- Cancer Topics
- Research Branches and Programmes
- Research Teams
- Knowledge Transfer
- Research Project Websites
- International Research Collaborations
- Useful Links
- Press Releases
- Featured News
- Videos and Podcasts
- Infographics and Photos
- Questions and Answers
- WHO Classification of Tumours
- IARC Monographs Programme
- IARC Handbooks of Cancer Prevention
- IARC Staff Journal Articles
- Scientific Meetings and Lectures
- IARC Seminar Series
- IARC/NCI Tumour Seminars
- Medals of Honour
- Professional Staff
- General service Staff
- Talent Pools
- Visiting Scientist and Postdoctoral Opportunities
- Postdoctoral Fellowships
- Call for Tenders
- Office of the Director
- Organization and Management
- Supporters and Friends
- IARC Newsletter
- Visitor Information
- Terms of use
- Privacy Policy
- iarc newsletter

World Cancer Report: Cancer Research for Cancer Prevention
DOWNLOAD PUBLICATION
Questions and answers, download pdf, what is the iarc world cancer report .
The new IARC World Cancer Report is the product of a collaboration between leading international scientists that describes multiple aspects of cancer research for cancer prevention. Starting with the latest trends in cancer incidence and mortality worldwide, this publication provides wide-ranging insights into cancer prevention based on the known causes of cancer, factors that determine how cancer develops, and the behaviour of different tumour types, and presents a broad scope of interventions to reduce the cancer burden from a global perspective. The scientific disciplines covered include descriptive epidemiology (the distribution of disease, and specifically cancer, within particular populations), cancer etiology (including infections, chemicals, radiation, metabolism and nutrition, and genetic factors), cellular and molecular biology, toxicology and pathology, behavioural and social sciences, public health, biostatistics, and informatics. Wild CP, Weiderpass E, Stewart BW, editors (2020). World Cancer Report: Cancer Research for Cancer Prevention. Lyon, France: International Agency for Research on Cancer. Available from: http://publications.iarc.fr/586 .
Who is this publication for?
World Cancer Report: Cancer Research for Cancer Prevention features the latest research from across multiple disciplines. It is aimed primarily at cancer researchers, academia, health professionals, and policy-makers, but this expert report remains accessible to a wider audience, including the general public, civil society, and the private sector.
What is the objective of the report?
The IARC World Cancer Report is the most comprehensive overview of relevant research yet available. This latest publication is part of a well-established series . Produced about every 5 years, World Cancer Report provides the latest evidence on cancer prevention and serves as an authoritative reference in the cancer research community. The volume editors of this new World Cancer Report are IARC Director Dr Elisabete Weiderpass, former IARC Director Dr Christopher P. Wild , and Professor Bernard W. Stewart of the University of New South Wales, Sydney, Australia.
What does the latest World Cancer Report include?
Based on how cancer is distributed worldwide, and differences between and within particular countries, this new assessment offers a comprehensive overview of the global cancer burden as a starting point for documenting all known options for cancer prevention through the latest research. The publication documents the causes of cancer, discussing infectious agents, alcohol consumption, metabolism and nutrition, physical activity, sedentary behaviour, and obesity as well as dietary carcinogens, occupational exposure, and the contamination of air, water, and soil, among other topics. The biological processes that affect cancer development are also presented, with a focus on sporadic cancer, genomics and susceptibility, gene–environment interactions, and DNA repair, as well as inflammation and its pivotal role in cancer pathogenesis, to name but a few. A full section is devoted to multiple chapters on the inequalities that affect the distribution of cancer within communities, clearly illustrating that in both high-income countries and low- and middle-income countries, there are groups of people in every community who are at a major disadvantage with respect to risk of cancer. Options for prevention include avoiding exposure to carcinogens, for example by smoking cessation, as well as vaccination, screening, monitoring those at high genetic risk, using therapeutics to reduce cancer risk, and emerging molecular technology for early diagnosis.
What’s new in this World Cancer Report ?
The impact of cancer on the global community can now be defined with greater precision than ever before. The causes of cancer are now better understood in terms of both the precise biological changes induced by causative agents and the characteristics of exposed people who prove to be susceptible to cancer development. This is the broad background against which both biological and sociological variables determine the distribution of cancer and, in many instances, its potential prevention. Factors determining cancer development and prevention The causes of cancer vary markedly in their character and impact. Cancer is just one of the diseases caused by tobacco smoking, but lung cancer and other cancer types caused by smoking are among the most lethal of such diseases. Millions of people are infected with human papillomavirus (HPV), Helicobacter pylori , or hepatitis B virus or hepatitis C virus, and are thus at risk of developing cervical cancer, stomach cancer, and liver cancer, respectively. Complex biological processes, including DNA repair, the occurrence of overweight or obesity, and the consequences of inflammation, are crucial determinants of cancer development. These processes are delineated in the new World Cancer Report . Although much is known about cancer causation, for many tumour types few, if any, relevant carcinogens have been identified. This applies to, for example, brain cancer and prostate cancer. For lung cancer, a broad spectrum of causes are known, beginning with active smoking and extending to second-hand smoke, certain occupations, and atmospheric pollution. Despite this, some individual cases of lung cancer have no evident cause. Such tumours, along with most cases of brain cancer and prostate cancer, are often described as sporadic. Another exciting first for the new World Cancer Report is a discussion of sporadic cancer and the biological principles that are thought to underpin the development of such cancer. Biological processes are common to all people, but the distribution of cancer in all countries is subject to socioeconomic differences. For the first time, inequalities as a determinant of cancer incidence and mortality are specifically addressed in a separate section of the new World Cancer Report . Previous World Cancer Reports described the disproportionately increasing burden of cancer in low- and middle-income countries, and this trend clearly persists. However, in all countries, irrespective of income grouping, sections of the communities are disadvantaged both in relation to circumstances of risk and with respect to prevention and treatment services. In the new World Cancer Report , separate chapters evaluate inequalities that affect cancer incidence in Africa, China, Europe, India, and the USA. Increasing options for cancer prevention Cancer prevention is often identified with community campaigns, such as those to promote smoking avoidance or cessation, to ensure that exposure to asbestos does not occur in the workplace and elsewhere, to prevent particular infections, and, particularly for fair-skinned people, to avoid deliberate sun exposure without sun protection. All these ways of preventing cancer remain relevant; they are proven to reduce cancer incidence, and research continues to demonstrate their efficacy. However, cancer prevention involves a far greater range of initiatives than avoiding exposure to known carcinogens. Perhaps the most effective means of cancer prevention, and one that has the prospect of eliminating one tumour type completely, is vaccination against human papillomavirus (HPV), which is the cause of cervical cancer. Vaccination against hepatitis B and C viruses also has a proven impact on the incidence of liver cancer in certain communities. The single greatest challenge to cancer prevention identified in the new World Cancer Report is overweight or obesity. Although the prevalence of overweight or obesity is readily identified with populations in high-income countries, this condition is now evident in many regions of the world. Multiple tumour types, including colorectal cancer and breast cancer, are attributable, at least in part, to overweight or obesity. The biological mechanisms by which overweight or obesity increases the risk of various tumour types are not yet fully explained. Altering community behaviour to reduce the prevalence of overweight or obesity is recognized as a means of preventing not only certain types of cancer but also other chronic diseases such as type 2 diabetes. For sporadic cancers in different organs (i.e. cancers for which no recognized exposure accounts for tumour development), options for prevention are emerging and are being evaluated by researchers. For multiple tumour types, World Cancer Report discusses population-based screening for detection of cancer at an early stage or of preconditions leading to cancer development. One chapter describes early diagnosis on the basis of tumour DNA detected in blood, and another describes how individual susceptibility to tumorigenesis may be determined using genomic data.
What is the difference between the WHO Report on Cancer and the IARC World Cancer Report ?
In May 2017, the cancer resolution ( WHA70.12 ) adopted at the Seventieth World Health Assembly requested WHO, in collaboration with IARC, to produce a comprehensive global report providing evidence-based public health- and policy-oriented guidance on cancer for WHO Member States. The outcome of this charge is the WHO Report on Cancer: Setting priorities, investing wisely and providing care for all . The WHO report complements the IARC World Cancer Report by synthesizing evidence to translate the latest knowledge into actionable policies to support governments to prevent and control cancer globally. These two complementary publications, launched jointly by WHO and IARC, will each contribute to an increased awareness, both professionally and in the wider community, of the lives affected by cancer, and what may be done, is being done, and should be done to decrease the impact of this disease.
What are the key messages in the IARC World Cancer Report ?
Cancer is the second most common cause of death worldwide, and the burden of cancer is increasing in all countries. This poses a rapidly growing threat to individuals, health systems, and economies globally. Countries must accelerate their multisectoral, evidence-based, and resource-appropriate responses now to avoid 7 million cancer deaths over the next decade. The cancer burden is predicted to nearly double over the next decade in low- and middle-income countries. If no additional action is taken, there will be millions of additional premature deaths from cancer over the next decade, and we will fail to achieve the United Nations Sustainable Development Goals target (Target 3.4) to reduce the total premature mortality from noncommunicable diseases, including cancer, by one third by 2030. The global cancer burden is expected to reach 29 million new cancer cases per year by 2040, a 62% increase on the estimated 18.1 million cancers in 2018. The increases in the cancer incidence burden will affect all countries, but the predicted increases will be proportionately greatest in low-income countries, due to known infectious agents, chemicals including tobacco, and obesity. World Cancer Report documents how the cancer burden continues to grow and emphasizes the need for urgent implementation of efficient prevention strategies to curb the disease. For cervical cancer, lung cancer, and most other cancer types, the relative incidence is greatest among those at socioeconomic disadvantage, particularly including ethnic and racial minorities and Indigenous populations. Cancer inequalities reflect the cultures and environments in which people are born, live, and work and the uneven application of preventive measures, both between and within countries. Vaccination and screening are effective for some cancer types but are differentially available. Most genomic data are from studies in individuals of European ancestry. In the future, the characterization of individual susceptibility to cancer and the closer identification of those at risk will enable precision cancer prevention.
Dr Elisabete Weiderpass, IARC Director, presents World Cancer Report: Cancer Research for Cancer Prevention
Professor bernard stewart presents world cancer report: cancer research for cancer prevention.
Published in section: Featured News
Publication date: 4 February, 2020, 6:50
Direct link: https://www.iarc.who.int/featured-news/new-world-cancer-report/
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 18 June 2009
Cancer prevention research — then and now
- Ann M. Bode 1 &
- Zigang Dong 1
Nature Reviews Cancer volume 9 , pages 508–516 ( 2009 ) Cite this article
3191 Accesses
127 Citations
7 Altmetric
Metrics details
Throughout history, humankind has won the battle against deadly diseases, including small pox and polio, by defeating them through prevention. Cancer prevention is a global priority, but studying history suggests that the journey towards achieving this goal is difficult and full of detours and roadblocks. Epidemiology and clinical evidence clearly indicate that specific genetic, environmental and behavioural factors are associated with an increased risk for cancer development. What can we learn from the past that is applicable to the reality of successful cancer prevention?
This is a preview of subscription content, access via your institution
Access options

