Appointments at Mayo Clinic
- Pregnancy week by week
- Fetal presentation before birth
The way a baby is positioned in the uterus just before birth can have a big effect on labor and delivery. This positioning is called fetal presentation.
Babies twist, stretch and tumble quite a bit during pregnancy. Before labor starts, however, they usually come to rest in a way that allows them to be delivered through the birth canal headfirst. This position is called cephalic presentation. But there are other ways a baby may settle just before labor begins.
Following are some of the possible ways a baby may be positioned at the end of pregnancy.

Head down, face down
When a baby is head down, face down, the medical term for it is the cephalic occiput anterior position. This the most common position for a baby to be born in. With the face down and turned slightly to the side, the smallest part of the baby's head leads the way through the birth canal. It is the easiest way for a baby to be born.
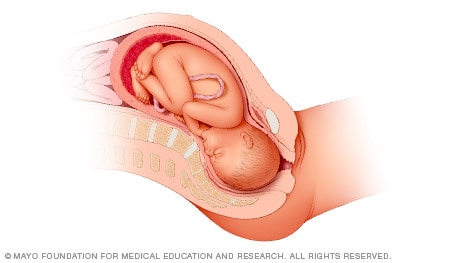
Head down, face up
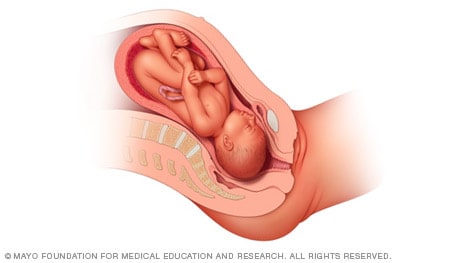
When a baby is head down, face up, the medical term for it is the cephalic occiput posterior position. In this position, it might be harder for a baby's head to go under the pubic bone during delivery. That can make labor take longer.
Most babies who begin labor in this position eventually turn to be face down. If that doesn't happen, and the second stage of labor is taking a long time, a member of the health care team may reach through the vagina to help the baby turn. This is called manual rotation.
In some cases, a baby can be born in the head-down, face-up position. Use of forceps or a vacuum device to help with delivery is more common when a baby is in this position than in the head-down, face-down position. In some cases, a C-section delivery may be needed.
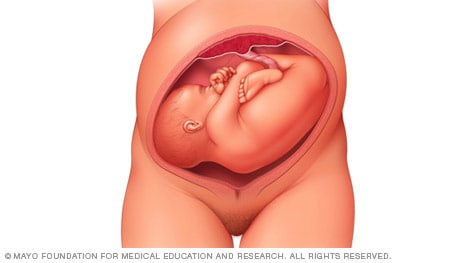
Frank breech
When a baby's feet or buttocks are in place to come out first during birth, it's called a breech presentation. This happens in about 3% to 4% of babies close to the time of birth. The baby shown below is in a frank breech presentation. That's when the knees aren't bent, and the feet are close to the baby's head. This is the most common type of breech presentation.
If you are more than 36 weeks into your pregnancy and your baby is in a frank breech presentation, your health care professional may try to move the baby into a head-down position. This is done using a procedure called external cephalic version. It involves one or two members of the health care team putting pressure on your belly with their hands to get the baby to roll into a head-down position.
If the procedure isn't successful, or if the baby moves back into a breech position, talk with a member of your health care team about the choices you have for delivery. Most babies in a frank breech position are born by planned C-section.

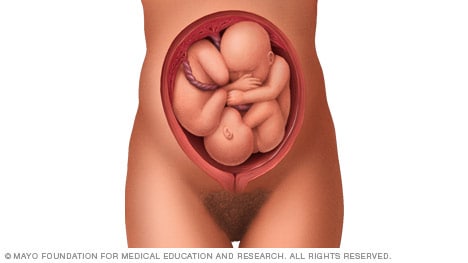
Complete and incomplete breech
A complete breech presentation, as shown below, is when the baby has both knees bent and both legs pulled close to the body. In an incomplete breech, one or both of the legs are not pulled close to the body, and one or both of the feet or knees are below the baby's buttocks. If a baby is in either of these positions, you might feel kicking in the lower part of your belly.
If you are more than 36 weeks into your pregnancy and your baby is in a complete or incomplete breech presentation, your health care professional may try to move the baby into a head-down position. This is done using a procedure called external cephalic version. It involves one or two members of the health care team putting pressure on your belly with their hands to get the baby to roll into a head-down position.
If the procedure isn't successful, or if the baby moves back into a breech position, talk with a member of your health care team about the choices you have for delivery. Many babies in a complete or incomplete breech position are born by planned C-section.

When a baby is sideways — lying horizontal across the uterus, rather than vertical — it's called a transverse lie. In this position, the baby's back might be:
- Down, with the back facing the birth canal.
- Sideways, with one shoulder pointing toward the birth canal.
- Up, with the hands and feet facing the birth canal.
Although many babies are sideways early in pregnancy, few stay this way when labor begins.
If your baby is in a transverse lie during week 37 of your pregnancy, your health care professional may try to move the baby into a head-down position. This is done using a procedure called external cephalic version. External cephalic version involves one or two members of your health care team putting pressure on your belly with their hands to get the baby to roll into a head-down position.
If the procedure isn't successful, or if the baby moves back into a transverse lie, talk with a member of your health care team about the choices you have for delivery. Many babies who are in a transverse lie are born by C-section.
If you're pregnant with twins and only the twin that's lower in the uterus is head down, as shown below, your health care provider may first deliver that baby vaginally.
Then, in some cases, your health care team may suggest delivering the second twin in the breech position. Or they may try to move the second twin into a head-down position. This is done using a procedure called external cephalic version. External cephalic version involves one or two members of the health care team putting pressure on your belly with their hands to get the baby to roll into a head-down position.
Your health care team may suggest delivery by C-section for the second twin if:
- An attempt to deliver the baby in the breech position is not successful.
- You do not want to try to have the baby delivered vaginally in the breech position.
- An attempt to move the baby into a head-down position is not successful.
- You do not want to try to move the baby to a head-down position.
In some cases, your health care team may advise that you have both twins delivered by C-section. That might happen if the lower twin is not head down, the second twin has low or high birth weight as compared to the first twin, or if preterm labor starts.
- Landon MB, et al., eds. Normal labor and delivery. In: Gabbe's Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed May 19, 2023.
- Holcroft Argani C, et al. Occiput posterior position. https://www.updtodate.com/contents/search. Accessed May 19, 2023.
- Frequently asked questions: If your baby is breech. American College of Obstetricians and Gynecologists https://www.acog.org/womens-health/faqs/if-your-baby-is-breech. Accessed May 22, 2023.
- Hofmeyr GJ. Overview of breech presentation. https://www.updtodate.com/contents/search. Accessed May 22, 2023.
- Strauss RA, et al. Transverse fetal lie. https://www.updtodate.com/contents/search. Accessed May 22, 2023.
- Chasen ST, et al. Twin pregnancy: Labor and delivery. https://www.updtodate.com/contents/search. Accessed May 22, 2023.
- Cohen R, et al. Is vaginal delivery of a breech second twin safe? A comparison between delivery of vertex and non-vertex second twins. The Journal of Maternal-Fetal & Neonatal Medicine. 2021; doi:10.1080/14767058.2021.2005569.
- Marnach ML (expert opinion). Mayo Clinic. May 31, 2023.
Products and Services
- A Book: Mayo Clinic Guide to a Healthy Pregnancy
- 3rd trimester pregnancy
- Fetal development: The 3rd trimester
- Overdue pregnancy
- Pregnancy due date calculator
- Prenatal care: Third trimester
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- NEW: Listen to Health Matters Podcast - Mayo Clinic Press NEW: Listen to Health Matters Podcast
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Healthy Lifestyle
Thank a researcher today
Their crucial work saves lives every day. Let Mayo Clinic researchers know they’re appreciated with a quick message.
Fetal Presentation, Position, and Lie (Including Breech Presentation)
- Key Points |
Abnormal fetal lie or presentation may occur due to fetal size, fetal anomalies, uterine structural abnormalities, multiple gestation, or other factors. Diagnosis is by examination or ultrasonography. Management is with physical maneuvers to reposition the fetus, operative vaginal delivery , or cesarean delivery .
Terms that describe the fetus in relation to the uterus, cervix, and maternal pelvis are
Fetal presentation: Fetal part that overlies the maternal pelvic inlet; vertex (cephalic), face, brow, breech, shoulder, funic (umbilical cord), or compound (more than one part, eg, shoulder and hand)
Fetal position: Relation of the presenting part to an anatomic axis; for vertex presentation, occiput anterior, occiput posterior, occiput transverse
Fetal lie: Relation of the fetus to the long axis of the uterus; longitudinal, oblique, or transverse
Normal fetal lie is longitudinal, normal presentation is vertex, and occiput anterior is the most common position.
Abnormal fetal lie, presentation, or position may occur with
Fetopelvic disproportion (fetus too large for the pelvic inlet)
Fetal congenital anomalies
Uterine structural abnormalities (eg, fibroids, synechiae)
Multiple gestation
Several common types of abnormal lie or presentation are discussed here.

Transverse lie
Fetal position is transverse, with the fetal long axis oblique or perpendicular rather than parallel to the maternal long axis. Transverse lie is often accompanied by shoulder presentation, which requires cesarean delivery.
Breech presentation
There are several types of breech presentation.
Frank breech: The fetal hips are flexed, and the knees extended (pike position).
Complete breech: The fetus seems to be sitting with hips and knees flexed.
Single or double footling presentation: One or both legs are completely extended and present before the buttocks.
Types of breech presentations
Breech presentation makes delivery difficult ,primarily because the presenting part is a poor dilating wedge. Having a poor dilating wedge can lead to incomplete cervical dilation, because the presenting part is narrower than the head that follows. The head, which is the part with the largest diameter, can then be trapped during delivery.
Additionally, the trapped fetal head can compress the umbilical cord if the fetal umbilicus is visible at the introitus, particularly in primiparas whose pelvic tissues have not been dilated by previous deliveries. Umbilical cord compression may cause fetal hypoxemia.

Predisposing factors for breech presentation include
Preterm labor
Uterine abnormalities
Fetal anomalies
If delivery is vaginal, breech presentation may increase risk of
Umbilical cord prolapse
Birth trauma
Perinatal death

Face or brow presentation
In face presentation, the head is hyperextended, and position is designated by the position of the chin (mentum). When the chin is posterior, the head is less likely to rotate and less likely to deliver vaginally, necessitating cesarean delivery.
Brow presentation usually converts spontaneously to vertex or face presentation.
Occiput posterior position
The most common abnormal position is occiput posterior.
The fetal neck is usually somewhat deflexed; thus, a larger diameter of the head must pass through the pelvis.
Progress may arrest in the second phase of labor. Operative vaginal delivery or cesarean delivery is often required.
Position and Presentation of the Fetus
If a fetus is in the occiput posterior position, operative vaginal delivery or cesarean delivery is often required.
In breech presentation, the presenting part is a poor dilating wedge, which can cause the head to be trapped during delivery, often compressing the umbilical cord.
For breech presentation, usually do cesarean delivery at 39 weeks or during labor, but external cephalic version is sometimes successful before labor, usually at 37 or 38 weeks.

