Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 23 August 2022

Assessment, management, and incidence of neonatal jaundice in healthy neonates cared for in primary care: a prospective cohort study
- Berthe A. M. van der Geest 1 , 2 ,
- Malou J. S. de Mol 1 , 2 ,
- Ivana S. A. Barendse 1 , 2 ,
- Johanna P. de Graaf 2 ,
- Loes C. M. Bertens 2 ,
- Marten J. Poley 3 , 4 ,
- Erwin Ista 5 , 6 ,
- René F. Kornelisse 1 ,
- Irwin K. M. Reiss 1 ,
- Eric A. P. Steegers 2 ,
- Jasper V. Been 1 , 2 , 7 &
STARSHIP Study Group
Scientific Reports volume 12 , Article number: 14385 ( 2022 ) Cite this article
12k Accesses
14 Citations
2 Altmetric
Metrics details
- Neonatology
- Paediatric research
Jaundice caused by hyperbilirubinaemia is a common phenomenon during the neonatal period. Population-based studies evaluating assessment, management, and incidence of jaundice and need for phototherapy among otherwise healthy neonates are scarce. We prospectively explored these aspects in a primary care setting via assessing care as usual during the control phase of a stepped wedge cluster randomised controlled trial.
We conducted a prospective cohort study embedded in the Screening and TreAtment to Reduce Severe Hyperbilirubinaemia in Infants in Primary care (STARSHIP) Trial. Healthy neonates were included in seven primary care birth centres (PCBCs) in the Netherlands between July 2018 and March 2020. Neonates were eligible for inclusion if their gestational age was ≥ 35 weeks, they were admitted in a PCBC for at least 2 days during the first week of life, and if they did not previously receive phototherapy. Outcomes were the findings of visual assessment to detect jaundice, jaundice incidence and management, and the need for phototherapy treatment in the primary care setting.
860 neonates were included of whom 608 (71.9%) were visibly jaundiced at some point during admission in the PCBC, with 20 being ‘very yellow’. Of the latter, four (20%) did not receive total serum bilirubin (TSB) quantification. TSB levels were not associated with the degree of visible jaundice (p = 0.416). Thirty-one neonates (3.6%) received phototherapy and none received an exchange transfusion. Five neonates did not receive phototherapy despite having a TSB level above phototherapy threshold.
Jaundice is common in otherwise healthy neonates cared for in primary care. TSB quantification was not always performed in very jaundiced neonates, and not all neonates received phototherapy when indicated. Quality improvement initiatives are required, including alternative approaches to identifying potentially severe hyperbilirubinaemia.
Trial registration: NL6997 (Dutch Trial Register; Old NTR ID 7187), registered 3 May 2018.
Similar content being viewed by others

Neonatal hyperbilirubinemia and bilirubin neurotoxicity in hospitalized neonates: analysis of the US Database
The cost-effectiveness of home phototherapy for hyperbilirubinemia in neonates: results from a randomized controlled trial
Validation of published rebound hyperbilirubinemia risk prediction scores during birth hospitalization after initial phototherapy: a retrospective chart review
Introduction.
Neonatal hyperbilirubinaemia is a common condition during the first days of life and typically presents as visible jaundice 1 . Hyperbilirubinaemia in the neonatal period is usually benign. In some neonates, unconjugated bilirubin may reach hazardous levels and cause acute bilirubin encephalopathy and later kernicterus spectrum disorder (KSD) when not timely recognised and treated 2 .
In several countries and settings, the first-line recognition of hyperbilirubinaemia is based on visual inspection of jaundice, followed by selective total serum bilirubin (TSB) quantification (i.e., if considered necessary). Transcutaneous bilirubin quantification is not widely used in the primary care setting. TSB levels are plotted on a nomogram to determine the need for treatment (Text Box 1 ) 3 , 4 , 5 . Phototherapy is a safe and effective treatment to decrease bilirubin levels and is usually applied in-hospital 1 . When bilirubin levels are extremely high or continue to increase despite intensive phototherapy, one or more exchange transfusions may be needed to decrease bilirubin levels.
Although neonatal jaundice is commonly observed, population-based data on the assessment, management, and incidence of visual jaundice and need for phototherapy among healthy neonates, especially if cared for in primary care, are scarce. Whereas the inaccuracy of visual inspection of jaundice to estimate TSB levels has previously been demonstrated 6 , 7 , the associations of visual jaundice assessment to the decision whether or not to quantify TSB, and of visual jaundice assessment to whether or not a neonate exceeded the individual phototherapy treatment threshold in primary care are unknown. Also, most population-based studies focus on hospitalised neonates having severe neonatal hyperbilirubinaemia or KSD 8 , 9 , 10 , 11 . Hence, these studies do not cover the complete scope of assessment, management, incidence, and burden of neonatal hyperbilirubinaemia. In addition, definitions of severe hyperbilirubinaemia vary, resulting in a wide variation in reported incidences of neonatal hyperbilirubinaemia between studies 12 , 13 , 14 , 15 , 16 , 17 .
The Screening and TreAtment to Reduce Severe Hyperbilirubinaemia in Primary care (STARSHIP) Trial is an ongoing factorial stepped-wedge cluster randomised controlled trial in seven Dutch primary care birth centres (PCBCs). It aims to assess the effectiveness of universal transcutaneous bilirubin (TcB) screening and of phototherapy applied in primary care 18 . See Text Box 2 . In each participating PCBC, the initial phase of the STARSHIP trial evaluates usual care (i.e. no interventions are implemented). This provides a unique opportunity to explore the assessment, management, and incidence of neonatal jaundice and phototherapy in primary care among children included during this initial phase.
Study design
Prospective cohort study embedded in the factorial stepped-wedge cluster randomised controlled STARSHIP Trial 18 .
In the Netherlands, most healthy neonates are either born in primary care or discharged to primary care (i.e. the home or a PCBC) within the first few hours to days of life 19 . A maternity care assistant (MCA) provides postpartum care to mother and neonate during daytime for the first 8 days after delivery 20 . The MCA is supervised by a community midwife, who visits the family at least three times in the first week 21 . The MCA assesses each day whether the neonate is visually jaundiced and if so, to which degree. The MCA is expected to consult the community midwife if she considers the neonate ‘too jaundiced’ or if she feels that there are other reasons to quantify TSB. Medical doctors are only involved in the care of otherwise healthy neonates if consulted by the community midwife. The current national multidisciplinary guideline on neonatal hyperbilirubinaemia does not include universal screening, but alternatively states that each involved perinatal healthcare professional should be aware of a neonate’s a priori risk for developing hyperbilirubinaemia and that this risk should be documented and communicated among all involved perinatal healthcare professionals 4 . According to the guideline, the healthcare provider may decide to have blood taken to quantify TSB levels if hyperbilirubinaemia is suspected based on visual inspection (e.g., a neonate is assessed ‘too jaundiced’). The guideline does not provide objective criteria for having TSB quantified 4 . One of the PCBCs and a small number of primary care midwifery practices participating in the STARSHIP Trial used selective transcutaneous bilirubin (TcB) screening (i.e., TcB quantification if a neonate is assessed ‘too jaundiced’, followed by TSB quantification if the TcB level is above or < 50 µmol/L below the phototherapy threshold). TSB levels are plotted on the Dutch TSB nomogram (Text Box 1 ), which is based on the American Academy of Pediatrics guidelines 3 . A paediatrician of a nearby affiliated hospital can be consulted when hyperbilirubinaemia is confirmed, and this is then usually treated in the hospital.
The STARSHIP Trial is conducted in seven PCBCs throughout the Netherlands where MCAs provide postpartum care, supervised by community midwives. Women can choose to receive their care either at home or in a PCBC if the neonate is healthy. Neonates included in the control phase of the STARSHIP Trial, when usual care was evaluated, were included in this cohort. The control phase of the STARSHIP Trial ran between 2 July 2018 and 8 March 2020 (Supplementary Table 1 ) 18 .
Text Box 1: the Dutch TSB nomogram
The Dutch TSB nomogram is adapted from the American Academy of Pediatrics 3 . Treatment thresholds are based on postnatal age and risk assessment. Gestational age (< 38 weeks or ≥ 38 weeks) and risk factors (blood group antagonism, haemolytic disease; birth asphyxia; suspicion of infection; drowsy or ill neonate; and serum albumin level below 30 g/L) are combined to assess the risk category: lower, medium, or higher risk. See Fig. 1 .
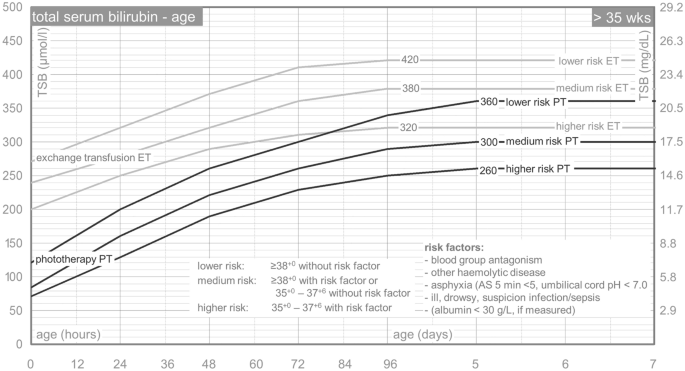
Phototherapy and exchange transfusion thresholds for neonates born after more than 35 weeks of gestation. TSB total serum bilirubin, PT phototherapy, ET exchange transfusion, AS Apgar score. Translated from Dutch. The Dutch nomogram is available at: http://babyzietgeel.nl/kinderarts/hulpmiddelen/diagnostiek/bilicurve35wkn.php .
Participants
Neonates were eligible for inclusion in the STARSHIP Trial if:
Born ≥ 35 + 0 weeks of gestation;
Admitted to a participating PCBC during the first week of life;
Expected to remain admitted to the PCBC for at least 2 days;
Signed informed consent from parent(s) or primary caregiver(s) was obtained.
Neonates were not eligible if:
The neonate previously received phototherapy;
Parents did not have sufficient understanding of the Dutch language to be able to comprehend the patient information form.
For the analyses presented in this manuscript, all neonates included in the control phase of the STARSHIP Trial were eligible. Inclusion of the neonates was performed at admission to the PCBC and irrespective of the degree of jaundice of the neonate.
Outcomes of this study are: findings of assessment of jaundice by MCAs (ranging from ‘not yellow at all’ to ‘very yellow’; in the Netherlands no standardised colour scale is used for visual jaundice assessment), the number of neonates in whom TSB was quantified; TSB level; management of neonatal hyperbilirubinaemia (i.e., what treatment is needed and what treatment is performed); the incidence of neonatal hyperbilirubinaemia and of receiving phototherapy treatment; and risk factors associated with receiving phototherapy. An overview of all variables used for the current analyses and definitions of variables is shown in Supplementary Table 2 .
Data sources
Baseline data regarding mother and neonate, and daily data regarding findings of screening and treatment of neonatal hyperbilirubinaemia were collected by MCAs of the participating PCBCs and by study personnel of the STARSHIP Trial and stored in a Limesurvey/Gemstracker database 22 . Additionally, parent(s) of all included neonates were asked to fill out a questionnaire, 2 weeks after discharge from the PCBC, that included questions regarding hospital admission for hyperbilirubinaemia. If a neonate was admitted to the hospital for neonatal hyperbilirubinaemia, additional information from the medical records regarding likely underlying causes, TSB levels, and treatment of hyperbilirubinaemia was requested from this hospital.
Statistical analysis
Analyses were performed using SPSS Statistics version 25.0. Data were summarised using descriptive statistics. Mean and standard deviation (SD) were calculated for continuous, normally distributed data. For non-normally distributed data, median and interquartile range (IQR) were calculated. As phototherapy treatment thresholds vary according to postnatal age and individual risk assessment for each neonate (Text Box 1 ), the difference between a neonate’s TSB level and the corresponding phototherapy threshold for each individual neonate was calculated 4 , 5 . In the absence of information on individual risk factors determining phototherapy thresholds, such as blood group incompatibility, the risk factor is generally considered to be absent. To compare whether or not TSB was quantified, and the difference between neonates’ TSB levels and corresponding phototherapy thresholds among neonates having different degrees of visual jaundice, χ 2 and Kruskal–Wallis test were performed as appropriate. Logistic regression was performed to analyse which risk factors were independently associated with hyperbilirubinaemia necessitating treatment. A p-value < 0.05 was considered to indicate statistical significance.