Danaei, G., Vander Hoorn, S., Lopez, A. D., Murray, C. J. & Ezzati, M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 366 , 1784–1793 (2005).
Article PubMed Google Scholar
Gurunluoglu, R. & Gurunluoglu, A. Paul of Aegina: landmark in surgical progress. World J. Surg. 27 , 18–25 (2003).
Ramazzini, B. De Morbis Artificium Diatriba (translated by Wright, W. C.) (Univ. of Chicago Press, Chicago,1940).
Google Scholar
Hill, J. Cautions Against the Immoderate Use of Snuff (Baldwin and Jackson, London, 1761).
Pott, P. Chirurgical Observations Relative to the Cataract, the Polypus of the Nose, the Cancer of the Scrotum, the Different Kinds of Ruptures and the Mortifications of the Toes and Feet (Haves, Clarke and Collins, London,1775).
Hutchinson, J. On some examples of arsenic-keratosis of the skin and of arsenic-cancer. Trans. Pathol. Soc. Lond. 39 , 352–393 (1888).
Rehn, L. Bladder tumours in fuchsin workers. Arch. Fuer Klin. Chirurgie 50 , 588–600 (1895).
Huff, J. Carcinogenicity Testing, Predicting, and Interpreting Chemical Effects (ed. Kitchin, K. T.) 21–123 (Dekker, New York, 1999).
Frieben, A. Demonstration eines Cancroid der rechten Handdruckens das sich nach langdauerneder Einwirkung von Roentgenstrahlen entwickelt hat. Fortschr. Roentgenstr. 6 , 106–111 (1902).
Sick, H. Karzinom der Haut das auf dem Boden eines Roentgenulcus enstanden its. Muench Med. Wochenschr. 50 , 1445 (1902).
Rous, P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J. Exp. Med. 13 , 397–411 (1911).
Article CAS PubMed PubMed Central Google Scholar
Yamagiwa, K. & Ichikawa, K. Experimental study of the pathogenesis of carcinoma. CA Cancer J. Clin. 27 , 174–181 (1977).
Article CAS PubMed Google Scholar
Hill, M. J. Changes and developments in cancer prevention. J. R. Soc. Health 121 , 94–97 (2001).
Article CAS Google Scholar
Gofman, J. W. & O'Connor, E. Cancer in the family: does each case require more than one cause? The likelihood of co-action. Committee for Nuclear Responsibility [online] ,; (1999).
Creech, H. J. Historical review of the American Association of Cancer Research, Inc., 1941–1978. Cancer Res. 39 , 1863–1890 (1979).
CAS PubMed Google Scholar
Adams, S. H. What can we do about cancer? The most vital and insistent question in the medical world. Ladies Home J. 30 , 21–22 (1913).
Cantor, D. Introduction: cancer control and prevention in the twentieth century. Bull . Hist. Med. 81 , 1–38 (2007).
Article Google Scholar
Cantor, D. Cancer, quackery and the vernacular meanings of hope in 1950s America. J. Hist. Med. Allied Sci. 61 , 324–368 (2006).
Wynder, E. L. & Graham, E. A. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J. Am. Med. Assoc. 143 , 329–336 (1950).
American Institute for Cancer Research. Food, nutrition and the prevention of cancer: a global perspective. World Cancer Research Fund/American Institute for Cancer Research [online], http://www.dietandcancerreport.org/ (1997).
American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. World Cancer Research Fund/American Institute for Cancer Research [online], http://www.dietandcancerreport.org/ (2007).
Doll, R. & Hill, A. B. Smoking and carcinoma of the lung; preliminary report. BMJ 2 , 739–748 (1950).
Hammond, E. C. & Horn, D. The relationship between human smoking habits and death rates: a follow-up study of 187,766 men. J. Am. Med. Assoc. 155 , 1316–1328 (1954).
Hammond, E. C. & Horn, D. Smoking and death rates; report on forty-four months of follow-up of 187,783 men. II. Death rates by cause. J. Am. Med. Assoc. 166 , 1294–1308 (1958).
Hammond, E. C. & Horn, D. Smoking and death rates; report on forty-four months of follow-up of 187,783 men. I. Total mortality. J. Am. Med. Assoc. 166 , 1159–1172 (1958).
Doll, R. & Hill, A. B. The mortality of doctors in relation to their smoking habits; a preliminary report. BMJ 1 , 1451–1455 (1954).
Doll, R. & Hill, A. B. Lung cancer and other causes of death in relation to smoking; a second report on the mortality of British doctors. BMJ 2 , 1071–1081 (1956).
Doll, R., Peto, R., Boreham, J. & Sutherland, I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 328 , 1519 (2004).
Article PubMed PubMed Central Google Scholar
Hammond, E. C. Cancer etiology: new prospective epidemiological study. CA Cancer J. Clin. 9 , 177–178 (1959).
Hammond, E. C. Smoking in relation to mortality and morbidity. Findings in first thirty-four months of follow-up in a prospective study started in 1959. J. Natl Cancer Inst. 32 , 1161–1188 (1964).
Royal College of Physicians. Smoking and Health (Pitman Medical, London, 1962).
Houseman, M. Smoking and health: the 1964 Surgeon General's report as a turning point in the anti-smoking movement. Harvard Health Policy Review 2 , 118–126 (2001).
Wagner, J. C., Sleggs, C. A. & Marchand, P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br. J. Ind. Med. 17 , 260–271 (1960).
CAS PubMed PubMed Central Google Scholar
IARC (ed.) Tobacco smoke and involuntary smoking. World Health Organization; International Agency for Research on Cancer [online], http://monographs.iarc.fr/ENG/Monographs/vol83/volume83.pdf (2002).
US Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Office of the Surgeon General [online], http://www.surgeongeneral.gov/library/secondhandsmoke/report/fullreport.pdf (2006).
Leborgne, R. Diagnosis of tumors of the breast by simple roentgenography; calcifications in carcinomas. Am. J. Roentgenol. Radium Ther. 65 , 1–11 (1951).
Sam, S. Evidence on screening for breast cancer from a randomized trial. Cancer 39 , 2772–2782 (1977).
Shapiro, S. Periodic screening for breast cancer: the HIP randomized controlled trial health insurance plan. J. Natl Cancer Inst. Monogr. 27–30 (1997).
Lilienfeld, A. M. The relationship of cancer of the female breast to artificial menopause and martial status. Cancer 9 , 927–934 (1956).
Jordan, V. C. Antitumour activity of the antioestrogen ICI 46,474 (tamoxifen) in the dimethylbenzanthracene (DMBA)-induced rat mammary carcinoma model. J. Steroid Biochem. 5 , 354 (1974).
Miki, Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1 . Science 266 , 66–71 (1994).
Wooster, R. et al. Localization of a breast cancer susceptibility gene, BRCA2 , to chromosome 13q12–13. Science 265 , 2088–2090 (1994).
Papanicolaou, G. New cancer diagnosis. Proceedings of the Third Race Betterment Conference 528–534 (1928).
Papanicolaou, G. N. & Traut, H. The diagnostic value of vaginal smears in carcinoma of the uterus. Am. J. Obstet. Gynecol. 42 , 193–206 (1941).
Wynder, E. L., Cornfield, J., Schroff, P. D. & Doraiswami, K. R. A study of environmental factors in carcinoma of the cervix. Am. J. Obstet. Gynecol. 68 , 1016–1047; discussion, 1048–1052 (1954).
Beral, V. Cancer of the cervix: a sexually transmitted infection? Lancet 1 , 1037–1040 (1974).
Hewitt, H. The vast investment in research into the role of viruses in human cancer has largely been wasted because there is still little firm evidence for such a role. Eur. J. Cancer Prev. 1 , 187–189 (1992).
Blumberg, B. S. & London, W. T. Hepatitis B virus and the prevention of primary hepatocellular carcinoma. N. Engl. J. Med. 304 , 782–784 (1981).
Durst, M., Gissmann, L., Ikenberg, H. & zur Hausen, H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc. Natl Acad. Sci. USA 80 , 3812–3815 (1983).
Barrasso, R., De Brux, J., Croissant, O. & Orth, G. High prevalence of papillomavirus-associated penile intraepithelial neoplasia in sexual partners of women with cervical intraepithelial neoplasia. N. Engl. J. Med. 317 , 916–923 (1987).
Schiffman, M. H. et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J. Natl Cancer Inst. 85 , 958–964 (1993).
Manos, M. M. et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 281 , 1605–1610 (1999).
Callaghan, J., Karim, S., Mortlock, S., Wintert, M. & Woodward, N. Hybrid capture as a means of detecting human papillomavirus DNA from liquid-based cytology specimens: a preliminary evaluation. Br. J. Biomed. Sci. 58 , 184–189 (2001).
Koutsky, L. A. et al. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 347 , 1645–1651 (2002).
Paavonen, J. et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 369 , 2161–2170 (2007).
[No authors listed.] New vaccine prevents cervical cancer. FDA Consum. 40 , 37 (2006).
Future II study group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 356 , 1915–1927 (2007).
Ault, K. A. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ : a combined analysis of four randomised clinical trials. Lancet 369 , 1861–1868 (2007).
Article PubMed CAS Google Scholar
Hockberger, P. E. A history of ultraviolet photobiology for humans, animals and microorganisms. Photochem. Photobiol. 76 , 561–579 (2002).
Findlay, G. M. Ultra-violet light and skin cancer. Lancet 1070–1073 (1928).
Bode, A. M. & Dong, Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE 2003, RE2 (2003).
Epstein, J. H. Photocarcinogenesis, skin cancer, and aging. J. Am. Acad. Dermatol. 9 , 487–502 (1983).
Fitzpatrick, T. B., Sober, A. J., Pearson, B. J. & Lew, R. in Research in Photobiology (ed. Castellani, A.) 485–490 (Plenum, New York, 1976).
Forbes, P. D., Davis, R. J. & Urbach, F. in Research in Photobiology (ed. Castellani, A.) 469–478 (Plenum, New York, 1976).
International Agency for Research on Cancer. Solar and ultraviolet radiation. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans [online], http://monographs.iarc.fr/ENG/Monographs/vol55/volume55.pdf (1992).
Kusewitt, D. F., Budge, C. L., Anderson, M. M., Ryan, S. L. & Ley, R. D. Frequency of ultraviolet radiation-induced mutation at the hprt locus in repair-proficient murine fibroblasts transfected with the denV gene of bacteriophage T4. Photochem. Photobiol. 58 , 450–454 (1993).
Ley, R. D. Photoreactivation in humans. Proc. Natl Acad. Sci. USA 90 , 4337 (1993).
Setlow, R. B. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc. Natl Acad. Sci. USA 71 , 3363–3366 (1974).
de Laat, A., van der Leun, J. C. & de Gruijl, F. R. Carcinogenesis induced by UVA (365-nm) radiation: the dose-time dependence of tumor formation in hairless mice. Carcinogenesis 18 , 1013–1020 (1997).
de Laat, J. M. & de Gruijl, F. R. The role of UVA in the aetiology of non-melanoma skin cancer. Cancer Surv. 26 , 173–191 (1996).
Gasparro, F. P. Sunscreens, skin photobiology, and skin cancer: the need for UVA protection and evaluation of efficacy. Environ. Health Perspect. 108 (Suppl. 1), 71–78 (2000).
Runger, T. M. Role of UVA in the pathogenesis of melanoma and non-melanoma skin cancer. A short review. Photodermatol. Photoimmunol. Photomed. 15 , 212–216 (1999).
Setlow, R. B. Spectral regions contributing to melanoma: a personal view. J. Investig. Dermatol. Symp. Proc. 4 , 46–49 (1999).
Greegor, D. H. Diagnosis of large-bowel cancer in the asymptomatic patient. JAMA 201 , 943–945 (1967).
Wolff, W. I. Colonoscopy: history and development. Am. J. Gastroenterol. 84 , 1017–1025 (1989).
Wynder, E. L. & Gori, G. B. Contribution of the environment to cancer incidence: an epidemiologic exercise. J. Natl Cancer Inst. 58 , 825–832 (1977).
Doll, R. & Peto, R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl Cancer Inst. 66 , 1191–1308 (1981).
Committee on Diet, Nutrition, and Cancer. Diet, Nutrition and Cancer. National Research Council [online], http://books.nap.edu/catalog.php?record_id=371#toc (1982).
Reddy, B. S. et al. Nutrition and its relationship to cancer. Adv. Cancer Res. 32 , 237–345 (1980).
Koop, C. E. The Surgeon General's Report on Nutrition and Health, US Department of Health and Human Services [online] , (1988).
Wattenberg, L. W. Chemoprophylaxis of carcinogenesis: a review. Cancer Res. 26 , 1520–1526 (1966).
Sporn, M. B. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 36 , 2699–2702 (1976).
Wattenberg, L. W. Chemoprevention of cancer. Cancer Res. 45 , 1–8 (1985).
Hong, W. K. & Sporn, M. B. Recent advances in chemoprevention of cancer. Science 278 , 1073–1077 (1997).
Fisher, B. et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl Cancer Inst. 90 , 1371–1388 (1998).
Fisher, B. et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl Cancer Inst. 97 , 1652–1662 (2005).
Vogel, V. G. et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295 , 2727–2741 (2006).
Cummings, S. R. et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 281 , 2189–2197 (1999).
Barrett-Connor, E. et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N. Engl. J. Med. 355 , 125–137 (2006).
Thompson, I. M., Klein, E. A., Lippman, S. M., Coltman, C. A. & Djavan, B. Prevention of prostate cancer with finasteride: US/European perspective. Eur. Urol. 44 , 650–655 (2003).
Kulkarni, G. S. et al. Evidence for a biopsy derived grade artifact among larger prostate glands. J. Urol. 175 , 505–509 (2006).
Redman, M. W. et al. Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach. Cancer Prev. Res. (Phila Pa) 1 , 174–181 (2008).
Pinsky, P., Parnes, H. & Ford, L. Estimating rates of true high-grade disease in the prostate cancer prevention trial. Cancer Prev. Res. (Phila Pa) 1 , 182–186 (2008).
Thun, M. J., Namboodiri, M. M. & Heath, C. W. Jr. Aspirin use and reduced risk of fatal colon cancer. N. Engl. J. Med. 325 , 1593–1596 (1991).
Steinbach, G. et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 342 , 1946–1952 (2000).
Bertagnolli, M. M. et al. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med. 355 , 873–884 (2006).
Arber, N. et al. Celecoxib for the prevention of colorectal adenomatous polyps. N. Engl. J. Med. 355 , 885–895 (2006).
Meyskens, F. L. Jr et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. (Phila Pa) 1 , 32–38 (2008).
Sporn, M. B. & Hong, W. K. Concomitant DFMO and sulindac chemoprevention of colorectal adenomas: a major clinical advance. Nature Clin. Pract. Oncol. 5 , 628–629 (2008).
Williams, R. W. The Natural History of Cancer With Special Reference to Its Causation and Prevention (Heinemann, London, 1908).
Lambe, W. Water and Vegetable Diet in Consumption, Scrofulla, Cancer, Asthma, and Other Chronic Diseases (Fowlers and Wells, New York, 1854).
The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 330 , 1029–1035 (1994).
Omenn, G. S. et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 334 , 1150–1155 (1996).
Omenn, G. S. et al. The β-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos-exposed workers. Cancer Res. 54 , 2038s–2043s (1994).
Albanes, D. et al. α-Tocopherol and β-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J. Natl Cancer Inst. 88 , 1560–1570 (1996).
Virtamo, J. et al. Incidence of cancer and mortality following α-tocopherol and β-carotene supplementation: a postintervention follow-up. JAMA 290 , 476–485 (2003).
Neuhouser, M. L. et al. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the β-carotene and retinol efficacy trial (CARET). Cancer Epidemiol. Biomarkers Prev. 12 , 350–358 (2003).
Goodman, G. E. et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping β-carotene and retinol supplements. J. Natl Cancer Inst. 96 , 1743–1750 (2004).
Taylor, P. R. & Albanes, D. Selenium, vitamin E, and prostate cancer — ready for prime time? J. Natl Cancer Inst. 90 , 1184–1185 (1998).
Brawley, O. W. & Parnes, H. Prostate cancer prevention trials in the USA. Eur. J. Cancer 36 , 1312–1315 (2000).
Kumar, N. B. & Besterman-Dahan, K. Nutrients in the chemoprevention of prostate cancer: current and future prospects. Cancer Control 6 , 580–586 (1999).
Lippman, S. M. et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301 , 39–51 (2009).
Alberts, D. S. et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians' Network. N. Engl. J. Med. 342 , 1156–1162 (2000).
Pierce, J. P. et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) study. Control Clin. Trials 23 , 728–756 (2002).
Pierce, J. P. et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA 298 , 289–298 (2007).
Blackburn, G. L. & Wang, K. A. Dietary fat reduction and breast cancer outcome: results from the Women's Intervention Nutrition Study (WINS). Am. J. Clin. Nutr. 86 , s878–s881 (2007).
Chlebowski, R. T. et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J. Natl Cancer Inst. 98 , 1767–1776 (2006).
Pierce, J. P. Diet and breast cancer prognosis: making sense of the Women's Healthy Eating and Living and Women's Intervention Nutrition Study trials. Curr. Opin. Obstet. Gynecol. 21 , 86–91 (2009).
Cummings, N. B. Women's health and nutrition research: US governmental concerns. J. Am. Coll. Nutr. 12 , 329–336 (1993).
Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin. Trials 19 , 61–109 (1998).
Prentice, R. L. et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 295 , 629–642 (2006).
Prentice, R. L. et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J. Natl Cancer Inst. 99 , 1534–1543 (2007).
Rohan, T. E. et al. Low-fat dietary pattern and risk of benign proliferative breast disease: a randomized, controlled dietary modification trial. Cancer Prev. Res. (Phila Pa) 1 , 275–284 (2008).
Wactawski-Wende, J. et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N. Engl. J. Med. 354 , 684–696 (2006).
Tomatis, L. & Huff, J. Evolution of cancer etiology and primary prevention. Environ. Health Perspect. 109 , A458–A460 (2001).
International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature 431 , 931–945 (2004).
Food and Drug Administration. Guidance for industry, investigators, and reviewers: exploratory IND studies. Center for Drug Evaluation and Research [online], http://www.fda.gov/cder/guidance/7086fnl.pdf (2006).
Kummar, S. et al. Phase 0 clinical trials: conceptions and misconceptions. Cancer J. 14 , 133–137 (2008).
Doroshow, J. H. & Parchment, R. E. Oncologic phase 0 trials incorporating clinical pharmacodynamics: from concept to patient. Clin. Cancer Res. 14 , 3658–3663 (2008).
van den Brandt, P. A., Botterweck, A. A. & Goldbohm, R. A. Salt intake, cured meat consumption, refrigerator use and stomach cancer incidence: a prospective cohort study (Netherlands). Cancer Causes Control 14 , 427–438 (2003).
La Vecchia, C., Franceschi, S. & Levi, F. Epidemiological research on cancer with a focus on Europe. Eur. J. Cancer Prev. 12 , 5–14 (2003).
Tsugane, S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 96 , 1–6 (2005).
Archer, V. E. Latitudinal variation of digestive tract cancers in the US and China. Nutr. Cancer 12 , 213–223 (1989).
Coggon, D., Barker, D. J., Cole, R. B. & Nelson, M. Stomach cancer and food storage. J. Natl Cancer Inst. 81 , 1178–1182 (1989).
Boeing, H. Epidemiological research in stomach cancer: progress over the last ten years. J. Cancer Res. Clin. Oncol. 117 , 133–143 (1991).
Cohen, A. J. & Roe, F. J. Evaluation of the aetiological role of dietary salt exposure in gastric and other cancers in humans. Food Chem. Toxicol. 35 , 271–293 (1997).
Download references
Acknowledgements
This work was supported by the Hormel Foundation and NIH grants CA027502, R37CA081064, CA077646, CA111536, CA1203889 and ES016548.
Author information
Authors and affiliations.
Ann M. Bode and Zigang Dong are at The Hormel Institute, University of Minnesota, 801 16th Ave NE, Austin, Minnesota 55912, USA.,
Ann M. Bode & Zigang Dong
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Zigang Dong .
Related links
National Cancer Institute Drug Dictionary
finasteride
FURTHER INFORMATION
Zigang Dong's homepage
At A Glance Budget
Cancer Prevention Overview
ClinicalTrials.gov
Division of Cancer Prevention History and Mission
Fruit and Vegetable Consumption Data and statistics
Important events in NCI History
Non-Profit Organizations Receiving Corporate Funding
Produce for Better Health Foundation
The Promise of Prevention and Early Diagnosis
WHO Cancer Fact Sheet
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Bode, A., Dong, Z. Cancer prevention research — then and now. Nat Rev Cancer 9 , 508–516 (2009). https://doi.org/10.1038/nrc2646
Download citation
Published : 18 June 2009
Issue Date : July 2009
DOI : https://doi.org/10.1038/nrc2646
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A potent estrogen receptor and microtubule specific purine-benzothiazole-based fluorescent molecular probe induces apoptotic death of breast cancer cells.
- Surajit Barman
- Subhajit Ghosh
- Surajit Ghosh
Scientific Reports (2022)
Differential gene expression and network analysis in head and neck squamous cell carcinoma
- Insan Habib
- Farah Anjum
- Md Imtaiyaz Hassan
Molecular and Cellular Biochemistry (2022)
A dual stimuli-responsive star-shaped nanocarrier as de novo drug delivery system for chemotherapy of solid tumors
- Sanaz Motamedi
- Bakhshali Massoumi
- Hamed Hamishehkar
Journal of Polymer Research (2020)
The pro-apoptotic effect of a Terpene-rich Annona cherimola leaf extract on leukemic cell lines
- Carl Ammoury
- Maria Younes
- Sandra Rizk
BMC Complementary and Alternative Medicine (2019)
Inhibition mechanism of cathepsin B by curcumin molecule: a DFT study
- C. Pitchumani Violet Mary
- S. Vijayakumar
Theoretical Chemistry Accounts (2019)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Scientific Scope

The National Cancer Institute Division of Cancer Prevention (DCP) is devoted to research on cancer prevention, interception, screening and early detection, and symptom science. To accomplish this, DCP provides funding and administrative support to clinical, population science, and laboratory researchers, community and multidisciplinary teams, and collaborative scientific networks.
Our Mission Statement
The Division of Cancer Prevention furthers the mission of the National Cancer Institute by leading, supporting, and promoting rigorous, innovative research and training to prevent cancer and its consequences to improve the health of all people.
DCP supports research for developing and validating new ways to prevent cancer and cancer-related deaths and reduce the burden of cancer and cancer therapies. This includes the creation and use of interventions that prevent carcinogenesis or intercept the carcinogenesis process before invasive cancer develops, as well as discovering and validating early detection biomarkers, developing screening technologies to find precancerous changes in tissues or identifying cancer when it can be successfully treated. DCP also supports research to manage or prevent symptoms caused by cancer and cancer treatment through understanding the cause of these debilitating side effects, improving methods of measuring their incidence, and creating new symptom relief interventions.
DCP supports bench-to-bedside research from basic research and preclinical development to clinical trials that demonstrate safety and efficacy/clinical utility. DCP supports effectiveness research, especially for high-risk populations. Developing a diverse, trans-disciplinary workforce is critical to reducing the population burden of cancer.
Cancer Early Detection Biomarker Development and Screening
DCP supports early detection and screening discovery, validation, and development to reduce cancer incidence and mortality, including:
- Morphology such as cytology for cervical cancer,
- Markers/biomarkers of precancer and cancer such as human papillomavirus (HPV) for cervical cancer, prostate specific antigen (PSA) for prostate, and fecal blood for colorectal cancer; and
- Imaging such as mammography for breast cancer and low-dose CT for lung cancer.
Screening for cervical, breast, lung, and colon cancers have proven effective for preventing and controlling cancer and are recommended by the U.S. Preventive Services Task Force (USPSTF). DCP-supported research has contributed to the evidence supporting their use and to limiting PSA testing. USPSTF recommends screening for hepatitis B and C, the leading liver cancer risk factors. Improved screening opportunities exist for these cancers by increasing their effectiveness and reducing their harms.
DCP is committed to identifying cancers that have no proven screening methods, such as the highly lethal pancreatic cancer. This includes supporting the discovery and validation of biomarkers for early cancer detection, and the development and evaluation of multi-cancer detection tests.
Intervention Development for Cancer Prevention
DCP supports research on the causes of and risk factors for cancer to translate these findings into new cancer prevention and interception strategies. This includes increasing knowledge of how internal and external factors contribute to cancer risk.
Internal factors – such as genetics, immunity and inflammation, hormones, and microbiome – are associated with cancer risk. External risk factors include infections (oncogenic viral and bacterial) and environmental carcinogens – such as smoke, air pollution, toxic waste, and radiation.
Elevated cancer risk can result from mutations and other inheritable factors. Non-biological factors like social determinants of health (SDH) also can elevate risk. People with elevated risk are more likely to develop cancer, and therefore more likely to benefit from a prevention intervention than people with average or low risk.
Cancer preventive agent discovery and development requires translation of risk factors into preventive interventions. This includes in vitro and in vivo studies cell lines to evaluate efficacy, toxicity, and biomarkers. Immunoprevention studies involve pathogenic vaccines and vaccines that target cancer-associated antigens along with studying adjuvants or immunomodulators to decrease immunosuppression.
Human studies and clinical trials focus on evaluating interventions to prevent or intercept pre-cancerous lesions; the pre-malignant progression to invasive cancer; recurrence of precancerous lesions; and the acquisition of or progression to cancer from carcinogenic infectious agents. Research involves reducing individual risk associated with genetic and non-genetic cancer-predisposing conditions.
Once ready these interventions move into early phase trials that evaluate components of diet; FDA-approved and investigational drugs; small molecules, vaccines, immunomodulators, biologics, biomarkers; and medical devices. Other research includes procedures such as risk-reducing surgery (e.g., mastectomy, removal of ovaries and fallopian tubes, etc.) and non-surgical ablative techniques directed at cancer biomarker modulation, cancer incidence and cancer risk reduction.
Precision Cancer Prevention
Precision cancer prevention – the use of biological data to identify those at risk of cancer and to inform targeted interventions, converges DCP’s investment in biomarkers for screening and early detection and preventive agents. Recent achievements include the Cancer Moonshot SM , the Pre-Cancer Atlas and the IOTN-Immunoprevention projects.
Symptom Science and Management
Intervention and strategies to prevent or lessen the symptoms of cancer or cancer treatment is another focus of our research. Improved symptom management impacts cancer survivors’ quality of life and allows completion of therapy that would otherwise be too toxic. This improves the likelihood that a person will survive longer, perhaps to receive the next-in-line therapy that is currently unavailable. Research spans:
- Defining basic mechanisms of cancer treatment-related toxicities and symptoms;
- Characterizing symptoms and chronic toxicities from cancer treatment in the clinic;
- Developing, verifying, and validating endpoints for assessing symptoms and chronic toxicities in symptom management clinical trials.
Creating a precision medicine model for symptom management and care (“precision symptom management”) in which knowledge of the person and disease informs how symptoms are prevented and treated, is the end goal.
DCP has a grant portfolio in quality of life, symptom science, and toxicity mitigation. Studies of symptom management and quality of life have been part of the NCI Community Oncology Research Program for decades.
Workforce Training
DCP has a longstanding commitment to training, education, and workforce development, starting with establishing the Cancer Prevention Fellowship Program (CPFP) in 1987. Through 35 years, CPFP has trained hundreds of scientists and clinicians, many of whom have taken on national and international leadership positions in academia, industry, and other settings. CPFP alumni (>300) across the country are in leadership positions at cancer centers, universities, government agencies, research firms, foundations, and policy organizations.

The Cancer Miracle Isn’t a Cure. It’s Prevention.
In the next few years, cancer will become the leading cause of death in the United States. Later in this century, it is likely to be the top cause of death worldwide. The shift marks a dramatic epidemiological transition: the first time in history that cancer will reign as humankind’s number-one killer.
It’s a good news/bad news story. Cancer is primarily a disease of aging, and the dubiously good news is that we are living long enough to experience its ravages. Cancer’s new ranking also reflects public health’s impressive gains against infectious disease, which held the top spot until the last century, and against heart disease, the current number one.
The bad news is that cancer continues to bring pain and sorrow wherever it strikes. Siddhartha Mukherjee titled his magisterial biography of cancer The Emperor of All Maladies , quoting a 19th-century surgeon. He left out the second part of the surgeon’s epithet: “the king of terrors.” Modern targeted treatments and immunotherapy have in some cases led to wondrous cures, and many malignancies are now caught early enough so that their sufferers can live out full lives. But advances in treatment alone will never be enough to fully stem the burden of cancer.
As every public health professional knows, on a population level, the only way to substantially reduce incidence and mortality for any disease is through prevention. And on a broad scale, we have made far less progress preventing cancer than preventing its predecessor scourges. We tamed infections with sanitation and vaccines, abetted by antibiotics. We tamed heart disease through smoking cessation, better medical management of risk factors such as high cholesterol, and improved interventions for a condition that has clear points of intervention and responds more readily to lifestyle changes.
Cancer is a different story. Even today, it continues to occupy our collective imagination as the king of terrors: insidious, capricious, relentless. Anyone who has suffered cancer, or has suffered alongside a loved one with the disease—a considerable portion of the population, given that more than one in three of us will be diagnosed with a malignancy during our lifetime—knows the anguish and helplessness that trail the diagnosis.
In 2015, a study in Science seemed to confirm our primal fear. It argued that only one-third of the variation in cancer risk in tissues is due to environmental assaults or inherited genetic predispositions. The majority of risk, the researchers concluded, was due to “bad luck”—random mutations during normal DNA replication.

