- Thermodynamics
- Law Of Conservation Of Mass

Law of Conservation of Mass
The law of conservation of mass states that mass within a closed system remains the same over time. Discover more about the law of conservation of mass, including its importance, equations, and some examples of this law in action.
What is the Law of Conservation of Mass?
The law of conservation of mass states that
“The mass in an isolated system can neither be created nor be destroyed but can be transformed from one form to another”.
According to the law of conservation of mass, the mass of the reactants must be equal to the mass of the products for a low energy thermodynamic process.
It is believed that there are a few assumptions from classical mechanics which define mass conservation. Later the law of conservation of mass was modified with the help of quantum mechanics and special relativity that energy and mass are one conserved quantity. In 1789, Antoine Laurent Lavoisier discovered the law of conservation of mass.
Formula of Law of Conservation of Mass
Law of conservation of mass can be expressed in the differential form using the continuity equation in fluid mechanics and continuum mechanics as:
- ρ is the density
- t is the time
- v is the velocity
- ▽ is the divergence
Related Articles:
- Law of Conservation of Momentum Derivation
- Mass And Weight
- Thermodynamic Processes
Law of Conservation of Mass Examples
- Combustion process: Burning of wood is a conservation of mass as the burning of wood involves Oxygen, Carbon dioxide, water vapor and ashes.
- Chemical reactions: To get one molecule of H 2 O (water) with the molecular weight of 10, Hydrogen with molecular weight 2 is added with Oxygen whose molecular weight is 8, thereby conserving the mass.
Law of Conservation of Mass Problems
Q1. 10 grams of calcium carbonate (CaCO 3 ) produces 3.8 grams of carbon dioxide (CO 2 ) and 6.2 grams of calcium oxide (CaO). Represent this reaction in terms of law of conservation of mass. Ans: According to law of conservation of mass: Mass of reactants = Mass of products ∴ 10 gram of CaCO 3 = 3.8 grams of CO 2 + 6.2 grams of CaO 10 grams of reactant = 10 grams of products
Hence, it is proved that the law of conservation of mass is followed by the above reaction.
Frequently Asked Questions – FAQs
Why is there no change in mass during chemical reactions.
During a chemical reaction, atoms are neither created nor destroyed. The atoms of the reactants are just rearranged to form products. Hence, there is no change in mass in a chemical reaction.
Verify law of conservation of mass with an experiment
According to the law of conservation of mass, during any physical or chemical change, the matter is neither created nor destroyed. However, it may change from one form to another. Below, we have listed an experiment that will help you verify the law of conservation of mass. Requirements: H-shaped tube, also known as Landolt’s tube; Sodium chloride solution; silver nitrate solution. Procedure: Sodium chloride solution is taken in one limb of the H-tube and silver nitrate solution in the other limb as shown in the figure. Both the limbs are now sealed and weighed. Now the tubes are averted so that the solutions can mix up together and react chemically. The reaction takes place and a white precipitate of silver chloride is obtained. The tube is weighed after the reaction has taken place. The mass of the tube is found to be exactly the same as the mass obtained before inverting the tube. This experiment clearly verifies the law of conservation of mass.
If energy is neither created nor destroyed, what is the ultimate source of energy?
What happens to the mass of a burned object.

Stay tuned with BYJU’S for more such interesting articles. Also, register to “BYJU’S – The Learning App” for loads of interactive, engaging Physics-related videos and unlimited academic assistance.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Physics related queries and study materials
Your result is as below
Request OTP on Voice Call
| PHYSICS Related Links | |
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Law of Conservation of Mass