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
- Cookie Preferences

- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
What Is Cephalic Position?
The ideal fetal position for labor and delivery
- Why It's Best
Risks of Other Positions
- Determining Position
- Turning a Fetus
The cephalic position is when a fetus is head down when it is ready to enter the birth canal. This is one of a few variations of how a fetus can rest in the womb and is considered the ideal one for labor and delivery.
About 96% of babies are born in the cephalic position. Most settle into it between the 32nd and 36th weeks of pregnancy . Your healthcare provider will monitor the fetus's position during the last weeks of gestation to ensure this has happened by week 36.
If the fetus is not in the cephalic position at that point, the provider may try to turn it. If this doesn't work, some—but not all—practitioners will attempt to deliver vaginally, while others will recommend a Cesarean (C-section).
Getty Images
Why Is the Cephalic Position Best?
During labor, contractions dilate the cervix so the fetus has adequate room to come through the birth canal. The cephalic position is the easiest and safest way for the baby to pass through the birth canal.
If the fetus is in a noncephalic position, delivery becomes more challenging. Different fetal positions have a range of difficulties and varying risks.
A small percentage of babies present in noncephalic positions. This can pose risks both to the fetus and the mother, and make labor and delivery more challenging. It can also influence the way in which someone can deliver.
A fetus may actually find itself in any of these positions throughout pregnancy, as the move about the uterus. But as they grow, there will be less room to tumble around and they will settle into a final position.
It is at this point that noncephalic positions can pose significant risks.
Cephalic Posterior
A fetus may also present in an occiput or cephalic posterior position. This means they are positioned head down, but they are facing the abdomen instead of the back.
This position is also nicknamed "sunny-side up."
Presenting this way increases the chance of a painful and prolonged delivery.
There are three different types of breech fetal positioning:
- Frank breech: The legs are up with the feet near the head.
- Footling breech: One or both legs is lowered over the cervix.
- Complete breech: The fetus is bottom-first with knees bent.
A vaginal delivery is most times a safe way to deliver. But with breech positions, a vaginal delivery can be complicated.
When a baby is born in the breech position, the largest part—its head—is delivered last. This can result in them getting stuck in the birth canal (entrapped). This can cause injury or death.
The umbilical cord may also be damaged or slide down into the mouth of the womb, which can reduce or cut off the baby's oxygen supply.
Some providers are still comfortable performing a vaginal birth as long as the fetus is doing well. But breech is always a riskier delivery position compared with the cephalic position, and most cases require a C-section.
Likelihood of a Breech Baby
You are more likely to have a breech baby if you:
- Go into early labor before you're full term
- Have an abnormally shaped uterus, fibroids , or too much amniotic fluid
- Are pregnant with multiples
- Have placenta previa (when the placenta covers the cervix)
Transverse Lie
In transverse lie position, the fetus is presenting sideways across the uterus rather than vertically. They may be:
- Down, with the back facing the birth canal
- With one shoulder pointing toward the birth canal
- Up, with the hands and feet facing the birth canal
If a transverse lie is not corrected before labor, a C-section will be required. This is typically the case.
Determining Fetal Position
Your healthcare provider can determine if your baby is in cephalic presentation by performing a physical exam and ultrasound.
In the final weeks of pregnancy, your healthcare provider will feel your lower abdomen with their hands to assess the positioning of the baby. This includes where the head, back, and buttocks lie
If your healthcare provider senses that the fetus is in a breech position, they can use ultrasound to confirm their suspicion.
Turning a Fetus So They Are in Cephalic Position
External cephalic version (ECV) is a common, noninvasive procedure to turn a breech baby into cephalic position while it's still in the uterus.
This is only considered if a healthcare provider monitors presentation progress in the last trimester and notices that a fetus is maintaining a noncephalic position as your delivery date approaches.
External Cephalic Version (ECV)
ECV involves the healthcare provider applying pressure to your stomach to turn the fetus from the outside. They will attempt to rotate the head forward or backward and lift the buttocks in an upward position. Sometimes, they use ultrasound to help guide the process.
The best time to perform ECV is about 37 weeks of pregnancy. Afterward, the fetal heart rate will be monitored to make sure it’s within normal levels. You should be able to go home after having ECV done.
ECV has a 50% to 60% success rate. However, even if it does work, there is still a chance the fetus will return to the breech position before birth.
Natural Methods For Turning a Fetus
There are also natural methods that can help turn a fetus into cephalic position. There is no medical research that confirms their efficacy, however.
- Changing your position: Sometimes a fetus will move when you get into certain positions. Two specific movements that your provider may recommend include: Getting on your hands and knees and gently rocking back and forth. Another you could try is pushing your hips up in the air while laying on your back with your knees bent and feet flat on the floor (bridge pose).
- Playing stimulating sounds: Fetuses gravitate to sound. You may be successful at luring a fetus out of breech position by playing music or a recording of your voice near your lower abdomen.
- Chiropractic care: A chiropractor can try the Webster technique. This is a specific chiropractic analysis and adjustment which enables chiropractors to establish balance in the pregnant person's pelvis and reduce undue stress to the uterus and supporting ligaments.
- Acupuncture: This is a considerably safe way someone can try to turn a fetus. Some practitioners incorporate moxibustion—the burning of dried mugwort on certain areas of the body—because they believe it will enhance the chances of success.
A Word From Verywell
While most babies are born in cephalic position at delivery, this is not always the case. And while some fetuses can be turned, others may be more stubborn.
This may affect your labor and delivery wishes. Try to remember that having a healthy baby, and staying well yourself, are your ultimate priorities. That may mean diverting from your best laid plans.
Speaking to your healthcare provider about turning options and the safest route of delivery may help you adjust to this twist and feel better about how you will move ahead.
Glezerman M. Planned vaginal breech delivery: current status and the need to reconsider . Expert Rev Obstet Gynecol. 2012;7(2):159-166. doi:10.1586/eog.12.2
Cleveland Clinic. Fetal positions for birth .
MedlinePlus. Breech birth .
UT Southwestern Medical Center. Can you turn a breech baby around?
The American College of Obstetricians and Gynecologists. If your baby is breech .
Roecker CB. Breech repositioning unresponsive to Webster technique: coexistence of oligohydramnios . Journal of Chiropractic Medicine . 2013;12(2):74-78. doi:10.1016/j.jcm.2013.06.003
By Cherie Berkley, MS Berkley is a journalist with a certification in global health from Johns Hopkins University and a master's degree in journalism.

- Mammary Glands
- Fallopian Tubes
- Supporting Ligaments
- Reproductive System
- Gametogenesis
- Placental Development
- Maternal Adaptations
- Menstrual Cycle
- Antenatal Care
- Small for Gestational Age
- Large for Gestational Age
- RBC Isoimmunisation
- Prematurity
- Prolonged Pregnancy
- Multiple Pregnancy
- Miscarriage
- Recurrent Miscarriage
- Ectopic Pregnancy
- Hyperemesis Gravidarum
- Gestational Trophoblastic Disease
- Breech Presentation
- Abnormal lie, Malpresentation and Malposition
- Oligohydramnios
- Polyhydramnios
- Placenta Praevia
- Placental Abruption
- Pre-Eclampsia
- Gestational Diabetes
- Headaches in Pregnancy
- Haematological
- Obstetric Cholestasis
- Thyroid Disease in Pregnancy
- Epilepsy in Pregnancy
- Induction of Labour
- Operative Vaginal Delivery
- Prelabour Rupture of Membranes
- Caesarean Section
- Shoulder Dystocia
- Cord Prolapse
- Uterine Rupture
- Amniotic Fluid Embolism
- Primary PPH
- Secondary PPH
- Psychiatric Disease
- Postpartum Contraception
- Breastfeeding Problems
- Primary Dysmenorrhoea
- Amenorrhoea and Oligomenorrhoea
- Heavy Menstrual Bleeding
- Endometriosis
- Endometrial Cancer
- Adenomyosis
- Cervical Polyps
- Cervical Ectropion
- Cervical Intraepithelial Neoplasia + Cervical Screening
- Cervical Cancer
- Polycystic Ovary Syndrome (PCOS)
- Ovarian Cysts & Tumours
- Urinary Incontinence
- Genitourinary Prolapses
- Bartholin's Cyst
- Lichen Sclerosus
- Vulval Carcinoma
- Introduction to Infertility
- Female Factor Infertility
- Male Factor Infertility
- Female Genital Mutilation
- Barrier Contraception
- Combined Hormonal
- Progesterone Only Hormonal
- Intrauterine System & Device
- Emergency Contraception
- Pelvic Inflammatory Disease
- Genital Warts
- Genital Herpes
- Trichomonas Vaginalis
- Bacterial Vaginosis
- Vulvovaginal Candidiasis
- Obstetric History
- Gynaecological History
- Sexual History
- Obstetric Examination
- Speculum Examination
- Bimanual Examination
- Amniocentesis
- Chorionic Villus Sampling
- Hysterectomy
- Endometrial Ablation
- Tension-Free Vaginal Tape
- Contraceptive Implant
- Fitting an IUS or IUD
Abnormal Fetal lie, Malpresentation and Malposition
Original Author(s): Anna Mcclune Last updated: 1st December 2018 Revisions: 12
- 1 Definitions
- 2 Risk Factors
- 3.2 Presentation
- 3.3 Position
- 4 Investigations
- 5.1 Abnormal Fetal Lie
- 5.2 Malpresentation
- 5.3 Malposition
The lie, presentation and position of a fetus are important during labour and delivery.
In this article, we will look at the risk factors, examination and management of abnormal fetal lie, malpresentation and malposition.
Definitions
- Longitudinal, transverse or oblique
- Cephalic vertex presentation is the most common and is considered the safest
- Other presentations include breech, shoulder, face and brow
- Usually the fetal head engages in the occipito-anterior position (the fetal occiput facing anteriorly) – this is ideal for birth
- Other positions include occipito-posterior and occipito-transverse.
Note: Breech presentation is the most common malpresentation, and is covered in detail here .
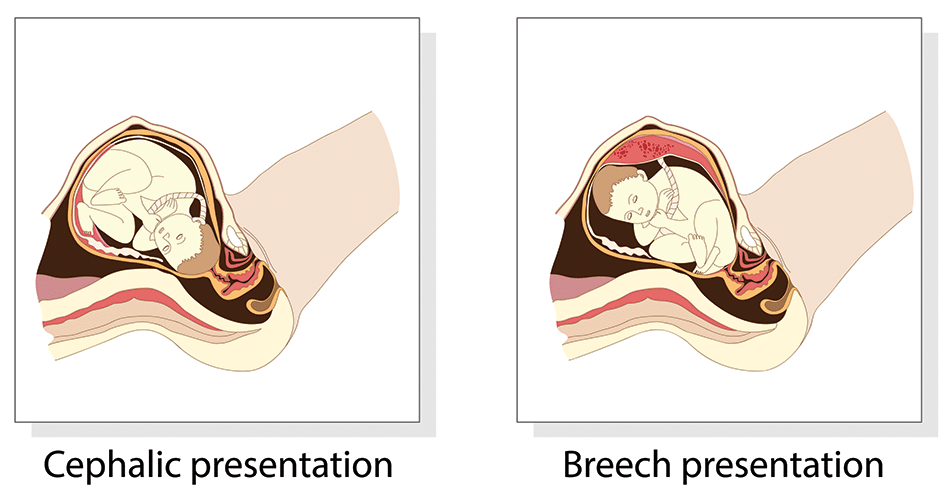
Fig 1 – The two most common fetal presentations: cephalic and breech.
Risk Factors
The risk factors for abnormal fetal lie, malpresentation and malposition include:
- Multiple pregnancy
- Uterine abnormalities (e.g fibroids, partial septate uterus)
- Fetal abnormalities
- Placenta praevia
- Primiparity
Identifying Fetal Lie, Presentation and Position
The fetal lie and presentation can usually be identified via abdominal examination. The fetal position is ascertained by vaginal examination.
For more information on the obstetric examination, see here .
- Face the patient’s head
- Place your hands on either side of the uterus and gently apply pressure; one side will feel fuller and firmer – this is the back, and fetal limbs may feel ‘knobbly’ on the opposite side
Presentation
- Palpate the lower uterus (above the symphysis pubis) with the fingers of both hands; the head feels hard and round (cephalic) and the bottom feels soft and triangular (breech)
- You may be able to gently push the fetal head from side to side
The fetal lie and presentation may not be possible to identify if the mother has a high BMI, if she has not emptied her bladder, if the fetus is small or if there is polyhydramnios .
During labour, vaginal examination is used to assess the position of the fetal head (in a cephalic vertex presentation). The landmarks of the fetal head, including the anterior and posterior fontanelles, indicate the position.
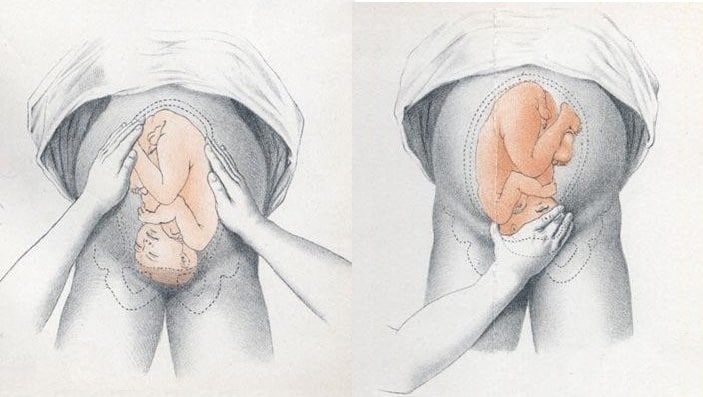
Fig 2 – Assessing fetal lie and presentation.
Investigations
Any suspected abnormal fetal lie or malpresentation should be confirmed by an ultrasound scan . This could also demonstrate predisposing uterine or fetal abnormalities.
Abnormal Fetal Lie
If the fetal lie is abnormal, an external cephalic version (ECV) can be attempted – ideally between 36 and 38 weeks gestation.
ECV is the manipulation of the fetus to a cephalic presentation through the maternal abdomen.
It has an approximate success rate of 50% in primiparous women and 60% in multiparous women. Only 8% of breech presentations will spontaneously revert to cephalic in primiparous women over 36 weeks gestation.
Complications of ECV are rare but include fetal distress , premature rupture of membranes, antepartum haemorrhage (APH) and placental abruption. The risk of an emergency caesarean section (C-section) within 24 hours is around 1 in 200.
ECV is contraindicated in women with a recent APH, ruptured membranes, uterine abnormalities or a previous C-section .
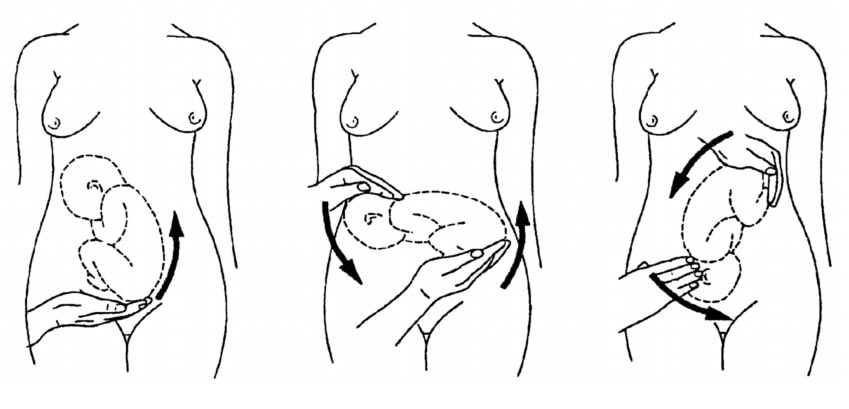
Fig 3 – External cephalic version.
Malpresentation
The management of malpresentation is dependent on the presentation.
- Breech – attempt ECV before labour, vaginal breech delivery or C-section
- Brow – a C-section is necessary
- If the chin is anterior (mento-anterior) a normal labour is possible; however, it is likely to be prolonged and there is an increased risk of a C-section being required
- If the chin is posterior (mento-posterior) then a C-section is necessary
- Shoulder – a C-section is necessary

