An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Nutrition and mental health: A review of current knowledge about the impact of diet on mental health
Mateusz grajek, karolina krupa-kotara, agnieszka białek-dratwa, karolina sobczyk, martina grot, oskar kowalski, wiktoria staśkiewicz.
- Author information
- Article notes
- Copyright and License information
Edited by: Andrew Scholey, Swinburne University of Technology, Australia
Reviewed by: Monica Carrera, Spanish National Research Council (CSIC), Spain; Yu Xiao, Hunan Agricultural University, China
*Correspondence: Karolina Krupa-Kotara, [email protected]
This article was submitted to Nutrition, Psychology and Brain Health, a section of the journal Frontiers in Nutrition
Received 2022 May 14; Accepted 2022 Jul 20; Collection date 2022.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Applied psychopharmacotherapy and psychotherapy do not always bring the expected results in the treatment of mental disorders. As a result, other interventions are receiving increasing attention. In recent years, there has been a surge in research on the effects of nutrition on mental status, which may be an important aspect of the prevention of many mental disorders and, at the same time, may lead to a reduction in the proportion of people with mental disorders. This review aims to answer whether and to what extent lifestyle and related nutrition affect mental health and whether there is scientific evidence supporting a link between diet and mental health. A review of the scientific evidence was conducted based on the available literature by typing in phrases related to nutrition and mental health using the methodological tool of the PubMed database. The literature search yielded 3,473 records, from which 356 sources directly related to the topic of the study were selected, and then those with the highest scientific value were selected according to bibliometric impact factors. In the context of current changes, urbanization, globalization, including the food industry, and changes in people’s lifestyles and eating habits, the correlations between these phenomena and their impact on mental state become important. Knowledge of these correlations creates potential opportunities to implement new effective dietary, pharmacological, therapeutic, and above all preventive interventions. The highest therapeutic potential is seen in the rational diet, physical activity, use of psychobiotics, and consumption of antioxidants. Research also shows that there are nutritional interventions that have psychoprotective potential.
Keywords: nutrition, mental health, diet, psychology of food, eating behavior
Inherent in urbanization and the accompanying technological and cultural development, the rush of life, the pursuit of self-actualization, and the resulting overstimulation and lack of time, affect the change in eating habits and the consumption of high-calorie and processed foods ( 1 ). We can consider them as factors influencing the development of civilization diseases, important from the point of view of public health. Among them, we cannot forget about depressive and anxiety disorders that are becoming a global epidemic ( 2 ). The number of people requiring assistance from a mental health professional is steadily increasing in Poland and worldwide. According to the International Health Metrics Evaluation (IHME), at the end of 2017, 13% of the world population suffered from mental disorders ( 3 ). The Wittchen et al. study shows that mental disorders affect 38% of the European population ( 4 ). By the end of 2019, about 1.6 million people in Poland had received psychiatric treatment ( 5 ). The situation was not improved by the COVID-19 pandemic and related sanitary restrictions, which led to the isolation of many people, with feelings of insecurity, sadness, anxiety, and misinformation ( 6 ). All this has made psychological and psychiatric help the most sought-after form of health support today. There are only about 4,300 practicing psychiatrists in Poland ( 7 ). Even fewer, only 455, are practicing child psychiatry specialists ( 8 ). Statistics are believed to be better in the psychological and psychotherapeutic support sector, although public opinion is still divided about this form of support. Moreover, registers of psychologists and psychotherapists are not common. The described phenomena lead to a transformation of the psychiatric care model and mental health support. The number of people receiving psychiatric treatment is expected to increase over the next decades. The applied psychopharmacotherapy and psychotherapy do not always bring the expected treatment result ( 9 ). As a result, other interventions are receiving increasing attention. In recent years, there has been a dramatic increase in research on the effects of nutrition on mental status, which may be an important aspect of the prevention of many mental disorders, and at the same time may lead to a reduction in the proportion of people with mental disorders.
Thus, this review aims to answer the question of whether and to what extent lifestyle and related nutrition affect mental health and whether there is scientific evidence supporting the diet and mental health relationship.
The question posed in the objective can be divided into specific questions according to which this review was divided.
Are there correlations between nutrition and mental health?
Are there psychoprotective food ingredients?
Are there nutritional interventions with proven preventive potential for mental disorders?
Review methodology
Methodology background.
The main aspect that guided the review works conducted was to look for nutritional recommendations in the cited works regarding nutrition as psychoprophylaxis and dietary management of psychiatric disorders. Unfortunately, the current state of knowledge on this topic, despite many studies, is still poor, so the authors decided to conduct a broad review of the most current knowledge in this area to identify those sources that address the described topic and gather in one place the available knowledge.
Review procedure
The review was conducted following good practices associated with conducting similar reviews. Literature items were searched by a team of researchers (authors) along with a library staff member trained in literature searching and EBM (evidence-based medicine) and HTA (health technology assessment). A preliminary search for items consistent with the topic and purpose of the review was conducted to identify the research field. After reviewing existing data, a keyword package was selected that seemed most relevant and consistent with the review topic.
Eligibility criteria
The primary eligibility criteria were the language of publication, years of research or review, publication status, and whether the authors were specialists in their field (or had other publications in a similar field). Regarding language, English-language articles were selected because this language seems to be universal in the scientific community. In addition, articles that were published after 2005 were included to make sure that the topic addressed was not a completely new field of research, but also to avoid very old data, because as is known from common practice, dietetics, as well as mental health expertise, are two of the most rapidly developing scientific fields. Additionally, articles were selected that were available in full-text on an open-access basis and had impact factor values.
Search strategy
A review of the scientific evidence was conducted based on the available literature by entering sample phrases (consistent with the MeSh dictionary) with Boole operators, logical operators (and, or, not), and special characters,: “psychodietetics,” “nutripsychiatry,” “diet,” “mental health,” “lifestyle,” “body weight,” “obesity,” “depression,” “mental disorders” (and various combinations thereof) using the methodological tool of the PubMed database. The PubMed database in this regard seems most appropriate because it is a methodological tool that allows searching for articles available in multiple scientific databases (such as Medline or Embase). Its use provides the opportunity to meet all expectations from the review (transparency, clarity, comprehensiveness, focus, uniformity, accessibility, coverage of the entire topic).
Sources selection
The literature search yielded 3,473 records, from which 356 sources directly related to the topic of the study were selected, and then those with the highest scientific value were selected according to eligibility criteria.
The accuracy, objectivity, validity, and relevance of the evidence were tested using questions consistent with the GRADE scale: Is the information reliable? Is the information free of mistakes? Has the information been properly substantiated? Is it possible to verify the information against other reliable sources? Who are the authors? Are they qualified to present information on the topic? Are they affiliated with reputable institutions working on the issue? Is the data source peer-reviewed? For what purpose was the information? Is the information an evidenced-based fact or constitutes an opinion? Is the information subject to risk? Can this risk be estimated? When was the information published? Is the information current or outdated? Is the timeliness relevant to the issue at hand? Does the information cover the entire issue? Does the information contain background data or does it explore the issue in depth? The final literature review was based on 110 sources, representing mainly scientific output after 2005 and important multicenter studies performed after 2015. The data obtained from the review are presented in descriptive and tabular form. In addition, 11 additional sources were used in preparing the background of the research problem and the theoretical introduction.
