- Frontiers in Medicine
- Research Topics

Transfusion Medicine and Blood
Total Downloads
Total Views and Downloads
About this Research Topic
Transfusion medicine is in perpetual evolution and has faced several challenges from donors screening to clinical practices through blood preparation. Nowadays, blood is mainly processed in its different components that are red blood cells, platelets, plasma and some therapeutics. This incredible story ...
Keywords : Blood, blood products, donors, hematology, transfusion medicine
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, recent articles, submission deadlines.
Submission closed.
Participating Journals
Total views.
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
Current advances in transfusion medicine: a 2019 review of selected topics from the AABB Clinical Transfusion Medicine Committee
Affiliations.
- 1 Transfusion Medicine Division, Department of Laboratory Medicine, University of Washington, Seattle, Washington.
- 2 Department of Pathology, University of California San Diego, La Jolla, California.
- 3 Department of Pediatrics, The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania.
- 4 Department of Pathology and Laboratory Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
- 5 NorthShore University Health System, Chicago, Illinois.
- 6 Transfusion Medicine Division, Department of Pathology, School of Medicine, Johns Hopkins University, Baltimore, Maryland.
- 7 Division of Hematology/Oncology, Simmons Cancer Institute at Southern Illinois University School of Medicine and Mississippi Valley Regional Blood Center, Springfield, Illinois, USA.
- 8 Pathology and Laboratory Medicine, University of Vermont Medical Center, Burlington, Vermont.
- 9 Vitalant, Scottsdale, Arizona.
- 10 Department of Pathology, New York University Grossman School of Medicine, New York, New York.
- 11 Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
- 12 Clinical Pathology Division, Department of Pathology, University of Utah, Salt Lake City, Utah.
- 13 Transfusion Medicine Service, Department of Pathology, University of New Mexico, Albuquerque, New Mexico.
- 14 Transfusion Medicine & Cellular Therapy, Department of Pathology & Cell Biology, Columbia University, New York, New York.
- 15 Department of Pathology, Stanford University, Stanford, California.
- 16 Division of Pediatric Critical Care, Washington University in St Louis, St Louis, Missouri, USA.
- 17 Office of Blood Research and Review, Food and Drug Administration, Silver Spring, Maryland.
- 18 Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, Minnesota.
- PMID: 32472580
- DOI: 10.1111/trf.15848
Background: The AABB Clinical Transfusion Medicine Committee (CTMC) compiles an annual synopsis of the published literature covering important developments in the field of transfusion medicine (TM) for the board of director's review. This synopsis is now made available as a manuscript published in TRANSFUSION.
Study design and methods: CTMC committee members review original manuscripts including TM-related topics published in different journals between late 2018 and 2019. The selection of topics and manuscripts are discussed at committee meetings and are chosen based on relevance and originality. After the topics and manuscripts are selected, committee members work in pairs to create a synopsis of the topics, which is then reviewed by two committee members. The first and senior authors of this manuscript assembled the final manuscript. Although this synopsis is comprehensive, it is not exhaustive, and some papers may have been excluded or missed.
Results: The following topics are included: infectious risks to the blood supply, iron donor studies, pre-transfusion testing interference and genotyping, cold agglutinin disease (CAD), HLA alloimmunization in platelet transfusions, patient blood management, updates to TACO and TRALI definitions, pediatric TM, and advances in apheresis medicine.
Conclusion: This synopsis provides easy access to relevant topics and may be useful as an educational tool.
© 2020 AABB.
Publication types
- Anemia, Hemolytic, Autoimmune* / etiology
- Anemia, Hemolytic, Autoimmune* / genetics
- Anemia, Hemolytic, Autoimmune* / immunology
- Anemia, Hemolytic, Autoimmune* / therapy
- Genotyping Techniques*
- HLA Antigens* / genetics
- HLA Antigens* / immunology
- Platelet Transfusion / adverse effects*
- Transfusion-Related Acute Lung Injury* / etiology
- Transfusion-Related Acute Lung Injury* / genetics
- Transfusion-Related Acute Lung Injury* / immunology
- Transfusion-Related Acute Lung Injury* / therapy
- HLA Antigens

Immunohematology and Transfusion Medicine
A Case Study Approach
- © 2018
- 2nd edition
- View latest edition
- Mark T. Friedman 0 ,
- Kamille A. West 1 ,
- Peyman Bizargity 2 ,
- Kyle Annen 3 ,
- Jeffrey S. Jhang 4
Icahn School of Medicine, Mount Sinai Health System, New York, USA
You can also search for this author in PubMed Google Scholar
Department of Transfusion Medicine, National Institutes of Health Clinical Center, Bethesda, USA
Department of Molecular & Human Genetics, Baylor College of Medicine, Houston, USA
Department of Pathology, Children’s Hospital Colorado, Aurora, USA
- Provides an interactive tool that makes blood banking and transfusion medicine memorable, practical and relevant
- New edition features over 20 new cases
- Written by experts in their fields
44k Accesses
This is a preview of subscription content, log in via an institution to check access.
Access this book
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
The latest edition of this volume features an extensively revised and expanded collection of immunohematology and transfusion medicine cases, comprised of clinical vignettes and antibody panels with questions based on each case. Arranged in a workbook format, the text presents cases based on real patient problems that are typically encountered and covers a number of common issues and challenging problems in blood banking and transfusion practice. Discussion and resolution of each case is provided in a separate answer section, including up-to-date information on pertinent advances in the field. This second edition also contains new cases on topics not previously covered, including types of compatibility testing, polyagglutination, hematopoietic stem cell transplantation, immunohematology test drug interference, granulocyte transfusion, heparin-induced thrombocytopenia, and the approach to the bloodless patient.
Written by experts in the field, Immunohematology and Transfusion Medicine: A Case Study Approach, Second Edition provides an interactive tool that makes blood banking and transfusion medicine memorable, practical, and relevant to residents and fellows.
Similar content being viewed by others

Transfusion Medicine


Modern Blood Banking

- Rh isoimmunization
- alloantibody
- autoantibody
Table of contents (57 chapters)
Front matter, basic single antibody identification: how hard can it be.