The STARSHIP Trial has been reviewed and approved by the Medical Research Ethics Committee of Erasmus MC Rotterdam, the Netherlands (MEC2017-473). The STARSHIP Trial was performed in accordance with the Declaration of Helsinki 23 .
Consent to participate
Parents provided written informed consent before participation of their neonate in the study.
Text Box 2: STARSHIP Trial
The Screening and TreAtment to Reduce Severe Hyperbilirubinaemia in Infants in Primary care (STARSHIP) Trial is a factorial stepped-wedge cluster randomised controlled trial. In the STARSHIP Trial, universal transcutaneous bilirubin screening and phototherapy in primary care are evaluated. The STARSHIP Trial is conducted in seven primary care birth centres (PCBCs) in the Netherlands. MCAs provide postpartum care supervised by community midwives in PCBCs. Medical doctors are not involved in providing care, although in some PCBCs they can be consulted if a problem arises.
According to the factorial stepped-wedge cluster design of the STARSHIP Trial, each PCBC is allocated to a predefined timeline with three phases. Each PCBC starts with a control phase in which all included neonates receive standard care according to the national multidisciplinary hyperbilirubinaemia guideline (i.e., visual inspection of jaundice and selected TSB quantification to screen for hyperbilirubinaemia, and phototherapy in the hospital if treatment is indicated) 4 . The control phase is followed by a second phase in which one intervention is implemented (i.e., transcutaneous bilirubin screening or phototherapy in the PCBC rather than in-hospital) and eventually by a final phase in which both interventions are implemented (i.e., transcutaneous bilirubin screening and phototherapy in the PCBC) 18 .
In total, 860 neonates were included in the control phase of the STARSHIP Trial. Baseline characteristics are shown in Table 1 . Median gestational age was 39.3 weeks (IQR 1.9) and mean birth weight was 3399 g (SD 487). Most neonates were born after a vaginal, non-instrumental delivery, had a Western ethnicity and a Rh D positive mother. Apgar score at 5 min was below 5 in 18 neonates (2.1%) and umbilical cord pH was below 7.0 in 11 (2.5%) out of 441 neonates in whom umbilical cord pH was quantified.
Assessment and incidence of neonatal hyperbilirubinaemia
The majority of neonates (n = 608, 71.9%) had some degree of jaundice at any point during admission in the PCBC; the maximum degree of jaundice was ‘slightly yellow’ in the vast majority of jaundiced neonates (n = 442, 72.7%). In most neonates, jaundice was first noted on postnatal day one or two (n = 390, 75.0% of neonates having some degree of jaundice); two neonates (0.3%) were jaundiced within 24 h after birth (i.e., on postnatal day 0). TSB was quantified at least once in 129 neonates (15.0%). Twenty-three neonates (2.7%) had a TSB level above the phototherapy threshold during PCBC admission, at a median age of 57 h (IQR 43) 4 . In an additional five neonates, TSB level was above phototherapy threshold after discharge home from the PCBC (postnatal age range: 40–142 h), see Table 2 .
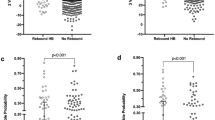
In total, 165 TcB and 171 TSB quantifications were performed during admission in the PCBC. Figure 2 shows the association between visual jaundice assessment by the MCA and whether or not TcB or TSB quantification was performed. Although there was a clear increase in the proportion of neonates having TcB or TSB quantified as jaundice was considered more severe (χ 2 trend test p < 0.001), still no TcB or TSB was quantified in 44% of the neonates considered ‘quite yellow’ and in 20% in of the neonates considered ‘very yellow’.
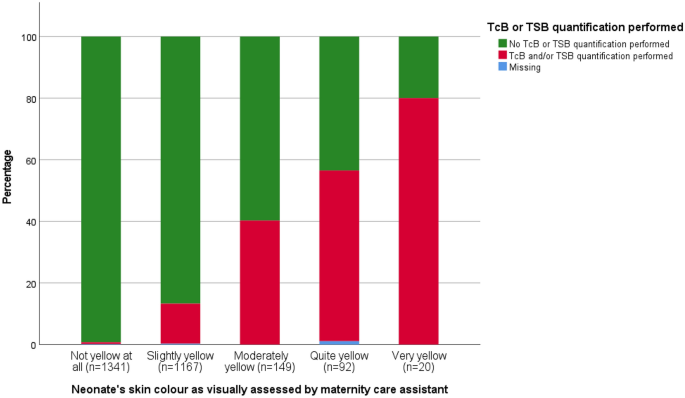
Proportion of assessment resulting in TcB or TSB being quantified according to degree of visible jaundice. TcB transcutaneous bilirubin, TSB total serum bilirubin.
The difference between individual phototherapy treatment thresholds and TSB levels according to the visually assessed degree of jaundice is shown in Fig. 3 . TSB was below the treatment threshold for all four assessments resulting in TSB being quantified in the absence of jaundice. There was no clear association between the degree of jaundice and the TSB level in those having TSB quantified (p = 0.416).
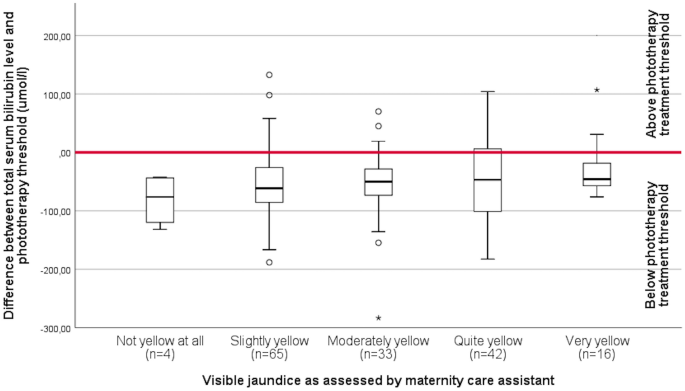
Difference between individual phototherapy treatment threshold and total serum bilirubin level according to degree of jaundice as visually assessed. The area above the red bar indicates a total serum bilirubin level above phototherapy treatment threshold.
Management of hyperbilirubinaemia
Table 3 shows the management of hyperbilirubinaemia in neonates who received treatment. During the control period of the STARSHIP Trial, 33 neonates (3.8%) had a TSB level above the phototherapy treatment threshold 4 . Phototherapy was performed in 31 neonates (3.6%) with a median duration of 22 h (IQR 22.5). Three neonates (0.3%) received phototherapy despite having a TSB level below the phototherapy threshold, whereas five neonates (0.6%) did not receive phototherapy despite having a TSB level above the phototherapy threshold 4 . TSB levels of the latter five exceeded phototherapy threshold with a maximum of 31 µmol/L (1.81 mg/dL). One of these neonates was admitted to the hospital for another reason than hyperbilirubinaemia treatment. TSB levels exceeded the threshold for exchange transfusion (with a maximum of 71 µmol/L; 4.15 mg/dL) during admission in the PCBC in three neonates (0.3%) and during hospital admission in one additional neonate (0.1%) 4 , 5 , but no exchange transfusions were performed. The neonates with TSB levels that exceeded the exchange transfusion threshold during admission in the PCBC were slightly yellow (n = 2) and very yellow (n = 1). None of these neonates had a TSB quantified in the PCBC prior to exceeding the exchange transfusion threshold.
Risk factors for receiving phototherapy treatment
Neonates who received phototherapy were more often born before 38 weeks of gestation when compared to neonates not receiving hyperbilirubinaemia treatment (56.7% vs. 12.8%; p < 0.001). The proportion of neonates born after an instrumental delivery was higher in the group receiving phototherapy than in the group not receiving phototherapy (26.7% vs. 8.3%; p = 0.004). Birth weight percentile 25 , perinatal asphyxia, Rh D incompatibility, type of feeding, sibling(s) who received phototherapy, and ethnicity were not significantly different between neonates who received phototherapy and those who did not (Table 4 ).
In our prospective cohort study evaluating the assessment, management, and incidence of neonatal hyperbilirubinaemia and the need for phototherapy among neonates cared for in primary care, we found that approximately 70% of neonates became jaundiced at any point during the first days of life and that 3.6% received treatment for hyperbilirubinaemia. However, not all neonates who had a TSB level that exceeded the phototherapy threshold received phototherapy. Also, TcB or TSB levels were not quantified in a substantial proportion of neonates assessed as moderately to severely jaundiced. Visual jaundice assessment was not reliable in estimating TSB levels.
To the best of our knowledge, this study is the first to prospectively describe the full scope of assessment, management, and corresponding incidence of hyperbilirubinaemia in otherwise healthy neonates cared for in primary care. This provides insight in the overall burden of neonatal hyperbilirubinaemia in primary care. We were able to identify neonates requiring phototherapy following discharge home by using parental questionnaires. Using parents as a source for data also has some pitfalls. First, if parents indicated that their neonate received phototherapy after discharge from the PCBC, this was not always in agreement with the actual data from the medical records in the hospital. Second, despite the prospective nature of the study, a proportion of included neonates had missing data, primarily due to missing parental questionnaires (17.3%). This may have led to an underestimation of the proportion of neonates who needed treatment. However, among the 711 (out of 860) neonates whose parents did respond, only five extra neonates who received treatment were identified using the questionnaires. Thus, we expect minimal influence of the missing data on this outcome. Neonates born after a C-section were overrepresented in our study (36% vs. 15% nationally) 26 , probably because their mothers were more likely to stay (longer) in the PCBC. As C-section is not known as a protective or risk factor for neonatal hyperbilirubinaemia, we expect negligible impact on our results. Additionally, the informed consent procedure may have induced selection (e.g., neonates whose parents refused participation in the STARSHIP Trial may have had other demographic characteristics). In contrast, overestimation of the proportion of neonates receiving hyperbilirubinaemia treatment in the whole population may have occurred as well. This is because we were dependent on parental consent for participation of their neonate in the STARSHIP trial and parents having a previous child with hyperbilirubinaemia may have been more likely to provide informed consent. Unfortunately, we were unable to assess the incidence of receiving phototherapy and associated risk factors (e.g., siblings having received phototherapy) among neonates without consent. Other findings may also have been influenced by the trial itself. Before the start of the STARSHIP Trial, all maternity care professionals were trained regarding neonatal hyperbilirubinaemia and study procedures. The training and the trial may have raised awareness on neonatal hyperbilirubinaemia, potentially resulting in a lower threshold to assess the neonate as jaundiced and to quantify TSB. From a clinical perspective, this can be considered a positive development in the context of preventing severe hyperbilirubinaemia.
The incidence of visible jaundice in our study is comparable to other studies in (near) term neonates in which 60–90% became jaundiced 27 , 28 , 29 . The finding that visual jaundice assessment is not reliable to estimate TSB levels is in line with other studies describing the inaccuracy of visual jaundice assessment 6 , 7 . Strikingly, in a substantial proportion of neonates being assessed as ‘quite yellow’ or ‘very yellow’, no TcB or TSB was quantified. This observation corresponds with a previous study among MCAs regarding neonatal hyperbilirubinaemia, which showed structural underestimation of TSB levels and common application of a so-called ‘wait-and-see approach’ in visibly jaundiced neonates 30 . Moreover, despite being strongly recommended by the national guideline 4 , TSB was not quantified in two neonates who developed visible jaundice within 24 h after birth. Also, five neonates did not receive phototherapy despite having a TSB level that exceeded the phototherapy threshold as defined by the national guideline 4 . Our evaluation of standard practice in this cohort highlights significant gaps in guideline application. In the current study, we did not prospectively explore the considerations underlying these decisions. Previous studies indicate that lack of knowledge on guideline recommendations 31 , 32 , and systematic underestimation of the severity of jaundice based on visual assessment likely contributed 30 . Other potential reasons for non-compliance may include a belief that the recommendations in the guideline do not reflect the best care for the neonate (e.g., a healthcare provider may consider the phototherapy thresholds too conservative as evidence on exact phototherapy thresholds is lacking 33 , and TSB quantification is avoided to keep the neonate in primary care), or practical challenges regarding feasibility of guideline compliance in daily practice. Research focused on these considerations may be useful to improve guideline adherence. Non-compliance to neonatal jaundice guidelines can have potentially severe consequences, as demonstrated by Rennie et al. in a Swedish study where KSD was (potentially) avoidable in 11 out of 13 neonates having KSD 9 . Additionally, a national audit indicated that non-compliance to the guideline was an important contributing factor to severe neonatal hyperbilirubinaemia in the Netherlands 34 .