Critical appraisal
In critically evaluating the sources, attention was paid to whether the articles appeared in peer-reviewed journals (by at least two reviewers) and whether they had an impact factor. As described above, 110 sources were eligible for final review. A limitation of the method adopted was primarily the exclusion of sources written in a language other than English. In addition, IF has many well-documented drawbacks as a research assessment tool and therefore is not the best way to evaluate the quality of individual research articles. Nevertheless, it was chosen because it is a synthetic indicator of a source’s impact on the field of science, and a journal that has it can more likely claim to be publishing credible scientific evidence. The review did not include so-called “grey literature”, i.e., literature that has not gone through the review process or that is internal to the university (theses, conference reports, government leaflets, newsletters, etc.). Despite their multiple values, these sources are characterized by a high risk of containing outdated knowledge ( Figure 1 ).
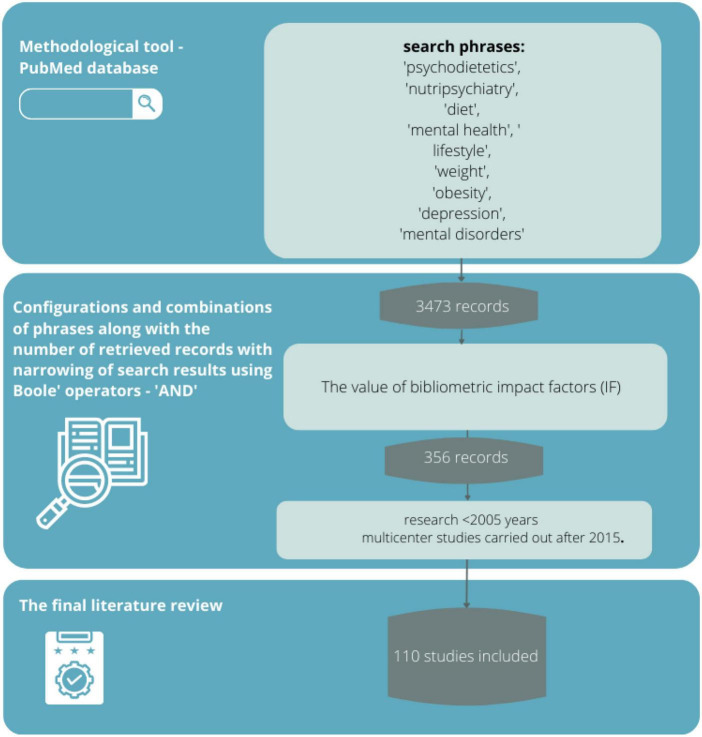
A flowchart of how to proceed in selecting bibliographic sources.
Q1: Are there correlations between nutrition and mental health?
Excess body weight is certainly an important social problem today. More than 0.7 billion people worldwide are obese, this is about 30% of the total population, and the number of obesity-related deaths is constantly increasing ( 10 ). We consume more and more processed, high-energy, and nutrient-poor foods. Consequently, we face problems of overweight and obesity with concomitant nutrient deficiencies (quantitative malnutrition) ( 11 ). Although the level of calories consumed is increasing, we are not taking in the recommended values of micro- and macroelements that play a significant role in the proper functioning of our nervous system – B vitamins, zinc, and magnesium. Additionally, we consume less fiber- and nutrient-rich vegetables and cereal products than recommended ( 10 , 11 ). Superimposing smoking, limited physical activity, and harmful alcohol consumption to the above dietary patterns, adversely affect health and development of mental disorders, including depression ( 10 ). Whose nutritional prevention is well documented in the literature ( 12 ).
The antioxidant system, which has been implicated in the development of psychiatric disorders, is relevant here ( 13 ) and its proper functioning depends on the presence of nutrients in food. In addition, the concentration of brain-derived neurotrophic factor (BDNF), which is involved in plasticity and neurodegenerative processes, depends on nutrients ( 14 ). Findings indicate a reduction in the incidence of depression and suicide with a healthy eating pattern ( 15 , 16 ). Randomized trials are emerging that evaluate the efficacy of dietary change as a form of treatment for depression ( 15 – 17 ). Selective food supplementation can be beneficial in the treatment of psychiatric disorders. Among them, compounds such as S-adenosylmethionine, N -acetylcysteine, zinc, and B vitamins including folic acid, and vitamin D are mentioned. Also, omega-3 unsaturated fatty acids have a wide range of effects. They participate in synaptogenesis by influencing receptor degradation and synthesis. They have an anti-inflammatory effect and inhibit apoptosis. They affect cell membrane function, BDNF action, and neurotransmitter reuptake ( 18 ). S-adenosylmethionine (SAM) is a compound formed from adenosine and methionine, which plays a key role in methylation processes. The results of studies show its antidepressant effects ( 19 ). The use of N -acetylcysteine influenced the effectiveness of therapy in schizophrenia, bipolar affective disorder, or trichotillomania. It has anti-inflammatory, antioxidant, and neuroprotective effects ( 20 ). Zinc deficiency, in turn, has been linked to the severity of depressive symptoms, and its supplementation included with antidepressants plays a role in mood stabilization. Zinc modulates cytokine activity and influences neurogenesis by affecting brain-derived neurotrophic factor levels ( 21 ). B vitamins play a role in the proper functioning of the nervous tissue. Folic acid (vitamin B9) deficiency has been associated with depressive symptoms and determined in subjects with mediocre responses to antidepressants ( 22 ). Low vitamin D levels were associated with a higher risk of schizophrenia and depression ( 23 ). It has been proven that vitamin D supplementation for a period of 3 months (4,000 IU/day for 1 month and 2,000 IU/day for 2 months) significantly reduced the severity of depression, irritability, fatigue, mood swings, sleep difficulties, weakness, and ability to concentrate in adolescents diagnosed with depression. This effect is supported by studies on animal models – vitamin D contributes to the plasticity of synapses, has a neuroprotective effect, supports the production of neurotrophic factors such as nerve growth factor (NGF) and regulates the function of the dopaminergic system. ( 24 ).
For the review, the results of the most important studies on the psychoprotective effects of bioactive components contained in foods (vitamins, minerals, omega-3, and more). have been collected in tabular form ( Table 1 ).
Review of selected studies on the psychoprotective effect of probiotics.
Source: Own compilation based on literature review.
Q2: Are there psychoprotective food ingredients?
The gut microbiota is estimated to form a complex ecosystem containing 1,014 microorganisms. It contains 3.3 million genes and outnumbers the human genome by about 150-fold. At the same time, it is built by more than a thousand different species of microorganisms ( 25 ). The gut-brain axis describing the bidirectional relationship between the gastrointestinal tract and the central nervous system uses several communication mechanisms. Mutual exchange of information can occur via the autonomic nervous system and the vagus nerve ( 26 ). Many of the effects of probiotics on mental status are associated with information transmission via the vagus nerve ( 27 ). Results from germ-free (GF) mice cultured under sterile conditions, devoid of detectable microorganisms, demonstrate the involvement of the gut microbiota in the proper formation and function of the endocrine system by influencing the development of the hypothalamic-pituitary-adrenal axis. The response to a stress stimulus as measured by glucocorticosteroid and adrenocorticotropin levels was significantly elevated in GF mice. It was normalized after gastrointestinal colonization with the Bifidobacterium infantis strain ( 28 ). Additionally, stress affects the formation and diversity of intestinal microflora ( 29 ). Another link of communication is the immune system. The microbiota is involved in the proper development of the gastrointestinal mucosal immune system ( 30 ). Bacterial antigens such as polysaccharide A, lipopolysaccharides, and thymic acids shape its proper functioning ( 31 ). The microbiota also produces neurotransmitters: gamma-aminobutyric acid, butyric acid, serotonin, dopamine, and short-chain fatty acids, which can directly affect the nervous system ( 32 ).