Malposition
90% of malpositions spontaneously rotate to occipito-anterior as labour progresses. If the fetal head does not rotate, rotation and operative vaginal delivery can be attempted. Alternatively a C-section can be performed.
- Usually the fetal head engages in the occipito-anterior position (the fetal occiput facing anteriorly) - this is ideal for birth
If the fetal lie is abnormal, an external cephalic version (ECV) can be attempted - ideally between 36 and 38 weeks gestation.
- Breech - attempt ECV before labour, vaginal breech delivery or C-section
Found an error? Is our article missing some key information? Make the changes yourself here!
Once you've finished editing, click 'Submit for Review', and your changes will be reviewed by our team before publishing on the site.
We use cookies to improve your experience on our site and to show you relevant advertising. To find out more, read our privacy policy .
Privacy Overview
The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations
Affiliations.
- 1 Department of Obstetrics and Gynecology, Salus Hospital, Brindisi, Italy. Electronic address: [email protected].
- 2 Department of Obstetrics and Gynecology, Santa Maria Hospital, GVM Care and Research, Bari, Italy, Laboratory of Human Anatomy, Department of Applied Mathematics, Moscow Institute of Physics and Technology (State University), Moscow, Russia.
- 3 Department of Obstetrics and Gynecology, Division of Experimental Endoscopic Surgery, Imaging, Technology, and Minimally Invasive Therapy, Laboratory of Human Anatomy, Department of Applied Mathematics, Moscow Institute of Physics and Technology (State University), Moscow, Russia; Department of Obstetrics and Gynecology, Vito Fazzi Hospital, Lecce, Italy.
- PMID: 29408571
- DOI: 10.1016/j.ajog.2018.01.028
Publication types
- Labor Presentation*
- Version, Fetal*