Most studies assessing the burden of neonatal hyperbilirubinaemia focused on severe neonatal hyperbilirubinaemia or on KSD 12 , 13 , 14 , 15 , 16 , 17 . Studies assessing the hospitalisation rate for neonatal hyperbilirubinaemia showed incidences for hyperbilirubinaemia treatment ranging from 0.55 to 2.62% 35 , 36 , 37 , 38 , 39 . The retrospective nature of these studies in which the researchers depended on correct registration of the diagnosis of hyperbilirubinaemia may have contributed to the lower published incidence. Studies having phototherapy use as secondary outcome when assessing the institution of a bilirubin screening programme found (slightly) higher percentages in their control group (4.2–6.1%) 38 , 39 , 40 . The difference in the percentage of neonates necessitating hyperbilirubinaemia treatment between these studies and ours may also be attributed to other hyperbilirubinaemia assessment and management strategies. In the Netherlands, neonates are typically screened visually for neonatal hyperbilirubinaemia, followed by selective TSB quantification; universal TcB or TSB screening is not performed. Additionally, in the Netherlands a relatively high proportion of neonates are cared for in primary care shortly after birth, where transcutaneous bilirubinometers are not widely used yet. Our current evaluation of care-as-usual indicates that TcB or TSB is often not quantified even in neonates who were considered quite yellow or very yellow. As such, it is possible that some neonates requiring phototherapy were not identified. In other countries, most neonates remain admitted in the hospital for several days after birth and TcB or TSB is quantified before discharge 3 , 15 .
Potential risk factors for developing severe hyperbilirubinaemia have been widely investigated 3 , 33 . Whereas gestational age < 38 weeks is a well-known risk factor, instrumental delivery itself is not widely investigated as risk factor 16 , 41 , 42 , 43 . Most studies focus on bruising and cephalic haematomas, that may arise from an instrumental delivery, which increases the risk for severe neonatal hyperbilirubinaemia 16 , 41 . Instrumental delivery may be a marker for another risk factor (e.g., large for gestational age; LGA) 44 . However, we did not find a higher LGA incidence in neonates receiving phototherapy. Other well-known risk factors for hyperbilirubinaemia, such as Rh D incompatibility, previous siblings who received phototherapy, and exclusive breastfeeding, did not differ significantly between neonates who received phototherapy and those who did not. This may in part be due to limited power.
Findings from this study are useful for perinatal healthcare providers in primary care as well as in secondary and tertiary care (e.g., if a neonate is admitted together with mother). Data on the incidence of jaundice and the need for hyperbilirubinaemia treatment can help raise awareness regarding the extent of the problem. This awareness should also include the inaccuracy of visual jaundice assessment. Although this inaccuracy has been demonstrated previously 29 , 45 , 46 , our current study indicates that many healthcare professionals still strongly rely on visual assessment, and this is in fact in line with the current Dutch guideline, which is now undergoing revision. Although not every case of severe hyperbilirubinaemia results in KSD, KSD is entirely preventable and should clearly be a never-event. As such, regarding severe hyperbilirubinaemia as a healthcare system failure may strengthen implementation of new strategies to prevent KSD 47 . More objective approaches to universal hyperbilirubinaemia screening, for example using a transcutaneous bilirubinometer, should be considered to improve early recognition of potentially severe hyperbilirubinaemia 3 , 48 , 49 . Even though the PCBCs and their healthcare professionals took part in a trial focused on hyperbilirubinaemia assessment and management, the recommendations of the national guideline regarding TSB quantification and start of phototherapy treatment were not adhered to in some cases. Hence, more knowledge regarding risk factors for hyperbilirubinaemia, when to quantify TSB, treatment thresholds, and adherence to the national guideline are important as well. Future research should focus on objective approaches of universal screening for potentially severe neonatal hyperbilirubinaemia in a primary care setting. The STARSHIP Trial will present results from implementing a universal screening programme in primary care using TcB in the next year or two.
In this prospective cohort study embedded in the STARSHIP Trial, assessment, management and incidence of neonatal jaundice and the need for phototherapy were evaluated. We demonstrated that the vast majority of neonates had some degree of jaundice during admission and that phototherapy was provided in 3.6% of neonates. Also, we showed that visual jaundice assessment was inaccurate in determining hyperbilirubinaemia and that compliance to the guideline requires improvement. We suggest that awareness regarding neonatal hyperbilirubinaemia and its potentially devastating consequences should be raised. Additionally, the benefits of objective universal screening to improve recognition of hyperbilirubinaemia need to be assessed in an attempt to reduce the burden of neonatal hyperbilirubinaemia.
Data availability
The anonymised datasets from the current study are available from the corresponding author on reasonable request.
Code availability
The syntaxes from the current analyses are available from the corresponding author on reasonable request.
Olusanya, B. O., Kaplan, M. & Hansen, T. W. R. Neonatal hyperbilirubinaemia: A global perspective. Lancet Child Adolesc. Health 2 , 610–620. https://doi.org/10.1016/S2352-4642(18)30139-1 (2018).
Article PubMed Google Scholar
Le Pichon, J. B., Riordan, S. M., Watchko, J. & Shapiro, S. M. The neurological sequelae of neonatal hyperbilirubinemia: Definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDs). Curr. Pediatr. Rev. 13 , 199–209. https://doi.org/10.2174/1573396313666170815100214 (2017).
Article CAS PubMed Google Scholar
American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114 , 297–316. https://doi.org/10.1542/peds.114.1.297 (2004).
Article Google Scholar
Nederlandse Vereniging voor Kindergeneeskunde, Kwaliteitsinstituut voor de Gezondheidszorg CBO. Guideline prevention, diagnosis and treatment of hyperbilirubinaemia among newborns born at a gestational age of more than 35 weeks. Richtlijn preventie, diagnostiek en behandeling van hyperbilirubinemie bij de pasgeborene, geboren na een zwangerschapsduur van meer dan 35 weken. https://www.nvk.nl/Portals/0/richtlijnen/hyperbili/richtlijnhyperbili.pdf (2008).
Paediatric Association of the Netherlands. Nederlandse Vereniging voor Kindergeneeskunde. Bilirubin nomograms >35 weeks. Bilicurve >35 wkn. [Paediatric Association of the Netherlands] Nederlandse Vereniging voor Kindergeneeskunde. http://babyzietgeel.nl/kinderarts/hulpmiddelen/diagnostiek/bilicurve35wkn.php (2008).
Lease, M. & Whalen, B. Assessing jaundice in infants of 35-week gestation and greater. Curr. Opin. Pediatr. 22 , 352–365. https://doi.org/10.1097/MOP.0b013e328339603f (2010).
Maisels, M. J. et al. The natural history of jaundice in predominantly breastfed infants. Pediatrics 134 , e340–345. https://doi.org/10.1542/peds.2013-4299 (2014).
Donneborg, M. L., Hansen, B. M., Vandborg, P. K., Rodrigo-Domingo, M. & Ebbesen, F. Extreme neonatal hyperbilirubinemia and kernicterus spectrum disorder in Denmark during the years 2000–2015. J. Perinatol. 40 , 194–202. https://doi.org/10.1038/s41372-019-0566-8 (2020).
Alkén, J., Håkansson, S., Ekéus, C., Gustafson, P. & Norman, M. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in Sweden. JAMA Netw. Open 2 , e190858. https://doi.org/10.1001/jamanetworkopen.2019.0858 (2019).
Article PubMed PubMed Central Google Scholar
Bjerre, J. V., Petersen, J. R. & Ebbesen, F. Surveillance of extreme hyperbilirubinaemia in Denmark. A method to identify the newborn infants. Acta Paediatr. 97 , 1030–1034. https://doi.org/10.1111/j.1651-2227.2008.00879.x (2008).
Bhutani, V. K. et al. Extreme hyperbilirubinemia and rescue exchange transfusion in California from 2007 to 2012. J. Perinatol. 36 , 853–857. https://doi.org/10.1038/jp.2016.106 (2016).
Gotink, M. J. et al. Severe neonatal hyperbilirubinemia in the Netherlands. Neonatology 104 , 137–142. https://doi.org/10.1159/000351274 (2013).
Sgro, M., Campbell, D. & Shah, V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ 175 , 587–590. https://doi.org/10.1503/cmaj.060328 (2006).
Sgro, M., Campbell, D. M., Kandasamy, S. & Shah, V. Incidence of chronic bilirubin encephalopathy in Canada, 2007–2008. Pediatrics 130 , e886–890. https://doi.org/10.1542/peds.2012-0253 (2012).
Barrington KJ, Sankaran K, Canadian Paediatric Society, Fetus and Newborn Committee. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants. Paediatr. Child Health 12 , 1B–12B. https://doi.org/10.1093/pch/12.5.401 (2007).
Kuzniewicz, M. W. et al. Risk factors for severe hyperbilirubinemia among infants with borderline bilirubin levels: A nested case-control study. J. Pediatr. 153 , 234–240. https://doi.org/10.1016/j.jpeds.2008.01.028 (2008).
Slusher, T. M. et al. Burden of severe neonatal jaundice: A systematic review and meta-analysis. BMJ Paediatr. Open 1 , e000105. https://doi.org/10.1136/bmjpo-2017-000105 (2017).
van der Geest, B. A. M. et al. Screening and treatment to reduce severe hyperbilirubinaemia in infants in primary care (STARSHIP): A factorial stepped-wedge cluster randomised controlled trial protocol. BMJ Open 9 , e028270. https://doi.org/10.1136/bmjopen-2018-028270 (2019).
Perined. Perinatal care in the Netherlands anno 2018: national perinatal figures and interpretation. Perinatale zorg in Nederland anno 2018: landelijke perinatale cijfers en duiding (Perined, 2019).
Google Scholar
Lagendijk, J., Steegers, E. A. P. & Been, J. V. Inequity in postpartum healthcare provision at home and its association with subsequent healthcare expenditure. Eur. J. Public Health 29 , 849–855. https://doi.org/10.1093/eurpub/ckz076 (2019).
de Boer, J. & Zondag, L. Multidisciplinary guideline postnatal care. Multidisciplinaire richtlijn Postnatale Zorg - Verloskundige basiszorg voor moeder en kind. Koninklijke Nederlandse Organisatie van Verloskundigen. https://www.knov.nl/serve/file/knov.nl/knov_downloads/2882/file/Postnatale_zorg_opgemaakte_versie_door_IB_md_10_aug_2018.pdf (2018).
Schmitz, C. LimeSurvey: An open source survey tool. LimeSurvey Project, Hamburg, Germany (2012).
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Guideline for Good Clinical Practice ICH (2016).
Statistics Netherlands. Centraal Bureau voor de Statistiek. Person with a western migration background. Statistics Netherlands Centraal Bureau voor de Statistiek. https://www.cbs.nl/en-gb/onze-diensten/methods/definitions/person-with-a-western-migration-background.2021 (2016).
Hoftiezer, L. et al. From population reference to national standard: New and improved birthweight charts. Am. J. Obstet. Gynecol. 220 , 383.e381–383.e317. https://doi.org/10.1016/j.ajog.2018.12.023 (2019).
Perined. Peristat. http://www.peristat.nl (2020).
Hansen, T. W. R. The epidemiology of neonatal jaundice. Pediatr. Med. https://doi.org/10.21037/pm-21-4 (2021).
Osborn, L. M., Reiff, M. I. & Bolus, R. Jaundice in the full-term neonate. Pediatrics 73 , 520–525 (1984).
