has no direct effect on children’s weight except through the junk food environment. One potential concern is that there may be unobserved factors that are correlated with both the likelihood of combined school attendance as well as BMI. For example, it is well known that states differ markedly in the prevalence of childhood obesity. But, states are also likely to differ in terms of factors that contribute to school grade span such as: (1) the size of the school-age population, (2) its distribution within the state, (3) differences in the educational systems and policies, as well as (4) education budgets. Similarly, school grade span can vary across urban versus rural areas (even within states), with the latter more likely to have combined schools largely because of a smaller school-age population. The inclusion of state and urbanicity dummies in our regressions controls for unobserved differences across states and across rural/urban areas that may be correlated with combined school attendance (or grade span, more generally) and BMI.
Another potential concern with this identification strategy is that variation in grade span exposes children to older peers who may influence obesogenic behaviors. Peers, defined broadly, have been shown to influence a wide range of adolescent behaviors and outcomes. 14 However, of particular relevance to our identification strategy is the literature examining a specific type of peer effect, namely, the effect of exposure to older peers due to school grade span.
Several studies have examined peer effects on academic, social-behavioral and substance use outcomes by leveraging variation in school grade span ( Clark and Folk 2007 ; Clark and Loheac 2007 ; Eisenberg 2004 ; Bedard and Do 2005 ; Cook et al 2008 ). Most studies compare students in the same grade who attend middle versus combined schools or middle versus elementary schools . 15 These studies generally find that 6 th or 7 th graders who attend middle school fare poorly compared to those who attend elementary or combined schools. 16 However, we are not aware of any studies that compare children in the same grade level who attend elementary versus combined schools . The exception is Rickles (2005) , whose findings suggest inconsistent effects of elementary versus combined schools attendance on achievement.
Furthermore, there is very limited evidence on the influence of older peers on food choices. Cullen and Zakeri (2004) compared changes in food consumption of 4 th graders who transitioned to middle school in 5 th grade and gained access to school snack bars to changes in food consumption of 5 th graders who were already in middle school. Fourth graders who transitioned to middle school consumed fewer healthy foods compared with the previous school year, but it is not clear whether this was due to the presence of older peers or the change in school food environment.
Overall, the literature suggests that the presence of older peers may adversely affect academic and social behavioral outcomes, but there is less evidence to support effects on their eating behaviors. Nevertheless, if such an effect exists, the potential bias in our IV estimates due to peer effects is likely to be upward. That is, 5 th graders might emulate older peers who are more likely to consume junk foods in school and would therefore tend to be overweight, independent of the school food environment. In that case, an insignificant finding is unlikely to be undermined.
4.2.2. Checks for Instrument Validity
Identification in our IV models relies on the assumption that, conditional on state and urbanicity dummies, the school’s grade span does not influence BMI except through differences in the availability of junk foods. Districts typically determine the grade span at the time of the schools’ opening based on a number of factors including transportation costs, length of bus ride, desired number of transitions, population size, site availability, preferred school size, and likelihood of parental involvement ( Paglin and Fager 1997 ) rather than children’s health outcomes. Changes in grade span over time are possible, but infrequent and similarly-motivated. For example, in our ECLS-K sample, less than 4 percent of the children who remained in the same school between kindergarten and fifth grade experienced a grade-span change from combined to elementary school or vice-versa. While unlikely, it is nevertheless possible that schools may change grade span in response to children’s physical size. Therefore, below we report results from several tests that support the validity of our instruments. These analyses are based on our preferred specification, which controls for the full set of covariates, including state and urbanicity dummies and baseline BMI.
First, we report first-stage estimates of the effect of our instrument – combined school attendance – on junk food availability in school. The first-stage estimates show that combined school attendance significantly increases the likelihood of junk food availability with an F-statistic on the instrument that exceeds 22 ( Table 2 ).
First Stage Regression Estimates of Junk Food Availability in Fifth Grade
Notes: Figures in brackets are robust standard errors clustered at the school level. Other covariates in the model include male, age (months), male*age, race/ethnicity, kindergarten BMI, mother’s education, income, private school dummy, categories for percent minority in school and school enrollment, and state and urbanicity dummies.
significant at 10%;
significant at 5%;
significant at 1%.
Second, since our instrument leverages across school variation we might be concerned that selection into different schools (or communities) might undermine the validity of our instrument. To test for differential selection into combined versus elementary schools, we regress BMI, obesity, test scores, social-behavioral outcomes, and parental involvement measured in kindergarten on combined school attendance in 5 th grade ( Table 3 ). 17 Because these outcomes are determined prior to exposure to school, these comparisons allow us to test for selection. The results suggest that, conditional on observed characteristics, combined school attendance is uncorrelated with pre-exposure BMI, obesity, test scores, social-behavioral outcomes and parental involvement.
Effect of Attending a Combined School on Kindergarten Outcomes
Notes: Each estimate represents a separate regression. Other covariates in the models include age, male, age*male, race/ethnicity, kindergarten BMI (not in model in Columns 1 and 2), mother’s education, income, private school dummy, categories for percent minority in school and school enrollment, and state and urbanicity dummies. Robust standard errors clustered at school level are shown in brackets. For reading, math, self control, and interpersonal skills, higher skills indicate better outcomes. For externalizing and internalizing behavior problems, higher scores indicate worse outcomes. Parent involvement is measured as the sum of the number of times/week that the parent engages in 9 activities with the child (e.g. reading books, talk about nature, do science projects, tell stories).
Third, another concern is that combined school attendance might generate peer effects on BMI, obesity, food consumption and physical activity, independent of junk food availability. We test for the presence of peer effects by regressing these outcomes on combined school attendance using only the sample of schools that do not offer junk foods ( Table 4 ). The results do not provide any support for peer effects on BMI, obesity, food consumption or physical activity. 18
Effect of Combined School Attendance on BMI, Obesity and Related Behaviors Without Junk Food Availability in Fifth Grade
Notes: Each estimate represents a separate regression. All models control for the full set of covariates. Robust standard errors clustered at school level are shown in brackets.
Overall, the instrument appears to be strongly predictive of junk food availability and there is no evidence that selection or peer effects threaten its validity.
