Confusion to Clarity: Definition of Terms in a Research Paper
Explore the definition of terms in research paper to enhance your understanding of crucial scientific terminology and grow your knowledge.
Have you ever come across a research paper and found yourself scratching your head over complex synonyms and unfamiliar terms? It’s a hassle as you have to fetch a dictionary and then ruffle through it to find the meaning of the terms.
To avoid that, an exclusive section called ‘ Definition of Terms in a Research Paper ’ is introduced which contains the definitions of terms used in the paper. Let us learn more about it in this article.

What Is The “Definition Of Terms” In A Research Paper?
The definition of terms section in a research paper provides a clear and concise explanation of key concepts, variables, and terminology used throughout the study.
In the definition of terms section, researchers typically provide precise definitions for specific technical terms, acronyms, jargon, and any other domain-specific vocabulary used in their work. This section enhances the overall quality and rigor of the research by establishing a solid foundation for communication and understanding.
Purpose Of Definition Of Terms In A Research Paper
This section aims to ensure that readers have a common understanding of the terminology employed in the research, eliminating confusion and promoting clarity. The definitions provided serve as a reference point for readers, enabling them to comprehend the context and scope of the study. It serves several important purposes:
- Enhancing clarity
- Establishing a shared language
- Providing a reference point
- Setting the scope and context
- Ensuring consistency
Benefits Of Having A Definition Of Terms In A Research Paper
Having a definition of terms section in a research paper offers several benefits that contribute to the overall quality and effectiveness of the study. These benefits include:
Clarity And Comprehension
Clear definitions enable readers to understand the specific meanings of key terms, concepts, and variables used in the research. This promotes clarity and enhances comprehension, ensuring that readers can follow the study’s arguments, methods, and findings more easily.
Consistency And Precision
Definitions provide a consistent framework for the use of terminology throughout the research paper. By clearly defining terms, researchers establish a standard vocabulary, reducing ambiguity and potential misunderstandings. This precision enhances the accuracy and reliability of the study’s findings.
Common Understanding
The definition of terms section helps establish a shared understanding among readers, including those from different disciplines or with varying levels of familiarity with the subject matter. It ensures that readers approach the research with a common knowledge base, facilitating effective communication and interpretation of the results.
Avoiding Misinterpretation
Without clear definitions, readers may interpret terms and concepts differently, leading to misinterpretation of the research findings. By providing explicit definitions, researchers minimize the risk of misunderstandings and ensure that readers grasp the intended meaning of the terminology used in the study.
Accessibility For Diverse Audiences
Research papers are often read by a wide range of individuals, including researchers, students, policymakers, and professionals. Having a definition of terms in a research paper helps the diverse audience understand the concepts better and make appropriate decisions.
Types Of Definitions
There are several types of definitions that researchers can employ in a research paper, depending on the context and nature of the study. Here are some common types of definitions:
Lexical Definitions
Lexical definitions provide the dictionary or commonly accepted meaning of a term. They offer a concise and widely recognized explanation of a word or concept. Lexical definitions are useful for establishing a baseline understanding of a term, especially when dealing with everyday language or non-technical terms.
Operational Definitions
Operational definitions define a term or concept about how it is measured or observed in the study. These definitions specify the procedures, instruments, or criteria used to operationalize an abstract or theoretical concept. Operational definitions help ensure clarity and consistency in data collection and measurement.
Conceptual Definitions
Conceptual definitions provide an abstract or theoretical understanding of a term or concept within a specific research context. They often involve a more detailed and nuanced explanation, exploring the underlying principles, theories, or models that inform the concept. Conceptual definitions are useful for establishing a theoretical framework and promoting deeper understanding.
Descriptive Definitions
Descriptive definitions describe a term or concept by providing characteristics, features, or attributes associated with it. These definitions focus on outlining the essential qualities or elements that define the term. Descriptive definitions help readers grasp the nature and scope of a concept by painting a detailed picture.
Theoretical Definitions
Theoretical definitions explain a term or concept based on established theories or conceptual frameworks. They situate the concept within a broader theoretical context, connecting it to relevant literature and existing knowledge. Theoretical definitions help researchers establish the theoretical underpinnings of their study and provide a foundation for further analysis.
Also read: Understanding What is Theoretical Framework
Types Of Terms
In research papers, various types of terms can be identified based on their nature and usage. Here are some common types of terms:
A key term is a term that holds significant importance or plays a crucial role within the context of a research paper. It is a term that encapsulates a core concept, idea, or variable that is central to the study. Key terms are often essential for understanding the research objectives, methodology, findings, and conclusions.
Technical Term
Technical terms refer to specialized vocabulary or terminology used within a specific field of study. These terms are often precise and have specific meanings within their respective disciplines. Examples include “allele,” “hypothesis testing,” or “algorithm.”
Legal Terms
Legal terms are specific vocabulary used within the legal field to describe concepts, principles, and regulations. These terms have particular meanings within the legal context. Examples include “defendant,” “plaintiff,” “due process,” or “jurisdiction.”
Definitional Term
A definitional term refers to a word or phrase that requires an explicit definition to ensure clarity and understanding within a particular context. These terms may be technical, abstract, or have multiple interpretations.
Career Privacy Term
Career privacy term refers to a concept or idea related to the privacy of individuals in the context of their professional or occupational activities. It encompasses the protection of personal information, and confidential data, and the right to control the disclosure of sensitive career-related details.
A broad term is a term that encompasses a wide range of related concepts, ideas, or objects. It has a broader scope and may encompass multiple subcategories or specific examples.
Also read: Keywords In A Research Paper: The Importance Of The Right Choice
Steps To Writing Definitions Of Terms
When writing the definition of terms section for a research paper, you can follow these steps to ensure clarity and accuracy:
Step 1: Identify Key Terms
Review your research paper and identify the key terms that require definition. These terms are typically central to your study, specific to your field or topic, or may have different interpretations.
Step 2: Conduct Research
Conduct thorough research on each key term to understand its commonly accepted definition, usage, and any variations or nuances within your specific research context. Consult authoritative sources such as academic journals, books, or reputable online resources.
Step 3: Craft Concise Definitions
Based on your research, craft concise definitions for each key term. Aim for clarity, precision, and relevance. Define the term in a manner that reflects its significance within your research and ensures reader comprehension.
Step 4: Use Your Own Words
Paraphrase the definitions in your own words to avoid plagiarism and maintain academic integrity. While you can draw inspiration from existing definitions, rephrase them to reflect your understanding and writing style. Avoid directly copying from sources.
Step 5: Provide Examples Or Explanations
Consider providing examples, explanations, or context for the defined terms to enhance reader understanding. This can help illustrate how the term is applied within your research or clarify its practical implications.
Step 6: Order And Format
Decide on the order in which you present the definitions. You can follow alphabetical order or arrange them based on their importance or relevance to your research. Use consistent formatting, such as bold or italics, to distinguish the defined terms from the rest of the text.
Step 7: Revise And Refine
Review the definitions for clarity, coherence, and accuracy. Ensure that they align with your research objectives and are tailored to your specific study. Seek feedback from peers, mentors, or experts in your field to further refine and improve the definitions.
Step 8: Include Proper Citations
If you have drawn ideas or information from external sources, remember to provide proper citations for those sources. This demonstrates academic integrity and acknowledges the original authors.
Step 9: Incorporate The Section Into Your Paper
Integrate the definition of terms section into your research paper, typically as an early section following the introduction. Make sure it flows smoothly with the rest of the paper and provides a solid foundation for understanding the subsequent content.
By following these steps, you can create a well-crafted and informative definition of terms section that enhances the clarity and comprehension of your research paper.
In conclusion, the definition of terms in a research paper plays a critical role by providing clarity, establishing a common understanding, and enhancing communication among readers. The definition of terms section is an essential component that contributes to the overall quality, rigor, and effectiveness of a research paper.
Also read: Beyond The Main Text: The Value Of A Research Paper Appendix
Join Our Fast-Growing Community Of Users To Revolutionize Scientific Communication!
Every individual needs a community to learn, grow, and nurture their hobbies, passions, and skills. But when you are a scientist, it becomes difficult to identify the right community that aligns with your goals, has like-minded professionals, and understands mutual collaboration.
If you are a scientist, looking for a great community, Mind the Graph is here. Join our fast-growing community of users to revolutionize scientific communication and build a healthy collaboration. Sign up for free.

Subscribe to our newsletter
Exclusive high quality content about effective visual communication in science.
Unlock Your Creativity
Create infographics, presentations and other scientifically-accurate designs without hassle — absolutely free for 7 days!
About Sowjanya Pedada
Sowjanya is a passionate writer and an avid reader. She holds MBA in Agribusiness Management and now is working as a content writer. She loves to play with words and hopes to make a difference in the world through her writings. Apart from writing, she is interested in reading fiction novels and doing craftwork. She also loves to travel and explore different cuisines and spend time with her family and friends.
Content tags
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

How to Write the Definition of Terms in Chapter 1 of a Thesis
Related Papers
Meta: Journal des traducteurs
Phaedra Royle
Rahmadi Nirwanto
The study is intended to describe the methods of defining terms found in the theses of the English Foreign Language (EFL) students of IAIN Palangka Raya. The method to be used is a mixed method, qualitative and quantitative. Quantitative approach was used to identify, describe the frequencies, and classify the methods of defining terms. In interpreting and explaining the types of method to be used, the writer used qualitative approach. In qualitative approach, data were described in the form of words and explanation. The findings show that there were two methods of defining terms, dictionary approach and athoritative reference.
Terminology
Blaise Nkwenti
Sead Spuzic
Eurasia Review
Mohamed Chtatou
Terminology is the field of lexicology (or the study of lexicon) that deals with specialized vocabularies and sets of terms related to particular fields (aviation terminology, medical terminology, stylistics, agriculture, etc.). Terminology as a new academic field is located at the boundary among linguistics, logic, theory of existence, information science and specialized areas of science and technology, and in the interdisciplinary area.
Erikas Kupciunas
During the past several decades, the theory of terminology has been a subject of debates in various circles. The views on terminology as a scientific discipline vary considerably. Currently, there are a number of treatments of this field and a number of debatable questions involved. Is terminology a science, or just a practice? does terminology have a status of separate scholarly discipline with its own theory or does it owe its theoretical assumptions to more consolidated disciplines?
Tomasz Michta
Chapter 1.1 of my book A Model for an English-Polish Systematic Dictionary of Chemical Terminology
Alia Channel
Claudia Dobrina
Practical Tools for Leaders and Teams
Terry Schmidt
RELATED PAPERS
Dalbir Judge
johatmi ayala q
Journal of Applied …
saeid ahmadjo
Marine Engineering
Kentaroh Watanabe
Physical Review A
Malaya Kumar Nayak
Joao Carlos
Stephanie Petrie
Internal Medicine
Midori Noguchi
ACTA ZOOLÓGICA MEXICANA (N.S.)
Julio C . Rojas
Ana Lucía Sánchez Montes
Conference Paper
Syed Muhammad Asim Ali Rizvi
Risk Analysis
Vincent Covello
PALOMA RUIZ VEGA
Koko Arianto Wardani
EPRA International Journal of Environmental Economics, Commerce and Educational Management
Shalini Ojha
Journal of Virology
Srinand Sreevatsan
2021 International Conference on Content-Based Multimedia Indexing (CBMI)
amir sohail
Bulletin of Education and Research
Circulation. Cardiovascular interventions
Olli Kajander
INGnosis Revista de Investigación Científica
Jaime Gutierrez Ascon
Martha Augoustinos
Applied Energy
Nguyen Hung
Prof. Vijay Aithekar
The Laryngoscope
Mohamed reda
Genes & development
Tatsuaki Kurosaki
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- USC Libraries
- Research Guides
Organizing Your Social Sciences Research Paper
Glossary of research terms.
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Applying Critical Thinking
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
This glossary is intended to assist you in understanding commonly used terms and concepts when reading, interpreting, and evaluating scholarly research. Also included are common words and phrases defined within the context of how they apply to research in the social and behavioral sciences.
- Acculturation -- refers to the process of adapting to another culture, particularly in reference to blending in with the majority population [e.g., an immigrant adopting American customs]. However, acculturation also implies that both cultures add something to one another, but still remain distinct groups unto themselves.
- Accuracy -- a term used in survey research to refer to the match between the target population and the sample.
- Affective Measures -- procedures or devices used to obtain quantified descriptions of an individual's feelings, emotional states, or dispositions.
- Aggregate -- a total created from smaller units. For instance, the population of a county is an aggregate of the populations of the cities, rural areas, etc. that comprise the county. As a verb, it refers to total data from smaller units into a large unit.
- Anonymity -- a research condition in which no one, including the researcher, knows the identities of research participants.
- Baseline -- a control measurement carried out before an experimental treatment.
- Behaviorism -- school of psychological thought concerned with the observable, tangible, objective facts of behavior, rather than with subjective phenomena such as thoughts, emotions, or impulses. Contemporary behaviorism also emphasizes the study of mental states such as feelings and fantasies to the extent that they can be directly observed and measured.
- Beliefs -- ideas, doctrines, tenets, etc. that are accepted as true on grounds which are not immediately susceptible to rigorous proof.
- Benchmarking -- systematically measuring and comparing the operations and outcomes of organizations, systems, processes, etc., against agreed upon "best-in-class" frames of reference.
- Bias -- a loss of balance and accuracy in the use of research methods. It can appear in research via the sampling frame, random sampling, or non-response. It can also occur at other stages in research, such as while interviewing, in the design of questions, or in the way data are analyzed and presented. Bias means that the research findings will not be representative of, or generalizable to, a wider population.
- Case Study -- the collection and presentation of detailed information about a particular participant or small group, frequently including data derived from the subjects themselves.
- Causal Hypothesis -- a statement hypothesizing that the independent variable affects the dependent variable in some way.
- Causal Relationship -- the relationship established that shows that an independent variable, and nothing else, causes a change in a dependent variable. It also establishes how much of a change is shown in the dependent variable.
- Causality -- the relation between cause and effect.
- Central Tendency -- any way of describing or characterizing typical, average, or common values in some distribution.
- Chi-square Analysis -- a common non-parametric statistical test which compares an expected proportion or ratio to an actual proportion or ratio.
- Claim -- a statement, similar to a hypothesis, which is made in response to the research question and that is affirmed with evidence based on research.
- Classification -- ordering of related phenomena into categories, groups, or systems according to characteristics or attributes.
- Cluster Analysis -- a method of statistical analysis where data that share a common trait are grouped together. The data is collected in a way that allows the data collector to group data according to certain characteristics.
- Cohort Analysis -- group by group analytic treatment of individuals having a statistical factor in common to each group. Group members share a particular characteristic [e.g., born in a given year] or a common experience [e.g., entering a college at a given time].
- Confidentiality -- a research condition in which no one except the researcher(s) knows the identities of the participants in a study. It refers to the treatment of information that a participant has disclosed to the researcher in a relationship of trust and with the expectation that it will not be revealed to others in ways that violate the original consent agreement, unless permission is granted by the participant.
- Confirmability Objectivity -- the findings of the study could be confirmed by another person conducting the same study.
- Construct -- refers to any of the following: something that exists theoretically but is not directly observable; a concept developed [constructed] for describing relations among phenomena or for other research purposes; or, a theoretical definition in which concepts are defined in terms of other concepts. For example, intelligence cannot be directly observed or measured; it is a construct.
- Construct Validity -- seeks an agreement between a theoretical concept and a specific measuring device, such as observation.
- Constructivism -- the idea that reality is socially constructed. It is the view that reality cannot be understood outside of the way humans interact and that the idea that knowledge is constructed, not discovered. Constructivists believe that learning is more active and self-directed than either behaviorism or cognitive theory would postulate.
- Content Analysis -- the systematic, objective, and quantitative description of the manifest or latent content of print or nonprint communications.
- Context Sensitivity -- awareness by a qualitative researcher of factors such as values and beliefs that influence cultural behaviors.
- Control Group -- the group in an experimental design that receives either no treatment or a different treatment from the experimental group. This group can thus be compared to the experimental group.
- Controlled Experiment -- an experimental design with two or more randomly selected groups [an experimental group and control group] in which the researcher controls or introduces the independent variable and measures the dependent variable at least two times [pre- and post-test measurements].