Article CAS Google Scholar
Keren, R., Tremont, K., Luan, X. & Cnaan, A. Visual assessment of jaundice in term and late preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 94 , F317–F322. https://doi.org/10.1136/adc.2008.150714 (2009).
van der Geest, B. A. M., Theeuwen, I. M., Reiss, I. K. M., Steegers, E. A. P. & Been, J. V. Assessing knowledge and skills of maternity care professionals regarding neonatal hyperbilirubinaemia: A nationwide survey. BMC Pregnancy Childbirth 21 , 63. https://doi.org/10.1186/s12884-020-03463-0 (2021).
Wakkee, M. et al. Knowledge, attitudes and use of the guidelines for the treatment of moderate to severe plaque psoriasis among Dutch dermatologists. Br. J. Dermatol. 159 , 426–432. https://doi.org/10.1111/j.1365-2133.2008.08692.x (2008).
Lugtenberg, M., Zegers-van Schaick, J. M., Westert, G. P. & Burgers, J. S. Why don’t physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 4 , 54. https://doi.org/10.1186/1748-5908-4-54 (2009).
National Collaborating Centre for Women's and Children's Health. Neonatal jaundice. National Institute for Health and Clinical Excellence. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0016565/pdf/PubMedHealth_PMH0016565.pdf (2010).
van der Geest, B. A. M. et al. Severe neonatal hyperbilirubinaemia: Lessons learnt from a national perinatal audit. Arch. Dis. Child Fetal Neonatal Ed. https://doi.org/10.1136/archdischild-2021-322891 (2022).
Eggert, L. D., Wiedmeier, S. E., Wilson, J. & Christensen, R. D. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics 117 , e855–e862. https://doi.org/10.1542/peds.2005-1338 (2006).
Burgos, A. E., Schmitt, S. K., Stevenson, D. K. & Phibbs, C. S. Readmission for neonatal jaundice in California, 1991–2000: Trends and implications. Pediatrics 121 , e864–869. https://doi.org/10.1542/peds.2007-1214 (2008).
Burke, B. L. et al. Trends in hospitalizations for neonatal jaundice and kernicterus in the United States, 1988–2005. Pediatrics 123 , 524–532. https://doi.org/10.1542/peds.2007-2915 (2009).
Wainer, S., Parmar, S. M., Allegro, D., Rabi, Y. & Lyon, M. E. Impact of a transcutaneous bilirubinometry program on resource utilization and severe hyperbilirubinemia. Pediatrics 129 , 77–86. https://doi.org/10.1542/peds.2011-0599 (2012).
Kuzniewicz, M. W., Escobar, G. J. & Newman, T. B. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics 124 , 1031–1039. https://doi.org/10.1542/peds.2008-2980 (2009).
Mah, M. P. et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics 125 , e1143–e1148. https://doi.org/10.1542/peds.2009-1412 (2010).
Newman, T. B., Xiong, B., Gonzales, V. M. & Escobar, G. J. Prediction and prevention of extreme neonatal hyperbilirubinemia in a mature health maintenance organization. Arch. Pediatr. Adolesc. Med. 154 , 1140–1147. https://doi.org/10.1001/archpedi.154.11.1140 (2000).
Maisels, M. J. & Kring, E. Length of stay, jaundice, and hospital readmission. Pediatrics 101 , 995–998. https://doi.org/10.1542/peds.101.6.995 (1998).
Keren, R. et al. A comparison of alternative risk-assessment strategies for predicting significant neonatal hyperbilirubinemia in term and near-term infants. Pediatrics 121 , e170–e179. https://doi.org/10.1542/peds.2006-3499 (2008).
Keren, R. et al. Identifying newborns at risk of significant hyperbilirubinaemia: A comparison of two recommended approaches. Arch. Dis. Child. 90 , 415–421. https://doi.org/10.1136/adc.2004.060079 (2005).
Article CAS PubMed PubMed Central Google Scholar
Moyer, V. A., Ahn, C. & Sneed, S. Accuracy of clinical judgment in neonatal jaundice. Arch. Pediatr. Adolesc. Med. 154 , 391–394. https://doi.org/10.1001/archpedi.154.4.391 (2000).
Riskin, A., Tamir, A., Kugelman, A., Hemo, M. & Bader, D. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia?. J. Pediatr. 152 (782–787), 787.e1–2. https://doi.org/10.1016/j.jpeds.2007.11.003 (2008).
Bhutani, V. K. & Johnson, L. A proposal to prevent severe neonatal hyperbilirubinemia and kernicterus. J. Perinatol. 29 , S61–S67. https://doi.org/10.1038/jp.2008.213 (2009).
Bhutani, V. K., Vilms, R. J. & Hamerman-Johnson, L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J. Perinatol. 30 (Suppl), S6-15. https://doi.org/10.1038/jp.2010.98 (2010).
Maisels, M. J. et al. Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics 124 , 1193–1198. https://doi.org/10.1542/peds.2009-0329 (2009).
Download references
Acknowledgements
We acknowledge the participants, their parent(s), and the personnel of the participating primary care birth centres for their efforts. We thank Martin Baartmans, Jolita Bekhof, Esther Bijl, Harry Buijs, Jan Erik Bunt, Peter Dijk, Marja Huizer, Christian Hulzebos, Ralph Leunissen, Beata Pazur, Ben Snoeren, Bente de Vries, and Leo Wewerinke for their support in initiating and conducting the STARSHIP Trial; Paul den Butter, Maurits Schreuder, and Gerdien Tramper-Stranders for providing data regarding hospital admissions; and Melissa Gouvernante, Janine Hageman, Joen IJsselmuiden, Kristel Kolloen, Deborah van Urk, Lisanne Vermeulen-Nieuwelink, Sanne van de Walle, Lina Wang, Senna Worlanyoh, and Miranda van Zwet for their help in data collection.
This work was supported by The Netherlands Organisation for Health Research and Development (ZonMw), grant number 843002805, and an Erasmus MC Efficiency Research grant, grant number 2016-16107. The funders had no role in the design of the study, data collection, analysis and interpretation of the data, writing the manuscript, nor in the decision to submit the manuscript for publication.
Author information
A list of authors and their affiliations appears at the end of the paper.
Authors and Affiliations
Division of Neonatology, Department of Paediatrics, Erasmus MC–Sophia Children’s Hospital, University Medical Centre Rotterdam, Rotterdam, The Netherlands
Berthe A. M. van der Geest, Malou J. S. de Mol, Ivana S. A. Barendse, René F. Kornelisse, Irwin K. M. Reiss & Jasper V. Been
Division of Obstetrics and Fetal Medicine, Department of Obstetrics and Gynaecology, Erasmus MC, University Medical Centre Rotterdam, Rotterdam, The Netherlands
Berthe A. M. van der Geest, Malou J. S. de Mol, Ivana S. A. Barendse, Johanna P. de Graaf, Loes C. M. Bertens, Eric A. P. Steegers & Jasper V. Been
Institute for Medical Technology Assessment (iMTA), Erasmus University Rotterdam, Rotterdam, The Netherlands
Marten J. Poley
Intensive Care and Department of Paediatric Surgery, Erasmus MC–Sophia Children’s Hospital, University Medical Centre Rotterdam, Rotterdam, The Netherlands
Department of Paediatrics, Intensive Care Unit, Erasmus MC–Sophia Children’s Hospital, University Medical Centre Rotterdam, Rotterdam, The Netherlands
Department of Internal Medicine, Nursing Science, Erasmus MC, University Medical Centre Rotterdam, Rotterdam, The Netherlands
Department of Public Health, Erasmus MC, University Medical Centre Rotterdam, Rotterdam, The Netherlands
Jasper V. Been
Department of Paediatrics, Maasstad Hospital, Rotterdam, The Netherlands
- Martin G. A. Baartmans
Department of Paediatrics, Isala–Amalia Children’s Clinic, Zwolle, The Netherlands
Jolita Bekhof
Primary Care Birth Centre Haga, The Hague, The Netherlands
Harry Buijs
Primary Care Birth Centre Maasstad, Rotterdam, The Netherlands
Department of Paediatrics, Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands
Jan Erik Bunt
Department of Neonatology, University Medical Centre Groningen–Beatrix Children’s Hospital, University of Groningen, Groningen, The Netherlands
Peter H. Dijk & Christian V. Hulzebos
Department of Paediatrics, Haaglanden Medical Centre Westeinde, The Hague, The Netherlands
Ralph W. J. Leunissen
Primary Care Birth Centre Fam, Tilburg, The Netherlands
Ben J. P. W. Snoeren
Primary Care Birth Centre Westeinde, The Hague, The Netherlands
Bente de Vries
Department of Paediatrics, Haga Hospital–Juliana Children’s Hospital, The Hague, The Netherlands
Leo Wewerinke
You can also search for this author in PubMed Google Scholar
- , Jolita Bekhof
- , Harry Buijs
- , Jan Erik Bunt
- , Peter H. Dijk
- , Christian V. Hulzebos
- , Ralph W. J. Leunissen
- , Ben J. P. W. Snoeren
- , Bente de Vries
- & Leo Wewerinke
Contributions
The STARSHIP Trial was designed by J.V.B., J.P.D.G., R.F.K., L.C.M.B., M.J.P., E.I., I.K.M.R., and E.A.P.S. Funding for the STARSHIP Trial was secured by J.V.B., J.P.D.G., R.F.K., I.K.M.R., and E.A.P.S. The members of the STARSHIP study group were involved in initiating and conducting the research in the participating PCBCs. B.A.M.v.d.G. and J.V.B. designed the current analysis of the control period of the STARSHIP Trial. Data was collected by B.A.M.v.d.G., M.J.S.d.M., and I.S.A.B. B.A.M.v.d.G., M.J.S.d.M., and I.S.A.B. analysed the data. B.A.M.v.d.G. and J.V.B. wrote the first version of the manuscript. All authors were involved in the interpretation of the data. The manuscript was critically revised and approved for final submission by all authors.
Corresponding authors
Correspondence to Berthe A. M. van der Geest or Jasper V. Been .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
van der Geest, B.A.M., de Mol, M.J.S., Barendse, I.S.A. et al. Assessment, management, and incidence of neonatal jaundice in healthy neonates cared for in primary care: a prospective cohort study. Sci Rep 12 , 14385 (2022). https://doi.org/10.1038/s41598-022-17933-2
Download citation
Received : 17 November 2021
Accepted : 03 August 2022
Published : 23 August 2022
DOI : https://doi.org/10.1038/s41598-022-17933-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative

This article is cited by
Neonatal jaundice detection in low-resource mexican settings: possibilities and barriers for innovation with mobile health.
- Gabriela Jiménez-Díaz
- Anders Aune
- Jennifer J. Infanti
BMC Health Services Research (2024)
The correlation between serum total bile acid and alanine aminotransferase of pregnant women and the disorders of neonatal hyperbilirubinemia-related amino acid metabolism
- Xizhenzi Fan
- Huijuan Rong
- Tianxiao Yu
BMC Pregnancy and Childbirth (2024)
Risk of childhood neoplasms related to neonatal phototherapy- a systematic review and meta-analysis
- Ilari Kuitunen
- Atte Nikkilä
- Anssi Auvinen
Pediatric Research (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

A Case of Obstructive Jaundice
Um caso de icterícia obstrutiva, nuno veloso, rogério godinho.
- Author information
- Article notes
- Copyright and License information
Corresponding author. [email protected]
Received 2013 Sep 13; Accepted 2014 Sep 24; Collection date 2015 Jan-Feb.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
An 84-year-old woman presented with a 2-day history of jaundice, fever and abdominal pain. Physical examination showed scleral icterus and right upper quadrant tenderness without inspiratory arrest at palpation (absent Murphy's sign). Laboratory workup revealed leukocytosis (12.4 × 10 3 μL), elevated C-reactive protein (8.3 mg/dL) and cholestasis (bilirubin 5.4 mg/dL, alkaline phosphatase 893 U/L, gamma-glutamyl transferase 1143 U/L) with elevated liver enzymes (aspartate aminotransferase 231 U/L, alanine aminotransferase 178 U/L). Abdominal ultrasound demonstrated a scleroatrophic gallbladder with cholelithiasis and an impacted large gallstone in the common bile duct with dilated common and intrahepatic bile ducts.