And though that study provoked torrents of criticism about whether its conclusions based on tissue studies could be spun up to populations, it’s true that cancer is the price we pay as organisms composed of trillions of cells. Cell division is an imperfect process; like a biological keyboard with a letter missing, it makes mistakes. For that reason, it is unlikely that cancer could ever be eradicated.
The reality of cancer lies somewhere between the public health ideal of perfect prevention and the depressing stochastics of bad luck. Current research suggests that at least half of cancer cases—estimates range from 30 percent to upward of 70 percent—could be prevented by applying what we already know. The other half of cancer cases—including the elusive and often deadly types often caught too late to make a difference, such as ovarian, pancreatic, and brain tumors—could be detected and potentially even prevented far earlier if basic science and promising diagnostic technologies received the sustained government support they need.
Put simply, cancer must be framed not just as a curable disease but equally as a preventable one. “We will always need good treatments,” says Timothy Rebbeck , the Vincent L. Gregory, Jr. Professor of Cancer Prevention at the Harvard T.H. Chan School of Public Health and Dana-Farber Cancer Institute, and director of the School’s Zhu Family Center for Global Cancer Prevention . “But we can’t treat our way out of this problem. In order to make a dent in a public health sense, we must prevent cancer.”
A Grim Tally
In 2019, according to the American Cancer Society, an estimated 1,762,450 people will be diagnosed with cancer in the United States and an estimated 606,880 will die of the disease. Globally, cancer killed an estimated 9.6 million people in 2018—more than malaria, tuberculosis, and HIV combined. In this century, cancer will become not only the leading cause of death worldwide (in 91 nations it already ranks as the first or second cause of death before age 70, according to the World Health Organization) but also the single biggest hurdle to boosting life expectancy in scores of nations.
The reasons for cancer’s ascendancy are complex. Part of the trend is demographic: The human population is both growing and aging each year, meaning more people are vulnerable to the disease, which takes advantage of the waning immune system and the accumulated DNA damage that accompanies aging. But cancer’s chief risk factors are also changing. While smoking is down in the United States, for example, it is up in Africa and the Eastern Mediterranean, as tobacco companies expand into new markets. And while cigarette use is the most important risk factor for cancer worldwide, cancer-causing infections, such as hepatitis and the human papilloma virus (HPV)—both preventable with vaccines—account for up to 25 percent of cancer cases in some low- and middle-income countries.
These shifting sands of causation are also evident in the United States. Over the past 25 years, while cancer deaths have risen in number as the population grows, the cancer death rate has steadily declined. As of 2016, the cancer mortality rate for men and women combined had fallen 27 percent from its peak in 1991. The engine behind this impressive public health feat was the decline in smoking, though early detection and improved treatments also played a role. In 1965, 42 percent of U.S. adults were cigarette smokers; in 2017, just 14 percent. Lung cancer death rates declined in tandem, falling 48 percent from 1990 to 2016 among men and 23 percent from 2002 to 2016 among women.
That public health victory is now in peril. In the next five to 10 years, experts say, the cancer-causing effects of obesity could actually reverse the downward trend ushered in by the decline in smoking. Indeed, obesity could soon become the number-one risk factor for cancer in the United States and eventually around the world. And given obesity’s seeming irreversibility, thwarting cancer’s concomitant rise will be exceedingly difficult. In the U.S., 39.5 percent of adults are now estimated to be obese and an additional 31.8 percent overweight.
Obesity is a well-established risk factor for at least 13 cancers. According to a 2019 report in The Lancet Public Health , excess body weight in the U.S. accounted for up to 60 percent of all endometrial cancers, 36 percent of gallbladder cancers, 33 percent of kidney cancers, 17 percent of pancreatic cancers, and 11 percent of multiple myelomas in 2014.
Increasing obesity among younger people may portend a bigger wave of cancer in the near future, according to the The Lancet Public Health study. In the U.S., the incidence significantly increased for six obesity-related cancers in young adults, with each successively younger generation suffering a higher rate of cancer than the previous generation. These cancer cases serve as sentinels for future disease in older people. In light of rising rates of colorectal cancer among young adults, a trend suggesting environmental factors, the American Cancer Society last year lowered its recommended age for people’s first cancer screening, from 50 to 45.
Calculating the Benefits of Prevention
Two kinds of prevention can substantially reduce cancer deaths. The first, and most important, is primary prevention: averting a malignancy by attacking its causes and promoting the factors that protect against it. Taxes on cigarettes and alcohol, vaccination against cancer-causing pathogens such as HPV and hepatitis B, promoting healthy eating and regular exercise: All are examples of primary prevention. Primary prevention works when social and economic conditions, the built environment, and the public health and medical systems work in concert to support it.
Secondary prevention controls cancer by screening to detect the disease at its earliest stages and, if necessary, intervening early in the course of the disease’s progression. Secondary prevention has helped bring down death rates of breast, cervical, and colorectal cancers, among others.
Long-term epidemiological studies have clarified which cancers are preventable and by how much, if specific risk factors were reduced. A 2016 report in JAMA Oncology by the Harvard Chan School’s Ed Giovannucci , professor of nutrition and epidemiology, and Minyang Song , assistant professor of clinical epidemiology and nutrition, found that 20–40 percent of cancer cases and about half of cancer deaths could potentially be prevented through lifestyle modification, including quitting smoking , avoiding heavy alcohol drinking, maintaining a body mass index of 18.5 to 27.5 , and exercising at moderate intensity for at least 150 minutes or at a vigorous intensity for at least 75 minutes every week. (An additional bonus is that promoting cancer’s protective risk factors could also prevent other common noncommunicable diseases, such as type 2 diabetes, heart disease, dementia, and depression.)
A 2018 study in Science —co-authored by Song, Giovannucci, and Harvard Chan’s Walter Willett , professor of epidemiology and nutrition—made an even more emphatic case for prevention. It noted that for cancers in which most of the driving genetic mutations are caused by the environment—such as lung cancers, melanomas, and cervical cancers—85 to 100 percent of new cases could be eliminated through smoking cessation, avoidance of ultraviolet radiation exposures, and vaccination against HPV, respectively.
“With such further research, we envision that cancer death rates could be reduced by 70 percent around the world, even without the development of any new therapies,” the authors concluded. “Such a reduction, similar to that for heart disease over the past six decades, will only come about if research priorities are changed.” Specifically, the authors argue for more support of molecular, behavioral, and policy research on prevention.
Even individuals at high inherited genetic risk for cancer can benefit from lifestyle change, adds Peter Kraft , professor of epidemiology at the Harvard Chan School. In 2016, Kraft published a paper in JAMA Oncology showing that U.S. women who were in the highest decile of breast cancer risk because of factors they could not alter—mostly genetics but also family history, height, and menstrual and/or reproductive history—actually benefited the most from a healthy lifestyle. In fact, the women who had the highest nonmodifiable risk but also kept their weight down, did not drink or smoke, and did not use menopausal hormone therapy had about the same breast cancer risk as an average woman in the general population.
“Although our day jobs are studying the genetics of cancer, genetics is not destiny, by any means,” says Kraft. “This is something we’ve seen consistently across many cancers—and many diseases generally. Even if you’re high-risk based on your genetics, there’s still plenty that you can do to reduce your risk. In fact, high-risk individuals are the people who seem to reap the biggest benefit from adopting healthy lifestyles.”
Cancer Clues across Two Dimensions
Should anyone still doubt that many cancers are preventable, the inarguable proof is how the disease plays out over time and space. Cancer rates and types can starkly change within a country and starkly vary between countries. These variations are not genetic—a small minority of cancers are directly attributable to known, death-dealing DNA mutations. Rather, they reflect external—and, in principle, modifiable—risk factors.
For example, lung cancer eclipsed all other cancers during most of the 20th century in the United States because per capita cigarette consumption shot up from 54 cigarettes a year in 1900 to 4,345 cigarettes in 1963, then fell to 2,261 in 1998. The initial upward trend was powered by corporate profiteering. The downward slope was powered by the landmark 1964 U.S. Surgeon General’s report on smoking and health, which firmly linked smoking and lung cancer and led to public education, indoor smoking bans, and higher tobacco taxes. Another instance of a breathtaking prevention success within a country took place in the 1980s and 1990s in Taiwan, which saw an 80 percent decline in liver cancer rates in birth cohorts that received hepatitis B vaccination early in life. (The most common causes of liver cancer are infection with the hepatitis B virus in Africa and East Asia, and the hepatitis C virus in the U.S. and Europe.) And Australia recently reported it is on course to completely eliminate cervical cancer in the coming decades through vaccinations.
The spatial dimension of cancer is equally revealing. When racial or ethnic groups migrate from one part of the world to another, their cancer risks quickly take on the local patterns. Between 1975 and 2003, for example, numerous studies looked at cancer incidence in U.S. Caucasians, immigrant groups, and matched controls. Among the populations studied were first- and second-generation Japanese immigrants, Asian American women, Vietnamese Americans, and Hmong refugees from Vietnam, Laos, and Thailand. Drawing on data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, the studies found that the kinds of cancers that were newly diagnosed among first-generation immigrants in the U.S. were nearly identical to the kinds in their native countries. But over subsequent generations, their cancer patterns became distinctly American. This was especially true for cancers related to hormones, such as breast, prostate, and ovarian cancers, and to cancers attributable to Westernized diets, such as colorectal malignancies.
Understanding Cancer’s Genesis
Given the fact that many cancers can be averted, what would it take to make the dream of prevention a reality?
First, scientists say, we must understand the earliest biological events that give rise to the birth of a cancer cell. While genomic analyses have provided a good molecular description of cancer, researchers still don’t understand how and when cells start to go rogue.
“Cancer initiation is much less well understood than the biology of cancer cells themselves,” says Brendan Manning , professor of genetics and complex diseases at the Harvard Chan School. “Cancer cells are doing things that normal cells do, only in an uncontrolled manner. So, how is cancer initiated? What are the brakes on early cancer? What are the challenges that the cancer cell faces in becoming a cancer cell? How does the cancer cell remove enough of those brakes so that it will become malignant?” Answering those questions will also shed light on the mechanisms by which apparent cancer risk factors, such as aging or obesity or chronic inflammation, trigger uncontrolled cell growth and progression to cancer, says Manning.
Manning’s lab explores how the body’s cells and tissues sense nutrient shifts in their local environment and adapt accordingly. “The cells in our body have the ability to acclimate to changes in nutrient availability, and this is achieved through special lines of communication—referred to as nutrient sensing pathways—that serve to tune cell metabolism to match these changes,” he says. “Understanding these fundamental mechanisms has provided us with key insights into how nutrient sensing becomes corrupted in human cancers, which universally exhibit alterations in cellular metabolism that underlie uncontrolled growth.”
Another biological unknown is the role of the microbiome—the trillions of microbes in and on our bodies—in human cancer. “These living organisms can at times be found right at the site of the cancer,” says Wendy Garrett , professor of immunology and infectious diseases at the Harvard Chan School. “We are beginning to see very provocative associations between the microbiome and cancer, and interesting molecular mechanisms—which are emerging from experiments with cells and in tissue cultures and preclinical mouse models—may explain these associations.”
One intriguing culprit on which Garrett and her colleagues are focusing is Fusobacterium nucleatum , normally a microbial denizen of the mouth. Garrett’s lab and others have shown that the bacterium is abundant in colon tumors. She wants to find out why, whether such bacteria are important early signals for carcinogenesis, and if any interventions—such as changing one’s everyday behaviors and exposures, including diet and tobacco use—map onto the microbiome and could potentially halt the disease process.
The microbiome is proving to be a vast and inviting landscape in cancer biology. In humans, gum disease caused by bacterial infections has been connected to higher risk of pancreatic cancer. In mice, lung tumors appear to alter nearby bacterial populations to help the tumors thrive—and antibiotics appear to shrink the tumors. Experiments in mice have even linked a disrupted gut microbiome to greater risk of invasive breast cancer.
“It’s possible that the cancers for which we currently don’t fully understand risk factors—such as pancreatic and ovarian cancer—might be tied to infections and therefore be preventable,” says Giovannucci. “Forty years ago, we didn’t know what caused stomach cancer. Now we know: the bacterium Helicobacter pylori .” H. pylori is treatable with antibiotics, and stomach cancer rates have dropped considerably as a result.
Prevention via Detection
With many tumors, there is a lag time of 20 years or more between the development of the first cancer cell and the onset of end-stage metastatic disease. Knowing each cancer’s basic biology could lead to a host of new technologies that register early biomarkers of the disease, potentially opening up new ways to head off malignancy before it spreads. That prospect would be transformative for the implacable cancers that don’t cause symptoms until they have reached their late and often incurable stages.
Among these promising biomarkers are proteins that signal early tumors, DNA or RNA, small molecules, circulating tumor cells, immune cells, and other infinitesimal biological entities. Scientists are also fashioning synthetically engineered biomarkers that harness the body’s own biology to spin off early signals of disease. “It’s a matter of screening technology getting refined enough so that you can find two suspicious molecules in four liters of blood which suggest you are at risk for or have already developed cancer,” says Rebbeck.
Sangeeta Bhatia, a biomedical researcher and early-detection pioneer, and the John J. and Dorothy Wilson Professor of Engineering at the Massachusetts Institute of Technology, injects nanoparticles into the bloodstream that respond to cancer-associated enzymes. When the particles find the enzyme for which they are designed, a chemical reaction produces “reporters”: synthetic chemicals eliminated in the urine that can tip off researchers to a nascent malignancy. Her lab is searching for highly specific biomarkers for often-elusive tumors of the ovary and lung and in colon metastasis. Clinical trials for the technology will begin later this year.