The Law of Conservation of Mass is a fundamental concept in chemistry, stating that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law, the mass of the reactants in a chemical reaction equals the mass of the products . Further, the number and type of atom s in a chemical reaction is the same before and after the reaction.
Definition and Statement of the Law of Conservation of Mass
The Law of Conservation of Mass was first articulated by Antoine Lavoisier in the late 18th century. It asserts that the total mass of a closed system remains constant over time. This principle is widely applicable in chemical reactions and also applies to other disciplines.
Applicability of the Law
The law holds true in chemical reactions under ordinary conditions. This is because chemical reactions only involve electrons and do not affect the identities of the parts of the atom .
However, the Law of Conservation of Mass does not hold in nuclear reactions, where mass can convert into energy (and vice versa) according to the principle of mass-energy equivalence as proposed by Einstein in the theory of relativity. This conversion occurs in nuclear fission and fusion reactions and some forms of radioactive decay.
Also, the law applies to isolated systems. If matter or energy enters or exits a system, mass may not be conserved.
Historical Overview
The concept of mass conservation dates back to ancient Greece. Mikhail Lomonsov, outlined the principle in 1756. Lavoisier gets credit for formalizing the law in 1773. His work disproved the then-popular theory of phlogiston , a supposed fire-like element released during combustion. Lavoisier demonstrated that combustion results from chemical reactions with oxygen, not from releasing a mysterious substance, and that the mass before and after the reaction was the same.
Examples in Chemical Reactions
Chemical reactions clearly illustrate the Law of Conservation of Mass. Chemists apply the law in balancing chemical equations.
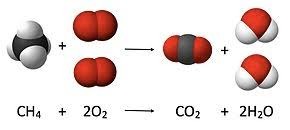
- Combustion: In a simple combustion reaction , such as burning methane (CH₄), the total mass of methane and oxygen equals the mass of the resulting carbon dioxide and water. CH 4 + 2O 2 → CO 2 + 2H 2 O (4 H, 1 C, 4 O atoms on each side of the reaction arrow.)
- Synthesis: When hydrogen and oxygen gases react to form water, the mass of the two gases equals the mass of the water produced. 2H 2 + O 2 → 2H 2 O (4 H and 2 O on both sides of the reaction arrow.)
Examples in Organisms
In biological systems, the law applies to metabolic processes. For example, in photosynthesis , plants convert carbon dioxide and water into glucose and oxygen. The total mass of carbon dioxide and water used equals the mass of glucose and oxygen produced:
6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2
On a larger scale, the law applies to the mass of a human body, which encompasses numerous chemical reactions occurring at once. If you maintain a constant weight, the mass you gain from breathing, eating, and drinking equals the mass lost through breathing, perspiration, urination, and defecation.
Examples in Ecosystems
In ecosystems, the law is evident in nutrient cycles, such as the carbon cycle. Carbon atoms are conserved as they move through different components of the ecosystem, including the atmosphere, hydrosphere, lithosphere, and biosphere. For example, the photosynthesis reaction takes carbon from the air and fixes it into a glucose molecule. Photosynthesis does not create mass, nor is any lost in the process.
- Okuň, Lev Borisovič (2009). Energy and Mass in Relativity Theory . World Scientific. ISBN 978-981-281-412-8.
- Pomper, Philip (1962). “Lomonosov and the Discovery of the Law of the Conservation of Matter in Chemical Transformations”. Ambix . 10 (3): 119–127. doi: 10.1179/amb.1962.10.3.119
- Whitaker, Robert D. (1975). “An historical note on the conservation of mass”. Journal of Chemical Education . 52 (10): 658. doi: 10.1021/ed052p658
Related Posts
This page has been archived and is no longer updated
The Conservation of Mass
The Law of Conservation of Mass
The Law of Conservation of Mass dates from Antoine Lavoisier's 1789 discovery that mass is neither created nor destroyed in chemical reactions. In other words, the mass of any one element at the beginning of a reaction will equal the mass of that element at the end of the reaction. If we account for all reactants and products in a chemical reaction, the total mass will be the same at any point in time in any closed system. Lavoisier's finding laid the foundation for modern chemistry and revolutionized science.