We now turn to our main results, which examine the effects of junk food availability on BMI and other outcomes. We first estimate basic OLS models of BMI and obesity, then augment with state fixed effects and baseline BMI to address omitted variable bias and selection, and finally estimate the IV and reduced form specifications (Section 5.1). In Section 5.2, we examine the sensitivity of our results to alternate measures of junk food availability and various sample restrictions. We also report findings from falsification tests. And finally, in Section 5.3, we describe results from ancillary regressions that explore the potential mechanisms underlying our BMI findings. In particular, we examine in-school and total consumption of selected foods and beverages and the availability of and participation in physical activity.
5.1. BMI and Obesity
Our main results focus on whether the availability of junk foods increases BMI and obesity among 5 th graders ( Table 5 ). Columns 1 and 4 in Panel A show the results of basic OLS regressions of log BMI and obesity, respectively, on junk food availability controlling for child, household, and school characteristics. 19 These regressions yield a statistically significant increase in both BMI and obesity when junk food is available, although the point estimates are small. The inclusion of state fixed effects and urbanicity dummies (Panel A, columns 2 and 5) and then baseline BMI measured in kindergarten (Panel A, columns 3 and 6) eliminates the significant coefficients. The fully-specified OLS models have very small, precisely estimated, and statistically insignificant point estimates.
Effects of Junk Food Availability on BMI and Obesity in Fifth Grade
Notes: N=9,380. Robust standard errors clustered at school level are shown in brackets. Other covariates in the model include male, age (months), male*age, race/ethnicity, kindergarten BMI, mother’s education, income, private school dummy, categories for percent minority in school and school enrollment, and state and urbanicity dummies. First stage results are shown in Table 2 .
However, the coefficients from these OLS models may be biased if junk food availability is related to unobserved determinants of children’s BMI. For example, districts with a large population of students at risk for obesity may adopt more stringent nutritional policies that reduce the availability of junk foods in school. In such situations, OLS regressions may show no significant relationship or even a negative relationship between junk food availability and BMI. OLS estimates might also suffer from attenuation bias due to the presence of measurement error in the junk food availability measures.
To address these issues, we estimate instrumental variables (IV) and reduced form regressions using grade span as the instrument: whether the 5 th grader attends a combined school with older peers. 20 The IV point estimates are relatively larger than the OLS estimates, but less precisely estimated rendering them statistically insignificant ( Table 5 , Panel B). 21 , 22 IV estimates from models that do not control for state and urbanicity dummies and baseline BMI (columns 1 and 4) are much larger than those in our preferred specification (Columns 3 and 6), although they are not statistically significantly different from each other. Even if the IV point estimates in our preferred specification (columns 3 and 6) were significant, they would represent only small increases in BMI and obesity of less than one-third of one percent. Hausman tests that check for the endogeneity of junk food availability by comparing estimates from the fully-specified OLS regression with the IV cannot reject the null hypothesis that both estimates are consistent. Therefore, we also report the reduced form estimates of BMI and obesity regressed directly on our instrument ( Table 5 , Panel C). The coefficients on the instrument are close to zero and very precisely estimated, which further confirm the null findings. Given concerns about unobserved heterogeneity in the OLS specifications and the larger standard errors in the IV specifications, the reduced form estimates are preferred.
5.2. Sensitivity and Falsification Checks
We conducted a number of sensitivity analyses to test the robustness of our findings. In this section, we report results from a few key analyses and then turn to falsification tests. 23 These analyses control for the full set of covariates, including state and urbanicity dummies and baseline BMI.
For the sensitivity analyses, we first re-estimate our BMI and obesity regressions with the two alternate measures of junk food availability ( Table 6 ). Both the child-reported measure of junk food availability and the school-administrator reported measure of competitive food outlet show no effect of junk food availability on BMI or obesity. Next, we re-estimate the models with the exclusion of three particular groups that might confound our instrument ( Table 7 ). First, because combined schools are much more likely to be private, our instruments may simply capture variation across public versus private schools students, even though the regressions control for private school attendance. We re-estimate the models on a sample that excludes children who attend private schools ( Table 7 , Panel A) and find no effects on BMI and obesity. 24 Second, even though Section 4.2.2 suggests there are no peer effects on BMI and related behaviors, we test the sensitivity of our results to exclusion of the oldest peers (e.g., grade 9 or higher), but still find no evidence of an effect on BMI and obesity ( Table 7 , Panel B). Finally, children who switch schools for unobservable reasons potentially related to junk food availability may bias our estimates, but estimates from models that exclude children who changed schools between kindergarten and fifth grade confirm no effects ( Table 7 , Panel C). The point estimates from the OLS, IV and reduced form regressions for these sensitivity checks are essentially zero, though less precisely estimated in the IV models. 25
Effects of Alternate Measures of Junk Food Availability on BMI and Obesity in Fifth Grade
Notes: N=9,380
Competitive Food Outlet measure captures whether school has vending machines, school stores, canteens, snack bars, or a la carte lines through which competitive foods are sold. Robust standard errors clustered at school level are shown in brackets. Other covariates in the model include male, age (months), male*age, race/ethnicity, kindergarten BMI, mother’s education, income, private school dummy, categories for percent minority in school and school enrollment, and state and urbanicity dummies.
Effects of Junk Food Availability on BMI and Obesity in Fifth Grade with Alternate Sample Restrictions
Notes: All models include the full set of covariates. Robust standard errors clustered at school level are shown in brackets. Hausman tests for consistency of OLS estimates could not be rejected in any case. The tests are not reported in the table.
significant at 1%
As falsification tests, we examined whether junk food availability in the fifth grade influenced children’s height in the fifth grade and their pre-exposure BMI. Height should clearly be unrelated. And indeed, the coefficients are essentially zero and insignificant ( Table 8 ). Because BMI and obesity in kindergarten is measured prior to exposure to junk foods in school, any effects would suggest unobserved heterogeneity. The OLS, IV and reduced form point estimates are close to zero (though the IV estimates are less precise) and the reduced form specifications also show no relationship ( Table 9 , Panel A). Results for BMI and obesity measured in first and third grade likewise confirm insignificant effects of junk food availability during fifth grade ( Table 9 , Panels B and C). However, because our data do not contain information on junk food availability prior to 5 th grade, these results are also consistent with the absence of junk foods in earlier grades.
Effect of Junk Food Availability in School on Height in Fifth Grade
Note: N=9,380. Robust standard errors clustered at school level are shown in brackets.
