Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 04 March 2024

Effects of acute phase intensive electrical muscle stimulation in COVID-19 patients requiring invasive mechanical ventilation: an observational case-control study
- Yohei Tsuchikawa 1 na1 ,
- Shinya Tanaka 1 na1 ,
- Daisuke Kasugai 2 ,
- Riko Nakagawa 1 ,
- Miho Shimizu 3 ,
- Takayuki Inoue 1 ,
- Motoki Nagaya 1 ,
- Takafumi Nasu 4 ,
- Norihito Omote 5 ,
- Michiko Higashi 2 ,
- Takanori Yamamoto 2 ,
- Naruhiro Jingushi 2 ,
- Atsushi Numaguchi 2 &
- Yoshihiro Nishida 1 , 6
Scientific Reports volume 14 , Article number: 5254 ( 2024 ) Cite this article
617 Accesses
4 Altmetric
Metrics details
- Infectious diseases
- Rehabilitation
- Respiratory tract diseases
We investigated the effects of acute-phase intensive electrical muscle stimulation (EMS) on physical function in COVID-19 patients with respiratory failure requiring invasive mechanical ventilation (IMV) in the intensive care unit (ICU). Consecutive COVID-19 patients requiring IMV admitted to a university hospital ICU between January and April 2022 (EMS therapy group) or between March and September 2021 (age-matched historical control group) were included in this retrospective observational case–control study. EMS was applied to both upper and lower limb muscles for up to 2 weeks in the EMS therapy group. The study population consisted of 16 patients undergoing EMS therapy and 16 age-matched historical controls (median age, 71 years; 81.2% male). The mean period until initiation of EMS therapy after ICU admission was 3.2 ± 1.4 days. The EMS therapy group completed a mean of 6.2 ± 3.7 EMS sessions, and no adverse events occurred. There were no significant differences between the two groups in Medical Research Council sum score (51 vs. 53 points, respectively; P = 0.439) or ICU mobility scale at ICU discharge. Addition of upper and lower limb muscle EMS therapy to an early rehabilitation program did not result in improved physical function at ICU discharge in severe COVID-19 patients.
Similar content being viewed by others
Physical function and mental health trajectories in COVID-19 patients following invasive mechanical ventilation: a prospective observational study
Hiromasa Yamamoto, Shinya Tanaka, … Yoshihiro Nishida
A case for inspiratory muscle training in SCI: potential role as a preventative tool in infectious respiratory diseases like COVID-19
Anne E. Palermo, Lawrence P. Cahalin & Mark S. Nash
Transcutaneous electrical diaphragmatic stimulation reduces the duration of invasive mechanical ventilation in patients with cervical spinal cord injury: retrospective case series
Gregory Lui Duarte, Aldrin Lucas Bethiol, … Antonio Luis Eiras Falcão
Introduction
The capacities of healthcare systems around the world have been stressed by the novel coronavirus disease 2019 (COVID-19) pandemic, which is caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Severe COVID-19 requiring invasive mechanical ventilation (IMV) has an estimated in-hospital mortality rate of approximately 45% 1 , with survivors often requiring prolonged IMV support in the intensive care unit (ICU) 2 . Such patients requiring prolonged IMV have prolonged ICU stays and require deep sedation, neuromuscular blockade, and/or placement in the prone position, which are significant risk factors for ICU-acquired weakness 3 , and have high rates of development of impairments in physical function, limited mobility, mental health, and quality of life after discharge from the ICU or from hospital 4 , 5 , 6 , 7 .
Early exercise with the active involvement of a physiotherapist is recommended after ICU discharge among patients with COVID-19 8 . Early mobilization and exercise appear to be essential for treatment of severe COVID-19, and recent studies demonstrated the safety and efficacy of early rehabilitation therapy in patients with severe COVID-19 treated in the ICU 9 . However, active early rehabilitation often cannot be performed due to limited medical resources, especially lack of personal protective equipment and personnel required for patients with obesity, severe physical dysfunction, and/or following IMV in the ICU 4 , 10 , 11 . Novel interventions are therefore required to prevent early injury and enhance functional recovery of patients with severe COVID-19 requiring treatment in the ICU. A meta-analysis of randomized controlled trials (RCTs) reported that the use of electrical muscle stimulation (EMS)—a method for safely inducing muscle contraction without requiring volitional effort and that does not evoke dyspnea—can reduce the incidence of ICU-acquired weakness in critically ill patients 12 , 13 , 14 , 15 . Therefore, EMS is expected to be effective and an adjunctive therapy or a bridge to rehabilitation in patients with COVID-19 16 , 17 , but its benefits are not clear. The present study was performed to determine whether intensive EMS add-on therapy could improve the muscle strength in patients with COVID-19 requiring IMV in the ICU compared with early rehabilitation alone.
Study cohorts
This single-center, retrospective observational, case–control study was performed in patients ≥ 18 years old admitted to the ICU of Nagoya University Hospital due to COVID-19 with respiratory failure requiring IMV between January and April 2022 (EMS therapy group) and age-matched controls admitted between March and September 2021 (historical control group) with length of stay > 24 h in the ICU. Patients who died in the ICU, who were not intubated, and who did not receive rehabilitation therapy in the ICU were excluded.
In all patients, COVID-19 diagnosis was confirmed by real-time polymerase chain reaction (PCR) for SARS-CoV-2 from any specimen. Our clinical setting and management of COVID-19 were reported previously 5 , 18 . Management of COVID-19 requiring IMV in the ICU was based on the “ABCDEF (Assess & manage pain, Both spontaneous awakening trials and spontaneous breathing trials, Choice of sedation and analgesia, Delirium assessment & management, Early mobilization and exercise, and Family engagement)” bundle 19 . Patients requiring < 4 L of O 2 were transferred to the general COVID-19 ward. Rehabilitation therapy was performed by a multidisciplinary critical care team. The first stage of rehabilitation performed in patients with Richmond Agitation Sedation Scale (RASS) score ≤ − 2 consisted of positioning or range of motion exercises. In patients whose condition stabilized, rehabilitation proceeded to the second stage consisting of sitting on the edge of the bed, standing, transferring to a chair, and active muscle training until discharge from the ICU.
Electrical muscle stimulation
EMS therapy was incorporated into the rehabilitation program in all patients in the EMS therapy group once they had progressed beyond the initial very acute phase after discontinuing neuromuscular blockade. Patients with skin lesions, cardiac pacemakers, infection or trauma of the extremities, those who were unable to walk before hospital admission, and those who could not speak Japanese were excluded from the EMS therapy group. EMS was applied to the bilateral upper and lower limb muscles (biceps brachii, quadriceps femoris, and gastrocnemius muscles: middle of the upper arm and approximately 2 cm above the cubital fossa for biceps brachii, approximately 5 cm below the inguinal fold and 3 cm above the upper patella border for the quadriceps femoris, and approximately 3 cm below the popliteal fossa and immediately above the proximal end of the Achilles tendon for the gastrocnemius muscles) with a stimulator (Solius; Minato Medical Science, Osaka, Japan) using self-adhesive surface electrodes (40 × 80 mm). The EMS intervention included as part of the standard rehabilitation therapy for patients with respiratory or circulatory failure and postoperative patients in the ICU in our institution was reported previously 20 , 21 , 22 . We applied EMS with a variable-frequency train that began with high-frequency bursts (200 Hz), followed by low-frequency stimulation (20 Hz), and EMS was applied as a symmetrical biphasic square wave with 0.4-s pulses of direct current followed by a 0.6-s pause. Pulse groups consisting of 10 impulse trains were delivered to unilateral muscle groups at 10-s intervals during the session, and the output current was adjusted to ensure visible muscle contraction. EMS was applied by trained physiotherapists for 30 min per day, 6 days per week, for up to 2 weeks until the discharge from the ICU. We set the discontinuation criteria during the EMS session as follows: (1) change in systolic blood pressure > ± 20 mmHg; (2) increase in heart rate > + 20 beats/min; (3) development of sustained ventricular arrhythmia, atrial fibrillation, and paroxysmal supraventricular tachycardia; (4) decrease in blood oxygen saturation > − 4%.
Data collection
The Coronavirus Clinical Characterisation Consortium Mortality Score was calculated for each patient on admission to the ICU 23 . The worst Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores, both of which were also calculated within 24 h after ICU admission, were used in the analyses. The clinical frailty scale was used to assess the degree of frailty prior to ICU admission, with scores ranging from 1 (very fit) to 9 (terminally ill) 24 .