So, can the psychoprotective effect of strains be used in nutritional intervention? It seems reasonable here to consider the possibility of implementing treatment with probiotic preparations containing selected bacterial strains that show positive effects on the human psyche. In this approach, “probiotic” is defined as living organisms that, when consumed in adequate amounts, have a beneficial effect on the functioning of the body ( 33 ). Ilya Metchnikov was awarded the Nobel Prize in 1908 for his research on probiotics. Among them, lactic acid bacteria are the most popular. Probiotics are mainly found in fermented dairy products, or pickled products ( 34 ). Prebiotics are non-digested food components whose fermentation in the gastrointestinal tract stimulates either bacterial growth or activity or affects both, leading to the development of beneficial intestinal microflora ( 35 ). Prebiotics can include ingredients such as inulin or fructooligosaccharides. Prebiotics may also have a beneficial effect by inhibiting the growth of pathogenic bacteria. Moreover, some research results show that prebiotics can reduce inflammation by modifying the composition of the microbiota ( 36 ). Synbiotics are ingredients that contain both prebiotics and probiotics. Such a constellation allows the use of synergistic effects of these preparations. In turn, psychobiotics are defined as microorganisms that are probiotics, that show positive effects in patients treated for mental disorders ( 37 ). They can often achieve their effect through the production of neurotransmitters such as gamma-aminobutyric acid, serotonin, or other substances with an effect on the cells of the nervous system such as short-chain organic acids: acetic, propionic, or butyric ( 36 ). Oral substitution of such probiotics as Lactobacillus helveticus and Bifidobacterium longum over a period of 1 month was associated with a reduction in symptoms of anxiety and depressive disorders and a reduction in stress levels as measured by the determination of cortisol levels in animal models ( 38 ). Currently, the most effective treatment of psychiatric disorders is achieved through the use of antidepressants, or antipsychotics. However, the additional use of psychobiotics to treat anxiety or depressive disorders may prove effective in the future. It is also worth noting that popular antidepressants and antipsychotics can affect the quality of gut flora and change the composition of the microbiome to a disadvantage by killing the cultures of bacteria living in the gastrointestinal tract ( 39 ).
For the review, the results of the most important studies on the psychoprotective effect of probiotics were collected in tabular form – Table 2 .
Review of selected studies on the psychoprotective effects of substances contained in food.
Factors such as genotype, intrauterine infections, developmental disorders, later traumatizing events, use of harmful psychoactive substances, and many others will influence the onset of psychiatric disorders. These factors influence not only the onset of the disorder but also its progression. Treating early conditions in psychiatry can result in a much better response to the treatment given and better functioning of patients. This fact can be particularly observed in studies on the early detection of psychotic disorders ( 40 ). Prevention in medicine, including psychiatry, requires knowledge of appropriate and useful tools that would allow detection of increased risk of mental illness and monitoring of the developing psychopathology of the disorder. McGorry et al. ( 41 ) proposed a four-stage model of the development of mental disorders. According to this model, serious mental disorders develop from high-risk states: grade 0 means the development of undifferentiated, general symptoms, such as slight anxiety, restlessness, depressive symptoms, or somatic symptoms lead to grade 1, in which types 1A and 1B can be distinguished according to their severity. Further progression of the disease results in the development of a first episode of the disorder and here we speak of stage 2, which is accompanied by persistent 7ncludims and frequent relapses. Grade 3 includes incomplete remission and regular and repeated relapses. Grade 4 in this context means treatment-resistant disorder. The worsening of a psychiatric disorder is determined by genetic and environmental factors, and it is the latter that seems to be the main target for preventive interventions in psychiatry. Some biomarkers in psychiatry are directly related to nutrition. The first of these is the hypothalamic-pituitary-adrenal axis (HPA). Reduced ability to cope with stress plays a role in the development of psychiatric disorders ( 42 ). It is known that traumatizing experiences in early childhood shape vulnerability to stress in later life ( 43 ). The normal functioning of the HPA axis is often altered in psychiatric disorders, and increased cortisol secretion is observed in affective and psychotic disorders. Additionally, antipsychotic drugs appear to decrease HPA axis activity ( 44 – 47 ). Furthermore, healthy individuals who were first-degree relatives of individuals with psychotic disorders were found to have HPA axis dysfunction with elevated cortisol levels ( 48 ). These studies show that the HPA axis appears to be an important biological marker of susceptibility to developing psychiatric disorders. In this context, its association with gut microbiota is not insignificant. Other potential biomarkers involved in the pathophysiology of psychiatric disorders are inflammation and oxidative stress ( 49 ). The inflammatory theory of depression development is gaining increasing attention, and elevated levels of proinflammatory cytokines are observed in depressive, psychotic, and manic states ( 50 , 51 ). Elevated levels of proinflammatory cytokines occur before the onset of de novo disorders, suggesting their role in the genesis of these disorders ( 52 ). An increase in oxidative stress in psychotic disorders with a decrease in glutathione and antioxidant enzymes has also been observed ( 53 ). The potential effectiveness of selective cyclooxygenase-2 antagonists in the treatment of bipolar affective disorder and schizophrenia has been demonstrated ( 51 , 54 ). The use of statins, which have anti-inflammatory and antioxidant properties, reduced the risk of depressive disorders ( 55 ). Polyunsaturated fatty acids are further potential biomarkers that may have applications in psychiatry. Omega-3 polyunsaturated fatty acids may play a role in the pathogenesis of affective and psychotic disorders ( 56 , 57 ). Their deficiency may be present in the early stages of psychotic disorders – stage 1b. Supplementation with omega-3 polyunsaturated fatty acids reduced the risk of psychotic disorders among individuals at high risk of developing them ( 58 ).