We performed an endoscopic retrograde cholangiopancreatography (ERCP) that clearly showed common hepatic duct compression by a large gallstone (20 mm) impacted in the cystic duct ( Fig. 1 ), compatible with the diagnosis of Mirizzi syndrome. Successful biliary decompression was performed by internal stenting ( Fig. 2 ) with subsequent patient referral to surgery (cholecystectomy plus closure of the fistula).
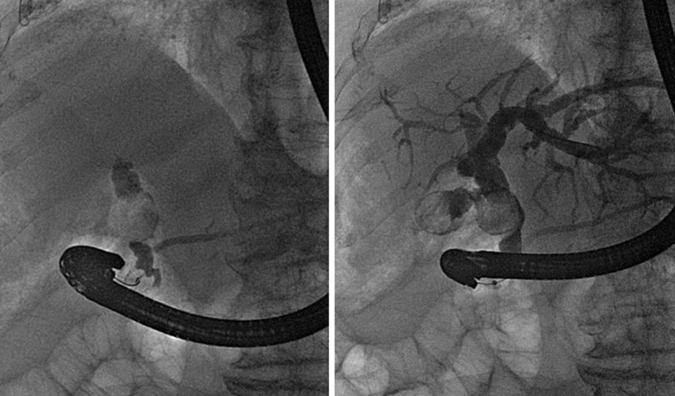
ERCP: cholangiography.
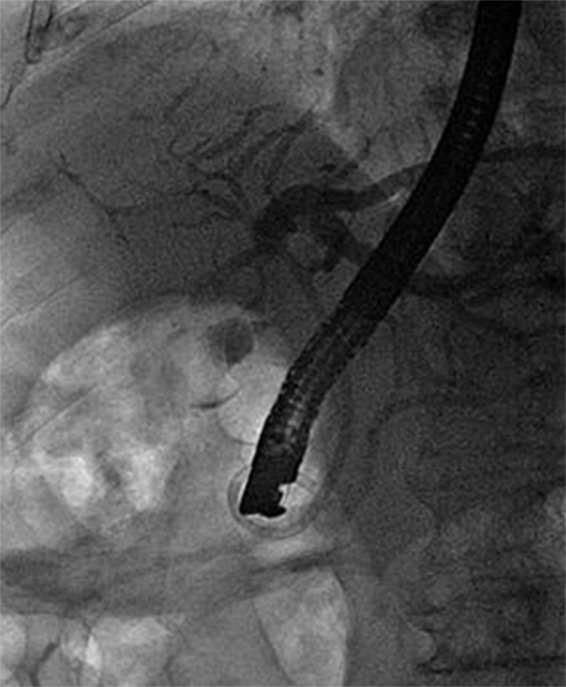
ERCP: internal stenting.
The Mirizzi syndrome refers to common hepatic duct obstruction caused by an extrinsic compression from an impacted stone in the cystic duct or Hartmann's pouch of the gallbladder. 1 The majority of the patients present the clinical triad of jaundice, fever, and right upper quadrant pain, showing in the laboratory evaluation elevations in the serum concentrations of alkaline phosphatase and bilirubin. 2
The Mirizzi syndrome is part of the differential diagnosis of obstructive jaundice and therefore the diagnostic approach usually begins with ultrasonography complemented by ERCP or magnetic resonance cholangiography.
A useful classification system takes into account the presence and extent of a cholecystobiliary fistula, due to erosion of the anterior or lateral wall of the common bile duct by impacted stones. 3
Surgery is the mainstay of therapy for Mirizzi syndrome. 4 ERCP treatment can be effective as a temporizing measure before surgery and can be definitive treatment for unsuitable surgical candidates.
Ethical disclosures
Protection of human and animal subjects.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data
The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent
The authors declare that no patient data appear in this article.
- 1. Alberti-Flor J.J., Iskandarani M., Jeffers L., Schiff E.R. Mirizzi syndrome. Am J Gastroenterol. 1985;80:822. [ PubMed ] [ Google Scholar ]
- 2. Binmoeller K.F., Thonke F., Soehendra N. Endoscopic treatment of Mirizzi's syndrome. Gastrointest Endosc. 1993;39:532. doi: 10.1016/s0016-5107(93)70165-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Csendes A., Díaz J.C., Burdiles P., Maluenda F., Nava O. Mirizzi syndrome and cholecystobiliary fistula: a unifying classification. Br J Surg. 1989;76:1139. doi: 10.1002/bjs.1800761110. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Kwon A.H., Inui H. Preoperative diagnosis and efficacy of laparoscopic procedures in the treatment of Mirizzi syndrome. J Am Coll Surg. 2007;204:409. doi: 10.1016/j.jamcollsurg.2006.12.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (604.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Maternal disease factors associated with neonatal jaundice: a case–control study
Youngjae yu, jinwha choi, myeong hoon lee, kanghyun kim, hyun mee ryu, hyun wook han.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Contributed equally.
Received 2021 Nov 17; Accepted 2022 Feb 28; Collection date 2022.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Neonatal jaundice is common, and despite the considerable medical costs associated with it, there are still few studies on the maternal factors associated with it. Identification of maternal factors associated with neonatal jaundice is very important in terms of prevention, screening and management of neonatal jaundice. The current study aimed to identify maternal disease factors associated with neonatal jaundice.
We compared the maternal disease diagnostic codes during pregnancy (study A) and 1 year before conception (study B) in mothers whose insurance claims data included newborns treated for neonatal jaundice before birth registration via the National Health Insurance Service–National Sample Cohort (control group). To decrease the effect of confounding variables, the neonatal jaundice and control groups were matched at a ratio of 1:10 via propensity score matching using covariates including age and income.
The matched samples for studies A and B included 4,026 and 3,278 (jaundice group: 366 and 298) delivery cases, respectively. In both studies, the jaundice group had a higher proportion of patients who underwent cesarean section than the control group. In study A, other diseases of the digestive system had the highest odds ratio (OR) (K92; adjusted OR: 14.12, 95% confidence interval [CI]: 2.70–82.26). Meanwhile, gastritis and duodenitis had the lowest OR (K29; adjusted OR: 0.39, 95% CI: 0.22–0.69). In study B, salpingitis and oophoritis had the highest OR (N70; adjusted OR: 3.33, 95% CI: 1.59–6.94). Heartburn had the lowest OR (R12; adjusted OR: 0.29, 95% CI:0.12–0.71).
Conclusions
This study identified maternal disease factors correlated with neonatal jaundice during pregnancy and 1 year before conception. Maternal risk factors for neonatal jaundice included syphilis and leiomyoma during pregnancy, and salpingo-oophoritis before pregnancy. The protective factors included infection, inflammatory diseases, and dyspepsia.
Keywords: Maternal disease factors, Neonatal jaundice, Prevention, Prediction, Disease network, National Health Insurance Service–National Sample Cohort
Backgrounds
Neonatal jaundice is a common disease [ 1 ]. In Korea, it is the most common cause of admission among newborns [ 2 ], and medical expenses correlated with jaundice exceeded $10 million in 2012 [ 3 ]. Moreover, it still poses global burden particularly in low- and middle-income countries where the immediate assessment of serum bilirubin concentration is challenging and treatment is often delayed [ 4 , 5 ]. Moreover, recent studies have reported that neonatal jaundice may be a risk factor for pediatric diseases such as asthma [ 6 , 7 ], autism spectrum disorders [ 8 , 9 ], attention deficit hyperactivity disorder (ADHD) [ 10 ], and epilepsy [ 11 ]. Therefore, identifying maternal risk factors for neonatal jaundice is important in providing cost-effective healthcare expenditure and predicting jaundice-associated diseases.
However, recent studies have not assessed these factors and only a few predisposing factors, including maternal age, race, primiparity, teenage pregnancy, diabetes mellitus, Rh incompatibility, ABO incompatibility, oxytocin use during labor, and breastfeeding, were identified [ 4 , 12 ]. These factors were demographic or pregnancy-related, and there has been no study to identify risk factors for neonatal jaundice related to the mother's own disease.
Many studies so far were cross-sectional studies [ 13 , 14 ], and nation-wide study focused on neonatal jaundice requiring the treatment in clinical situation has not been done well. In Korea, all citizens are covered by the National Health Insurance [ 15 ], and a database for claims data has been established [ 16 ]. Moreover, the antenatal care (ANC) coverage of married women approaches about 100%, and the average number of antenatal care visits is over 13 times [ 17 ]. Based on the high ANC coverage and the longitudinal data on individuals, the study on the gestation or antenatal period can be conducted appropriately. Hence, the current study aimed to analyze the maternal disease risk factors for neonatal jaundice during pregnancy and 1 year before conception using data from the National Health Insurance claims database.
Data source and variables
The National Health Insurance Service–National Sample Cohort (NHIS-NSC) established by the National Health Insurance Service in South Korea was used [ 16 ]. This database (DB) is a representative sample that randomly selected 1 million people, accounting for about 2.2% of the Korean population in 2002. Moreover, it contains sample data obtained from 2002 to 2013 [ 18 ]. In this study, the qualification DB and treatment DB of the NHIS-NSC were used. Five variables of the qualification DB (patient ID, sex, year, age, and income rank), five variables of statement data (patient ID, claim number, visit date, principal diagnosis, and additional diagnosis), and three variables of type of disease data (claim number, visit date, and diagnosis) in the treatment DB were utilized. Age is divided into 19 groups from 0 to 85 years old at 5-year intervals (age 0, 1–4, 5–9, …, and over 84). As the age of participants considered in this study was 15–49 years old, it was regrouped subsequently into three groups with ages 15–24, 25–34, and 35–49. The income rank is divided into 11 groups at deciles with medical aid beneficiaries, and it was regrouped into 5 groups at 20% intervals. Variables about diagnosis were distinguished using the Korean Standard Classification of Diseases, version 6 (KCD-6), which is the Korean modified version of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10).
All methods were carried out in accordance with relevant guidelines and regulations.
Data preprocessing and case selection
The principal diagnosis and additional diagnosis of statement data were integrated into one diagnostic variable and were then merged with the type of disease data according to claim number. Based on the merged diagnosis data, details regarding delivery and age of the participants (15–49 years old) at the year of delivery date were extracted. Then, the pregnancy records of patients who had data about delivery were collected. In cases in which treatment for neonatal jaundice were provided before birth registration, when insurance claims were made by the mother, the diagnosis of neonatal jaundice is included in the mother’s record. Hence, these cases were included in the jaundice group. Cases with diagnostic codes correlated with neonatal jaundice within 4 weeks after the delivery date were included in the jaundice group. Meanwhile, the control group included cases in which the diagnosis of neonatal jaundice was not attached to the mother.
In this study, KCD-6 codes related to delivery [ 19 – 21 ], pregnancy [ 19 , 22 , 23 ] and neonatal jaundice were selected to identify each event. Preterm delivery and multiple gestation were defined as at least one record of the related codes within 4 weeks before and after the delivery date.
As the NHIS-NSC included a sample established from the claims data, but not designed for the study, the diagnoses entered in the records did not always indicate new-onset diseases. The same diagnosis codes might have been recorded repeatedly. In such a case, it was counted as one. If the same person delivered several times, each delivery was considered an independent case. The minimum interval from delivery to diagnosis of the next pregnancy was 4 weeks. Considering that periviable birth is defined as delivery during at least 20 weeks of gestation [ 24 ], the minimum interval from the previous to the next delivery was 24 weeks. The maximum duration from the diagnosis of pregnancy to delivery was 44 weeks [ 25 ]. After cross-joining pregnancy and delivery records, the joint records were listed chronologically. The date of pregnancy diagnosis was defined as the visit date of the first pregnancy record among all pregnancy records. Cases with diagnostic codes related to abortion (O00-O08, pregnancy with abortive outcome) [ 22 , 23 , 26 ] or stillbirths (O36.4, maternal care for intrauterine death; Z37.1, single stillbirth; Z37.4, twins, both stillborn; and Z37.7, other multiple births, all stillborn) [ 19 , 22 , 26 ] up to 4 weeks after the delivery date were excluded. Deliveries assigned with codes including O82 and O84.2 (multiple delivery, all via cesarean section) were considered as cesarean section. Further, deliveries assigned with codes such as O80, O81, O83, and O84.0 (multiple delivery, all spontaneous) and O84.1 (multiple delivery, all using forceps and vacuum extractor) were considered as vaginal. If the two types of delivery were present, cesarean section (O82, O84.2) was prioritized. Cases in which the type of delivery was not identified were excluded.