Effects of Junk Food Availability on BMI and Obesity in Kindergarten, First, and Third Grade
Notes: Each estimate represents a separate regression. All models include the full set of covariates. Robust standard errors clustered at school level are shown in brackets.
5.3. Effects of Junk Food Availability on Food Consumption and Physical Activity
The consistent lack of significant findings for BMI and obesity raises questions regarding how the energy balance equation is affected by junk food availability. While we cannot measure children’s energy intake and expenditure explicitly with these data, we can examine whether junk food availability influences general food consumption patterns and physical activity. Unlike BMI and obesity, the consumption and physical activity measures are based on parents’ and children’s reports . As a result, they are subject to measurement error and consequently produce noisier estimates particularly for the IV models. Nevertheless, they represent our best opportunity for understanding important mechanisms underlying our null finding. Therefore, for the in-school junk food purchases, total consumption, and physical activity analyses, we focus mainly on the reduced form results (though we provide OLS results for comparison). 26
5.3.1 In-School Purchases and Overall Consumption
One potential explanation for our null findings for BMI and obesity may be that availability does not impact overall food consumption. This may happen for several different reasons. First, young children may not purchase significant amounts of junk food in school either due to limited access to such foods or fewer discretionary resources to purchase them. Second, children may not change their total consumption of junk food because junk food purchased in school simply substitutes for junk food brought from home. Or third, children may not change their overall consumption during the day, but simply substitute between junk food consumed in-school and out-of-school.
Unfortunately, we cannot completely separate out these possible explanations because the ECLS-K does not provide us with full information about the daily dietary intake of each child. However, we do have information about in-school purchases of foods with sugar, salty snacks, and sweetened beverages for those children with in-school availability. We also have total (in-school plus out-of-school) consumption of soda, fast food, and a variety of healthy foods for all children in the sample. While not definitive, we can use this information to gain some insight into underlying eating behaviors and lend support for our BMI and obesity findings.
Not surprisingly, our analysis of in-school consumption of junk foods does confirm that children purchase junk food when it is available. 27 The OLS estimates show a significant relationship for purchases of all types of junk food when junk foods are available in schools ( Table 10 , Panel A). And the reduced form estimates show that children in combined schools are between 5 and 9 percentage points more likely to purchase junk foods compared to those in elementary schools Table 10 , Panel B).
Effect of Junk Food Availability on In-School Junk Food Purchases in Fifth Grade
Notes: N=9380. Each estimate represents a separate regression. Dependent variables in columns (1)–(3) are dichotomous and capture whether any purchase of that item was made in school during the last week. All regressions include the full set of covariates. Robust standard errors clustered at school level are shown in brackets.
To provide a sense of the caloric contribution of these purchases, we multiplied the increase in the probability of purchase from attending a combined school by the median number of times that food was purchased among children who purchased at least once, by the number of the calories per unit. 28 Summing across the three junk food groups yields 50 calories per week (7 calories per day) from in-school junk food purchases. The caloric contribution of in-school purchases is much higher (435 calories per week or 62 calories per day for the median child) among children who purchase these foods (as opposed to merely having them available). But even the 62 calories per day represents less than a quarter (23 percent) of the daily discretionary calorie allowance (267 calories) for a moderately active fifth grader. 29
It is possible that children substitute in-school purchases for snacks brought from home or eaten at home either due to satiation or parental monitoring. With our simple dietary recall measures, we cannot explicitly test the nature of potential substitution. We can, however, examine the total intake of soda and fast food consumed in and out of school. Soda is of particular interest because it is the only item for which children were asked about both their in-school and total consumption separately. Fast food, on the other hand, does not correspond exactly to the in-school snack food consumption categories. We find that junk food availability is not associated with significant increases in children’s total consumption of soda or fast foods ( Table 11 , Columns 1 and 2). 30 The OLS regressions show negative, though generally insignificant, estimates. 31 More importantly, the reduced form estimates confirm that there is no relationship between combined school attendance and total consumption of soda and fast food. The fact that children who consume soda and other junk food in schools show no evidence of an increase in total consumption provides support for the substitution hypothesis. This finding is also consistent with the literature, which indicates that only 27 percent of soda and sweetened drinks consumed in elementary schools are bought at school compared to 67 percent brought from home ( Briefel et al 2009 ).
Effect of Junk Food Availability on Total Consumption of Selected Unhealthy and Healthy Foods in Fifth Grade
Notes: N=9380. Each estimate represents a separate regression. Dependent variable captures the number of times the food or beverage item was consumed during the last 7 days. All models include the full set of covariates. Robust standard errors clustered at school level are shown in brackets.
While BMI is a widely-used outcome measure, it does not capture nutritional changes. Just because children are not gaining weight does not mean that their diets are not adversely affected by junk food availability. If children are consuming junk food in lieu of healthy foods, there may still be concerns about their nutrition. Columns 3 through 8 of Table 11 examine whether children with in-school availability of junk foods consume less milk, green salad, carrots, potatoes, other vegetables, and fruit. The OLS results show no significant associations with junk food availability. Moreover, reduced form regressions also show that combined school attendance does not significantly impact total consumption of the healthy foods. 32
Physical Activity
The absence of any effects of junk food availability on BMI despite the in-school purchases of junk food also raises questions regarding potential compensatory changes in the availability of and participation in physical activity. For example, revenues from junk food sales may be used to fund playgrounds or pay for physical education instructors. Or it may be that combined schools simply offer more opportunities for physical activity due to their scale and organization relative to elementary schools. Another possibility is that parents or children may increase children’s physical activity to balance junk food intake. If physical activity is greater, then we may find no change in BMI or obesity despite an increase in caloric intake.
OLS and reduced form estimates for school- and parent-reported physical activity measures are reported in Table 12 . OLS estimates show no relationship between junk food availability and minutes per week of physical education at school, minutes per week of recess at school, and parent-reported participation in physical activity (measured as the number of days per week that the child engaged in exercise that causes rapid heart beat for 20 continuous minutes or more). The reduced form regressions show no significant effects of combined school attendance on minutes per week of physical education instruction. Children attending combined school have fewer minutes of recess ( Table 12 , Column 2), but slightly higher days of parent-reported physical activity ( Table 12 , Column 3) though neither finding is statistically significant at.conventional levels. Overall, the regressions do not provide consistent evidence that increased energy expenditure explains the null finding for BMI and obesity.