- Mark T. Friedman, Kamille A. West, Peyman Bizargity, Kyle Annen, Jeffrey S. Jhang
Rhesus Pieces
Crossmatch crisscross, buried treasure, i can “see” clearly now, you really “oughta” get this, what the kell, are you kidding, hide and seek, the transfusion reaction, what’s this junk, playing with enzymes, the platelet transfusion, differential alloadsorption, hey, how did that antibody get there, i can’t stop the hemolysis, authors and affiliations.
Mark T. Friedman, Jeffrey S. Jhang
Kamille A. West
Department of Molecular & Human Genetics, Baylor College of Medicine, Houston, USA
Peyman Bizargity
About the authors
Mark T. Friedman, DO
Mount Sinai Health System
Icahn School of Medicine
Blood Bank and Transfusion Service
Department of Pathology
New York NY
National Institutes of Health Clinical Center
Department of Transfusion Medicine
Bethesda, MD
Peyman Bizargity, MD
Baylor College of Medicine
Department of Molecular and Human Genetics
Houston, TX
Kyle Annen, DO
Children’s Hospital Colorado
Jeffrey S. Jhang, MD
New York, NY
Bibliographic Information
Book Title : Immunohematology and Transfusion Medicine
Book Subtitle : A Case Study Approach
Authors : Mark T. Friedman, Kamille A. West, Peyman Bizargity, Kyle Annen, Jeffrey S. Jhang
DOI : https://doi.org/10.1007/978-3-319-90960-8
Publisher : Springer Cham
eBook Packages : Medicine , Medicine (R0)
Copyright Information : Springer International Publishing AG, part of Springer Nature 2018
eBook ISBN : 978-3-319-90960-8 Published: 01 August 2018
Edition Number : 2
Number of Pages : XXII, 335
Number of Illustrations : 12 b/w illustrations, 5 illustrations in colour
Topics : Hematology , Blood Transfusion Medicine
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Strengthening quality management system: An assessment of awareness and knowledge in trainees in blood bank
Manisha shrivastava, seema navaid, shweta mishra.
- Author information
- Article notes
- Copyright and License information
Address for correspondence: Dr. Manisha Shrivastava, All India Institute of Medical Science, Saket Nagar, Bhopal, Madhya Pradesh, India. E-mail: [email protected]
Received 2018 Dec 13; Accepted 2019 Mar 3; Issue date 2022 Jan-Jun.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
OBJECTIVES:
The objective of this study was to assess the changes in knowledge concerning quality management system (QMS) among the participants before and after attending a QMS training.
After obtaining the ethical approval, a retrospective study was designed to evaluate the effectiveness of QMS. Fifty participants from district blood banks of three different states participated in the study organized at two different periods. After obtaining informed consent, the participants were subjected to set of questionnaire containing 45 questions (35 multiple choice and 10 subjective questions) as pretest on quality standards and were again subjected to posttest questionnaire containing the same set of questions after 5 days of workshop. Twenty minutes were granted to solve the questions. Each question was given one mark. There was no negative marking. An assessment of knowledge gained during training was evaluated by comparing the scores of pre- and post-assessment.
Of the total 50 delegates, 29 were trained in the month of July (Training I) and 21 were trained in the month of November (Training II). There were 96% ( n = 48) males and 4% ( n = 2) females. In both the training sessions, that is, Training I and II, the mean scores of objective questions in pretest were 13.629 ± 6.58 and 9.34 ± 5.74, and after the training, the posttest scores increased significantly to 17.77 ± 7.05 and 14.34 ± 7.09, respectively. Paired Student's t -test was applied which showed statistically significant increment in knowledge ( P = 0.001).
CONCLUSIONS:
There was a significant positive change in the knowledge of the participants after attending QMS workshop.
Keywords: Blood banks, hemovigilance, quality management systems, transfusion medicine
Introduction
The blood transfusion services are an essential component of health-care system that works with a motive to ensure availability, accessibility, and adequacy to provide quality blood products. Transfusion of blood is a life-saving procedure. At every step from donor screening to transfusion of blood unit required a quality management system (QMS) and any lowering in quality would reflect adversely on the final product. Profound theoretical and practical knowledge is required to ensure the optimal clinical use of blood and blood products.[ 1 , 2 ] Various studies have been conducted to assess knowledge and practice of blood transfusion among medical personnel and nursing staff suggesting that the lack of training in transfusion practices is detrimental to patient safety.[ 3 , 4 , 5 , 6 , 7 ] The competent performance in transfusion medicine becomes an essential requirement to prevent possible complications and transfusion reactions as lack of knowledge in this field can reduce transfusion safety and can cause significant harm to the patient.[ 8 ] Based on the principle of delivering quality health-care services, the National Blood Transfusion Council (NBTC) and the National AIDS Control Organization (NACO) have conducted baseline assessment of NACO supported blood bank and concluded that many blood bank in the country are not aware of the standard quality management practices for various procedures that are performed in blood transfusion services.[ 9 , 10 ] To achieve the quality in blood transfusion services, the NACO conducted training sessions on strengthening QMS at its recognized nodal training centers. Trainings are aimed first to enhance the knowledge of the individuals and later to monitor the improvement thereof in the service where the trainings have focused. The objective of this study was to assess the changes in knowledge concerning QMS among the participants before and after attending a QMS training.
After obtaining the ethical approval from the Institutional Ethical Committee, a retrospective study was designed to evaluate the effectiveness of QMS. Fifty participants from district blood banks of three different states Madhya Pradesh, Bihar, and Jharkhand participated in the National QMS workshop organized at two different periods of time at the Department of Transfusion Medicine, Bhopal Memorial Hospital and Research Center, Bhopal, which is a recognized nodal training center for NBTC and NACO, in the month of July 2017 and November 2017. For the convenience of analysis, the training in the month of July including the participants of Madhya Pradesh was named as Training 1 and training in the month of November including the participants of Jharkhand and Bihar was named as Training II. After taking informed consent from the participants, they were subjected to a predesigned questionnaire on quality standards containing questions on relevant topics of the workshop which were approved by the NACO and sent by the QMS training team of NACO.[ 9 ] The workshops conducted were of 5 days, respectively, covering the basics of QMS in blood banks through lectures delivered by experts as well as group activities and interactive sessions. The participants were again subjected to posttest questionnaire containing the same set of questions. Twenty minutes were granted to solve the 45 questions containing 35 multiple choice questions (MCQ) and 10 subjective questions. Each question whether MCQ or subjective was given one mark. The MCQs were evaluated on the basis of choosing the correct choice of answer, whereas all the subjective questions were evaluated on the basis of standard answer sheet, and after summing up the scores, the subjective questions were graded on a three-level scale of minimum knowledge (0–5 marks), medium knowledge (5–10 marks), and adequate knowledge (10–15 marks). There was no negative marking. An assessment of knowledge gained during training was evaluated by comparing the scores of pre- and post-assessment.