The intestinal barrier is composed of several layers, including the intestinal microflora, mucus layer, intestinal epithelium, and elements of the circulatory, immune, nervous, and lymphatic systems. The layer of epithelial cells, mainly enterocytes connected by tight junctions, is the most important for the intestinal barrier ( 59 ). Its main function is to regulate the absorption of nutrients, electrolytes, and water from the gastrointestinal lumen into the blood or lymphatic system and prevent the penetration of pathogens from the gastrointestinal lumen. Factors such as stress, pro-inflammatory factors, dysbacteriosis of the intestinal microflora, alcohol, or antibiotics may cause excessive permeability of the intestinal barrier ( 60 – 62 ). Currently, the microbiota and its diversity as a trigger for generalized inflammation are gaining great importance ( 61 ) Under the influence of the impaired functioning of the barrier, the migration of bacteria from the lumen of the gastrointestinal tract occurs, which activates the cells of the immune system affecting the functioning of the immune, endocrine and nervous systems ( 62 ). It has been observed that patients with depression have elevated IgA and IgM immunoglobulins against lipopolysaccharides of the bacterial microbiome ( 63 ). The current study indicates the use of a dietary inflammatory index, which assesses the effect of the entire diet or individual dietary components on the concentration of inflammatory markers. The results of a systematic review by Chen et al. ( 64 ) indicate that a higher dietary inflammatory index is associated with an increased risk of common psychiatric disorders, including symptoms of depression, anxiety, distress, and schizophrenia. Of particular importance is the novel finding from the dose-response analysis that a 1 unit increase in the dietary inflammatory index was associated with a 6% higher risk of depressive symptoms. Similar relationships have been observed by Firth et al. ( 63 ), particularly in schizophrenia – individuals who consume more pro-inflammatory foods and less anti-inflammatory foods are more predisposed to psychiatric disorders. At this point, it is important to look at the relationship between diet and the proper functioning of the intestinal barrier. It turns out that it is not without significance in maintaining homeostasis. A diet consisting of fast food and highly processed foods is associated with increased intestinal barrier permeability ( 65 , 66 ).

Q3: Are there nutritional interventions with proven preventive potential for mental disorders?
Epidemiological studies have shown that diet impacts mental health, and intervention studies confirm this relationship ( 17 ). The challenge for “nutritional psychiatry” is to produce comprehensive, consistent, and scientifically rigorous evidence-based studies that define the role of diet and nutrients in different aspects of mental health ( 67 – 70 ). Overall, few randomized trials investigate the effectiveness of dietary change in mental health treatment. One intervention study to date involved a 12-week Mediterranean diet. This study reported significant improvements in mood and reduced anxiety in adults with major depression ( 71 ) More recent RCTs – HELFIMED ( 72 ) and PREDI_DEP ( 73 ) have confirmed the benefits of a Mediterranean-style diet for mental health in depression. In contrast to these studies, in the MooDFOOD RCT, multiple nutrient supplementation did not reduce episodes of major depression in overweight or obese adults with subsyndromal depressive symptoms. This study found that multinutrient supplements containing omega-3 PUFAs, vitamin D, folic acid, and selenium neither reduced depressive symptoms, anxiety symptoms nor improved health utility indices ( 74 ). Similar results regarding the lack of effect on mental state improvement were obtained in a review of the literature in the context of vitamin D ( 75 ). For omega-3 PACs, one RCT including people with mild to moderate depression found no beneficial effect of omega-3 PACs on depressive symptoms ( 76 ). No effect of folic acid supplementation in combination with vitamin B 6 and B 12 on the onset of depression was found in older men ( 77 ) and older women ( 78 ). Furthermore, Rayman et al. ( 79 ) found no effect of selenium supplementation on mood in older people. Overall, the studies available to date, do not support the use of nutritional supplementation to prevent depression.
However, many studies confirm that higher dietary quality in adulthood is associated with a reduced risk of cognitive decline ( 17 ). Additionally, the intake of antioxidant polyphenols in older adults is associated with improved cognitive ability ( 80 – 82 ). Another study showed that a Mediterranean diet supplemented with olive oil and nuts was associated with improved cognitive function in an older population ( 83 ).
Therefore, we undertook an analysis of diets that could potentially affect mental health such as the MIND diet, the Mediterranean diet, and the ketogenic diet.
The MIND diet is a dietary recommendation to counteract neurodegenerative brain changes and improve nervous system function. This diet is beneficial for cognitive decline in the aging process, as well as for the prevention and progression of neurodegenerative diseases, including Alzheimer’s disease ( 84 ). The MIND diet combines the principles of the Mediterranean diet and the DASH diet, which are based on a high intake of vegetables, fruits, nuts, whole grain cereal products, olive oil, fish, and seafood, and moderate consumption of dry red wine with meals ( 85 ). Studies prove the positive effects of the DASH and Mediterranean diets on other diseases such as diabetes, cancer, and obesity ( 86 – 89 ).
Long-term observations confirm that adherence to the Mediterranean diet reduces the risk of developing neurological disorders by up to 28% compared to the use of other diets ( 83 ). Adherence to the MIND diet was significantly associated with a lower chance of depression and psychological distress, but not anxiety, in the entire study population ( 90 ). Like the Mediterranean diet and the DASH diet, the MIND diet emphasizes natural plant-based foods and limited intake of animal and high-fat foods, especially of animal origin. However, there are some differences between the MIND diet and the DASH diet, and the Mediterranean diet. For example, leafy green vegetables and especially berries are unique components of the MIND diet that are not included in the Mediterranean and DASH diets ( 90 ). The MIND diet does not focus on a high intake of fruit, dairy products, and potatoes. Another difference between MIND and the DASH and Mediterranean diets concerns fish consumption. In MIND, individuals consuming as little as 1 portion of fish per week receive a positive result, whereas, in the Mediterranean and DASH diets, larger amounts of fish would need to be consumed to achieve a result ( 91 ). The MIND diet significantly slows cognitive decline with age ( 92 ). The Mediterranean diet has also been shown to have a protective effect on anxiety and mental stress ( 93 ).
Mental illnesses are associated with numerous metabolic disorders in the brain and co-occur with many other metabolic disorders such as obesity, diabetes, and CVD. The ketogenic diet is an evidence-based treatment for epilepsy that has been shown to have profound effects on brain metabolism and neurotransmitter function. In a ketogenic diet, as much as 80 percent of energy can come from fat. This proportion sounds like a deal-breaker for healthy eating, but it turns out that ketones formed from fats can alleviate epileptic seizures unresponsive to anticonvulsant drug therapy ( 83 ). In the case of mitochondrial epilepsy, reports on the effects of the ketogenic diet are conflicting. In a study by El Sabbagh et al. ( 94 ), no patients on a ketogenic diet achieved no significant reduction in seizure frequency epileptic seizures. In contrast, a study by Kang et al. ( 95 ) involving 14 patients showed that the use of a ketogenic diet in 10 of them reduced the frequency of epileptic seizures by more than 50%, and in 7 patients, epileptic seizures ceased. In the analysis, there were improvements in symptoms including mood, cognitive function, communication skills, energy, anxiety, and auditory and visual hallucinations ( 90 ). Other reported benefits included positive biometric changes such as improvements in lipid profile, weight reduction, positive change in blood glucose, and reduction in HbA1c. These benefits may facilitate the management of comorbidities and improve overall health and well-being ( 93 ). This highlights that advances in nutritional psychiatry are important and it will be important to replicate, refine and scale up dietary intervention studies targeting the prevention and treatment of common mental health disorders. In addition, there is an unmet need for more randomized, controlled clinical trials ( 118 – 121 ).
Strengths and limitations
There is still little work in the scientific space that summarizes the major findings related to the impact of nutrition on mental health, especially, as this review does, highlighting the importance of nutrition in psychoprevention and pointing to the psychoprotective effects of nutrients. The primary limitation of the presented review of research on the relationship between diet and mental health is the plethora of studies on the topic. The plethora of studies here does not mean that they all address the issue presented in this manuscript. Much of the work that was searched and queried assumes a relationship between nutrition and the psyche, but these tend to be very superficial opinions that are not scientifically grounded. The authors are aware that in the face of such a large body of research, important reports may have been overlooked, but it should be noted that every effort was made to ensure that this review was conducted fairly, taking into account large, multi-center research projects and highlighting the major research streams in psychodietetics and nutripsychiatry.