Effects of Junk Food Availability on Physical Education, Recess and Physical Activity in Fifth Grade
Notes: Each estimate represents a separate regression. All models include the full set of covariates as well as the baseline (kindergarten) measure of the dependent variable. Robust standard errors clustered at school level are shown in brackets.
6. Conclusion
Junk food availability is a prominent issue for middle and high schools in the U.S. However, there is also widespread legislation and regulation targeting junk foods even in elementary school ( Trust for American’s Health 2009 ). Young children’s access to junk foods in school is an important concern due to the strong correlation between childhood overweight and obesity in adolescence and adulthood ( Institute of Medicine 2005 ). In this paper, we examined whether junk food availability increased BMI and obesity among a national sample of 5th graders. Those 5th graders who attend a combined school are much more likely to have junk food availability relative to those in elementary school. While estimates from naïve models that only control for a limited set of covariates suggest a positive association between junk foods in school and BMI and obesity, fully-specified OLS models that control for BMI at school entry and state fixed-effects demonstrate no statistically or economically significant relationships among these young children. Likewise, the IV and reduced form models, which are not subject to the potential bias undermining OLS models, confirm the null findings for BMI and obesity. These results are not sensitive to various robustness checks including alternate measures of junk food availability and sample restrictions.
Finally, we provide further support for the null findings by examining in-school and overall food consumption patterns as well as physical activity. The null effects on BMI and obesity cannot be explained entirely by limited access or limited discretionary resources among young children because 5 th graders do purchase junk food when it is available in schools. However, our results suggest that the caloric contribution of in-school purchases is likely to be small. Moreover, we find no evidence of significant changes in the overall frequency of consumption of soda and fast food, which is consistent with children substituting in-school purchases of junk food for that taken from or eaten at home. Alternative explanations such as compensatory changes children’s consumption of healthy foods and in their opportunities for and participation in physical activity do not appear to play a significant role in explaining our null findings for BMI and obesity.
Our findings may have implications in the current economic environment. Half of the states are projecting budget shortfalls that threaten staffing, compensation, extracurricular activities, and policy initiatives such as mandated limits on class size. 33 Many schools subsidize their funding with revenue from the sale of junk foods. In total, elementary schools earn approximately $442 million annually from junk food sales ( Institute of Medicine 2007 ). In light of our findings, certain policy measures, such as outright bans on junk food sales (at least among elementary school children), might appear premature given that they remove a key source of discretionary funds.
While our results are robust, we caution that we could not consider the full range of consequences of junk food availability. Not only are the dietary intake measures in the ECLS-K limited, but we are also not able to examine whether related health outcomes such as diet quality or dental caries are influenced by junk food availability. Also, we are unable to examine the generalizability of our findings to older children who may have greater junk food access and intake both in and outside school. And finally, we could not consider whether exclusive contracts between schools and beverage/snack companies influence students’ food choices in the longer run through product or brand recognition. Additional research is necessary to fully understand the potential consequences before costly legislation is implemented. Such research might also consider the consequences of junk food regulations on school finances and the extent to which these financial consequences could be mitigated by the sale of more nutritious alternatives or through alternative financing mechanisms.
Means by Attendance in Elementary Versus Combined School and by Private/Public
Notes: N=9,380.
differences in means are statistically significant at the 5% level.
Effect of Grade-Span on Academic and Social-Behavioral Outcomes Among Schools Without Junk Food Availability in Fifth Grade
Acknowledgments
This research was funded by grants from the Robert Wood Johnson Foundation’s Healthy Eating Research Program, NIH R01 HD057193, the Bing Center for Health Economics at RAND, and the RAND Labor and Population Program. All opinions are those of the authors and do not represent opinions of the funding agencies.
Other studies have examined the effects of price reductions, increases in availability, and promotion of low-fat foods in secondary schools on sales and purchases of these foods ( French et al 2004 , 2001 , 1997a , 1997b , Jeffery et al 1994 ) as well as their consumption ( Perry et al 2004 ) within experimental settings and found positive effects.
Kubik et al (2003) find that a la carte availability in school is negatively associated with overall intake of fruits and vegetables and positively associated with total and saturated fat intake among 7 th graders attending 16 Minneapolis-St Paul schools. Using the same data, Kubik et al (2005) show that using competitive foods as rewards and incentives is positively associated with BMI.
Also, using the ECLS-K, Fernandes (2008) found small positive associations between soda availability in schools and both in-school and overall soda consumption of fifth graders.
Their results for the other school policies, pouring rights contracts, and food and beverage advertisements are smaller and less precise.
For example, California’s first nutrition policy (SB 677) implemented beverage standards for elementary and middle schools, not high schools.
All sample sizes have been rounded to the nearest 10 per the ECLS-K’s restricted-use data agreement.
Obesity is defined as BMI greater than the 95 th percentile for age and gender on the Center for Disease Control growth charts.
Sweets include candy, ice cream, cookies, brownies or other sweets; salty snack foods include potato chips, corn chips, Cheetos, pretzels, popcorn, crackers or other salty snacks, and sweetened beverages include soda pop, sports drinks or fruit drinks that are not 100 percent juice.
To validate the ECLS-K estimates, we examined the Third School Nutrition and Dietary Assessment Study (SNDA-III), which collected 24-hour dietary recall from 2,300 children attending a nationally representative sample of public schools in 2005. Similar to the ECLS-K, eighty percent of elementary school children reported no competitive food purchases. Among children who made a purchase, the median daily caloric intake from these foods was 185 calories. The SNDA estimate is higher than our ECLS-K estimates (62 calories reported in Section 5) because it includes healthy foods purchased from competitive food venues: for example, milk was by far the most popular item purchased from competitive food venues and yogurt also ranked highly.
The “potatoes” category excluded French fries, fried potatoes, and potato chips.
The questionnaire separately asked about availability of high- and low-fat options for baked foods, salty snacks, and ice cream/frozen yogurt/sherbert. We include both the low- and high-fat options in our measure, however, in sensitivity analyses, we used only the high-fat versions to construct our school-administrator based measure of junk food availability and found results to be similar.