Statistical analysis
Statistical analysis was done for calculations of means, percentages, and ranges. The comparison of means was done using paired sample Student's t -test at 95% confidence interval, and P < 0.05 was considered statistically significant.
Of the total 50 delegates, 29 delegates were trained in the month of July (Training I) and 21 delegates were trained in the month of November (Training II). There were 96% ( n = 48) males and 4% ( n = 2) females. All participants consented to fill the pretest and posttest questionnaire, and informed consent was obtained from the participants. Combining the participants of both the training sessions, there were total 48% ( n = 24) medical officers and 52% ( n = 26) laboratory technicians registered as participants [ Table 1 ]. Figure 1 represents that, in both the trainings (Training I and II), the mean scores of objective questions in pretest were 13.629 ± 6.58 and 9.34 ± 5.74, and after the training, the posttest scores increased to 17.77 ± 7.05 and 14.34 ± 7.09, respectively. The mean pretest scores of objective questions of laboratory technicians and medical officers were 13.92 ± 5.37 and 20.18 ± 4.60 and were increased to mean posttest scores of 21.9259 ± 4.62 and 23.0455 ± 4.64, respectively [ Table 2 ]. The mean rise in the score of laboratory technicians and medical officers increased to 8.0% and 2.86%, respectively, with an overall rise in the scores of 4.6%. Paired Student's t -test was applied to find the significant difference before and after test, and statistically significant increment was seen in knowledge ( P = 0.001).
Demographic characteristics
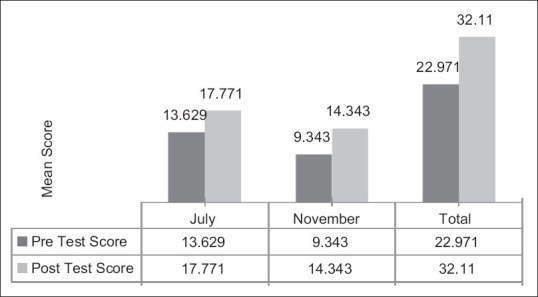
Mean correct knowledge before and after test in July and November
Mean correct knowledge before and after test in Training I and Training II
SD=Standard deviation, S=Significant, HS=Highly significant
As evident from Table 3 , the scores of subjective questions were also increased significantly in both the training sessions. In Training I, maximum participants 96.55% ( n = 28) had a pretest score the sum between 0–5 while they reduced to 48.27% ( n = 14) in posttest, whereas none of the participants scored the sum between 11–15 in pretest and the achievement of this increased up to 10.34% ( n = 3) in posttest. Similarly, in Training II, 95.23% ( n = 20) maximum number of participants scored the sum between 0–5 which reduced to such an extent that no participant scored the minimum score of 0–5 in posttest, whereas the maximum scores of sum between11and 15, of the participants, increased from none in pretest to 38.05% ( n = 8) in posttest [ Table 4 ]. Chi-square test was applied to find the significant difference before and after test. There was a statistically significant increment seen in knowledge ( P = 0.001).
Pre- and post-subjective test scores (number range)
HS=Highly significant
Overall increase in knowledge in both the groups
SD=Standard deviation, HS=Highly significant
Table 5 suggests the comparison of the knowledge of subjective questions between medical officers and laboratory technicians in both the training sessions. The knowledge of subjective questions in medical officers was higher in Training I in pretest score and was found statistically significant whereas the posttest scores showed almost equal increment in the knowledge of both. However, in Training II, there was no significant difference between the knowledge of the medical officers and laboratory technicians in pretest as well as in posttest. While comparing both the scores of both the trainings, the participants in Training II showed a higher compatibility to the training as the rise in score is higher in Training II.
Comparison of pre- and post-subjective test score in both the groups
S=Significant, NS=Not significant, SD=Standard deviation
As evident from Table 6 , in Training sessions I and II, the pretest scores of Q13 and Q16 suggest that the delegates were well aware about the administrative part of the blood banks, including the knowledge of accessibility of standard operating protocol and quality of audit, whereas there was lack of knowledge in defining quality, implementation, intention, and direction of quality in blood banks. However, in posttest assessment, the knowledge about quality audit, blood storage, quality monitoring of blood components, documentation, stock control, and blood cold chain increased significantly as 82.75% delegates answered Q2, Q27, Q29, and 86.2% delegates answered Q16, Q24, Q25, Q30, and Q32 correctly in Training I, whereas in addition to some similar questions as in Training I knowledge about quality policy, document control, quality assurance, and assessment was significantly increased in Training II as 100% delegates answered Q8, Q15, Q21, Q22, Q23, Q25, Q27, and Q30 correctly.
Question-wise scores of the participants in pre- and post-test assessment
QMS is an integral part of blood banks as blood consists of living cells and is being used for pharmaceutical purposes and comes under the regulation of pharmaceutical production rules. The present study was aimed to assess the improvement in knowledge of the participants participated in QMS training. This training program in QMS was a predesigned activity-based training which incorporated the maximum involvement of the participants in the hands-on training, including group activities, homework assessments, interactive assessments, and laboratory demonstration including the quality issues related to blood banking. The training of clinical staff in blood banks is a national mandate and is being regularly conducted throughout the country. As there is paucity of studies related to the assessment of knowledge of the all clinical staff working in blood transfusion whereas no study has been published in India or abroad to find the effectiveness of these QMS training programs; however, the World Health Organization (WHO) reported a workshop conducted in Pakistan, 2014 on the WHO training on quality management and revealed an increment in knowledge of the delegates after an uniform workshop being organized at four different places of the country. The participants scored between 70%–80% in the postcourse assessment as compared with the scores 40%–50% in the precourse assessment.[ 11 ] The present study also revealed that there was a significant increase in knowledge among the delegates participated in both the training of QMSs as the scores increased from 46.99% to 61.82% in Training I and from 49.25% to 68.29% in Training II. Similarly, Kaur et al. in their study also reported the rise in the mean score of posttraining assessment to 85.4% from 51% in the pretraining, which was statistically significant while training the clinicians for transfusion practices.[ 12 ]
Clark et al. reported an improvement in compliance with the national guidelines to over 95% in six out of seven of the recommendations on the best practice was seen 18 months after the initial intervention and suggested that education is the primary requisite of those who prescribe and administer transfusions.[ 13 ]
As this is an era of evidence-based practice, regular workshops and their assessment become a mandatory to bring forth the ethics in clinical practices and ensure quality. Furthermore, various researches favor training and workshop and an important tool in medical education as well as for promoting ethical and evidence-based practice.[ 14 , 15 , 16 ] The pre- and post-assessment of the workshop is an effective method to find the effectiveness of the training program or workshop module and also helps in improving the instructor's ability.[ 14 ] However, to assess the QMS training prospective research studies needs to be planned to fulfill the research gap.