Physical function and clinical outcomes
Physical function was evaluated in each patient at the time of discharge from the ICU. Muscle strength was determined based on the Medical Research Council (MRC) sum score, which assesses the strength of each muscle group in the upper and lower limbs with scores for each muscle group ranging from 0 to 5 and higher scores indicating greater muscle strength (total score range: 0 = worst to 60 = best, minimal clinically important difference 4 points) 3 , 25 ; MRC sum score < 48 points was taken as the definition of muscle weakness 26 . Handgrip strength was also measured to assess muscle strength with the patient performing two maximal isometric voluntary contractions of each hand for 3 s with the elbow joint fixed at 90° flexion in the supine position using a Jamar dynamometer set to the second handle position (DHD-1 Digital Hand Dynamometer; Saehan Corporation, Seoul, South Korea). The greatest strength expressed as an absolute value (kg) was used in the analyses. The grip and release test and foot tapping test, involving measurement of the number of times the patient could flex and stretch the fingers of each hand in 10 s and tap the sole of each foot in 10 s while keeping the heel in contact with the floor and with the knees at 90° flexion, were performed with the patient in the supine position to evaluate upper and lower peripheral extremity motor function, respectively 27 , 28 . The analyses were performed using the highest scores obtained for both grip and release test and foot tapping test.
Clinical outcomes, including length of stay in the ICU, unplanned readmission to the ICU, and the location of hospital discharge (i.e., home or to another department/institution/ward/facility), were included in the analysis. At ICU discharge, we calculated the ICU mobility scale score for each patient determined on an 11-point ordinal scale ranging from 0 (lying/passive exercises in bed) to 10 (independent ambulation). The time taken to first mobilization (defined as ICU mobility scale score ≥ 3, i.e., sitting on the edge of the bed or higher) was assessed 29 .
Statistical analysis
Continuous variables are expressed as the median and interquartile range (IQR), and categorical variables are expressed as numbers and percentages. Differences between groups were evaluated by the Mann–Whitney U test for continuous variables and Fisher’s exact test for dichotomous variables. The primary outcome was MRC sum score at ICU discharge.
Statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY) and R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria). In all analyses, a two-tailed P < 0.05 was taken to indicate statistical significance.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Nagoya University Hospital, and was performed in accordance with the tenets of the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Informed patient consent was obtained, and the patients agreed to reveal their facial photos for academic purposes. All participants were informed that they were free to opt out of participation in the study at any time.
During the study period from March 2021 to April 2022, 110 consecutive critically ill patients with laboratory-confirmed COVID-19 were admitted to the ICU of Nagoya University Hospital. The final analysis was performed using data from 16 patients in the EMS therapy group and 16 age-matched historical controls with a median age of 71 years (81.2% male) (Fig. 1 ). There were no significant differences in baseline clinical characteristics between the two groups, except in vaccination status, SOFA score, and APACHE II score (Table 1 ).
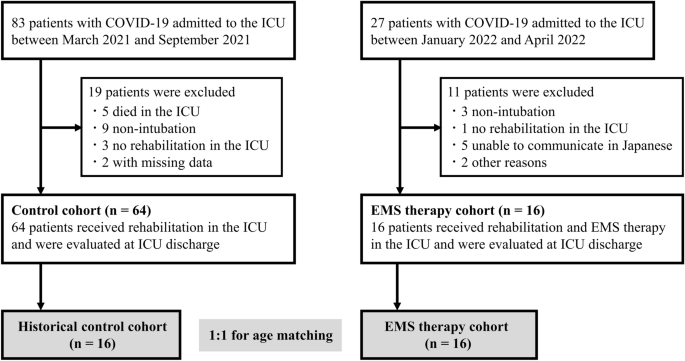
Flow diagram for inclusion of patients in the study. COVID-19, coronavirus disease 2019; EMS, electrical muscle stimulation; ICU, intensive care unit.
In the EMS therapy group, EMS therapy was initiated 3.2 ± 1.4 days after ICU admission, and patients completed a mean of 6.2 ± 3.7 EMS sessions (median, 5 sessions; total, 99 sessions) (Fig. 2 ). Five patients completed the 2-weeks of EMS intervention before ICU discharge. Two patients dropped out because they complained of muscle discomfort induced by EMS. Thus, the completion rate of the planned sessions until ICU discharge was 87.5%. EMS was applied to the biceps brachii, quadriceps femoris, and the gastrocnemius muscles at intensities of 30 ± 10 milliampere peak (mAp), 51 ± 9 mAp, and 37 ± 9 mAp, respectively. Patients with severe COVID-19, including those on extracorporeal membrane oxygenation (ECMO) support or placement in the prone position, received EMS therapy (Fig. S1 ). No alterations in vital signs (heart/respiratory rate, blood pressure, and blood oxygen saturation) or adverse events occurred during EMS. There were no cases of hospital-acquired SARS-CoV-2 infection among the medical staff during the study period.
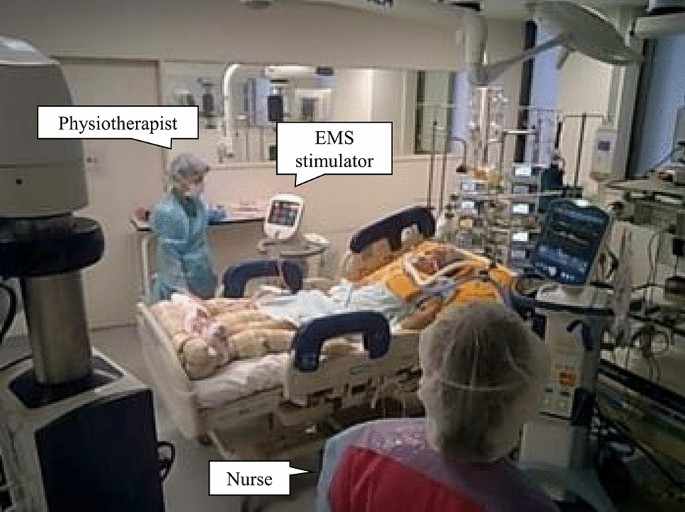
Electrical muscle stimulation for patients with severe COVID-19. COVID-19, coronavirus disease 2019; EMS, electrical muscle stimulation. This figure was provided after informed consent and permission were received from the patient.
There was no significant difference in median MRC sum score at discharge from the ICU between the EMS therapy group and historical controls (51 points [IQR 42–55] vs. 53 points [IQR 46–59], respectively; P = 0.439). Physical function at ICU discharge, including rates of MRC sum score < 48 points (31% vs. 25%, respectively; P = 0.680) and handgrip strength (7.3 kg [IQR 4.2–15.1] vs. 11.6 kg [IQR 8.4–15.9], respectively; P = 0.239), showed no significant differences between the two groups (Table 2 ). There were no significant differences in clinical outcomes, including number of days taken to first mobilization, number of ventilator-free days, length of stay in the ICU, ICU mobility scale at ICU discharge, and rate of discharge home between the two groups (Table 2 ).
This study showed that EMS therapy of the muscles of the upper and lower extremities added to early rehabilitation compared with early rehabilitation alone in patients admitted to the ICU due to severe COVID-19 with respiratory failure, and did not result in improved global muscle strength as assessed by the MRC score at ICU discharge, and was not associated with any adverse events. There were also no significant differences in important clinical outcomes, such as the number of ventilator-free days and ICU mobility scale at ICU discharge, between the EMS therapy group and age-matched historical controls.
Consistent with previous studies in critically ill patients, EMS was initiated a mean of 3.2 ± 1.4 days after ICU admission for COVID-19 patients with IMV, ECMO, and/or placement in the prone position, and was accompanied by neither effects on vital signs nor adverse events in the present study 13 , suggesting that acute-phase intensive EMS therapy is safe for use in critically ill COVID-19 patients admitted to the ICU. However, our findings were inconsistent with a previous meta-analysis indicating that EMS reduces ICU-acquired weakness and increases muscle strength during ICU admission 13 . As these previous studies did not discuss administration of EMS to patients with COVID-19, it was not possible to perform direct comparisons of the effects of EMS with the present study.