We rely mainly on the first measure of junk food availability because it is the most specific with respect to the quality of foods and because school-level policies regarding junk food availability are frequently set by school principals and staff ( Gordon et al 2007a ). We prefer this measure over the simple dichotomy of having any (unregulated) competitive food outlets because the outlet-based measure does not differentiate the type of foods sold (e.g. milk vs. soda). We also prefer it over the child-report because children who do not consume junk foods are less likely to accurately report availability and because children reported only the availability of any sweets, salty snacks, or sweetened beverages, but did not differentiate specific items (e.g. low-fat vs. high-fat).
The value of reduced form regressions has been highlighted by Angrist and Krueger (2001) and, more recently, Chernozhukov and Hansen (2008) formally show that the test for instrument irrelevance in the reduced form regression can be viewed as a weak-instrument-robust test of the hypothesis that the coefficient on the endogenous variable in the structural equation is zero.
This literature examines peer effects on a wide range of outcomes including substance use ( Lundborg 2006 ; Eisenberg 2004 ; Case and Katz 1991 ; Gaviria and Raphael 2001 ), crime ( Case and Katz 1991 ; Glaeser, Sacerdote, and Scheinkman 1996 ; Regnerus 2002 ), teenage pregnancy ( Crane 1991 ; Evans, Oates and Schwab 1992 ), discipline ( Cook et al 2008 ), academic achievement ( Hanushek et al 2003 ; Cook et al 2008 ), adolescent food choices ( Perry, Kelder, Komro 1993 ; Cullen et al 2001 ; French et al 2004 ) and weight ( Trogdon, Nonnemaker and Pais 2008 ).
However, Clark and Loheac (2007) estimate how substance use behavior of students within the same school who are one year older influences adolescent substance use and find a positive relationship.
One exception is Eisenberg (2004) who finds that 7 th and 8 th graders who attend schools with older peers are no more likely to use substances relative to those who attend schools with younger peers.
We also examined unadjusted differences in children’s individual, family and school characteristics during the 5 th grade (see Appendix Table A3 ). There were slight differences for some of the covariates. However, there was no overall pattern in the socioeconomic factors that would threaten the validity of the IV approach: that is, some differences imply better BMI outcomes for one group and others worse. For example, in our sample, elementary school students are more likely to be Hispanic and Asian while combined school students are more likely to be white. There are no differences in the share that are Black. Similarly, there is no consistent pattern in maternal education. Elementary school students are more likely to have poorly and highly educated mothers (less than high school, more than Bachelors).
To check whether these null findings are merely due to lack of power instead of absence of peer effects, we estimated the same models using social-behavioral outcomes and test scores as dependent variables because the literature finds evidence of peer effects on these outcomes. We were able to identify statistically significant peer effects on social-behavioral outcomes (but not test scores), which suggests that lack of power is an unlikely explanation for the finding of null peer effects on BMI and related outcomes.
In all models, we estimate robust standard errors clustered at the school level.
In alternate analyses, we used continuous measures of the highest and lowest grades in the school as instruments. In these over-identified models, both instruments had a strong positive association with junk food availability (i.e. increases in the highest and lowest grades available at the school were strongly predictive of junk food availability). This approach yielded qualitatively similar results as the exactly-identified models (available upon request).
The IV regressions were also estimated without baseline BMI. The point estimates, first-stage F-statistics, and Hausman tests yield similar results (available upon request).
A concern with our IV specification estimated via two-stage least-squares is that our first stage models do not account for the dichotomous nature of the treatment variable ( Maddala 1983 ). Estimates from binary treatment effect IV models confirm that the effects of junk food availability on BMI are neither substantive nor significant (available upon request).
We also conducted additional sensitivity analyses not reported here. First, given that we do not know the exposure to junk food in previous grades and given concerns that genetic susceptibility may not have a constant proportional effect on BMI at every point in the life cycle, we controlled for 1 st or 3 rd grade BMI instead of BMI in Kindergarten and obtained similar results. Second, inclusion of controls for school meal participation did not change our findings. Third, we used BMI z-scores as the dependent variable to accurately control for age and gender influences on BMI and obtained qualitatively similar results. Fourth, we estimated quantile regressions to test whether the effects of junk food availability varied across the BMI distribution, but found no evidence for heterogeneous effects. Finally, we also re-estimated our BMI and obesity models separately for each gender. The results for junk food availability mirrored those for the full sample. The OLS, IV, and RF models show no significant effects of junk food availability for either boys or girls. Still we may be concerned about differential peer effects, for example, if girls are influenced by older peers’ concerns about body image, which would bias our IV estimates downward. Restricting the sample to those boys and girls attending schools without junk food availability, the coefficients from the reduced form were nearly identical to those based on the full sample of boys and girls, which suggests that peer effects are not an issue even when regressions are gender-specific.
Estimates based only on the sample of private schools yield small and statistically insignificant effects of competitive food availability on BMI in both OLS and IV specifications, although the F-statistics for the instrument in the first stage were smaller (Results available upon request).
Hausman tests cannot reject the consistency of fully-specified OLS estimates in any of our sensitivity checks.
Although not shown, the IV (Wald) estimates are easily calculated by dividing the reduced form estimates in Table 10 – Table 12 by 0.2 (first stage estimate from Table 2 ). The IV coefficients are never significant in part due to the larger standard errors in the regressions of reported eating behaviors and physical activity.
We dichotomize the in-school purchase variables and estimate linear probability models since much of the variation in junk food purchases at school occurs on the extensive margin.
The median number of times an item is purchased in school among children who purchase at least once is 1.5 times (1–2 times per week). We assume that salty snacks add 140 calories (typical calories from a bag of potato chips), sweets add 200 calories (typically calories from a candy bar), and soda adds 150 calories. Given the limitations of the consumption data in the ECLS-K, we caution the reader to treat these caloric intake calculations as approximations.
Discretionary calories are the difference between an individual’s total energy requirement and the energy necessary to meet nutrient requirements. According to Dietary Guidelines for Americans, the discretionary allowance for a 2000 calorie diet is 267 calories. See: http://www.health.gov/dietaryguidelines/dga2005/document/html/chapter2.htm#table3 accessed August 22, 2008.
The total consumption variables are not dichotomized because there is sufficient variation on the intensive margin.