“Ultimately, we’d like to be in a place where you could do a urine test on a paper strip for a defined set of cancers,” Bhatia says. Other scientists envision, in the more distant future, continuous monitoring of cancer risk through smart toilets, wearables such as diagnostic imaging bras, and other passive and noninvasive technologies.
In clinical medicine, the value of screening tests is gauged by their sensitivity and specificity. Sensitivity measures a test’s ability to identify people who have the condition that is being tested for; a highly sensitive test will not generate false-negative results. Specificity measures a test’s ability to identify people who do not have the condition that is being tested for; a highly specific test will not generate false-positive results.
All the futuristic approaches described above require knowing that a technology’s molecular quarry is made by a certain kind of cancer cell and only that cancer cell—that is, the screening test must be highly specific. Since many tiny malignancies never go on to become metastatic disease—because the immune system reins in such cells—the ideal biomarker would not only tip off doctors to the presence of a cancer or precancer but also predict whether the suspect cells are aggressive or slow-growing. “[O]ne can imagine a day when healthy individuals are routinely tested for these biomarkers to detect early cancers, along with lipid concentrations to detect early cardiac disease, at periodic visits to their physicians,” the Harvard Chan School scientists wrote in Science in 2018.
Before liquid biopsies, “smart tattoos” that light up in the presence of cancer cells, small ingestibles that monitor the gastrointestinal tract, and other early-detection tests that sample blood, urine, saliva, or the breath can ever become part of the annual physical, they will have to be honed to the point of 99.9 percent accuracy or higher, similar to the accuracy of the early-pregnancy urine tests available at any drugstore. That is, they must be both highly sensitive and highly specific. This high degree of accuracy prevents false negative or false positive results when the test is used in large numbers of people.
Such tests could also help doctors decide whom to monitor more closely for cancer. “Advances in biomarker testing could help us better risk-stratify the population,” says Jane Kim, professor of health decision science at the Harvard Chan School. “The whole point of screening is to pull out the people who are at lowest risk and focus your attention on those at highest risk. Today, with cervical and even colorectal cancer, there is a prevention mechanism: You remove precancerous lesions before they develop into cancer. But with breast cancer, you need early detection, because there are no really strong prevention mechanisms. Risk-stratifying patients would help efficiently identify high-risk patients through prevention and early detection.”
Validating today’s candidate biomarkers will partly depend on long-term cohort studies—such as the Nurses’ Health Study —that have followed healthy volunteers over decades, collected biological material from these volunteers, and tracked the natural course of diseases as the participants aged. To speed the clinical validation of such early diagnostic tests, researchers will first try them out on people at high genetic risk for various cancers, for whom the tests have a higher likelihood of detecting an abnormality and making an impact.
“Combining basic science and cohort studies would also facilitate the discovery and validation of new biomarkers,” says Manning. “If you’re banking molecular information from blood and tissue, and the data changes over time, you can look back retrospectively at thousands of patient outcomes and see if the changes predicted an outcome or might be related to that outcome. Basic science holds the key to determining how that identified biomarker links back to the disease state and whether it is contributing to the disease’s onset—perhaps as a risk factor—or is a consequence of the disease.”
But being able to find an early cancer or predict its progression is not enough. “The key thing is that you have an intervention and that it’s actionable,” says Rebbeck. Such interventions might include surgery, cancer vaccines, anti-inflammatory drugs, a standard chemoprevention treatment, tinkering with the body’s microbiome, or even lifestyle change. “If you detect an early cancer biomarker but cannot act on it, then it may just produce anxiety,” he says. “There is a quote from Sophocles that we sometimes use: ‘Knowledge is but sorrow when wisdom profits not.’”
From Science to Action
Just as crucial will be translating new scientific insights into public health practice—a field known as implementation science. “Public health impact is efficacy times reach,” says Karen Emmons , professor of social and behavioral sciences at the Harvard Chan School. “We often develop interventions without thinking about the end users and what could get in the way of true impact, so shame on us as a field. As a scientific community, we think, rather arrogantly, ‘Well, we’ve shown that colorectal cancer screening is important—why don’t community health centers just make sure that everybody has colorectal cancer screening? It’s clear that vaccines are important—why aren’t all kids getting HPV vaccine?’ But the real question is: How do you structure systems to make those goals possible?”
Today’s cancer prevention and detection efforts regularly fall short in their impact. Although HPV vaccination administered in preadolescence, before a teen becomes sexually active, theoretically prevents some 90 percent of cervical cancers, the U.S. vaccination rate among adolescents is low. In 2017, only 42 percent of girls and 31 percent of boys received the two recommended doses before their 13th birthday. Similarly, in 2015, only 50 percent of women ages 40 years and older reported having a mammogram within the previous year, and only 64 percent within the previous two years.
Even the most well-established intervention against the most formidable cancer threat in the U.S.—lung cancer—is only fitfully used. “For some time after we started doing lung cancer screening for smokers, we didn’t also do smoking cessation with them,” says Emmons. “Even today, we still do it inconsistently. Now how stupid is that?”
Alan Geller , senior lecturer on social and behavioral sciences at the Harvard Chan School, has seen up close how the failure to translate science into action and policy leads to health disparities. “All of my work now is trying to ask the big question of who unnecessarily dies from preventable diseases,” he says. “Smoking rates are at best stabilizing among low-income people in the U.S.—but they’re stabilizing at 30 to 33 percent of the adult population. Among the well-to-do, smoking rates have for years been well below 10 percent. It’s not a racial disparity —it’s an income disparity, because the smoking rate among whites and African Americans is exactly the same. So we should target low-income people. Public health needs to go where high-risk people are.”
Geller adds that with smoking, four strategies could substantially reduce cancer deaths. “First would be to work really hard in the U.S. South, where smoking rates are double those in the North. Second would be working among people with mental health issues, because 41 percent of all smokers have diagnosed mental health conditions. Third would be figuring out how we could intervene with people who have GEDs [general education diplomas, also known as high school equivalency certificates]; 14 million people in the United States have one, and as a group their smoking rates are 40 percent. And fourth would be working with people in public housing—figuring out how their doctors and housing providers can give them access to nicotine replacement therapy, which is extraordinarily inexpensive, and how they can use community health workers and patient navigators. Those are all beautiful, low-cost, public health models for smoking cessation and lung cancer prevention.”
It’s almost a public health truism that when breakthrough medical advances hit the market, they disproportionately benefit people of means and thus widen health disparities. This divide is brutally apparent with cancer. From 2012 to 2016, for example, death rates in the poorest U.S. counties were two times higher for cervical cancer and 40 percent higher for male lung and liver cancers compared with rates in the richest counties. Poverty is also linked with lower rates of routine cancer screening, later stage at diagnosis, and a lower likelihood of receiving the best treatment.
“There are still parts of this nation where the rates of cervical cancer mirror those in developing countries—not developed countries,” notes Susan Curry, distinguished professor of health management and policy and dean emerita of the College of Public Health at the University of Iowa, and immediate past chair of the U.S. Preventive Services Task Force. “Are there barriers to screening within the population eligible to be screened? Are there barriers in terms of the organization and availability of screening? Are there barriers in terms of, you can get screened, but if you don’t have the means to follow up on a positive test or don’t understand what that is, then screening is for naught? We can pinpoint some pretty disturbing disparities. But how much are we investing in the intervention science that we need to close those gaps?”
These divergences are writ larger on the global stage. Earlier this year, The Lancet Global Health published a damningly titled article: “Cervical cancer: lessons learned from neglected tropical diseases.” The malignancy claims 310,000 lives annually around the globe, making it the fourth-most-common cancer killer of women. “[C]ervical cancer is not a disease of the past—it is a disease of the poor,” the authors state. They go on to list the hurdles that cervical cancer—which could virtually be eliminated from the planet with vaccination and screening—shares with neglected tropical diseases: Both accompany poverty; strike populations mostly overlooked by policymakers; are associated with stigma and discrimination; strongly affect female morbidity and mortality; tend to be neglected in clinical research and technological development; and can be controlled, prevented, and conceivably eliminated through currently available solutions that are cheap and effective.
It’s worth noting that in Africa, more people die from cancer than from malaria . And while overall cancer death rates have been rising in Africa—and will double in the next 20 years—malaria death rates are dropping because of concerted efforts to prevent and treat the infection.
A 2009 study in the journal Cancer Epidemiology, Biomarkers & Prevention underscored the fact that the newest and best cancer preventions disproportionately benefit people of means. The study found that the more knowledge, technology, and effective medical interventions there are for a given disease—that is, the more amenable a disease is to early detection and cure—the wider its disparities, because people who have knowledge, income, and useful social relations stand a better chance of surviving. By contrast, with diseases where effective medical interventions are absent or negligible, such as ovarian or pancreatic cancers, social and economic resources are of limited use, and survival differences between the most and least socially advantaged people are minimal.
“When you look at cancers that are preventable, as soon as something comes online to screen or prevent, you start to get pretty sharp disparities by race, ethnicity, and income,” says Emmons. “Colon cancer is a great example. Before sigmoidoscopy and colonoscopy screening came on board, there were actually slightly higher rates of colon cancer in whites than there were in blacks. Literally within three years after these screening tools were introduced, colon cancer rates among whites fell dramatically, but the rates in blacks did not. You see this over and over again.”
Such health inequities represent lives lost to cancer. When Emmons looks at new technologies, she asks: “What is the user perspective? How will the new technology interface with places where lower-income populations get their care? What does the technology mean for population health management, as opposed to managing the health of an individual? If you don’t pay attention to how these technologies are utilized across racial and economic lines, you wind up with persistent disparities that we shouldn’t tolerate.”
The Prevention Mindset
In the 1970s, a New Yorker cartoon depicted two stereotypical (for that era) male scientists standing before a blackboard scrawled with complicated equations. In the middle of these obscure scribbles is the phrase: “THEN A MIRACLE OCCURS….”
So it goes with cancer. “A cure for cancer” is our cultural synonym for a miracle. But as Curry points out, “We’re still waiting for that miracle.” When cancer treatments work, as they increasingly do, they seem indeed miraculous. But often, they come too late. The real miracle would be to prevent cancer from ever striking.
“Prevention is very hard,” Rebbeck concedes. “People want to think about cure. They say we need to cure cancer—and if someone has cancer, you absolutely want to cure it. But what’s not gotten into the public mindset is that we need to prevent cancer so that nobody needs to be cured.”
“For decades, success in cancer control has been ‘just around the corner,’” wrote Tom Frieden, the then-commissioner of the New York City Department of Health and Mental Hygiene, in 2008 in The Oncologist . Frieden, who went on to lead the U.S. Centers for Disease Control and Prevention (CDC), added, “Yet, to wage a true war on cancer, we must expand our approach to give preventive interventions at least as much focus as medical treatment.” Pointedly, he added that such a goal would require correcting the imbalance between “money invested in cancer treatment and money invested in cancer prevention.”
Currently, those two streams of funding are wildly unequal. In fiscal year 2018, the last year for which data is available, only 5.7 percent of the National Cancer Institute (NCI) budget was allotted to cancer prevention and control. Today, even the money for treatment research and other programs may be whittled back. The proposed fiscal year 2020 budget for the NCI is $5.2 billion—nearly $900 million less than the enacted 2019 budget. At the CDC, the proposed budget for cancer prevention and control was trimmed by more than $34 million—a 9 percent cut from last year. Globally, cancer prevention research is allotted an estimated 2 to 9 percent of global cancer research funding.
“The biggest unknown in cancer prevention is how to sustain proven, effective, and lifesaving preventive efforts over the long run,” says Howard Koh , the Harvey V. Fineberg Professor of the Practice of Public Health Leadership at the Harvard Chan School and the Harvard Kennedy School; former assistant secretary for health for the U.S. Department of Health and Human Services; and former commissioner of public health for the Commonwealth of Massachusetts. “Prevention should be integral, not optional. But in government, prevention budgets are always the first items to be cut and the last to be restored.”
Some researchers go so far as to argue that government research funding should be shifted somewhat from treatment to prevention—because solving the front end of the problem will save countless more lives. Others disagree, arguing that cancer will never go away completely and that, even today, we only know how to prevent about half of cancer cases. “You can take the pie and divide it differently or increase the pie,” says Curry. She would like to see more support for front-line public health. “Clearly, we need more dissemination science. There’s a huge gap between what we know and what we do.”
Manning insists that bench science is just as important in prevention. “In most cases, the biggest breakthroughs in biomedical research, including cancer biology, are made using reductionist approaches in which you’re isolating one aspect of the broader biology,” he says. “Stripping a biological problem down to its essence is key. We need to keep funding research that allows us to understand with detail and accuracy the aspects of biology that are important for cancer initiation. But right now, there is an overemphasis at the NIH [National Institutes of Health] and at NCI on supporting research that purports to be directly translatable or is seemingly translatable to treatment for an existing cancer, rather than on understanding how cancer begins.”
Shoe-leather population research and high-tech bench science: Both will be needed to stop cancer’s unabated rise.
Shaping Public Opinion
Desperate entreaties for increased support of cancer prevention are nothing new. In 1929, James Ewing, the director of cancer research at Memorial Hospital in New York City, wrote in Public Health Reports : “It is only within the last few years that cancer has been considered a public health problem. I suppose that the old attitude was due to the fact that cancer is not an infectious disease; also largely because of the popular notion that it is not preventable; and probably also, to a large extent, to the feeling, fairly well grounded, that the disease is incurable.” Ewing hoped for a change in public attitudes. “[C]ancer is a public health problem of the first importance, because many of the forms of cancer are preventable, and if the public were thoroughly informed, a definite reduction in the incidence of cancer might follow.”
Ninety years later, most people still do not grasp that point. Nor do they see that with robust research, the incidence of today’s more elusive and frightening cancers could also fall. In the 2017 American Institute for Cancer Research’s Cancer Risk Awareness Survey, for example, fewer than half of Americans recognized that alcohol, processed meat, high amounts of red meat, low amounts of fruits and vegetables, and not enough physical activity all have clear links to cancer development. And contradicting scientific evidence, they tended to blame cancer on factors they couldn’t control rather than on those they could. Nuclear power ranked eighth as a perceived cause of cancer, for example, and food additives ninth. Obesity—which may soon become the top modifiable risk factor for cancer—ranked 16th.
As Frieden explained in 2008 in The Oncologist , cancer-causing agents “are not primarily trace chemicals found in food, water, or air, but instead are the major constituents of what humans consume voluntarily. These agents are best viewed as toxins, and public policies can substantially reduce our exposure to them.”
A Moon Shot for Prevention
In 1969, the Citizens Committee for the Conquest of Cancer, inspired by the success that year of the Apollo 11 space mission and propelled by the indomitable philanthropist Mary Lasker, conceived of a “moon shot” for cancer. That December, the group ran a full-page ad in The Washington Post and The New York Times : “Mr. Nixon: You can cure cancer.” At the time, a cure was perceived to be imminent.

President Richard Nixon’s grandiloquent response in his 1971 State of the Union address: “The time has come in America when the same kind of concentrated effort that split the atom and took man to the moon should be turned toward conquering this dread disease. Let us make a total national commitment to achieve this goal.”
But the War on Cancer, as the moon shot was called, didn’t reach its goal. Partly, that was because “cure” was an erroneous target. Cancer is not one disease, but more than 200. “We talk about a ‘cure’ for cancer, but no one would ever use the term ‘cure’ for infectious disease—they would talk about a cure for AIDS or TB or malaria,” says the Harvard Chan School’s Giovannucci. “You have to think about these diseases one by one.” More fundamentally, the War on Cancer failed because it spent far too little on cancer prevention and cancer prevention research.
There are many reasons why prevention research is unenticing. Most societies are reactive, not proactive. The final phases of research on treatment are simpler than research on prevention. Curing a patient with advanced disease is more dramatic than preventing disease in a healthy person. And perhaps most conspicuously, treatments earn far higher profits than do new diagnostics or prevention measures.
Yet every great public health success has overcome those entrenched obstacles. “The way I message this to lawmakers is that our well-being is a gift; we can’t take good health for granted, and prevention is a powerful way to protect that gift. When prevention works, you can enjoy the miracle of a perfectly normal, healthy day,” says Koh. “When I interact with lawmakers, I often ask about whether they have experienced the pain of losing a loved one when it could have been prevented. That usually humanizes the conversation and gives it relevance and immediacy.”
A cure for cancer is our culture’s threadbare metaphor for a miracle. But a cancer prevented is even better than a cancer cured. When cancer becomes our leading cause of death—as it soon will—cancer prevention will become our leading cause of life.
Madeline Drexler is editor of Harvard Public Health .
News from the School