There have been few studies of the effects of EMS therapy in patients with COVID-19. In a previous RCT, application of EMS to the gastrocnemius muscles for up to 14 days was accompanied only by improvements in lower extremity muscle condition, e.g., ankle muscle strength and endurance, in critically ill patients with COVID-19 admitted to the ICU 30 . In another study, application of EMS to the quadriceps femoris muscles for 7 consecutive days only increased muscle strength assessed according to the MRC score and function in patients with severe COVID-19 during ICU admission, although they did not include a control group for comparison 31 . The results of the present study indicated that the application of EMS to the biceps brachii, quadriceps femoris, and gastrocnemius muscles for up to 2 weeks (median 5 days) was not accompanied by a decrease in occurrence of ICU-acquired weakness (i.e., MRC score < 48 points) and improved physical function and mobility at discharge from the ICU in patients with COVID-19 requiring IMV. Early additional muscle exercise may not improve muscle function in the most fragile patients with severe inflammation-induced muscle protein breakdown 25 . As a previous RCT suggested that the application of EMS for 7 days was required to prevent muscle atrophy and weakness in critically ill patients 32 , the duration of treatment in the present study may not have been sufficient to observe improvements in the outcomes of our patients. The effects of EMS therapy on physical function may have been attenuated by the mobilization program in the present study as we compared the effects of early rehabilitation with addition of EMS to early rehabilitation alone and more than 75% of our patients could sit on the edge of the bed or better before discharge from the ICU. Moreover, as more than 70% of our patients had MRC score ≥ 48 points at discharge from the ICU and the highest possible score is 60 points, this suggests that a ceiling effect 33 may have prevented detection of differences between groups. Further studies are required to determine the optimal frequency and duration of EMS therapy and the most suitable method for physical assessment to improve clinical outcomes in patients with severe COVID-19 requiring ICU admission.
This study had several limitations, the most important of which was the small sample size, which may have been underpowered for detection of some of the clinical characteristics and outcomes. In addition, this was not a RCT but compared data from patients before and after the introduction of EMS therapy in our hospital. Because of several advances in the treatment of critically ill patients that affect the type of treatment used, it may be risky to compare data with historical controls. Further RCTs are required to determine the effects of EMS. In addition, the optimal EMS configurations and parameters for patients with severe COVID-19 remain to be determined. As we measured physical function only at discharge from the ICU, the effects of EMS may have been influenced by physical function before admission. Therefore, it was considered necessary to perform an initial assessment upon awakening to observe differences between the two time points. We did not assess oedema, blood flow, and basal metabolic rate. In addition, we did not measure muscle mass or examine mental health, which may be important considerations in patients with severe COVID-19. Finally, this was a single-center study in a population of Asian patients, thus limiting the generalizability of our findings to other populations. However, the single-center setting ensured that similar sedation and ventilator weaning protocols were applied in both groups, and so may also be seen as a strength of this study.
Conclusions
The results of the present study indicated the safety of EMS therapy in critically ill patients with COVID-19 in the ICU setting, but adding EMS of the upper and lower muscles to a standardized early rehabilitation program did not improve either physical function or clinical outcomes at discharge from the ICU in patients with COVID-19 requiring IMV.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Coronavirus disease 2019
Severe acute respiratory syndrome coronavirus 2
Invasive mechanical ventilation
Intensive care unit
Randomized controlled trial
Acute Physiology and Chronic Health Evaluation II
Sequential Organ Failure Assessment
Medical Research Council
Interquartile range
Lim, Z. J. et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am. J. Respir. Crit. Care Med. 203 , 54–66. https://doi.org/10.1164/rccm.202006-2405OC (2021).
Article CAS PubMed PubMed Central Google Scholar
Botta, M. et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): A national, multicentre, observational cohort study. Lancet Respir. Med. 9 , 139–148. https://doi.org/10.1016/s2213-2600(20)30459-8 (2021).
Article CAS PubMed Google Scholar
Hermans, G. & Van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 19 , 274. https://doi.org/10.1186/s13054-015-0993-7 (2015).
Article PubMed PubMed Central Google Scholar
McWilliams, D. et al. Rehabilitation levels in patients with COVID-19 admitted to intensive care requiring invasive ventilation. An observational study. Ann. Am. Thorac. Soc. 18 , 122–129. https://doi.org/10.1513/AnnalsATS.202005-560OC (2021).
Yamamoto, H. et al. Physical function and mental health trajectories in COVID-19 patients following invasive mechanical ventilation: A prospective observational study. Sci. Rep. 13 , 14529. https://doi.org/10.1038/s41598-023-41684-3 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar
van Gassel, R. J. J. et al. Functional outcomes and their association with physical performance in mechanically ventilated coronavirus disease 2019 survivors at 3 months following hospital discharge: A cohort study. Crit. Care Med. 49 , 1726–1738. https://doi.org/10.1097/ccm.0000000000005089 (2021).
Elaraby, A., Shahein, M., Bekhet, A. H., Perrin, P. B. & Gorgey, A. S. The COVID-19 pandemic impacts all domains of quality of life in Egyptians with spinal cord injury: A retrospective longitudinal study. Spinal Cord 60 , 757–762. https://doi.org/10.1038/s41393-022-00775-0 (2022).
Thomas, P. et al. Physiotherapy management for COVID-19 in the acute hospital setting: Clinical practice recommendations. J. Physiother. 66 , 73–82. https://doi.org/10.1016/j.jphys.2020.03.011 (2020).
Stutz, M. R. et al. Early rehabilitation feasibility in a COVID-19 ICU. Chest 160 , 2146–2148. https://doi.org/10.1016/j.chest.2021.05.059 (2021).
Dubb, R. et al. Barriers and strategies for early mobilization of patients in intensive care units. Ann. Am. Thorac. Soc. 13 , 724–730. https://doi.org/10.1513/AnnalsATS.201509-586CME (2016).
Article PubMed Google Scholar
Hodgson, C. L., Capell, E. & Tipping, C. J. Early mobilization of patients in intensive care: Organization, communication and safety factors that influence translation into clinical practice. Crit. Care 22 , 77. https://doi.org/10.1186/s13054-018-1998-9 (2018).
Renner, C. et al. Guideline on multimodal rehabilitation for patients with post-intensive care syndrome. Crit. Care 27 , 301. https://doi.org/10.1186/s13054-023-04569-5 (2023).
Nakanishi, N. et al. Effect of neuromuscular electrical stimulation in patients with critical illness: An updated systematic review and meta-analysis of randomized controlled trials. Crit. Care Med. 51 , 1386–1396. https://doi.org/10.1097/ccm.0000000000005941 (2023).
Dolbow, D. R., Gorgey, A. S., Johnston, T. E. & Bersch, I. Electrical stimulation exercise for people with spinal cord injury: A healthcare provider perspective. J. Clin. Med. 12 , 3150. https://doi.org/10.3390/jcm12093150 (2023).
Bekhet, A. H. et al. Effects of electrical stimulation training on body composition parameters after spinal cord injury: A systematic review. Arch. Phys. Med. Rehabil. 103 , 1168–1178. https://doi.org/10.1016/j.apmr.2021.09.004 (2022).
Burgess, L. C. et al. Effect of neuromuscular electrical stimulation on the recovery of people with COVID-19 admitted to the intensive care unit: A narrative review. J. Rehabil. Med. 53 , jrm00164. https://doi.org/10.2340/16501977-2805 (2021).
Nakamura, K., Nakano, H., Naraba, H., Mochizuki, M. & Hashimoto, H. Early rehabilitation with dedicated use of belt-type electrical muscle stimulation for severe COVID-19 patients. Crit. Care 24 , 342. https://doi.org/10.1186/s13054-020-03080-5 (2020).
Kasugai, D. et al. Usefulness of respiratory mechanics and laboratory parameter trends as markers of early treatment success in mechanically ventilated severe coronavirus disease: A single-center pilot study. J. Clin. Med. 10 , 11. https://doi.org/10.3390/jcm10112513 (2021).
Article CAS Google Scholar
Marra, A., Ely, E. W., Pandharipande, P. P. & Patel, M. B. The ABCDEF bundle in critical care. Crit. Care Clin. 33 , 225–243. https://doi.org/10.1016/j.ccc.2016.12.005 (2017).
Nakanishi, N., Takashima, T. & Oto, J. Muscle atrophy in critically ill patients: A review of its cause, evaluation, and prevention. J. Med. Invest. 67 , 1–10. https://doi.org/10.2152/jmi.67.1 (2020).
Iwatsu, K. et al. Neuromuscular electrical stimulation may attenuate muscle proteolysis after cardiovascular surgery: A preliminary study. J. Thorac. Cardiovasc. Surg. 153 , 373–379. https://doi.org/10.1016/j.jtcvs.2016.09.036 (2017).