- Correlation -- a common statistical analysis, usually abbreviated as r, that measures the degree of relationship between pairs of interval variables in a sample. The range of correlation is from -1.00 to zero to +1.00. Also, a non-cause and effect relationship between two variables.
- Covariate -- a product of the correlation of two related variables times their standard deviations. Used in true experiments to measure the difference of treatment between them.
- Credibility -- a researcher's ability to demonstrate that the object of a study is accurately identified and described based on the way in which the study was conducted.
- Critical Theory -- an evaluative approach to social science research, associated with Germany's neo-Marxist “Frankfurt School,” that aims to criticize as well as analyze society, opposing the political orthodoxy of modern communism. Its goal is to promote human emancipatory forces and to expose ideas and systems that impede them.
- Data -- factual information [as measurements or statistics] used as a basis for reasoning, discussion, or calculation.
- Data Mining -- the process of analyzing data from different perspectives and summarizing it into useful information, often to discover patterns and/or systematic relationships among variables.
- Data Quality -- this is the degree to which the collected data [results of measurement or observation] meet the standards of quality to be considered valid [trustworthy] and reliable [dependable].
- Deductive -- a form of reasoning in which conclusions are formulated about particulars from general or universal premises.
- Dependability -- being able to account for changes in the design of the study and the changing conditions surrounding what was studied.
- Dependent Variable -- a variable that varies due, at least in part, to the impact of the independent variable. In other words, its value “depends” on the value of the independent variable. For example, in the variables “gender” and “academic major,” academic major is the dependent variable, meaning that your major cannot determine whether you are male or female, but your gender might indirectly lead you to favor one major over another.
- Deviation -- the distance between the mean and a particular data point in a given distribution.
- Discourse Community -- a community of scholars and researchers in a given field who respond to and communicate to each other through published articles in the community's journals and presentations at conventions. All members of the discourse community adhere to certain conventions for the presentation of their theories and research.
- Discrete Variable -- a variable that is measured solely in whole units, such as, gender and number of siblings.
- Distribution -- the range of values of a particular variable.
- Effect Size -- the amount of change in a dependent variable that can be attributed to manipulations of the independent variable. A large effect size exists when the value of the dependent variable is strongly influenced by the independent variable. It is the mean difference on a variable between experimental and control groups divided by the standard deviation on that variable of the pooled groups or of the control group alone.
- Emancipatory Research -- research is conducted on and with people from marginalized groups or communities. It is led by a researcher or research team who is either an indigenous or external insider; is interpreted within intellectual frameworks of that group; and, is conducted largely for the purpose of empowering members of that community and improving services for them. It also engages members of the community as co-constructors or validators of knowledge.
- Empirical Research -- the process of developing systematized knowledge gained from observations that are formulated to support insights and generalizations about the phenomena being researched.
- Epistemology -- concerns knowledge construction; asks what constitutes knowledge and how knowledge is validated.
- Ethnography -- method to study groups and/or cultures over a period of time. The goal of this type of research is to comprehend the particular group/culture through immersion into the culture or group. Research is completed through various methods but, since the researcher is immersed within the group for an extended period of time, more detailed information is usually collected during the research.
- Expectancy Effect -- any unconscious or conscious cues that convey to the participant in a study how the researcher wants them to respond. Expecting someone to behave in a particular way has been shown to promote the expected behavior. Expectancy effects can be minimized by using standardized interactions with subjects, automated data-gathering methods, and double blind protocols.
- External Validity -- the extent to which the results of a study are generalizable or transferable.
- Factor Analysis -- a statistical test that explores relationships among data. The test explores which variables in a data set are most related to each other. In a carefully constructed survey, for example, factor analysis can yield information on patterns of responses, not simply data on a single response. Larger tendencies may then be interpreted, indicating behavior trends rather than simply responses to specific questions.
- Field Studies -- academic or other investigative studies undertaken in a natural setting, rather than in laboratories, classrooms, or other structured environments.
- Focus Groups -- small, roundtable discussion groups charged with examining specific topics or problems, including possible options or solutions. Focus groups usually consist of 4-12 participants, guided by moderators to keep the discussion flowing and to collect and report the results.
- Framework -- the structure and support that may be used as both the launching point and the on-going guidelines for investigating a research problem.
- Generalizability -- the extent to which research findings and conclusions conducted on a specific study to groups or situations can be applied to the population at large.
- Grey Literature -- research produced by organizations outside of commercial and academic publishing that publish materials, such as, working papers, research reports, and briefing papers.
- Grounded Theory -- practice of developing other theories that emerge from observing a group. Theories are grounded in the group's observable experiences, but researchers add their own insight into why those experiences exist.
- Group Behavior -- behaviors of a group as a whole, as well as the behavior of an individual as influenced by his or her membership in a group.
- Hypothesis -- a tentative explanation based on theory to predict a causal relationship between variables.
- Independent Variable -- the conditions of an experiment that are systematically manipulated by the researcher. A variable that is not impacted by the dependent variable, and that itself impacts the dependent variable. In the earlier example of "gender" and "academic major," (see Dependent Variable) gender is the independent variable.
- Individualism -- a theory or policy having primary regard for the liberty, rights, or independent actions of individuals.
- Inductive -- a form of reasoning in which a generalized conclusion is formulated from particular instances.
- Inductive Analysis -- a form of analysis based on inductive reasoning; a researcher using inductive analysis starts with answers, but formulates questions throughout the research process.
- Insiderness -- a concept in qualitative research that refers to the degree to which a researcher has access to and an understanding of persons, places, or things within a group or community based on being a member of that group or community.
- Internal Consistency -- the extent to which all questions or items assess the same characteristic, skill, or quality.
- Internal Validity -- the rigor with which the study was conducted [e.g., the study's design, the care taken to conduct measurements, and decisions concerning what was and was not measured]. It is also the extent to which the designers of a study have taken into account alternative explanations for any causal relationships they explore. In studies that do not explore causal relationships, only the first of these definitions should be considered when assessing internal validity.
- Life History -- a record of an event/events in a respondent's life told [written down, but increasingly audio or video recorded] by the respondent from his/her own perspective in his/her own words. A life history is different from a "research story" in that it covers a longer time span, perhaps a complete life, or a significant period in a life.
- Margin of Error -- the permittable or acceptable deviation from the target or a specific value. The allowance for slight error or miscalculation or changing circumstances in a study.
- Measurement -- process of obtaining a numerical description of the extent to which persons, organizations, or things possess specified characteristics.
- Meta-Analysis -- an analysis combining the results of several studies that address a set of related hypotheses.
- Methodology -- a theory or analysis of how research does and should proceed.
- Methods -- systematic approaches to the conduct of an operation or process. It includes steps of procedure, application of techniques, systems of reasoning or analysis, and the modes of inquiry employed by a discipline.
- Mixed-Methods -- a research approach that uses two or more methods from both the quantitative and qualitative research categories. It is also referred to as blended methods, combined methods, or methodological triangulation.
- Modeling -- the creation of a physical or computer analogy to understand a particular phenomenon. Modeling helps in estimating the relative magnitude of various factors involved in a phenomenon. A successful model can be shown to account for unexpected behavior that has been observed, to predict certain behaviors, which can then be tested experimentally, and to demonstrate that a given theory cannot account for certain phenomenon.
- Models -- representations of objects, principles, processes, or ideas often used for imitation or emulation.
- Naturalistic Observation -- observation of behaviors and events in natural settings without experimental manipulation or other forms of interference.
- Norm -- the norm in statistics is the average or usual performance. For example, students usually complete their high school graduation requirements when they are 18 years old. Even though some students graduate when they are younger or older, the norm is that any given student will graduate when he or she is 18 years old.
- Null Hypothesis -- the proposition, to be tested statistically, that the experimental intervention has "no effect," meaning that the treatment and control groups will not differ as a result of the intervention. Investigators usually hope that the data will demonstrate some effect from the intervention, thus allowing the investigator to reject the null hypothesis.
- Ontology -- a discipline of philosophy that explores the science of what is, the kinds and structures of objects, properties, events, processes, and relations in every area of reality.
- Panel Study -- a longitudinal study in which a group of individuals is interviewed at intervals over a period of time.
- Participant -- individuals whose physiological and/or behavioral characteristics and responses are the object of study in a research project.
- Peer-Review -- the process in which the author of a book, article, or other type of publication submits his or her work to experts in the field for critical evaluation, usually prior to publication. This is standard procedure in publishing scholarly research.
- Phenomenology -- a qualitative research approach concerned with understanding certain group behaviors from that group's point of view.
- Philosophy -- critical examination of the grounds for fundamental beliefs and analysis of the basic concepts, doctrines, or practices that express such beliefs.
- Phonology -- the study of the ways in which speech sounds form systems and patterns in language.
- Policy -- governing principles that serve as guidelines or rules for decision making and action in a given area.
- Policy Analysis -- systematic study of the nature, rationale, cost, impact, effectiveness, implications, etc., of existing or alternative policies, using the theories and methodologies of relevant social science disciplines.
- Population -- the target group under investigation. The population is the entire set under consideration. Samples are drawn from populations.
- Position Papers -- statements of official or organizational viewpoints, often recommending a particular course of action or response to a situation.
- Positivism -- a doctrine in the philosophy of science, positivism argues that science can only deal with observable entities known directly to experience. The positivist aims to construct general laws, or theories, which express relationships between phenomena. Observation and experiment is used to show whether the phenomena fit the theory.
- Predictive Measurement -- use of tests, inventories, or other measures to determine or estimate future events, conditions, outcomes, or trends.
- Principal Investigator -- the scientist or scholar with primary responsibility for the design and conduct of a research project.
- Probability -- the chance that a phenomenon will occur randomly. As a statistical measure, it is shown as p [the "p" factor].
- Questionnaire -- structured sets of questions on specified subjects that are used to gather information, attitudes, or opinions.
- Random Sampling -- a process used in research to draw a sample of a population strictly by chance, yielding no discernible pattern beyond chance. Random sampling can be accomplished by first numbering the population, then selecting the sample according to a table of random numbers or using a random-number computer generator. The sample is said to be random because there is no regular or discernible pattern or order. Random sample selection is used under the assumption that sufficiently large samples assigned randomly will exhibit a distribution comparable to that of the population from which the sample is drawn. The random assignment of participants increases the probability that differences observed between participant groups are the result of the experimental intervention.
- Reliability -- the degree to which a measure yields consistent results. If the measuring instrument [e.g., survey] is reliable, then administering it to similar groups would yield similar results. Reliability is a prerequisite for validity. An unreliable indicator cannot produce trustworthy results.
- Representative Sample -- sample in which the participants closely match the characteristics of the population, and thus, all segments of the population are represented in the sample. A representative sample allows results to be generalized from the sample to the population.
- Rigor -- degree to which research methods are scrupulously and meticulously carried out in order to recognize important influences occurring in an experimental study.
- Sample -- the population researched in a particular study. Usually, attempts are made to select a "sample population" that is considered representative of groups of people to whom results will be generalized or transferred. In studies that use inferential statistics to analyze results or which are designed to be generalizable, sample size is critical, generally the larger the number in the sample, the higher the likelihood of a representative distribution of the population.
- Sampling Error -- the degree to which the results from the sample deviate from those that would be obtained from the entire population, because of random error in the selection of respondent and the corresponding reduction in reliability.
- Saturation -- a situation in which data analysis begins to reveal repetition and redundancy and when new data tend to confirm existing findings rather than expand upon them.
- Semantics -- the relationship between symbols and meaning in a linguistic system. Also, the cuing system that connects what is written in the text to what is stored in the reader's prior knowledge.
- Social Theories -- theories about the structure, organization, and functioning of human societies.
- Sociolinguistics -- the study of language in society and, more specifically, the study of language varieties, their functions, and their speakers.
- Standard Deviation -- a measure of variation that indicates the typical distance between the scores of a distribution and the mean; it is determined by taking the square root of the average of the squared deviations in a given distribution. It can be used to indicate the proportion of data within certain ranges of scale values when the distribution conforms closely to the normal curve.
- Statistical Analysis -- application of statistical processes and theory to the compilation, presentation, discussion, and interpretation of numerical data.
- Statistical Bias -- characteristics of an experimental or sampling design, or the mathematical treatment of data, that systematically affects the results of a study so as to produce incorrect, unjustified, or inappropriate inferences or conclusions.
- Statistical Significance -- the probability that the difference between the outcomes of the control and experimental group are great enough that it is unlikely due solely to chance. The probability that the null hypothesis can be rejected at a predetermined significance level [0.05 or 0.01].
- Statistical Tests -- researchers use statistical tests to make quantitative decisions about whether a study's data indicate a significant effect from the intervention and allow the researcher to reject the null hypothesis. That is, statistical tests show whether the differences between the outcomes of the control and experimental groups are great enough to be statistically significant. If differences are found to be statistically significant, it means that the probability [likelihood] that these differences occurred solely due to chance is relatively low. Most researchers agree that a significance value of .05 or less [i.e., there is a 95% probability that the differences are real] sufficiently determines significance.
- Subcultures -- ethnic, regional, economic, or social groups exhibiting characteristic patterns of behavior sufficient to distinguish them from the larger society to which they belong.
- Testing -- the act of gathering and processing information about individuals' ability, skill, understanding, or knowledge under controlled conditions.
- Theory -- a general explanation about a specific behavior or set of events that is based on known principles and serves to organize related events in a meaningful way. A theory is not as specific as a hypothesis.
- Treatment -- the stimulus given to a dependent variable.
- Trend Samples -- method of sampling different groups of people at different points in time from the same population.
- Triangulation -- a multi-method or pluralistic approach, using different methods in order to focus on the research topic from different viewpoints and to produce a multi-faceted set of data. Also used to check the validity of findings from any one method.
- Unit of Analysis -- the basic observable entity or phenomenon being analyzed by a study and for which data are collected in the form of variables.
- Validity -- the degree to which a study accurately reflects or assesses the specific concept that the researcher is attempting to measure. A method can be reliable, consistently measuring the same thing, but not valid.
- Variable -- any characteristic or trait that can vary from one person to another [race, gender, academic major] or for one person over time [age, political beliefs].
- Weighted Scores -- scores in which the components are modified by different multipliers to reflect their relative importance.
- White Paper -- an authoritative report that often states the position or philosophy about a social, political, or other subject, or a general explanation of an architecture, framework, or product technology written by a group of researchers. A white paper seeks to contain unbiased information and analysis regarding a business or policy problem that the researchers may be facing.
Elliot, Mark, Fairweather, Ian, Olsen, Wendy Kay, and Pampaka, Maria. A Dictionary of Social Research Methods. Oxford, UK: Oxford University Press, 2016; Free Social Science Dictionary. Socialsciencedictionary.com [2008]. Glossary. Institutional Review Board. Colorado College; Glossary of Key Terms. Writing@CSU. Colorado State University; Glossary A-Z. Education.com; Glossary of Research Terms. Research Mindedness Virtual Learning Resource. Centre for Human Servive Technology. University of Southampton; Miller, Robert L. and Brewer, John D. The A-Z of Social Research: A Dictionary of Key Social Science Research Concepts London: SAGE, 2003; Jupp, Victor. The SAGE Dictionary of Social and Cultural Research Methods . London: Sage, 2006.
- << Previous: Independent and Dependent Variables
- Next: 1. Choosing a Research Problem >>
- Last Updated: Apr 20, 2024 2:57 PM
- URL: https://libguides.usc.edu/writingguide
Scientific Research and Methodology
2.2 conceptual and operational definitions.
Research studies usually include terms that must be carefully and precisely defined, so that others know exactly what has been done and there are no ambiguities. Two types of definitions can be given: conceptual definitions and operational definitions .
Loosely speaking, a conceptual definition explains what to measure or observe (what a word or a term means for your study), and an operational definitions defines exactly how to measure or observe it.
For example, in a study of stress in students during a university semester. A conceptual definition would describe what is meant by ‘stress.’ An operational definition would describe how the ‘stress’ would be measured.
Sometimes the definitions themselves aren’t important, provided a clear definition is given. Sometimes, commonly-accepted definitions exist, so should be used unless there is a good reason to use a different definition (for example, in criminal law, an ‘adult’ in Australia is someone aged 18 or over ).
Sometimes, a commonly-accepted definition does not exist, so the definition being used should be clearly articulated.