The Law of Conservation of Mass holds true because naturally occurring elements are very stable at the conditions found on the surface of the Earth. Most elements come from fusion reactions found only in stars or supernovae. Therefore, in the everyday world of Earth, from the peak of the highest mountain to the depths of the deepest ocean, atoms are not converted to other elements during chemical reactions. Because of this, individual atoms that make up living and nonliving matter are very old and each atom has a history. An individual atom of a biologically important element, such as carbon, may have spent 65 million years buried as coal before being burned in a power plant, followed by two decades in Earth's atmosphere before being dissolved in the ocean, and then taken up by an algal cell that was consumed by a copepod before being respired and again entering Earth's atmosphere (Figure 1). The atom itself is neither created nor destroyed but cycles among chemical compounds. Ecologists can apply the law of conservation of mass to the analysis of elemental cycles by conducting a mass balance. These analyses are as important to the progress of ecology as Lavoisier's findings were to chemistry.
Life and the Law of Conservation of Mass
Ecosystems can be thought of as a battleground for these elements, in which species that are more efficient competitors can often exclude inferior competitors. Though most ecosystems contain so many individual reactions, it would be impossible to identify them all, each of these reactions must obey the Law of Conservation of Mass — the entire ecosystem must also follow this same constraint. Though no real ecosystem is a truly closed system, we use the same conservation law by accounting for all inputs and all outputs. Scientists conceptualize ecosystems as a set of compartments (Figure 2) that are connected by flows of material and energy. Any compartment could represent a biotic or abiotic component: a fish, a school of fish, a forest, or a pool of carbon. Because of mass balance, over time the amount of any element in any one of these compartments could hold steady (if inputs = outputs), increase (if inputs > outputs), or decrease (if inputs 2 . Mass balance ensures that the carbon formerly locked up in biomass must go somewhere; it must reenter some other compartment of some ecosystem. Mass balance properties can be applied over many scales of organization, including the individual organism, the watershed, or even a whole city (Figure 4).
Mass Balance of Elements in Organisms
Each organism has a unique, relatively fixed, elemental formula, or composition determined by its form and function. For instance, large size or defensive structures create particular elemental demands. Other biological factors such as rapid growth can also influence elemental composition. Ribonucleic acid (RNA) is the biomolecular template used in protein synthesis. RNA has a high phosphorus content (~9% by mass), and in microbes and invertebrates RNA accounts for a large fraction of an organism's total phosphorus content. As a result, fast-growing organisms such as bacteria (which can double more than 6 times per day) have especially high phosphorus content and therefore demands. By contrast, among vertebrates structural materials such as bones (made of calcium phosphate) account for the majority of an organism's phosphorus content. Among mammals, black-tailed deer ( Odocoileus columbianus ; Figure 6) have a relatively high phosphorus demand due to their annual investment in calcium- and phosphorus-rich antlers. Failure to meet elemental demands can lead to poor health, limited reproduction, and even extinction. The extinction of the majestic Irish Elk ( Megaloceros giganteus ) is thought to have been caused by the shortened growing season that occurred during the last ice age, which reduced the availability of the calcium and phosphorus these animals needed to grow their enormous antlers.
Obtaining the resources required for metabolism, growth, and reproduction is one of the central challenges of life. Animals, particularly those that feed on plants (herbivores) or detritus (detritivores), often consume diets that do not include enough of the nutrients they need. The struggle to obtain nutrients from poor quality diets influences feeding behavior and digestive physiology and has led to epic migrations and seemingly bizarre behavior such as geophagy (feeding on materials such as clay and chalk). For example, the seasonal mass migration of Mormon crickets ( Anabrus simplex ) across western North America in search of two nutrients: protein and salt. Researchers have shown that the crickets stop walking once their demand for protein is met (Figure 7).
The flip side of the struggle to obtain scarce resources is the need to get rid of excess substances. Herbivores often consume a diet rich in carbon — think potato chips, few nutrients but lots of energy. Some of this material can be stored internally, but this is a limited option and excess carbon storage can be harmful, just as obesity is harmful to humans. Thus, animals have several mechanisms for getting rid of excess elements. Excess nutrients are released in feces or urine or sometimes it is respired (i.e., released as carbon dioxide). This release of excess nutrients can influence both food webs and nutrient cycles.
Mass Balance in Watersheds