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

- Health Topics
- Drugs & Supplements
- Medical Tests
- Medical Encyclopedia
- About MedlinePlus
- Customer Support
Your baby in the birth canal
During labor and delivery, your baby must pass through your pelvic bones to reach the vaginal opening. The goal is to find the easiest way out. Certain body positions give the baby a smaller shape, which makes it easier for your baby to get through this tight passage.
The best position for the baby to pass through the pelvis is with the head down and the body facing toward the mother's back. This position is called occiput anterior.
Information
Certain terms are used to describe your baby's position and movement through the birth canal.
FETAL STATION
Fetal station refers to where the presenting part is in your pelvis.
- The presenting part. The presenting part is the part of the baby that leads the way through the birth canal. Most often, it is the baby's head, but it can be a shoulder, the buttocks, or the feet.
- Ischial spines. These are bone points on the mother's pelvis. Normally the ischial spines are the narrowest part of the pelvis.
- 0 station. This is when the baby's head is even with the ischial spines. The baby is said to be "engaged" when the largest part of the head has entered the pelvis.
- If the presenting part lies above the ischial spines, the station is reported as a negative number from -1 to -5.
In first-time moms, the baby's head may engage by 36 weeks into the pregnancy. However, engagement may happen later in the pregnancy, or even during labor.
This refers to how the baby's spine lines up with the mother's spine. Your baby's spine is between their head and tailbone.
Your baby will most often settle into a position in the pelvis before labor begins.
- If your baby's spine runs in the same direction (parallel) as your spine, the baby is said to be in a longitudinal lie. Nearly all babies are in a longitudinal lie.
- If the baby is sideways (at a 90-degree angle to your spine), the baby is said to be in a transverse lie.
FETAL ATTITUDE
The fetal attitude describes the position of the parts of your baby's body.
The normal fetal attitude is commonly called the fetal position.
- The head is tucked down to the chest.
- The arms and legs are drawn in towards the center of the chest.
Abnormal fetal attitudes include a head that is tilted back, so the brow or the face presents first. Other body parts may be positioned behind the back. When this happens, the presenting part will be larger as it passes through the pelvis. This makes delivery more difficult.
DELIVERY PRESENTATION
Delivery presentation describes the way the baby is positioned to come down the birth canal for delivery.
The best position for your baby inside your uterus at the time of delivery is head down. This is called cephalic presentation.
- This position makes it easier and safer for your baby to pass through the birth canal. Cephalic presentation occurs in about 97% of deliveries.
- There are different types of cephalic presentation, which depend on the position of the baby's limbs and head (fetal attitude).
If your baby is in any position other than head down, your doctor may recommend a cesarean delivery.
Breech presentation is when the baby's bottom is down. Breech presentation occurs about 3% of the time. There are a few types of breech:
- A complete breech is when the buttocks present first and both the hips and knees are flexed.
- A frank breech is when the hips are flexed so the legs are straight and completely drawn up toward the chest.
- Other breech positions occur when either the feet or knees present first.
The shoulder, arm, or trunk may present first if the fetus is in a transverse lie. This type of presentation occurs less than 1% of the time. Transverse lie is more common when you deliver before your due date, or have twins or triplets.
CARDINAL MOVEMENTS OF LABOR
As your baby passes through the birth canal, the baby's head will change positions. These changes are needed for your baby to fit and move through your pelvis. These movements of your baby's head are called cardinal movements of labor.
- This is when the widest part of your baby's head has entered the pelvis.
- Engagement tells your health care provider that your pelvis is large enough to allow the baby's head to move down (descend).
- This is when your baby's head moves down (descends) further through your pelvis.
- Most often, descent occurs during labor, either as the cervix dilates or after you begin pushing.
- During descent, the baby's head is flexed down so that the chin touches the chest.
- With the chin tucked, it is easier for the baby's head to pass through the pelvis.
Internal Rotation
- As your baby's head descends further, the head will most often rotate so the back of the head is just below your pubic bone. This helps the head fit the shape of your pelvis.
- Usually, the baby will be face down toward your spine.
- Sometimes, the baby will rotate so it faces up toward the pubic bone.
- As your baby's head rotates, extends, or flexes during labor, the body will stay in position with one shoulder down toward your spine and one shoulder up toward your belly.
- As your baby reaches the opening of the vagina, usually the back of the head is in contact with your pubic bone.
- At this point, the birth canal curves upward, and the baby's head must extend back. It rotates under and around the pubic bone.
External Rotation
- As the baby's head is delivered, it will rotate a quarter turn to be in line with the body.
- After the head is delivered, the top shoulder is delivered under the pubic bone.
- After the shoulder, the rest of the body is usually delivered without a problem.
Alternative Names
Shoulder presentation; Malpresentations; Breech birth; Cephalic presentation; Fetal lie; Fetal attitude; Fetal descent; Fetal station; Cardinal movements; Labor-birth canal; Delivery-birth canal
Barth WH. Malpresentations and malposition. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe's Obstetrics: Normal and Problem Pregnancies . 8th ed. Philadelphia, PA: Elsevier; 2021:chap 17.
Kilpatrick SJ, Garrison E, Fairbrother E. Normal labor and delivery. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe's Obstetrics: Normal and Problem Pregnancies . 8th ed. Philadelphia, PA: Elsevier; 2021:chap 11.
Review Date 11/10/2022
Updated by: John D. Jacobson, MD, Department of Obstetrics and Gynecology, Loma Linda University School of Medicine, Loma Linda, CA. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.
Related MedlinePlus Health Topics
- Childbirth Problems
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Pregnancy and Fetal Development: Cephalic Presentation and Other Descriptive Ultrasonographic Findings from Clinically Healthy Bottlenose Dolphins ( Tursiops truncatus ) under Human Care
Pietro saviano, letizia fiorucci, francesco grande, roberto macrelli, alessandro troisi, angela polisca, riccardo orlandi.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Received 2020 Apr 3; Accepted 2020 May 22; Collection date 2020 May.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/ ).
Simple Summary
Ultrasound data are vital for monitoring and detecting problems in pregnancies, and although there is a significant amount of data for domestic species, data for marine mammals are scarce. In domestic species, the use of ultrasonography to monitor a pregnancy usually has the following aims: fetal movements, fetal heart rates, measurements of the skull and the thorax for the prediction of the birth date interval, the morphological aspects of the fetal organs, the appearance of the umbilical cord, and the placentation. The purpose of this study is to provide to the clinician additional relevant data on fetal development and well-being during a dolphin pregnancy that may also be useful for wild population monitoring. This study is the result of a retrospective analysis of 192 ultrasound scans over 10 years that, for the first time, describes the sonographic findings of the bottlenose dolphin organogenesis and their correlation with the stage of pregnancy, as well as the calf presentation at birth, according to its position within the uterus, and moreover a complete literature review.
Ultrasonography is widely used in veterinary medicine for the diagnosis of pregnancy, and can also be used to monitor abnormal pregnancies, embryonic resorption, or fetal abortion. Ultrasonography plays an important role in modern-day cetacean preventative medicine because it is a non-invasive technique, it is safe for both patient and operator, and it can be performed routinely using trained responses that enable medical procedures. Reproductive success is an important aspect of dolphin population health, as it is an indicator of the future trajectory of the population. The aim of this study is to provide additional relevant data on feto-maternal ultrasonographic monitoring in bottlenose dolphin ( Tursiops truncatus ) species, for both the clinicians and for in situ population studies. From 2009 to 2019, serial ultrasonographic exams of 11 healthy bottlenose dolphin females kept under human care were evaluated over the course of 16 pregnancies. A total of 192 ultrasound exams were included in the study. For the first time, the sonographic findings of the bottlenose dolphin organogenesis and their correlation with the stage of pregnancy are described. Furthermore, this is the first report that forecasts the cephalic presentation of the calf at birth, according to its position within the uterus.
Keywords: ultrasonography, bottlenose dolphins ( Tursiops truncatus ), normal appearance, pregnancy, fetal position, fetal development, fetal welfare
1. Introduction
A preventative medicine program is one of the key factors in health evaluation for ensuring the welfare of dolphins under human care. Ultrasonography (US) plays an important role in modern-day cetacean preventative medicine because it is a non-invasive technique, it is safe for both patient and operator, and it can be performed routinely using trained responses that enable medical procedures [ 1 , 2 ]. Ultrasound data are vital for monitoring and detecting problems in pregnancies, and while there is a significant amount of data for domestic species [ 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 ], data for marine mammals are scarce [ 12 , 13 , 14 ]. In domestic species, US is often used for the early identification of fetal pathologies and reabsorption, for example, altered/slowed down growth, loss of fetal fluids with decrease in the size of the vesicle and alteration of its shape, absence of heartbeat, blurring of margins and alteration of normal fetal anatomy, and detachment of the placenta from the uterine wall [ 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 ]. In marine mammal medicine, in the past, US was used only to confirm a pregnancy; however, an increasing number of facilities are now monitoring gestation by US, and further studies are now emerging with additional reference ranges.
Reproductive success is an important aspect of dolphin population health, as it is an indicator of the future trajectory of the population [ 15 , 16 ]. Pregnancy determination for wild dolphins, including differentiation of pregnancy stage, is possible during capture–release health assessments through application of diagnostic ultrasound to evaluate fetal development and viability, estimate gestational age, and measure anatomical structures [ 15 , 16 ]. The use of ultrasound for systematic pregnancy determination provides a useful tool for measuring an important component of reproductive success. Application of this approach for conservation of wild populations benefits from the establishment of baseline values, such as the estimates provided herein for the reference population of bottlenose dolphins [ 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ].
The brightness (B)-mode technique is based on a process in which focused beams are iteratively sent into the body and the received waves are used to form an image scan-line, covering line-by-line the region of interest. The use of it to monitor bottlenose dolphin pregnancy dates back to the early 1990s, when Williamson et al. (1990) diagnosed pregnancy in a limited number of subjects ( n = 4), at approximately the fourth month of gestation, being able to visualize fetal movements and fluids. Periodic monitoring allowed the authors to observe fetal vitality through the observation of cardiac mechanics and to carry out measurements both of the cranial diameter (on the front–occipital axis) and of the thoracic diameter, obtaining linear growth diagrams [ 23 ]. The authors showed difficulties in obtaining clear ultrasonographic images, both because the dolphins were uncooperative during the exam due to scarce training, and due to the features of the transducers used at the time. As regards the positioning of the transducer in the first months of gestation, the midline between the genital opening and the navel was used as a landmark, obtaining images in cross section, whereas in late pregnancy the probe was placed longitudinally, at 10–20 cm from the ventral midline [ 23 ]. Stone et al. (1999) observed a similar pattern of linear growth in bottlenose dolphins by measuring bi-parietal and thoracic fetal diameters from week 46 up to 1 week after delivery [ 24 ].
Lacave in her work developed an easy-to-use computer program to provide better birth prediction for regularly scanned dolphins during their gestation and to predict dolphin delivery dates, even with only one or two ultrasound scans of their animals [ 25 ]. Measurement of bi-parietal diameters is only possible when the head is distinguishable from the rest of the body; the head is presented ultrasonographically as a symmetrical ovoid structure and the bi-parietal diameters are measured where they reach their maximum amplitude [ 25 ]. For thoracic diameters, Lacave et al. (2004) used, as a reference point, the section where all cardiac chambers, symmetrically surrounded by the lungs, appear in the ultrasound image in the same section and where pectoral fins are also frequently visible. Lacave showed how the diameters of the skull increase more slowly than the thoracic diameters, and that the thoracic diameters represent the limit of accuracy in late pregnancy [ 25 ].
Sklansky et al. (2010) recognize the utility of fetal echocardiography as a safe technique able to evaluate the cardiovascular system in the bottlenose dolphin, especially in the period between the eighth and the ninth month of gestation. As in humans, this technique allows us to identify congenital cardiac anomalies [ 26 , 27 , 28 ] and to identify the possible causes of perinatal mortality risk associated with physiological abnormalities and cardiac hemodynamics [ 29 , 30 ]. In most cases, the optimal visualization of the fetal heart is obtained by positioning the pregnant female in lateral decubitus, homolaterally to the uterine horn in which the fetus is housed [ 26 , 27 ]. The optimal window is located near the maternal navel, proximal to the dorsal and caudal–ventral fin compared to the caudal fin. As expected, the cardiac dimensions increased with the approaching birth; passing from 3 to 6 cm of the 9th month up to 8–9 cm of the 10th month [ 26 , 27 ].
Recently, Ivancic et al. (2020) developed a protocol for feto-maternal ultrasonographic monitoring in bottlenose dolphins. In their work, a total of 203 US exams were performed during a 7-year period to monitor 16 pregnancies. The authors reported normal measurements and descriptive findings correlated with a positive outcome—fetal bi-parietal diameter, thoracic width in dorsal and transverse planes, thoracic height in a sagittal plane, aortic diameter, and blubber thickness all demonstrated a high correlation with date of gestation [ 21 ].
Umbilical cord accidents were diagnosed in the same dolphin in three consecutive pregnancies in a study by García-Párraga et al. (2014). The trans-abdominal ultrasound evaluation revealed the presence of a wrap of the umbilical cord around the fetal peduncle. All pregnancies ended in in utero death of fetuses and their expulsion [ 31 ]. In addition, an omphalocele (an abdominal wall defect at the base of the umbilical cord) in an approximately 16-week-old fetus was detected in the clinical case reported by Smith et al. (2013), thanks to the US prenatal examination. Color Doppler was utilized to study the blood flow within the omphalocele, as well as diagnose an associated anomaly of the umbilical cord, which contained three vessels instead of four [ 32 ]. Finally, the case of meconium aspiration syndrome (MAS) in a male neonate of bottlenose dolphin who died immediately after birth was reported by Tanaka et al. in 2014. At necropsy, a knot was found in the umbilical cord [ 33 ]. The lungs showed diffuse intra-alveolar edema, hyperemic congestion, and atelectasis due to meconium aspiration with mild inflammatory cell infiltration. Although the exact cause of MAS in this case was unknown, fetal hypoxia due possibly to the umbilical knot might have been associated with MAS, which is the first report in dolphins. MAS due to perinatal asphyxia should be taken into account as a possible cause of neonatal mortality and stillbirth of dolphin calves [ 33 ].
Valuable information about the ontogeny of the body systems and their development in cetacean species, the precise time intervals of such developments, and any distinctive growth trajectories are basically unknown, because descriptions are based on occasional recoveries of embryos and fetuses and it very difficult to acquire complete ontogenic series [ 34 , 35 , 36 , 37 , 38 , 39 ]. Fetal abnormalities have been observed in cetaceans, as in other species. Brook, in 1994, for the first time, described the ultrasound diagnosis of an anencephaly, a lethal form of cephalic axial skeletal-neuronal disraphism, in a Tursiops aduncus fetus [ 34 ]. The fetal skull base appeared disproportionately small, and the cranium could not be identified. Fetal heart motion could be detected throughout the gestation. After a period of 357 days after conception, an uncomplicated, spontaneous delivery produced a stillborn male anencephalic calf. The abnormality was associated with various factors including respiratory tract infection in early gestation and folic acid deficiency. This case illustrates the ability of US to provide assessment of fetal morphology and growth, information not available by other means [ 34 ]. Ultrasonography proved to be appropriate to monitor fetal development, placenta and fetal membranes, and also to identify umbilical cord defects, thanks to the monitoring of its position and perfusion. This method has the advantage that it can identify abnormalities at early gestational stages [ 21 , 34 ], and that regular measurements allow monitoring of fetal growth and provide a more accurate prediction of expected delivery [ 25 ], so that adequate arrangements can be made in good time. The aim of this retrospective study was to provide additional relevant descriptive findings on feto-maternal ultrasonographic monitoring protocols in bottlenose dolphin species, thanks to the analysis of 192 ultrasound exams during a 10-year period. The data obtained may be very useful for the future clinical practice for managed population and in situ population studies, as it can be used to improve the understanding of the pathophysiology of reproductive failure.