Additionally, it was observed that in the current state of scientific knowledge, few large meta-analyses are treating the effects of food and diet on mental health. Therefore, it is difficult to discuss the effectiveness of introducing nutritional interventions among people with mental disorders or treating nutrition as the only means of prevention. Furthermore, the primary threat to interventions of this type is the difficulty in monitoring dietary patterns or intake of specific components. In addition, their absorption and metabolism are also dependent on many factors that rarely have a consistent course. Therefore, it is postulated that further research should be directed toward the creation of unambiguous dietary recommendations for mental health problems.
In recent decades, the relationship between nutrition and patients’ mental status has been underappreciated, as evidenced by the lack of research conducted before the 21st century in this area of knowledge – cited in this review. In recent years, this trend has been reversed, with research in psychodietetics and nutripsychiatry gaining popularity. In the context of current changes, urbanization, globalization, including the food industry, and changes in people’s lifestyles and eating habits, correlations between these phenomena and their impact on psychological status are becoming important. Exploring these correlations creates potential opportunities to implement new effective dietary, pharmacological, therapeutic, and above all preventive interventions ( Figure 2 ).

Links between nutrition and mental health.
Author contributions
MATG: conceptualization. MATG and KK-K: investigation and methodology. KS and AB-D: data curation. MATG: writing – original draft preparation. MATG, KK-K, MARG, and AB-D: writing – review and editing. KS and AB-D: supervision. KK-K: project administration. WS: conducting an additional literature review, creating tables summarizing current knowledge of psychobiotics and psychoprotective food ingredients, and revising the work. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- 1. Hidaka BH. Depression as a disease of modernity: Explanations for increasing prevalence. J Affect Disord. (2012) 140:205–14. 10.1016/j.jad.2011.12.036 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Baxter AJ, Patton G, Scott KM, Degenhardt L, Whiteford HA. Global epidemiology of mental disorders: what are we missing? PLoS One. (2013) 8:e65514. 10.1371/journal.pone.0065514 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. IHME. Data visualizations. Seattle, WA: IHME; (2020) [ Google Scholar ]
- 4. Wittchen HU, Fuetsch M, Sonntag H, Müller N, Liebowitz M. Disability and quality of life in pure and comorbid social phobia. Findings from a controlled study. Eur Psychiatry. (2000) 15:46–58. 10.1016/s0924-9338(00)00211-x [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. NPOZP. Narodowy program ochrony zdrowia psychicznego na lata 2017-2022. Rozporządzenie rady ministrów z dnia 8 lutego 2017 roku. Warsaw: Ministerstwo Zdrowia; (2017) [ Google Scholar ]
- 6. Hawes MT, Szenczy AK, Klein DN, Hajcak G, Nelson BD. Increases in depression and anxiety symptoms in adolescents and young adults during the COVID-19 pandemic. Psychol Med. (2021) 5:1–9. 10.1017/S0033291720005358 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Notes From Poland. Stigmatisation and medication: Poland’s outdated approach to mental health. New York, NY: NFP; (2020). [ Google Scholar ]
- 8. Pawłowski T, Kiejna A. Pathways to psychiatric care and reform of the public health care system in Poland. Eur Psychiatry. (2004) 19:168–71. 10.1016/j.eurpsy.2003.09.009 [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. (2008) 5:e45. 10.1371/journal.pmed.0050045 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Parker E, Goldman J, Moshfegh A. America’s nutrition report card: comparing WWEIA, NHANES 2007-2010 Usual nu-trient intakes to dietary reference intakes. FASEB J. (2014) 28:384.2. [ Google Scholar ]
- 11. Opie RS, Itsiopoulos C, Parletta N, Sanchez-Villegas A, Akbaraly TN, Ruusunen A, et al. Dietary recommendations for the prevention of depression. Nutr Neurosci. (2017) 20:161–71. 10.1179/1476830515Y.0000000043 [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. (2013) 34:167–77. 10.1016/j.tips.2013.01.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Diniz BS, Mendes-Silva AP, Silva LB, Bertola L, Vieira MC, Ferreira JD, et al. Oxidative stress markers imbalance in late-life depression. J Psychiatr Res. (2018) 102:29–33. 10.1016/j.jpsychires.2018.02.023 [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Guimarães LR, Jacka FN, Gama CS, Berk M, Leitão-Azevedo CL, Belmonte de Abreu MG, et al. Serum levels of brain-derived neurotrophic factor in schizophrenia on a hypocaloric diet. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:1595–8. 10.1016/j.pnpbp.2008.06.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. (2014) 99:181–97. 10.3945/ajcn.113.069880 [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: Shared pathology and treatment? Br. J. Clin. Pharmacol . (2011) 71:365–76. 10.1111/j.1365-2125.2010.03830.x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the “SMILES” trial). BMC Med. (2017) 15:23. 10.1186/s12916-017-0791-y [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Mischoulon D, Freeman MP. Omega-3 fatty acids in psychiatry. Psychiatr Clin North Am. (2013) 36:15–23. 10.1016/j.psc.2012.12.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Sarris J, Papakostas GI, Vitolo O, Fava M, Mischoulon D. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. (2014) 164:76–81. 10.1016/j.jad.2014.03.041 [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan A, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. (2013) 11:200. 10.1186/1741-7015-11-200 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Lai J, Moxey A, Nowak G, Vashum K, Bailey K, McEvoy M. The efficacy of zinc supplementation in depression: systematic review of randomised controlled trials. J Affect Disord. (2012) 136:e31–9. 10.1016/j.jad.2011.06.022 [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Fava M, Mischoulon D. Folate in depression: efficacy, safety, differences in formulations, and clinical issues. J Clin Psychiatry. (2009) 70:12–7. 10.4088/JCP.8157su1c.03 [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. (2013) 34:47–64. 10.1016/j.yfrne.2012.07.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Brouwer-Brolsma EM, Dhonukshe-Rutten RA, van Wijngaarden JP, van der Zwaluw NL, Sohl E, In’t Veld PH, et al. Low vitamin D status is associated with more depressive symptoms in dutch older adults. Eur J Nutr. (2016) 55:1525–34. 10.1007/s00394-015-0970-6 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. (2012) 489:220–30. 10.1038/nature11550 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. (2004) 16:55–9. 10.1111/j.1743-3150.2004.00476.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. (2014) 817:115–33. 10.1007/978-1-4939-0897-4_5 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalam-ic-pituitary-adrenal system for stress response in mice. J Physiol. (2004) 558:263–75. 10.1113/jphysiol.2004.063388 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Bangsgaard Bendtsen KM, Krych L, Sørensen DB, Pang W, Nielsen DS, Josefsen K, et al. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One. (2012) 7:e46231. 10.1371/journal.pone.0046231 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Mayer L. Mucosal immunity. Pediatrics. (2003) 111:1595–600. [ PubMed ] [ Google Scholar ]