Ecologists have often used naturally delineated ecosystems, such as lakes or watersheds, for applying mass balances. A forested watershed receives inputs of carbon through photosynthesis, inputs of nitrogen from nitrogen-fixing bacteria, as well as through the deposition of atmospheric nitrogen, inputs of phosphorus from the slow weathering of bedrock, and inputs of water from precipitation. Outputs include gaseous pathways (e.g., H 2 O losses through evapotranspiration, CO 2 production as respiration, N 2 produced by denitrifying bacteria) and dissolved pathways (nutrients and carbon dissolved in stream water). Outputs also include material transport across ecosystem boundaries, such as the movement of migratory animals or harvesting trees in a forest.
The Hubbard Brook Experimental Forest in the White Mountains of New Hampshire, USA, has been the site of ecosystem mass balance studies since the 1960s. This landscape has similar-sized, discreet watersheds drained by streams and underlain by impermeable bedrock. By installing V-notch weirs, investigators could precisely and continuously measure stream discharge. By measuring the concentration of nutrients and ions in stream water, they could quantify the losses of these materials from the ecosystem. After calculating inputs to the ecosystem (by sampling precipitation, dry deposition, and nitrogen fixation), they could also construct mass balances. Additionally, researchers could experimentally manipulate these watersheds to measure the effects of disturbance on nutrient retention. In 1965, an entire experimental watershed was whole-tree harvested, resulting in large increases in nitrate and calcium losses relative to an uncut reference watershed (Figure 8). By studying inputs and outputs, an understanding of the internal functioning of the ecosystem within the watershed was obtained.
Figure 8: An experimental reference watershed at the Hubbard Brook Experimental Forest in the White Mountains of New Hampshire, USA Researchers have manipulated entire watersheds, for example by whole-tree harvesting, and then monitored losses of various elements. The whole-tree harvesting of watershed 2 in 1965 affected the uptake and loss of nutrients and elements within the forest ecosystem and was followed by high loss rates of nitrate, hydrogen ions, and calcium ions in stream waters for several years. (Stream chemistry data were provided by G. E. Likens with funding from the National Science Foundation and The A. W. Mellon Foundation.) © 2011 US Forest Service .
Mass Balance in Human-Dominated Ecosystems
Mass balance constraints apply everywhere, even to highly altered ecosystems such as cities or agricultural fields. Cities import food, fuel, water, and other materials and export materials such as manufactured goods. Cities also produce large quantities of waste products — with solid waste sent to landfills, CO 2 (and other pollutants) produced from the combustion of fossil fuels being released to the atmosphere. Nutrients from sewage and from fertilizer runoff can end up in rivers where they will fertilize downstream aquatic ecosystems.
Human agricultural systems can also be analyzed using a mass-balance, ecosystem approach. Traditional agricultural practices emphasized efficiency, with most production staying on the farm — food for livestock was produced on the farm, food for farmers' families was produced on the farm, and plant and animal waste was composted for use as fertilizer on the farm. As a result, the amount of material cycling within the farm "ecosystem" was large relative to the inputs and outputs to the system (a relatively closed ecosystem). By contrast, modern industrial agriculture emphasizes maximizing yields over efficiency. Farmers import fertilizer in large amounts (often far exceeding the amounts that crops can use) and grow and export commodity crops. Ironically, in these highly open ecosystems (where inputs and outputs can far exceed internal cycling), food for farmers' families must often be imported as well. Highly productive agricultural systems are critical in feeding the world's growing human population, but as many of the ingredients of modern agriculture (e.g., water, petroleum, phosphorus) become increasingly limiting over the next century (due to depleted geologic deposits), we will be faced with the challenge of increasing the efficiency of these systems. Just as the constraints of mass balance provide a useful tool for ecologists in studying natural ecosystems, mass balance also ensures that the increase in human population and material consumption that has characterized the past 200 years cannot continue indefinitely.
References and Recommended Reading
Chapin, F. S. et al . Principles of Terrestrial Ecosystem Ecology . New York, NY: Springer, 2002.
Likens, G. E. & Bormann, F. H. Biogeochemistry of a Forested Ecosystem . 2nd ed. New York, NY: Springer-Verlag, 1995.
Moen, R. A. et al . Antler growth and extinction of Irish Elk. Evolutionary Ecology Research 1, 235–249 (1999).
Sterner, R. W. & Elser, J. J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere . Princeton, NJ: Princeton University Press, 2002.
Simpson, S. J. et al. Cannibal crickets on a forced march for protein and salt. Proceedings of the National Academy of Sciences of the USA 103, 4152-4156 (2006).
Article History
Flag inappropriate.