2. Materials and Methods
2.1. study animals.
This study is the result of a retrospective analysis of 192 ultrasound scans obtained during the routine pregnancy check of bottlenose dolphins and from the marine mammal ultrasound consulting work in 11 different facilities over 10 years. All the examinations were included in the preventative medicine protocol of each facility, and no additional examinations were performed. From 2009 to 2019, serial ultrasonographic exams of 11 healthy bottlenose dolphin females (average age: 18 ± 7 years; min–max: 9 to 36 years) kept under human care were evaluated over the course of 16 pregnancies. Three dams were pluriparous. The calves born were 10 males and 6 females; of these, 2 females died 9 days after birth due to a respiratory disease. Neither case was that of cephalic birth. Inclusion criteria involved all pregnancies ended with the birth of alive calves that survived at least 48 h after the delivery. Exclusion criteria included any pre-existing health conditions that could have affected pregnancy, abortion, or any significant health conditions that occurred during pregnancy and required initiation of treatment by the attending clinician. A total of 192 ultrasound exams were included in the study. All examined animals were trained routinely for medical behaviors, including US. Lateral and ventral abdominal scanning was performed using seawater for acoustic coupling. For the study, the urogenital area was explored and the reproductive organs, ovaries, and uterus were evaluated. Uterine fluid echogenicity was assessed as anechoic, hypo-echoic, or hyper-echoic, as well as for the presence of echoic free-floating particles. Umbilical cord vasculature was assessed in cross section. Color Doppler confirmed vascular flow.
2.2. Ultrasonography: Instrumentation and Methodology
A portable SonoSite 180 Plus with a 2–5 MHz convex probe, an Esaote Mylab 25 gold with convex probe 2–5 MHz, an Aloka 900 with convex probe 2–5 MHz, and a General Electrics Logiq V2 with a 2–5 MHz curvilinear transducer were used to evaluate the dolphins during pregnancy. During the exam, the machine was covered with a transparent plastic bag to avoid accidental contact of the device with salt water. To avoid direct sunlight, a dark-colored bag was used to cover the instrument. The probe was waterproof. Acoustic gel was unnecessary because water provides an excellent medium through which to conduct ultrasound waves. Still images obtained were stored in DICOM (Digital Imaging and Communications in Medicine) format, whereas videos were also recorded using an external hard-disk.
2.3. Statistical Analyses
For each fetus, the time required for the appearance of each organ studied was analyzed. The quantitative variable “organ onset time” was introduced and a descriptive statistic expressed—average organ onset time with relative standard deviation. In addition, left or right ovulation rates and podalic or cephalic birth rates were analyzed. Medcalc, version 11.6.0.0, was used to analyze the data.
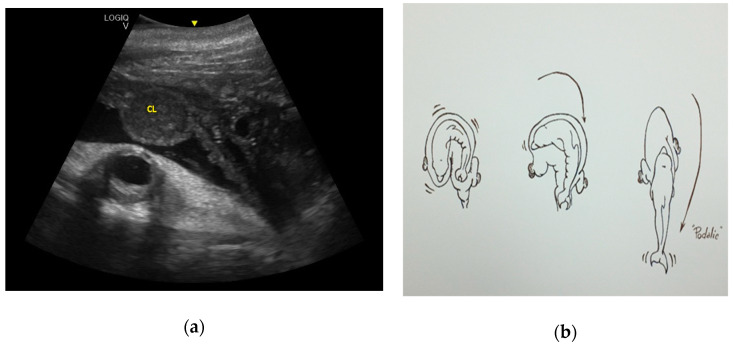
Considering the 16 pregnancies, the percentage of ovulation in the left ovary was 68.75%, whereas the ovulation in the right ovary was 31.25%, and it was of interest that two dams always ovulated in the right ovary. Maximum corpus luteum (CL) longitudinal diameter was 3.63 cm and transversal diameter was 3.02 cm ( Figure 1 ), even though the diameter may vary according to laterality. The results concerning the gonadal activity correspond to previous studies [ 18 , 19 , 20 ].
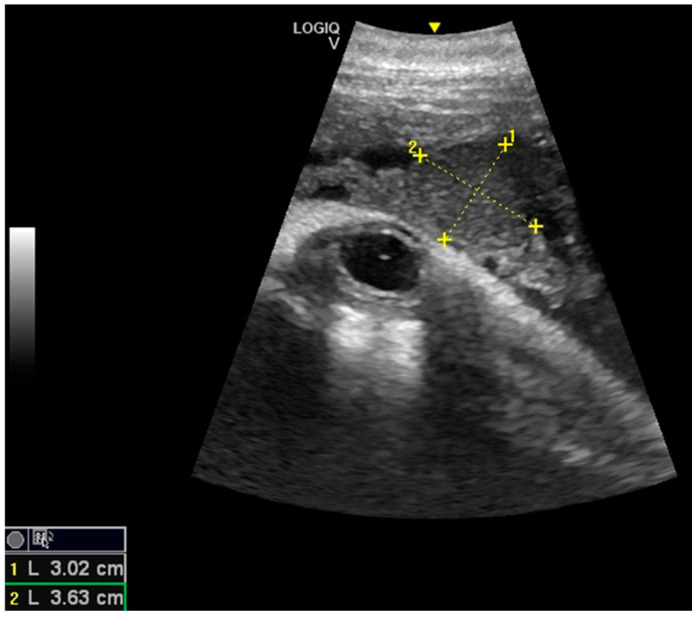
Corpus luteum (CL) with measurements.
The embryonic vesicle was recognizable at 29 ± 3 days post-ovulation on the apex of the uterine horn as a roundish structure with an average diameter of 1.21 cm with an anechoic content. In the following week, it was possible to recognize the embryo inside it as an elongated hyperechoic structure ( Figure 2 ).
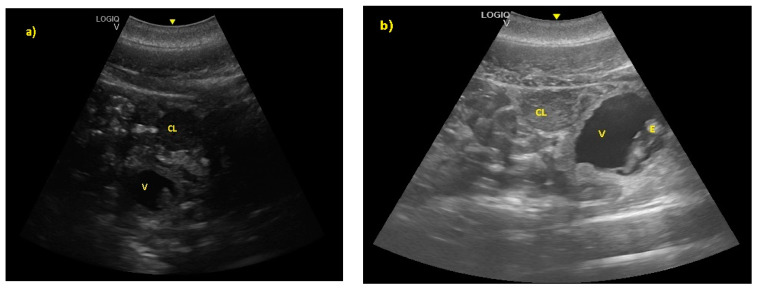
( a ) The embryonic vesicle at 38 ± 2 days post-ovulation appeared as a roundish structure with an anechoic content and a hyper-echoic structure inside (V), under the CL. ( b ) At 52 ± 3 days, the embryo was perfectly recognizable (E).
The distinction between head and trunk was visible starting from 68 ± 5 days after ovulation. From the 216 ± 5 days of gestation, measurements started to be hard to realize with accuracy. In fact, in the evaluations after this last period, the position/orientation of the fetus and its size meant that it was not possible to take reliable measurements thereafter. Starting from 68 ± 5 days after ovulation, the embryonic cardiac mechanisms were displayed as a point of maximum fluctuation of the echoes. The heart rate was measured because the cardiac mechanics became visible and remained constant between 155 and 198 bpm until the ninth month of pregnancy ( Figure 3 ). During the last 3 months, it stabilized at 140 bpm, to reach 85 ± 5 bpm in the last 2 weeks of gestation. The first abdominal organs to be visualized were the stomach and the urinary bladder (98 ± 3 and 110 ± 2 days of gestation, respectively), which appeared as distinct and anechoic cavities. It was also possible to recognize the eye as an anechoic cavitary structure ( Figure 4 ).
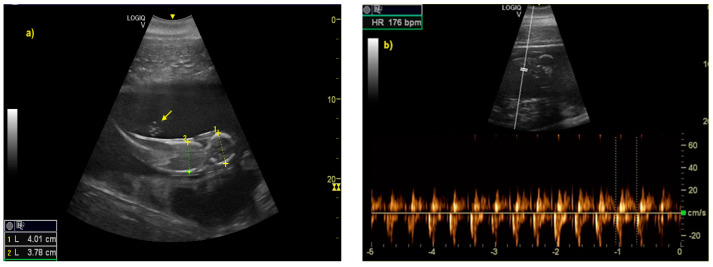
( a ) The distinction between head and trunk was clear between 68 ± 5, up to the 216 ± 5 day of gestation. It allowed measurement of bi-parietal diameters. The umbilical cord was already easy guessed (as indicated by yellow arrow). ( b ) The fetal heart rate (HR) was measured once the cardiac mechanics became visible.
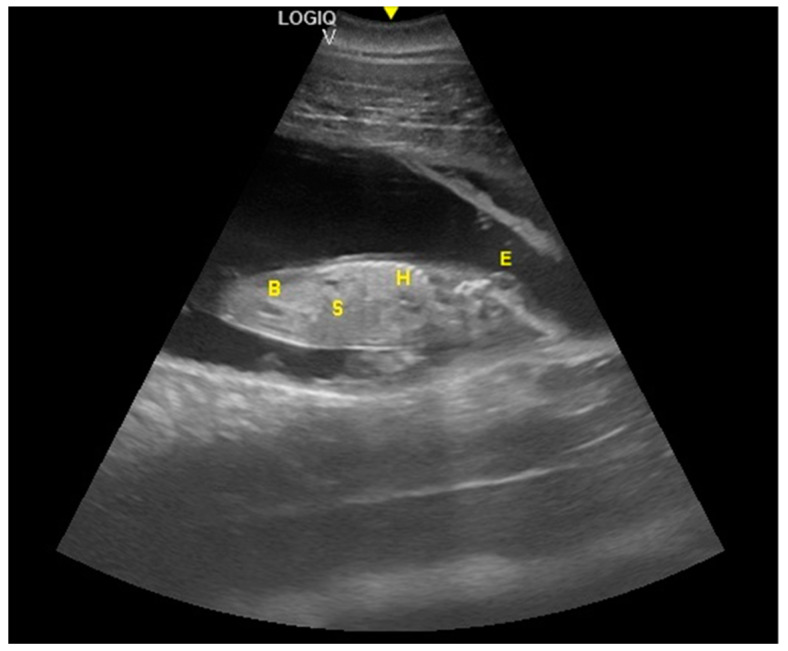
Fetal stomach (S) and fetal urinary bladder (B) were the first abdominal organs to be visualized, and appeared as distinct and anechoic cavities. The heart was recognizable as an anechoic cavity (H) and the embryonic cardiac mechanics were displayed as a point of maximum fluctuation of the echoes. The eye appeared as an anechoic cavitary structure (E).
The distinction between thorax and abdomen and, thus, the presence of the diaphragm, was seen at 92 ± 5 days of gestation. A clear distinction between lungs and liver was identified at 112 ± 5 days, whereas the ribs were visible at 153 ± 5 days. At 167 ± 3 days of gestation, the dorsal fin was visible as a hyper-echoic triangular structure in the dorsal portion of the trunk. During the same period, it was possible to identify the teeth. Even if the umbilical cord was easily guessed previously (as shown in Figure 3 ) from the 119 ± 6 day of gestation, it was clear as a hyper-echoic cordoniform structure, and it was important to evaluate the internal vascular components and the absence of knots or torsions until the birth ( Figure 5 ). Furthermore, it was possible to notice, between the 149th day and the 230th day of gestation, that the eye was open, the lens was visible, and eyelid movements were also detectable ( Figure 6 ).
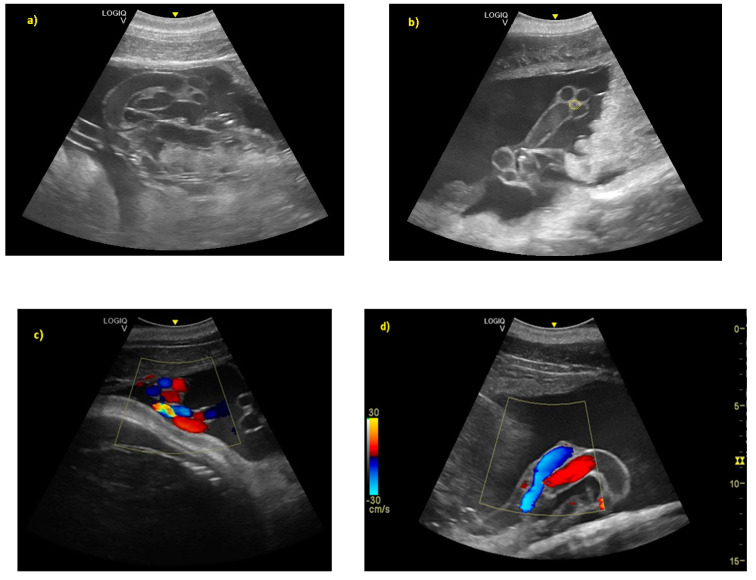
( a ) From day 119 ± 6 of gestation, the umbilical cord was clear as a hyper-echoic cordoniform structure. ( b ) The small hypo-echoic central cavity is the urachus, shown by the yellow circle. ( c ) Color Doppler demonstrating flow within the umbilical vasculature: ( d ) the umbilical veins (in blue), and the umbilical arteries (in red).
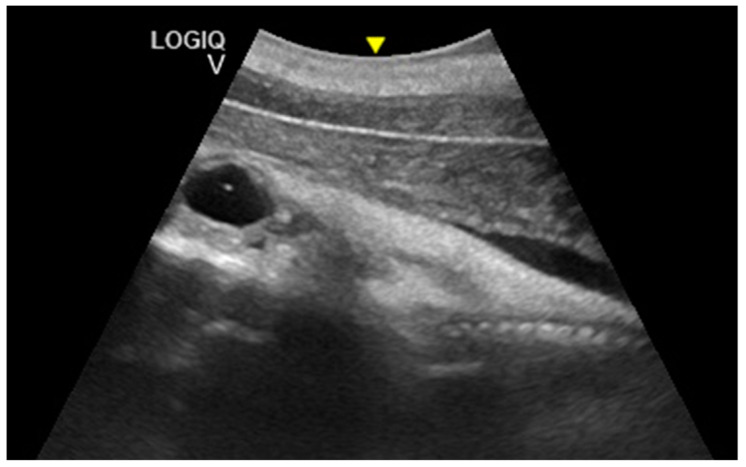
From day 110 ± 2 of gestation, the eye appeared as anechoic cavitary structures, whereas starting from the 149th day, the lens was also visible. At 167 ± 3 days of gestation, it was possible to identify the teeth.
The intestine was visualized at 189 ± 5 days of gestation. The cardiac chambers were visualized 194 ± 5 days after ovulation, and after about 3 weeks, the vascular structures (aorta and caudal vena cava) departing from them were visualized ( Figure 7 ).
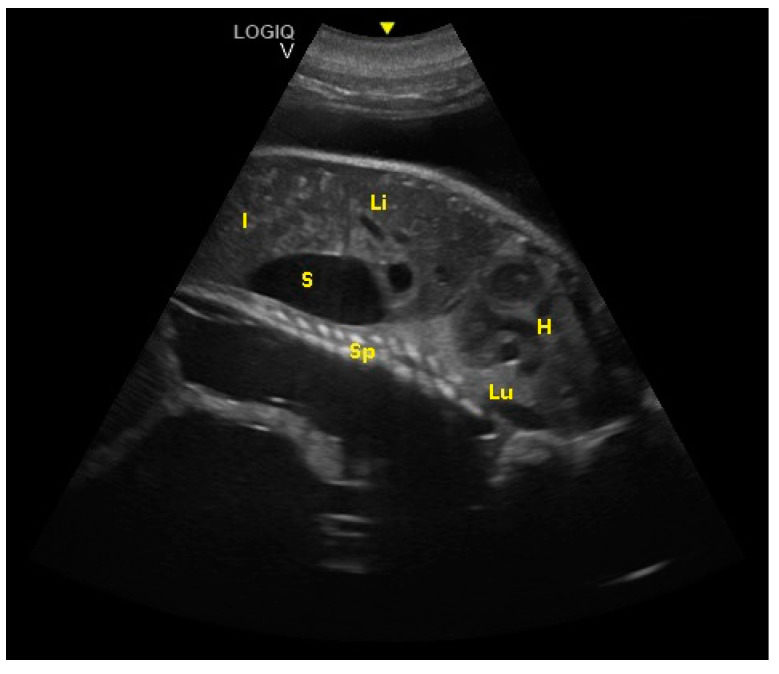
Fetal spinal cord (Sp), stomach (S), liver (Li), intestine (I), lungs (Lu), and heart (H) were visualized at 194 ± 5 days of gestation.
From the 230th day of gestation, it was possible to observe a ventral flexion of the caudal fin, a hyper-echoic structure, in contact with the abdomen. From the 245th to the 288th day of gestation, it was possible to recognize the thyroid and thymus ( Figure 8 ). During the last 3 months of gestation, it was possible to identify the kidneys.
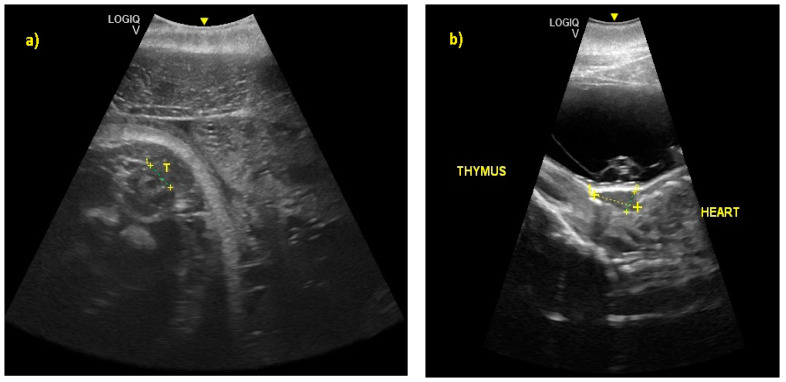
( a ) At 245 ± 2 to 288 ± 2 days of gestation, it was possible to recognize the thyroid (T) and ( b ) the thymus, respectively.
In addition, it was possible to identify the genitalia and sex the fetus (males have a tri-lobed structure, whereas females have an apricot-shaped structure, as shown in Figure 9 ).
( a ) An ultrasound image of female genitals (apricot-shaped) by SonoSite 180 Plus with a 2–5 MHz convex probe; ( b ) the same area caught with General Electrics Logiq V2, with a 2–5 MHz convex probe.
Starting from the 301th post-ovulation day until the end of pregnancy, it is very difficult to obtain images of the caudal portions of the fetus, due to the folded position it assumes within the maternal uterus. To the authors’ knowledge, this is the first study that reports the sonographic descriptive findings of the bottlenose dolphin organogenesis and their correlation with the stage of pregnancy as shown in Figure 10 ).
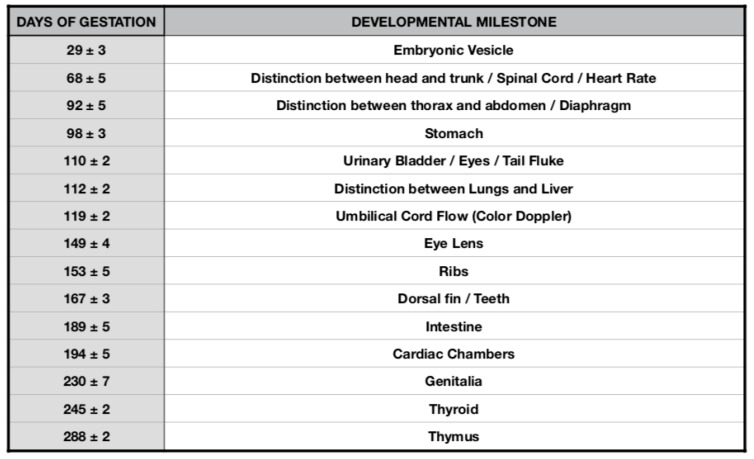
Bottlenose dolphin ( Tursiops truncatus ) organogenesis timeline table.
Allantoic and amniotic fluid are distinguishable in all examinations starting from the last trimester of pregnancy. Allantoic fluid appears as an anechoic fluid and the amniotic fluid appears as a hyper-echoic fluid, with an increasing number of echoic particles during the last period of pregnancy ( Figure 11 ).
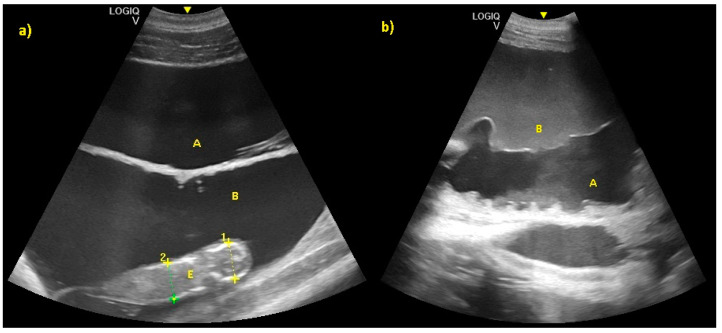
( a ) Allantoic (A) and amniotic fluid (B) in early stage of pregnancy. ( b ) Allantoic fluid appears as an anechoic fluid (A), and the amniotic fluid appears as a hyper-echoic fluid, with an increasing amount of echoic particles during the last period of pregnancy (B).
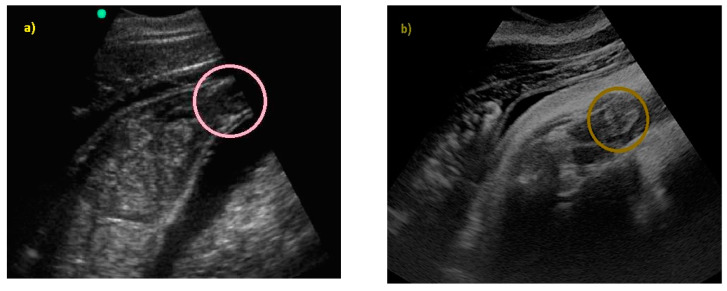
Finally, fetal position was evaluated throughout the gestational period. Considering the 16 pregnancies, the percentage of fluke presentation was 93.75%, whereas the head presentation was 6.25%. It is interesting to note that they were all successful cephalic deliveries. According to the results of the present study, it is possible to predict the calf presentation at birth, considering its position in the uterus during the last trimester. Using the CL as a reference, if the fetus skull is located close to the CL, it will have a podalic position at birth ( Figure 12 ).
( a ) Using the CL as a reference, if the fetus skull is located close to the CL, it will have a podalic position at birth, ( b ) as shown in the image.
However, if the tail fluke is located close to the CL, it will have a cephalic position at birth ( Figure 13 ).
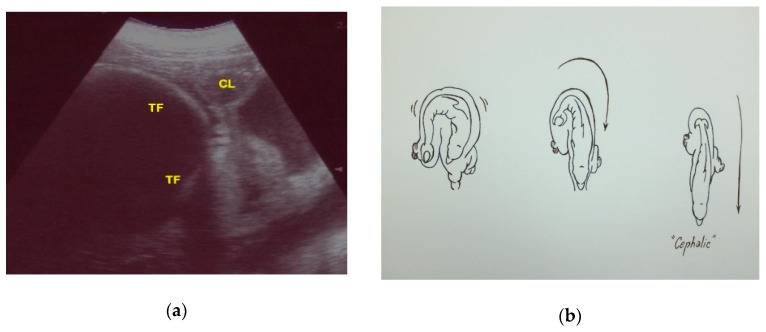
( a ) Using the CL as a reference, if the tail fluke (TF) is located close to the CL, it will have a cephalic position at birth, ( b ) as shown in the image.
To the authors’ knowledge, the present study reports the first bottlenose dolphin cephalic presentation documented by US.
4. Discussion
In the present retrospective study, we describe, by US, the genesis of the fetal organs (stomach, bladder, lungs, eye, intestine) and structures (column, appendices), and note that their appearance can be used to estimate the gestational period in the dolphin, in the absence of further information, as described in other species [ 40 ]. To the authors’ knowledge, this is the first study that reports the sonographic descriptive findings of bottlenose dolphin organogenesis and their correlation with the stage of pregnancy. The heart, while appearing visible already in the early stages of gestation, was displayed optimally between the eighth and the ninth month, allowing exclusion of the presence of detectable pathologies. As reported by Sedmera et al. (2003), there is a scant amount of data regarding heart development in cetaceans [ 41 ]. In their study, the authors examined samples from a unique collection of embryonic dolphin specimens macroscopically and histologically to learn more about normal cardiac development in the spotted dolphin. It was found that during the spotted dolphin’s 280 days of gestation, the heart completes septation at about 35 days. However, substantial trabecular compaction, which normally occurs in terrestrial mammals, as well as in humans at around the same time period, was delayed until day 60, when coronary circulation became established. By day 80, however, the heart gained a compacted, characteristic shape, with a single apex [ 41 ]. Considering the bottlenose dolphin’s 385 days of gestation, around 68 ± 5 days after ovulation, the results of the present study show how the heart was recognizable by US, and the embryonic cardiac mechanics were displayed as a point of maximum fluctuation of the echoes. An accurate index of fetus welfare is the fetal heart rate [ 26 , 27 , 28 , 29 , 30 ]. The heart rate was measured once the cardiac mechanics became visible, and remained constant between 155 and 198 bpm until the ninth month of pregnancy. During the last 3 months, it stabilized at 140 bpm, to reach 85 ± 5 bpm in the last 2 weeks of gestation.
The distinction between thorax and abdomen and, thus, the presence of the diaphragm, was seen between 92 ± 5 days of gestation, whereas the clear distinction between lungs and liver was identified at 112 ± 5 days of gestation. The correlation between the dolphin fetus organogenesis and the gestational period may help the clinician to identify the proximity of the delivery and any suspected anomaly in fetal development. In human literature, normal fetal lung and lung/liver echogenicity relationships facilitate early diagnosis of congenital bronchopulmonary abnormalities, and a decrease in the fetal lung/liver echogenicity has been shown to predict respiratory distress in newborns [ 21 ]. Regarding the two calves who died 9 days after birth due to a respiratory disease, there are no data that suggested lung alteration identified by ultrasound throughout the pregnancy. The two clinical cases had fetal development comparable with the other fetuses examined. The necropsy of both calves revealed postpartum pathological process as cause of death.
The umbilical cord has also been documented in ultrasound images, thus being able to exclude the presence of twisting nodes or echographically detectable defects of the same conditions that proved fatal in the bottlenose dolphins [ 31 , 32 , 33 ]. Color Doppler may be utilized to study the blood flow, as well as diagnose an associated anomaly of the umbilical cord, such as in the case of an onphalocele that contained three vessels instead of four [ 32 ]. It is worth mentioning that four vessels are needed as normal presentation, as shown in Figure 4 .
As described in other animals such as horses [ 42 ], during the last 20 days of pregnancy, amniotic fluid shows an increase of the echogenicity compared with allantoic fluid. Allantoic fluid has to remain completely anechoic during all the stages of pregnancy. Amniotic and allantoic fluids increase their volume during pregnancy, giving protection to the fetus. At the time of birth, allantoic fluid dilates the pathways of birth, whereas amniotic fluid has the function of lubricating the fetus [ 43 ]. The allantoic liquid is composed mainly of the urine of the fetus and has an anechoic aspect throughout the pregnancy [ 43 ]. The amniotic fluid envelops the fetus, and epithelial cells and meconium are accumulated in it, causing a progressive increase of its echogenicity compared to the allantoic liquid [ 43 ]. As confirmed by the present study, the difference in echogenicity between these two liquids reaches the highest level in the last 20 days of pregnancy because of the increase in the number of echogenic particles in the amniotic fluid. The authors hypothesize that these observations could be used to determine the approximate date of birth. Echogenic particles may indicate pathology and fetal stress when associated with infective debris, however, increased fluid echogenicity is not always predictive of pathology [ 21 ]. In all pregnancies evaluated in the present study, no changes in the allantoic fluid were observed throughout the gestation period. The results of the present study agree with recently published data by Ivancic et al. (2020) and provide further support for the newly established reference ranges [ 21 ].
Fetal position is another aspect to evaluate throughout gestation. In fact, keeping both the CL and the fetus in the same scan, it is possible to evaluate how, during the last three months of pregnancy, the relative topographical position of the head to the CL of the fetus is related to a podalic presentation at birth, which is the normal condition in this species (93.75% of cases). On the contrary, when the tail of the fetus is topographically related to the CL in the same image, it is related to a cephalic birth (6.25%). It is known that a cetacean fetus can change its position in the uterus during pregnancy, but usually moves into a tail-first position by the last months [ 36 , 44 ]. Head presentation of a fetus is reported for several delphinid species, and is thought to be an adaptation to the pregnancy and delivery in the aquatic environment [ 19 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 ]. It is repeatedly observed under human care—the rate of cephalic delivery was found to be 7% in killer whales ( Orcinus orca ) and 1.2% in bottlenose dolphins [ 19 ]. The labor of eight captive finless porpoises ( Neophocaena asiaeorientalis asiaeorientalis and Neophocaena asiaeorientalis sunameri ) were described by Deng et al. in 2019. The duration of parturition and the time of particular events in the parturition process were recorded for both podalic and cephalic births. Cephalic births were shorter than podalic births, and the calf position at birth did not seem to have a negative effect on its survival [ 47 ]. Successful cephalic delivery in a bottlenose dolphin under human care is described in detail by Essapian (1963)—stage 2 of the parturition lasted 22 min [ 49 ]. The result corresponds to that of the present study; in fact, the cephalic birth mentioned here also had a duration of 22 min. Even if it is indicated as a complicating factor, such cases are not referred to as pathologies [ 49 ]. In the wild, head presentation of a fetus was observed in a stranded dead white-beaked dolphin ( Lagenorhynchus albirostris ) [ 50 ]; it had ruptured the uterine wall, and thus the head presentation could be a cause of both dystocia and maternal death [ 50 ]. Numerous cases of cephalic delivery have been consistently reported for belugas ( Delphinapterus leucas ) in both nature and under human care; a rate of head-first delivery in captive belugas of 14% has been reported [ 51 , 52 , 53 ]. Head presentation seems to be more widespread in belugas than in any cetacean species. The cephalic presentation of a fetus in cetaceans is not a pathology but a natural variation. This idea is also supported by the documented cases of successful cephalic deliveries and by the occurrence of head presentations both in the wild and under human care. Nonetheless, head presentation of a fetus in a small cetacean can increase the risk of trauma or dystocia. In addition, a change of presentation at an advanced stage can be a pathology, such as that described by Baker and Martin in 1992 [ 54 ]. To the authors’ knowledge, the present study reports the first bottlenose dolphin cephalic presentation documented by US. An accurate and early ultrasound diagnosis of the presentation at delivery can help the clinician to adapt the intervention protocol to the needs of the case and, thus, minimize the risk of reproductive failure. The results of the present study reinforce the importance of monitoring health and fetal vitality throughout the duration of pregnancy and at the time of delivery by ultrasound.