Example 2.2 (Operational and conceptual definitions) Players and fans have become more aware of concussions and head injuries in sport. A Conference on concussion in sport developed this conceptual definition ( McCrory et al. 2013 ) :
Concussion is a brain injury and is defined as a complex pathophysiological process affecting the brain, induced by biomechanical forces. Several common features that incorporate clinical, pathologic and biomechanical injury constructs that may be utilised in defining the nature of a concussive head injury include: Concussion may be caused either by a direct blow to the head, face, neck or elsewhere on the body with an “impulsive” force transmitted to the head. Concussion typically results in the rapid onset of short-lived impairment of neurological function that resolves spontaneously. However, in some cases, symptoms and signs may evolve over a number of minutes to hours. Concussion may result in neuropathological changes, but the acute clinical symptoms largely reflect a functional disturbance rather than a structural injury and, as such, no abnormality is seen on standard structural neuroimaging studies. Concussion results in a graded set of clinical symptoms that may or may not involve loss of consciousness. Resolution of the clinical and cognitive symptoms typically follows a sequential course. However, it is important to note that in some cases symptoms may be prolonged.
While this is all helpful… it does not explain how to identify a player with concussion during a game.
Rugby decided on this operational definition ( Raftery et al. 2016 ) :
… a concussion applies with any of the following: The presence, pitch side, of any Criteria Set 1 signs or symptoms (table 1)… [ Note : This table includes symptoms such as ‘convulsion,’ ‘clearly dazed,’ etc.]; An abnormal post game, same day assessment…; An abnormal 36–48 h assessment…; The presence of clinical suspicion by the treating doctor at any time…
Example 2.3 (Operational and conceptual definitions) Consider a study requiring water temperature to be measured.
An operational definition would explain how the temperature is measured: the thermometer type, how the thermometer was positioned, how long was it left in the water, and so on.

Example 2.4 (Operational definitions) Consider a study measuring stress in first-year university students.
Stress cannot be measured directly, but could be assessed using a survey (like the Perceived Stress Scale (PSS) ( Cohen et al. 1983 ) ).
The operational definition of stress is the score on the ten-question PSS. Other means of measuring stress are also possible (such as heart rate or blood pressure).
Meline ( 2006 ) discusses five studies about stuttering, each using a different operational definition:
- Study 1: As diagnosed by speech-language pathologist.
- Study 2: Within-word disfluences greater than 5 per 150 words.
- Study 3: Unnatural hesitation, interjections, restarted or incomplete phrases, etc.
- Study 4: More than 3 stuttered words per minute.
- Study 5: State guidelines for fluency disorders.
A study of snacking in Australia ( Fayet-Moore et al. 2017 ) used this operational definition of ‘snacking’:
…an eating occasion that occurred between meals based on time of day. — Fayet-Moore et al. ( 2017 ) (p. 3)
A study examined the possible relationship between the ‘pace of life’ and the incidence of heart disease ( Levine 1990 ) in 36 US cities. The researchers used four different operational definitions for ‘pace of life’ (remember the article was published in 1990!):
- The walking speed of randomly chosen pedestrians.
- The speed with which bank clerks gave ‘change for two $20 bills or [gave] two $20 bills for change.’
- The talking speed of postal clerks.
- The proportion of men and women wearing a wristwatch.
None of these perfectly measure ‘pace of life,’ of course. Nonetheless, the researchers found that, compared to people on the West Coast,
… people in the Northeast walk faster, make change faster, talk faster and are more likely to wear a watch… — Levine ( 1990 ) (p. 455)
Glossary of Key Research Terms
This glossary provides definitions of many of the terms used in the guides to conducting qualitative and quantitative research. The definitions were developed by members of the research methods seminar (E600) taught by Mike Palmquist in the 1990s and 2000s.

Citation Information
Members of the Research Methods Seminar (E600) taught by Mike Palmquist in the 1990s and 2000s. (1994-2024). Glossary of Key Terms. The WAC Clearinghouse. Colorado State University. Available at https://wac.colostate.edu/repository/writing/guides/.
Copyright Information
Copyright © 1994-2024 Colorado State University and/or this site's authors, developers, and contributors . Some material displayed on this site is used with permission.
- Office of Clinical Trials
- Office of Research Integrity
- Office of Sponsored Programs
- Office of Undergraduate Research
- International Gaming Institute
- Nevada Institute of Personalized Medicine
- National Supercomputing Institute
- Science and Engineering Building
- Harry Reid Center
- Policies & Forms
- Faculty Awards
- Councils & Committees
- Faculty/Staff Directory
- Research Electronic Data Capture
- Directories
Quick Links
- Directories Home
- Colleges, Schools, and Departments
- Administrative Units
- Research Centers and Institutes
- Resources and Services
- Employee Directory
- Contact UNLV
- Social Media Directory
- UNLV Mobile Apps
- Research Home
- Division Units
Definition of Terms
- Info for Researchers
- Information for Research Subjects
- Institutional Review Boards
- Policies and Regulation
- Additional Resources
- Human Subjects Home
- Information For:
- Researchers
- Research Subjects
- Report a Concern/Provide Input
Any untoward occurrence in a research participant. The occurrence need not have a clear causal relationship with the individual’s participation in the research; an AE can be any unfavorable and unintended sign, symptom, event, or occurrence affecting a participant’s physical, mental, social, financial, legal, or psychological well-being. An unanticipated AE should be reported to the committee as soon as possible after it is identified.
Agreement by an individual not competent to give legally valid informed consent (e.g., a child or cognitively impaired person) to participate in research. An assent is typically paired with permission from a parent or guardian, and together they comprise the informed consent to participate.
An officer of an institution with the authority to speak for and legally commit the institution to adherence to the requirements of the federal regulations regarding the involvement of human subjects in biomedical and behavioral research.
A statement of basic ethical principles governing research involving human subjects issued by the National Commission for the Protection of Human Subjects in 1979. View a summary of the Belmont Report . The Belmont Report principles permeate human subjects research to this day.
An ethical principle discussed in the Belmont Report that entails an obligation to protect persons from harm. The principle of beneficence can be expressed in two general rules: 1) do not harm; and 2) protect from harm by maximizing possible benefits and minimizing possible risks of harm.
A valued or desired outcome associated with a research project. Anticipated benefits may express the probability that subjects and society may benefit from the research procedures. Research may benefit the individual or society as a whole. If research will not benefit individuals, it is required to provide a reasonable likelihood of resulting in benefits to society. UNLV’s human research application requests information about the direct benefits accruing to the research participants and to society. Compensation and incentives given to participants are not considered benefit.
This is a certificate issued by the National Institutes of Health that protects identifiable research information of a sensitive nature from forced disclosure. It is typically requested when the researcher believes his/her research objectives could not be met without this form of protection.
Persons who have not attained the legal age for consent to treatment or procedures involved in the research, as determined under the applicable law of the jurisdiction in which the research will be conducted [45 CFR 46 46.401(a)]. In Nevada, individuals younger than 18 years of age are considered children for most research situations, and informed consent then consists of the child’s assent and the parent’s permission.(See “Assent.”)
The act of forcing or compelling one to take action against one’s will. Coercion can be overt or perceived, and it can occur when the researcher is in a position of authority or power over the subject (for example, teachers over students or physicians over patients). It can also occur when incentives become so great that the participant will only participate to attain the incentive.
Having either a psychiatric disorder (e.g., psychosis, neurosis, personality or behavior disorders, or dementia) or a developmental disorder (e.g., mental retardation) that affects cognitive or emotional functions to the extent that capacity for judgment and reasoning is significantly diminished. Others, including persons under the influence of or dependent on drugs or alcohol, those suffering from degenerative diseases affecting the brain, terminally ill patients, and persons with severely disabling physical handicaps, may also be compromised in their ability to make decisions in their best interests.
Human subjects research projects conducted by more than one institution. Each institution is responsible for safeguarding the rights and welfare of human subjects. Arrangements for joint review, relying upon one qualified IRB, or similar arrangements are acceptable. (Please contact the ORI-HS staff if this situation occurs; they can assist with the arrangements.)
Payment for participation in research. Compensation should be appropriate for the amount of effort involved, and not excessive and thereby coercive. Compensation is NOT considered a benefit.
Technically, a legal term, used to denote capacity to act on one’s own behalf; the ability to understand information presented, to appreciate the consequences of acting (or not acting) on that information, and to make a choice. (See also: Incompetence, Incapacity)
Pertains to the treatment of information that an individual has disclosed in a relationship of trust and with the expectation that it will not be divulged to others without permission in ways that are inconsistent with the understanding of the original disclosure.
Defined as a set of conditions in which an investigator’s judgment concerning a primary interest (e.g., subject welfare, integrity of research) could be biased by a secondary interest (e.g., personal or financial gain). See information regarding UNLV’s Conflict of Interest/Compensated Outside Services Policy .
See “Informed Consent.”
Subject(s) used for comparison who are not given the treatment under study or who do not have a given condition, background, or risk factor that is the object of study. Control conditions may be concurrent (occurring more or less simultaneously with the condition under study) or historical (preceding the condition under study). When the present condition of subjects is compared with their own condition on a prior regimen or treatment, the study is considered historically controlled.
The other primary scholar or researcher involved in conducting the research. Co-PIs must also meet the UNLV PI eligibility requirements.
Giving subjects previously undisclosed information about the research project following completion of their participation in research.
A code of ethics for clinical research approved by the World Medical Association in 1964 and widely adopted by medical associations in various countries. It was revised most recently in 2008.
Any study that is not truly experimental (e.g., quasi-experimental studies, correlational studies, record reviews, case histories, and observational studies).
A legal status conferred upon persons who have not yet attained the age of legal competency as defined by state law (for such purposes as consenting to medical care), but who are entitled to treatment as if they had by virtue of assuming adult responsibilities such as marriage, procreation, or being self-supporting and not living at home. (See also “Mature Minor.”)
Fair or just; used in the context of selection of subjects to indicate that the benefits and burdens of research are fairly distributed.
The code of federal regulations (45 CFR 46.101(b)) identifies several categories of minimal risk research as exempt from the Federal Policy for the Protection of Research Subjects. This determination must not be made by the PI, but by the IRB or someone appointed by the IRB. For more information, see the U.S. Health and Human Services website, “ Exempt Research and Research That May Undergo Expedited Review .”
The code of federal regulations (45 CFR 46.110 and 21 CFR 56.110) identifies several categories of minimal risk research that may be reviewed through an expedited review process. For more information, see the U.S. Health and Human Services website on “ Guidance on Expedited Review Procedures .”
This act defines the rights of students and parents concerning reviewing, amending, and disclosing educational records and requires written permission to disclose personally identifiable information from a student’s education record, except under certain circumstances such as an order of subpoena. 1
The federal policy that provides regulations for the involvement of human subjects in research. The policy applies to all research involving human subjects conducted, supported, or otherwise subject to regulation by any federal department or agency that takes appropriate administrative action to make the policy applicable to such research. Currently, 16 federal agencies have adopted this policy, commonly referred to as “The Federal Policy,” but also known as the “Common Rule.”
A formal written, binding commitment that is submitted to the Department of Health and Human Services (DHHS) Office of Human Research Protections (OHRP) in which an institution agrees to comply with applicable regulations governing research with human subjects and stipulates the procedures through which compliance will be achieved. UNLV’s assurance number is FWA00002305.
Review of proposed research at a convened meeting at which a majority of the membership of the IRB are present, including at least one member whose primary concerns are in nonscientific areas. For the research to be approved, it must receive the approval of a majority of those members present at the meeting. Generally, studies that undergo full board review are studies involving greater than minimal risk, risky, or novel procedures or vulnerable populations.
An individual who is authorized under applicable state or local law to give permission on behalf of a child for general medical care. In Nevada, under NRS 159.0805, guardians may not give permission for a child to enter into a research study unless a court order has been obtained.
The rule which protects the privacy of individually identifiable health information. The privacy rule provides federal protections for personal health information held by covered entities and gives patients specific rights with respect to that information.
Individuals whose physiological or behavioral characteristics and responses are the object of study in a research project. Under the federal regulations, human subjects are defined as living individual(s) about whom an investigator conducting research obtains: (1) data through intervention or interaction with the individual; or (2) identifiable private information.
Federal regulations define identifiable to mean that the identity of the individual subject is or may readily be ascertained by the investigator or may be associated with the information.
This refers to a person’s mental status and means inability to understand information presented, to appreciate the consequences of acting (or not acting) on that information, and to make a choice. The term is often used as a synonym for incompetence.
A legal term meaning inability to manage one’s own affairs, and often used as a synonym for incapacity.
A person’s voluntary agreement, based upon adequate knowledge and understanding of relevant information, to participate in research or to undergo a diagnostic, therapeutic, or preventive procedure. In giving informed consent, subjects may not waive or appear to waive any of their legal rights, or release or appear to release the investigator, the sponsor, the institution, or agents thereof from liability for negligence.
Institutional research (also called internal research) is the gathering of data from or about UNLV students, faculty, and staff by university offices or organizations, with the sole intent of using the data for internal informational purposes or for required data-collection purposes. This data would not be made generalizable. Examples include surveys to improve university services or procedures; ascertain the opinions, experiences, or preferences of the university community; or to provide necessary information to characterize the university community. This kind of data gathering does not require IRB review unless respondents are queried about sensitive aspects of their own behavior. For debatable projects, investigators should submit an exclusion review form to the ORI-HS.
A specially constituted, federally mandated review body established or designated by an entity to protect the welfare of human subjects recruited to participate in biomedical or behavioral research. UNLV has two IRBs – Social/Behavioral and Biomedical.
The federal regulations define interaction as “communication or interpersonal contact between investigator and subject.”
The federal regulations define intervention as both physical procedures by which data are gathered (for example, venipuncture) and manipulations of the subject or the subject’s environment that are performed for research purposes.
This refers to a researcher conducting the project. Investigators can be principal investigators or co-principal investigators. Students are always listed as student investigators.
A formal agreement between UNLV and another FWA-holding institution that allows the one IRB to serve as the “IRB of Record” for protocols involving collaborative research between UNLV and the other institution.
A term utilized when an institution assumes the IRB responsibilities for a human subject research protocol conducted at another institution. An IRB authorization agreement signed by institutional officials at both institutions is required.
An ethical principle discussed in the Belmont Report requiring fairness in distribution of burdens and benefits; those that bear the burdens of research should also receive the benefits. There must be fair and equitable selection of subjects.
A person authorized either by statute or by court appointment to make decisions on behalf of another person. In human subjects research, an individual or judicial or other body authorized under applicable law to consent on behalf of a prospective subject to the subject’s participation in the procedure(s) involved in the research.
Someone who has not reached adulthood (as defined by state law) but who may be treated as an adult for certain purposes (e.g., consenting to medical care). Note that a mature minor is not necessarily an emancipated minor. (See also “Emancipated Minor.”)
A risk is minimal when the probability and magnitude of harm or discomfort anticipated in the proposed research are not greater, in and of themselves, than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests. For example, the risk of drawing a small amount of blood from a healthy individual for research purposes is no greater than the risk of doing so as part of routine physical examination. Note: The definition of minimal risk for research involving prisoners differs somewhat from that given for non-institutionalized adults.
Any change to an IRB-approved study protocol, regardless of the level of review it receives initially.
A federally mandated member of an Institutional Review Board who has no ties to the parent institution, its staff, or faculty. This individual is usually from the local community (e.g., business person, attorney, or teacher).
A code of research ethics developed during the trials of Nazi war criminals following World War II and widely adopted as a standard during the 1950s and 1960s for protecting human subjects.
The office within the Department of Health and Human Services that is responsible for implementing DHHS regulations (45CFR46) governing research involving human subjects.
The UNLV office, formerly known as the Office for the Protection of Research Subjects (OPRS), that serves as an administrative hub for the UNLV IRB’s oversight of human subjects research.
The agreement of parent(s) to the participation of their child in research.
The scientist or scholar with primary responsibility for the design and conduct of a research project. See UNLV’s PI Eligibility Policy for those who are eligible for automatic PI status and how to apply for PI status.
An individual involuntarily confined in a penal institution, including persons: 1) sentenced under a criminal or civil statue; 2) detained pending arraignment, trial, or sentencing; and 3) detained in other facilities (e.g., for drug detoxification or treatment of alcoholism) under statutes or commitment procedures providing such alternatives to criminal prosecution or incarceration in a penal institution. Note that this includes adjudicated youth.