In this study, two studies, study A and B, were conducted. One was about diseases during ANC (study A), and the second was about diseases 1 year before ANC (study B). In study A, claims data from each pregnancy diagnosis date to day 1 before the delivery date were extracted. Cases that had no record in the ANC period, other than diagnostic codes correlated with pregnancy, were excluded to identify possible maternal risk factors. In study B, claims data from 1 year before each pregnancy diagnosis date to day 1 before the pregnancy diagnosis date were extracted. In the analyses of both two studies, the only first three characters of the diagnosis codes were used.
Statistical analysis
The two-sided Fisher’s exact test with 95% CI for the categorical variables was performed. The t -test was used to assess continuous variables. To decrease the effect of confounding variables, the jaundice and control groups were matched at a ratio of 1:10 via propensity score matching (PSM) with nearest neighbor matching. Age at the time of delivery and income at the time of pregnancy diagnosis were considered covariates. MatchIt package [ 27 ] was used to perform PSM. The results obtained by repeating PSM 1,000 times by randomly shuffling the order of records were used for the analysis of matched samples. The average number of cases, odds ratio, and p -value were calculated only for significant findings from the 1,000 results obtained using PSM. If the odds ratio was infinite, it was excluded from the average. Diseases that have more than 900 significant results, with a mean odds ratio of > 1 and a mean p -value of < 0.05, were considered a risk factor. Moreover, those with a mean odds ratio of < 1 and a mean p -value of < 0.05 were considered a protective factor. Conditional logistic regression analyses, adjusted for preterm delivery, delivery mode, multiple gestation and ANC duration, were performed for the diseases which have more than 900 significant results in the univariable analyses. Survival package [ 28 ] was used to perform conditional logistic regression analyses.
Results with a lower bound of > 1 or an upper bound of < 1 and a p -value of < 0.05 were considered significant. igraph package [ 29 ] was used to make a network image for the identified risk/protective factors. R (version 3.6.2) [ 30 ] was used in all analyses.
Demographic characteristics of the participants
Figure 1 shows the flowchart of the case selection process. 555,474 of 560,645 women, included in NHIS-NSC from 2002 to 2013, had significant claims data about diagnoses. 67,967 of those 555,474 women had claims data related to diagnosis of delivery and were 15 to 49 years old at the time of the delivery. 65,442 participants of them, who had delivery records, had claims data related to the diagnosis of pregnancy. With pairing the delivery and pregnancy records, 91,477 delivery cases (64,723 women), satisfied with the time intervals which were defined as inclusion criteria in this study, were identified. 116 cases of 91,477 delivery cases were excluded as the delivery modes were not identified, and 131 cases of abortion or stillbirth were excluded subsequently. The participants with several delivery cases were included in each step unless all cases were excluded. Among 91,230 delivery cases, 5,111 cases incomplete on qualification DB around the gestation period and 7,800 cases that had no diagnosis records except pregnancy or delivery during ANC were excluded to consist of the sample for study A. For study B, 12,691 incomplete cases and 4,418 cases with no diagnosis records during ANC were excluded.
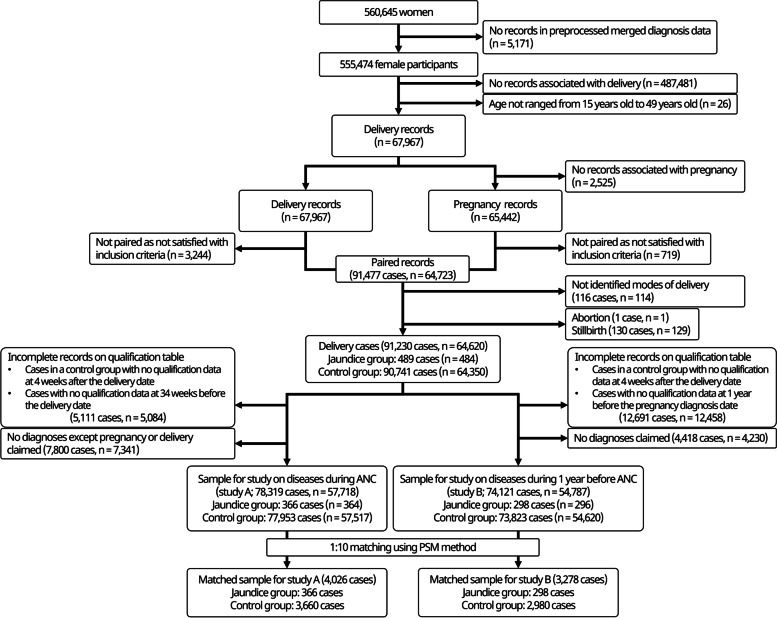
Flowchart of the inclusion and exclusion process. ANC, antenatal care; PSM, propensity score matching
The sample in study A included 78,319 cases ( n = 57,718). Among them, 366 cases ( n = 364) were included in the jaundice group and 77,953 cases ( n = 57,517) in the control group. The sample in study B had 74,121 cases ( n = 54,787). Among them, 298 cases ( n = 296) were included in the jaundice group and 73,823 cases ( n = 54,620) in the control group. The n value indicated the number of mothers, not delivery cases. If a mother has delivered several times, it can be included in both the jaundice and control groups. Thus, the total number of patients in the jaundice and control groups did not correspond to the total population. The jaundice group accounted for 0.47% (366 in 78,319 cases) and 0.40% (298 in 74,121 cases) of all delivery cases in studies A and B, respectively. There was a significant difference in terms of income at the time of pregnancy diagnosis, multiple gestation, and ANC duration between the two groups in study A, but not in study B. However, the Cochran–Armitage trend test result (chi-square test for trend in proportion) for income was significant in studies A and B ( p -value = 0.002 and 0.010, respectively). There was a significant difference in the mode of delivery between the two groups in studies A and B (Table 1 ).
Demographic data of the unmatched samples
The number and ratio of cases in each group were presented as N (%), except for the duration of ANC that was expressed as mean ± standard deviation days
ANC Antenatal care
* Significance at p -value of < 0.05
a age at the time of delivery, b income at the time of pregnancy diagnosis
The matched sample for study A had 4,026 cases (jaundice group: 366, control group: 3,660), and that for study B had 3,278 cases (jaundice group: 298, control group: 2,980). All the 1,000 matched samples significantly differed in terms of the type of delivery in both two studies (Table 2 ). There was also a significant difference in ANC duration in all the 1,000 matched samples of study A.
Demographic data of the matched samples
The number and ratio of cases in each group were presented as N (%), except for the duration of ANC that was expressed as mean ± standard deviation days. The average number of cases and mean p -values from 1,000 matched samples were presented
* Significance at p -value < 0.05
a P -value from the 670 matched samples were significant, with a mean of 0.027
b P -value from the 15 matched samples were significant, with a mean of 0.031
c P -value from the 541 matched samples were significant, with a mean of 0.032
Odds ratios for each diagnosis code in the unmatched samples
Tables 3 and 4 show the unadjusted odds ratios for neonatal jaundice according to disease that showed significant results in unmatched samples for studies A and B, respectively.
Maternal diseases during antenatal care and their unadjusted odds ratio for neonatal jaundice obtained from the unmatched samples (study A)
The number and ratio of cases in each group are presented as N (%)
CI Confidence interval
Maternal diseases during 1 year before antenatal care and their unadjusted odds ratio for neonatal jaundice obtained from the unmatched samples (study B)
The number and ratio of cases in each group were expressed as N (%)
In study A, obstetrical tetanus (A34) had the largest OR (212.33, 95% CI: 2.71–14,121.54). Fever of other and unknown origin (R50) had the lowest OR (0.23, 95% CI: 0.03–0.84).
In study B, polyarteritis nodosa and related conditions (M30) had the largest OR (95% CI: 6.35-infinite). However, there was only case in the jaundice group. Pain associated with micturition (R30) had the lowest OR (0, 95% CI: 0–0.92).
Acute bronchitis (J20), vasomotor and allergic rhinitis (J30), gastritis and duodenitis (K29), dyspepsia (K30), and alopecia areata (L63) showed significance in the unmatched samples of the two studies. Among them, the OR of alopecia areata (L63) was > 1.
Risk and protective factors in the matched samples
For diseases that showed significance more than 900 times in 1000 times of PSM, the average number of cases and average odds ratio are depicted in Fig. 2 (network image) and Table 5 . Adjusted ORs were calculated for the diseases which have more than 900 significant results in the univariable analyses, as the primary outcome of this study.
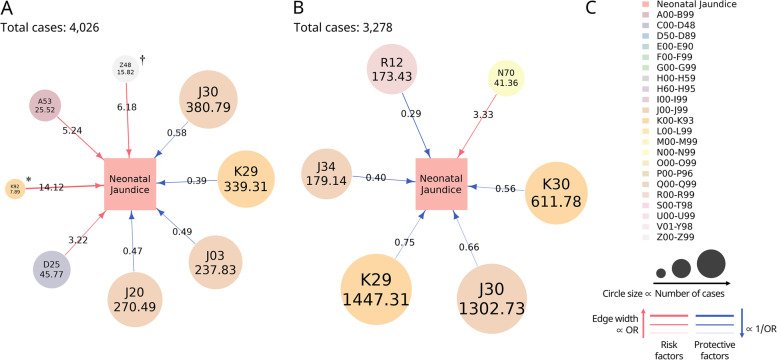
Disease network image about maternal risk factors and protective factors for neonatal jaundice . A Maternal diseases during ANC associated with neonatal jaundice (Study A). B Maternal diseases during 1 year before ANC associated with neonatal jaundice (Study B). C Index. Risk factors are illustrated as red lines and protective factors as blue lines. The average odds ratio is represented by number on the line and the average number of cases as the number in the circle. The major classification of diagnosis codes is represented in a different color. The circle size is proportional to the number of cases. The edge width is proportional to the odds ratio in the case of risk factors and inversely proportional to the odds ratio in the case of protective factors. ANC, antenatal care; A53, other and unspecified syphilis; D25, leiomyoma of the uterus; D62, acute posthemorrhagic anemia; J03, acute tonsillitis; J20, acute bronchitis; J30, vasomotor and allergic rhinitis; J34, other disorders of the nose and nasal sinuses; K29, gastritis and duodenitis; K30, dyspepsia; K92, other diseases of the digestive system; N70, salpingitis and oophoritis; R12, heartburn; Z48, other surgical follow-up care. * K92, 7.89; † Z48, 15.82
Maternal diseases associated with neonatal jaundice identified from the matched samples in studies A and B
The number and ratio of cases in each group were presented as N (%). The average number of cases, average odds ratio, and mean p -values for significant results from 1,000 matched samples were calculated. Conditional logistic regression analyses, adjusted for preterm delivery, delivery mode, multiple gestation and ANC duration, were performed for the diseases showing more than 900 significant results in the univariable analyses
In study A, among the probable risk factors, the disease with the highest OR was other diseases of digestive system (K92; adjusted OR: 14.12, 95% CI: 2.70–82.26), which was present in 0.20% of all matched cases, and the incidence of leiomyoma of the uterus was the highest (D25; adjusted OR: 3.22, 95% CI: 1.59–6.52), accounting for 1.14% of all matched cases. Among the probable protective factors, gastritis and duodenitis had the lowest OR (K29; adjusted OR: 0.39, 95% CI: 0.22–0.69), accounting for 8.43% of all matched cases, and the incidence of vasomotor and allergic rhinitis was the highest (J30; adjusted OR: 0.58, 95% CI: 0.37–0.92), which accounted for 9.46% of all matched cases (Fig. 2 a).
