An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Non-operative management for abdominal solidorgan injuries: A literature review
Amonpon kanlerd, karikarn auksornchart, piyapong boonyasatid.
- Author information
- Article notes
- Copyright and License information
Corresponding author. [email protected]
Received 2021 Feb 4; Revised 2021 Jul 18; Accepted 2021 Jul 26; Issue date 2022 Sep.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
The philosophy of abdominal injury management is currently changing from mandatory exploration to selective non-operative management (NOM). The patient with hemodynamic stability and absence of peritonitis should be managed non-operatively. NOM has an overall success rate of 80%–90%. It also can reduce the rate of non-therapeutic abdominal exploration, preserve organ function, and has been defined as the safest choice in experienced centers. However, NOM carries a risk of missed injury such as hollow organ injury, diaphragm injury, and delayed hemorrhage. Adjunct therapies such as angiography with embolization, endoscopic retrograde cholangiopancreatography with stenting, and percutaneous drainage could increase the chances of successful NOM. This article aims to describe the evolution of NOM and define its place in specific abdominal solid organ injury for the practitioner who faces this problem.
Keywords: Nonoperative management of abdominal injury, Abdominal injury, Management of abdominal injury, Abdominal solidorgan injury
Introduction
The philosophy of abdominal injury management is currently changing from the era of mandatory exploration to the age of selective non-operative management (NOM). Currently, NOM is the standard treatment for an abdominal trauma patient with hemodynamic stability, which has a success rate of approximately 80%–90%. 1 NOM can reduce the rate of non-therapeutic abdominal exploration and has been defined as the safest choice in the experienced centers which equipped with available surgeons, operating rooms, intensive care units, and other supporting resources. However, NOM has the risk of missed injury such as hollow organ injury, diaphragm injury, and delayed hemorrhage.
Evolution of NOM
The non-therapeutic laparotomy rate in the penetrating anterior abdominal trauma patients who are treated with mandatory abdominal exploration is 25%–40% and is the same in blunt abdominal injury. This rate is higher at 75%–80% in penetrating flank and back and 15%–27% in abdominal gunshot. 2 The high non-therapeutic laparotomy rate was associated with high unnecessary health costs, loss of resources, and increased morbidity. In 1951, it was reported that 5 infants died following splenectomy. 3 After that, several pediatric surgeons suggested careful splenectomy in pediatric splenic injury patients. Both pediatric patient and adult patient with splenic injury can be successfully managed by non-operative method and it has been found that spleen bleeding can stop spontaneously and can also heal itself. In 1984, a survey of NOM in blunt splenic injury found that the success rate of NOM was 30% and the mortality rate was 12.3%, mainly from severe head injuries. 4 In 1989, Cogbill et al. 5 reported multicenter experience on NOM in blunt splenic injury, of which 83% were successful in adults and 98% in children. A current evidence-based study demonstrates over 95% of blunt splenic injury are treated non-operatively with a 10% failure rate. 6 The first report of NOM in blunt liver injury was published in 1979. 7 Meyer et al. 8 reported that a benefit of CT scan is that it may be used to select blunt hepatic injury cases for NOM. Patients with minor parenchymal injury, without estimated intraperitoneal blood (or less than 250 mL) on CT scan or shock do not need subsequent laparotomy. 8 NOM has become the standard of care in any grade of blunt hepatic injury with hemodynamic stability, and has a success rate of 90%–95%. 9
The first prospective randomized study comparing mandatory exploration with selective NOM in penetrating trauma was in 1996. 10 The results showed 17% of patients in NOM group needed delayed laparotomy. Also, NOM reduced hospital stays, and a successful NOM saved $2800. Renze et al. 11 reported successful NOM with gunshot to the right abdomen during 1990–1993. Then in 1997, a prospective protocol-guide study of NOM in anterior abdominal gunshot found that the rate of non-therapeutic exploration and negative exploration was 2.2% and 8.6%, respectively, and 92/106 (86.8%) patients were successfully managed non-operatively. 12 The first study to demonstrate successful NOM in penetrating isolated solid organ injury took place in 2006, and they found that overall success rate of selective NOM was 27%. For the individual organs, the liver had success rate of 28.7% and the kidney had 14.9%, and the spleen had the lowest success rate of 3.5%. 13
The current definition of NOM according to international consensus conference (ICC) in 2018 is “an initial non-surgical management strategy of a solid organ injury which usually consists of observation, but may include use of endovascular, percutaneous, or endoscopic procedures.’’ 14 The principle of NOM is to promote spontaneous hemostasis, maintain clot formation, enhance healing, and preserve organ functions. NOM is a useful protocol and has strong evidence of benefit in blunt abdominal solid organ injuries, such as the liver, spleen, pancreas, and kidney injuries. However, caution is urged when using NOM in blunt intestinal hematoma, blunt high-grade pancreatic injury, penetrating renal injury and penetrating splenic injury. 15 NOM should be attempted in an institute where a 24-h operating room (OR) is available, and an intensive monitoring can be provided. All candidate patients for NOM must have contrast-enhanced CT scan to identify the injured organ and grade the severity. Some patients require intensive monitoring such as high-grade injury, multiple organ injuries, pediatric, advanced age, and multiple co-morbidities. 16 A summary of NOM recommendations is shown in Table 1 .
Summary of recommendations for NOM in abdominal solid organ injuries.
NOM: non-operative management; AAST: the American Association for the Surgery of Trauma; OIS: the organ injury scales; PDI: pancreatic duct injury; OR: operating room; PA: pseudoaneurysm; CE: contrast extravasation; AVF: arteriovenous fistula; RAT: renal artery thrombosis; RAD: renal artery dissection; ERCP: endoscopic retrograde cholangio-pancreatography.
Some experts suggested to follow up imaging within 1 week after injury in a hepatic injury patient with high-risk for complications such as high-grade injury, central lobes injury (segment IV, V, VIII), post main hepatic artery embolization.
Some experts suggested repeating imaging in large perinephric hematomas that may obscure urine leakage in 48 h. CT scan is not accurately able to predict the failure of conservative treatment.
The risk for infectious complications (urinary tract infection, urosepsis, and perinephric abscess) such as presence of devitalized tissue, presence of urinoma, associated bowel and pancreatic injury, multiple co-morbidities, and immunosuppression.