In spite of the advantages provided by ultrasonographic surveys of dolphins, the procedure has some limitations compared to the same examination carried out in species commonly investigated in clinical practice [ 1 , 2 ]:
it must be conducted by an expert operator;
it depends on the features of the device;
animals must be trained for the voluntary medical behavior;
the animals must remain in water, which may not be safe for the instrumentation;
the external environment (and the light level, in particular) negatively affects results.
However, the difficulties listed above can be overcome, given the value of the information that can be gained, as the methods have been successfully applied to both under human care and wild dolphins.
5. Conclusions
This study adds further findings to the ultrasonographic monitoring protocol of bottlenose dolphin pregnancy. On the basis of the review of the literature, this is the first study that describes the sonographic data of bottlenose dolphin organogenesis and their correlation with the stage of pregnancy. As described in other species [ 40 ], these data could be used to estimate the gestational period in the dolphin in the absence of further information (such as measurements that allow derivation of linear growth diagrams, or a known ovulation date). These findings may be useful for investigations of stranding dolphins, providing data on prenatal development. These data are otherwise difficult to establish in wild cetaceans, as the precise time intervals of such developments and any distinctive growth trajectories in most cetacean species are basically unknown [ 34 , 35 , 36 , 37 , 38 , 39 ]. Furthermore, this is the first report that describes by ultrasonography the cephalic presentation of the calf at birth, according to its position within the uterus. The results of the present study reinforce the importance of monitoring health and fetal vitality throughout the duration of pregnancy and at the time of delivery by ultrasound. Reproductive success is vital in sustaining cetacean populations, and the systematic use of ultrasound for pregnancy monitoring provides a useful tool for assessing reproductive success, including for free-ranging dolphins. During capture–release health assessments, it is possible through application of diagnostic ultrasound to evaluate fetal development and viability, estimate gestational age, and measure anatomical structures [ 16 ]. This wild population conservation approach benefits from the findings of studies of the population of bottlenose dolphins under human care. Deviations from the normal findings during pregnancy could be related to the alteration of the health status and the well-being of the fetus or the mother, and, if detected sufficiently early, could result in timely therapeutic intervention for animals under human care. Thus, findings related to a reproductive failure in the wild may also be elucidated. However, further investigation will be necessary, carried out with a greater number of subjects, in order to validate the results obtained and to apply these diagnostic methods to other cetacean species.
Acknowledgments
We would like to thank the University of Perugia, Faculty of Veterinary Medicine (Dipartimento di Medicina Veterinaria, Università di Perugia, 06124, Perugia, Italy) for their support and for making this study possible. We are especially grateful to all of the staff of veterinarians and trainers who dedicate themselves daily to dolphin welfare. Their dedication and teamwork make medical behavior, investigation, and conservation projects possible.
Author Contributions
Conceptualization, P.S., L.F., F.G., A.T., A.P., and R.O.; data curation, R.M.; formal analysis, P.S., F.G., and L.F.; methodology, P.S., F.G., and R.O.; supervision, P.S. and L.F.; writing—original draft, L.F.; writing—review and editing, P.S., L.F., F.G., R.M., A.T., A.P., and R.O. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
- 1. Instrumentation and Methodology. [(accessed on 24 May 2020)]; Available online: https://www.amazon.nl/Handbook-Ultrasonography-Dolphins-Abdomen-English-ebook/dp/B00BRXUSPG .
- 2. Fiorucci L., García-Párraga D., Macrelli R., Grande F., Flanagan C., Rueca F., Busechian S., Bianchi B., Arbelo M., Saviano P. Determination of the main reference values in ultrasound examination of the gastrointestinal tract in clinically healthy bottlenose dolphins (Tursiops truncatus) Aquat. Mamm. 2015;41:284–294. doi: 10.1578/AM.41.3.2015.284. [ DOI ] [ Google Scholar ]
- 3. England G.C.W. Ultrasound evaluation of pregnancy and spontaneous embryonic resorption in the bitch. J. Small Anim. Pract. 1992;33:430–436. doi: 10.1111/j.1748-5827.1992.tb01197.x. [ DOI ] [ Google Scholar ]
- 4. England G.C.W. Ultrasonographic Assessment of Abnormal Pregnancy. J. Small Anim. Pract. 1998;28:849–868. doi: 10.1016/S0195-5616(98)50081-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. England G.C.W., Russo M. Ultrasonographic characteristic of early pregnancy failure in bitches. Theriogenology. 2006;66:1694–1698. doi: 10.1016/j.theriogenology.2006.01.028. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Aissi A., Alloui N., Slimani C., Touri S. Preliminary study of the early ultrasonographic diagnosis of pregnancy and fetal development in dog. J. Anim. Vet. Adv. 2008;7:607–611. [ Google Scholar ]
- 7. Nyland T.G., Mattoon J.S. Veterinary Diagnostic Ultrasound. WB Saunders; Philadelphia, PA, USA: 1995. Physical principles, instrumentation and safety of Diagnostic Ultrasound; pp. 3–18. [ Google Scholar ]
- 8. Root K.M.V. Pregnancy diagnosis and abnormalities of pregnancy in the dog. Theriogenology. 2005;64:755–765. doi: 10.1016/j.theriogenology.2005.05.024. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Luvoni C.G., Beccaglia M. The prediction of parturition date in canine pregnancy. Reprod. Dom. Anim. 2006;41:27–32. doi: 10.1111/j.1439-0531.2006.00641.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Lamm C.G., Makoloski C.L. Current avances in gestation and parturition in cats and dogs. Vet. Clin. N. Am. Small Anim. Pract. 2012;42:445–456. doi: 10.1016/j.cvsm.2012.01.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Davidson A.P., Baker T.W. Reproductive ultrasound of the bitch and queen. Top. Companion Anim. Med. 2009;24:55–63. doi: 10.1053/j.tcam.2008.11.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Adams G.P., Plotka E.D., Asa C.S., Ginther O.J. Feasibility of characterizing reproductive events in large, non-domestic species by transrectal ultrasonic imaging. Zoo Biol. 1991;10:247–253. doi: 10.1002/zoo.1430100308. [ DOI ] [ Google Scholar ]
- 13. Harrison R.J., Ridgway S.H. Gonadal activity in some bottlenose dolphins (Tursiops truncatus) J. Zool. 1971;165:355–366. doi: 10.1111/j.1469-7998.1971.tb02193.x. [ DOI ] [ Google Scholar ]
- 14. Brook F.M. Ultrasonographic imaging of the reproductive organs of the female bottlenose dolphin, Tursiops truncatus aduncus. Reproduction. 2001;121:419–428. doi: 10.1530/rep.0.1210419. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Neuenhoff R.D., Cowan D.F., Whitehead H., Marshall C. Prenatal data impacts common bottlenose dolphin (Tursiops truncatus) growth parameters estimated by length-at-age curves. Mar. Mamm. Sci. 2011;27:195–216. doi: 10.1111/j.1748-7692.2010.00394.x. [ DOI ] [ Google Scholar ]
- 16. Kellar N.M., Speakman T.R., Smith C.R., Lane S.M., Balmer B.C., Trego M.L., Catelani K.N., Robbins M.N., Allen C.D., Wells R.S., et al. Low reproductive success rates of common bottlenose dolphins Tursiops truncatus in the northern Gulf of Mexico following the Deepwater Horizon disaster (2010–2015) Endanger. Species Res. 2017;33:143–158. doi: 10.3354/esr00775. [ DOI ] [ Google Scholar ]
- 17. Wells R.S., Smith C.R., Sweeney J.C., Townsend F.I., Fauquier D.A., Stone R., Langan J., Schwacke L.H., Rowles T.K. Fetal survival of common bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Aquat. Mamm. 2014;40:252. doi: 10.1578/AM.40.3.2014.252. [ DOI ] [ Google Scholar ]
- 18. Robeck T.R., Curry B.E., McBain J.F., Kraemer D.C. Reproductive biology of the bottlenose dolphin (Tursiops truncatus) and the potential application of advanced reproductive technologies. J. Zoo Wildl. Med. 1994;25:321–336. [ Google Scholar ]
- 19. Robeck T.R., Atkinson S.K.C., Brook F. Reproduction. In: Dierauf L.A., Gulland F.M.D., editors. CRC Handbook of Marine Mammals Medicine. 2th ed. CRC Press; Baca Raton, FL, USA: 2001. pp. 193–236. [ Google Scholar ]
- 20. Robeck T.R., Steinman K.J., Yoshioka M., Jensen E., O’Brien J.K., Katsumata E., Gili C., McBain J.F., Sweeney J., Monfort S.L. Estrous cycle characterisation and artificial insemination using frozen-thawed spermatozoa in the Bottlenose dolphin (Tursiops Truncatus) Reproduction. 2005;129:659–674. doi: 10.1530/rep.1.00516. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Ivančič M., Gomez F.M., Musser W.B., Barratclough A., Meegan J.M., Waitt S.M., Llerenas A.C., Jensen E.C., Smith C.R. Ultrasonographic findings associated with normal pregnancy and fetal well-being in the bottlenose dolphin (Tursiops truncatus) Vet. Radiol. Ultrasound. 2020;2020:1–12. doi: 10.1111/vru.12835. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Barratclough A., Gomez F.M., Morey J.S., Deming A., Parry C., Meegan J.M., Carlin K.P., Schwacke L., Venn-Watson S., Jensen E.D., et al. Pregnancy profiles in the common bottlenose dolphin (Tursiops truncatus): Clinical biochemical and hematological variations during healthy gestation and a successful outcome. Theriogenology. 2020;142:92–103. doi: 10.1016/j.theriogenology.2019.09.028. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Williamson P., Gales N.J., Lister S. Use of real-timeB-mode ultrasound for pregnancy diagnosis and measurement of fetal growth rate in captive bottlenose dolphins (Tursiops truncatus) J. Reprod. Fertil. 1990;88:543–548. doi: 10.1530/jrf.0.0880543. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Stone L.R., Johnson R.L., Sweeney J.C., Lewis M.L. Fetal ultrasonography in dolphins with emphasis on gestational aging. In: Fowler M.E., Miller. R.E., editors. Zoo and Wild Animal Medicine: Current Therapy 4. WB Saunders; Philadelphia, PA, USA: 1999. pp. 501–506. [ Google Scholar ]
- 25. Lacave G., Eggermont M., Verslycke T., Kinoshita R. Prediction from ultrasonographic measurements of the expected delivery date in two species of bottlenose. Vet. Rec. 2004;154:228–233. doi: 10.1136/vr.154.8.228. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Sklansky M. Fetal cardiovascular malformations and arrhythmias. In: Creasy R.K., Resnik R., Iams J.D., Lockwood C.J., Moore T.R., editors. Creasy and Resnik’s Maternal-Fetal Medicine. 6th ed. Elsevier Saunders; Philadelphia, PA, USA: 2009. pp. 305–345. [ Google Scholar ]
- 27. Sklansky M., Renner M., Clough P., Levine G., Campbell M., Stone R., Schmitt T., Chang R.K., Shannon-Rodriguez J. Fetal Echocardiographic Evaluation of the Bottlenose Dolphin (Tursiops truncatus) J. Zoo Wildl. Med. 2010;41:35–43. doi: 10.1638/2009-0061.1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Rychik J., Ayres N., Cuneo B., Gotteiner N., Hornberger L., Spevak P., Van der Veld M. American Society of Echocardiography guidelines and standards for performance of the fetal echocardiogram. J. Am. Soc. Echocardiogr. 2004;17:803–810. doi: 10.1016/j.echo.2004.04.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Makikallio K., Räsänen J., Mäkikallio T., Vuolteenaho O., Huhta J.C. Human fetal cardiovascular profile score and neonatal outcome in intrauterine growth restriction Ultrasound. Obstet. Gynecol. 2008;31:48–54. doi: 10.1002/uog.5210. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Powell J., Archibald R., Cross C., Rotstein D., Soop V., McFee W. Multiple congenital cardiac abnormalities in an Atlantic bottlenose dolphin (Tursiops truncatus) J. Wildl. Dis. 2009;45:839–842. doi: 10.7589/0090-3558-45.3.839. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. García-Párraga D., Brook F., Crespo-Picazo J.L., Valls M., Penadés M., Ortega J., Corpa J.M. Recurrent umbilical cord accidents in a bottlenose dolphin, Tursiops truncatus. Dis. Aquat. Org. 2014;108:177–180. doi: 10.3354/dao02711. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Smith C.R., Wong S.K., Jensen E.D., Venn-Watson S.K. Fetal omphalocele in a common bottlenose dolphin (Tursiops truncatus) J. Zoo Wildl. Med. 2013;44:87–92. doi: 10.1638/1042-7260-44.1.87. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Tanaka M., Izawa T., Kuwamura M., Ozaki M., Nakao T., Ito S., Yamate J. A case of meconium aspiration syndrome in a bottlenose dolphin (Tursiops truncatus) calf. J. Vet. Med. Sci. 2014;76:81–84. doi: 10.1292/jvms.13-0227. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Brook F.M. Ultrasound Diagnosis of Anencephaly in the Fetus of a Bottlenose Dolphin (Tursiops aduncas) J. Zoo Wildl. Med. 1994;25:569–574. [ Google Scholar ]
- 35. Thewisen J.G.M., Heyning J.E. Embryogenesis and development in Stenella atenuatta and other cetaceans. In: Miller D.L., editor. Reproductive Biology and Phylogeny in Cetacea, Whales, Dolphins and Porpoises. Science Publishers; Enflield, NH, USA: 2007. pp. 307–330. [ Google Scholar ]
- 36. Reidenberg J.S., Laitman J.T. Prenatal development in cetaceans. In: Perrin W.F., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. 2nd ed. Academic Press; San Diego, CA, USA: 2009. pp. 220–230. [ Google Scholar ]
- 37. Cozzi B., Huggenberger S., Oelschläger H. Anatomy of Dolphins: Insights into Body Structure and Function. Academic Press; London, UK: 2017. pp. 1–438. [ Google Scholar ]
- 38. Sterba O., Klima M., Schildger B. Embryology of dolphins. Staging and ageing of embryos and fetuses of some cetaceans. Adv. Anat. Embryol. Cell Biol. 2000;157:1–133. [ PubMed ] [ Google Scholar ]
- 39. Zarzosa G.R., Fernández A.L., Gomariz F.M., Cano F.G., Laguía M.S., Espinosa A.A., de los Ríos y Loshuertos A.G. A Study of the Head during Prenatal and Perinatal Development of Two Fetuses and One Newborn Striped Dolphin (Stenella coeruleoalba) Using Dissections, Sectional Anatomy, CT, and MRI: Anatomical and Functional Implications in Cetaceans and Terrestrial Mammals. Animals. 2019;9:1139. doi: 10.3390/ani9121139. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Michel E., Spörri M., Ohlerth S., Reichler L. Prediction of parturition date in the bitch and queen. Reprod. Domest. Anim. 2011;46:926–932. doi: 10.1111/j.1439-0531.2011.01763.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Sedmera D., Misek I., Milan K., Thompson R.P. Heart Development in the Spotted Dolphin (Stenella attenuata) Anat. Rec. 2003;273A:687–699. doi: 10.1002/ar.a.10086. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Adams-Brendemuehl C., Pipers F.S. Antepartum evaluations of the equine fetus. J. Reprod. Fertil. 1987;35:565–573. [ PubMed ] [ Google Scholar ]
- 43. Capellini I., Venditti C., Barton R.A. Placentation and Maternal Investment in Mammals. Am. Nat. 2011;177:86–98. doi: 10.1086/657435. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Berta A., Sumich J.L., Kovacs K.M. Marine Mammal Evolutionary Biology. 2nd ed. Academic Press; San Diego, CA, USA: 2005. Cetacean evolution and systematics; pp. 165–209. [ Google Scholar ]
- 45. Smith C.R., Wong S.K., Jensen E.D., Ruiz C., Nollens H. Overview of neonatal management techniques at the U.S. Navy Marine Mammal Program. Int. Assoc. Aquat. Anim. Med. Proc. Nassau. Bahamas. 2006;37:164–167. [ Google Scholar ]
- 46. Slijper E.J. Functional morphology of the reproductive system in Cetacea. In: Norris K.S., Berkeley, L.A., editor. Whales, Dolphins and Porpoises. University of California Press; Berkeley, CA, USA: 1966. pp. 277–319. [ Google Scholar ]
- 47. Deng X., Hao Y., Serres A., Wang K., Wang D. Position at Birth and Possible Effects on Calf Survival in Finless Porpoises (Neophocaena asiaeorientalis) Aquat. Mamm. 2019;45:4. doi: 10.1578/AM.45.4.2019.411. [ DOI ] [ Google Scholar ]
- 48. Gol’din P.E. Case of cephalic presentation of fetus in a harbor porpoise (Phocoena phocoena), with notes on other aquatic mammals. Vestn. zool. 2011;45:33–38. [ Google Scholar ]
- 49. Essapian F.K. Observations on Abnormalities of Parturition in Captive Bottle-Nosed Dolphins, Tursiops truncatus, and Concurrent Behavior of Other Porpoises. J. Mammal. 1963;44:405–414. doi: 10.2307/1377210. [ DOI ] [ Google Scholar ]
- 50. Hart P., Van der Kemp J.S. Cephalic presentation observed in a white-beaked dolphin, Lagenorhynchus albirostris. Lutra. 1999;41:21–24. [ Google Scholar ]
- 51. Vladykov V.D. Études sur les mammifères aquatiques. III. Chasse, biologie et valeur économique du marsouin blanc ou béluga (Delphinapterus leucas) du fleuve et du golfe du Saint-Laurent. Département des pêcheries de la province de Québec; Québec, QC, Canada: 1944. p. 194. [ Google Scholar ]
- 52. Doan K.H., Douglas C.W. Beluga of the Churchill region of Hudson Bay. Bull. Fish. Res. Board Can. 1953;98:1–27. [ Google Scholar ]
- 53. Kleinenberg S.E., Yablokov A.V., Bel’kovich V.M., Tarasevich M.N. Beluga: The Experience of Monographic Species Study. Nauka; Moscow, Russia: 1964. p. 456. [ Google Scholar ]
- 54. Baker J.R., Martin A.R. Causes of mortality and parasites and incidental lesions in harbour porpoises (Phocoena phocoena) from British waters. Vet. Rec. 1992;130:554–558. doi: 10.1136/vr.130.25.554. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (8.0 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Most Beautiful Metro Stations in Moscow