Nurturing student entrepreneurs

40 years after Bhopal disaster, suffering continues

Meet the Dean


Making health care more affordable
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Omega-3 fatty acids for breast cancer prevention and survivorship
Carol j fabian, bruce f kimler, stephen d hursting.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Issue date 2015.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Women with evidence of high intake ratios of the marine omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) relative to the omega-6 arachidonic acid have been found to have a reduced risk of breast cancer compared with those with low ratios in some but not all case–control and cohort studies. If increasing EPA and DHA relative to arachidonic acid is effective in reducing breast cancer risk, likely mechanisms include reduction in proinflammatory lipid derivatives, inhibition of nuclear factor-κB-induced cytokine production, and decreased growth factor receptor signaling as a result of alteration in membrane lipid rafts. Primary prevention trials with either risk biomarkers or cancer incidence as endpoints are underway but final results of these trials are currently unavailable. EPA and DHA supplementation is also being explored in an effort to help prevent or alleviate common problems after a breast cancer diagnosis, including cardiac and cognitive dysfunction and chemotherapy-induced peripheral neuropathy. The insulin-sensitizing and anabolic properties of EPA and DHA also suggest supplementation studies to determine whether these omega-3 fatty acids might reduce chemotherapy-associated loss of muscle mass and weight gain. We will briefly review relevant omega-3 fatty acid metabolism, and early investigations in breast cancer prevention and survivorship.
Introduction
Although the predominant driving force in breast carcinogenesis has been thought to be hormonal, cytokine production and inflammation are also being recognized as important in breast cancer development and progression [ 1 , 2 ]. A progressive increase in activated macrophages and T cells is observed between normal breast tissue, proliferative breast disease, and breast cancer [ 3 , 4 ]. The stimulus for the increase in inflammatory cell infiltration observed with proliferative breast disease and breast cancer is unknown but probably has varying etiologies including immunogenic gene alterations in epithelial cells [ 5 ], reaction to breakdown of basement membrane components [ 4 ], and for obese women excess cytokine production from dysfunctional adipocytes [ 6 ].
The long-chain omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are important in generating bioactive lipid mediators important in inflammation resolution [ 7 ]. As key components of phospholipid membranes and lipid rafts that serve to organize or separate molecules, these fatty acids also affect cell signaling thought to impact breast carcinogenesis [ 8 - 12 ]. The ability of long-chain omega-6 fatty acids to modulate inflammation and other physiologic processes is dependent on concomitant levels of the proinflammatory omega-6 arachidonic acid (AA) as well as an individual’s genetic makeup governing lipid metabolism [ 13 - 16 ].
Interest in the use of supplementary omega-3 fatty acids to reduce risk of cancer and other chronic debilitating conditions, including cardiovascular disease and cognitive impairment, stems from several longstanding avenues of investigation: 1) an increased incidence of breast cancer and heart disease in western societies with low omega-3:omega-6 fatty acid intake ratios; 2) a very low incidence of these two conditions in populations with high marine omega-3 fatty acid intake (Japan and natives of Alaska and Greenland); 3) a dramatic increase in the incidence of breast cancer and cardiovascular disease in cohorts from low-incidence populations who migrate to western countries and/or adopt a western diet [ 15 , 17 ]; and 4) the demonstrated importance of adequate DHA in retinal and brain development and cognitive function [ 18 , 19 ].
Although the ideal total omega 3:omega-6 intake ratio has not been defined, a ratio approaching 1:1 or 1:2 similar to that of precivilized man is generally accepted as associated with a low incidence of diseases characterized by chronic inflammation, and therefore is desirable [ 16 , 20 ]. By the early 1900s the omega 3:omega-6 intake ratio in the United States was estimated at 1:5, probably due to the high dietary content of corn oil products and corn-fed animals. Today, largely due to the >1,000-fold increase in use of soybean oil in the last several decades, the dietary omega 3:omega-6 intake ratio is now 1:10 or lower [ 16 , 21 ]. Although much of the imbalance is probably due to the increase in omega-6 consumption, it has been suggested that the most practical remedy may actually be to increase long-chain or marine omega-3 intake rather than to attempt to markedly reduce omega-6 intake [ 22 , 23 ].
We will briefly review omega-3 and omega-6 fatty acid metabolism and function, preclinical mechanistic and prevention studies, as well as selected case–control and prospective cohort studies, and ongoing trials relevant to breast cancer prevention. Reports dealing with omega-3 fatty acids and breast cancer recurrence as well as other relevant survivorship topics including insulin resistance and obesity, cardiovascular disease and cognition will also be discussed.
What are omega-3 and omega-6 fatty acids and how do they work?
Omega-3 and omega-6 fatty acids are a group of essential polyunsaturated fatty acids (PUFAs) that play important roles in cell membrane structure, fluidity, and cell signaling [ 13 ]. The designation 3 or 6 is structural, referring to the double bond on the third or sixth carbon respectively from the methyl group [ 13 ]. The most abundant dietary PUFAs are the short-chain omega-3 alpha linolenic acid (ALA) and the omega-6 linoleic acid (LA), most often ingested as plant oils. The longer chain omega-3 PUFAs EPA and DHA, commonly referred to as marine fatty acids, are most efficiently obtained from fatty cold water fish such as salmon, whereas the long-chain omega-6 fatty acid AA is obtained most efficiently from eggs, poultry, and meat [ 24 - 26 ] (see Figure 1 ). Unless EPA, DHA, and AA are directly ingested, they must be derived from ALA and LA, respectively. In general, the desaturases and elongases have a greater affinity for ALA than LA but, due to the general 10-fold higher intake of LA, generally more AA than EPA and DHA is formed [ 24 ].
Dietary sources and general metabolic pathway for omega-6 and omega-3 polyunsaturated fatty acids, leading to proinflammatory and anti-inflammatory products respectively.
Whether ingested or synthesized, PUFAs are either oxidized for fuel, stored in triacylglycerol, taken up in phospholipid membranes for eventual use as substrates by cyclooxygenase (COX) and lipoxygenase (LOX) enzymes, or used as ligands for G receptors [ 26 ]. Neither LA nor ALA is readily converted to bioactive lipid products due to low uptake into phospholipid membranes. However, 5 to 10% of both LA and ALA can be converted to the longer chain PUFAs that are readily taken up in phospholipid membranes and form the substrates for conversion to bioactive lipid products by COX and LOX enzymes [ 26 ] (see Figure 2 ).
Metabolic pathways for omega-6 and omega-3 fatty acids that result in a variety of inflammation mediators and cell function effectors. Proinflammatory (red) and anti-inflammatory or less inflammatory (green) molecules are denoted within ellipses. Other molecules are indicated that are likely to promote (red) or repress (green) neoplastic processes. Cyclooxygenase (blue) and lipoxygenase (yellow) enzymatic processes are indicated. AA, arachidonic acid; ALA, alpha linolenic acid; COX, cyclooxygenase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDHA, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyoctadecadienoic acid; HPETE, hydroperoxyeicosatetraenoic acid; LA, linoleic acid; LOX, lipoxygenase; LT, leukotriene; LX, lipoxin; HODE, hydroxyoctadecadienoic acid; HX, hepoxilin; MaR, maresin; PD1, protectin D1; PG, prostaglandin; Rv, resolvin; TX, thromboxane.
The omega-6 PUFA AA and its derivatives are important in a diverse set of physiologic functions including initiation and sustainment of inflammation (for example, T-cell and monocyte activation, chemotaxis), platelet aggregation, endothelial adhesion molecules, ovulation, parturition, and muscle strength. The omega-3 fatty acids EPA and DHA and their derivatives are important for retina and brain development, cognitive function, and in the production of minimally inflammatory eicosanoids as well inflammation resolving mediators termed resolvins and various tissue protectins [ 20 , 22 ]. Although most of the bioactive lipid mediators of interest are a result of COX and LOX enzyme activity on the long-chain PUFAs EPA, DHA, and AA, 15-LOX acts on the short chain LA to form 13(S)-hydroxyoctadecadienoic acid, which is probably carcinogenic and is known to increase mammary tumor proliferation [ 22 ] (see Figure 2 ). EPA and DHA compete with AA as substrates for COX and LOX enzymes although EPA is a poorer substrate than AA, at least for COX [ 24 ].
Upon inflammatory stimulus, the enzyme phospholipase A2 releases AA from phospholipid membranes of monocytes and predominantly proinflammatory derivatives are produced (Figure 2 ). COX-1 and COX-2 enzymes are responsible for AA-derived prostaglandin E 2 and other series-two prostaglandins and thromboxanes [ 15 , 24 ]. 5-LOX, 12-LOX, and 15-LOX are responsible for generation of the series-four leukotrienes and lipoxins. Leukotrienes have chemotactic and other effects on inflammatory cells.
In general, the action of COX and LOX enzymes on the omega-3 fatty acids EPA and DHA is to produce eicosanoids with less affinity for the corresponding receptors as well as resolvins that block inflammatory cell recruitment and promote phagocytosis. The net effect if EPA and DHA are present in sufficient amounts relative to AA is anti-inflammatory or inflammation resolving. The action of COX on EPA gives rise to the series-three prostaglandins and thromboxanes, whereas actions of 5-LOX and 15-LOX ultimately produce the series-five leukotrienes and resolvins. LOX enzymes are also responsible for the DHA-derived resolvins and eventual production of neuroprotectins [ 15 , 26 ] (see Figure 2 ).
Recommended and average intakes and sources of omega-3 and omega-6 fatty acids
No dietary reference intake has been established for EPA and DHA. Although the dietary reference intake for omega-3 ALA of 1.1 g/day for women [ 27 ] is achieved by the average intake in the United States of 1.3 g/day, this is only about 1/10 of the 13 to 15 g daily intake of omega-6 LA [ 28 ].
Given the general health benefits increasingly recognized for EPA and DHA, many organizations have made recommendations for direct intake of 200 to 500 mg/day EPA + DHA for adult general health in the form of fish or fish oil, krill oil, or algae oil supplements (see Table 1 ). Average intakes of EPA and DHA combined, usually from fish or supplements, is ~100 mg/day or 0.1 to 0.2% of calories. Intake of AA is ~250 mg/day, generally from eggs, meat, and poultry [ 21 , 25 , 28 ].
Recommended intakes of EPA + DHA by cohort and organization
CHD, coronary heart disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; TG, triglyceride.
The omega-3 fatty acid intakes recommended for healthy individuals are not likely to be effective in chronic inflammatory conditions, given the level of omega-6 fatty acids in our diets [ 15 , 23 , 25 , 28 ]. If the ratio of EPA + DHA to AA in blood or tissue is the key factor [ 25 , 28 ], an intake of ~2 to 3 g/day combined EPA and DHA, or at least 2% of calories, is likely to be needed to result in a tissue level ratio of EPA + DHA to AA that approaches or exceeds unity. Doses generally exceeding 2 g/day combined EPA + DHA are needed to reduce prostaglandin E 2 levels [ 26 ] and doses of 3 to 3.5 g/day combined EPA + DHA are most often used in the treatment of hypertriglyceridemia or inflammatory disorders such as rheumatoid arthritis [ 15 , 29 ]. No tolerable upper limit has been set for EPA and DHA, although the US Food and Drug Administration recognizes doses of up to 3 g/day as safe and the European Safety Union up to 5 g/day as safe [ 30 ]. Side effects of fish oil supplements or EPA + DHA ethyl esters include fishy burps, dyspepsia, gas, and diarrhea [ 15 , 29 ].
Primary sources of EPA and DHA are fish and supplements, which vary dramatically in their content. DHA is generally present in equal or higher amounts than EPA in seafood but the total amount of EPA and DHA as well as the ratios of EPA to DHA vary by supplement, and in many over-the-counter supplements EPA may be almost twice as high as DHA (see Tables 2 and 3 ). Fatty cold water fish such as salmon, herring, and mackerel have the highest levels of DHA and EPA, with lower levels in shellfish and many popular freshwater fish. A total of 2.4 g EPA + DHA can be obtained from a 4 oz (114 g) serving of wild Atlantic salmon but one would have to eat 8 oz (227 g) canned pink salmon, 1 lb (0.45 kg) halibut, or 5 lb (2.27 kg) shrimp to obtain the same amount (see Table 2 ) [ 31 ].
Dietary sources of EPA + DHA
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Amounts of EPA and DHA in commonly available supplements
How might EPA and DHA act to prevent breast cancer? Preclinical mechanistic studies
Most of the work assessing how EPA and DHA might work to reduce breast cancer risk has been performed in in vitro or in transgenic mouse models and is far from conclusive. However, the predominant mechanisms are thought to be: a reduction in proinflammatory eicosanoids and an increase in inflammation-resolving derivatives as detailed previously (Figure 2 ); a reduction in oncogenic protein signaling through disruption of plasma membrane lipid rafts; a reduction in cytokine production; and an increase in apoptosis following activation of plasma membrane GRP120 protein receptor, which along with activation of peroxisome proliferator-activated receptor gamma blocks nuclear factor-κB translocation to the nucleus [ 8 , 9 ].
EPA and DHA disrupt lipid rafts, sphingolipid/cholesterol-enriched microdomains of plasma membranes that optimize signaling by concentrating proteins. Lipid rafts are particularly important for several tyrosine kinase receptors, and reduction in epidermal growth factor receptor and human epidermal growth factor-2 receptor level and activation has been demonstrated in transformed and malignant cells [ 10 - 12 ]. A decrease in epidermal growth factor receptor and human epidermal growth factor-2 signaling would be expected to reduce proliferation, and a decrease in Ki-67 has indeed been observed in benign and malignant mammary tissue after EPA and DHA supplementation in most preclinical models [ 32 - 35 ].
Nuclear factor-κB nuclear translocation and signaling is reduced via the agonist effects of EPA and DHA on peroxisome proliferator-activated receptor gamma as well as interaction with the G protein receptor GPR120, with expected reduction in inhibitors of apoptosis as well as cytokines, adhesion molecules, and metalloproteases [ 9 ]. Additional preclinical studies suggest that EPA and DHA increase expression of BRCA1/2, phosphatase and tensin homolog (PTEN), and other proteins associated with cell cycle control and DNA repair [ 32 , 36 , 37 ].
Preclinical models of mammary cancer prevention
Studies in rodent models find that increasing the ratio of total omega-3:omega-6 in feed to >1 (usually with EPA + DHA between 8 and 25% of calories) reduces mammary cancer incidence and multiplicity by 20 to 35% [ 22 , 32 , 37 - 41 ] . Reductions in tumor incidence have been observed in the estrogen receptor-negative MMTV-HER-2/neu transgenic mice [ 39 , 40 ], the estrogen receptor-positive NMU rat model [ 32 , 37 ], and the estrogen receptor-positive DMBA rat model [ 41 - 43 ]. The minimum dose of marine omega-3 fatty acids for effect is not clear and may vary by animal model, source of EPA and DHA (fish oil versus ethyl esters), and total amount and type of fat in the diet. Other important experimental conditions include when in an animal’s lifespan supplementation is started (younger may be more protective than older), and whether agent is added to feed or administered by gavage as omega-3 fatty acids are readily oxidized once exposed to light [ 38 ]. Several preclinical studies suggest that EPA/DHA supplementation may be most optimal for prevention of estrogen receptor-positive breast cancer when used with another chemoprevention agent such as vitamin D [ 41 ], a selective estrogen receptor modulator [ 43 ], or celecoxib [ 42 ].
Human studies
Results of case–control and cohort studies have to date been variable, probably reflecting the heterogeneity of cohorts, methods used to assess omega-3 and omega-6 exposure, time from exposure when measures were taken, dose, and response endpoint.
Pharmacodynamics
Omega-3 and omega 6 fatty acids are incorporated at different rates in different tissues and tissue components. Levels as a percentage of total fatty acids vary tremendously between tissues/organs although, with supplementation, levels of EPA and DHA rise in a fairly proportional manner [ 44 ]. Substantial increases in monocyte membrane DHA and EPA and decreases in monocyte AA may be seen as early as 1 week after beginning supplementation and do not change dramatically over the ensuing several weeks [ 9 , 26 ]. The time to EPA maximum uptake is ~2 weeks in plasma triglycerides, 3 weeks in serum cholesterol esters, ~2 months in red blood cells (RBCs), and >12 months for most types of adipose tissue. The highest levels of EPA and DHA in the blood are generally in the RBC membranes (RBC phospholipids), plasma phospholipids, and cholesterol esters and platelets, although mononuclear cells also contain appreciable amounts [ 45 ]. The concentration of EPA and DHA in subcutaneous or breast adipose is 1/10 or less of that in the blood compartments [ 44 ]. DHA is generally much higher than EPA in most body organs, including the brain and retina, but its incorporation into RBCs lags behind EPA [ 26 , 28 , 29 , 46 ]. Women generally have higher levels of EPA and DHA than men following equivalent dosing, and older women have higher levels than younger women [ 45 ]. Individuals who take fish oil supplements tend to take them daily whereas consumption of fish may be more intermittent. Browning and colleagues determined in a 12-month study of adults taking identical weekly doses of EPA + DHA that those taking continuous daily doses had higher EPA and DHA levels in monocytes and platelets than those taking intermittent doses [ 47 ].
For clinical trials, chronic exposure is generally assessed by measuring EPA, DHA, and AA RBC phospholipids, although some investigators feel that monocyte or platelet phospholipid measures are superior to those in RBCs [ 29 ].
Case–control studies
Results of case–control studies, particularly when questionnaires are used as the primary measure of exposure, are mixed, probably reflective of the accuracy of recall and food frequency questionnaires in estimating dietary intake. There is no significant association between total fish intake and breast cancer particularly in populations where total fish and fatty fish consumption tends to be low [ 48 , 49 ]. EPA and DHA content varies tremendously by type of fish, which may not be well specified in the questionnaires. However, two case–control studies (one from Mexico and another from the United States) using dietary recall instruments suggest breast cancer risk reduction in premenopausal women with higher intakes of omega-3 fatty acids from diet and supplements [ 50 , 51 ].
Measurement of the fatty acid composition in blood cell membranes (phospholipids) and adipose is thought to be a good indicator of chronic exposure to omega-3 and omega-6 fatty acids and thus avoids some of the problems with dietary recall. A nested case–control study within a prospective cohort of women in Shanghai China, a population with relatively high fish intake, found that total omega-3 fatty acids and EPA in red cells were associated with significantly lower risk of proliferative breast disease and breast cancer [ 52 , 53 ]. Similar findings were reported in a Japanese cohort where total omega-3, EPA, and DHA in red cells was inversely associated with breast cancer risk [ 54 ]. Another case–control study suggested reduced risk of breast cancer with higher ratios of omega-3 to omega-6 in breast adipose [ 55 ]. No association was reported between the risk biomarker mammographic breast density and omega-3 fatty acids [ 56 ].
Prospective cohort studies of omega-3 fatty acids and breast cancer risk
A meta-analysis of 16 prospective cohort studies examining marine omega-3 intake suggests a reduction in breast cancer risk when individuals with highest intakes are compared with those with lowest intakes of marine PUFA (EPA, docosapentaenoic acid, and DHA) in the diet or the diet plus supplements [ 57 ]. The method for assessment of marine PUFA exposure varied from dietary questionnaire to blood or tissue n-3 PUFA assessment. Overall the relative risk for highest exposure was 0.86 (95% confidence interval, 0.97 to 1.03). The affect appeared strongest for marine PUFA in postmenopausal women but there were fewer premenopausal women studied [ 57 ]. In three of the largest studies – the Singapore Chinese Health Study [ 58 ], the Japanese Collaborative Cohort Study [ 59 ], and the Vitamins and Lifestyle (VITAL) study from western Washington state [ 60 ] – there was a significant reduction in relative risk in the individual trials ranging from 31 to 50%. Current use of fish oil supplements (generally 300 mg EPA + DHA or more per capsule) in the VITAL trial in women aged >50 years was associated with a 32% reduction in risk of breast cancer (hazard ratio, 0.68; 95% confidence interval, 0.50 to 0.92) [ 60 , 61 ].
Eight studies were available for dose–response analysis, which showed that a 0.1 g/day increment and/or 0.1% of energy intake increments were associated with a 5% reduction in breast cancer risk [ 57 ]. In this same meta-analysis no association was observed between total fish intake, total PUFA or ALA (the shorter chain omega-3 fatty acid) intake and breast cancer risk [ 57 ].
A recent meta-analysis combined six prospective nested case–control studies and five cohort studies in which the omega-3:omega-6 intake ratio and/or omega-3:omega-6 ratio in serum phospholipids was known. There were over 274,000 women, and more than 8,300 breast cancer events. Their conclusions were that each 1/10 increment in the dietary n-3:n-6 ratio was associated with a 6% reduction in breast cancer risk, and amongst US subjects each 1/10 increment in the serum n-3:n-6 phospholipid ratio was associated with a 27% reduction in breast cancer risk [ 49 ].
Interventional studies for primary prevention of breast cancer
Although one is not likely to achieve EPA + DHA intake in human trials at the same percentage of calories as in animal prevention trials, doses of EPA and DHA ethyl esters up to ~7 g/day given to healthy women are well tolerated [ 62 ]. A dose of 3.4 g/day DHA + EPA ethyl esters, providing ~ 2% of calories, is US Food and Drug Administration approved for treatment of hypertriglyceridemia. Importantly, this dose should produce an EPA + DHA:AA ratio approaching equivalence and thus provide an anti-inflammatory effect.
Human studies in healthy individuals show little effect of marine PUFA on blood inflammatory biomarkers, although a recent randomized trial in healthy young adults given 0, 300, 600, 900, or 1,800 mg/day EPA + DHA for 5 months showed a marginal decrease in serum tumor necrosis factor alpha ( P = 0.08) but no change in interleukin-6 [ 63 ].
Human studies in inflammatory disorders show little evidence of a systemic anti-inflammatory effect such as reduction of cytokines or prostaglandin E 2 levels with doses of combined EPA + DHA less than ~3.5 g/day and/or EPA-alone doses <2.7 g/day [ 26 , 64 ]. However, experts in this area suggest that systemic measures of cytokines in inflammatory conditions are likely to be insensitive compared with measuring conditions in the tissue of interest [ 65 ].
Signori and colleagues are conducting a trial of raloxifene 30 mg, raloxifene 60 mg, Lovaza™ (GlaxoSmithKline) 4 g, Lovaza™ 4 g + raloxifene 30 mg, or no intervention in postmenopausal women with >25% breast density. No change with Lovaza™ has been found in the first 46 women in secondary endpoint blood risk biomarkers such as insulin-like growth factor I and insulin-like growth factor-binding protein 3 or the inflammatory marker high-sensitivity C-reactive protein [ 66 ].
We have completed separate pilot studies of 3.4 g/day EPA + DHA ethyl esters (4 g Lovaza™) administered for 6 months to explore effects on benign breast tissue risk biomarkers for breast cancer in premenopausal and postmenopausal women at increased risk for breast cancer. Favorable modulation of several tissue risk biomarkers for breast cancer was observed [ 67 , 68 ].
A study of particular interest is the ongoing VITAL trial ( NCT01169259 ) which aims to randomize over 28,000 men and women to vitamin D3 (2,000 IU/day), omega-3 fatty acids (840 mg EPA + DHA), both, or none, with a primary outcome of reduction in risk for cancer, stroke, and other diseases. Eligible women must be age 55 years and over.
Omega-3 fatty acids and breast cancer survivorship
There is also interest in EPA and DHA for improvement of outcomes after a diagnosis of breast cancer. Breast cancer recurrence, cardiovascular events, weight gain and obesity, bone density loss, and chemotherapy-associated cognitive impairment and peripheral neuropathy are common concerns during the survivorship period. Although there is little in the way of definitive interventional trials, we will review here some of the more interesting preliminary results.
EPA and DHA and reduction of breast cancer recurrence
Higher intakes of EPA and DHA from dietary sources were reported to be associated with a 25% reduction in breast cancer recurrence and improved overall mortality in a large cohort of over 3,000 women with early stage breast cancer followed for a median of 7 years [ 69 ]. One reason for this observation may be enhancement of at least some types of chemotherapeutic cytotoxicity, which has been reported for concomitant administration of DHA with anthracyclines [ 70 , 71 ]. This enhanced cytotoxicity probably results from alteration in membrane lipid rafts, which increases surface expression and clustering of the death receptor CD95 in mammary cancer cell lines treated with EPA and DHA and doxorubicin [ 72 ]. Improved outcome with DHA added to chemotherapy in a small phase II trial has been reported in metastatic breast cancer patients [ 73 ]. This observation raises the question of whether cardiac toxicity might also be increased by adding EPA or DHA to anthracyclines, but this does not appear to be the case at least in rats [ 74 ].
EPA and DHA to reduce cardiac events
Cardiac events are the second most common cause of mortality in women with breast cancer, and the most common cause of death for women with stage I breast cancer over the age of 65. EPA and DHA reduce triglycerides and platelet aggregation and are thought to have an anti-arrhythmic effect. EPA and DHA supplementation have been noted to be associated with reduced cardiac deaths in the general population [ 75 , 76 ]. A highly purified prescription strength form of ~3.4 g/day EPA and DHA (Lovaza™, formerly omacor, 4 g/day) is US Food and Drug Administration approved for treatment of hypertriglyceridemia and has been shown to reduce triglycerides and nonhigh-density lipoproteins to a greater extent than a statin alone in individuals with mixed dyslipidemia and triglycerides >200 mg/dl [ 77 ]. This highly purified prescription formulation has also been shown to reduce cardiac events and mortality in individuals with a prior myocardial infarction at lower doses of 1 g/day [ 77 ]. However, a recent secondary prevention trial with 1 g/day EPA and DHA compared with 1 g/day olive oil did not show any cardioprotective effect [ 78 ]. A recent meta-analysis of EPA and DHA in moderate doses also showed no benefit [ 79 ]. The cause of these discrepancies is open to speculation. Possibilities include the following: 1) a lack of additional benefit for EPA + DHA in women with cardiac disease already on optimal medical management; 2) the placebo, often olive oil, may also have cardiovascular benefit; 3) or the highly purified forms of EPA + DHA may have special properties such as lower reactive oxygen species than less purified forms of fish oil [ 80 ]. Trials such as the VITAL trial in women without a prior history of heart disease will be of great interest.
EPA and DHA to reduce bone density loss and arthralgias
Loss of bone density and increased fracture rate are a side effect of premature menopause caused by cytotoxic chemotherapy or surgical ovarian ablation in premenopausal women or use of aromatase inhibitors in postmenopausal women. EPA and DHA probably inhibit RANK ligand and osteoclast formation [ 81 ]. A small randomized pilot trial suggests that 3 g/day EPA and DHA inhibits bone reabsorption in individuals taking aromatase inhibitors [ 82 ]. The anti-inflammatory activity and beneficial effects of EPA and DHA on rheumatoid arthritis have led to a clinical trial of high-dose EPA and DHA versus placebo in women who have aromatase inhibitor-induced arthralgias. This cooperative group study of 262 women has been reported in abstract form and no benefit was observed [ 83 ]. A small randomized trial of omega-3 fatty acids to protect against taxane-induced neuropathy suggests benefit [ 84 ] and further studies are needed.
EPA and DHA to prevent insulin resistance and sarcopenic weight gain
EPA and DHA help prevent obesity and insulin resistance particularly in animal models fed a high-fat diet [ 85 , 86 ], but effects in humans have yet to be proven. Sarcopenic weight gain is common during adjuvant chemotherapy for breast cancer. The anabolic effects of EPA and DHA might help reduce muscle mass loss and weight gain during treatment and weight gain following diagnosis, but studies in this area have yet to be conducted [ 87 , 88 ]. Results of the Muscle Mass, Omega-3, Diet, Exercise and Lifestyle (MODEL) trial in healthy individuals aged >70 years examining the effects of 90 minutes of exercise weekly, vitamin D3 (2,000 IU/day) or 1 g EPA and DHA daily are awaited with interest [ 89 ].
EPA and DHA and cognition
Cognitive abnormalities are observed in 20 to 70% of women after chemotherapy depending on the agents used, intensity and duration of treatment, predisposing factors, and type and scoring of cognitive tests [ 90 , 91 ]. DHA is the most abundant PUFA in the brain and is involved in multiple functions including cell signaling, neurogenesis, neuroprotection, and learning and memory [ 18 ]. A number of epidemiologic studies show a 40 to 50% reduction in risk of multicause dementia with increased dietary intake of DHA or increased blood levels of DHA [ 19 ]. In meta-analyses, DHA supplementation improves attention, processing speed and immediate recall, learning, and memory in individuals with cognitive impairment without dementia but not in those with dementia [ 92 , 93 ]. Probable mechanisms include suppression of oxidative stress [ 94 ], decreases in proinflammatory lipid derivatives from AA, an increase in inflammation resolving and protective lipid derivatives, enhanced production of neurotransmitters [ 95 ], and reduced production and accumulation of amyloid B peptide toxin [ 19 ]. Doses of DHA administered as supplements for cognitive improvement are generally in the range of 1,800 mg/day. Studies utilizing DHA or DHA + EPA as a neuroprotectant during chemotherapy are needed.
The inflammation-resolving properties and favorable effects of EPA and DHA on oncogenic proteins, as well as on the cardiovascular, bone, and central nervous system, make them excellent candidates for primary and secondary breast cancer prevention trials for individuals at increased risk as well as breast cancer survivors. Interventional trials in these cohorts are ongoing.
Abbreviations
Arachidonic acid
Alpha linolenic acid
Cyclooxygenase
Docosahexaenoic acid
Eicosapentaenoic acid
Linoleic acid
Lipoxygenase
Polyunsaturated fatty acid
Red blood cell
Vitamins and lifestyle
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Carol J Fabian, Email: [email protected].
Bruce F Kimler, Email: [email protected].
Stephen D Hursting, [email protected].
- 1. Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19:6074–83. doi: 10.1158/1078-0432.CCR-12-2603. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Baumgarten SC, Minireview FJ. Inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol Endocrinol. 2012;26:360–71. doi: 10.1210/me.2011-1302. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol. 2006;59:972–7. doi: 10.1136/jcp.2005.031252. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Pollard J. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–30. doi: 10.1189/jlb.1107762. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. McDermott RS, Beuvon F, Pauly M, Pallud C, Vincent-Salomon A, Mosseri V, et al. Tumor antigens and antigen-presenting capacity in breast cancer. Pathobiology. 2002;70:324–9. doi: 10.1159/000071272. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5S. doi: 10.1093/ajcn/83.2.461S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012;97:73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88:43–7. doi: 10.1016/j.plefa.2012.03.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Calder PC. n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc. 2013;72:326–36. doi: 10.1017/S0029665113001031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Ravacci GR, Brentani MM, Tortelli T, Jr, Torrinhas RS, Saldanha T, Torres EA, et al. Lipid raft disruption by docosahexaenoic acid induces apoptosis in transformed human mammary luminal epithelial cells harboring HER-2 overexpression. J Nutr Biochem. 2013;24:505–15. doi: 10.1016/j.jnutbio.2012.02.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Lee EJ, Yun UJ, Koo KH, Sung JY, Shim J, Ye SK, et al. Down-regulation of lipid raft-associated onco-proteins via cholesterol-dependent lipid raft internalization in docosahexaenoic acid-induced apoptosis. Biochim Biophys Acta. 1841;2014:190–203. doi: 10.1016/j.bbalip.2013.10.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Rogers KR, Kikawa KD, Mouradian M, Hernandez K, McKinnon KM, Ahwah SM, et al. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis. 2010;31:1523–30. doi: 10.1093/carcin/bgq111. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol. 2011;668:S50–8. doi: 10.1016/j.ejphar.2011.05.085. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Wen ZH, Su YC, Lai PL, Zhang Y, Xu YF, Zhao A, et al. Critical role of arachidonic acid-activated mTOR signaling in breast carcinogenesis and angiogenesis. Oncogene. 2013;32:160–70. doi: 10.1038/onc.2012.47. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Yates CM, Calder PC, Ed RG. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–81. doi: 10.1016/j.pharmthera.2013.10.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–7. doi: 10.1016/j.biopha.2006.07.080. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Friborg JT, Melbye M. Cancer patterns in Inuit populations. Lancet Oncol. 2008;9:892–900. doi: 10.1016/S1470-2045(08)70231-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Su H-M. Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance neuroprotection. J Nutr Biochem. 2010;21:364–73. doi: 10.1016/j.jnutbio.2009.11.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Cole GM, Ma QL, Frautschy SA. Omega-3 fatty acids and dementia. Prostaglandins Leukot Essent Fatty Acids. 2009;81:213–21. doi: 10.1016/j.plefa.2009.05.015. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Simopoulos AP. Evolutionary aspects of omega-3 fatty acids in the food supply. Prostaglandins Leukot Essent Fatty Acids. 1999;60:421–9. doi: 10.1016/S0952-3278(99)80023-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–62. doi: 10.3945/ajcn.110.006643. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Rose D, Connolly J. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83:217–44. doi: 10.1016/S0163-7258(99)00026-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Simonsen N, van't Veer P, Strain JJ, Martin-Moreno JM, Huttunen JK, Navajas JF, et al. EURAMIC study. European Community Multicenter Study on Antioxidants, Myocardial Infarction, and Breast Cancer. Am J Epidemiol. 1998;147:342–52. doi: 10.1093/oxfordjournals.aje.a009456. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77:937–46. doi: 10.1016/j.bcp.2008.10.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. International Society for the Study of Fatty Acids and Lipids, ISSFAL, alpha-linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–62. doi: 10.1111/j.1365-2125.2012.04374.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Flock MR, Harris WS, Kris-Etherton PM. Long-chain omega-3 fatty acids: time to establish a dietary reference intake. Nutr Rev. 2013;71:692–707. doi: 10.1111/nure.12071. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–76S. doi: 10.1093/ajcn/83.6.1467S. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96:748–58. doi: 10.3945/ajcn.112.041343. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. European Food Safety Authority Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA) EFSA J. 2012;10:2815–82. [ Google Scholar ]
- 31. Addendum A. EPA and DHA Content of Fish Species. United States Department of Agriculture. 2004. www.health.gov/dietaryguidelines/dga2005/report/HTML/G2_Analyses.htm # omegafish. Accessed 10 Mar 2015.
- 32. Jiang W, Zhu Z, McGinley JN, El Bayoumy K, Manni A, Thompson HJ. Identification of a molecular signature underlying inhibition of mammary carcinoma growth by dietary N-3 fatty acids. Cancer Res. 2012;72:3795–806. doi: 10.1158/0008-5472.CAN-12-1047. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Manni A, Richie JP, Jr, Xu H, Washington S, Aliaga C, Cooper TK, et al. Effects of fish oil and tamoxifen on preneoplastic lesion development and biomarkers of oxidative stress in the early stages of N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. Int J Oncol. 2011;39:1153–64. doi: 10.3892/ijo.2011.1133. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Manna S, Janarthan M, Ghosh B, Rana B, Rana A, Chatterjee M. Fish oil regulates cell proliferation, protect DNA damages and decrease HER-2/neu and c-Myc protein expression in rat mammary carcinogenesis. Clin Nutr. 2010;29:531–7. doi: 10.1016/j.clnu.2009.12.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Yee LD, Agarwal D, Rosol TJ, Lehman A, Tian M, Hatton J, et al. The inhibition of early stages of HER-2/neu-mediated mammary carcinogenesis by dietary n-3 PUFAs. Mol Nutr Food Res. 2013;57:320–7. doi: 10.1002/mnfr.201200445. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Bernard-Gallon D, Vissac-Sabatier C, Antoine-Vincent D, Rio PG, Maurizis JC, Fustier P, et al. Differential effects of n-3 and n-6 polyunsaturated fatty acids on BRCA1 and BRCA2 gene expression in breast cell lines. Br J Nutr. 2002;87:281–9. doi: 10.1079/BJN2002522. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Jourdan M-L, Mahéo K, Barascu A, Goupille C, De Latour MP, Bougnoux P, et al. Increased BRCA1 protein in mammary tumours of rats fed marine ω-3 fatty acids. Oncol Rep. 2007;17:713–9. [ PubMed ] [ Google Scholar ]
- 38. Signori C, El-Bayoumy K, Russo J, Thompson HJ, Richie JP, Hartman TJ, et al. Chemoprevention of breast cancer by fish oil in preclinical models: trials and tribulations. Cancer Res. 2011;71:6091–6. doi: 10.1158/0008-5472.CAN-11-0977. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. MacLennan MB. Mammary tumor development is directly inhibited by lifelong n-3 polyunsaturated fatty acids. J Nutr Biochem. 2013;24:388–95. doi: 10.1016/j.jnutbio.2012.08.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Yee LD, Young DC, Rosol TJ, VanBuskirk AM, Clinton SK. Dietary (n-3) polyunsaturated fatty acids inhibit HER-2/neu induced breast cancer in mice independently of the PPARγ ligand rosiglitazone. J Nutr. 2005;135:983–8. doi: 10.1093/jn/135.5.983. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Chatterjee M, Janarthan M, Manivannan R, Rana A, Chatterjee M. Combinatorial effect of fish oil (Maxepa) and 1alpha,25-dihydroxyvitamin D(3) in the chemoprevention of DMBA-induced mammary carcinogenesis in rats. Chem Biol Interact. 2010;188:102–10. doi: 10.1016/j.cbi.2010.06.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Negi AK, Kansal S, Bhatnagar A, Agnihotri N. Alteration in apoptosis and cell cycle by celecoxib and/or fish oil in 7,12-dimethyl benzene (α) anthracene-induced mammary carcinogenesis. Tumour Biol. 2013;34:3753–64. doi: 10.1007/s13277-013-0959-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Manni A, Richie JP, Jr, Xu H, Washington S, Aliaga C, Bruggeman R, et al. Influence of omega-3 fatty acids on tamoxifen-induced suppression of rat mammary carcinogenesis. Int J Cancer. 2014;134:1549–57. doi: 10.1002/ijc.28490. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–22. [ PubMed ] [ Google Scholar ]
- 45. Walker CG, Browning LM, Mander AP, Madden J, West AL, Calder PC, et al. Age and sex differences in the incorporation of EPA and DHA into plasma fractions, cells and adipose tissue in humans. Br J Nutr. 2014;111:679–89. doi: 10.1017/S0007114513002985. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Kopecky J, Rossmeisl M, Flachs P, Kuda O, Brauner P, Jilkova Z, et al. n-3 PUFA: bioavailability and modulation of adipose tissue function. Proc Nutr Soc. 2009;68:361–9. doi: 10.1017/S0029665109990231. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Browning LM, Walker CG, Mander AP, West AL, Gambell J, Madden J, et al. Compared with daily, weekly n-3 PUFA intake affects the incorporation of eicosapentaenoic acid and docosahexaenoic acid into platelets and mononuclear cells in humans. J Nutr. 2014;144:667–72. doi: 10.3945/jn.113.186346. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Terry PD, Rohan TE, Wolk A. Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr. 2003;77:532–43. doi: 10.1093/ajcn/77.3.532. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Yang B, Ren XL, Fu YQ, Gao JL, Li D. Ratio of n-3/n-6 PUFAs and risk of breast cancer: a meta-analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer. 2014;14:105. doi: 10.1186/1471-2407-14-105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Chajès V, Torres-Mejía G, Biessy C, Ortega-Olvera C, Angeles-Llerenas A, Ferrari P, et al. ω-3 and ω-6 polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiol Biomarkers Prev. 2012;21:319–26. doi: 10.1158/1055-9965.EPI-11-0896. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Goodstine SL, Zheng T, Holford TR, Ward BA, Carter D, Owens PH, et al. Dietary (n-3)/(n-6) fatty acid ratio: possible relationship to premenopausal but not postmenopausal breast cancer risk in U.S. women. J Nutr. 2003;133:1409–14. doi: 10.1093/jn/133.5.1409. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Shannon J, King IB, Moshofsky R, Lampe JW, Gao DL, Ray RM, et al. Erythrocyte fatty acids and breast cancer risk: a case–control study in Shanghai. China Am J Clin Nutr. 2007;85:1090–7. doi: 10.1093/ajcn/85.4.1090. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Shannon J, King IB, Lampe JW, Gao DL, Ray RM, Lin MG, et al. Erythrocyte fatty acids and risk of proliferative and nonproliferative fibrocystic disease in women in Shanghai. China Am J Clin Nutr. 2009;89:265–76. doi: 10.3945/ajcn.2008.26077. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Kuriki K, Hirose K, Wakai K, Matsuo K, Ito H, Suzuki T, et al. Breast cancer risk and erythrocyte compositions of n-3 highly unsaturated fatty acids in Japanese. Int J Cancer. 2007;121:377–85. doi: 10.1002/ijc.22682. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Bagga D, Anders KH, Wang HJ, Glaspy JA. Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr Cancer. 2002;42:180–5. doi: 10.1207/S15327914NC422_5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Hudson AG, Reeves KW, Modugno F, Wilson JW, Evans RW, Vogel VG, et al. Erythrocyte omega-6 and omega-3 fatty acids and mammographic breast density. Nutr Cancer. 2013;65:410–6. doi: 10.1080/01635581.2013.760744. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346:f3706. doi: 10.1136/bmj.f3706. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Gago-Dominguez M, Yuan JM, Sun CL, Lee HP, Yu MC. Opposing effects of dietary n-3 and n-6 fatty acids on mammary carcinogenesis: the Singapore Chinese Health Study. Br J Cancer. 2003;89:1686–92. doi: 10.1038/sj.bjc.6601340. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Wakai K, Tamakoshi K, Date C, Fukui M, Suzuki S, Lin Y, et al. Dietary intakes of fat and fatty acids and risk of breast cancer: a prospective study in Japan. Cancer Sci. 2005;96:590–9. doi: 10.1111/j.1349-7006.2005.00084.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Brasky TM, Lampe JW, Potter JD, Patterson RE, White E. Specialty supplements and breast cancer risk in the VITamins And Lifestyle (VITAL) Cohort. Cancer Epidemiol Biomarkers Prev. 2010;19:1696–708. doi: 10.1158/1055-9965.EPI-10-0318. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Kris-Etherton PM, Grieger JA, Etherton TD. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids. 2009;81:99–104. doi: 10.1016/j.plefa.2009.05.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y, et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr. 2010;91:1185–94. doi: 10.3945/ajcn.2009.29036. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Flock MR, Skulas-Ray AC, Harris WS, Gaugler TL, Fleming JA, Kris-Etherton PM. Effects of supplemental long-chain omega-3 fatty acids and erythrocyte membrane fatty acid content on circulating inflammatory markers in a randomized controlled trial of healthy adults. Prostaglandins Leukot Essent Fatty Acids. 2014;91:161–8. doi: 10.1016/j.plefa.2014.07.006. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Yusof HM, Cawood AL, Ding R, Williams JA, Napper FL, Shearman CP, et al. Limited impact of 2 g/day omega-3 fatty acid ethyl esters (Omacor®) on plasma lipids and inflammatory markers in patients awaiting carotid endarterectomy. Mar Drugs. 2013;11:3569–81. doi: 10.3390/md11093569. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66:237–59. doi: 10.1017/S0029665107005472. [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Signori C, DuBrock C, Richie JP, Prokopczyk B, Demers LM, Hamilton C, et al. Administration of omega-3 fatty acids and raloxifene to women at high risk of breast cancer: interim feasibility and biomarkers analysis from a clinical trial. Eur J Clin Nutr. 2012;66:878–84. doi: 10.1038/ejcn.2012.60. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Fabian CJ, Kimler BF, Petroff BK, Zalles CM, Metheny T, Nydegger JL, et al. High dose omega-3 fatty acid supplementation modulates breast tissue biomarkers in post-menopausal women at high risk for development of breast cancer [abstract]. Cancer Res. 2013;73:P4-10-01. doi:10.1158/0008-5472.SABCS13-P4-10-01.
- 68. Fabian CF, Kimler BF, Petroff BK, Zalles CM, Metheny T, Box JA, et al. High dose omega-3 fatty acid (FA) supplementation modulates breast tissue biomarkers in pre-menopausal women at high risk for development of breast cancer [abstract]. J Clin Oncol. 2013;31 Suppl:abstract 1515.
- 69. Patterson RE. Marine fatty acid intake is associated with breast cancer prognosis. J Nutr. 2011;141:201–6. doi: 10.3945/jn.110.128777. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Kang KS, Wang P, Yamabe N, Fukui M, Jay T, Zhu BT. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010296. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Colas S, Mahéo K, Denis F, Goupille C, Hoinard C, Champeroux P, et al. Sensitization by dietary docosahexaenoic acid of rat mammary carcinoma to anthracycline: a role for tumor vascularization. Clin Cancer Res. 2006;12:5879–86. doi: 10.1158/1078-0432.CCR-06-0386. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Ewaschuk JB, Newell M, Field CJ. Docosahexanoic acid improves chemotherapy efficacy by inducing CD95 translocation to lipid rafts in ER(−) breast cancer cells. Lipids. 2012;47:1019–30. doi: 10.1007/s11745-012-3717-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101:1978–85. doi: 10.1038/sj.bjc.6605441. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Germain E, Bonnet P, Aubourg L, Grangeponte MC, Chajès V, Bougnoux P. Anthracycline-induced cardiac toxicity is not increased by dietary omega-3 fatty acids. Pharmacol Res. 2003;47:111–7. doi: 10.1016/S1043-6618(02)00287-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Calder PC, Yaqoob P. Marine omega-3 fatty acids and coronary heart disease. Curr Opin Cardiol. 2012;27:412–9. doi: 10.1097/HCO.0b013e328353febd. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Baum SJ, Kris-Etherton PM, Willett WC, Lichtenstein AH, Rudel LL, Maki KC, et al. Fatty acids in cardiovascular health and disease: a comprehensive update. J Clin Lipidol. 2012;6:216–34. doi: 10.1016/j.jacl.2012.04.077. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Bays H. Clinical overview of omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids. Am J Cardiol. 2006;98:71i–6i. doi: 10.1016/j.amjcard.2005.12.029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. ORIGIN Trial Investigators. Bosch J, Gerstein HC, Dagenais GR, Díaz R, Dyal L, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–18. doi: 10.1056/NEJMoa1203859. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–33. doi: 10.1001/2012.jama.11374. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Harris WS, Shearer GC. Omega-6 fatty acids and cardiovascular disease: friend or foe? Circulation. 2014;130:1562–4. doi: 10.1161/CIRCULATIONAHA.114.012534. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Bonnet N, Somm E, Rosen CJ. Diet and gene interactions influence the skeletal response to polyunsaturated fatty acids. Bone. 2014;68C:100–7. doi: 10.1016/j.bone.2014.07.024. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Hutchins-Wiese HL, Picho K, Watkins BA, Li Y, Tannenbaum S, Claffey K, et al. High-dose eicosapentaenoic acid and docosahexaenoic acid supplementation reduces bone resorption in postmenopausal breast cancer survivors on aromatase inhibitors: a pilot study. Nutr Cancer. 2014;66:68–76. doi: 10.1080/01635581.2014.847964. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Hershman DL, Unger JM, Crew KD, Dakhil SR, Awad D, Greenlee H, et al. Omega-3 fatty acids for aromatase inhibitor-induced musculoskeletal symptoms in women with early-stage breast cancer (SWOG S0927) [abstract]. J Clin Oncol. 2014;32:5 s Suppl: abstract 9532.
- 84. Ghoreishi Z, Esfahani A, Djazayeri A, Djalali M, Golestan B, Ayromlou H, et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer. 2012;12:355. doi: 10.1186/1471-2407-12-355. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Flock MR, Rogers CJ, Prabhu KS, Kris-Etherton PM. Immunometabolic role of long-chain omega-3 fatty acids in obesity-induced inflammation. Diabetes Metab Res Rev. 2013;29:431–45. doi: 10.1002/dmrr.2414. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138–46. doi: 10.1097/MCO.0b013e3283218299. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Buckley JD, Howe PRC. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity – a review. Nutrients. 2010;2:1212–30. doi: 10.3390/nu2121212. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Di Girolamo FG, Situlin R, Mazzucco S, Valentini R, Toigo G, Biolo G. Omega-3 fatty acids and protein metabolism: enhancement of anabolic interventions for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17:145–50. doi: 10.1097/MCO.0000000000000032. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. McDonald C, Bauer J, Capra S, Coll J. The muscle mass, omega-3, diet, exercise and lifestyle (MODEL) study – a randomised controlled trial for women who have completed breast cancer treatment. BMC Cancer. 2014;14:264. doi: 10.1186/1471-2407-14-264. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–56. doi: 10.1002/cncr.25098. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102–13. doi: 10.3109/09540261.2013.864260. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6:456–64. doi: 10.1016/j.jalz.2010.01.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Mazereeuw G, Lanctôt KL, Chau SA, Swardfager W, Herrmann N. Effects of ω-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol Aging. 2012;33:1482.e17-29. doi: 10.1016/j.neurobiolaging.2011.12.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Ye S, Tan L, Ma J, Shi Q, Li J. Polyunsaturated docosahexaenoic acid suppresses oxidative stress induced endothelial cell calcium influx by altering lipid composition in membrane caveolar rafts. Prostaglandins Leukot Essent Fatty Acids. 2010;83:37–43. doi: 10.1016/j.plefa.2010.02.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75:259–69. doi: 10.1016/j.plefa.2006.07.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (638.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