Shimizu, M. et al. Cardiac rehabilitation in severe heart failure patients with impella 5.0 support via the subclavian artery approach prior to left ventricular assist device implantation. J. Pers. Med. 13 , 630. https://doi.org/10.3390/jpm13040630 (2023).
Knight, S. R. et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 370 , m3339. https://doi.org/10.1136/bmj.m3339 (2020).
Rockwood, K. et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 173 , 489–495. https://doi.org/10.1503/cmaj.050051 (2005).
Fossat, G. et al. Effect of in-bed leg cycling and electrical stimulation of the quadriceps on global muscle strength in critically ill adults: A randomized clinical trial. Jama 320 , 368–378. https://doi.org/10.1001/jama.2018.9592 (2018).
Schefold, J. C. et al. Muscular weakness and muscle wasting in the critically ill. J. Cachexia Sarcopenia Muscle 11 , 1399–1412. https://doi.org/10.1002/jcsm.12620 (2020).
Ono, K. et al. Myelopathy hand new clinical signs of cervical cord damage. J. Bone Joint Surg. Br. 69 , 215–219. https://doi.org/10.1302/0301-620x.69b2.3818752 (1987).
Numasawa, T. et al. Simple foot tapping test as a quantitative objective assessment of cervical myelopathy. Spine (Phila Pa 1976) 37 , 108–113. https://doi.org/10.1097/BRS.0b013e31821041f8 (2012).
Hodgson, C. et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung 43 , 19–24. https://doi.org/10.1016/j.hrtlng.2013.11.003 (2014).
Zulbaran-Rojas, A. et al. Safety and efficacy of electrical stimulation for lower-extremity muscle weakness in intensive care unit 2019 Novel Coronavirus patients: A phase I double-blinded randomized controlled trial. Front. Med. (Lausanne) 9 , 1017371. https://doi.org/10.3389/fmed.2022.1017371 (2022).
Righetti, R. F. et al. Neuromuscular electrical stimulation in patients with severe COVID-19 associated with sepsis and septic shock. Front. Med. (Lausanne) 9 , 751636. https://doi.org/10.3389/fmed.2022.751636 (2022).
Silva, P. E. et al. Neuromuscular electrical stimulation in critically ill traumatic brain injury patients attenuates muscle atrophy, neurophysiological disorders, and weakness: A randomized controlled trial. J. Intensive Care 7 , 59. https://doi.org/10.1186/s40560-019-0417-x (2019).
Terwee, C. B. et al. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 60 , 34–42. https://doi.org/10.1016/j.jclinepi.2006.03.012 (2007).
Download references
Acknowledgements
They thank all the Nagoya University Hospital for their support.
This work was supported by the Research funding from the Hori Science and Arts Foundation, and the Japan Society for the Promotion of Science Grant-in-Aid (JSPS KAKENHI, Grant No. 20K19375).
Author information
These authors contributed equally: Yohei Tsuchikawa and Shinya Tanaka.
Authors and Affiliations
Department of Rehabilitation, Nagoya University Hospital, Nagoya, Japan
Yohei Tsuchikawa, Shinya Tanaka, Riko Nakagawa, Takayuki Inoue, Motoki Nagaya & Yoshihiro Nishida
Department of Emergency and Critical Care Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan
Daisuke Kasugai, Michiko Higashi, Takanori Yamamoto, Naruhiro Jingushi & Atsushi Numaguchi
Department of Rehabilitation, Mie University Hospital, Tsu, Japan
Miho Shimizu
Department of Rehabilitation, Juko Osu Hospital, Nagoya, Japan
Takafumi Nasu
Department of Respiratory Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan
Norihito Omote
Department of Orthopaedic Surgery, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya, 466-8560, Japan
Yoshihiro Nishida
You can also search for this author in PubMed Google Scholar
Contributions
Y.T. and S.T. contributed equally to this work. Y.T., S.T., and D.K. contributed to the conception or design of the work. R.N., M.S., T.I., M.N., T.N., N.O., M.H., T.Y., and N.J. contributed to the acquisition, analysis, or interpretation of data for the work. Y.T. and S.T. drafted the manuscript. A.N., Y.G., and Y.N. critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Corresponding author
Correspondence to Yoshihiro Nishida .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary figure s1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Tsuchikawa, Y., Tanaka, S., Kasugai, D. et al. Effects of acute phase intensive electrical muscle stimulation in COVID-19 patients requiring invasive mechanical ventilation: an observational case-control study. Sci Rep 14 , 5254 (2024). https://doi.org/10.1038/s41598-024-55969-8
Download citation
Received : 29 October 2023
Accepted : 29 February 2024
Published : 04 March 2024
DOI : https://doi.org/10.1038/s41598-024-55969-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neuromuscular electrical stimulation
- Physical performance
- Intensive care unit acquired weakness
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.

Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Modifying the Medical Research Council grading system through Rasch analyses
Collaborators.
- PeriNomS Study Group : A A Barreira , D Bennett , P Y K van den Bergh , V Bril , G Devigili , R D Hadden , A F Hahn , H-P Hartung , R A C Hughes , I Illa , H Katzberg , A J van der Kooi , J-M Léger , R A Lewis , M P T Lunn , O J M Nascimento , E Nobile-Orazio , L Padua , J Pouget , M M Reilly , I van Schaik , B Smith , M de Visser , D Walk
Affiliation
- 1 Spaarne Hospital Hoofddorp, Spaarnepoort 1, Hoofddorp, The Netherlands.
- PMID: 22189568
- PMCID: PMC3338921
- DOI: 10.1093/brain/awr318
The Medical Research Council grading system has served through decades for the evaluation of muscle strength and has been recognized as a cardinal feature of daily neurological, rehabilitation and general medicine examination of patients, despite being respectfully criticized due to the unequal width of its response options. No study has systematically examined, through modern psychometric approach, whether physicians are able to properly use the Medical Research Council grades. The objectives of this study were: (i) to investigate physicians' ability to discriminate among the Medical Research Council categories in patients with different neuromuscular disorders and with various degrees of weakness through thresholds examination using Rasch analysis as a modern psychometric method; (ii) to examine possible factors influencing physicians' ability to apply the Medical Research Council categories through differential item function analyses; and (iii) to examine whether the widely used Medical Research Council 12 muscles sum score in patients with Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy would meet Rasch model's expectations. A total of 1065 patients were included from nine cohorts with the following diseases: Guillain-Barré syndrome (n = 480); myotonic dystrophy type-1 (n = 169); chronic inflammatory demyelinating polyradiculoneuropathy (n = 139); limb-girdle muscular dystrophy (n = 105); multifocal motor neuropathy (n = 102); Pompe's disease (n = 62) and monoclonal gammopathy of undetermined related polyneuropathy (n = 8). Medical Research Council data of 72 muscles were collected. Rasch analyses were performed on Medical Research Council data for each cohort separately and after pooling data at the muscle level to increase category frequencies, and on the Medical Research Council sum score in patients with Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. Disordered thresholds were demonstrated in 74-79% of the muscles examined, indicating physicians' inability to discriminate between most Medical Research Council categories. Factors such as physicians' experience or illness type did not influence these findings. Thresholds were restored after rescoring the Medical Research Council grades from six to four options (0, paralysis; 1, severe weakness; 2, slight weakness; 3, normal strength). The Medical Research Council sum score acceptably fulfilled Rasch model expectations after rescoring the response options and creating subsets to resolve local dependency and item bias on diagnosis. In conclusion, a modified, Rasch-built four response category Medical Research Council grading system is proposed, resolving clinicians' inability to differentiate among its original response categories and improving clinical applicability. A modified Medical Research Council sum score at the interval level is presented and is recommended for future studies in Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy.
- Biomedical Research*
- Child, Preschool
- Health Planning Councils / standards*
- Health Planning Councils / statistics & numerical data
- Infant, Newborn
- Middle Aged
- Muscle Strength / physiology*
- Muscular Diseases / classification
- Muscular Diseases / diagnosis*
- Muscular Diseases / epidemiology
- Muscular Diseases / physiopathology*
- Young Adult
Grants and funding
- G0601943/MRC_/Medical Research Council/United Kingdom
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Acute Crit Care
- v.34(1); 2019 Feb
Pulmonary and Physical Rehabilitation in Critically Ill Patients
Myung hun jang.