Control over the extent, timing, and circumstances of disclosing personal information (physical, behavioral, or intellectual) with others.
Defined by the federal regulations to include information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place. It also includes information that has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (e.g., a medical record). Private information must be individually identifiable (i.e., the identity of the subject is or may readily be ascertained by the investigator or associated with the information) in order for the acquisition of the information to constitute research involving human subjects.
Studies designed to observe outcomes or events that occur subsequent to the identification of the group of subjects to be studied. Prospective studies need not involve manipulation or intervention but may be purely observational or involve only the collection of data.
Applies to survey research conducted in schools and states that parents have the right to inspect surveys and questionnaires distributed within schools. This amendment also specifies that parental permission must be obtained to have minors participate in surveys that disclose certain types of sensitive information. 1
The formal design or plan of an experiment or research study; specifically, the plan submitted to an IRB for review and to an agency for research support. The protocol includes a description of the research design or methodology to be employed, the eligibility requirements for prospective subjects and controls, the treatment regimen(s), and the proposed methods of analysis that will be performed on the collected data.
A systematic investigation (i.e., the gathering and analysis of information) designed to develop or contribute to generalizable knowledge.
An ethical principle discussed in the Belmont Report requiring that individual autonomy be respected and persons with diminished autonomy be protected.
Research conducted by reviewing records from the past (e.g., birth and death certificates, medical records, school records, or employment records) or by obtaining information about past events elicited through interviews or surveys. Case control studies are an example of this type of research. This requires IRB review, as long as it involves private information about humans.
The probability of harm or injury (physical, psychological, social, or economic) occurring as a result of participation in a research study. Both the probability and magnitude of possible harm may vary from minimal to significant. Risks include immediate risks of study participation as well as risks of long-term effects.
This involves two types of data: 1) data collected by someone other than the principal investigator for a research or non-research purpose, or 2) data that was collected by the principal investigator, but when collected was not intended to be used for human subjects research. For data to be considered secondary data, the data must exist prior to the initiation of the current research study or be “on the shelf” at the time of study initiation. Principal investigators must submit and receive approval for use of secondary human subjects data prior to initiation of the project.
A visit by agency officials, representatives, or consultants to the location of a research activity to assess the adequacy of IRB protection of human subjects or the capability of personnel to conduct the research.
“Participant” is the preferred term since it more correctly portrays the participatory aspects of research. Sometimes “subject” more accurately describes the role.
Free of coercion, duress, or undue inducement or influence. Used in the research context to refer to a subject’s decision to participate (or to continue to participate) in a research activity.
- Visit the University of Nebraska–Lincoln
- Apply to the University of Nebraska–Lincoln
- Give to the University of Nebraska–Lincoln
Search Form
Key research terms.
bias: any influence that may distort the results of a research study and lead to error; the loss of balance and accuracy in the use of research methods.
case study: presentation of data about selected settings, persons, groups or events. Data can have been gathered using variety of different research methods (e.g., questionnaire, observation, historical or literary analysis). Is chiefly descriptive and analytical, usually based on qualitative data, though statistics such as survey findings may be included.
causal relationship: relationship between variables where movements in one or more variable(s) are held to cause changes in the other (s).
coded data: data are put into groups or categories, such as age groups, and each category is given a code number. Data are usually coded for convenience, speed, and handling to enable statistical analysis. construct: a mental state that can’t be directly observed or manipulated, such as love, intelligence, hunger, feeling warm, and aggression; a concept developed (constructed) for describing relations among or between phenomena or for other research purposes.
construct validity: the degree to which the study actually measures and manipulates the elements that the researcher claims to be measuring and manipulating. If the operational definitions of the constructs are poor, the study will not have good construct validity. For example, a test claiming to measure “aggressiveness” would not have construct validity if it really measured assertiveness.
internal validity: the degree to which the study demonstrates that a particular factor caused a change in behavior. If a study lacks internal validity, the researcher may falsely believe that a factor causes an effect when it really doesn’t. Most studies involving humans do not have internal validity because they can’t rule out the possibility that some other factor may have been responsible for the effect.
controls: processes used to make uniform or constant the conditions for carrying out an investigation.
control group: in experimental research, the group or item which does not receive the treatment or intervention under investigation and is used to compare outcomes with the one that does. correlation: the extent to which two or more factors vary in relationship to one another; the extent of association between two or more variables. Correlation does not equal causation. For example, might suggest relationship between academic success and self-esteem, but cannot prove that a change in first variable causes a change in second variable. correlation coefficient: a measure of the degree of relationship between two variables. It usually lies between +1 (showing a perfect positive relationship), 0 (showing no relationship), to -1.0 (showing a perfect negative relationship). dependent variable: variable thought to be determined or influenced by others.
experiment: a special type of study (not all studies are experiments!) that allows researchers to determine the cause of an effect; usually involves randomly assigning participants to groups.
external validity: the degree to which the results of the study can be generalized to other places, people, or times.
hypothesis: a proposition which research sets out to prove or disprove: “experimental” where the hypothesis is a positive statement, or “null” where statement contains a negative.
independent variable: a variable that researcher believes precedes, influences or predicts the dependent variable.
informed consent: giving potential participants information about the study, especially in terms of factors that might lead them to refuse to be in the study, before they decide whether to participate. Institutional Review Board (IRB): a committee of at least five members--one of whom must be a nonscientist--that review proposed research and monitor approved research in an effort to protect human research participants.
literature review: often the first step in the research process, it is a review of the literature on and around the subject of inquiry. Its main purposes are to avoid duplication, to identify gaps in research and to place the researcher’s approach within the work and approaches of others.
primary/secondary sources: primary sources are original firsthand records or materials relating to an event or happening. They may include, for example, official minutes of meetings, diaries, verbatim transcripts of interviews, completed questionnaires or records of the results of experiments. Secondary sources are accounts bases upon these, which usually offer an interpretation, commentary, analysis, or restatement of the primary sources. They can include, for example, books, journal articles, and conference papers.
qualitative data: information gathered in narrative, non-numerical form (e.g., transcript of an interview). Qualitative research used for exploratory (hypothesis-generating) purposes or explaining puzzling quantitative results, while quantitative methods are used to test hypotheses.
quantitative data: information gathered in numerical form. reliability: extent to which the same result will be repeated/achieved by using the same measure.
statistical significance: tests used to estimate the likelihood that the finding in a sample is true of the population from which the sample is derived and not due to chance.
simple experiment: used to establish cause and effect, so this type often used to determine effect of treatment. Participants randomly assigned to either control group with no treatment, while the experimental group receives treatment.
validity: extent to which research findings can be said to be accurate and reliable; degree to which conclusions are justified. Internal validity is extent to which researchers can show that they have evidence for the statements they make; external validity refers to a study’s generalizability.
Writing Help
Where to find a research paper definition of terms sample.
When writing your research paper, you want to ensure that attention is given to the minutest of details. A definition of terms may not be deemed necessary for some students, especially those who prefer taking the easier route. However, incorporating a definition of terms can greatly enhance your research paper.
Benefits of a Definition of Terms
- This is a useful place to include technical terms in your topic or your research question.
- You can clarify the definition of a term especially if it has different meanings. Include the definition according to how it will be used throughout your research.
- Makes it easy for someone to consult to revisit the definition of a term instead of searching through the paper to try and locate it.
- Remember your paper is written not only for your professor but also for a general audience. You want to ensure that the general public is able to read your research paper and understand technical terminology and jargons.
This being said, if you have never seen a research paper with a definition of terms, you can find here. Otherwise to find samples of definition of terms, you can consider doing the following:
- Use several different research samples that your professor can provide you. From these samples, pick out the ones that contain a definition of terms.
- Use the internet and plug the terms into your favorite search engines. If you do choose the option of using the Internet, find here useful samples.
- Make use of a handbook for research papers which normally have samples there that you can copy and utilize as a guide.
A Guide For Your Definition of Terms
When you go through the definition of terms samples that you can find here, take note that this is not a place for you to add just any terms. This is a place where you define those terms of a technical nature to the research, a term that you would not want your audience to misinterpret. If this will not add any value to your research paper, then you do not have to include a definition of terms which is optional.
- Biography paper suggestions
- Looking for narrative research paper examples
Useful Links
- MyHomeworkDone
- ThesisHelpers
- MyPaperWriter
Institutional Review Board (IRB)
Definition of terms.
The Mayo Clinic Institutional Review Board's definition of terms explains legal definitions related to research guidelines and the protection of human research subjects, including advocate, conflict of interest, emergency treatment, informed consent and more.
- Abbreviated investigational device exemption requirements. The FDA considers an investigation of a nonsignificant risk device to have an approved IDE when an IRB concurs with the initial nonsignificant risk determination of the sponsor or investigator and approves the study. Therefore, both the sponsor and the investigator are expected to comply with 21 CFR 812.2(b), the abbreviated FDA requirements for IDEs. If the study is investigator initiated, then the investigator is also acting as the sponsor.
- Accrual. The number of subjects who have completed or are actively in the process of completing a study at Mayo Clinic. This does not include screen failures. It does include withdrawals. Example: You enroll 100 to accrue 25.
- Administrative hold. A voluntary action initiated by the principal investigator whenever specific research activities, including subject enrollment, are placed temporarily on hold.
- Adverse event. An untoward or undesirable experience associated with the use of a medical product, such as a drug, device or biologic, in a patient or research subject.
- Advocate. An individual who has the background and experience to act in, and agrees to act in, the best interest of the child for the duration of the child's participation in the clinical investigation.
- Agent. For purposes of this document, an institution's employees or agents refers to individuals who (1) act on behalf of the institution, (2) exercise institutional authority or responsibility, or (3) perform institutionally designated activities. Employees and agents can include staff, students, contractors and volunteers, among others, regardless of whether these individuals are receiving compensation. A student's affiliation with an academic institution makes them an agent of that institution; and thus the academic institution is engaged in the research regardless of where the research takes place.
- Allegation of noncompliance. An unproven assertion of noncompliance.
- Alternate member. Alternate IRB committee members may be designated, as needed, for regular voting members. The appointment of alternate members should be based on expertise similar to that of the regular voting member. An alternate member may vote only when the regular voting member is absent.
- Anonymous data. Information that was previously recorded or collected without any of the 18 identifiers as defined by HIPAA, and no code is assigned that would allow data to be traced to an individual.
- Assent. A child's affirmative agreement to participate in research. Mere failure to object should not, absent affirmative agreement, be construed as assent.
- Assured institution. An institution with an FWA that has filed with the federal OHRP. Employees and agents of the institution holding an approved FWA are covered whenever they are involved in the conduct of the research covered by the FWA. Employees and agents are individuals performing institutionally designated activities and acting on behalf of the institution or exercising institutional authority or responsibility.
- Belmont Report. Report by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research identifying the basic ethical principles underlying the conduct of research involving human subjects, that is, respect for persons, beneficence and justice.
- Beneficence. Persons are treated in an ethical manner not only by respecting their decisions and protecting them from harm but also by making efforts to secure their well-being. Such treatment falls under the principle of beneficence. Two general rules have been formulated as expressions of beneficent actions (Belmont Report, 1978): (1) Do no harm, and (2) maximize possible benefits and minimize possible harms.
- Blood and blood products for transfusion and or manufacturing into other products.
- Allergenic extracts, which are used for both diagnosis and treatment, such as allergy shots.
- Human cells and tissues used for transplantation, such as tendons, ligaments and bone.
- Gene therapies.
- Cellular therapies.
- Tests to screen potential blood donors for infectious agents, such as HIV.
- In general, the term "drugs" includes therapeutic biological products.
- Children. People who have not attained the legal age for consent to treatments or procedures involved in the research under the applicable law of the jurisdiction in which the research will be conducted.
- Clinical investigation. The FDA has defined clinical investigation to be synonymous with research. The FDA defines clinical investigation as any experiment that involves a test article and one or more human subjects and that either must meet the requirements for prior submission to the FDA or the results of which are intended to be later submitted to, or held for inspection by, the FDA as part of an application for a research or marketing permit.
- Coded information and data. Identifying information that would enable the investigator to readily ascertain the identity of the individual to whom the private information or specimens pertain has been replaced with a number, letter, symbol or combination thereof and a key to decipher the code exists, enabling linkage of the identifying information to the private information or specimens.
- Is or will be an investigator or member of the research team (that is, listed on the IRB application)
- Has an immediate family member (that is, spouse, dependent children) or personal relationship with an individual serving as the principal investigator or co-principal investigator of the research
- Has a financial or managerial interest in a sponsoring entity or product being evaluated or provided by a commercial entity in the research, as defined by Mayo Clinic Conflict of Interest Policy
- Has received or will receive compensation with value (as defined by Mayo Clinic Conflict of Interest Policy) that may be affected by the outcome of the research project
- Has a proprietary interest in the research, such as a nonprovisional patent application, patent, trademark, copyright or licensing agreement as defined by Mayo Clinic Conflict of Interest Policy
- Has a nonfinancial interest (personal circumstance, ethical belief or other factor) that may be conflicting, for example, the IRB member has an interest that they believe conflicts with their ability to review a project objectively
- Has responsibility for institutional business development, such as raising funds or garnering support for research or as an officer within the Department of Development
- Consent capacity. An individual's ability to understand and process information relevant to making an informed, voluntary decision to participate in research. Several kinds of information are crucial to such decisions, including an understanding of the purpose of the study, its experimental nature, risks and anticipated benefits, the right to withdraw, alternatives to participation, confidentiality protections, and the safeguards used to minimize risks. A wide variety of diseases, disorders, conditions, situations and injuries can affect a person's ability to understand such information, to weigh the advantages and disadvantages of participation in research, and to reach an informed decision regarding study participation.
- Consent document. A structured, written description in lay terms of relevant research project information. The written consent document is not consent itself; it is the record of what has been communicated to a prospective subject. It is the document, based on a template provided by the IRB and approved by the IRB, that ensures all regulatory elements are present and communicated to a potential subject. When signed by the potential subject, the consent document is a record of the receipt of research-related information by the subject. It also serves as reference material for the subject as the research project progresses. It is not legally binding, and the subject may choose to withdraw consent at any time.
- Consultant. A scientist or nonscientist from within or external to Mayo Clinic who has special expertise to act — at the request of the IRB — as an ad hoc reviewer of a human research project application. These individuals have access to all documents relevant to the specific project under review and may participate in the deliberations and make recommendations on the project but may not vote and are not counted toward quorum.
- Continuing noncompliance. Continuing noncompliance is a pattern of noncompliance that continues to occur after a report of noncompliance and a corrective action plan have been reviewed and approved by the IRB. Continuing noncompliance may also be a pattern of noncompliance that continues to occur after the IRB has directed the investigator to correct the issue(s).
- Continuing review. Periodic review of research activities at intervals appropriate to the degree of risk but not less than once a year. The criteria for approval are defined by federal regulations.
- Cooperative research project. Research projects that involve more than one institution as defined by federal regulations.
- Coordinating center. An institution, department or center that agrees to be responsible for the conduct and administrative or coordinating functions of a multicenter research project.
- Co-principal investigator (co-PI). Mayo Clinic investigator who plays a key role in scientific development and conduct of the study. The co-PI collaborates with the principal investigator who has overall responsibility for study conduct at Mayo Clinic (all campuses). Conditions of eligibility for the role of co-PI are the same as those for a PI. Students, residents, fellows and research temporary professional personnel (appointees) are not eligible to be a PI or co-PI on IRB or IACUC-approved research.
- Covered entity. HIPAA regulations apply to health plans, health care clearinghouses and health care providers who transmit health information. Any individual creating or accessing PHI for the delivery of health care at Mayo Clinic is within the covered entity.
- Necessarily deviates from devices generally available or from an applicable performance standard or premarket approval requirement in order to comply with the order of an individual physician or dentist;
- Is not generally available to, or generally used by, other physicians or dentists;
- Is not generally available in finished form for purchase or for dispensing upon prescription;
- Is not offered for commercial distribution through labeling or advertising; and
- Is intended for use by an individual patient named in the order of a physician or dentist and is to be made in a specific form for that patient, or is intended to meet the special needs of the physician or dentist in the course of professional practice (such as a particular operating tool).