Email your Friend

- | Lead Editor:

Within this Subject (24)
- Basic (13)
- Intermediate (5)
- Advanced (6)
Other Topic Rooms
- Ecosystem Ecology
- Physiological Ecology
- Population Ecology
- Community Ecology
- Global and Regional Ecology
- Conservation and Restoration
- Animal Behavior
- Teach Ecology
- Earth's Climate: Past, Present, and Future
- Terrestrial Geosystems
- Marine Geosystems
- Scientific Underpinnings
- Paleontology and Primate Evolution
- Human Fossil Record
- The Living Primates
© 2014 Nature Education
- Press Room |
- Terms of Use |
- Privacy Notice |

Visual Browse
Please log in to save materials. Log in
- Resource Library

Education Standards
Wyoming science content and performance standards.
Learning Domain: Matter and Its Interactions
Standard: Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
Next Generation Science Standards
Science Domain: Physical Sciences
Topic: Chemical Reactions
Standard: Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction. [Clarification Statement: Emphasis is on using mathematical ideas to communicate the proportional relationships between masses of atoms in the reactants and the products, and the translation of these relationships to the macroscopic scale using the mole as the conversion from the atomic to the macroscopic scale. Emphasis is on assessing students’ use of mathematical thinking and not on memorization and rote application of problem-solving techniques.] [Assessment Boundary: Assessment does not include complex chemical reactions.]
Balancing Chemical Equations
Changing matter not weight, chapter 7 notes, law of conservation of mass lab, mathematically balancing equations, law of conservation of mass.
This unit dicusses the law of conservation of mass, as well as teaches the fundamentals of balancing chemical equations.
Students determine the portion of original mass of gum that is swallowed for sugar and sugar-free gum.
Matter is not created nor destroyed; it simply changes from one form to another. This law of conservation of mass challenges elementary students’ ideas about matter, because many children may think that matter is created or destroyed in a chemical reaction. In this lesson, students will challenge their preconceptions about matter by experimenting with physical and chemical changes to determine that the total weight of the matter does not change. Students will use math to show that the total weight of matter is equal to the sum of the weight of its component parts, and they will graph this information to show that the weight of matter is conserved during physical and chemical changes.
Notes PowerPoint
Follow the Powerpoint, and take notes. Make sure to include all vocabulary.
Balancing Chemical Equations Article
This lesson will reinforce your knowledge about chemical formulas and introduce the concept of balanced chemical equations.
Balancing Chemical Equations Activity
How do you know if a chemical equation is balanced? What can you change to balance an equation? Play a game to test your ideas!
This provides a mathematical method of balancing chemical equations as an alternative to the conventional method (by inspection)
Version History

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

- Is mathematics a physical science?
- Why does physics work in SI units?

conservation of mass
Our editors will review what you’ve submitted and determine whether to revise the article.
- Royal Society of Chemistry - Conservation of mass
- VIVA Open Publishing - Conservation of Energy and Conservation of Mass
- New York University - Department of Mathematics - Conservation of mass and momentum
- Chemistry LibreTexts - Conservation of Mass - There is No New Matter
- Nasa - Conservation of Mass
- conservation of mass - Student Encyclopedia (Ages 11 and up)
conservation of mass , principle that the mass of an object or collection of objects never changes, no matter how the constituent parts rearrange themselves. Mass has been viewed in physics in two compatible ways. On the one hand, it is seen as a measure of inertia, the opposition that free bodies offer to forces: trucks are harder to move and to stop than less massive cars. On the other hand, mass is seen as giving rise to gravitational force, which accounts for the weight of an object: trucks are heavier than cars. The two views of mass are generally considered equivalent. Thus, from the perspective of either inertial mass or gravitational mass, according to the principle of mass conservation, different measurements of the mass of an object taken under various circumstances should always be the same.

With the advent of relativity theory (1905), the notion of mass underwent a radical revision. Mass lost its absoluteness. The mass of an object was seen to be equivalent to energy , to be interconvertible with energy, and to increase significantly at exceedingly high speeds near that of light. The total energy of an object was understood to comprise its rest mass as well as its increase of mass caused by high speed. The rest mass of an atomic nucleus was discovered to be measurably smaller than the sum of the rest masses of its constituent neutrons and protons. Mass was no longer considered constant, or unchangeable. In both chemical and nuclear reactions, some conversion between rest mass and energy occurs, so that the products generally have smaller or greater mass than the reactants. The difference in mass, in fact, is so slight for ordinary chemical reactions that mass conservation may be invoked as a practical principle for predicting the mass of products. Mass conservation is invalid, however, for the behaviour of masses actively involved in nuclear reactors, in particle accelerators, and in the thermonuclear reactions in the Sun and stars. The new conservation principle is the conservation of mass-energy. See also energy, conservation of ; Einstein’s mass-energy relation.