1 Department of Rehabilitation Medicine, Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
Myung-Jun Shin
2 Department of Rehabilitation Medicine, Pusan National University School of Medicine, Busan, Korea
Yong Beom Shin
Some patients admitted to the intensive care unit (ICU) because of an acute illness, complicated surgery, or multiple traumas develop muscle weakness affecting the limbs and respiratory muscles during acute care in the ICU. This condition is referred to as ICU-acquired weakness (ICUAW), and can be evoked by critical illness polyneuropathy (CIP), critical illness myopathy (CIM), or critical illness polyneuromyopathy (CIPNM). ICUAW is diagnosed using the Medical Research Council (MRC) sum score based on bedside manual muscle testing in cooperative patients. The MRC sum score is the sum of the strengths of the 12 regions on both sides of the upper and lower limbs. ICUAW is diagnosed when the MRC score is less than 48 points. However, some patients require electrodiagnostic studies, such as a nerve conduction study, electromyography, and direct muscle stimulation, to differentiate between CIP and CIM. Pulmonary rehabilitation in the ICU can be divided into modalities intended to remove retained airway secretions and exercise therapies intended to improve respiratory function. Physical rehabilitation, including early mobilization, positioning, and limb exercises, attenuates the weakness that occurs during critical care. To perform mobilization in mechanically ventilated patients, pretreatment by removing secretions is necessary. It is also important to increase the strength of respiratory muscles and to perform lung recruitment to improve mobilization in patients who are weaned from the ventilator. For these reasons, pulmonary rehabilitation is important in addition to physical therapy. Early recognition of CIP, CIM, and CIPNM and early rehabilitation in the ICU might improve patients’ functional recovery and outcomes.
INTRODUCTION
In recent years, awareness has grown regarding the importance and necessity of intensive care unit (ICU) rehabilitation, both in Korea and worldwide, and accumulating evidence has shown that ICU rehabilitation has a positive impact on patients’ prognosis, quality of life, and return to normal life. Moreover, efficient ICU rehabilitation is facilitated by multidisciplinary evaluations and the establishment of systematic rehabilitation teams. As interest in ICU rehabilitation has emerged in Korea, it has been actively implemented in some leading hospitals, but the wider expansion of ICU rehabilitation to other hospitals has been hindered by a lack of awareness of its necessity, as well as concrete issues such as medical fees, facility limitations, and staffing [ 1 ]. Thus, we conducted a literature review regarding ICU rehabilitation to assess the initial approach to ICU patients and methods of evaluating them from the perspective of rehabilitation medicine, as well as the effectiveness and practical applications of ICU rehabilitation.
ICU-ACQUIRED WEAKNESS
The occurrence of generalized muscle weakness, including weakness of the limb and respiratory muscles, during ICU admission with no causes other than acute illness is defined as ICU-acquired weakness (ICUAW) [ 2 ]. The pathogenesis of ICUAW is complex, with functional and structural involvement of the muscles and nerves. Critical illness myopathy (CIM), critical illness polyneuropathy (CIP), or both (critical illness polyneuromyopathy [CIPNM]) during the course of critical illness are the most common causes of neuromuscular weakness in ICUAW, which is frequently implicated in reduced physical function due to quadriplegia and failure to wean from the ventilator [ 3 ]. Although the onset of isolated CIP is still controversial, the incidence of CIM and CIPNM has been reported to be 25%–83%, depending on the underlying critical illness [ 4 , 5 ].
Risk Factors
Sepsis, systemic inflammatory response syndrome, and multiple organ failure are important risk factors for the development of ICUAW [ 6 ]. Hyperglycemia is an independent risk factor for ICUAW; thus, controlling glycemia through intensive insulin therapy and early mobilization can decrease ICUAW, which is also effective for reducing the duration of mechanical ventilation [ 7 , 8 ]. Because long-term immobilization and mechanical ventilation can cause severe limb muscle atrophy, it is necessary to reduce the duration of immobilization by ensuring early mobilization [ 6 ]. The effects of using corticosteroids and neuromuscular blocking agents in critically ill patients with ICUAW are controversial, and they are expected to affect complex pathways in ways that depend on factors such as dose, timing, glycemic control, and neuromuscular complications [ 2 , 9 ]. Age is an independent risk factor for ICUAW, and premorbid physiological muscle reserve may play an important role [ 2 ]. Decreased skeletal muscle mass at the time of ICU admission is another important risk factor for mortality and complications, and this factor merits particular consideration in elderly patients [ 10 , 11 ]. Because ICU admission occurs unexpectedly, functional assessment is limited, so the likelihood of ICUAW can be predicted by sarcopenia evaluation in the early stages of treatment. Images obtained from computed tomography performed at the time of admission can be referred to; alternatively, ultrasonography can be used to evaluate muscle mass at the early stage of hospitalization or to monitor muscle loss that worsens during the treatment [ 12 , 13 ].
Critical Features and Diagnosis
In patients with CIP, distal axonal sensory-motor polyneuropathy develops in the limbs and respiratory muscles, while the facial muscles are preserved. The involvement of the limbs is symmetrical on both sides, and CIP is characterized by more severe weakness in the lower limbs and more severe distal weakness than proximal weakness, accompanied by sensory abnormalities [ 6 ]. In patients with CIM, proximal weakness is more severe than distal weakness, sensory abnormalities are preserved, muscle atrophy depends on the duration of illness, and facial weakness occurs rarely. In both conditions, muscle stretch reflexes are preserved in the early stage, but diminish as weakness progresses [ 3 ]. The diagnosis of ICUAW is confirmed via clinical features, and predisposing factors based on a physical examination and causes other than critical illness should be excluded. Performing an accurate physical examination is difficult, and in patients showing slow recovery after severe muscle weakness, an electrodiagnostic evaluation can be considered [ 6 ].
Manual muscle testing
A clinical diagnosis of ICUAW can be made through a bedside evaluation of muscle strength. The Medical Research Council (MRC) scale is used for manual muscle testing. It assesses the strength of the muscle groups of the upper and lower limbs, and an MRC sum score of less than 48 out of 60 points indicates an ICUAW diagnosis [ 4 ]. However, the MRC scale has the disadvantages of poor discrimination and a ceiling effect, and it is limited in that it does not distinguish between CIP and CIM. Among other methods of muscle testing, the use of handheld dynamometry and handgrip dynamometry can be considered ( Figure 1 ). Both methods are good to excellent in terms of interrater reliability and must be applied in patients who are at least grade 3 on the MRC scale [ 14 , 15 ]. However, there is a lack of evidence regarding the diagnostic criteria of ICUAW, and more research results are needed. When evaluating critically ill patients, the cooperation, adequacy, and motivation of the patient influence the accuracy of the results, and due to restrictions in the ICU environment itself, there are limitations in accurate muscle testing. To overcome such disadvantages, strictly standardized methods should be used. Prior to a muscle strength evaluation, patients whose volitional muscle strength can be accurately evaluated should be selected based on their level of cooperation (i.e., the standardized 5 questions [S5Q] scale or the confusion assessment method for the intensive care unit) ( Figure 2 ). In addition, it is necessary to ensure that the patient remains in a consistent position, including limb positions, joint angles, and hand position of the tester, and it is also necessary to standardize the contraction time, number of repetitions, and rest periods between tests [ 14 ]. Respiratory muscle strength can also be evaluated in patients with ICUAW. Maximum inspiratory pressure can be used to assess inspiratory muscle strength, and the unidirectional valve method can be used for an accurate assessment, from which the possibility of success of weaning can be predicted [ 16 ].

Assessment of muscle strength with a handgrip dynamometer (A) and handheld dynamometry (B).

Sample sheet used for muscle strength testing through the Medical Research Council (MRC) sum score and the standardized 5 questions (S5Q) at Pusan National University Hospital. For values that are difficult to assess due to peripheral or central nervous lesions, amputation, or orthopedic reasons, the values of the same muscle group on the opposite side or in proximity on the ipsilateral side are extrapolated. EP: extrapolation.