Preventing Cancer
Although 1 in 5 men and 1 in 6 women worldwide develop some type of cancer during their lifetime, those diagnosed are living longer than ever, thanks to screening and early detection, vaccinations , and improvements in treatment. However, even for cancers with effective treatment options, prevention has the greatest potential to reduce the burden of cancer in the general population. [1]
Because each person is exposed to unique environmental and lifestyle factors, cancer risk can vary. Although some factors cannot be controlled (such as inherited genetic mutations), there is a range of modifiable environmental and lifestyle factors that can help reduce the risk of developing cancer.
According to the World Health Organization (WHO), a 30-40% cancer burden can be attributed to lifestyle risk factors such as tobacco smoking, alcohol consumption, a diet low in fruit and vegetables, overweight and obesity, and physical inactivity. [2] In a 2018 report by the World Cancer Research Fund (WCRF) and the American Institute of Cancer Research (AICR), not-for-profit organizations that lead a network of cancer prevention charities with a global reach, 10 cancer prevention recommendations on diet and nutrition were developed. These recommendations were based on the continuous update project of evidence in cancer research, which summarizes current evidence with relevant papers from randomized controlled trials and cohort studies. [3] Taken together, they promote a lifestyle consisting of a healthy dietary pattern, physical activity, and weight management. This may not only help reduce the risk of cancer but may also contribute to the prevention of obesity and other chronic diseases such as type 2 diabetes and cardiovascular disease.
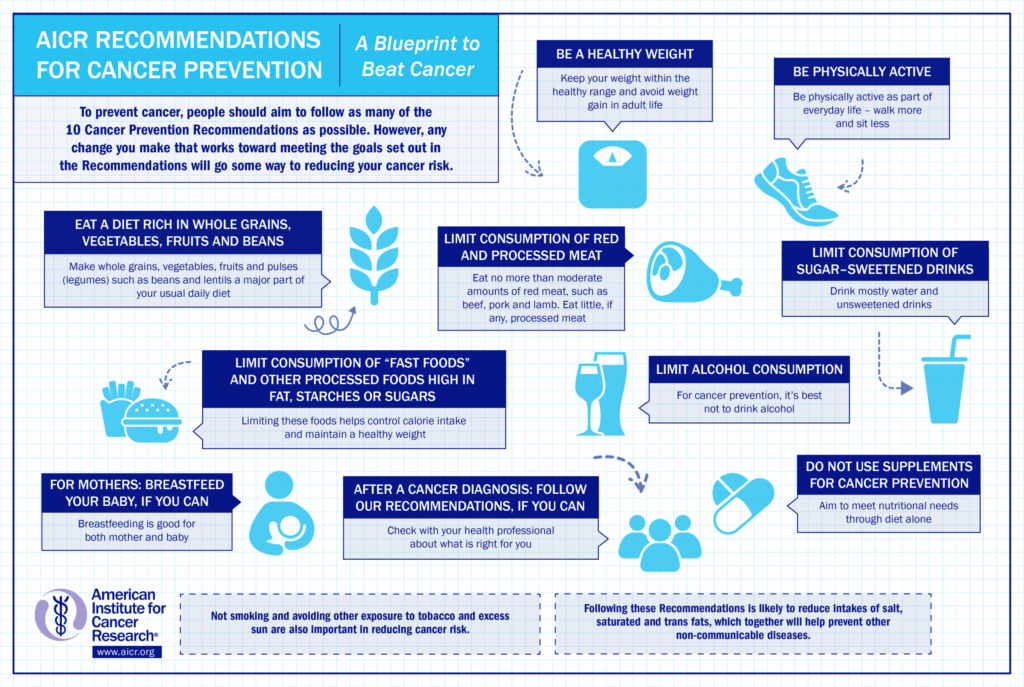
Here is a closer look at some of their recommendations:
Maintain a healthy weight
In a meta-analysis conducted by the WCRF/AICR, there is convincing evidence that carrying extra fat mass, marked by a higher body mass index (BMI), greater waist circumference, and greater waist-to-hip ratio, significantly increases the risk of several cancers: [4]
- BMI measures one’s weight in relation to height. A BMI between 18.5-25 is classified as normal, 25.1-29.9 overweight, and 30 or higher obese. Each increase of 5 points in BMI was associated with a 50% higher risk of endometrial cancer, 48% higher risk of esophageal adenocarcinoma, 30% higher risk of kidney cancer, 30% higher risk of liver cancer, 12% higher risk for postmenopausal breast cancer, 10% higher risk of pancreatic cancer, and 5% higher risk of colorectal cancer.
- Each 4-inch increase in waist circumference was shown to increase the risk of esophageal adenocarcinoma by 34%, pancreatic cancer by 11%, postmenopausal breast cancer by 11%, kidney cancer by 11%, endometrial cancer by 5%, and colorectal cancer by 2%.
- A waist-to-hip ratio (WHR) measures one’s waist size divided by hip size. People who carry more weight in the belly (apple shape) are at higher risk for cancer and other chronic diseases than those who carry more weight in the hips (pear shape). The WHO recommends a healthy WHR to be 0.9 or less in men and 0.85 or less in women. Each 0.1 unit increase in waist-hip ratio significantly increased the risk of esophageal adenocarcinoma by 38%, kidney cancer by 26%, endometrial cancer by 21%, pancreatic cancer by 19%, postmenopausal breast cancer by 10%, and colorectal cancer by 2%.
Significant increasing weight in adult life is a convincing cause of postmenopausal breast cancer and endometrial cancer.
- Each 11-pound increase in weight gain during adulthood was significantly associated with a 16% higher risk of endometrial cancer, and 6% higher risk of postmenopausal breast cancer.
Incidence of obesity-related cancers is also rising in young adults . Researchers from the American Cancer Society collected data from 25 state cancer registries for people ages 25-84 years diagnosed with any cancer from 1995 to 2014. [5] Over this time period, incidence rates of several obesity-related cancers—including colorectal, kidney, and pancreatic—increased significantly in the youngest age group, ages 25-49 years (with the sharpest increases in progressively younger ages). Although the incidence of these cancers also rose in older age groups, the rate of increase was much smaller.
Be physically active