Data safety monitoring board (DSMB). An independent committee set up specifically to monitor data throughout the duration of a study to determine if continuation of the study is appropriate scientifically and ethically. Factors that suggest a DSMB is needed:
- A large study population.
- Multiple study sites. It is more difficult to recognize a pattern of increased or unusual problems or events when investigators treat small fractions of the population separately.
- Highly toxic therapies or dangerous procedures.
- High expected rates of morbidity or mortality in the study population.
- High chance of early termination of the study.
DSMB membership is usually composed of experts in the fields of medicine and science that are applicable to the study — statistical experts, lay representatives and others who can offer an unbiased assessment of the study progress.
- Data safety monitoring plan (DSMP). A quality-assurance plan for a research study. A DSMP is meant to ensure that each clinical investigation has a system for appropriate oversight and monitoring of the conduct of the clinical investigation. The purpose of a DSMP is to ensure the safety of the participants, the validity of the data and integrity of the study, and the appropriate termination of studies for which significant benefits or risk has been uncovered or when it appears that the investigation cannot be concluded successfully. A DSMP is commensurate with the risks involved with the research study. The DSMP may include a DSMB.
- Data use agreement. An agreement into which Mayo Clinic and the investigator enter with the intended recipient of a limited data set that establishes the ways in which the information in the limited data set may be used and how it will be protected.
- dbGaP. The National Institutes of Health (NIH) Genome-Wide Association Studies (GWAS) data repository is designated as the database of genotypes and phenotypes (dbGaP) at the National Center for Biotechnology Information (NCBI), a component of the National Library of Medicine (NLM).
- Dead fetus. A fetus that exhibits neither heartbeat, spontaneous respiratory activity, spontaneous movement of voluntary muscles nor pulsation of the umbilical cord.
- The code is not derived from or related to the information about the individual;
- The code could not be translated to identify the individual; and
- The covered entity does not use or disclose the code for other purposes or disclose the mechanism for re-identification.
- The geographic unit formed by combining all ZIP codes with the same three initial digits contains more than 20,000 people, and
- The initial three digits of a ZIP code for all such geographic units containing 20,000 or fewer people are changed to 000
- All elements of dates (except year) for dates directly related to an individual, including birth date, admission date, discharge date, and date of death; and all ages over 89 and all elements of dates (including year) indicative of such age, except that such ages and elements may be aggregated into a single category of age 90 or older
- Phone numbers
- Fax numbers
- Electronic mail addresses
- Social Security numbers
- Medical record numbers
- Health plan beneficiary numbers
- Account numbers
- Certificate and license numbers
- Vehicle identifiers and serial numbers, including license plate numbers
- Device identifiers and serial numbers
- Web Uniform Resource Locators (URLs)
- Internet Protocol (IP) address numbers
- Biometric identifiers, including finger and voice prints
- Full-face photographic images and any comparable images
- Any other unique identifying number, characteristic or code (note this does not mean the unique code assigned by the investigator to code the data)
- Delivery. Complete separation of the fetus from the woman by expulsion or extraction or any other means.
- Recognized in the official United States Pharmacopeia and National Formulary (USP-NF) or the United States Pharmacopeia, or any supplement to them;
- Intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment or prevention of disease in humans or other animals; or
- Intended to affect the structure or any function of the body of humans or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of humans or other animals and which is not dependent on being metabolized for the achievement of any of its primary intended purposes.
- Disclosure of PHI. The release, transfer or provision of access to or divulging in any manner of information outside the covered entity.
- Documentation. The act or an instance of furnishing or authenticating with documents. Documentation of informed consent includes use of a written consent form approved by the IRB and signed and dated by the subject or the subject's legally authorized representative.
- Emergency research. Planned research involving human subjects who have a life-threatening medical condition that necessitates urgent intervention (for which available treatments are unproved or unsatisfactory), and who, because of their condition (such as traumatic brain injury) cannot provide informed consent.
- Emergency treatment IDE. A mechanism through the FDA for providing eligible participants with investigational devices for the treatment of an immediate serious or life-threatening illness for which there are no satisfactory alternatives.
- Emergency treatment IND. A mechanism through the FDA for providing eligible participants with investigational drugs, agents or biologics for the treatment of an immediate serious or life-threatening illness for which there are no satisfactory alternatives.
- Life-threatening includes both life-threatening and severely debilitating diseases or conditions where likelihood of death is high unless the course of the disease is interrupted, and diseases or conditions with potentially fatal outcomes, where the endpoint of clinical trial analysis is survival.
- The criteria for life-threatening do not require the condition to be immediately life-threatening or to immediately result in death. Rather, the subjects must be in a life-threatening situation requiring intervention before review at a convened IRB meeting is feasible.
- Severely debilitating: Diseases or conditions that cause major irreversible morbidity, such as blindness; loss of arm, leg, hand or foot; loss of hearing; paralysis; or stroke.
- Data about the subjects of the research through intervention or interaction with them;
- Identifiable private information about the subjects of the research;
- The informed consent of human subjects for the research; or
- When the institution receives a direct federal award to conduct human subject research, even when all activities involving human subjects are carried out by a subcontractor (that is, employees or agents of another institution). See Engagement of Institutions in Human Subjects Research (2008) .
- Enrollment. Occurs when an eligible, informed potential participant undergoes the initial informed consent process and voluntarily agrees to participate in a research project. Example: You enroll 100 to accrue 25. See also Accrual.
- Exempt human subjects research. Studies determined by the IRB to meet the exempt criteria as defined by the federal regulations. Exempt studies do not require periodic review by the IRB unless a change in the project is planned.
- Expedited review. A review of research involving human subjects by the IRB chair or by one or more experienced reviewers designated by the chair from among members of the IRB in accordance with the requirements set forth in 45 CFR 46.110.
- Experimental subject as defined by the Department of Defense. Research involving a human being as an experimental subject is an activity for research purposes in which there is an intervention or interaction with a human being for the primary purpose of obtaining data regarding the effect of the intervention or interaction [32 CFR.210.102 (f) reference (c)]. Examples include, but are not limited to, a physical procedure, a drug, a manipulation of the subject or subject's environment, and the withholding of an intervention that would have been undertaken if not for the research purpose.
- Expired study. When continuing review of the research does not occur prior to the end of the approval period specified by the IRB, IRB approval expires automatically. The study expires on the date specified on the approval letter and the consent document. No activities can occur after the expiration date.
- External UPIRTSO. A problem or event involving research subjects enrolled by other institutions in multicenter research projects that fall under the purview of an external IRB, that is, not the Mayo Clinic IRB.
- Federalwide assurance (FWA). A formal, written, binding attestation in which an institution ensures the U.S. Department of Health and Human Services that it will comply with applicable regulations governing research with human subjects.
- Fetus. The product of conception from implantation until delivery.
- Final report. A report the principal investigator may elect to submit to the IRB to serve as a final record of any pertinent activity since the last continuing review report and to record research project completion.
- Fluctuating capacity. Capacity to consent may alter as a function of the natural course of an illness, response to treatment, effects of medication, general physical health and other factors. Therefore, a participant's ability to provide ongoing informed consent must be reevaluated periodically throughout the course of their participation in a study.
- Food and Drug Administration (FDA). The regulatory authority in the United States that oversees the pharmaceutical and medical device industries. The FDA is responsible for ensuring that the drugs and medical devices marketed in the U.S. are safe and have a greater benefit than risk when used according to manufacturer's directions.
- Full committee review. Studies reviewed by the full, convened IRB committee with a recorded vote and corresponding minutes to document the discussion.
- Genome-wide association study (GWAS). The National Institutes of Health policy defines GWAS as any study of genetic variation across the entire human genome that is designed to identify genetic associates with observable traits or the presence or absence of a disease or condition. This policy applies to genome-wide association research using genetic materials and data collected both prospectively and retrospectively.
- Greater than minimal risk. The research involves more than minimal risk to subjects.
- Guardian. An individual who is authorized under applicable state or local law to consent on behalf of a child to general medical care when general medical care includes participation in research.
- HIPAA authorization. A customized document or form that gives permission to use specified PHI for a specific purpose, or to disclose PHI to a third party specified by the investigator other than for treatment, payment or health care operations.
- Human biospecimens. A quantity of tissue, blood, urine or other human-derived material. A single biopsy may generate several biospecimens, including multiple paraffin blocks or frozen biospecimens. The molecular makeup of such specimens reflects the physiological or pathological condition of the person from whom they derive; therefore, they provide sensitive and specific insight into the biological state of the donor. A biospecimen can include subcellular structures (such as DNA), cells, tissue (such as bone, muscle, connective tissue and skin), organs (such as liver, bladder, heart and kidney), blood, gametes, embryos, fetal tissue, and waste (such as urine and stool). Portions or aliquots of a biospecimen are referred to as samples. (Derived from National Cancer Institute Best Practices for Biospecimen Research.)
- Humanitarian use device exemption (HDE). An FDA approval for a physician to use a HUD in clinical treatment or as the subject of a clinical investigation.
- Humanitarian use device (HUD). A device that is intended to benefit patients by treating or diagnosing a disease or condition that affects fewer than 4,000 individuals a year in the U.S..
- Human specimen research repository. A collection of human specimens and associated data for research purposes, the physical structure where the collection is stored, and all relevant processes and procedures.
- Intervention: Includes both physical procedures through which data are gathered (for example, venipuncture) and manipulations of the subjects' environment that are performed for research purposes.
- Interaction: Includes communication or interpersonal contact with a subject or their private identifiable information.
- Private information: Includes information about behavior that occurs in a setting in which an individual can reasonably expect that no observation or recording is taking place. It includes information that has been provided for specific purposes by an individual and that the individual can reasonably expect will not be made public (such as a medical record). Private information must be individually identifiable in order to be considered information to constitute research involving human subjects. This may include identifiable private information obtained from a primary subject about a third party.
- Research involving a human being as an experimental subject. An activity for research purposes in which there is an intervention or interaction with a living individual for the primary purpose of obtaining data regarding the effect of the intervention or interaction.
- Research involving human subjects. An activity that both includes a systematic investigation designed to develop or contribute to generalizable knowledge and involves a living individual about whom an investigator conducting research obtains data through intervention or interaction with the individual or identifiable private information.
- Test article: Any drug (including a biological product for human use), medical device for human use, human food additive, color additive, electronic product or any other article subject to FDA regulation.
- Human subject identifier. Any word, number, symbol, or combination of words, numbers or symbols that can be used by a third party to uniquely identify an individual, such as name, Social Security number, address or patient registration number that is provided for use in a research protocol.
- Impaired consent capacity. Impaired consent capacity may involve partial impairment, impairment that fluctuates over time or complete impairment. For example, consent capacity can be affected by a wide range of disorders and conditions, such as dementia, stroke, traumatic brain injury, developmental disorders, serious mental illness, intoxication and delirium.
- Individually identifiable health information. Any information collected from an individual (including demographics) that is created or received by a health care provider, health plan, employer or health care clearinghouse that relates to the past, present or future physical or mental health or condition of an individual, the provision of health care to an individual, or the past, present or future payment for the provision of health care to an individual and that identifies the individual or for which there is reasonable basis to believe that the information can be used to identify the individual.
- Informed consent. An ongoing process of communication between the participant and the study team. Informed consent is a continuing process through which a participant, after having been informed, voluntarily confirms their willingness to participate in a research project and can demonstrate understanding of all aspects of the research project that are relevant to the participant's decision to participate.
- Institutional official (IO). The IO who is the signatory on the FWA filed with the OHRP to ensure compliance with regulations governing protection of human subjects. The OHRP requires the institutional official to be a high-level official who has the authority to represent the institution named in the FWA.
- Institutional review board (IRB). A specifically constituted review body established or designated by an entity to protect the rights and welfare of human subjects recruited to participate in biomedical or behavioral or social science research.
- Interaction. Includes communication or interpersonal contact with a subject or their private identifiable information.
- Intervention. Physical procedures (such as venipuncture) through which data are gathered and manipulations of the subject or the subject's environment that are performed for research purposes.
- Investigational agent. A pharmaceutical form of an active ingredient or placebo being tested or used as a reference in a clinical trial. This includes products with a marketing authorization when used or assembled (formulated or packaged) in a way different from the approved form, products used for an unapproved indication, or products used to gain further information about an approved use.
- Investigational device. A device, including a transitional device, that is the object of an investigation.
- Investigational device exemption (IDE). Application document submitted to the FDA proposing human clinical research to study an unapproved significant risk device or a cleared or approved device for use other than its approved indication or intent. The FDA grants permission so a device that otherwise would be required to comply with a performance standard or to have premarket approval can be shipped lawfully for the purpose of conducting investigations of that device. This FDA permission is evidenced by the assignment of an IDE number.
- Investigational drugs or investigational biologics. New drugs or biologics that have not yet been approved by the FDA or approved drugs that have not yet been approved for a new use and are in the process of being tested for safety and effectiveness.
- Investigational new drug (IND). Application document submitted to the FDA proposing human clinical research to study an unapproved drug or an approved product for a new indication or in a new patient population in a research study. This includes new drugs that have not yet been approved by the FDA or approved drugs that have not yet been approved for a new use and are in the process of being tested for safety and effectiveness. This FDA permission is evidenced by the assignment of an IND number by the FDA or the granting of an IND exemption.
- IRB authorization agreement (IAA). A formal, written agreement in which the reviewing IRB agrees to serve as the IRB of record for a relying organization.
- IRB of record. A reviewing IRB that assumes IRB responsibilities for another organization and is designated to do so through an approved FWA on file with the federal OHRP. Note: Commercial IRBs will not have FWAs but must be registered with the OHRP.
- Label. The FDA-approved label is the official description of a drug or biologic product that includes indication (what the product is used for); who should take it; adverse events (side effects); instructions for uses in pregnancy, children and other populations; and safety information for the patient. Labels are often found inside product packaging.
- Legally authorized representative (LAR). An individual or judicial or other body authorized under applicable law to consent on behalf of a prospective subject to the subject's participation in the procedures involved in the research.
- Legally effective informed consent. A potential participant has been provided enough information to make a decision, the potential participant has the capacity to make a decision, the potential participant understands the consequences of his or her decision, and the potential participant can communicate that decision.
- Limited data set. A limited data set allows retention of specific elements of identifying private information: geographic subdivision, town, city, state, ZIP code, dates, age. Limited data sets are not considered to be de-identified information.
- Local research context. Knowledge of the institution and community environment in which human subjects research will be conducted.
- Material transfer agreement (MTA). A contract that governs the transfer of tangible research materials between two organizations when the recipient intends to use the materials for their own research purposes.
- Mayo Clinic. Mayo Clinic locations in Arizona, Florida and Rochester; Mayo Clinic Health System; and all owned and affiliated clinics, hospitals and entities.
- Listed in the online FDA database Devices@FDA .
- Intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment or prevention of disease, in humans or other animals, or
- Intended to affect the structure or any function of the body of humans or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of humans or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes [21 U.S.C. 321(h)].
- Minimal risk. The probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.
- Minimal risk for prisoners. The probability and magnitude of physical or psychological harm that is normally encountered in the daily lives or in the routine medical, dental or psychological examination of healthy persons.
- Modification. Any change to an IRB-approved study protocol regardless of the level of review it receives initially.
- Neonate. A newborn 0 to 28 days old.
- Nonaffiliated member. Any IRB member who is not currently affiliated with Mayo Clinic and whose immediate family members are not affiliated with Mayo Clinic. Examples of Mayo Clinic affiliation include employment, participation as a student in a Mayo Clinic academic program, or receipt of post-employment benefits, such as health and wellness or a pension.
- Noncompliance. An act or omission in the conduct or oversight of human subject research that represents a failure to follow (1) federal, state or local regulations, (2) institutional policies relevant to human subject research, (3) the approved research plan, and/or (4) the determinations of the IRB.
- Nonsignificant risk (NSR) device study. A study of a device that does not meet the definition for a significant risk device and does not present a potential for serious risk to the health, safety or welfare of participants.
- Non-UPIRTSO. A reportable event that does not meet the Mayo Clinic IRB's definition of a UPIRTSO. See also Unanticipated problem involving risk to subjects or others (UPIRTSO).
- Nonviable neonate. A neonate after delivery that, although living, is not viable.