In addition to direct measurements of muscle strength, ultrasound can be used to assess muscle thickness and ultrasonic echogenicity, thereby serving as a straightforward method to detect changes of muscle mass in critically ill patients [ 17 ]. Although ultrasound has limitations for diagnosing ICUAW [ 18 ], a close correlation was shown between ICU patient function and changes in the thickness of limb muscle and echogenicity; thus, ultrasound is an important and convenient tool that can be used to monitor muscle conditions during critical care [ 19 ]. Lean body mass can be easily predicted and assessed using bioelectrical impedance analysis (BIA). The phase angle (PA) values computed using current (R) and reactance (X c ), which can be directly measured, are less affected by the hydration status of the patient than other BIA parameters [ 20 ]. The PA value signifies general cellular health and can substitute for lean body mass [ 21 ]; since low PA values are closely correlated with high mortality, PA can be used as a prognostic marker in critically ill patients. In BIA, weight is an important factor, but as there are limitations in accurately measuring patients’ weight in the ICU, caution should be used when interpreting the results.
Electrodiagnostic studies
An electrodiagnostic evaluation, such as a nerve conduction study (NCS) or electromyography is necessary to diagnose CIP and CIM, and direct muscle stimulation can be additionally used to distinguish between CIP and CIM [ 22 ]. In NCSs, nerve conduction velocity is mostly normal, while the amplitude of compound muscle action potentials (CMAPs) is reduced. In patients with CIP, sensory nerve action potentials (SNAPs) are also reduced, but the test results of ICU patients who have edema should be carefully interpreted ( Table 1 ) [ 2 ]. Because there are restrictions regarding the time and cost required to perform full electrophysiological testing in the ICU environment, the amplitudes of peroneal nerve CMAPs and sural nerve SNAPs should be selectively tested by a screening test, and additional tests can be considered in case of abnormal findings [ 23 , 24 ].
Electrophysiological findings of ICUAW
ICUAW: intensive care unit-acquired weakness; CMAP: compound muscle action potential; LLN: lower limit of normal; SNAP: sensory nerve action potential; MUAP: motor-unit action potential.
EARLY REHABILITATION IN THE ICU
Feasibility and safety.
Due to immobilization throughout the course of critical illness, neuromuscular weakness and impairment of physical function often occur. Early rehabilitation has been proven to be safe, feasible, and important; in fact, it can be safely performed without adverse events, even when the patient uses a mechanical ventilator and undergoes continuous renal replacement therapy, extracorporeal membrane oxygenation, or femoral catheterization [ 25 , 26 ]. In addition, passive and active range of motion (ROM) exercises in neuro-ICU patients with normal or elevated intracranial pressure can be safely performed without affecting the intracranial pressure [ 27 ]. Although removal of the endotracheal tube, feeding tube, or chest tube; hemodynamic instability such as hypotension, hypertension, or desaturation; and falling can occur during rehabilitation, these events are preventable with careful patient monitoring and skilled staff who are capable of evaluating physiological changes during rehabilitation [ 28 , 29 ]. Prerehabilitation screening criteria to identify patients for whom rehabilitation is suitable and criteria for discontinuing rehabilitation should be established for each ICU ( Table 2 ) [ 30 ]. Furthermore, because the culture of the ICU acts as a barrier at most centers, awareness of the importance of early mobilization and rehabilitation and a structured multidisciplinary effort based on smooth communication are necessary [ 1 ].
Examples of safety criteria for starting and stopping rehabilitation in the intensive care unit
SpO 2 : peripheral capillary oxygen saturation; FiO 2 : fraction of inspired oxygen; PEEP: positive expiratory end pressure.
Efficacy and Benefits
Evidence for the effects of active mobilization and rehabilitation in the ICU continues to be accumulated. It is known that ICU rehabilitation has a positive effect on patients’ prognosis, as assessed by improvements in functional status such as exercise capacity, muscle strength, and walking ability at discharge, as well as reductions in the duration of mechanical ventilation, length of the ICU stay, and length of hospital stay [ 31 - 34 ]. Furthermore, conducting very early mobilization in acute stroke patients in the neuro-ICU resulted in a rapid recovery of walking ability [ 35 ]. However, the results of previous studies are not fully consistent, as various factors affect mortality and length of hospital stay and rehabilitation requirements vary across studies. Therefore, there is a continuing need for studies to determine the effects of rehabilitation based on the disease and ICU status of patients.
PULMONARY REHABILITATION
The aims of pulmonary rehabilitation in the ICU are to recover voluntary respiration of the patient by clearing airway secretions, reducing the work of breathing, improving respiratory function, and enhancing inflation of the lungs [ 36 ]. In addition, in patients with chest trauma, normalization of abnormal chest wall movements and restoration of the ventilatory capacity of collapsed lungs help with proper sputum removal and prevent additional pulmonary complications. Finally, the purpose of pulmonary rehabilitation is to restore the patient’s normal lung function ( Table 3 ) [ 37 , 38 ].
Goals of pulmonary rehabilitation in the intensive care unit
Exercises for Respiratory Muscle Function
Deep breathing exercises and incentive spirometry
Deep breathing exercises introduce air into the lungs using negative pressure generated by the patient’s diaphragm instead of the accessory respiratory muscles. This can restore atelectasis, improve oxygenation and lung recruitment, increase functional residual capacity and tidal volume, and potentially help clear secretions [ 38 ]. Incentive spirometry (IS) has been widely used to prevent pulmonary complications and to improve lung function in nonambulatory surgical patients. Deep breathing exercises can more effectively induce maximal inspiration when patients receive visual feedback (inspired flow or volume) through IS [ 39 ]. Generally, patients perform five to 10 repetitions of a sequence of performing deep breathing slowly, holding the breath for 2 to 3 seconds, and then exhaling slowly. If sputum needs to be released, it is spit out by coughing at the end of the session ( Figure 3A ).

Incentive spirometry (A) and threshold inspiratory muscle training (B) for intensive care patients.
Respiratory muscle training
Weakness or fatigue of respiratory muscles and the diaphragm is an important factor in patients who fail to be weaned from mechanical ventilation. Fatigue can occur when the load of the inspiratory muscles is excessively increased due to the increase of airway resistance and decrease of lung compliance, or when there is an imbalance between respiratory muscles [ 40 ]. Additionally, prolonged ventilation itself promotes atrophy of the diaphragm and leads to its functional decrease [ 41 ]. Inspiratory muscle training can improve both inspiratory and expiratory muscle strength and reduce the duration of ventilation and weaning. However, additional research is needed to confirm the effects of training on clinical outcomes, and specific guidelines for how each training method can be used to improve strength and endurance are also necessary [ 42 ]. Threshold loading is the most general method used to determine training intensity. The threshold can be determined based on the maximum respiratory pressure (MIP) measured using the ventilator or a respiratory pressure meter. Threshold loading can be set between 20% and 50% of MIP, and generally, five sets with 6–10 breaths per set are performed once or twice a day. The threshold can be gradually increased as the patient’s inspiratory muscle strength improves ( Figure 3B ) [ 43 ].
Management of Airway Secretions
Positioning and mobilization
Positioning and mobilization enhance ventilation and ventilation/perfusion (V/Q) mismatch, thereby assisting in oxygenation [ 36 ]. In patients with unilateral lung disease, positioning the affected lung at the uppermost location increases recruitment and promotes drainage from the lung segment, so lung function and atelectasis can be improved [ 44 ]. Moreover, in ventilated patients, the functional residual capacity and oxygenation improve and work is reduced in a seated position of greater than 30° [ 45 ] because the displacement of the rib cage increases, resulting in a positive effect on minute ventilation, respiratory rate, tidal volume, and inspiratory flow rate [ 38 ]. Together with other chest physiotherapy modalities, early mobilization can result in a decreased extubation failure rate and shorter durations of mechanical ventilation and ICU stay [ 46 , 47 ] ( Figure 4 ). Further details on mobilization are discussed below.

Treatment modalities for airway secretions. RFC: residual functional capacity; HFO: high frequency oscillation; IPV: intrapulmonary percussive ventilation; CPAP: continuous positive airway pressure; EPAP: expiratory positive airway pressure; PEP: positive expiratory pressure; NIV: noninvasive ventilation; MIE: mechanical insufflation-exsufflation.
Hyperinflation
The aim of lung hyperinflation is to prevent pulmonary collapse or re-expansion of collapsed alveoli by improving gas exchange through promoting secretion removal and increasing lung compliance. The cardiovascular stability of the patient must be ensured before treatment because of the large tidal volume and increased intrathoracic pressure inherent to hyperinflation. The specific protocol in each ICU varies, but the basic method is to induce expiratory flow after sufficient inspiration and 2–3 seconds of inspiratory hold. To reduce the risk of barotrauma, it is necessary to use a manometer connected to a circuit. Methods include manual hyperinflation (MHI) and ventilator hyperinflation; the sputum removal effect of both methods is known to be similar, but MHI is advantageous in that the physiotherapist receives feedback through the resuscitator bag to estimate lung compliance [ 38 , 48 ].