There is evidence from a 2009 meta-analysis of 52 epidemiologic studies showing that the most physically active individuals had a 24% lower risk of colon cancer than those who were the least physically active. [6] A 2013 meta-analysis of 31 prospective studies showed that the average breast cancer risk reduction associated with physical activity was 12%, and the protective effect was stronger for postmenopausal women. [7] After menopause, women who increase their physical activity may also have a lower risk of breast cancer than women who do not. [8]
Sedentary behaviors, such as spending an extended amount of time sitting, reclining, or lying down, may also increase cancer risk. [9] The review of research on sedentary behavior and risk of endometrial, colon, and lung cancers found that the highest versus lowest levels of sedentary time increased risks of these cancers by a statistically significant range of 20-35%. [10]
Eat a healthful diet

- Limit alcohol consumption. [13] There is strong evidence that consumption of alcoholic drinks is a cause of cancers of the mouth, pharynx and larynx, esophagus (squamous cell carcinoma), liver, colorectum, and breast (particularly postmenopause). Every 10 grams of alcohol (as ethanol) consumed per day elevated the risks of these cancers by 4-25%. The evidence shows that alcoholic drinks of all types have a similar impact on cancer risk. This recommendation therefore covers all types of alcoholic drinks, whether beer, wine, spirits (liquors), or any other alcoholic drinks, as well as other alcohol sources.
- Eat a diet rich in whole grains, vegetables, fruit, and beans. [14] Make whole grains , vegetables , fruit , and pulses (legumes) such as beans and lentils a major part of your daily diet. There is strong evidence that eating whole grains protects against colorectal cancer, and that eating foods containing dietary fiber protects against colorectal cancer, weight gain, overweight, and obesity, which, as described above, increases the risk of many cancers.
- Limit “fast” foods. [15] Fast foods are readily available convenience foods that tend to be energy-dense and are often consumed in large portions. Most of the evidence on fast foods is from studies looking at burgers, fried chicken, French fries, and high-calorie drinks (containing sugar, such as soda; or unhealthy fats, such as shakes). There is strong evidence that diets containing higher amounts of fast foods and other processed foods high in unhealthy fats, starches, or sugars, as well as consuming a “Western type” diet (characterized by a high amount of added sugars, meat, and fat), are causes of weight gain, overweight, and obesity, which are a risk factor for many cancers.
- Limit red and processed meat . [16,17] Red meat includes all types of muscle meat from a mammal, including beef, veal, pork, lamb, mutton, horse, and goat. Processed meat has been transformed through salting, curing, fermentation, smoking, or other processes to enhance flavor or improve preservation. Although these products are often made from red meat (i.e., ham, salami, bacon, and some sausages such as frankfurters and chorizo), other meats can also be processed (i.e., turkey bacon, chicken sausage, and deli-sliced chicken). While both red and processed meat were suggested to increase the risk of a number of other cancers , the evidence was most convincing for colorectal cancer. The risk of colorectal cancer increased by 16% with every 50g/day of processed meat intake, and by 12% with every 100g/day of red meat intake. Because meat can be a valuable source of nutrients, in particular protein, iron, zinc, and vitamin B12, the recommendation is to limit rather than completely avoid minimally processed red meat. However, poultry and seafood are generally healthier sources of protein as well as many of these other nutrients. Very little, if any, processed meat should be consumed.
- Limit sugar-sweetened drinks. [18] There is convincing evidence that sugar-sweetened drinks is a cause of weight gain, overweight, and obesity in both children and adults, especially when consumed frequently or in large portions. As noted above, obesity increases the risk of many cancers.
Avoiding tobacco and excess sun exposure
The recommendations also emphasize that not smoking and avoiding other exposure to tobacco and excess sun are also important in reducing cancer risk.
Cancer survivorship
Although evidence is not strong enough to reach firm conclusions, there are indications of links between lifestyle factors and cancer survivorship with improved quality of life and longer survival, especially for more common cancers. For example, maintaining a healthy weight, being physically active, eating foods containing fiber, and having a lower intake of saturated fat appear to lead to better survival after a breast cancer diagnosis.[14] Unless otherwise advised, and if they can, all cancer patients and survivors are advised to follow the WCRF/AICR Cancer Prevention Recommendations as long as possible after the acute stage of treatment.
These recommendations are also helpful for managing or preventing other chronic diseases after a cancer diagnosis. A study of survivors of the 20 most common cancers revealed that, even after controlling for the overlapped risk factors for cancer and cardiovascular disease such as excessive weight and smoking, survivors of most site-specific cancers had an increased risk for cardiovascular diseases compared with that of the general population. [15] Evidence has shown that certain cancer treatments, for example anthracycline and trastuzumab, can lead to a higher risk of disease and death from cardiovascular complications. [16-18]
- Cancer: Overview, Types, and Risk Factors
- Prevention Information and Cancer Fact Finder Tool from the Zhu Family Center for Global Cancer Prevention at the Harvard Chan School of Public Health
- WHO – International Agency for Research on Cancer. “Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018.” https://www.who.int/cancer/PRGlobocanFinal.pdf
- Ullrich A. Cancer Control: Knowledge Into Action: WHO Guide for Effective Programmes . World Health Organization, 2007.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018 . “ Recommendations and public health and policy implications. “
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018 . “ Body fatness and weight gain and the risk of cancer. “
- Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. The Lancet Public Health . 2019 Mar 1;4(3):e137-47.
- Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. British journal of cancer . 2009 Feb;100(4):611-6.
- Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast cancer research and treatment . 2013 Feb;137(3):869-82.
- Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Archives of internal medicine . 2010 Oct 25;170(19):1758-64.
- Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SF, Altenburg TM, Chinapaw MJ. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. International Journal of Behavioral Nutrition and Physical Activity . 2017 Dec;14(1):1-7.
- 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services, 2018.
- Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. Journal of the Academy of Nutrition and Dietetics . 2015 May 1;115(5):780-800.
- Grosso G, Bella F, Godos J, Sciacca S, Del Rio D, Ray S, Galvano F, Giovannucci EL. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutrition reviews . 2017 Jun 1;75(6):405-19.
- Ervik M, Lam F, Ferley J, et al. Cancer Today. 2016. International Agency for Research on Cancer. Available from http://gco.iarc.fr/taody . Accessed on 2019/4/15.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018 . “ Diet, nutrition, physical activity and breast cancer survivors. “
- Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, dos-Santos-Silva I, Smeeth L, Bhaskaran K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. The Lancet . 2019 Sep 21;394(10203):1041-54.
- Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, Piña IL. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation . 2018 Feb 20;137(8):e30-66.
- Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, Habel LA. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. Journal of the National Cancer Institute . 2012 Sep 5;104(17):1293-305.
- McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovascular drugs and therapy . 2017 Feb 1;31(1):63-75.
Last reviewed March 2021
Online Help
Our 24/7 cancer helpline provides information and answers for people dealing with cancer. We can connect you with trained cancer information specialists who will answer questions about a cancer diagnosis and provide guidance and a compassionate ear.
Chat live online
Select the Live Chat button at the bottom of the page
Call us at 1-800-227-2345
Available any time of day or night
Our highly trained specialists are available 24/7 via phone and on weekdays can assist through online chat. We connect patients, caregivers, and family members with essential services and resources at every step of their cancer journey. Ask us how you can get involved and support the fight against cancer. Some of the topics we can assist with include:
- Referrals to patient-related programs or resources
- Donations, website, or event-related assistance
- Tobacco-related topics
- Volunteer opportunities
- Cancer Information
For medical questions, we encourage you to review our information with your doctor.
Prostate Cancer
- What Is Prostate Cancer?
- Key Statistics for Prostate Cancer
- What’s New in Prostate Cancer Research?
- Prostate Cancer Risk Factors
- What Causes Prostate Cancer?
- Genetic Counseling and Testing for Prostate Cancer Risk
Can Prostate Cancer Be Prevented?
- Can Prostate Cancer Be Found Early?
- Screening Tests for Prostate Cancer
- American Cancer Society Recommendations for Prostate Cancer Early Detection
- Insurance Coverage for Prostate Cancer Screening
- Signs and Symptoms of Prostate Cancer
- Tests to Diagnose and Stage Prostate Cancer
- Prostate Cancer Stages
- Risk Groups and Lab Tests to Help Determine Risk from Localized Prostate Cancer
- Survival Rates for Prostate Cancer
- Questions to Ask About Prostate Cancer
- Observation or Active Surveillance for Prostate Cancer
- Surgery for Prostate Cancer
- Radiation Therapy for Prostate Cancer
- Cryotherapy, HIFU, and Other Ablative Treatments for Prostate Cancer
- Hormone Therapy for Prostate Cancer
- Chemotherapy for Prostate Cancer
- Immunotherapy for Prostate Cancer
- Targeted Drug Therapy for Prostate Cancer
- Treatments for Prostate Cancer Spread to Bones
- Considering Treatment Options for Early Prostate Cancer
- Initial Treatment of Prostate Cancer, by Stage and Risk Group
- Following PSA Levels During and After Prostate Cancer Treatment
- Treating Prostate Cancer That Doesn’t Go Away or Comes Back After Treatment
- Living as a Prostate Cancer Survivor
- If You Have Prostate Cancer
- Prostate Cancer Videos
- Prostate Cancer Quiz
There is no sure way to prevent prostate cancer. Many prostate cancer risk factors , such as age, race, and family history, can’t be controlled. But there are some things you can do that might lower your risk of prostate cancer.
Body weight, physical activity, and diet
Vitamin, mineral, and other supplements.
The effects of body weight, physical activity, and diet on prostate cancer risk aren’t completely clear, but there are things you can do that might lower your risk.
Some studies have found that men with excess body weight have a higher risk of developing advanced prostate cancer or prostate cancer that is more likely to be fatal.
Although not all studies agree, several have found a higher risk of prostate cancer in men whose diets are high in dairy products and calcium .
For now, the best advice about diet and activity to possibly reduce the risk of prostate cancer is to:
- Get to and stay at a healthy weight.
- Be physically active.
- Follow a healthy eating pattern, which includes a variety of colorful fruits and vegetables and whole grains, and avoids or limits red and processed meats, sugar-sweetened beverages, and highly processed foods.
It may also be sensible to limit calcium supplements and to not get too much calcium in the diet. (This does not mean that men who are being treated for prostate cancer should not take calcium supplements if their doctor recommends them.)
To learn more, see the American Cancer Society Guideline for Diet and Physical Activity for Cancer Prevention .
Vitamin E and selenium: Some early studies suggested that taking vitamin E or selenium supplements might lower prostate cancer risk.
But a large study known as the Selenium and Vitamin E Cancer Prevention Trial (SELECT) found that neither vitamin E nor selenium supplements lowered prostate cancer risk . In fact, men in the study taking the vitamin E supplements were found to have a slightly higher risk of prostate cancer.
Soy and isoflavones: Some early research has suggested possible benefits from soy proteins (called isoflavones) in lowering prostate cancer risk. Several studies are now looking more closely at the possible effects of these proteins.
Taking any supplement could have both risks and benefits. Before starting vitamins or other supplements, talk with your doctor.
Some drugs might help reduce the risk of prostate cancer.
5-alpha reductase inhibitors
5-alpha reductase is an enzyme in the body that changes testosterone into dihydrotestosterone (DHT), the main hormone that causes the prostate to grow. Drugs called 5-alpha reductase inhibitors , such as finasteride and dutasteride , block this enzyme from making DHT. These drugs are used to treat benign prostatic hyperplasia (BPH), a non-cancerous growth of the prostate.
Large studies of both of these drugs have tested if they might also be useful in lowering prostate cancer risk. In these studies, men taking either drug were less likely to develop prostate cancer after several years than men getting an inactive placebo.
When the results were looked at more closely, the men who took these drugs had fewer low-grade prostate cancers, but they had about the same risk of higher-grade prostate cancers, which are more likely to grow and spread. It’s not clear if these drugs can lower the risk of dying from prostate cancer, as men in these studies had similar survival rates whether or not they took one of these drugs.
These drugs can cause sexual side effects such as lowered sexual desire and erectile dysfunction (impotence), as well as the growth of breast tissue in some men. But they can help with urinary problems from BPH, such as trouble urinating and leaking urine (incontinence).
These drugs aren’t approved by the FDA specifically to help lower prostate cancer risk, although doctors can prescribe them “ off label ” for this use. Men who want to know more about these drugs should discuss them with their doctors.
Some research suggests that men who take a daily aspirin might have a lower risk of getting and dying from prostate cancer. But more research is needed to show if the possible benefits outweigh the risks. Long-term aspirin use can have side effects, including an increased risk of bleeding in the digestive tract. While aspirin can also have other health benefits, at this time most doctors don’t recommend taking it just to try to lower prostate cancer risk.
Other drugs
Other drugs and dietary supplements that might help lower prostate cancer risk are now being studied. But so far, no drug or supplement has been found to be helpful in studies large enough for experts to recommend them.

The American Cancer Society medical and editorial content team
Our team is made up of doctors and oncology certified nurses with deep knowledge of cancer care as well as editors and translators with extensive experience in medical writing.
Klein EA, Thompson IM Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA . 2011; 306:1549.
National Cancer Institute. Physician Data Query (PDQ). Prostate Cancer Prevention. 2023. Accessed at https://www.cancer.gov/types/prostate/hp/prostate-prevention-pdq on June 30, 2023.
Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin . 2020;70(4). doi:10.3322/caac.21591. Accessed at https://onlinelibrary.wiley.com/doi/full/10.3322/caac.21591 on June 30, 2023.
Sartor AO. Chemoprevention strategies in prostate cancer. UpToDate. 2023. Accessed at https://www.uptodate.com/contents/chemoprevention-strategies-in-prostate-cancer on June 30, 2023.
Sartor AO. Risk factors for prostate cancer. UpToDate. 2023. Accessed at https://www.uptodate.com/contents/risk-factors-for-prostate-cancer on June 30, 2023.
Last Revised: November 22, 2023
American Cancer Society medical information is copyrighted material. For reprint requests, please see our Content Usage Policy .
American Cancer Society Emails
Sign up to stay up-to-date with news, valuable information, and ways to get involved with the American Cancer Society.
More in Prostate Cancer
- About Prostate Cancer
- Causes, Risk Factors, and Prevention
- Early Detection, Diagnosis, and Staging
- After Treatment
Help us end cancer as we know it, for everyone.

If this was helpful, donate to help fund patient support services, research, and cancer content updates.

- SUBSCRIBE TO EMAIL
- Weather
Search location by ZIP code
Grant funds research to improve cancer outcomes for indigenous people.
Tribal nations and the University of Oklahoma have partnered in a multimillion-dollar effort to fight cancer among Native Americans.
- Copy Link Copy {copyShortcut} to copy Link copied!