There is still not enough high-quality literature available to reach a consensus about: (1) How frequent and how long to make hemoglobin measurements and abdominal examination? (2) When is the best time to resume oral intake? (3) How long to restrict a patient's activity? (4) When is the best time to initiate chemoprophylaxis for venous thromboembolism (VTE)? and (5) How long to observe in intensive care units or hospital? Many guidelines recommend measuring hemoglobin level every 6–8 h, and also suggest examining the abdomen by the same investigator. The patient should not be allowed to eat and drink for at least 24 h after admission in case any hollow organ injury was missed. The interval of restricted patient activity is proportional to the severity of organ injury, but there is no need for absolute restriction in bed. The patient should be allowed to ambulate with light activity in the hospital and instructed to refrain from substantial activity including sport before discharge. A retrospective cohort study in 2008 found the timing of mobilization of the patient with blunt solid organ injuries did not contribute to delayed hemorrhage requiring laparotomy and they suggested the protocol with strict rest in bed was unnecessary. 17 Many centers start initially with a sequential compression device for mechanical prevention of deep vein thrombosis. Afterward, chemoprophylaxis is initiated as soon as possible after the patient has hemodynamic stability. A study in 2011 showed chemoprophylaxis of VTE did not increase the failure rate of NOM nor the need of blood transfusion. 18 In low-grade solid organ injuries, the recent trend is to shorten the length of hospital stay, but in high-grade injuries there is still the need for extended the length of hospital stay to make sure there are no serious complications.
NOM is currently the standard care for blunt hepatic injury patients with hemodynamic stability without signs of peritonitis or other indications for surgery. In high-grade blunt liver injury, the success rate of NOM is >90%. Approximately, 25% of cases require intervention to manage a complication of NOM, and the risk of rebleeding is higher in grade IV/V liver injury. 14 A systematic review in 2015 calculated a pool of failure rate of 9.5% in any grade of blunt liver injury which was managed with NOM. They also found no effect of age, sex, initial heart rate, volume of blood transfusion, liver injury grading, but associated with extra-abdominal injuries, abdominal CT scan findings, and overall injury severity score (ISS) are the risk factors of failure of NOM. 9 A blunt hepatic injury combined with brain injury is also not currently a contraindication for NOM. Hommes et al. 19 performed a prospective study of NOM in blunt hepatic trauma, and they found no significant effect of low Glasgow coma scale score on the risk of failure of NOM. A CT scan has 97% sensitivity, 98.7% specificity in hepatic injury, and can detect associated intra- or retro-peritoneal lesions and has the benefit of identifying hepatic vascular injuries such as active contrast extravasation, pseudoaneurysm, arteriovenous fistula, and vessel truncation. These findings are the indication for angiography with embolization (AE). 20 AE is a currently useful adjunctive method to control hepatic hemorrhage and can be used as an adjunct procedure before or during the operation in a hybrid OR if the patient has hemodynamic instability. AE is also useful after a damage control operation, and as a primary treatment in a hemodynamically stable patient with CT signs of hepatic vascular injury. The failure rate of AE is 13%-20%, and the efficacy of controlling hepatic hemorrhage is 83%. 21 AE has no benefit in high-grade liver injury without CT signs of hepatic vascular injury. 14
There is still no consensus about how long patients with blunt liver injury should be admitted to the hospital. Park et al. 22 reported the optimum length of in-patient observation for blunt hepatic injury. The median length of hospital stay was 1.9 days for all grades. They concluded that the length of hospital stay should be based on clinical criteria, and the patient can be safely discharged in the presence of normal abdominal signs and stable hemoglobin regardless of grading of injury. Tiberio et al. 23 studied the median healing time of blunt liver injury, and they found the time to complete resolution of hematomas was 6, 45.5, and 108 days for grades I, II and III hepatic injuries, respectively and the median time to complete healing of liver lacerations were 29, 34, and 77.5 days for grades II, III, and IV, respectively. Some experts suggest limiting substantial activity and contact sport for at least 1 month based on an animal experiment. 24 ICC suggest not routinely performing follow-up CT due to only 0.5% of cases needing intervention based on follow-up CT findings. 14 The latest study about follow-up imaging for blunt hepatic trauma found only 1 of 920 cases non-clinically indicated follow-up CT scan showed a complication that required intervention. 25
Risk of complication after NOM in blunt hepatic injury is dependent on the severity, with a 1% risk in grade III and higher up to 63% in grade V. 14 Complications following NOM can be classified into (1) acute or early post-treatment complications such as hepatic necrosis, abscess, bile leakage, biloma, hemobilia, and delayed bleeding; (2) late complications such as gall stone, vascular outflow tract obstruction, and biliary fistula. Biliary complications have a 3.2% occurrence rate in all hepatic injury. 26 Associated bile duct injury is a salient cause of biliary complications and commonly found with high-grade injury or central hepatic injury. Clinical manifestation of biliary complications such as jaundice, abdominal pain, abdominal distension, fever, systemic inflammation or sepsis, feeding intolerance, and elevated liver enzymes usually present within a week of injury or procedure. Hepatobiliary iminodiacetic acid scan has a 100% sensitivity and specificity to detect bile leakage and bile duct injury. 27 Additional diagnostic modalities such as CT scan, abdominal ultrasonography, magnetic resonance cholangiopancreatography (MRCP), percutaneous transcatheter cholangiography, and endoscopic retrograde cholangiopancreatography (ERCP) can be used to diagnose these complications. 19 , 28 Currently there are still no consensus about the time to preform follow-up imaging after NOM in liver injury, but it is suggested to have follow-up imaging for a patient with non-specific abdominal complaint, developing jaundice, abruptly elevated liver enzymes, and high risks for biliary complications such as high-grade injury, central hepatic injury and post main hepatic artery embolization. Minor bile duct injuries mostly heal spontaneously within 14 days and there is no need for interventions, but major bile duct injury or continuous bile leakage could be managed with ERCP and biliary stent drainage. Bilomas can be managed with percutaneous drainage (PCD) as the first step and ERCP with stent for persistent leakage. Biliary complications rarely need operative management except in patients who failed conservative management or for uncontrolled sepsis. 29 Liver abscess is a rare complication with a 4% post-NOM incidence but has a 10% risk of mortality. 14 An abscess can occur within 1 week after injury or may present late within a month — hepatic abscesses following trauma are usually single and involve the right lobe more than the left. The most common pathogen is gram-negative bacteria such as Klebsiella pneumoniae, Proteus mirabilis, Eikenella corrodens, and Anaerobic streptococci . PCD is the mainstay treatment modality, when the abscess is larger than 5 cm and in a patient with sepsis. Systemic antibiotic choices are usually adjusted with the cultured microbial results and may need 4–6 weeks. 30 , 31 Peritoneal inflammatory syndrome is a clinical syndrome consisting of mild abdominal pain, high-grade fever (>38.5 °C), tachycardia, mild tachypnea, leukocytosis, and elevated C-reactive protein level. It is caused by bile irritation to the peritoneal surface and induces an inflammatory cascade. This complication mostly presents within the first week after injury. Laparoscopic peritoneal washout is the choice of treatment for this complication. 32
NOM in penetrating liver injury is currently acceptable in many institutes. The study in 2019 on gunshot injury to the liver found a 4.9% of failure rate. 33 Navsaria et al. 34 reported success rate of NOM in liver gunshot as high as 94.4% with no deaths. Selective NOM in penetrating liver injury should be attempted only in a high-volume or high-experience center. The patient who meets criteria for selective NOM must have a contrast-enhanced CT scan and may need diagnostic laparoscopy to confirm a diagnosis and exclude hollow organ injury or diaphragm injury. A summary of NOM for hepatic injury is in Fig. 1 .