In study B, the possible risk factor was salpingitis and oophoritis (N70; adjusted OR: 3.33, 95% CI: 1.59–6.94), which accounted for 1.26% of all matched cases. Among the probable protective factors, heartburn had lowest OR (R12; adjusted OR: 0.29, 95% CI: 0.12–0.71), which accounted for 5.29% of all cases, and the incidence of gastritis and duodenitis (K29; adjusted OR: 0.75, 95% CI: 0.58–0.95) was the highest, which accounted for 44.15% of all cases (Fig. 2 b).
There was no common risk factor in both studies. However, the common protective factors included vasomotor and allergic rhinitis (J30) and gastritis and duodenitis (K29).
Many diseases were identified as significant disease factors from unmatched samples in Table 3 and 4 . Because these results were obtained from the unmatched samples, bias should be considered to interpret results. However, diseases with a genetic factor, such as disorders of glycoprotein metabolism from the unmatched sample of study B, may be attributed to the low incidence of these diseases. Therefore, these rare diseases are needed to be verified in a larger population. Meanwhile, some diseases identified from unmatched samples showed significance in hundreds of matched samples, but less than 900. These were not considered as risk factors in this study but may have a weak association with neonatal jaundice. For example, among the disease with OR > 1, alopecia areata showed significance in more than 700 matched samples of study B, which indicates that it may be a maternal risk factor before ANC. In previous studies, alopecia areata was associated with oxidative stress [ 31 , 32 ]. A decrease in oxidative stress is associated with low serum bilirubin levels [ 33 , 34 ].
The risk factors for neonatal jaundice included syphilis, surgical follow-up care, leiomyoma of uterus, and other diseases of the digestive system during ANC. Based on previous studies [ 35 , 36 ], congenital syphilis increases the risk of neonatal jaundice. According to the significant results of surgical follow-up during pregnancy, the type, purpose, and timing of surgery should be identified to explain the relationship between surgical follow-up and neonatal jaundice, which would be limitation of this study using claims data. Leiomyoma can be an extension of the association between leiomyoma as well as preterm birth and cesarean delivery [ 37 , 38 ].
Other diseases of the digestive system (K92) include hematemesis, melena, and gastrointestinal hemorrhage. However, considering the medical practice of registering a diagnosis, different diseases of the digestive system were included, and the incidence was low. Hence, the significant was low.
Most protective factors identified in study A were associated with infection and inflammation. Recent studies have shown the inverse association between bilirubin and inflammation [ 39 – 41 ]. Thus, inflammation may be associated with the low bilirubin levels, which could decrease the levels of unconjugated bilirubin transferred to neonates.
The pre-pregnancy maternal disease associated with neonatal jaundice was salpingo-oophoritis. Gastritis, dyspepsia, and heartburn are diagnoses associated with the gastrointestinal system. Notably, the OR value for neonatal jaundice was < 1.
The proportion of neonatal jaundice to total delivery cases was 0.40%–0.47%, which was different from its known incidence (30%–80%) [ 42 – 46 ]. This finding could be attributed to the fact that this study was based on neonatal jaundice recorded in the mothers’ claims data. Although prematurity is a risk factor for neonatal jaundice, there was no significant difference in terms of its occurrence between both groups [ 4 , 12 ]. Since newborns born prematurely are admitted to the neonatal intensive care unit, the diagnosis of neonatal jaundice is rarely applied to the mother. Therefore, jaundice in premature infants may not have been well reflected in this study. Contrary to a known risk factor for neonatal jaundice, vaginal delivery was less common in the jaundice group [ 47 – 49 ]. One possible reason for that may be the differences in the gut microbiota of newborns according to the type of delivery. Infants born via cesarean section have a lower number of Bifidobacterium and Bacteroides than infants born via vaginal delivery [ 50 – 52 ]. Bacteroides reduces unconjugated bilirubin to urobilinoids [ 53 ], and an association between the decreased number of Bifidobacterium and the elevated levels of bilirubin has been reported [ 50 ]. In terms of gut microbiota, cesarean section can be a potential risk factor for neonatal jaundice. The duration of ANC in the jaundice group of study A was significantly short, thereby indicating differences in pregnancy duration or delayed pregnancy diagnosis. A previous study reported the association between the late recognition of pregnancy and adverse outcomes such as neonatal intensive care admission [ 54 ].
Identification of the maternal gestational period or pre-pregnancy disease associated with neonatal jaundice may be helpful in counseling mothers preparing for pregnancy or pregnant mothers. For mothers with risk factors, it can predict jaundice for future babies and provide useful information for things to keep in mind after birth. In addition, neonatal jaundice can be prevented through prevention and management of maternal diseases related to neonatal jaundice.
Integration with research on maternal diseases may lead to the development of prenatal care programs to prevent neonatal jaundice. Moreover, it is thought that a more detailed correlation can be derived if the study is conducted including the maternal medication (especially, folic acid and iron, which are essential to take before and during pregnancy). The possible risk factors of certain child diseases including maternal disorders must be assessed from a long-term perspective. Therefore, follow-up studies with a disease network connecting diseases (from maternal to the child disorders) must be performed to assess the association between neonatal jaundice and other pediatric and maternal diseases.
This is the first study to analyze the association between maternal disease not related to pregnancy and neonatal jaundice. Maternal risk factors suggested in this study, including syphilis, leiomyoma, and salpingo-oophoritis, are differentiated from well-known risk factors for neonatal jaundice, such as diabetes mellitus, and suggest there may be unknown pathophysiology. While machine learning-based studies on the prediction of neonatal jaundice required information of neonates such as total serum bilirubin [ 55 , 56 ], the risk factors identified from the method used in this study can be evaluated with only maternal history before pregnancy or delivery. Another strength includes a large sample size based on health insurance data that covers almost all citizens.
This study had several limitations associated with the use of claims data. The diagnostic code for insurance claims could be differ from the actual diagnosis [ 57 – 59 ]. To ensure data integrity, although mild, the study included mothers who were insured for diseases other than those associated with pregnancy and delivery. The diagnosis codes used in this study were based on KCD-6, the Korean modified version of ICD-10, and numerous medical conditions, including symptoms, are included in the codes. Therefore, statistically significant diagnosis codes about symptoms obtained in this study, such as dyspepsia and heartburn, do not represent the diagnosis of a specific disease. The details about the surgery what other surgical follow-up care referred could not be identified precisely from claims data, which was also a limitation of this study.
This study has identified that maternal risk factors for neonatal jaundice were syphilis and leiomyoma during pregnancy, and salpingo-oophoritis before pregnancy, and protective factors were infection and inflammatory diseases, and dyspepsia. This has shown significant information that can be used for risk management and the prediction and prevention of neonatal jaundice before or during pregnancy. Furthermore it is necessary to study not only maternal diseases related to neonatal jaundice, but also studies including maternal medication history and long-term prognosis.
Acknowledgements
Not applicable.
Authors’ contributions
YJY and JWC are co-first authors and contributed equally. They conceived and designed the study. YJY, JWC, MHL, KHK, HMR and HWH performed the data collection and curation. YJY and JWC performed data analysis. YJY and JWC conducted statistical analyses. YJY, JWC, MHL, KHK, HMR and HWH performed data interpretation and wrote the paper. All authors reviewed the final manuscript. The author(s) read and approved the final manuscript.
This work was supported by grants from Institute of Information & communications Technology Planning & Evaluation (IITP) funded by the Korea government (MSIT) (No. 2019000224, Development of Information Protection Source Technology), Brain Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Science, ICT & Future Planning (No. 2019M3C7A1032262), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No.2020R1F1A1068423) and the Bio Industry Technology Development Program(No. 20015086) from the Ministry of Trade, Industry & Energy(MOTIE, Korea), Information and Communications Promotion Fund through the National IT Industry Promotion. Agency (NIPA), funded by the Ministry of Science and ICT (MSIT), Republic of Korea (No. 20002033324071212100101AU), Korea University Guro Hospital (KOREA RESEARCH-DRIVEN HOSPITAL) funded by Korea University Medicine (No. K2117371) and (HI21C1779) from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the regulation that the National Health Insurance Service–National Sample Cohort (NHIS-NSC) data is not provided directly to researchers and can only be accessed via the NHIS analysis system.
Declarations
Ethics approval and consent to participate.
This study was approved by the institutional review board of CHA Bundang Medical Center, CHA University (CHAMC 2021–04-049). The need for informed consent from the participants was waived by the institutional review board of CHA Bundang Medical Center, CHA University as the study used anonymized claims data.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Youngjae Yu and Jinwha Choi are co-first authors and contributed equally to this work.
- 1. Jaundice in newborn babies under 28 days: Clinical guideline [CG98] [ https://www.nice.org.uk/guidance/cg98 ]
- 2. Kim MJ. Readmission of late preterm infants after discharge from nursery. Clin Exp Pediatr. 2009;52(8):888–892. [ Google Scholar ]
- 3. 2012 National Health Insurance Statistics Yearbook [ https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020045020000&brdScnBltNo=4&brdBltNo=2305&pageIndex=1 ]
- 4. Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health. 2018;2(8):610–620. doi: 10.1016/S2352-4642(18)30139-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Slusher TM, Zamora TG, Appiah D, Stanke JU, Strand MA, Lee BW, Richardson SB, Keating EM, Siddappa AM, Olusanya BO. Burden of severe neonatal jaundice: a systematic review and meta-analysis. BMJ Paediatr Open. 2017;1(1):e000105. doi: 10.1136/bmjpo-2017-000105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Kuzniewicz MW, Niki H, Walsh EM, McCulloch CE, Newman TB. Hyperbilirubinemia, phototherapy, and childhood asthma. Pediatrics. 2018;142(4):e20180662. doi: 10.1542/peds.2018-0662. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Ku MS, Sun HL, Sheu JN, Lee HS, Yang SF, Lue KH. Neonatal jaundice is a risk factor for childhood asthma: a retrospective cohort study. Pediatr Allergy Immunol. 2012;23(7):623–628. doi: 10.1111/j.1399-3038.2012.01345.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Lozada LE, Nylund CM, Gorman GH, Hisle-Gorman E, Erdie-Lalena CR, Kuehn D. Association of autism spectrum disorders with neonatal hyperbilirubinemia. Glob Pediatr Health. 2015;2:2333794x15596518. doi: 10.1177/2333794X15596518. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Maimburg RD, Bech BH, Vaeth M, Møller-Madsen B, Olsen J. Neonatal jaundice, autism, and other disorders of psychological development. Pediatrics. 2010;126(5):872–878. doi: 10.1542/peds.2010-0052. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Wei CC, Chang CH, Lin CL, Chang SN, Li TC, Kao CH. Neonatal jaundice and increased risk of attention-deficit hyperactivity disorder: a population-based cohort study. J Child Psychol Psychiatry. 2015;56(4):460–467. doi: 10.1111/jcpp.12303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Maimburg RD, Olsen J, Sun Y. Neonatal hyperbilirubinemia and the risk of febrile seizures and childhood epilepsy. Epilepsy Res. 2016;124:67–72. doi: 10.1016/j.eplepsyres.2016.05.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med. 2001;344(8):581–590. doi: 10.1056/NEJM200102223440807. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Boskabadi H, Rakhshanizadeh F, Zakerihamidi M. Evaluation of maternal risk factors in neonatal hyperbilirubinemia. Arch Iran Med. 2020;23(2):128–140. [ PubMed ] [ Google Scholar ]