Various studies have been conducted to assess the knowledge of interns and nurses in working or involved in transfusion services.[ 5 , 6 , 17 ] Hijji et al. in their study reported a deficit of knowledge in the nurses working in blood transfusion services.[ 5 ] Similar results addressed in a survey conducted on Jordian nurses and were found detrimental to patient safety.[ 6 ] Further, a study conducted on nursing students showed a lack of knowledge in transfusion services, which was fund to be improved after training, similar to the present study.[ 17 ]
The Pyramid Model was also described as a tool to manage the quality systems, which include a four-level model including the quality policy, annual plans, job descriptions, standard operating procedures (SOPs) and emergency operating procedures description, and record maintenance.[ 18 ] The present study also included the questions related to quality policy, SOPs, documentations, and job responsibilities, and it was found that the participants were well compatible in maintaining documents, following SOP, and audits as between approximately 75% of participants answered the questions related to these topics correctly whereas they lacked in practical quality management. Furthermore, the participants lack information about the implementation, intention, and direction and overall organization in relation to quality as <41% of the participants answered the questions related to these topics. It is an essential requirement for quality, safety, and efficacy of blood and blood products to ensure well-equipped blood centers with adequate infrastructure and trained workforce. To ensure uniformly ethical practice, good quality practice, and effective clinical use of blood in blood banks, it is necessary to train clinical staff. While moving toward total quality management to attain maximum safety, the requirements of good manufacturing practices and implementation of the quality system have offered challenges to the organization and management of blood transfusion service.[ 9 ] This study also included the questions related to quality monitoring of processed blood component and safety issues such as maintaining blood cold chain, identification of patients, and about the storage area for blood and blood components, and it was found that the participants were well aware of these questions and majority approximately 60% and above answered the questions correctly in pretest assessment in both the trainings; however, the scores increased further after the training. Unlike this study, Hijji et al. in their study reported the lack of knowledge related to the appropriate identification of patients.[ 6 ]
The training in QMS along with the other quality issues and protocols also addresses the concept of hemovigilance. It is a continuous need to aware the transfusion team about hemovigilance to avoid undesired transfusion reaction and to keep the laws and tools in place.[ 19 ] The present study also addressed the concept of hemovigilance in training and <51% of participants answered the question correctly in pretest assessment while it improved to 84% after training.
Transfusion services in developed world such as the USA are highly costly and efficient; however, in the developing world like ours, transfusion therapy is facing the continuous concern related to blood safety issues.[ 20 ] The WHO has identified blood safety as a health issue requiring high priority and has developed a comprehensive program and guidelines to ensure the quality management in blood banks and also considered it important to provide comprehensive, appropriate, and effective training is for all blood transfusion service staff and other health-care professionals involved in blood transfusion which also includes the distance learning modules.[ 21 ]
In India, in spite of good training programs for QMSs in blood banks by NACO, the effectiveness of these QMS training programs has not been assessed and provides a research gap.[ 10 ]
Conclusions
There was a significant positive change in the knowledge of the participants after attending QMS workshop. This study will help to develop the roadmap to the implementation of QMS in blood banks to improve consistency in all its activities and aware the participants for the enhancement of quality of the blood banks. A prospective study can be planed and also a standardized questionnaire can be developed to implement similar assessment throughout the country. Thus, it would be helpful to facilitate to focus the course on areas of particular need.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.
Assessment Questionnaire
QMT 1.5 PRE--COURSE ASSESSMENT QUESTIONS
Name: ____________________________________________________________
Total Questions: 45 Time: 20 minutes
Mark the correct answer to each question
1 The quality of a product or a service denotes:
a High cost
b Fitness for the purpose
c Quick results and efficacious products
d Sophistication and complexity of the process
a Internal Services Office
b International Organisation for Standardisation
c International Safety Organization
d Instant Solutions Offer
3 The relationship between the results achieved and the resources used is:
a Efficiency
b Effectiveness
c Precision
d Verification
4 The initial draft of a standard operating procedure should be written by:
a Person performing the procedure
b Quality manager
c Technical head of the blood bank
d An expert committee
5 A system of activities that uses resources to transform inputs into outputs is defined as:
a Procedure
d Performance
6 The fulfilment of a requirement is defined as:
a Conformity
b Characteristic
7 The implementation of quality in blood banks is the responsibility of:
a The quality manager only
b The technical head of the blood bank only
c External auditors
d All staff members of the blood bank
8 A quality policy is officially endorsed and approved by the:
a Top management of the blood bank
b Quality Manager
d Technical professionals of the blood bank
9 The overall intentions and direction of an organization in relation to quality, as formally expressed by top management is:
a Quality objective
b Quality policy
c Quality management system
d Quality planning
10 A document stating the quality policy and describing the quality system of an organization is called:
a Quality manual
b Guidelines
c Specifications
d Quality plan
11 A job description includes all of the following except:
a Key tasks to be performed
b Minimum qualifications and experience
c Position in the organization's organogram
d Career advancement prospects
12 Standard operating procedures (SOPs):
a Are guidelines for screening of transfusion-transmissible infections
b May be used by some staff members sometimes
c Are designed to help newly recruited and inexperienced technical staff to
develop confidence and acquire skills
d Must be followed strictly by all staff members at all times
13 SOPs should be accessible to:
a Senior staff only
b All relevant staff all the time
c Staff when they encounter problems in performing procedure
d All staff only on demand
14 The following documents need to be controlled:
b Standard operating procedures
c Donor records
d List of approved suppliers
e All of the above
15 The part of quality assurance that ensures that products are consistently produced and controlled to quality standards appropriate to their intended use is called:
a Good Manufacturing Practice (GMP)
b Good Laboratory Practice (GLP)
c Good Clinical Practice (GCP)
d Internal quality control
16 A quality audit is:
a A systematic, independent and documented examination to determine whether quality activities comply with planned arrangements
b An evaluation of conformity by observation and judgement
c An activity that ensures correct financial procedures
17 Competency assessment of staff includes all the following except:
a Written evaluation
b Review of work records
c Testing of unknown samples
d Gross salary received
e Problem solving skills
18 A stock card is characterized by the following except:
a A simple and efficient stock control system
b A record of the order, delivery and use of each item
c Decides next order and quantity to order
d Ensures excessive stocks are always available
e Helps at each time of issue, order or delivery of stock
19 The following essential information should be retained for stock control except:
a Minimum stock level
b Minimum order
c Code number of consumables
d Test in which consumable is to be used
20 The method most suitable for ordering consumables with a long expiry period if you have sufficient resources and storage space is:
a Bulk order
b Standing order
c Order as required
21 Which of the following does not apply to an external quality assessment (EQA) scheme?
a Organized by an external agency
b Does not require follow up
d Compares performance at different sites
22 Material received by a participating blood bank for external quality assessment should be analyzed:
a By the quality manager alone
b By the most skilled worker
c With specially procured and exclusive reagents
d In the same manner as routine work
23 A unique number must be assigned to each donation of blood. To which of the following should this number be attached?
a The primary collection bag only
b The primary and all secondary collection bags only
c The primary, all secondary collection bags and all specimen tubes used only
d The primary, all secondary collection bags, all specimen tubes used and donation record
24 The following applies to storage areas for blood and blood components:
a Quarantined components should be stored with non-conforming blood components
b Tested (available) units should be stored separately from partially tested or untested (quarantined) blood components
c Quarantined components should be stored with expired blood components
25 Quality monitoring of processed blood components is performed to:
a Find reasons not to make blood components
b Research new techniques for making blood components
c Ensure that the final product meets specifications and that the process is "in control"
d Keep the quality manager happy
26 The identification of a patient receiving transfusion should be carried out:
a By the patient's bedside immediately before transfusion
b At the nurses' station before transfusion
c During the transfusion
d After the transfusion
27 The documentation required in the preparation of blood components includes:
a Approved SOPs and records of all key activities ranging from the receipt of whole blood to the distribution of released components to hospitals and blood banks for compatibility testing
b Validation protocol for testing for transfusion-transmissible infections
c Crossmatching results
d Training records for staff working in the Quality Department
28 Documented procedures for the recall of blood components must enable:
a Recall of all components/component pool related to the donation that caused an adverse reaction
b Recall of the initial component that caused the adverse reaction
c Awareness that the component caused an adverse reaction
29 Recall of a product should lead to:
a Notification of the donor staff
b No further action
c An investigation, with corrective action to prevent recurrence
d Notification of the components preparation staff
30 It is important to have a stock control system for reagents because:
a It ensures that reagents are validated properly
b It helps you in monitoring the rate of usage of items, and the reliability of your supplier which, in turn will help prevent an out-of-stock situation
c It is an extra system to keep people busy
d It is a new system that management wants implemented
31 Record-keeping in the laboratory is essential in meeting the requirements of:
a Good laboratory practice
b Good record-keeping practice
c Good testing practice
d Good housekeeping practice
32 A “blood cold chain” is:
a A metal link that is kept in the refrigerator
b The storage of products in a refrigerator and/or freezer
c A system for storing and transporting blood and plasma in an appropriate way to maintain all its functions
d A cold climate
33 The following are NOT essential parts of the blood cold chain:
a Equipment for the storage and transportation of blood
b People who manage the storage and transportation of blood
c People and equipment, resulting in an adequate blood cold chain
d Maintenance of blood storage equipment
e Control of the stock of blood available for use
34 A Haemovigilance programme is concerned with:
a Investigation of transfusion-related incidents
b Haemoglobin level of a donor
c Haemoglobin test
d Efficiency of staff
35 The customers of the BTS at the clinical interface are:
b Clinicians
c Patients and clinicians
36 Write full form of the following
a. NABH....................
b. QCI.....................
c. MRM....................
d. QMS....................
37 Name of Standards of Blood Bank and lay down by which organization?
........................................
.......................................
38 Frequency of Internal Audit is
....................................
39 Write and describe type of Internal Audits
40 Activities includes in Process Control Clause of Blood Bank
41 Write the Clauses covered in Blood Bank Standards:
42 Write the steps when non conformity is detected
43 How can you improve performance of your Blood Bank?
44 Prepare an organogram of your Blood Bank
45 Write down hierarchy system of documents in QMS
- 1. Panzer S, Engelbrecht S, Cole-Sinclair MF, Wood EM, Wendel S, Biagini S, et al. Education in transfusion medicine for medical students and doctors. Vox Sang. 2013;104:250–72. doi: 10.1111/j.1423-0410.2012.1661.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Friedman MT. Blood transfusion practices: A little consistency please. Blood Transfus. 2011;9:362–5. doi: 10.2450/2011.0007-11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Saillour-Glénisson F, Tricaud S, Mathoulin-Pélissier S, Bouchon B, Galpérine I, Fialon P, et al. Factors associated with nurses' poor knowledge and practice of transfusion safety procedures in Aquitaine, France. Int J Qual Health Care. 2002;14:25–32. doi: 10.1093/intqhc/14.1.25. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Reza PA, Aziz SV, Ali MA, Marjan MH, Reza TM. Evaluation of knowledge of healthcare workers in hospitals of Zabol city on proper methods of blood and components transfusion. Asian J Transfus Sci. 2009;3:78–81. doi: 10.4103/0973-6247.53878. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Hijji B, Parahoo K, Hussein MM, Barr O. Knowledge of blood transfusion among nurses. J Clin Nurs. 2013;22:2536–50. doi: 10.1111/j.1365-2702.2012.04078.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Hijji BM, Oweis AE, Dabbour RS. Measuring knowledge of blood transfusion: A survey of Jordanian nurses. Am Int J Contemp Res. 2012;2:77–94. [ Google Scholar ]
- 7. Aslani Y, Etemadyfar S, Noryan K. Nurses' knowledge of blood transfusion in medical training centers of Shahrekord University of medical science in 2004. Iran J Nurs Midwifery Res. 2010;15:141. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Ferreira O, Martinez EZ, Mota CA, Silva AM. Evaluation of knowledge about hemotherapy and transfusional care of nurses. Rev Bras Hematol E Hemoter. 2007;29:160–7. [ Google Scholar ]
- 9. National Blood Transfusion Council, National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India. [Last accessed on 2018 Oct 30]. Available from: http://www.naco.gov.in/national-blood-transfusion-council-nbtc-0 .