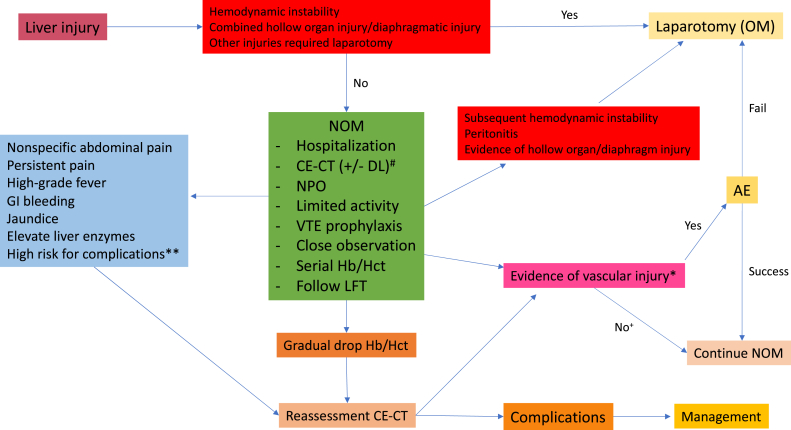
Summary of nonoperative management for hepatic injury. # DL is an optional investigation in penetrating liver injury ∗ Evidence of vascular injury on CT scan included active contrast extravasation, pooling of contrast, pseudoaneurysm, arteriovenous fistula and vessel truncation. ∗∗ High risk for complication included high-grade liver injury (AAST IV-VI), central lobes involvement (segment IV, V, VIII), post AE at main hepatic artery. + No with conditions: If reassessment CT in the patient who suspected rebleeding, searching for other sources of bleeding required and transfusion needed. OM: operative management; NOM: non-operative management; CE-CT: contrast-enhanced computed tomography; DL: diagnostic laparoscopy; NPO: nil per os; VTE: venous thromboembolism; Hb: hemoglobin; Hct: hematocrit; LFT: liver function test; AE: angiography with embolization; GI: gastrointestinal.
NOM is the standard treatment for any grade of blunt splenic injury, accompanied by hemodynamic stability without peritonitis or associated abdominal injuries requiring surgery. 14 Unnecessary splenectomy in trauma may carry the risk of overwhelming post-splenectomy infections with a high mortality rate of 50%–70%. 6 The failure rate of NOM in blunt splenic injury is approximately 10%. Risk factors contributing to the failure of NOM are high-grade injury, presence of large hemoperitoneum, active bleeding signs on admission CT scan, age of the patient, and concomitant solid organ injury. 35 A multi-institutional study on blunt splenic injury reported the failure rate of NOM increases with the grading of injury, 4.8% in grade I to 75% in grade V injury. 36 Harbrecht et al. 37 found that the patients aged more than 55 years old had 2.5 times risk of failure of NOM compared to those younger than 55 years. A prospective cohort study of NOM in blunt splenic injury reported no significant difference in failure rate of NOM, splenectomy rate, complications and mortality in case of reduced consciousness and normal consciousness group. They concluded NOM can be used safely in case of reduced consciousness with blunt splenic injury. 38 A retrospective study of NOM in 8166 blunt splenic injury with traumatic brain injury cases demonstrated the low failure rate of NOM even in a high-grade injury and no difference in mortality among the patients with and without traumatic brain injury. 39 A study in 2010 reported a significant increase in the failure rate of NOM in combined injuries compared to isolated splenic injury (11.6% vs. 5.8%), increased blood transfusion requirement and also increased mortality. 40 The risk of delayed bleeding in blunt splenic trauma is as high as 20% in grade III injuries, 50% in patients with active contrast extravasation, and 70% in patients with large hemoperitoneum. 14 A meta-analysis study on the role of AE as an adjunct to NOM in blunt splenic injury considered the use of AE in high-grade injury (grade IV-V) but should not be used in low-grade injury and found no significant difference in the failure rate, length of hospital stay, blood transfusion requirement, and mortality between all grades of blunt splenic injury patients who received AE and those who did not. 41 The study showed a 15% risk rate of developing contrast extravasations on initial CT scan in blunt splenic injury with hemodynamic stability, and 67% risk rate of NOM failure in patients who received AE compared to 93% observed without AE. 42 AE has recently been recommended as an adjunct to NOM in blunt splenic injury of any grade with contrast extravasations on initial CT scan, or in high-grade injury with/without signs of active bleeding. The theoretically, proximal embolized splenic artery may lead to a large splenic infarction and cause splenic malfunction. However, some recent reports showed that proximal splenic AE has a smaller area of infarction compared to distal splenic AE, and proximal splenic AE did not affect the long-term immunologic function of the spleen. 43 , 44 Proximal splenic AE is technically faster, easier, and cheaper than distal splenic AE, but currently, there is still no consensus on where the appropriate point is to AE in blunt splenic injury.