- 31. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. (2004) 118:229–41. 10.1016/j.cell.2004.07.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Lyte M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes. (2014) 5:381–9. 10.4161/gmic.28682 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Grot M, Krupa-Kotara K, Wypych-Ślusarska A, Grajek M, Białek-Dratwa A. The concept of intrauterine programming and the development of the neonatal microbiome in the prevention of SARS-CoV-2 infection. Nutrients. (2022) 14:1702. 10.3390/nu14091702 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Mackowiak PA. Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health. (2013) 1:52. 10.3389/fpubh.2013.00052 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Durchschein F, Petritsch W, Hammer HF. Diet therapy for inflammatory bowel diseases: The established and the new. World J Gastroenterol. (2016) 22:2179–94. 10.3748/wjg.v22.i7.2179 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. (2015) 12:303–10. 10.1038/nrgastro.2015.47 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation ( Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. (2011) 105:755–64. 10.1017/S0007114510004319 [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation ( Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. (2011) 2:256–61. 10.4161/gmic.2.4.16108 [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Cussotto S, Clarke G, Dinan TG, Cryan JF. Psychotropics and the microbiome: A chamber of secrets. Psychopharmacology. (2019) 236:1411–32. 10.1007/s00213-019-5185-8 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Hegelstad WT, Larsen TK, Auestad B, Evensen J, Haahr U, Joa I, et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am J Psychiatry. (2012) 169:374–80. 10.1176/appi.ajp.2011.11030459 [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. McGorry PD, Nelson B, Goldstone S, Yung AR. Clinical staging: a heuristic and practical strategy for new research and better health and social outcomes for psychotic and related mood disorders. Can J Psychiatry. (2010) 55:486–97. 10.1177/070674371005500803 [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Phillips LJ, McGorry PD, Garner B, Thompson KN, Pantelis C, Wood SJ, et al. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. (2006) 40:725–41. 10.1080/j.1440-1614.2006.01877.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. (2013) 38:111–23. 10.1038/npp.2012.149 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Belvederi Murri M, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology. (2016) 63:327–42. 10.1016/j.psyneuen.2015.10.014 [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Pariante CM, Dazzan P, Danese A, Morgan KD, Brudaglio F, Morgan C, et al. Increased pituitary volume in antipsychotic-free and antipsychotic-treated patients of the AEsop first-onset psychosis study. Neuropsychopharmacology. (2005) 30:1923–31. 10.1038/sj.npp.1300766 [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Cohrs S, Röher C, Jordan W, Meier A, Huether G, Wuttke W, et al. The atypical antipsychotics olanzapine and quetiapine, but not haloperidol, reduce ACTH and cortisol secretion in healthy subjects. Psychopharmacology. (2006) 185:11–8. 10.1007/s00213-005-0279-x [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Collip D, Nicolson NA, Lardinois M, Lataster T, van Os J, Myin-Germeys I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol Med. (2011) 41:2305–15. 10.1017/S0033291711000602 [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry. (2011) 70:663–71. 10.1016/j.biopsych.2011.04.013 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. (2011) 24:519–25. 10.1097/YCO.0b013e32834b9db6 [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Wadee AA, Kuschke RH, Wood LA, Berk M, Ichim L, Maes M. Serological observations in patients suffering from acute manic episodes. Hum Psychopharmacol. (2002) 17:175–9. 10.1002/hup.390 [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, et al. Clinical implications of the cytokine hy-pothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother Psychosom. (2010) 79:323–5. 10.1159/000319530 [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. (2009) 19:220–30. 10.1016/j.conb.2009.05.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, et al. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psycho-Pharmacol. (2008) 23:87–94. 10.1002/hup.912 [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Müller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. Celecoxib treatment in an early stage of schizophrenia: Results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. (2010) 121:118–24. 10.1016/j.schres.2010.04.015 [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. (2006) 163:969–78. 10.1176/ajp.2006.163.6.969 [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. (1998) 30:193–208. 10.1016/s0920-9964(97)00151-5 [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. (2010) 67:146–54. 10.1001/archgenpsychiatry.2009.192 [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. (2009) 87:101–8. 10.2527/jas.2008-1339 [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, et al. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. (1999) 112:137–46. 10.1242/jcs.112.1.137 [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA. (2014) 111:E4485–93. 10.1073/pnas.1415174111 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Tulstrup MV, Christensen EG, Carvalho V, Linninge C, Ahrné S, Højberg O, et al. Antibiotic treatment affects intestinal permeability and gut microbial composition in wistar rats dependent on antibiotic class. PLoS One. (2015) 10:e0144854. 10.1371/journal.pone.0144854 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. (2008) 29:117–24. [ PubMed ] [ Google Scholar ]
- 63. Firth J, Veronese N, Cotter J, Shivappa N, Hebert JR, Ee C, et al. What is the role of dietary inflammation in severe mental illness? A review of observational and experimental findings. Front Psychiatry. (2019) 10:350. 10.3389/fpsyt.2019.00350 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Chen GQ, Peng CL, Lian Y, Wang BW, Chen PY, Wang GP. Association between dietary inflammatory index and mental health: A systematic review and dose-response meta-analysis. Front Nutr. (2021) 5:662357. 10.3389/fnut.2021.662357 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Sánchez-Villegas A, Toledo E, de Irala J, Ruiz-Canela M, Pla-Vidal J, Martínez-González MA. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. (2012) 15:424–32. 10.1017/S1368980011001856 [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Adan RAH, van der Beek EM, Buitelaar JK, Cryan JF, Hebebrand J, Higgs S, et al. Nutritional psychiatry: Towards im-proving mental health by what you eat. Eur Neuropsychopharmacol. (2019) 29:1321–32. 10.1016/j.euroneuro.2019.10.011 [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Marx W, Moseley G, Berk M, Jacka F. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. (2017) 76:427–36. 10.1017/S0029665117002026 [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP, et al. Nutritional medicine as main-stream in psychiatry. Lancet Psychiatry. (2015) 2:271–4. 10.1016/S2215-0366(14)00051-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP, et al. International society for nutri-tional psychiatry research consensus position statement: nutritional medicine in modern psychiatry. World Psychiatry. (2015) 14:370–1. 10.1002/wps.20223 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Jacka FN, O’Neil A, Itsiopoulos C, Opie R, Mohebbi M, Castle D, et al. The SMILES trial: An important first step. BMC Med. (2018) 16:237. 10.1186/s12916-018-1228-y [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr Neurosci. (2019) 22:474–87. 10.1080/1028415X.2017.1411320 [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Sánchez-Villegas A, Cabrera-Suárez B, Molero P, González-Pinto A, Chiclana-Actis C, Cabrera C, et al. Preventing the recurrence of depression with a Mediterranean diet supplemented with extra-virgin olive oil. The PREDI-DEP trial: study protocol. BMC Psychiatry. (2019) 19:63. 10.1186/s12888-019-2036-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Berk M, Jacka FN. Diet and depression-from confirmation to implementation. JAMA. (2019) 321:842–3. 10.1001/jama.2019.0273 [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Bot M, Brouwer IA, Roca M, Kohls E, Penninx BWJH, Watkins E, et al. Effect of multinutrient supplementation and food-related behavioral activation therapy on prevention of major depressive disorder among overweight or obese adults with subsyndromal depressive symptoms: The MooDFOOD randomized clinical trial. JAMA. (2019) 321:858–68. 