- 10. Blood Transfusion Services, National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India. [Last accessed on 2018 Oct 30]. Available from: http://www.naco.gov.in/blood-transfusion-services .
- 11. World Health Organization. A Joint Project of The OPEC Fund for International Development (OFID) and World Health Organization. [Last accessed on 2018 Nov 02]. Available from: http://www.who.int/bloodsafety/transfusion_services/joint_project/en/
- 12. Kaur P, Kaur G, Kaur R, Sood T. Assessment of impact of training in improving knowledge of blood transfusion among clinicians. Transfus Med Hemother. 2014;41:222–6. doi: 10.1159/000362896. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Clark P, Rennie I, Rawlinson S. Quality improvement report: Effect of a formal education programme on safety of transfusions. BMJ. 2001;323:1118–20. doi: 10.1136/bmj.323.7321.1118. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Rajadhyaksha V. Training for clinical research professionals: Focusing on effectiveness and utility. Perspect Clin Res. 2010;1:117–9. doi: 10.4103/2229-3485.71767. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Ajay S, Bhatt A. Training needs of clinical research associates. Perspect Clin Res. 2010;1:134–8. doi: 10.4103/2229-3485.71771. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Shrivastava M, Shah N, Navaid S. Assessment of change in knowledge about research methods among delegates attending research methodology workshop. Perspect Clin Res. 2018;9:83–90. doi: 10.4103/picr.PICR_41_17. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Flood LS, Higbie J. A comparative assessment of nursing students' cognitive knowledge of blood transfusion using lecture and simulation. Nurse Educ Pract. 2016;16:8–13. doi: 10.1016/j.nepr.2015.05.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. van der Tuuk Adriani WP, Sibinga S. The pyramid model as a structured way of quality management. Asian J Transfus Sci. 2008;2:6–8. doi: 10.4103/0973-6247.39503. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Carneiro-Proietti AB. Hemovigilance: A system to improve the whole transfusion chain. Rev Bras Hematol Hemoter. 2013;35:158–9. doi: 10.5581/1516-8484.20130045. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Dhingra N. Blood safety in the developing world and WHO initiatives. Vox Sang. 2002;83(Suppl 1):173–7. doi: 10.1111/j.1423-0410.2002.tb05295.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. World Health Organization. Quality Management Training for Blood Transfusion Services. World Health Organization. [Last accessed on 2018 Nov 02]. Available from: http://www.who.int/bloodsafety/publications/qmp_toolkit/en/
- View on publisher site
- PDF (676.0 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Bibliography
- More Referencing guides Blog Automated transliteration Relevant bibliographies by topics
- Automated transliteration
- Relevant bibliographies by topics
- Referencing guides
Dissertations / Theses on the topic 'Blood bank'
Create a spot-on reference in apa, mla, chicago, harvard, and other styles.
Consult the top 50 dissertations / theses for your research on the topic 'Blood bank.'
Next to every source in the list of references, there is an 'Add to bibliography' button. Press on it, and we will generate automatically the bibliographic reference to the chosen work in the citation style you need: APA, MLA, Harvard, Chicago, Vancouver, etc.
You can also download the full text of the academic publication as pdf and read online its abstract whenever available in the metadata.
Browse dissertations / theses on a wide variety of disciplines and organise your bibliography correctly.
Williams, Rosalind. "Blood in the archive : rethinking the public umbilical cord blood bank." Thesis, University of York, 2015. http://etheses.whiterose.ac.uk/12189/.
Fletcher, Andrew, Olivia Luzzi, and Jennifer R. Hunt. "Resources Management: Efficient Utilization of Blood Transfusion: Lessons from the Blood Bank." Digital Commons @ East Tennessee State University, 2017. https://dc.etsu.edu/etsu-works/8209.
Dodson, Patricia W. "The Blood Bank: A Collection of Short Stories." VCU Scholars Compass, 2016. http://scholarscompass.vcu.edu/etd/4214.
Bloom, Connor. "The Feasibility of Whole-Blood-System Genotyping: A Case Study using the San Diego Blood Bank." Scholarship @ Claremont, 2019. https://scholarship.claremont.edu/cmc_theses/2110.
Santiago, Karina Basso [UNESP]. "Monitoramento da qualidade de hemocomponentes produzidos no Hemocecentro da FMB - UNESP." Universidade Estadual Paulista (UNESP), 2011. http://hdl.handle.net/11449/88108.
Schörner, Everaldo José. "Guia nacional para implantação de banco de sangue com fenótipos raros: uma proposta para a hemorrede pública brasileira." Universidade de São Paulo, 2015. http://www.teses.usp.br/teses/disponiveis/17/17155/tde-27072015-053713/.
Santiago, Karina Basso. "Monitoramento da qualidade de hemocomponentes produzidos no Hemocecentro da FMB - UNESP /." Botucatu : [s.n.], 2011. http://hdl.handle.net/11449/88108.
Sonaglio, Franciele. "Avaliação bioquímica in vitro do concentrado de eritrócitos felino Armazenado em soluções de cpda-1 e cpd/sagm durante 35 dias." reponame:Biblioteca Digital de Teses e Dissertações da UFRGS, 2014. http://hdl.handle.net/10183/96918.
Blasi, Brugué Carles. "Advances in feline transfusion medicine." Doctoral thesis, Universitat Autònoma de Barcelona, 2021. http://hdl.handle.net/10803/673650.