There is still no consensus on how long is enough and safe to discharge from the hospital. A study of 2660 failed NOM in 23,532 patients with blunt splenic injury found that most of the failed NOM patients (95%) usually failed within the first 72 h of admission. They also found a few patients failed within 3–5 days of hospitalization, which suggested observing the patients at least 3–5 days in the hospital is necessary. 45 The notion of repeat imaging after NOM is still controversial. Recent literature highlights a risk of delayed development of pseudoaneurysm as high as 20.8%, even the patients have received primary AE. The presence of pseudoaneurysm carries a risk of rebleeding and may cause failure of NOM. 46 ICC considered repeat imaging in patients with a risk of developing late vascular injuries (grade III-V) within 48–72 h after admission. 14 There is still no consensus on when to return to regular activity. A study of 97 blunt splenic injury patients who received post-discharge CT scan found 10% worsened with 2 cases requiring subsequent splenectomy. Low-grade injuries had reduced mean healing time compared to the high-grade injuries (12.5 days vs. 37.2 days). In high-grade injuries, they found that 80% of patients completely healed within 75 days of the injury. They suggested observation of the patient until healing can be confirmed (at least 8–10 weeks). 47 Some experts suggested to preform imaging examination on the athletes with high-grade splenic injuries repeatedly to confirm their healing, before allowing them back into the game. 35 There is still no substantial evidence supporting the benefit of pre-splenectomy vaccination in trauma patients. The US Centers for Disease Control recommend giving the vaccine to prevent post-splenectomy infections in asplenic patients within 2 weeks. 48 While World Society of Emergency Surgery Guidelines for adult splenic trauma suggests starting the vaccine no sooner than 14 days after splenectomy or spleen total vascular occlusion. 49 The appropriate primary vaccine should include the pneumococcal vaccine, Hemophilus influenza vaccine, Neisseria meningitides , and the influenza vaccine.
The feasibility of NOM in penetrating splenic injury is still questionable. The latest systematic review in 2019 included 608 penetrating splenic injury cases with 123 NOM cases from 5 studies, and they found overall failure rate was 18%. They demonstrated that NOM for penetrating splenic injury in highly selected patients has been utilized in well-equipped and experienced centers, and not associated with increased morbidity or mortality. Data from this study did not include basically-equipped and inexperienced centers. 50 Currently, selective NOM in penetrating splenic injury is still cautioned except in high-volume, well-equipped, and highly-experienced centers. A summary of NOM for splenic injury is in Fig. 2 .
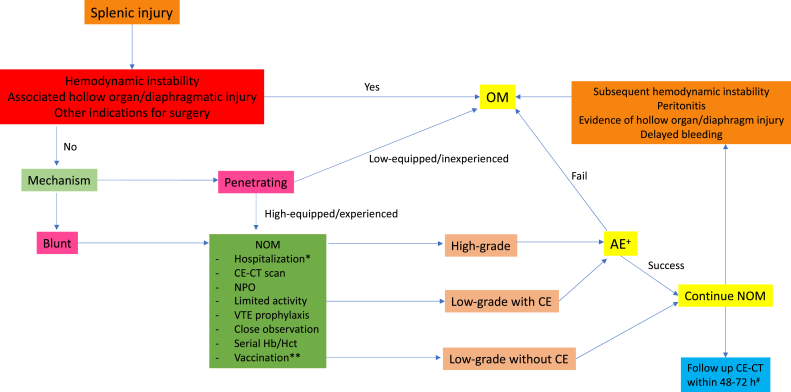
Summary of nonoperative management for splenic injury. ∗ Some experts suggested to hospitalized the patient at least 5 days. ∗∗ No consensus about pre-splenectomy vaccination, optional due to risk of failure NOM. + Location (proximal vs. distal splenic artery) and technique upon an interventionist preference. # Considered in high-risk for delayed hemorrhage (grade III-V), contrast-enhanced ultrasound might be use as alternative in the patient who concerns about cumulative radiation exposure. OM: operative management; NOM: non-operative management; CE-CT: contrast-enhanced computed tomography; NPO: nil per os; VTE: venous thromboembolism; Hb: hemoglobin; Hct: hematocrit; CE: contrast extravasations; AE: angiography with embolization.
Genitourinary tract injury comprises 0.3%-3.5% of all injuries and 10% of all abdominal traumas. This injury is mostly from blunt mechanism (70%–95%), more than 80% are concomitant injuries and predominately in young males. 51 NOM in renal trauma is acceptable using in low-grade injuries (grades I-III) with hemodynamic stability, but currently attracting interest in high-grade injuries too. Data from the American association for the surgery of trauma genitourinary trauma study in 2018 demonstrated 80% currently utilized NOM for high-grade renal trauma, but the rate of subsequent nephrectomy remained up to 13%. The risk factors contributing to NOM failure are grade V injury and penetrating trauma. 52 The gold-standard diagnostic modality for renal trauma is a four-phase CT scan of abdomen and pelvis including non-contrast, arterial, nephrogenic, and pyelographic phases. 53 , 54 This type of imaging carries benefits in accurate grading of renal injury, defining preexisting renal pathology, identifying the function of the uninjured kidney and demonstrating associated abdominal organ injuries. Presence of contrast leakage or contrast pooling in pyelographic phase, ipsilateral hydronephrosis, ipsilateral delayed excretory phase all demonstrate a collecting system injury with urine leakage, which is an indication for interventions such as ureteric stenting, percutaneous nephrostomy, or percutaneous drainage, but not an absolute contraindication to NOM. 54 The ureteric stenting should be the first step in treating urine leakage, but if not successful, retrograde stenting via percutaneous nephrostomy is reasonable. The ureteric stent should be left within the ureter at least 3 weeks or until radiographic confirmation of complete resolution. Percutaneous drainage should be considered in cases of enlarging urinomas, small urinomas with clinical sepsis, increasing pain, ileus and fistula formation. 53 However, in cases of proximal collecting system avulsion (renal pelvis or proximal ureter) that shows large medial urinoma or gross contrast leakage in pyelographic phase combined with non-opacified distal part of ureter and where the patient failed minimally invasive techniques to control urine leakage, these types of injury usually need operative intervention. 