Visiting Moscow? Get yourself a metro card and explore Moscow’s beautiful metro stations. Moscow’s world-famous metro system is efficient and a great way to get from A to B. But there is more to it; Soviet mosaic decorations, exuberant halls with chandeliers, colourful paintings and immense statues. Moscow’s metro is an attraction itself, so take half a day and dive into Moscow’s underground!
The best thing to do is to get on the brown circle (number 5) line since the most beautiful metro stations are situated on this line. The only exception is the metro stop Mayakovskaya one the green line (number 2). My suggestion is to get a map, mark these metro stops on there and hop on the metro. It helps to get an English > Russian map to better understand the names of the stops. At some of the metro stops, the microphone voice speaks Russian and English so it’s not difficult at all.
Another thing we found out, is that it’s worth taking the escalator and explore the other corridors to discover how beautiful the full station is.
Quick hotel suggestion for Moscow is the amazing Brick Design Hotel .
These are my favourite metro stations in Moscow, in order of my personal preference:
1. Mayakovskaya Station
The metro station of Mayakovskaya looks like a ballroom! Wide arches, huge domes with lamps and mosaic works make your exit of the metro overwhelming. Look up and you will see the many colourful mosaics with typical Soviet pictures. Mayakovskaya is my personal favourite and is the only stop not on the brown line but on the green line.