- Notification. Process of notifying research subjects of changes in the research by letter or phone.
- Office for Human Research Protection (OHRP). The office under the Department of Health and Human Services (HHS) responsible for implementing HHS regulations (45 CFR 46) governing biomedical and behavioral or social science research involving human subjects.
- Oral (verbal) consent. A spoken presentation of the elements of informed consent to the prospective subject or their legally authorized representative. The presentation may be based on information contained within an oral consent script or the written consent document. Oral consent is often associated with waiving the documentation of consent. Oral consent is usually recorded in the research project files.
- Permission. The agreement of parents or guardians to the participation of their child or ward in research.
- Pregnancy. Encompasses the period of time from implantation until delivery. A woman shall be assumed to be pregnant if she exhibits any of the pertinent presumptive signs of pregnancy, such as a missed menses, until the results of a pregnancy test are negative or until delivery.
- Preparatory to research. Any action taken in assessing the research question or hypothesis, such as accessing medical records or querying databases for any type of individually identifiable health information, or any activity in which PHI is accessed to prepare a research protocol.
- Supervising the research process.
- Taking responsibility for ensuring that key study personnel are properly trained and qualified and have appropriate facilities and resources to conduct the research.
- Ensuring adherence to the study protocol.
- Monitoring the informed consent process.
- Communicating regularly and effectively with the research staff.
- Taking responsibility for protecting the safety and welfare of research subjects.
- Prisoner. Prisoner means any individual involuntarily confined or detained in a penal institution. The term is intended to encompass individuals sentenced to such an institution under a criminal or civil statute, individuals detained in other facilities by virtue of statutes or commitment procedures that provide alternatives to criminal prosecution or incarceration in a penal institution, and individuals detained pending arraignment, trial or sentencing.
- Prisoner of war. Any person captured, detained, held or otherwise under the control of Department of Defense (DOD) personnel (military and civilian, or contractor employee) except DOD personnel held for law enforcement purposes (DOD directive 3216.2, section 4.42).
- Privacy versus confidentiality. Privacy is about people and their choice to share personal information. It is a right in health care and research. Confidentiality is about data. It is the investigator's obligation to protect subjects' information.
- Private information. Information about behavior that occurs in a setting in which an individual can reasonably expect that no observation or recording is taking place. It includes information that has been provided for specific purposes by an individual and that the individual can reasonably expect will not be made public, such as a medical record. Private information must be individually identifiable in order to be considered information to constitute research involving human subjects. This may include identifiable private information obtained from a primary subject about a third party.
- Protected health information (PHI). Individually identifiable health information recorded in any form, or media that is created or received by a health care provider.
- Major protocol violation/deviation. Any change that affects the rights and welfare of subjects and others, increases risks to subjects and others, decreases potential benefits, compromises the integrity or validity of the research, or represents willful or knowing misconduct.
- Minor protocol violation/deviation. Any change that did not increase the risk or decrease the benefit or significantly affect the subject's rights, safety or welfare and/or the integrity of research data (for example, a routine lab missed at a visit and redrawn, shortening the duration between a planned study visit; using an outdated HIPAA form or consent form when there are no differences between the two forms other than the approval date).
- Reconsenting. Process of notifying research subjects of changes in the research, including documentation of the subjects' continued informed consent through signature on a revised written consent form.
- Recruitment. Recruitment, a component of the consent process, is the process of distributing or presenting information that describes the research project and eligibility criteria so that a prospective subject may consider enrollment.
- Relying organization. A relying organization has entered into an IRB authorization agreement with another organization's IRB.
- Reportable event. A process (with an associated IRB form) used by investigator to report any problem or event or other act or omission to the IRB that in their opinion is a UPIRTSO.
- Research activities. Research activity includes all contact with the research subject (such as enrolling subjects, intervention or interaction), data collection and data analysis.
- Research as defined by the Department of Health and Human Services. A systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.
- Research as defined by the Department of Defense (DOD). An activity that both includes a systematic investigation designed to develop or contribute to generalizable knowledge and involves a living individual about whom an investigator conducting research obtains data through intervention or interaction with the individual or identifiable private information, or activities covered by section 32 CFR 219.101 (including exempt research involving human subjects) and DOD Instruction 3216.02.
- Must meet the requirements for prior submission to the FDA under section 505(i) of the Federal Food, Drug, and Cosmetic Act, meaning any use of a drug other than the use of an approved drug in the course of medical practice
- Must meet the requirements for prior submission to the Food and Drug Administration under section 520(g) of the Federal Food, Drug, and Cosmetic Act, meaning any activity that evaluates the safety or effectiveness of a device
- Any activity the results of which are intended to be later submitted to, or held for inspection by, the FDA as part of an application for a research or marketing permit
- Research involving a human being as an experimental subject as defined by the Department of Defense (DOD). An activity for research purposes in which there is an intervention or interaction with a living individual for the primary purpose of obtaining data regarding the effect of the intervention or interaction. Research involving a human being as an experimental subject is a subset of research involving human subjects. This definition relates only to the application of section 980 of Title 10 USC; it does not affect the application of 32 CFR 219. This definition does not include activities that are not considered research involving human subjects, activities that meet the exemption criteria at section 32 CFR 219.101(b), and research involving the collection or study of existing data, documents, records or specimens from living individuals. Section 980 of Title 10 USC imposes limitations on waiving informed consent when using DOD-appropriated funds. Section 980 of Title 10 USC is applicable only to DOD-funded research involving a human being as an experimental subject. Section 980 of Title 10 USC is not applicable to exempt research involving human subjects.
- Screen failures. Subjects who consented to participate in research but who were disqualified during screening procedures.
- Serious adverse event. Any untoward medical occurrence that at any dose results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or results in a congenital anomaly or birth defect.
- Serious noncompliance. Any noncompliance that results in or has the potential to (a) substantially compromise the rights and welfare of subjects, (b) substantially impact the integrity and validity of the study data, and (c) compromise the integrity and effectiveness of the Mayo Clinic Human Research Protection Program.
- Short-form consent document. A written consent document stating that the elements of consent have been presented orally. A witness to the oral presentation is required.
- Significant new information. Information that indicates a significant new serious risk or increased severity of known risk, or a safety issue that requires immediate action.
- Is intended as an implant;
- Is used in supporting or sustaining human life, or otherwise prevents impairment of human health;
- Is of substantial importance in diagnosing, curing, mitigating or treating disease, or otherwise prevents impairment of human health; or
- Otherwise presents a potential for serious risk to the health, safety or welfare of a participant.
Sponsor-investigator. An individual who both initiates and conducts an investigation and under whose immediate direction:
- The investigational drug is administered or dispensed, or
- The investigational device is administered, dispensed or used.
The term does not include any person other than an individual.
- Study expiration. If IRB approval of a specific study expires before continuing review and approval occur, investigators must stop all research activities involving human subjects related to that study except where they judge that it is in the best interests of already enrolled subjects to continue to participate. When investigators make this judgment, they must promptly notify the IRB. When the IRB reviews the investigator's decision, it may decide whether it is in the best interests of already enrolled subjects to continue to participate in the research by considering the best interests of subjects either one at a time or as a group. If an IRB determines that it is not in the best interests of already enrolled subjects to continue to participate, investigators must stop all human subjects research activities, including intervening or interacting with subjects or obtaining or analyzing identifiable private information about human subjects. Investigators may resume the human subjects research activity once continuing review and approval by the IRB have occurred.
- Surrogate consent. Consent obtained from the participant's legally authorized representative (LAR).
- Inappropriate involvement of human subjects in research
- Violation of the rights or welfare of human subjects or others
- Serious or continuing noncompliance with federal regulations or IRB policies
- New information regarding increased risk to human subjects or others
- Termination for cause. An action initiated by the IRB to stop permanently some or all research procedures.
- Test article. Any investigational drug, biologic product — such as blood or a vaccine — or medical device for human use.
- Therapeutic misconception. This term is used to describe the assumption by research participants that decisions about their care are being made solely with their benefit in mind. Therapeutic misconception can be defined as the situation in which participants or LARs either overestimate the direct therapeutic benefits that may be gained by participation in the research or underestimate the risks, thereby compromising their ability to provide or maintain a voluntary and knowing informed consent. Investigators must be especially careful to make participants and their families or caretakers aware of the differences between individualized treatment versus research and the separate and distinct roles of the clinician and the research investigator.
- Treatment investigational device exemption (IDE). A mechanism through the FDA for providing eligible participants with investigational devices for the treatment of a serious or life-threatening illness for which there are no satisfactory alternatives.
- Treatment investigational new drug (IND). A mechanism through the FDA for providing eligible participants with investigational drugs for the treatment of a serious or life-threatening illness for which there are no satisfactory alternatives.
- Serious: Serious problems or events that result in significant harm (which may be physical, psychological, financial, social, economic or legal) or places subjects or others at a greater risk of harm than was previously known or recognized. Note that actual harm need not have occurred for there to be a change in the risk-to-benefit ratio.
- Not already described as a potential risk in the approved informed consent
- Not already described as a potential risk in the approved protocol
- Not listed in the investigator's brochure
- Not part of an underlying disease
- Occurring at an increased frequency or at an increased severity than expected
- Related: A problem or event is related if it is possibly related to the research procedures.
- Viable. As it pertains to the neonate, means being able, after delivery, to survive (given the benefit of available medical therapy) to the point of independently maintaining a heartbeat and respiration.
- Vulnerable populations in research. Vulnerable populations may include (but are not limited to) individuals who are pregnant; prisoners; individuals who have been involuntarily committed to a medical facility; children; subordinates such as students, trainees and employees; individuals who are economically or educationally disadvantaged; individuals who have a language barrier; individuals with a cognitive disability; and individuals with an illness for which all standard treatment options have been exhausted. Federal regulations state that "when some or all of the subjects are likely to be vulnerable to coercion or undue influence, such as children, prisoners, pregnant women, mentally disabled persons, or economically or educationally disadvantaged persons, additional safeguards have been included in the study to protect the rights and welfare of these subjects" [45 CFR 46.111(b)]. FDA regulations expressly identify "mentally disabled persons" as a vulnerable category of subjects in clinical investigations for which IRBs may need to assume increased responsibilities [21 CFR 56.107(a) and 56.111(b)].
- Ward. A child who is placed in the legal custody of the state or other agency, institution or entity, consistent with applicable federal, state or local law.
- Withdrawals. Subjects who were accrued but later withdrew from the study, either before or after receiving a study drug, device or intervention. This does not include screen failures.
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Clinical Trials
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Lesson 21: Definition of Terms
A word or phrase used to describe a thing or to express concept, especially In a particular kind of language or branch of study.
Guidelines in defining terms:
1. Definition of terms works like a glossary but have a different twist. It is placed on the beginning of the research paper to tell the meaning of the terms used in the said paper.
2. Only terms, words, or phrases which have special or unique meanings in the study are defined.
3. There are two types of definition of terms. Conceptual and Operational Terms.
Theoretical Definition are based be taken from encyclopedias, books, magazines and newspaper article, dictionaries, and other publications but the researcher must acknowledge his/her sources.
Conceptual Definition are based on how the researcher may develop his own definition from the characteristics of the term define.
4. The term should be arranged alphabetically .
5. When the definition are taken from encyclopedias, books, magazine and newspaper articles, dictionaries and other publications, the researcher must acknowledge his sources .
Definition of terms
Theoretical Definition
Knowledge - the fact or condition of knowing something with familiarity gained through experience or association.
Conceptual Definition
Knowledge - it is a condition of being aware to a certain problem-cyberbullying.
Multidisciplinary Methods for Exploring Organizations
Bias : a lack of balance and accuracy in the use of research methods. It can appear at any phase of research, from deciding on a sampling frame, sampling, to data collection and analysis. Bias also arises in the identity of the researcher through assumptions and ideas related to his or her own culture that may influence data collection and analysis. Bias interfere with the extent to which results are valid and accurate, whether or not the research is reliable, and the potential for results to be representative of, or generalizable to, a wider population. Click here to access a brief article from the National Institutes of Health on research bias.
Case Study : the collection and presentation of in-depth information about a specific individual, group, or community. Often these data represent the subjective experiences of an individual or group. Click here to access more information on the case study approach to research.
Causality : the relation between cause and effect. Causality is the agency that links one process or event (the cause) with another process, state, or event (the effect). The first of these is normally understood to be at least partly responsible for the occurrence of the second, thus the second is dependent upon the first. Causality is an abstraction based upon experience that is used to show and explain how change happens in the world. Below is a very useful video explaining causality and how it relates to research.
Cultural Relativism : the idea that cultures are value-neutral. This means that rather than various cultures being a better or worse ways of organizing behavior, they are simply different. In anthropology, this idea has been used to make sense out of behaviors and values that seem alien or morally wrong to an outside observer; it has also been used to raise awareness of the potential for bias by an observer. The concept has been debated in anthropology and has raised concern that it inherently leads to moral relativism. Most modern anthropologists use the idea of cultural relativism as a way to bracket off one’s own cultural assumptions and biases to the extent possible. Here is a brief article on cultural relativism by anthropologist Clifford Geertz.
Data : factual information, collected through systematic methods, that is used as a basis for reasoning and analysis of a phenomenon.
Deductive Reasoning: a type of reasoning in which conclusions are formulated about particulars from general or universal premises. Here’s Monty Python’s take on deductive reasoning.
Dependent Variable: a variable that varies due, at least in part, to the impact of the independent variable. In other words, its value “depends” on the value of the independent variable. For example, in the variables “gender” and “academic major,” academic major is the dependent variable, meaning that your major cannot determine whether you are male or female, but your gender might indirectly lead you to favor one major over another. Check out the video under the entry for independent variables for more information on the difference between dependent and independent variables.
Emic : an approach to the study or description of a language or culture that focuses on its internal elements and logic and their functioning rather than in terms of any existing external scheme. The term can also refer to the native explanation for a behavior or cultural pattern. The video below will help you to understand the differences between emic and etic perspectives as they are understood by cultural anthropologists.
Etic : an approach to the study or description of a language or culture that is general, nonstructural, and objective in its perspective. It is typically explanations for behavior from the perspective of the scientist/researcher observing a culture or language.
Epistemology: theory of knowledge that questions how we know things, how knowledge is constructed, and what constitutes valid knowledge. Here is a very detailed definition/discussion of epistemology from the Stanford online dictionary of philosophy.
Ethnography: method for studying study groups and/or cultures over an extended period of time using a variety of qualitative (and sometimes quantitative) research techniques. Ethnography employs participant observation, which is intended to allow researchers to understand a group through immersion into its lifestyles. This allows for a detailed, in-depth, understanding of human experience. Check out the TEDx video below for a nice discussion of the use of ethnography in business.
Field Studies : research studies carried out in natural settings, rather than in laboratories, classrooms, or other structured environments.
Focus Groups : small, roundtable discussion groups charged with examining or discussing topics or problems associated with a research project. In some cases, these may also involve discussion of solutions to identified problems. Focus groups usually consist of 4-12 participants and are guided by moderators to keep the discussion moving and collect data. Here is more on focus group research from the Robert Wood Johnson Foundation.
Grounded Theory: an approach to research in which theories emerge from observing a group rather than being brought to the context of observation. Theories are grounded in the group’s observable experiences and interpretations, but researchers add their own insight into why those experiences exist. Click here to access the website Grounded Theory Online .
Hypothesis : a tentative explanation or educated guess based on theory or observation that is used to predict a causal relationship between variables. Click here to review some examples of hypotheses .
Independent Variable: the conditions or variables of an experiment that are systematically manipulated by the researcher or a variable that is not impacted by the dependent variable, but that itself impacts the dependent variable. Check out the video below for more information on the difference between dependent and independent variables.
Inductive Reasoning: a type of reasoning in which a generalized conclusion is formulated generated based on particular instances. Below is a video on the difference between inductive and deductive approaches to reasoning.
Naturalistic Observation: observation of behaviors and events in natural settings rather than in experimental contexts that involve manipulation of variables or other types of interference.
Ontology: a discipline of philosophy that explores the science of what is, the kinds and structures of objects, properties, events, processes, and relations in every area of reality. Click here for a detailed discussion of logic and ontology from the Stanford online dictionary of philosophy.
Organization : For the purposes of MMEO, and organization is an institutionalized structure that is formed for a specific purpose. Examples of organizations are businesses, academic institutions, religious institutions, or government institutions.