14 Baghdanian et al. 55 studied 162 CT scans in renal trauma, and they found 15% risk of renal vascular injury and 13.6% risk of collecting system injury. In the collecting system injury group, 50% could not demonstrate urine leakage on initial CT scan. This group usually had a more massive perinephric hematoma compared to patients who could demonstrate urine leakage on initial CT scan. They suggest repeating CT scan in patients with large perinephric hematoma and deep parenchymal injury may obscure a collecting system injury. The American Urological Association Education and Research, Inc. suggests repeating CT scan in high-grade renal injury (grades IV-V) and patients who have signs and symptoms of complications such as high-grade fever, persistent/worsening back pain, on-going blood loss, intermittent gross hematuria, hypertension, and abdominal distension after 48 h of admission. Routine use of dimercaptosuccinic acid scan, or other renal functional scans are not advised. 53
AE can decrease the risk of nephrectomy 78% in grade IV injuries and 83% in grade V, but may require more than 1 intervention. 14 Indication for AE is any grade of kidney injury with evidence of active bleeding on CT scan such as contrast extravasation, contrast blushing, pseudoaneurysm and arteriovenous fistula, and in non-self-limited gross hematuria. 54 Other criteria to assign the patient to AE are high-grade injury with the extent of hematoma, perirenal hematoma rim distance >25 mm and rupture of Gerota fascia. 14 , 56 , 57 Literature describes some of the CT-scan findings that may predict the need of AE in renal injuries such as large hematoma area, huge hematoma to kidney ratio, marked difference between hematoma and kidney area, and long perirenal hematoma rim distance. 58 Renal artery thrombosis and dissection following trauma can be treated with angiography through endovascular stenting. It should be performed within 4 h of warm ischemic time for better outcomes. There is still a high rate of renal loss if CT scan shows a completely non-perfused kidney before treatment. 14 , 15 There is a 5% risk of renovascular hypertension following renal artery injury. Refractory hypertension may occur in rare cases, such as Page kidney. If the perinephric collection presents, percutaneous drainage is the treatment of choice and nephrectomy can be used if it fails. 59 Incidence of infectious complications such as urinary tract infection, urosepsis, and perinephric abscess following renal trauma is low at 5%, but some clinical risk factors are associated with infectious complications; presence of devitalized tissue, large urinoma, associated bowel and pancreatic injury, multiple comorbidities, and immunosuppression. A patient with these risk factors may benefit from prophylactic antibiotics. There is still no consensus on this issue and no standard prophylaxis recommendation. 14
NOM in penetrating renal injury is currently acceptable in cases with hemodynamic stability. Penetrating injury has a higher risk of nephrectomy, failure of AE, and multiorgan injuries compared to a blunt mechanism. A prospective study in 75 penetrating kidney injury patients with 47 patients treated by NOM, found a 100% success rate of NOM, with less morbidity (9%), and also found a penetrating renal injury had a high risk of nephrectomy (24.3%). 60 The latest study in 2019 about selective NOM in renal gunshot found a 10.2% failure rate of NOM. The authors also demonstrated the benefits of selective NOM in shorter length of hospital stay, fewer complications, reduction in need for nephrectomy, and absence of association with mortality compared to operative management. 61 A summary of NOM for kidney injury is in Fig. 3 .
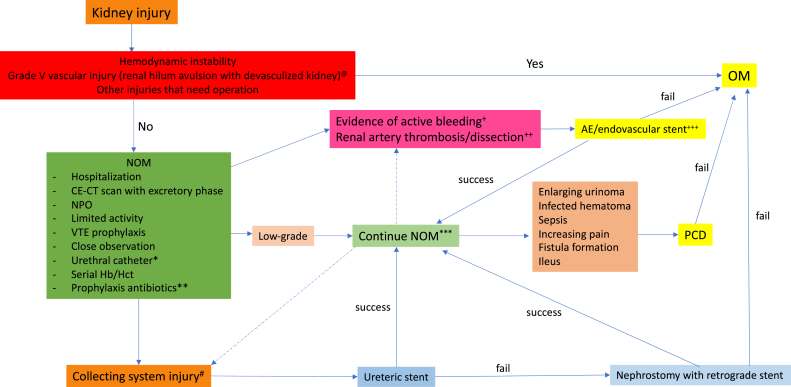
Summary of nonoperative management for kidney injury. @ Due to low renal salvage rate and high complications rate many experts suggested to manage operatively. ∗ Should be left in place until evidence of collecting system injury healed. ∗∗ No consensus but suggested in high-risk to develop infectious complications such as presence of devitalized tissue, large urinoma, associated bowel and pancreatic injury, multiple comorbidities, and immunosuppression. ∗∗∗ Suggested repeating imaging within 48 h in high-risk cases such as large perinephric hematoma and deep parenchymal injury that may obscure a collecting system injury, high-grade renal injury (grade IV-V), high-grade fever, persistent/worsening back pain, on-going blood loss, intermittent gross hematuria, hypertension, and abdominal distention. + Evidence of active bleeding such as vascular contrast extravasation, contrast blushing, pseudoaneurysm, and arteriovenous fistula; some experts suggested to perform AE in high-risk features on CT-scan such as high-grade injury with extent hematoma, perirenal rim distance >25 mm and ruptured Gerota fascia. ++ Endovascular stenting must be performed within 4 h of warm ischemic time, high renal loss rate even endovascular treatment. +++ May need more than one intervention. # Signs of collecting system injury such as presence of contrast leakage or contrast pooling in pyelographic phase, ipsilateral hydronephrosis, and ipsilateral delayed excretory phase; some experts suggested to shift to operative management in the patient with evidence of proximal collecting system avulsion. OM: operative management; NOM: non-operative management; CE-CT: contrast-enhanced computed tomography; NPO: nil per os; VTE: venous thromboembolism; Hb: hemoglobin; Hct: hematocrit; AE: angiography with embolization; PCD: percutaneous drainage.