- 14. Olusanya BO, Osibanjo FB, Slusher TM. Risk factors for severe neonatal hyperbilirubinemia in low and middle-income countries: a systematic review and meta-analysis. PloS One. 2015;10(2):e0117229. doi: 10.1371/journal.pone.0117229. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24(1):63–71. doi: 10.1093/heapol/czn037. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Cheol SS, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. 2017;46(3):799–800. doi: 10.1093/ije/dyw253. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Kim S-K, Kim Y-K, Kim H, Park J, Son C, Choi Y, Kim Y, Lee G, Yoon A. The 2012 national survey on fertility, family health & welfare in Korea. Seoul: Korea Institute for Health and Social Affairs; 2012. pp. 2012–2054. [ Google Scholar ]
- 18. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. doi: 10.1093/ije/dyv319. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Moon HY, Kim MR, Hwang DS, Jang JB, Lee J, Shin JS, Ha I, Lee YJ. Safety of acupuncture during pregnancy: a retrospective cohort study in Korea. BJOG. 2020;127(1):79–86. doi: 10.1111/1471-0528.15925. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Nam JY, Choi Y, Kim J, Cho KH, Park EC. The synergistic effect of breastfeeding discontinuation and cesarean section delivery on postpartum depression: a nationwide population-based cohort study in Korea. J Affect Disord. 2017;218:53–58. doi: 10.1016/j.jad.2017.04.048. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Lee SY, Lee SW, Kong IG, Oh DJ, Choi HG. Pregnancy does not increase the risk of sudden sensorineural hearing loss: a national cohort study. Laryngoscope. 2020;130(4):E237–E242. doi: 10.1002/lary.28170. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Park KY, Kwon HJ, Wie JH, Lee HH, Cho SB, Kim BJ, Bae JM. Pregnancy outcomes in patients with vitiligo: a nationwide population-based cohort study from Korea. J Am Acad Dermatol. 2018;79(5):836–842. doi: 10.1016/j.jaad.2018.02.036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Yuk JS, Shin JY, Park WI, Kim DW, Shin JW, Lee JH. Association between pregnancy and adnexal torsion: a population-based, matched case-control study. Medicine (Baltimore) 2016;95(24):e3861. doi: 10.1097/MD.0000000000003861. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. American College of Obstetricians and Gynecologists. Society for Maternal-Fetal Medicine Obstetric care consensus no. 6: periviable birth. Obstet Gynecol. 2017;130(4):e187–199. doi: 10.1097/AOG.0000000000002352. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. American College of Obstetricians and Gynecologists Practice bulletin no. 146: management of late-term and postterm pregnancies. Obstet Gynecol. 2014;124(2 Pt 1):390–396. doi: 10.1097/01.AOG.0000452744.06088.48. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Kwak MY, Lee SM, Lee TH, Eun SJ, Lee JY, Kim Y. Accessibility of prenatal care can affect inequitable health outcomes of pregnant women living in obstetric care underserved areas: a nationwide population-based study. J Korean Med Sci. 2019;34(1):1–9. doi: 10.3346/jkms.2019.34.e8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28. [ Google Scholar ]
- 28. Therneau T: A Package for Survival Analysis in R. R Package Version 3.2–13.(2021). [ https://CRAN.R-project.org/package=survival ]
- 29. The igraph software package for complex network research [ https://www.researchgate.net/profile/Gabor-Csardi/publication/221995787_The_Igraph_Software_Package_for_Complex_Network_Research/links/0c96051d301a30f265000000/The-Igraph-Software-Package-for-Complex-Network-Research.pdf ]
- 30. R: A language and environment for statistical computing [ https://www.r-project.org/ ]
- 31. Bakry OA, Elshazly RM, Shoeib MA, Gooda A. Oxidative stress in alopecia areata: a case-control study. Am J Clin Dermatol. 2014;15(1):57–64. doi: 10.1007/s40257-013-0036-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Prie BE, Voiculescu VM, Ionescu-Bozdog OB, Petrutescu B, Iosif L, Gaman LE, Clatici VG, Stoian I, Giurcaneanu C. Oxidative stress and alopecia areata. J Med Life. 2015;8 Spec Issue(Spec Issue):43–46. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Dani C, Poggi C, Pratesi S. Bilirubin and oxidative stress in term and preterm infants. Free Radic Res. 2019;53(1):2–7. doi: 10.1080/10715762.2018.1478089. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Dani C, Martelli E, Bertini G, Pezzati M, Filippi L, Rossetti M, Rizzuti G, Rubaltelli FF. Plasma bilirubin level and oxidative stress in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003;88(2):F119–123. doi: 10.1136/fn.88.2.F119. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Chawla V, Pandit PB, Nkrumah FK. Congenital syphilis in the newborn. Arch Dis Child. 1988;63(11):1393–1394. doi: 10.1136/adc.63.11.1393. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Adachi E, Koibuchi T, Okame M, Sato H, Kikuchi T, Koga M, Nakamura H, Iwamoto A, Fujii T. Liver dysfunction in patients with early syphilis: a retrospective study. J Infect Chemother. 2013;19(1):180–182. doi: 10.1007/s10156-012-0440-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Lai J, Caughey AB, Qidwai GI, Jacoby AF. Neonatal outcomes in women with sonographically identified uterine leiomyomata. J Matern Fetal Neonatal Med. 2012;25(6):710–713. doi: 10.3109/14767058.2011.572205. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Coronado GD, Marshall LM, Schwartz SM. Complications in pregnancy, labor, and delivery with uterine leiomyomas: a population-based study. Obstet Gynecol. 2000;95(5):764–769. doi: 10.1016/s0029-7844(99)00605-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Vítek L, Tiribelli C. Bilirubin, Intestinal Integrity, the Microbiome, and Inflammation. N Engl J Med. 2020;383(7):684–686. doi: 10.1056/NEJMcibr2013250. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Zheng JD, He Y, Yu HY, Liu YL, Ge YX, Li XT, Li X, Wang Y, Guo MR, Qu YL, et al. Unconjugated bilirubin alleviates experimental ulcerative colitis by regulating intestinal barrier function and immune inflammation. World J Gastroenterol. 2019;25(15):1865–1878. doi: 10.3748/wjg.v25.i15.1865. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Vogel ME, Zucker SD. Bilirubin acts as an endogenous regulator of inflammation by disrupting adhesion molecule-mediated leukocyte migration. Inflamm Cell Signal. 2016;3(1):e1178. doi: 10.14800/ics.1178. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Tikmani SS, Warraich HJ, Abbasi F, Rizvi A, Darmstadt GL, Zaidi AK. Incidence of neonatal hyperbilirubinemia: a population-based prospective study in Pakistan. Trop Med Int Health. 2010;15(5):502–507. doi: 10.1111/j.1365-3156.2010.02496.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Scrafford CG, Mullany LC, Katz J, Khatry SK, LeClerq SC, Darmstadt GL, Tielsch JM. Incidence of and risk factors for neonatal jaundice among newborns in southern Nepal. Trop Med Int Health. 2013;18(11):1317–1328. doi: 10.1111/tmi.12189. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Burke BL, Robbins JM, Bird TM, Hobbs CA, Nesmith C, Tilford JM. Trends in hospitalizations for neonatal jaundice and kernicterus in the United States, 1988–2005. Pediatrics. 2009;123(2):524–532. doi: 10.1542/peds.2007-2915. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Final Results of Birth Statistics in 2012 [ http://kostat.go.kr/portal/eng/pressReleases/8/1/index.board ]
- 46. Health Insurance Review & Assessment Service [ http://opendata.hira.or.kr/op/opc/olap3thDsInfo.do ]
- 47. Brits H, Adendorff J, Huisamen D, Beukes D, Botha K, Herbst H, Joubert G. The prevalence of neonatal jaundice and risk factors in healthy term neonates at National District Hospital in Bloemfontein. Afr J Prim Health Care Fam Med. 2018;10(1):e1–e6. doi: 10.4102/phcfm.v10i1.1582. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Gregory KD, Jackson S, Korst L, Fridman M. Cesarean versus vaginal delivery: whose risks? Whose benefits? Am J Perinatol. 2012;29(1):7–18. doi: 10.1055/s-0031-1285829. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Benedetto C, Marozio L, Prandi G, Roccia A, Blefari S, Fabris C. Short-term maternal and neonatal outcomes by mode of delivery. A case-controlled study. Eur J Obstet Gynecol Reprod Biol. 2007;135(1):35–40. doi: 10.1016/j.ejogrb.2006.10.024. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Zhou S, Wang Z, He F, Qiu H, Wang Y, Wang H, Zhou J, Zhou J, Cheng G, Zhou W, et al. Association of serum bilirubin in newborns affected by jaundice with gut microbiota dysbiosis. J Nutr Biochem. 2019;63:54–61. doi: 10.1016/j.jnutbio.2018.09.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, Retetangos C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev. 2010;86(Suppl 1):13–15. doi: 10.1016/j.earlhumdev.2010.01.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. doi: 10.1542/peds.2005-2824. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Tiribelli C, Ostrow JD. Intestinal flora and bilirubin. J Hepatol. 2005;42(2):170–172. doi: 10.1016/j.jhep.2004.12.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Ayoola AB, Stommel M, Nettleman MD. Late recognition of pregnancy as a predictor of adverse birth outcomes. Am J Obstet Gynecol. 2009;201(2):156.e151–156. doi: 10.1016/j.ajog.2009.05.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Daunhawer I, Kasser S, Koch G, Sieber L, Cakal H, Tütsch J, Pfister M, Wellmann S, Vogt JE. Enhanced early prediction of clinically relevant neonatal hyperbilirubinemia with machine learning. Pediatr Res. 2019;86(1):122–127. doi: 10.1038/s41390-019-0384-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Ferreira D, Oliveira A, Freitas A. Applying data mining techniques to improve diagnosis in neonatal jaundice. BMC Med Inform Decis Mak. 2012;12(1):1–6. doi: 10.1186/1472-6947-12-143. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Strom BL. Data validity issues in using claims data. Pharmacoepidemiol Drug Saf. 2001;10(5):389–392. doi: 10.1002/pds.610. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104(3):157–163. [ PubMed ] [ Google Scholar ]
- 59. Iqbal SA, Isenhour CJ, Mazurek G, Truman BI. Diagnostic code agreement for electronic health records and claims data for tuberculosis. Int J Tuberc Lung Dis. 2020;24(7):706–711. doi: 10.5588/ijtld.19.0792. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
- View on publisher site
- PDF (1.3 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
Dr. Akash Gupta (Pediatrics): A 16-year-old boy was admitted to this hospital in autumn because of jaundice and abnormal results on liver-function tests. The patient had been well until 4 days ...
A 2-day-old, 2.68-kg term male neonate is brought to the emergency department with lethargy, poor feeding, and significant generalized jaundice. He was born via spontaneous vaginal delivery at home to a gravida 4, para 3 Amish woman under the supervision of a midwife, at an estimated gestational age of 39 weeks after an uncomplicated pregnancy with scant prenatal care. Jaundice was noticed 7 ...
A recent multicenter study examined the causes of severe acute liver injury in ... HAV infection is an important consideration in this case. However, in this patient, jaundice had progressed over ...
Case 34-2019: A 16-Year-Old Boy with Jaundice. Dr. Akash Gupta (Pediatrics): A 16-year-old boy was admitted to this hospital in au-tumn because of jaundice and abnormal results on liver-function ...
Jaundice, also known as hyperbilirubinemia,[1] is a yellow discoloration of the body tissue resulting from the accumulation of an excess of bilirubin. Deposition of bilirubin happens only when there is an excess of bilirubin, a sign of increased production or impaired excretion. The normal serum levels of bilirubin are less than 1mg/dl; however, the clinical presentation of jaundice as scleral ...
Abstract. Obstructive jaundice (OJ) is a common problem in daily clinical practice. However, completely understanding the pathophysiological changes in OJ remains a challenge for planning current and future management. The effects of OJ are widespread, affecting the biliary tree, hepatic cells, liver function, and causing systemic complications.
The incidence of visible jaundice in our study is comparable to other studies in (near) term neonates in which 60-90% became jaundiced 27,28,29. ... A nested case-control study. J.
ERCP: internal stenting. The Mirizzi syndrome refers to common hepatic duct obstruction caused by an extrinsic compression from an impacted stone in the cystic duct or Hartmann's pouch of the gallbladder. 1 The majority of the patients present the clinical triad of jaundice, fever, and right upper quadrant pain, showing in the laboratory ...
An estimated 60%-80% of term newborns (>37 0/7 weeks) will present with some level of jaundice. 1, 2 Jaundice is derived from the French word jaune, meaning yellow, and describes the skin taking on a yellow hue. 2 Physiologic jaundice is a mild and self-limiting form and the most common etiology. 3 It can be challenging for clinicians to differentiate the type without an appropriate ...
The matched sample for study A had 4,026 cases (jaundice group: 366, control group: 3,660), and that for study B had 3,278 cases (jaundice group: 298, control group: 2,980). All the 1,000 matched samples significantly differed in terms of the type of delivery in both two studies (Table (Table2). 2). There was also a significant difference in ...