Participant observation : a form of qualitative research that involves participating in the activities of the people being observed as a way of developing an experience-near understanding of their behaviors and ideas.
Phenomenology: a qualitative research approach that focuses on meaning expressed by individuals through their lived experience of a particular idea, concept, or event. This link will take you to more information on phenomenology .
Probability : the likelihood that a phenomenon will occur randomly. As a statistical measure, it is represented as p.
Qualitative research: a systematic approach to creating knowledge about how people interpret their surroundings, construct meaning, and interpret the meanings they construct. Qualitative research relies upon subtle and complex techniques of observation, recording data, and writing to develop an interpretive framework for analyzing and explaining why people do what they do and think what they think.
Quantitative research: Quantitative research focuses on identifying objective measurements of phenomena such as human behavior. In human subjects research it makes use of statistical, mathematical, and numerical analysis of empirical data collected using instruments such as questionnaires or through analyzing and manipulating pre-existing statistical data using computational techniques. Quantitative research uses numerical data to draw general conclusions across groups of people as a way of explaining particular behaviors or phenomena. This link to a site as USC will give you more details on quantitative research .
Questionnaire : structured groups of questions used to gather information, attitudes, or opinions. Questionnaires can be either quantitative, including forced-choice questions, or qualitative, including open-ended questions.
Random Sampling : a process used in research to draw a sample of a population that does not reflect any pattern or order beyond chance.
Reliability : the extent to which a research method yields consistent results. If the observational or measurement instrument is reliable, then administering it to similar groups should yield similar results. Reliability is a prerequisite for validity. If a data collection approach is unreliable, then cannot produce trustworthy results.
Rigor: degree to which research methods are carefully designed and carried out.
Sample : any population researched in a study. In many studies, researchers often try to select a “sample population” that is believed to be representative of the behaviors or other qualities (race, ethnicity, gender) of people for whom results will be generalized. This video will help you understand different types of sampling and the goals in sampling.
Sampling Error : the degree to which the results from the sample deviate from those that would be obtained from the entire population. This can be a result of random error in the selection of participants and any corresponding reduction in reliability that arises as a result of that error.
Standard Deviation : a measure used to quantify how much variation or dispersion there is in a set of data values. A low standard deviation means that the data points tend to be close to the mean; a high standard deviation means the data points are spread out over a wider range of values and further from the mean.
Statistical Analysis : application of statistical methods and theory to the collection, presentation, and interpretation of numerical data.
Statistical Significance: in any experiment or observation that involves using a sample from a population, statistical significance refers to the likelihood that a behavior or set of behaviors is due to chance. The probability that the null hypothesis can be rejected at a predetermined significance level [0.05 or 0.01].
Theory: a general explanation about a specific behavior or set of events that is based on known principles and serves to organize related events in a meaningful way. A theory is not as specific as a hypothesis.
Triangulation: a multi-method or pluralistic approach to research that uses a variety of methods to collect data from different viewpoints. This produces a complex and multi-faceted data set that helps in checking the validity of findings.
Unit of Analysis: the thing being observed, analyzed, and for which data are collected in the form of variables.
Validity — the degree to which a study accurately represents and assesses the specific phenomenon a researcher wants to measure. This brief video will help you to understand the difference between validity and reliability in research.
Variable: any characteristic or trait that can vary from person to person. Race, gender, education level, hair color, age, political beliefs, religion are all examples of variables. This link will take you to a website that provides more detail on variables.

Psychotherapist's Guide to Socratic Dialogue pp 43–57 Cite as
Definition of Terms
- Mohammad Sadegh Montazeri 2
- First Online: 19 August 2022
471 Accesses
The term has categories such as ambiguous or unambiguous, clear or unclear, vague or exact.
If we can give a precise definition, many ambiguities in the client’s mind remove. Then, clients can think more clearly. For example, terms such as failure, success, and happiness are vague in the client’s thoughts.
I will introduce methods for defining terms in this chapter. We try definition to be coextensive. In Socratic dialogue, we can use violation examples to delineate terms. Using violation examples helps to have a neither limited nor extended definition.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
We apply the word mode in schema therapy.
In this case, if I analyze the client logically, I will corrupt the rapport. However, since we had many dialogues about modes and the client was aware of her schema modes, my interpretation helped empathize with the client. She found out that I had realized her mental status.
I do not validate aggressive behavior, but I verify the feelings of the client.
Microbes have some subclasses, such as bacteria and viruses. However, it is not required to explain this classification to the client.
Hurley, P. J., & Watson, L. (2016). A concise introduction to logic (13th ed.). Cengage Learning.
Google Scholar
Kreeft, P. (2010). Socratic logic: A logic text using Socratic method, Platonic questions & Aristotelian principles . St Augustine’s Press.
Overholser, J. C. (2018). The Socratic method of psychotherapy . Columbia University Press.
Book Google Scholar
Plato. (380 BC). Laches or courage . Retrieved from https://www.sacred-texts.com/cla/plato/laches.htm
Download references
Author information
Authors and affiliations.
Golestan University, Gorgan, Iran
Mohammad Sadegh Montazeri
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter.
Montazeri, M.S. (2022). Definition of Terms. In: Psychotherapist's Guide to Socratic Dialogue. Springer, Cham. https://doi.org/10.1007/978-3-031-07972-6_4
Download citation
DOI : https://doi.org/10.1007/978-3-031-07972-6_4
Published : 19 August 2022
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-07971-9
Online ISBN : 978-3-031-07972-6
eBook Packages : Behavioral Science and Psychology Behavioral Science and Psychology (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- QuestionPro

- Solutions Industries Gaming Automotive Sports and events Education Government Travel & Hospitality Financial Services Healthcare Cannabis Technology Use Case NPS+ Communities Audience Contactless surveys Mobile LivePolls Member Experience GDPR Positive People Science 360 Feedback Surveys
- Resources Blog eBooks Survey Templates Case Studies Training Help center
Home Market Research
Sample: Definition, Types, Formula & Examples
How often do researchers look for the right survey respondents, either for a market research study or an existing survey in the field? The sample or the respondents of this research may be selected from a set of customers or users that are known or unknown.
You may often know your typical respondent profile but don’t have access to the respondents to complete your research study. At such times, researchers and research teams reach out to specialized organizations to access their panel of respondents or buy respondents from them to complete research studies and surveys.
These could be general population respondents that match demographic criteria or respondents based on specific criteria. Such respondents are imperative to the success of research studies.
This article discusses in detail the different types of samples, sampling methods, and examples of each. It also mentions the steps to calculate the size, the details of an online sample, and the advantages of using them.
Content Index
- What is a sample?
Probability sampling methodologies with examples
Non-probability sampling methodologies with examples.
- How to determine a sample size
- Calculating sample size
- Sampling advantages
What is a Sample?
A sample is a smaller set of data that a researcher chooses or selects from a larger population using a pre-defined selection bias method. These elements are known as sample points, sampling units, or observations.
Creating a sample is an efficient method of conducting research . Researching the whole population is often impossible, costly, and time-consuming. Hence, examining the sample provides insights the researcher can apply to the entire population.
For example, if a cell phone manufacturer wants to conduct a feature research study among students in US Universities. An in-depth research study must be conducted if the researcher is looking for features that the students use, features they would like to see, and the price they are willing to pay.
This step is imperative to understand the features that need development, the features that require an upgrade, the device’s pricing, and the go-to-market strategy.
In 2016/17 alone, there were 24.7 million students enrolled in universities across the US. It is impossible to research all these students; the time spent would make the new device redundant, and the money spent on development would render the study useless.
Creating a sample of universities by geographical location and further creating a sample of these students from these universities provides a large enough number of students for research.
Typically, the population for market research is enormous. Making an enumeration of the whole population is practically impossible. The sample usually represents a manageable size of this population. Researchers then collect data from these samples through surveys, polls, and questionnaires and extrapolate this data analysis to the broader community.
LEARN ABOUT: Survey Sampling
Types of Samples: Selection methodologies with examples
The process of deriving a sample is called a sampling method. Sampling forms an integral part of the research design as this method derives the quantitative and qualitative data that can be collected as part of a research study. Sampling methods are characterized into two distinct approaches: probability sampling and non-probability sampling.
Probability sampling is a method of deriving a sample where the objects are selected from a population-based on probability theory. This method includes everyone in the population, and everyone has an equal chance of being selected. Hence, there is no bias whatsoever in this type of sample.
Each person in the population can subsequently be a part of the research. The selection criteria are decided at the outset of the market research study and form an important component of research.
LEARN ABOUT: Action Research
Probability sampling can be further classified into four distinct types of samples. They are:
- Simple random sampling: The most straightforward way of selecting a sample is simple random sampling . In this method, each member has an equal chance of participating in the study. The objects in this sample population are chosen randomly, and each member has the same probability of being selected. For example, if a university dean would like to collect feedback from students about their perception of the teachers and level of education, all 1000 students in the University could be a part of this sample. Any 100 students can be selected randomly to be a part of this sample.
- Cluster sampling: Cluster sampling is a type of sampling method where the respondent population is divided into equal clusters. Clusters are identified and included in a sample based on defining demographic parameters such as age, location, sex, etc. This makes it extremely easy for a survey creator to derive practical inferences from the feedback. For example, if the FDA wants to collect data about adverse side effects from drugs, they can divide the mainland US into distinctive cluster analysis , like states. Research studies are then administered to respondents in these clusters. This type of generating a sample makes the data collection in-depth and provides easy-to-consume and act-upon, insights.
- Systematic sampling: Systematic sampling is a sampling method where the researcher chooses respondents at equal intervals from a population. The approach to selecting the sample is to pick a starting point and then pick respondents at a pre-defined sample interval. For example, while selecting 1,000 volunteers for the Olympics from an application list of 10,000 people, each applicant is given a count of 1 to 10,000. Then starting from 1 and selecting each respondent with an interval of 10, a sample of 1,000 volunteers can be obtained.
- Stratified random sampling: Stratified random sampling is a method of dividing the respondent population into distinctive but pre-defined parameters in the research design phase. In this method, the respondents don’t overlap but collectively represent the whole population. For example, a researcher looking to analyze people from different socioeconomic backgrounds can distinguish respondents by their annual salaries. This forms smaller groups of people or samples, and then some objects from these samples can be used for the research study.
LEARN ABOUT: Purposive Sampling
The non-probability sampling method uses the researcher’s discretion to select a sample. This type of sample is derived mostly from the researcher’s or statistician’s ability to get to this sample.
This type of sampling is used for preliminary research where the primary objective is to derive a hypothesis about the topic in research. Here each member does not have an equal chance of being a part of the sample population, and those parameters are known only post-selection to the sample.
We can classify non-probability sampling into four distinct types of samples. They are:
- Convenience sampling: Convenience sampling , in easy terms, stands for the convenience of a researcher accessing a respondent. There is no scientific method for deriving this sample. Researchers have nearly no authority over selecting the sample elements, and it’s purely done based on proximity and not representativeness.
This non-probability sampling method is used when there is time and costs limitations in collecting feedback. For example, researchers that are conducting a mall-intercept survey to understand the probability of using a fragrance from a perfume manufacturer. In this sampling method, the sample respondents are chosen based on their proximity to the survey desk and willingness to participate in the research.
- Judgemental/purposive sampling: The judgemental or purposive sampling method is a method of developing a sample purely on the basis and discretion of the researcher purely, based on the nature of the study along with his/her understanding of the target audience. This sampling method selects people who only fit the research criteria and end objectives, and the remaining are kept out.
For example, if the research topic is understanding what University a student prefers for Masters, if the question asked is “Would you like to do your Masters?” anything other than a response, “Yes” to this question, everyone else is excluded from this study.
- Snowball sampling: Snowball sampling or chain-referral sampling is defined as a non-probability sampling technique in which the samples have rare traits. This is a sampling technique in which existing subjects provide referrals to recruit samples required for a research study.
For example, while collecting feedback about a sensitive topic like AIDS, respondents aren’t forthcoming with information. In this case, the researcher can recruit people with an understanding or knowledge of such people and collect information from them or ask them to collect information.
- Quota sampling: Quota sampling is a method of collecting a sample where the researcher has the liberty to select a sample based on their strata. The primary characteristic of this method is that two people cannot exist under two different conditions. For example, when a shoe manufacturer would like to understand millennials’ perception of the brand with other parameters like comfort, pricing, etc. It selects only females who are millennials for this study as the research objective is to collect feedback about women’s shoes.
How to determine a Sample Size
As we have learned above, the right sample size determination is essential for the success of data collection in a market research study. But is there a correct number for the sample size? What parameters decide the sample size? What are the distribution methods of the survey?
To understand all of this and make an informed calculation of the right sample size, it is first essential to understand four important variables that form the basic characteristics of a sample. They are:
- Population size: The population size is all the people that can be considered for the research study. This number, in most cases, runs into huge amounts. For example, the population of the United States is 327 million. But in market research, it is impossible to consider all of them for the research study.
- The margin of error (confidence interval): The margin of error is depicted by a percentage that is a statistical inference about the confidence of what number of the population depicts the actual views of the whole population. This percentage helps towards the statistical analysis in selecting a sample and how much sampling error in this would be acceptable.
LEARN ABOUT: Research Process Steps
- Confidence level: This metric measures where the actual mean falls within a confidence interval. The most common confidence intervals are 90%, 95%, and 99%.
- Standard deviation: This metric covers the variance in a survey. A safe number to consider is .5, which would mean that the sample size has to be that large.
Calculating Sample Size
To calculate the sample size, you need the following parameters.
- Z-score: The Z-score value can be found here .
- Standard deviation
- Margin of error
- Confidence level
To calculate use the sample size, use this formula:
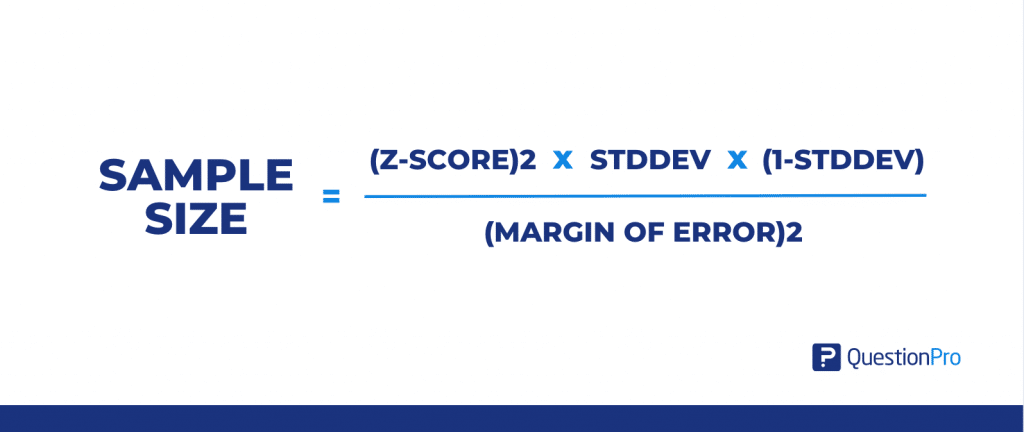
Sample Size = (Z-score)2 * StdDev*(1-StdDev) / (margin of error)2
Consider the confidence level of 90%, standard deviation of .6 and margin of error, +/-4%
((1.64)2 x .6(.6)) / (.04)2
( 2.68x .0.36) / .0016
.9648 / .0016
603 respondents are needed and that becomes your sample size.
Try our sample size calculator to give population, margin of error calculator , and confidence level.
LEARN MORE: Population vs Sample
Sampling Advantages
As shown above, there are many advantages to sampling. Some of the most significant advantages are:
- Reduced cost & time: Since using a sample reduces the number of people that have to be reached out to, it reduces cost and time. Imagine the time saved between researching with a population of millions vs. conducting a research study using a sample.
- Reduced resource deployment: It is obvious that if the number of people involved in a research study is much lower due to the sample, the resources required are also much less. The workforce needed to research the sample is much less than the workforce needed to study the whole population .
- Accuracy of data: Since the sample indicates the population, the data collected is accurate. Also, since the respondent is willing to participate, the survey dropout rate is much lower, which increases the validity and accuracy of the data.