10.1001/jama.2019.0556 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Okereke OI, Singh A. The role of vitamin D in the prevention of late-life depression. J Affect Disord. (2016) 198:1–14. 10.1016/j.jad.2016.03.022 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Ford AH, Flicker L, Thomas J, Norman P, Jamrozik K, Almeida OP. Vitamins B12, B6, and folic acid for onset of depressive symptoms in older men: results from a 2-year placebo-controlled randomized trial. J Clin Psychiatry. (2008) 69:1203–9. 10.4088/JCP.v69n0801 [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Okereke OI, Cook NR, Albert CM, Van Denburgh M, Buring JE, Manson JE. Effect of long-term supplementation with folic acid and B vitamins on risk of depression in older women. Br J Psychiatry. (2015) 206:324–31. 10.1192/bjp.bp.114.148361 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Valls-Pedret C, Lamuela-Raventós RM, Medina-Remón A, Quintana M, Corella D, Pintó X, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. (2012) 29:773–82. 10.3233/JAD-2012-111799 [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Rayman M, Thompson A, Warren-Perry M, Galassini R, Catterick J, Hall E, et al. Impact of selenium on mood and quality of life: a randomized, controlled trial. Biol Psychiatry. (2006) 59:147–54. 10.1016/j.biopsych.2005.06.019 [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Witte AV, Kerti L, Margulies DS, Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. (2014) 34:7862–70. 10.1523/JNEUROSCI.0385-14.2014 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Anton SD, Embry C, Marsiske M, Lu X, Doss H, Leeuwenburgh C, et al. Safety and metabolic outcomes of resveratrol supplementation in older adults: results of a twelve-week, placebo-controlled pilot study. Exp Gerontol. (2014) 57:181–7. 10.1016/j.exger.2014.05.015 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martínez-González MÁ, et al. Mediterranean Diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern Med. (2015) 175:1094–103. 10.1001/jamainternmed.2015.1668 [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. (2015) 11:1015–22. 10.1016/j.jalz.2015.04.011 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Dutta R. Role of MIND diet in preventing dementia and Alzheimer’s disease. ARC J Neurosci. (2019) 4:1–8. 10.20431/2456-057X.0403001 [ DOI ] [ Google Scholar ]
- 85. Said MS, El Sayed IT, Ibrahim EE, Khafagy GM. Effect of DASH Diet Versus healthy dietary advice on the estimated atherosclerotic cardiovascular disease risk. J Prim Care Community Health. (2021) 12:2150132720980952. 10.1177/2150132720980952 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. (2019) 11:338. 10.3390/nu11020338 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ Res. (2019) 124:779–98. 10.1161/CIRCRESAHA.118.313348 [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Salas-Salvadó J, Becerra-Tomás N, García-Gavilán JF, Bulló M, Barrubés L. Mediterranean diet and cardiovascular disease prevention: What do we know? Prog Cardiovasc Dis. (2018) 61:62–7. 10.1016/j.pcad.2018.04.006 [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Salari-Moghaddam A, Keshteli AH, Mousavi SM, Afshar H, Esmaillzadeh A, Adibi P. Adherence to the MIND diet and prevalence of psychological disorders in adults. J Affect Disord. (2019) 256:96–102. 10.1016/j.jad.2019.05.056 [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Hodge A, Almeida OP, English DR, Giles GG, Flicker L. Patterns of dietary intake and psychological distress in older Australians: benefits not just from a Mediterranean diet. Int Psychogeriatr. (2013) 25:456–66. 10.1017/S1041610212001986 [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Olveira C, Olveira G, Espildora F, Girón RM, Vendrell M, Dorado A, et al. Mediterranean diet is associated on symptoms of depression and anxiety in patients with bronchiectasis. Gen Hosp Psychiatry. (2014) 36:277–83. 10.1016/j.genhosppsych.2014.01.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Norwitz NG, Dalai SS, Palmer CM. Ketogenic diet as a metabolic treatment for mental illness. Curr Opin Endocrinol Diabetes Obes. (2020) 27:269–74. 10.1097/MED.0000000000000564 [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Tillery EE, Ellis KD, Threatt TB, Reyes HA, Plummer CS, Barney LR. The use of the ketogenic diet in the treatment of psychiatric disorders. Ment Health Clin. (2021) 11:211–9. 10.9740/mhc.2021.05.211 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. El Sabbagh S, Lebre AS, Bahi-Buisson N, Delonlay P, Soufflet C, Boddaert N, et al. Epileptic phenotypes in children with respiratory chain disorders. Epilepsia. (2010) 51:1225–35. 10.1111/j.1528-1167.2009.02504.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Kang HC, Lee YM, Kim HD. Mitochondrial disease and epilepsy. Brain Dev. (2013) 35:757–61. 10.1016/j.braindev.2013.01.006 [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Gazerani P. Migraine and diet. Nutrients. (2020) 12:1658. 10.3390/nu12061658 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Cater RJ, Chua GL, Erramilli SK, Keener JE, Choy BC, Tokarz P, et al. Structural basis of omega-3 fatty acid transport across the blood-brain barrier. Nature. (2021) 595:315–9. 10.1038/s41586-021-03650-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Parikh M, Maddaford TG, Austria JA, Aliani M, Netticadan T, Pierce GN. Dietary flaxseed as a strategy for improving human health. Nutrients. (2019) 11:1171. 10.3390/nu11051171 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Park M, Choi J, Lee HJ. Flavonoid-rich orange juice intake and altered gut microbiome in young adults with depressive symptom: A randomized controlled study. Nutrients. (2020) 12:1815. 10.3390/nu12061815 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Mulati A, Ma S, Zhang H, Ren B, Zhao B, Wang L, et al. Sea-buckthorn flavonoids alleviate high-fat and high-fructose diet-induced cognitive impairment by inhibiting insulin resistance and neuroinflammation. J Agric Food Chem. (2020) 68:5835–46. 10.1021/acs.jafc.0c00876 [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J Cell Physiol. (2017) 232:2359–72. 10.1002/jcp.25518 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Fernández MJF, Valero-Cases E, Rincon-Frutos L. Food components with the potential to be used in the therapeutic approach of mental diseases. Curr Pharm Biotechnol. (2019) 20:100–13. 10.2174/1389201019666180925120657 [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Dogan-Sander E, Mergl R, Willenberg A, Baber R, Wirkner K, Riedel-Heller SG, et al. Inflammation and the association of vitamin D and depressive symptomatology. Nutrients. (2021) 13:1972. 10.3390/nu13061972 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Godos J, Currenti W, Angelino D, Mena P, Castellano S, Caraci F, et al. Diet and Mental health: review of the recent updates on molecular mechanisms. Antioxidants Basel. (2020) 9:346. 10.3390/antiox9040346 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Burton-Freeman BM, Sandhu AK, Edirisinghe I. Red raspberries and their bioactive polyphenols: cardiometabolic and neuronal health links. Adv Nutr. (2016) 7:44–65. 10.3945/an.115.009639 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Diop L, Guillou S, Durand H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutr Res. (2008) 28:1–5. 10.1016/j.nutres.2007.10.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Wallace CJK, Foster JA, Soares CN, Milev RV. The effects of probiotics on symptoms of depression: protocol for a Dou-ble-blind randomized placebo-controlled trial. Neuropsychobiology. (2020) 79:108–16. 10.1159/000496406 [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin Nutr. (2019) 38:522–8. 10.1016/j.clnu.2018.04.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Rudzki L, Ostrowska L, Pawlak D, Malus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus plantarum 299v de-creases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. (2019) 100:213–22. 10.1016/j.psyneuen.2018.10.