Mechanical insufflation-exsufflation
Mechanical insufflation-exsufflation (MIE) is a commonly used method to remove excessive sputum from patients with neuromuscular abnormalities and is applicable to patients who do not effectively expectorate sputum due to an impaired cough. Similar to the general cough principle, the lung is inflated to a large volume with positive pressure. Subsequently, a negative pressure is quickly applied to induce sputum removal. In addition to sputum removal, MIE has the advantages of maintaining lung compliance, respiratory muscle length, and thoracic rib cage mobility. Manual techniques such as assisted cough or thoracoabdominal thrusts may be applied by the therapist along with MIE to facilitate sputum release. The use of MIE improves the likelihood of extubation in patients requiring tracheostomy and reduces the length of the postextubation ICU stay. In addition, when MIE is used in patients in whom a noninvasive ventilator (NIV) fails, it has the advantages of easier NIV adaptation and enhancement of its effects [ 49 , 50 ]. The use of MIE requires caution in patients with an undrained pneumothorax, major cardiovascular instability, or emphysematous bullae, as well as in patients with head trauma, as it may affect intracranial pressure or cerebral perfusion pressure [ 51 ].
Percussion and vibrations
In percussion, cupped hands or the palm cup is used to manually clap the affected lung and perform postural drainage. This allows shifting of secretions from the peripheral airway to the central airway, enhancing airway clearance. Vibrations can be performed manually or using mechanical devices, and conducting chest oscillation and compression together during the expiratory phase can improve peak expiratory flow rates by more than 50% ( Figure 5A ) [ 52 ].

Postural drainage with high-frequency oscillation (A) and the Acapella device (B) for secretion removal.
Oscillatory positive-expiratory-pressure devices
Flutter and Acapella (Smiths Medical, Carlsbad, CA, USA) devices combine positive expiratory pressure therapy and highfrequency oscillations in the airway. By generating oscillations with sustained expiratory pressure, they reduce airway collapse and improve mucus discharge, enhancing lung function and oxygenation [ 53 ]. When using the Flutter device, the breath is held for 2–3 seconds after deep inspiration, and oscillations are then induced by performing slow expiration through the Flutter valve. In general, three sets of 10–15 exhalations are performed over 12–20 minutes, and after completing each set, the “huff” cough maneuver is performed to promote sputum removal [ 54 ]. Depending on the patient’s condition, this can be performed three to four times a day. The Acapella device is characterized by using a mask or mouthpiece as the interface and is applied with a nebulizer ( Figure 5B ).
Intrapulmonary percussive ventilation
Intrapulmonary percussive ventilation (IPV) allows simultaneous positive pressure, high-frequency oscillations, and aerosol delivery. IPV can reduce the work of breathing by increasing sputum removal and lung expansion. IPV can be effectively and safely applied in patients with cystic fibrosis, bronchiectasis, chronic obstructive pulmonary disease exacerbation, and acute respiratory failure, as well as in tracheostomized patients [ 55 ]. Generally, the treatment time is short (less than 15 minutes), and IPV can be repeated several times a day. Similar to MIE, cardiac output can decrease due to an increase of intrathoracic pressure, and adverse effects such as barotrauma and volutrauma should be considered.
Chest Physiotherapy in Chest Trauma Patients
In patients with chest trauma such as rib fractures and pulmonary contusions, the effort of inspiration increases and maximal cough strength decreases, thereby reducing lung volume and promoting atelectasis. In addition, the increased immobility period and the medications prescribed due to trauma depress the respiratory drive and hinder the recovery of lung function. For such reasons, the risk of pulmonary complications after trauma is especially high [ 37 ].
IS has not been found to have a positive effect on preventing pulmonary complications or shortening the length of hospital stay, but the incidence of acute respiratory failure may be high if the vital capacity measured by IS at the time of admission is low [ 56 , 57 ]. The use of IS starting with the initial stage of trauma allows monitoring of the patient’s lung volume changes, and a sudden decrease in inspiratory volume during treatment suggests the possibility of atelectasis, pneumonia, or other pulmonary complications. The active cycle of breathing technique (ACBT) is a breathing method that helps air clearance by assisting lung ventilation and prevents infection; ACBT involves breathing control and a forced expiration technique that utilizes thoracic expansion, deep breathing, and huffing. The addition of ACBT to conventional pulmonary rehabilitation showed a significant effect on pain reduction after chest trauma [ 58 ].
PHYSICAL ACTIVITY AND EARLY MOBILIZATION
Physical activity and exercise in the ICU should be of appropriate intensity and type according to the patient’s condition. Thus, accurate evaluation of the patient’s cooperation level, muscle strength, joint mobility, functional status, and cardiopulmonary reserve should precede exercises, and the rehabilitation goal should be determined based on this assessment [ 59 ]. Guidelines are not defined for evaluation tools or goals, and the appropriate activity stage is determined by stepping up or down according to the guidelines of each hospital or ICU [ 60 ].
Uncooperative Patient
Positioning
Basic positioning (i.e., lateral rotational therapy) can be performed to prevent soft tissue contracture, joint contracture, peripheral nerve compression, and pressure ulcerations. The upright position can be used to increase lung volume and further improve gas exchange, although caution is required to prevent adverse effects to the cardiopulmonary system. To safely change the position of sedated patients or heavy care patients, lift use can be considered [ 59 ]. Although there is a lack of evidence for the interval of position change, it is generally performed at 2- to 4-hour intervals, which can help reduce the incidence of pulmonary complications such as nosocomial pneumonia and atelectasis [ 61 ].
Passive ROM and stretching
Passive ROM or stretching exercises are an important treatment method to maintain the ROM and soft tissue length in patients incapable of voluntary movement. By using a continuous passive motion device, it is possible to better prevent contracture and preserve the architecture of muscle fibers [ 62 ]. Moreover, patients with severe burns, trauma, and those with central nerve damage are at a high risk for soft tissue contracture, so an additional orthosis, such as an ankle-foot orthosis, can be used to prevent joint contracture and reduce muscle tone [ 36 ].
Cycle ergometer
With technological advancements, passive cycle ergometers can also be used in sedated, immobile, and bedridden patients, which can help maintain ROM. Even after the patient recovers consciousness, active-assisted and active-resistive modes can be used to perform muscle strengthening exercises in the lower limbs, and the bedside ergometer can be used to improve quadriceps force and increase exercise capacity after ICU discharge ( Figure 6 ) [ 32 ].

Cycle ergometer for active and passive cycling in the intensive care unit.
Neuromuscular electrical stimulation
Few treatment options exist for preventing the progression of muscle atrophy during prolonged immobilization periods. Neuromuscular electrical stimulation (NMES) is advantageous because it can be applied irrespective of the patient’s level of interaction or posture. The use of NMES promotes muscular microcirculation, delays muscle atrophy during the immobility period, and improves muscle strength and endurance [ 63 , 64 ]. NMES can be applied together with bed leg cycling, but the functional results of patients after discharge remains controversial [ 65 ]. In addition, it is necessary to establish a consensus for appropriate protocols to determine the optimal current, contraction characteristics, and targeted muscle.
Tilt table treatment
A tilt table provides partial weight bearing, which can assist in the gradual transition from bed rest to bearing the full body weight [ 29 ]. Although the evidence level for tilt table intervention is not high, it can help improve lower limb strength, prevent ankle joint contracture, and enhance patient arousal. In the beginning, only tilting without an arm support can provide effective exercise [ 66 , 67 ].
Cooperative Patient
Mobilization
If the patient’s response to external stimulation is appropriate and cooperation is good (i.e., S5Q ≥3), then transitioning from passive exercise to active exercise can be attempted [ 68 ]. Mobilization generally includes sitting on the edge of the bed, moving from the bed to the chair, standing next to the bed, walking on the spot, and walking with or without ambulatory assisting devices. Particularly, during the standing and walking stages, caution should be taken to prevent monitoring lines, catheters, or the urinary bag from disconnecting [ 59 ]. Early and protocol-based mobilization is important for functional recovery and shortening the length of hospital stay, but insufficient evidence still exists for neuro-ICU patients [ 69 ].