Negative binomial models with a binary treatment variable to account for the count-data distribution of the total consumption variable and the binary nature of junk food availability produced qualitatively similar results. (Results available upon request).
Given the limitations of the ECLS-K’s consumption variables, we again examined the SNDA-III data and found no evidence that combined school attendance increases total caloric intake.
“Schools expect budget cuts as economy sours: State problems, decline in property values eat away at district funds”. Available at: http://www.msnbc.msn.com/id/23116409/ (Accessed February 10, 2009).
Contributor Information
Ashlesha Datar, RAND Corporation, 1776 Main Street, P.O. Box 2138, Santa Monica, CA 90407, USA, [email protected], Phone: 1-310-393-0411 x7367, Fax: 1-310-260-8161.
Nancy Nicosia, RAND Corporation, 20 Park Plaza, 7th Floor, Suite 720, Boston, MA 02116, USA, [email protected], Phone: 1-617-338-2059 x4227.
- Anderson PM, Butcher KM. Reading, Writing, and Refreshments: Are School Finances Contributing to Children’s Obesity? Journal of Human Resources. 2006;41(3):467–494. [ Google Scholar ]
- Anderson ML, Matsa DA. Are Restaurants Really Supersizing America? American Economic Journal: Applied Economics. 2011;3(1):152–188. [ Google Scholar ]
- Angrist J, Krueger A. Instrumental Variables and the Search for Identification: From Supply and Demand to Natural Experiments. Journal of Economic Perspectives. 2001;15(4):69–85. [ Google Scholar ]
- Bedard K, Do C. Are Middle Schools More Effective? The Impact of School Structure on Student Outcomes. Journal of Human Resources. 2005;40(3):660–682. [ Google Scholar ]
- Black J. Senate drops measure to greatly reduce sugar and fat in food at schools. Washington Post: 2007. Dec 15, p. A02. [ Google Scholar ]
- Boehmer TK, Brownson RC, Haire–Joshu D, Dreisinger ML. Patterns of childhood obesity prevention legislation in the United States. Prev Chronic Disease. 2007;4(3):A56. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Briefel RR, Crepinsek MK, Cabili C, Wilson A, Gleason PM. School food environments and practices affect dietary behaviors of US public school children. Journal of the American Dietetic Association. 2009;109(2 Suppl):S91–S107. doi: 10.1016/j.jada.2008.10.059. [ DOI ] [ PubMed ] [ Google Scholar ]
- Case AC, Katz L. The Company You Keep: The Effects of Family and Neighborhood on Disadvantaged Youths. National Bureau of Economic Research Working paper. 1991 # 3705. [ Google Scholar ]
- Chernozhukov V, Hansen C. An IV Model of Quantile Treatment Effects. Econometrica. 2005;73(1):245–261. [ Google Scholar ]
- Chernozhukov V, Hansen C. The Reduced Form: A Simple Approach to Inference with Weak Instruments. Economics Letters. 2008;100:68–71. [ Google Scholar ]
- Clark C, Folk J. Mimeo. Milledgeville, GA: Georgia College and State University; 2007. Do Peers Matter? Evidence from the Sixth Grade Experiment. [ Google Scholar ]
- Clark AE, Loheac Y. “It wasn’t me, it was them!” Social influence in risky behavior by adolescents. Journal of Health Economics. 2007;26:763–784. doi: 10.1016/j.jhealeco.2006.11.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cook P, MacCoun R, Muschkin C, Vigdor JL. The Negative Impacts of Starting Middle School in Sixth Grade. Journal of Policy Analysis and Management. 2008;27(1):104–121. [ Google Scholar ]
- Crane J. The Epidemic Theory of Ghettos and Neighborhood Effects on Dropping Out and Teenage Childbearing. American Journal of Sociology. 1991;96(5):1226–1260. [ Google Scholar ]
- Cullen KW, Eagan J, Baranowski T, et al. Effect of à la carte and snack bar foods at school on children’s lunchtime intake of fruits and vegetables. Journal of American Dietetic Association. 2000;100:1482–1486. doi: 10.1016/s0002-8223(00)00414-4. 2000. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cullen KW, Zakeri I. Fruits, vegetables, milk, and sweetened beverages consumption and access to a la carte/snack bar meals at school. Am J Public Health. 2004 Mar;94(3):463–467. doi: 10.2105/ajph.94.3.463. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cullen KW, Baranowski T, Rittenberry L, et al. Child-reported family and peer influences on fruit, juice and vegetable consumption: reliability and validity of measures. Health Education Research. 2001 Apr;16(2):187–200. doi: 10.1093/her/16.2.187. [ DOI ] [ PubMed ] [ Google Scholar ]
- Eisenberg D. Mimeo. Ann Arbor: University of Michigan; 2004. Peer Effects for Adolescent Substance Use: Do They Really Exist? [ Google Scholar ]
- Evans WM, Oates WE, Schwab RM. Measuring Peer Group Effects: A Study of Teenage Behavior. Journal of Political Economy. 1992;100(5):966–991. [ Google Scholar ]
- Fernandes M. The Effect of Soft Drink Availability in Elementary Schools on Consumption. Journal of the American Dietetic Association. 2008;108(9):1445–1452. doi: 10.1016/j.jada.2008.06.436. [ DOI ] [ PubMed ] [ Google Scholar ]
- Finkelstein DM, Hill EL, Whitaker RC. School food environments and policies in US public schools. Pediatrics. 2008;122(1):e251–e259. doi: 10.1542/peds.2007-2814. [ DOI ] [ PubMed ] [ Google Scholar ]
- French S, Story M, Fulkerson JA, Hannan P. An environmental intervention to promote lower fat food choices in secondary schools: Outcomes from the TACOS study. American Journal of Public Health. 2004;94:1507–1512. doi: 10.2105/ajph.94.9.1507. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- French SA, Jeffery RW, Story M, et al. Pricing and promotion effects on low-fat vending snack purchases: the CHIPS Study. Am J Public Health. 2001;91(1):112–117. doi: 10.2105/ajph.91.1.112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- French SA, Jeffery RW, Story M, Hannan P, Snyder MP. A pricing strategy to promote low-fat snack choices through vending machines. Am J Public Health. 1997;87(5):849–851. doi: 10.2105/ajph.87.5.849. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- French SA, Story M, Jeffery RW, et al. Pricing strategy to promote fruit and vegetable purchase in high school cafeterias. J Am Diet Assoc. 1997;97:1008–1010. doi: 10.1016/S0002-8223(97)00242-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- Froelich M, Melly B. [Retrieved 2009-02-10];Estimation of quantile treatment effects with STATA: mimeo. 2008 from http://www.alexandria.unisg.ch/publications/46580 .