Pancreatic injury is infrequent (0.2%–12% of abdominal injuries), 62 but has high morbidity at 53% and significant mortality rate at 21.2%. 63 More than 24 h delay in diagnosis of pancreatic injuries is associated with 66%–100% morbidity and 11%–16% mortality. 14 Major pancreatic duct injury (PDI) can activate the inflammatory cascade due to pancreatic enzymes leaking and causing severe local inflammation, pancreatic necrosis, digestion of adjacent structures, superimposed infection, systemic inflammatory response, and finally remote organ failure. Contrast-enhanced CT scan is the diagnostic modality of choice in hemodynamically stable, blunt abdominal trauma to diagnose pancreatic injury. It has up to 60% sensitivity to detect pancreatic injury and 54% sensitivity to detect PDI. CT scan has high specificity to detect PDI at 90%–94%. 64 MRCP is currently the gold-standard for PDI diagnosis. However, ERCP is the tool has the highest sensitivity for PDI diagnosis, and additionally provides pancreatic duct interventions. ERCP can improve the success of NOM in pancreatic injury but has a risk of complication of 5%–15% and lack of available personnel in an emergency setting. 14 NOM is currently acceptable as a first line treatment for low-grade pancreatic injury (American Association for the Surgery of Trauma grades I-II) without any associated injuries that require operation. 14 , 62 NOM in low-grade pancreatic injury has near 100% success rate, and low mortality with a 20% reported complication rate. 62 Complications of NOM in pancreatic injury are pancreatitis, pseudocyst and fistula formation. If PDI cannot be excluded on initial CT scan, it is reasonable to perform further investigation such as MRCP or ERCP. Because PDI can change the grading of injury, the management pathway and PDI intervention can improve the success of treatment. There is still no strong evidence to support use of NOM in grade III/IV pancreatic injury. Regarding the high complication rate from delayed management of PDI, operative management still plays the major role in grades III/IV pancreatic injury. Failure rate of NOM in grade III/IV pancreatic injury is up to 10%–50% with a complication rate of 30%. 62 Only few successful NOMs in grade III/IV pancreatic injury have been reported, but it should be chosen in selective cases and in the hands of highly-experienced endoscopists who can perform advanced pancreatic duct interventions. ERCP with pancreatic duct interventions such as transpapillary stenting or sphincterotomy are reasonable in hemodynamically stable patients with contained pancreatic enzyme leakage, and in a center with available resources. Surgical management should be completed rapidly in case of failure interventions. One complication following pancreatic duct interventions in acute settings is pancreatic duct stricture. In a case of pancreatic duct stricture, ERCP with pancreatic duct stent is the treatment of choice. In late phase, ERCP combined with intervention radiology such as CT-guided drainage is valuable and useful in management of pancreatic enzyme leakage, pseudocyst, peripancreatic abscess and pancreatic fistula. 14
Multiple organ injuries
Compared to single solid organ injury, multiple organ injuries have a higher failure rate, associated hollow organ injury, morbidity, and mortality rate. 65 A study in 2005 reported the failure rate of NOM in combined liver and splenic injury (15.4%) is significantly higher than that in isolated liver (6.1%) or splenic injury (7.5%). 66 In 2008, a study of 46 multiple solid organ injury patients found a 25% failure rate of NOM. They also found many parameters such as high lactate level, high solid organ injury score, necessity for blood and crystalloid transfusion, and drop in the hematocrit in the first hour of admission associated with failure of NOM in multiple organ injuries. 67 Nance et al. 68 demonstrated a high incidence of associated hollow organ injury in multiple solid organ injuries (33%–50%) compared to single organ injury (7%). The latest retrospective cohort in 2017 found multiple organ injuries had a high risk of complications (pneumonia and sepsis), increased need of blood transfusion, greater number of operations required, prolonged length of hospital stay, and increased ventilator days compared to single organ injury. They also found that the mortality rate between the two groups was comparable. 69 Nowadays, multiple solid organ injury is not a contraindication for NOM, but the physician should be aware of the high failure rate and risk of missed injury, especially hollow organ injury.
NOM is widely used in abdominal solid organ injury, especially in blunt mechanism, and there is also increasing interest in penetrating injury. The benefits of NOM in solid organ injury are decreasing risk of non-therapeutic laparotomy and preserved organ function. NOM is currently indicated in hemodynamically stable patients who are void of indications for immediate laparotomy, and within the capability of the institute. The physician should be aware of the failure rate, missed hollow organ and diaphragm injury, and delayed hemorrhage during NOM. Adjunct therapies such as AE, ERCP, and PCD could help and increase the chance of success of NOM. However, prompt operation should be prepared and should not be delayed.
Ethical statement
Not applicable.

Declaration of competing interest
The authors have no conflict of interest.
Acknowledgments
I wish to thank Norman Mangnall of Faculty of Medicine, Thammasat University (Thailand) for his help in preparing the English manuscript.
Author contributions
Kanlerd A. made contributions in literatures search, data interpretation, draft writing, critical revision and final approval of the final version for submitted. Karikarn A. made contributions in literatures search, data interpretation, and draft writing and Piyapong B. made contributions in draft writing, and critical revision.
Peer review under responsibility of Chinese Medical Association.
- 1. Raza M., Abbas Y., Devi V., et al. Non operative management of abdominal trauma - a 10 years review. World J Emerg Surg. 2013;8:14. doi: 10.1186/1749-7922-8-14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Feliciano D.V. Abdominal trauma revisited. Am Surg. 2017;83:1193–1202. [ PubMed ] [ Google Scholar ]
- 3. King H., Shumacker HB. Splenic studies. I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg. 1952;136:239–242. doi: 10.1097/00000658-195208000-00006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Bitseff E.L., Adkins R.B., Jr. Splenic trauma: a trial at selective management. South Med J. 1984;77:1286–1290. [ PubMed ] [ Google Scholar ]
- 5. Cogbill T.H., Moore E.E., Jurkovich G.J., et al. Nonoperative management of blunt splenic trauma: a multicenter experience. J Trauma. 1989;29:1312–1317. doi: 10.1097/00005373-198910000-00002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Olthof D.C., van der Vlies C.H., Goslings J.C. Evidence-based management and controversies in blunt splenic trauma. Curr Trauma Rep. 2017;3:32–37. doi: 10.1007/s40719-017-0074-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Lambeth W., Rubin B.E. Nonoperative management of intrahepatic hemorrhage and hematoma following blunt trauma. Surg Gynecol Obstet. 1979;148:507–511. [ PubMed ] [ Google Scholar ]