Choose Your Test
- Search Blogs By Category
- College Admissions
- AP and IB Exams
- GPA and Coursework
2 Easy Examples of the Law of Conservation of Mass
General Education

Chemistry is an important subject that you’ll definitely need to know if you’re planning to pursue a chemistry or other science major in college. One thing you should be familiar with is the law of conservation of mass. What is it? And how is it used in chemistry?
Keep reading to learn what the law of conservation of mass is and how it came to be. We will also give you some law of conservation of mass examples to help you understand the concept better.
What Is the Law of Conservation of Mass?
First off, exactly what is the law of conservation of mass? This law states that in a closed system, matter can neither be created nor destroyed—it can only change form.
Put differently, the amount, or mass, of matter in an isolated system will always be constant regardless of any chemical reactions or physical changes that take place. (Note that an isolated or closed system is one that does not interact with its environment.)
This law is important in chemistry, particularly when combining different materials and testing the reactions between them.
In chemistry, the law of conservation of mass states that the mass of the products (the chemical substances created by a chemical reaction) will always equal the mass of the reactants (the substances that make the chemical reaction).
Think of it as being similar to balancing an algebraic equation. Both sides around an equal sign might look different (for example, 6 a + 2 b = 20), but they still represent the same total quantity. This is similar to how the mass must be constant for all matter in a closed system—even if that matter changes form!
But how does the law of conservation of mass work?
When a substance undergoes a chemical reaction, you might assume that some or even all of the matter present is disappearing, but, in actuality, it's simply changing form.
Think about when a liquid turns into a gas. You might think that the matter (in this case, the liquid) has simply vanished. But if you were to actually measure the gas, you'd find that the initial mass of the liquid hasn’t actually changed. What this means is that the substance, which is now a gas, still has the same mass it had when it was a liquid (yes—gas has mass, too!).
What Is the History Behind the Law of Conservation of Mass?
Though many people, including the ancient Greeks, laid the scientific groundwork necessary for the discovery of the law of conservation of mass, it is French chemist Antoine Lavoisier (1743-1794) who is most often credited as its discoverer. This is also why the law is occasionally called Lavoisier’s law.

In the late 1700s, Lavoisier proved through experimentation that the total mass does not change in a chemical reaction, leading him to declare that matter is always conserved in a chemical reaction.
Lavoisier’s experiments marked the first time someone clearly tested this idea of the conservation of matter by measuring the masses of materials both before and after they underwent a chemical reaction.
Ultimately, the discovery of the law of conservation of mass was immensely significant to the field of chemistry because it proved that matter wasn’t simply disappearing (as it appeared to be) but was rather changing form into another substance of equal mass.
What Are Some Law of Conservation of Mass Examples?
Law of conservation of mass examples are useful for visualizing and understanding this crucial scientific concept. Here are two examples to help illustrate how this law works.

Example 1: The Bonfire/Campfire
One common example you’ll come across is the image of a bonfire or campfire.
Picture this: you’ve gathered some sticks with friends and lit them with a match. After a couple of toasted marshmallows and campfire songs, you realize that the bonfire, or campfire, you've built has completely burned down. All you’re left with is a small pile of ashes and some smoke.
Your initial instinct might be to assume that some of the campfire's original mass from the sticks has somehow vanished. But it actually hasn’t —i t’s simply transformed!
In this scenario, as the sticks burned, they combined with oxygen in the air to turn into not just ash but also carbon dioxide and water vapor. As a result, If we measured the total mass of the wooden sticks and the oxygen before setting the sticks on fire, we'd discover that this mass is equal to the mass of the ashes, carbon dioxide, and water vapor combined.

Example 2: The Burning Candle
A similar law of conservation of mass example is the image of a burning candle.
For this example, picture a regular candle, with wax and a wick. Once the candle completely burns down, though, you can see that there is definitely far less wax than there was before you lit it. This means that some of the wax (not all of it, as you’ve likely noticed with candles you’ve lit in real life!) has been transformed into gases —namely, water vapor and carbon dioxide.
As the previous example with the bonfire has shown, no matter (and therefore no mass) is lost through the process of burning.
Recap: What Is the Law of Conservation of Mass?
The law of conservation of mass is a scientific law popularized and systematized by the 18th-century French chemist Antoine Lavoisier.
According to the law, in an isolated system, matter cannot be created or destroyed — only changed. This means that the total mass of all substances before a chemical reaction will equal the total mass of all substances after a chemical reaction. Simply put, matter (and thus mass) is always conserved, even if a substance changes chemical or physical form.
Knowing this scientific law is important for the study of chemistry, so if you plan to get into this field, you'll definitely want to understand what the law of conservation of mass is all about!
What’s Next?
Are there other science topics you want to review? Then you're in luck! Our guides will teach you loads of useful topics, from how to convert Celsius to Fahrenheit , to what the density of water is , to how to balance chemical equations .
Need help identifying stylistic techniques in a book you're reading for English class? Let our comprehensive list of the most important literary devices lend you a hand!
Trending Now
How to Get Into Harvard and the Ivy League
How to Get a Perfect 4.0 GPA
How to Write an Amazing College Essay
What Exactly Are Colleges Looking For?
ACT vs. SAT: Which Test Should You Take?
When should you take the SAT or ACT?
Get Your Free