Carvalho, Thiago Vianna de. "Desenvolvimento de estratégia de genotipagem para discriminação de alelos antitéticos do sistema de grupo sanguíneo Diego utilizando pool de DNA." Universidade de São Paulo, 2018. http://www.teses.usp.br/teses/disponiveis/17/17155/tde-19072018-141223/.
Lopes, Renata Vernay. "Elaboração de um manual de reserva de concentrados de hemácias para cirurgias eletivas no Hospital de Base do Distrito Federal." Universidade de São Paulo, 2018. http://www.teses.usp.br/teses/disponiveis/17/17155/tde-08012019-154356/.
Meissner-Roloff, Madelein. "Prerequisites for establishing a public human UCB SCB; assessment of public acceptance and resistance of UCB to HIV." Thesis, University of Pretoria, 2012. http://hdl.handle.net/2263/24166.
Borges, Ivo Ricardo Fernandes. "Caraterização estatística dos valores percentuais de sangue do grupo AB0 colhido pelo Centro Regional de Sangue de Lisboa e respetivo emparelhamento com a distribuição a nível hospitalar." Master's thesis, Faculdade de Ciências Médicas, 2013. http://hdl.handle.net/10362/11012.
Passos, Paula Renata Machado. "Associação entre os níveis citoplasmáticos da enzima aldeído desidrogenase (ALDH) e a capacidade proliferativa \"in vitro\" das células progenitoras hematopoéticas de sangue de cordão umbilical e placentário." Universidade de São Paulo, 2018. http://www.teses.usp.br/teses/disponiveis/17/17155/tde-13092018-160308/.
Binobaid, Abdulmajeed. "Riyadh Blood Banks Distribution System." Digital Commons at Loyola Marymount University and Loyola Law School, 2014. https://digitalcommons.lmu.edu/etd/377.
Zanelli, Ana Paula Rocha Diniz. "Avaliação da viabilidade financeira do banco do sangue de cordão umbilical do Hemocentro de Ribeirão Preto." Universidade de São Paulo, 2017. http://www.teses.usp.br/teses/disponiveis/17/17155/tde-07062017-132339/.
Meira, Katia Milena Gonçalves. "Utilização da tecnologia Data Warehousing e da ferramenta OLAP para apoiar a captação de doadores de sangue: estudo de caso no Hemonúcleo Regional de Jáu." Universidade de São Paulo, 2004. http://www.teses.usp.br/teses/disponiveis/18/18140/tde-01082017-115123/.
Otani, Márcia Mitiko. "Programa de avaliação externa para os testes de triagem sorológica de doadores de bancos de sangue dos centros de referência da América Latina: utilização de multipainel específico." Universidade de São Paulo, 2003. http://www.teses.usp.br/teses/disponiveis/9/9136/tde-07072010-104629/.
Custer, Brian Scott. "Blood safety and resource allocation : economic analyses of donated blood safety initiatives /." Thesis, Connect to this title online; UW restricted, 2003. http://hdl.handle.net/1773/7964.
Bray, Timothy John. "The rational use of blood in India : intervention to promote good transfusion practice." Thesis, London School of Hygiene and Tropical Medicine (University of London), 2001. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.274939.
Bruce, Lesley J. "A study of human erythrocyte band 3 variants." Thesis, University of Bristol, 1994. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.240590.
Leipoldt, Edmund Johann. "Alternative blood risk categorization models for South Africa." Thesis, Bloemfontein : Central University of Technology, Free State, 2008. http://hdl.handle.net/11462/107.
Bianchi, Juliana Vieira dos Santos. "Genotipagem de grupos sanguíneos por meio de microarranjos líquidos." Universidade de São Paulo, 2016. http://www.teses.usp.br/teses/disponiveis/99/99131/tde-19042016-145530/.
Van, Heerden Marchell. "Evaluation of the effectiveness of strategic planning in the blood transfusion services in South Africa." Thesis, Port Elizabeth Technikon, 2000. http://hdl.handle.net/10948/27.
Chen, Jinyan, and 陈锦艳. "Residual risks estimating models of transmission of HBV, HIV and HCV with different assays : lesson for screening strategies for Chinese blood banks." Thesis, The University of Hong Kong (Pokfulam, Hong Kong), 2013. http://hdl.handle.net/10722/193767.
Giacomini, Luana. "Elementos para a organização do trabalho em hemoterapia com vistas à fidelização do doador voluntário de sangue." reponame:Repositório Institucional da FURG, 2007. http://repositorio.furg.br/handle/1/2763.
Kurunlahti, M. (Mauno). "Association of impaired blood supply with painful lumbar disc degeneration." Doctoral thesis, University of Oulu, 2003. http://urn.fi/urn:isbn:9514270436.
Rund, Robin Lindsay. "Study of elective surgical blood usage at Groote Schuur Hospital." Thesis, University of Cape Town, 1992. http://hdl.handle.net/11427/25796.
Che, Alexis Pun Kit. "Studies of band 3 rotational mobility in normal and ovalocytic human red blood cell membranes by transient dichroism." Thesis, University of Essex, 1994. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.241212.
Rydstedt, Linn. "”No Blood Should Hold us Back” : en genusanalys av Libresses mensskyddsreklam i Sverige från år 2010 till 2016." Thesis, Stockholms universitet, JMK, 2017. http://urn.kb.se/resolve?urn=urn:nbn:se:su:diva-138537.
Sanchez, Arianni Rondelli. "Validação de teste ELISA para pesquisa de anticorpos anti-MSP119 de Plasmodium vivax visando à aplicação em serviços hemoterápicos do Brasil em áreas não endêmicas para malária." Universidade de São Paulo, 2014. http://www.teses.usp.br/teses/disponiveis/99/99131/tde-05052015-140550/.
Mququ, Mpumzi H. "A survey of customer satisfaction, expectations and perceptions as a measure of service quality in SANBS." Thesis, Rhodes University, 2006. http://hdl.handle.net/10962/d1003888.
Olney, Christine M. "Back massage : long term effects and dosage determination for persons with pre-hypertension and hypertension." [Tampa, Fla] : University of South Florida, 2007. http://purl.fcla.edu/usf/dc/et/SFE0001923.
Peres, Aline Almeida. "Processo de punção venosa na captação e transfusão de sangue e trauma vascular periférico na perspectiva do binômio usuário-profissional." Universidade Federal de Juiz de Fora (UFJF), 2016. https://repositorio.ufjf.br/jspui/handle/ufjf/4051.