- 8. Meyer A.A., Crass R.A., Lim R.C., et al. Selective nonoperative management of blunt liver injury using computed tomography. Arch Surg. 1985;120:550–554. doi: 10.1001/archsurg.1985.01390290032005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Boese C.K., Hackl M., Muller L.P., et al. Nonoperative management of blunt hepatic trauma: a systematic review. J Trauma Acute Care Surg. 2015;79:654–660. doi: 10.1097/TA.0000000000000814. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Leppaniemi A.K., Haapiainen R.K. Selective nonoperative management of abdominal stab wounds: a prospective, randomized study. World J Surg. 1996;20:1101–1106. doi: 10.1007/s002689900168. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Renz B.M., Feliciano D.V. Gunshot wounds to the right thoracoabdomen: a prospective study of nonoperative management. J Trauma. 1994;37:737–744. doi: 10.1097/00005373-199411000-00007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Demetriades D., Velmahos G., Cornwell E., 3rd, et al. Selective nonoperative management of gunshot wounds of the anterior abdomen. Arch Surg. 1997;132:178–183. doi: 10.1001/archsurg.1997.01430260076017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Demetriades D., Hadjizacharia P., Constantinou C., et al. Selective nonoperative management of penetrating abdominal solid organ injuries. Ann Surg. 2006;244:620–628. doi: 10.1097/01.sla.0000237743.22633.01. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Cimbanassi S., Chiara O., Leppaniemi A., et al. Nonoperative management of abdominal solid-organ injuries following blunt trauma in adults: results from an International Consensus Conference. J Trauma Acute Care Surg. 2018;84:517–531. doi: 10.1097/TA.0000000000001774. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Stawicki S.P.A. Trends in nonoperative management of traumatic injuries - a synopsis. Int J Crit Illn Inj Sci. 2017;7:38–57. doi: 10.4103/IJCIIS.IJCIIS_7_17. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Leppaniemi A. Nonoperative management of solid abdominal organ injuries: from past to present. Scand J Surg. 2019;108:95–100. doi: 10.1177/1457496919833220. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. London J.A., Parry L., Galante J., et al. Safety of early mobilization of patients with blunt solid organ injuries. Arch Surg. 2008;143:972–976. doi: 10.1001/archsurg.143.10.972. discussion 977. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Eberle B.M., Schnuriger B., Inaba K., et al. Thromboembolic prophylaxis with low molecular-weight heparin in patients with blunt solid abdominal organ injuries undergoing non-operative management: current practice and outcomes. J Trauma. 2011;70:141–147. doi: 10.1097/TA.0b013e3182032f45. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Hommes M., Navsaria P.H., Schipper I.B., et al. Management of blunt liver trauma in 134 severely injured patients. Injury. 2015;46:837–842. doi: 10.1016/j.injury.2014.11.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Li M., Yu W.K., Wang X.B., et al. Non-operative management of isolated liver trauma. Hepatobiliary Pancreat Dis Int. 2014;13:545–550. doi: 10.1016/s1499-3872(14)60049-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Lee Y.H., Wu C.H., Wang L.J., et al. Predictive factors for early failure of transarterial embolization in blunt hepatic injury patients. Clin Radiol. 2014;69:e505–511. doi: 10.1016/j.crad.2014.08.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Parks N.A., Davis J.W., Forman D., et al. Observation for nonoperative management of blunt liver injuries: how long is long enough? J Trauma. 2011;70:626–629. doi: 10.1097/TA.0b013e31820d1c69. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Tiberio G.A.M., Portolani N., Coniglio A., et al. Evaluation of the healing time of non-operatively managed liver injuries. Hepato-Gastroenterology. 2008;55(84):1010–1012. [ PubMed ] [ Google Scholar ]
- 24. Dulchavsky S.A., Lucas C.E., Ledgerwood A.M., et al. Efficacy of liver wound healing by secondary intent. J Trauma. 1990;30:44–48. doi: 10.1097/00005373-199001000-00007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Mebert R.V., Schnuriger B., Candinas D., et al. Follow-up imaging in patients with blunt splenic or hepatic injury managed nonoperatively. Am Surg. 2018;84:208–214. [ PubMed ] [ Google Scholar ]
- 26. Stassen N.A., Bhullar I., Cheng J.D., et al. Nonoperative management of blunt hepatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S288–S293. doi: 10.1097/TA.0b013e318270160d. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Wahl W.L., Brandt M.M., Hemmila M.R., et al. Diagnosis and management of bile leaks after blunt liver injury. Surgery. 2005;138:742–747. doi: 10.1016/j.surg.2005.07.021. discussion 747-748. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Yuan K.C., Wong Y.C., Fu C.Y., et al. Screening and management of major bile leak after blunt liver trauma: a retrospective single center study. Scand J Trauma Resuscitation Emerg Med. 2014;22:26. doi: 10.1186/1757-7241-22-26. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Hommes M., Nicol A.J., Navsaria P.H., et al. Management of biliary complications in 412 patients with liver injuries. J Trauma Acute Care Surg. 2014;77:448–451. doi: 10.1097/TA.0000000000000335. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Hsieh C.H. Comparison of hepatic abscess after operative and nonoperative management of isolated blunt liver trauma. Int Surg. 2002;87:178–184. [ PubMed ] [ Google Scholar ]
- 31. Lok K.H., Li K.F., Li K.K., et al. Pyogenic liver abscess: clinical profile, microbiological characteristics, and management in a Hong Kong hospital. J Microbiol Immunol Infect. 2008;41:483–490. [ PubMed ] [ Google Scholar ]
- 32. Letoublon C., Chen Y., Arvieux C., et al. Delayed celiotomy or laparoscopy as part of the nonoperative management of blunt hepatic trauma. World J Surg. 2008;32:1189–1193. doi: 10.1007/s00268-007-9439-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Schellenberg M., Benjamin E., Piccinini A., et al. Gunshot wounds to the liver: No longer a mandatory operation. J Trauma Acute Care Surg. 2019;87:350–355. doi: 10.1097/TA.0000000000002356. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Navsaria P., Nicol A., Krige J., et al. Selective nonoperative management of liver gunshot injuries. Eur J Trauma Emerg Surg. 2019;45:323–328. doi: 10.1007/s00068-018-0913-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Zarzaur B.L., Rozycki G.S. An update on nonoperative management of the spleen in adults. Trauma Surg Acute Care Open. 2017;2 doi: 10.1136/tsaco-2017-000075. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Peitzman A.B., Heil B., Rivera L., et al. Blunt splenic injury in adults: multi-institutional study of the Eastern Association for the Surgery of Trauma. J Trauma. 2000;49 doi: 10.1097/00005373-200008000-00002. 177-187-189. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Harbrecht B.G., Peitzman A.B., Rivera L., et al. Contribution of age and gender to outcome of blunt splenic injury in adults: multicenter study of the eastern association for the surgery of trauma. J Trauma. 2001;51:887–895. doi: 10.1097/00005373-200111000-00010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Teuben M., Spijkerman R., Blokhuis T., et al. Nonoperative management of splenic injury in closely monitored patients with reduced consciousness is safe and feasible. Scand J Trauma Resuscitation Emerg Med. 2019;27:108. doi: 10.1186/s13049-019-0668-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Dhillon N.K., Barmparas G., Thomsen G.M., et al. Nonoperative management of blunt splenic trauma in patients with traumatic brain injury: feasibility and outcomes. World J Surg. 2018;42:2404–2411. doi: 10.1007/s00268-018-4494-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Malhotra A.K., Carter R.F., Lebman D.A., et al. Preservation of splenic immunocompetence after splenic artery angioembolization for blunt splenic injury. J Trauma. 