2. Komsomolskaya Station
Komsomolskaya metro station is famous for its yellow ceiling. An average museum is nothing compared to this stop. Splendour all over the place, black and gold, mosaic – again – and enormous chandeliers that made my lamp at home look like a toy.

3. Novoslobodskaya Station
The pillars in the main hall of Novoslobodskaya metro station have the most colourful stained glass decorations. The golden arches and the golden mosaic with a naked lady holding a baby in front of the Soviet hammer and sickle, make the drama complete.

4. Prospect Mira Station
The beautiful chandeliers and the lines in the ceiling, make Prospekt Mira an architectural masterpiece.

5. Belorusskaya Station
Prestigious arches, octagonal shapes of Socialistic Soviet Republic mosaics. The eyecatcher of Belorusskaya metro station, however, is the enormous statue of three men with long coats, holding guns and a flag.

6. Kiyevskaya Station
The metro station of Kiyevskaya is a bit more romantic than Belorusskaya and Prospect Mira. Beautiful paintings with classical decorations.

7. Taganskaya Station
At the main hall Taganskaya metro station you will find triangle light blue and white decorations that are an ode to various Russians that – I assume – are important for Russian history and victory. There is no need to explore others halls of Taganskaya, this is it.

8. Paveletskaya Station
Another and most definitely the less beautiful outrageous huge golden mosaic covers one of the walls of Paveletskaya. I would recommend taking the escalator to the exit upstairs to admire the turquoise dome and a painting of the St Basil’s Cathedral in a wooden frame.

Travelling with Moscow’s metro is inexpensive. You can have a lot of joy for just a few Rubbles.
- 1 single journey: RMB 50 – € 0,70
- 1 day ticket: RMB 210 – € 2,95
Like to know about Moscow, travelling in Russia or the Transsiberian Train journey ? Read my other articles about Russia .
- 161 Shares
You may also like
Hunting for the best coffee in irkutsk, amsterdam forest: a day trip for nature..., a romantic amalfi coast road trip itinerary, complete weekend city guide to maastricht, olkhon island: siberian sunsets over lake baikal, 8 great reasons to visit mongolia in..., trans-siberian railway travel guide, all you need to know for your..., food & drinks in moscow, why we love grünerløkka in oslo.
Wow! It is beautiful. I am still dreaming of Moscow one day.
It’s absolutely beautiful! Moscow is a great city trip destination and really surprised me in many ways.
My partner and I did a self guided Moscow Metro tour when we were there 2 years ago. So many breathtaking platforms…I highly recommend it! Most of my favorites were along the Brown 5 line, as well. I also loved Mayakovskaya, Arbatskaya, Aleksandrovski Sad and Ploshchad Revolyutsii. We’re heading back in a few weeks and plan to do Metro Tour-Part 2. We hope to see the #5 stations we missed before, as well as explore some of the Dark Blue #3 (Park Pobedy and Slavyansky Bul’var, for sure), Yellow #8 and Olive #10 platforms.
That’s exciting Julia! Curious to see your Metro Tour-Part 2 experience and the stations you discovered.
Leave a Comment Cancel Reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .

IMAGES
VIDEO
COMMENTS
Cephalic occiput posterior. Your baby is head down with their face turned toward your belly. This can make delivery a bit harder because the head is wider this way and more likely to get stuck ...
Head Down, Facing Down (Cephalic Presentation) This is the most common position for babies in-utero. In the cephalic presentation, the baby is head down, chin tucked to chest, facing their mother's back. This position typically allows for the smoothest delivery, as baby's head can easily move down the birth canal and under the pubic bone ...
Frank breech. When a baby's feet or buttocks are in place to come out first during birth, it's called a breech presentation. This happens in about 3% to 4% of babies close to the time of birth. The baby shown below is in a frank breech presentation. That's when the knees aren't bent, and the feet are close to the baby's head.
0 station. This is when the baby's head is even with the ischial spines. The baby is said to be "engaged" when the largest part of the head has entered the pelvis. ... Cephalic presentation occurs in about 97% of deliveries. There are different types of cephalic presentation, which depend on the position of the baby's limbs and head (fetal ...
Head first (called vertex or cephalic presentation) Facing backward (occiput anterior position) Spine parallel to mother's spine (longitudinal lie) Neck bent forward with chin tucked. Arms folded across the chest . If the fetus is in a different position, lie, or presentation, labor may be more difficult, and a normal vaginal delivery may not ...
The cephalic presentation can be further categorized based on the degree of flexion of the fetal head: ... Station. In addition to the fetal lie, presentation, and position, the level or station of the presenting part in the maternal pelvis is an important factor in the labor process. For this assessment, the ischial tuberosities of the ...
The movement of the fetus to cephalic presentation is called head engagement.It occurs in the third trimester.In head engagement, the fetal head descends into the pelvic cavity so that only a small part (or none) of it can be felt abdominally. The perineum and cervix are further flattened and the head may be felt vaginally. [2] Head engagement is known colloquially as the baby drop, and in ...
In breech presentation, the presenting part is a poor dilating wedge, which can cause the head to be trapped during delivery, often compressing the umbilical cord. For breech presentation, usually do cesarean delivery at 39 weeks or during labor, but external cephalic version is sometimes successful before labor, usually at 37 or 38 weeks.
To understand the mechanism of labor for a cephalic presentation. To understand the meaning of the following germs: Presentation, position, lie, station, effacement, dilatation. To understand the phases and stages of labor. To understand the following abnormalities of labor: Prolonged latent phase, arrest of dilatation, and arrest of descent.
Fetal presentation, or how your baby is situated in your womb at birth, is determined by the body part that's positioned to come out first, and it can affect the way you deliver. At the time of delivery, 97 percent of babies are head-down (cephalic presentation). But there are several other possibilities, including feet or bottom first (breech ...
The cephalic position is when a fetus is head down when it is ready to enter the birth canal. This is one of a few variations of how a fetus can rest in the womb and is considered the ideal one for labor and delivery. About 96% of babies are born in the cephalic position. Most settle into it between the 32nd and 36th weeks of pregnancy.
Fetal position reflects the orientation of the fetal head or butt within the birth canal. The bones of the fetal scalp are soft and meet at "suture lines." Over the forehead, where the bones meet, is a gap, called the "anterior fontanel," or "soft spot." This will close as the baby grows during the 1st year of life, but at birth, it is open.
This type of cephalic presentation may require a C/Section if the attitude cannot be changed. (d) Hyperextended. In reference to the cephalic position, the fetus head is extended all the way back. ... Station. This refers to the depth that the presenting part has descended into the pelvis in relation to the ischial spines of the mother's ...
Definitions. Lie - the relationship between the long axis of the fetus and the mother. Longitudinal, transverse or oblique. Presentation - the fetal part that first enters the maternal pelvis. Cephalic vertex presentation is the most common and is considered the safest. Other presentations include breech, shoulder, face and brow.
The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2018 May;218 (5):540-541. doi: 10.1016/j.ajog.2018.01.028.
0 station. This is when the baby's head is even with the ischial spines. The baby is said to be "engaged" when the largest part of the head has entered the pelvis. ... Cephalic presentation occurs in about 97% of deliveries. There are different types of cephalic presentation, which depend on the position of the baby's limbs and head (fetal ...
The cephalic presentation of a fetus in cetaceans is not a pathology but a natural variation. This idea is also supported by the documented cases of successful cephalic deliveries and by the occurrence of head presentations both in the wild and under human care. Nonetheless, head presentation of a fetus in a small cetacean can increase the risk ...
4. Prospect Mira Station. The beautiful chandeliers and the lines in the ceiling, make Prospekt Mira an architectural masterpiece. 5. Belorusskaya Station. Prestigious arches, octagonal shapes of Socialistic Soviet Republic mosaics. The eyecatcher of Belorusskaya metro station, however, is the enormous statue of three men with long coats ...