Find Your Target SAT Score
Free Complete Official SAT Practice Tests
How to Get a Perfect SAT Score, by an Expert Full Scorer
Score 800 on SAT Math
Score 800 on SAT Reading and Writing
How to Improve Your Low SAT Score
Score 600 on SAT Math
Score 600 on SAT Reading and Writing
Find Your Target ACT Score
Complete Official Free ACT Practice Tests
How to Get a Perfect ACT Score, by a 36 Full Scorer
Get a 36 on ACT English
Get a 36 on ACT Math
Get a 36 on ACT Reading
Get a 36 on ACT Science
How to Improve Your Low ACT Score
Get a 24 on ACT English
Get a 24 on ACT Math
Get a 24 on ACT Reading
Get a 24 on ACT Science
Stay Informed
Get the latest articles and test prep tips!

Hannah received her MA in Japanese Studies from the University of Michigan and holds a bachelor's degree from the University of Southern California. From 2013 to 2015, she taught English in Japan via the JET Program. She is passionate about education, writing, and travel.
Ask a Question Below
Have any questions about this article or other topics? Ask below and we'll reply!
Law of Conservation of Mass
Defining the law of conservation of mass in the field of chemistry
imagenavi, Getty Images
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chemistry is a physical science that studies matter and energy and how they interact. When studying these interactions, it's important to understand the law of conservation of mass.
Key Takeaways: Conservation of Mass
- Simply stated, the law of conservation of mass means matter cannot be created or destroyed, but it can change forms.
- In chemistry, the law is used to balance chemical equations. The number and type of atoms must be the same for both reactants and products.
- Credit for discovering the law may be given to either Mikhail Lomonosov or Antoine Lavoisier.
Law of Conservation of Mass Definition
The law of conservation of mass is that in a closed or isolated system, matter cannot be created or destroyed. It can change forms but is conserved.
Law of Conservation of Mass in Chemistry
In the context of the study of chemistry, the law of conservation of mass says that in a chemical reaction , the mass of the products equals the mass of the reactants .
To clarify: An isolated system does not interact with its surroundings. Therefore, the mass contained in that isolated system will remain constant, regardless of any transformations or chemical reactions that occur—while the result may be different than what you had in the beginning, there can't be any more or less mass than what you had before the transformation or reaction.
The law of conservation of mass was crucial to the progression of chemistry, as it helped scientists understand that substances did not disappear as a result of a reaction (as they may appear to do); rather, they transform into another substance of equal mass.
History credits multiple scientists with discovering the law of conservation of mass. Russian scientist Mikhail Lomonosov noted it in his diary as a result of an experiment in 1756. In 1774, French chemist Antoine Lavoisier meticulously documented experiments that proved the law. The law of conservation of mass is known by some as Lavoisier's Law.
In defining the law, Lavoisier stated, "Atoms of an object cannot be created or destroyed, but can be moved around and be changed into different particles."
- Okuň, Lev Borisovič (2009). Energy and Mass in Relativity Theory . World Scientific. ISBN 978-981-281-412-8.
- Whitaker, Robert D. (1975). "An historical note on the conservation of mass." Journal of Chemical Education . 52 (10): 658. doi: 10.1021/ed052p658
- The Law of Conservation of Energy Defined
- The Major Laws of Chemistry
- Balancing Chemical Equations
- Stoichiometry Definition in Chemistry
- First Law of Thermodynamics Definition
- Graham's Law Definition
- Balanced Equation Definition and Examples
- How to Calculate the Density of a Gas
- Law of Constant Composition in Chemistry
- The Formula for Boyle's Law
- Graham's Law Example: Gas Diffusion-Effusion
- Charles' Law Example Problem
- What Is the Formula for Charles' Law?
- Law of Definite Proportions Definition
- Gay-Lussac's Law Definition
- Gram Molecular Mass Definition
Conservation of Energy and Mass
The law of conservation of mass states that in a chemical reaction mass is neither created nor destroyed. For example, the carbon atom in coal becomes carbon dioxide when it is burned. The carbon atom changes from a solid structure to a gas but its mass does not change. Similarly, the law of conservation of energy states that the amount of energy is neither created nor destroyed. For example, when you roll a toy car down a ramp and it hits a wall, the energy is transferred from kinetic energy to potential energy.
Teach about the conservation of energy and mass with these classroom resources.