Aerobic exercises and resistive muscle training
In addition to the mobilization techniques discussed above, aerobic exercises and strengthening exercises can be included. Endurance training using a bed cycle ergometer is common, and in patients with limited movement due to multiple fractures, such as lower limb fractures, an upper body ergometer can be used ( Figure 7A ). Resistive muscle training increases muscle mass and force generation. For an exercise effect, three sets of eight to ten repetitions with an intensity of 50%–70% of one repetition maximum within the patient’s tolerance are performed [ 36 ]. For resistive training, exercise tools such as elastic bands and pulleys can be used to perform exercises in bed ( Figure 7B , ,C) C ) [ 59 ]. When using tools such as the Borg Rate of Perceived Exertion, the patient’s perceived fatigue can be assessed before, during, and after exercise to monitor the patient’s exercise intensity [ 68 ].

(A) Upper-body aerobic exercise with fitness equipment. (B, C) Resistive training of upper extremities and bridging exercise using elastic band.
Critically Ill Trauma Patients
Early mobilization in trauma patients has many limitations due to specific factors related to injuries and their treatment, and less evidence exists for functional outcomes. However, since the risks of prolonged immobilization and bed rest are evident, the effects of early mobilization may be enhanced when polytrauma patients are assessed jointly with the involved trauma specialists to ensure safe exercise [ 70 ]. In addition, early goal-directed mobilization in trauma patients improves mobilization during hospitalization, reduces the length of the ICU stay, and assists in enhancing functional mobility after discharge [ 71 ].
CONCLUSIONS
The safety, feasibility, and treatment effects of ICU rehabilitation in critically ill patients are well proven. The most important factor in ICU rehabilitation is to increase therapeutic effects through a multidisciplinary approach in which the treatment goals are planned and shared. The rehabilitation team should consist of an ICU physiatrist, physiotherapist, respiratory therapist, and occupational therapist. Additionally, the physiatrist should lead the team by focusing on the patient’s functional evaluation and rehabilitation plan. To make ICU rehabilitation more widespread in Korea, it is necessary to address practical issues, such as the absence of an ICU rehabilitation fee and the lack of medical staff.
KEY MESSAGES
▪ Critically ill patients experiencing weakness or difficulty weaning from the ventilator in the intensive care unit (ICU) need to be evaluated for ICU-acquired weakness (ICUAW).
▪ Early pulmonary and physical rehabilitation prevents ICUAW and ensures better outcomes for critically ill patients.
▪ A multidisciplinary approach is important, as is the role of the ICU physiatrist during ICU rehabilitation.
Acknowledgments
This work was supported by clinical research grant from Pusan National University Hospital in 2019, Busan, Korea.
The patients provided written informed consent for the publication and the use of their images.
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: MJS. Data curation: MHJ. Formal analysis: YBS. Funding acquisition: MJS. Methodology: YBS. Project administration: YBS. Visualization: MHJ. Writing - original draft: MHJ. Writing - review & editing: MHJ.

IMAGES
VIDEO
COMMENTS
Medical Research Council (MRC)-sumscore evaluates global muscle strength. Manual strength of six muscle groups (shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension, and ankle dorsiflexion) is evaluated on both sides using MRC scale. Summation of scores gives MRC-sumscore, ranging from 0 to 60.
A measure of global peripheral muscle strength, the Medical Research Council sum score (MRC-SS), which ranges from 0 (complete paralysis) to 60 (normal strength) , has been widely used, with scores less than 48 providing the basis for diagnosing ICU-AW . As with all volitional measures of muscle strength, however, a patient's inability to ...
To examine interobserver agreement, two observers independently measured Medical Research Council (MRC) sum-score (n = 75) and handgrip strength (n = 46) in a cross-sectional ICU sample.
The median MRC sum scores for each observer were 55 (IQR, 49 to 58) and 56 (IQR, 50 to 58). The continuous outcome of the MRC sum score differed by 10% or more between observers for 7 (23%) of the 30 patients. The intraclass correlation coefficient of the sum score was 0.83 (95% CI, 0.67 to 0.93).
Clinical evaluation of muscle strength. Current guidelines recommend a clinical diagnosis of ICUAW, made by bedside evaluation of the muscle strength with the use of the Medical Research Council (MRC) sum score .
A diagnosis of ICUAW is achieved by manually testing the muscle strength using the Medical Research Council (MRC) scale or by measuring handgrip strength using a dynamometer. MRC muscle strength is assessed in 12 muscle groups (Figure 2): a summed score below 48/60 designates ICUAW or significant weakness, and an MRC score below 36/48 indicates ...
The Medical Research Council (MRC) muscle scale is a commonly used bedside measure ... from a sample of 391 participants enrolled in the parent study. 1 Twenty participants were enrolled in this substudy for muscle strength evaluation, including 15 male participants ... Although the MRC sum score (which incorporates strength scores of 6 muscle ...
Medical Research Council (MRC)-sumscore evaluates global muscle strength. Manual strength of six muscle groups (shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension, and ankle dorsiflexion) is evaluated on both sides using MRC scale. Summation of scores gives MRC-sumscore, ranging from 0 to 60. This score was ...
Manual muscle testing (MMT), an established and easily performed tool in clinical routine, typically measures muscle strength according to the six-point Medical Research Council (MRC) score.
For interventional studies, reliable measurements of muscle force in the intensive care unit (ICU) are needed. Methods: To examine interobserver agreement, two observers independently measured Medical Research Council (MRC) sum-score (n = 75) and handgrip strength (n = 46) in a cross-sectional ICU sample. Results: The intraclass correlation ...
Muscle strength was determined based on the Medical Research Council (MRC) sum score, which assesses the strength of each muscle group in the upper and lower limbs with scores for each muscle ...
The MRC grading system provides the following grades: 0, paralysis; 1, only a trace or flicker of muscle contraction is seen or felt; 2, muscle movement is possible with gravity eliminated; 3, muscle movement is possible against gravity; 4, muscle strength is reduced, but movement against resistance is possible and 5, normal strength.
4. Hermans G, Clerckx B, Vanhullebusch T, et al: Interobserver agreement of Medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle Nerve 2012; 45: 18-25 5. Baldwin CE, Paratz JD, Bersten AD: Muscle strength assessment in critically ill patients with
ate with muscle function [2]. Medical Research Council (MRC)-sumscore evaluates global muscle strength. Manual strength of six muscle groups (shoulder abduction, elbow flexion, wrist exten-sion, hip flexion, knee extension, and ankle dorsiflexion) is evaluated on both sides using MRC scale. Summation of scores gives MRC-sumscore, ranging from 0 ...
A previous study showed that the MRC-sum score is a reliable tool for evaluating sarcopenia in patients after stroke. 21 Manual strength of the six muscle groups (shoulder abductor, elbow flexor ...
Muscle strength testing is an important component of the physical exam that can reveal information about neurologic deficits. It is used to evaluate weakness and can be effective in differentiating true weakness from imbalance or poor endurance. It may be referred to as motor testing, muscle strength grading, manual muscle testing, or many other synonyms. The muscle strength evaluation may be ...
The Medical Research Council grading system has served through decades for the evaluation of muscle strength and has been recognized as a cardinal feature of daily neurological, rehabilitation and general medicine examination of patients, despite being respectfully criticized due to the unequal width of its response options.
Secondary outcomes include performance-based measures of muscle strength (Medical Research Council Sum Score) 34 35 and function (30 s sit-to-stand test, 36 2 min walk test, 37 ) each of which ...
It remains unknown whether variation of scores on the Medical Research Council (MRC) scale for muscle strength is associated with operator-independent techniques: dynamometry and surface electromyography (sEMG). This study aimed to evaluate whether the scores of the MRC strength scale are associated with instrumented measures of torque and muscle activity in post-stroke survivors with severe ...
Medical Research Council (MRC)-sumscore evaluates global muscle strength. Manual strength of six muscle groups (shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension, and ankle dorsiflexion) is evaluated on both sides using MRC scale. Summation of scores gives MRC-sumscore, ranging from 0 to 60.
Muscular strength should be tested using the validated Medical Research Council (MRC) scale [6,16] (Table 1). (b) In the comatose patient, neurological assessment considers level of arousal ...
A clinical diagnosis of ICUAW can be made through a bedside evaluation of muscle strength. The Medical Research Council (MRC) scale is used for manual muscle testing. It assesses the strength of the muscle groups of the upper and lower limbs, and an MRC sum score of less than 48 out of 60 points indicates an ICUAW diagnosis . However, the MRC ...