- Gaviria A, Raphael S. School Based Peer Effects and Juvenile Behavior. Review of Economics and Statistics. 2001;83(2):257–268. [ Google Scholar ]
- Glaeser EL, Sacerdote B, Scheinkman JA. Crime and Social Interactions. Quarterly Journal of Economics. 1996;111(2):507–548. [ Google Scholar ]
- Gordon A, Crepinsek MK, Nogales R, Elizabeth Condon. School Nutrition Dietary Assessment Study-III: Volume I: School Foodservice, School Food Environment, and Meals Offered and Served. 2007a Report No. CN-07-SNDA-III. [ Google Scholar ]
- Gordon A, Crepinsek MK, Nogales R, Elizabeth Condon. School Nutrition Dietary Assessment Study-III: Volume II: Student Participation and Dietary Intakes. 2007b Report No. CN-07-SNDA-III, November. [ Google Scholar ]
- Gresham F, Elliott S. Social skills rating system. Circle Pines, MN: American Guidance Service; 1990. [ Google Scholar ]
- Hanushek EA, Kain JF, Markman JM, Rivkin SG. Does peer ability affect student achievement? Journal of Applied Econometrics. 2003;18(5):527–544. [ Google Scholar ]
- Harnack L, Snyder P, Story M, et al. Availability of a la carte food items in junior and senior high schools: a needs assessment. J Am Diet Assoc. 2000;100:701–703. doi: 10.1016/S0002-8223(00)00204-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- Institute of Medicine. Committee on Prevention of Obesity in Children and Youth, Preventing Childhood Obesity:Health in the Balance. Washington, D.C: National Academies Press; 2005. [ Google Scholar ]
- Institute of Medicine. Nutrition Standards For Foods in Schools: Leading the Way Towards Healthier Youth. Washington, D.C.: The National Academies Press; 2007. [ Google Scholar ]
- Jeffery RW, French SA, Raether C, Baxter JE. An environmental intervention to increase fruit and salad purchases in a cafeteria. Prev Med. 1994;23:788–792. doi: 10.1006/pmed.1994.1135. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kubik MY, Lytle LA, Hannan PJ, et al. The association of the school food environment with dietary behaviors of young adolescents. Am J Public Health. 2003;93:1168–1173. doi: 10.2105/ajph.93.7.1168. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lundborg P. Having the wrong friends? Peer effects in adolescent substance use. Journal of Health Economics. 2006;25(2):214–233. doi: 10.1016/j.jhealeco.2005.02.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- Maddala GS. Limited Dependent and Qualitative Variables in Econometrics. Cambridge University Press; 1983. [ Google Scholar ]
- Ogden CL, Carroll MD, et al. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [ DOI ] [ PubMed ] [ Google Scholar ]
- Paglin C, Fager J. Grade configuration: Who goes where? By request series. Portland, OR: Northwest Regional Educational Laboratory; 1997. [ Google Scholar ]
- Perry CL, Bishop DB, Taylor GL, et al. A Randomized School Trial of Environmental Strategies to Encourage Fruit and Vegetable Consumption among Children. Health Educ Behav. 2004;31:65. doi: 10.1177/1090198103255530. [ DOI ] [ PubMed ] [ Google Scholar ]
- Perry CL, Kelder SH, Komro KA. In: The social world of adolescents: families, peers, school and the community. SG Millstein, AC Petersen, EO Nightingale., editors. New York, NY: Oxford University Press; 1993. pp. 73–96. Promoting the Health of Adolescents: New Directions for the Twenty-First Century. [ Google Scholar ]
- Rickles JH. Achievement Gains for Students in Schools with Alternative Grade Spans. Planning, Assessment and Research Division Publication No. 251. Los Angeles Unified School District, Los Angeles, CA: Program Evaluation and Research Branch; 2005. [ Google Scholar ]
- Regnerus MD. Friends’ influence on adolescent theft and minor delinquency: A developmental test of peer-reported effects. Social Science Research. 2002;31:681–705. [ Google Scholar ]
- Story M, Hayes M, Kalina B. Availability of foods in high schools: is there cause for concern? J Am Diet Assoc. 1996;96:123–126. doi: 10.1016/S0002-8223(96)00039-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- Templeton SB, Marlette MA, Panemangalore M. Competitive foods increase the intake of energy and decrease the intake of certain nutrients by adolescents consuming school lunch. J Am Diet Assoc. 2005 Feb;105(2):215–220. doi: 10.1016/j.jada.2004.11.027. [ DOI ] [ PubMed ] [ Google Scholar ]
- Tourangeau K, Nord C, Lê T, et al. U.S. Department of Education. Washington, DC: National Center for Education Statistics; 2006. Early childhood longitudinal study, kindergarten class of 1998–99 (ECLS-K), Combined User’s Manual for the ECLS-K Fifth-Grade Data Files and Electronic Codebooks (NCES 2006–032) [ Google Scholar ]
- Trogdon JG, Nonnemaker J, Pais J. Peer effects in adolescent overweight. Journal of Health Economics. 2008;27(5):1388–1399. doi: 10.1016/j.jhealeco.2008.05.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- Trust for American’s Health. F as in Fat. How Obesity Policies are Failing in America. Tech. rep. 2009 Trust for American’s Health. [ Google Scholar ]
- Wechsler H, Brener NC, Kuester S, Miller C. Food service and foods and beverages available at school: results from the School Health Policies and Programs Study 2000. J Sch Health. 2001;71:313–324. doi: 10.1111/j.1746-1561.2001.tb03509.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wolraich ML, Wilson DB, White JW. The effect of sugar on behavior or cognition in children: a meta-analysis. JAMA. 1995;274:1617–1621. doi: 10.1001/jama.1995.03530200053037. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (373.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
Obesity in childhood is the most challenging public health issue in the twenty-first century. It has emerged as a pandemic health problem worldwide. The children who are obese tend to stay obese in adulthood and prone to increased risk for diabetes and cardiac problems at a younger age. Childhood obesity is associated with increased morbidity and premature death.[1] Prevention of obesity in ...