GET LOCAL BREAKING NEWS ALERTS
The latest breaking updates, delivered straight to your email inbox.
>> Download the KOCO 5 App
Indigenous people in the state are more likely to get cancer and die from the disease than non-Native people, according to the OU Health Stephenson Cancer Center. In Oklahoma, American Indian and Alaska Native populations experience a 36% higher incidence of cancer and a 73% higher death rate than the rest of the U.S. population.
But a team at OU is hoping to change that with grant-funded research.
"We’re so excited about it, because we think it allows us to do something, we think, right now," Dorothy Rhoades, director of the Native American Center for Cancer Health Equity, said.
The National Institutes of Health awarded a 5-year, $17 million grant to improve cancer outcomes for Indigenous people.
Rhoades, the doctor leading the charge, said the grant focuses on three major areas of research: cancer prevention, screening and car coordination.
"If you look at some nationwide statistics, it suggests that Native American rates are lower, but a lot of those data are flawed by racial misclassification," Rhoades said. "(For) example, on my birth certificate, I wasn’t identified as American Indian."
Get the latest news stories of interest by clicking here.
OU said 16 different tribal nations are in support of the research, but they hope their work goes beyond that.
"We’re viewing this as a starting point for the future, so when five years is over, we’re hoping to have more – more scope, more reach," Rhoades said.
Top Headlines
- Motorcyclist taken to hospital after crash in southwest Oklahoma City
- Pirates take over Massachusetts house in 13-year-old's amazing Halloween display
- Possible suspension of several teaching certificates on Oklahoma Board of Education's agenda
- Oklahoma being impacted by deadly E. coli outbreak linked to McDonald’s burger
- Boy arrested, accused of shooting a 15-year-old girl near a Norman elementary school
Obesity and Cancer
What is obesity.
Obesity is a disease in which a person has an unhealthy amount and/or distribution of body fat ( 1 ). Compared with people of healthy weight, those with overweight or obesity are at greater risk for many diseases, including diabetes , high blood pressure , cardiovascular disease , stroke , and at least 13 types of cancer, as well as having an elevated risk of death from all causes ( 2 – 5 ).
To determine if someone has obesity, researchers commonly use a measure known as the body mass index (BMI). BMI is calculated by dividing a person’s weight (in kilograms) by their height (in meters) squared (commonly expressed as kg/m 2 ). BMI provides a more accurate measure of obesity than weight alone, and for most people it is a good (although imperfect) indicator of body fatness.
The National Heart Lung and Blood Institute has a BMI calculator for adults . The standard weight categories based on BMI for adults ages 20 years or older are:
The Centers for Disease Control and Prevention (CDC) has a BMI percentile calculator for children and teens . Overweight and obesity for people younger than 20 years old, whose BMI can change significantly as they grow, are based on CDC’s BMI-for-age growth charts .
Measurements that reflect the distribution of body fat are sometimes used along with BMI as indicators of obesity and disease risks. These measurements include waist circumference, waist-to-hip ratio (the waist circumference divided by the hip circumference), waist-to-height ratio, and fat distribution as measured by dual-energy X-ray absorptiometry (DXA or DEXA) or imaging with CT or PET .
These measures are used because the distribution of fat is increasingly understood to be relevant to disease risks. In particular, visceral fat—fat that surrounds internal organs—seems to be more dangerous, in terms of disease risks, than overall fat or subcutaneous fat (the layer just under the skin).
How common are obesity and severe obesity?
Obesity and severe obesity have become more common in the United States in recent years ( 7 ).
- In 2011, 27.4% of adults ages 18 or older had obesity or severe obesity.
- By contrast, in 2020, 31.9% of adults ages 18 or older had obesity or severe obesity.
The percentage of children and adolescents ages 2–19 years with obesity or severe obesity has also increased ( 6 ).
- In 2011–2012, 16.9% of 2–19-year-olds had obesity and 5.6% had severe obesity.
- By contrast, in 2017–2018, 19.3% of 2–19-year-olds had obesity and 6.1% had severe obesity.
According to the CDC, the prevalence of obesity in the United States differs among racial and ethnic groups ( 7 ). In 2020, the proportions of adults ages 18 years or older with obesity or severe obesity were:
- Non-Hispanic Black, 41.6%
- American Indian/Alaska Native, 38.8%
- Hawaiian/Pacific Islander, 38.5%
- Hispanic, 36.6%
- Non-Hispanic White, 30.7%
- Asian, 11.8%
In 2017–2018, the proportions of obesity among children and adolescents ages 2–19 years were ( 6 ):
- Mexican American, 26.9%
- Hispanic, 25.6%
- Non-Hispanic Black, 24.2%
- Non-Hispanic White, 16.1%
- Non-Hispanic Asian, 8.7%
The prevalence of obesity has increased more quickly recently, possibly due to the COVID-19 pandemic ( 8 ). CDC has state-level estimates of adult obesity prevalence in the United States .
What is known about the relationship between obesity and cancer?
Nearly all of the evidence linking obesity to cancer risk comes from large cohort studies, a type of observational study. However, data from observational studies cannot definitively establish that obesity causes cancer. That is because people with obesity or overweight may differ from people without these conditions in ways other than their body fat, and it is possible that these other differences—rather than their body fat—explain their increased cancer risk.
An International Agency for Research on Cancer (IARC) Working Group concluded that there is consistent evidence that higher amounts of body fat are associated with an increased risk of a number of cancers. The table below shows the risks reported in representative studies.
People who have a higher BMI at the time their cancer is diagnosed ( 29 ) or who have survived cancer ( 30 , 31 ) have higher risks of developing a second, unrelated cancer (a second primary cancer ).
How might obesity increase the risk of cancer?
Several possible mechanisms have been suggested to explain how obesity might increase the risks of some cancers ( 32 , 33 ).
- Fat tissue (also called adipose tissue) produces excess amounts of estrogen , high levels of which have been associated with increased risks of breast, endometrial, ovarian, and some other cancers.
- People with obesity often have increased blood levels of insulin and insulin-like growth factor -1 (IGF-1). High levels of insulin, a condition known as hyperinsulinemia, is due to insulin resistance and precedes the development of type 2 diabetes, another known cancer risk factor. High levels of insulin and IGF-1 may promote the development of colon, kidney, prostate, and endometrial cancers ( 34 ).
- People with obesity often have chronic inflammatory conditions such as gallstones or non-alcoholic fatty liver disease. These conditions can cause oxidative stress , which leads to DNA damage ( 35 ) and increases the risk of biliary tract and other cancers ( 36 ).
- Fat cells produce hormones called adipokines that can stimulate or inhibit cell growth. For example, the level of an adipokine called leptin in the blood increases with increasing body fat, and high levels of leptin can promote aberrant cell proliferation . Another adipokine, adiponectin, is less abundant in people with obesity than in people with a healthy weight and may have antiproliferative effects that protect against tumor growth.
- Fat cells may also have direct and indirect effects on other cell growth and metabolic regulators, including mammalian target of rapamycin (mTOR) and AMP-activated protein kinase.
Other possible mechanisms by which obesity could affect cancer risk include impaired tumor immunity and changes in the mechanical properties of the scaffolding tissue that surrounds developing tumors ( 37 ).
In addition to biological effects, obesity can lead to difficulties in screening and management. For example, women with overweight or obesity have an increased risk of cervical cancer compared with women of healthy weight, likely due to less effective cervical cancer screening in these individuals ( 38 ).
How many cancer cases may be due to obesity?
A nationwide cross-sectional study using BMI and cancer incidence data from the US Cancer Statistics database estimated that each year in 2011 to 2015 among people ages 30 and older, about 37,670 new cancer cases in men (4.7%) and 74,690 new cancer cases in women (9.6%) were due to excess body weight (overweight, obesity, or severe obesity) ( 39 ). The percentage of cases attributed to excess body weight varied widely across cancer types and was as high as 51% for liver or gallbladder cancer and 49.2% for endometrial cancer in women and 48.8% for liver or gallbladder cancer and 30.6% for esophageal adenocarcinoma in men.
Globally, a 2019 study found that in 2012, excess body weight accounted for approximately 3.9% of all cancers (544,300 cases), with the burden of these cancer cases higher for women (368,500 cases) than for men (175,800 cases) ( 40 ). The proportion of cancers due to excess body weight varied from less than 1% in low-income countries to 7% or 8% in some high-income Western countries and in Middle Eastern and Northern African countries.
Does losing weight lower the risk of cancer?
Most of the data about whether losing weight reduces cancer risk comes from cohort and case–control studies . Observational studies of obesity and cancer risk should be interpreted with caution because they cannot definitively establish that obesity causes cancer and people who lose weight may differ in other ways from people who do not.
Some of these studies have found decreased risks of breast, endometrial, colon, and prostate cancers among people with obesity who had lost weight. For example, in one large prospective study of postmenopausal women, intentional loss of more than 5% of body weight was associated with lower risk of obesity-related cancers, especially endometrial cancer ( 41 ). However, unintentional weight loss was not associated with cancer risk in this study.
A follow-up study of weight and breast cancer in the Women’s Health Initiative ( 42 ) found that, for women who were already overweight or obese at the beginning of the study, weight change (either gain or loss) was not associated with breast cancer risk during follow-up. However, in a study that pooled data from 10 cohorts, sustained weight loss was associated with lower breast cancer risk among women 50 years and older ( 43 ).
To better understand the relationship between weight loss among people with obesity and cancer risk, some researchers are examining cancer risk in people with obesity who have undergone bariatric surgery (surgery performed on the stomach or intestines to provide maximum and sustained weight loss). Studies have found that bariatric surgery among people with obesity, particularly women, is associated with reduced risks of cancer overall ( 44 ); of hormone-related cancers, such as breast, endometrial, and prostate cancers ( 45 ); and of obesity-related cancers, such as postmenopausal breast cancer, endometrial cancer, and colon cancer ( 46 ).
How does obesity affect cancer survivors?
Most of the evidence about obesity in cancer survivors comes from people who were diagnosed with breast, prostate, or colorectal cancer. Research indicates that obesity may worsen several aspects of cancer survivorship, including quality of life , cancer recurrence , cancer progression , prognosis (survival), and risk of certain second primary cancers ( 29 , 30 , 47 , 48 ).
For example, obesity is associated with increased risks of treatment-related lymphedema in breast cancer survivors ( 49 ) and of incontinence in prostate cancer survivors treated with radical prostatectomy ( 50 ). In a large clinical trial of patients with stage II and stage III rectal cancer, those with a higher baseline BMI (particularly men) had an increased risk of local recurrence ( 51 ). Death from multiple myeloma is 50% more likely for people with the highest levels of obesity compared with people at healthy weight ( 52 ).
Is weight loss after a cancer diagnosis beneficial for people with overweight or obesity?
Most studies of this question have focused on breast cancer. Several randomized clinical trials in breast cancer survivors have reported weight loss interventions that resulted in both weight loss and beneficial changes in biomarkers that have been linked to the association between obesity and prognosis ( 53 , 54 ).
However, there is little evidence about whether weight loss reduces the risk of breast cancer recurrence or death ( 55 ). The NCI-sponsored Breast Cancer WEight Loss (BWEL) Study , an ongoing randomized phase III trial, is examining whether participating in a weight loss program after breast cancer diagnosis affects invasive disease-free survival and recurrence in overweight and obese women ( 56 ).
What research is being done on obesity and cancer?
Many studies are exploring mechanisms that link obesity and cancer ( 34 , 57 ). One research area involves understanding the role of the microbes that live in the human gastrointestinal tract (collectively called the gut microbiota, or microbiome ) in both type 2 diabetes and obesity. Both diseases are associated with dysbiosis, an imbalance in the community of these microbes. For example, the gut microbiomes of people with obesity differ from and are less diverse than those of people of healthy weight. Imbalances in the gut microbiota are associated with inflammation , altered metabolism , and genotoxicity, which may in turn be related to cancer.
Researchers are also studying how obesity alters the tumor microenvironment , which may play a role in cancer progression . For example, studies in mouse models show that obesity (induced by feeding mice a high-fat diet) creates a competition for lipids between tumor cells and T cells that makes the T cells less effective at fighting the cancer ( 58 ).
Another area of investigation is the role of insulin receptor signaling in cancer. Many cancer cells express elevated levels of IR-A, a form of the insulin receptor that has a high affinity for insulin and related growth factors. Researchers are investigating how these factors contribute to metabolic disease and cancer and whether they may be useful targets for therapeutic interventions to prevent obesity-related cancers.
Investigators are also exploring whether the associations of obesity with cancer risk and outcomes vary by race or ethnicity ( 59 ). Also, researchers are investigating whether different cutoffs for overweight and obesity should be used for different racial/ethnic groups. For example, the World Health Organization (WHO) has suggested the alternate thresholds of 23.0 and 27.5 kg/m 2 for overweight and obesity for people of Asian ancestry ( 60 ).
The NCI Cohort Consortium is an extramural–intramural partnership that combines more than 50 prospective cohort studies from around the world with more than seven million participants. The studies are gathering information on body mass index, waist circumference, and other measures of adiposity from each cohort. The large size of the consortium will allow researchers to get a better sense of how obesity-related factors relate to less common cancers , such as cancers of the thyroid, gallbladder, head and neck, and kidney.
Another area of study is focused on developing more precise and effective interventions to prevent weight gain and weight regain after weight loss. This area of research includes two NIH-based initiatives—the Accumulating Data to Optimally Predict Obesity Treatment (ADOPT) Core Measures ( 61 ) and the Trans-NIH Consortium of Randomized Controlled Trials of Lifestyle Weight Loss Interventions ( 62 )—both of which aim to identify predictors of successful weight loss and maintenance and to incorporate information on genetic, psychosocial, behavioral, biological, and environmental factors into predictive profiles to enable more precise and, ultimately, more effective weight loss interventions.
NCI supports research on obesity and cancer risk through a variety of activities, including large cooperative initiatives, web and data resources, epidemiologic and basic science studies, and dissemination and implementation resources. For example, the Transdisciplinary Research on Energetics and Cancer (TREC) initiative supports ongoing training workshops for postdocs and early career investigators to enhance the ability to produce innovative and impactful transdisciplinary research in energetics and cancer and clinical care. The Trans-NCI Obesity and Cancer Working Group promotes the exchange of information and cross-cutting interests in obesity and cancer research within NCI by identifying and sharing state-of-the-science knowledge about obesity and cancer to document what is known and what is needed to move the science forward.

IMAGES
COMMENTS
Cancer Prevention Research publishes articles that focus on cancer prevention, preclinical, clinical, and translational research, with special attention given to molecular discoveries and an emphasis on building a translational bridge between the basic and clinical sciences. Read More About the Journal.
Cancer Prevention Overview; Research; Causes and Prevention Research For Healthy Adults, Taking Multivitamins Daily Is Not Associated with a Lower Risk of Death. Posted: June 26, 2024. ... When it comes to cancer prevention, primary care clinicians play a critical role. But there are many barriers to integrating cancer prevention into primary care.
The NCI Cancer Prevention Clinic (CPC) conducts research on cancer prevention, early detection, and screening for groups of people at high risk of cancer, including individuals with a family history of cancer or known cancer-causing mutations. The clinic supports the development of novel agents and the repurposing of therapeutic agents to ...
The Cancer Prevention and Early Detection Facts & Figures, 2023-2024 is an educational companion for "Updated Review of Major Cancer Risk Factors and Screening Test Use in the United States, with a Focus on Changes During the COVID-19 Pandemic," a scientific paper published in Cancer Epidemiology, Biomarkers, and Prevention. Updated information is provided in the 2024 Tables & Figures Only ...
The growing global burden of cancer is rapidly exceeding the current cancer control capacity. More than 19 million new cancer cases were diagnosed in 2020 worldwide, and 10 million people died of cancer.1 By 2040, that burden is expected to increase to around 30 million new cancer cases annually and 16 million deaths from cancer according to the Global Cancer Observatory.
The new IARC is the product of a collaboration between leading international scientists that describes multiple aspects of cancer research for cancer prevention. Starting with the latest trends in cancer incidence and mortality worldwide, this publication provides wide-ranging insights into cancer prevention based on the known causes of cancer ...
Cancer prevention includes treatments, procedures and lifestyle changes that aim to reduce the risk that cancer will develop in an individual. Some cancer prevention strategies are well known and ...
About Cancer Prevention Research Scope. Cancer Prevention Research publishes original studies, reviews, and commentaries in the fields of cancer prevention and interception. The journal comprises preclinical, clinical, translational, and implementation research, with special attention given to molecular discoveries and an emphasis on building translational and mechanistic bridges between the ...
The Principles and Practice of Cancer Precision Prevention Studies. Tue, November 12, 2024: 11:00a.m. ET - 12:00p.m. ET. The Division of Cancer Prevention (DCP) conducts and supports research to determine a person's risk of cancer and to find ways to reduce the risk. This.
About. The NCI Cancer Prevention Clinic (CPC) conducts research in cancer prevention, early detection and screening for groups at high risk of cancer. Housed in the NCI Center for Cancer Research, the clinic combines expertise from the Division of Cancer Prevention (DCP) with that of intramural investigators from the Center for Cancer Research ...
The revival of interest in cancer prevention research in the 1980s up to the present day was certainly accelerated by the discovery of important associations between cancer development and ...
Normally considered a disease of genetic origin, research over the last several decades has established beyond doubt that various epigenetic/environmental factors play an important role in the development and/or progression of cancer [1,2]. ... the significance of these results in relation to cancer prevention is yet to be understood ...
Scientific Scope. The National Cancer Institute Division of Cancer Prevention (DCP) is devoted to research on cancer prevention, interception, screening and early detection, and symptom science. To accomplish this, DCP provides funding and administrative support to clinical, population science, and laboratory researchers, community and ...
Building on MD Anderson's decades at the forefront of cancer prevention research, Be Well Communities uses best practices to deploy science-based cancer prevention strategies to communities with the greatest need. It brings individuals and organizations together to promote wellness and to address modifiable risk factors for cancer.
Globally, cancer prevention research is allotted an estimated 2 to 9 percent of global cancer research funding. "The biggest unknown in cancer prevention is how to sustain proven, effective, and lifesaving preventive efforts over the long run," says Howard Koh, the Harvey V. Fineberg Professor of the Practice of Public Health Leadership at ...
Online ISSN 1940-6215. Print ISSN 1940-6207. AACR Journals. Blood Cancer Discovery. Cancer Discovery. Cancer Epidemiology, Biomarkers & Prevention. Cancer Immunology Research. Cancer Prevention Research. Cancer Research.
Cancer prevention is action taken to lower the chance of getting cancer. In 2023, about 1.9 million people will be diagnosed with cancer in the United States. In addition to the physical problems and emotional distress caused by cancer, the high costs of care are also a burden to patients, their families, and to the public. By preventing cancer, the number of new cases of cancer is lowered.
This is summarized in our 10 Cancer Prevention Recommendations. Keep your weight within the healthy range and avoid weight gain in adult life. The evidence linking body fatness to cancer is overwhelming and has grown stronger over the past decade. Be physically active as part of everyday life.
Short answer - yes! For over a decade, researchers have investigated the effect of AICR's Cancer Prevention Recommendations on cancer risk and other health outcomes. Studies have shown that following AICR's diet and lifestyle recommendations lower cancer risk, risk of other chronic diseases and help people live a longer and healthier life ...
10 Cancer Prevention Recommendations - American Institute for Cancer Research. When you include the American Institute for Cancer Research in your estate plans, you make a major difference in the fight against cancer. Corporate Champions who partner with the American Institute for Cancer Research stand at the forefront of the fight against cancer.
Primary prevention trials with either risk biomarkers or cancer incidence as endpoints are underway but final results of these trials are currently unavailable. EPA and DHA supplementation is also being explored in an effort to help prevent or alleviate common problems after a breast cancer diagnosis, including cardiac and cognitive dysfunction ...
In a 2018 report by the World Cancer Research Fund (WCRF) and the American Institute of Cancer Research (AICR), not-for-profit organizations that lead a network of cancer prevention charities with a global reach, 10 cancer prevention recommendations on diet and nutrition were developed. These recommendations were based on the continuous update ...
There are ongoing programs at NCI that support prevention and early detection research in different cancers, including breast cancer. Examples include: The Cancer Biomarkers Research Group, which promotes research in cancer biomarkers and manages the Early Detection Research Network (EDRN). EDRN is a network of NCI-funded institutions that are ...
Some research suggests that men who take a daily aspirin might have a lower risk of getting and dying from prostate cancer. But more research is needed to show if the possible benefits outweigh the risks. ... Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4 ...
About Cancer Clinical Research; Research Needs People from All Backgrounds; Personal Stories; Clinical Trials Information. What Are Clinical Trials? How Clinical Trials Work; Paying for Clinical Trials; Safety and Clinical Trials; Why Participate in a Trial; Find a Clinical Trial; What are Observational Studies? Participate in Screening Studies ...
Foods that Fight Cancer - research shows that a diet filled with a variety of vegetables, fruits, whole grains, beans and other plant foods helps lower risk for many cancers. ... our expert dietitians weigh in on some well-known diet trends and the impact they have on cancer prevention. Flexitarian Diet. Lacto-Ovo Vegetarian Diet. Mediterranean ...
The National Institutes of Health awarded a 5-year, $17 million grant to improve cancer outcomes for Indigenous people. Rhoades, the doctor leading the charge, said the grant focuses on three major areas of research: cancer prevention, screening and car coordination.
Multiple kinds of genetic changes can lead to cancer. One genetic change, called a DNA mutation or genetic variant, is a change in the DNA code, like a typo in the sequence of DNA letters.. Some variants affect just one DNA letter, called a nucleotide.A nucleotide may be missing, or it may be replaced by another nucleotide.
Breast Cancer Research and Treatment 2015; 150(2):405-413. [PubMed Abstract] Brinton LA, Scoccia B, Moghissi KS, et al. Long-term relationship of ovulation-stimulating drugs to breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention 2014; 23(4):584-593. [PubMed Abstract] Practice Committee of the American Society for Reproductive Medicine.
Obesity is a disease in which a person has an unhealthy amount and/or distribution of body fat ().Compared with people of healthy weight, those with overweight or obesity are at greater risk for many diseases, including diabetes, high blood pressure, cardiovascular disease, stroke, and at least 13 types of cancer, as well as having an elevated risk of death from all causes (2 - 5).