2010;69:1126–1131. doi: 10.1097/TA.0b013e3181f9fa1e. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Crichton J.C.I., Naidoo K., Yet B., et al. The role of splenic angioembolization as an adjunct to nonoperative management of blunt splenic injuries: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2017;83:843–934. doi: 10.1097/TA.0000000000001649. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Bhullar I.S., Frykberg E.R., Tepas J.J., 3rd, et al. At first blush: absence of computed tomography contrast extravasation in Grade IV or V adult blunt splenic trauma should not preclude angioembolization. J Trauma Acute Care Surg. 2013;74:105–111. doi: 10.1097/TA.0b013e3182788cd2. discussion 111-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Bessoud B., Duchosal M.A., Siegrist C.A., et al. Proximal splenic artery embolization for blunt splenic injury: clinical, immunologic, and ultrasound-Doppler follow-up. J Trauma. 2007;62:1481–1486. doi: 10.1097/TA.0b013e318047dfb8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Bessoud B., Denys A., Calmes J.M., et al. Nonoperative management of traumatic splenic injuries: is there a role for proximal splenic artery embolization? AJR Am J Roentgenol. 2006;186:779–785. doi: 10.2214/AJR.04.1800. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Smith J., Armen S., Cook C.H., et al. Blunt splenic injuries: have we watched long enough? J Trauma. 2008;64:656–663. doi: 10.1097/TA.0b013e3181650fb4. discussion 663-665. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Haan J.M., Marmery H., Shanmuganathan K., Mirvis S.E., Scalea T.M. Experience with splenic main coil embolization and significance of new persistent pseudoaneurysm: reembolize, operate, or observe. J Trauma. 2007;63:615–619. doi: 10.1097/TA.0b013e318142d244. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Savage S.A., Zarzaur B.L., Magnotti L.J., et al. The evolution of blunt splenic injury: resolution and progression. J Trauma. 2008;64:1085–1092. doi: 10.1097/TA.0b013e31816920f1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Sinwar P.D. Overwhelming post splenectomy infection syndrome - review study. Int J Surg. 2014;12:1314–1316. doi: 10.1016/j.ijsu.2014.11.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Coccolini F., Montori G., Catena F., et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg. 2017;12:40. doi: 10.1186/s13017-017-0151-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Teuben M., Spijkerman R., Pfeifer R., et al. Selective non-operative management for penetrating splenic trauma: a systematic review. Eur J Trauma Emerg Surg. 2019;45:979–985. doi: 10.1007/s00068-019-01117-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Erlich T., Kitrey N.D. Renal trauma: the current best practice. Ther Adv Urol. 2018;10:295–303. doi: 10.1177/1756287218785828. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Keihani S., Xu Y., Presson A.P., et al. Contemporary management of high-grade renal trauma: results from the American association for the surgery of trauma genitourinary trauma study. J Trauma Acute Care Surg. 2018;84:418–425. doi: 10.1097/TA.0000000000001796. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Morey A.F., Brandes S., Dugi D.D., 3rd, et al. Urotrauma: AUA guideline. J Urol. 2014;192:327–335. doi: 10.1016/j.juro.2014.05.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Coccolini F., Moore E.E., Kluger Y., et al. Kidney and uro-trauma: WSES-AAST guidelines. World J Emerg Surg. 2019;14:54. doi: 10.1186/s13017-019-0274-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Baghdanian A.H., Baghdanian A.A., Armetta A., et al. Utility of MDCT findings in predicting patient management outcomes in renal trauma. Emerg Radiol. 2017;24:263–272. doi: 10.1007/s10140-016-1473-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Charbit J., Manzanera J., Millet I., et al. What are the specific computed tomography scan criteria that can predict or exclude the need for renal angioembolization after high-grade renal trauma in a conservative management strategy? J Trauma. 2011;70:1219–1228. doi: 10.1097/TA.0b013e31821180b1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Lin W.C., Lin C.H., Chen J.H., et al. Computed tomographic imaging in determining the need of embolization for high-grade blunt renal injury. J Trauma Acute Care Surg. 2013;74:230–235. doi: 10.1097/TA.0b013e318270e156. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Nuss G.R., Morey A.F., Jenkins A.C., et al. Radiographic predictors of need for angiographic embolization after traumatic renal injury. J Trauma. 2009;67:578–582. doi: 10.1097/TA.0b013e3181af6ef4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Chouhan J.D., Winer A.G., Johnson C., et al. Contemporary evaluation and management of renal trauma. Can J Urol. 2016;23:8191–8197. [ PubMed ] [ Google Scholar ]
- 60. Moolman C., Navsaria P.H., Lazarus J., et al. Nonoperative management of penetrating kidney injuries: a prospective audit. J Urol. 2012;188:169–173. doi: 10.1016/j.juro.2012.03.009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Schellenberg M., Benjamin E., Piccinini A., et al. Selective nonoperative management of renal gunshot wounds. J Trauma Acute Care Surg. 2019;87:1301–1307. doi: 10.1097/TA.0000000000002475. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Ho V.P., Patel N.J., Bokhari F., et al. Management of adult pancreatic injuries: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82:185–199. doi: 10.1097/TA.0000000000001300. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Kuza C.M., Hirji S.A., Englum B.R., et al. Pancreatic injuries in abdominal trauma in US adults: analysis of the national trauma data bank on management, outcomes, and predictors of mortality. Scand J Surg. 2020;109:193–204. doi: 10.1177/1457496919851608. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Phelan H.A., Velmahos G.C., Jurkovich G.J., et al. An evaluation of multidetector computed tomography in detecting pancreatic injury: results of a multicenter AAST study. J Trauma. 2009;66:641–647. doi: 10.1097/TA.0b013e3181991a0e. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Galvan D.A., Peitzman A.B. Failure of nonoperative management of abdominal solid organ injuries. Curr Opin Crit Care. 2006;12:590–594. doi: 10.1097/MCC.0b013e328010d4ad. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Robinson W.P., 3rd, Ahn J., Stiffler A., et al. Blood transfusion is an independent predictor of increased mortality in nonoperatively managed blunt hepatic and splenic injuries. J Trauma. 2005;58:437–445. doi: 10.1097/01.ta.0000153935.18997.14. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Yanar H., Ertekin C., Taviloglu K., et al. Nonoperative treatment of multiple intra-abdominal solid organ injury after blunt abdominal trauma. J Trauma. 2008;64:943–948. doi: 10.1097/TA.0b013e3180342023. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Nance M.L., Peden G.W., Shapiro M.B., et al. Solid viscus injury predicts major hollow viscus injury in blunt abdominal trauma. J Trauma. 1997;43:223–618. doi: 10.1097/00005373-199710000-00009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. El-Menyar A., Abdelrahman H., Al-Hassani A., et al. Single versus multiple solid organ injuries following blunt abdominal trauma. World J Surg. 2017;41:2689–2696. doi: 10.1007/s00268-017-4087-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1014.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