- Intensive & exhaustive data: Since there are lesser respondents, the data collected from a sample is intense and thorough. More time and effort are given to each respondent rather than collecting data from many people.
- Apply properties to a larger population: Since the sample is indicative of the broader population, it is safe to say that the data collected and analyzed from the sample can be applied to the larger population, which would hold true.
To collect accurate data for research, filter bad panelists, and eliminate sampling bias by applying different control measures. If you need any help arranging a sample audience for your next market research project, contact us at [email protected] . We have more than 22 million panelists across the world!
In conclusion, a sample is a subset of a population that is used to represent the characteristics of the entire population. Sampling is essential in research and data analysis to make inferences about a population based on a smaller group of individuals. There are different types of sampling, such as probability sampling, non-probability sampling, and others, each with its own advantages and disadvantages.
Choosing the right sampling method depends on the research question, budget, and resources is important. Furthermore, the sample size plays a crucial role in the accuracy and generalizability of the findings.
This article has provided a comprehensive overview of the definition, types, formula, and examples of sampling. By understanding the different types of sampling and the formulas used to calculate sample size, researchers and analysts can make more informed decisions when conducting research and data unit of analysis .
Sampling is an important tool that enables researchers to make inferences about a population based on a smaller group of individuals. With the right sampling method and sample size, researchers can ensure that their findings are accurate and generalizable to the population.
Utilize one of QuestionPro’s many survey questionnaire samples to help you complete your survey.
When creating online surveys for your customers, employees, or students, one of the biggest mistakes you can make is asking the wrong questions. Different businesses and organizations have different needs required for their surveys.
If you ask irrelevant questions to participants, they’re more likely to drop out before completing the survey. A questionnaire sample template will help set you up for a successful survey.
LEARN MORE SIGN UP FREE
MORE LIKE THIS

21 Best Customer Advocacy Software for Customers in 2024
Apr 19, 2024

10 Quantitative Data Analysis Software for Every Data Scientist
Apr 18, 2024
11 Best Enterprise Feedback Management Software in 2024

17 Best Online Reputation Management Software in 2024
Apr 17, 2024
Other categories
- Academic Research
- Artificial Intelligence
- Assessments
- Brand Awareness
- Case Studies
- Communities
- Consumer Insights
- Customer effort score
- Customer Engagement
- Customer Experience
- Customer Loyalty
- Customer Research
- Customer Satisfaction
- Employee Benefits
- Employee Engagement
- Employee Retention
- Friday Five
- General Data Protection Regulation
- Insights Hub
- Life@QuestionPro
- Market Research
- Mobile diaries
- Mobile Surveys
- New Features
- Online Communities
- Question Types
- Questionnaire
- QuestionPro Products
- Release Notes
- Research Tools and Apps
- Revenue at Risk
- Survey Templates
- Training Tips
- Uncategorized
- Video Learning Series
- What’s Coming Up
- Workforce Intelligence
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
The polarization in today’s Congress has roots that go back decades
It’s become commonplace among observers of U.S. politics to decry partisan polarization in Congress . Indeed, a Pew Research Center analysis finds that, on average, Democrats and Republicans are farther apart ideologically today than at any time in the past 50 years.
But the dynamics behind today’s congressional polarization have been long in the making. The analysis of members’ ideological scores finds that the current standoff between Democrats and Republicans is the result of several overlapping trends that have been playing themselves out – and sometimes reinforcing each other – for decades.
- Both parties have grown more ideologically cohesive. There are now only about two dozen moderate Democrats and Republicans left on Capitol Hill, versus more than 160 in 1971-72.
- Both parties have moved further away from the ideological center since the early 1970s. Democrats on average have become somewhat more liberal, while Republicans on average have become much more conservative.
- The geographic and demographic makeup of both congressional parties has changed dramatically. Nearly half of House Republicans now come from Southern states, while nearly half of House Democrats are Black, Hispanic or Asian/Pacific Islander.
The Center’s analysis is based on DW-NOMINATE , a method that uses lawmakers’ roll-call votes to place them in a two-dimensional ideological space. It is designed to produce scores that are comparable across time. This analysis focuses on the first dimension, which is essentially the economic and governmental aspects of the familiar left-right spectrum and ranges from 1 (most conservative) to -1 (most liberal). (For more details on DW-NOMINATE and this analysis’ geographical definitions, read “How we did this.”)
This analysis is based on DW-NOMINATE, a method of scaling lawmakers’ ideological positions based on their roll-call votes. It is the latest iteration of a procedure first developed by political scientists Keith T. Poole and Howard Rosenthal in the early 1980s.
DW-NOMINATE places each lawmaker on a two-dimensional scale, much like a standard x-y graph. The first (“horizontal”) dimension is essentially the same as the economic and governmental aspects of the familiar left-liberal/right-conservative political spectrum. The second (“vertical”) dimension typically picks up crosscutting issues that have divided the major parties at various times in American history, such as slavery, currency policy, immigration, civil rights and abortion. But as Poole noted in 2017 , since about 2000 that second dimension has faded in significance, to the point where congressional activity has “collapse[d] into a one-dimensional, near-parliamentary voting structure … almost every issue is voted along ‘liberal-conservative’ … lines.”
Accordingly, like most political science work that employs DW-NOMINATE scores, this analysis focuses on the primary liberal/conservative scale. That scale runs from -1 (most liberal) to 1 (most conservative). Each lawmaker is assigned a value between those endpoints based on their voting record; the scores are designed to be comparable between Congresses and across time.
In mid-February 2022, we downloaded DW-NOMINATE data for all senators and representatives from the 92nd Congress (1971-72) to the current 117th Congress. We excluded nonvoting delegates from the analysis, as well as lawmakers who officially served but (due to health issues, resignation or other factors) didn’t have a voting record that could be analyzed and scored for a given Congress. We did include all other lawmakers who served at any time during a given Congress, including those who died mid-term; those appointed to temporarily fill Senate seats who only served for part of a term; and those who left Congress early to fill some other office, such as a Cabinet position. (We also included all House speakers, even if they didn’t have an analyzable voting record. For many years, the tradition in the House has been for speakers to vote only on very significant matters or if their vote will be decisive.)
Lawmakers who changed parties in mid-Congress were classified by whichever label they wore for the longest time. Independents were analyzed as part of whichever major party they caucused with, with the exception of Rep. Justin Amash of Michigan during the 116th Congress. (Amash left the Republican Party in mid-2019 , and for most of his final term did not caucus with either major party.)
In our discussion of “Southern Democrats” and “Southern Republicans,” we defined “the South” as the 11 states that comprised the Confederacy during the Civil War, most of which were dominated politically by Democrats for generations after Reconstruction ended. Southern Democrats, however, were ideologically and demographically quite distinct from Democrats in the rest of the country, so they merited separate study (and we wanted to see if today’s Southern Republicans are similarly distinctive). We chose to use the former Confederate states as our definition of “the South,” as the states that made up the so-called “Solid South” varied somewhat over time and we wanted a consistent, relatively objective definition.
Our analysis of the changing racial and ethnic composition of lawmakers was based on data from the U.S. House of Representatives’ archives .
Between the 92nd Congress of 1971-72 and the current 117th Congress, both parties in both the House and the Senate have shifted further away from the center, but Republicans more so. House Democrats, for example, moved from about -0.31 to -0.38, meaning that over time they’ve become modestly more liberal on average. House Republicans, by contrast, moved from 0.25 to nearly 0.51, a much bigger increase in the conservative direction.
As Democrats have grown more liberal over time and Republicans much more conservative, the “middle” – where moderate-to-liberal Republicans could sometimes find common ground with moderate-to-conservative Democrats on contentious issues – has vanished.
Five decades ago, 144 House Republicans were less conservative than the most conservative Democrat, and 52 House Democrats were less liberal than the most liberal Republican, according to the analysis. But that zone of ideological overlap began to shrink, as conservative Democrats and liberal Republicans – increasingly out of step with their caucuses and their constituents – either retired, lost reelection bids or, in a few cases, switched parties.
Since 2002, when Republican Rep. Constance Morella of Maryland was defeated for reelection and GOP Rep. Benjamin Gilman of New York retired, there’s been no overlap at all between the least liberal Democrats and the least conservative Republicans in the House. In the Senate, the end of overlap came in 2004, when Democrat Zell Miller of Georgia retired.
Ever since, the gaps between the least conservative Republicans and least liberal Democrats in both the House and Senate have widened – making it ever less likely that there’s any common ground to find.
The ideological shifts in the congressional parties have occurred alongside – and, perhaps to some extent, because of – geographic and demographic shifts in their composition.
In 1971-72, representatives from the 11 former Confederate states made up nearly a third (31.4%) of all the House Democrats who served in that Congress. Those Southern representatives were notably less liberal than Democrats from elsewhere in the country: Their average DW-NOMINATE score was -0.144, versus -0.388 for non-Southern House Democrats.
Over time, though, Southern Democrats became both fewer in number and more liberal – to the point where today, they account for only 22% of the House Democratic caucus, but ideologically are almost indistinguishable from their non-Southern colleagues (average scores of -0.383 and -0.381, respectively).
On the Republican side of the aisle, almost the exact opposite trend has occurred. Southerners made up less than 15% of the House GOP caucus 50 years ago but comprise about 42% of it today. And while Republicans in general have become more conservative, that’s been especially true of Southern Republicans in the House: Their DW-NOMINATE score has moved from about 0.29 (only slightly to the right of non-Southern Republicans) in 1971-72 to 0.57 in the current Congress, versus about 0.46 today for non-Southern House Republicans. (These trends are similar in the Senate, although only four of the 22 senators from former Confederate states are currently Democrats.)
The racial and ethnic makeup of both parties’ Southern lawmakers has changed considerably. In 1971-72, according to House records , only 12 African Americans served in the House and one in the Senate, and none were from the South. Of the five Hispanics in the House, two were from Texas (the lone Hispanic senator was from New Mexico). And the only Asian Americans or Pacific Islanders in Congress were Hawaii’s two senators (one Democrat, one Republican) and two representatives (both Democrats).
In the current Congress, 24 of the 50 House Democrats from the South are African American; seven are Hispanic; and two are Asian Americans or Pacific Islanders. (Rep. Bobby Scott of Virginia is of both African American and Filipino descent.) One of the four Democratic senators from the South (Raphael Warnock of Georgia) is African American. In contrast, only one of the 91 Southern House Republicans is Black (Byron Donalds of Florida); four others are Hispanic. One of the GOP’s 18 Southern senators is Black (Tim Scott of South Carolina) and two are Hispanic (Ted Cruz of Texas and Marco Rubio of Florida).
Note: This is an updated version of a post originally published June 12, 2014.

Most Popular
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy

IMAGES
VIDEO
COMMENTS
A key term is a term that holds significant importance or plays a crucial role within the context of a research paper. It is a term that encapsulates a core concept, idea, or variable that is central to the study. Key terms are often essential for understanding the research objectives, methodology, findings, and conclusions.
STEP ONE Select the. STEP TWO Use the. STEP TWO Example: In this. STEP THREE You can. STEP THREE You can. STEP FOUR Open the. STEP FOUR Use a. STEP FOUR Complete one. STEP FIVE On half.
The study is intended to describe the methods of defining terms found in the theses of the English Foreign Language (EFL) students of IAIN Palangka Raya. The method to be used is a mixed method, qualitative and quantitative. Quantitative approach was used to identify, describe the frequencies, and classify the methods of defining terms.
Accuracy-- a term used in survey research to refer to the match between the target population and the sample. Affective Measures-- procedures or devices used to obtain quantified descriptions of an individual's feelings, emotional states, or dispositions. Aggregate-- a total created from smaller units. For instance, the population of a county ...
Let us pretend we are doing research on nurturing international business research through global value chains literature. You do not need to include definitions for research, business, international, global, etc. These terms are common knowledge and are mostly understood the same way by everyone.
2.2 Conceptual and operational definitions. Research studies usually include terms that must be carefully and precisely defined, so that others know exactly what has been done and there are no ambiguities. Two types of definitions can be given: conceptual definitions and operational definitions. Loosely speaking, a conceptual definition explains what to measure or observe (what a word or a ...
limit the abstract to one typed page; (b) maintain the scholarly language used throughout the. dissertation; (c) keep the abstract concise, accurate, and readable; (d) use correct English; (e) ensure each sentence adds value to the reader's understanding of the research; and (f) use the full.
research terminologies in educational research. It provides definitions of many of the terms used in the guidebooks to conducting qualitative, quantitative, and mixed methods of research. The terms are arranged in alphabetical order. Abstract A brief summary of a research project and its findings. A summary of a study that
This glossary provides definitions of many of the terms used in the guides to conducting qualitative and quantitative research. The definitions were developed by members of the research methods seminar (E600) taught by Mike Palmquist in the 1990s and 2000s. ... Process used in research to draw a sample of a population strictly by chance ...
Answer: Operational definition of terms refers to a detailed explanation of the technical terms and measurements used during data collection. This is done to standardize the data. Whenever data is being collected, it is necessary to clearly define how to collect the data. Data that is not defined runs the risk of being inconsistent and might ...
A valued or desired outcome associated with a research project. Anticipated benefits may express the probability that subjects and society may benefit from the research procedures. Research may benefit the individual or society as a whole. If research will not benefit individuals, it is required to provide a reasonable likelihood of resulting ...
Research Terms and Definitions. 1. Delimitations: address how the study will be narrowed in scope. 2. Descriptive statistics: those statistics that describe, organize, and summarize data (frequencies, percentages, descriptions of central tendency and descriptions of relative position). 3.
hypothesis: a proposition which research sets out to prove or disprove: "experimental" where the hypothesis is a positive statement, or "null" where statement contains a negative. independent variable: a variable that researcher believes precedes, influences or predicts the dependent variable. informed consent: giving potential ...
Otherwise to find samples of definition of terms, you can consider doing the following: Use several different research samples that your professor can provide you. From these samples, pick out the ones that contain a definition of terms. Use the internet and plug the terms into your favorite search engines. If you do choose the option of using ...
Definition of Terms. The Mayo Clinic Institutional Review Board's definition of terms explains legal definitions related to research guidelines and the protection of human research subjects, including advocate, conflict of interest, emergency treatment, informed consent and more. Abbreviated investigational device exemption requirements.
Terms. A word or phrase used to describe a thing or to express concept, especially In a particular kind of language or branch of study. Guidelines in defining terms: 1. Definition of terms works like a glossary but have a different twist. It is placed on the beginning of the research paper to tell the meaning of the terms used in the said paper.
Research methods are specific procedures for collecting and analyzing data. Developing your research methods is an integral part of your research design. When planning your methods, there are two key decisions you will make. First, decide how you will collect data. Your methods depend on what type of data you need to answer your research question:
Glossary. Bias: a lack of balance and accuracy in the use of research methods. It can appear at any phase of research, from deciding on a sampling frame, sampling, to data collection and analysis. Bias also arises in the identity of the researcher through assumptions and ideas related to his or her own culture that may influence data collection ...
Abstract. The term has categories such as ambiguous or unambiguous, clear or unclear, vague or exact. If we can give a precise definition, many ambiguities in the client's mind remove. Then, clients can think more clearly. For example, terms such as failure, success, and happiness are vague in the client's thoughts.
Sample Definition of Terms - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. sample only of a part of thesis chapter 1
conducting qualitative and quantitative research. The definitions were developed by members of the research methods seminar (E600) taught by Mike Palmquist in the 1990s and 2000s. Accuracy: A term used in survey research to refer to the match between the target population and the sample. ANCOVA (Analysis of Co-Variance): Same method as ANOVA, but
Quantitative Research Sample Definition of Terms. Accessibility - the quickness and convenience with which customers can find a retailer. It considers characteristics including a store's accessibility, parking accessibility, hours of operation, closeness to other retailers, and telephone and internet connection.
Reduced cost & time: Since using a sample reduces the number of people that have to be reached out to, it reduces cost and time. Imagine the time saved between researching with a population of millions vs. conducting a research study using a sample. Reduced resource deployment: It is obvious that if the number of people involved in a research study is much lower due to the sample, the ...
The polarization in today's Congress has roots that go back decades. It's become commonplace among observers of U.S. politics to decry partisan polarization in Congress. Indeed, a Pew Research Center analysis finds that, on average, Democrats and Republicans are farther apart ideologically today than at any time in the past 50 years.