IMAGES
VIDEO
COMMENTS
Represent this reaction in terms of law of conservation of mass. Ans: According to law of conservation of mass: Mass of reactants = Mass of products. ∴ 10 gram of CaCO 3 = 3.8 grams of CO 2 + 6.2 grams of CaO. 10 grams of reactant = 10 grams of products. Hence, it is proved that the law of conservation of mass is followed by the above reaction.
The Law of Conservation of Mass is a fundamental concept in chemistry, stating that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law, the mass of the reactants in a chemical reaction equals the mass of the products. Further, the number and type of atom s in a ...
Answer the following questions by applying the Law of Conservation of Matter. Show your work. Determine whether each of the following statements is consistent with or inconsistent with the laws of conservation of mass. (a) 127 g of Cu reacts with 34 g of O 2 to produce 159 g of CuO. Inconsistent
Law of Conservation of Mass. This burning campfire example illustrates a very important law in science: the law of conservation of mass. This law states that matter cannot be created or destroyed. Even when matter goes through a physical or chemical change, the total mass of matter always remains the same. Q: How could you show that the mass of ...
The Law of Conservation of Mass dates from Antoine Lavoisier's 1789 discovery that mass is neither created nor destroyed in chemical reactions. In other words, the mass of any one element at the ...
After the reaction is complete and the materials separated, we find that we have formed 143.4 grams of silver chloride and 85.0 grams of sodium nitrate, giving us a total mass of 228.4 grams for the products. So, the total mass of reactants equals the total mass of products, a proof of the law of conservation of mass.
The law of conservation of mass can only be formulated in classical mechanics, in which the energy scales associated with an isolated system are much smaller than , where is the mass of a typical object in the system, measured in the frame of reference where the object is at rest, and is the speed of light.. The law can be formulated mathematically in the fields of fluid mechanics and ...
This law of conservation of mass challenges elementary students' ideas about matter, because many children may think that matter is created or destroyed in a chemical reaction. In this lesson, students will challenge their preconceptions about matter by experimenting with physical and chemical changes to determine that the total weight of the ...
Matter can change form through physical and chemical changes, but through any of these changes, matter is conserved. The same amount of matter exists before and after the change—none is created or destroyed. This concept is called the Law of Conservation of Mass. In a physical change, a substance's physical properties may change, but its ...
conservation law. mass. conservation of mass-energy. conservation of mass, principle that the mass of an object or collection of objects never changes, no matter how the constituent parts rearrange themselves. Mass has been viewed in physics in two compatible ways. On the one hand, it is seen as a measure of inertia, the opposition that free ...
Example 2: The Burning Candle. A similar law of conservation of mass example is the image of a burning candle. For this example, picture a regular candle, with wax and a wick. Once the candle completely burns down, though, you can see that there is definitely far less wax than there was before you lit it. This means that some of the wax (not ...
Key Takeaways: Conservation of Mass. Simply stated, the law of conservation of mass means matter cannot be created or destroyed, but it can change forms. In chemistry, the law is used to balance chemical equations. The number and type of atoms must be the same for both reactants and products. Credit for discovering the law may be given to ...
Conservation of Mass. Mass of products equals mass of the reactants. Estimated7 minsto complete. Progress. Practice Conservation of Mass. Practice.
The law of conservation of mass states that in a chemical reaction mass is neither created nor destroyed. For example, the carbon atom in coal becomes carbon dioxide when it is burned. The carbon atom changes from a solid structure to a gas but its mass does not change. Similarly, the law of conservation of energy states that the amount of energy is neither created nor destroyed. For example ...