Childhood Obesity 3 Childhood Obesity: Turning a Risk Factor into a Solution Obesity is a critical health problem that is increasing worldwide, and in the United States in particular. In 2012, The Center for Disease Control and Prevention (CDC) identified obesity as a leading cause of death of adults in the US, second only to heart disease, and
Overweight or obesity (OW/OB) affects just under half of the children and adolescents in the United States [1], tracks into adulthood and is associated with significant mental and physical ...
️ 9 Tips for Writing a Childhood Obesity Essay. In many countries, obesity is becoming the leading cause of death. From an overabundance of unhealthy food to a tendency to be less active, the habits of parents often transfer to their children, resulting in the acuteness of a childhood obesity essay.
obesity. In this essay, we document trends in children's obesity and examine the under-lying causes of the obesity epidemic. We begin by discussing definitions of over-weight and obesity, noting some potential problems. We document trends in adult and childhood obesity, both worldwide and in the United States, over the past three
Effects of Childhood Obesity. Childhood obesity can have serious consequences for a child's physical and emotional health, both in the short and long term. Some of the effects of obesity in children include: Increased risk of developing type 2 diabetes, high blood pressure, and high cholesterol.
In 2019, the World Obesity Federation estimated there . would be 206 million children and adolescents aged 5-19 years living with obesity in 2025, and 254 million in 2030. 1. Of the 42 countries each estimated to have more than 1 million children with obesity in 2030, the top ranked are China, followed by India, the USA, Indonesia,
Clinical Comorbidities of Obesity in Children Growth and Puberty. Excess weight gain in children can influence growth and pubertal development ().Childhood obesity can cause prepubertal acceleration of linear growth velocity and advanced bone age in boys and girls ().Hyperinsulinemia is a normal physiological state during puberty, but children with obesity can have abnormally high insulin ...
Childhood obesity is a serious medical condition that affects children and adolescents. It's caused by lifestyle issues, such as too much food and too little exercise, and can lead to health problems like diabetes, high blood pressure and low self-esteem.
The data may indicate that there is only a limited direct intrauterine effect of maternal obesity on childhood obesity; rather, genetic effects inherited from the mother or father, or both, and/or ...
Reducing obesity in adolescent and young adult females could be expected to reduce childhood obesity by 10-22%, with ongoing effects for subsequent generations. Food banks and medically tailored meals (low-fat or low-glycemic index meals with reduced calories) may be successful in reducing obesity among adolescents at highest risk ( 76 ).
The use of medication for childhood obesity is also limited by side effects, cost, and uncertainty about their long-term safety. 3,37 Bariatric Surgery Bariatric surgery is effective in selected adolescents with severe obesity who fit the criteria in Table 2 38 and was recently endorsed by a policy statement from the American Academy of ...
The editorial discusses the rising prevalence and consequences of obesity in children and adolescents worldwide, especially during the COVID-19 pandemic. It highlights the need for multifaceted interventions, policies, and research to address the biological, commercial, and social determinants of obesity.
Learn about the causes, prevention, and treatment of childhood obesity, a serious health problem affecting 1 in 5 children in the US. Find out how to monitor your child's BMI, make healthy lifestyle changes, and get support from your community.
Looking forward, we need more research to find the best ways to prevent and manage child obesity. By continuing to study why it happens and seeing how different solutions work, we can improve and adjust our strategies to fit different groups of people. Solving child obesity is something we all need to work on together - health professionals, policymakers, teachers, and families.
Childhood obesity is one of the foremost threats to population health in the United States (U.S.). 1, 2 Childhood obesity refers to a body mass index (BMI) at or greater than 95 th percentile for age and sex, while childhood overweight is BMI at or greater than 85 th to less than 95 th percentile for age and sex. 3 Globally, the mean ...
Looking for a good effects of obesity essay? 🔥 Check our paper example to find out about the main cause of obesity, social effects, and consequences of obesity. IvyPanda® Free Essays. ... Child and Adolescent Obesity: Causes and Consequences, Prevention and Management. United Kingdom: Cambridge University Press, 2002. Print.
Find free essay examples and topic ideas on childhood obesity, a serious medical condition that affects millions of children in the US and worldwide. Learn about the causes, consequences, prevention and management of obesity, as well as the role of parents, schools and society.
Essay Example: Abstract There is growing concern about the state of children's health. Every year there is an increase in the number of overweight and obese children. ... Childhood obesity effects every area of that child's life and will continue to affect that child as he or she grows to adulthood. Causes of Childhood Obesity.
Obesity in children and adolescents is a global health issue with increasing prevalence in low-income and middle-income countries (LMICs) as well as a high prevalence in many high-income countries. 1 Obesity during childhood is likely to continue into adulthood and is associated with cardiometabolic and psychosocial comorbidity as well as premature mortality. 2-4 The provision of effective ...
Childhood obesity is a serious problem in the US, putting children and adolescents at risk for ... Popkin BM. Screen time and physical activity during adolescence: longitudinal effects on obesity in young adulthood. Int J Behav Nutr Phys Act [Internet]. 2007. [cited 2020 Nov 16];4(1):26. ... If you have cited papers that have been retracted ...
A commentary on the global challenge of childhood obesity and its health effects, especially in low- and middle-income countries. It highlights the need for prevention strategies, early interventions, and implementation research across sectors and contexts.
Girls may be more sensitive to obesity-related effects of ACEs than boys, sexual abuse appears to have a greater effect on childhood obesity than other ACEs, and co-occurrence of multiple ACEs may be associated with greater childhood obesity risk. Further, the effect of ACEs on development of childhood obesity may take 2-5 years to manifest.
Learn how to model healthy eating, move more, set sleep routines, limit screen time, support ECE settings, and find a family healthy weight program. Childhood obesity is a complex disease with many contributing factors and health risks, but there are many ways to help children have a healthy weight and set up lifelong healthy habits.
We re-estimate the models on a sample that excludes children who attend private schools (Table 7, Panel A) and find no effects on BMI and obesity. 24 Second, even though Section 4.2.2 suggests there are no peer effects on BMI and related behaviors, we test the sensitivity of our results to exclusion of the oldest peers (e.g., grade 9 or higher ...