Rodrigues, Patrícia Isabel da Silva. "Proposta de abordagem para gestão de ocorrências em Serviços de Sangue." Master's thesis, Faculdade de Ciências Médicas, 2013. http://hdl.handle.net/10362/10859.
Cho, Kisoo. "Characteristics of North Korean Music under Juche philosophy with reference to the Revolutionary Opera Sea of Blood and the Moranbong band / Two examination concerts." Diss., University of Pretoria, 2016. http://hdl.handle.net/2263/60438.
Koffi, Kouakou Hyacinthe Privat. "Sistemi per Autotrasfusione: Stato dell'Arte e Tecnologia a confronto." Bachelor's thesis, Alma Mater Studiorum - Università di Bologna, 2012. http://amslaurea.unibo.it/3595/.
Costa, Maykon Luiz Nascimento. "Avaliação da viabilidade da implementação da tecnologia de gelificação na produção de insumos para diagnóstico." Universidade Tecnológica Federal do Paraná, 2017. http://repositorio.utfpr.edu.br/jspui/handle/1/2805.
Thompson, Kelly. "Characterizing the Chondrodystrophic Canine Intervertebral Disc in Health and Disease." The Ohio State University, 2019. http://rave.ohiolink.edu/etdc/view?acc_num=osu157428888276191.
Papenfuss, Kylara A. "Regulated Deficit Irrigation of 'Montmorency' Tart Cherry." DigitalCommons@USU, 2010. https://digitalcommons.usu.edu/etd/535.
Thorell, Eva. "Physical Fitness and Pregnancy." Doctoral thesis, Uppsala universitet, Allmänmedicin och preventivmedicin, 2013. http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-203630.
Pavlík, Dušan. "Ultrazvukový měřič rychlosti toku krve." Master's thesis, Vysoké učení technické v Brně. Fakulta elektrotechniky a komunikačních technologií, 2011. http://www.nusl.cz/ntk/nusl-219209.
Mülek, Melanie [Verfasser], and Petra [Gutachter] Högger. "Distribution and metabolism of constituents and metabolites of a standardized maritime pine bark extract (Pycnogenol®) in human serum, blood cells and synovial fluid of patients with severe osteoarthritis / Melanie Mülek. Gutachter: Petra Högger." Würzburg : Universität Würzburg, 2016. http://d-nb.info/1112040978/34.
Neves, Soraya Andreassa. "Banco de sangue de cordão umbilical e placentário: proposta de sistema híbrido brasileiro." Universidade Tecnológica Federal do Paraná, 2012. http://repositorio.utfpr.edu.br/jspui/handle/1/305.
Kameníček, Robert. "Ultrazvukový indikátor toku krve." Master's thesis, Vysoké učení technické v Brně. Fakulta elektrotechniky a komunikačních technologií, 2010. http://www.nusl.cz/ntk/nusl-218785.
Splittstoesser, Riley Emiel. "Inflammatory Responses to Combinations of: Mental Load, Repetitive Lifting and Subject Personality." The Ohio State University, 2016. http://rave.ohiolink.edu/etdc/view?acc_num=osu1479763594134482.
Derakhshan, Jamal Jon. "Innovations Involving Balanced Steady State Free Precession MRI." Cleveland, Ohio : Case Western Reserve University, 2009. http://rave.ohiolink.edu/etdc/view?acc_num=case1247256364.
Yamamoto, Celia Regina Furucho. ""Diagnóstico da doença de Chagas em bancos de sangue: linfoproliferação, detecção de anticorpos e estudo epidemiológico em indivíduos com provas sorológicas inconclusivas"." Universidade de São Paulo, 2006. http://www.teses.usp.br/teses/disponiveis/5/5134/tde-03052006-133432/.
Goenarjo, Roman. "Effect of age, vascular parameters, physical activity, and cardiorespiratory fitness on executive performances : role of cerebral oxygenation." Thesis, Poitiers, 2020. http://www.theses.fr/2020POIT2252.
林明智. "A simulation approach to setting the levels for a hospital blood bank." Thesis, 1986. http://ndltd.ncl.edu.tw/handle/52296250453782870931.

COMMENTS
Impact of using different blood donor subpopulations and models on the estimation of transfusion transmission residual risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus in Zimbabwe.
The following topics will be included: - History of blood transfusion - Donors, screening and infectious markers (including demographic evolution) - Immunohematology - Labile blood products (red blood cells, platelets and plasma) - New products in transfusion: synthetic cells, blood-derived products, cellular therapies - Epidemiology of blood ...
advancement in blood banking is absolutely essential for technical staff for successful implementation of blood banking protocols and timely delivery of blood components to needy patients.
Current Topics in Blood Banking. By Elizabeth Walker | August 24 2021. May 18, 2018 — Save The Date. An educational program for medical lab scientists, residents, fellows and faculty, designed to discuss topics related to blood banking, hemostasis, quality and management. CE credits offered for medical lab scientists. View Site.
Results: The following topics are included: infectious risks to the blood supply, iron donor studies, pre-transfusion testing interference and genotyping, cold agglutinin disease (CAD), HLA alloimmunization in platelet transfusions, patient blood management, updates to TACO and TRALI definitions, pediatric TM, and advances in apheresis medicine.
Interests: blood transfusion; patient blood management; blood banking; immunohematology; cell therapy; transplantation. Special Issues, Collections and Topics in MDPI journals. Special Issue Information. Dear Colleagues, Blood banking and transfusion practices have evolved considerably over the years.
This book provides a comprehensive overview of immunohematology and transfusion medicine through over 50 cases based on real patient problems that are typically encountered, covering a number of common issues and challenging problems in blood banking and transfusion practice.
It is an essential requirement for quality, safety, and efficacy of blood and blood products to ensure well-equipped blood centers with adequate infrastructure and trained workforce. To ensure uniformly ethical practice, good quality practice, and effective clinical use of blood in blood banks, it is necessary to train clinical staff.
Struggling with your thesis topic in blood banking is a common challenge due to the vast amount of information available and high standards required. Blood banking covers many complex areas of study from transfusion medicine to donor recruitment.
List of dissertations / theses on the topic 'Blood bank'. Scholarly publications with full text pdf download. Related research topic ideas.