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Heidarzadeh-Rad N, Gökmen-Özel H, Kazemi A, Almasi N, Djafarian K. Effects of a psychobiotic supplement on serum brain-derived neurotrophic factor levels in depressive patients: A post hoc analysis of a randomized clinical trial. J Neurogastroenterol Motil. (2020) 26:486–95. 10.5056/jnm20079 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Agahi A, Hamidi GA, Daneshvar R, Hamdieh M, Soheili M, Alinaghipour A, et al. Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front Neurol. (2018) 15:662. 10.3389/fneur.2018.00662 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front Aging Neurosci. (2016) 10:256. 10.3389/fnagi.2016.00256 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 113. Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin Nutr. (2019) 38:2569–75. 10.1016/j.clnu.2018.11.034 [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Wallis A, Ball M, Butt H, Lewis DP, McKechnie S, Paull P, et al. Open-label pilot for treatment targeting gut dysbiosis in myalgic encephalomyelitis/chronic fatigue syndrome: neuropsychological symptoms and sex comparisons. J Transl Med. (2018) 16:24. 10.1186/s12967-018-1392-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 115. Hwang YH, Park S, Paik JW, Chae SW, Kim DH, Jeong DG, et al. Efficacy and Safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, random-ized, double-blind, placebo-controlled clinical trial. Nutrients. (2019) 11:305. 10.3390/nu11020305 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 116. Kobayashi Y, Kinoshita T, Matsumoto A, Yoshino K, Saito I, Xiao JZ. Bifidobacterium breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: An open-label, single-arm study. J Prev Alzheimers Dis. (2019) 6:70–5. 10.14283/jpad.2018.32 [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Kobayashi Y, Kuhara T, Oki M, Xiao JZ. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef Microbes. (2019) 10:511–20. 10.3920/BM2018.0170 [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Nanri A, Mizoue T, Noda M, Takahashi Y, Kirii K, Inoue M, et al. Magnesium intake and type II diabetes in Japanese men and women: the Japan public health center-based prospective study. Eur J Clin Nutr. (2010) 64:1244–7. 10.1038/ejcn.2010.138 [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Ibsen DB, Levitan EB, Åkesson A, Gigante B, Wolk A. The dietary approach to stop hypertension (DASH) diet is associated with a lower risk of heart failure: A cohort study. Eur J Prev Cardiol. (2022) 29:1114–23. 10.1093/eurjpc/zwac003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Chatterton ML, Mihalopoulos C, O’Neil A, Itsiopoulos C, Opie R, Castle D, et al. Economic evaluation of a dietary inter-vention for adults with major depression (the “SMILES” trial). BMC Public Health. (2018) 18:599. 10.1186/s12889-018-5504-8 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 121. Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: A randomised controlled trial. Br J Nutr. (2008) 99:421–31. 10.1017/S0007114507801097 [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.3 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Nutritional psychiatry: Towards improving mental health by what you eat
Affiliations.
- 1 Department of Translational Neurosciences, University Medical Center Utrecht, Universiteitsweg 100, 3584 CG Utrecht, the Netherlands; Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Medicinaregatan 11, SE-405 30 Gothenburg, Sweden. Electronic address: [email protected].
- 2 Danone Nutricia Research, Utrecht, the Netherlands; Department of Pediatrics, University Medical Centre Groningen, Groningen, the Netherlands.
- 3 Department of Cognitive Neuroscience, Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, the Netherlands; Karakter Child and Adolescent Psychiatry, Nijmegen, the Netherlands.
- 4 Department of Anatomy & Neuroscience and APC Microbiome Ireland, University College Cork, Ireland.
- 5 Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital Essen, University of Duisburg-Essen, Essen, Germany.
- 6 Suzanne Higgs School of Psychology, University of Birmingham, Birmingham, UK.
- 7 Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Medicinaregatan 11, SE-405 30 Gothenburg, Sweden. Electronic address: [email protected].
- PMID: 31735529
- DOI: 10.1016/j.euroneuro.2019.10.011
Does it matter what we eat for our mental health? Accumulating data suggests that this may indeed be the case and that diet and nutrition are not only critical for human physiology and body composition, but also have significant effects on mood and mental wellbeing. While the determining factors of mental health are complex, increasing evidence indicates a strong association between a poor diet and the exacerbation of mood disorders, including anxiety and depression, as well as other neuropsychiatric conditions. There are common beliefs about the health effects of certain foods that are not supported by solid evidence and the scientific evidence demonstrating the unequivocal link between nutrition and mental health is only beginning to emerge. Current epidemiological data on nutrition and mental health do not provide information about causality or underlying mechanisms. Future studies should focus on elucidating mechanism. Randomized controlled trials should be of high quality, adequately powered and geared towards the advancement of knowledge from population-based observations towards personalized nutrition. Here, we provide an overview of the emerging field of nutritional psychiatry, exploring the scientific evidence exemplifying the importance of a well-balanced diet for mental health. We conclude that an experimental medicine approach and a mechanistic understanding is required to provide solid evidence on which future policies on diet and nutrition for mental health can be based.
Keywords: Cognition; Dietary intervention; Early life nutrition; Nutrients; Nutritional psychiatry; Obesity.
Copyright © 2019 The Author(s). Published by Elsevier B.V. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
- Gastrointestinal Microbiome / physiology*
- Mental Disorders / diet therapy*
- Mental Disorders / prevention & control
- Mental Health* / trends
- Meta-Analysis as Topic
- Nutritional Status / physiology*
- Psychiatry / methods*
- Psychiatry / trends
- Randomized Controlled Trials as Topic / methods

IMAGES
VIDEO
COMMENTS
Can nutrition affect your mental health? A growing research literature suggests the answer could be yes. Western-style dietary habits, in particular, come under special scrutiny in much of this research.
Abstract. Nutritional psychiatry is an emerging field that explores the relationship between diet and mental health. Research focuses on dietary patterns, gut microbiome health, and the impact of individual nutrients on mental health. Nutritional and lifestyle interventions can complement psychopharmacology and psychotherapy in clinical practice.
This review aims to answer whether and to what extent lifestyle and related nutrition affect mental health and whether there is scientific evidence supporting a link between diet and mental health.
An increasing number of studies are revealing that diet and nutrition are critical not only for physiology and body composition, but also have significant effects on mood and mental well-being. In particular, Western dietary habits have been the object of several research studies focusing on the relationship between nutrition and mental health.
How the foods you eat affect your mental health. Serotonin is a neurotransmitter that helps regulate sleep and appetite, mediate moods, and inhibit pain. Since about 95% of your serotonin is produced in your gastrointestinal tract, and your gastrointestinal tract is lined with a hundred million nerve cells, or neurons, it makes sense that the ...
While the determining factors of mental health are complex, increasing evidence indicates a strong association between a poor diet and the exacerbation of mood disorders, including anxiety and depression, as well as other neuropsychiatric conditions.